Introduction
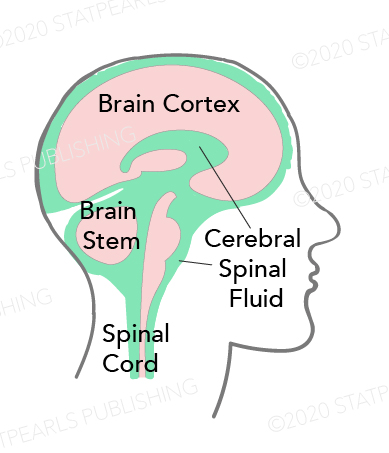
Cerebrospinal fluid (CSF) is a clear, plasma-like fluid that surrounds the central nervous system (CNS), occupying the central spinal canal, ventricular system, and subarachnoid space (see Image. Cerebrospinal Fluid Distribution). CSF has essential biochemical and mechanical functions. Production occurs at a rate of approximately 500 mL per day. Estimates suggest a total volume of 125 to 150 mL in the body at any given time. Based on the rate of production and absorption, which varies among individuals, the total volume may be replenished approximately every 7.5 hours. Cerebral atrophy may increase the total CSF volume to as much as 350 mL in older individuals.[1]
The majority of CSF is produced in the cerebral ventricles by the choroid plexus and the ependymal cells. From the lateral ventricles, CSF flows into the 3rd ventricle, then enters the subarachnoid space via the median aperture of Magendie and the 2 lateral apertures of Luschka. CSF also passes through the foramen magnum to reach the spinal subarachnoid space and through the central canal of the spinal cord. Reabsorption into the venous circulation occurs primarily through arachnoid villi (arachnoid granulations) in the arachnoid mater, although more recent evidence supports additional absorption through dural lymphatics and alternative pathways (see Image. Cerebrospinal Fluid Circulation).
Clinical analysis of this fluid is possible through lumbar puncture. Examination of CSF obtained via lumbar puncture can assist in generating a differential diagnosis by identifying abnormalities in its composition.[2][3]
Normal CSF pressure ranges from 8 to 15 mm Hg in the supine position, reflecting the tightly regulated balance between CSF production and resorption. An imbalance between production and absorption or obstruction of circulation can lead to CSF accumulation and elevated intracranial pressure (ICP).
Structure and Function
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Structure and Function
CSF originates by active secretion from the choroid plexus epithelium. The classical theory attributes approximately 80% of CSF production to the choroid plexus and the remaining 20% to secretion by endothelial cells of the blood-brain barrier (BBB). More recent theories propose extrachoroidal sources that contribute to CSF production either in addition to or as an alternative to the choroid plexus. These theories suggest a fluid influx across the BBB mediated by ion and solute cotransporters, accompanied by osmotic water movement.
The extensive surface area of cerebral capillaries supports substantial fluid entry into the interstitial space, which partially drains into the ventricular system. Arterioles located in the subarachnoid space express aquaporin water channels and sodium-potassium-chloride cotransporters (NKCC1), findings that support the possibility of local CSF secretion at these sites.
After its formation, CSF flows from the lateral ventricles through the foramina of Monro into the 3rd ventricle. From the 3rd ventricle, the fluid continues through the cerebral aqueduct of Sylvius into the 4th ventricle. From the 4th ventricle, the majority of CSF exits the ventricular system via the paired lateral foramina of Luschka and the single medial foramen of Magendie, entering the subarachnoid space and its associated cisterns. A smaller volume continues into the central canal of the spinal cord.
The subarachnoid space is situated between the arachnoid membrane and the pia mater and contains CSF located outside the ventricles. The arachnoid membrane adheres to the dura mater and forms the outer boundary of the subarachnoid space. This membrane is composed of fibroblasts connected by tight junctions involving claudin-11, which help contain the CSF within the compartment. Arachnoid trabeculae extend into the subarachnoid space and anchor to the pia mater. The pia consists of a single layer of flattened cells that closely appose the underlying astrocytic processes. Unlike the arachnoid membrane, the pia does not restrict solute exchange.
In the subarachnoid spaces, CSF flows over the surface of the cerebral cortex before reentering the blood circulation. The rate of CSF egress generally matches the rate of its formation.
According to the classical theory, CSF is absorbed into the bloodstream through arachnoid granulations that project through the dura mater into the sagittal dural venous sinus. Arachnoid villi and granulations form as protrusions of the arachnoid membrane extending through gaps in the dura into venous sinuses and lacunae. These structures differ primarily in size, with granulations representing the larger formations. Only a minority of current researchers accept this pathway as the primary route of CSF egress. More recent evidence identifies dural lymphatic vessels in the dorsal and basal dura as major absorption sites. These vessels drain CSF to the cervical lymphatic system.
