Introduction
Salzmann nodular corneal degeneration (SNCD) is a relatively rare, slowly progressive, non-inflammatory condition of the cornea that is often underrecognized and frequently misdiagnosed due to its subtle early manifestations and overlapping features with other corneal disorders. In 1925, Austrian ophthalmologist Maximilian Salzmann described a series of patients with bluish-white corneal nodules secondary to phlyctenular or trachomatous keratitis.[1] Although initially termed as Salzmann nodular corneal dystrophy, SNCD was later redefined as an indolent, degenerative condition. SNCD is characterised by the formation of fibrocellular outgrowth anterior to the basement membrane, resulting in elevated nodules most commonly involving the mid-periphery of the cornea.[2] The disease was initially thought to be a variant of corneal dystrophy associated with phlyctenular and trachomatous keratitis. However, as clinical and histopathological understanding evolved, the term Salzmann nodular dystrophy was refined to Salzmann nodular degeneration to better reflect its acquired, degenerative nature.
SNCD may be either primary or secondary. Primary Salzmann, the most common form, is typically not associated with any other ocular surface disease. However, these patients typically have coexisting meibomian gland dysfunction.[3] Infrequently, SNCD has been observed in cases with chronic ocular surface inflammation, such as vernal keratoconjunctivitis, trachoma, interstitial keratitis, and dry eyes, as well as in non-inflammatory conditions, including epithelial basement membrane dystrophy and chronic rigid contact lens use.[4]
SNCD typically occurs in the fifth to sixth decade of life, though it has been reported in individuals aged 4 to 90.[2][4] Although most cases are bilateral, it may present asymmetrically.[5] SNCD predominantly affects women and may affect the White population more often.[5] Spontaneous remission is unlikely, but symptoms typically improve with lubrication and topical anti-inflammatory therapy.[5][6][7][8]
Surgical options in refractory cases include manual excision, phototherapeutic keratectomy (PTK) with or without the use of topical mitomycin-C, and lamellar or penetrating keratoplasty.[5][6] Though many patients are asymptomatic, SNCD may cause significant morbidity and contribute to the degradation of vision.[3][5][7][9][10] The prognosis is generally favorable; however, prompt recognition and management of SNCD, along with its underlying etiologies, is critical, as no cases of spontaneous remission have been reported.[6]
SNCD is characterized by the development of discrete, elevated, bluish-white, superficial nodules, typically situated in the mid-peripheral or peripheral cornea. These nodules consist of collagenous fibrocellular material deposited anterior to Bowman's layer and the epithelial basement membrane. The overlying epithelium remains intact but may show irregularity or thinning. The condition is often bilateral but asymmetrical and may be either primary (idiopathic) or secondary to chronic ocular surface inflammation or trauma.[7]
To understand the pathogenesis of SNCD, it is essential to appreciate the anatomical structure of the cornea. The cornea is composed of 5 primary layers—the epithelium, Bowman's layer, stroma, Descemet's membrane, and endothelium. The epithelial basement membrane, situated beneath the epithelium, plays a crucial role in maintaining corneal homeostasis and facilitating repair. In SNCD, pathology predominantly occurs in the anterior cornea, with fibrocellular deposits accumulating between the epithelial basement membrane and Bowman's layer, or replacing Bowman's layer altogether.[11]
Histologically, the nodules in SNCD demonstrate replacement of Bowman's layer by extracellular matrix material, including collagen types I and III, and are often accompanied by activated keratocytes and myofibroblasts. This process reflects a reparative or reactive response to chronic epithelial stress, inflammation, or trauma, rather than a genetically determined dystrophy. This distinction is important, as SNCD is not inherited and does not follow a predictable pattern of progression typical of true dystrophies.[12]
SNCD can be broadly categorized into 2 types—primary (idiopathic) and secondary. In primary SNCD, there is no identifiable preceding ocular pathology. Primary SNCD typically presents in middle-aged women and may be associated with coexisting meibomian gland dysfunction or ocular surface dryness, although not directly caused by any specific ocular condition. This form is more common and typically arises without an apparent trigger. In contrast, secondary SNCD develops in the context of chronic ocular surface disease or corneal structural abnormalities. Several ocular conditions have been implicated in its pathogenesis, including vernal keratoconjunctivitis, trachoma, interstitial keratitis, and chronic blepharitis. Patients with severe dry eye disease or limbal stem cell deficiency are also at elevated risk. Other predisposing factors include recurrent epithelial erosions, a history of ocular trauma or surgery, and long-term contact lens wear, all of which contribute to chronic irritation and corneal remodeling. Additionally, epithelial basement membrane dystrophy has been associated with SNCD, likely due to persistent epithelial irregularity and defective wound healing responses. Understanding the classification and associated conditions of SNCD is essential for targeted management and prevention of recurrence.[13]
The pathophysiology likely involves chronic low-grade inflammation and recurrent epithelial microtrauma, leading to aberrant healing and subepithelial fibrosis. The exact molecular mechanisms are not yet fully understood, but they involve dysregulated wound healing, the activation of matrix metalloproteinases (MMPs), and the stimulation of fibroblasts. SNCD typically manifests in individuals aged 40 to 70, though cases have been reported in children as young as 4 and adults older than 90. SNCD shows a clear female predominance and is more frequently reported among the Caucasian population. The condition is bilateral in over 60% of cases, but it may present with highly asymmetrical features. Due to its often indolent course, many cases remain undiagnosed unless the nodules affect the visual axis or cause ocular discomfort.[14]
Patients with SNCD may remain asymptomatic, particularly in the early stages or when the nodules are located outside the visual axis, thus sparing the central vision. However, as the disease progresses or when the nodules encroach upon the optical zone, a variety of symptoms may develop. A common complaint is a persistent foreign body sensation, often caused by elevated nodules that disrupt the smooth corneal surface. Decreased visual acuity can result from direct obstruction of the visual axis or secondary to irregular corneal astigmatism induced by the nodules. Patients may also experience fluctuating vision, particularly in association with tear film instability. Photophobia and ocular irritation are frequently reported, especially when there is concomitant ocular surface inflammation. In some cases, blurred vision may be exacerbated by surface irregularities that alter the cornea's refractive contour. These symptoms collectively impact the patient's visual function and quality of life, emphasizing the importance of timely diagnosis and tailored management.[7]
The nodules are typically 1 to 3 mm in diameter, round to oval in shape, firm, and elevated. These nodules often appear bluish-white due to underlying stromal haze and are most frequently located in the midperipheral or superior cornea. In some cases, central involvement can significantly impair visual function. Diagnosis is primarily clinical, based on slit-lamp biomicroscopy. Nodules appear elevated, smooth, and opalescent. Adjacent corneal thinning or superficial vascularization may occasionally be observed. Corneal topography may show irregular astigmatism, and anterior segment optical coherence tomography (AS-OCT) can confirm subepithelial location, epithelial thinning, and loss of Bowman's layer. IVCM may reveal increased subepithelial reflectivity, activated keratocytes, and stromal scarring.[6]
The differential diagnosis of SNCD includes several ocular surface and corneal conditions that present with similar clinical features, particularly subepithelial corneal opacities or nodules. The presence of Salzmann-like nodules observed in interstitial keratitis is an important differential diagnosis, where chronic inflammation can lead to subepithelial fibrosis and nodule formation, mimicking SNCD. Band keratopathy, characterized by calcium deposition in the superficial cornea, may also present with gray-white opacities. However, these typically follow a horizontal interpalpebral distribution and have a distinct crystalline appearance. Epithelial basement membrane dystrophy, particularly when presenting with elevated map-dot-fingerprint patterns, can be mistaken for Salzmann nodules due to the irregularities on the anterior corneal surface. Pingueculae that extend onto the cornea can cause localized elevation and opacity, leading to diagnostic confusion, especially in the nasal interpalpebral region. Lastly, peripheral hypertrophic subepithelial corneal degeneration (PHSCD), a condition with peripheral subepithelial fibrosis and opacification, should be considered, particularly when nodules are located in the peripheral cornea. Accurate clinical assessment, aided by slit-lamp biomicroscopy and anterior segment imaging, is crucial for distinguishing these entities and ensuring appropriate management.[7]
SNCD is a slowly progressive condition, but it may remain stable for many years. Spontaneous regression is rare. Some patients may experience episodic symptom exacerbation due to concurrent ocular surface disease. Without treatment, nodules may enlarge and coalesce, contributing to more pronounced vision loss. However, many patients adapt well with minimal intervention. Symptom severity does not always correlate with nodule size. Even small lesions located centrally may significantly degrade vision due to irregular astigmatism or localized tear film disruption.[15]
Management strategies for SNCD are individualized and primarily depend on the severity of symptoms, the anatomical location of the nodules, and the presence of underlying or associated ocular surface disease. In mild cases, conservative treatment is often effective, typically involving regular use of lubricating eye drops to alleviate discomfort and maintain the stability of the tear film. Anti-inflammatory therapy, such as topical corticosteroids or cyclosporine, may be employed to control subclinical or overt inflammation. Additionally, addressing contributory conditions such as meibomian gland dysfunction or chronic blepharitis is critical in halting progression and preventing recurrence.[15]
In cases where conservative management fails or vision is significantly impaired due to central nodular encroachment or irregular astigmatism, surgical intervention becomes necessary. Superficial keratectomy involves the manual excision of nodules and is the first-line surgical approach in many patients. PTK, performed with an excimer laser, enables precise ablation and smoothing of the corneal surface, often yielding excellent visual outcomes. The adjunctive use of mitomycin-C may reduce recurrence by limiting fibroblastic activity. In advanced cases with deeper stromal involvement or a history of previous surgical failure, lamellar or penetrating keratoplasty may be required to restore visual function. Regardless of the chosen approach, long-term management of the ocular surface is crucial for minimizing the risk of recurrence and optimizing surgical outcomes.[16]
Postoperative recurrence is possible, especially in cases with ongoing ocular surface inflammation. Long-term lubrication and anti-inflammatory therapy may help reduce the risk of recurrence. Unlike infectious or neoplastic conditions, SNCD does not exhibit contiguous tissue spread. However, the disease can progress centripetally, especially in the presence of unaddressed risk factors. Nodules may increase in size or number, and new lesions may form in adjacent corneal areas.
The prognosis for SNCD is generally favorable. Vision can often be preserved or restored with timely and appropriate treatment—most patients who undergo superficial keratectomy or PTK experience significant symptomatic and visual improvement. However, recurrence rates vary and depend largely on the adequacy of management of coexisting ocular surface disease. SNCD is a clinically significant yet underrecognized degenerative corneal disorder. Although indolent, its impact on visual function and quality of life can be profound, particularly when lesions encroach on the visual axis or induce irregular astigmatism. Early recognition, accurate diagnosis, and a tailored approach to management are essential. Understanding the anatomical basis, natural course, and associated risk factors is key for clinicians to provide evidence-based, interprofessional care aimed at preventing vision loss and minimizing recurrence.[17]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
The most commonly cited risk factors for SNCD in ocular surface inflammatory conditions include dry eye, chronic blepharitis, meibomian gland dysfunction, phlyctenular keratoconjunctivitis, pterygium, trauma, and vernal keratoconjunctivitis.[1][2][4][5][6][5][7] Additionally, contact lens wear also appears to be a risk factor, accounting for 34.2% of 152 eyes in one study.[5] Mechanisms may involve a disruption of stem cell turnover, the reduced rate of exfoliation of the central corneal epithelium due to the physical barrier of the CL, and poor tear film stability over the cornea.[18][19][20]
Surgical wounds to the cornea, such as from cataract extraction, radial keratotomy, penetrating keratoplasty, or laser in situ keratomileusis (LASIK), may increase the risk of developing SNCD.[10][21][22][23] In a 2010 study involving 180 eyes with SNCD, 27.2% had a history of previous ocular surgery.[5] Post-LASIK SNCD may occur due to postoperative dry eye disease secondary to a disruption of corneal nerves, uneven corneal tissue in the area of the flap, or the mere presence of surgical trauma.[10][21][24][25][26][27][28] Often, these post-LASIK Salzmann nodules responded to conservative treatment alone; however, 2 published cases required superficial keratectomy.[26][28] SNCD in posterior keratoconus after a penetrating corneal injury has also been reported.[29]
SNCD has been reported in association with several systemic diseases, although a definitive causal relationship remains unproven. Notably, SNCD has been observed in patients with Crohn disease, dermatopathia pigmentosa reticularis, Kabuki syndrome, Kartagener syndrome, Ehlers-Danlos syndrome, thyroid eye disease, and Dandy-Walker syndrome.[13][30][31] Although no specific gene abnormalities have been identified in association with SNCD, several cases have been reported in which SNCD occurs in consecutive generations, with an autosomal dominant inheritance pattern in 2 cases.[18][32] A unique genetic link in SNCD, or possibly a missense mutation in the TGF-β gene, which is the genetic basis for other corneal dystrophies, may play a role.[32]
Table 1. Etiological Classification of Superficial Nodular Corneal Disease
|
Etiological Category |
Specific Causes/Associations |
|
Primary (idiopathic) |
|
|
Chronic inflammatory ocular conditions |
|
|
Tear film abnormalities |
|
|
Structural corneal abnormalities |
|
|
Iatrogenic/traumatic causes |
|
|
Contact lens-related causes |
|
|
Corneal dystrophies |
|
Epidemiology
SNCD typically occurs in the fifth to sixth decade of life, though it has been reported in individuals aged 4 to 90.[2][4] The largest study, which included 180 eyes, established a mean age of 60.8 years, with a range of 13 to 92 years.[5] A bimodal age distribution has also been reported, with the most affected individuals present in the fifth or eighth decades.[3] SNCD is more common in women, who accounted for 78% of the patients in Salzmann's original series, which has been reproduced in subsequent reports.[3][5][24][27] The female preponderance in SNCD has been linked to hormonal influences, as most affected women are middle-aged, whereas men tend to be older.[8][33] Studies have shown that SNCD occurs bilaterally more than 50% of the time, though severity may be asymmetric.[3][5][8][3]
SNCD is an uncommon, slowly progressive, non-inflammatory degenerative corneal condition. The true prevalence of SNCD is difficult to ascertain due to its indolent nature and frequently asymptomatic presentation, especially when the nodules are located away from the visual axis. Although comprehensive population-based data are limited, SNCD is increasingly recognized in routine clinical ophthalmic practice, particularly among patients with chronic ocular surface disorders and those undergoing refractive evaluation.[34]
SNCD is most commonly diagnosed in the fifth to sixth decades of life, although the reported age range spans from childhood (as young as 4) to older patients (up to 90). The degenerative changes are generally insidious and may take years to become clinically apparent. Pediatric cases are rare and typically associated with secondary forms due to predisposing inflammatory or traumatic causes. A consistent observation across multiple studies is the predominance of SNCD among females, with some literature suggesting a female-to-male ratio of approximately 2:1. The underlying reason for this sex disparity is not fully understood but may be related to hormonal influences, differences in meibomian gland function, and a higher prevalence of dry eye disease and autoimmune disorders among women.[35]
SNCD tends to be bilateral in most cases but is often asymmetrical in presentation. In unilateral cases, an identifiable predisposing factor such as trauma, surgery, or localized ocular surface disease is typically present. Nodules typically occur in the mid-peripheral or peripheral cornea, though they may extend centrally in advanced cases.[36] There is limited literature examining ethnic or racial predisposition to SNCD. However, some studies and case series suggest that White or Caucasian individuals may be disproportionately affected. Whether this is due to genetic predisposition, environmental exposures, or referral bias remains unclear and warrants further epidemiological investigation.[13]
Several ocular and systemic risk factors have been associated with an increased likelihood of developing SNCD:
- Ocular surface inflammation: Chronic conditions such as vernal keratoconjunctivitis, trachoma, and blepharitis are frequently reported in patients with secondary SNCD.
- Dry eye disease: A significant proportion of patients with SNCD exhibit signs of aqueous-deficient or evaporative dry eye, including meibomian gland dysfunction.
