Introduction
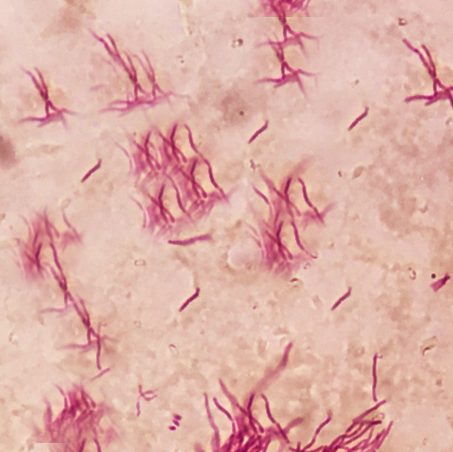
Nocardia is a gram-variable, aerobic, weakly acid-fast bacterium that infrequently causes ocular disease, most often as a corneal infection (see Image. Filamentous Nocardia). Misdiagnosis or delayed recognition of Nocardia keratitis commonly stems from nonspecific clinical features that resemble those produced by more prevalent pathogens.[1] The organism often responds poorly to standard empiric agents for bacterial keratitis, including fluoroquinolones, leading to treatment delays and avoidable visual morbidity.[2]
A rare but potentially vision-threatening corneal infection, Nocardia keratitis typically follows direct inoculation of the epithelium by the pathogen, which is found in soil, decaying vegetation, and water (see Image. Nocardia Keratitis). While Nocardia most frequently causes pulmonary or cutaneous disease, ocular involvement may arise from systemic spread or primary infection. Corneal entry usually occurs through epithelial defects associated with trauma, contact lens wear, ocular surface disease, or prior surgery, underscoring the relevance of both environmental and iatrogenic risk factors.[3]
Nocardia keratitis accounts for fewer than 2% of bacterial keratitis cases in temperate climates but appears more frequently in tropical and subtropical regions where agricultural exposure and humid conditions favor organism growth. The risk increases among patients with impaired ocular surface defenses, including those with dry eye, neurotrophic keratitis, prior herpetic infection, and immunodeficiency. Although systemic immunocompromise is not required for corneal involvement, chronic topical steroid use and systemic diseases such as diabetes mellitus may contribute to more severe disease courses.[4]
Clinically, Nocardia keratitis presents insidiously, with patients reporting photophobia, foreign-body sensation, tearing, and blurred vision developing over several days to weeks. On slit-lamp examination, the hallmark feature is a multifocal, superficial stromal infiltrate with a characteristic “wreath-like” or “serpiginous” pattern and satellite lesions radiating from a central nidus. Corneal epithelial defects often appear irregular and overlay thin, yellowish stromal infiltrates. A dense perineural infiltrate—radial, bead-like accumulations along corneal nerves—is another suggestive finding, though not pathognomonic. Hypopyon formation is uncommon but, when present, may indicate deeper anterior chamber involvement.[5]
Rapid, accurate diagnosis remains challenging. Standard smear techniques using gram, modified acid-fast (eg, Kinyoun), and Giemsa stains can reveal delicate, branching filamentous organisms. However, low organism load and atypical morphology may lead to misidentification as fungal filaments or other actinomycetes. Culture on selective media such as blood agar, Sabouraud dextrose agar (SDA), and Lowenstein-Jensen medium supports the growth of Nocardia colonies, typically chalky, matte, and white to tan over 3 to 5 days. Species-level identification may require biochemical assays, high-performance liquid chromatography, or molecular methods such as polymerase chain reaction (PCR) and 16S rRNA gene sequencing—tools that enable faster turnaround times and precise speciation to guide antimicrobial therapy.[6]
Effective treatment depends on the timely initiation of targeted topical and, when needed, systemic antibiotics. Historically, sulfonamides, particularly topical trimethoprim-sulfamethoxazole (TMP-SMX), were used based on Nocardia’s susceptibility profile. Topical amikacin 2.5% is now widely considered first-line therapy for its potent in vitro and in vivo activity, superior corneal penetration, and favorable safety profile. A typical regimen includes hourly instillation during the acute phase, followed by tapering over 4 to 6 weeks, depending on clinical response.
Systemic TMP-SMX (160/800 mg twice daily) may be added for deep stromal involvement or scleral extension due to its complementary ocular tissue penetration. Alternative agents, such as topical linezolid, clarithromycin, or minocycline, may be used in cases of amikacin resistance, intolerance, or disseminated disease, highlighting the need for species-specific susceptibility data.[7]
Despite targeted therapy, Nocardia keratitis may remain refractory, with prolonged healing and risk of corneal thinning, perforation, and scarring. Surgical interventions, including epithelial debridement, lamellar keratectomy, and therapeutic penetrating keratoplasty (TPK), are often required for persistent infiltrates, progressive stromal melt, or impending perforation. Adjunctive strategies such as corneal cross-linking have been evaluated in small case series to slow collagen degradation and microbial proliferation, though controlled clinical trials are lacking.[8]
Outcomes vary widely and depend on diagnostic timing, species virulence, stromal depth of infection, host immune status, and the prompt initiation of effective antimicrobial therapy. Visual prognosis ranges from near-complete recovery with early treatment to severe impairment or globe loss in advanced or mismanaged cases. The high morbidity of Nocardia keratitis underscores the need for clinical vigilance, especially in at-risk individuals such as contact lens users, agricultural workers, and those with ocular surface disease.[9]
From a systems perspective, Nocardia keratitis exposes key deficiencies in current ophthalmic practice, including the following:
- Underrecognition due to clinical overlap with fungal keratitis
- Limited access to rapid species-level diagnostics
- Inconsistent empiric antibiotic regimens lacking Nocardia coverage
Addressing these gaps requires interprofessional collaboration among ophthalmologists, microbiologists, pharmacists, and primary care providers. Optimizing outcomes depends on streamlining diagnostic workflows, standardizing laboratory protocols, and disseminating guideline-based treatment strategies.[10]
The expanding use of molecular diagnostics and the emergence of antimicrobial resistance demand continued surveillance and prospective studies. Comparative trials of topical agents, novel drug-delivery platforms (eg, antibiotic-loaded contact lenses or intracorneal implants), and evaluation of adjunctive therapies such as collagen cross-linking offer promising avenues for investigation. The potential utility of immunomodulators, biologics targeting inflammatory mediators, and photodynamic therapy remains largely unexplored, presenting opportunities to personalize treatment for this uncommon infection.[11]
Nocardia keratitis presents significant diagnostic and therapeutic challenges with potentially severe visual consequences. A clear understanding of its epidemiology, clinical features, microbiology, and treatment is essential for managing corneal infections, especially in settings with high environmental exposure risk or ocular surface compromise. Improved outcomes depend on early recognition, targeted antimicrobial therapy, timely surgical intervention when warranted, and sustained interprofessional collaboration. Ongoing education, research, and quality-improvement efforts will be critical to advancing care and reducing the global burden of Nocardia keratitis.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Although originally misclassified as a fungus, Nocardia is a filamentous, aerobic, gram-positive bacterium with weak acid-fast staining characteristics. Over 80 Nocardia species have been identified, many of which are environmental organisms commonly found in water, soil, dust, mud, and decaying vegetation. As Nocardia does not form part of the normal ocular flora, keratitis caused by Nocardia arises exclusively through environmental exposure and subsequent inoculation.
Historically, Nocardia asteroides was the species most frequently isolated in cases of Nocardia keratitis. However, advances in molecular diagnostics, including PCR and 16S rRNA sequencing, have revealed that other species, such as Nocardia arthritidis, may be more prevalent in specific populations.[12]
Environmental exposure plays a major role in disease acquisition. Nearly half of Nocardia keratitis cases involve a history of ocular contact with soil or plant material.[13] The most common risk factor is corneal trauma, which accounted for 25% of cases in one series.[14] Additional risk factors include prior ocular surgeries such as laser-assisted in situ keratomileusis (LASIK) or photorefractive keratectomy (PRK), topical corticosteroid use, and extended contact lens wear, particularly when associated with improper hygiene.[15][16][17]
Although pulmonary and cutaneous infections are more typical of Nocardia, direct inoculation into the cornea through a compromised epithelium can result in keratitis. The organism's ability to persist in diverse environmental niches underscores the importance of both host factors and exposure risk in pathogenesis.
Microbial Features
Nocardia species are filamentous, beaded, branching rods that stain weakly acid-fast on modified Ziehl-Neelsen stain. These organisms are slow-growing, forming chalky white to tan colonies on blood agar within 3 to 5 days. Multiple species cause infection, each exhibiting different antimicrobial susceptibilities.
Environmental Exposures
Common sources of exposure include vegetative trauma, such as from thorns or wood splinters, and transferring Nocardia from soil to the cornea. Contact lens–related microtrauma from contaminated lenses or solutions is another risk factor. Agricultural workers in tropical and subtropical climates face higher exposure due to increased environmental burden. Ocular surgery or minor injuries causing epithelial defects also facilitate infection.[18]
Host Susceptibility
Conditions compromising the ocular surface, including dry eye, neurotrophic keratitis, and prior herpetic infections, increase vulnerability. Topical corticosteroids cause local immunosuppression, while systemic immunocompromise, such as diabetes mellitus, HIV, systemic steroids, or chemotherapy, further elevates risk. Contact lens wear contributes to the pathology via both microtrauma and contamination of lens cases.[19]
Pathogenesis
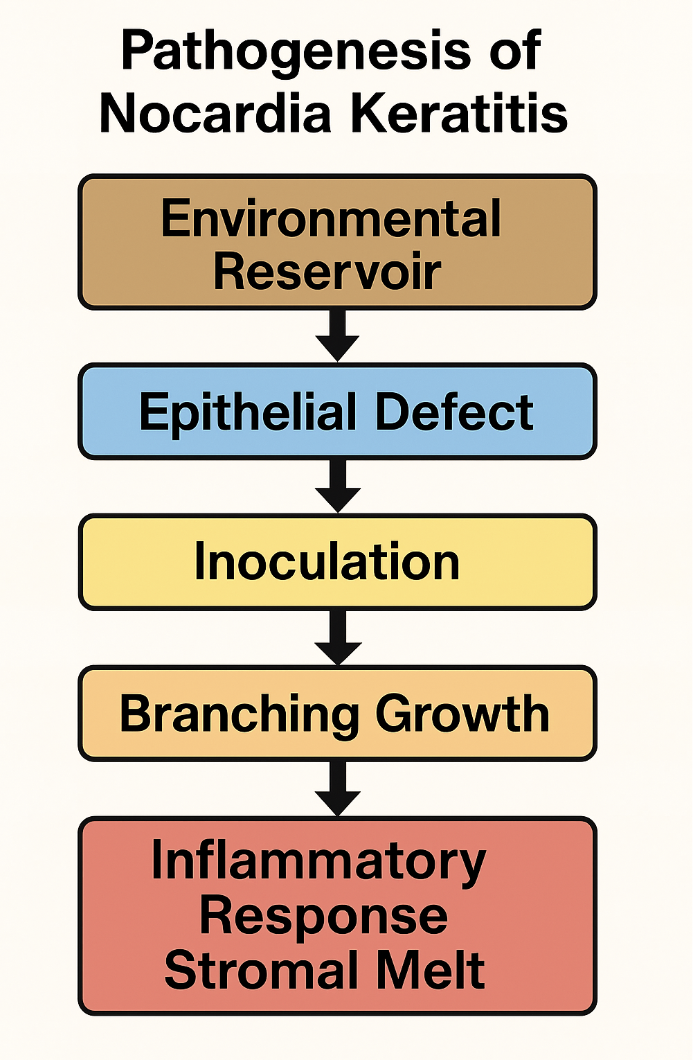
Infection begins with an epithelial breach allowing direct inoculation of Nocardia into the corneal stroma. The bacteria grow as branching filaments and may spread perineurally, triggering an inflammatory response. This process results in characteristic “wreath-like” stromal infiltrates with satellite lesions. Without treatment, progressive stromal melt, corneal perforation, and hypopyon formation can occur.
Nocardia Species Commonly Implicated in Keratitis
Several Nocardia species cause keratitis with distinct geographic distributions and clinical profiles. The Nocardia asteroides complex is the most common worldwide and generally remains susceptible to amikacin and TMP-SMX. Nocardia brasiliensis, found primarily in tropical and subtropical regions, tends to cause aggressive infections that often require prolonged therapy. Nocardia farcinica occurs rarely worldwide but is known for resistance to TMP-SMX. Nocardia otitidiscaviarum, seen mainly in Asia and Latin America, is frequently linked to contact lens case contamination and shows variable antimicrobial susceptibility.
Epidemiology
Nocardia species are more prevalent in the soil of South Asia, contributing to a higher incidence of Nocardia keratitis in this region.[20][21] Studies from South India report that Nocardia accounts for 1.7% (in Hyderabad) to 8.34% (in Tamil) of bacterial keratitis cases.[22][23] Bacterial keratitis overall tends to occur more frequently in men, young adults, and individuals residing in rural areas, likely reflecting increased risk of minor corneal trauma and environmental exposure. Despite its relative rarity and diagnostic challenges, Nocardia keratitis remains a clinically significant cause of infectious keratitis, with key epidemiological features characterized through case series and multicenter reviews.
Global Incidence and Geographic Distribution
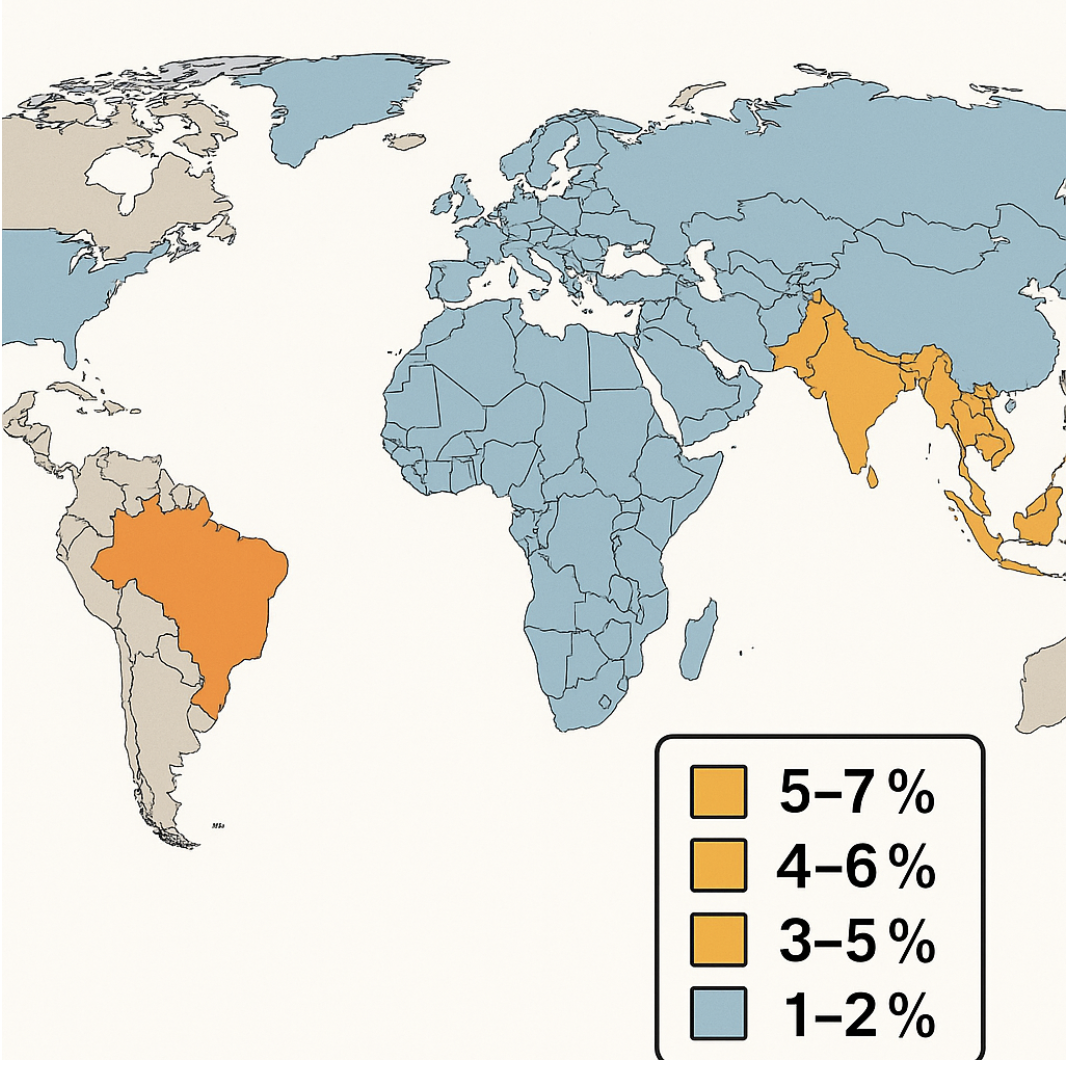
Nocardia species account for approximately 1% to 2% of all culture-positive bacterial keratitis cases in temperate climates. However, in tropical and subtropical regions, particularly in South and Southeast Asia, parts of Africa, and Central and South America, the proportion rises to 3% to 8%. This increase reflects both environmental factors and heightened agricultural exposures.[24] Notable regional hotspots include India, where some tertiary centers report that Nocardia causes 5% to 7% of bacterial keratitis cases. Similarly, case series from Thailand and the Philippines document rates of 4% to 6%, often associated with rice-paddy trauma. In Brazil, agricultural workers who sustain vegetative injuries face a higher risk, with Nocardia comprising approximately 3% of keratitis isolates (see Image. Epidemiology of Nocardia).
