Introduction
Ultrasound is a cornerstone imaging modality for evaluating the urinary tract due to its noninvasive nature, absence of ionizing radiation, broad availability, no contrast agents required, point-of-care usage, and real-time diagnostic capabilities.[1][2] This imaging modality enables effective visualization of the kidneys, ureters (when dilated), urinary bladder, and scrotum, playing a pivotal role in the initial and follow-up assessment of a wide range of urological conditions.[3] Common clinical indications include hematuria, suspected obstruction, urinary tract infections, nephrolithiasis, renal and testicular neoplasms, testicular pain (torsion), and lower urinary tract dysfunction, including urinary retention.[4][5][6]
Ultrasonographic imaging is based on the principle that low-density (hypoechoic) structures reflect less high-frequency sound waves than surrounding tissue, whereas high-density (hyperechoic) elements and organs are highly reflective.[7] Hollow structures, such as simple cysts, are considered anechoic and do not reflect sound waves.[7]
The amount and magnitude of the returning sound waves are directly related to the density of the tissue scanned.[7] The elapsed time since the sound was generated corresponds to the depth or distance from the transducer of the structure causing the reflection.[7] The ultrasound machine assembles an image based on these reflections, with highly dense entities such as bones or stones appearing white, whereas anechoic structures such as simple cysts appear black.[7]
- Color Doppler presents vascular flow velocity and directional data. A computer uses red to indicate flow towards the transducer, whereas blue indicates flow in the opposite direction.[7][8][9]
- Brighter colors indicate a higher flow rate and velocity.[7][8][9]
- A third color, typically green or yellow, can be used to indicate turbulence.
- Pulsed wave Doppler is a way of presenting flow velocity data on a timeline.[7][10]
- Duplex ultrasound is the continuous presentation of pulsed-wave Doppler and standard ultrasound imaging.[7][11]
The transducer shape and frequency are selected based on the depth of the structure being studied, the desired field width, and the resolution required. Higher frequencies do not penetrate as deeply but provide better resolution.[7]
As a first-line imaging tool, ultrasound is especially valuable in populations where minimizing radiation exposure is critical, including children, pregnant individuals, and patients with recurrent renal stone disease.[2][4][5][12][13] Advances in sonographic technology, including high-resolution probes, color Doppler imaging, contrast-enhanced ultrasound, and elastography, have improved the modality's sensitivity and specificity for various pathologies.[14] Although computed tomography (CT) remains the gold standard for many diagnostic scenarios, ultrasound is often the initial imaging study performed, particularly in emergency, primary care, and outpatient settings.[2][4][5][6]
Point-of-care ultrasound (POCUS) further enhances accessibility and diagnostic speed, especially in acute presentations such as flank pain, urinary retention, suprapubic discomfort, bladder fullness, hematuria, and in patients with acute kidney injury.[2][4][5][15] When performed by trained healthcare providers, urinary tract ultrasound facilitates timely diagnosis, reduces unnecessary imaging, and supports safer, more efficient clinical decision-making.[16] This review describes the sonographic assessment of the urinary tract, including indications, techniques, findings, and applications in general, emergency, and point-of-care contexts.
Please see StatPearls' companion resource, "Transrectal Ultrasonography and Image-Guided Biopsies of the Prostate," for further information (see Image. Transrectal Ultrasound Guided Biopsy of the Prostate).
Anatomy and Physiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Anatomy and Physiology
The urinary tract consists of the kidneys, ureters, bladder, and urethra, which function collectively to filter blood, regulate fluid and electrolyte balance, and excrete metabolic waste as urine. These structures, particularly the kidneys and bladder, are well-suited for ultrasound evaluation and are routinely visualized with high diagnostic confidence.
Adrenal Glands
The adrenal glands are small, paired, triangular organs located on the superior surface of each kidney.[17] These organs measure approximately 2 × 5 cm and lie within the superior renal fascia and inside the perirenal space. The right adrenal is more pyramidal in shape, whereas the left adrenal is more crescentic.[17]
Each adrenal gland consists of a capsule, a cortex—comprising zona glomerulosa, zona fasciculata, and zona reticularis—and a central medulla.[18]
- The zona glomerulosa is the outermost layer of the adrenal cortex, responsible for producing mineralocorticoids such as aldosterone.[19]
- The zona fasciculata is the middle layer and the largest layer of the adrenal cortex, which manufactures and secretes glucocorticoids, primarily cortisol.[20]
- The zona reticularis is the innermost layer of the adrenal cortex, which manufactures adrenal androgens, such as dehydroepiandrosterone.[18]
- The adrenal medulla produces catecholamines.
The adrenal glands are roughly located between the 11th and 12th ribs.[17] The right adrenal gland is below the liver and posterior to the inferior vena cava. The left adrenal is superior to the splenic vessels, medial to the spleen, and lateral to the abdominal aorta. Both adrenal glands are below and anterior to the diaphragm.[17]
Kidneys
The kidneys are retroperitoneal organs located between the T12 and L4 vertebrae. Due to the overlying liver, the right kidney is typically positioned slightly lower than the left.[21]
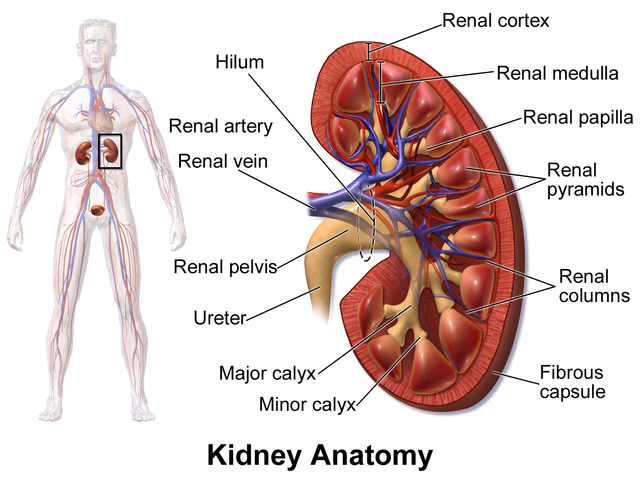
Each kidney is composed of a cortex, medulla, and central collecting system (see Image. Kidney Anatomy).[21]
The renal hilum is composed of (from anterior to posterior) the renal artery, the renal vein, and the renal pelvis.[21][22] This structure is rotated approximately 30° posteriorly from the coronal plane.[21][22] Sonographically, the renal cortex appears homogeneous and is usually isoechoic or mildly hypoechoic relative to the adjacent liver or spleen.[23][24]
The medullary pyramids are hypoechoic, triangular structures arranged around the central sinus. The renal sinus, which contains the calyces, renal pelvis, major vessels, lymphatics, and fat, appears hyperechoic on ultrasound due to the echogenicity of fat.[14] Without obstruction, reflux, or a congenital defect, the collecting system remains nondilated and may be partially obscured within the echogenic sinus fat.[25] The kidney is surrounded by Gerota's fascia and echogenic retroperitoneal fat.[21]
When viewed in the coronal plane, the upper pole of each kidney is angled 15° medial to the lower pole. The mass of the psoas muscle pushes the lower poles of the kidneys anteriorly compared to the upper poles.[21]
The ultrasound probe should be aligned with the kidney’s long axis to obtain optimal longitudinal views and accurately identify the midsagittal plane.
Penis
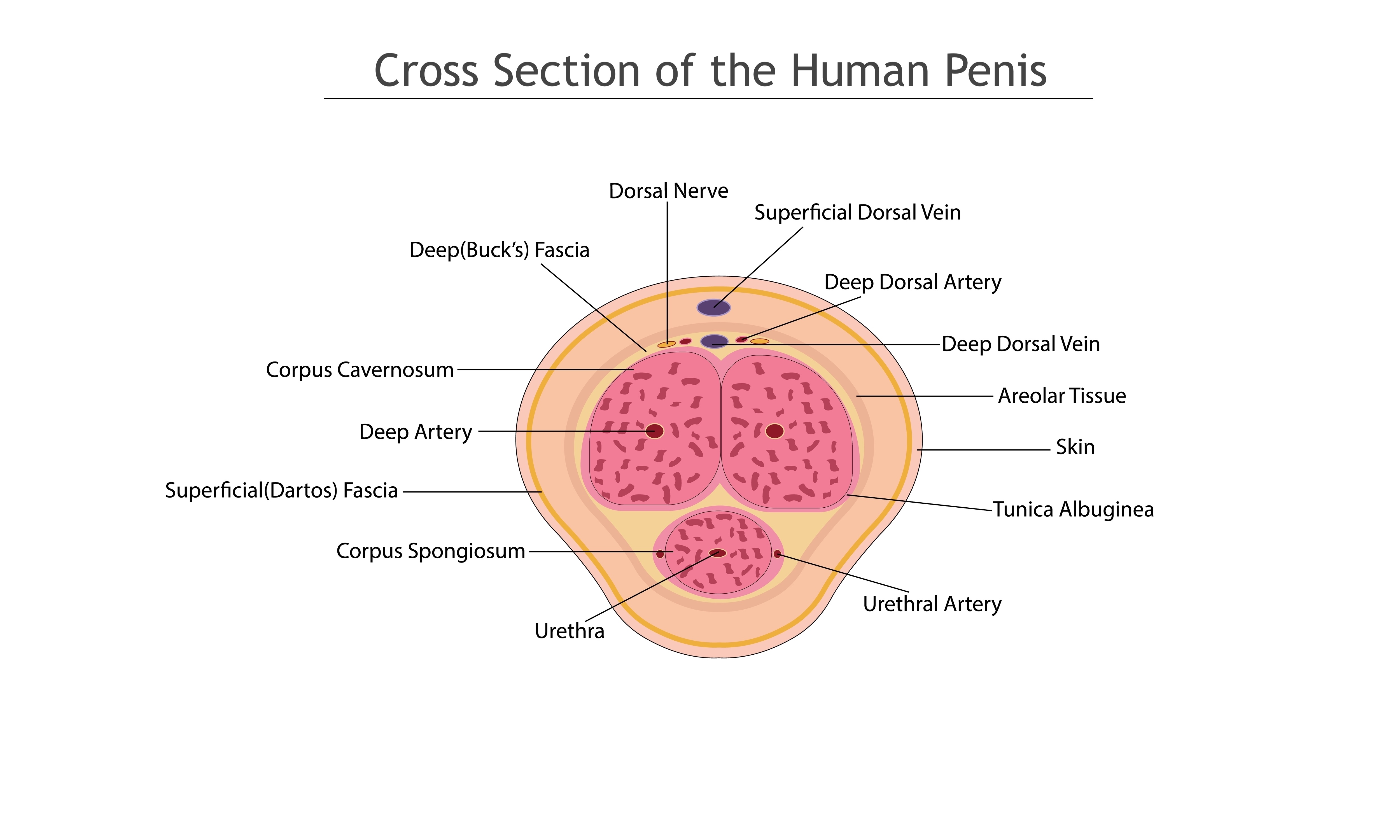
The penis is the male sexual organ used for reproduction and urination. This organ comprises 2 paired dorsal cylindrical corpora (the erection bodies) and the ventral corpus spongiosum (urethra and glans).[26] The body of the penis, which has no muscles, is mostly comprised of the erection bodies, urethra, glans, supporting skin, connective tissue, nerves, lymphatics, and blood vessels.[26]
The penile tunica albuginea covers each corpora cavernosa separately and the spongiosum.[26] Buck's fascia (deep fascia of the penis) surrounds both erectile bodies as well as the urethra as a single structure and is immediately superficial to the tunica albuginea.[26] See Image. Transverse Section of the Penis showing Corpus Cavernosum Urethrae, Urethra, and Septum.
The arterial blood supply to the penis derives from the internal pudendal arteries, which include the paired dorsal arteries of the penis located on either side of the deep dorsal vein.[27] A dorsal penile nerve is just lateral to each of the dorsal arteries. All of these structures are beneath Buck's fascia.[27] Another large, midline superficial dorsal vein is found superficial to Buck's fascia but below the Dartos fascia.[27] Each corpora cavernosa has a central cavernosal artery running inside at its center.
The deep dorsal vein of the penis drains the venous blood from the cavernosal bodies, whereas the superficial dorsal vein receives venous drainage from the skin and penile subcutaneous tissue.[26] See Image. Cross Section of the Human Penis.
Please see StatPearls' companion resources, "Anatomy, Abdomen and Pelvis, Prostate" and "Physiology, Male Reproductive System," for more information (see Image. Median Sagittal Section of the Male Penis).
Scrotum and Testicles
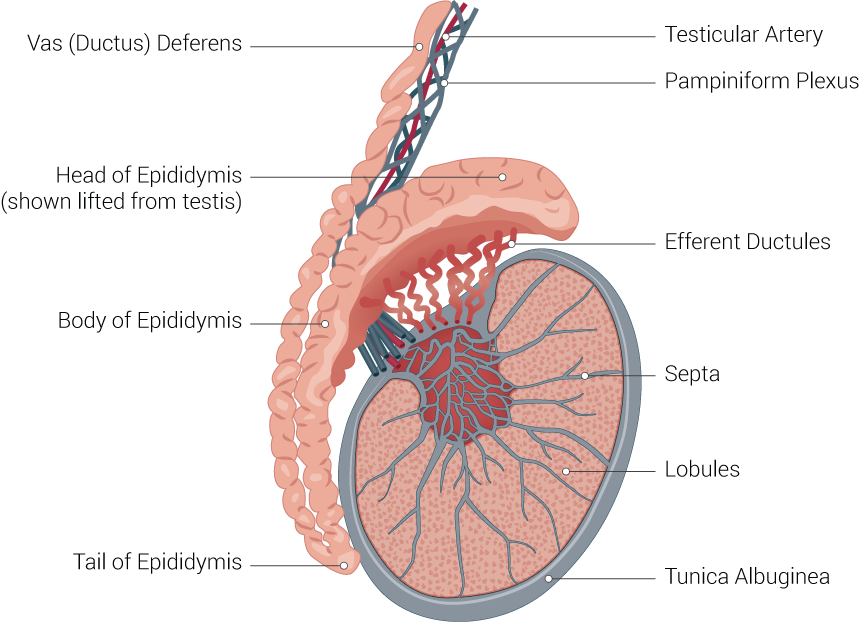
The scrotum and testicles constitute an external part of the male reproductive system located below the penis and anterior to the rectum and perineum. The scrotum is a thick sac (0.8 mm) composed of skin and muscle, which surrounds the testicles in 2 compartments, separated by a septum.[28] Internally, the tunica vaginalis consists of 2 layers. The parietal layer covers the interior surface of the scrotal wall, and the visceral layer covers the testicle.[28][29] Structures inside the scrotum include the external spermatic fascia, testes, epididymides, spermatic cord, and vas deferens (see Image. Testicle Illustration).[28]
The testicles are normally surrounded by a small amount of fluid trapped between the parietal and visceral layers of the tunica vaginalis.[29] Excessive fluid in this area constitutes a hydrocele.[29]
Ureters
The ureters arise from the renal pelvis and descend medially and inferiorly through the retroperitoneum. On ultrasound, they are normally not visible unless dilated. The distal ureters enter the bladder at the ureterovesical junctions, located at the posterior-inferior aspect of the bladder. When imaged in the transverse plane, the ureterovesical junctions may appear as symmetric protrusions extending into the bladder lumen. Intermittent ureteral jets can be visualized at these sites using color Doppler, reflecting normal urine flow.[30]
Urinary Bladder
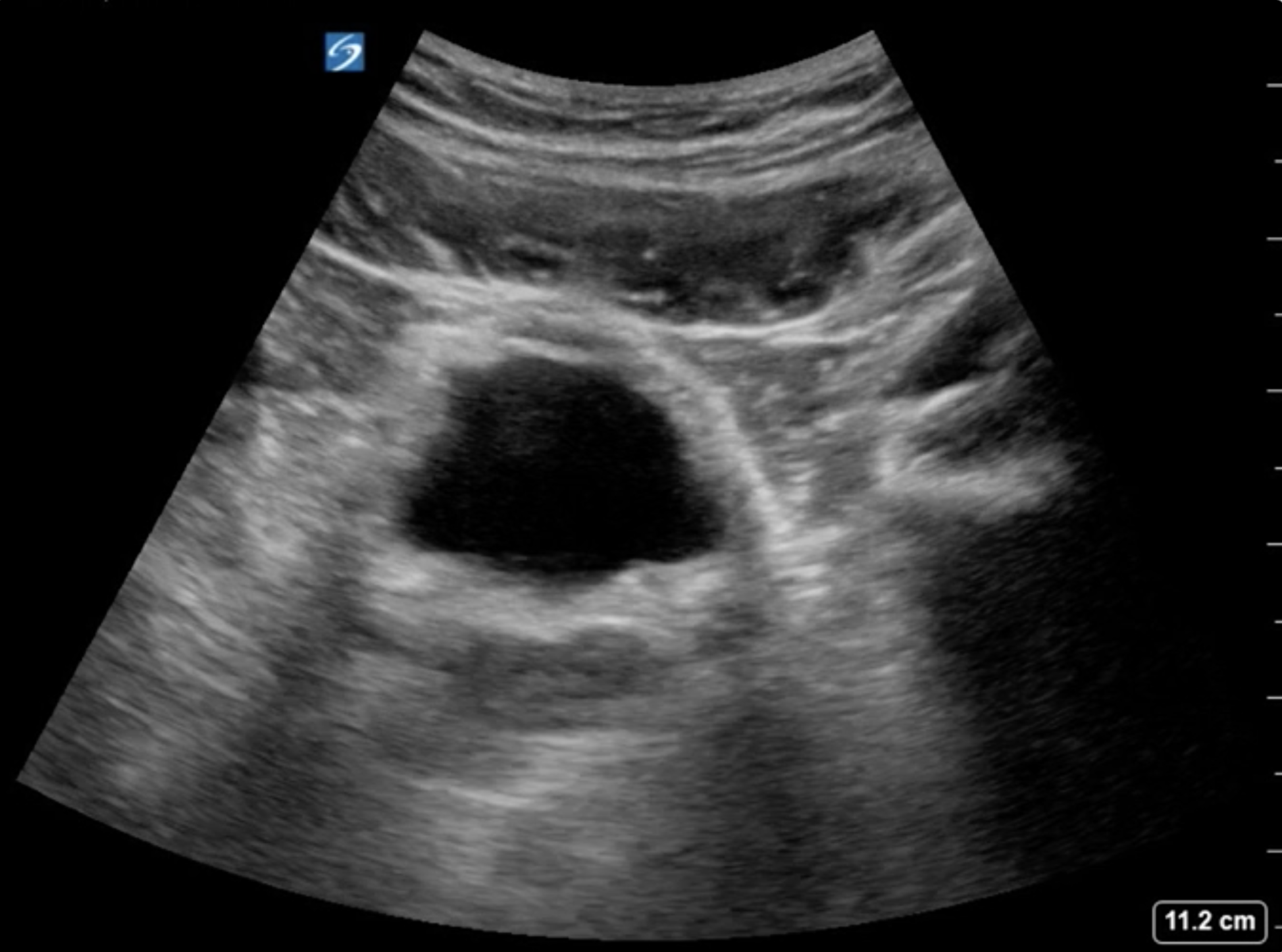
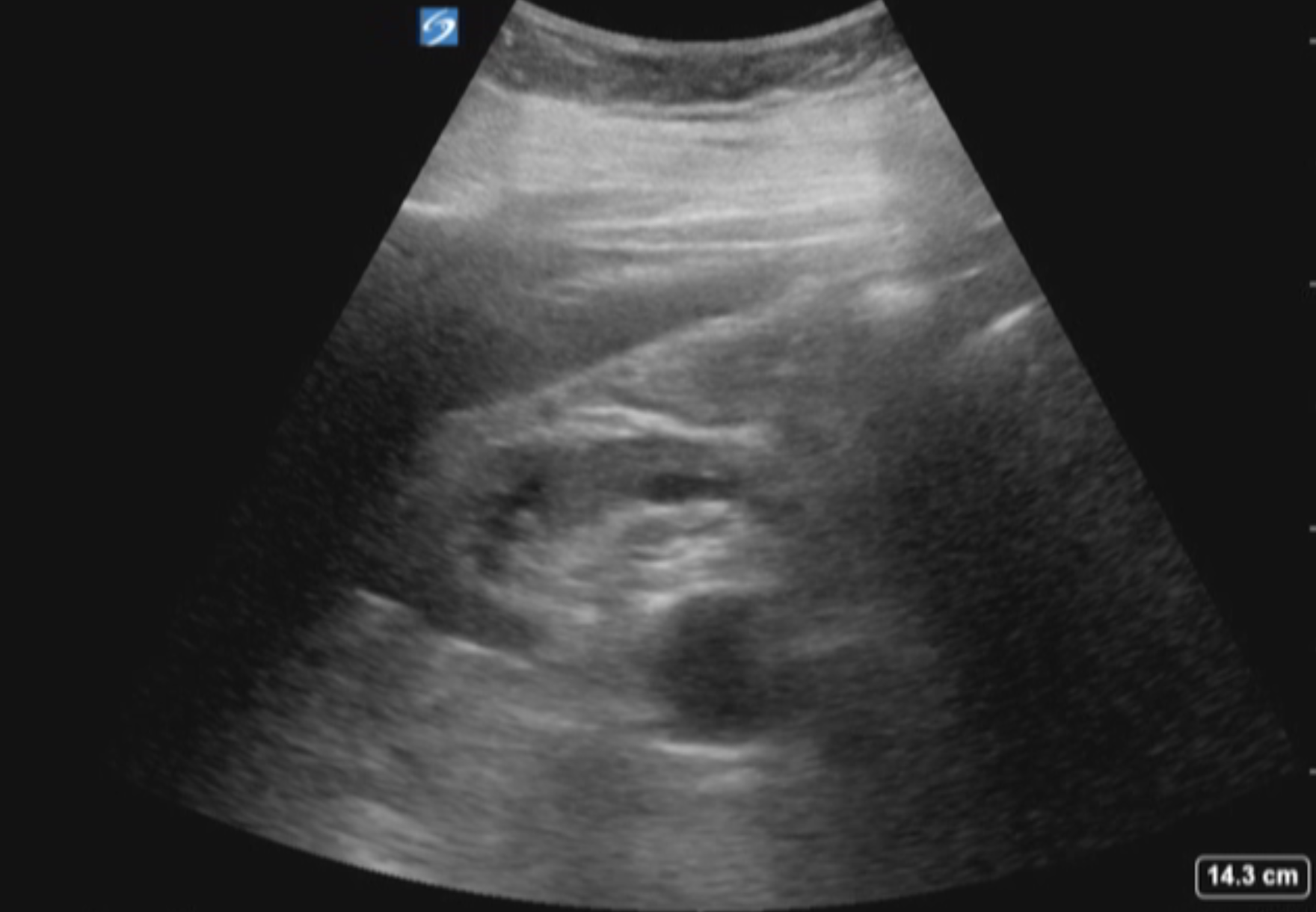
The urinary bladder is a muscular, extraperitoneal organ located just posterior to the pubic symphysis. When adequately distended, it appears as a well-defined, thin-walled, anechoic structure on ultrasound.[31] In the transverse view, the bladder is typically rectangular, whereas in the sagittal view, it takes on a more triangular or dome-shaped appearance (see Image. Ultrasound of Bladder in Transverse View). The wall should measure less than 5 mm when fully distended.[32][33] The bladder serves as a key landmark for evaluating lower urinary tract anatomy and assessing urinary retention.
A thorough understanding of normal urinary tract anatomy and its sonographic appearance is essential for accurately identifying pathology and avoiding false-positive or false-negative findings during ultrasound evaluation.
Indications
Ultrasound is frequently used as a first-line imaging modality to guide the clinical management of various urinary tract conditions in emergency and outpatient settings.
