Introduction
Extracorporeal membrane oxygenation (ECMO) is a life support modality for adults and children with life-threatening cardiac and pulmonary failure that is refractory to conventional therapy or unresponsive to cardiopulmonary resuscitation (CPR).[1][2] The ECMO circuit consists of a pump and an oxygenator that temporarily replace cardiac and pulmonary function, allowing time for organ recovery.[2]
According to the Extracorporeal Life Support Organization (ELSO), ECMO was used in 151,683 patients through 2020, including 45,205 neonates, 30,743 children, and 75,735 adults. In 1990, ECMO was available in 83 centers; by 2020, the number of ECMO centers had grown to 492. As of July 2024, more than 185,000 cumulative ECMO runs have been reported across over 1200 centers worldwide, reflecting the rapid global expansion of this therapy.[3][4][ECLS International Summary of Statistics]
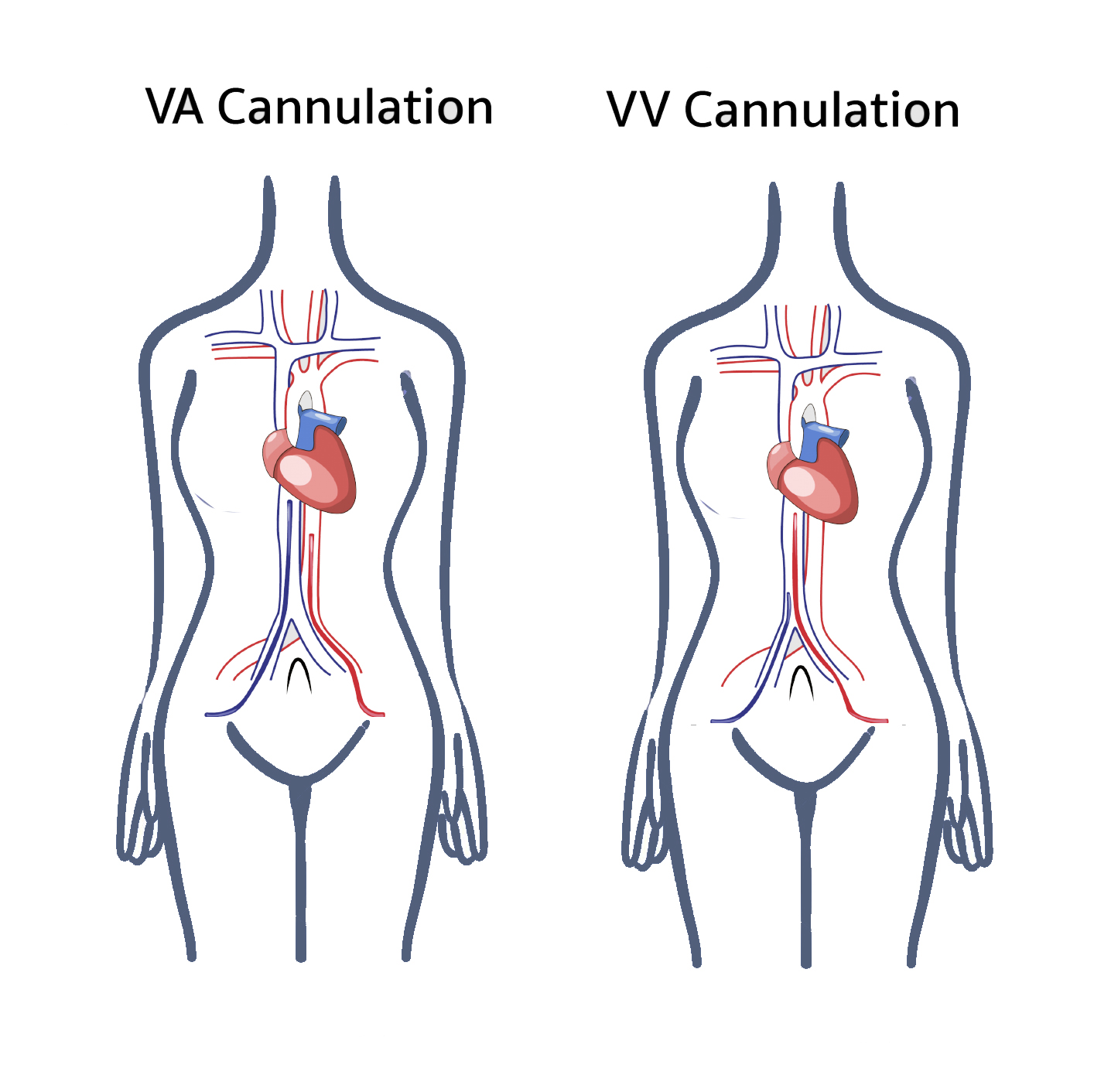
Venovenous (VV) ECMO provides respiratory support, whereas venoarterial (VA) ECMO supports both cardiac and respiratory function.[3][4]
ECMO is a supportive therapy rather than a disease-modifying intervention. In 1944, Kolff and Berk demonstrated blood oxygenation using cellophane chambers in an artificial kidney. In 1953, Gibbon applied artificial oxygenation and perfusion during the first successful open-heart operation. Early oxygenators included film and bubble designs, both associated with intravascular hemolysis, systemic inflammation, platelet destruction, and embolization.[5]
In 1956, Clowes and Basler developed the first prototype membrane oxygenator suitable for cardiopulmonary bypass (CPB).[6] Rashkind used a bubble oxygenator in 1965 to treat neonatal respiratory failure. In 1969, Dorson et al reported the use of a membrane oxygenator in infant CPB. Baffes et al described ECMO use during infant cardiac surgery in 1970. In 1972, Hill et al reported the first use of ECMO for adult respiratory failure, and Bartlett et al achieved the first successful neonatal ECMO case in 1975 for severe respiratory distress.[7]
From the 1980s to the early 2000s, ECMO circuits typically used either silicone membrane or polypropylene hollow fiber oxygenators.[8] These oxygenators were prone to plasma leakage, prompting the development of polymethylpentene oxygenators. The newer generation polymethylpentene models are more durable, provide improved gas exchange, and result in less blood trauma.[9][10] A recent single-center pilot study comparing 4 polymethylpentene oxygenators found low oxygenator failure rates, but notable differences in resistance and post-oxygenator PaO2, emphasizing the need to tailor device choice to patient-specific requirements during long ECMO runs.[11]
Kolobow and colleagues analyzed ECMO outcomes in a National Institutes of Health trial conducted in 1981. That same year, Gattinoni et al demonstrated successful ECMO use in a large population of patients with acute respiratory distress syndrome (ARDS). By 1987, Gattinoni's group reported approximately 50% survival.[12]
In 1994, a randomized controlled trial by Morris et al did not show improved outcomes with low-flow VV ECMO versus conventional ventilation in ARDS. Survival in the control group was 42%, compared to 33% in the ECMO group. Interest in ECMO resurged after publication of the CESAR trial in 2009, which randomized 180 patients across 68 centers. CESAR demonstrated significantly improved outcomes—including reduced mortality and disability—among patients with severe respiratory failure treated with ECMO versus conventional management.[13]
ECMO use expanded dramatically during the COVID-19 pandemic. Large-scale ELSO registry analyses have confirmed hospital survival rates of 48% to 60% in patients with severe viral ARDS when ECMO is initiated at experienced centers.[14]
Anatomy and Physiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Anatomy and Physiology
ECMO consists of a circuit where blood is drained through a catheter from the venous vascular system, circulated in a pump outside the body, and reinfused into the other venous or arterial vascular system, depending on the ECMO circuit type, for circulation in the body.[15]
Cannulas made of plastic tubes are placed in veins or arteries in the groin, neck, or chest. A catheter withdraws blood from the cannula through veins that consist of high carbon dioxide (CO2) and low oxygen (O2) content. Deoxygenated blood extracted from the venous catheter gets transferred to the oxygenator with the help of a pump. An oxygenator works as an artificial lung that maintains the CO2 extraction and oxygenation flow rate. Air and oxygen flow through the hollow fibers in the oxygenator. As the blood passes through tiny fibers, oxygen leaves the fibers and replaces carbon dioxide in the red blood cells. CO2 then enters the fiber and is removed in the exhaust gas. Oxygenated blood is delivered through the catheter back to the patient.[2]
Two basic types of ECMO are used—VV ECMO and VA ECMO. VV ECMO provides respiratory support only, whereas VA ECMO bypasses the heart and lungs, making it the preferred option for patients with cardiogenic shock or cardiac arrest with failed therapies (see Image. Traditional VA and VV ECMO cannulation).[3] Hybrid configurations such as veno-arteriovenous (V-AV) ECMO are increasingly used to tailor support when combined respiratory and partial cardiac assistance is required, although they remain below 10% of total adult ECMO runs worldwide.[16]
Venovenous Extracorporeal Membrane Oxygenation
Types of cannula:
- Single venous cannula: A single venous cannula facilitates the extraction of blood from the vena cava or right atrium, transferred to the ECMO circuit, and returned to the right atrium. Placement is typically performed percutaneously via the right jugular vein using the Seldinger technique.
- Advantages include:
- Only one cannulation site in the neck, avoiding groin lines.
- Allows early mobilization and ambulation once extubated.
- Flexible, kink-resistant design.
- Disadvantages include:
- Greater sensitivity to patient movement, which can affect flow.
- Smaller French (Fr) sizes, limiting peak circuit flow.
