Neuroanatomy, Pupillary Light Reflexes and Pathway
Neuroanatomy, Pupillary Light Reflexes and Pathway
Introduction
The visual (retino-thalamo-cortical) and pupillary light reflex (PLR) pathways are essential for the eye to perceive and respond to environmental changes. Signals pass through multiple relays from the cornea to the brain, and lesions along either pathway can produce visual dysfunction. Key structures include the cornea, lens, retina, optic nerve (cranial nerve II), optic chiasm, optic tract, pretectal nuclei, Edinger-Westphal nuclei, oculomotor nerve (cranial nerve III), and pupillary muscles.
Understanding the visual and PLR pathways enables clinicians to localize lesions within the afferent or efferent limbs by evaluating direct and consensual pupillary responses. This anatomical insight aids in diagnosing pathologies of the optic and oculomotor nerves and midbrain. Surgeons familiar with these pathways can better protect parasympathetic and sympathetic fibers during orbital, optic canal, or midbrain surgeries, helping preserve pupillary and visual function.
Structure and Function
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Structure and Function
Anatomical Components of the Visual and Pupillary Light Reflex Pathways
The visual and pupillary reflex pathways work in concert to process environmental light and adjust ocular responses instantaneously. These mechanisms preserve visual precision and protect ocular tissues through involuntary control of the pupil. Central to this regulation is the PLR, which translates retinal stimulation into precise responses in the midbrain to modulate pupil diameter. The critical anatomical components are discussed below.
Cornea
The cornea is the transparent anterior layer of the eye and the first structure encountered by incoming light. Owing to the significant difference in refractive index between air and corneal tissue, this structure accounts for approximately 65% of the eye’s total refractive power. Despite its major role in focusing light, the cornea’s optical power is fixed and does not accommodate to changes in focus.[1][2]
Iris and Pupil
The iris, the colored part of the eye located just behind the anterior chamber, surrounds the pupil—a dynamic opening that controls the amount of light entering the eye. Acting like an adjustable diaphragm, the iris regulates pupil size through 2 involuntary muscles: the sphincter pupillae, which constricts the pupil under parasympathetic control, and the dilator pupillae, which dilates the pupil via sympathetic innervation. This modulation protects retinal tissue from excessive light exposure while optimizing visual acuity across varying lighting conditions.[3]
Lens
The lens and ciliary muscle act together to enable accommodation, allowing precise retinal focus for objects at varying distances. The accommodation reflex occurs when contraction of the ciliary muscle relaxes the zonular fibers attached to the lens. This relaxation allows the lens to become more spherical, increasing its axial thickness and dioptric power, thereby bringing the focus, initially behind the retina, onto the retina. The ciliary muscle receives parasympathetic input via the short ciliary nerve, and its contraction enables the eye to accommodate to nearer objects. Tension on the zonular fibers increases when the ciliary muscle relaxes, causing the lens to flatten and reducing its dioptric power.
The ability to accommodate progressively declines with age. Presbyopia, a condition commonly affecting older individuals, arises as the lens becomes denser and less flexible, impairing its ability to change shape and focus on near objects.[4][5][6]
Retina
Light entering the retina traverses multiple inner retinal layers before reaching the outer segments of rods and cones, the specialized regions where phototransduction occurs. Photon absorption initiates a cascade within these photoreceptors, leading to cell hyperpolarization and a decrease in glutamate release. This reduction modulates synaptic transmission to downstream bipolar and horizontal cells, which in turn relay signals to ganglion cells. The axons of ganglion cells form the retinal nerve fiber layer and project through the optic nerve.
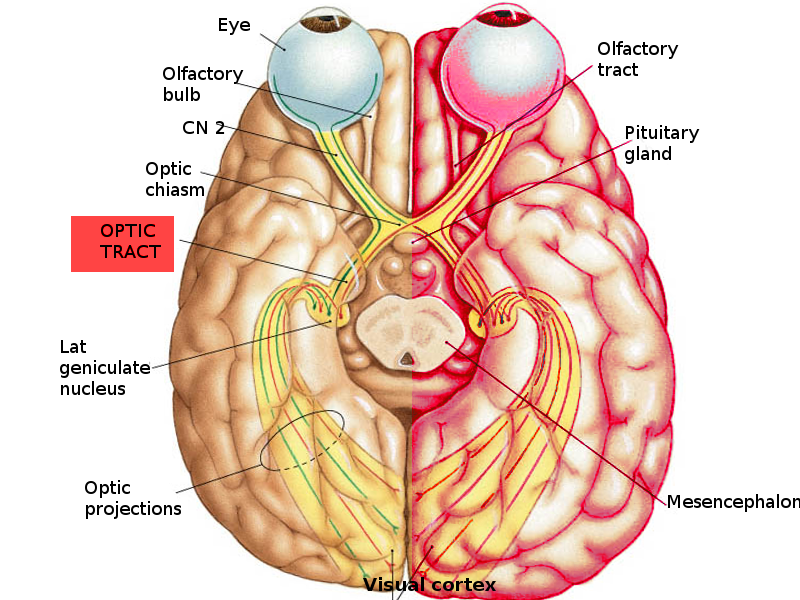
Optic Nerve, Chiasm, and Tracts
The optic nerve consists of unmyelinated axons of retinal ganglion cells that converge at the optic disc. The absence of photoreceptors at the disc produces a physiologic blind spot in the visual field. Optic nerve axons become myelinated by oligodendrocytes as they traverse the lamina cribrosa posteriorly.