Adrenal Glands
Adrenal ultrasonography is indicated when adrenal neoplasms, incidental nodules, or tumors are observed in other imaging studies and when suspected adrenal endocrine abnormalities are observed.[34] Ultrasound is a convenient and readily available imaging method that avoids ionizing radiation and can be performed at the point of care. However, CT scans and magnetic resonance imaging (MRI) provide more definitive results. Ultrasonography is the preferred imaging technique for suspected adrenal disorders, particularly in children and neonates.[34]
Indications for adrenal ultrasonography include the following:[35]
- Abdominal masses in children
- Adrenal hemorrhage
- Adrenal insufficiency
- Adrenal nodules identified during other imaging (incidentalomas)
- Congenital adrenal hyperplasia
- Cushing syndrome
- Functional adrenal disorders
- Hematomas
- Hyperaldosteronism
- Hypertension due to endocrinopathies
- Laparoscopic adrenal ultrasonography for adrenalectomy surgeries
- Metastatic disease in patients with malignancies
- Monitoring and tracking of adrenal neoplasms identified on other imaging
- Neuroblastoma
- Pheochromocytoma
- Suspected adrenal nodules, masses, tumors, or neoplasms
Foley Catheter Placement and Verification
Ultrasound can be used to confirm the correct placement of a Foley catheter by visualizing the hyperechoic surface of the catheter balloon within the bladder lumen.[24] Fluid can be added through the catheter to distend the bladder and help with visualization. Ultrasound may also be used to guide Foley placement in cases of challenging transurethral catheterization, especially in men.[36][37]
Hematuria
Hematuria, whether microscopic or gross, is a common indication for ultrasound, particularly in the outpatient or initial triage setting. Ultrasound is widely used as the initial imaging investigation of patients with microscopic hematuria (≥3 red blood cells/high-power field on microscopic urinalysis) because it is safe, convenient, cost-effective, widely available, free of ionizing radiation, does not require potentially nephrotoxic intravenous contrast, and can detect significant renal and urinary pathology.[38][39][40]
In the setting of gross hematuria with normal renal imaging, bladder ultrasound may detect intraluminal masses, clots, stones, or bladder wall irregularities, particularly in patients at risk for urothelial carcinoma. However, the resolution of ultrasound is insufficient to identify smaller lesions (<5 mm), and it does not visualize anterior bladder wall lesions well, so a cystoscopy is still required.[39][41][42]
High-risk individuals, such as patients aged 60 or older, those with a history of greater than 30 pack-years smoking, those with greater than 25 red blood cells/high-power field, or those with a history of gross hematuria, should undergo a CT urogram (abdomen and pelvis CT with and without IV contrast) for comprehensive imaging investigation and cystoscopy.[38][39][41]
Prostate
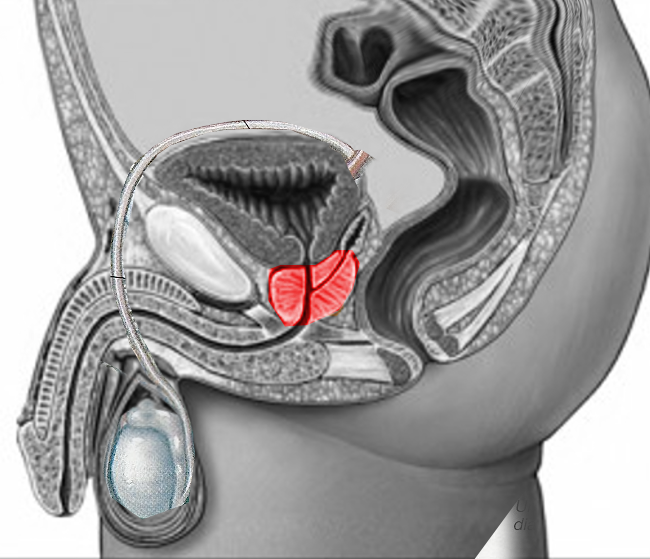
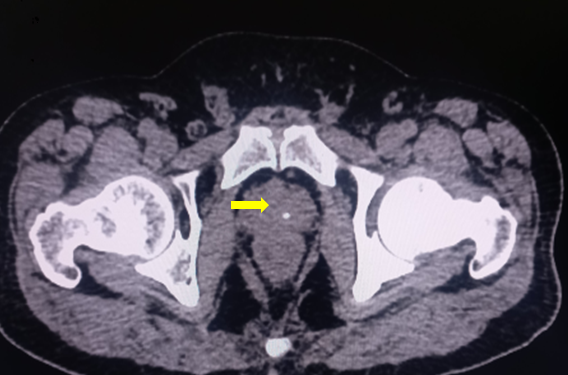
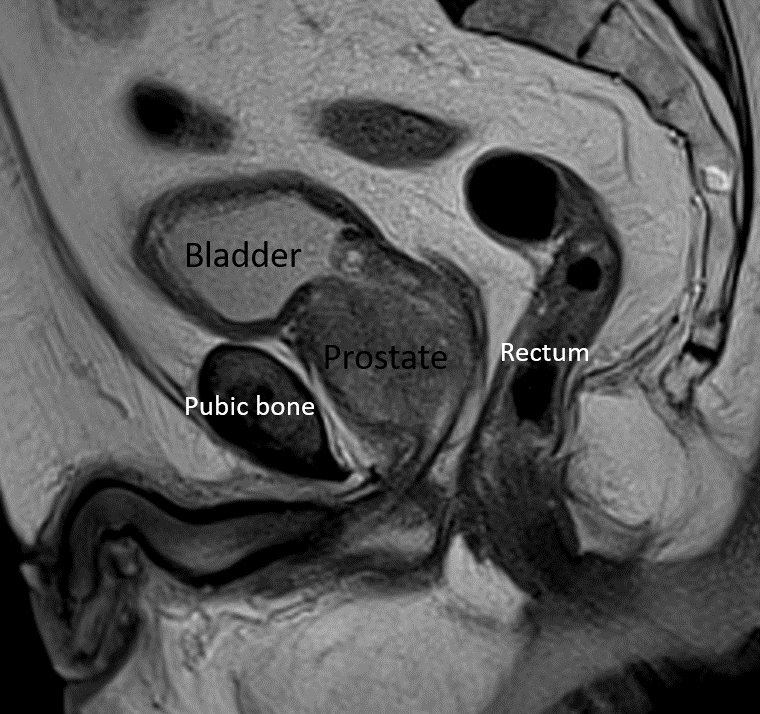
Please see StatPearls' companion resource "Transrectal Ultrasonography and Image-Guided Biopsies of the Prostate," for further information (see Images. Sagittal Section of Male Pelvis Showing Prostate Gland With Foley Catheter Placement, Axial CT Scan of Pelvis Showing Prostate Gland, Midsagittal Image of Prostate on MRI).
Penis
Ultrasound can be used in some penile emergencies, such as possible penile fractures, traumatic injuries, and in the evaluation of Peyronie's disease.[43][44] Color Doppler ultrasound is sometimes used for the evaluation of penile vascular flow in patients with erectile dysfunction, especially in those who have failed oral and topical therapy.[45][46][47]
Ultrasound may also be useful in dorsal penile vein thrombosis, foreign bodies in the penis or urethra, unexplained penile masses, priapism, and urethral abnormalities such as diverticula, cysts, or abscesses.
Kidneys
Ultrasound is highly effective for identifying hydronephrosis, simple or complex cysts, calculi larger than 4 mm, and solid renal masses, as well as for tracking their progress over time.[48][49] Ultrasound is often the first imaging modality used for the kidneys due to its convenience, availability, low cost, lack of ionizing radiation, painless usage, and immediate results.
- Simple renal cysts appear as anechoic, well-circumscribed lesions with posterior acoustic enhancement.[4][48][49]
- Solid or complex cystic masses may exhibit internal echoes, septations, calcifications, or vascular flow on Doppler, warranting further imaging with contrast-enhanced ultrasonography, CT, or MRI.[40][50]
- The vascularity of renal masses can be determined using Doppler technology.
Contrast-enhanced ultrasonography can help characterize renal neoplasms, especially complex cystic lesions, but is not as useful for solid lesions due to overlapping diagnostic characteristics.[51] Ultrasound intravenous contrast uses microbubbles to enhance the visualization of blood flow with ultrasound.[52] The microbubbles are composed of a shell of albumin, galactose, lipid, or suitable polymer filled with a dense, hydrophobic gas, such as perfluorocarbons and sulfur hexafluoride.[53] Microbubbles are readily visualized on ultrasound and are designed to allow slow gas diffusion from their capsule, providing sufficient persistence for clinical use.[53]
Characteristics such as the degree and type of enhancement, degree of heterogeneity, washout rate, and the presence of ring or annular enhancement can help characterize solid renal lesions.[54][55]
Contrast-enhanced ultrasonography is superior to standard ultrasonography and CT scanning in evaluating complex cystic renal lesions.[56] This technique is better at identifying wall thickness, visualizing internal septa, and detecting solid components within these complex cystic lesions.[56] Contrast-enhanced ultrasonography is particularly well-suited for patients with significant renal failure, as the contrast agents used for ultrasonography are typically not nephrotoxic.[56]
Superb microvascular imaging is a newer Doppler ultrasound technique with enhanced sensitivity for detecting slow blood flow in renal masses.[57][58][59][60] This imaging technique enables visualization of blood flow characteristics within microvessels of the neoplasm.[58][59][60][59] Superb microvascular imaging has the potential to differentiate between benign and malignant renal neoplasms just based on the vascular patterns.[58][59][60] This technique may also have a role in evaluating the vascularity of transplanted kidneys.[61]
Ultrasound is highly operator-dependent, and visualization of the retroperitoneum, renal vasculature, and hilum is limited.[52] Visualization of renal lesions can also be challenging in patients with a high body mass index, bowel gas interposition, isoechoic neoplasms, or smaller masses (<3.5 cm).[52] The detection rate and visualization of renal lesions are far superior with CT scanning compared to ultrasound until the lesions reach at least 3.5 cm in size, at which point the 2 modalities are roughly equivalent.[52]
The renal resistive index is calculated from arterial vascular flow readings in the kidney using spectral Doppler measurements at the corticomedullary junction (arcuate arteries) or adjacent to the medullary pyramids (interlobar arteries).[62] The formula is (PSV − EDV) / PSV with a normal range of 0.50 to 0.70, where PSV is peak systolic velocity and EDV is end-diastolic velocity.[63] Increased renal resistive indices are most closely associated with ureteral obstruction from calculi but may also be elevated in various other kidney disorders, such as renal vascular disease and kidney failure.[62][64][65]
A difference of more than 0.1 between the 2 kidneys indicates obstructive uropathy, such as from an obstructing ureteral calculus.[5][62][63][66][67][68][69][70] Immediately after an acute ureteral obstruction from a stone, the immediate renal reaction is prostaglandin-mediated vasodilation. This phase typically lasts less than 2 hours, followed by a decrease in renal arterial blood flow and an increase in vascular resistance in the kidney due to mechanical factors and the release of vasoconstrictive regulatory pathways, including kallikrein-kinin, prostaglandin-thromboxane, and renin-angiotensin systems.[62][71][72] A higher unilateral renal resistive index reflects this difference.[62]
The renal resistive index, especially the difference between the resistive indices of the 2 kidneys, is a particularly sensitive and specific test in diagnosing obstruction from acute renal colic due to a ureteral calculus in pregnant women, where intravenous urography and ionizing radiation are discouraged.[63][73][74][75] A renal resistive difference of 0.04 or more is considered significant in this population.[73]
Ultrasonography and renal resistive index can be used to verify the patency of double J ureteral stents, to differentiate obstructive from nonobstructive hydronephrosis, and to detect signs of early kidney transplant rejection or acute kidney injury.[62][64][65][76][77][78][79][80][81] An increased renal resistive index indicates decreased renal vascular perfusion and may also suggest changes in systemic hemodynamics and possible subclinical atherosclerosis, which makes renal ultrasonography valuable in the workup of primary hypertension.[65][82]
The pulsatility index, similar to the resistive index, is calculated using the formula (PSV − minimum diastolic velocity) / mean velocity, with a normal range of 0.50 to 0.70.[83][84]
Indications for renal ultrasonography include the following:
- Abdominal trauma
- Active surveillance for renal lesions, such as an angiomyolipoma
- Acute kidney injury
- Back or flank pain that is unexplained or acute (possible abscess, pyelonephritis, or renal colic from ureterolithiasis)
- Confirmation of double J stent placement
- Febrile urinary tract infection (UTI) to rule out hydronephrosis and possible obstructive pyelonephritis
- Follow-up evaluations after reimplantation surgery
- Further evaluation of a renal lesion seen on other imaging
- Hematuria (gross or microscopic)
- History of hydronephrosis or ureteral reimplantation surgery
- Possible abscess, pyelonephritis, or renal colic
- Preoperative evaluation of kidneys before renal procedures (percutaneous nephrostomy), renal tumor ablation, cyst aspiration/sclerotherapy, and biopsies.
- Renal transplant evaluation and monitoring
- Right-sided varicoceles
- Tracking of known urinary and renal calculi
Testicular Pain and Scrotal Masses
Ultrasonography is the imaging modality of choice for visualizing and diagnosing intrascrotal lesions and masses, as well as investigating and evaluating testicular pain, such as a possible testicular torsion.[85][86] Color Doppler ultrasonography has proven to be very accurate for diagnosing testicular torsion.[87][88][89]
Ultrasonography can also differentiate epididymal cysts (spermatoceles) from solid masses and tumors. This technique can measure the diameter of dilated scrotal varices in varicoceles, examine impalpable testicles in patients with hydroceles, determine whether a traumatized testis is ruptured, diagnose testicular torsion, identify testicular malignancies, indicate testicular infections (orchitis, epididymitis), visualize the testicle after trauma, and verify vascular flow to the testicle.[90][91][92][93]
Scrotal (testicular) ultrasonography is the diagnostic procedure of choice for testicular pain or scrotal masses and can also be useful to evaluate male infertility.[85][86]
Indications for scrotal ultrasonography include the following:
- Abnormal scrotal sac (hydrocele or spermatocele)
- Abnormal scrotal wall lesion
- Unexplained retroperitoneal lymphadenopathy
- History of testicular cancer, leukemia, or lymphoma
- Infertility
- Possible testicular torsion
- Scrotal abscess
- Scrotal mass identified by other imaging
- Scrotal swelling
- Scrotal or testicular mass on examination
- Scrotal surgery follow-up
- Testicular nodule or lump
- Testicular pain (chronic and acute)
- Testicular trauma
- Varicocele
Ureteral Obstruction, Hydronephrosis, and Vesicoureteral Reflux
Evaluation of suspected ureteral obstruction, particularly in the setting of flank pain due to nephrolithiasis, is one of the most common indications for urinary tract ultrasound.[14][94][95] Patients often present with acute, colicky pain radiating to the groin, which may be accompanied by hematuria, nausea, or vomiting.[96] The hallmark sonographic finding of obstruction is hydronephrosis, which appears as an anechoic dilatation of the renal collecting system within the echogenic renal sinus and, possibly, the associated ureter.[14][95]
Grading of hydronephrosis is somewhat subjective and is generally classified as follows:
- Mild: Preservation of renal papillae with early calyceal dilatation.[4][15][23]
- Moderate: Blunting of calyces and obliteration of papillae, often producing a bear paw appearance.[4][15][23]
- Severe: Coalescence of dilated calyces into a single fluid-filled space, with compression of a thinned renal cortex (<1 cm).[4][15][23]
Hydronephrosis is not specific to ureterolithiasis and may also result from extrinsic compression of the ureter, such as that caused by pregnancy, retroperitoneal tumors, or pelvic masses; anatomical abnormalities such as ureteral strictures; or lower urinary tract dysfunction, including urinary retention, benign prostatic hyperplasia, or neurogenic bladder.[97][98]
In children, contrast-enhanced voiding urosonography is a safe, sensitive, and well-established method for diagnosing and monitoring vesicoureteral reflux without patient radiation exposure.[99][100][101][102][103][104][105][106][107] Evidence suggests that contrast-enhanced voiding urosonography may be superior to conventional voiding cystourethrography in diagnosing and grading pediatric vesicoureteral reflux; however, the reported false negative rate of 3% is a concern.[103][106][108]
Contrast-enhanced ultrasonography can also be used to evaluate the urethra and lower urinary tract, as well as a possible alternative to fluoroscopy in video urodynamics.[109][110][111][112][113]
Differentiating Hydronephrosis from Similar Appearing Mimics
Clinicians must distinguish hydronephrosis from normal anatomic variants or other anechoic structures to avoid misinterpretation, misdiagnosis, and inappropriate treatment.
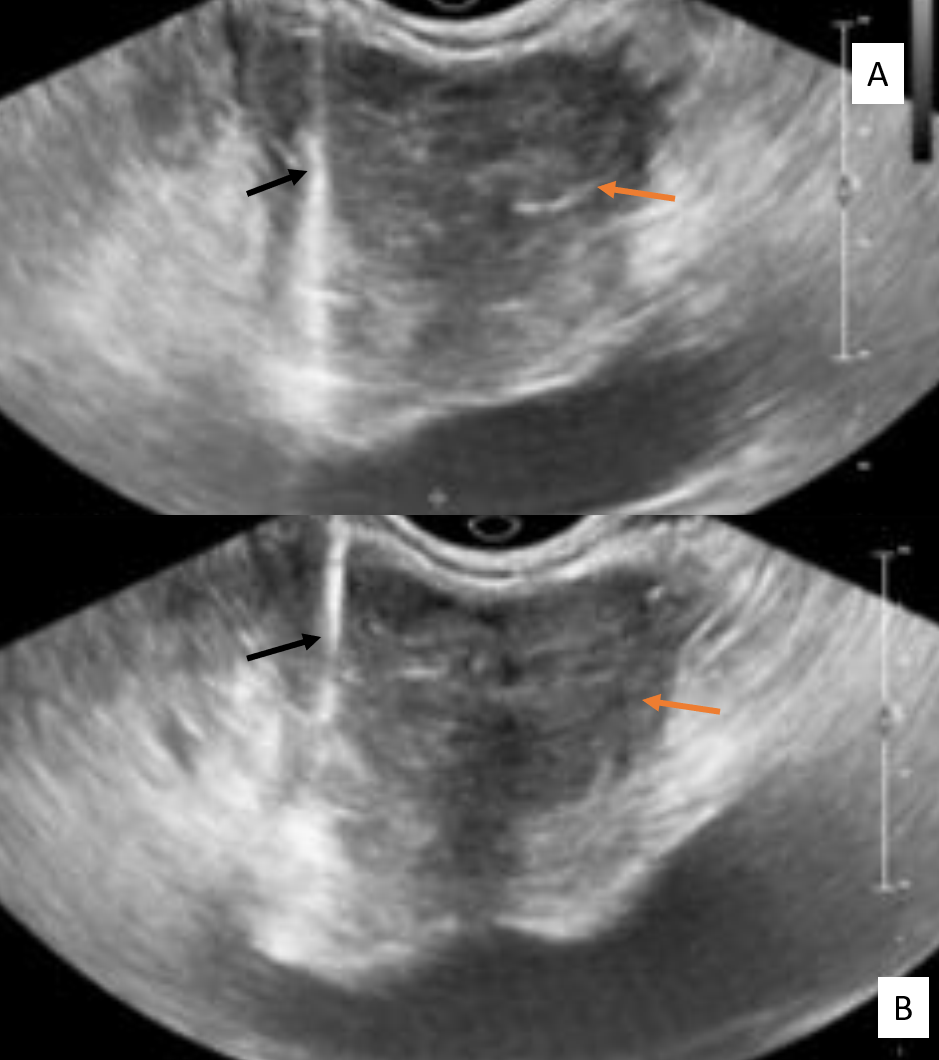
- An extrarenal pelvis, found in about 10% of the population, may appear as an isolated anechoic structure medial to the hilum and should not be mistaken for collecting system dilatation (see Image. Extrarenal Pelvis on Ultrasound).[114]
- Color Doppler can help identify hilar vessels and rule out other structures that can simulate hydronephrosis.[115]
- This technique achieves this by identifying blood flow in major arteries and veins, distinguishing stagnant, dilated calyces from blood vessels with blood flow, and diagnosing pseudohydronephrosis, which suggests dilation without obstruction.[115]
- Hydronephrosis demonstrates an elevation in the renal resistive index that is not found in mimicking structures, such as an extrarenal pelvis or a parapelvic cyst.[116][117]
- Hydroureteronephrosis technically refers to the dilation of the renal pelvis and its associated ureter; however, the term hydronephrosis is commonly used for both conditions.
- Parapelvic cysts, found in up to 1.5% of individuals, are well-defined, non-communicating anechoic cystic structures in the renal sinus.[4][15]
- Renal pyramids are triangular, hypoechoic areas found in the medulla, bounded by the cortex, which do not distort renal architecture.[23][118]
Urinary Bladder and Retention of Urine
Ultrasound of the urinary bladder is commonly performed to assess post-void residual volume and to diagnose urinary retention in patients with suprapubic pain or urinary difficulties.[119][120][121] Ultrasound is a rapid and reliable method for evaluating bladder volume in such situations. In the transverse plane, the width (W) and depth (D) of the bladder are measured; in the sagittal plane, the height (H) is recorded.[122] Bladder volume is estimated by multiplying these dimensions together with a correction coefficient for the bladder shape (Please refer to the Technique or Treatment section for more information).[123]
This information helps guide decisions regarding catheterization and monitoring of post-void residual volumes. Generally, a post-void residual volume of 150 mL or less is considered acceptable, whereas a volume of 400 mL or more is considered significant and may indicate urinary retention.[119] A significantly distended bladder in the presence of hydronephrosis may indicate a lower urinary tract obstruction.[124]
A ureterocele is a congenital anomaly characterized by ballooning of the distal ureter into the bladder lumen, resulting in a sac-like protrusion.[125] This structural defect can lead to ureteral obstruction, which can progress to kidney damage, urinary tract infections, and other problems.[125] Diagnosis is commonly made during prenatal ultrasonography or other imaging during a UTI workup.[125]
Indications for bladder ultrasonography include the following:
- Assessment of posterior urethral valves using contrast-enhanced voiding urosonogram
- Bladder neoplasm
- Bladder volume measurement
- Benign prostatic hyperplasia (to identify changes associated with outlet obstruction)
- Confirmation of Foley catheter position
- Evaluation of intravesical clot burden (hematoma)
- Foreign bodies in the bladder
- Hematuria evaluation
- Investigation for distal ureteral dilation and/or distal ureteral calculi
- Measurement of bladder volume in urinary retention
- Measurement of post-void residual urine volume
- Pelvic fluid collections
- Prostate imaging when the transrectal approach is not available (such as after rectal cancer)
- Suprapubic tube placement assistance
- Ureterocele evaluation and tracking
- Urinary retention
- Vesicoureteral reflux assessment with contrast-enhanced voiding video urosonogram
Urinary Calculi
Although most of the ureter is not reliably visualized due to overlying bowel gas and stool, stones can sometimes be detected at the ureteropelvic junction, within the renal pelvis, or at the ureterovesical junction.[126][127] Stones appear as hyperechoic foci with posterior acoustic shadowing.[4][128] Color Doppler may reveal a twinkle artifact, a rapidly alternating signal that enhances stone detection, which is highly predictive of urolithiasis, especially when combined with a finding of acoustic shadowing in patients with urinary stones sized 5 mm or more. (see Video. Nephrolithiasis, Ultrasound).[129][130][131][132][133][134][135][136]
Ultrasound can reliably identify larger renal calculi (>5 mm), but its sensitivity is significantly reduced for stones smaller than 4 mm, and it tends to overestimate the size of smaller stones it detects.[129][137][138][139][140] Adding a flat plate abdominal x-ray (KUB) to ultrasonography can substantially enhance the usefulness of the ultrasound, as the KUB is more likely to visualize smaller calcific stones. In contrast, ultrasound can detect even radiolucent calculi, such as those composed of uric acid.[129][139][141][142] Sizeable calculi easily observed on ultrasound that are not visible on the KUB strongly suggest a radiolucent stone.
Stones at the ureterovesicle junction, the most common location for impaction of ureteral calculi due to their narrow ureteral caliber, may cause an identifiable focal protrusion into the bladder wall and can occasionally be directly visualized on a transverse bladder scan.[14]
The absence of a ureteral jet on Doppler may suggest complete obstruction, although variability in jet timing limits its reliability as a standalone finding.[30][143][144][145]
Ultrasound has significant limitations in assessing urinary tract calculi, particularly in directly visualizing small stones (<4 mm) and overestimating calculus size. Therefore, a non-contrast CT (the gold standard) should be pursued if clinical suspicion is high or when ultrasound findings are inconclusive.[128][137]
Hydronephrosis from a ureteral calculus can be observed on ultrasonography, even if the stone itself may not be visualized with this modality. A high renal resistive index suggests an obstruction in a patient with apparent renal colic with or without hydronephrosis.[5][62][66][67][68] Ureteral dilation from hydronephrosis may also be observed. The absence of hydronephrosis may suggest that there is no acute obstruction; however, this is not definitive.
If clinical findings or resistive index measurements suggest a stone but the ultrasound is negative, CT imaging should be performed for confirmation.
Contraindications
There are no absolute contraindications to performing abdominal or pelvic ultrasound, including urinary tract ultrasound. The procedure is noninvasive, does not involve ionizing radiation, and is generally safe for all patient populations, including pregnant individuals and children.[12][13]
Relative limitations may arise if inadequate acoustic windows are encountered due to obesity, excessive bowel gas, or poor patient cooperation.[146] Ultrasonography is very operator-dependent, which may lead to suboptimal or variable imaging quality at times. These factors can all hinder diagnostic accuracy by reducing image quality.
In such situations, alternative imaging modalities, such as CT or MRI, should be considered to complete the diagnostic evaluation.
The only relative contraindication to ultrasound evaluation is the need for urgent surgical intervention, where even a brief delay for an ultrasonic assessment is inadvisable.