- Requires precise echo-guided placement to ensure accurate placement of the superior and inferior vena cava catheters.
- Higher risk of recirculation, where oxygenated blood is withdrawn back into the drainage lumen instead of reaching systemic circulation—often caused by cannula malposition or displacement.
- Recent registry data indicate that bicaval dual-lumen cannulas allow out-of-bed mobilization in up to 60% of patients on VV ECMO while reducing pressure-related skin injury compared with 2-site cannulation.[17]
- Advantages include:
- Double venous cannula: In double venous cannulation for ECMO, one cannula is placed in the common femoral vein for drainage, while the infusion cannula is positioned either in the right internal jugular vein or the femoral vein for return of oxygenated blood. This configuration enables venous flow either from the right atrium to the inferior vena cava or in the reverse direction. Most centers use multistage catheters for drainage via the right internal jugular vein, with oxygenated blood returned through the femoral vein cannula. This approach significantly reduces the risk of recirculation.
Venoarterial Extracorporeal Membrane Oxygenation
Types of cannula:
- Peripheral cannula: Blood drainage from the right atrium or vena cava and infusion of blood to either femoral, axillary, or carotid arteries.
- Central cannula: Blood drainage from the right atrium or vena cava and infusion of blood to the ascending aorta. Central cannulation is preferred in patients who have undergone cardiotomy, as cannulas used for CPB can be transferred to the ECMO circuit.
In case of a right ventricular assist device (VAD), oxygenated blood from ECMO is infused into the pulmonary artery, and blood bypasses the right heart.
In case of emergency or cardiogenic shock, femoral access is preferred. To decrease the ischemia to the ipsilateral lower extremity, a cannula can be inserted distal to the femoral artery or posterior tibial artery to perfuse the distal extremity or provide retrograde flow.. A recent meta-analysis demonstrated that placement of a distal perfusion catheter—particularly when used prophylactically—nearly halves the incidence of acute limb ischemia after femoral VA ECMO cannulation.[19]
In case of peripheral artery disease or prior femoral reconstruction, femoral arterial cannulation is unsuitable. Therefore, the right common carotid artery or axillary artery should be considered.
In addition to respiratory support, VA ECMO provides hemodynamic support. The ECMO circuit is connected in parallel to the heart and lungs.
Indications
Inclusion Criteria for Extracorporeal Cardiopulmonary Resuscitation
- Age younger than 70 years
- Cardiopulmonary arrest to first CPR less than 5 minutes
- Witnessed arrest
- Ventricular fibrillation, paroxysmal ventricular tachycardia, or pulseless electrical activity as the initial cardiac rhythm
- Recurrent ventricular fibrillation or intermittent return of spontaneous circulation
- Absence of comorbidities such as end-stage heart failure, chronic obstructive pulmonary disease, liver failure, end-stage renal failure, or terminal irreversible illness
- No known aortic valve incompetence
ECMO is increasingly used for prolonged support in intensive care units. In certain specialized centers, ECMO is initiated as part of CPR during cardiac arrest.[2] The 2023 American Heart Association Focused Update on Adult Advanced Cardiovascular Life Support states that extracorporeal CPR is reasonable for selected in-hospital or refractory out-of-hospital arrests, provided that flow can be established within 60 minutes and trained teams are available.[20]
Indications for Venoarterial Extracorporeal Membrane Oxygenation
- VA ECMO is used to provide both respiratory and cardiac support.[21]
- Cardiac conditions with low cardiac output (cardiac index <2 L/min/m) and hypotension (systolic blood pressure <90 mm Hg) despite inotropic and intra-aortic balloon pump (IABP) support.
- Cardiogenic shock secondary to either acute coronary syndrome, refractory cardiac arrhythmia, sepsis-induced cardiac depression, myocarditis, pulmonary embolism, drug toxicity, cardiac trauma, anaphylaxis, acute decompensated heart failure, or septic shock—conditions in which cardiac activity is compromised and unable to pump out adequate blood to meet the body's demand.
- Periprocedural for high-risk cardiac interventions.
- Postoperative heart failure: Inability to wean from CPB after cardiac surgery. Postoperatively, ECMO can be beneficial by providing cardiac rest and supporting recovery following surgery.
- Post-heart transplant: After heart or lung-heart transplantation in cases of primary graft failure.
- Bridge to long-term VAD support or bridge to heart/lung transplant.
A 2024 National Inpatient Sample analysis (n = 13,106) showed that each 12-hour delay from admission to VA ECMO initiation increased adjusted in-hospital mortality by 6%; initiation after 24 hours was associated with an odds ratio of 1.20 for death versus initiation within 24 hours, reinforcing the importance of timely deployment.[22]
Indications for Venovenous Extracorporeal Membrane Oxygenation
- VV ECMO is used for respiratory support in individuals who do not respond to mechanical ventilation or any acute, potentially reversible respiratory failure.[21]
- ARDS secondary to either severe bacterial or viral pneumonia, including COVID-19 or aspiration pneumonitis. ECMO bypasses the compromised activity of the lungs and maintains oxygenation and ventilation by removing CO2.[2]
- COVID-19 severe respiratory failure: ARDS due to SARS-CoV-2 infection when prolonged mechanical ventilatory support fails. In some cases, when ventilation fails, ECMO support (VV ECMO) has been initiated.[5]
- Extracorporeal assistance to support the lung in cases of airway obstruction and pulmonary contusion. (barotrauma), smoke inhalation, drowning, air leak syndrome, hypercapnia, or hypoxic respiratory failure.
- Status asthmaticus.
- Massive hemoptysis or pulmonary hemorrhage.
- Bridge to lung transplant.
- Support for lung resections in unstable patients.
Prone positioning while on VV ECMO is safe; the 2023 PRONECMO randomized trial found no difference in 60-day ECMO-free survival versus supine management but confirmed feasibility and similar 90-day mortality, supporting its selective use for oxygenation improvement.[23]
Contraindications
Absolute Contraindications to Extracorporeal Membrane Oxygenation
- Unwitnessed cardiac arrest
- Prolonged CPR without adequate tissue perfusion
- Not a transplant or VAD support candidate
- Unrepaired aortic dissection
- Severe aortic regurgitation
- Unrecoverable severe brain injury
- Disseminated malignancy
- Severe organ dysfunction, for example, emphysema/cirrhosis/renal failure
- Peripheral vascular disease in cases of peripheral VA ECMO
- Lethal chromosomal abnormalities
- Pulmonary hypertension (mean pulmonary artery pressure >50 mm Hg) or cardiogenic failure: VV ECMO is contraindicated
Relative Contraindications to Extracorporeal Membrane Oxygenation
- Advanced age
- Pre-existing chronic illness with a long-term poor prognosis
- Mechanical ventilation lasting more than 14 days
- Frailty (clinical frailty scale ≥3) is increasingly flagged as a relative contraindication in contemporary ECMO selection frameworks.[24]
- Severe obesity (body mass index ≥50 kg m²) is associated with lower weaning success; however, a recent systematic review of VV ECMO cases found survival comparable to nonobese cohorts, supporting case-by-case assessment rather than absolute exclusion.[25]
Equipment
Table 1. Comparison of VV ECMO and VA ECMO
|
VV ECMO |
VA ECMO |
|
Requires venous cannulation |
Arterial and venous cannulation |
|
Maintains pulmonary blood flow and increases mixed venous pO2 |
Decreases pulmonary arterial pressure as it bypasses the pulmonary circulation |
|
Does not assist systemic circulation; no cardiac support is provided |
Cardiac support assists in systemic circulation |
|
Not useful in right ventricular failure |
Can be useful in right ventricular failure |
|
The perfusion rate requirement is high |
Perfusion rate requirement is low |
|
Compared to VA ECMO, lower PaO2 |
Higher PaO2 achievement |
|
ECMO circuit connection in series to the heart and lungs |
ECMO circuit connection is parallel to the heart and lungs |
|
Fewer complications |
More complications |
References for the table.[1][3]
Key Components of an Extracorporeal Membrane Oxygenation Circuit
The ECMO circuit consists of a drainage cannula, a return cannula, a pump, and a heat/gas exchanger.[4]
Pump: The 2 types of driving force (pump) of ECMO are roller and centrifugal pumps. Centrifugal pumps contain plastic cones or impellers that rotate around 3000 rpm, generating up to 900 mm Hg of forward pressure that propels the blood. The negative pressure of around 400 to 500 mm Hg is responsible for fewer microemboli and fewer cavitations. Blood flow in the pump is dependent on preload and afterload. In case of hypovolemia, the inlet pressure becomes more negative to maintain the speed of the pump; however, this results in a decrease in blood flow rate. Systemic vascular resistance changes the circuit flow and the speed of the pump in VA ECMO.[26]
Magnetically levitated centrifugal pumps introduced in 2022 to 2023 deliver equivalent flow at lower shear stress. A prospective adult ECMO study reported significantly lower plasma-free hemoglobin and reduced unplanned circuit change-outs compared to conventional bearing pumps.[27]
The roller pump consists of tubing compressed by rollers. The rotating arm has rollers that compress the tube and propel the blood. In case of hypovolemia, pump speed and flow rate decrease. Roller pumps are not controlled by the afterload; therefore, in VA ECMO, changes in the systemic vascular resistance do not influence blood pumping. Roller pumps are less expensive, safer, and more reliable, but microembolization shedding can occur due to the production of high negative pressure.