The optic nerves from both eyes converge at the optic chiasm, located anterior to the pituitary gland, and continue as the optic tracts (see Image. Visual Pathway). Nasal retinal fibers decussate at the chiasm to join the contralateral tract, whereas temporal retinal fibers remain uncrossed and proceed ipsilaterally. Therefore, each optic tract contains temporal fibers from the ipsilateral retina and nasal fibers from the contralateral retina. This organization allows the left cerebral hemisphere to process input from the right visual field and the right hemisphere to process input from the left field.[7][8]
Lateral Geniculate Nucleus
Most optic tract fibers synapse in the ipsilateral lateral geniculate nucleus (LGN) of the thalamus, where image processing occurs. A smaller subset of fibers bypasses the LGN and projects to the pretectal nucleus of the midbrain, contributing to the PLR. From the LGN, axons project to the primary visual cortex via the geniculocalcarine tract, forming 2 major bundles, the temporal and parietal radiations. The temporal radiations, also known as the Meyer loop, transmit information from the contralateral superior visual field, while the parietal radiations, or the Baum loop, carry information from the contralateral inferior visual field.
Calcarine Cortex
Electrochemical signals terminate in the calcarine cortex (Brodmann area 17), the primary visual cortex of the occipital lobe. Further integration in adjacent visual association areas (Brodmann areas 18 and 19) enables higher-order perception and recognition of visual stimuli.
Mechanism of the Pupillary Light Reflex
The PLR pathway parallels the visual pathway, but the optic tract fibers involved terminate in the pretectal nucleus of the midbrain rather than the LGN of the thalamus. Nasal retinal fibers decussate at the optic chiasm and project to the contralateral pretectal nucleus, whereas temporal retinal fibers project to the ipsilateral pretectal nucleus (see Image. Pupillary Light Reflex Neural Pathway).
Each pretectal nucleus projects bilaterally to the Edinger-Westphal nuclei of the oculomotor nerve. Activation of the Edinger-Westphal nuclei initiates the efferent limb of the reflex by generating action potentials in preganglionic parasympathetic neurons. These axons travel through the oculomotor nerve to synapse with postganglionic neurons in the ciliary ganglion. The short ciliary nerves arising from the ciliary ganglion then stimulate the pupillary sphincter muscle, producing pupillary constriction.
Illumination of one eye produces a consensual response in the contralateral eye because nasal retinal fibers cross at the optic chiasm to reach the contralateral pretectal nucleus, and each pretectal nucleus projects bilaterally to both Edinger-Westphal nuclei. Each Edinger-Westphal nucleus controls ipsilateral pupillary constriction, and, together, they mediate the bilateral PLR.
In dim light, pupillary dilation occurs through contraction of the pupillary dilator muscle. This response is mediated by postganglionic sympathetic fibers that travel via the long ciliary nerves.[9][10][11]
Embryology
Ocular development is a highly coordinated process that begins around the 3rd week of gestation and continues through the 10th week, although structures such as the retina and optic nerve continue to mature after birth. The eye arises from tissues derived from the neuroectoderm, surface ectoderm, mesoderm, and neural crest, with each contributing to distinct anatomical components.
The earliest visible event is the appearance of optic grooves on either side of the developing forebrain. These grooves evaginate to form paired optic vesicles, which originate from the neuroectoderm of the diencephalon. As the optic vesicle extends laterally toward the surface ectoderm, inductive interactions trigger thickening of the overlying ectoderm to form the lens placode. This specialized ectodermal region subsequently invaginates to create the lens pit and ultimately pinches off as the lens vesicle, which develops into the crystalline lens and the anterior corneal epithelium.[12]
Concurrently, the distal portion of the optic vesicle invaginates to form a double-walled optic cup, while the proximal portion constricts to become the optic stalk. The inner layer of the optic cup differentiates into the neural retina, containing photoreceptors, bipolar cells, ganglion cells, and interneurons. The outer layer develops into the retinal pigment epithelium, which is essential for photoreceptor maintenance and visual cycle metabolism. The rim of the optic cup gives rise to the ciliary body and iris, including the sphincter and dilator pupillae muscles, which derive from neural crest cells and neuroectoderm.[13]
The optic stalk serves as the conduit for developing axons of retinal ganglion cells, which extend centrally toward the brain. These axons eventually form the optic nerve, with myelination by oligodendrocytes beginning after birth and progressing from the optic chiasm toward the globe, terminating at the lamina cribrosa.