Equipment
Ultrasound of the urinary tract is typically performed using a low-frequency (2-5 MHz) curvilinear transducer, which provides adequate depth penetration for evaluating the kidneys, bladder, and surrounding structures in most adult patients.[31][146] This transducer is optimal for imaging retroperitoneal organs due to its wider footprint and deeper tissue penetration.[15][25]
In pediatric patients or when assessing superficial structures, such as the bladder in thin individuals or transplanted kidneys, a high-frequency linear transducer (5-12 MHz) may be used to achieve improved imaging and spatial resolution.[31]
Modern ultrasound systems often incorporate color and spectral Doppler capabilities, which are essential for assessing renal perfusion, evaluating suspected vascular abnormalities, and visualizing ureteral jets. Some systems also offer contrast-enhanced ultrasound functionality for characterizing renal masses.[147] Additionally, although still emerging in routine practice, elastography may be available for research or advanced diagnostic applications, particularly in patients with chronic kidney disease or renal transplant follow-up.[148]
For point-of-care applications, portable ultrasound machines with curvilinear probes and Doppler functionality are commonly used and offer sufficient image quality for most diagnostic needs when performed by trained and experienced clinicians.
Personnel
Ultrasound of the urinary tract may be performed by a range of trained healthcare professionals, including radiologists, sonographers, urologists, nephrologists, emergency physicians, intensivists, and other clinicians credentialed in POCUS. Competency in performing and interpreting renal and bladder ultrasound requires appropriate training and supervised experience to ensure diagnostic accuracy and patient safety.
According to the American College of Emergency Physicians, clinicians should complete a minimum of 25-50 supervised renal and bladder ultrasound examinations to achieve basic competency in these studies.[149] Additional practice and correlation with radiologic interpretations are recommended to maintain and refine skills, particularly in recognizing less common or subtle findings.
In institutions with formal ultrasound credentialing programs, ultrasound studies may be performed by certified sonographers and interpreted by board-certified radiologists. In point-of-care settings, clinicians performing bedside urinary tract ultrasound must maintain proficiency through continued education, quality assurance programs, and adherence to institutional guidelines for review, documentation and image storage.
Preparation
Ultrasound of the urinary tract should be performed with the patient in a supine position, with the abdomen and pelvis fully exposed to allow unobstructed access for transducer placement.[4] A towel or drape may be used to protect the patient's clothing and maintain modesty. A generous application of ultrasound gel helps eliminate air between the transducer and skin surface, improving acoustic coupling and image quality.
Warmed ultrasound gel should be used whenever possible for patient comfort. Applying the gel only to the area being scanned is typically preferable, as this helps minimize air bubbles that can interfere with ultrasound transmission and detection.
Right-handed operators should position the ultrasound machine on the patient's right side for optimal ergonomics, allowing for easy visualization of the screen while scanning with the right hand and adjusting machine controls with the left.
The room should be dimly lit to enhance screen visibility and reduce glare. The patient should be instructed to remain still and breathe normally during the scan.
When assessing the urinary bladder, the patient should ideally have it full to improve visualization of the bladder contour, wall thickness, interior lining, neoplasms, foreign bodies, and the ureterovesical junctions. Ultrasound scanning can be performed in urgent or emergent scenarios, regardless of bladder volume; however, the sonographer should note any suboptimal conditions.
Cooperation in pediatric patients can be improved by involving parents, providing clear instructions, and using distraction techniques when appropriate.
Technique or Treatment
Ultrasound of the urinary tract is typically performed using a low-frequency curvilinear transducer (2-5 MHz) to achieve adequate depth penetration and resolution for abdominal and pelvic structures.[15] A phased-array transducer may be used as an alternative to a curvilinear probe, particularly in cases where a limited acoustic window is present.
The ultrasound system should be set to the Abdominal preset, which optimizes image parameters for structures such as the kidneys and bladder.[35] A higher frequency should be used for neonates, children, and thinner adult patients, as well as for the penis, prostate, scrotum, testicles, and adrenal glands.[35]
The American Urological Association Consensus Statement on Urologic Ultrasound Utilization recommends using transducers with frequencies ranging from 3.0 to 5.0 MHz for abdominal scanning, 6.0 to 9 MHz for transrectal scanning, and 7.0 to 12.0 MHz for genital ultrasonography.
Adrenal Glands
The adrenal glands can be identified in approximately 80% of abdominal ultrasound examinations; however, the reality is that this may be a bit optimistic.[35][150] The echogenicity of the adrenal glands is quite similar to that of the retroperitoneal fat that is immediately adjacent. Visualization also depends on the quality of the ultrasound equipment being used, the acoustic window, individual patient characteristics such as obesity, and the examiner's experience.[35] Visualizing the adrenal glands with ultrasonography is even more difficult in patients with fatty livers, increased stomach or intestinal gas, and obesity.[35]
Although ultrasound of the adrenals should be included as part of any abdominal ultrasound examination, CT and MRI scans are preferred for evaluating adrenal disorders and follow-up studies if the ultrasound examination suggests any pathology or is equivocal. If a prominent adrenal gland is visualized on ultrasound, it should be considered suspicious, and further imaging with either CT or MRI should be considered.
Transabdominal ultrasonography can be used to monitor adrenal masses and neoplasms that have been previously identified but not surgically treated.
Fortunately, even normal-sized adrenal glands are typically easily observed in neonates.[35] This visibility is due to their relatively large size compared to the kidneys, the limited amount of retroperitoneal fat, and the close proximity of the transducer, which allows the use of higher-frequency probes, providing better resolution.[35]
Tips for adrenal ultrasound examinations include using a higher-frequency transducer to optimize image resolution in thinner and smaller patients.[35] Extra care should be taken for proper patient positioning and angulation of the transducer. Multiple planes and angles should be checked to examine the adrenals. Examiners should be aware of anatomical variants that may be mistaken for pathology.[35] Examination of the adrenals should be attempted with every abdominal ultrasound examination, as this may identify unsuspected pathology and provide additional experience to the ultrasonographers.[35]
Harmonic and spatial (3D) compound imaging are helpful and recommended for imaging the adrenal glands.[35] Harmonic imaging produces better contrast, whereas 3D imaging provides improved visualization of complex anatomical variances and is particularly helpful in identifying neoplasms in the more challenging left-sided adrenal gland.[35] A CT or MRI examination should be considered when the imaging is inconclusive or suspicious.[35]
Shear wave elastography permits better differentiation between cystic and solid lesions. This technique analyzes the speed of shear waves in tissues, which is determined by the elasticity of the tissue.[151] Extremely rapid ultrasound images (about 20,000/s) are taken, and tissue elasticity is visualized as a color map on B-mode imaging.[151] As shear waves do not progress through fluids, solid tumors appear with a colored elastographic signal, whereas cystic structures do not.[151]
Contrast-enhanced ultrasonography of the adrenals helps visualize their vascularity but has not been shown to reliably differentiate benign from malignant lesions in all cases.[35][152]
Normal adrenal glands appear as triangular, homogeneous, hypoechoic organs with a distinct capsule on ultrasonography. The 2 glands have similar echogenicity with no focal masses or adjacent lymphadenopathy.[35][153]
Adrenal pathology may appear as follows on ultrasonography:[35]
- Adrenal hyperplasia appears as enlarged and irregular glands with a thickened cortex.[154]
- Adrenal nodular hyperplasia shows vascular enhancement at the lesion's periphery, whereas adenomas demonstrate a mixed or central vascularization pattern.[35][152]
- Benign adenomas appear with well-defined borders and homogeneous echogenicity.[150] These tumors are typically isoechoic to the adrenal gland, hypoechoic to the perinephric fat, and hypovascular.[150] Calcifications, necrosis, and hemorrhage are possible but unusual and may be observed in larger adenomas.[150]
- Cystic lesions of the adrenals (cysts, hematomas, hemorrhage, or necrosis) on ultrasound are hypoechoic or anechoic regions within the gland.[155]
- Left-sided adenomas may be misdiagnosed as an accessory spleen if their connection to the adrenal gland is unclear.[150]
- Adrenal involvement in lymphoma occurs in up to 25% of patients, most frequently with non-Hodgkin's lymphoma. Almost 50% of patients have bilateral disease.
- On ultrasound, they appear hypoechoic; however, areas of bleeding or necrosis appear hyperechoic.
- Metastatic lesions smaller than 3 cm are homogeneous and indistinguishable from normal adrenal tissue. Larger neoplasms often demonstrate heterogeneity due to hemorrhage or central necrosis along with irregular margins.[150] Metastatic lesions are frequently bilateral and demonstrate increased vascularity.[150]
- Pheochromocytomas appear relatively large and well-defined on ultrasound. Although solid, they may be homogeneous or heterogeneous.[156] These tumors typically demonstrate hypervascularity.
- There may be bleeding into the adrenal medulla that appears as a round or ovoid echogenic central mass.[156]
- The liver on the right and the spleen on the left can be used as acoustic windows to help visualize the adrenals.
Primary adrenal cortical carcinomas are rare lesions with a variable appearance. Smaller lesions may have an ultrasonic appearance similar to normal adrenals. A thin echogenic capsule may be present. Larger lesions are often quite large, sometimes exceeding 8 cm in diameter.[150] Most primary adrenal cancers are over 6 cm when they are discovered clinically.[157] Approximately 55% of adrenal cortical carcinomas are functional.[150][158]
These malignancies are irregularly shaped; may contain calcifications, necrosis, or hemorrhage; and may sometimes demonstrate surrounding tissue invasion.[150][159] In some cases, they can mimic pheochromocytomas.[150] Color Doppler imaging may demonstrate internal afferent blood vessels or hypervascularization.[150]
Adrenal incidentalomas are any newly discovered solid adrenal neoplasms greater than 1 cm.[160] A CT or MRI scan should be used to evaluate them, and their functional status should be determined.[35][160]
Adrenal incidentalomas, typically non-functioning adrenal adenomas, are overwhelmingly benign, with malignancies in less than 10% of cases.[160][161] The most common malignant adrenal neoplasms include metastases, typically from the breast, colon, kidney, liver, and stomach, as well as lymphomas.[160][162]
Adrenal incidentalomas less than 1 cm in size do not require further investigation.[160][163][160] Adrenal incidentalomas are bilateral in over 15% of patients, and about 10% are functional, meaning they secrete androgens, catecholamines, mineralocorticoids, or steroids.[160][164][165][166]
A non-contrast CT scan showing a homogeneous neoplasm with a density of no more than 10 Hounsfield units (HU) indicates a benign adenoma, and no further imaging is required, regardless of size.[160][161] However, a functional screening for hyperaldosteronism, hypercortisolism, and possibly pheochromocytoma should still be performed.[160][167]
Additional CT, FDG-PET/CT, or MRI imaging is suggested for incidentalomas larger than 4 cm, homogeneous lesions with densities between 11 and 20 HU, or heterogeneous neoplasms smaller than 4 cm with HU densities greater than 20.[160][161][168] Adrenal neoplasms larger than 4 cm, those that are not homogeneous, or those with a density greater than 20 HU require surgical intervention due to the increased risk of malignancy.[160][161][168]
Ultrasonography, with and without contrast enhancement, is being evaluated for its ability to help differentiate between benign and malignant adrenal neoplasms.[160][169]
Intraoperative laparoscopic ultrasonography of the adrenal glands has been found to be useful in adrenalectomy surgery. Due to its excellent visualization characteristics, a linear side-viewing or flexible side-viewing transducer with a frequency range of 7.5 to 10 MHz is recommended.[170] The flexible transducer is generally preferred. Color Doppler ultrasonography can be used to identify blood vessels.
Intraoperative laparoscopic ultrasonography is particularly helpful for right-sided adrenal neoplasms, markedly obese patients, and smaller adrenal nodules. This technique also aids in assessing the degree of possible tumor infiltration into surrounding tissues, delineating surgical planes, and identifying the left renal vein.[171][172][173][174][175] Intraoperative laparoscopic ultrasonography offers enhanced surgical precision, reduces blood loss, shortens hospital stays, and decreases complications.[174] This technique is particularly helpful when performing partial adrenalectomies.[176][177][178]
Bladder
Bladder ultrasonography typically uses a low-frequency curvilinear transducer (3.5-5 MHz), which provides a wider field of view with sufficient penetration to visualize the entire bladder. A phased array transducer may also be used.
Bladder imaging begins with the transducer placed just superior to the pubic symphysis, with the indicator directed superiorly to obtain a sagittal view.[25] The probe is then rotated 90° counterclockwise, with the indicator pointing to the patient's right, to acquire a transverse view. Fanning through the bladder in this plane allows assessment of the full volume and contour.
The ureterovesicle junction, located at the posteroinferior aspect of the bladder, should be examined for distal ureteral stones, which may appear as hyperechoic foci with posterior shadowing. Color Doppler imaging can aid in visualizing ureteral jets, which indicate ureteral patency.
In a full bladder, the urine appears anechoic and fills most of the anterior screen. The bladder wall should be evaluated for smoothness, thickness, and the presence of any mural or intraluminal abnormalities such as clots, diverticula, or masses.[4][31]
Bladder stones are readily visualized on bladder ultrasound examinations.[179] These stones appear as well-demarcated, highly echogenic structures with intense posterior acoustic shadowing, which are mobile and move as the patient changes position.[179]
Bladder tumors are generally too small to be detected on ultrasound unless they are sizable; therefore, cystoscopy is required to complete the urological evaluation of hematuria.
The bladder wall should measure less than 5 mm in thickness.[180] A uniformly thickened bladder suggests bladder overactivity, cystitis, or outlet obstruction, such as from benign prostatic hyperplasia or a urethral stricture.[181] The normal maximum bladder capacity is about 500 mL, and the post-void residual urine volume should be less than 150 mL.[119]
An enlarged median lobe of the prostate can extend into the bladder lumen. If large enough, this can cause obstructive uropathy or create a ball-valve effect, making urination difficult. A mass at the bladder neck or base, as observed on imaging of the bladder, is often an extension of the prostatic median lobe, which is frequently misinterpreted by radiology.
Trabeculations of the urinary bladder wall typically occur with bladder outlet obstruction but may also be observed with prolonged overactivity. These trabeculations are caused by hypertrophied individual muscle bundles in the bladder wall that have hypertrophied at different rates, creating irregularities. Over time, these changes can progress to deep cells and, eventually, diverticula, outpouchings of the bladder mucosa and submucosa that extend outside the external contour of the bladder and are devoid of muscle.
Ultrasound can detect trabeculations and diverticulae, as the neck of the diverticulum is easily observed, and color Doppler imaging can determine if there is internal stagnation.
Ureteroceles are congenital anomalies where the distal ureter balloons out, extending into the bladder lumen, forming a sac-like structure.[125] This sac-like structure can cause ureteral obstruction, which can progress to kidney damage, urinary tract infections, and other problems.[125] Diagnosis is often made via prenatal ultrasonography or other imaging during a UTI workup.[125]
A ureterocele appears as a thin-walled, fluid-filled cystic mass of variable size located inside the urinary bladder near the ureterovesical junction.[125] These ureteroceles are often associated with duplicated kidneys.[125] Ureteroceles may be obstructive and cause hydroureteronephrosis with ureteral dilation.[125] On imaging, ureteroceles can present with a bladder-in-bladder appearance or exhibit a cobra head sign.[125] If symptoms or renal deterioration are present, ureteroceles are generally managed surgically.[125]
For bladder volume estimation, especially in cases of urinary retention, height (superior-inferior), width (lateral), and depth (anterior-posterior) are measured in centimeters (see Image. Bladder Dimensions Visualized on Ultrasound). A volume formula is applied by multiplying these values by a correction coefficient, as determined by the overall shape of the bladder.[122][123] A universal or standard correction coefficient of 0.7 can be used. However, more accurate measurements are obtained using a customized coefficient value based on each patient's bladder shape, ranging from 0.56 (spherical bladder) to 0.92 (cuboid).[123]
The standard formula for calculating the bladder volume is [Bladder volume (mL) = Height (cm) × Width (cm) × Depth (cm) × Correction coefficient (standard is 0.7)].[123]
Kidneys
For renal ultrasonography, a low-frequency (2-5 MHz) curvilinear transducer is preferred in most adults. A higher frequency (5-12 MHz) may be used in children, superficial transplanted kidneys, pelvic kidneys, or very thin patients.
If applicable, a renal evaluation should begin with the asymptomatic side to establish a normal baseline and identify patient-specific anatomical features.
- For the right kidney, the patient is in a supine position. The transducer is placed in the mid-axillary line, just below the costal margin, with the probe indicator directed superiorly. The right kidney is best visualized in a coronal oblique plane, using the liver as an acoustic window, and appears medial and inferior to the liver on the screen.[4][15]
- For the left kidney, the transducer is positioned in the posterior axillary line, typically higher than the right side due to the superior position of the left kidney. The patient is typically in the right lateral decubitus position to minimize bowel interference. Visualization is often more challenging due to overlying bowel gas and the absence of a large acoustic window, such as the liver. The spleen may assist by providing a partial acoustic window.[15][25]
To improve image acquisition and reduce rib shadowing, the transducer can be rotated obliquely to align with intercostal spaces, rotating counterclockwise on the right and clockwise on the left. Having the patient take and hold a deep breath may widen the intercostal spaces, allowing the kidneys to move into a more favorable position. Placing the patient in the right lateral decubitus position can improve access in complex left kidney visualization cases.
Each kidney should be scanned in longitudinal and transverse planes, using a slow fanning motion to evaluate the entire organ from anterior to posterior. Key assessment features include renal size (dimensions), cortical thickness, corticomedullary differentiation, echogenicity, and the presence of hydronephrosis, cysts, stones, or masses.
- Both kidneys should be roughly the same size. A difference in length of more than 1.5 cm is considered abnormal.
- The renal cortical thickness should be measured. Approximately 1.5 cm is considered normal, whereas a cortical thickness of less than 1 cm is worrisome for cortical thinning.
- The parenchyma of the renal cortex should be homogeneous and hypoechoic or isoechoic compared to that of the spleen or liver. The renal hilum is more echogenic than the renal cortex due to its large vessels, sinus fat, and collecting system. The kidney's medullary pyramids are typically hypoechoic compared to the renal cortex.
- The upper poles of the kidneys are typically closer together than the lower poles, as the upper pole of each kidney is tilted or angled towards the midline. If this is reversed, with the lower poles closer together, it suggests a horseshoe kidney.[182]
- Renal ultrasound is not very sensitive for visualizing renal calculi smaller than 3 mm. However, it can identify hydronephrosis, measure the resistive index, and is the preferred imaging study for pregnant and pediatric patients who present with possible renal colic.
Parenchymal renal scarring is almost always observed over the renal pyramids, frequently affecting the compound calyces, and often appears at the poles. The renal cortex is thin or absent when there is a parenchymal scar, which is typically visualized as an acute depression of the otherwise smooth renal outline.[183]
Such scars are almost always related to kidney infections and are often associated with vesicoureteral reflux in childhood.[184] A renal nuclear scan is a more sensitive imaging modality for detecting parenchymal scarring, although contrast-enhanced ultrasonography may also be helpful.[185][186][187] Standard ultrasound alone is considered insufficient to detect renal parenchymal scarring.[188]
Cysts of the kidney are often found incidentally on ultrasound examinations of the kidneys. The following 3 criteria identify and define a simple cyst:[189]
- Anechoic interior
- Thin, smooth, and well-demarcated walls
- Posterior acoustic enhancement beyond the cyst
Simple renal cysts do not require additional imaging or testing. However, solid masses and complex cysts typically necessitate a CT scan (preferred) or MRI scan, either with or without IV contrast or contrast-enhanced ultrasonography, to assess vascularity and identify tissue vascular enhancement.[190][191][192][193][194][195] Such vascular enhancement suggests a likely malignancy.[194][196]
The Bosniak classification scoring system was developed for characterizing renal cystic lesions on CT but has been expanded and updated to describe cystic neoplasms observed on ultrasound, especially when IV contrast enhancement is used to determine vascularity.[197][198][199][200][201][202][203][204][205] The Bosniak classification system is described as follows:
- Class I: A benign simple cyst with a very thin wall without septa, calcifications, or solid components. Density similar to water (≤15 HU) with no enhancement after IV contrast administration. Malignant potential is approximately 0%. No follow-up imaging is required.
- Class II: There are 2 sub-categories:
- A benign simple cyst with a few thin walls and septa. May have fine calcifications but no vascular enhancement after IV contrast.
- Homogeneous high-attenuating (>70 HU) masses ≤3 cm in size with sharp margins. No vascular enhancement after IV contrast. Malignant potential is extremely low at less than 1%. Follow-up imaging is unnecessary but may be suggested 6 or 12 months later in selected patients to confirm the diagnosis and classification.
- Class IIF: An indeterminate designation between classifications II and III, with 2 subtypes:
- Cysts with multiple thin septa with smooth, minimally thickened walls that may have nodular calcifications. There may be an impression of some mild enhancement.
- High attenuation masses larger than 3 cm in size but with no vascular enhancement after IV contrast administration. The estimated malignant potential is 5%. Follow-up imaging with CT or ultrasound is recommended at 6 months and then annually for 5 years.
- Class III: The cyst demonstrates a multilocular structure with thickened walls or septa, irregular calcifications, or measurable vascular enhancement more than 10 HU. These cysts have an estimated malignancy rate of 55%. Close follow-up or surgical excision with partial or total nephrectomy is recommended based on lesion size as follows:
- Active surveillance if the lesion is less than or equal to 2 cm.
- Active surveillance, percutaneous ablation, or surgical excision if the lesion is 2 to 4 cm.
- Surgical excision if the lesion is larger than 4 cm.
- Class IV: These cysts are typically large nodular lesions with enhancing solid or necrotic components. These cysts have an extremely high malignant potential, ranging from 91% to 100%. Surgery consisting of a partial or total nephrectomy is recommended.
Parapelvic cysts can sometimes be mistaken for hydronephrosis. One way to differentiate a parapelvic cyst from hydronephrosis is to look at its orientation. Parapelvic cysts have their long axis pointing between the renal pyramids, whereas in hydronephrosis, the long axis points to the renal pyramid and papilla. Renal resistive index can also differentiate a parapelvic cyst and an extrarenal pelvis (with normal or low resistive index) from true hydronephrosis with its higher resistive index.
Pyelonephritis typically appears as a unilateral enlargement of the affected kidney with increased (brighter) echogenicity and decreased (darker) corticomedullary differentiation. Doppler ultrasonography may show decreased blood flow to affected areas, and abscesses may also be visible. Hypoechoic areas appear darker on ultrasound, indicating edema or inflammatory changes. Hyperechoic areas that appear brighter may indicate hemorrhage.
Ultrasound is highly recommended for patients with acute pyelonephritis, especially when they are unresponsive to appropriate medical therapy, as it can identify hydronephrosis and an increased renal resistive index, suggesting potentially life-threatening obstructive pyelonephritis (pyonephrosis) requiring urgent surgical drainage.[206]
Ultrasound in patients with renal failure typically shows more hyperechoic parenchyma (relative to the liver), reduced cortical thickness, and a smaller kidney overall.[207][208] However, older individuals naturally have a thinning of the renal cortex and smaller kidneys.[209][210][211]
Solid renal masses are often initially detected by ultrasonography, which is frequently the first imaging modality used for suspected kidney neoplasms. As ultrasound is a noninvasive, convenient, economical imaging modality that avoids radiation exposure, it is recommended for initial imaging of suspected renal neoplasms before resorting to CT or MRI.
Doppler ultrasonic imaging can assess tumor vascularity, and the diagnosis can be initially made by ultrasonography, such as benign angiomyolipomas, which demonstrate characteristic hyperechoic fatty components that can be confirmed by other imaging.[194][212]
CT and MRI are more sensitive, but ultrasound is more convenient and less costly, as it avoids unnecessary radiation exposure and performs extremely well for tracking renal neoplasms when monitored closely under an active surveillance protocol. The latest American Urological Association guideline on evaluating microhematuria recommends renal ultrasonography as the preferred imaging modality for the kidneys in low- and intermediate-risk patients with microhematuria.[213][214]
Renal ultrasound also has an intraoperative role. This method can be used to localize intraparenchymal masses in partial nephrectomy surgery, as contrast-enhanced ultrasonography and Doppler imaging improve tumor visualization.[215][216][217][218][219] Imaging is projected onto console screens for real-time visualization.
Ultrasound guidance is sometimes used to establish percutaneous access to the renal collecting system for nephrostomy tube placement or for percutaneous nephrolithotomy. This technique has the advantage of not using ionizing radiation but requires some degree of hydronephrosis as a target and may not be feasible in very obese patients.[220]
Penile Urethra
For the evaluation of the penis and urethra, the preferred probes are linear transducers with a maximum frequency of 12 to 15 MHz.[27]
To visualize the urethra, sterile ultrasound gel or 2% lidocaine jelly is injected gently through the urethral meatus using a 60 mL catheter-tip syringe. A small urethral catheter may also be used. About 20 mL is needed to fill the urethra. Filling the bladder provides a useful landmark. If any strictures are found, their location, length, diameter, and distance from the external sphincter or other recognizable landmark should be noted.[221]
There is increasing use of contrast-enhanced urethral ultrasonography, particularly in pediatrics. The contrast agent is instilled into the bladder for voiding studies or directly into the urethra for retrograde urethrosonography.