Seasonal Trends
Incidence of Nocardia keratitis peaks during the monsoon and postmonsoon seasons in tropical regions, coinciding with increased agricultural activity and greater exposure to soil and water. In temperate climates, cases tend to cluster from late spring to early autumn, periods marked by heightened outdoor recreation and gardening.
Demographic Characteristics
The median age at presentation ranges from 45 to 60 years, though cases affect individuals from young adulthood to the elderly. A male predominance of 60% to 70% is consistently observed, likely due to higher rates of outdoor occupational exposure. Occupationally, up to 50% to 65% of cases involve agricultural or rural workers, with a history of direct soil contact, such as farming or horticulture.
Predisposing Exposures and Host Factors
Vegetative trauma, such as from thorn or wood splinter injuries, is reported in 30% to 50% of patients. Contact lens wear contributes to 10% to 20% of cases, often linked to poor lens hygiene or overnight use. Ocular surface diseases, including dry eye and neurotrophic keratitis, as well as topical corticosteroid use, each appear in 15% to 25% of reported cases. Systemic immunosuppression, such as in diabetes, chronic steroid use, or HIV infection, is present in 10% to 15% of patients but is not necessary for infection to occur.
Species Distribution
The Nocardia asteroides complex predominates, accounting for more than 60% of ocular isolates. Next is Nocardia brasiliensis, which comprises 20% to 25%, and Nocardia farcinica, responsible for 5% to 10% of cases. Geographic variation is evident, with N. brasiliensis being relatively more common in tropical agricultural settings.
The prevalence of Nocardia species varies by region. In South Asia, particularly India, Nocardia accounts for 5% to 7% of bacterial keratitis cases, with N. asteroides and N. brasiliensis being the predominant species. Similarly, in Southeast Asia, where Nocardia causes 4% to 6% of cases, N. asteroides and N. brasiliensis remain the most common isolates. In sub-Saharan Africa, Nocardia accounts for 2% to 4% of bacterial keratitis cases, with N. asteroides predominating. South America reports 3% to 5% of bacterial keratitis cases caused by Nocardia, mainly involving N. asteroides and N. farcinica. In temperate regions such as Europe and North America, Nocardia keratitis is less frequent, comprising 1% to 2% of cases, and is primarily caused by N. asteroides.
Pathophysiology
Nocardia keratitis is a slow-growing infection (see Image. Pathogenesis of Nocardia Keratitis). A case series reported a mean time to presentation of 24.5 days (± 22.2 days) after symptom onset, underscoring the organism’s indolent course and the diagnostic delays it often causes.[25] Infection typically begins with an epithelial breach caused by trauma (eg, vegetative matter or contact lens-related microabrasions), surgery, or preexisting ocular surface disease. This breach creates a portal of entry through which Nocardia adheres using filamentous, mycolic-acid-rich surface adhesins that bind to components of the extracellular matrix, such as fibronectin and laminin.[26]
Once in the corneal stroma, Nocardia grows as branching, beaded filaments that resist phagocytosis both by size and by virtue of virulence factors such as trehalose 6,6’-dimycolate. This glycolipid interferes with phagosome-lysosome fusion, reducing lysosomal enzyme activity.[27][28] Additionally, catalase and superoxide dismutase detoxify reactive oxygen species (ROS), allowing intracellular survival within polymorphonuclear leukocytes.[29] These properties, combined with filamentous morphology, enable the organisms to evade host defenses and persist within tissue.
The organisms may track along corneal nerves, producing a radial, perineural spread seen clinically as characteristic wreath-like, bead-like infiltrates. Tissue damage is compounded by enzymatic secretion of proteases and phospholipases, which degrade stromal collagen and cell membranes, resulting in stromal melt and, in severe cases, corneal perforation.[30] Concurrently, neutrophil degranulation amplifies tissue destruction through the release of ROS and matrix metalloproteinases (e.g., MMP-8, MMP-9), contributing to ulceration.
The innate immune response is characterized by neutrophil infiltration, with these cells attempting to phagocytose smaller filaments and releasing ROS and proteolytic enzymes. Macrophages, in turn, release proinflammatory cytokines such as interleukin 1 and tumor necrosis factor α, which promote leukocyte recruitment and increase vascular permeability. The adaptive response involves T helper type 1-mediated interferon γ secretion, which activates macrophages for intracellular killing. Antibodies directed against Nocardia surface antigens enhance opsonization, but immune complex formation may exacerbate tissue damage.[31] A hypopyon may form in severe infections, reflecting leukocyte accumulation in the anterior chamber and deeper extension of inflammation.[32]
Failure to control infection is often due to delayed recognition, owing to the organism’s slow growth and resemblance to fungal elements, and inadequate early treatment. Even after bacterial clearance, persistent inflammation driven by neutrophils may lead to ongoing stromal melt. Healing occurs via fibrosis, often leaving dense stromal scarring that compromises visual acuity. In advanced cases, corneal thinning and perforation may necessitate TPK. Understanding the interplay between Nocardia's virulence mechanisms and the host immune response highlights the importance of early identification, initiation of targeted antimicrobials (eg, topical amikacin), and careful modulation of inflammation to preserve corneal structure and vision.[33]
Histopathology
The histopathological examination of corneal tissue in Nocardia keratitis reveals features reflecting both the organism's distinctive filamentous morphology and the host’s layered immune response. Loss of the epithelium overlying the infiltrate is typically observed, which may be accompanied by reactive hyperplasia at ulcer margins as the epithelium attempts regeneration.[34] Within the stroma, sheets of polymorphonuclear leukocytes fill the lamellae, often accompanied by focal liquefactive necrosis and collagen degradation, forming “punched-out” cavities.[35] In chronic lesions, granulomatous inflammation with epithelioid histiocytes and multinucleated giant cells may also be present.
The organisms appear as thin (0.5–1 µm), beaded, branching filaments that may fragment into short rods. These filaments are often seen interspersed within inflammatory foci or aligned along stromal lamellae and perineural spaces. The close association of these pathogens with degenerating nerve sheaths explains the radial “grain-of-sand” infiltrates seen clinically.
Anterior stromal sections typically reveal dense neutrophilic infiltrates and cellular debris, with filamentous organisms clustered in wreath-like foci. Deeper stromal layers may exhibit architectural disruption from necrosis and, in some cases, granulomatous inflammation consistent with chronicity. The endothelial surface is usually spared, although variable anterior chamber exudates may be seen.[36]
Special stains are essential for identifying Nocardia. Gram staining may show weakly gram-positive, thin, branching filaments, though these can be faint. The modified Ziehl-Neelsen stain is more definitive, revealing weakly acid-fast red filaments and helping distinguish Nocardia from non-acid-fast organisms. Gomori methenamine silver (GMS) and PAS (periodic acid–Schiff) also highlight the organisms, though less specifically—GMS stains them black, and PAS imparts a bright magenta hue. Hematoxylin and eosin (H&E) typically reveals pale basophilic filament fragments within a dense neutrophilic background.
Differential diagnoses include fungal keratitis, where organisms appear as thicker, septate hyphae with wider-angle branching and stronger staining with GMS; Actinomyces infection, which shows gram-positive filaments but lacks acid-fastness and may present with sulfur granules; and Acanthamoeba keratitis, identified by cysts and trophozoites, though perineuritis is usually less pronounced.[37]
Key diagnostic points include routine use of the modified Ziehl-Neelsen stain when filamentous bacteria are suspected, recognition of perineural organism spread as a hallmark of Nocardia, and correlation with culture or PCR for species confirmation.[38]
Toxicokinetics
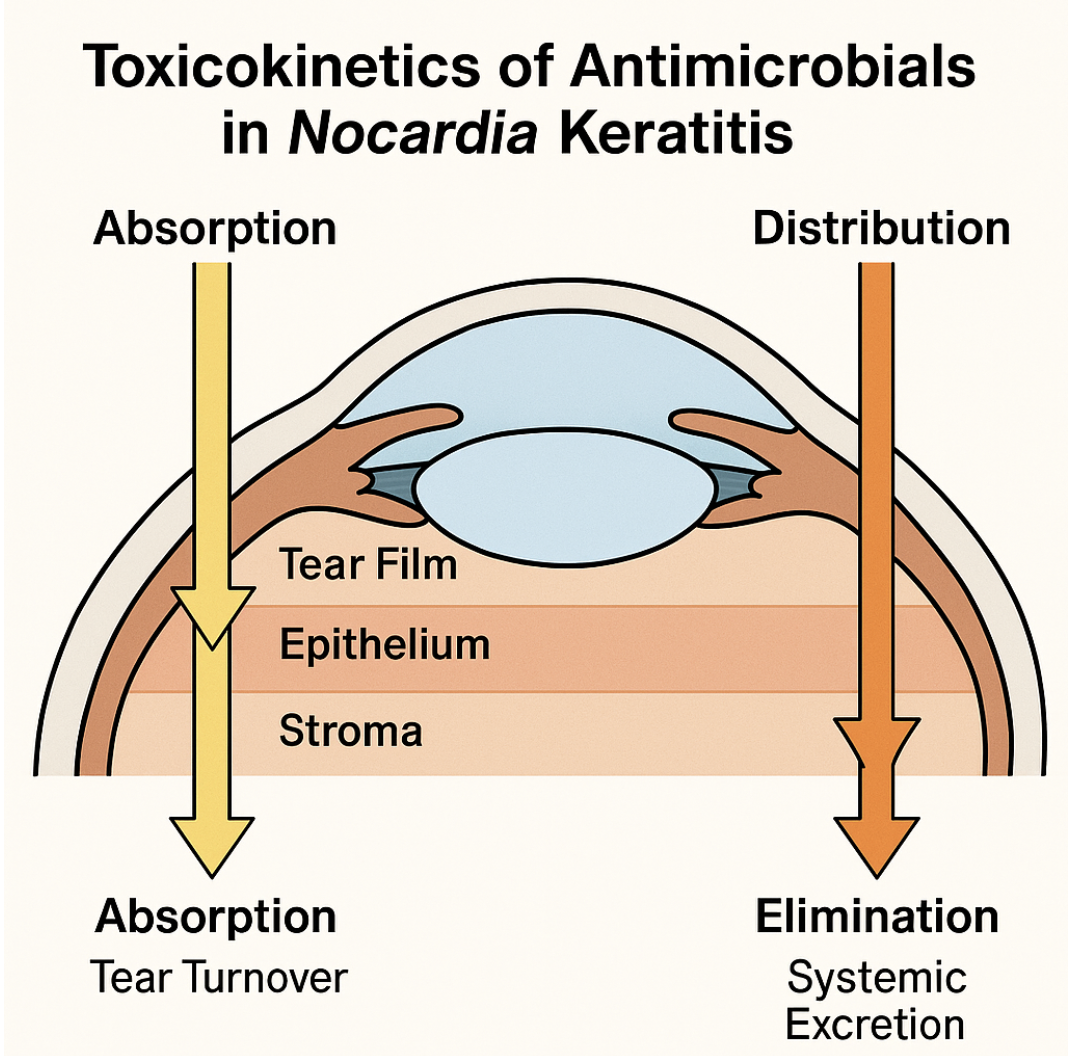
Topical and systemic antimicrobials used in Nocardia keratitis exhibit distinct absorption, distribution, metabolism, and elimination (ADME) profiles that influence therapeutic efficacy and toxicity (see Image. Toxicokinetics of Nocardia Treatment). Topical amikacin (2.5%) rapidly distributes across the tear film, achieving peak concentrations (Cmax) within minutes. Partial binding to the corneal epithelium occurs, with permeation enhanced by frequent dosing. Measurable drug levels reach the corneal stroma within 1 to 2 hours (Tmax), attaining approximately 15 to 20 µg/g tissue. However, tear turnover limits contact time, with a tear half-life of about 5 minutes, necessitating hourly instillation during acute infection phases.[39]
Systemic TMP-SMX exhibits oral bioavailability exceeding 85%, with peak plasma levels reached within 1 to 4 hours. Both TMP and SMX penetrate inflamed corneal and aqueous humor tissues, achieving approximately 50% of plasma concentrations. TMP, being lipophilic, attains higher ocular tissue levels than SMX, though both contribute synergistically to antimicrobial effects.[40][41]
Topically, amikacin concentrates predominantly in anterior corneal layers with minimal systemic absorption, whereas systemic TMP-SMX distributes to ocular tissues via uveal blood flow. Amikacin is not locally metabolized and remains active until elimination, which primarily occurs through tear drainage and the nasolacrimal duct. TMP undergoes partial hepatic acetylation, while SMX is metabolized by hepatic cytochrome P450 enzymes through N-acetylation and hydroxylation. Both TMP and SMX are eliminated renally as parent compounds and metabolites, with half-lives of approximately 10 hours and 11 hours, respectively.
Ocular toxicity of topical amikacin includes epithelial toxicity manifesting as punctate keratopathy and conjunctival irritation, though systemic toxicity is minimal. TMP may cause rare local irritation but carries risks of hyperkalemia and bone marrow suppression systemically. SMX is associated with photosensitivity rash and severe hypersensitivity reactions such as Stevens–Johnson syndrome (SJS), as well as crystalluria.
Table 1. Pharmacokinetic Parameters of Amikacin and TMP-SMX
|
Parameter |
Amikacin (Topical 2.5%) |
TMP (Oral 160 mg) |
SMX (Oral 800 mg) |
|
Cmax (ocular) |
~20 µg/g (stroma) |
~3 µg/mL (aqueous) |
~10 µg/mL (aqueous) |
|
Tmax |
1-2 hours |
2-3 hours |
2-4 hours |
|
Half-life |
Tear film: 5 min |
8-10 hours |
10-12 hours |
|
Elimination |
Tear drainage |
Renal excretion |
Renal excretion |
Table 2. Toxicity Profile of Amikacin and TMP-SMX
|
Drug |
Adverse Effect |
Mechanism / Notes |
|
Amikacin |
Punctate keratopathy |
Direct epithelial toxicity at high concentration |
|
Amikacin |
Conjunctival hyperemia |
Local irritation, transient |
|
Trimethoprim |
Hyperkalemia |
Possible inhibition of distal tubular K secretion |
|
Trimethoprim |
Bone marrow suppression |
Folate antagonism |
|
Sulfamethoxazole |
Hypersensitivity reactions |
Immune-mediated (rash, SJS) |
|
Sulfamethoxazole |
Crystalluria |
Low solubility in acidic urine |
Optimizing dosing schedules to maximize corneal concentrations while minimizing toxicity, such as hourly topical amikacin with regular monitoring for epithelial damage, combined with systemic TMP-SMX for deeper infections, ensures effective eradication of Nocardia with a favorable safety profile.