Mesoderm contributes to the formation of the sclera, extraocular muscles, and portions of the vascular tunic. Neural crest cells migrate into the anterior segment to generate the corneal stroma and endothelium, the trabecular meshwork, and the connective tissue components of the uvea.[14]
The developing eye is initially nourished by the hyaloid vascular system, composed of the hyaloid artery and vein, which pass through the optic stalk into the vitreous cavity to supply the lens and inner retina. With maturation, the retinal vasculature develops and the hyaloid system regresses, leaving the central retinal artery and vein as its adult derivatives.[15]
This developmental sequence is regulated by a network of transcription factors and signaling molecules, including PAX6, SOX2, OTX2, MITF, and SHH. Disruption at any stage can lead to congenital ocular malformations, including anophthalmia, microphthalmia, colobomas from incomplete closure of the embryonic fissure, and anterior segment dysgenesis resulting from abnormal neural crest migration.[16][17]
Blood Supply and Lymphatics
The ophthalmic artery is the 1st branch of the internal carotid artery and supplies the majority of ocular structures. Accompanied by the optic nerve, it passes through the optic canal to enter the orbit. The artery's major branches include the central retinal artery and anterior and posterior ciliary arteries. The central retinal artery penetrates the meninges from the inferior aspect of the optic nerve and emerges at the optic disc to supply the inner retinal layers. The posterior ciliary arteries supply the optic nerve head, choroid, and outer retinal layers, while the anterior ciliary arteries provide the principal blood supply to the iris and ciliary body.
Venous drainage of the retina occurs through the central retinal vein, which runs temporal to the central retinal artery near the optic nerve head. Blood from the central retinal vein empties into the superior ophthalmic vein and subsequently into the cavernous sinus. The choroid drains via the vortex veins, which converge into the superior and inferior ophthalmic veins before reaching the cavernous sinus.[18]
Nerves
The optic nerve is the primary pathway for transmitting visual information to the brain. The PLR requires both the optic and oculomotor nerves, which act as the afferent and efferent limbs, respectively, to constrict the pupil in response to light. The oculomotor nerve also innervates the ciliary muscle, responsible for lens accommodation, and the sphincter pupillae muscle, which controls pupillary constriction. In contrast, the dilator pupillae muscle is innervated by postganglionic sympathetic fibers originating from the superior cervical ganglion. These fibers travel along the long ciliary nerve, a branch of the nasociliary nerve of the ophthalmic division of the trigeminal nerve (cranial nerve V).
Muscles
Two intraocular muscles regulate pupillary size within the PLR pathway: the sphincter pupillae and the dilator pupillae. The coordinated activity of these muscles enables dynamic adjustment of pupil size to optimize visual performance across varying light environments.[19]
The sphincter pupillae, composed of concentric circular fibers, receives parasympathetic innervation via the short ciliary nerves arising from the ciliary ganglion. Contraction of this muscle produces pupillary constriction (miosis), reducing retinal light exposure in response to increased illumination. In contrast, the dilator pupillae consists of radially oriented smooth muscle fibers supplied by sympathetic postganglionic fibers from the superior cervical ganglion, which travel with the long ciliary nerves. Contraction of the dilator pupillae induces pupillary dilation (mydriasis), increasing retinal illumination under low-light conditions.[20]
Surgical Considerations
Surgical procedures involving structures of the PLR pathway require careful planning to avoid damage that could impair pupillary function. Anterior segment surgeries, including cataract extraction, corneal transplantation, glaucoma filtering procedures, and refractive surgeries, pose risks to the iris sphincter and dilator muscles. Intraoperative trauma, thermal injury, or inadvertent manipulation may cause irregular pupil shape, impaired constriction, abnormal dilation, or permanent mydriasis from sphincter muscle injury.
Extraocular surgeries near the orbital apex or cavernous sinus, such as orbital decompression or tumor resection, may endanger sympathetic and parasympathetic fibers essential for pupillary control. Neurosurgical procedures addressing lesions near the midbrain, particularly in the region of the Edinger-Westphal nucleus or pretectal nuclei, demand precise technique and vigilant monitoring, as injury to these structures can produce permanent deficits in the PLR pathway. Understanding the anatomical relationships within this pathway is critical for preoperative planning, patient counseling, and intraoperative decision-making, supporting preservation of both visual function and pupillary integrity.[21][22]
Clinical Significance
The visual and PLR pathways are complex, coordinated systems where multiple components participate with precision. Even one lesion in the pathway can severely deteriorate the quality of vision. The location of the lesion is associated with the extent and type of vision deficit (see Images. Visual Field Defects From Anterior Pathway Lesions; Visual Field Defects From Posterior Pathway Lesions).