A urethral lesion may be found in up to 15% of penile traumas.[27][222][223] If blood is observed at the urethral meatus, an imaging evaluation of the urethra is necessary.[224] Retrograde urethrography is the most commonly performed procedure, although contrast-enhanced ultrasound may also be used. The latter avoids ionizing radiation and can be performed more quickly, even while waiting for x-ray availability.[223] MRI may also be used, but it is time-consuming, costly, and generally restricted to equivocal cases when alternative imaging is inconclusive.[27]
Penis
As an external, superficial organ, the penis is particularly well suited to ultrasonic examination.[27][225] As such, it is probably underutilized. Linear transducers with a higher frequency range of 12 to 15 MHz are typically used for penile ultrasonography.[27]
The corpora cavernosa appear as well-delineated cylindrical structures with moderate homogeneous echogenicity.[47] These structures are less echogenic than the corpus spongiosum.[47] The penile septum is visualized as an area of posterior acoustic shadowing between the 2 corpora.[47][226][227] Buck's fascia and the tunica albuginea appear as bright (dense) lines, with the tunica measuring about 2 mm thick when the penis is flaccid.[27][47]
The cavernosal arteries can be observed in the center of each corpus as thin tubular structures with echogenic walls.[47] The normal diameter of the cavernosal arteries is 0.3 to 0.5 mm (flaccid) and more than 0.7 mm when erect.[47] The dorsal penile veins appear as anechoic compressible tubules and typically have detectable flow on color Doppler ultrasonography (Fig. 3).[47]
In the evaluation of erectile dysfunction, particularly before consideration of a penile prosthesis surgical implant, an intracorporal injection of vasoactive drugs (eg, alprostadil, papaverine, or a combination such as trimix: alprostadil, papaverine, and phentolamine) is given.[228] Cavernosal arterial diameters are measured before and 5 minutes after the injection, and the clinical response is noted.
Left and right cavernosal peak systolic velocity (PSV) and end-diastolic velocity (EDV) are recorded, and the resistive index (RI) is calculated.[45][46][47]
- The PSV is the maximum flow rate during systole.[46][47]
- The EDV is the residual flow at the end of diastole.[46][47]
- The RI indicates the peripheral resistance to blood flow in the penis. The formula is RI = (PSV − EDV) / PSV.[46][47]
- The PSV is the most accurate indicator of penile arterial disease.
- A PSV value of 35 cm/s or more is considered normal, whereas a PSV determination of less than 25 cm/s is diagnostic of severe arterial insufficiency.[46][47]
- PSV values between 25 and 35 cm/s are considered intermediate or marginal for diagnosing arteriogenic erectile dysfunction.[27][46][47][225][229]
- A PSV of 30 to 35 cm/s is probably acceptable or borderline, whereas a PSV of 20 to 25 cm/s is marginal and may be abnormal.[225]
- A difference in PSV readings between the 2 cavernosal arteries of more than 10 cm/s is suggestive of erectile dysfunction due to arterial insufficiency.[46][47][230]
- The EDV and RI reflect the activity of the veno-occlusive mechanism.[46][47] Normal cutoff values are an EDV of less than 5 cm/s with a RI greater than 0.8.[46][47][231][232]
- In patients with arterial insufficiency, venous competence cannot be assessed using Doppler ultrasonography.[27]
- An arterial acceleration time greater than 110 ms, failure to visualize the helicine arteries on power Doppler, and incomplete or inadequate penile rigidity after intracorporeal vasoactive injection are indicative of arteriogenic erectile dysfunction.[225]
- Damped waveforms and high-velocity jets on penile Doppler ultrasonography suggest proximal artery stenosis.[230]
- Arteriogenic erectile dysfunction is also an independent risk factor for cardiovascular disease, especially as erectile dysfunction typically occurs several years before any cardiac events.[47][233][234][235][236][237][238][239][240]
Central cavernosal shear wave elastography has been used to objectively quantify penile rigidity in the evaluation of erectile dysfunction.[241][242][243][244] Patients with vasculogenic erectile dysfunction demonstrated much higher central shear wave elastography values both before (flaccid) and after vasoactive intracorporal injections than those with erectile dysfunction from other causes.[241][242][243][244]
Priapism is the abnormal condition where the penis develops a prolonged, involuntary erection in the absence of appropriate stimulation.[245] The immediate diagnostic need is to identify whether the priapism is ischemic (low-flow) or nonischemic (high-flow).[27][245] Ischemic priapism is a true surgical emergency and requires prompt treatment to prevent permanent damage to the penis, such as cavernosal fibrosis.[245][246]
Penile ultrasound demonstrates fibrosis of the corpora cavernosa as fine, echogenic strands close to the cavernosal arteries.[47] The fibrosis replaces the normal sinusoids.[47] There are generally minimal changes after the injection of vasoactive drugs, and Doppler ultrasound typically shows venous dysfunction.[47]
Penile ultrasound is highly valuable in differentiating ischemic from nonischemic pathologies.[247] The diagnostic accuracy of this modality is such that it has been proposed as an alternative to cavernosal blood gas analysis for distinguishing between ischemic and nonischemic priapism.[247] Penile ultrasonography is particularly helpful when used for confirmation of blood flow after a shunting procedure for ischemic priapism, as penile edema and inflammation of the cavernosa can simulate a residual erection.[45][247]
- In ischemic priapism, the cavernosal arteries demonstrate minimal EDV due to very high vascular resistance.[248][249]
- In nonischemic priapism, the PSV of the cavernosal artery is extremely elevated (>50 cm/s), along with a high EDV.[231][232] Venous drainage is enhanced, and internal fistulas show high-velocity flow with turbulence.
- These results can quickly differentiate ischemic (low-flow) from nonischemic (high-flow) priapism.[27]
Ultrasound is a valuable tool for evaluating penile trauma.[27][250] The modality is safe, accessible, inexpensive, and can provide information not immediately available otherwise.[27][250] Any interruption of the echogenic line of the tunica albuginea indicates a penile corporal rupture (penile fracture), signifying a tear in the tunica albuginea.[251] Ultrasound can quickly establish this diagnosis and identify the location and extent of the injury, which can otherwise be difficult to determine due to the swelling and hematoma accompanying this injury.[27][252] Ultrasound demonstrates any associated injury to the urethra and penile dorsal vein.[27][222][223][253]
Peyronie disease is a benign, progressive disorder of the penile tunica albuginea that causes localized fibrosis, scarring, plaque formation, and curvature of the penis during erections.[254][255] Penile ultrasonography is the imaging modality of choice for evaluating Peyronie disease.[255][256] The presence, size, and thickness of Peyronie plaques in the penile tunica albuginea and any calcifications can be detected by penile ultrasound.[255][256][257] Peyronie plaques appear as hyperechoic localized thickening of the penile tunica albuginea.[27] Calcifications greatly increase the echogenicity of the plaques and will demonstrate acoustic shadowing.[27][255][258]
In addition to identifying, measuring, and tracking Peyronie plaques over time, ultrasound can also be used to determine the involvement of the neurovascular bundle within the lesion, which is an important consideration for patients considering surgery.[27][255]
Elastography can identify Peyronie plaques even if they are non-palpable and not visualized on standard ultrasonography.[259]
Using vasoactive intracorporeal injections allows for an assessment of the penile vascular potential.[228] Patients identified with arterial insufficiency and venous leak disorders are unlikely to have good erectile function from grafting procedures alone and likely require a penile prosthesis.[256][257] Ultrasonography can also track the condition's progress when using non-surgical therapies.[256][257]
Prostate
Please see StatPearls' companion resources, "Prostate Imaging" and "Transrectal Ultrasonography and Image-Guided Biopsies of the Prostate," for further information (see Image. Transrectal Ultrasound Guided Biopsy of the Prostate).
Scrotum, Testicles, and Epididymis
Ultrasonography is the preferred diagnostic imaging modality for evaluating scrotal masses and testicular pain.[260] A high-frequency (7.5-10 MHz) linear array transducer is typically used, as it provides detailed images of the scrotal contents, which are relatively superficial.[260]
Epidermoid cysts are rare, benign testicular neoplasms that are characterized by multiple concentric layers of keratinizing squamous epithelium and keratinous debris, giving them a solid hypoechoic appearance.[261][262] These cysts are typically round with concentric hyperechoic layers internally, giving them an onion skin appearance, and lack detectable internal vascular flow on color Doppler ultrasonography.[261][262] Although they are the most common benign neoplasm of the testes, they only account for 1% to 2% of all testicular masses.[261][263][264]
Although they can mimic testicular cancers on imaging, the lack of tumor markers, the characteristic onion skin appearance on ultrasound, and the lack of internal vascularity on color Doppler imaging are typically sufficient to make the diagnosis.[261]
Epididymal cysts (spermatoceles) are observed as anechoic structures, typically near the head of the epididymis. The falling snow sign may be observed using color Doppler, and internal septations may be present.[265] Hydroceles are also anechoic and surround the testicle completely, whereas spermatoceles are typically smaller and separate from it.[29][266]
Scrotal ultrasonography has a role in the evaluation of male infertility and varicoceles as well.[267][268][269][270] Generally, the diameter of a varicose vein of more than 2.95 mm indicates a clinically significant varicocele.[271][272][273][274]
Testicular microlithiasis was once thought to be associated with testicular cancer but has since been found to be a relatively common incidental finding that has no such malignant association.[275][276][277]
Superb microvascular imaging has been found helpful in evaluating vascularity in undescended testes.[278][279][280]
Epididymitis is visualized on ultrasound as an enlarged, hypervascular epididymis that may be hypoechoic or heterogeneous.[86] Increased blood flow on color Doppler imaging is characteristic.[86] Reactive hydroceles and scrotal wall thickening may also be observed, and their presence can support the diagnosis.[86] Spread of these findings into the testis is termed epididymoorchitis.[86]
Solid paratesticular tumors of the epididymis include adenomatoid neoplasms (73%), leiomyomas (11%), and cystadenomas (9%).[281][282][283] These paratesticular tumors are rare and comprise only about 5% of all intrascrotal neoplasms.[281]
Paratesticular neoplasms generally have well-defined borders; however, other characteristics, such as size, echogenicity, and vascularity, are variable, making a definitive diagnosis difficult solely from ultrasonography.
- Adenomatoid tumors are the most common benign neoplasms of the epididymis. These tumors are typically homogeneous on ultrasound with minimal internal vascularity on color Doppler imaging, although this is variable.[284][285] Adenomatoid tumors are most often found in the tail of the epididymis, are asymptomatic, and typically occur in middle-aged patients.[286]
- Epididymal papillary cystadenomas are often associated with von Hippel-Lindau disease. Approximately 67% of patients with cystadenomas were reported to have von Hippel-Lindau disease. Sonographically, it may appear solid (when its cystic components are small) or primarily cystic. These neoplasms tend to be hypervascular.[287][288][289]
- Leiomyomas are benign tumors of the epididymis that are often bulky.[281]
Although ultrasound alone cannot reliably distinguish between these different paratesticular neoplasms, sonography can reliably differentiate intratesticular neoplasms—which are associated with a high likelihood of malignancy—from paratesticular lesions, which are overwhelmingly benign (approximately 95%).[290][291] Tumor size greater than 1.5 cm and detectable vascular flow on color Doppler imaging are generally suggestive of possible malignancy.[292] When a solid mass involves intratesticular and paratesticular structures, the differential diagnosis includes leukemia, lymphoma, metastatic disease, sarcoidosis, and tuberculosis, which can also affect both regions.[293][292]
MRI imaging can be performed if further investigation of the lesion is necessary.[285][294] If the benign nature of these paratesticular lesions cannot be reliably determined otherwise, surgical exploration with an intraoperative frozen section is warranted.[295]
Testicular Cancer
Testicular neoplasms typically appear as heterogeneously hypoechoic solid lesions with lobulations, well-defined borders, and identifiable internal blood flow on Doppler ultrasound imaging.[86] Cystic structures and irregular margins may also be observed, which may contain internal echogenic foci from calcifications, fibrosis, or hemorrhage.[86] These features are not diagnostic for testicular malignancies and may be found in various benign entities.[86]
The sonographic appearance of testicular cancer is highly variable, and it is not currently possible to reliably characterize histological types based on ultrasonographic appearance alone, as this requires radical orchiectomy surgery and careful histological examination.[86] However, as summarized below, several histological types of testicular neoplasms tend to demonstrate specific ultrasonographic characteristics.
- Choriocarcinomas are relatively solid, heterogeneous masses with internal calcifications, necrosis, and hemorrhage.
- Embryonal cell cancers are typically heterogeneous with poorly defined borders that blend into the adjacent normal testicular tissue.
- Leydig cell tumors are generally small and hypoechoic. Cystic changes may be present.
- Lymphomas of the testes are visualized as discrete hypoechoic masses with increased blood flow on color Doppler imaging. If the testis is completely involved with lymphoma, this may only be apparent by comparison with the contralateral testicle.
- Seminomas typically appear hypoechoic and uniform, although larger neoplasms may exhibit more lobulations and heterogeneity. They are the most common germ-cell testicular cancers.
- Sertoli cell tumors are typically round, lobulated, and well-demarcated.
- Teratomas appear as well-defined complex masses with cystic changes and may contain calcifications.
- Yolk sac neoplasms may have calcified regions or cystic areas.
Patients with extensive metastatic disease may infrequently develop areas of calcification or fibrosis in a normal-sized or atrophic testis, which develops from involuted malignancy.[296]
Testicular cysts are well-demarcated, homogeneously hypoechoic lesions with posterior acoustic enhancement on ultrasound imaging.[86] Complex testicular cysts have some degree of internal echogenicity. However, all cysts lack internal vascularity on color Doppler imaging.[86]
Testicular torsion is most commonly observed in adolescents who develop acute unilateral scrotal pain.[297] This condition is caused by inadequate fixation of the testicle inside the scrotum, with the tunica vaginalis joining the spermatic cord higher (more superiorly) than normal.[297] This anatomical variation allows the superior end of the testicle to drop down into the scrotal sac, causing the bell clapper deformity, which is usually bilateral.[86][298]
Intrascrotal rotation of the testicle compromises the blood supply, leading to ischemia, acute pain, and necrosis if not repaired expeditiously (optimally, within 6 hours of onset).[297] A definitive diagnosis can be made when there is no detectable blood flow on color Doppler imaging of the affected testicle with normal blood flow on the contralateral side.[86][297] Color Doppler imaging of the testicle is far superior to radionuclide imaging, which was used previously. Ultrasound is not only more reliable diagnostically, but it is also readily available in emergency departments and can be performed much more quickly.[297][298]
The whirlpool sign is a reliable sonographic finding characteristic of testicular torsion, appearing as an acute, sudden change in the course of the spermatic cord with a spiral or twisting appearance between the testicle and the external inguinal ring.[299][300][301][302][303][304]
A tortuous and redundant spermatic cord may also be observed.[299] In patients with acute unilateral scrotal pain, the redundant cord is typically found bunched up and lying on top of the testicle or in the scrotal sac, suggesting abnormal attachment of the tunica vaginalis.[299]
Torsion of the appendix testis is typically diagnosed clinically based on the history and the blue dot sign, but ultrasonography can help confirm the diagnosis.[86][305] Normally, the appendix testis typically measures less than 5.6 mm and exhibits minimal or no detectable blood flow on color Doppler imaging.[305][306][307] As the appendix testis may not be easily visualized on ultrasound, normal blood flow to the affected testicle can at least effectively rule out testicular torsion. The appendix testis is generally visualized in about 80% of cases.[307]
A torsed appendix testis appears enlarged (>5.6 mm), avascular, and may be surrounded by a hyperemic epididymis, often with posterior enhancement.[305][308]
Varicoceles are dilated veins of the pampiniform plexus of the spermatic cord caused by dysfunctional valves of the internal spermatic veins, typically on the left side.[309] Normal-sized veins are typically 0.5 to 1.5 mm in diameter, whereas veins larger than 3 mm are considered pathological and clinically significant.[309][310][311][312][311] Varicoceles are typically found above and lateral to the testicle. Ultrasonography is typically not required for varicoceles, as the diagnosis is typically made clinically based on clinical examination findings such as a bag-of-worms appearance.[309]
Ultrasonography should be performed with the patient in a supine position.[309] The scrotum should be elevated and supported by placing a rolled towel underneath.[313] Warm ultrasound gel is suggested. Higher-frequency transducers (greater than 7.5 MHz) are recommended for improved image quality.[313]
Varicoceles are typically found above and lateral to the testicle. A Valsalva maneuver may show reflux (reverse blood flow) on color Doppler and accentuate the veins, making them easier to identify.[310] On ultrasound, varicoceles appear as multiple hypoechoic (dark), tortuous, tubular structures and may show a complex pattern.[309] Varicoceles are often described as multiple, anechoic, serpiginous, tubular structures.[93][313]
The finding of an isolated right-sided varicocele has traditionally been associated with the possibility of a right renal carcinoma with a tumor thrombus extending into the inferior vena cava, resulting in the varicocele.[309] Although this is quite unlikely, performing a quick renal ultrasound examination is recommended either separately or at the time of the varicocele ultrasonography.[273][309][314][315][316][317][318][319][320]
Ultrasound Artifacts
- Anisotropy refers to the phenomenon in which ultrasonic reflections are detected only when the transducer is precisely perpendicular to the structure being examined. This effect occurs with particularly smooth surfaces, which appear bright when the transducer is at 90°, but dark at other angles.[7][321][322][323][324]
- Edging artifacts occur when ultrasonic waves hit a curved surface at a critical angle. This unique surface reflection of sound waves along its surface does not return any reflections to the transducer and is observed on the ultrasound image as a dark line.[325] This edging artifact is helpful in differentiating the upper pole of the testis from the head of the epididymis.[321][325]
- Enhancement occurs when tissue immediately behind a very hypoechoic structure appears unusually bright due to increased transmission.[321]
- Resolution artifact results in loss of detail, causing two adjacent structures to appear as one, particularly notable in kidney stones.[326]
- Shadowing (acoustic shadowing) is the opposite of the enhancement artifact. This artifact occurs when a structure absorbs or reflects a large proportion of the sound waves, resulting in tissues behind the structure receiving insufficient ultrasonic waves to be visible, which appears extremely dark or black.[129] Acoustic shadowing is caused by refraction and reflective effects at the margins between a curved object and its surrounding tissues.[321][327] Phase cancellation also contributes to this effect.[327]
- Speckle artifacts occur close to the transducer due to interference from tissue ultrasound scatterers.[326]
- When using color Doppler imaging, twinkling artifacts appear as rapidly alternating colors behind an irregular hyperechoic structure, such as a kidney stone.[130][328][329][330][331] This artifact is caused by ultrasonic sound waves interacting with the irregular surface of the stone, producing temporary microbubbles.[328][329][330] This twinkling artifact is the most sensitive ultrasound modality for identifying small stones.[329]
- Shadowing and twinkling artifacts are particularly useful in identifying calcifications in urological organs such as kidney stones.[5][331]
- Other artifacts include comet tail, focal enhancement, grating lobe, multipath, range ambiguity, refraction, ring down, section thickness, side lobe, and speed errors.[326]
Complications
Ultrasound of the urinary tract is a safe, noninvasive diagnostic modality with no known biological risks at standard diagnostic energy levels. Unlike imaging modalities that involve ionizing radiation or intravenous contrast, ultrasound carries virtually no systemic complications and is well tolerated across all patient populations, including children, pregnant individuals, and those with renal impairment.
Minor discomfort may occur during the examination, particularly when firm transducer pressure is applied over tender areas such as the flanks or suprapubic region. Additionally, the application of gel may feel cold or mildly uncomfortable to some patients, though this is easily mitigated by warming the gel beforehand and promptly removing any residue after the examination.
There are no procedural complications specific to urinary tract ultrasound, and adverse effects are exceedingly rare. Proper patient communication, good positioning, gentle technique, and attention to patient comfort help ensure a well-tolerated and effective examination.
Clinical Significance
Ultrasound of the urinary tract is a rapid, cost-effective, and noninvasive imaging modality that plays a critical role in evaluating a wide range of urological conditions. This technique is particularly valuable in the diagnosis of hydronephrosis, where it demonstrates high specificity for moderate-to-severe obstruction.[5] When performed by experienced healthcare providers, its sensitivity in detecting obstructive uropathy and nephrolithiasis is significantly improved, making it a reliable tool in both emergency and outpatient settings.[4][5][6][332][333]
Importantly, ultrasound can expedite clinical decision-making, especially in the assessment of flank pain, hematuria, urinary retention, and suspected renal or bladder masses. This technique enables prompt identification of pathology without the risks associated with ionizing radiation or intravenous contrast, which is particularly advantageous for pregnant patients, children, and those with impaired renal function.
By serving as a first-line diagnostic tool, urinary tract ultrasonography can reduce reliance on CT, minimize radiation exposure, and support more judicious use of advanced imaging. The real-time nature of urinary tract ultrasonography also allows for immediate bedside application in point-of-care settings, facilitating faster diagnoses and more efficient care delivery.
Enhancing Healthcare Team Outcomes
Ultrasound of the urinary tract is a powerful diagnostic tool that supports timely, patient-centered decision-making across a broad range of clinical settings. The safety, accessibility, and effectiveness of urinary tract ultrasound make it an ideal first-line modality for evaluating flank pain, hematuria, urinary retention, and suspected urinary tract obstruction. However, the effective use of ultrasound depends not only on the technical skills of individual clinicians but also on the strength of interprofessional collaboration among the healthcare team.
Competent performance and interpretation of urinary tract ultrasound require the coordinated effort of physicians, advanced practice providers, nurses, sonographers, radiologists, and allied healthcare professionals. Each team member plays a vital role in delivering accurate and efficient care. Clinicians and advanced practitioners must develop strong ultrasound acquisition and interpretation skills or collaborate closely with imaging specialists. Nurses contribute to patient preparation, positioning, and comfort, while also ensuring continuity of care before and after imaging. Radiologists and sonographers bring advanced expertise in identifying subtle or incidental findings, helping to distinguish clinically relevant pathology from benign variants such as parapelvic cysts or extrarenal pelvis.
Effective interprofessional communication ensures that ultrasound findings are contextualized appropriately and followed by timely management decisions. For example, incidental findings—such as renal cysts or bladder wall irregularities—often require nuanced judgment and shared decision-making between emergency physicians, radiologists, urologists, and primary care providers to avoid unnecessary testing while ensuring patient safety.
The adoption of POCUS dramatically expands immediate access to diagnostic imaging, especially in time-sensitive situations or underserved environments. When integrated into structured workflows, POCUS allows for faster diagnoses, reduces length of stay, limits exposure to ionizing radiation, and enhances the overall efficiency of care delivery.[15]
By fostering a team-based culture, encouraging cross-disciplinary training, and prioritizing shared accountability, the healthcare team can ensure that ultrasound of the urinary tract is applied effectively and ethically. This interprofessional approach leads to more accurate diagnoses, safer imaging practices, improved patient satisfaction, and better clinical outcomes across diverse healthcare environments.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
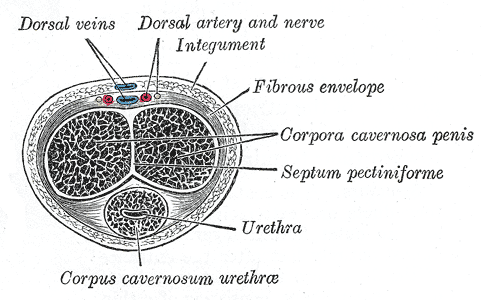
Transverse Section of the Penis showing Corpus Cavernosum Urethrae, Urethra, Septum Pectiniforme, Corpora Cavernosa Penis, and Fibrous Envelope
Henry Vandyke Carter, Public Domain, via Wikimedia Commons
(Click Image to Enlarge)
Median Sagittal Section of the Male Pelvis. This illustration shows the anatomic relationships between the symphysis pubis, sacrum, coccyx, retrovesical pouch (retrovesical excavation), ejaculatory duct, ductus deferens, bladder, penis, scrotum, ureter, urethra, scrotum, bulb, anal canal, and external urethral orifice.
Henry Vandyke Carter, Public Domain, via Wikimedia Commons
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Video to Play)
Nephrolithiasis, Ultrasound. The image shows a normal kidney with grades of hydronephrosis, twinkle artifact, and ureterovesical junction stone. UVJ, ureterovesical junction.