- Contact lens wear: Particularly long-term rigid gas-permeable contact lens users have shown an increased incidence, possibly due to mechanical microtrauma and reduced oxygen transmission.
- Previous ocular surgery or trauma: Individuals with a history of penetrating keratoplasty, radial keratotomy, or LASIK may develop secondary SNCD, often at sites of surgical incisions.
- Corneal dystrophies: Epithelial basement membrane dystrophy has been observed to coexist with SNCD in a subset of patients.[37]
With the increased use of high-resolution anterior segment imaging (eg, AS-OCT and confocal microscopy), ophthalmologists are now more adept at detecting subtle nodular changes even in early or atypical presentations. Additionally, the growth of corneal refractive surgery has contributed to heightened detection of SNCD during preoperative evaluations, as nodules may influence corneal curvature and refractive outcomes. SNCD is generally nonprogressive or slowly progressive. Many patients remain stable for years without significant visual disturbance. However, in the presence of ongoing ocular surface inflammation, nodules may enlarge, migrate toward the visual axis, and lead to visual impairment, necessitating surgical intervention. Although SNCD remains a relatively rare corneal degeneration, Increased awareness among clinicians and advancements in diagnostic modalities have led to more frequent diagnoses. The condition shows a female predominance, typically affects individuals in middle to late adulthood, and is often bilateral but asymmetric. Identification of underlying inflammatory or structural ocular pathologies is crucial in understanding the epidemiological profile of this disorder.[38]
Pathophysiology
The exact mechanism of SNCD development is unknown; however, multiple theories have been proposed. Histological studies have revealed hyaline plaques between the epithelium and Bowman's layer, resembling changes observed in degenerative pannus or old corneal scars, suggesting a potential role for chronic inflammation or immune processes. External irritation, trauma, or chronic exposure to sunlight and environmental irritants are thought to stimulate fibroblast overgrowth and hyaline degeneration. Additionally, prolonged epithelial remodeling and wound healing responses, marked by subepithelial fibrosis, MMP-2 activity, and disruption of Bowman's layer, are believed to contribute to nodule formation. Mechanical disruption of the barrier between the epithelium and stroma from trauma, surgery, or chronic corneal irritation from ocular surface diseases, such as meibomian gland disease or aqueous-deficient dry eye, leads to prolonged wound repair and remodeling of the epithelium, thus creating the conditions for epithelial cell differentiation and anterior shift of stromal fibroblasts, along with excess deposition of extracellular material.[7][8] Subsequent deposition of extracellular matrix by fibroblasts and other cells within the nodules follows. Moreover, ground substances on collagen fibers in the nodules are stimulated by chronic exposure to sunlight or environmental irritants.[2][23][39][23][40] Decreased PAX6 and DSG1 protein expression has also been found in the corneal epithelium of patients with SNCD.[41]
Wilson and Dupps reviewed atypical cases of SNCD that developed years after keratorefractive surgeries.[42] Their findings support a pathophysiological model in which damage to the epithelial basement membrane allows pro-fibrotic cytokines, such as transforming growth factor-beta, to penetrate the subepithelial space, stimulating the transformation of fibrocytes or corneal fibroblasts into myofibroblasts. These cells generate disorganized extracellular matrix, forming the characteristic subepithelial opacities of SNCD. When the injury involves deeper structures, such as Bowman's layer, as often occurs in LASIK, the fibrotic changes can extend into the anterior stroma, making treatment more complex and less amenable to conventional superficial keratectomy. The authors propose Salzmann's subepithelial fibrosis as a more accurate term for such presentations. SNCD is a non-inflammatory, degenerative corneal disorder characterized by the formation of bluish-white or grayish nodules anterior to the Bowman's layer. These nodules result from chronic degenerative changes in the corneal epithelium and subepithelial layers. The exact mechanisms underlying the formation of these nodules remain incompletely understood, but recent histopathological, immunohistochemical, and imaging studies have provided insight into the pathophysiological processes involved.[43]
Anatomical Basis
The cornea comprises 5 primary layers—epithelium, Bowman's layer, stroma, Descemet's membrane, and endothelium. In SNCD, the pathology is confined primarily to the anterior corneal stroma and subepithelial zone, often with disruption or complete absence of the Bowman's layer beneath the nodular lesions. The nodules are situated between the basal epithelial cells and the anterior stroma, and are composed of fibrocellular tissue with extracellular matrix deposition.[44]
Role of Chronic Irritation and Inflammation
The pathogenesis is closely linked to chronic ocular surface inflammation or mechanical irritation.
- In secondary SNCD, conditions such as vernal keratoconjunctivitis, chronic blepharitis, dry eye disease, and trachoma lead to a sustained low-grade inflammatory state. Such inflammation promotes the release of proinflammatory cytokines (eg, interleukin-1 and tumor necrosis factor-alpha) and MMPs that degrade the standard extracellular matrix and stimulate aberrant wound healing responses.
- In primary (idiopathic) SNCD, even in the absence of overt ocular inflammation, subtle chronic epithelial microtrauma from meibomian gland dysfunction or subclinical dry eye may activate similar pathways.[45]
Epithelial-Stromal Interaction and Fibrosis
In SNCD, repetitive epithelial injury or stress leads to dysregulated wound healing, characterized by the following:
- Loss of Bowman's layer integrity.
- Migration of basal epithelial cells into the subepithelial space.
- Activation of keratocytes in the anterior stroma, which transdifferentiate into myofibroblasts.
- The secretion of abnormal extracellular matrix proteins, including collagen types I, III, and V, as well as fibronectin, contributes to the formation of nodules.[46]
- Lack of normal basement membrane regeneration further perpetuates fibrosis.
This sequence results in fibrocellular nodules that gradually accumulate over time, often without significant vascularization or immune cell infiltration, reflecting the non-inflammatory but degenerative nature of the disease.
Histopathological Findings
Microscopic evaluation of excised nodules reveals the following features:
- Epithelial hyperplasia or thinning overlying the nodules.
- Subepithelial fibrous tissue containing disorganized collagen bundles.
- Absence or disruption of the Bowman's membrane.
- Presence of fibroblasts and myofibroblasts within the nodules.
- Occasional deposition of hyaline-like material, often Periodic acid-Schiff–positive but Congo red-negative (excluding amyloid), has been observed. These findings are consistent with a chronic reparative process rather than an acute inflammatory or dystrophic mechanism.[47]
Contributions of Contact Lens Wear and Surgery
- Long-term use of rigid gas-permeable contact lenses can cause microtrauma to the corneal surface, leading to subepithelial fibrosis and the formation of nodules.
- Surgical trauma, particularly from penetrating keratoplasty, radial keratotomy, or refractive procedures (eg, LASIK and PRK), may induce abnormal healing responses and contribute to the formation of secondary SNCD at the wound sites.[48]
Biomechanical Changes
The formation of elevated nodules distorts the regular corneal surface, particularly when nodules encroach upon the visual axis. This distortion can:
- Alter the tear film stability, contributing to symptoms of ocular surface irritation.
- Induce irregular astigmatism, degrading visual quality.
- Compromise corneal transparency, especially in advanced cases with stromal involvement.[49]
Natural Course and Progression
The nodules tend to be indolent and slowly progressive. However, in the absence of intervention, especially in cases with active ocular surface disease, the nodules may:
- Increase in number and size.
- Become more elevated.
- Migrate centrally and interfere with the optical zone.
There is no evidence of spontaneous regression; however, stabilization is common with appropriate treatment of the underlying causes.
SNCD is a fibrodegenerative corneal process initiated by chronic epithelial microtrauma and perpetuated by impaired epithelial-stromal interaction and abnormal wound healing. The histological hallmark includes subepithelial fibrocellular nodules with variable epithelial changes and disruption of Bowman's layer. Whether arising idiopathically or in association with ocular surface disease, the pathophysiology of SNCD reflects the cornea's maladaptive repair mechanism, making early recognition and management of the inciting factors crucial in preventing visual morbidity.[50]
Histopathology
Histological examination typically shows thinning of the epithelium overlying a nodule. Disruption of the Bowman's layer may be observed with duplication of the epithelial basement membrane.[7] Other findings include a decreased cell density and deposition of matrix material within the nodule, anterior stromal scarring, and an irregular epithelial-stromal interface. There are no pathognomonic histological features of SNCD, and the features that are present are also present in other pathologies, such as degenerative pannus or corneal scars secondary to inflammation or trauma, requiring appropriate clinical correlation.[7]
The mechanism underlying the development of SNCD and subepithelial matrix deposition appears to involve the function of the corneal epithelium itself. The cells of the limbus differ from the cells of the central cornea in structure and function. The limbal epithelium contains stem cells and transient amplifying cells, which are not completely differentiated but have a high potential for proliferation. Typically, the central cornea contains completely differentiated epithelium and should not contain stem cells. Immunohistochemical analysis has shown that the epithelium in SNCD shows similar markers to those of the undifferentiated transient amplifying cells in the limbus.[51]
High levels of staining for CK19 and enolase, markers of the metabolically more active migrating epithelium, suggest that the epithelium that overlies SNCD nodules is composed of cells that are not terminally differentiated and are more comparable to limbal transient amplifying cells than the surrounding corneal epithelium. Healthy central corneal cells stain strongly for CK3/12, a marker for terminally differentiated corneal epithelium. Only the superficial epithelial layers overlying Salzmann nodules stain positive for CK3/12. The cellular elements of the Salzmann nodules stain positively for matrix-metalloproteinase-2 (MMP-2), which is characteristically expressed in the central corneal epithelium only during the remodeling phase of wound repair.[10]
MMP-2 functions to break down type 4 collagen, the primary component of the corneal epithelial basement membrane. The expression of MMP-2 by the basal epithelial cells in Salzmann nodules appears to contribute to the destruction of the Bowman's layer in an ongoing wound remodeling process.[40] These histopathological findings support the clinical association of chronic ocular surface disease with SNCD.
Toxicokinetics
Toxicokinetics typically refers to the ADME (absorption, distribution, metabolism, and excretion) of toxic substances in the body. Although SNCD is fundamentally a degenerative and nontoxic condition, there are some relevant connections to consider when examining ocular surface exposure to topical or environmental agents that may contribute to or exacerbate the disease, particularly in secondary SNCD. In this context, the toxicokinetic framework applies primarily to exogenous agents, as outlined below.
Absorption
Topical ophthalmic agents, environmental toxins, and preservatives (eg, benzalkonium chloride in eye drops) can be absorbed across the corneal epithelium and into the stroma. This absorption may lead to several issues, including:
- Trigger epithelial injury.
- Disrupts tear film and corneal surface homeostasis.
- Promote chronic low-grade inflammation leading to fibrocellular proliferation.[52]
Distribution
Once absorbed, these agents may:
- Accumulate in the superficial corneal tissues.
- Alter corneal stromal cell behavior by modifying extracellular matrix production.
- Enhance local cytokine production or oxidative stress—factors implicated in SNCD pathogenesis.[53]
Metabolism
The cornea possesses limited enzymatic activity. Metabolism of exogenous substances occurs minimally within corneal and conjunctival cells. Persistent presence of unprocessed agents (eg, preservatives, contact lens solutions, and pollutants) may lead to several issues, including:
- Chronic irritation.
- Delayed epithelial repair.
- Promotion of abnormal healing cascades, as observed in SNCD.[54]
Excretion and Clearance
Tears and conjunctival vasculature play a role in clearing topical or environmental exposures. However, in dry eye disease or ocular surface dysfunction (often comorbid with SNCD), clearance may be impaired. Prolonged retention of irritants exacerbates epithelial-stromal interactions, fueling nodule development.
Although SNCD is not classically viewed through a toxicokinetic lens, chronic exposure to topical drugs, preservatives, environmental pollutants, or prolonged contact lens use may result in toxic effects on the ocular surface. These agents, through impaired clearance or cumulative damage, can disturb the epithelial and stromal integrity, setting the stage for the degenerative remodeling observed in SNCD. Understanding these pathways may aid in preventing disease progression, particularly in cases of secondary SNCD.[55]
History and Physical
Most patients with SNCD are asymptomatic. As the disease progresses, patients typically present with symptoms such as foreign body sensation, irritation, tearing, or photophobia.[3][5][8] In one study, decreased visual acuity was the most common presenting symptom, though acuity was typically not severely reduced.[5] In another study, the visual disturbance was the most common indication for surgery, necessitating 85% of all procedures.[3]
Nodule location influences patients' primary complaint, whereby more central nodules are associated with decreased or distorted vision and more peripheral nodules with foreign body sensation. Visual distortions are caused by the involvement of the visual axis by nodules, irregular astigmatism, hyperopia, or mechanical disturbances to the tear film.[9][10] Peripherally located nodules can cause flattening of the central cornea.[10] This flattening induces a hyperopic change, whereby the astigmatism in each quadrant involved increases by 0.38 D.[5]
Obtaining a detailed history and performing a thorough physical examination are essential in diagnosing SNCD and identifying contributing factors and complications. As SNCD is a slowly progressive degenerative disorder of the cornea, patients often remain asymptomatic for years until nodules encroach upon the visual axis or induce irregular astigmatism. Therefore, eliciting subtle signs and symptoms, as well as associated ocular conditions, becomes crucial during evaluation.[8]
Patient History
Symptom onset and progression: Patients may report gradual worsening of vision or ocular discomfort. In primary SNCD, the onset is insidious and often bilateral, with a slow course over years. Secondary SNCD may follow chronic inflammatory eye diseases or ocular trauma.[56]
Visual complaints: Symptoms vary depending on the size and location of nodules. Common complaints include the following:
- Blurred vision
- Fluctuating visual acuity
- Glare or halos, especially at night
- Ghost images (monocular diplopia)
- Photophobia [57]
Ocular surface symptoms: Patients commonly report the following complaints:
- Foreign body sensation
- Dryness and burning
- Redness
- Irritation [58]
Past ocular history: The following details should be elicited during history-taking:
- History of vernal or atopic keratoconjunctivitis
- Previous ocular surgery or trauma
- Chronic blepharitis or meibomian gland dysfunction
- Contact lens use (exceptionally rigid lenses)
- Recurrent corneal erosions or epithelial basement membrane dystrophy [59]
Systemic history: Although SNCD is primarily ocular, associations with autoimmune diseases or systemic inflammation (eg, Stevens-Johnson syndrome and ocular cicatricial pemphigoid) should be ruled out in atypical presentations.
Physical Examination
The ophthalmic examination typically reveals the following features:
- Elevated, whitish-blue nodules in the corneal mid-periphery
- Nodules located between Bowman's layer and the epithelium
- Clear intervening corneal stroma
- Flattening or indentation of the surrounding cornea
- Absence of inflammation [60]
Slit-lamp findings include the following:
- Nodules appear gray-white, translucent, or chalky
- Surface may be smooth or irregular
- Nodules may coalesce in advanced cases
- Fluorescein staining is typically absent unless epithelial breakdown occurs
Corneal topography shows localized irregular astigmatism with decreased corneal regularity index. This technique is particularly helpful in assessing visual axis involvement.
AS-OCT reveals elevated subepithelial masses with normal underlying stroma, aiding in the differentiation from other causes of corneal opacities.[61]
Table 2. Clinical Features of Salzmann Nodular Corneal Degeneration
|
Parameter |
Typical Findings |
|
Age of onset |
50-70 years (range: 4-90 years) |
|
Laterality |
Often bilateral; may be asymmetrical |
|
Gender predilection |
Female predominance |
|
Symptoms |
Blurred vision, photophobia, irritation, and foreign body sensation |
|
Nodule characteristics |
Elevated, whitish, and mid-peripheral; subepithelial |
|
Visual axis involvement |
May lead to decreased acuity and monocular diplopia |
|
Associated conditions |
Meibomian gland dysfunction, dry eye, vernal keratoconjunctivitis, and epithelial basement membrane dystrophy |
|
AS-OCT findings |
Subepithelial hyperreflective nodules and intact Bowman's layer |
|
Fluorescein staining |
Typically negative unless epithelial erosion |
Abbreviation: AS-OCT, anterior segment optical coherence tomography.