Optic Nerve
A lesion of the optic nerve anterior to the optic chiasm produces vision loss in the ipsilateral eye. For example, injury to the right optic nerve causes right anopia, eliminating the entire visual field of the right eye. Such lesions disrupt reflex function since the optic nerve also contributes to the afferent limb of the PLR. Pupillary constriction is absent in both eyes when light is directed into the affected eye, as the retinal signal cannot reach central processing centers. In contrast, illumination of the unaffected eye produces bilateral pupillary constriction, confirming that the optic nerve, rather than the oculomotor nerve, is compromised.
Oculomotor Nerve
Damage to the oculomotor nerve impairs parasympathetic output, leaving sympathetic activity unopposed. Clinical findings include pupillary dilation, loss or reduction of the PLR, and impaired lens accommodation. Light directed into the eye with a damaged oculomotor nerve produces a retinal signal that reaches both Edinger-Westphal nuclei, but only the contralateral pupil constricts because the efferent pathway on the affected side is disrupted. Illumination of the contralateral eye also results in constriction only of that eye, with no response from the eye with the oculomotor lesion.
Visual System
Lesions of the optic chiasm result in bitemporal hemianopia. The most common causes are pituitary tumors, given the close relationship of the chiasm to the pituitary gland, and aneurysms of the anterior communicating artery. Nasal retinal fibers, which transmit information from the temporal visual fields, decussate at the optic chiasm, while temporal retinal fibers remain uncrossed. Injury at this site selectively disrupts the crossing nasal fibers, producing loss of the temporal visual fields in both eyes. The temporal visual field projects onto the nasal retina since the retinal image is inverted and reversed.
Damage posterior to the chiasm, at the optic tract, produces contralateral homonymous hemianopia. Each optic tract contains fibers from the ipsilateral temporal retina and contralateral nasal retina. For example, injury to the right optic tract interrupts the right temporal retinal fibers and left nasal retinal fibers, resulting in loss of the left visual field in both eyes (left homonymous hemianopia). Optic tract lesions may arise from tumors, demyelinating disease, or infarction involving the anterior portion of the posterior cerebral artery or the posterior communicating artery.
LGN lesions produce contralateral homonymous hemianopia. This structure functions as the primary thalamic relay for visual signals. The LGN has a layered organization receiving segregated inputs from each eye. Thus, damage to this structure may cause sectoral or wedge-shaped visual field defects rather than complete hemianopia. LGN pathology may result from thalamic infarction, neoplasm, or demyelinating disease.
Injury to the optic radiations produces visual field loss that depends on the affected portion. The optic radiations extend from the LGN to the primary visual cortex. Inferior fibers, known as the Meyer loop, course anteriorly into the temporal lobe and convey information from the superior visual quadrants. Temporal lobe lesions involving the Meyer loop cause contralateral superior quadrantanopia (“pie in the sky” defect). Superior fibers pass more directly through the parietal lobe and carry inferior visual field information. Lesions in this region produce contralateral inferior quadrantanopia (“pie on the floor” defect).
Damage to the primary visual cortex (striate cortex) in the occipital lobe results in contralateral homonymous hemianopia. Posterior cerebral artery occlusion often results in macular sparing, as the occipital pole representing the macula commonly receives dual blood supply from both the posterior and middle cerebral arteries. Bilateral involvement of the visual cortex can lead to cortical blindness with preserved PLRs.
Other Issues
Pupillary Light Reflex Evaluation in Trauma Settings
The PLR is an essential element of neurological assessment in trauma, reflecting brainstem function and the integrity of afferent and efferent pathways. Increased intracranial pressure can impair this reflex by compressing brainstem centers, often causing sluggish or absent pupillary responses, which serve as a critical indicator in trauma for monitoring neurological deterioration.