Additional clearance pathways include perineuronal spaces surrounding cranial and spinal nerves, through which CSF exits the intracranial compartment. As large cerebral vessels exit the skull, CSF permeates their adventitial layers and enters the perivascular lymphatics. Spinal nerve root sheaths contribute to clearance via paravertebral lymphatics and the epidural venous plexuses.[4]
CSF appears clear and colorless, with a density ranging from 1.003 to 1.008 g/cm³.[5] The average volume of CSF in the body is approximately 140 mL, distributed across the ventricular system (approximately 35 mL), spinal canal (30-70 mL), and subarachnoid spaces.[6] Compared to plasma, CSF contains lower concentrations of glucose, protein, and potassium, but a higher concentration of chloride.
Glymphatic System
The brain contains a fluid transport system with structural and functional similarities to the lymphatic system, referred to as the "glymphatic" (glial-lymphatic) system. This system consists of a network of perivascular spaces that surround cerebral blood vessels. Specialized astrocytic processes, known as endfeet, envelop these vessels.
CSF enters the brain along periarterial spaces, driven by arterial pulsations. Astrocytic endfeet express high densities of aquaporin 4 water channels, which mediate the movement of fluid from periarterial spaces into the extracellular fluid compartment (interstitium) of the brain parenchyma. Waste-laden interstitial fluid then drains along perivenous pathways toward the subarachnoid space, eventually reaching the meningeal lymphatic vessels and cervical lymph nodes (see Image. Glymphatic System).
Glymphatic transport increases during nonrapid eye movement (NREM) sleep and decreases during wakefulness. This reduction in flow during wakefulness is thought to prevent excess glutamate diffusion, which could disrupt synaptic precision. The glymphatic system facilitates the clearance of metabolic byproducts and maintains extracellular homeostasis.[7]
Through convective bulk flow, the glymphatic system removes soluble proteins and metabolic waste to the meningeal lymphatics and cervical lymph nodes. This system may also support nutrient delivery by transporting solutes that lack dedicated BBB transporters. In addition, glymphatic flow may allow neurotransmitters and neurohormones to travel beyond their release sites, enabling brain-wide distribution of peptides and hormones released into CSF.
Cerebrospinal Fluid Functions
CSF performs several vital functions that contribute to the structural integrity, physiological stability, and immune defense of the CNS. The distribution and composition of CSF enable dynamic interactions between neural tissue, vasculature, and the immune system.
Buoyant support and protective cushioning
CSF serves several essential functions, including buoyant support of the brain and spinal cord. The brain has considerable mass (approximately 1,500 g) but remains structurally malleable. Because CSF surrounds the brain, it dissipates downward force, reducing mechanical stress and helping preserve cerebral architecture. This buoyant suspension also decreases tension on exiting nerve roots when CSF volume is adequate.[8]
The fluid properties of CSF contribute to its role in protecting the brain from injury during sudden head movements. Rapid acceleration or deceleration of the skull can transmit damaging forces to the brain. CSF minimizes this risk by acting as a cushion and absorbing mechanical shocks.
Homeostasis
CSF contributes to homeostatic regulation within the CNS by stabilizing temperature and maintaining osmotic balance. Electrolyte composition regulates osmotic pressure, preserving normal CSF pressure and supporting adequate cerebral perfusion. Metabolic waste products diffuse into CSF and are cleared through absorption at the arachnoid granulations into the venous system, with a portion also exiting through lymphatic pathways. In addition to clearance, CSF facilitates the distribution of nutrients, metabolites, and toxins, functioning in a manner analogous to a lymphatic system. Additional roles include neurodevelopmental signaling, chemical buffering, and regulation of homeostatic hormonal communication.[9][10]
Nutrition
CSF contains glucose, proteins, lipids, and electrolytes that support the essential nutritional demands of the CNS. CSF solute content complements vascular nutrient delivery, particularly in regions with limited BBB permeability.
Immune function
CSF contains immunoglobulins and mononuclear cells that contribute to CNS immune defense. Through drainage to the cervical lymph nodes, CSF facilitates immunosurveillance by enabling antigen sampling and cellular trafficking from the CNS.
Embryology
The timing of initial CSF production remains unknown. Neurulation in week 4 encloses amniotic fluid within the neural tube, forming the first CSF-filled cavity. Flexion of the neural tube and regional cellular proliferation generate the ventricles, which gradually expand. This ventricular enlargement plays a critical role in brain development. The choroid plexus forms by week 7. The subarachnoid space also emerges around week 7 and becomes externally sealed by tight junctions between arachnoid epithelial cells.