History and Physical
History
A detailed history and thorough ocular examination are essential for all patients with suspected ocular infection to enable early recognition and appropriate management of Nocardia keratitis. Clinicians should carefully inquire about risk factors, including recent travel to Asia, which raises suspicion for this infection. The symptoms are often nonspecific and include severe ocular pain, blepharospasm, photophobia, eyelid swelling, and gradual vision changes. The onset is typically insidious, evolving over days to weeks, with symptoms progressively worsening despite empiric broad-spectrum antibiotics.
Patients frequently report moderate pain, less severe than that seen in Pseudomonas keratitis but more persistent than fungal infections, along with tearing, foreign-body sensation, and mucopurulent discharge that may be scant in the early stages.[42] A history of corneal trauma with vegetative matter such as thorns, twigs, or soil is documented in 30% to 50% of cases. Other precipitating factors include contact lens wear with poor hygiene or contaminated solutions and recent ocular procedures such as LASIK, pterygium excision, or corneal cross-linking.[43]
Past ocular history may reveal ocular surface disease like dry eye or neurotrophic keratitis, prior infections (eg, bacteria, fungi, Acanthamoeba), or chronic use of topical corticosteroids and immunosuppressive drops.[44] Systemic risk factors include immunosuppression due to diabetes mellitus, systemic steroids, chemotherapy, or HIV, as well as occupational exposure to soil and organic matter through farming, gardening, or construction work. Treatment history should detail prior antibiotic regimens and any therapeutic or bandage contact lens use.[45]
Ocular Examination
On slit-lamp examination, Nocardia keratitis typically presents with patchy anterior stromal infiltrates arranged in a characteristic wreath-like or serpiginous pattern, often accompanied by satellite lesions. These infiltrates are usually located in the midperiphery of the cornea, adjacent to the site of corneal trauma.[46] Occasionally, the infection may present as nonspecific punctate epitheliopathy or an ulcer with margins studded with superficial yellow, pinhead-sized infiltrates. Although infiltrates are typically superficial, extension into the deeper stroma can occur.
The eyelids and adnexa may show mild edema or erythema, while the conjunctiva often exhibits injection, possible chemosis, and early mucopurulent discharge. The presence of foreign bodies should be investigated. Corneal epithelial defects are irregular and highlighted by fluorescein staining. Perineural infiltration may be seen as beading along corneal nerves, best visualized with a high-magnification slit beam. Anterior chamber inflammation with cells and flare is common, with occasional small hypopyon in advanced cases. Intraocular pressure (IOP) is often normal or mildly reduced, likely related to pain-induced blepharospasm.
Peripheral anterior segment evaluation should include inspection of the iris and angle to rule out adhesions or synechiae if the anterior chamber deepens. Fundus examination should confirm the absence of posterior segment involvement. B-scan ultrasonography is indicated if the media are opaque. Ancillary bedside tests can support the diagnosis and include corneal sensitivity testing to identify neurotrophic components; tear film break-up time and Schirmer test to assess for dry eye contributing to epithelial compromise; and point-of-care microscopy using gram and modified acid-fast stains to detect filamentous organisms.
A superficial, yellow-white, serpiginous or “wreath-like” stromal infiltrate with satellite lesions in the setting of vegetative trauma or contact lens wear should prompt a high index of suspicion for Nocardia keratitis. Insidious progression despite broad-spectrum antibiotic therapy is a key clinical red flag, underscoring the importance of early direct smear and culture. Accurate documentation of the infiltrate’s exact size, location, and depth using slit-lamp calipers and serial photography is critical for monitoring therapeutic response.[47]
Evaluation
Clinical diagnosis of Nocardia keratitis could theoretically be made based solely on the symptoms and signs elicited through a thorough history and physical examination. However, the gold standard remains the inoculation and growth of Nocardia species in culture media following corneal scraping or biopsy. These colonies typically grow on blood or chocolate agar and SDA. Given Nocardia’s slow growth rate, clinicians must inform the microbiology laboratory of the suspicion for Nocardia to ensure that culture plates are observed for a sufficient period—growth often appears within 5 to 7 days, but cultures should be maintained for at least 2 weeks to reduce the risk of false negatives. Importantly, the detection of Nocardia is clinically significant, as it is not considered a common laboratory contaminant.
Several diagnostic strategies beyond traditional culture are available for evaluating suspected Nocardia keratitis. A retrospective analysis has identified cases where early diagnosis was made using gram or 1% acid-fast (Ziehl-Neelsen) staining.
In vivo confocal microscopy (IVCM) serves as a noninvasive and real-time imaging modality capable of visualizing corneal structures. On IVCM, Nocardia appears as filamentous, highly reflective structures that may resemble fungal elements. A history of ocular trauma involving organic material can help contextualize these findings. Notably, the diagnostic accuracy of IVCM is highly dependent on the examiner’s experience. In addition, molecular techniques, including PCR, gene sequencing, DNA sequencing, and pyrosequencing, are increasingly recognized for their sensitivity and specificity in identifying Nocardia DNA within ocular tissues.[48][49][50]
Diagnostic evaluations should follow national and international guidelines to ensure both diagnostic accuracy and appropriate therapeutic targeting. According to the Preferred Practice Pattern (PPP) of the American Academy of Ophthalmology (AAO) and the guidelines of the British Royal College of Ophthalmologists (RCOphth) on microbial keratitis, corneal scrapings should be obtained using a sterile Kimura spatula or blade under topical anesthesia. Samples should be collected from both the ulcer edge and base. Smears prepared from these samples may include gram staining to detect weakly gram-positive branching filaments, modified Ziehl-Neelsen staining (Kinyoun technique) to identify weakly acid-fast Nocardia rods, and Giemsa or modified Giemsa staining to highlight beaded filaments.[51]
Following smear preparation, cultures should be established on blood agar (incubated at 35-37°C with 5% CO2), SDA (to exclude fungal pathogens), and optionally on Lowenstein-Jensen or buffered charcoal yeast extract media to optimize Nocardia recovery. Cultures should be incubated for 3 to 7 days before a negative result is confirmed. Species identification may involve biochemical assays or matrix-assisted laser desorption ionization–time of flight (MALDI-TOF) mass spectrometry, and can be confirmed through 16S rRNA sequencing when available.[52]
Real-time PCR-based diagnostics targeting the 16S rRNA gene provide rapid detection and species-level identification within 24 to 48 hours and are available in many reference laboratories. These molecular tools are particularly valuable when clinical suspicion is high but culture resources are limited or delayed.[53]
Imaging technologies such as confocal microscopy and anterior-segment optical coherence tomography (AS-OCT) may also aid diagnosis. Although no universally mandated imaging guideline exists, the European Society of Cataract and Refractive Surgeons supports the adjunctive use of imaging in cases of suspected infectious keratitis. Confocal microscopy allows for in vivo visualization of fine, branching filaments within the superficial to midstroma, and has a sensitivity approaching 85%. AS-OCT is especially useful for measuring the depth of corneal ulcers, monitoring stromal thinning, and guiding surgical planning. Corneal sensitivity testing with a Cochet-Bonnet esthesiometer may also be performed to assess for a neurotrophic component in patients with reduced corneal sensation.
Systemic evaluation is warranted when concern arises for disseminated Nocardia infection, particularly in immunocompromised individuals. Per the Infectious Diseases Society of America (IDSA) guidelines on nocardiosis, chest imaging should be pursued if the patient presents with respiratory symptoms or is known to be immunosuppressed. A chest x-ray may serve as an initial screening tool, with chest computed tomography indicated if the radiograph is abnormal or clinical suspicion remains high, for example, if nodules or cavitary lesions are suspected.
Baseline laboratory work-up should include a complete blood count (CBC) and renal and hepatic panels, especially before initiating systemic therapy. HIV testing and blood glucose measurement are recommended to assess for potential immunosuppression. If neurological symptoms such as headache or focal deficits are present, a brain magnetic resonance imaging should be performed to evaluate for central nervous system involvement.[54]
Ancillary laboratory studies may include antimicrobial susceptibility testing of cultured Nocardia isolates using broth microdilution methods, as outlined in the Clinical and Laboratory Standards Institute (CLSI) M24 guidelines. This testing informs the selection of appropriate topical agents, such as amikacin, as well as systemic agents like TMP-SMX or linezolid. Inflammatory markers, such as erythrocyte sedimentation rate and C-reactive protein (CRP), may be elevated in systemic infections, though these are nonspecific. Blood cultures should be drawn if the patient is febrile or shows signs of systemic involvement.[55]
Clinically, early and adequate corneal sampling is essential to tailor therapy effectively and is recommended by both the AAO and British guidelines. Confocal microscopy may provide critical diagnostic information when culture facilities are not readily available. A complete systemic evaluation should be conducted in immunocompromised patients or when ocular disease remains refractory to initial treatment. If the patient does not demonstrate improvement within 48 to 72 hours of initiating targeted therapy, repeat imaging and microbiologic evaluation should be promptly pursued.[56]
Treatment / Management
Amikacin 2% to 2.5% administered hourly remains the 1st-line treatment for Nocardia keratitis, demonstrating excellent in vitro activity, good corneal penetration, and low resistance rates. A study of 20 ocular Nocardia isolates found that only 55% responded to ciprofloxacin, while all were susceptible to amikacin.[57] Given that culture results require 5 to 7 days, empiric therapy should commence promptly when clinical suspicion is high.
Once Nocardia is identified (by smear, culture, or PCR), switching to specific anti-nocardial agents is prudent.[58] Topical amikacin typically continues until infection resolution, with a mean duration of approximately 38 days of daily administration. The dosing protocol includes hourly instillation during the initial 48 to 72 hours, tapering to every 2 hours by days 4 to 7, then reducing to 4 times daily for 4 to 6 weeks, guided by clinical response and stromal healing.
Second-line treatments for amikacin-resistant Nocardia include topical tobramycin or gentamicin 1.4%, imipenem 0.5%, and moxifloxacin 0.5%. Alternative or adjunctive topical agents comprise linezolid 0.2% administered hourly during the acute phase, clarithromycin 1% 4 times daily, and minocycline 1% 4 times daily.[59][60][59] Sulfonamides, such as topical trimethoprim-sulfamethoxazole, have fallen out of favor for topical use due to significantly higher MICs compared to amikacin.
Systemic antibiotic therapy plays a role in cases with deep stromal involvement, scleral extension, or immunocompromised status, as recommended by the IDSA. TMP-SMX dosed at TMP 8 mg/kg/day plus SMX 40 mg/kg/day in divided doses (eg, 160/800 mg adult formulation twice daily) typically continues for 4 to 6 weeks. Regular monitoring of CBC, renal function, and electrolytes every 1 to 2 weeks facilitates early detection of cytopenias, hyperkalemia, or renal impairment. Linezolid, at 600 mg orally every 12 hours, serves as an alternative for TMP-SMX-resistant isolates or patients with sulfa allergy, with treatment duration limited to 21 days to minimize hematologic toxicity.
Topical corticosteroids should be avoided until Nocardia has been excluded or adequate targeted therapy is underway, as corticosteroids exacerbate filamentous infections and increase the risk of corneal perforation and endophthalmitis.[61] Secondary scleritis may arise from Nocardia extension beyond the cornea, particularly following ocular trauma. Such cases warrant immediate surgical debridement prior to culture or PCR confirmation. Alongside topical amikacin 2%, scleritis treatment involves subconjunctival injection of 100 mg amikacin.[62] Persistent scleritis despite repeated injections may necessitate initiation of oral TMP-SMX at 160 mg/800 mg hourly.(B3)
Surgical intervention becomes necessary in the presence of medication nonresponsiveness, progressive corneal thinning, or significant scleral involvement, particularly in cases of impending or frank perforation, stromal melt persisting beyond 48 to 72 hours of targeted therapy, or dense central scarring threatening vision, as outlined by the RCOphth. Procedures include lamellar keratectomy, penetrating keratoplasty, and conjunctival flap.[63] Lamellar keratectomy facilitates the removal of infected superficial stroma while preserving the Descemet membrane and may enhance topical antibiotic penetration.[64] (B3)
TPK involves full-thickness grafting with at least a 1 mm clear margin around the infiltrate and is preferably performed early to prevent intractable melt, accompanied by intensive anti-nocardial therapy before and after surgery. Conjunctival flaps or tectonic grafts address small perforations or melts unsuitable for TPK.[65] Though the literature contains few reports on the surgical management of Nocardia keratitis, existing outcomes are generally favorable.
Best practices according to the AAOPPP for Infectious Keratitis, the RCOphth Microbial Keratitis Guidelines, and the IDSA Nocardiosis Recommendations inform the management approach. Initial empiric therapy includes hourly fortified topical antibiotics with broad-spectrum coverage, such as vancomycin 50 mg/mL combined with ceftazidime 50 mg/mL, prior to microbiological identification.
Adjunctive measures include gentle epithelial debridement to improve drug penetration, therapeutic bandage contact lenses to promote comfort after epithelial healing (avoiding use during acute stromal melt), and experimental corneal collagen cross-linking using the epi-off protocol in recalcitrant cases to arrest stromal melt.[66](B3)
Monitoring involves daily to twice-daily clinical examinations assessing infiltrate size, depth, epithelial closure, and anterior chamber reaction. Serial photography and AS-OCT document stromal thinning and guide therapy tapering. Repeat cultures become essential if no improvement occurs by 72 hours or if secondary infection is suspected. Systematic evaluation for disseminated nocardiosis, including pulmonary, cutaneous, or central nervous system involvement, remains critical in immunocompromised patients, in accordance with IDSA guidance.[67]
Successful management hinges on rapid identification, prompt initiation of high-frequency topical amikacin (with or without systemic TMP-SMX), vigilant monitoring for toxicity and clinical response, and timely surgical intervention to preserve globe integrity and optimize visual outcomes.[68](B3)
Differential Diagnosis
Nocardia keratitis typically presents insidiously with superficial “wreath-like” infiltrates, radial satellite lesions, and mild to moderate pain, often following trauma. On slit-lamp examination, lesions may occasionally appear as cotton-wool infiltrates with irregular, feathery margins—an atypical pattern resembling fungal keratitis.[69][70] Both Nocardia and fungal infections may exhibit satellite lesions and filamentous structures in culture, contributing to diagnostic confusion.[71]
Distinguishing features favoring Nocardia include a history of trauma involving organic matter, a prolonged disease course, and a superficial granular lesion appearance. Laboratory diagnosis relies on the identification of gram-positive, weakly acid-fast, branching filaments and slow-growing chalky colonies, confirmed by modified Ziehl-Neelsen staining. Treatment centers on hourly topical amikacin 2.5%, with systemic TMP-SMX for deep or scleral involvement. Steroids should be avoided until adequate antimicrobial coverage is established. Keratoplasty remains an option in refractory cases.