Manual pupil evaluation with a penlight is widely used but is subjective and influenced by confounders such as drug effects or ocular trauma. Automated pupillometry provides objective, quantitative measurements with greater reliability. Studies demonstrate that the PLR, in combination with Glasgow Coma Scale scores, predicts outcomes in traumatic brain injury, although no single assessment method has shown clear superiority in prognostic accuracy. (Source: Butt et al, 2021)
Infectious Disorders Affecting Pupillary Responses
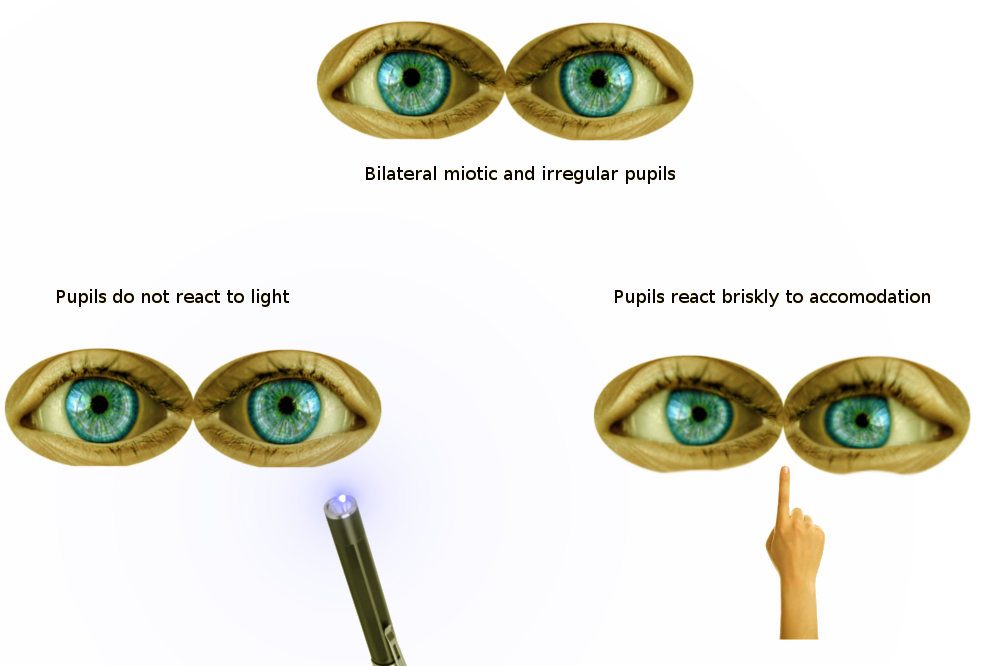
Several infections can impair the PLR by targeting specific regions of the reflex pathway. In neurosyphilis, damage to midbrain interneurons in the pretectal nucleus produces the Argyll Robertson pupil, characterized by absent or markedly reduced light reflex with preserved constriction to near stimulus, a phenomenon known as light-near dissociation (see Image. Findings of Argyll Robertson Pupils on Pupillary Reflex Exam).[23]
Herpes zoster and cytomegalovirus infections can lead to retinitis affecting retinal ganglion cells, producing relative afferent pupillary defects (RAPD), where the affected eye shows reduced constriction when a light is swung from the healthy to the diseased eye.[24] Cryptococcal infection can inflame the optic nerve, impairing the afferent limb of the reflex. Lyme disease may cause optic neuritis, also damaging the afferent pathway and altering PLR.[25]
Toxidromes
The PLR is an important diagnostic tool in evaluating toxicity cases because many substances alter pupil size through their effects on autonomic pathways. Careful assessment of this reflex in this context helps clinicians identify specific toxidromes, distinguish drug effects from structural neurologic lesions, and guide acute management in patients presenting with altered consciousness or abnormal pupils.
Mydriasis may result from anticholinergics such as atropine and scopolamine, which block muscarinic receptors of the iris sphincter, or from sympathomimetics like cocaine and amphetamines, which enhance norepinephrine activity at the dilator muscle. In contrast, miosis is characteristic of opioids such as morphine and heroin, which stimulate the Edinger-Westphal nucleus to increase parasympathetic outflow. Benzodiazepines and barbiturates, through potentiation of γ-aminobutyric acid receptor type A (GABA-A) activity in the central nervous system, generally cause sedation without marked or consistent changes in pupillary diameter, but their central depressant effects may blunt the reactivity of the PLR.[26]
Multiple Sclerosis
Multiple sclerosis is a chronic, relapsing-remitting, immune-mediated disorder of the central nervous system marked by demyelination and axonal loss, most often affecting the optic nerves, brainstem, spinal cord, and periventricular white matter.[27] Optic neuritis is a frequent manifestation, causing visual loss and contributing to PLR abnormalities.