Evaluation
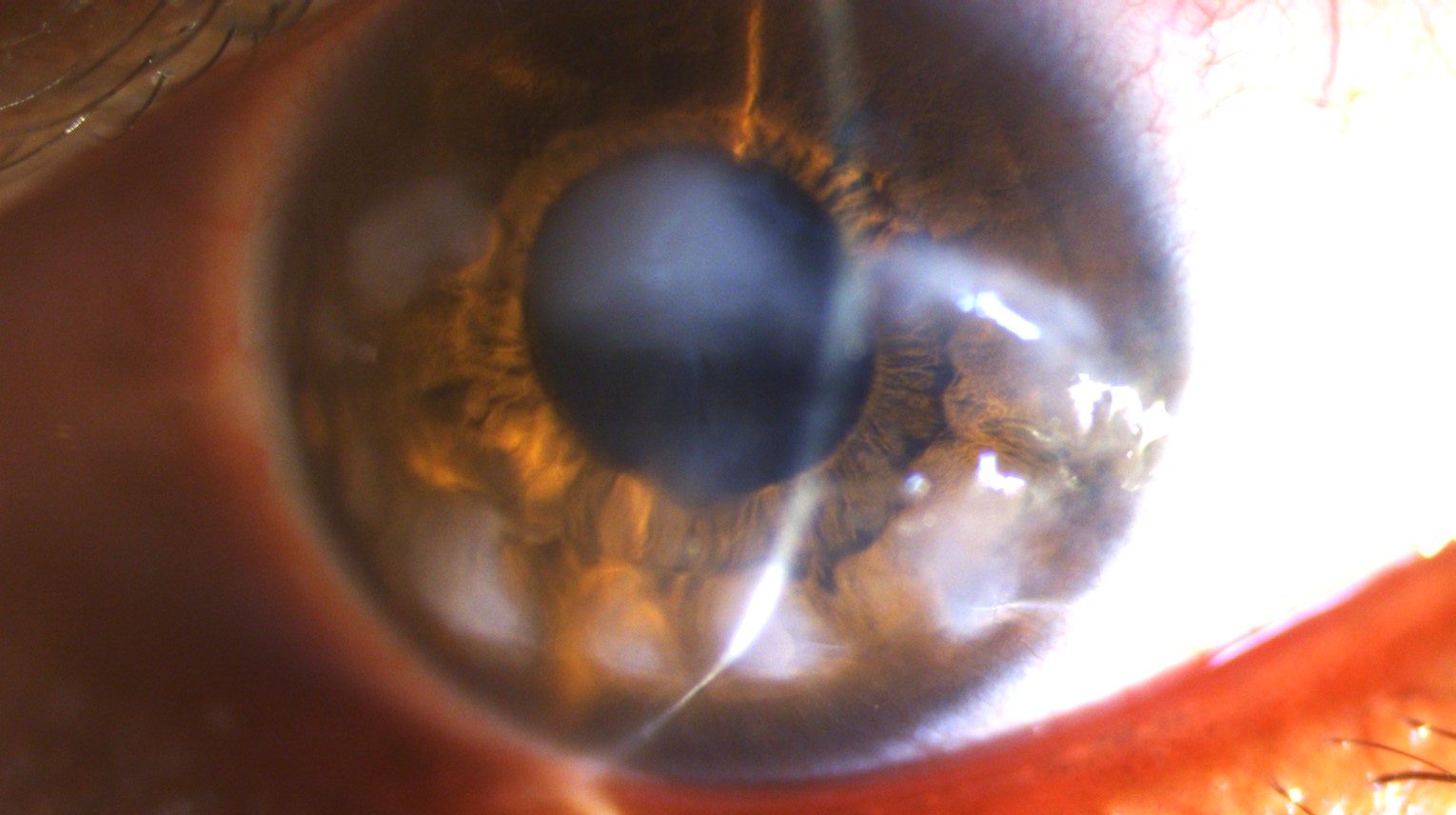
The diagnosis of SNCD is typically made clinically through a slit-lamp examination. Slit-lamp examination reveals nodules that are typically smaller than 2 mm but may also be as large as 4 × 2.5 mm.[6][62] The nodules may appear in any part of the cornea, and their location depends on the underlying risk factor. For example, nodules that develop post-LASIK are often located in the mid-periphery, overlying the flap interface.[10] See Image. Slit-Lamp Photograph of a Patient Suffering From Primary SNCD.
Another common pattern is the 3 and 9 o'clock location of the nodules in patients with a history of contact lens wear.[3] Nodules are bluish-white to gray and are typically round but may also be conical or prismatic.[6] Peripheral SNCD is more common than central.[8] Opacities are most often found in the superior-nasal quadrant, followed by the superior-temporal.[10] Nodules are typically discrete from one another but may fuse if they are adjacent to each other. Several cases of ring-like peripheral confluent SNCD have been reported.[5][63] Other findings include decreased tear breakup time, reduced tear lake, and negative fluorescein staining of the nodules.[10][64]
In one study, each nodule was associated with an adjacent neovascular anterior stromal pannus that did not enter the nodule.[10] In a series of 10 patients, a Bell or inverse Bell phenomenon was correlated with nodule location; patients with superior nodules were found to have an inverse Bell phenomenon and vice versa.[20]
Although most cases are accurately diagnosed through clinical examination, the diagnosis may be supported by ancillary investigations, such as high-frequency UBM, in vivo confocal microscopy (IVCM), AS-OCT, or corneal topography and tomography. UBM of peripheral nodules in a post-LASIK patient showed differentiated epithelium, Bowman's layer, and LASIK flap, localizing the nodules to within the margin of the flaps and revealing the destruction of the underlying Bowman's layer.[10] UBM demonstrated that the nodules were made up of an abnormally thin layer of epithelium overlying a hyperreflective substance.[10] Other findings include hyperechoic superficial corneal thickening that spares the stromal layer, as well as a well-defined demarcation line.
Biometric values obtained through corneal topography are influenced by the presence of SNCD, leading to inaccurate intraocular lens calculations, with errors as high as 3.2 D reported in a study.[65] These changes may be attributable to alterations in lamellar stromal anatomy, nodular elevation above the corneal surface, and tear film disruption.[65]
AS-OCT is a noninvasive imaging modality that can be helpful in the diagnosis, management, and follow-up of SNCD. AS-OCT shows bright, hyperreflective, subepithelial deposits above the Bowman's layer that are covered by an abnormally thin layer of epithelium. AS-OCT can perform noninvasive optical biopsies that have been shown to correlate with the histopathology of the disease.[63] This technique enables in vivo histological analysis of nodules in the early stages without requiring tissue sampling.[63]
Using IVCM allows morphological and quantitative analysis of the microstructure of Salzmann nodules. Normal epithelial cells imaged show a honeycomb-shaped arrangement with bright borders and dark bodies. The overlying epithelium nodules, as shown by IVCM, have an irregular morphology, consisting of elongated, polygonal cells of varying sizes.[66][67][68] More deeply, the keratocytes in the superficial stroma showed prominent, hyperreflective nuclei consistent with cells in a more activated state.[51]
The corneal stroma within the nodule appeared disorganized, with an increase in the hyperreflective extracellular background.[63] Analysis of the deeper stroma revealed very prominent nerves, highlighted by an unusual pattern of grouped keratocytes. The Descemet membrane and the endothelium were of normal appearance.[67][68]
A careful, stepwise evaluation is essential for confirming SNCD, gauging its functional impact, and distinguishing it from look-alike corneal pathologies. The assessment combines targeted history-taking, meticulous slit-lamp examination, and selective anterior-segment imaging.
Comprehensive Clinical Examination
- Visual acuity and refraction: Measure best-corrected distance and near acuity. Perform manifest and cycloplegic refractions to reveal irregular astigmatism or monocular diplopia caused by nodules encroaching on the visual axis.
- Slit-lamp biomicroscopy: Identify elevated, bluish-white or gray nodules lying anterior to Bowman's layer. Note their size (1-3 mm), location (typically mid-peripheral superior cornea), surface smoothness, and any overlying epithelial defects or punctate staining.
- Fluorescein staining and tear-film evaluation: Although the epithelium is generally intact, tear breakup time often decreases over nodular elevations, explaining fluctuating vision.
- Eyelid and meibomian gland assessment: Look for blepharitis, meibomian gland dysfunction, or Demodex infestation that can sustain ocular-surface inflammation and drive secondary SNCD.[69]
Anterior-Segment Imaging
Table 3. Diagnostic Modalities and Key Findings in Salzmann Nodular Corneal Degeneration
|
Modality |
Key Findings in SNCD |
Utility |
|
AS-OCT |
Hyperreflective subepithelial plaques anterior to or replacing Bowman's layer; normal or mildly thinned overlying epithelium; and clear underlying stroma |
Confirms depth, differentiates from stromal dystrophies or band keratopathy |
|
Scheimpflug or Placido topography |
Localized inferior or mid-peripheral steepening, increased surface irregularity index, and asymmetric astigmatism |
Quantifies optical impact, guides refractive counseling and surgical planning. |
|
Corneal pachymetry |
Slight focal thinning beneath nodules; global pachymetry is typically within normal limits |
Useful pre-PTK or keratoplasty |
|
In vivo confocal microscopy |
Hyperreflective fibrous tissue with activated keratocytes; absent inflammatory infiltrate |
Research/tertiary centers—adds microstructural confirmation |
|
Wavefront aberrometry |
Elevated higher-order aberrations (eg, coma and trefoil) in visually significant lesions |
Explains poor quality of vision despite 20/20 Snellen acuity |
Abbreviations: AS-OCT, anterior segment optical coherence tomography; SNCD, Salzmann nodular corneal degeneration; PTK, phototherapeutic keratectomy.
Ancillary Tests
- Keratometry/keratograph: Reveals skewed mires and irregular cylinders >1 D, corroborating visual complaints.
- Tear osmolarity and inflammatory markers (eg, MMP-9): Abnormal in concomitant dry eye disease; treating tear-film instability may halt progression.
- Routine microbiology/serology: Not required unless atypical inflammation or associated infection (eg, interstitial keratitis) is suspected.[70]
Diagnostic Pitfalls and Differentials
Given that SNCD presents with superficial opacities similar to other conditions, maintaining a high index of suspicion is essential in the following scenarios:
- Nodules contain chalky calcium (band keratopathy)—AS-OCT shows a hyperreflective line in Bowman's layer rather than discrete plaques.
- Central corneal guttata or stromal edema are present—consider Fuchs endothelial dystrophy.
- Nodules are peripheral, crescentic, and vascularized—rule out Terrien marginal degeneration.[71][72]
Functional Assessment and Grading
Table 4. Grading of Salzmann Nodular Corneal Degeneration Severity Across Centers
|
Grade |
Description |
Management Implication |
|
I (mild) |
Scattered mid-peripheral nodules; no visual-axis involvement; and minimal symptoms |
Lubricants, lid hygiene, and treat meibomian gland disease/dry eye |
|
II (moderate) |
Nodules enlarge or approach the visual axis and induce >1 D irregular astigmatism |
Consider superficial keratectomy or PTK if symptoms persist |
|
III (severe) |
Central coalescent nodules, marked irregularity, or prior recurrence post-excision |
PTK with mitomycin-C or lamellar/penetrating keratoplasty |
Reference for the table.[44]
Abbreviation: PTK, phototherapeutic keratectomy.
Key Take-Home Points
- Patient history, slit-lamp examination, AS-OCT, and topography should be combined to confirm diagnosis and quantify visual impact.
- Concomitant ocular-surface disease should be continually evaluated and managed; treating the underlying condition reduces the recurrence.
- Imaging not only differentiates SNCD from mimickers but also guides surgical depth and optical zone planning for PTK or keratoplasty.
This thorough evaluation framework equips clinicians and interprofessional eye-care teams to detect SNCD early, stratify severity, and choose evidence-based interventions that preserve visual function and patient quality of life.[73]
Treatment / Management
Management choices are influenced by the size and location of nodules, as well as the severity of symptoms. Asymptomatic patients may be treated with observation alone, whereas mildly symptomatic patients typically respond to conservative management. In contrast, severe cases may require surgical intervention. Visual disturbance is the most common indication for surgery.[5][3][9] However, continually symptomatic nodules, despite conservative management, may also require surgical management.[3][5][8] Additionally, patients with multiple quadrants affected are more likely to require surgery.[5](B2)
Conservative Management
In most cases, patients respond to conservative medical therapy. The use of lubricants, warm compresses, lid massage, and lid hygiene often provides symptomatic relief, especially in the context of dry eye disease. A study found that conservative therapy was effective enough to avoid surgery in 77.3% of cases.[5] Initial treatment should include the use of preservative-free lubricant drops. Other medical options to improve ocular inflammation include topical steroids; topical nonsteroidal anti-inflammatory drugs; immunomodulators, such as cyclosporine and lifitegrast; and doxycycline.[8] Post-LASIK SNCD has been treated with punctal plugs, in addition to loteprednol and cyclosporine, with a resolution of symptoms after 6 months.[10] If nodules occur in the setting of contact lens wear, cessation or reduction of lens wear may relieve symptoms. Contact lens fit should be reviewed.(B2)
Nodulectomy/Superficial Keratectomy
Manual excision and superficial keratectomy are the most straightforward surgical approaches for managing SNCD.[5][74] The epithelium is locally removed or, in the setting of epithelial membrane dystrophy or poorly visible nodules, epithelial debridement can be performed, with or without the use of alcohol.[75] The edge of the nodule is grasped using forceps and pulled toward the periphery or limbus.[5][8][9] With an edge of the nodule peeled back, a surgical plane can be established. Manual separation or blunt dissection is typically adequate for removal at this point.[7] If a surgical plane is not present, Calibri forceps, along with a bladed instrument, can be used to create a lamellar plane at the edge of the nodule.[9](B2)
If adhesions are present between the nodule and the limbus, typically due to a fibrovascular pannus, scissors may be used to amputate the nodule close to the limbus. After removal of the nodule, the underlying stroma is typically regular and smooth.[6] This presentation contrasts PHSCD, a condition that involves peripheral Salzmann-like opacities. Removal of a PHSCD lesion may be more challenging than its SNCD counterpart; bundles of subepithelial anchoring fibrils, as well as blood vessels, can complicate the establishment of a surgical plane.[76][77] Borgia and associates described intraoperative optical coherence tomography–assisted superficial keratectomy to enhance precision in Salzmann nodule removal by providing real-time visualization and enabling accurate dissection while preserving healthy corneal tissue.[43](B2)
Some patients with SNCD are left with significant defects in the Bowman's layer. These irregularities, if present, may be treated with a diamond burr or PTK.[78][79][80] Recurrences are more common in cases with deeper defects in the Bowman's layer and superficial stroma.[6] In a set of 41 cases that underwent superficial keratectomy, one report indicated a 22% recurrence rate. A study utilized superficial keratectomy followed by amniotic membrane transplantation; however, the nodule recurred. Although the procedure was repeated, recurrence occurred again. Published data on similar cases remain limited.[81](B2)
Mitomycin-C is an alkylating agent that interrupts DNA synthesis, inducing apoptosis in fibroblasts.[82] Mitomycin-C, used intraoperatively, targets the active fibrocytes and keratocytes within the stroma to halt the progression of Salzmann nodules and prevent recurrence after excision.[83][84] In a study involving 30 eyes, Bowers et al removed nodules via manual keratectomy and applied a mitomycin-C-soaked sponge to the area of degeneration twice, each time for 10 seconds, followed by irrigation with a balanced salt solution. No recurrence was reported for 28±15 months.[84] (B2)
Phototherapeutic Keratectomy
PTK is a viable management option for the treatment of SNCD. In a study, Trufanov and Riks found that both PTK and anterior automated lamellar keratoplasty (ALK) effectively improve vision in patients with central corneal opacities due to Salzmann's nodular degeneration.[44] PTK is preferred for superficial lesions due to faster healing and re-epithelisation, whereas ALK with a 200 μm head provides more consistent astigmatism outcomes for deeper stromal involvement. Unlike the typical hyperopic effect observed with PTK in other conditions, PTK in Salzmann's nodular degeneration usually results in a myopic shift, likely due to the midperipheral location of the nodules and asymmetric tear film pooling before surgery.[85]
Keratoplasty
In rare cases, lamellar keratoplasty (LK) is required for sufficient resolution of SNCD. A comparison of ALK to PTK for the treatment of SNCD found that the overall change in visual acuity was comparable between the 2 groups; however, PTK had a lower rate of complications.[86] Cases of recurrent SNCD after keratoplasty have been reported.[8][87] Cases that necessitate PK are exceedingly rare, typically in the setting of extensive concomitant disease or intraoperative perforation during LK.(B2)
Newer Advances
Newer antifibrotic agents are being studied for their potential role in the management of SNCD. Subbot and colleagues found that pirfenidone and cyclosporine A significantly reduce myofibroblast proliferation, metabolic activity, and alpha-smooth muscle actin expression in corneal cell cultures derived from patients with Salzmann's nodular degeneration.[45] These findings suggest their potential as antifibrotic agents in developing targeted, pathogenetically driven treatments for SNCD and related corneal fibrotic disorders.