Although the precise mechanisms of subarachnoid space formation remain unclear, capillaries appear central to both CSF secretion and absorption.[11] Communication between the ventricles and the subarachnoid space begins with the formation of the foramen of Magendie at month 4 and the foramina of Luschka by month 6. The arachnoid villi begin forming at approximately 35 weeks, with continued maturation throughout the first 18 months of life.
Blood Supply and Lymphatics
The anterior choroidal artery, a branch of the internal carotid artery, and the lateral posterior choroidal artery, a branch of the posterior cerebral artery, provide the primary arterial supply to the choroid plexus of the lateral ventricles. The choroid plexus of the 3rd and 4th ventricles receives arterial blood from the paired medial posterior choroidal arteries, which arise from the posterior cerebral artery, and from the posterior inferior cerebellar arteries, respectively. Meningeal lymphatic vessels appear to serve as the primary lymphatic drainage system for CSF.
Surgical Considerations
Neurosurgical interventions involving the ventricular system, particularly external ventriculostomy, require meticulous aseptic technique. Breach of sterile barriers can permit pathogen entry, increasing the risk for meningitis or ventriculitis if infection reaches the CSF.
Cerebrospinal Fluid Leak
The detection of CSF leaks relies on biochemical markers unique to CSF. β2-transferrin, a desialylated isoform of the iron-binding glycoprotein transferrin, is found exclusively in CSF, ocular fluids, and perilymph. The presence of β2-transferrin in a fluid specimen confirms the diagnosis of a CSF leak with high specificity.[12]
Ventricular Access
The Kocher point provides access to the frontal horn of the lateral ventricle. This entry is located anterior to the motor strip, most commonly on the right side to avoid the dominant hemisphere in most patients. Surface landmarks place the insertion site 2 to 3 cm lateral to the midline, along the midpupillary line, and at least 1 cm anterior to the coronal suture, corresponding to approximately 11 cm posterior to the nasion. Catheter insertion is directed perpendicular to the cortical surface, aiming toward the medial canthus of the ipsilateral eye in the medial plane and the external auditory meatus in the anterior-posterior plane.[13] CSF is typically encountered at a ventricular depth of less than 5 to 7 cm, with a reduced depth of 3 to 4 cm in cases of significant ventricular dilation.
Other commonly described surgical approaches to the ventricular system include the Keen, Dandy, and Frazier points, as well as the Paine technique.[14][15][16] Selection among these approaches depends on clinical context, ventricular anatomy, and surgeon preference.
Clinical Significance
Lumbar Puncture
Lumbar puncture, also widely known as spinal tap, is a key clinical procedure used to access and evaluate CSF. The technique involves inserting a long needle into the subarachnoid space of the lumbar spine, typically below the termination of the spinal cord. In most individuals, the conus medullaris ends at approximately the L2 level, while the thecal sac extends to the level of S2. After needle placement, the stylet is removed to allow CSF sampling.
Measurement of opening CSF pressure is an essential component of the procedure. Elevated opening pressure may indicate hydrocephalus, intracranial mass, or idiopathic intracranial hypertension (IIH). CSF removal may precipitate brain herniation in the presence of an intracranial space-occupying lesion. Thus, intracranial mass lesions and associated mass effect must be excluded prior to lumbar puncture.
Neuroimaging with computed tomography (CT) of the head or magnetic resonance imaging (MRI) of the brain is necessary to rule out such pathology. Low CSF pressure also carries clinical significance and may result from leakage into surrounding tissues. CSF leakage can occur following trauma, previous lumbar puncture, or spontaneously in the absence of an identifiable cause.
CSF obtained via lumbar puncture can undergo several diagnostic analyses. Since CSF is normally transparent, color is a key observation. Cloudy fluid may suggest infection, while red or xanthochromic discoloration may indicate the presence of blood. Cultures may be performed to identify infectious organisms, particularly in suspected cases of meningitis. Molecular testing may reveal abnormal protein levels or other markers of disease. These analyses assist in diagnosing a broad range of CNS disorders.[17]
Normal CSF is clear, with pressure ranging from 8 to 15 mm Hg. Glucose concentrations typically range from 50 to 80 mg/dL or measure approximately 2/3 of the corresponding blood glucose level. Protein levels are normally between 15 and 45 mg/dL. Mononuclear cell counts fall between 0 and 5 cells/mm³.
In bacterial meningitis, CSF may appear cloudy or purulent, with elevated protein, low glucose, elevated lactate, and marked neutrophil pleocytosis. CSF is clear in viral meningitis but may show slightly decreased glucose, moderate protein elevation, and lymphocytic pleocytosis. In subarachnoid hemorrhage, CSF may contain more than 100 red blood cells/mm³ or demonstrate xanthochromia.