Contributed by MK Herbst, MD
(Click Image to Enlarge)
References
Nuraj P, Hyseni N. The Diagnosis of Obstructive Hydronephrosis with Color Doppler Ultrasound. Acta informatica medica : AIM : journal of the Society for Medical Informatics of Bosnia & Herzegovina : casopis Drustva za medicinsku informatiku BiH. 2017 Sep:25(3):178-181. doi: 10.5455/aim.2017.25.178-181. Epub [PubMed PMID: 29114110]
Orso D, Peric D, Di Gioia CC, Comisso I, Bove T, Ban A, Fonda F, Federici N. Renal and Genitourinary Ultrasound Evaluation in Emergency and Critical Care: An Overview. Healthcare (Basel, Switzerland). 2024 Jul 7:12(13):. doi: 10.3390/healthcare12131356. Epub 2024 Jul 7 [PubMed PMID: 38998890]
Level 3 (low-level) evidenceMoslemi MK, Mahfoozi B. Urologist-operated ultrasound and its use in urological outpatient clinics. Patient preference and adherence. 2011 Jan 24:5():85-8. doi: 10.2147/PPA.S17132. Epub 2011 Jan 24 [PubMed PMID: 21423592]
Kim DJ, Bell CR, Sheppard G. Genitourinary Ultrasound. Emergency medicine clinics of North America. 2024 Nov:42(4):819-838. doi: 10.1016/j.emc.2024.05.007. Epub 2024 Aug 17 [PubMed PMID: 39326990]
Wong C, Teitge B, Ross M, Young P, Robertson HL, Lang E. The Accuracy and Prognostic Value of Point-of-care Ultrasound for Nephrolithiasis in the Emergency Department: A Systematic Review and Meta-analysis. Academic emergency medicine : official journal of the Society for Academic Emergency Medicine. 2018 Jun:25(6):684-698. doi: 10.1111/acem.13388. Epub 2018 Mar 25 [PubMed PMID: 29427476]
Level 1 (high-level) evidenceVijayakumar V, Rama Krishnan KK, Bala P, S V, T P, Das P. Decoding Acute Scrotum: Diagnostic Accuracy of Ultrasound in Urgent Clinical Settings. Cureus. 2024 Oct:16(10):e71011. doi: 10.7759/cureus.71011. Epub 2024 Oct 7 [PubMed PMID: 39507162]
Borowy CS, Mukhdomi T. Sonography Physical Principles And Instrumentation. StatPearls. 2025 Jan:(): [PubMed PMID: 33620787]
Meola M, Ibeas J, Lasalle G, Petrucci I. Basics for performing a high-quality color Doppler sonography of the vascular access. The journal of vascular access. 2021 Nov:22(1_suppl):18-31. doi: 10.1177/11297298211018060. Epub 2021 Jul 28 [PubMed PMID: 34320855]
Level 2 (mid-level) evidenceOdak M, De Jesus O. Vascular Technology Color Flow Imaging. StatPearls. 2025 Jan:(): [PubMed PMID: 33232016]
Russ MK, Lafata NM, Robertson SH, Samei E. Pulsed wave Doppler ultrasound: Accuracy, variability, and impact of acquisition parameters on flow measurements. Medical physics. 2023 Nov:50(11):6704-6713. doi: 10.1002/mp.16774. Epub 2023 Oct 4 [PubMed PMID: 37793117]
Tafti D, Singh V, Cheung ME, Firstenberg MS. Duplex Ultrasound. StatPearls. 2025 Jan:(): [PubMed PMID: 29083769]
Alshoabi SA. Association between grades of Hydronephrosis and detection of urinary stones by ultrasound imaging. Pakistan journal of medical sciences. 2018 Jul-Aug:34(4):955-958. doi: 10.12669/pjms.344.14602. Epub [PubMed PMID: 30190760]
Sayed AO, Zeidan NS, Fahmy DM, Ibrahim HA. Diagnostic reliability of pediatric appendicitis score, ultrasound and low-dose computed tomography scan in children with suspected acute appendicitis. Therapeutics and clinical risk management. 2017:13():847-854. doi: 10.2147/TCRM.S134153. Epub 2017 Jul 6 [PubMed PMID: 28740395]
Hansen KL, Nielsen MB, Ewertsen C. Ultrasonography of the Kidney: A Pictorial Review. Diagnostics (Basel, Switzerland). 2015 Dec 23:6(1):. doi: 10.3390/diagnostics6010002. Epub 2015 Dec 23 [PubMed PMID: 26838799]
Roy-Burman P, Zhang K, Smallwood N. Point-of-Care Ultrasound of the Urinary Tract. The Medical clinics of North America. 2025 Jan:109(1):47-62. doi: 10.1016/j.mcna.2024.07.007. Epub 2024 Oct 15 [PubMed PMID: 39567103]
Herbst MK, Rosenberg G, Daniels B, Gross CP, Singh D, Molinaro AM, Luty S, Moore CL. Effect of provider experience on clinician-performed ultrasonography for hydronephrosis in patients with suspected renal colic. Annals of emergency medicine. 2014 Sep:64(3):269-76. doi: 10.1016/j.annemergmed.2014.01.012. Epub 2014 Mar 11 [PubMed PMID: 24630203]
Level 2 (mid-level) evidenceMegha R, Wehrle CJ, Kashyap S, Leslie SW. Anatomy, Abdomen and Pelvis: Adrenal Glands (Suprarenal Glands). StatPearls. 2025 Jan:(): [PubMed PMID: 29489211]
Dutt M, Wehrle CJ, Jialal I. Physiology, Adrenal Gland. StatPearls. 2025 Jan:(): [PubMed PMID: 30725945]
Scott JH, Menouar MA, Dunn RJ. Physiology, Aldosterone. StatPearls. 2025 Jan:(): [PubMed PMID: 29261963]
Chourpiliadis C, Aeddula NR. Physiology, Glucocorticoids. StatPearls. 2025 Jan:(): [PubMed PMID: 32809732]
Soriano RM, Penfold D, Leslie SW. Anatomy, Abdomen and Pelvis: Kidneys. StatPearls. 2025 Jan:(): [PubMed PMID: 29494007]
Trivedi S, Sharma U, Rathore M, John MR. Comprehensive Study of Arrangement of Renal Hilar Structures and Branching Pattern of Segmental Renal Arteries: An Anatomical Study. Cureus. 2023 Jul:15(7):e42165. doi: 10.7759/cureus.42165. Epub 2023 Jul 19 [PubMed PMID: 37602117]
Taus PJ, Manivannan S, Dancel R. Bedside Assessment of the Kidneys and Bladder Using Point of Care Ultrasound. POCUS journal. 2022:7(Kidney):94-104. doi: 10.24908/pocus.v7iKidney.15347. Epub 2022 Feb 1 [PubMed PMID: 36896106]
Koratala A, Bhattacharya D, Kazory A. Point of care renal ultrasonography for the busy nephrologist: A pictorial review. World journal of nephrology. 2019 Jun 28:8(3):44-58. doi: 10.5527/wjn.v8.i3.44. Epub [PubMed PMID: 31363461]
Niyyar VD, O'Neill WC. Point-of-care ultrasound in the practice of nephrology. Kidney international. 2018 May:93(5):1052-1059. doi: 10.1016/j.kint.2017.11.032. Epub 2018 Feb 22 [PubMed PMID: 29477241]
Sam P, LaGrange CA. Anatomy, Abdomen and Pelvis, Penis. StatPearls. 2025 Jan:(): [PubMed PMID: 29489230]
Fernandes MAV, de Souza LRMF, Cartafina LP. Ultrasound evaluation of the penis. Radiologia brasileira. 2018 Jul-Aug:51(4):257-261. doi: 10.1590/0100-3984.2016.0152. Epub [PubMed PMID: 30202130]
Garcia RA, Sajjad H. Anatomy, Abdomen and Pelvis, Scrotum. StatPearls. 2025 Jan:(): [PubMed PMID: 31751083]
Huzaifa M, Moreno MA. Hydrocele. StatPearls. 2025 Jan:(): [PubMed PMID: 32644551]
de Bessa J Jr, Dénes FT, Chammas MC, Cerri L, Monteiro ED, Buchpiguel CA, Cerri GG, Srougi M. Diagnostic accuracy of color Doppler sonographic study of the ureteric jets in evaluation of hydronephrosis. Journal of pediatric urology. 2008 Apr:4(2):113-7. doi: 10.1016/j.jpurol.2007.10.013. Epub 2008 Jan 14 [PubMed PMID: 18631905]
American Institute of Ultrasound in Medicine, American Urological Association. AIUM practice guideline for the performance of an ultrasound examination in the practice of urology. Journal of ultrasound in medicine : official journal of the American Institute of Ultrasound in Medicine. 2012 Jan:31(1):133-44 [PubMed PMID: 22215782]
Level 1 (high-level) evidenceHakenberg OW, Linne C, Manseck A, Wirth MP. Bladder wall thickness in normal adults and men with mild lower urinary tract symptoms and benign prostatic enlargement. Neurourology and urodynamics. 2000:19(5):585-93 [PubMed PMID: 11002301]
Franco G, De Nunzio C, Leonardo C, Tubaro A, Ciccariello M, De Dominicis C, Miano L, Laurenti C. Ultrasound assessment of intravesical prostatic protrusion and detrusor wall thickness--new standards for noninvasive bladder outlet obstruction diagnosis? The Journal of urology. 2010 Jun:183(6):2270-4. doi: 10.1016/j.juro.2010.02.019. Epub 2010 Apr 18 [PubMed PMID: 20400138]
Aderotimi TS, Kraft JK. Ultrasound of the adrenal gland in children. Ultrasound (Leeds, England). 2021 Feb:29(1):48-56. doi: 10.1177/1742271X20951915. Epub 2020 Aug 20 [PubMed PMID: 33552228]
Słapa RZ, Jakubowski WS, Dobruch-Sobczak K, Kasperlik-Załuska AA. Standards of ultrasound imaging of the adrenal glands. Journal of ultrasonography. 2015 Dec:15(63):377-87. doi: 10.15557/JoU.2015.0035. Epub 2015 Dec 28 [PubMed PMID: 26807295]
Joseph R, Huber M, Leeson B, Leeson K. Ultrasound-guided Placement of a Foley Catheter Using a Hydrophilic Guide Wire. Clinical practice and cases in emergency medicine. 2018 May:2(2):143-146. doi: 10.5811/cpcem.2017.12.37045. Epub 2018 Mar 14 [PubMed PMID: 29849285]
Level 3 (low-level) evidenceKameda T, Murata Y, Fujita M, Isaka A. Transabdominal ultrasound-guided urethral catheterization with transrectal pressure. The Journal of emergency medicine. 2014 Feb:46(2):215-9. doi: 10.1016/j.jemermed.2013.08.072. Epub 2013 Nov 5 [PubMed PMID: 24199721]
Level 3 (low-level) evidenceLinder BJ, Bass EJ, Mostafid H, Boorjian SA. Guideline of guidelines: asymptomatic microscopic haematuria. BJU international. 2018 Feb:121(2):176-183. doi: 10.1111/bju.14016. Epub 2017 Nov 2 [PubMed PMID: 28921833]
Barocas DA, Boorjian SA, Alvarez RD, Downs TM, Gross CP, Hamilton BD, Kobashi KC, Lipman RR, Lotan Y, Ng CK, Nielsen ME, Peterson AC, Raman JD, Smith-Bindman R, Souter LH. Microhematuria: AUA/SUFU Guideline. The Journal of urology. 2020 Oct:204(4):778-786. doi: 10.1097/JU.0000000000001297. Epub 2020 Jul 23 [PubMed PMID: 32698717]
Leão LRS, Mussi TC, Yamauchi FI, Baroni RH. Common pitfalls in renal mass evaluation: a practical guide. Radiologia brasileira. 2019 Jul-Aug:52(4):254-261. doi: 10.1590/0100-3984.2018.0007. Epub [PubMed PMID: 31435088]
Leslie SW, Hamawy K, Saleem MO. Gross and Microscopic Hematuria. StatPearls. 2025 Jan:(): [PubMed PMID: 30480952]
Ozden E, Turgut AT, Turkolmez K, Resorlu B, Safak M. Effect of bladder carcinoma location on detection rates by ultrasonography and computed tomography. Urology. 2007 May:69(5):889-92 [PubMed PMID: 17482928]
Wilkins CJ, Sriprasad S, Sidhu PS. Colour Doppler ultrasound of the penis. Clinical radiology. 2003 Jul:58(7):514-23 [PubMed PMID: 12834634]
Di Serafino M, Acampora C, Iacobellis F, Schillirò ML, Borzelli A, Barbuto L, Oliva G, Pezzullo F, Segreto S, Vallone G, Romano L. Ultrasound of scrotal and penile emergency: how, why and when. Journal of ultrasound. 2021 Jun:24(2):211-226. doi: 10.1007/s40477-020-00500-8. Epub 2020 Jul 11 [PubMed PMID: 32654040]
Flores JM, West M, Mulhall JP. Efficient use of penile Doppler ultrasound for investigating men with erectile dysfunction. The journal of sexual medicine. 2024 Aug 1:21(8):734-739. doi: 10.1093/jsxmed/qdae070. Epub [PubMed PMID: 39091226]
Elgendi K, Zulia N, Beilan J. A Review on Penile Doppler and Ultrasonography for Erectile Dysfunction. Current urology reports. 2023 Feb:24(2):69-74. doi: 10.1007/s11934-022-01135-4. Epub 2022 Nov 22 [PubMed PMID: 36417045]
Varela CG, Yeguas LAM, Rodríguez IC, Vila MDD. Penile Doppler Ultrasound for Erectile Dysfunction: Technique and Interpretation. AJR. American journal of roentgenology. 2020 May:214(5):1112-1121. doi: 10.2214/AJR.19.22141. Epub 2020 Jan 28 [PubMed PMID: 31990215]
Mazziotti S, Cicero G, D'Angelo T, Marino MA, Visalli C, Salamone I, Ascenti G, Blandino A. Imaging and Management of Incidental Renal Lesions. BioMed research international. 2017:2017():1854027. doi: 10.1155/2017/1854027. Epub 2017 May 31 [PubMed PMID: 28642870]
Whelan TF. Guidelines on the management of renal cyst disease. Canadian Urological Association journal = Journal de l'Association des urologues du Canada. 2010 Apr:4(2):98-9 [PubMed PMID: 20368890]
Leveridge MJ, Bostrom PJ, Koulouris G, Finelli A, Lawrentschuk N. Imaging renal cell carcinoma with ultrasonography, CT and MRI. Nature reviews. Urology. 2010 Jun:7(6):311-25. doi: 10.1038/nrurol.2010.63. Epub 2010 May 18 [PubMed PMID: 20479778]
King KG. Use of Contrast Ultrasound for Renal Mass Evaluation. Radiologic clinics of North America. 2020 Sep:58(5):935-949. doi: 10.1016/j.rcl.2020.05.002. Epub 2020 Jun 17 [PubMed PMID: 32792125]
Stakhovskyi O, Yap SA, Leveridge M, Lawrentschuk N, Jewett MA. Small renal mass: what the urologist needs to know for treatment planning and assessment of treatment results. AJR. American journal of roentgenology. 2011 Jun:196(6):1267-73. doi: 10.2214/AJR.10.6336. Epub [PubMed PMID: 21606288]
Ajmal S. Contrast-Enhanced Ultrasonography: Review and Applications. Cureus. 2021 Sep:13(9):e18243. doi: 10.7759/cureus.18243. Epub 2021 Sep 24 [PubMed PMID: 34712527]
Zhang W, Wang J, Chen L, Shi J. Qualitative and quantitative assessment of non-clear cell renal cell carcinoma using contrast-enhanced ultrasound. BMC urology. 2024 Jun 17:24(1):129. doi: 10.1186/s12894-024-01514-8. Epub 2024 Jun 17 [PubMed PMID: 38886684]
Level 2 (mid-level) evidenceLiu Y, Kan Y, Zhang J, Li N, Wang Y. Characteristics of contrast-enhanced ultrasound for diagnosis of solid clear cell renal cell carcinomas ≤4 cm: A meta-analysis. Cancer medicine. 2021 Dec:10(23):8288-8299. doi: 10.1002/cam4.4365. Epub 2021 Nov 1 [PubMed PMID: 34725960]
Level 1 (high-level) evidenceXue LY, Lu Q, Huang BJ, Ma JJ, Yan LX, Wen JX, Wang WP. Contrast-enhanced ultrasonography for evaluation of cystic renal mass: in comparison to contrast-enhanced CT and conventional ultrasound. Abdominal imaging. 2014 Dec:39(6):1274-83. doi: 10.1007/s00261-014-0171-4. Epub [PubMed PMID: 24929667]
Xiao XY, Chen X, Guan XF, Wu H, Qin W, Luo BM. Superb microvascular imaging in diagnosis of breast lesions: a comparative study with contrast-enhanced ultrasonographic microvascular imaging. The British journal of radiology. 2016 Oct:89(1066):20160546. doi: 10.1259/bjr.20160546. Epub 2016 Aug 16 [PubMed PMID: 27529640]
Level 2 (mid-level) evidenceMao Y, Mu J, Zhao J, Zhao L, Xin X. The value of superb microvascular imaging in differentiating benign renal mass from malignant renal tumor: a retrospective study. The British journal of radiology. 2018 Feb:91(1082):20170601. doi: 10.1259/bjr.20170601. Epub 2017 Nov 28 [PubMed PMID: 29125337]
Level 2 (mid-level) evidenceRoussel E, Campi R, Amparore D, Bertolo R, Carbonara U, Erdem S, Ingels A, Kara Ö, Marandino L, Marchioni M, Muselaers S, Pavan N, Pecoraro A, Beuselinck B, Pedrosa I, Fetzer D, Albersen M, On Behalf Of The European Association Of Urology Eau Young Academic Urologists Yau Renal Cancer Working Group. Expanding the Role of Ultrasound for the Characterization of Renal Masses. Journal of clinical medicine. 2022 Feb 19:11(4):. doi: 10.3390/jcm11041112. Epub 2022 Feb 19 [PubMed PMID: 35207384]
Leong JY, Wessner CE, Kramer MR, Forsberg F, Halpern EJ, Lyshchik A, Torkzaban M, Morris A, Byrne K, VanMeter M, Trabulsi EJ, Lallas CD, Eisenbrey JR. Superb Microvascular Imaging Improves Detection of Vascularity in Indeterminate Renal Masses. Journal of ultrasound in medicine : official journal of the American Institute of Ultrasound in Medicine. 2020 Oct:39(10):1947-1955. doi: 10.1002/jum.15299. Epub 2020 Apr 20 [PubMed PMID: 32309889]
Gürbüz AF, Keven A, Elmalı A, Toru S, Apaydın A, Çeken K. A comparison between the superb microvascular imaging technique and conventional Doppler ultrasound in evaluating chronic allograft damage in renal transplant recipients. Diagnostic and interventional radiology (Ankara, Turkey). 2023 Mar 29:29(2):212-218. doi: 10.5152/dir.2022.21555. Epub 2023 Jan 27 [PubMed PMID: 36960635]
Tublin ME, Bude RO, Platt JF. Review. The resistive index in renal Doppler sonography: where do we stand? AJR. American journal of roentgenology. 2003 Apr:180(4):885-92 [PubMed PMID: 12646425]
Granata A, Andrulli S, Bigi MQ, Pozzoni P, Fiorini F, Logias F, Figuera M, Basile A, Fiore CE. Predictive role of duplex Doppler ultrasonography in the diagnosis of acute renal obstruction in patients with unilateral renal colic. Clinical nephrology. 2009 Jun:71(6):680-6 [PubMed PMID: 19473637]
Haitsma Mulier JLG, Rozemeijer S, Röttgering JG, Spoelstra-de Man AME, Elbers PWG, Tuinman PR, de Waard MC, Oudemans-van Straaten HM. Renal resistive index as an early predictor and discriminator of acute kidney injury in critically ill patients; A prospective observational cohort study. PloS one. 2018:13(6):e0197967. doi: 10.1371/journal.pone.0197967. Epub 2018 Jun 11 [PubMed PMID: 29889830]
Darabont R, Mihalcea D, Vinereanu D. Current Insights into the Significance of the Renal Resistive Index in Kidney and Cardiovascular Disease. Diagnostics (Basel, Switzerland). 2023 May 10:13(10):. doi: 10.3390/diagnostics13101687. Epub 2023 May 10 [PubMed PMID: 37238172]
Platt JF, Rubin JM, Ellis JH. Distinction between obstructive and nonobstructive pyelocaliectasis with duplex Doppler sonography. AJR. American journal of roentgenology. 1989 Nov:153(5):997-1000 [PubMed PMID: 2679004]
Kavakli HS, Koktener A, Yilmaz A. Diagnostic value of renal resistive index for the assessment of renal colic. Singapore medical journal. 2011 Apr:52(4):271-3 [PubMed PMID: 21552789]
Geavlete P, Georgescu D, Cauni V, Niţa G. Value of duplex Doppler ultrasonography in renal colic. European urology. 2002 Jan:41(1):71-8 [PubMed PMID: 11999470]
Piazzese EM, Mazzeo GI, Galipò S, Fiumara F, Canfora C, Angiò LG. The renal resistive index as a predictor of acute hydronephrosis in patients with renal colic. Journal of ultrasound. 2012 Dec:15(4):239-46. doi: 10.1016/j.jus.2012.10.003. Epub 2012 Oct 14 [PubMed PMID: 23730388]
Sayani R, Ali M, Shazlee K, Hamid RS, Hamid K. Functional evaluation of the urinary tract by duplex Doppler ultrasonography in patients with acute renal colic. International journal of nephrology and renovascular disease. 2012:5():15-21. doi: 10.2147/IJNRD.S27628. Epub 2011 Dec 30 [PubMed PMID: 22334796]
Ryan PC, Maher KP, Murphy B, Hurley GD, Fitzpatrick JM. Experimental partial ureteric obstruction: pathophysiological changes in upper tract pressures and renal blood flow. The Journal of urology. 1987 Sep:138(3):674-8 [PubMed PMID: 3625876]
Klahr S. Pathophysiology of obstructive nephropathy: a 1991 update. Seminars in nephrology. 1991 Mar:11(2):156-68 [PubMed PMID: 2034924]
Shokeir AA, Mahran MR, Abdulmaaboud M. Renal colic in pregnant women: role of renal resistive index. Urology. 2000 Mar:55(3):344-7 [PubMed PMID: 10699607]
Atar M, Bozkurt Y, Soylemez H, Penbegul N, Sancaktutar AA, Bodakci MN, Hatipoglu NK, Hamidi C, Ozler A. Use of renal resistive index and semi-rigid ureteroscopy for managing symptomatic persistent hydronephrosis during pregnancy. International journal of surgery (London, England). 2012:10(10):629-33. doi: 10.1016/j.ijsu.2012.10.006. Epub 2012 Nov 13 [PubMed PMID: 23159361]
Simonsen JA, Graumann O, Toft A, Henriques CU, Walter S. [Diagnosis and treatment of symptomatic hydronephrosis in pregnancy]. Ugeskrift for laeger. 2015 Sep 14:177(38):V06140360 [PubMed PMID: 26376416]
Wu H, Liu K, Darko IN, Xu X, Li L, Xing C, Mao H. Predictive value of renal resistive index for the onset of acute kidney injury and its non-recovery: A systematic review and meta-analysis . Clinical nephrology. 2020 Apr:93(4):172-186. doi: 10.5414/CN109979. Epub [PubMed PMID: 32017700]
Level 1 (high-level) evidenceWei Q, Zhu Y, Zhen W, Zhang X, Shi Z, Zhang L, Zhou J. Performance of resistive index and semi-quantitative power doppler ultrasound score in predicting acute kidney injury: A meta-analysis of prospective studies. PloS one. 2022:17(6):e0270623. doi: 10.1371/journal.pone.0270623. Epub 2022 Jun 28 [PubMed PMID: 35763514]
Level 1 (high-level) evidencePlatt JF, Ellis JH, Rubin JM. Renal transplant pyelocaliectasis: role of duplex Doppler US in evaluation. Radiology. 1991 May:179(2):425-8 [PubMed PMID: 2014285]
Platt JF, Rubin JM, Ellis JH, DiPietro MA. Duplex Doppler US of the kidney: differentiation of obstructive from nonobstructive dilatation. Radiology. 1989 May:171(2):515-7 [PubMed PMID: 2649925]
Schwenger V, Hinkel UP, Nahm AM, Morath C, Zeier M. Color doppler ultrasonography in the diagnostic evaluation of renal allografts. Nephron. Clinical practice. 2006:104(3):c107-12 [PubMed PMID: 16837783]
Imankulov S, Doskali M, Oskenbaeva K, Ibadildina A, Baigenzhin A, Doskaliyev Z. Evaluation of Kidney Allograft in the Early Posttransplant Period Using Ultrasonography. Experimental and clinical transplantation : official journal of the Middle East Society for Organ Transplantation. 2015 Nov:13 Suppl 3():62-5 [PubMed PMID: 26640915]