Additional Considerations
For the most accurate biometric data to be obtained, SNCD should be removed before cataract extraction. SNCD-induced refractive sequelae may adversely affect biometry measurements, K values, and consequently intraocular lens power calculations.[5][65](B2)
Postoperative Care and Recurrence Prevention
Postoperative care plays a pivotal role in minimizing recurrence after superficial keratectomy or PTK. The ocular surface should be optimized with the following:
- Preservative-free lubricants to support epithelial healing
- Prophylactic topical antibiotics for 5-7 days
- Topical corticosteroids tapered over 4-6 weeks to control fibroblastic proliferation
- Temporary use of bandage contact lenses in cases of large epithelial defects
- Close follow-up during the first postoperative month to monitor re-epithelialization and rule out infectious keratitis
Recurrence is often associated with incomplete excision of nodular tissue or untreated underlying ocular surface disease. Long-term suppression of inflammation using topical cyclosporine, lifitegrast, or low-dose steroids may help prevent recurrence, particularly in patients with chronic meibomian gland disease, dry eye, or blepharitis.[88]
International Guidelines and Best Practices
Although no universal guideline exists solely for SNCD, consensus-based approaches and recommendations from corneal subspecialty societies such as the American Academy of Ophthalmology and European Society of Cataract and Refractive Surgeons emphasize the following principles:
- Conservative therapy is the first-line treatment for asymptomatic or mild cases
- Preference for PTK over lamellar keratoplasty when the lesion is superficial
- Use of mitomycin-C intraoperatively for recurrent or aggressive cases to reduce fibroblast proliferation
- Preoperative imaging (eg, AS-OCT and topography) to guide treatment planning and laser ablation depth
- Patient education and involvement in long-term ocular surface care [89] (A1)
Role of Interprofessional Collaboration
Effective management of SNCD requires a coordinated interprofessional approach.
- Ophthalmologists (corneal specialists) lead surgical and diagnostic decision-making.
- Optometrists assist with refraction, contact lens adjustments after surgery, and the early recognition of recurrence.
- Primary care physicians or rheumatologists may be involved if autoimmune associations are suspected.
- Nurses and ocular surface technicians play a role in perioperative care, postoperative counseling, and procedural assistance.
- Pharmacists ensure appropriate formulation of topical agents (eg, preservative-free steroids and compounded cyclosporine)
- Patient educators support adherence to long-term lid hygiene and artificial tear regimens [90]
Differential Diagnosis
SNCD may resemble several other corneal disorders, making accurate differentiation essential. The following differential diagnosis should be considered when making the diagnosis of SNCD.
- Bullous keratopathy
- Typically associated with Descemet membrane folds, corneal stromal edema, and raised intraocular pressure (IOP). Bullae may be differentiated from Salzmann nodules at the slit lamp by the presence of water.
- Climatic droplet keratopathy
- Golden-yellow or translucent droplets in the interpalpebral area bilaterally.
- Corneal amyloidosis
- Primary amyloidosis, characterized by gelatinous drops such as dystrophy or primary familial amyloidosis, presents with a bilateral raised multinodular mound of amyloid in the central cornea. These deposits are typically subepithelial.[6]
- Secondary amyloidosis presents in the area of past trauma as a yellow-white to pink, waxy nodular lesion.
- Corneal keloid
- These lesions are typically observed in younger age groups, with a history of inflammation, trauma, or in association with Lowe syndrome.
- Salzmann's subepithelial fibrosis [42]
- A clinically distinct form of SNCD characterized by dense subepithelial corneal opacities that often extend into Bowman's layer and anterior stroma. This form typically arises following surgical or traumatic epithelial basement membrane injury and may require more extensive surgical management than classic SNCD.
- Hereditary hypertrophic scarring
- Nodular scarring after minor trauma or surgery. Difficult to differentiate from SNCD at the slit lamp.
- Peripheral hypertrophic subepithelial corneal degeneration [91]
- Salzmann-like corneal opacities localized to the palpebral aperture.
- Typically bilateral and involve the nasal and the temporal cornea
Table 5. Differential Diagnosis for Salzmann Nodular Corneal Degeneration
|
Condition |
Key Features |
Distinguishing Factors from SNCD |
|
Interstitial keratitis |
Inflammatory stromal opacities; vascularization is common |
Typically deeper stromal involvement; often associated with systemic or infectious etiology (eg, syphilis and herpes simplex virus) |
|
Band keratopathy |
Calcium deposition in Bowman's layer; gray-white horizontal band |
Interpalpebral location; stains with Alizarin red; visible calcific plaques |
|
Epithelial basement membrane dystrophy |
Map-dot-fingerprint patterns; recurrent erosions |
Appears as geographic opacities, basement membrane changes; no true nodules |
|
Pingueculae or pterygium with corneal involvement |
Conjunctival fibrovascular growth extending onto the cornea |
Originates nasally or temporally; associated with sun exposure; typically vascularized |
|
Peripheral hypertrophic subepithelial corneal degeneration |
Peripheral subepithelial fibrosis; opacities with vascular pannus |
Typically bilateral, symmetric, peripheral; more extensive subepithelial fibrosis; vascularization more pronounced |
|
Corneal scars (traumatic/infectious) |
Localized or diffuse corneal opacity following trauma or infection |
History of prior trauma or keratitis; deeper stromal involvement; irregular contour |
|
Spheroidal degeneration |
Amber-colored spherical deposits in the superficial stroma |
Often interpalpebral; associated with UV exposure; found in older individuals |
|
Anterior basement membrane dystrophy |
Similar to epithelial basement membrane dystrophy; irregular epithelium |
More diffuse epithelial involvement; no true nodules; frequent erosions |
Abbreviation: SNCD, Salzmann nodular corneal degeneration.
Pertinent Studies and Ongoing Trials
SNCD has been the subject of several retrospective and prospective studies that have shaped the current management protocols. Although randomized controlled trials are limited due to the rarity and indolent nature of the disease, observational studies and interventional case series have provided robust clinical insights.
Bowers et al (2009) conducted a prospective interventional study on 30 eyes with SNCD undergoing superficial keratectomy with intraoperative application of mitomycin-C. The study demonstrated excellent outcomes, with no recurrence observed over a mean follow-up period of 28 months. This study supports the use of mitomycin-C in reducing recurrence rates after excision.[84]
Sharma et al (2012) compared PTK with ALK. Both interventions improved visual acuity in patients with centrally located SNCD nodules. PTK demonstrated a faster healing time and a favorable safety profile, whereas ALK was preferred for deeper lesions to achieve better refractive control.[86]
Borgia et al (2021) introduced intraoperative AS-OCT–guided superficial keratectomy, enabling precise dissection and real-time assessment of nodule depth. This approach reduced the risk of residual fibrosis and recurrence.[43]
Subbot et al (2022) investigated the antifibrotic effects of pirfenidone and cyclosporine A on cultured SNCD keratocytes. Their findings demonstrated downregulation of α-SMA expression and myofibroblast transformation, suggesting a potential role in modulating pharmacological disease.[45]
Ongoing trials registered on ClinicalTrials.gov are evaluating the long-term efficacy of topical immunomodulators such as lifitegrast and low-dose topical corticosteroids for subclinical inflammation in early SNCD. Although results are pending, these therapies have the potential to redefine first-line treatment strategies for early disease.
These studies collectively contribute to the evolution of evidence-based recommendations for both conservative and surgical approaches in managing SNCD. They emphasize the importance of individualized therapy tailored to lesion location, depth, and associated ocular surface inflammation.
Treatment Planning
Effective treatment planning for SNCD requires a comprehensive, individualized approach tailored to the patient's clinical evaluation, symptom severity, nodule characteristics, and visual demands. The overarching goal is to preserve or restore visual function while minimizing recurrence and procedural morbidity.
Step 1: Clinical Assessment
- Visual acuity testing, slit-lamp examination, and documentation of nodule location, size, and depth using AS-OCT or ultrasound biomicroscopy (UBM) are essential.
- Assess for associated ocular surface conditions, such as meibomian gland dysfunction, dry eye disease, contact lens intolerance, and prior ocular surgery (eg, LASIK and PRK).
- Identify the patient's functional complaints, such as glare, photophobia, and fluctuating vision, especially if nodules involve or distort the visual axis.[3]
Step 2: Determine Management Pathway
- Asymptomatic or peripheral nodules: Observation and conservative treatment.
- Mild-to-moderate symptoms: Initiate medical management.
- Central, symptomatic, or visually significant nodules: Plan for surgical intervention.[92]
Step 3: Medical Management (First-line Therapy)
- Preservative-free lubricants to improve tear film and ocular surface comfort.
- Topical corticosteroids (eg, loteprednol) or immunomodulators (eg, cyclosporine A and lifitegrast) for inflammatory control.
- Address underlying contributors:
- Blepharitis: Practice lid hygiene and use warm compresses.
- Meibomian gland dysfunction: Lipid-based lubricants, oral doxycycline if needed.
- Contact lens overuse: Temporary cessation or lens refitting.[93]
Step 4: Surgical Planning (When Medical Management Fails)
- Superficial keratectomy: Indicated for elevated, visually significant nodules. The procedure can be performed under topical anesthesia. Intraoperative AS-OCT guidance should be considered for precision.
- Adjunctive mitomycin-C: Applied intraoperatively to reduce recurrence, especially in younger patients or cases with recurrent nodules.
- PTK: Best for central or irregular stromal surface nodules causing higher-order aberrations.
- Lamellar keratoplasty: Reserved for deep stromal involvement or failed superficial keratectomy/PTK.
- Amniotic membrane transplantation: Considered when epithelial healing is delayed or the ocular surface is severely compromised.[94]
Step 5: Postoperative Care
- Topical corticosteroids, antibiotics, and frequent lubricants in the early postoperative period.
- Tapering of steroids should be based on resolution of inflammation and epithelial healing.
- Follow-up schedule:
- Day 1: Assess for epithelial defect, inflammation.
- 1 Week: Monitor healing, comfort.
- Monthly (up to 6 months): Evaluate for recurrence, visual rehabilitation.
Step 6: Long-Term Surveillance and Counseling
- Educate patients on the chronic nature of the condition.
- Emphasize the importance of long-term lid hygiene, avoidance of ocular trauma, and regular follow-up, especially for patients with a history of prior ocular surgeries or contact lens wear.
- Counsel regarding the potential for recurrence, particularly in high-risk cases (eg, limbal involvement and recurrent epithelial erosions).[95]
Toxicity and Adverse Effect Management
Management of SNCD often involves both pharmacological and surgical interventions, each of which carries the potential for adverse effects. Proactive identification and management of these toxicities is crucial to optimizing patient outcomes and avoiding iatrogenic complications.
Pharmacologic Toxicities
Topical corticosteroids:
- Potential toxicities:
- Elevated IOP → steroid-induced glaucoma
- Cataract formation (posterior subcapsular)
- Delayed corneal epithelial healing
- Management:
- Limit duration and frequency
- Use low-potency agents (eg, loteprednol)
- Regular IOP monitoring in long-term users
- Taper gradually under supervision[96]
Topical cyclosporine A and Lifitegrast:
- Potential toxicities:
- Local irritation, burning, redness, and stinging on instillation
- Transient blurred vision
- Management:
- Administer artificial tears to reduce discomfort
- Educate patients about the delayed onset of effect (6-8 weeks)
- Monitor compliance and reinforce proper technique [97]
Oral doxycycline:
- Potential toxicities:
- Gastrointestinal upset
- Photosensitivity
- Esophageal irritation
- Management:
- Prescribe with food (but not dairy)
- Advise sun protection
- Recommend taking with a full glass of water and an upright posture[98]
Mitomycin-C:
- Potential toxicities:
- Corneal endothelial cell toxicity
- Delayed epithelial healing
- Scleral thinning (rare)
- Management:
- Apply intraoperatively in a controlled, timed manner (eg, 0.02% for 10-30 seconds)
- Rinse thoroughly with balanced salt solution
- Avoid in thin corneas or limbal stem cell deficiency
- Use judiciously with an ophthalmic surgeon's expertise [99]
Surgical Complications and Toxicities
Superficial keratectomy:
- Risks:
- Corneal haze or scarring
- Recurrence of nodules
- Delayed epithelial healing
- Management:
- Ensure proper dissection plane
- Postoperative use of topical steroids and lubricants
- Diamond burr polishing or PTK for residual surface irregularity [100]
Phototherapeutic keratectomy:
- Risks:
- Hyperopic shift or unexpected refractive changes
- Stromal haze
- Delayed epithelialization
- Management:
- Predict refractive shifts (typically myopic in SNCD)
- Use lower ablation depths
- Apply topical steroids and lubricants postoperatively
- Consider bandage contact lenses for comfort [101]
Lamellar or penetrating keratoplasty:
- Risks:
- Graft rejection
- Infection
- Astigmatism
- Management:
- Routine follow-up and graft monitoring
- Use of topical immunosuppressants and antibiotics
- Spectacle or contact lens correction for postoperative refractive errors [102]
Contact Lens–Related Adverse Effects
In patients with long-term contact lens wear contributing to or complicating SNCD:
- Risks:
- Hypoxia-induced corneal changes
- Infection (eg, microbial keratitis)
- Mechanical trauma
- Management:
- Temporary discontinuation
- Refitting with high Dk lenses
- Daily disposable lenses to reduce protein buildup [103]
Delayed or Missed Diagnosis
- Adverse outcome:
- Unnecessary visual impairment
- Misdiagnosis as dystrophies, keratitis, or degenerations (eg, band keratopathy)
- Management:
- High index of suspicion in patients with chronic ocular surface inflammation and subepithelial opacities
- AS-OCT for accurate diagnosis
- Histopathological examination in atypical cases
Although SNCD is typically a benign and indolent disease, both medical and surgical interventions carry a risk of toxicity and adverse effects. A tailored, cautious, and evidence-based approach—along with patient education and regular monitoring—can mitigate complications and improve long-term outcomes. The integration of imaging tools, such as AS-OCT, and surgical aids, such as intraoperative optical coherence tomography, can further reduce procedural risks.[72]
Staging
Although there is no universally accepted formal staging system for SNCD, clinicians have informally stratified the disease into stages based on clinical severity, nodule characteristics, and visual impact, which aids in treatment decisions and prognostication. A proposed practical staging framework includes:
Table 6. Proposed Clinical Staging of Salzmann Nodular Corneal Degeneration
|
Stage |
Clinical Features |
Visual Impact |
Recommended Management |
|
Stage I (subclinical/early) |
Small, flat, peripheral, or paracentral nodules; minimal or no symptoms; incidental finding during examination |
None or mild (outside visual axis) |
Observation, artificial tears, and ocular surface optimization |
|
Stage II (symptomatic/moderate) |
Enlarging nodules within or encroaching on the visual axis; associated ocular surface inflammation |
Mild-to-moderate visual disturbance; fluctuating vision |
Lubricants and topical anti-inflammatory agents are used to treat coexisting ocular surface diseases. |
|
Stage III (advanced/visually significant) |
Prominent, elevated nodules within the central visual axis; irregular astigmatism; surface breakdown possible |
Moderate-to-severe visual loss |
Surgical intervention (superficial keratectomy with or without mitomycin-C/PTK) |
|
Stage IV (refractory/recurrent) |
Recurrence after prior surgery; multiple quadrants involved; associated scarring or stromal thinning |
Severe vision impairment; recurrent symptoms |
Repeat superficial keratectomy, PTK, or lamellar keratoplasty |
Abbreviation: PTK, phototherapeutic keratectomy.