Hydrocephalus
Hydrocephalus is a significant clinical disorder involving abnormal accumulation of CSF within the ventricles, typically resulting in elevated ICP. In normal pressure hydrocephalus, ventricular dilation may occur without a corresponding elevation in pressure. CSF overproduction is a rare cause and is most often associated with choroid plexus papillomas or carcinomas. Most cases result from impaired CSF reabsorption.
Hydrocephalus due to decreased CSF reabsorption is broadly categorized into 2 functional subtypes: obstructive (noncommunicating) and nonobstructive (communicating). Obstructive hydrocephalus arises from a physical blockage in CSF flow proximal to the arachnoid granulations. Aqueductal stenosis, for example, may lead to disproportionate enlargement of the lateral and 3rd ventricles relative to the 4th, a pattern often referred to as "triventricular hydrocephalus." Obstructive causes may also include intracranial mass lesions. Communicating hydrocephalus occurs when CSF reabsorption is impaired at the level of the arachnoid granulations, without a discrete obstruction in ventricular flow.
Etiologies of hydrocephalus are broadly classified as congenital or acquired. Congenital causes include Chiari malformations. In Chiari I malformation, 4th ventricle outlet obstruction may occur, while Chiari II malformation is commonly associated with myelomeningocele. Other congenital etiologies include X-linked hydrocephalus, primary aqueductal stenosis, and Dandy-Walker malformation.
Acquired hydrocephalus may result from infection, the most common cause of communicating hydrocephalus. Additional acquired causes include posthemorrhagic changes following subarachnoid or intraventricular hemorrhage, neurosarcoidosis, postoperative changes in pediatric patients undergoing intracranial procedures, and space-occupying intracranial lesions that produce obstructive hydrocephalus. Common lesion locations associated with obstructive hydrocephalus include posterior fossa masses near the cerebral aqueduct, suprasellar masses compressing the 3rd ventricle, and colloid cysts obstructing CSF flow at the foramen of Monro.
Treatment of hydrocephalus involves addressing the underlying obstruction to CSF flow. Diversion of CSF through a shunt system may be necessary when obstruction cannot be resolved or hydrocephalus recurs. Shunts most commonly redirect CSF to the peritoneal cavity, though alternative drainage sites include the pleural cavity, gallbladder, and right atrium of the heart.[18][19]
Aquaporin 4 is the primary water channel in the brain and is expressed in ependymal cells lining the ventricular system as well as perivascular astrocytes that contribute to the BBB.[20] Emerging evidence supports a central role for aquaporin 4 in CSF production and circulation, identifying it as a potential future therapeutic target in hydrocephalus management.[21][22]
Idiopathic Intracranial Hypertension
IIH is characterized by elevated ICP in the absence of an identifiable structural, vascular, or infectious cause. Lumbar puncture typically confirms increased CSF pressure, with normal CSF composition. Patients may present with headache, visual disturbances, pulsatile tinnitus, nausea, vomiting, or focal neurologic symptoms. Diagnosis relies on clinical history and examination, but neuroimaging and lumbar puncture are required to exclude secondary causes. Brain MRI with venography is essential to rule out intracranial mass lesions and cerebral venous sinus thrombosis.
IIH primarily affects women of childbearing age with obesity and has an estimated annual incidence of 2.4 per 100,000. An opening pressure exceeding 25 cm of CSF confirms the diagnosis. The only proven disease-modifying treatment is weight loss, which should be initiated promptly. Acetazolamide may improve visual fields and reduce headache frequency. In fulminant or refractory cases, CSF diversion or optic nerve sheath fenestration may be required to preserve vision. IIH carries a risk of permanent visual loss if left untreated.[23]
Chiari Malformations
Chiari malformation comprises a group of structural abnormalities involving the hindbrain, including the cerebellum, pons, and medulla oblongata.[24] Two major types are recognized based on clinical and radiographic characteristics.
Chiari type I malformation, originally described by Hans Chiari, occurs in approximately 1 in 1,000 births and is more prevalent than type II malformations. This condition often arises from an idiopathic or syndromic reduction in the volume of the posterior fossa, resulting in downward displacement of the cerebellar tonsils into the spinal canal.
Chiari type II malformation, also known as Arnold-Chiari malformation, is consistently associated with myelomeningocele. Leakage of CSF through the spinal defect leads to herniation of hindbrain structures into the spinal canal, disrupting normal CSF dynamics and potentially resulting in subarachnoid adhesions.