Table 2. Comparison of Centrifugal and Roller Pumps in ECMO
|
Features |
Centrifugal Pump |
Roller Pump |
|
Description |
Nonocclusive |
Occlusive |
|
Effect of Afterload |
Affected by afterload |
Not affected by afterload |
|
Advantages |
|
|
|
Disadvantages |
|
|
Reference for the table.[3]
Oxygenators: Membrane oxygenators function similarly to the lungs, having the characteristic of either microporous polypropylene hollow fiber or non-microporous silicone rubber. Less particulate and gas embolization by membrane oxygenators compared to bubbles also allows superior control of blood gases.[28][29] During CPB, polypropylene hollow fiber oxygenators are used. Compared to silicone membrane oxygenators, polypropylene hollow fiber oxygenators are superior due to small priming volume, higher gas transfer, and lower resistance. A new generation of oxygenators developed from polymethyl pentene has shown improved gas exchange efficiency and a reduction in the need for red blood cell and platelet transfusions.[10][30]
Cannulae and Tubing: The drainage cannula is suggested to be 23 to 25 F, and the return cannula should be 17 to 21 F. Sidebotham et al suggested a 25-F multi-stage femoral venous cannula, which has numerous side holes that provide drainage for both VV and VA ECMO, particularly useful in patients who require a flow of more than 6 L/min.[26] In the ECMO circuit, polyvinyl tubing and polycarbonate connectors are used. Medical-grade polyvinyl tubing is popular due to its compatibility with blood, flexibility, smoothness, transparency, resistance to kinking, and collapse characteristics. In VA ECMO, cannulation may be central or peripheral, whereas in VV ECMO, it is typically peripheral. Central access through sternotomy is performed by a cardiothoracic surgeon, and peripheral access can be performed percutaneously in the intensive care unit or cardiac catheterization laboratory.[31]
In ECMO circuits, heparin can be coated through ionic or covalent bonds, but its effectiveness is not very well established. Heparin/platelet factor 4 antibodies are considered for the development of heparin-induced thrombocytopenia type II.[32] Activation of inflammatory mediators, such as the complement activation pathway, can occur due to direct contact of blood with the ECMO circuit. Several reports have indicated a decrease in C3a and C5b-9 complement levels due to heparin coating; however, the inflammatory response remains unchanged, and no convincing evidence has been established regarding the impact of heparin coating on reducing inflammation.[33]
- Anticoagulation for ECMO: Monitoring anticoagulation is essential for ECMO management. The balance between reducing platelet and thrombin activation to prevent thrombosis and providing sufficient clotting to prevent bleeding is the goal of anticoagulation.[34] Activated clotting time (ACT) is the most widely used test to monitor anticoagulation.[35][36] Accuracy of the ACT result varies by age, sample size, temperature, hemodilution, degree of hypothermia, antithrombin level, platelet dysfunction, maturity of the coagulation system, coagulopathy, and ongoing synthesis of thrombin.[37][38] The suggested range for ACT during ECMO is 180 to 220s. Baird et al mentioned a mean ACT of 227±50 seconds on 604 pediatric patients, but the range was extensive, from 158 to 620 seconds. Another option to measure anticoagulation is to obtain the heparin concentration. The use of heparin concentration is less sensitive to the changes in clotting factors and platelets. Fewer studies have evaluated anticoagulation monitoring using heparin concentration compared to those using ACT measurement.[39]
Thromboelastography has been used by several medical centers to assess the coagulation profile, including the measurement of strength and dissolution of clots in case of fibrinolysis. To monitor anticoagulation through heparin, activated partial thromboplastin time (APTT) is most widely used, except in case of CPB, when a high heparin dose is required. ECMO requires high heparin doses; therefore, APTT is considered a valuable tool for anticoagulation assessment. In contrast, ACT has been found to poorly correlate with APTT, as ACT cannot distinguish between low and moderate levels of anticoagulation.[34][40]
Heparin is the first-line anticoagulant for ECMO due to its wide availability, rapid onset of action, easy reversibility, and good tolerance in pediatric and adult patients. For ECMO, a heparin dosage of 20 to 70 U/kg/h is recommended. Dosage varies in adult and pediatric patients based on metabolic rates and thrombin generation. With prolonged use of ECMO, consumption of antithrombin can reduce heparin responsiveness. Argatroban is an alternative anticoagulant in patients with heparin allergies or a history of heparin-induced thrombocytopenia, with a starting dose of 0.2 to 0.5 mcg/kg/min. Koster et al reported the successful use of bivalirudin—a direct thrombin inhibitor similar to argatroban—for a patient with myocardial failure who developed heparin-induced thrombocytopenia during ECMO.[41] The dose of bivalirudin is 0.025 to 0.05 mg/kg/min. The 2021 ELSO Adult & Pediatric Anticoagulation Guideline now recommends maintaining anti-factor Xa levels between 0.3 and 0.7 IU/mL for routine heparin management, using ACT chiefly for trend monitoring and troubleshooting.[42]
Personnel
The ECMO team typically includes a cardiothoracic or vascular surgeon, an interventional cardiologist who performs the cannulation, an intensivist, a perfusionist, an ECMO specialist, a respiratory therapist, and a bedside nurse.[43] An ECMO specialist is a technical specialist trained to handle the ECMO circuit according to the patient's clinical needs under the guidance and supervision of an ECMO-trained clinician.
A qualitative study of 23 Australian centers highlighted that successful programs provide 24/7 on-site cover and aim for a 1:1 ECMO specialist-to-patient ratio during the first 24 hours of support, identifying staffing and training as key determinants of safety and sustainability.[44]
A US single-center analysis comparing 107 adult runs found that nurse-led (advanced practice-provider) ECMO management delivered survival and complication rates equivalent to a perfusionist-led model while lowering personnel costs, thereby supporting flexible staffing in resource-limited settings.[45]
Preparation
Preparation consists of the following steps:
- Insert the cannula.
- Connect to the circuit (removal of all air from the circuit).
- Check for gases in the circuit.
- Make sure that there are no air bubbles in the circuit, membrane, or connections.
- Connect sweep gas flow to the oxygenator and start at 3 to 4 L/min (titrate to CO2 on arterial blood gas)
- Increase rotations per minute to generate adequate positive pressure in the return limb of the circuit.
- Clamps on the circuit are removed to ensure the antegrade blood flow of ECMO.
- Increase rotations per minute to achieve 3 to 4 L/min ECMO blood flow.
- Discontinue mechanical chest compressions in patients experiencing cardiac arrest.
Following a cannulation placement, the position is confirmed by fluoroscopy or echocardiography in VA ECMO. Vasopressors should be titrated to maintain a mean arterial pressure above 60 mm Hg for proper organ perfusion and below 80 mm Hg to prevent the development of left ventricular (LV) distention. Endotracheal tube placement; central venous access; sedation; analgesia; bedside ultrasound to identify possible complications, such as pneumothorax, thoracic or abdominal bleeding; targeted temperature control; 12-lead electrocardiogram; chest x-ray; end-tidal CO2; laboratory blood test, including blood crossmatch, are recommended as part of extracorporeal CPR management.
No definite timing has been defined before the initiation of extracorporeal CPR. Initiation of cannulation is recommended to start in 10 to 20 minutes after the failed resuscitation efforts, as too late initiation can increase the risk of hypoxic brain injury or other organs. The recommended goal to establish ECMO flow is within 1 hour of cardiac arrest.[2][20][2]
If the patient is not at a facility where ECMO is performed, the patient should be transported to the nearest center as early as possible. The emergency medicine service should continue high-quality CPR without interruptions. An automated cardiac massage device is proven to be equally effective as human chest compressions.[2]
The cannulation phase starts with preparing the femoral area with an antiseptic solution. External cardiac massage should be continued with an automated mechanical compression device during the cannulation phase. Cannulation should be performed by a multidisciplinary team, including surgeons, intensivists, cardiologists, or emergency clinicians.
Technique or Treatment
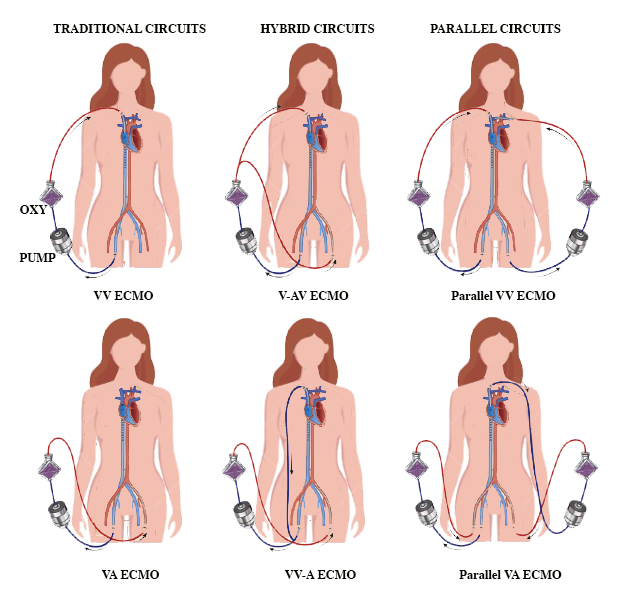
There are 4 types of ECMO circuits—traditional, hybrid, and parallel ECMO circuits (see Image. Types of ECMO Circuits).