- Imaging tools: AS-OCT is valuable for assessing depth, morphology, and surgical planning.
- Histopathology may be considered in atypical presentations.
- Recurrent SNCD may demonstrate deeper stromal involvement and may require more aggressive surgical planning.
- Staging helps in determining candidacy for surgical intervention versus continued medical management.[104]
Prognosis
Prognosis is good with the treatment of underlying factors. Although asymptomatic cases may be observed without progression, cases of spontaneous regression have not been reported. Varying rates of recurrence have been reported.[3][6][5] However, treatment of the underlying etiology may decrease the potential for further nodule formation.[3]
The prognosis of SNCD is generally favorable, particularly with the timely identification and management of underlying ocular surface disorders. Most patients respond well to both conservative and surgical interventions, mainly when inflammation and ocular surface disease are addressed concurrently.
Asymptomatic cases—especially those with peripheral nodules that do not involve the visual axis—can often be monitored without intervention, without evidence of progression. However, spontaneous regression of nodules has not been reported in the literature, and untreated cases may ultimately lead to visual impairment due to progressive irregular astigmatism, tear film instability, or scarring.[13]
Recurrence rates vary significantly depending on the type of treatment and the presence of persistent underlying risk factors. Reported recurrence rates range from 0% to 22% following superficial keratectomy, with recurrence being more common in cases involving deeper stromal involvement or inadequate management of the predisposing etiology.
Importantly, addressing the primary cause—such as blepharitis, dry eye disease, or contact lens misuse—has been shown to reduce the risk of recurrence and prevent the formation of new nodules. The use of adjunctive therapies, such as intraoperative mitomycin-C or anti-fibrotic agents, may further reduce the risk of recurrence in surgical cases.[16]
Visual prognosis is excellent in most cases after treatment, with many patients achieving significant improvement in both uncorrected and best-corrected visual acuity. Refractive stability is typically restored following resolution of nodules, although some patients may require spectacle or contact lens correction for residual astigmatism. Overall, SNCD is a benign but potentially vision-impairing condition with good outcomes when managed appropriately.[105]
Complications
Complications secondary to SNCD include the following:
- Irritation of the ocular surface leading to photophobia, tearing, and blepharospasm
- Secondary recurrent corneal erosions [106]
- Salzmann's nodules cause a flattening of the cornea and induce tremendous local irregularities, leading to hyperopia and astigmatism in many cases.[64] Due to their elevated nature, the nodules accentuate the flattening that typically occurs from the center to the periphery. Moreover, when located paracentrally, they produce central pooling of tears, which flattens measured corneal curvature.[107] Surgical removal of these nodules can often reverse the hyperopia or further cause a myopic shift.
- Superficial corneal scarring
SNCD is generally a slowly progressive and benign condition. However, if left untreated or in cases of recurrence, several ocular complications may arise. These complications can result from the disease process itself or as sequelae of surgical interventions. The location, size, and number of nodules influence both symptom severity and the risk of complications.[46]
Table 7. Complications of Salzmann Nodular Corneal Degeneration
|
Complication |
Description |
Associated Cause |
|
Visual impairment |
Blurred or fluctuating vision due to central nodules or astigmatism |
Mid-peripheral or central nodule location |
|
Irregular astigmatism |
Induced by nodular distortion of corneal curvature |
Multiple or large nodules |
|
Foreign body sensation and photophobia |
Resulting from elevated nodules and tear film disruption |
Elevated nodules affecting the eyelid blink |
|
Recurrent corneal erosion |
Due to epithelial instability over nodules |
Chronic mechanical stress or trauma |
|
Superficial keratitis or dry eye exacerbation |
Secondary to tear film disturbance |
Tear film irregularity and ocular surface inflammation |
|
Infection (postoperative or erosion) |
Risk of microbial keratitis through epithelial breaks |
Post-surgical epithelial defects |
|
Recurrence of nodules |
Nodules reappear after surgical excision |
Incomplete removal or persistent risk factors |
|
Post-surgical haze or scarring |
Stromal haze or irregular healing after keratectomy or PTK |
Deep excisions or poor wound healing |
|
Refractive changes (myopic/hyperopic shift) |
Unpredictable changes in refraction post-PTK or keratectomy |
Asymmetric ablation or healing response |
|
Graft rejection or failure |
Rare but possible in lamellar or penetrating keratoplasty |
Immune-mediated or surgical complications |
|
Persistent epithelial defect |
Delayed healing or failure of re-epithelialization post-surgery |
Underlying ocular surface disease and mitomycin-C exposure [96] |
Abbreviation: PTK, phototherapeutic keratectomy.
Postoperative and Rehabilitation Care
Effective postoperative care following surgical interventions for SNCD—such as superficial keratectomy, PTK, or lamellar keratoplasty—is crucial for promoting corneal healing, minimizing complications, and preventing recurrence. A structured rehabilitation plan enhances visual recovery and patient comfort while reducing inflammation and the risk of recurrence.
Immediate Postoperative Care (Day 0-7)
- Topical antibiotics (eg, moxifloxacin 0.5%) are applied 4-6 times daily to prevent infection.
- Topical corticosteroids (eg, loteprednol or fluorometholone) reduce postoperative inflammation. Tapering is guided by clinical response.
- Lubricating eye drops (preservative-free) are prescribed frequently to support epithelial healing and restore tear film stability.
- A bandage contact lens may be applied post-superficial keratectomy or PTK to protect the healing epithelium and alleviate discomfort.
- Pain management includes oral analgesics and the use of cold compresses.[43]
Intermediate Care (1-4 Weeks)
- Continue topical corticosteroids with a gradual taper over 2-4 weeks, depending on the type of surgical procedure and the severity of inflammation.
- Monitor for epithelial healing using fluorescein staining during follow-up visits.
- Assess visual acuity and corneal clarity every week to guide further therapy.[13]
Long-Term Rehabilitation (1-3 Months and Beyond)
- Tear film optimization: Continue frequent lubricants, consider punctal plugs if symptoms persist.
- Anti-inflammatory modulation: For cases with chronic inflammation or autoimmune background, cyclosporine 0.05% or lifitegrast may be added.
- Refractive correction: Post-surgical astigmatism or irregularities may require spectacle correction, rigid gas-permeable lenses, or scleral lenses.
- Monitoring for recurrence: Clinical reviews are conducted every 3-6 months to detect new nodules or recurrence early.
- Patient education: Emphasis on maintaining eyelid hygiene, managing blepharitis or meibomian gland dysfunction, and avoiding ocular trauma or contact lens overuse.[108]
Rehabilitation Goals
- Achieve a stable, clear corneal surface.
- Minimize recurrence by controlling underlying ocular surface disorders.
- Restore optimal visual function.
- Maintain ocular comfort and quality of life.[109]
Consultations
Proper multidisciplinary collaboration and timely referrals are crucial in optimizing the management and outcomes of SNCD, particularly in cases that are recurrent, atypical, or vision-threatening. The following is an overview of clinical consultations that may be warranted throughout the course of SNCD:
Cornea and Ocular Surface Specialists
- Primary consultant for diagnosis and management.
- Responsibilities:
- Confirm diagnosis using slit-lamp biomicroscopy and AS-OCT.
- Evaluate the indication for surgical versus conservative treatment.
- Perform procedures such as superficial keratectomy, PTK, or keratoplasty.
- Monitor for recurrence and complications.[110]
Oculoplastic Surgeons
- Indication:
- When SNCD is associated with lid abnormalities such as entropion, lagophthalmos, or lid margin keratinization.
- Role:
- Correct structural eyelid disorders contributing to chronic surface irritation or exposure keratopathy.[111]
Contact Lens Specialists/Optometrists
- Indication:
- Postoperative visual rehabilitation is particularly important in cases with induced irregular astigmatism or central corneal flattening.
- Role:
- Fit rigid gas-permeable, hybrid, or scleral lenses.
- Optimize best-corrected visual acuity and comfort.[112]
Immunologists/Rheumatologists
- Indication:
- If SNCD is associated with an underlying autoimmune disease (eg, Sjögren's syndrome and ocular cicatricial pemphigoid).
- Role:
- Systemic evaluation for autoimmune markers.
- Initiate or adjust systemic immunosuppressive therapy if indicated.[110]
Pediatric Ophthalmologists
- Indication:
- In rare pediatric cases (juvenile SNCD) or when associated with vernal keratoconjunctivitis.
- Role:
- Guide long-term control of allergic conjunctivitis and ocular surface inflammation.
- Prevent amblyopia in young patients with significant visual axis involvement.[113]
Ocular Surface Disease Unit (Multidisciplinary)
- Recommended for:
- Complex or recurrent SNCD cases.
- Team members may include:
- Cornea specialists, immunologists, optometrists, oculoplastic surgeons, and dry eye specialists.
- Role:
- Holistic management, including anti-inflammatory therapy, surface lubrication, and immune modulation.
- Address multifactorial etiologies, including meibomian gland disease, dry eye, and contact lens intolerance.[114]
Low Vision and Vision Rehabilitation Specialists
- Indication:
- For patients with irreversible visual impairment despite surgical or optical correction.
- Role:
- Prescribe visual aids (eg, magnifiers and telescopes).
- Provide adaptive training and counseling to improve functional independence.[115]
Ophthalmic Pathologists (if biopsy performed)
- Indication:
- When the diagnosis is uncertain, atypical, or nodules recur despite therapy.
- Role:
- Histopathological confirmation of SNCD.
- Exclude masquerading lesions such as corneal amyloidosis or dysplastic changes.[116]
Table 8. Consultation Workflow Summary Table
|
Consultant |
Indication |
Role |
|
Cornea specialists |
All SNCD cases |
Diagnosis, medical/surgical treatment, and follow-up |
|
Oculoplastic surgeons |
Eyelid malpositions and exposure keratopathy |
Correct eyelid abnormalities |
|
Contact lens specialists |
Post-surgical visual rehab and irregular astigmatism |
Fit specialty lenses |
|
Immunologists/rheumatologists |
Autoimmune associations |
Systemic work-up and immunosuppression |
|
Pediatric ophthalmologists |
Pediatric SNCD and vernal keratoconjunctivitis |
Prevent amblyopia and control inflammation |
|
Multidisciplinary ocular surface unit |
Refractory/recurrent SNCD |
Comprehensive management |
|
Low vision specialists |
Irreversible visual loss |
Adaptive aids and visual rehabilitation |
|
Ophthalmic pathologists |
Diagnostic uncertainty and recurrence |
Histopathologic diagnosis |
Abbreviation: SNCD, Salzmann nodular corneal degeneration.
Deterrence and Patient Education
The most frequently cited factors in the development of SNCD include meibomian gland disease, dry eye syndrome, and prolonged contact lens wear.[5] In patients who have early signs of ocular inflammation, ocular lubrication with preservative-free lubricant drops, eyelid hygiene, and patient education to prevent contact lens overwear are essential.
Deterrence and patient education are essential aspects of long-term management and the prevention of recurrence in SNCD. As the disease is often chronic and associated with modifiable ocular surface insults, patient awareness and compliance significantly influence the clinical outcome.
Education on the Nature of Disease
- Patients should be informed that SNCD is a benign, non-infectious, and slowly progressive corneal condition.
- Although some cases are asymptomatic and stable, others may progress or lead to visual impairment, particularly when the visual axis is involved.[8]
Identification and Avoidance of Triggers
- Educate patients about risk factors contributing to nodule formation, including:
- Chronic ocular surface inflammation (eg, blepharitis and dry eye)
- Poor contact lens hygiene or overuse
- Recurrent trauma or prior ocular surgery
- Recommend modifications to these behaviors to prevent recurrence or progression.[36]
Eyelid and Ocular Surface Hygiene
- Daily lid hygiene, including the use of warm compresses and gentle lid scrubs, can help reduce the risk of chronic blepharitis and meibomian gland dysfunction.
- The importance of preserving the tear film should be emphasized through the consistent use of preservative-free lubricants and the avoidance of eye rubbing.[117]
Compliance With Medical Therapy
- Counsel patients to adhere to prescribed topical medications such as:
- Lubricants
- Anti-inflammatory drops (eg, corticosteroids, cyclosporine, and lifitegrast)
- Antibiotic-steroid combinations in specific cases
- Emphasize proper administration techniques and potential adverse effects to enhance compliance.[118]
Postoperative Care Awareness
- In surgically treated patients, education should focus on:
- The importance of follow-up to detect recurrence or complications.
- Use of protective eyewear postoperatively.
- Adherence to prescribed postoperative medications and restrictions on eye rubbing or lens use.[119]
Contact Lens Counseling
- Encourage proper lens hygiene, fitting, and replacement schedules.
- For patients whose SNCD is linked to lens use, temporary discontinuation or transition to daily disposables or scleral lenses may be advised.[120]
Regular Follow-Up
- Patients should understand the importance of periodic ophthalmic examinations to monitor for:
- Recurrence of nodules
- Progression affecting the visual axis
- Surface irregularities causing astigmatism [121]
Education Materials and Support
- Provide brochures or digital resources about SNCD.
- Offer guidance to patients about when to seek immediate care (eg, sudden vision loss, pain, or photophobia).[122]
Lifestyle and Environmental Adjustments
- Recommend protection from dust, pollutants, and excessive screen time, which may exacerbate dry eye.
- Recommend using humidifiers in dry environments to support ocular surface health.
Encouraging Self-Monitoring
- Patients should be taught to self-monitor for:
- Fluctuations in vision
- New onset of irritation or photophobia
- Decreased tolerance to contact lenses
By empowering patients through comprehensive education, clinicians can significantly reduce recurrence, improve treatment outcomes, and promote a better quality of life for individuals with SNCD.[123]
Pearls and Other Issues
Clinical Pearls
- SNCD is often underdiagnosed due to its subtle, peripheral, or asymptomatic presentation. A high index of suspicion is required in patients with longstanding dry eye or post-refractive surgery visual fluctuations.
- Blue light slit-lamp examination enhances visualization of the bluish-gray, elevated nodules, which are pathognomonic.
- In patients with visual complaints without corneal opacity, perform topography or AS-OCT to detect subclinical nodules and assess their impact on the tear film or corneal regularity.
- Disruption of the tear film over nodules significantly contributes to visual symptoms, which explains the success of therapies targeting the ocular surface.[124]
Disposition
- Most patients with SNCD can be managed on an outpatient basis, with long-term follow-up to monitor for progression or recurrence.
- Surgical intervention, when necessary, can typically be performed under topical or local anesthesia, resulting in a rapid recovery.
- Patients with recurrent nodules may benefit from adjunctive intraoperative mitomycin-C or superficial PTK to delay recurrence.[125]
Pitfalls
- Misdiagnosis: SNCD can be confused with band keratopathy, pannus, epithelial basement membrane dystrophy, or Salzmann-like changes in chronic inflammation. A careful clinical and slit-lamp examination can help distinguish.