Viazzi F, Leoncini G, Derchi LE, Pontremoli R. Ultrasound Doppler renal resistive index: a useful tool for the management of the hypertensive patient. Journal of hypertension. 2014 Jan:32(1):149-53. doi: 10.1097/HJH.0b013e328365b29c. Epub [PubMed PMID: 24172238]
Barić D. Why pulsatility still matters: a review of current knowledge. Croatian medical journal. 2014 Dec:55(6):609-20 [PubMed PMID: 25559832]
Petersen LJ, Petersen JR, Talleruphuus U, Ladefoged SD, Mehlsen J, Jensen HA. The pulsatility index and the resistive index in renal arteries. Associations with long-term progression in chronic renal failure. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 1997 Jul:12(7):1376-80 [PubMed PMID: 9249772]
Langan RC, Puente MEE. Scrotal Masses. American family physician. 2022 Aug:106(2):184-189 [PubMed PMID: 35977130]
Kühn AL, Scortegagna E, Nowitzki KM, Kim YH. Ultrasonography of the scrotum in adults. Ultrasonography (Seoul, Korea). 2016 Jul:35(3):180-97. doi: 10.14366/usg.15075. Epub 2016 Feb 24 [PubMed PMID: 26983766]
Alexander CE, Warren H, Light A, Agarwal R, Asif A, Chow BJ, Clement K, Chan V, Zimmermann E, Khadhouri S, Eyskens PJ, Shah TT, Nathan A, Byrnes K, Bhatt N, Mani N, Yuhong Yuan C, Sidhu PS, Takwoingi Y, Kasivisvanathan V. Ultrasound for the Diagnosis of Testicular Torsion: A Systematic Review and Meta-analysis of Diagnostic Accuracy. European urology focus. 2025 May 13:():. pii: S2405-4569(25)00104-X. doi: 10.1016/j.euf.2025.04.026. Epub 2025 May 13 [PubMed PMID: 40368720]
Level 1 (high-level) evidenceDahlgren N, Sundström G, Wagenius M, Navntoft A, Nilsson C. A validation of ultrasound as a diagnostic tool for the detection of testicular torsion. Acta radiologica (Stockholm, Sweden : 1987). 2025 Aug:66(8):878-884. doi: 10.1177/02841851251331560. Epub 2025 Apr 15 [PubMed PMID: 40232242]
Level 1 (high-level) evidenceHodler J, Kubik-Huch RA, von Schulthess GK, Wibmer AG, Vargas HA. Imaging of Testicular and Scrotal Masses: The Essentials. Diseases of the Abdomen and Pelvis 2018-2021: Diagnostic Imaging - IDKD Book. 2018:(): [PubMed PMID: 31314374]
Mirochnik B, Bhargava P, Dighe MK, Kanth N. Ultrasound evaluation of scrotal pathology. Radiologic clinics of North America. 2012 Mar:50(2):317-32, vi. doi: 10.1016/j.rcl.2012.02.005. Epub 2012 Mar 3 [PubMed PMID: 22498445]
Muttarak M, Chaiwun B. Painless scrotal swelling: ultrasonographical features with pathological correlation. Singapore medical journal. 2005 Apr:46(4):196-201; quiz 202 [PubMed PMID: 15800728]
Philips S, Nagar A, Dighe M, Vikram R, Sunnapwar A, Prasad S. Benign non-cystic scrotal tumors and pseudotumors. Acta radiologica (Stockholm, Sweden : 1987). 2012 Feb 1:53(1):102-11. doi: 10.1258/ar.2011.110185. Epub 2011 Oct 24 [PubMed PMID: 22025740]
Sommers D, Winter T. Ultrasonography evaluation of scrotal masses. Radiologic clinics of North America. 2014 Nov:52(6):1265-81. doi: 10.1016/j.rcl.2014.07.014. Epub 2014 Aug 29 [PubMed PMID: 25444105]
Smith-Bindman R, Aubin C, Bailitz J, Bengiamin RN, Camargo CA Jr, Corbo J, Dean AJ, Goldstein RB, Griffey RT, Jay GD, Kang TL, Kriesel DR, Ma OJ, Mallin M, Manson W, Melnikow J, Miglioretti DL, Miller SK, Mills LD, Miner JR, Moghadassi M, Noble VE, Press GM, Stoller ML, Valencia VE, Wang J, Wang RC, Cummings SR. Ultrasonography versus computed tomography for suspected nephrolithiasis. The New England journal of medicine. 2014 Sep 18:371(12):1100-10. doi: 10.1056/NEJMoa1404446. Epub [PubMed PMID: 25229916]
Level 2 (mid-level) evidenceLeo MM, Langlois BK, Pare JR, Mitchell P, Linden J, Nelson KP, Amanti C, Carmody KA. Ultrasound vs. Computed Tomography for Severity of Hydronephrosis and Its Importance in Renal Colic. The western journal of emergency medicine. 2017 Jun:18(4):559-568. doi: 10.5811/westjem.2017.04.33119. Epub 2017 May 15 [PubMed PMID: 28611874]
Türk C, Petřík A, Sarica K, Seitz C, Skolarikos A, Straub M, Knoll T. EAU Guidelines on Diagnosis and Conservative Management of Urolithiasis. European urology. 2016 Mar:69(3):468-74. doi: 10.1016/j.eururo.2015.07.040. Epub 2015 Aug 28 [PubMed PMID: 26318710]
Thotakura R, Anjum F. Hydronephrosis and Hydroureter. StatPearls. 2025 Jan:(): [PubMed PMID: 33085364]
Rishor-Olney CR, Hinson MR. Obstructive Uropathy. StatPearls. 2025 Jan:(): [PubMed PMID: 32644347]
Ntoulia A, Aguirre Pascual E, Back SJ, Bellah RD, Beltrán Salazar VP, Chan PKJ, Chow JS, Coca Robinot D, Darge K, Duran C, Epelman M, Ključevšek D, Kwon JK, Sandhu PK, Woźniak MM, Papadopoulou F. Contrast-enhanced voiding urosonography, part 1: vesicoureteral reflux evaluation. Pediatric radiology. 2021 Nov:51(12):2351-2367. doi: 10.1007/s00247-020-04906-8. Epub 2021 Mar 31 [PubMed PMID: 33787945]
Ntoulia A, Back SJ, Shellikeri S, Poznick L, Morgan T, Kerwood J, Christopher Edgar J, Bellah RD, Reid JR, Jaramillo D, Canning DA, Darge K. Contrast-enhanced voiding urosonography (ceVUS) with the intravesical administration of the ultrasound contrast agent Optison™ for vesicoureteral reflux detection in children: a prospective clinical trial. Pediatric radiology. 2018 Feb:48(2):216-226. doi: 10.1007/s00247-017-4026-3. Epub 2017 Nov 27 [PubMed PMID: 29181582]
Sofia C, Solazzo A, Cattafi A, Chimenz R, Cicero G, Marino MA, D'angelo T, Manti L, Condorelli E, Ceravolo G, Mazziotti S, Ascenti G. Contrast-enhanced voiding urosonography in the assessment of vesical-ureteral reflux: the time has come. La Radiologia medica. 2021 Jul:126(7):901-909. doi: 10.1007/s11547-021-01360-w. Epub 2021 May 5 [PubMed PMID: 33954899]
Ye ZL, Zhang LH, Zhu L, Chen WJ, Xu D, Lin N. Application of contrast-enhanced ultrasound in the surgical treatment of vesicoureteral reflux in children. Pediatric surgery international. 2023 Nov 25:40(1):10. doi: 10.1007/s00383-023-05605-9. Epub 2023 Nov 25 [PubMed PMID: 38006461]
Yousefifard M, Toloui A, Rafiei Alavi SN, Madani Neishaboori A, Ahmadzadeh K, Ghelichkhani P, Safari S, Abbasi A, Ataei N, Hosseini M. Contrast-enhanced voiding urosonography, a possible candidate for the diagnosis of vesicoureteral reflux in children and adolescents; a systematic review and meta-analysis. Journal of pediatric urology. 2022 Feb:18(1):61-74. doi: 10.1016/j.jpurol.2021.10.023. Epub 2021 Nov 3 [PubMed PMID: 34801413]
Level 1 (high-level) evidenceRubelj K, Oletić L, Valent Morić B, Trutin I. OUR EXPERIENCE WITH CONTRAST-ENHANCED VOIDING UROSONOGRAPHY. Acta clinica Croatica. 2021 Jun:60(2):184-190. doi: 10.20471/acc.2021.60.02.03. Epub [PubMed PMID: 34744267]
Velasquez M, Emerson MG, Diaz E, Kennedy W, Rubesova E, Barth RA. The learning curve of contrast-enhanced 'microbubble' voiding urosonography-validation study. Journal of pediatric urology. 2019 Aug:15(4):385.e1-385.e6. doi: 10.1016/j.jpurol.2019.04.015. Epub 2019 Apr 19 [PubMed PMID: 31133505]
Level 1 (high-level) evidenceKim D, Choi YH, Choi G, Lee S, Lee S, Cho YJ, Lim SH, Kang HG, Cheon JE. Contrast-enhanced voiding urosonography for the diagnosis of vesicoureteral reflux and intrarenal reflux: a comparison of diagnostic performance with fluoroscopic voiding cystourethrography. Ultrasonography (Seoul, Korea). 2021 Oct:40(4):530-537. doi: 10.14366/usg.20157. Epub 2021 Feb 10 [PubMed PMID: 33887876]
Hu S, Tian Y, He M. Determining the optimal contrast-enhanced voiding urosonography technique for vesicoureteral reflux in children and adolescents: a systematic review and network meta-analysis. Frontiers in pediatrics. 2025:13():1472382. doi: 10.3389/fped.2025.1472382. Epub 2025 Apr 25 [PubMed PMID: 40352609]
Level 1 (high-level) evidenceDarge K. Voiding urosonography with US contrast agent for the diagnosis of vesicoureteric reflux in children: an update. Pediatric radiology. 2010 Jun:40(6):956-62. doi: 10.1007/s00247-010-1623-9. Epub 2010 Apr 30 [PubMed PMID: 20432014]
Barnewolt CE, Acharya PT, Aguirre Pascual E, Back SJ, Beltrán Salazar VP, Chan PKJ, Chow JS, Coca Robinot D, Darge K, Duran C, Ključevšek D, Kwon JK, Ntoulia A, Papadopoulou F, Woźniak MM, Piskunowicz M. Contrast-enhanced voiding urosonography part 2: urethral imaging. Pediatric radiology. 2021 Nov:51(12):2368-2386. doi: 10.1007/s00247-021-05116-6. Epub 2021 Aug 13 [PubMed PMID: 34386854]
Kapral N, Kern NG, Corbett ST, Leroy SV, Daugherty RJ. Viability of contrast-enhanced voiding urosonography as an alternative to fluoroscopy during video urodynamics. Pediatric radiology. 2023 Jul:53(8):1713-1719. doi: 10.1007/s00247-023-05619-4. Epub 2023 Mar 7 [PubMed PMID: 36879049]
Kennady E, Hutchison D, Corbett ST, Leroy S, Morgan K, Daugherty R, Kapral N, Kern NG. Novel use of contrast enhanced ultrasonography with urodynamics in children: A comparison study to fluoroscopy. Neurourology and urodynamics. 2024 Mar:43(3):711-718. doi: 10.1002/nau.25422. Epub 2024 Feb 14 [PubMed PMID: 38356366]
Benya EC. Contrast-enhanced voiding urosonography for video urodynamics: feasible but not perfect. Pediatric radiology. 2023 Jul:53(8):1720-1721. doi: 10.1007/s00247-023-05634-5. Epub 2023 Mar 3 [PubMed PMID: 36867205]
Roic AC, Milošević D, Turudić D, Roic G. An innovative diagnostic procedure in children: videourodynamics with contrast-enhanced voiding urosonography. Journal of ultrasound. 2023 Jun:26(2):583-587. doi: 10.1007/s40477-022-00721-z. Epub 2022 Nov 22 [PubMed PMID: 36417175]
Koratala A, Bhattacharya D. Extrarenal pelvis mimicking hydronephrosis: a case for caution. Clinical case reports. 2017 Oct:5(10):1720-1721. doi: 10.1002/ccr3.1143. Epub 2017 Aug 24 [PubMed PMID: 29026582]
Level 3 (low-level) evidenceTrunz LM, Balasubramanya R. Doppler Renal Assessment, Protocols, and Interpretation. StatPearls. 2025 Jan:(): [PubMed PMID: 34283501]
Apoku IN, Ayoola OO, Salako AA, Idowu BM. Ultrasound evaluation of obstructive uropathy and its hemodynamic responses in southwest Nigeria. International braz j urol : official journal of the Brazilian Society of Urology. 2015 May-Jun:41(3):556-61. doi: 10.1590/S1677-5538.IBJU.2014.0197. Epub [PubMed PMID: 26200551]
Koratala A, Alquadan KF. Parapelvic cysts mimicking hydronephrosis. Clinical case reports. 2018 Apr:6(4):760-761. doi: 10.1002/ccr3.1431. Epub 2018 Feb 21 [PubMed PMID: 29636957]
Level 3 (low-level) evidenceEmamian SA, Nielsen MB, Pedersen JF, Ytte L. Sonographic evaluation of renal appearance in 665 adult volunteers. Correlation with age and obesity. Acta radiologica (Stockholm, Sweden : 1987). 1993 Sep:34(5):482-5 [PubMed PMID: 8369185]
Ballstaedt L, Leslie SW, Woodbury B. Bladder Post Void Residual Volume. StatPearls. 2025 Jan:(): [PubMed PMID: 30969661]
Dougherty JM, Leslie SW, Aeddula NR. Male Urinary Retention: Acute and Chronic. StatPearls. 2025 Jan:(): [PubMed PMID: 30860734]
Leslie SW, Rawla P, Dougherty JM. Female Urinary Retention. StatPearls. 2025 Jan:(): [PubMed PMID: 30860732]
Bih LI, Ho CC, Tsai SJ, Lai YC, Chow W. Bladder shape impact on the accuracy of ultrasonic estimation of bladder volume. Archives of physical medicine and rehabilitation. 1998 Dec:79(12):1553-6 [PubMed PMID: 9862299]
Kuzmić AC, Brkljacić B, Ivanković D. The impact of bladder shape on the ultrasonographic measurement of bladder volume in children. Pediatric radiology. 2003 Aug:33(8):530-4 [PubMed PMID: 12802537]
Nixon G, Blattner K, Muirhead J, Kerse N. Rural point-of-care ultrasound of the kidney and bladder: quality and effect on patient management. Journal of primary health care. 2018 Dec:10(4):324-330. doi: 10.1071/HC18034. Epub [PubMed PMID: 31039961]
Level 2 (mid-level) evidenceGhanem K, Leslie SW, Badreldin AM. Ureterocele. StatPearls. 2025 Jan:(): [PubMed PMID: 37983348]
Glazer K, Brea IJ, Leslie SW, Vaitla P. Ureterolithiasis. StatPearls. 2025 Jan:(): [PubMed PMID: 32809509]
Lescay HA, Jiang J, Leslie SW, Tuma F. Anatomy, Abdomen and Pelvis Ureter. StatPearls. 2025 Jan:(): [PubMed PMID: 30422575]
Ray AA, Ghiculete D, Pace KT, Honey RJ. Limitations to ultrasound in the detection and measurement of urinary tract calculi. Urology. 2010 Aug:76(2):295-300. doi: 10.1016/j.urology.2009.12.015. Epub 2010 Mar 5 [PubMed PMID: 20206970]
Hamamoto S, Inoue T, Okada S, Taguchi K, Yasui T. Application of ultrasound imaging in the treatment of urinary tract stones. Journal of medical ultrasonics (2001). 2023 Aug 12:():. doi: 10.1007/s10396-023-01343-6. Epub 2023 Aug 12 [PubMed PMID: 37572224]
Nabheerong P, Kengkla K, Saokaew S, Naravejsakul K. Diagnostic accuracy of Doppler twinkling artifact for identifying urolithiasis: a systematic review and meta-analysis. Journal of ultrasound. 2023 Jun:26(2):321-331. doi: 10.1007/s40477-022-00759-z. Epub 2023 Jan 27 [PubMed PMID: 36705851]
Level 1 (high-level) evidenceNicolau C, Claudon M, Derchi LE, Adam EJ, Nielsen MB, Mostbeck G, Owens CM, Nyhsen C, Yarmenitis S. Imaging patients with renal colic-consider ultrasound first. Insights into imaging. 2015 Aug:6(4):441-7. doi: 10.1007/s13244-015-0396-y. Epub 2015 May 21 [PubMed PMID: 25994497]
Abdel-Gawad M, Kadasne RD, Elsobky E, Ali-El-Dein B, Monga M. A Prospective Comparative Study of Color Doppler Ultrasound with Twinkling and Noncontrast Computerized Tomography for the Evaluation of Acute Renal Colic. The Journal of urology. 2016 Sep:196(3):757-62. doi: 10.1016/j.juro.2016.03.175. Epub 2016 Apr 8 [PubMed PMID: 27063853]
Level 2 (mid-level) evidencePuttmann K, Dajusta D, Rehfuss AW. Does twinkle artifact truly represent a kidney stone on renal ultrasound? Journal of pediatric urology. 2021 Aug:17(4):475.e1-475.e6. doi: 10.1016/j.jpurol.2021.03.026. Epub 2021 Mar 30 [PubMed PMID: 33867287]
Gliga ML, Chirila CN, Podeanu DM, Imola T, Voicu SL, Gliga MG, Gliga PM. Twinkle, twinkle little stone: an artifact improves the ultrasound performance! Medical ultrasonography. 2017 Jun 17:19(3):272-275. doi: 10.11152/mu-984. Epub [PubMed PMID: 28845492]
Bacha R, Manzoor I, Gilani SA, Khan AI. Clinical Significance of Twinkling Artifact in the Diagnosis of Urinary Stones. Ultrasound in medicine & biology. 2019 Dec:45(12):3199-3206. doi: 10.1016/j.ultrasmedbio.2019.08.015. Epub 2019 Sep 16 [PubMed PMID: 31537388]
Shrestha R, Shakya RM, Khan A A. Bedside Ultrasound in the Emergency Department to Detect Hydronephrosis for the Evaluation of Suspected Ureteric Colic. Kathmandu University medical journal (KUMJ). 2016 Apr-Jun:14(54):172-176 [PubMed PMID: 28166076]
Ganesan V, De S, Greene D, Torricelli FC, Monga M. Accuracy of ultrasonography for renal stone detection and size determination: is it good enough for management decisions? BJU international. 2017 Mar:119(3):464-469. doi: 10.1111/bju.13605. Epub 2016 Aug 17 [PubMed PMID: 27459091]
Alahmadi AE, Aljuhani FM, Alshoabi SA, Aloufi KM, Alsharif WM, Alamri AM. The gap between ultrasonography and computed tomography in measuring the size of urinary calculi. Journal of family medicine and primary care. 2020 Sep:9(9):4925-4928. doi: 10.4103/jfmpc.jfmpc_742_20. Epub 2020 Sep 30 [PubMed PMID: 33209823]
Brisbane W, Bailey MR, Sorensen MD. An overview of kidney stone imaging techniques. Nature reviews. Urology. 2016 Nov:13(11):654-662. doi: 10.1038/nrurol.2016.154. Epub 2016 Aug 31 [PubMed PMID: 27578040]
Level 3 (low-level) evidenceKrakhotkin DV, Chernylovskyi VA, Sarica K, Tsaturyan A, Liatsikos E, Makevicius J, Iglovikov NY, Pikhovkin DN. Diagnostic value ultrasound signs of stones less than or equal to 10 mm and clinico-radiological variants of ureteric colic. Asian journal of urology. 2023 Jan:10(1):39-49. doi: 10.1016/j.ajur.2022.03.015. Epub 2022 Nov 13 [PubMed PMID: 36721697]
Kaul I, Moore S, Barry E, Pareek G. Renal Imaging in Stone Disease: Which Modality to Choose? Rhode Island medical journal (2013). 2023 Dec 1:106(11):31-35 [PubMed PMID: 38015782]
Kanno T, Kubota M, Funada S, Okada T, Higashi Y, Yamada H. The Utility of the Kidneys-ureters-bladder Radiograph as the Sole Imaging Modality and Its Combination With Ultrasonography for the Detection of Renal Stones. Urology. 2017 Jun:104():40-44. doi: 10.1016/j.urology.2017.03.019. Epub 2017 Mar 21 [PubMed PMID: 28341578]
Mostbeck GH, Zontsich T, Turetschek K. Ultrasound of the kidney: obstruction and medical diseases. European radiology. 2001:11(10):1878-89 [PubMed PMID: 11702120]
Pepe P, Motta L, Pennisi M, Aragona F. Functional evaluation of the urinary tract by color-Doppler ultrasonography (CDU) in 100 patients with renal colic. European journal of radiology. 2005 Jan:53(1):131-5 [PubMed PMID: 15607864]
Delair SM, Kurzrock EA. Clinical utility of ureteral jets: disparate opinions. Journal of endourology. 2006 Feb:20(2):111-4 [PubMed PMID: 16509793]
Level 3 (low-level) evidenceWieczorek AP, Woźniak MM, Tyloch JF. Errors in the ultrasound diagnosis of the kidneys, ureters and urinary bladder. Journal of ultrasonography. 2013 Sep:13(54):308-18. doi: 10.15557/JoU.2013.0031. Epub 2013 Sep 30 [PubMed PMID: 26674139]
Tamai H, Takiguchi Y, Oka M, Shingaki N, Enomoto S, Shiraki T, Furuta M, Inoue I, Iguchi M, Yanaoka K, Arii K, Shimizu Y, Nakata H, Shinka T, Sanke T, Ichinose M. Contrast-enhanced ultrasonography in the diagnosis of solid renal tumors. Journal of ultrasound in medicine : official journal of the American Institute of Ultrasound in Medicine. 2005 Dec:24(12):1635-40 [PubMed PMID: 16301719]
Cè M, Felisaz PF, Alì M, Re Sartò GV, Cellina M. Ultrasound elastography in chronic kidney disease: a systematic review and meta-analysis. Journal of medical ultrasonics (2001). 2023 Jul:50(3):381-415. doi: 10.1007/s10396-023-01304-z. Epub 2023 Apr 25 [PubMed PMID: 37186192]
Level 1 (high-level) evidence. Ultrasound Guidelines: Emergency, Point-of-Care and Clinical Ultrasound Guidelines in Medicine. Annals of emergency medicine. 2017 May:69(5):e27-e54. doi: 10.1016/j.annemergmed.2016.08.457. Epub [PubMed PMID: 28442101]
Fan J, Tang J, Fang J, Li Q, He E, Li J, Wang Y. Ultrasound imaging in the diagnosis of benign and suspicious adrenal lesions. Medical science monitor : international medical journal of experimental and clinical research. 2014 Nov 3:20():2132-41. doi: 10.12659/MSM.890800. Epub 2014 Nov 3 [PubMed PMID: 25363391]
Słapa RZ, Kasperlik-Załuska AA, Migda B, Jakubowski WS. Shear wave elastography of adrenal masses is feasible and may help to differentiate between solid and cystic lesions - an initial report. Endokrynologia Polska. 2014:65(2):119-24. doi: 10.5603/EP.2014.0017. Epub [PubMed PMID: 24802735]
Dietrich CF, Ignee A, Barreiros AP, Schreiber-Dietrich D, Sienz M, Bojunga J, Braden B. Contrast-enhanced ultrasound for imaging of adrenal masses. Ultraschall in der Medizin (Stuttgart, Germany : 1980). 2010 Apr:31(2):163-8. doi: 10.1055/s-0028-1109357. Epub 2009 Apr 28 [PubMed PMID: 19401979]
Kim KW, Kim JK, Choi HJ, Kim MH, Lee J, Cho KS. Sonography of the adrenal glands in the adult. Journal of clinical ultrasound : JCU. 2012 Jul-Aug:40(6):357-63. doi: 10.1002/jcu.21947. Epub 2012 May 15 [PubMed PMID: 22585678]
Kok HK, Sherlock M, Healy NA, Doody O, Govender P, Torreggiani WC. Imaging features of poorly controlled congenital adrenal hyperplasia in adults. The British journal of radiology. 2015 Sep:88(1053):20150352. doi: 10.1259/bjr.20150352. Epub 2015 Jul 2 [PubMed PMID: 26133223]
Badawy M, Gaballah AH, Ganeshan D, Abdelalziz A, Remer EM, Alsabbagh M, Westphalen A, Siddiqui MA, Taffel MT, Itani M, Shaaban AM, Elsayes KM. Adrenal hemorrhage and hemorrhagic masses; diagnostic workup and imaging findings. The British journal of radiology. 2021 Nov 1:94(1127):20210753. doi: 10.1259/bjr.20210753. Epub 2021 Aug 31 [PubMed PMID: 34464549]
Raja A, Leung K, Stamm M, Girgis S, Low G. Multimodality imaging findings of pheochromocytoma with associated clinical and biochemical features in 53 patients with histologically confirmed tumors. AJR. American journal of roentgenology. 2013 Oct:201(4):825-33. doi: 10.2214/AJR.12.9576. Epub [PubMed PMID: 24059371]
Wajchenberg BL, Albergaria Pereira MA, Medonca BB, Latronico AC, Campos Carneiro P, Alves VA, Zerbini MC, Liberman B, Carlos Gomes G, Kirschner MA. Adrenocortical carcinoma: clinical and laboratory observations. Cancer. 2000 Feb 15:88(4):711-36 [PubMed PMID: 10679640]
Lucon AM, Pereira MA, Mendonça BB, Halpern A, Wajchenbeg BL, Arap S. Pheochromocytoma: study of 50 cases. The Journal of urology. 1997 Apr:157(4):1208-12 [PubMed PMID: 9120903]