PLR disruption reflects autonomic pathway damage, producing reduced constriction amplitude, delayed latency, and slowed pupil responses. Parasympathetic impairment is more common than sympathetic involvement, and PLR abnormalities provide a noninvasive biomarker of autonomic dysfunction, aiding diagnosis and monitoring of multiple sclerosis.[28]
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
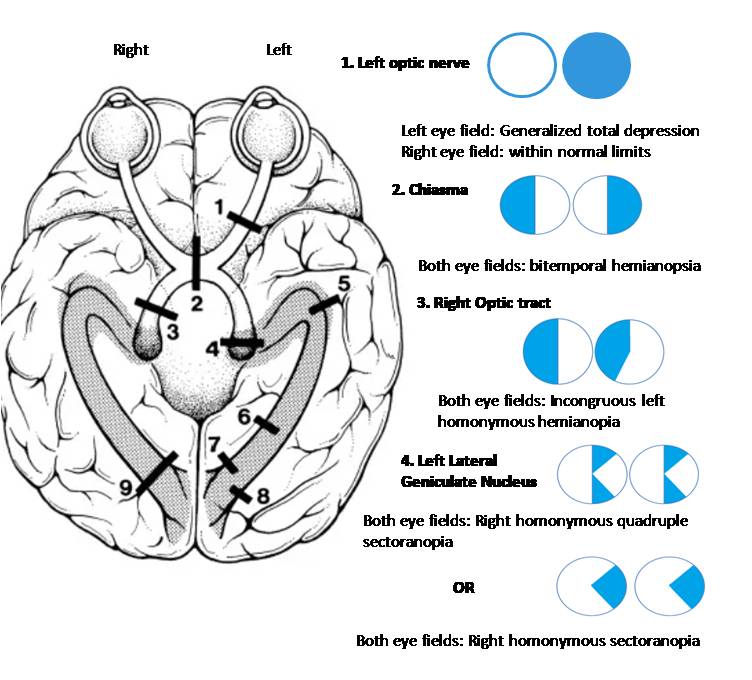
Visual Field Defects From Anterior Pathway Lesions. This illustration shows the characteristic visual field defects caused by lesions in the initial part of the visual pathway. A lesion of the optic nerve (1) leads to monocular visual loss, which is complete blindness in one eye, as all nerve fibers from that eye are severed. A lesion at the optic chiasm (2) interrupts the crossing nasal fibers from both eyes, resulting in bitemporal hemianopsia, or loss of vision in the outer half of each visual field. Damage to the optic tract (3) or lateral geniculate nucleus (4) causes incongruous homonymous hemianopia, a defect affecting the same side of the visual field in both eyes. The incongruity means the size and shape of the defect differ slightly between the two eyes.
Contributed by Surabhi Ruia, MS
(Click Image to Enlarge)
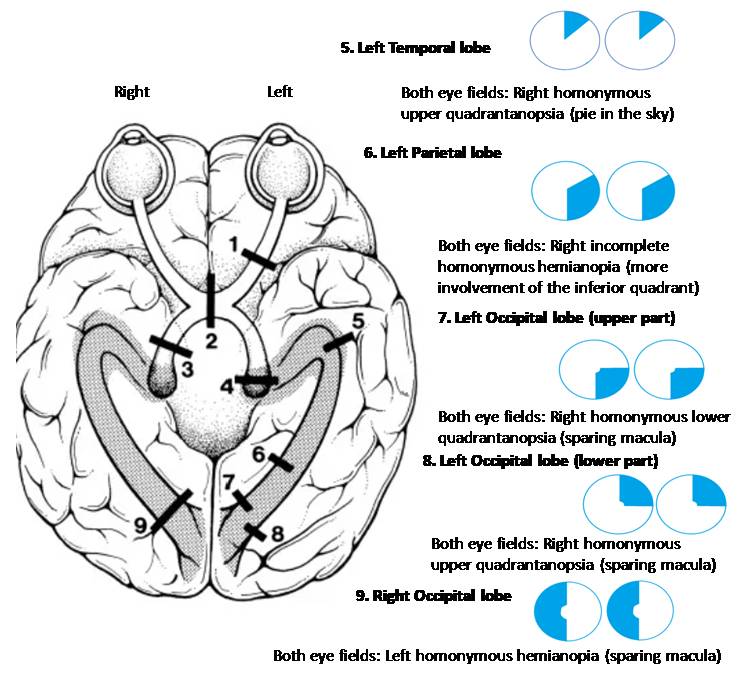
Visual Field Defects From Posterior Pathway Lesions. This image details the visual field defects that result from lesions in the brain's visual cortex. A lesion in the left temporal lobe (5), which contains the Meyer loop, results in a right homonymous superior quadrantanopia (often called "pie in the sky"). A lesion in the left parietal lobe (6) causes an incomplete right homonymous hemianopia, often with greater involvement of the inferior quadrant. Damage to the left occipital lobe (7, 8) produces a right homonymous hemianopia with macular sparing, a condition where the central part of the visual field remains intact due to the dual blood supply to the macula's representation in the occipital pole. The specific quadrantanopia (upper vs. lower) depends on whether the superior or inferior part of the occipital lobe is affected. Finally, a lesion in the right occipital lobe (9) results in left homonymous hemianopia with macular sparing.