- Over-treatment: Avoid aggressive surgery in asymptomatic or peripherally located nodules. Conservative therapy is often sufficient.
- Inadequate management of ocular surface disease after surgery can lead to recurrence, delayed healing, or the development of new nodules.[13]
Prevention
- Early recognition and control of chronic ocular surface inflammation, especially blepharitis and dry eye, can prevent the development or recurrence of nodules.
- Patient counseling regarding proper contact lens hygiene and limited exposure to environmental irritants is vital in minimizing exacerbating factors.
- Adjunctive therapies, such as mitomycin-C or amniotic membrane transplantation, administered during surgery in select cases, may reduce recurrence in high-risk eyes.[126]
Other Considerations
- No standardized staging system exists for SNCD; however, efforts to classify it based on nodule location (central vs. peripheral), size, number, and impact on vision may guide treatment planning.
- Emerging therapies, including targeted antifibrotic agents such as pirfenidone and topical immunomodulators, represent a promising future direction in managing chronic or recurrent SNCD.
- Longitudinal studies are needed to establish recurrence rates, long-term outcomes of surgical interventions, and the role of adjuvant medical therapies.[127]
Enhancing Healthcare Team Outcomes
Assessment of corneal pathology may involve optometrists and ophthalmologists to ensure that patients have adequate access to eye care, thereby helping to prevent vision deterioration due to SNCD. Effective management of SNCD necessitates a collaborative, interprofessional healthcare approach that aligns with principles of patient-centered care, safety, and optimal visual outcomes. Given its chronic nature and links to systemic and ocular surface comorbidities, coordinated care improves both diagnosis and management while reducing recurrence rates.[5]
Role of Ophthalmologists (Cornea Specialists/General Eye Physicians)
- Leads diagnosis and staging using slit-lamp examination, corneal imaging (eg, AS-OCT and topography), and clinical history.
- Determines need for conservative versus surgical management.
- Ensures continuity of care through patient education, follow-up, and coordination with allied healthcare personnel.
- Ensures ethical, informed consent, particularly for surgical options such as superficial keratectomy or PTK.[128]
Optometrists and Vision Rehabilitation Specialists
- Support visual rehabilitation post-treatment.
- Perform refraction to assess vision pre- and post-nodulectomy.
- Provide tailored contact lens fitting advice, especially in cases where SNCD is associated with long-term lens wear.
- May detect early signs of recurrence through routine visual performance assessments.[120]
Nursing and Ophthalmic Technicians
- Conduct preliminary evaluations, including visual acuity, IOP measurement, and a detailed history.
- Educate patients on medication adherence, ocular hygiene, and postoperative care.
- Monitor for signs of adverse effects or recurrence.
- Facilitate communication between the patient and other team members for timely escalation of care.[129]
Pharmacists
- Verify prescriptions for topical medications such as steroids, nonsteroidal anti-inflammatory drugs, and immunomodulators (eg, cyclosporine and lifitegrast).
- Counsel patients on potential adverse effects and the importance of compliance, especially with immunosuppressive agents and ocular lubricants.
- Ensure appropriate dosing, especially for intraoperative mitomycin C, to minimize toxic exposures.[130]
Clinical Coordinators and Administrative Staff
- Schedule and track follow-up appointments, surgical bookings, and long-term monitoring.
- Help with insurance approvals for surgical interventions or advanced diagnostics such as PTK or amniotic membrane transplantation.
- Facilitate interdisciplinary referrals when needed (eg, to rheumatologists in autoimmune-related cases).[108]
Ethics and Patient-Centered Strategy
- Ensure all treatments are aligned with the patient's values and preferences, particularly in elective surgical interventions.
- Discuss risks, benefits, and recurrence potential clearly with patients.
- Maintain confidentiality, especially when working with electronic records across multiple care points.[131]
Interprofessional Communication
- Shared electronic medical records allow seamless updates and communication between optometrists, ophthalmologists, and nurses.
- The use of structured communication tools, such as SBAR (Situation, Background, Assessment, Recommendation), enhances decision-making and patient safety.
- Regular interdisciplinary case reviews for patients undergoing surgical intervention or with complex, recurrent SNCD.[132]
Outcomes and Team Performance
- Coordinated care results in:
- Reduced recurrence rates through optimized ocular surface management.
- Enhanced patient satisfaction and adherence to treatment.
- Improved surgical outcomes through timely intervention and proper rehabilitation.
- Fewer medication-related adverse effects due to pharmacist oversight.
This team-based, ethical, and collaborative approach ensures that SNCD management is efficient, holistic, and responsive to patient needs, improving visual prognosis and quality of life.[133]
Media
(Click Image to Enlarge)
References
REBEL HH. [Again on the study reform; Comments on the above article by W. Salzmann]. Deutsche zahnarztliche Zeitschrift. 1947 Jan 15:2(2):44-7 [PubMed PMID: 20242449]
Frising M, Pitz S, Olbert D, Kriegsmann J, Lisch W. Is hyaline degeneration of the cornea a precursor of Salzmann's corneal degeneration? The British journal of ophthalmology. 2003 Jul:87(7):922-3 [PubMed PMID: 12812904]
Level 3 (low-level) evidenceFarjo AA, Halperin GI, Syed N, Sutphin JE, Wagoner MD. Salzmann's nodular corneal degeneration clinical characteristics and surgical outcomes. Cornea. 2006 Jan:25(1):11-5 [PubMed PMID: 16331034]
Level 2 (mid-level) evidenceMaharana PK, Sharma N, Das S, Agarwal T, Sen S, Prakash G, Vajpayee RB. Salzmann's Nodular Degeneration. The ocular surface. 2016 Jan:14(1):20-30. doi: 10.1016/j.jtos.2015.08.006. Epub 2015 Oct 17 [PubMed PMID: 26462409]
Graue-Hernández EO, Mannis MJ, Eliasieh K, Greasby TA, Beckett LA, Bradley JC, Schwab IR. Salzmann nodular degeneration. Cornea. 2010 Mar:29(3):283-9. doi: 10.1097/ICO.0b013e3181b7658d. Epub [PubMed PMID: 20098304]
Level 2 (mid-level) evidenceDas S, Link B, Seitz B. Salzmann's nodular degeneration of the cornea: a review and case series. Cornea. 2005 Oct:24(7):772-7 [PubMed PMID: 16160490]
Level 2 (mid-level) evidenceParanjpe V, Galor A, Monsalve P, Dubovy SR, Karp CL. Salzmann nodular degeneration: prevalence, impact, and management strategies. Clinical ophthalmology (Auckland, N.Z.). 2019:13():1305-1314. doi: 10.2147/OPTH.S166280. Epub 2019 Jul 25 [PubMed PMID: 31413538]
Hamada S, Darrad K, McDonnell PJ. Salzmann's nodular corneal degeneration (SNCD): clinical findings, risk factors, prognosis and the role of previous contact lens wear. Contact lens & anterior eye : the journal of the British Contact Lens Association. 2011 Aug:34(4):173-8. doi: 10.1016/j.clae.2011.02.004. Epub 2011 Feb 23 [PubMed PMID: 21349758]
Oster JG, Steinert RF, Hogan RN. Reduction of hyperopia associated with manual excision of Salzmann's nodular degeneration. Journal of refractive surgery (Thorofare, N.J. : 1995). 2001 Jul-Aug:17(4):466-9 [PubMed PMID: 11472006]
Level 3 (low-level) evidenceVanderBeek BL, Silverman RH, Starr CE. Bilateral Salzmann-like nodular corneal degeneration after laser in situ keratomileusis imaged with anterior segment optical coherence tomography and high-frequency ultrasound biomicroscopy. Journal of cataract and refractive surgery. 2009 Apr:35(4):785-7. doi: 10.1016/j.jcrs.2008.09.033. Epub [PubMed PMID: 19304107]
Level 3 (low-level) evidenceWilson SE. Bowman's layer in the cornea- structure and function and regeneration. Experimental eye research. 2020 Jun:195():108033. doi: 10.1016/j.exer.2020.108033. Epub 2020 Apr 24 [PubMed PMID: 32339517]
Yam GH, Pi S, Du Y, Mehta JS. Posterior corneoscleral limbus: Architecture, stem cells, and clinical implications. Progress in retinal and eye research. 2023 Sep:96():101192. doi: 10.1016/j.preteyeres.2023.101192. Epub 2023 Jun 29 [PubMed PMID: 37392960]
Roszkowska AM, Azzaro C, Calderone A, Spinella R, Schiano-Lomoriello D, Mencucci R, Wylęgała A. Salzmann Nodular Degeneration in Ocular and Systemic Diseases. Journal of clinical medicine. 2024 Aug 20:13(16):. doi: 10.3390/jcm13164900. Epub 2024 Aug 20 [PubMed PMID: 39201042]
Pastar I, Balukoff NC, Marjanovic J, Chen VY, Stone RC, Tomic-Canic M. Molecular Pathophysiology of Chronic Wounds: Current State and Future Directions. Cold Spring Harbor perspectives in biology. 2023 Apr 3:15(4):. doi: 10.1101/cshperspect.a041243. Epub 2023 Apr 3 [PubMed PMID: 36123031]
Level 3 (low-level) evidenceTheotoka D, Morkin MI, Naranjo A, Dubovy SR, Karp CL. Spontaneous regression of ocular surface squamous neoplasia: Possible etiologic mechanisms in cancer resolution. The ocular surface. 2020 Jul:18(3):351-353. doi: 10.1016/j.jtos.2020.03.001. Epub 2020 Mar 10 [PubMed PMID: 32169482]
Salari F, Beikmarzehei A, Liu G, Zarei-Ghanavati M, Liu C. Superficial Keratectomy: A Review of Literature. Frontiers in medicine. 2022:9():915284. doi: 10.3389/fmed.2022.915284. Epub 2022 Jul 6 [PubMed PMID: 35872789]
Moniz N, Fernandez ST. Efficacy of phototherapeutic keratectomy in various superficial corneal pathologies. Journal of refractive surgery (Thorofare, N.J. : 1995). 2003 Mar-Apr:19(2 Suppl):S243-6 [PubMed PMID: 12699182]
Singer AR, Pahl S, Lang HM, Ruprecht KW. [A familial anterior corneal degeneration: clinical aspects, histopathology and differential diagnosis]. Klinische Monatsblatter fur Augenheilkunde. 1998 Aug:213(2):104-7 [PubMed PMID: 9782469]
Level 3 (low-level) evidenceGUPTA JS, CHATTERJEE A, KUMAR K. INVERSE BELL'S PHENOMENON AS A PROTECTIVE MECHANISM. American journal of ophthalmology. 1965 May:59():931-3 [PubMed PMID: 14288945]
Nissirios NJ, Barsam A, Nadji E, Donnenfeld ED, Perry HD. Relationship of Bell phenomenon with Salzmann nodular degeneration. Cornea. 2013 Jul:32(7):939-42. doi: 10.1097/ICO.0b013e318281722b. Epub [PubMed PMID: 23442252]
Moshirfar M, Marx DP, Barsam CA, Mohebali J, Mamalis N. Salzmann's-like nodular degeneration following laser in situ keratomileusis. Journal of cataract and refractive surgery. 2005 Oct:31(10):2021-5 [PubMed PMID: 16338579]
Level 3 (low-level) evidenceWerner LP, Issid K, Werner LP, Pouliquen Y, Legeais JM, Renard G. Salzmann's corneal degeneration associated with epithelial basement membrane dystrophy. Cornea. 2000 Jan:19(1):121-3 [PubMed PMID: 10632022]
Level 3 (low-level) evidenceWood TO. Salzmann's nodular degeneration. Cornea. 1990 Jan:9(1):17-22 [PubMed PMID: 2297989]
Lim MC, Chan WK. Salzmann nodular degeneration after laser in situ keratomileusis. Cornea. 2009 Jun:28(5):577-8. doi: 10.1097/ICO.0b013e31818c2e79. Epub [PubMed PMID: 19421036]
Level 3 (low-level) evidenceMoshirfar M, Chang JC, Mamalis N. Salzmann nodular degeneration after laser in situ keratomileusis. Cornea. 2010 Jul:29(7):840-1. doi: 10.1097/ICO.0b013e3181c378df. Epub [PubMed PMID: 20489596]
Level 3 (low-level) evidenceStem MS, Hood CT. Salzmann nodular degeneration associated with epithelial ingrowth after LASIK treated with superficial keratectomy. BMJ case reports. 2015 Jan 6:2015():. doi: 10.1136/bcr-2014-207776. Epub 2015 Jan 6 [PubMed PMID: 25564642]
Level 3 (low-level) evidenceHopping GC, Somani AN, Vaidyanathan U, Liu H, Barnes JR, Ronquillo YC, Hoopes PC, Moshirfar M. Myopic regression and recurrent Salzmann nodule degeneration after laser in situ keratomileusis in Ehlers Danlos Syndrome. American journal of ophthalmology case reports. 2020 Sep:19():100729. doi: 10.1016/j.ajoc.2020.100729. Epub 2020 May 4 [PubMed PMID: 32426553]
Level 3 (low-level) evidenceHe X, Donaldson KE. Superficial keratectomy for Salzmann nodular degeneration following laser in situ keratomileusis. Canadian journal of ophthalmology. Journal canadien d'ophtalmologie. 2019 Jun:54(3):e149-e151. doi: 10.1016/j.jcjo.2018.07.002. Epub 2018 Oct 19 [PubMed PMID: 31109505]
Song P, Yu X, Pang C. Salzmann nodular degeneration in posterior keratoconus: a case report. BMC ophthalmology. 2022 Mar 12:22(1):117. doi: 10.1186/s12886-022-02268-3. Epub 2022 Mar 12 [PubMed PMID: 35279119]
Level 3 (low-level) evidenceKammoun S, Maaloul K, Mkaouar F, Jallouli M, Bahloul Z, Trigui A. A rare association of Salzmann's nodular degeneration of cornea and dermatopathia pigmentosa reticularis. Journal francais d'ophtalmologie. 2022 Dec:45(10):e466-e469. doi: 10.1016/j.jfo.2022.05.017. Epub 2022 Oct 19 [PubMed PMID: 36272866]
Goel R, Bodh SA, Sardana K, Goel A. Dermatopathia Pigmentosa Reticularis with Salzmann's nodular degeneration of cornea: A rare association. Nepalese journal of ophthalmology : a biannual peer-reviewed academic journal of the Nepal Ophthalmic Society : NEPJOPH. 2015 Jan-Jun:7(1):79-81. doi: 10.3126/nepjoph.v7i1.13175. Epub [PubMed PMID: 26695611]