Traditional Extracorporeal Membrane Oxygenation Circuits
Venovenous extracorporeal membrane oxygenation:
- Cannula configurations
- Femoro-atrial: Drainage cannula inserted via the femoral vein with the tip advanced to the diaphragm, and return cannula placed in the right internal jugular vein with the tip at the superior vena cava-right atrium junction. Rich et al performed a comparison between atrio-femoral and femoro-atrial flow for VV ECMO and suggested that femoro-atrial cannulation resulted in less recirculation and improved flow.[46]
- Femoro-femoral: Drainage cannula in the femoral vein that is advanced to the distal inferior vena cava, and return cannula placed in the contralateral femoral vein that is advanced to the right atrium.
- Double-lumen drainage and return cannula, which is placed in the jugular vein with the tip advanced to the inferior vena cava.
Opening of the atrial cannula should be directed towards the tricuspid valve. The position of the cannula should be checked every day or in case of a significant drop in ECMO flow. During right ventricular failure, switching over to VA ECMO is encouraged. Recent cohort data showed that properly positioned bicaval dual-lumen cannulas permit early mobilization and are associated with low recirculation rates when daily ultrasound confirms tip location.[17]
Peripheral venoarterial extracorporeal membrane oxygenation:
- The drainage cannula can be placed ipsilateral or contralateral to the femoral vein, and the return cannula can be inserted into the femoral artery.[47]
- The arterial and venous cannula should be placed in different groins. The arterial cannula should be inserted via an end-to-side graft, with only the tip advanced, to reduce the risk of distal limb ischemia.
- If the Seldinger technique is used, peripheral VA ECMO is discouraged in cases of peripheral vascular disease.
- Monitoring for compartment syndrome is required.
- In cases of LV dysfunction, poor ventricular contractility leads to distention of the left ventricle. In such a scenario, switching over to central VA ECMO is advised.
- Routine placement of a distal perfusion catheter during femoral VA ECMO halves the incidence of acute limb ischemia in contemporary series.[19]
Central venoarterial extracorporeal membrane oxygenation:
- In case of LV dysfunction, the addition of the LV vent to the ECMO circuit is recommended.
- The arterial and venous cannula should be inserted through separate incisions to facilitate temporary chest closure and promote early patient ambulation.
Hybrid Extracorporeal Membrane Oxygenation Circuits
Veno-arteriovenous extracorporeal membrane oxygenation:
- Venous drainage is achieved through a venous cannula and a return cannula to both arterial and venous circulation.
- In VV ECMO patients, the addition of an arterial cannula is achieved through either the femoral or axillary artery. In VA ECMO, additions of venous return cannula are made through either the right internal jugular vein or the femoral vein.[48]
- The additional cannula is connected to the existing ECMO circuit with a Y-connector.
- This technique can be used to manage Harlequin syndrome or to treat profound upper body hypoxia.
Venovenous-arterial extracorporeal membrane oxygenation:
- This type requires 2 or more drainage cannulas. An additional venous drainage cannula may be inserted into the right internal jugular vein in case of the existing femoral venous cannula, or the contralateral femoral vein can also be used. The left subclavian vein is another alternative.
- This technique is useful for patients with ongoing end-organ malperfusion.
- Stohr et al reported improved outcomes in ARDS patients treated with V-AV ECMO compared to those treated with isolated VV or VA ECMO. Mortality mentioned in various literature ranges from 27% to 61%.[49][16][50]
Parallel Extracorporeal Membrane Oxygenation Circuits
- In case of VV ECMO for respiratory failure, when the inability to capture an adequate fraction of cardiac output results in refractory hypoxia, an additional drainage and a return cannula connected to a separate circuit provides additional oxygenation.
- With VA ECMO, when flows are insufficient to provide adequate end-organ perfusion, an additional VA ECMO circuit provides additional systemic flow.
- In case of cardiac and respiratory failure, in addition to traditional VA and VV ECMO circuits, parallel circuits serve as an alternative to V-AV ECMO.
- Adequate distance between the drainage and return cannula of both circuits is recommended to prevent cross-circulation. Additionally, the possibility of capturing the majority of cardiac output, which reduces blood flow through the heart and lungs, should be considered.
In general, survival is lower with hybrid or parallel circuits compared to traditional circuits (32% with V-AV compared to 60% with VV support; 32% with V-AV support compared to 41% with VA support).[16]
Post-Extracorporeal Membrane Oxygenation Placement Care
- ECMO circuit blood flow should be continuously monitored. A decrease in flow indicates possible intra-abdominal or thoracic hemorrhage, cardiac tamponade, or left ventricular distention with pulmonary congestion.
- Refractory arrhythmias should be controlled with electric cardioversion. In case of LV distention, LV venting through an IABP, Impella, or atrial septostomy should be considered.
- Mechanical ventilation with a positive end-expiratory pressure of greater than 10 cm H2O is suggested to decrease the LV afterload and prevent or treat pulmonary edema. The mixture of ECMO fresh gas with air and oxygen prevents hyperoxia. Targeted temperature management with a heat exchanger, 33 to 36 °C for an initial 24 hours, followed by gradual rewarming to 37 °C, is suggested. Suggested ventilator settings include peak inflation pressure of 20 to 25 cm H2O, FiO2 of less than 0.5, respiratory rate of 4 to 8 breaths/minute, and tidal volume of less than 100 mL.[3]
- To prevent limb ischemia in case of VA ECMO, the distal perfusion cannula should be inserted on the side of the arterial cannulation.
- Cardiac arrests should be treated with cardiac catheterization post-ECMO cannulation.
- Post-cardiac arrest imaging with computed tomography of the brain, pulmonary angiography, and the abdomen/pelvis is recommended when etiology is unclear or if there is a decline in ECMO blood flow. Early echocardiography should be performed to assess ventricular function and valvular assessment.[2]
- IABP is considered the first choice when mechanical circulatory support is indicated. IABP increases pulsatility, decreases the ventricular afterload, and improves coronary perfusion. Doll et al reported improved survival with IABP.[51] For VV ECMO, IABP improves alveolar aeration and avoids ventilator-induced lung injury.[26] For peripheral ECMO, IABP is discouraged, as peripheral arterial blood flow from the femoral arterial cannula can compete with IABP.[21]
Table 3. Settings and Goals for the Initial Stage of ECMO
|
Parameter |
Target Range |
|
Circuit flow |
50-80 mL/kg/min |
|
Sweep gas flow |
50-80 mL/kg/min |
|
pH |
7.35-7.45 |
|
Fractional inspired oxygen |
100% |
|
Inlet pressure (centrifugal pump) |
>100 mm Hg |
|
Arterial carbon dioxide tension |
35-45 mm Hg |
|
Arterial oxygen saturation |
VV: 85%-92%, VA: >95% |
|
Oxygen saturation (drainage cannula) |
>65% |
|
Oxygen saturation (return cannula) |
100% |
|
Mixed venous oxygen saturation |
>65% |
|
Mean arterial pressure |
65-95 mm Hg |
|
Hematocrit |
30-40% |
|
Platelet count |
>100,000/mm3 |
Weaning off Extracorporeal Membrane Oxygenation
- In case of VV ECMO, assessment of native respiratory function is performed by altering the gas flow through the ECMO circuit between 5 and 65 %. Recovery of pulmonary functions takes around 1 to 3 weeks. Signs of improvement include a progressive increase in SaO2 greater than SvO2, improvement in SaO2 for a given circuit flow or requirement of a reduced circuit flow to achieve the target SaO2, and improvement in chest x-ray findings or lung compliance.
- Weaning is considered when the gas exchange can be maintained with a low FiO2 (<30%), fresh gas flow rates of less than 2 L/min, a respiratory rate of less than 25 breaths/min, and positive end-expiratory pressure of less than 15 cm H2O.
- In case of VA ECMO, once the etiology of cardiac arrest is addressed and native cardiac function is restored, weaning off ECMO can be planned. Factors indicating cardiac recovery include decreasing central venous and/or pulmonary pressures, increasing blood pressure that requires vasodilators, return of pulsatility on the arterial pressure waveform, or a decrease in pO2 by a right radial arterial line.
- Cardiac function assessment is performed by reducing flows in the ECMO circuit, changing the ventilator and oxygen flow settings, and increasing the heparin dose requirement to prevent thrombosis at low ECMO flow rates. To assess cardiac function and response to reduced flow rates, a transesophageal echocardiogram is used.
- Inotropic weaning is typically started several hours before the weaning. Reduction in-circuit flow should be 1 to 2 L/min. Weaning off VA ECMO is achieved with a gradual reduction in blood flow until the flow of 0.5 to 1.0 L/min is met. Close hemodynamic and serial echocardiographic monitoring is required. For temporary separation of ECMO from the patient, clamping or arteriovenous bridging within the circuit is used.
- After 1 to 2 hours of minimal or no support, if the patient is stable, they can be decannulated in a surgical setting.[3] On completion of successful weaning, the flow should be increased to 2 L/min until decannulation to prevent thrombosis development in the ECMO circuit.
- There is no definite timeline for weaning and decannulation; however, ECMO support typically lasts around 3 to 4 days.
- Premature decannulation can lead to the need for recannulation, hemodynamic compromise, cardiovascular collapse, or death. Unnecessary prolongation of ECMO is a futile effort; in fact, it increases morbidity and mortality.[2] After 2 days of ECMO, if the patient's ejection fraction remains less than 30%, it likely indicates unsuccessful weaning.[52]
- For patients who fail to recover myocardial function for weaning and decannulation and are neurologically intact, mechanical support with a VAD or heart transplantation should be considered.