Level 3 (low-level) evidenceNg L, Libertino JM. Adrenocortical carcinoma: diagnosis, evaluation and treatment. The Journal of urology. 2003 Jan:169(1):5-11 [PubMed PMID: 12478091]
Grazzini G, Pradella S, De Litteris F, Galluzzo A, Anichini M, Treballi F, Bicci E, Miele V. Adrenal Mass Evaluation: Suspicious Radiological Signs of Malignancy. Cancers. 2025 Feb 28:17(5):. doi: 10.3390/cancers17050849. Epub 2025 Feb 28 [PubMed PMID: 40075696]
Fassnacht M, Tsagarakis S, Terzolo M, Tabarin A, Sahdev A, Newell-Price J, Pelsma I, Marina L, Lorenz K, Bancos I, Arlt W, Dekkers OM. European Society of Endocrinology clinical practice guidelines on the management of adrenal incidentalomas, in collaboration with the European Network for the Study of Adrenal Tumors. European journal of endocrinology. 2023 Jul 20:189(1):G1-G42. doi: 10.1093/ejendo/lvad066. Epub [PubMed PMID: 37318239]
Level 1 (high-level) evidenceYoung WF Jr. Clinical practice. The incidentally discovered adrenal mass. The New England journal of medicine. 2007 Feb 8:356(6):601-10 [PubMed PMID: 17287480]
Expert Panel on Urologic Imaging, Wang ZJ, Nikolaidis P, Khatri G, Dogra VS, Ganeshan D, Goldfarb S, Gore JL, Gupta RT, Hartman RP, Heilbrun ME, Lyshchik A, Purysko AS, Savage SJ, Smith AD, Wolfman DJ, Wong-You-Cheong JJ, Lockhart ME. ACR Appropriateness Criteria® Indeterminate Renal Mass. Journal of the American College of Radiology : JACR. 2020 Nov:17(11S):S415-S428. doi: 10.1016/j.jacr.2020.09.010. Epub [PubMed PMID: 33153554]
Arnaldi G, Masini AM, Giacchetti G, Taccaliti A, Faloia E, Mantero F. Adrenal incidentaloma. Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologica. 2000 Oct:33(10):1177-89 [PubMed PMID: 11004718]
Udare A, Agarwal M, Siegelman E, Schieda N. CT and MR imaging of acute adrenal disorders. Abdominal radiology (New York). 2021 Jan:46(1):290-302. doi: 10.1007/s00261-020-02580-w. Epub [PubMed PMID: 32451675]
Mayo-Smith WW, Song JH, Boland GL, Francis IR, Israel GM, Mazzaglia PJ, Berland LL, Pandharipande PV. Management of Incidental Adrenal Masses: A White Paper of the ACR Incidental Findings Committee. Journal of the American College of Radiology : JACR. 2017 Aug:14(8):1038-1044. doi: 10.1016/j.jacr.2017.05.001. Epub 2017 Jun 23 [PubMed PMID: 28651988]
Rowe NE, Kumar RM, Schieda N, Siddiqi F, McGregor T, McAlpine K, Violette PD, Bathini V, Eng M, Izard J, Jana K, Kutikov A, Mayer W. Canadian Urological Association guideline: Diagnosis, management, and followup of the incidentally discovered adrenal mass. Canadian Urological Association journal = Journal de l'Association des urologues du Canada. 2023 Feb:17(2):12-24. doi: 10.5489/cuaj.8248. Epub [PubMed PMID: 36849113]
Level 2 (mid-level) evidenceWilson MP, Randhawa S, Bao B, Croutze R, Murad MH, McInnes MDF, Low G. Impact of Size Thresholds on the Diagnosis of Incidental Adrenal Lesions: A Systematic Review and Meta-Analysis. Journal of the American College of Radiology : JACR. 2024 Jan:21(1):107-117. doi: 10.1016/j.jacr.2023.04.027. Epub 2023 Aug 25 [PubMed PMID: 37634790]
Level 1 (high-level) evidenceAggarwal A, Das CJ. Contrast-enhanced ultrasound in evaluation of adrenal lesions with CT/MRI correlation. The British journal of radiology. 2021 Apr 1:94(1120):20201170. doi: 10.1259/bjr.20201170. Epub 2021 Jan 13 [PubMed PMID: 33439758]
Matin SF, Gill IS. Laparoscopic ultrasonography. Journal of endourology. 2001 Feb:15(1):87-92 [PubMed PMID: 11248925]
Lucas SW, Spitz JD, Arregui ME. The use of intraoperative ultrasound in laparoscopic adrenal surgery: the Saint Vincent experience. Surgical endoscopy. 1999 Nov:13(11):1093-8 [PubMed PMID: 10556445]
Brunt LM, Bennett HF, Teefey SA, Moley JF, Middleton WD. Laparoscopic ultrasound imaging of adrenal tumors during laparoscopic adrenalectomy. American journal of surgery. 1999 Dec:178(6):490-5 [PubMed PMID: 10670859]
Sebastian M, Rudnicki J. Recommendation for laparoscopic ultrasound guided laparoscopic left lateral transabdominal adrenalectomy. Gland surgery. 2020 Jun:9(3):689-694. doi: 10.21037/gs.2020.03.35. Epub [PubMed PMID: 32775258]
Mihai I, Boicean A, Dura H, Teodoru CA, Bratu DG, Ichim C, Todor SB, Bacalbasa N, Bereanu AS, Hașegan A. Intraoperative Ultrasound Guidance in Laparoscopic Adrenalectomy: A Retrospective Analysis of Perioperative Outcomes. Diagnostics (Basel, Switzerland). 2025 Apr 1:15(7):. doi: 10.3390/diagnostics15070898. Epub 2025 Apr 1 [PubMed PMID: 40218249]
Level 2 (mid-level) evidenceSopiński J, Kuzdak K. Navigation with the use of intraoperative ultrasonography in videoscopic adrenal surgery. Polski przeglad chirurgiczny. 2012 Aug:84(8):399-405. doi: 10.2478/v10035-012-0067-3. Epub [PubMed PMID: 22985702]
Pautler SE, Choyke PL, Pavlovich CP, Daryanani K, Walther MM. Intraoperative ultrasound aids in dissection during laparoscopic partial adrenalectomy. The Journal of urology. 2002 Oct:168(4 Pt 1):1352-5 [PubMed PMID: 12352391]
Heniford BT, Iannitti DA, Hale J, Gagner M. The role of intraoperative ultrasonography during laparoscopic adrenalectomy. Surgery. 1997 Dec:122(6):1068-73; discussion 1073-4 [PubMed PMID: 9426421]
Balescu I, Arnautu O, Grasu M, Badiu C, Tomulescu V, Copăescu C. Partial Adrenalectomy - Arguments for the Minimally Invasive Surgical Approach. Chirurgia (Bucharest, Romania : 1990). 2019 Sept-Oct:114(5):611-621. doi: 10.21614/chirurgia.114.5.611. Epub [PubMed PMID: 31670637]
Leslie SW, Sajjad H, Murphy PB. Bladder Stones. StatPearls. 2025 Jan:(): [PubMed PMID: 28722973]
Shermadou ES, Rahman S, Leslie SW. Anatomy, Abdomen and Pelvis: Bladder. StatPearls. 2025 Jan:(): [PubMed PMID: 30285360]
Ng M, Leslie SW, Baradhi KM. Benign Prostatic Hyperplasia. StatPearls. 2025 Jan:(): [PubMed PMID: 32644346]
Kirkpatrick JJ, Leslie SW. Horseshoe Kidney. StatPearls. 2025 Jan:(): [PubMed PMID: 28613757]
Drakonaki EE, Sudoł-Szopińska I, Sinopidis C, Givissis P. High resolution ultrasound for imaging complications of muscle injury: Is there an additional role for elastography? Journal of ultrasonography. 2019:19(77):137-144. doi: 10.15557/JoU.2019.0020. Epub 2019 Jun 28 [PubMed PMID: 31355586]
Bernstein J, Arant BS Jr. Morphological characteristics of segmental renal scarring in vesicoureteral reflux. The Journal of urology. 1992 Nov:148(5 Pt 2):1712-4 [PubMed PMID: 1433595]
Finkelstein JB, Rague JT, Chow J, Venna A, Logvinenko T, Nelson CP, Lee RS. Accuracy of Ultrasound in Identifying Renal Scarring as Compared to DMSA Scan. Urology. 2020 Apr:138():134-137. doi: 10.1016/j.urology.2020.01.019. Epub 2020 Jan 28 [PubMed PMID: 32004557]
Sinha MD, Gibson P, Kane T, Lewis MA. Accuracy of ultrasonic detection of renal scarring in different centres using DMSA as the gold standard. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2007 Aug:22(8):2213-6 [PubMed PMID: 17442740]
Banker H, Sheffield EG, Cohen HL. Nuclear Renal Scan. StatPearls. 2025 Jan:(): [PubMed PMID: 32965907]
Tasker AD, Lindsell DR, Moncrieff M. Can ultrasound reliably detect renal scarring in children with urinary tract infection? Clinical radiology. 1993 Mar:47(3):177-9 [PubMed PMID: 8386074]
Sigmon DF, Shikhman R, Nielson JL. Renal Cyst (Archived). StatPearls. 2025 Jan:(): [PubMed PMID: 29261909]
Agnello F, Albano D, Micci G, Di Buono G, Agrusa A, Salvaggio G, Pardo S, Sparacia G, Bartolotta TV, Midiri M, Lagalla R, Galia M. CT and MR imaging of cystic renal lesions. Insights into imaging. 2020 Jan 3:11(1):5. doi: 10.1186/s13244-019-0826-3. Epub 2020 Jan 3 [PubMed PMID: 31900669]
Masino F, Eusebi L, Bertolotto M, Pizzileo SM, Pizzolorusso F, Sortino G, Pitoni L, Santarelli S, Galosi AB, Guglielmi G. Contrast-enhanced ultrasound in renal cystic lesions: an update. Journal of medical ultrasonics (2001). 2024 Oct:51(4):635-647. doi: 10.1007/s10396-024-01489-x. Epub 2024 Aug 20 [PubMed PMID: 39164480]
Srivastava S, Dhyani M, Dighe M. Contrast-enhanced ultrasound (CEUS): applications from the kidneys to the bladder. Abdominal radiology (New York). 2024 Nov:49(11):4092-4112. doi: 10.1007/s00261-024-04388-4. Epub 2024 Jun 17 [PubMed PMID: 38884782]
Gerst S, Hann LE, Li D, Gonen M, Tickoo S, Sohn MJ, Russo P. Evaluation of renal masses with contrast-enhanced ultrasound: initial experience. AJR. American journal of roentgenology. 2011 Oct:197(4):897-906. doi: 10.2214/AJR.10.6330. Epub [PubMed PMID: 21940577]
Skelton WP, Leslie SW, Guzman N. Renal Mass. StatPearls. 2025 Jan:(): [PubMed PMID: 33620838]
Tsili AC, Andriotis E, Gkeli MG, Krokidis M, Stasinopoulou M, Varkarakis IM, Moulopoulos LA, Oncologic Imaging Subcommittee Working Group of the Hellenic Radiological Society. The role of imaging in the management of renal masses. European journal of radiology. 2021 Aug:141():109777. doi: 10.1016/j.ejrad.2021.109777. Epub 2021 May 15 [PubMed PMID: 34020173]
Pandey J, Syed W. Renal Cancer. StatPearls. 2025 Jan:(): [PubMed PMID: 32644401]
Bosniak MA. The current radiological approach to renal cysts. Radiology. 1986 Jan:158(1):1-10 [PubMed PMID: 3510019]
Spiesecke P, Thiemann J, Conen P, Clevert DA. Contrast enhanced ultrasound of cystic renal lesions, from diagnosis up to treatment. Clinical hemorheology and microcirculation. 2024:88(s1):S21-S33. doi: 10.3233/CH-248102. Epub [PubMed PMID: 39365320]
Nicolau C, Bunesch L, Sebastia C. Renal complex cysts in adults: contrast-enhanced ultrasound. Abdominal imaging. 2011 Dec:36(6):742-52. doi: 10.1007/s00261-011-9727-8. Epub [PubMed PMID: 21461960]
Rübenthaler J, Bogner F, Reiser M, Clevert DA. Contrast-Enhanced Ultrasound (CEUS) of the Kidneys by Using the Bosniak Classification. Ultraschall in der Medizin (Stuttgart, Germany : 1980). 2016 Jun:37(3):234-51. doi: 10.1055/s-0042-104646. Epub 2016 Apr 8 [PubMed PMID: 27058636]
Lerchbaumer MH, Putz FJ, Rübenthaler J, Rogasch J, Jung EM, Clevert DA, Hamm B, Makowski M, Fischer T. Contrast-enhanced ultrasound (CEUS) of cystic renal lesions in comparison to CT and MRI in a multicenter setting. Clinical hemorheology and microcirculation. 2020:75(4):419-429. doi: 10.3233/CH-190764. Epub [PubMed PMID: 32039837]
Graumann O, Osther SS, Karstoft J, Hørlyck A, Osther PJ. Bosniak classification system: a prospective comparison of CT, contrast-enhanced US, and MR for categorizing complex renal cystic masses. Acta radiologica (Stockholm, Sweden : 1987). 2016 Nov:57(11):1409-1417. doi: 10.1177/0284185115588124. Epub 2016 Jul 12 [PubMed PMID: 26019242]
Goksu SY, Leslie SW, Khattar D. Renal Cystic Disease. StatPearls. 2025 Jan:(): [PubMed PMID: 32119391]
Silverman SG, Pedrosa I, Ellis JH, Hindman NM, Schieda N, Smith AD, Remer EM, Shinagare AB, Curci NE, Raman SS, Wells SA, Kaffenberger SD, Wang ZJ, Chandarana H, Davenport MS. Bosniak Classification of Cystic Renal Masses, Version 2019: An Update Proposal and Needs Assessment. Radiology. 2019 Aug:292(2):475-488. doi: 10.1148/radiol.2019182646. Epub 2019 Jun 18 [PubMed PMID: 31210616]
Sevcenco S, Spick C, Helbich TH, Heinz G, Shariat SF, Klingler HC, Rauchenwald M, Baltzer PA. Malignancy rates and diagnostic performance of the Bosniak classification for the diagnosis of cystic renal lesions in computed tomography - a systematic review and meta-analysis. European radiology. 2017 Jun:27(6):2239-2247. doi: 10.1007/s00330-016-4631-9. Epub 2016 Oct 19 [PubMed PMID: 27761710]
Level 1 (high-level) evidenceSabih A, Leslie SW. Complicated Urinary Tract Infections. StatPearls. 2025 Jan:(): [PubMed PMID: 28613784]
Faubel S, Patel NU, Lockhart ME, Cadnapaphornchai MA. Renal relevant radiology: use of ultrasonography in patients with AKI. Clinical journal of the American Society of Nephrology : CJASN. 2014 Feb:9(2):382-94. doi: 10.2215/CJN.04840513. Epub 2013 Nov 14 [PubMed PMID: 24235286]
Khati NJ, Hill MC, Kimmel PL. The role of ultrasound in renal insufficiency: the essentials. Ultrasound quarterly. 2005 Dec:21(4):227-44 [PubMed PMID: 16344727]
Denic A, Glassock RJ, Rule AD. Structural and Functional Changes With the Aging Kidney. Advances in chronic kidney disease. 2016 Jan:23(1):19-28. doi: 10.1053/j.ackd.2015.08.004. Epub [PubMed PMID: 26709059]
Level 3 (low-level) evidenceHommos MS, Glassock RJ, Rule AD. Structural and Functional Changes in Human Kidneys with Healthy Aging. Journal of the American Society of Nephrology : JASN. 2017 Oct:28(10):2838-2844. doi: 10.1681/ASN.2017040421. Epub 2017 Aug 8 [PubMed PMID: 28790143]
Karam Z, Tuazon J. Anatomic and physiologic changes of the aging kidney. Clinics in geriatric medicine. 2013 Aug:29(3):555-64. doi: 10.1016/j.cger.2013.05.006. Epub [PubMed PMID: 23849007]
Rout P, Leslie SW. Renal Angiomyolipoma. StatPearls. 2025 Jan:(): [PubMed PMID: 36256751]
Barocas DA, Lotan Y, Matulewicz RS, Raman JD, Westerman ME, Kirkby E, Pak LJ, Souter L. Updates to Microhematuria: AUA/SUFU Guideline (2025). The Journal of urology. 2025 May:213(5):547-557. doi: 10.1097/JU.0000000000004490. Epub 2025 Feb 27 [PubMed PMID: 40013563]
Taylor JI, Souter LH, Barocas DA, Boorjian SA, Raman JD, Lotan Y. Diagnostic Imaging in the Evaluation of Asymptomatic Microhematuria: Systematic Review and Meta-analysis. The Journal of urology. 2023 Jun:209(6):1099-1106. doi: 10.1097/JU.0000000000003395. Epub 2023 Mar 8 [PubMed PMID: 36883858]
Level 1 (high-level) evidenceBukavina L, Mishra K, Calaway A, Ponsky L. Robotic Partial Nephrectomy: Update on Techniques. The Urologic clinics of North America. 2021 Feb:48(1):81-90. doi: 10.1016/j.ucl.2020.09.013. Epub 2020 Nov 5 [PubMed PMID: 33218596]
Li L, Niu D, Yang C, Liang CZ. Contrast-enhanced and conventional ultrasound images of renal wounds after partial nephrectomy. American journal of translational research. 2023:15(8):5508-5518 [PubMed PMID: 37692962]
Senel S, Koudonas A, Ahmadzada J, Rassweiler J, Gözen AS. Is intraoperative ultrasonography necessary in laparoscopic partial nephrectomy for exophytic tumours? Minimally invasive therapy & allied technologies : MITAT : official journal of the Society for Minimally Invasive Therapy. 2023 Dec:32(6):341-344. doi: 10.1080/13645706.2023.2233611. Epub 2023 Aug 1 [PubMed PMID: 37525989]
Das MK, Rohith G, Mandal S, Gaur AS, Nayak P, Kumaraswamy S, Tarigopula V, Dheroo DK, Tripathy S. Intraoperative ultrasonography (IOUS)-guided vs conventional laparoscopic nephrectomy: a randomised control trial. BJU international. 2024 Jan:133(1):71-78. doi: 10.1111/bju.16136. Epub 2023 Aug 2 [PubMed PMID: 37470129]
Level 1 (high-level) evidenceQin B, Hu H, Lu Y, Wang Y, Yu Y, Zhang J, Zhang Z, Gao H, Wang Q, Wang S. Intraoperative ultrasonography in laparoscopic partial nephrectomy for intrarenal tumors. PloS one. 2018:13(4):e0195911. doi: 10.1371/journal.pone.0195911. Epub 2018 Apr 26 [PubMed PMID: 29698427]
Level 2 (mid-level) evidenceYoung M, Leslie SW. Percutaneous Nephrostomy. StatPearls. 2025 Jan:(): [PubMed PMID: 29630257]
Morey AF, McAninch JW. Sonographic staging of anterior urethral strictures. The Journal of urology. 2000 Apr:163(4):1070-5 [PubMed PMID: 10737469]
Bertolotto M, Quaia E, Mucelli FP, Ciampalini S, Forgács B, Gattuccio I. Color Doppler imaging of posttraumatic priapism before and after selective embolization. Radiographics : a review publication of the Radiological Society of North America, Inc. 2003 Mar-Apr:23(2):495-503 [PubMed PMID: 12640162]
Bhatt S, Kocakoc E, Rubens DJ, Seftel AD, Dogra VS. Sonographic evaluation of penile trauma. Journal of ultrasound in medicine : official journal of the American Institute of Ultrasound in Medicine. 2005 Jul:24(7):993-1000; quiz 1001 [PubMed PMID: 15972714]
Tullington JE, Blecker N. Lower Genitourinary Trauma. StatPearls. 2025 Jan:(): [PubMed PMID: 32491459]
Aversa A, Crafa A, Greco EA, Chiefari E, Brunetti A, La Vignera S. The penile duplex ultrasound: How and when to perform it? Andrology. 2021 Sep:9(5):1457-1466. doi: 10.1111/andr.13029. Epub 2021 May 27 [PubMed PMID: 33960127]
Mihmanli I, Kantarci F. Erectile dysfunction. Seminars in ultrasound, CT, and MR. 2007 Aug:28(4):274-86 [PubMed PMID: 17874651]
Golijanin D, Singer E, Davis R, Bhatt S, Seftel A, Dogra V. Doppler evaluation of erectile dysfunction - part 1. International journal of impotence research. 2007 Jan-Feb:19(1):37-42 [PubMed PMID: 16625230]
Belew D, Klaassen Z, Lewis RW. Intracavernosal Injection for the Diagnosis, Evaluation, and Treatment of Erectile Dysfunction: A Review. Sexual medicine reviews. 2015 Mar:3(1):11-23. doi: 10.1002/smrj.35. Epub 2015 Oct 19 [PubMed PMID: 27784568]
Raza SA, Jhaveri KS. Imaging in male infertility. Radiologic clinics of North America. 2012 Nov:50(6):1183-200. doi: 10.1016/j.rcl.2012.08.006. Epub [PubMed PMID: 23122045]
Golijanin D, Singer E, Davis R, Bhatt S, Seftel A, Dogra V. Doppler evaluation of erectile dysfunction - part 2. International journal of impotence research. 2007 Jan-Feb:19(1):43-8 [PubMed PMID: 16625229]
Patel P, Masterson T, Ramasamy R. Penile duplex: clinical indications and application. International journal of impotence research. 2019 Jul:31(4):298-299. doi: 10.1038/s41443-018-0074-y. Epub 2018 Sep 7 [PubMed PMID: 30194373]
Jung DC, Park SY, Lee JY. Penile Doppler ultrasonography revisited. Ultrasonography (Seoul, Korea). 2018 Jan:37(1):16-24. doi: 10.14366/usg.17022. Epub 2017 Jun 10 [PubMed PMID: 28736428]
El-Sakka AI, Morsy AM. Screening for ischemic heart disease in patients with erectile dysfunction: role of penile Doppler ultrasonography. Urology. 2004 Aug:64(2):346-50 [PubMed PMID: 15302492]
Gupta N, Herati A, Gilbert BR. Penile Doppler ultrasound predicting cardiovascular disease in men with erectile dysfunction. Current urology reports. 2015 Mar:16(3):16. doi: 10.1007/s11934-015-0482-1. Epub [PubMed PMID: 25677231]
Kawanishi Y, Lee KS, Kimura K, Koizumi T, Nakatsuji H, Kojima K, Yamamoto A, Numata A, Sogou T. Screening of ischemic heart disease with cavernous artery blood flow in erectile dysfunctional patients. International journal of impotence research. 2001 Apr:13(2):100-3 [PubMed PMID: 11426348]
Mostafaei H, Mori K, Hajebrahimi S, Abufaraj M, Karakiewicz PI, Shariat SF. Association of erectile dysfunction and cardiovascular disease: an umbrella review of systematic reviews and meta-analyses. BJU international. 2021 Jul:128(1):3-11. doi: 10.1111/bju.15313. Epub 2021 Jan 9 [PubMed PMID: 33260254]
Level 1 (high-level) evidenceZhao B, Hong Z, Wei Y, Yu D, Xu J, Zhang W. Erectile Dysfunction Predicts Cardiovascular Events as an Independent Risk Factor: A Systematic Review and Meta-Analysis. The journal of sexual medicine. 2019 Jul:16(7):1005-1017. doi: 10.1016/j.jsxm.2019.04.004. Epub 2019 May 16 [PubMed PMID: 31104857]
Level 1 (high-level) evidenceDurukan E, Jensen CFS, Dons M, Sengeløv M, Landler NE, Skaarup KG, Højbjerg Lassen MC, Johansen ND, Østergren PB, Sønksen J, Fode M, Biering-Sørensen T. Prevalence of cardiac dysfunction in men with erectile dysfunction: the EDcard study. BJU international. 2025 Sep:136(3):537-544. doi: 10.1111/bju.16824. Epub 2025 Jun 19 [PubMed PMID: 40537936]
An J, Xiang B, Peng J, Li D. Understanding the erectile dysfunction-cardiovascular disease connection: clinical and pathophysiological insights. Sexual medicine reviews. 2025 Jul 3:13(3):406-422. doi: 10.1093/sxmrev/qeaf014. Epub [PubMed PMID: 40153594]
Level 3 (low-level) evidenceMei Y, Chen Y, Wang X, Xu R, Xu R, Feng X. Association between erectile dysfunction and the predicted 10-year risk for atherosclerosis cardiovascular disease among U.S. men: a population-based study from the NHANES 2001-2004. Frontiers in endocrinology. 2024:15():1442904. doi: 10.3389/fendo.2024.1442904. Epub 2024 Dec 17 [PubMed PMID: 39741880]
Singh P, Sharma AP, Chowhan PS, Gorsi U, Mavuduru RS, Rehsi SS, Lal A, Mete UK. Establishing the role of shear wave elastography in differentiating corporal rigidity between vasculogenic versus non-vasculogenic erectile dysfunction patients. Ultrasound (Leeds, England). 2025 Feb 24:():1742271X241275207. doi: 10.1177/1742271X241275207. Epub 2025 Feb 24 [PubMed PMID: 40012699]