Contributed by Surabhi Ruia, MS
(Click Image to Enlarge)
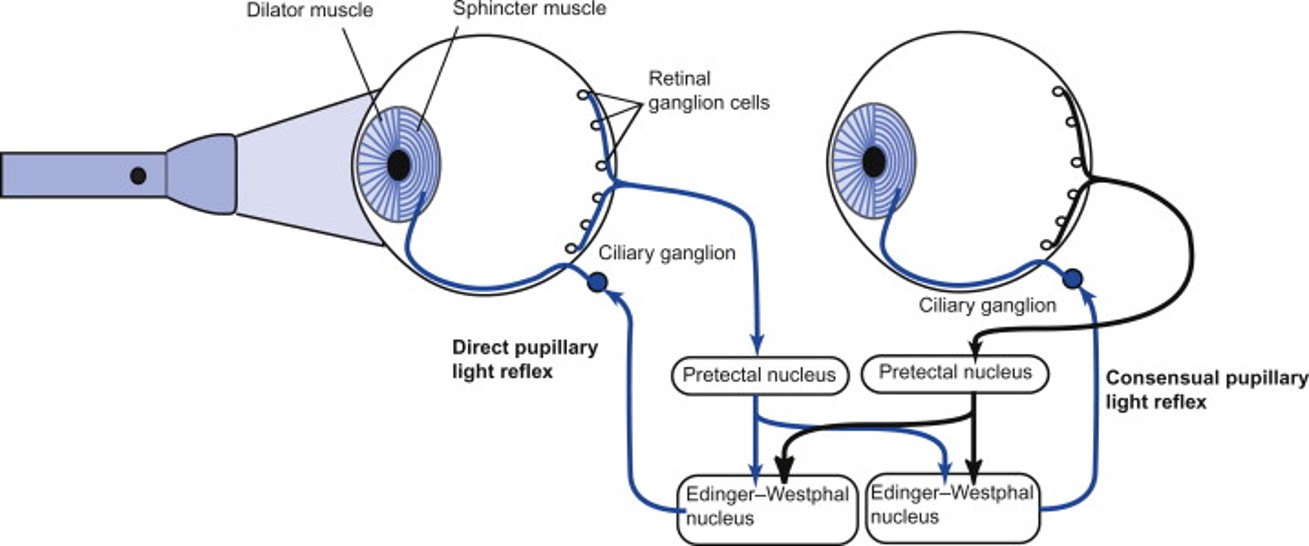
Pupillary Light Reflex Neural Pathway. This diagram depicts the neural circuitry of the pupillary light reflex, showing how light activates retinal ganglion cells, sending signals via the optic nerve to the pretectal nuclei. Bilateral projections connect these nuclei to the Edinger-Westphal nuclei, which give rise to parasympathetic fibers traveling to the ciliary ganglia. Postganglionic fibers innervate the iris sphincter muscle, mediating pupil constriction. The image illustrates the direct and consensual pupillary light reflexes and the coordinated response controlling pupil size.
Obtained from: McDougal, D., & Gamlin, P. (2008). Pupillary control pathways. In Elsevier eBooks (pp. 521–536). https://doi.org/10.1016/b978-012370880-9.00282-6
(Click Image to Enlarge)
References
Prasad S, Galetta SL. Anatomy and physiology of the afferent visual system. Handbook of clinical neurology. 2011:102():3-19. doi: 10.1016/B978-0-444-52903-9.00007-8. Epub [PubMed PMID: 21601061]
Level 3 (low-level) evidenceSridhar MS. Anatomy of cornea and ocular surface. Indian journal of ophthalmology. 2018 Feb:66(2):190-194. doi: 10.4103/ijo.IJO_646_17. Epub [PubMed PMID: 29380756]
Bouffard MA. The Pupil. Continuum (Minneapolis, Minn.). 2019 Oct:25(5):1194-1214. doi: 10.1212/CON.0000000000000771. Epub [PubMed PMID: 31584534]
Smith AM, Czyz CN. Neuroanatomy, Cranial Nerve 2 (Optic). StatPearls. 2025 Jan:(): [PubMed PMID: 29939684]
Hejtmancik JF, Shiels A. Overview of the Lens. Progress in molecular biology and translational science. 2015:134():119-27. doi: 10.1016/bs.pmbts.2015.04.006. Epub 2015 May 27 [PubMed PMID: 26310153]
Level 3 (low-level) evidenceWolffsohn JS, Davies LN. Presbyopia: Effectiveness of correction strategies. Progress in retinal and eye research. 2019 Jan:68():124-143. doi: 10.1016/j.preteyeres.2018.09.004. Epub 2018 Sep 19 [PubMed PMID: 30244049]
Kidd D. The optic chiasm. Clinical anatomy (New York, N.Y.). 2014 Nov:27(8):1149-58. doi: 10.1002/ca.22385. Epub 2014 May 14 [PubMed PMID: 24824063]
Ireland AC, Carter IB. Neuroanatomy, Optic Chiasm. StatPearls. 2025 Jan:(): [PubMed PMID: 31194427]
Belliveau AP, Somani AN, Dossani RH. Pupillary Light Reflex. StatPearls. 2025 Jan:(): [PubMed PMID: 30725865]
McDougal DH, Gamlin PD. Autonomic control of the eye. Comprehensive Physiology. 2015 Jan:5(1):439-73. doi: 10.1002/cphy.c140014. Epub [PubMed PMID: 25589275]
Level 3 (low-level) evidenceRuskell GL. Access of autonomic nerves through the optic canal, and their orbital distribution in man. The anatomical record. Part A, Discoveries in molecular, cellular, and evolutionary biology. 2003 Nov:275(1):973-8 [PubMed PMID: 14533171]
Giger FA, Houart C. The Birth of the Eye Vesicle: When Fate Decision Equals Morphogenesis. Frontiers in neuroscience. 2018:12():87. doi: 10.3389/fnins.2018.00087. Epub 2018 Feb 21 [PubMed PMID: 29515359]
Fuhrmann S. Eye morphogenesis and patterning of the optic vesicle. Current topics in developmental biology. 2010:93():61-84. doi: 10.1016/B978-0-12-385044-7.00003-5. Epub [PubMed PMID: 20959163]