Fungal keratitis progresses gradually with feathery, branching “fluffy” infiltrates and satellite lesions, often associated with dry-appearing ulcers. Diagnosis depends on identifying septate or nonseptate hyphae on potassium hydroxide (KOH) or Calcofluor smears and culture growth on SDA. Management typically involves topical natamycin 5% or voriconazole 1%, with systemic antifungals reserved for deep extension and adjunctive debridement as needed.[72]
Moraxella keratitis affects vulnerable populations such as patients with diabetes, excessive alcohol consumption, or malnutrition.[73] This pathogen usually causes peripheral ulcers with angular stromal infiltrates and associated blepharitis, frequently causing mild pain and localizing to the inferior cornea without spread. Both Moraxella and Nocardia grow on blood and chocolate agar. However, Moraxella stains as a gram-negative diplococcus and is oxidase-positive, in contrast to gram-positive filamentous Nocardia. Treatment involves topical fluoroquinolones or fortified cefuroxime, alongside lid hygiene management.[74]
Mycobacterial keratitis, caused by aerobic, acid-fast, gram-positive bacilli, manifests as slow-developing corneal infections. On slit-lamp examination, this organism may exhibit white infiltrates with characteristic “snowflake” or “cracked windshield” appearances. Both Nocardia and mycobacteria stain positive with Ziehl-Neelsen methods and grow on blood agar. However, mycobacteria uniquely grow on Lowenstein-Jensen medium. Management differs from Nocardia keratitis and requires targeted antimycobacterial therapy.[75]
Other bacterial mimickers include Pseudomonas aeruginosa, which presents with rapid, fulminant corneal ulcers characterized by dense central necrosis, copious purulent discharge, and severe pain. Gram-negative rods grow rapidly on blood and MacConkey agar, with hallmark epithelial defects featuring undermined edges. Treatment requires urgent, aggressive hourly fortified tobramycin combined with ceftazidime or fluoroquinolones to prevent perforation.[76]
Staphylococcus and Streptococcus species cause acute focal stromal abscesses with mucopurulent discharge and conjunctival hyperemia. These gram-positive cocci can be differentiated by culture morphology and catalase and coagulase tests. Management includes topical flucloxacillin or cefazolin, adjusted per sensitivity testing, with systemic antibiotics considered for severe infections.[77]
Corynebacterium diphtheriae infection produces a grayish membrane and ulcers with peripheral pseudomembranes, often accompanied by pharyngitis. Diagnosis relies on identifying club-shaped gram-positive bacilli with metachromatic granules on Albert staining. Treatment includes topical erythromycin, systemic penicillin, antitoxin administration, and membrane debridement.[78]
Acanthamoeba keratitis manifests with severe pain disproportionate to clinical signs, ring infiltrates, perineural lesions, and a history of contact lens wear. Diagnosis involves detecting double-walled cysts on KOH or Calcofluor staining, culture on nonnutrient agar with E. coli, or confocal microscopy. Treatment entails prolonged topical therapy with polyhexamethylene biguanide (PHMB) or chlorhexidine, with propamidine adjuncts.[79]
Viral keratitis includes herpes simplex (HSV) and varicella-zoster virus infections. HSV keratitis features dendritic or geographic ulcers with decreased corneal sensation and recurrent episodes, confirmed by fluorescein staining, multinucleated giant cells on smear, or PCR. Treatment consists of topical antivirals (trifluridine, ganciclovir), oral acyclovir, and cautious steroid use under antiviral cover.[80] Varicella-zoster keratitis shows pseudodendrites, lid vesicles, ocular pain, and the Hutchinson sign, diagnosed by PCR or immunofluorescence. Management includes oral acyclovir or valacyclovir, topical antivirals, and control of postherpetic inflammation.[81]
Immune-mediated keratitides such as Mooren ulcer and peripheral ulcerative keratitis (PUK) present with progressive peripheral stromal ulceration and sectoral conjunctival injection, respectively. Mooren ulcer involves undermined edges and intense pain with negative cultures, responding rapidly to systemic immunosuppression, including high-dose steroids, conjunctival resection, and cyclosporine.[82] PUK associates with systemic autoimmune diseases, confirmed by positive test results for antineutrophil cytoplasmic antibody (ANCA), antinuclear antibody (ANA), or rheumatoid factor. Management targets underlying vasculitis with topical and systemic steroids and immunosuppressants.[83]
Neurotrophic keratopathy causes painless epithelial defects and geographic ulcers with reduced corneal sensitivity, diagnosed by decreased Cochet-Bonnet esthesiometry and absence of pathogens on smear or culture. Treatment focuses on preservative-free lubricants, tarsorrhaphy, and recombinant nerve growth factor.
Exposure keratopathy arises from eyelid malposition and lagophthalmos, leading to inferior punctate erosions and tear-film breakup. Diagnosis is clinical with routine microbiology. Management includes lubricants, eyelid taping, or implantation of gold weights.[84]
Contact lens-associated red eye (CLARE) features diffuse superficial infiltrates, moderate pain, and a history of overnight lens wear, with negative cultures and rapid steroid responsiveness. Management involves lens discontinuation, topical antibiotics combined with short-course steroids, and lid hygiene.
Sterile marginal keratitis presents with peripheral infiltrates separated by a lucid interval, minimal discharge, and blepharitis. Cultures are negative, and rapid steroid response is characteristic. Treatment includes lid hygiene and topical antibiotic-steroid combinations.[85]
Any atypical or indolent keratitis, particularly with branching or wreath-like superficial infiltrates, warrants directed smears (including modified Ziehl-Neelsen stain) and comprehensive cultures to differentiate Nocardia from bacterial, fungal, viral, parasitic, and immune-mediated mimickers. Early and accurate diagnosis guides tailored antimicrobial or immunosuppressive therapy, optimizing patient outcomes.
Pertinent Studies and Ongoing Trials
Published data on Nocardia keratitis remain limited, but select reports provide valuable insights into its clinical features, causative species, and treatment outcomes. The following studies illustrate common diagnostic challenges and therapeutic responses.
A 1997 retrospective review of 16 culture-proven cases of Nocardia keratitis found that it accounted for 1.7% of bacterial keratitis, with “wreath-like” infiltrates observed in 37% of patients. All cases responded well to topical amikacin. A subsequent series from 2010 involving 19 patients seen between 2006 and 2008 at a tertiary hospital used 16S rRNA sequencing to identify 6 uncommon Nocardia species, reporting 100% susceptibility to both amikacin and tobramycin and noting trauma as a predisposing factor in 88% of cases.
Several case reports further illustrate the variable presentation and treatment response. One 2012 case initially misdiagnosed as fungal keratitis resolved completely after the introduction of fortified topical amikacin at 14 mg/mL. A 2011 report described Nocardia transvalensis keratitis in a traveler returning from Thailand, confirmed via 16S rRNA PCR, which responded to a combination of intensive topical amikacin and oral doxycycline. A 2018 case series emphasized diagnostic delays and advocated for the use of confocal microscopy and molecular testing to avoid misdiagnosis. Additionally, a 2021 report described an unusual case mimicking superior limbic keratoconjunctivitis and HSV keratitis; early smear and culture confirmed Nocardia, allowing successful treatment with amikacin.
These reports emphasize the importance of early suspicion, appropriate culture techniques, and amikacin-based therapy. Accurate speciation and prompt intervention remain critical to optimizing outcomes in Nocardia keratitis.
Treatment Planning
A structured, phase-based treatment approach to Nocardia keratitis ensures timely initiation, appropriate escalation, and measurable clinical benchmarks. This framework aligns with recommendations from the AAO and the RCOphth.
Phase 1: Initial Empiric Phase (Day 0-1)
Management begins with corneal scrapings for gram staining, modified Ziehl-Neelsen staining, culture, and PCR, followed immediately by hourly administration of broad-spectrum fortified antibiotics, typically vancomycin combined with ceftazidime. Steroids should be withheld during this phase. At 24 hours, clinicians assess ulcer size and depth, ocular discharge, pain levels, and initial smear findings to evaluate response and direct the next steps.
Phase 2: Targeted Therapy Phase (Day 1-7)
Upon microbiological identification of Nocardia, therapy should shift to topical amikacin 2.5% administered hourly. Systemic TMP-SMX is recommended in cases involving deep stromal extension, scleral spread, or systemic immunosuppression. Epithelial debridement may be performed to enhance drug penetration. Clinical review at 48 to 72 hours should document infiltrate shrinkage, ideally targeting at least a 10% reduction in diameter daily, alongside signs of epithelial healing and absence of drug-induced toxicity, such as punctate keratopathy.
Phase 3: Consolidation Phase (Weeks 2-4)
As clinical improvement becomes evident, the frequency of topical amikacin may be tapered from every 2 hours to 4 times daily. Systemic TMP-SMX should be continued for a total duration of 4 to 6 weeks. Lubricants may be introduced, and epithelial debridement discontinued. Weekly follow-up focuses on the resolution of stromal haze, absence of new satellite lesions, and quieting of anterior chamber inflammation.
Phase 4: Refractory or Escalation Phase (Day 7-10 if unresponsive)
If no improvement is observed within 7 to 10 days, repeat microbiological studies are essential to exclude coinfection or antimicrobial resistance. Alternative topical agents such as linezolid 0.2% or clarithromycin 1% may be initiated. Corneal cross-linking may be considered as an off-label adjunct. In the presence of stromal melt or signs of impending perforation, surgical options must be promptly evaluated.
Phase 5: Surgical Intervention Phase (As Needed)
Surgical management depends on the extent and location of the pathology. Lamellar keratectomy is suited for persistent superficial infiltrates, while deep stromal involvement or central nonhealing ulcers may necessitate TPK. In smaller peripheral melts, a tectonic graft or conjunctival flap may be appropriate. During the perioperative period, intensive topical amikacin should be maintained, systemic therapy adjusted as needed, and graft clarity monitored closely to detect any signs of recurrence.
Phase 6: Rehabilitation and Long-Term Follow-Up (Week 6 and beyond)
Once the infection is fully controlled, antimicrobial agents may be tapered over 6 to 8 weeks. Topical corticosteroids should only be introduced when the risk of recurrence is minimal, typically postoperatively and under close supervision. Visual rehabilitation through spectacles or contact lenses can then be initiated. Monthly follow-up is advised until a stable corneal scar is achieved, with routine assessment of visual acuity, refraction, and ocular surface integrity.[86]
Several principles underlie this approach. Rapid diagnostics, such as PCR or confocal microscopy, can significantly shorten the time to targeted therapy. Toxicity monitoring is essential, both via slit-lamp examination for signs of amikacin-induced keratopathy and systemic laboratory evaluation for TMP-SMX adverse effects. Importantly, a lack of at least 10% daily reduction in infiltrate size by days 3 to 4 should trigger early reevaluation. In cases requiring surgery, earlier intervention often yields superior structural and visual outcomes compared to delayed procedures.
Toxicity and Adverse Effect Management
Effective treatment of Nocardia keratitis often relies on intensive topical amikacin and systemic TMP-SMX, with adjunctive or second-line use of agents such as linezolid. However, these therapies carry a substantial risk of ocular and systemic toxicities. Prompt recognition and proactive management of adverse effects are essential to preserve treatment adherence and prevent morbidity.[87]
Ocular Toxicity
Topical amikacin 2.5%, when administered hourly, may lead to punctate epithelial erosions, commonly referred to as “amikacin keratopathy,” along with conjunctival hyperemia and punctate staining. These changes typically emerge within 48 to 72 hours of high-frequency dosing. When such toxicity is observed, the dosing frequency should be reduced (eg, hourly to every 2 hours), and preservative-free lubricants should be introduced at least 4 times daily. In cases of small, persistent epithelial defects, a temporary therapeutic soft contact lens may be applied to promote healing. If epithelial damage is severe or persistent, alternative topical agents such as linezolid 0.2% or clarithromycin 1% should be considered.[88]
Topical linezolid can cause conjunctival irritation or blurred vision, with onset and severity varying by individual. Management involves reducing the dosing frequency, typically from 4 to 3 times daily, and adding lubricants. If symptoms are intolerable, the drug should be discontinued in favor of amikacin or clarithromycin, depending on patient tolerance and response.[89]
Epithelial debridement, while useful in the acute phase to enhance drug penetration, may delay reepithelialization if overused. This treatment should be limited to visibly necrotic epithelium and discontinued once the infection is controlled, with priority shifting to lubrication and use of a bandage contact lens to support surface recovery.
Systemic Toxicity
TMP–SMX is associated with a range of systemic adverse effects, including bone marrow suppression (manifesting as pancytopenia), hyperkalemia, renal impairment, and dermatologic reactions such as rash or SJS. Monitoring should include a CBC with differential at baseline and then every 1 to 2 weeks, as well as serum potassium and creatinine at similar intervals. Liver function tests should be checked monthly. Mild cytopenias or electrolyte abnormalities may be managed by reducing the dose (by 25%-50%) or extending the dosing interval, while moderate to severe toxicity, including significant cytopenias or cutaneous reactions, requires immediate discontinuation and substitution with alternatives like linezolid. Adequate hydration and avoidance of concurrent nephrotoxic medications are critical to mitigating renal risk.
Systemic linezolid, typically dosed at 600 mg twice daily, carries risks of myelosuppression (specifically thrombocytopenia) and peripheral or optic neuropathy, especially with use beyond 14 days. Weekly CBCs are recommended, along with close monitoring for visual symptoms such as blurring or color vision changes. Duration should be limited to no more than 21 days when possible. If thrombocytopenia or signs of neuropathy develop, linezolid should be discontinued and replaced with an alternative agent, such as minocycline.
Doxycycline and minocycline, used occasionally as systemic adjuncts, are generally well-tolerated but may cause photosensitivity and gastrointestinal upset. These side effects are usually managed with clinical monitoring and patient education, including advice on sun protection and administration with food. The agent should be replaced if symptoms persist.
General Principles for Toxicity Management
Education plays a central role in toxicity prevention. Patients should be counseled from the outset about expected side effects, particularly local irritation, and the importance of continuing therapy despite mild discomfort. Preservative-free lubricants should be initiated alongside treatment to minimize ocular surface irritation.[90]
Dosing adjustments should be guided by both symptom severity and laboratory findings. For topical agents, tapering should follow epithelial recovery, and therapy should never be abruptly discontinued while infection is active. Instead, substitution with a better-tolerated alternative is preferred. For systemic medications, a dose reduction of 25% to 50% is appropriate for mild laboratory abnormalities. Moderate to severe toxicity warrants drug cessation and replacement.[91]
Interprofessional oversight is critical. Pharmacists should be involved in identifying drug interactions—for example, the risk of hyperkalemia when TMP-SMX is used with angiotensin-converting enzyme inhibitors—while primary care or internal medicine input is helpful for systemic toxicity surveillance and intervention.[92] In cases of intolerance to 1st-line agents, 2nd-line options include topical clarithromycin, minocycline, and systemic linezolid or doxycycline, guided by in vitro susceptibility testing.[93]
Regular slit-lamp examination enables early identification of epithelial toxicity, allowing timely tapering of amikacin without compromising antimicrobial efficacy. Weekly blood counts and electrolyte panels during TMP-SMX therapy can prevent serious hematologic or renal complications. Limiting the duration of systemic linezolid and closely monitoring for visual symptoms are key to avoiding potentially irreversible neuropathies.
Staging
A structured severity staging system helps guide treatment intensity, anticipate complications, and align prognosis with the extent of corneal involvement. This 4-stage classification integrates depth of infiltration, lesion size, and clinical complications to determine therapeutic urgency.
In Stage I, the disease is limited to the epithelium, presenting with superficial punctate keratitis or small geographic epithelial defects without stromal involvement. Symptoms are typically mild. Management includes epithelial debridement if needed and initiation of topical amikacin 4 times daily, with close monitoring to detect stromal progression.
Stage II is characterized by stromal involvement confined to the superficial 3rd of the cornea. Patients may develop classic “wreath-like” infiltrates, sometimes accompanied by satellite lesions, moderate photophobia, and tearing. Treatment involves hourly topical amikacin 2.5%, with systemic TMP-SMX added if the lesion exceeds 2 mm or if satellite foci are present. Epithelial debridement may be used to facilitate antibiotic penetration.
In Stage III, the infiltrate extends into the middle to deep stroma, often accompanied by persistent pain, early stromal melt, and sometimes a small hypopyon. Intensive hourly topical amikacin is essential, in combination with systemic TMP-SMX. Adjunctive topical linezolid or clarithromycin may be required if initial therapy proves inadequate. Serial AS-OCT is helpful to track stromal thinning and guide escalation.