Dementia
Failure of the glymphatic system may represent a final common pathway to dementia. Sleep disruption and aging impair glymphatic clearance and promote the accumulation of extracellular proteins. CSF production and lymphatic drainage may also decline with advanced age or microvascular disease. Reduced perivascular CSF flow results in protein stagnation, promoting misfolding and seeding of amyloid-β, τ, and α-synuclein. These aggregates then spread through perivascular and extracellular pathways in a prion-like manner. The deposition of these pathologic proteins triggers local inflammation and neuronal loss within vulnerable brain regions.
Spontaneous Intracranial Hypotension
Spontaneous intracranial hypotension results from spinal CSF leakage due to dural tears, diverticula, or CSF-venous fistula. This condition affects approximately 4 per 100,000 individuals annually. Most patients develop orthostatic headache, which begins or worsens upon standing and improves with recumbency.
Prompt brain and spinal MRI should be obtained early in the course of symptoms to detect extradural fluid collections. Brain MRI with contrast often reveals diffuse meningeal enhancement, engorged venous sinuses, and brain sagging. Spinal MRI may demonstrate longitudinal epidural collections, which are typically caused by discogenic microspurs or dural tears. Patient positioning for myelography depends on the anatomic extent of the fluid collection.
CT myelography, digital subtraction myelography, and ultrafast CT myelography are highly sensitive for detecting leaks in this setting. A CSF-venous fistula is suspected when no epidural collection is seen on MRI. In these cases, CT myelography and digital subtraction myelography performed in the lateral decubitus position improve visualization of the fistula.
Initial management includes bed rest, hydration, and caffeine administration. Nontargeted epidural blood patches are typically offered within 2 weeks of diagnosis. Persistent symptoms after patching warrant further evaluation with myelography to localize the leak and plan targeted treatment. Options include targeted epidural blood patching, transvenous embolization (for CSF-venous fistula), or surgical repair. Potential complications include subdural hematoma from cerebral sagging, cerebral venous thrombosis, and superficial siderosis.[25][26]
Media
(Click Image to Enlarge)
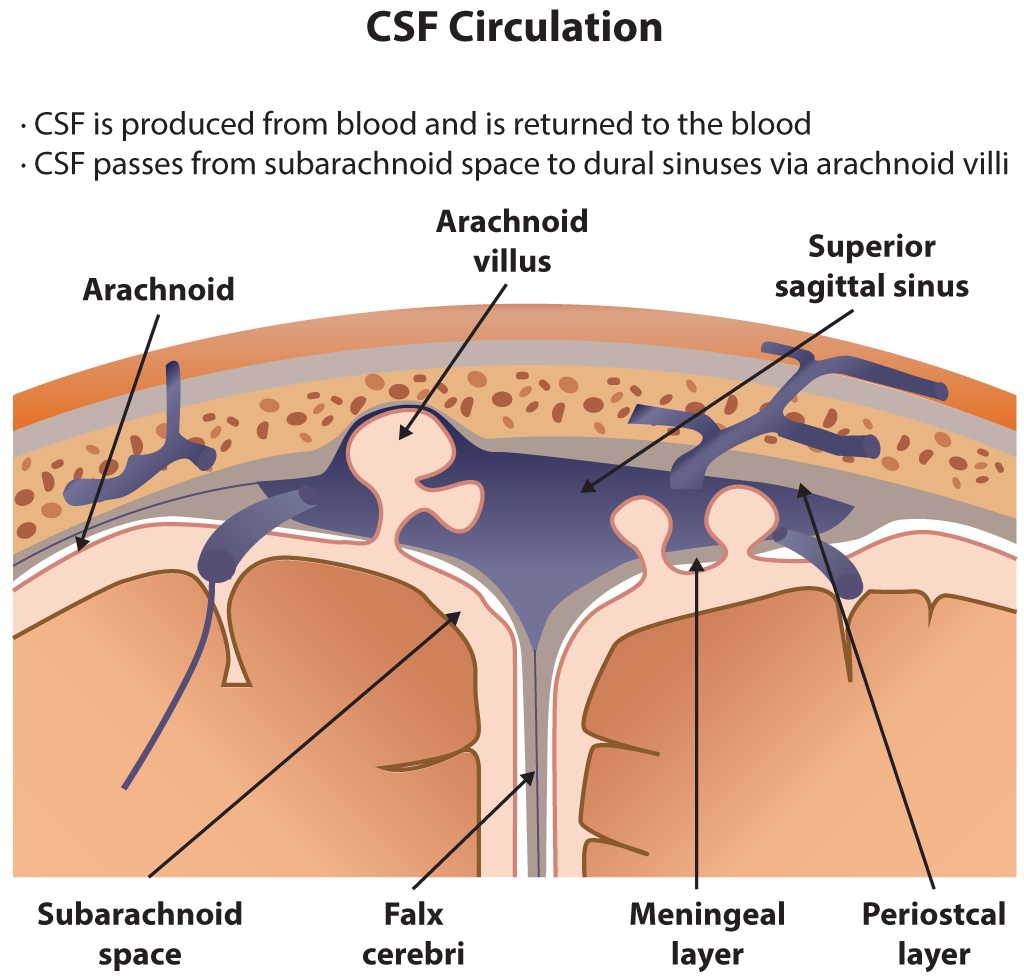
Cerebrospinal Fluid Circulation. The illustration depicts cerebrospinal fluid circulation through the subarachnoid space, arachnoid villi, and superior sagittal sinus. Key anatomical structures include the falx cerebri, meningeal and periosteal layers of the dura mater, and the arachnoid membrane.