- For some patients who cannot be weaned and are not candidates for VAD or heart transplantation, terminal decannulation and palliative care should be considered. In case of brain death, discussion of organ donation is also advised.
Complications
Bleeding
Bleeding is the most common and life-threatening complication of ECMO, as it can lead to intracranial hemorrhage or bleeding in the lungs or gastrointestinal tract. Factors responsible for bleeding include systemic heparinization, fibrinolysis, hemodilution of clotting factors, platelet dysfunction, uremia, or hepatic dysfunction. Within minutes of initiation of ECMO, activation of the contact and fibrinolytic system and consumption and dilution of coagulant factors can occur.[53] Robinson et al reported that thrombocytopenia may result from platelet adherence to surface fibrinogen and activation, aggregation, and clumping of platelets, followed by a drop in platelet counts.[54]
Aubron et al reported that 17% of VV ECMO and 34% of VA ECMO required surgery for bleeding from ECMO complications. Management of life-threatening hemorrhage may involve decreasing or stopping heparin, infusion of platelets, and administration of clotting factors such as activated factor VII. Wittenstein et al noted that factor VIIa has been associated with fatal thrombosis in several cases.[55] Stallion et al mentioned that transfusion of platelets causes only a temporary increase in platelet count.[56]
Prevention is better than a cure, as proven here as well. Avoidance of invasive or surgical procedures is recommended wherever possible. Platelet count greater than 150,000/mm³, fibrinogen greater than 200 mg/L, prothrombin ratio less than 1.5, and lower ACT are recommended. Temporary cessation of heparin infusion or another anticoagulant such as bivalirudin is recommended.[41] In case of fibrinolysis, elevated D-dimer or appearance of thromboelastography is useful, and the use of antifibrinolytic such as tranexamic acid, aminocaproic acid, and aprotinin is suggested.[57][58][59]
A 2022 ELSO-Registry study of greater than 29,000 adult runs showed major bleeding in 30% of cases—22% during VV support and 31% during VA support—confirming hemorrhage as the leading source of ECMO morbidity.[60]
Pulmonary hemorrhage is controlled with steroids or bronchoscopy in addition to the measures mentioned above. Between 10% and 15% of patients with ARDS on ECMO develop Intracerebral hemorrhage or infarction, which is responsible for 43% of ECMO-related deaths. Plasma-free hemoglobin levels exceeding 10% indicate hemolysis in patients; therefore, frequent monitoring is recommended.[1]
Intracardiac Thrombosis
Peripheral cannulation through the femoral artery and vein used in VA ECMO can predispose to retrograde blood flow to the ascending aorta. As a result, stasis of blood in the ventricle and inadequate left ventricular output lead to intracardiac thrombus formation.
Gas Embolism
With a centrifugal pump, a large negative pressure of up to 100 mm Hg is generated between the pump head and the drainage cannula. Gas embolism results from the entry of air from this part of the ECMO circuit.[26]
Thromboembolism
Systemic thromboembolism is an infrequent complication observed in VA ECMO more than VV ECMO. Vigilant observation of the ECMO circuit for any signs of clot formation and heparin infusion to maintain target ACT prevents thromboembolism.
Mechanical Complications
Clot formation in the ECMO circuit is a very common complication. Factors responsible for clot formation include pulmonary or systemic emboli, oxygenator failure, or consumption coagulopathy. To prevent clot formation, heparin-coated ECMO circuits are frequently used.[61]
Heparin-Induced Thrombocytopenia
Heparin-induced thrombocytopenia is an uncommon complication, particularly associated with long-term use of ECMO. Disseminated intravascular coagulation has also been reported with ECMO use. Management involves cessation of heparin infusion and switching to non-heparin anticoagulants such as bivalirudin or argatroban.[1]
Neurological Complications
Common neurological complications include seizures, infarctions, or intracranial hemorrhages. Unlike bleeding, a predisposition to blood clot formation increases the risk of stroke in this population.[4] Ischemic stroke or intracranial hemorrhage may occur secondary to coagulopathy, systemic heparinization, thrombocytopenia, systemic hypertension, or ligation of the carotid artery and jugular vein.[62]
A 2023 registry analysis of 37,473 patients treated with VV ECMO documented acute brain injury in 7% (2% stroke, 4% intracranial hemorrhage); these events doubled hospital mortality, underscoring the value of systematic neuro-monitoring.[63]
Renal Failure and Oliguria
Acute tubular necrosis can occur in the initial phase of ECMO and may require dialysis or hemofiltration.[3]
Gastrointestinal Tract Complications
Gastrointestinal tract hemorrhage may occur secondary to stress, ischemia, or bleeding tendencies. Prolonged fasting or total parenteral nutrition, hemolysis, and diuretics while on ECMO may predispose to hyperbilirubinemia and biliary calculi.
Sepsis
ECMO represents a foreign body that increases the chance of infection. Patients with postcardiotomy cardiogenic shock are more prone to develop an infection than other patients treated with ECMO.[64]
Metabolic Complications
Electrolyte disturbance, hypo- or hyperglycemia, alterations in the concentration of drugs secondary to increased volume of distribution, and a decrease in liver/kidney function may also be observed.
Cannula-Related Complications
Potential cannula-related complications observed in VA ECMO include cannula malposition; vessel perforation; hemorrhage; incorrect location, such as venous cannula within the artery; arterial dissection; pseudoaneurysm; and limb ischemia. These complications can be life-threatening and may require cannulation revision, such as displacement of the cannula.[5] The down-flow cannula can be inserted into the superficial femoral artery during percutaneous cannulation of the common femoral artery to prevent limb ischemia. Dacron graft can be sutured to the common femoral artery, and a cannula can be inserted into the graft during open procedures.[21] In case of severe leg ischemia, if required, fasciotomy should be performed.[51]
Hypoxia
In case of VA ECMO, peripheral insertion of the catheter into the femoral artery perfuses more to the lower extremities and abdominal viscera, leading to hypoxia of the upper extremities, brain, and heart. Therefore, oxygen saturation should be monitored in both the upper and lower extremities. In case of poor oxygen saturation in the upper extremities, oxygenated blood is infused into the right atrium.[1] During VV ECMO, inadequate circuit flow may cause hypoxia. Other possible causes include sepsis, recirculation, inadequate sedation, iatrogenic overheating, seizures, or overfeeding.[3]
Hypotension
In VA ECMO, hypotension is often due to reduced vascular tone, whereas in VV ECMO, it may result from reduced vascular tone, decreased preload, or impaired cardiac function. Sepsis is also a significant contributing factor and may necessitate inotropic support.
Others
Additional complications observed during ECMO resuscitation include reintubation, tracheostomy, LV distention, fatal arrhythmias, and pressure ulcers.[1][3]
Clinical Significance
ECMO is useful for treating cardiac failure in adults who cannot wean from cardiac bypass or have heart failure that cannot be treated with a VAD.[65] In cases of irreversible cardiac failure, patients with a cardiac index of less than 2 L/min/mm2, systolic blood pressure of less than 90 mm Hg, and lactic acidosis despite inotropic support and IABP should be considered for ECMO. ECMO resuscitation can serve as a bridge to heart transplantation or ventricular-assisted devices.
ECMO should be considered in case of life-threatening but potentially reversible respiratory failure.[66] The contact of blood components and the surface of the CPB circuit causes an inflammatory response and free radical–mediated pulmonary damage. Moreover, ischemia-reperfusion during CPB also predisposes to CPB-induced lung injury.[67]
Survival rates after ARDS indicate that 67% of patients were successfully weaned off ECMO and 52% survived to hospital discharge, as noted by Hemmila et al. Similar findings were reported in the CESAR trial. In H1N1 influenza–related ARDS, mortality was 23.7% in patients treated with ECMO compared to 52.5% without ECMO.
The use of VA ECMO in cardiac arrest, cardiogenic shock, or failure to wean from CPB after cardiac surgery has reported a 20% to 30% survival. Shin et al reported increased survival with minimum neurological impairment with ECMO support post-cardiac arrest compared to conventional CPR. Chen et al also reported improved survival rates and minimal disability at discharge, 30 days, and 1-year survival.
In case of progressive organ dysfunction, such as liver dysfunction or neurological decline, some patients may deteriorate despite ECMO support. In such instances, discontinuation of ECMO and initiation of hospice care are recommended.[68] The average cost of the ECMO procedure is $73,122, and the total average hospital cost is $212,142.[69]
Enhancing Healthcare Team Outcomes
In patients with SARS-CoV-2, data were collected from 40 patients aged 22 to 64 who required ECMO support in severe respiratory failure. A single-access, dual-stage right atrium to pulmonary artery cannula was used. The primary outcome was survival following safe discontinuation of ventilatory and ECMO support. All patients were successfully discontinued from ECMO support after a mean duration of 2.6 days from ECMO initiation. Thus, a single-access, dual-stage cannula offered better direct pulmonary artery flow, improved oxygenation and ventilation, and early mobility. To prevent thrombosis, all patients received systemic anticoagulation, as patients with COVID-19 are prone to developing severe thrombosis.