Stage IV denotes complicated or perforated cases. Clinical findings may include a large hypopyon, scleral spread, secondary glaucoma, or signs of impending or actual perforation. These cases require urgent surgical management, whether by lamellar or penetrating keratoplasty, depending on the depth and location, while maintaining intensive perioperative antibiotic coverage and close IOP and ocular surface support.
This classification provides a practical framework for tailoring therapy to disease severity, enabling clinicians to escalate or de-escalate treatment efficiently. Early recognition of stage progression remains critical to minimizing structural damage and preserving vision.
Prognosis
With appropriate treatment, Nocardia keratitis generally heals rapidly, leaving behind stromal scarring and some peripheral vascularization. Visual acuity often improves when the infection responds well to medical therapy, and most patients remain asymptomatic with no recurrence. In a case series, nearly all patients regained visual acuity better than 20/25 following prompt diagnosis and treatment.[94] Poorer outcomes are associated with older age and scleral extension of the infection.
The prognosis of Nocardia keratitis varies widely depending on several critical factors: time from symptom onset to accurate diagnosis, depth and size of the corneal infiltrate, immune status of the patient, and the promptness and adequacy of medical and surgical management. In large series from tertiary centers, superficial infections, confined to the epithelium or anterior 1/3 of the stroma and treated within the 1st week, achieve best-corrected visual acuity of 20/40 or better in over 70% of cases, typically with only mild residual stromal haze. In contrast, deeper stromal involvement, particularly infiltrates larger than 3 mm or those extending into the mid or posterior stroma, carries a 2- to 3-fold increased risk of corneal thinning and perforation, even with intensive topical amikacin and systemic TMP-SMX.
Surgical intervention, most commonly TPK, is required in approximately 15% to 25% of eyes with deep or progressive disease. Graft clarity at 1 year post-TPK is achieved in about 60%, though outcomes may be compromised by postoperative rejection or recurrence of infection at the graft–host interface.
Immunocompromised patients, such as those receiving systemic corticosteroids or with poorly controlled diabetes, tend to have prolonged healing, higher rates of scleral extension, and poorer visual outcomes, highlighting the need for comprehensive systemic evaluation and management. Early recognition through prompt smear and culture—and confocal microscopy when available—along with rapid initiation of targeted antimicrobial therapy is critical; each 24-hour delay in starting amikacin increases the risk of deep stromal involvement and surgical intervention by nearly 20%.
Complications
Undiagnosed or poorly managed Nocardia keratitis may extend into the adjacent sclera, manifesting as necrotizing scleritis with hemorrhage, abscess formation, and engorgement of episcleral veins. Initial management includes debridement, topical amikacin, and subconjunctival amikacin injections, as outlined previously.
In rare cases, Nocardia keratitis progresses to endophthalmitis, although this form more commonly arises from hematogenous spread in immunocompromised individuals with extraocular Nocardia infection. Endophthalmitis typically presents with marked anterior chamber inflammation, hypopyon, and yellow-white nodules on the iris. Visual outcomes are generally poor, but prompt diagnosis and aggressive topical and intravitreal antibiotic therapy may preserve vision.
Published literature lacks incidence data for scleral or intraocular extension as a direct complication of Nocardia keratitis. However, substantial delays in diagnosis and treatment can lead to corneal perforation or irreversible vision loss.
Corneal thinning and perforation result from stromal collagen degradation due to proteolytic enzymes released by both Nocardia and activated neutrophils. Clinically, patients may develop progressive ulcer enlargement, Descemetocele formation, and anterior chamber shallowing. Management hinges on urgent tissue support. Small perforations may be sealed with tissue glue, while larger defects often require tectonic grafts or TPK.[95]
Corneal scarring and irregular astigmatism typically follow healing by fibrosis after stromal necrosis, leaving dense, irregular scar tissue. Central scars or visual axis haze can significantly impair visual acuity, often necessitating rigid gas-permeable lenses or optical keratoplasty for rehabilitation.[96]
Hypopyon and secondary anterior uveitis arise from a robust inflammatory response, with leukocyte accumulation in the anterior chamber. This reaction increases the risk of posterior synechiae and secondary glaucoma. Once infection control is established, carefully timed topical corticosteroids help limit synechiae and minimize permanent iris damage.
Secondary glaucoma frequently develops due to obstruction of aqueous outflow by inflammatory debris and fibrin; corticosteroids may further elevate IOP. Management includes serial IOP monitoring and use of aqueous suppressants such as β-blockers and carbonic anhydrase inhibitors, while prostaglandin analogues are generally avoided during active inflammation.[97]
Scleral extension and endophthalmitis may occur via direct spread through limbal vasculature or perforation sites, leading to posterior segment involvement. Clinically, patients exhibit ocular pain, vitritis, and marked vision reduction. Treatment requires high-dose systemic TMP-SMX, intravitreal amikacin, and, in some cases, pars plana vitrectomy.[98]
Therapeutic failure and recurrence often stem from delayed diagnosis, drug resistance, suboptimal dosing, or premature cessation of therapy. Confirming antibiotic susceptibility, completing the full 4- to 6-week treatment course, and repeating cultures when clinical deterioration occurs are essential preventive measures.[99]
Graft failure and rejection may complicate cases requiring TPK, particularly when infection recurs at the graft–host margin or immunologic rejection is triggered by postoperative corticosteroid use. Continued intensive antimicrobial therapy and judicious steroid administration help mitigate these risks.[100]
Visual rehabilitation poses additional challenges. Rigid gas-permeable lenses improve visual quality in eyes with irregular astigmatism. Optical keratoplasty may be necessary for central scars, and amniotic membrane transplantation can enhance ocular surface health in cases with severe dryness or neurotrophic keratopathy. Proactive identification and management of emerging complications, especially stromal melt and IOP elevation, remain critical to preserving ocular structure and function.
Postoperative and Rehabilitation Care
Successful surgical intervention, whether lamellar keratectomy, TPK, or conjunctival flap, marks the beginning of a critical postoperative and rehabilitation phase. Careful management during this period enhances graft survival, maintains globe integrity, and supports visual recovery.[101]
During the immediate postoperative period (day 0–7), intensive topical antimicrobial therapy is essential. Amikacin 2.5% should be continued hourly for the first 48 to 72 hours, followed by a gradual taper according to graft appearance and microbial status. In cases with persistently positive intraoperative cultures, adjunctive topical linezolid or clarithromycin may be added while awaiting susceptibility results. Topical corticosteroids should not be initiated until at least 72 hours postoperatively and only after confirming complete infection control. Prednisolone acetate 1% may be started 4 times daily and tapered over 8 to 12 weeks, depending on the level of inflammation and graft clarity.[102]
Supportive measures include preservative-free artificial tears 4 times daily to preserve epithelial integrity. A bandage contact lens may be considered once the corneal epithelium has stabilized to alleviate discomfort.[103] IOP should be checked daily, with elevations managed using topical β-blockers or carbonic anhydrase inhibitors. Cycloplegic agents such as homatropine twice daily help deepen the anterior chamber and reduce ciliary spasm.[104] Patients should wear an eye shield at all times. Head-of-bed elevation must be maintained to reduce periorbital edema. An eye patch must be used at night to prevent inadvertent trauma.[105]
Between weeks 2 and 4, suture evaluation becomes essential. Tension should be assessed at 2 to 3 weeks, and overly tight sutures causing astigmatism or vascularization may be selectively loosened. Interrupted sutures may be removed individually starting at 6 to 8 weeks, guided by corneal topography and graft stability.[106] Amikacin may be tapered to 4 times daily by week 2 and discontinued entirely if the clinical response remains stable. Steroids should be reduced to twice daily by week 4, depending on inflammation and absence of recrudescence.
Patients with persistent ocular surface dryness may benefit from nighttime erythromycin ointment. Concurrent lid margin disease requires aggressive management with hygiene, warm compresses, and topical azithromycin. Best-corrected visual acuity should be documented weekly, and early visual rehabilitation may include a trial of spectacles or low-vision aids to address irregular astigmatism.[107]
In the late rehabilitation phase (months 2–6), refractive stability should be confirmed by corneal topography at months 2 and 4. Rigid gas-permeable or scleral lenses may be fitted once refractive change stabilizes, defined as less than 0.50 D variation over 4 weeks. Steroids may be tapered to once daily by month 3, with a maintenance dose of low-concentration prednisolone acetate (0.125%-0.25%) every other day in cases of vascularization or low-grade inflammation. Topical cyclosporine 0.05% twice daily may be introduced when chronic low-grade inflammation or graft vascularization is observed, to help modulate ongoing immune activity. When indicated, selective suture removal or phototherapeutic keratectomy can refine the graft surface. If the fellow eye remains visually compromised by scarring, candidacy for optical penetrating keratoplasty should be reassessed.
Systemic management should continue if the initial infection demonstrated deep stromal or scleral extension. TMP-SMX must be maintained for a minimum of 4 weeks postoperatively in such cases. Monthly monitoring of blood counts and renal function remains necessary until systemic therapy concludes.[108]
Long-term follow-up focuses on preventing recurrence and detecting graft rejection. Patients should be counseled on warning signs, including pain, photophobia, and sudden vision loss. Routine visits every 3 months are appropriate once stability is achieved, with increased frequency if immunosuppressive therapy is modified. Visual rehabilitation strategies must be tailored to functional needs. Patients with persistent best-corrected visual acuity below 20/60 despite optical correction should be referred to low-vision services for assistive devices, such as magnifiers or electronic aids. Protective eyewear should be recommended to minimize occupational or recreational risks. Psychosocial support remains a key component of long-term care. Prolonged recovery may contribute to anxiety or depression, warranting referral to support groups or rehabilitation services.[109]
An interprofessional, staged approach to postoperative care, including antimicrobial stewardship, calibrated anti-inflammatory therapy, and proactive surface and suture management, supports graft survival. Individualized visual rehabilitation then helps restore functional vision following Nocardia keratitis.
Consultations
Effective management of Nocardia keratitis depends on timely, coordinated care across multiple specialties. This section discusses essential consultations, their roles, and the optimal timing and content of communication throughout the treatment course.
A positive or suspected Nocardia smear prompts early coordination with clinical microbiology. The team guides specimen collection, selects appropriate culture media, and performs gram and modified Ziehl-Neelsen stains. Preliminary smear results must be communicated promptly. PCR or 16S rRNA testing is expedited as needed, with susceptibility data and minimum inhibitory concentration (MIC) interpretation provided within 72 hours. The ophthalmologist should alert the laboratory immediately upon identifying filamentous organisms and follow up as results become available.[110]
Infectious diseases consultation becomes essential when stromal invasion, scleral extension, or systemic immunosuppression complicates the infection. This team recommends systemic therapy, typically with TMP-SMX or linezolid alternatives, and monitors for hematologic, renal, or hepatic toxicities. Coordination includes baseline and follow-up laboratory evaluations, beginning within 24 hours of treatment initiation and continuing with weekly reviews.
Corneal and anterior segment surgeons determine the need for epithelial debridement, lamellar keratectomy, or TPK. Preoperatively, these specialists review AS-OCT and culture results, as well as ulcer size, with the primary ophthalmologist. Postoperatively, anterior segment surgeons guide suture management and monitor graft clarity with daily examinations, gradually spacing visits as the graft stabilizes.[111]
Pharmacy support ensures accurate dosing of topical and systemic agents, especially high-risk medications such as amikacin, TMP-SMX, and linezolid. Pharmacists review potential drug interactions and recommend dose adjustments based on renal or hepatic function. Communication begins at therapy initiation and continues throughout treatment, particularly when laboratories prompt dosing changes or interaction risks.[112]
Internal medicine or primary care physicians manage underlying systemic conditions such as diabetes, HIV, or autoimmune disease. These teams coordinate comprehensive metabolic and immunologic workups early in the course and oversee long-term outpatient management, including adjustments to medications that may affect infection control or wound healing.[113]
When significant corneal scarring or irregular astigmatism persists following resolution of infection, low-vision and rehabilitation services initiate visual rehabilitation. Around weeks 4 to 6, once refraction stabilizes, patients are evaluated for rigid gas-permeable or scleral lenses. These services also provide training in visual aids such as magnifiers and electronic devices, with ongoing support tailored to individual needs.[114]
Occupational and social work teams assess logistical and financial barriers to adherence, such as cost, transportation, or limited dexterity. These professionals coordinate home health services for drop administration and connect patients with financial assistance and vision-related support programs. Evaluations are ideally performed early during hospitalization or the intensive treatment phase.[115]
Key Consultation Workflows
When a smear reveals filamentous branching organisms, the ophthalmologist promptly contacts the microbiology laboratory to initiate Nocardia-specific culture protocols and notifies the pharmacy to prepare fortified amikacin. If deep stromal or scleral extension is present, or the patient is immunosuppressed, an infectious diseases consultation occurs within the first 24 hours to initiate systemic TMP-SMX and tailor dosing. Concurrently, internal medicine optimizes comorbid conditions, such as hyperglycemia, to support corneal healing.
Surgical collaboration becomes essential when infiltrates fail to regress by days 5 to 7. The ophthalmologist and corneal surgeon jointly review AS-OCT and clinical photographs to determine the need for lamellar keratectomy or early TPK, minimizing the risk of perforation.
By weeks 4 to 6, once the infection stabilizes, referral to low-vision and occupational therapy ensures timely rehabilitation. These services facilitate access to visual aids, mobility training, and psychosocial support, helping patients recover function and maintain quality of life.
Integrated consultation across microbiology, infectious diseases, surgery, pharmacy, internal medicine, rehabilitation, and social services enables precise diagnostics, antimicrobial optimization, timely intervention, and long-term recovery planning. This collaborative model enhances anatomical preservation and visual outcomes in patients recovering from Nocardia keratitis.
Deterrence and Patient Education
Preventing Nocardia keratitis begins with minimizing corneal exposure to environmental reservoirs and reinforcing host defenses. Individuals engaged in agricultural or gardening work should wear well-fitting polycarbonate safety goggles or face shields when handling soil, decaying vegetation, or compost. During home repairs or woodworking, protective eyewear helps prevent vegetative splinters from entering the eye while cutting wood, drilling, or sanding. When the corneal epithelium is compromised, patients must avoid swimming pools, hot tubs, and natural bodies of water to reduce microbial exposure.[116]
Strict adherence to prescribed wear schedules is essential. Patients must not sleep in contact lenses unless instructed by their ophthalmologist. Lens cases should be rinsed daily with fresh, sterile solution, air-dried upright on a clean surface, and replaced every 1 to 3 months. Only multipurpose or hydrogen peroxide–based disinfection systems approved by the U.S. Food and Drug Administration should be used, and “topping off” old solutions must be avoided. Proper hand hygiene—washing and drying thoroughly before inserting, removing, or handling lenses—is critical to prevent contamination.
To support epithelial defense, patients with dry eye or blepharitis should use preservative-free lubricating drops or gels in conjunction with warm compresses and eyelid hygiene. Topical corticosteroids must be used only under direct ophthalmic supervision to avoid local immunosuppression and potential exacerbation of infection.[117]
Empowering patients with practical skills and early-warning awareness fosters adherence and facilitates prompt recognition of recurrence. Patients should be taught to monitor for new or worsening redness, pain, tearing, photophobia, or blurred vision, even if symptoms appear mild. A written highlight sheet listing “Red-Flag Symptoms” and a 24-hour clinic contact number should be provided.
The “drop-and-press” method should be demonstrated: the head is tilted back, the lower lid pulled down to form a pouch, 1 drop instilled, then the nasolacrimal sac pressed for 90 seconds to enhance ocular absorption. The use of alarms or smartphone applications is recommended to remind patients of hourly drops during the acute phase, with daily check-off tools advised as dosing frequency tapers. Systemic antibiotics should be taken with or after meals to reduce gastrointestinal upset, and the full 4- to 6-week course must be completed even if symptoms improve.