Contributed by B Parker
(Click Image to Enlarge)
(Click Image to Enlarge)
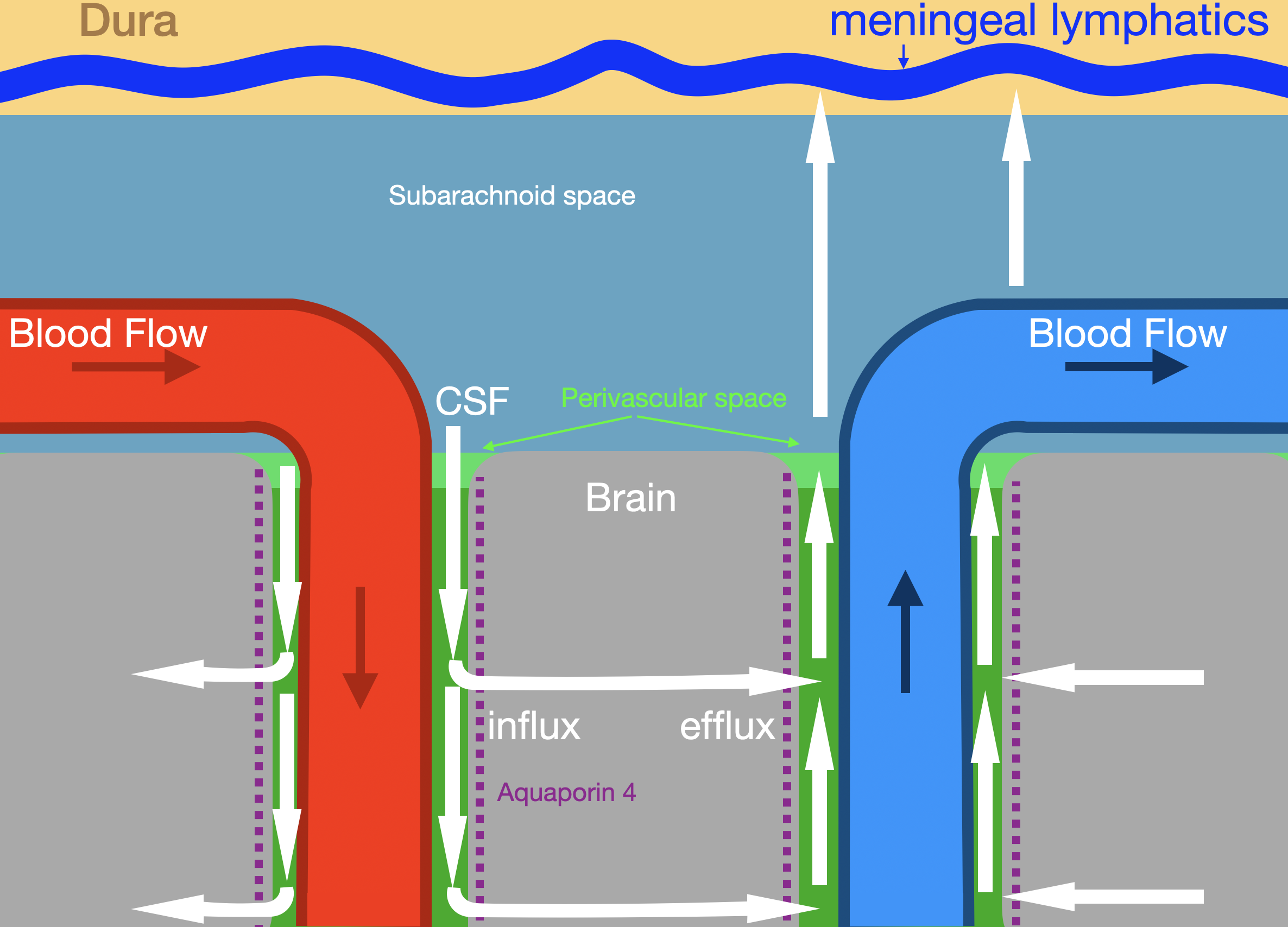
Glymphatic System. The illustration shows glymphatic fluid flow (white arrows) along periarterial and perivenous pathways. Labeled structures include the dura, meningeal lymphatics, subarachnoid space, brain parenchyma, arterial and venous circulation, and aquaporin 4 channels mediating influx and efflux.