Overall, the study showed promising outcomes with most patients surviving and being discharged home without any oxygen support. Complications were minimal, with no incidence of ischemic stroke and no requirement for inotropic support or tracheostomy. However, this study was limited to 40 subjects, single-access, dual-stage VV ECMO with early extubation. Ongoing studies are necessary to define the long-term outcomes of this approach further.[5]
Nursing, Allied Health, and Interprofessional Team Interventions
Cardiovascular Management
Maintenance of intravascular volume and systemic perfusion is essential. Urine output, central venous pressure, body weight, and physical signs of perfusion were used to assess the volume status. Inotropic support, such as epinephrine, norepinephrine, or dopamine, or mechanical support with peripheral Impella or percutaneous atrial septostomy should be considered to sustain a good cardiac output. Echocardiography should be performed to monitor the heart's condition, rule out thrombosis, evaluate changes in ECMO flow, or assess worsening hemodynamics.
Pulmonary Management
Patients on ECMO should have daily radiographs, endotracheal suctioning every 4 to 6 hours depending on the secretions, frequent posture changes, and flexible bronchoscopy when required.
Renal System Management
- Oliguric phase: During the oliguric phase, typically within the first 24 to 48 hours of ECMO initiation, an acute inflammatory response may trigger capillary leak and intravascular volume depletion, leading to oliguria and acute tubular necrosis.
- Diuretic phase: The diuretic phase generally begins after 48 hours, which is the earliest sign of recovery. Diuretics are required if oliguria persists for more than 48 to 72 hours.
If renal failure does not improve, continuous renal replacement therapy can be initiated. Kielstein et al reported that approximately 60% of patients on ECMO require continuous renal replacement therapy (CRRT), and 3-month survival after CRRT for acute kidney injury is 17% versus 53% without CRRT. These findings suggest that the need for CRRT in ECMO-associated acute kidney injury is associated with increased mortality.
Central Nervous System Management
Regular neurological examinations are recommended to prevent paralysis, and incorporating sedation vacations is encouraged. A low threshold for initiating imaging studies should be maintained in cases of clinical suspicion, and aggressive management for seizures is recommended.
Infection Control
Strict monitoring for signs of infection or sepsis is crucial. Obtaining pan cultures weekly or whenever infection is suspected is recommended to guide timely treatment.
Hematologic Considerations
For patients on ECMO, maintaining a hemoglobin level above 8 g/dL is recommended. Platelet transfusion is encouraged to maintain a platelet count above 100,000/mcL. ACT should be maintained between 180 and 240 seconds to avoid bleeding complications.
Nutrition, Fluid, and Electrolytes
Nutrition requirements should be maintained using hyperalimentation techniques. Total parenteral nutrition delivers fluids, electrolytes, vitamins, minerals, glucose, proteins (amino acids), and often lipids (fat) into the vein. Close monitoring of fluids and electrolytes, such as potassium, magnesium, phosphorus, and ionized calcium, is advised. For the first 3 days on ECMO due to fluid resuscitation, fluid retention, and oliguria, the patient's weight is expected to increase.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Makdisi G, Wang IW. Extra Corporeal Membrane Oxygenation (ECMO) review of a lifesaving technology. Journal of thoracic disease. 2015 Jul:7(7):E166-76. doi: 10.3978/j.issn.2072-1439.2015.07.17. Epub [PubMed PMID: 26380745]
Richardson ASC, Tonna JE, Nanjayya V, Nixon P, Abrams DC, Raman L, Bernard S, Finney SJ, Grunau B, Youngquist ST, McKellar SH, Shinar Z, Bartos JA, Becker LB, Yannopoulos D, Bˇelohlávek J, Lamhaut L, Pellegrino V. Extracorporeal Cardiopulmonary Resuscitation in Adults. Interim Guideline Consensus Statement From the Extracorporeal Life Support Organization. ASAIO journal (American Society for Artificial Internal Organs : 1992). 2021 Mar 1:67(3):221-228. doi: 10.1097/MAT.0000000000001344. Epub [PubMed PMID: 33627592]
Level 3 (low-level) evidenceLafç G, Budak AB, Yener AÜ, Cicek OF. Use of extracorporeal membrane oxygenation in adults. Heart, lung & circulation. 2014 Jan:23(1):10-23. doi: 10.1016/j.hlc.2013.08.009. Epub 2013 Sep 1 [PubMed PMID: 24144910]
Hadaya J, Benharash P. Extracorporeal Membrane Oxygenation. JAMA. 2020 Jun 23:323(24):2536. doi: 10.1001/jama.2020.9148. Epub [PubMed PMID: 32463441]
Liu JY, Merkow RP, Cohen ME, Bilimoria K, Ko CY, Sweeney JF, Sharma J. Association of Weekend Effect With Recovery After Surgery. JAMA surgery. 2020 Oct 1:155(10):988-990. doi: 10.1001/jamasurg.2020.2618. Epub [PubMed PMID: 32845300]
Bartlett RH, Isherwood J, Moss RA, Olszewski WL, Polet H, Drinker PA. A toroidal flow membrane oxygenator: four day partial bypass in dogs. Surgical forum. 1969:20():152-3 [PubMed PMID: 5383034]
Level 3 (low-level) evidenceHill JD, O'Brien TG, Murray JJ, Dontigny L, Bramson ML, Osborn JJ, Gerbode F. Prolonged extracorporeal oxygenation for acute post-traumatic respiratory failure (shock-lung syndrome). Use of the Bramson membrane lung. The New England journal of medicine. 1972 Mar 23:286(12):629-34 [PubMed PMID: 5060491]
Peek GJ, Killer HM, Reeves R, Sosnowski AW, Firmin RK. Early experience with a polymethyl pentene oxygenator for adult extracorporeal life support. ASAIO journal (American Society for Artificial Internal Organs : 1992). 2002 Sep-Oct:48(5):480-2 [PubMed PMID: 12296566]
Level 2 (mid-level) evidenceKhoshbin E, Roberts N, Harvey C, Machin D, Killer H, Peek GJ, Sosnowski AW, Firmin RK. Poly-methyl pentene oxygenators have improved gas exchange capability and reduced transfusion requirements in adult extracorporeal membrane oxygenation. ASAIO journal (American Society for Artificial Internal Organs : 1992). 2005 May-Jun:51(3):281-7 [PubMed PMID: 15968960]
Level 2 (mid-level) evidenceThiara AP, Hoel TN, Kristiansen F, Karlsen HM, Fiane AE, Svennevig JL. Evaluation of oxygenators and centrifugal pumps for long-term pediatric extracorporeal membrane oxygenation. Perfusion. 2007 Sep:22(5):323-6 [PubMed PMID: 18416217]
Level 2 (mid-level) evidenceModi SP, D'Aloiso B, Palmer A, Smith S, Arlia P, Anselmi M, Sanchez P, Ramanan R. Comparative analysis of oxygenator dysfunction in polymethylpentene oxygenators: A pilot study. Perfusion. 2025 May:40(4):933-940. doi: 10.1177/02676591241268402. Epub 2024 Aug 1 [PubMed PMID: 39089248]
Level 2 (mid-level) evidenceGattinoni L, Pesenti A, Mascheroni D, Marcolin R, Fumagalli R, Rossi F, Iapichino G, Romagnoli G, Uziel L, Agostoni A. Low-frequency positive-pressure ventilation with extracorporeal CO2 removal in severe acute respiratory failure. JAMA. 1986 Aug 15:256(7):881-6 [PubMed PMID: 3090285]
Peek GJ, Mugford M, Tiruvoipati R, Wilson A, Allen E, Thalanany MM, Hibbert CL, Truesdale A, Clemens F, Cooper N, Firmin RK, Elbourne D, CESAR trial collaboration. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet (London, England). 2009 Oct 17:374(9698):1351-63. doi: 10.1016/S0140-6736(09)61069-2. Epub 2009 Sep 15 [PubMed PMID: 19762075]
Level 1 (high-level) evidenceBarbaro RP, MacLaren G, Boonstra PS, Combes A, Agerstrand C, Annich G, Diaz R, Fan E, Hryniewicz K, Lorusso R, Paden ML, Stead CM, Swol J, Iwashyna TJ, Slutsky AS, Brodie D, Extracorporeal Life Support Organization. Extracorporeal membrane oxygenation for COVID-19: evolving outcomes from the international Extracorporeal Life Support Organization Registry. Lancet (London, England). 2021 Oct 2:398(10307):1230-1238. doi: 10.1016/S0140-6736(21)01960-7. Epub 2021 Sep 29 [PubMed PMID: 34599878]
Skinner SC, Hirschl RB, Bartlett RH. Extracorporeal life support. Seminars in pediatric surgery. 2006 Nov:15(4):242-50 [PubMed PMID: 17055954]