Bedding and towels must be kept clean, dusty or moldy environments avoided, and the use of indoor air purifiers considered. Swimming, hot tub use, and dusty work should be avoided until the ophthalmologist provides clearance. To support corneal healing, adequate hydration and a diet rich in vitamins A and C should be emphasized, along with tight glycemic control in diabetic patients.[118]
Follow-up visits should be scheduled every 1 to 2 days during the acute phase, with clinical photographs reviewed to illustrate healing progress. Patients are encouraged to call immediately if a dose is missed, side effects occur (eg, rash, severe irritation), or new symptoms develop. A written action plan outlining after-hours contact information and criteria for emergency care, such as sudden vision loss or severe pain, should be provided.
By integrating these deterrence measures and patient-centered education strategies, clinicians can substantially reduce the risk of Nocardia keratitis, detect recurrences early, and ensure safe, effective therapy completion. Consistent communication and ongoing support remain essential to maintaining patient adherence and optimizing outcomes.
Pearls and Other Issues
Pearls and Other Issues in Nocardia Keratitis Management
The following are the most important points to remember when evaluating and managing Nocardia keratitis:
-
Think “wreath” early: A superficial “wreath-like” or serpiginous pattern of small, yellow-white stromal infiltrates, often with radiating satellite lesions, is highly suggestive of Nocardia in the appropriate clinical context. Early recognition of this hallmark pattern, especially in patients with vegetative trauma or contact lens wear, should prompt directed smears (including modified Ziehl-Neelsen) and culture, thereby shortening time to targeted therapy.
-
Do not confuse Nocardia with fungi: Under low magnification, Nocardia filaments may resemble fungal hyphae. Always perform a modified acid-fast stain. Nocardia’s weakly acid-fast red filaments contrast with the blue background, distinguishing them from true fungal elements.
-
Use aggressive, prolonged therapy: Unlike many bacterial keratitides, Nocardia requires sustained, high-frequency topical amikacin (hourly initial dosing) for at least 4 to 6 weeks, with systemic TMP-SMX for deep or scleral involvement. Premature tapering underestimates the organism’s resilience and increases the risk of recurrence.
-
Monitor for amikacin toxicity: Hourly amikacin can cause punctate epithelial erosions (“amikacin keratopathy”). Clinicians should monitor for new corneal staining and, if present, reduce dosing frequency (eg, to every 2–3 hours) and add preservative-free lubricants rather than discontinuing therapy.[119]
-
Use adjunctive imaging wisely. Confocal microscopy can visualize fine filaments in vivo, which is helpful when cultures are delayed or negative. AS-OCT quantifies stromal thinning, guiding decisions on debridement versus surgical intervention.[120]
-
Beware of resistant variants: Although amikacin resistance in ocular Nocardia is rare, certain species (eg, N. farcinica) may have higher MICs. When clinical response lags, clinicians must obtain species-level identification and susceptibility testing to consider alternatives such as linezolid or clarithromycin.[121]
-
Consider host factors: Local immunosuppression (topical steroids, ocular surface disease) and systemic conditions (diabetes, HIV) prolong and deepen infection. Coordinate with primary care or endocrinology to optimize systemic health.
-
Understand that surgical timing matters: Early lamellar keratectomy or TPK, ideally before frank perforation, yields better outcomes than “rescue” surgery. Plan intervention when progressive thinning is evident rather than waiting for impending rupture.[122]
-
Plan visual rehabilitation early: Dense central scars often induce irregular astigmatism. Once refraction stabilizes, engage low-vision specialists early to fit rigid gas-permeable or scleral lenses and consider optical keratoplasty for visual axis scars after infection clearance.[123]
-
Recognize that research gaps remain: No randomized trials define optimal topical concentrations or durations. Novel delivery modalities (eg, drug-eluting contact lenses, intrastromal injections) and adjuncts (eg, corneal cross-linking) remain investigational but warrant further study.
The above pearls emphasize the unique diagnostic challenges, the need for aggressive treatment, and the importance of interprofessional coordination to optimize outcomes in patients with Nocardia keratitis.
Nocardia represents a rare and frequently misdiagnosed cause of ocular infection, with keratitis as the most common manifestation. The pathognomonic sign on slit-lamp examination is patchy anterior stromal infiltrates arranged in a wreath pattern. Diagnosis relies on corneal scrapings and culture, which often require 5 to 7 days to yield results. First-line treatment consists of topical amikacin, while corticosteroids should be avoided to prevent exacerbation.
Enhancing Healthcare Team Outcomes
Patients with Nocardia keratitis often seek initial evaluation from a primary care or emergency medicine provider. Immediate referral to an ophthalmologist is essential. Delays can result in permanent vision loss. Coordination among the pathologist, ophthalmologist, and microbiologist is critical for the implementation of appropriate isolation and identification techniques.
Close ophthalmic follow-up is needed to assess clinical improvement on slit-lamp examination following medical or surgical intervention. Primary care clinicians, including nursing staff, should counsel contact lens users on proper hygiene practices. Patients must avoid water exposure while wearing lenses, wash their hands before handling lenses, and store lenses in clean, fresh solution.
Any episode of painful, red eye should prompt urgent evaluation by an eye specialist. Pharmacists with infectious disease expertise should assist in selecting and dosing antimicrobials, screening for harmful drug interactions, and relaying findings to the team. Only through open communication and active collaboration among interprofessional team members can the morbidity of Nocardia keratitis be reduced and visual outcomes improved.
Optimizing outcomes in Nocardia keratitis depends on structured interprofessional collaboration, clearly defined communication pathways, and continuous quality improvement. Standardized care protocols, including early corneal sampling checklists, empiric-to-targeted therapy algorithms, and escalation criteria based on disease stage, ensure every team member adheres to the same evidence-based roadmap from initial presentation through visual rehabilitation. Regular interprofessional rounds involving ophthalmologists, microbiologists, infectious disease specialists, pharmacists, and nursing staff enable real-time assessment of clinical status, laboratory data, and drug monitoring parameters. These discussions support rapid therapeutic adjustments and facilitate early detection of complications.
Timely communication further improves care coordination. Shared electronic care plans and secure messaging platforms enable immediate dissemination of preliminary smear or PCR results to the full team, narrowing the time gap between diagnosis and initiation of high-frequency topical amikacin or systemic antibiotics. Embedded electronic medical record alerts, such as reminders for twice-weekly CBCs during TMP-SMX therapy or slit-lamp examinations for epithelial toxicity after 72 hours of hourly drops, reinforce adherence to monitoring protocols and reduce treatment-related morbidity.
Targeted interprofessional training sessions, integrating didactic instruction, case-based reviews, and hands-on workshops in corneal debridement techniques and confocal microscopy interpretation, foster shared competencies and reinforce mutual respect across disciplines. Pharmacists may lead in-service sessions on compounding fortified antibiotics and managing drug interactions, while microbiologists can demonstrate best practices in smear preparation and stain interpretation. Simulation-based drills for emergent complications, such as impending perforation requiring urgent keratoplasty or acute ocular toxicity, strengthen team coordination under pressure.
Finally, continuous audit and feedback cycles, tracking metrics such as time to targeted therapy, frequency of TPK, and final visual acuity, guide iterative improvements in care protocols. Regular morbidity and mortality conferences that engage representatives from all services promote a culture of shared learning, accountability, and collective stewardship of patient outcomes. By integrating standardized clinical pathways, real-time communication systems, interprofessional education, and data-driven quality improvement, healthcare teams can significantly enhance structural and functional recovery in patients confronting the formidable challenge of Nocardia keratitis.[124]
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Behaegel J, Ní Dhubhghaill S, Koppen C. Diagnostic Challenges in Nocardia Keratitis. Eye & contact lens. 2018 Sep:44 Suppl 1():S370-S372. doi: 10.1097/ICL.0000000000000462. Epub [PubMed PMID: 29219900]
Sridhar MS, Gopinathan U, Garg P, Sharma S, Rao GN. Ocular nocardia infections with special emphasis on the cornea. Survey of ophthalmology. 2001 Mar-Apr:45(5):361-78 [PubMed PMID: 11274691]
Level 3 (low-level) evidenceClaudia MA, Zuhria I. A complex case of Nocardia keratitis: challenges in diagnosis and therapy. Revista do Instituto de Medicina Tropical de Sao Paulo. 2025:67():e19. doi: 10.1590/S1678-9946202567019. Epub 2025 Mar 17 [PubMed PMID: 40105634]
Level 3 (low-level) evidenceLimbu S, Sharma SP, Kaur H, Malhotra C. Mirror mirror on the wall: in vivo confocal microscopy of Nocardia keratitis replicating microbiological staining. Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2024 Dec:30(12):1551-1552. doi: 10.1016/j.cmi.2024.08.013. Epub 2024 Aug 24 [PubMed PMID: 39187127]
Srujana D, Shankar S, Anand KB, Agrawal M, Singhal A, Kumar A, Kochhar D. Nocardia keratitis: A clinical diagnosis with successful outcome. Oman journal of ophthalmology. 2024 May-Aug:17(2):261-263. doi: 10.4103/ojo.ojo_175_23. Epub 2024 Jun 27 [PubMed PMID: 39132098]
Sara M, Hui A, Yasir M, Peguda HK, Kalaiselvan P, Willcox M. Intrastromal Corneal Ring Implants Associated Bacterial Infections. Current eye research. 2024 Oct:49(10):1012-1020. doi: 10.1080/02713683.2024.2354438. Epub 2024 May 23 [PubMed PMID: 38780797]
Alhemyari MH, Satarasi P, Joseph J, Bagga B. Nocardia keratitis after small incision lenticule extraction (SMILE). BMJ case reports. 2024 May 15:17(5):. pii: e259486. doi: 10.1136/bcr-2023-259486. Epub 2024 May 15 [PubMed PMID: 38749526]
Level 3 (low-level) evidenceGuo X, Zhang Z, Chen Q, Wang L, Xu X, Wei Z, Zhang Y, Chen K, Wang Z, Lu X, Liang Q. Whole Genome Sequencing Highlights the Pathogenic Profile in Nocardia Keratitis. Investigative ophthalmology & visual science. 2024 Mar 5:65(3):26. doi: 10.1167/iovs.65.3.26. Epub [PubMed PMID: 38502137]
Chaidaroon W, Sawetwong P, Manochomphu S. A Case of Nocardia africana-Related Keratitis. Case reports in ophthalmology. 2023 Jan-Dec:14(1):507-512. doi: 10.1159/000533906. Epub 2023 Oct 6 [PubMed PMID: 37901618]
Level 3 (low-level) evidenceBhanot K, Jefferys S, Clipstone K, Guest S, Blanch RJ. Contact lens-related complications in austere conditions among military personnel: a systematic review. BMJ military health. 2023 Sep 12:():. pii: e002476. doi: 10.1136/military-2023-002476. Epub 2023 Sep 12 [PubMed PMID: 37699733]
Level 1 (high-level) evidenceLedbetter EC, Schlesener BN, Demeter EA. Nocardia and Streptomyces keratitis in dogs: In vivo detection of filamentous bacteria by confocal microscopy. Veterinary ophthalmology. 2023 May:26(3):211-218. doi: 10.1111/vop.13077. Epub 2023 Feb 25 [PubMed PMID: 36840607]
Yin X, Liang S, Sun X, Luo S, Wang Z, Li R. Ocular nocardiosis: HSP65 gene sequencing for species identification of Nocardia spp. American journal of ophthalmology. 2007 Oct:144(4):570-3 [PubMed PMID: 17698022]
DeCroos FC, Garg P, Reddy AK, Sharma A, Krishnaiah S, Mungale M, Mruthyunjaya P, Hyderabad Endophthalmitis Research Group. Optimizing diagnosis and management of nocardia keratitis, scleritis, and endophthalmitis: 11-year microbial and clinical overview. Ophthalmology. 2011 Jun:118(6):1193-200. doi: 10.1016/j.ophtha.2010.10.037. Epub 2011 Jan 26 [PubMed PMID: 21276615]
Level 2 (mid-level) evidenceSridhar MS, Sharma S, Reddy MK, Mruthyunjay P, Rao GN. Clinicomicrobiological review of Nocardia keratitis. Cornea. 1998 Jan:17(1):17-22 [PubMed PMID: 9436875]
Level 2 (mid-level) evidencePérez-Santonja JJ, Sakla HF, Abad JL, Zorraquino A, Esteban J, Alió JL. Nocardial keratitis after laser in situ keratomileusis. Journal of refractive surgery (Thorofare, N.J. : 1995). 1997 May-Jun:13(3):314-7 [PubMed PMID: 9183766]
Level 3 (low-level) evidenceFaramarzi A, Feizi S, Javadi MA, Rezaei Kanavi M, Yazdizadeh F, Moein HR. Bilateral nocardia keratitis after photorefractive keratectomy. Journal of ophthalmic & vision research. 2012 Apr:7(2):162-6 [PubMed PMID: 23275825]
Level 3 (low-level) evidenceParsons MR, Holland EJ, Agapitos PJ. Nocardia asteroides keratitis associated with extended-wear soft contact lenses. Canadian journal of ophthalmology. Journal canadien d'ophtalmologie. 1989 Apr:24(3):120-2 [PubMed PMID: 2659153]