Contributed by Konstantinos Margetis MD, PhD
References
Yamada S, Mase M. Cerebrospinal Fluid Production and Absorption and Ventricular Enlargement Mechanisms in Hydrocephalus. Neurologia medico-chirurgica. 2023 Apr 15:63(4):141-151. doi: 10.2176/jns-nmc.2022-0331. Epub 2023 Mar 1 [PubMed PMID: 36858632]
Javed K, Reddy V, Lui F. Neuroanatomy, Choroid Plexus. StatPearls. 2025 Jan:(): [PubMed PMID: 30844183]
Shenoy SS, Lui F. Neuroanatomy, Ventricular System. StatPearls. 2025 Jan:(): [PubMed PMID: 30422527]
Rasmussen MK, Mestre H, Nedergaard M. Fluid transport in the brain. Physiological reviews. 2022 Apr 1:102(2):1025-1151. doi: 10.1152/physrev.00031.2020. Epub 2021 May 5 [PubMed PMID: 33949874]
Brodbelt A, Stoodley M. CSF pathways: a review. British journal of neurosurgery. 2007 Oct:21(5):510-20 [PubMed PMID: 17922324]
Kohn MI, Tanna NK, Herman GT, Resnick SM, Mozley PD, Gur RE, Alavi A, Zimmerman RA, Gur RC. Analysis of brain and cerebrospinal fluid volumes with MR imaging. Part I. Methods, reliability, and validation. Radiology. 1991 Jan:178(1):115-22 [PubMed PMID: 1984289]
Level 1 (high-level) evidenceNedergaard M, Goldman SA. Glymphatic failure as a final common pathway to dementia. Science (New York, N.Y.). 2020 Oct 2:370(6512):50-56. doi: 10.1126/science.abb8739. Epub [PubMed PMID: 33004510]
Kimelberg HK. Water homeostasis in the brain: basic concepts. Neuroscience. 2004:129(4):851-60 [PubMed PMID: 15561403]
Level 3 (low-level) evidenceRedzic ZB, Segal MB. The structure of the choroid plexus and the physiology of the choroid plexus epithelium. Advanced drug delivery reviews. 2004 Oct 14:56(12):1695-716 [PubMed PMID: 15381330]
Level 3 (low-level) evidenceBrown PD, Davies SL, Speake T, Millar ID. Molecular mechanisms of cerebrospinal fluid production. Neuroscience. 2004:129(4):957-70 [PubMed PMID: 15561411]
Level 3 (low-level) evidenceSakka L, Coll G, Chazal J. Anatomy and physiology of cerebrospinal fluid. European annals of otorhinolaryngology, head and neck diseases. 2011 Dec:128(6):309-16. doi: 10.1016/j.anorl.2011.03.002. Epub 2011 Nov 18 [PubMed PMID: 22100360]
Papadea C, Schlosser RJ. Rapid method for beta2-transferrin in cerebrospinal fluid leakage using an automated immunofixation electrophoresis system. Clinical chemistry. 2005 Feb:51(2):464-70 [PubMed PMID: 15608153]
Level 3 (low-level) evidenceGhajar JB. A guide for ventricular catheter placement. Technical note. Journal of neurosurgery. 1985 Dec:63(6):985-6 [PubMed PMID: 4056916]
Huang KT, Chavakula V, Gormley WB. Keen's Point for External Ventricular Drainage in Traumatic Brain Injury Patients: An Uncommon Indication for An Old Technique. World neurosurgery. 2017 Jun:102():694.e1-694.e7. doi: 10.1016/j.wneu.2017.03.145. Epub 2017 Apr 8 [PubMed PMID: 28400228]
Meybodi AT, Meybodi KT. Letter: Craniometrics and Ventricular Access: A Review of Kocher's, Kaufman's, Paine's, Menovksy's, Tubbs', Keen's, Frazier's, Dandy's, and Sanchez's Points. Operative neurosurgery (Hagerstown, Md.). 2020 Jul 1:19(1):E104. doi: 10.1093/ons/opaa104. Epub [PubMed PMID: 32348479]
Level 3 (low-level) evidenceMatsuzaki H, Otsuka T, Uekawa K, Nakagawa T, Tsubota N. Use of Paine's Technique: Projecting Puncture Point to the Skull and Skin. World neurosurgery. 2017 Aug:104():45-47. doi: 10.1016/j.wneu.2017.04.173. Epub 2017 May 9 [PubMed PMID: 28499903]