Papanikolaou T, Goel S, Jayamanne DG, Mudhar H, Desai SP. Familial pattern of Salzmann-type nodular corneal degeneration--a four generation series. The British journal of ophthalmology. 2010 Nov:94(11):1543. doi: 10.1136/bjo.2009.177055. Epub 2010 Aug 1 [PubMed PMID: 20679082]
Level 3 (low-level) evidenceMathers WD, Stovall D, Lane JA, Zimmerman MB, Johnson S. Menopause and tear function: the influence of prolactin and sex hormones on human tear production. Cornea. 1998 Jul:17(4):353-8 [PubMed PMID: 9676904]
Guglielmetti S, Kirton A, Reinstein DZ, Carp GI, Archer TJ. Repair of Irregularly Irregular Astigmatism by Transepithelial Phototherapeutic Keratectomy. Journal of refractive surgery (Thorofare, N.J. : 1995). 2017 Oct 1:33(10):714-719. doi: 10.3928/1081597X-20170721-04. Epub [PubMed PMID: 28991341]
Hirose K, Murakami G, Minowa T, Kura H, Yamashita T. Lateral ligament injury of the ankle and associated articular cartilage degeneration in the talocrural joint: anatomic study using elderly cadavers. Journal of orthopaedic science : official journal of the Japanese Orthopaedic Association. 2004:9(1):37-43 [PubMed PMID: 14767703]
Britten-Jones AC, Wang MTM, Samuels I, Jennings C, Stapleton F, Craig JP. Epidemiology and Risk Factors of Dry Eye Disease: Considerations for Clinical Management. Medicina (Kaunas, Lithuania). 2024 Sep 5:60(9):. doi: 10.3390/medicina60091458. Epub 2024 Sep 5 [PubMed PMID: 39336499]
Leonardi A, Di Zazzo A, Cutrupi F, Iaccarino L. Dry eye and systemic diseases. Saudi journal of ophthalmology : official journal of the Saudi Ophthalmological Society. 2025 Jan-Mar:39(1):5-13. doi: 10.4103/sjopt.sjopt_182_24. Epub 2025 Jan 2 [PubMed PMID: 40182960]
Thomas BJ, Galor A, Nanji AA, El Sayyad F, Wang J, Dubovy SR, Joag MG, Karp CL. Ultra high-resolution anterior segment optical coherence tomography in the diagnosis and management of ocular surface squamous neoplasia. The ocular surface. 2014 Jan:12(1):46-58. doi: 10.1016/j.jtos.2013.11.001. Epub 2013 Nov 9 [PubMed PMID: 24439046]
Yoon KC, Park YG. Recurrent Salzmann's nodular degeneration. Japanese journal of ophthalmology. 2003 Jul-Aug:47(4):401-4 [PubMed PMID: 12842211]
Level 3 (low-level) evidenceStone DU, Astley RA, Shaver RP, Chodosh J. Histopathology of Salzmann nodular corneal degeneration. Cornea. 2008 Feb:27(2):148-51. doi: 10.1097/ICO.0b013e31815a50fb. Epub [PubMed PMID: 18216567]
Stachon T, Fries FN, Li Z, Daas L, Nagy ZZ, Seitz B, Szentmáry N. Decreased PAX6 and DSG1 Protein Expression in Corneal Epithelium of Patients with Epithelial Basal Membrane Dystrophy, Salzmann Nodular Degeneration, and Pterygium. Journal of clinical medicine. 2025 Feb 21:14(5):. doi: 10.3390/jcm14051456. Epub 2025 Feb 21 [PubMed PMID: 40094891]
Wilson SE, Dupps WJ. Salzmann's Nodular Degeneration in Refractive Surgery: The Earlier Hit Hypothesis of EBM Injury-Related Fibrosis of the Subepithelial Space and Deeper Corneal Extension. Journal of refractive surgery (Thorofare, N.J. : 1995). 2024 May:40(5):e279-e290. doi: 10.3928/1081597X-20240322-02. Epub 2024 May 1 [PubMed PMID: 38717084]
Borgia A, Airaldi M, Menassa N, Kaye S, Romano V. Intraoperative optical coherence tomography-assisted superficial keratectomy: A novel surgical technique for Salzmann nodular degeneration. European journal of ophthalmology. 2025 Sep:35(5):1938-1941. doi: 10.1177/11206721251346278. Epub 2025 Jun 6 [PubMed PMID: 40474661]
Trufanov SV, Riks IA. [Surgical treatment of central corneal opacities in Salzmann's degeneration]. Vestnik oftalmologii. 2025:141(2):75-84. doi: 10.17116/oftalma202514102175. Epub [PubMed PMID: 40353544]
Subbot AM, Krakhmaleva DA, Malozhen SA, Osipyan GA, Emets EV, Tokareva VV, Krivulina DA. [Evaluation of the efficacy of antifibrotic drugs on cell cultures in Salzmann's nodular degeneration]. Vestnik oftalmologii. 2024:140(6):80-89. doi: 10.17116/oftalma202414006180. Epub [PubMed PMID: 39731240]
Moshirfar M, Moin KA, Ronquillo Y. Peripheral Hypertrophic Subepithelial Corneal Degeneration. StatPearls. 2025 Jan:(): [PubMed PMID: 39383284]
Ramos MF, Baker J, Atzpodien EA, Bach U, Brassard J, Cartwright J, Farman C, Fishman C, Jacobsen M, Junker-Walker U, Kuper F, Moreno MCR, Rittinghausen S, Schafer K, Tanaka K, Teixeira L, Yoshizawa K, Zhang H. Nonproliferative and Proliferative Lesions of the Ratand Mouse Special Sense Organs(Ocular [eye and glands], Olfactory and Otic). Journal of toxicologic pathology. 2018:31(3 Suppl):97S-214S. doi: 10.1293/tox.31.97S. Epub 2018 Jul 28 [PubMed PMID: 30158741]
Zhang XH, Li X. Effect of rigid gas permeable contact lens on keratoconus progression: a review. International journal of ophthalmology. 2020:13(7):1124-1131. doi: 10.18240/ijo.2020.07.17. Epub 2020 Jul 18 [PubMed PMID: 32685402]
Satitpitakul V, Taweekitikul P, Puangsricharern V, Kasetsuwan N, Reinprayoon U, Kittipibul T. Alteration of corneal biomechanical properties in patients with dry eye disease. PloS one. 2021:16(7):e0254442. doi: 10.1371/journal.pone.0254442. Epub 2021 Jul 12 [PubMed PMID: 34252118]
Riguetto CM, Barbosa EB, Atihe CC, Reis F, Alves M, Zantut-Wittmann DE. Ocular Surface Disease Related to the Inflammatory and Non-Inflammatory Phases of Thyroid Eye Disease. Clinical ophthalmology (Auckland, N.Z.). 2023:17():3465-3475. doi: 10.2147/OPTH.S430861. Epub 2023 Nov 15 [PubMed PMID: 38026592]
Eberwein P, Hiss S, Auw-Haedrich C, Sundmacher R, Hauer K, Boehringer D, Meier P, Reinhard T. Epithelial marker expression in Salzmann nodular degeneration shows characteristics of limbal transient amplifying cells and alludes to an involvement of the epithelium in its pathogenesis. Acta ophthalmologica. 2010 Aug:88(5):e184-9. doi: 10.1111/j.1755-3768.2010.01887.x. Epub 2010 Jun 25 [PubMed PMID: 20583999]
Dixit R, Riviere J, Krishnan K, Andersen ME. Toxicokinetics and physiologically based toxicokinetics in toxicology and risk assessment. Journal of toxicology and environmental health. Part B, Critical reviews. 2003 Jan-Feb:6(1):1-40 [PubMed PMID: 12587252]
Torricelli AA, Wilson SE. Cellular and extracellular matrix modulation of corneal stromal opacity. Experimental eye research. 2014 Dec:129():151-60. doi: 10.1016/j.exer.2014.09.013. Epub 2014 Oct 1 [PubMed PMID: 25281830]
Teo AWJ, Zhang J, Zhou L, Liu YC. Metabolomics in Corneal Diseases: A Narrative Review from Clinical Aspects. Metabolites. 2023 Mar 3:13(3):. doi: 10.3390/metabo13030380. Epub 2023 Mar 3 [PubMed PMID: 36984820]
Level 3 (low-level) evidencePatel S, Mittal R, Kumar N, Galor A. The environment and dry eye-manifestations, mechanisms, and more. Frontiers in toxicology. 2023:5():1173683. doi: 10.3389/ftox.2023.1173683. Epub 2023 Aug 23 [PubMed PMID: 37681211]
Raharja A, Whitefield L. Clinical approach to vision loss: a review for general physicians. Clinical medicine (London, England). 2022 Mar:22(2):95-99. doi: 10.7861/clinmed.2022-0057. Epub [PubMed PMID: 35304366]
Chen H, Mao X, Xu D, Guo C, Dai J. The dynamic changes and influencing factors of visual symptoms after small incision lenticule extraction. BMC ophthalmology. 2023 May 19:23(1):223. doi: 10.1186/s12886-023-02964-8. Epub 2023 May 19 [PubMed PMID: 37208645]
Shah S, Jani H. Prevalence and associated factors of dry eye: Our experience in patients above 40 years of age at a Tertiary Care Center. Oman journal of ophthalmology. 2015 Sep-Dec:8(3):151-6. doi: 10.4103/0974-620X.169910. Epub [PubMed PMID: 26903719]
Kaur K, Gurnani B. Vernal Keratoconjunctivitis. StatPearls. 2025 Jan:(): [PubMed PMID: 35015458]
Sekeroglu HT, Simsek F, Erdem E, Harbiyeli II, Yagmur M, Ersoz R. Coexistence of ocular cicatricial pemphigoid with Stevens Johnson syndrome. International journal of ophthalmology. 2013:6(3):411-2. doi: 10.3980/j.issn.2222-3959.2013.03.30. Epub 2013 Jun 18 [PubMed PMID: 23826544]
Pellegrini M, Bernabei F, Moscardelli F, Vagge A, Scotto R, Bovone C, Scorcia V, Giannaccare G. Assessment of Corneal Fluorescein Staining in Different Dry Eye Subtypes Using Digital Image Analysis. Translational vision science & technology. 2019 Nov:8(6):34. doi: 10.1167/tvst.8.6.34. Epub 2019 Dec 12 [PubMed PMID: 31857917]
Marcon AS, Rapuano CJ. Excimer laser phototherapeutic keratectomy retreatment of anterior basement membrane dystrophy and Salzmann's nodular degeneration with topical mitomycin C. Cornea. 2002 Nov:21(8):828-30 [PubMed PMID: 12410046]
Level 3 (low-level) evidenceHurmeric V, Yoo SH, Galor A, Canto AP, Wang J. Atypical presentation of Salzmann nodular degeneration diagnosed with ultra-high-resolution optical coherence tomography. Ophthalmic surgery, lasers & imaging : the official journal of the International Society for Imaging in the Eye. 2011 Dec 8:42 Online():e122-5. doi: 10.3928/15428877-20111201-05. Epub 2011 Dec 8 [PubMed PMID: 22150601]
Level 3 (low-level) evidenceJaworski A, Arvanitis A. Salzmann's nodular degeneration of the cornea. Clinical & experimental optometry. 1999 Jan-Feb:82(1):14-16 [PubMed PMID: 12482303]
Goerlitz-Jessen MF, Gupta PK, Kim T. Impact of epithelial basement membrane dystrophy and Salzmann nodular degeneration on biometry measurements. Journal of cataract and refractive surgery. 2019 Aug:45(8):1119-1123. doi: 10.1016/j.jcrs.2019.03.014. Epub 2019 Jun 4 [PubMed PMID: 31174985]
Linke S, Kugu C, Richard G, Katz T. An in vivo confocal microscopic analysis of Salzmann's nodular degeneration: pre- and post-surgical intervention. Acta ophthalmologica. 2009 Mar:87(2):233-4. doi: 10.1111/j.1755-3768.2008.01243.x. Epub [PubMed PMID: 19292857]
Level 3 (low-level) evidenceKu JY, Grupcheva CN, McGhee CN. Microstructural analysis of Salzmann's nodular degeneration by in vivo confocal microscopy. Clinical & experimental ophthalmology. 2002 Oct:30(5):367-8 [PubMed PMID: 12213164]
Level 3 (low-level) evidenceMeltendorf C, Bühren J, Bug R, Ohrloff C, Kohnen T. Correlation between clinical in vivo confocal microscopic and ex vivo histopathologic findings of Salzmann nodular degeneration. Cornea. 2006 Jul:25(6):734-8 [PubMed PMID: 17077670]
Level 3 (low-level) evidenceKaur K, Gurnani B. Cycloplegic and Noncycloplegic Refraction. StatPearls. 2025 Jan:(): [PubMed PMID: 35593830]
Kook KY, Jin R, Li L, Yoon HJ, Yoon KC. Tear Osmolarity and Matrix Metallopeptidase-9 in Dry Eye Associated with Sjögren's Syndrome. Korean journal of ophthalmology : KJO. 2020 Jun:34(3):179-186. doi: 10.3341/kjo.2019.0145. Epub [PubMed PMID: 32495525]
Nishant P, Gurnani B, Singh P, Sinha S, Kaur K, Kumar A, Sinha RK. Current concepts and recent trends in endothelial keratoplasty. World journal of transplantation. 2025 Jun 18:15(2):102507. doi: 10.5500/wjt.v15.i2.102507. Epub [PubMed PMID: 40535500]
Kumar A, Sahu V, Misra S, Rathore V, Gurnani B, Kaur K. Changes in various ocular parameters of patients undergoing hemodialysis. European journal of ophthalmology. 2025 Jun 18:():11206721251349681. doi: 10.1177/11206721251349681. Epub 2025 Jun 18 [PubMed PMID: 40530962]
Ong Tone S, Kocaba V, Böhm M, Wylegala A, White TL, Jurkunas UV. Fuchs endothelial corneal dystrophy: The vicious cycle of Fuchs pathogenesis. Progress in retinal and eye research. 2021 Jan:80():100863. doi: 10.1016/j.preteyeres.2020.100863. Epub 2020 May 8 [PubMed PMID: 32438095]
Scorsetti MM, Eguiza VS, Durán JA. Manual Superficial Keratectomy Is the First Choice Treatment for Salzmann Nodular Degeneration. Cornea. 2024 Jun 1:43(6):716-719. doi: 10.1097/ICO.0000000000003413. Epub 2023 Oct 26 [PubMed PMID: 37889535]
Roszkowska AM, Colosi P, De Grazia L, Mirabelli E, Romeo G. One year outcome of manual alcohol-assisted removal of Salzmann's nodular degeneration. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2009 Oct:247(10):1431-4. doi: 10.1007/s00417-009-1154-y. Epub 2009 Aug 6 [PubMed PMID: 19657667]
Level 3 (low-level) evidenceGore DM, Iovieno A, Connell BJ, Alexander R, Meligonis G, Dart JK. Peripheral hypertrophic subepithelial corneal degeneration: nomenclature, phenotypes, and long-term outcomes. Ophthalmology. 2013 May:120(5):892-8. doi: 10.1016/j.ophtha.2012.10.037. Epub 2013 Mar 6 [PubMed PMID: 23474249]
Level 3 (low-level) evidenceSchargus M, Kusserow C, Schlötzer-Schrehardt U, Hofmann-Rummelt C, Schlunck G, Geerling G. Peripheral hypertrophic subepithelial corneal degeneration presenting with bilateral nasal and temporal corneal changes. Eye (London, England). 2015 Jan:29(1):88-97. doi: 10.1038/eye.2014.236. Epub 2014 Oct 3 [PubMed PMID: 25277306]
Malta JB, Soong HK. Diamond burr superficial keratectomy in the treatment of visually-significant anterior corneal lesions. Arquivos brasileiros de oftalmologia. 2008 May-Jun:71(3):415-8 [PubMed PMID: 18641833]
Level 2 (mid-level) evidenceDas S, Langenbucher A, Pogorelov P, Link B, Seitz B. Long-term outcome of excimer laser phototherapeutic keratectomy for treatment of Salzmann's nodular degeneration. Journal of cataract and refractive surgery. 2005 Jul:31(7):1386-91 [PubMed PMID: 16105611]