Level 3 (low-level) evidenceGurnani B, Kaur K. Pythium Keratitis. StatPearls. 2025 Jan:(): [PubMed PMID: 34424645]
Gurnani B, Christy J, Narayana S, Rajkumar P, Kaur K, Gubert J. Retrospective multifactorial analysis of Pythium keratitis and review of literature. Indian journal of ophthalmology. 2021 May:69(5):1095-1101. doi: 10.4103/ijo.IJO_1808_20. Epub [PubMed PMID: 33913840]
Level 2 (mid-level) evidenceGoel S, Kanta S. Prevalence of Nocardia species in the soil of Patiala area. Indian journal of pathology & microbiology. 1993 Jan:36(1):53-60 [PubMed PMID: 8354557]
Level 3 (low-level) evidenceTrichet E, Cohen-Bacrie S, Conrath J, Drancourt M, Hoffart L. Nocardia transvalensis keratitis: an emerging pathology among travelers returning from Asia. BMC infectious diseases. 2011 Oct 31:11():296. doi: 10.1186/1471-2334-11-296. Epub 2011 Oct 31 [PubMed PMID: 22040176]
Level 3 (low-level) evidenceGarg P, Rao GN. Corneal ulcer: diagnosis and management. Community eye health. 1999:12(30):21-3 [PubMed PMID: 17491983]
Lalitha P. Nocardia keratitis. Current opinion in ophthalmology. 2009 Jul:20(4):318-23. doi: 10.1097/ICU.0b013e32832c3bcc. Epub [PubMed PMID: 19387343]
Level 3 (low-level) evidenceTandra P, Mitra S, Madduri B, Tarini S. Macrophomina phaseolina keratitis: treatment approach for poor response to topical natamycin. BMJ case reports. 2025 May 15:18(5):. pii: e260237. doi: 10.1136/bcr-2024-260237. Epub 2025 May 15 [PubMed PMID: 40379289]
Level 3 (low-level) evidenceGarg P. Fungal, Mycobacterial, and Nocardia infections and the eye: an update. Eye (London, England). 2012 Feb:26(2):245-51. doi: 10.1038/eye.2011.332. Epub 2011 Dec 16 [PubMed PMID: 22173077]
Mosenia A, Nguyen AH, Mandel MR, Seitzman GD. Nocardia sienata: a new causative species of infectious keratitis. BMJ case reports. 2022 Mar 25:15(3):. doi: 10.1136/bcr-2021-247850. Epub 2022 Mar 25 [PubMed PMID: 35338040]
Level 3 (low-level) evidenceSilva CL, Tincani I, Brandão Filho SL, Faccioli LH. Mouse cachexia induced by trehalose dimycolate from Nocardia asteroides. Journal of general microbiology. 1988 Jun:134(6):1629-33 [PubMed PMID: 3065451]
Level 3 (low-level) evidenceFatahi-Bafghi M. Nocardiosis from 1888 to 2017. Microbial pathogenesis. 2018 Jan:114():369-384. doi: 10.1016/j.micpath.2017.11.012. Epub 2017 Nov 13 [PubMed PMID: 29146497]
Beaman BL, Black CM, Doughty F, Beaman L. Role of superoxide dismutase and catalase as determinants of pathogenicity of Nocardia asteroides: importance in resistance to microbicidal activities of human polymorphonuclear neutrophils. Infection and immunity. 1985 Jan:47(1):135-41 [PubMed PMID: 3880721]
Hegazy S, Phan T. Keratitis caused by Nocardia farcinica in a contact lens wearer. Infectious medicine. 2022 Jun:1(2):143-145. doi: 10.1016/j.imj.2022.04.001. Epub 2022 Apr 28 [PubMed PMID: 38073875]
Ong HS, Sharma N, Phee LM, Mehta JS. Atypical microbial keratitis. The ocular surface. 2023 Apr:28():424-439. doi: 10.1016/j.jtos.2021.11.001. Epub 2021 Nov 10 [PubMed PMID: 34768003]
Guo XY, Wang ZQ, Chen KX, Zhang Y, Wei ZY, Liang QF. [Analysis of clinical manifestations and imaging characteristics of in vivo confocal microscopy for Nocardia keratitis]. [Zhonghua yan ke za zhi] Chinese journal of ophthalmology. 2023 Apr 11:59(4):279-287. doi: 10.3760/cma.j.cn112142-20221001-00480. Epub [PubMed PMID: 37012591]
Gurnani B, Kaur K, Venugopal A, Srinivasan B, Bagga B, Iyer G, Christy J, Prajna L, Vanathi M, Garg P, Narayana S, Agarwal S, Sahu S. Pythium insidiosum keratitis - A review. Indian journal of ophthalmology. 2022 Apr:70(4):1107-1120. doi: 10.4103/ijo.IJO_1534_21. Epub [PubMed PMID: 35325996]
Gurnani B, Feroze KB, Patel BC. Neurotrophic Keratitis. StatPearls. 2025 Jan:(): [PubMed PMID: 28613758]
Gurnani B, Kaur K, Agarwal S, Lalgudi VG, Shekhawat NS, Venugopal A, Tripathy K, Srinivasan B, Iyer G, Gubert J. Pythium insidiosum Keratitis: Past, Present, and Future. Ophthalmology and therapy. 2022 Oct:11(5):1629-1653. doi: 10.1007/s40123-022-00542-7. Epub 2022 Jul 5 [PubMed PMID: 35788551]
Meera S, Aishwarya B, Sreelatha S, Nair KP. Nocardia puris: A rare cause of keratitis. Indian journal of pathology & microbiology. 2021 Jan-Mar:64(1):210-211. doi: 10.4103/IJPM.IJPM_760_19. Epub [PubMed PMID: 33433448]
Verner A, Durrani A, Kowalski RP, Jhanji V. A Case of Nocardia farcinica Keratitis in a Pediatric Contact Lens Wearer. Eye & contact lens. 2020 Mar:46(2):e11-e12. doi: 10.1097/ICL.0000000000000594. Epub [PubMed PMID: 31453821]
Level 3 (low-level) evidenceBellala MM, Tandra PS, Bagga B, Madduri B. Nocardia keratitis presenting as an anterior chamber ball of exudates and its management. BMJ case reports. 2023 Feb 21:16(2):. doi: 10.1136/bcr-2022-251647. Epub 2023 Feb 21 [PubMed PMID: 36810335]
Level 3 (low-level) evidenceSizar O, Rahman S, Sundareshan V. Amikacin. StatPearls. 2025 Jan:(): [PubMed PMID: 28613658]
Feiz V, Nijm L, Glickman RD, Morse LS, Telander DG, Park SS, Polage CR, Christiansen SM, Moshirfar M. Vitreous and Aqueous Penetration of Orally Administered Trimethoprim-Sulfamethoxazole Combination in Humans. Cornea. 2013 Oct:32(10):1315-20. doi: 10.1097/ICO.0b013e318298ddf8. Epub [PubMed PMID: 23928948]
Santos G, Delgado E, Silva B, Braz BS, Gonçalves L. Topical Ocular Drug Delivery: The Impact of Permeation Enhancers. Pharmaceutics. 2025 Mar 31:17(4):. doi: 10.3390/pharmaceutics17040447. Epub 2025 Mar 31 [PubMed PMID: 40284442]
Gurnani B, Kaur K. Anti-infective therapies for Pythium insidiosum keratitis. Expert review of anti-infective therapy. 2024 Oct:22(10):805-817. doi: 10.1080/14787210.2024.2403146. Epub 2024 Sep 13 [PubMed PMID: 39268901]
Gurnani B, Kaur K. Contact Lenses. StatPearls. 2025 Jan:(): [PubMed PMID: 35593861]
Gurnani B, Kaur K. Bacterial Keratitis. StatPearls. 2025 Jan:(): [PubMed PMID: 34662023]
Gurnani B, Kaur K. Contact Lens–Related Complications. StatPearls. 2025 Jan:(): [PubMed PMID: 36512659]
Johansson B, Fagerholm P, Petranyi G, Claesson Armitage M, Lagali N. Diagnostic and therapeutic challenges in a case of amikacin-resistant Nocardia keratitis. Acta ophthalmologica. 2017 Feb:95(1):103-105. doi: 10.1111/aos.13182. Epub 2016 Aug 29 [PubMed PMID: 27572657]
Level 3 (low-level) evidenceLorin MI, Palazzi DL, Turner TL, Ward MA. What is a clinical pearl and what is its role in medical education? Medical teacher. 2008:30(9-10):870-4. doi: 10.1080/01421590802144286. Epub [PubMed PMID: 18821165]
Brown JM, Pham KN, McNeil MM, Lasker BA. Rapid identification of Nocardia farcinica clinical isolates by a PCR assay targeting a 314-base-pair species-specific DNA fragment. Journal of clinical microbiology. 2004 Aug:42(8):3655-60 [PubMed PMID: 15297512]
Patel JB, Wallace RJ Jr, Brown-Elliott BA, Taylor T, Imperatrice C, Leonard DG, Wilson RW, Mann L, Jost KC, Nachamkin I. Sequence-based identification of aerobic actinomycetes. Journal of clinical microbiology. 2004 Jun:42(6):2530-40 [PubMed PMID: 15184431]
Galor A, Hall GS, Procop GW, Tuohy M, Millstein ME, Jeng BH. Rapid species determination of Nocardia keratitis using pyrosequencing technology. American journal of ophthalmology. 2007 Jan:143(1):182-3 [PubMed PMID: 17188068]
Level 3 (low-level) evidenceLin A, Rhee MK, Akpek EK, Amescua G, Farid M, Garcia-Ferrer FJ, Varu DM, Musch DC, Dunn SP, Mah FS, American Academy of Ophthalmology Preferred Practice Pattern Cornea and External Disease Panel. Bacterial Keratitis Preferred Practice Pattern®. Ophthalmology. 2019 Jan:126(1):P1-P55. doi: 10.1016/j.ophtha.2018.10.018. Epub 2018 Oct 23 [PubMed PMID: 30366799]
Sandven P, Lassen J. Importance of selective media for recovery of yeasts from clinical specimens. Journal of clinical microbiology. 1999 Nov:37(11):3731-2 [PubMed PMID: 10523586]
Harris KA, Brown JR. Diagnostic yield of broad-range 16s rRNA gene PCR varies by sample type and is improved by the addition of qPCR panels targeting the most common causative organisms. Journal of medical microbiology. 2022 Dec:71(12):. doi: 10.1099/jmm.0.001633. Epub [PubMed PMID: 36748452]
Feigin DS. Nocardiosis of the lung: chest radiographic findings in 21 cases. Radiology. 1986 Apr:159(1):9-14 [PubMed PMID: 3952335]
Level 3 (low-level) evidenceCaso Coelho V, Pereira Neves SD, Cintra Giudice M, Benard G, Lopes MH, Sato PK. Evaluation of antimicrobial susceptibility testing of Nocardia spp. isolates by broth microdilution with resazurin and spectrophotometry. BMC microbiology. 2021 Dec 5:21(1):331. doi: 10.1186/s12866-021-02394-w. Epub 2021 Dec 5 [PubMed PMID: 34865615]
Tavakoli M, Hossain P, Malik RA. Clinical applications of corneal confocal microscopy. Clinical ophthalmology (Auckland, N.Z.). 2008 Jun:2(2):435-45 [PubMed PMID: 19668734]
Reddy AK, Garg P, Kaur I. Speciation and susceptibility of Nocardia isolated from ocular infections. Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2010 Aug:16(8):1168-71. doi: 10.1111/j.1469-0691.2009.03079.x. Epub 2009 Oct 14 [PubMed PMID: 19832708]
Lafont E, Conan PL, Rodriguez-Nava V, Lebeaux D. Invasive Nocardiosis: Disease Presentation, Diagnosis and Treatment - Old Questions, New Answers? Infection and drug resistance. 2020:13():4601-4613. doi: 10.2147/IDR.S249761. Epub 2020 Dec 22 [PubMed PMID: 33376366]
Patel R, Sise A, Al-Mohtaseb Z, Garcia N, Aziz H, Amescua G, Pantanelli SM. Nocardia asteroides Keratitis Resistant to Amikacin. Cornea. 2015 Dec:34(12):1617-9. doi: 10.1097/ICO.0000000000000634. Epub [PubMed PMID: 26418432]
Erkin EF, Günenç U, Oner FH, Gelal A, Erkin Y, Güven H. Penetration of amikacin into aqueous humor of rabbits. Ophthalmologica. Journal international d'ophtalmologie. International journal of ophthalmology. Zeitschrift fur Augenheilkunde. 2001 Jul-Aug:215(4):299-302 [PubMed PMID: 11399939]
Feld R. Vancomycin as part of initial empirical antibiotic therapy for febrile neutropenia in patients with cancer: pros and cons. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 1999 Sep:29(3):503-7 [PubMed PMID: 10530436]
Cunha LPD, Juncal V, Carvalhaes CG, Leão SC, Chimara E, Freitas D. Nocardial scleritis: A case report and a suggested algorithm for disease management based on a literature review. American journal of ophthalmology case reports. 2018 Jun:10():1-5. doi: 10.1016/j.ajoc.2018.01.018. Epub 2018 Jan 10 [PubMed PMID: 29780901]
Level 3 (low-level) evidenceTseng SH, Chen JJ, Hu FR. Nocardia brasiliensis keratitis successfully treated with therapeutic lamellar keratectomy. Cornea. 1996 Mar:15(2):165-7 [PubMed PMID: 8925664]
Level 3 (low-level) evidenceShah P, Zhu D, Culbertson WW. Therapeutic Femtosecond Laser-Assisted Lamellar Keratectomy for Multidrug-Resistant Nocardia Keratitis. Cornea. 2017 Nov:36(11):1429-1431. doi: 10.1097/ICO.0000000000001318. Epub [PubMed PMID: 28834821]
Gurnani B, Kaur K. Therapeutic Keratoplasty. StatPearls. 2025 Jan:(): [PubMed PMID: 37276297]
Gurnani B, Kaur K, Lalgudi VG, Kundu G, Mimouni M, Liu H, Jhanji V, Prakash G, Roy AS, Shetty R, Gurav JS. Role of artificial intelligence, machine learning and deep learning models in corneal disorders - A narrative review. Journal francais d'ophtalmologie. 2024 Sep:47(7):104242. doi: 10.1016/j.jfo.2024.104242. Epub 2024 Jul 15 [PubMed PMID: 39013268]
Level 3 (low-level) evidenceGurnani B, Natarajan R, Mohan M, Kaur K. Breaking-Down Barriers: Proposal of Using Cellulose Biosynthesis Inhibitors and Cellulase Enzyme as a Novel Treatment Modality for Vision Threatening Pythium Insidiosum Keratitis. Clinical ophthalmology (Auckland, N.Z.). 2024:18():765-776. doi: 10.2147/OPTH.S450665. Epub 2024 Mar 11 [PubMed PMID: 38495678]
Ni N, Srinivasan M, McLeod SD, Acharya NR, Lietman TM, Rose-Nussbaumer J. Use of adjunctive topical corticosteroids in bacterial keratitis. Current opinion in ophthalmology. 2016 Jul:27(4):353-7. doi: 10.1097/ICU.0000000000000273. Epub [PubMed PMID: 27096374]
Level 3 (low-level) evidenceLiu S, Li Y, Song T, Zhang J, Zhang P, Luo H, Zhang S, Niu Y, Xu T, He S. The Pathogen Adaptation of HLA Alleles and the Correlation with Autoimmune Diseases in the Han Chinese. Genomics, proteomics & bioinformatics. 2025 Apr 29:():. pii: qzaf038. doi: 10.1093/gpbjnl/qzaf038. Epub 2025 Apr 29 [PubMed PMID: 40300101]
Lalitha P, Tiwari M, Prajna NV, Gilpin C, Prakash K, Srinivasan M. Nocardia keratitis: species, drug sensitivities, and clinical correlation. Cornea. 2007 Apr:26(3):255-9 [PubMed PMID: 17413948]
Level 2 (mid-level) evidenceSrinivasan M, Sharma S. Nocardia asteroides as a cause of corneal ulcer. Case report. Archives of ophthalmology (Chicago, Ill. : 1960). 1987 Apr:105(4):464 [PubMed PMID: 3551895]
Level 3 (low-level) evidenceGurnani B, Kaur K. Comment on: Clinical and mycological profile of fungal keratitis from North and North-East India. Indian journal of ophthalmology. 2023 Jun:71(6):2607-2608. doi: 10.4103/ijo.IJO_1655_22. Epub [PubMed PMID: 37322697]
Level 3 (low-level) evidenceMarioneaux SJ, Cohen EJ, Arentsen JJ, Laibson PR. Moraxella keratitis. Cornea. 1991 Jan:10(1):21-4 [PubMed PMID: 2019103]
Level 3 (low-level) evidenceSoleimani M, Najafabadi SJ, Razavi A, Tabatabaei SA, Mirmoosavi S, Asadigandomani H. Clinical characteristics, predisposing factors, and management of moraxella keratitis in a tertiary eye hospital. Journal of ophthalmic inflammation and infection. 2024 Jul 30:14(1):36. doi: 10.1186/s12348-024-00417-x. Epub 2024 Jul 30 [PubMed PMID: 39080177]