Level 3 (low-level) evidenceShah A, Dave S, Goerlich CE, Kaczorowski DJ. Hybrid and parallel extracorporeal membrane oxygenation circuits. JTCVS techniques. 2021 Aug:8():77-85. doi: 10.1016/j.xjtc.2021.02.024. Epub 2021 Feb 24 [PubMed PMID: 34401820]
Noe C, Rottmann FA, Bemtgen X, Supady A, Wengenmayer T, Staudacher DL. Dual lumen cannulation and mobilization of patients with venovenous extracorporeal membrane oxygenation. Artificial organs. 2023 Oct:47(10):1654-1662. doi: 10.1111/aor.14604. Epub 2023 Jul 7 [PubMed PMID: 37358935]
Memon S, Drosou ME, Caroline M, Casanova E, Gnall EM. Feasibility and outcomes with subclavian vein access for crescent jugular dual lumen catheter for venovenous extracorporeal membrane oxygenation in COVID-19 related acute respiratory distress syndrome. Perfusion. 2024 Mar:39(2):304-309. doi: 10.1177/02676591221137760. Epub 2022 Nov 12 [PubMed PMID: 36373765]
Level 2 (mid-level) evidenceGouchoe DA, Chaurasia S, Henn MC, Whitson BA, Mokadam NA, Mast D, Satyapriya S, Vallakati A, Ganapathi AM. Does Size Matter? The Effect of Size of Distal Perfusion Catheter on Acute Limb Ischemia: A Meta-Analysis. ASAIO journal (American Society for Artificial Internal Organs : 1992). 2024 Oct 1:70(10):853-860. doi: 10.1097/MAT.0000000000002178. Epub 2024 Mar 6 [PubMed PMID: 38446827]
Level 1 (high-level) evidencePerman SM, Elmer J, Maciel CB, Uzendu A, May T, Mumma BE, Bartos JA, Rodriguez AJ, Kurz MC, Panchal AR, Rittenberger JC, American Heart Association. 2023 American Heart Association Focused Update on Adult Advanced Cardiovascular Life Support: An Update to the American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2024 Jan 30:149(5):e254-e273. doi: 10.1161/CIR.0000000000001194. Epub 2023 Dec 18 [PubMed PMID: 38108133]
Marasco SF, Lukas G, McDonald M, McMillan J, Ihle B. Review of ECMO (extra corporeal membrane oxygenation) support in critically ill adult patients. Heart, lung & circulation. 2008:17 Suppl 4():S41-7. doi: 10.1016/j.hlc.2008.08.009. Epub 2008 Oct 29 [PubMed PMID: 18964254]
Jentzer JC, Drakos SG, Selzman CH, Owyang C, Teran F, Tonna JE. Timing of Initiation of Extracorporeal Membrane Oxygenation Support and Outcomes Among Patients With Cardiogenic Shock. Journal of the American Heart Association. 2024 Feb 6:13(3):e032288. doi: 10.1161/JAHA.123.032288. Epub 2024 Jan 19 [PubMed PMID: 38240232]
Schmidt M, Hajage D, Lebreton G, Dres M, Guervilly C, Richard JC, Sonneville R, Winiszewski H, Muller G, Beduneau G, Mercier E, Roze H, Lesouhaitier M, Terzi N, Thille AW, Laurent I, Kimmoun A, Combes A, PRONECMO Investigators, the REVA Network, and the International ECMO Network (ECMONet). Prone Positioning During Extracorporeal Membrane Oxygenation in Patients With Severe ARDS: The PRONECMO Randomized Clinical Trial. JAMA. 2023 Dec 26:330(24):2343-2353. doi: 10.1001/jama.2023.24491. Epub [PubMed PMID: 38038395]
Level 1 (high-level) evidenceKaraoğlanoğlu S, Akgün M. Is Extracorporeal Membrane Oxygenation a Panacea? The Eurasian journal of medicine. 2023 Dec:55(1):S27-S34. doi: 10.5152/eurasianjmed.2023.23269. Epub [PubMed PMID: 39128031]
Reid TD, Crespo Regalado R, Carlson R, Schneider A, Boone JS, Hockran S, Butler LR, Perez DL, Holloway AD, Nguyen PG, Gallaher J, Charles AG, Raff L. Outcomes in Obese Adult Veno-Venous Extracorporeal Membrane Oxygenation: A Systematic Review. ASAIO journal (American Society for Artificial Internal Organs : 1992). 2024 Feb 1:70(2):86-92. doi: 10.1097/MAT.0000000000002068. Epub 2023 Oct 17 [PubMed PMID: 37850988]
Level 1 (high-level) evidenceSidebotham D, McGeorge A, McGuinness S, Edwards M, Willcox T, Beca J. Extracorporeal membrane oxygenation for treating severe cardiac and respiratory failure in adults: part 2-technical considerations. Journal of cardiothoracic and vascular anesthesia. 2010 Feb:24(1):164-72. doi: 10.1053/j.jvca.2009.08.002. Epub 2009 Oct 28 [PubMed PMID: 19875307]
Condello I. Magnetic levitation pumps for cell-free hemoglobin prevention during VV ECMO. Critical care (London, England). 2022 Mar 29:26(1):86. doi: 10.1186/s13054-022-03963-9. Epub 2022 Mar 29 [PubMed PMID: 35351168]
Blauth CI, Smith PL, Arnold JV, Jagoe JR, Wootton R, Taylor KM. Influence of oxygenator type on the prevalence and extent of microembolic retinal ischemia during cardiopulmonary bypass. Assessment by digital image analysis. The Journal of thoracic and cardiovascular surgery. 1990 Jan:99(1):61-9 [PubMed PMID: 2294366]
Pearson DT. Gas exchange: bubble and membrane oxygenators. Seminars in thoracic and cardiovascular surgery. 1990 Oct:2(4):313-9 [PubMed PMID: 2128689]
Haworth WS. The development of the modern oxygenator. The Annals of thoracic surgery. 2003 Dec:76(6):S2216-9 [PubMed PMID: 14667689]
Spina R, Forrest AP, Adams MR, Wilson MK, Ng MK, Vallely MP. Veno-arterial extracorporeal membrane oxygenation for high-risk cardiac catheterisation procedures. Heart, lung & circulation. 2010 Dec:19(12):736-41. doi: 10.1016/j.hlc.2010.08.015. Epub 2010 Sep 24 [PubMed PMID: 20869915]
Level 3 (low-level) evidenceMielck F, Quintel M. Extracorporeal membrane oxygenation. Current opinion in critical care. 2005 Feb:11(1):87-93 [PubMed PMID: 15659951]
Level 3 (low-level) evidenceRanucci M, Mazzucco A, Pessotto R, Grillone G, Casati V, Porreca L, Maugeri R, Meli M, Magagna P, Cirri S, Giomarelli P, Lorusso R, de Jong A. Heparin-coated circuits for high-risk patients: a multicenter, prospective, randomized trial. The Annals of thoracic surgery. 1999 Apr:67(4):994-1000 [PubMed PMID: 10320241]
Level 1 (high-level) evidenceOliver WC. Anticoagulation and coagulation management for ECMO. Seminars in cardiothoracic and vascular anesthesia. 2009 Sep:13(3):154-75. doi: 10.1177/1089253209347384. Epub [PubMed PMID: 19767408]
Baird CW, Zurakowski D, Robinson B, Gandhi S, Burdis-Koch L, Tamblyn J, Munoz R, Fortich K, Pigula FA. Anticoagulation and pediatric extracorporeal membrane oxygenation: impact of activated clotting time and heparin dose on survival. The Annals of thoracic surgery. 2007 Mar:83(3):912-9; discussion 919-20 [PubMed PMID: 17307433]
Level 2 (mid-level) evidenceMartindale SJ, Shayevitz JR, D'Errico C. The activated coagulation time: suitability for monitoring heparin effect and neutralization during pediatric cardiac surgery. Journal of cardiothoracic and vascular anesthesia. 1996 Jun:10(4):458-63 [PubMed PMID: 8776637]
Guzzetta NA, Bajaj T, Fazlollah T, Szlam F, Wilson E, Kaiser A, Tosone SR, Miller BE. A comparison of heparin management strategies in infants undergoing cardiopulmonary bypass. Anesthesia and analgesia. 2008 Feb:106(2):419-25, table of contents. doi: 10.1213/01.ane.0000297290.03501.db. Epub [PubMed PMID: 18227295]
Level 1 (high-level) evidenceCodispoti M, Mankad PS. Management of anticoagulation and its reversal during paediatric cardiopulmonary bypass: a review of current UK practice. Perfusion. 2000 Jun:15(3):191-201 [PubMed PMID: 10866420]
Level 3 (low-level) evidenceNuttall GA, Oliver WC, Santrach PJ, Bryant S, Dearani JA, Schaff HV, Ereth MH. Efficacy of a simple intraoperative transfusion algorithm for nonerythrocyte component utilization after cardiopulmonary bypass. Anesthesiology. 2001 May:94(5):773-81; discussion 5A-6A [PubMed PMID: 11388527]
Level 1 (high-level) evidenceDe Waele JJ, Van Cauwenberghe S, Hoste E, Benoit D, Colardyn F. The use of the activated clotting time for monitoring heparin therapy in critically ill patients. Intensive care medicine. 2003 Feb:29(2):325-8 [PubMed PMID: 12594595]
Koster A, Weng Y, Böttcher W, Gromann T, Kuppe H, Hetzer R. Successful use of bivalirudin as anticoagulant for ECMO in a patient with acute HIT. The Annals of thoracic surgery. 2007 May:83(5):1865-7 [PubMed PMID: 17462416]
Level 3 (low-level) evidenceMcMichael ABV, Ryerson LM, Ratano D, Fan E, Faraoni D, Annich GM. 2021 ELSO Adult and Pediatric Anticoagulation Guidelines. ASAIO journal (American Society for Artificial Internal Organs : 1992). 2022 Mar 1:68(3):303-310. doi: 10.1097/MAT.0000000000001652. Epub [PubMed PMID: 35080509]