Level 2 (mid-level) evidenceGermundsson J, Fagerholm P. Phototherapeutic keratectomy in Salzmann's nodular degeneration. Acta ophthalmologica Scandinavica. 2004 Apr:82(2):148-53 [PubMed PMID: 15043531]
Level 3 (low-level) evidenceKnox DL, Snip RC, Stark WJ. The keratopathy of Crohn's disease. American journal of ophthalmology. 1980 Dec:90(6):862-5 [PubMed PMID: 7446674]
Level 3 (low-level) evidenceBell K, de Padua Soares Bezerra B, Mofokeng M, Montesano G, Nongpiur ME, Marti MV, Lawlor M. Learning from the past: Mitomycin C use in trabeculectomy and its application in bleb-forming minimally invasive glaucoma surgery. Survey of ophthalmology. 2021 Jan-Feb:66(1):109-123. doi: 10.1016/j.survophthal.2020.05.005. Epub 2020 May 23 [PubMed PMID: 32450159]
Level 3 (low-level) evidenceFini ME, Girard MT, Matsubara M. Collagenolytic/gelatinolytic enzymes in corneal wound healing. Acta ophthalmologica. Supplement. 1992:(202):26-33 [PubMed PMID: 1322008]
Level 3 (low-level) evidenceBowers PJ Jr, Price MO, Zeldes SS, Price FW Jr. Superficial keratectomy with mitomycin-C for the treatment of Salzmann's nodules. Journal of cataract and refractive surgery. 2003 Jul:29(7):1302-6 [PubMed PMID: 12900236]
Level 2 (mid-level) evidenceMahler SBC, Adams C, Daas L, Langenbucher A, Seitz B. Phototherapeutic Keratectomy for Salzmann's Nodular Degeneration. What Effect Does the Choice of Excimer Laser Have on Treatment Success? Klinische Monatsblatter fur Augenheilkunde. 2023 Nov:240(11):1262-1268. doi: 10.1055/a-1788-3819. Epub 2022 May 18 [PubMed PMID: 35584772]
Sharma N, Prakash G, Titiyal JS, Vajpayee RB. Comparison of automated lamellar keratoplasty and phototherapeutic keratectomy for Salzmann nodular degeneration. Eye & contact lens. 2012 Mar:38(2):109-11. doi: 10.1097/ICL.0b013e318243e813. Epub [PubMed PMID: 22249432]
Level 2 (mid-level) evidenceSinha R, Chhabra MS, Vajpayee RB, Kashyap S, Tandon R. Recurrent Salzmann's nodular degeneration: report of two cases and review of literature. Indian journal of ophthalmology. 2006 Sep:54(3):201-2 [PubMed PMID: 16921221]
Level 3 (low-level) evidenceMoshirfar M, Wang Q, Theis J, Porter KC, Stoakes IM, Payne CJ, Hoopes PC. Management of Corneal Haze After Photorefractive Keratectomy. Ophthalmology and therapy. 2023 Dec:12(6):2841-2862. doi: 10.1007/s40123-023-00782-1. Epub 2023 Aug 21 [PubMed PMID: 37603162]
Zhang JH, Ramke J, Lee CN, Gordon I, Safi S, Lingham G, Evans JR, Keel S. A Systematic Review of Clinical Practice Guidelines for Cataract: Evidence to Support the Development of the WHO Package of Eye Care Interventions. Vision (Basel, Switzerland). 2022 Jun 20:6(2):. doi: 10.3390/vision6020036. Epub 2022 Jun 20 [PubMed PMID: 35737423]
Level 1 (high-level) evidenceBudning A, El-Defrawy S. Interprofessional collaboration in eye health care. Canadian journal of ophthalmology. Journal canadien d'ophtalmologie. 2016 Jun:51(3):130-2. doi: 10.1016/j.jcjo.2016.04.007. Epub [PubMed PMID: 27316255]
Auteri N, Presa M, Pierson K, Kurji K, Cheung A, Holland E. Peripheral hypertrophic subepithelial corneal degeneration versus Salzmann's nodular degeneration: A clinical and surgical comparison. The ocular surface. 2022 Jan:23():71-73. doi: 10.1016/j.jtos.2021.11.009. Epub 2021 Nov 24 [PubMed PMID: 34838722]
Khan T, Usman Y, Abdo T, Chaudry F, Keddissi JI, Youness HA. Diagnosis and management of peripheral lung nodule. Annals of translational medicine. 2019 Aug:7(15):348. doi: 10.21037/atm.2019.03.59. Epub [PubMed PMID: 31516894]
Burrow MK, Gurnani B, Patel BC. Keratoconjunctivitis. StatPearls. 2025 Jan:(): [PubMed PMID: 31194419]
Wang ZW, Yin XF, Wang CX, Wang HZ, Zhou SY. Anterior segment optical coherence tomography for superficial keratectomy. Photodiagnosis and photodynamic therapy. 2024 Aug:48():104237. doi: 10.1016/j.pdpdt.2024.104237. Epub 2024 Jun 12 [PubMed PMID: 38871017]
Fung AT, Tran T, Lim LL, Samarawickrama C, Arnold J, Gillies M, Catt C, Mitchell L, Symons A, Buttery R, Cottee L, Tumuluri K, Beaumont P. Local delivery of corticosteroids in clinical ophthalmology: A review. Clinical & experimental ophthalmology. 2020 Apr:48(3):366-401. doi: 10.1111/ceo.13702. Epub 2020 Jan 22 [PubMed PMID: 31860766]
Brown AC, Nataneli N. Salzmanns Nodular Corneal Degeneration. StatPearls. 2025 Jan:(): [PubMed PMID: 32809519]
Ames P, Galor A. Cyclosporine ophthalmic emulsions for the treatment of dry eye: a review of the clinical evidence. Clinical investigation. 2015:5(3):267-285 [PubMed PMID: 25960865]
Tan KR, Magill AJ, Parise ME, Arguin PM, Centers for Disease Control and Prevention. Doxycycline for malaria chemoprophylaxis and treatment: report from the CDC expert meeting on malaria chemoprophylaxis. The American journal of tropical medicine and hygiene. 2011 Apr:84(4):517-31. doi: 10.4269/ajtmh.2011.10-0285. Epub [PubMed PMID: 21460003]
Zare M, Jafarinasab MR, Feizi S, Zamani M. The effect of mitomycin-C on corneal endothelial cells after photorefractive keratectomy. Journal of ophthalmic & vision research. 2011 Jan:6(1):8-12 [PubMed PMID: 22454700]
Moshirfar M, West WB Jr, Milner DC, McCabe SE, Ronquillo YC, Hoopes PC. Delayed Epithelial Healing with Corneal Edema and Haze After Photorefractive Keratectomy Using Intraoperative Mitomycin C. International medical case reports journal. 2021:14():863-870. doi: 10.2147/IMCRJ.S342774. Epub 2021 Dec 24 [PubMed PMID: 34992474]
Level 3 (low-level) evidenceAmm M, Duncker GI. Refractive changes after phototherapeutic keratectomy. Journal of cataract and refractive surgery. 1997 Jul-Aug:23(6):839-44 [PubMed PMID: 9292665]
Gurnani B, Kaur K. Pythium Keratitis. StatPearls. 2025 Jan:(): [PubMed PMID: 34424645]
Gurnani B, Kaur K. Contact Lens–Related Complications. StatPearls. 2025 Jan:(): [PubMed PMID: 36512659]
Shan J, DeBoer C, Xu BY. Anterior Segment Optical Coherence Tomography: Applications for Clinical Care and Scientific Research. Asia-Pacific journal of ophthalmology (Philadelphia, Pa.). 2019 MarchApril 01:8(2):146-157. doi: 10.22608/APO.201910. Epub [PubMed PMID: 31020820]
Venkateswaran N, Luna RD, Gupta PK. Ocular surface optimization before cataract surgery. Saudi journal of ophthalmology : official journal of the Saudi Ophthalmological Society. 2022 Apr-Jun:36(2):142-148. doi: 10.4103/sjopt.sjopt_190_21. Epub 2022 Aug 29 [PubMed PMID: 36211316]
Khaireddin R, Katz T, Baile RB, Richard G, Linke SJ. Superficial keratectomy, PTK, and mitomycin C as a combined treatment option for Salzmann's nodular degeneration: a follow-up of eight eyes. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2011 Aug:249(8):1211-5. doi: 10.1007/s00417-011-1643-7. Epub 2011 Apr 5 [PubMed PMID: 21465289]
Level 2 (mid-level) evidenceKoch DD. Impact of Salzmann's lesions on corneal curvature. Journal of cataract and refractive surgery. 1995 Mar:21(2):111-2 [PubMed PMID: 7791043]
Messina M, Poddi M, De Santi N, Bianchi E, Cagini C. Combined superficial keratectomy, alcohol delamination and amniotic membrane patch with fibrin glue in Salzmann nodular degeneration. European journal of ophthalmology. 2024 Jul:34(4):1281-1285. doi: 10.1177/11206721241251890. Epub 2024 Apr 26 [PubMed PMID: 38676318]
Thorgrimson JL, Hessert DD. Salzmann's Nodular Degeneration in a Pilot Applicant. Aerospace medicine and human performance. 2023 May 1:94(5):400-403. doi: 10.3357/AMHP.6184.2023. Epub [PubMed PMID: 37069748]
Level 3 (low-level) evidenceGurnani B, Kaur K. Changing drug regimen during COVID-19 pandemic lockdown: An experience from a Tertiary Eye Care Hospital as a cornea specialist. Indian journal of pharmacology. 2020 Sep-Oct:52(5):435-436. doi: 10.4103/ijp.IJP_688_20. Epub [PubMed PMID: 33283776]
Gurnani B, Kaur K. Anti-infective therapies for Pythium insidiosum keratitis. Expert review of anti-infective therapy. 2024 Oct:22(10):805-817. doi: 10.1080/14787210.2024.2403146. Epub 2024 Sep 13 [PubMed PMID: 39268901]
Gurnani B, Kaur K. Contact Lenses. StatPearls. 2025 Jan:(): [PubMed PMID: 35593861]
Gurnani B, Kaur K. Intricacies and solutions for interpretation of microbiologic samples of Pythium insidiosum keratitis. Indian journal of ophthalmology. 2024 Apr 1:72(4):602-603. doi: 10.4103/IJO.IJO_1573_23. Epub 2024 Mar 28 [PubMed PMID: 38546474]
Agarwal S. Collaboration in ocular surface diseases: Bridging specialties for better care. Indian journal of ophthalmology. 2025 Apr 1:73(4):465. doi: 10.4103/IJO.IJO_539_25. Epub 2025 Mar 27 [PubMed PMID: 40146133]
Kaur K, Gurnani B. Low Vision Aids. StatPearls. 2025 Jan:(): [PubMed PMID: 36256771]
Shields CL, Shields JA. Tumors of the conjunctiva and cornea. Indian journal of ophthalmology. 2019 Dec:67(12):1930-1948. doi: 10.4103/ijo.IJO_2040_19. Epub [PubMed PMID: 31755426]
Eom Y, Na KS, Hwang HS, Cho KJ, Chung TY, Jun RM, Ko BY, Chun YS, Kim HS, Song JS. Clinical efficacy of eyelid hygiene in blepharitis and meibomian gland dysfunction after cataract surgery: a randomized controlled pilot trial. Scientific reports. 2020 Jul 16:10(1):11796. doi: 10.1038/s41598-020-67888-5. Epub 2020 Jul 16 [PubMed PMID: 32678131]
Level 1 (high-level) evidenceJavidi H, Poonit N, Patel RP, Barry RJ, Rauz S, Murray PI. Adherence to Topical Medication in Patients with Inflammatory Eye Disease. Ocular immunology and inflammation. 2021 Jul 4:29(5):890-895. doi: 10.1080/09273948.2019.1699122. Epub 2020 Jan 16 [PubMed PMID: 31944132]
Lopez Montes T, Gurnani B, Stokkermans TJ. Assessment of the Watery Eye. StatPearls. 2025 Jan:(): [PubMed PMID: 36508543]
Lievens CW, Cilimberg KC, Moore A. Contact lens care tips for patients: an optometrist's perspective. Clinical optometry. 2017:9():113-121. doi: 10.2147/OPTO.S139651. Epub 2017 Aug 11 [PubMed PMID: 30214367]
Level 3 (low-level) evidenceWeiss JS, Rapuano CJ, Seitz B, Busin M, Kivelä TT, Bouheraoua N, Bredrup C, Nischal KK, Chawla H, Borderie V, Kenyon KR, Kim EK, Møller HU, Munier FL, Berger T, Lisch W. IC3D Classification of Corneal Dystrophies-Edition 3. Cornea. 2024 Apr 1:43(4):466-527. doi: 10.1097/ICO.0000000000003420. Epub 2024 Feb 12 [PubMed PMID: 38359414]
Digre KB, Brennan KC. Shedding light on photophobia. Journal of neuro-ophthalmology : the official journal of the North American Neuro-Ophthalmology Society. 2012 Mar:32(1):68-81. doi: 10.1097/WNO.0b013e3182474548. Epub [PubMed PMID: 22330853]
Al-Dossary SK. Environmental and Occupational Triggers of Dry Eye Symptoms in the Ahsa Region of Saudi Arabia: A Cross-Sectional Study. Clinical ophthalmology (Auckland, N.Z.). 2024:18():2427-2438. doi: 10.2147/OPTH.S474832. Epub 2024 Aug 29 [PubMed PMID: 39224176]
Level 2 (mid-level) evidenceKaur RP, Gurnani B, Kaur K. Intricate insights into immune response in dry eye disease. Indian journal of ophthalmology. 2023 Apr:71(4):1248-1255. doi: 10.4103/IJO.IJO_481_23. Epub [PubMed PMID: 37026255]
Gurnani B, Kaur K, Gireesh P, Balakrishnan L, Mishra C. Evaluating the novel role of ChatGPT-4 in addressing corneal ulcer queries: An AI-powered insight. European journal of ophthalmology. 2025 Sep:35(5):1531-1541. doi: 10.1177/11206721251337290. Epub 2025 Apr 28 [PubMed PMID: 40295112]
Chaudhary S, Ghimire D, Basu S, Agrawal V, Jacobs DS, Shanbhag SS. Contact lenses in dry eye disease and associated ocular surface disorders. Indian journal of ophthalmology. 2023 Apr:71(4):1142-1153. doi: 10.4103/IJO.IJO_2778_22. Epub [PubMed PMID: 37026246]
Gurnani B, Kaur K. Navigating the challenges of infective keratitis: A critical analysis of treatment and diagnostic approaches. Indian journal of ophthalmology. 2024 Aug 1:72(8):1227-1228. doi: 10.4103/IJO.IJO_706_24. Epub 2024 Jul 29 [PubMed PMID: 39078975]
Venkateswaran N, Galor A, Wang J, Karp CL. Optical coherence tomography for ocular surface and corneal diseases: a review. Eye and vision (London, England). 2018:5():13. doi: 10.1186/s40662-018-0107-0. Epub 2018 Jun 12 [PubMed PMID: 29942817]
Pathak M, Sahu V, Kumar A, Kaur K, Gurnani B. Current Concepts and Recent Updates of Optical Biometry- A Comprehensive Review. Clinical ophthalmology (Auckland, N.Z.). 2024:18():1191-1206. doi: 10.2147/OPTH.S464538. Epub 2024 May 2 [PubMed PMID: 38711575]
Gurnani B, Badri T, Hafsi W. Phthiriasis Palpebrarum. StatPearls. 2025 Jan:(): [PubMed PMID: 29083779]
Gurnani B, Kaur K, Lalgudi VG, Kundu G, Mimouni M, Liu H, Jhanji V, Prakash G, Roy AS, Shetty R, Gurav JS. Role of artificial intelligence, machine learning and deep learning models in corneal disorders - A narrative review. Journal francais d'ophtalmologie. 2024 Sep:47(7):104242. doi: 10.1016/j.jfo.2024.104242. Epub 2024 Jul 15 [PubMed PMID: 39013268]
Level 3 (low-level) evidenceRobertson ST, Rosbergen ICM, Burton-Jones A, Grimley RS, Brauer SG. The Effect of the Electronic Health Record on Interprofessional Practice: A Systematic Review. Applied clinical informatics. 2022 May:13(3):541-559. doi: 10.1055/s-0042-1748855. Epub 2022 Jun 1 [PubMed PMID: 35649501]
Level 1 (high-level) evidenceChorny A, Gershoni A, Mahler O, Sorkin N, Nahum Y, Sella R, Bahar I, Livny E. Corneal pseudoectasia: a case series. International ophthalmology. 2024 Feb 7:44(1):17. doi: 10.1007/s10792-024-02992-3. Epub 2024 Feb 7 [PubMed PMID: 38321320]
Level 2 (mid-level) evidence