Özütemiz C, Rykken JB. Lumbar puncture under fluoroscopy guidance: a technical review for radiologists. Diagnostic and interventional radiology (Ankara, Turkey). 2019 Mar:25(2):144-156. doi: 10.5152/dir.2019.18291. Epub [PubMed PMID: 30774095]
Graff-Radford NR, Jones DT. Normal Pressure Hydrocephalus. Continuum (Minneapolis, Minn.). 2019 Feb:25(1):165-186. doi: 10.1212/CON.0000000000000689. Epub [PubMed PMID: 30707192]
van Steenoven I, Majbour NK, Vaikath NN, Berendse HW, van der Flier WM, van de Berg WDJ, Teunissen CE, Lemstra AW, El-Agnaf OMA. α-Synuclein species as potential cerebrospinal fluid biomarkers for dementia with lewy bodies. Movement disorders : official journal of the Movement Disorder Society. 2018 Nov:33(11):1724-1733. doi: 10.1002/mds.111. Epub 2018 Nov 15 [PubMed PMID: 30440090]
Amiry-Moghaddam M, Ottersen OP. The molecular basis of water transport in the brain. Nature reviews. Neuroscience. 2003 Dec:4(12):991-1001 [PubMed PMID: 14682361]
Level 3 (low-level) evidenceIgarashi H, Suzuki Y, Kwee IL, Nakada T. Water influx into cerebrospinal fluid is significantly reduced in senile plaque bearing transgenic mice, supporting beta-amyloid clearance hypothesis of Alzheimer's disease. Neurological research. 2014 Dec:36(12):1094-8. doi: 10.1179/1743132814Y.0000000434. Epub 2014 Aug 1 [PubMed PMID: 25082552]
Level 3 (low-level) evidenceTrillo-Contreras JL, Toledo-Aral JJ, Echevarría M, Villadiego J. AQP1 and AQP4 Contribution to Cerebrospinal Fluid Homeostasis. Cells. 2019 Feb 24:8(2):. doi: 10.3390/cells8020197. Epub 2019 Feb 24 [PubMed PMID: 30813473]
Mollan SP, Davies B, Silver NC, Shaw S, Mallucci CL, Wakerley BR, Krishnan A, Chavda SV, Ramalingam S, Edwards J, Hemmings K, Williamson M, Burdon MA, Hassan-Smith G, Digre K, Liu GT, Jensen RH, Sinclair AJ. Idiopathic intracranial hypertension: consensus guidelines on management. Journal of neurology, neurosurgery, and psychiatry. 2018 Oct:89(10):1088-1100. doi: 10.1136/jnnp-2017-317440. Epub 2018 Jun 14 [PubMed PMID: 29903905]
Level 3 (low-level) evidenceMohammad SA, Osman NM, Ahmed KA. The value of CSF flow studies in the management of CSF disorders in children: a pictorial review. Insights into imaging. 2019 Jan 28:10(1):3. doi: 10.1186/s13244-019-0686-x. Epub 2019 Jan 28 [PubMed PMID: 30689061]
Cheema S, Anderson J, Angus-Leppan H, Armstrong P, Butteriss D, Carlton Jones L, Choi D, Chotai A, D'Antona L, Davagnanam I, Davies B, Dorman PJ, Duncan C, Ellis S, Iodice V, Joy C, Lagrata S, Mead S, Morland D, Nissen J, Pople J, Redfern N, Sayal PP, Scoffings D, Secker R, Toma AK, Trevarthen T, Walkden J, Beck J, Kranz PG, Schievink W, Wang SJ, Matharu MS. Multidisciplinary consensus guideline for the diagnosis and management of spontaneous intracranial hypotension. Journal of neurology, neurosurgery, and psychiatry. 2023 Oct:94(10):835-843. doi: 10.1136/jnnp-2023-331166. Epub 2023 May 5 [PubMed PMID: 37147116]
Level 3 (low-level) evidenceCheema S, Mehta D, Qureshi A, Sayal P, Kamourieh S, Davagnanam I, Matharu M. Spontaneous intracranial hypotension. Practical neurology. 2024 Mar 19:24(2):98-105. doi: 10.1136/pn-2023-003986. Epub 2024 Mar 19 [PubMed PMID: 38135500]