Level 3 (low-level) evidenceWilliams AL, Bohnsack BL. Neural crest derivatives in ocular development: discerning the eye of the storm. Birth defects research. Part C, Embryo today : reviews. 2015 Jun:105(2):87-95. doi: 10.1002/bdrc.21095. Epub 2015 Jun 4 [PubMed PMID: 26043871]
Lutty GA, McLeod DS. Development of the hyaloid, choroidal and retinal vasculatures in the fetal human eye. Progress in retinal and eye research. 2018 Jan:62():58-76. doi: 10.1016/j.preteyeres.2017.10.001. Epub 2017 Nov 2 [PubMed PMID: 29081352]
Zagozewski JL, Zhang Q, Eisenstat DD. Genetic regulation of vertebrate eye development. Clinical genetics. 2014 Nov:86(5):453-60. doi: 10.1111/cge.12493. Epub 2014 Sep 25 [PubMed PMID: 25174583]
Level 3 (low-level) evidenceTorres M, Gómez-Pardo E, Gruss P. Pax2 contributes to inner ear patterning and optic nerve trajectory. Development (Cambridge, England). 1996 Nov:122(11):3381-91 [PubMed PMID: 8951055]
Level 3 (low-level) evidenceHayreh SS. Orbital vascular anatomy. Eye (London, England). 2006 Oct:20(10):1130-44 [PubMed PMID: 17019411]
Graw J. Eye development. Current topics in developmental biology. 2010:90():343-86. doi: 10.1016/S0070-2153(10)90010-0. Epub [PubMed PMID: 20691855]
Level 3 (low-level) evidenceDE GROOT SG, GEBHARD JW. Pupil size as determined by adapting luminance. Journal of the Optical Society of America. 1952 Jul:42(7):492-5 [PubMed PMID: 14939111]
Dohlman JC, Yoon MK. Principles of Protection of the Eye and Vision in Orbital Surgery. Journal of neurological surgery. Part B, Skull base. 2020 Aug:81(4):381-384. doi: 10.1055/s-0040-1714077. Epub 2020 Aug 7 [PubMed PMID: 33072480]
Mombaerts I. A pupil called Worry: Mydriasis in orbital surgery. Clinical neurology and neurosurgery. 2025 Feb:249():108737. doi: 10.1016/j.clineuro.2025.108737. Epub 2025 Jan 11 [PubMed PMID: 39827733]
Dichter SL, Khan Suheb MZ, Shubert GS. Argyll Robertson Pupil. StatPearls. 2025 Jan:(): [PubMed PMID: 30725864]
Tao BK, Soor D, Micieli JA. Herpes zoster in neuro-ophthalmology: a practical approach. Eye (London, England). 2024 Aug:38(12):2327-2336. doi: 10.1038/s41433-024-03030-3. Epub 2024 Mar 27 [PubMed PMID: 38538778]
Thomsen ML, Raposo FM, Greenberg PB, Janigian RH, Gaitanis MM, Hunter AM. Diagnostic Testing for Patients With Suspected Ocular Manifestations of Lyme Disease. Federal practitioner : for the health care professionals of the VA, DoD, and PHS. 2025 Jan:42(1):58-61. doi: 10.12788/fp.0547. Epub 2025 Jan 15 [PubMed PMID: 40529603]
Dhingra D, Kaur S, Ram J. Illicit drugs: Effects on eye. The Indian journal of medical research. 2019 Sep:150(3):228-238. doi: 10.4103/ijmr.IJMR_1210_17. Epub [PubMed PMID: 31719293]
Pongratz V, Bussas M, Schmidt P, Grahl S, Gasperi C, El Husseini M, Harabacz L, Pineker V, Sepp D, Grundl L, Wiestler B, Kirschke J, Zimmer C, Berthele A, Hemmer B, Mühlau M. Lesion location across diagnostic regions in multiple sclerosis. NeuroImage. Clinical. 2023:37():103311. doi: 10.1016/j.nicl.2022.103311. Epub 2023 Jan 5 [PubMed PMID: 36623350]
Gil-Casas A, Piñero DP, Molina-Martín A. Dynamic Pupillary Response in Multiple Sclerosis Patients with and without Optic Neuritis. Biomedicines. 2023 Dec 17:11(12):. doi: 10.3390/biomedicines11123332. Epub 2023 Dec 17 [PubMed PMID: 38137553]