Broadway DC, Kerr-Muir MG, Eykyn SJ, Pambakian H. Mycobacterium chelonei keratitis: a case report and review of previously reported cases. Eye (London, England). 1994:8 ( Pt 1)():134-42 [PubMed PMID: 8013708]
Level 3 (low-level) evidenceAbdipour Mehrian SR, Noushadi F, Pourasghar Y, Farkarian A, Meftah E, Homayounifar F, Amanati A. Prevalence, microbiology, and antimicrobial susceptibility profile of bacterial skin and soft tissue infections in pediatric patients with malignancies at a referral teaching hospital in Shiraz, Iran. BMC infectious diseases. 2025 May 16:25(1):707. doi: 10.1186/s12879-025-11059-2. Epub 2025 May 16 [PubMed PMID: 40380338]
Gurnani B, Kaur K. Navigating the challenges of infective keratitis: A critical analysis of treatment and diagnostic approaches. Indian journal of ophthalmology. 2024 Aug 1:72(8):1227-1228. doi: 10.4103/IJO.IJO_706_24. Epub 2024 Jul 29 [PubMed PMID: 39078975]
Gross N, Brodard I, Overesch G, Kittl S. Genetic basis of β-lactam resistance in Corynebacterium auriscanis and association with otitis externa in dogs and cats. Veterinary microbiology. 2025 Jun:305():110526. doi: 10.1016/j.vetmic.2025.110526. Epub 2025 Apr 24 [PubMed PMID: 40319560]
Papa V, Bodicoat DH, Duarte AA, Dart JKG, De Francesco M. The Natural History of Acanthamoeba Keratitis: A Systematic Literature Review. Ophthalmology and therapy. 2025 Jul:14(7):1369-1383. doi: 10.1007/s40123-025-01152-9. Epub 2025 May 5 [PubMed PMID: 40323557]
Level 1 (high-level) evidenceAhmad B, Gurnani B, Patel BC. Herpes Simplex Keratitis. StatPearls. 2025 Jan:(): [PubMed PMID: 31424862]
Wang J, Rabiee B, Patel C, Jafri M, Hussain H, Chaudhry A, Chaudhry I, Kamoun L, Chaudhry I, Oh L, Bobat FI, Shukla D, Farooq AV. Herpesvirus Infections of the Corneal Endothelium. Microorganisms. 2025 Mar 28:13(4):. doi: 10.3390/microorganisms13040778. Epub 2025 Mar 28 [PubMed PMID: 40284615]
Meddahi M, Denoyer A, Pouillard F, Didier K, Boulagnon-Rombi C. [Case report of a severe Mooren's ulcer]. Annales de pathologie. 2025 May:45(3):233-236. doi: 10.1016/j.annpat.2024.08.004. Epub 2024 Oct 30 [PubMed PMID: 39482225]
Level 3 (low-level) evidenceRebolloso-González AU, González-De la Mora DLÁ. [Peripheral ulcerative keratitis with necrotizing scleritis, manifestation of granulomatosis with polyangiitis]. Revista medica del Instituto Mexicano del Seguro Social. 2025 Mar 3:63(2):e6015. doi: 10.5281/zenodo.14617182. Epub 2025 Mar 3 [PubMed PMID: 40279461]
Gurnani B, Somani AN, Moshirfar M, Patel BC. Fuchs Endothelial Dystrophy. StatPearls. 2025 Jan:(): [PubMed PMID: 31424832]
Awh CC, Knapp C 3rd, Unwala RD, Lee EH, See CW. Marginal corneal infiltrates as an ocular manifestation of acute generalized exanthematous pustulosis. American journal of ophthalmology case reports. 2023 Dec:32():101953. doi: 10.1016/j.ajoc.2023.101953. Epub 2023 Nov 9 [PubMed PMID: 38045987]
Level 3 (low-level) evidenceAdre E, Maestre-Mesa J, Durkee H, Arboleda A, Flynn H Jr, Amescua G, Parel JM, Miller D. Nocardia keratitis: amikacin nonsusceptibility, risk factors, and treatment outcomes. Journal of ophthalmic inflammation and infection. 2022 Mar 5:12(1):11. doi: 10.1186/s12348-022-00287-1. Epub 2022 Mar 5 [PubMed PMID: 35247126]
Kalavathy CM, Parmar P, Ramalingam K, Kaliamurthy J, Jesudasan CA, Thomas PA. Trimethoprim-sulphamethoxazole therapy in Nocardia keratitis. Clinical & experimental ophthalmology. 2004 Aug:32(4):424-8 [PubMed PMID: 15281980]
Kato H, Hagihara M, Hirai J, Sakanashi D, Suematsu H, Nishiyama N, Koizumi Y, Yamagishi Y, Matsuura K, Mikamo H. Evaluation of Amikacin Pharmacokinetics and Pharmacodynamics for Optimal Initial Dosing Regimen. Drugs in R&D. 2017 Mar:17(1):177-187. doi: 10.1007/s40268-016-0165-5. Epub [PubMed PMID: 28063020]
Tu EY, Jain S. Topical linezolid 0.2% for the treatment of vancomycin-resistant or vancomycin-intolerant gram-positive bacterial keratitis. American journal of ophthalmology. 2013 Jun:155(6):1095-1098.e1. doi: 10.1016/j.ajo.2013.01.010. Epub 2013 Feb 26 [PubMed PMID: 23453280]
Foley P, Kerdraon YA, Hogden JP, Shumack S, Spelman L, Sebaratnam DF, Su CS, Katelaris CH. Dupilumab-associated ocular surface disease: An interdisciplinary decision framework for prescribers in the Australian setting. The Australasian journal of dermatology. 2022 Nov:63(4):421-436. doi: 10.1111/ajd.13924. Epub 2022 Sep 20 [PubMed PMID: 36125089]
Siberry GK, Abzug MJ, Nachman S, Brady MT, Dominguez KL, Handelsman E, Mofenson LM, Nesheim S, Panel on Opportunistic Infections in HIV-Exposed and HIV-Infected Children, National Institutes of Health, Centers for Disease Control and Prevention, HIV Medicine Association of the Infectious Diseases Society of America, Pediatric Infectious Diseases Society, American Academy of Pediatrics. Guidelines for the prevention and treatment of opportunistic infections in HIV-exposed and HIV-infected children: recommendations from the National Institutes of Health, Centers for Disease Control and Prevention, the HIV Medicine Association of the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the American Academy of Pediatrics. The Pediatric infectious disease journal. 2013 Nov:32 Suppl 2(0 2):i-KK4. doi: 10.1097/01.inf.0000437856.09540.11. Epub [PubMed PMID: 24569199]
Antoniou T, Gomes T, Juurlink DN, Loutfy MR, Glazier RH, Mamdani MM. Trimethoprim-sulfamethoxazole-induced hyperkalemia in patients receiving inhibitors of the renin-angiotensin system: a population-based study. Archives of internal medicine. 2010 Jun 28:170(12):1045-9. doi: 10.1001/archinternmed.2010.142. Epub [PubMed PMID: 20585070]
Brown-Elliott BA, Nash KA, Wallace RJ Jr. Antimicrobial susceptibility testing, drug resistance mechanisms, and therapy of infections with nontuberculous mycobacteria. Clinical microbiology reviews. 2012 Jul:25(3):545-82. doi: 10.1128/CMR.05030-11. Epub [PubMed PMID: 22763637]
Sridhar MS, Sharma S, Garg P, Rao GN. Treatment and outcome of nocardia keratitis. Cornea. 2001 Jul:20(5):458-62 [PubMed PMID: 11413397]
Level 2 (mid-level) evidenceVemuganti GK, Murthy SI, Das S. Update on pathologic diagnosis of corneal infections and inflammations. Middle East African journal of ophthalmology. 2011 Oct:18(4):277-84. doi: 10.4103/0974-9233.90128. Epub [PubMed PMID: 22224015]
Danjoux JP, Fraenkel G, Wai D, Conway M, Eckstein R, Lawless M. Corneal scarring and irregular astigmatism following refractive surgery in a corneal transplant. Australian and New Zealand journal of ophthalmology. 1998 Feb:26(1):47-9 [PubMed PMID: 9524031]
Kaur K, Zeppieri M, Gurnani B. Primary Congenital Glaucoma. StatPearls. 2025 Jan:(): [PubMed PMID: 34662067]
Parkash RO, Gurnani B, Kaur K, Parkash TO, Sharma T. Assessing the validity of flap motility sign in predicting the extent of anterior capsular tears in phacoemulsification. Indian journal of ophthalmology. 2023 Aug:71(8):3095-3099. doi: 10.4103/IJO.IJO_2552_22. Epub [PubMed PMID: 37530287]
Gurnani B, Kaur K, Chaudhary S, Kaur RP, Nayak S, Mishra D, Balakrishnan H, Parkash RO, Morya AK, Porwal A. Pediatric corneal transplantation: techniques, challenges, and outcomes. Therapeutic advances in ophthalmology. 2024 Jan-Dec:16():25158414241237906. doi: 10.1177/25158414241237906. Epub 2024 Mar 25 [PubMed PMID: 38533487]
Level 3 (low-level) evidenceGurnani B, Czyz CN, Mahabadi N, Havens SJ. Corneal Graft Rejection. StatPearls. 2025 Jan:(): [PubMed PMID: 30085585]
Gurnani B, Kaur K. Penetrating Keratoplasty. StatPearls. 2025 Jan:(): [PubMed PMID: 37276324]
Fung AT, Tran T, Lim LL, Samarawickrama C, Arnold J, Gillies M, Catt C, Mitchell L, Symons A, Buttery R, Cottee L, Tumuluri K, Beaumont P. Local delivery of corticosteroids in clinical ophthalmology: A review. Clinical & experimental ophthalmology. 2020 Apr:48(3):366-401. doi: 10.1111/ceo.13702. Epub 2020 Jan 22 [PubMed PMID: 31860766]
Sharma N, Sah R, Priyadarshini K, Titiyal JS. Contact lenses for the treatment of ocular surface diseases. Indian journal of ophthalmology. 2023 Apr:71(4):1135-1141. doi: 10.4103/IJO.IJO_17_23. Epub [PubMed PMID: 37026245]
Noecker RJ. The management of glaucoma and intraocular hypertension: current approaches and recent advances. Therapeutics and clinical risk management. 2006 Jun:2(2):193-206 [PubMed PMID: 18360593]
Level 3 (low-level) evidenceMahan M, Purt B. Ocular Trauma Prevention Strategies and Patient Counseling. StatPearls. 2025 Jan:(): [PubMed PMID: 35593844]
Pagano L, Shah H, Al Ibrahim O, Gadhvi KA, Coco G, Lee JW, Kaye SB, Levis HJ, Hamill KJ, Semeraro F, Romano V. Update on Suture Techniques in Corneal Transplantation: A Systematic Review. Journal of clinical medicine. 2022 Feb 18:11(4):. doi: 10.3390/jcm11041078. Epub 2022 Feb 18 [PubMed PMID: 35207352]
Level 1 (high-level) evidenceSarhan AR, Fares U, Al-Aqaba MA, Miri A, Otri AM, Said DG, Dua HS. Rapid suture management of post-keratoplasty astigmatism. Eye (London, England). 2010 Apr:24(4):540-6. doi: 10.1038/eye.2009.146. Epub 2009 Jun 12 [PubMed PMID: 19521426]
Binder PS. Selective suture removal can reduce postkeratoplasty astigmatism. Ophthalmology. 1985 Oct:92(10):1412-6 [PubMed PMID: 3906491]
Feroze KB, Gurnani B, O'Rourke MC. Transient Loss of Vision. StatPearls. 2025 Jan:(): [PubMed PMID: 28613595]
Baron S, McMurray DN. Mycobacteria and Nocardia. Medical Microbiology. 1996:(): [PubMed PMID: 21413269]
Cao B, Gonugunta VT, Radhakrishnan N, Lalitha P, Gurnani B, Kaur K, Iyer G, Agarwal S, Srinivasan B, Keenan JD, Prajna NV. Outcomes of Pythium keratitis: a meta-analysis of individual patient data. Current ophthalmology reports. 2022 Dec:10(4):198-208. doi: 10.1007/s40135-022-00302-7. Epub 2022 Nov 10 [PubMed PMID: 37250102]
Level 1 (high-level) evidenceGurnani B, Narayana S, Christy J, Rajkumar P, Kaur K, Gubert J. Successful management of pediatric pythium insidiosum keratitis with cyanoacrylate glue, linezolid, and azithromycin: Rare case report. European journal of ophthalmology. 2022 Sep:32(5):NP87-NP91. doi: 10.1177/11206721211006564. Epub 2021 Mar 28 [PubMed PMID: 33779337]
Level 3 (low-level) evidenceSatlin MJ, Hoover DR, Glesby MJ. Glycemic control in HIV-infected patients with diabetes mellitus and rates of meeting American Diabetes Association management guidelines. AIDS patient care and STDs. 2011 Jan:25(1):5-12. doi: 10.1089/apc.2010.0237. Epub [PubMed PMID: 21214374]
Gurnani B, Kaur K, Sivakumar P, Bhandari S. Retrospective analysis of low vision assistive products - A 6-year review. Saudi journal of ophthalmology : official journal of the Saudi Ophthalmological Society. 2023 Jan-Mar:37(1):32-37. doi: 10.4103/sjopt.sjopt_253_21. Epub 2023 Mar 9 [PubMed PMID: 36968774]
Level 2 (mid-level) evidenceVelez M, Lugo-Agudelo LH, Patiño Lugo DF, Glenton C, Posada AM, Mesa Franco LF, Negrini S, Kiekens C, Spir Brunal MA, Roberg AB, Cruz Sarmiento KM. Factors that influence the provision of home-based rehabilitation services for people needing rehabilitation: a qualitative evidence synthesis. The Cochrane database of systematic reviews. 2023 Feb 10:2(2):CD014823. doi: 10.1002/14651858.CD014823. Epub 2023 Feb 10 [PubMed PMID: 36780267]
Level 1 (high-level) evidenceGoel R, Malik K. Protective eyewear for agricultural workers. Community eye health. 2015:28(91):53 [PubMed PMID: 26989316]
Agarwal P, Craig JP, Rupenthal ID. Formulation Considerations for the Management of Dry Eye Disease. Pharmaceutics. 2021 Feb 3:13(2):. doi: 10.3390/pharmaceutics13020207. Epub 2021 Feb 3 [PubMed PMID: 33546193]
Gurnani B, Kaur K, Gireesh P, Balakrishnan L, Mishra C. Evaluating the novel role of ChatGPT-4 in addressing corneal ulcer queries: An AI-powered insight. European journal of ophthalmology. 2025 Apr 28:():11206721251337290. doi: 10.1177/11206721251337290. Epub 2025 Apr 28 [PubMed PMID: 40295112]
Okoye GS, Bonabe D, Obasi CU, Munikrishna D, Osho F, Mutali M, Ogwumu K, Oke-Ifidon EO, Nathan IG, Enaholo ES, Suleman AI, Chukwuyem C, Enang AE, Oji RC, Ogechukwu VN, Chidera SP, Ogechukwu HC, Kaur K, Gurnani B. Visual outcomes and complications after phacoemulsification and small incision manual cataract surgery in two eye hospitals. Journal francais d'ophtalmologie. 2025 Jan:48(1):104353. doi: 10.1016/j.jfo.2024.104353. Epub 2024 Nov 18 [PubMed PMID: 39561679]
Murugan SRB, Sanjay S, Somanath A, Mahendradas P, Patil A, Kaur K, Gurnani B. Artificial Intelligence in Uveitis: Innovations in Diagnosis and Therapeutic Strategies. Clinical ophthalmology (Auckland, N.Z.). 2024:18():3753-3766. doi: 10.2147/OPTH.S495307. Epub 2024 Dec 14 [PubMed PMID: 39703602]
Wang C, Sun Q, Yan J, Liao X, Long S, Zheng M, Zhang Y, Yang X, Shi G, Zhao Y, Wang G, Pan J. The species distribution and antimicrobial resistance profiles of Nocardia species in China: A systematic review and meta-analysis. PLoS neglected tropical diseases. 2023 Jul:17(7):e0011432. doi: 10.1371/journal.pntd.0011432. Epub 2023 Jul 10 [PubMed PMID: 37428800]
Level 1 (high-level) evidenceMaghsoudlou P, Sood G, Gurnani B, Akhondi H. Cornea Transplantation. StatPearls. 2025 Jan:(): [PubMed PMID: 30969512]
Vohra V, Tuteja S, Gurnani B, Chawla H. Collagen Cross Linking for Keratoconus. StatPearls. 2025 Jan:(): [PubMed PMID: 32965942]
Schnider C, Yuen L, Rampat R, Zhu D, Dhallu S, Trinh T, Gurnani B, Abdelmaksoud A, Bhogal-Bhamra G, Wolffsohn JS, Naroo SA. BCLA CLEAR presbyopia: Management with intraocular lenses. Contact lens & anterior eye : the journal of the British Contact Lens Association. 2024 Aug:47(4):102253. doi: 10.1016/j.clae.2024.102253. Epub 2024 Jul 2 [PubMed PMID: 39068141]