Moll V, Teo EY, Grenda DS, Powell CD, Connor MJ Jr, Gartland BT, Zellinger MJ, Bray HB, Paciullo CA, Kalin CM, Wheeler JM, Nguyen DQ, Blum JM. Rapid Development and Implementation of an ECMO Program. ASAIO journal (American Society for Artificial Internal Organs : 1992). 2016 May-Jun:62(3):354-8. doi: 10.1097/MAT.0000000000000331. Epub [PubMed PMID: 26735556]
Ross P, Watterson J, Fulcher BJ, Linke NJ, Nicholson AJ, Ilic D, Hodgson CL. Nursing workforce, education, and training challenges to implementing extracorporeal membrane oxygenation services in Australian intensive care units: A qualitative substudy. Australian critical care : official journal of the Confederation of Australian Critical Care Nurses. 2023 Jan:36(1):114-118. doi: 10.1016/j.aucc.2021.12.003. Epub 2022 Jan 10 [PubMed PMID: 35016842]
Level 2 (mid-level) evidenceDhamija A, Kakuturu J, Schauble D, Hayanga HK, Jacobs JP, Badhwar V, Hayanga JWA. Outcome and Cost of Nurse-Led vs Perfusionist-led Extracorporeal Membrane Oxygenation. The Annals of thoracic surgery. 2022 Apr:113(4):1127-1134. doi: 10.1016/j.athoracsur.2021.04.095. Epub 2021 May 25 [PubMed PMID: 34043952]
Gattinoni L, Kolobow T, Damia G, Agostoni A, Pesenti A. Extracorporeal carbon dioxide removal (ECCO2R): a new form of respiratory assistance. The International journal of artificial organs. 1979 Jul:2(4):183-5 [PubMed PMID: 287656]
Level 3 (low-level) evidenceStroud MH, Okhuysen-Cawley R, Jaquiss R, Berlinski A, Fiser RT. Successful use of extracorporeal membrane oxygenation in severe necrotizing pneumonia caused by Staphylococcus aureus. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies. 2007 May:8(3):282-7 [PubMed PMID: 17417120]
Level 3 (low-level) evidenceBrasseur A, Scolletta S, Lorusso R, Taccone FS. Hybrid extracorporeal membrane oxygenation. Journal of thoracic disease. 2018 Mar:10(Suppl 5):S707-S715. doi: 10.21037/jtd.2018.03.84. Epub [PubMed PMID: 29732190]
Biscotti M, Lee A, Basner RC, Agerstrand C, Abrams D, Brodie D, Bacchetta M. Hybrid configurations via percutaneous access for extracorporeal membrane oxygenation: a single-center experience. ASAIO journal (American Society for Artificial Internal Organs : 1992). 2014 Nov-Dec:60(6):635-42. doi: 10.1097/MAT.0000000000000139. Epub [PubMed PMID: 25232764]
Werner NL, Coughlin M, Cooley E, Haft JW, Hirschl RB, Bartlett RH, Mychaliska GB. The University of Michigan Experience with Veno-Venoarterial Hybrid Mode of Extracorporeal Membrane Oxygenation. ASAIO journal (American Society for Artificial Internal Organs : 1992). 2016 Sep-Oct:62(5):578-83. doi: 10.1097/MAT.0000000000000405. Epub [PubMed PMID: 27347710]
Doll N, Kiaii B, Borger M, Bucerius J, Krämer K, Schmitt DV, Walther T, Mohr FW. Five-year results of 219 consecutive patients treated with extracorporeal membrane oxygenation for refractory postoperative cardiogenic shock. The Annals of thoracic surgery. 2004 Jan:77(1):151-7; discussion 157 [PubMed PMID: 14726052]
Fiser SM, Tribble CG, Kaza AK, Long SM, Zacour RK, Kern JA, Kron IL. When to discontinue extracorporeal membrane oxygenation for postcardiotomy support. The Annals of thoracic surgery. 2001 Jan:71(1):210-4 [PubMed PMID: 11216748]
Plötz FB, van Oeveren W, Bartlett RH, Wildevuur CR. Blood activation during neonatal extracorporeal life support. The Journal of thoracic and cardiovascular surgery. 1993 May:105(5):823-32 [PubMed PMID: 7683735]
Robinson TM, Kickler TS, Walker LK, Ness P, Bell W. Effect of extracorporeal membrane oxygenation on platelets in newborns. Critical care medicine. 1993 Jul:21(7):1029-34 [PubMed PMID: 8319460]
Bui JD, Despotis GD, Trulock EP, Patterson GA, Goodnough LT. Fatal thrombosis after administration of activated prothrombin complex concentrates in a patient supported by extracorporeal membrane oxygenation who had received activated recombinant factor VII. The Journal of thoracic and cardiovascular surgery. 2002 Oct:124(4):852-4 [PubMed PMID: 12324751]
Level 3 (low-level) evidenceStallion A, Cofer BR, Rafferty JA, Ziegler MM, Ryckman FC. The significant relationship between platelet count and haemorrhagic complications on ECMO. Perfusion. 1994:9(4):265-9 [PubMed PMID: 7981464]
Level 2 (mid-level) evidenceDownard CD, Betit P, Chang RW, Garza JJ, Arnold JH, Wilson JM. Impact of AMICAR on hemorrhagic complications of ECMO: a ten-year review. Journal of pediatric surgery. 2003 Aug:38(8):1212-6 [PubMed PMID: 12891495]
Level 2 (mid-level) evidenceBrunet F, Mira JP, Belghith M, Lanore JJ, Schlumberger S, Toulon P, Dhainaut JF. Effects of aprotinin on hemorrhagic complications in ARDS patients during prolonged extracorporeal CO2 removal. Intensive care medicine. 1992:18(6):364-7 [PubMed PMID: 1281849]
Level 3 (low-level) evidencevan der Staak FH, de Haan AF, Geven WB, Festen C. Surgical repair of congenital diaphragmatic hernia during extracorporeal membrane oxygenation: hemorrhagic complications and the effect of tranexamic acid. Journal of pediatric surgery. 1997 Apr:32(4):594-9 [PubMed PMID: 9126762]
Willers A, Swol J, Buscher H, McQuilten Z, van Kuijk SMJ, Ten Cate H, Rycus PT, McKellar S, Lorusso R, Tonna JE. Longitudinal Trends in Bleeding Complications on Extracorporeal Life Support Over the Past Two Decades-Extracorporeal Life Support Organization Registry Analysis. Critical care medicine. 2022 Jun 1:50(6):e569-e580. doi: 10.1097/CCM.0000000000005466. Epub 2022 Feb 3 [PubMed PMID: 35167502]
Thelin S, Bagge L, Hultman J, Borowiec J, Nilsson L, Thorelius J. Heparin-coated cardiopulmonary bypass circuits reduce blood cell trauma. Experiments in the pig. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 1991:5(9):486-91 [PubMed PMID: 1931093]
Level 3 (low-level) evidenceSmedira NG, Moazami N, Golding CM, McCarthy PM, Apperson-Hansen C, Blackstone EH, Cosgrove DM 3rd. Clinical experience with 202 adults receiving extracorporeal membrane oxygenation for cardiac failure: survival at five years. The Journal of thoracic and cardiovascular surgery. 2001 Jul:122(1):92-102 [PubMed PMID: 11436041]
Kalra A, Bachina P, Shou BL, Hwang J, Barshay M, Kulkarni S, Sears I, Eickhoff C, Bermudez CA, Brodie D, Ventetuolo CE, Whitman GJR, Abbasi A, Cho SM. Utilizing Machine Learning to Predict Neurological Injury in Venovenous Extracorporeal Membrane Oxygenation Patients: An Extracorporeal Life Support Organization Registry Analysis. Research square. 2023 Dec 22:():. pii: rs.3.rs-3779429. doi: 10.21203/rs.3.rs-3779429/v1. Epub 2023 Dec 22 [PubMed PMID: 38196631]
O'Neill JM, Schutze GE, Heulitt MJ, Simpson PM, Taylor BJ. Nosocomial infections during extracorporeal membrane oxygenation. Intensive care medicine. 2001 Aug:27(8):1247-53 [PubMed PMID: 11511935]
Level 2 (mid-level) evidenceSchuerer DJ, Kolovos NS, Boyd KV, Coopersmith CM. Extracorporeal membrane oxygenation: current clinical practice, coding, and reimbursement. Chest. 2008 Jul:134(1):179-84. doi: 10.1378/chest.07-2512. Epub [PubMed PMID: 18628221]
Sidebotham D, McGeorge A, McGuinness S, Edwards M, Willcox T, Beca J. Extracorporeal membrane oxygenation for treating severe cardiac and respiratory disease in adults: Part 1--overview of extracorporeal membrane oxygenation. Journal of cardiothoracic and vascular anesthesia. 2009 Dec:23(6):886-92. doi: 10.1053/j.jvca.2009.08.006. Epub [PubMed PMID: 19944353]
Level 3 (low-level) evidenceSuzuki T. Additional lung-protective perfusion techniques during cardiopulmonary bypass. Annals of thoracic and cardiovascular surgery : official journal of the Association of Thoracic and Cardiovascular Surgeons of Asia. 2010 Jun:16(3):150-5 [PubMed PMID: 20930674]
Makdisi T, Makdisi G. Extra corporeal membrane oxygenation support: ethical dilemmas. Annals of translational medicine. 2017 Mar:5(5):112. doi: 10.21037/atm.2017.01.38. Epub [PubMed PMID: 28361077]
Harvey MJ, Gaies MG, Prosser LA. U.S. and International In-Hospital Costs of Extracorporeal Membrane Oxygenation: a Systematic Review. Applied health economics and health policy. 2015 Aug:13(4):341-57. doi: 10.1007/s40258-015-0170-9. Epub [PubMed PMID: 25894740]
Level 1 (high-level) evidence