Zhang Y, Zhou W, Wu X, Zhao S, Zhang X. Role of shear wave elastography measured in the flaccid state in predicting arteriogenic erectile dysfunction. Andrologia. 2021 May:53(4):e13996. doi: 10.1111/and.13996. Epub 2021 Feb 1 [PubMed PMID: 33527468]
Zhang J, Zhou W, Zhang Y, Zhang W, Zhang C. A Novel Method to Quantify Penile Arterial Blood Supply Using Shear Wave Elastography During Penile Duplex Ultrasound in Men with Erectile Dysfunction. Medical science monitor : international medical journal of experimental and clinical research. 2022 Mar 26:28():e935232. doi: 10.12659/MSM.935232. Epub 2022 Mar 26 [PubMed PMID: 35338109]
Lee JY, Jung DC, Lee S, Kang NG, Oh YT, Han K. Stiffness of the Central Corpus Cavernosum on Shear-Wave Elastography Is Inversely Correlated with the Penile Rigidity Score in Patients with Erectile Dysfunction. The world journal of men's health. 2021 Jan:39(1):123-130. doi: 10.5534/wjmh.190094. Epub 2020 Jan 9 [PubMed PMID: 32009308]
Silberman M, Stormont G, Leslie SW, Hu EW. Priapism. StatPearls. 2025 Jan:(): [PubMed PMID: 29083574]
Avery LL, Scheinfeld MH. Imaging of penile and scrotal emergencies. Radiographics : a review publication of the Radiological Society of North America, Inc. 2013 May:33(3):721-40. doi: 10.1148/rg.333125158. Epub [PubMed PMID: 23674771]
Wakrim S, Ziouziou I, Ralph D, Khabbal Y. Penile Doppler ultrasound study in priapism: A systematic review. Progres en urologie : journal de l'Association francaise d'urologie et de la Societe francaise d'urologie. 2022 Jan:32(1):61-69. doi: 10.1016/j.purol.2021.03.009. Epub 2021 Jul 3 [PubMed PMID: 34229947]
Level 1 (high-level) evidenceQureshi JM, Wood H, Feldman M. High flow priapism on color Doppler ultrasound. The Journal of urology. 2013 Jun:189(6):2312-3. doi: 10.1016/j.juro.2013.03.007. Epub 2013 Mar 7 [PubMed PMID: 23473902]
Bertolotto M, Zappetti R, Pizzolato R, Liguori G. Color Doppler appearance of penile cavernosal-spongiosal communications in patients with high-flow priapism. Acta radiologica (Stockholm, Sweden : 1987). 2008 Jul:49(6):710-4. doi: 10.1080/02841850802027026. Epub [PubMed PMID: 18568565]
Nicola R, Carson N, Dogra VS. Imaging of traumatic injuries to the scrotum and penis. AJR. American journal of roentgenology. 2014 Jun:202(6):W512-20. doi: 10.2214/AJR.13.11676. Epub [PubMed PMID: 24848844]
Hulsmans FJ, Castelijns JA, Reeders JW, Tytgat GN. Review of artifacts associated with transrectal ultrasound: understanding, recognition, and prevention of misinterpretation. Journal of clinical ultrasound : JCU. 1995 Oct:23(8):483-94 [PubMed PMID: 7499519]
Level 3 (low-level) evidenceNomura JT, Sierzenski PR. Ultrasound diagnosis of penile fracture. The Journal of emergency medicine. 2010 Apr:38(3):362-5. doi: 10.1016/j.jemermed.2008.03.010. Epub 2008 Oct 4 [PubMed PMID: 18835513]
Scott SE, Langenohl R, Crisostomo-Wynne T, Kang C. Penile Dorsal Vein Rupture Identified by Emergency Department Ultrasound. Clinical practice and cases in emergency medicine. 2021 Feb:5(1):121-122. doi: 10.5811/cpcem.2020.10.49631. Epub [PubMed PMID: 33560969]
Level 3 (low-level) evidenceSandean DP, Leslie SW, Lotfollahzadeh S. Peyronie Disease. StatPearls. 2025 Jan:(): [PubMed PMID: 32809463]
Bertolotto M, Pavlica P, Serafini G, Quaia E, Zappetti R. Painful penile induration: imaging findings and management. Radiographics : a review publication of the Radiological Society of North America, Inc. 2009 Mar-Apr:29(2):477-93. doi: 10.1148/rg.292085117. Epub [PubMed PMID: 19325060]
McCauley JF, Dean RC. Diagnostic utility of penile ultrasound in Peyronie's disease. World journal of urology. 2020 Feb:38(2):263-268. doi: 10.1007/s00345-019-02928-y. Epub 2019 Oct 12 [PubMed PMID: 31606787]
Kalokairinou K, Konstantinidis C, Domazou M, Kalogeropoulos T, Kosmidis P, Gekas A. US Imaging in Peyronie's Disease. Journal of clinical imaging science. 2012:2():63. doi: 10.4103/2156-7514.103053. Epub 2012 Oct 31 [PubMed PMID: 23230545]
Prando D. New sonographic aspects of peyronie disease. Journal of ultrasound in medicine : official journal of the American Institute of Ultrasound in Medicine. 2009 Feb:28(2):217-32 [PubMed PMID: 19168771]
Richards G, Goldenberg E, Pek H, Gilbert BR. Penile sonoelastography for the localization of a non-palpable, non-sonographically visualized lesion in a patient with penile curvature from Peyronie's disease. The journal of sexual medicine. 2014 Feb:11(2):516-20. doi: 10.1111/jsm.12396. Epub 2013 Nov 22 [PubMed PMID: 24261857]
Tyloch JF, Wieczorek AP. Standards for scrotal ultrasonography. Journal of ultrasonography. 2016 Dec:16(67):391-403. doi: 10.15557/JoU.2016.0039. Epub 2016 Dec 30 [PubMed PMID: 28138410]
Bennett GL, Garcia RA. Benign intratesticular dermoid cyst: sonographic findings. AJR. American journal of roentgenology. 2002 Nov:179(5):1315-7 [PubMed PMID: 12388520]
Dogra VS, Gottlieb RH, Rubens DJ, Oka M, Di Sant Agnese AP. Testicular epidermoid cysts: sonographic features with histopathologic correlation. Journal of clinical ultrasound : JCU. 2001 Mar-Apr:29(3):192-6 [PubMed PMID: 11329161]
Kim W, Rosen MA, Langer JE, Banner MP, Siegelman ES, Ramchandani P. US MR imaging correlation in pathologic conditions of the scrotum. Radiographics : a review publication of the Radiological Society of North America, Inc. 2007 Sep-Oct:27(5):1239-53 [PubMed PMID: 17848688]
Loya AG, Said JW, Grant EG. Epidermoid cyst of the testis: radiologic-pathologic correlation. Radiographics : a review publication of the Radiological Society of North America, Inc. 2004 Oct:24 Suppl 1():S243-6 [PubMed PMID: 15486244]
Sista AK, Filly RA. Color Doppler sonography in evaluation of spermatoceles: the "falling snow" sign. Journal of ultrasound in medicine : official journal of the American Institute of Ultrasound in Medicine. 2008 Jan:27(1):141-3 [PubMed PMID: 18096740]
Rew KT, Langan RC, Hadj-Moussa M, Heidelbaugh JJ. Men's Health: Scrotal and Testicular Conditions. FP essentials. 2021 Apr:503():23-27 [PubMed PMID: 33856180]
Sakamoto H, Saito K, Shichizyo T, Ishikawa K, Igarashi A, Yoshida H. Color Doppler ultrasonography as a routine clinical examination in male infertility. International journal of urology : official journal of the Japanese Urological Association. 2006 Aug:13(8):1073-8 [PubMed PMID: 16903932]
Armstrong JM, Keihani S, Hotaling JM. Use of Ultrasound in Male Infertility: Appropriate Selection of Men for Scrotal Ultrasound. Current urology reports. 2018 May 28:19(8):58. doi: 10.1007/s11934-018-0810-3. Epub 2018 May 28 [PubMed PMID: 29808325]
Baigorri BF, Dixon RG. Varicocele: A Review. Seminars in interventional radiology. 2016 Sep:33(3):170-6. doi: 10.1055/s-0036-1586147. Epub [PubMed PMID: 27582603]
Pilatz A, Altinkilic B, Köhler E, Marconi M, Weidner W. Color Doppler ultrasound imaging in varicoceles: is the venous diameter sufficient for predicting clinical and subclinical varicocele? World journal of urology. 2011 Oct:29(5):645-50. doi: 10.1007/s00345-011-0701-4. Epub 2011 May 24 [PubMed PMID: 21607575]
Metin A, Bulut O, Temizkan M. Relationship between the left spermatic vein diameter measured by ultrasound and palpated varicocele and Doppler ultrasound findings. International urology and nephrology. 1991:23(1):65-8 [PubMed PMID: 1938219]
Hoekstra T, Witt MA. The correlation of internal spermatic vein palpability with ultrasonographic diameter and reversal of venous flow. The Journal of urology. 1995 Jan:153(1):82-4 [PubMed PMID: 7966798]
Bertolotto M, Freeman S, Richenberg J, Belfield J, Dogra V, Huang DY, Lotti F, Markiet K, Nikolic O, Ramanathan S, Ramchandani P, Rocher L, Secil M, Sidhu PS, Skrobisz K, Studniarek M, Tsili A, Turgut AT, Pavlica P, Derchi LE, Members of the ESUR-SPIWG WG. Ultrasound evaluation of varicoceles: systematic literature review and rationale of the ESUR-SPIWG Guidelines and Recommendations. Journal of ultrasound. 2020 Dec:23(4):487-507. doi: 10.1007/s40477-020-00509-z. Epub 2020 Jul 27 [PubMed PMID: 32720266]
Level 1 (high-level) evidenceLotti F, Studniarek M, Balasa C, Belfield J, De Visschere P, Freeman S, Kozak O, Markiet K, Ramanathan S, Richenberg J, Secil M, Skrobisz K, Tsili AC, Bertolotto M, Rocher L. The role of the radiologist in the evaluation of male infertility: recommendations of the European Society of Urogenital Radiology-Scrotal and Penile Imaging Working Group (ESUR-SPIWG) for scrotal imaging. European radiology. 2025 Feb:35(2):752-766. doi: 10.1007/s00330-024-10964-5. Epub 2024 Jul 31 [PubMed PMID: 39083089]
Balawender K, Orkisz S, Wisz P. Testicular microlithiasis: what urologists should know. A review of the current literature. Central European journal of urology. 2018:71(3):310-314. doi: 10.5173/ceju.2018.1728. Epub 2018 Aug 21 [PubMed PMID: 30386652]
Lundager KS, Frandsen RH, Durukan E, Belhouche NZ, Jensen CFS, Busch Østergren P, Sønksen J, Fode M. Detection of germ cell neoplasia in situ and testicular cancer risk in men with testicular microlithiasis: Real world results through 10 years. Andrology. 2025 May 21:():. doi: 10.1111/andr.70071. Epub 2025 May 21 [PubMed PMID: 40395097]
Richenberg J, Belfield J, Ramchandani P, Rocher L, Freeman S, Tsili AC, Cuthbert F, Studniarek M, Bertolotto M, Turgut AT, Dogra V, Derchi LE. Testicular microlithiasis imaging and follow-up: guidelines of the ESUR scrotal imaging subcommittee. European radiology. 2015 Feb:25(2):323-30. doi: 10.1007/s00330-014-3437-x. Epub 2014 Oct 15 [PubMed PMID: 25316054]
Keçeli M, Keskin Z, Keskin S. Comparison of Superb Microvascular Imaging With Other Doppler Methods in Assessment of Testicular Vascularity in Cryptorchidism. Ultrasound quarterly. 2020 Dec:36(4):363-370. doi: 10.1097/RUQ.0000000000000533. Epub [PubMed PMID: 32956243]
Durmaz MS, Sivri M. Comparison of superb micro-vascular imaging (SMI) and conventional Doppler imaging techniques for evaluating testicular blood flow. Journal of medical ultrasonics (2001). 2018 Jul:45(3):443-452. doi: 10.1007/s10396-017-0847-9. Epub 2017 Dec 16 [PubMed PMID: 29248966]
Ayaz E, Ayaz M, Önal C, Yıkılmaz A. Seeing the Unseen: Evaluating Testicular Vascularity in Neonates by Using the Superb Microvascular Imaging Ultrasound Technique. Journal of ultrasound in medicine : official journal of the American Institute of Ultrasound in Medicine. 2019 Jul:38(7):1847-1854. doi: 10.1002/jum.14882. Epub 2018 Dec 13 [PubMed PMID: 30548880]
Cakıroğlu B, Ozcan F, Ateş L, Aksoy SH. Leiomyoma of the epididymis treated with partial epididymectomy. Urology annals. 2014 Oct:6(4):356-8. doi: 10.4103/0974-7796.141005. Epub [PubMed PMID: 25371616]
Beccia DJ, Krane RJ, Olsson CA. Clinical management of non-testicular intrascrotal tumors. The Journal of urology. 1976 Oct:116(4):476-9 [PubMed PMID: 802862]
Spark RP. Leiomyoma of epididymis. Archives of pathology. 1972 Jan:93(1):18-21 [PubMed PMID: 5061684]
Lee JC, Bhatt S, Dogra VS. Imaging of the epididymis. Ultrasound quarterly. 2008 Mar:24(1):3-16. doi: 10.1097/RUQ.0b013e318168f116. Epub [PubMed PMID: 18362528]
Farah M, Song M, Mahmalji W. Epididymal Adenomatoid Tumour: A Case Report. Cureus. 2023 Oct:15(10):e47505. doi: 10.7759/cureus.47505. Epub 2023 Oct 23 [PubMed PMID: 37908693]
Level 3 (low-level) evidenceSangoi AR, McKenney JK, Schwartz EJ, Rouse RV, Longacre TA. Adenomatoid tumors of the female and male genital tracts: a clinicopathological and immunohistochemical study of 44 cases. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2009 Sep:22(9):1228-35. doi: 10.1038/modpathol.2009.90. Epub 2009 Jun 19 [PubMed PMID: 19543245]
Level 3 (low-level) evidenceLopez O, Bahmad HF, Delgado R, Cordon BH, Poppiti R, Howard L. Papillary cystadenoma of the epididymis. Autopsy & case reports. 2022:12():e2021374. doi: 10.4322/acr.2021.374. Epub 2022 Apr 14 [PubMed PMID: 35496736]
Level 3 (low-level) evidenceOdrzywolski KJ, Mukhopadhyay S. Papillary cystadenoma of the epididymis. Archives of pathology & laboratory medicine. 2010 Apr:134(4):630-3 [PubMed PMID: 20367315]
Lissens P, Michiels H, Geysens P, Van de Voorde W, Lauweryns J. Papillary cystadenoma of the epididymis: a case report. Acta urologica Belgica. 1995 Mar:63(1):109-12 [PubMed PMID: 7725985]
Level 3 (low-level) evidenceGiyanani VL, Hennigan DB, Fowler M, Sanders TJ. Sonographic findings in leiomyoma of postorchiectomy scrotum. Urology. 1985 Feb:25(2):204-6 [PubMed PMID: 3881873]
Frates MC, Benson CB, DiSalvo DN, Brown DL, Laing FC, Doubilet PM. Solid extratesticular masses evaluated with sonography: pathologic correlation. Radiology. 1997 Jul:204(1):43-6 [PubMed PMID: 9205221]
Alleman WG, Gorman B, King BF, Larson DR, Cheville JC, Nehra A. Benign and malignant epididymal masses evaluated with scrotal sonography: clinical and pathologic review of 85 patients. Journal of ultrasound in medicine : official journal of the American Institute of Ultrasound in Medicine. 2008 Aug:27(8):1195-202 [PubMed PMID: 18645078]
Hamidi M, Nikolaidis P, Miller FH, Casalino DD, Hammond NA, Horowitz JM, Gabriel H. Lesions Without Borders: Scrotal Lesions That Involve Both the Intratesticular and Extratesticular Regions. AJR. American journal of roentgenology. 2018 Feb:210(2):W70-W79. doi: 10.2214/AJR.17.18369. Epub [PubMed PMID: 29355401]
Guo K, Tian R, Liu L, Du C, Li F, Wang H. Adenomatoid Tumor of the Tunica Albuginea in a Boy: A Case Report and Literature Review. Case reports in urology. 2015:2015():935193. doi: 10.1155/2015/935193. Epub 2015 May 27 [PubMed PMID: 26106502]
Level 3 (low-level) evidenceSubik MK, Gordetsky J, Yao JL, di Sant'Agnese PA, Miyamoto H. Frozen section assessment in testicular and paratesticular lesions suspicious for malignancy: its role in preventing unnecessary orchiectomy. Human pathology. 2012 Sep:43(9):1514-9. doi: 10.1016/j.humpath.2011.11.013. Epub 2012 Mar 8 [PubMed PMID: 22406369]
Samnani S, Alimohamed N. Case - "Burned-out" testicular tumor: A rare entity with diagnostic dilemma. Canadian Urological Association journal = Journal de l'Association des urologues du Canada. 2022 Nov:16(11):E572-E574. doi: 10.5489/cuaj.7879. Epub [PubMed PMID: 35704933]
Level 3 (low-level) evidenceSchick MA, Sternard BT. Testicular Torsion. StatPearls. 2025 Jan:(): [PubMed PMID: 28846325]
Caesar RE, Kaplan GW. Incidence of the bell-clapper deformity in an autopsy series. Urology. 1994 Jul:44(1):114-6 [PubMed PMID: 7913779]
Bandarkar AN, Blask AR. Testicular torsion with preserved flow: key sonographic features and value-added approach to diagnosis. Pediatric radiology. 2018 May:48(5):735-744. doi: 10.1007/s00247-018-4093-0. Epub 2018 Feb 21 [PubMed PMID: 29468365]
Vijayaraghavan SB. Sonographic differential diagnosis of acute scrotum: real-time whirlpool sign, a key sign of torsion. Journal of ultrasound in medicine : official journal of the American Institute of Ultrasound in Medicine. 2006 May:25(5):563-74 [PubMed PMID: 16632779]
Prando D. Torsion of the spermatic cord: the main gray-scale and doppler sonographic signs. Abdominal imaging. 2009 Sep-Oct:34(5):648-61. doi: 10.1007/s00261-008-9449-8. Epub [PubMed PMID: 18709404]
Arce JD, Cortés M, Vargas JC. Sonographic diagnosis of acute spermatic cord torsion. Rotation of the cord: a key to the diagnosis. Pediatric radiology. 2002 Jul:32(7):485-91 [PubMed PMID: 12107581]
Baud C, Veyrac C, Couture A, Ferran JL. Spiral twist of the spermatic cord: a reliable sign of testicular torsion. Pediatric radiology. 1998 Dec:28(12):950-4 [PubMed PMID: 9880639]
Esposito F, Di Serafino M, Mercogliano C, Vitale V, Sgambati P, Vallone G. The "whirlpool sign", a US finding in partial torsion of the spermatic cord: 4 cases. Journal of ultrasound. 2014 Dec:17(4):313-5. doi: 10.1007/s40477-014-0095-4. Epub 2014 Jul 3 [PubMed PMID: 25368691]
Level 3 (low-level) evidencePomajzl AJ, Leslie SW. Appendix Testis Torsion. StatPearls. 2025 Jan:(): [PubMed PMID: 31550101]
Boettcher M, Bergholz R, Krebs TF, Wenke K, Treszl A, Aronson DC, Reinshagen K. Differentiation of epididymitis and appendix testis torsion by clinical and ultrasound signs in children. Urology. 2013 Oct:82(4):899-904. doi: 10.1016/j.urology.2013.04.004. Epub 2013 Jun 2 [PubMed PMID: 23735611]
Level 2 (mid-level) evidenceJohnson KA, Dewbury KC. Ultrasound imaging of the appendix testis and appendix epididymis. Clinical radiology. 1996 May:51(5):335-7 [PubMed PMID: 8641095]
Lev M, Ramon J, Mor Y, Jacobson JM, Soudack M. Sonographic appearances of torsion of the appendix testis and appendix epididymis in children. Journal of clinical ultrasound : JCU. 2015 Oct:43(8):485-9. doi: 10.1002/jcu.22265. Epub 2015 Feb 20 [PubMed PMID: 25704247]
Leslie SW, Sajjad H, Siref LE. Varicocele. StatPearls. 2025 Jan:(): [PubMed PMID: 28846314]
Lehner K, Ingram C, Bansal U, Baca C, Balasubramanian A, Thirumavalavan N, Scovell JM, Rajanahally S, Pollard M, Lipshultz LI. Color Doppler ultrasound imaging in varicoceles: Is the difference in venous diameter encountered during Valsalva predictive of palpable varicocele grade? Asian journal of urology. 2023 Jan:10(1):27-32. doi: 10.1016/j.ajur.2021.12.006. Epub 2022 Jan 4 [PubMed PMID: 36721684]
Karami M, Mazdak H, Khanbabapour S, Adibi A, Nasr N. Determination of the best position and site for color Doppler ultrasonographic evaluation of the testicular vein to define the clinical grades of varicocele ultrasonographically. Advanced biomedical research. 2014:3():17. doi: 10.4103/2277-9175.124647. Epub 2014 Jan 9 [PubMed PMID: 24592367]
Stahl P, Schlegel PN. Standardization and documentation of varicocele evaluation. Current opinion in urology. 2011 Nov:21(6):500-5. doi: 10.1097/MOU.0b013e32834b8698. Epub [PubMed PMID: 21926627]
Level 3 (low-level) evidenceBelay RE, Huang GO, Shen JK, Ko EY. Diagnosis of clinical and subclinical varicocele: how has it evolved? Asian journal of andrology. 2016 Mar-Apr:18(2):182-5. doi: 10.4103/1008-682X.169991. Epub [PubMed PMID: 26780869]
El-Saeity NS, Sidhu PS. "Scrotal varicocele, exclude a renal tumour". Is this evidence based? Clinical radiology. 2006 Jul:61(7):593-9 [PubMed PMID: 16784945]
Horrow MM. Further Workup for Isolated Right-Sided Varicocele. AJR. American journal of roentgenology. 2019 Aug:213(2):W95. doi: 10.2214/AJR.19.21339. Epub [PubMed PMID: 31328994]
Gleason A, Bishop K, Xi Y, Fetzer DT. Isolated Right-Sided Varicocele: Is Further Workup Necessary? AJR. American journal of roentgenology. 2019 Apr:212(4):802-807. doi: 10.2214/AJR.18.20077. Epub 2019 Feb 19 [PubMed PMID: 30779666]
Elmer DeWitt M, Greene DJ, Gill B, Nyame Y, Haywood S, Sabanegh E Jr. Isolated Right Varicocele and Incidence of Associated Cancer. Urology. 2018 Jul:117():82-85. doi: 10.1016/j.urology.2018.03.047. Epub 2018 Apr 9 [PubMed PMID: 29649544]
Itani M, Kipper B, Corwin MT, Burgan CM, Fetzer DT, Shenoy-Bhangle AS, Althubaity A, Loehfelm TW, Middleton WD, Fananapazir G. Right-sided scrotal varicocele and its association with malignancy: a multi-institutional study. Abdominal radiology (New York). 2021 May:46(5):2140-2145. doi: 10.1007/s00261-020-02840-9. Epub 2020 Nov 5 [PubMed PMID: 33151361]
Hanna GB, Byrne D, Townell N. Right-sided varicocele as a presentation of right renal tumours. British journal of urology. 1995 Jun:75(6):798-9 [PubMed PMID: 7613843]
Level 3 (low-level) evidenceBonfitto M, Kimura LSM, Godoy JMP, Zeratti Filho M, Spessoto LCF, Facio FN Jr. Does right-sided varicocele indicate a right-sided kidney tumor? Archivio italiano di urologia, andrologia : organo ufficiale [di] Societa italiana di ecografia urologica e nefrologica. 2019 Mar 29:91(1):53-54. doi: 10.4081/aiua.2019.1.53. Epub 2019 Mar 29 [PubMed PMID: 30932432]
Grogan SP, Mount CA. Ultrasound Physics and Instrumentation. StatPearls. 2025 Jan:(): [PubMed PMID: 34033355]
Kossoff G. Basic physics and imaging characteristics of ultrasound. World journal of surgery. 2000 Feb:24(2):134-42 [PubMed PMID: 10633140]
Terris MK, Klaassen Z. Office-based ultrasound for the urologist. The Urologic clinics of North America. 2013 Nov:40(4):637-47. doi: 10.1016/j.ucl.2013.07.006. Epub 2013 Sep 7 [PubMed PMID: 24182981]
Martino P, Galosi AB, Bitelli M, Consonni P, Fiorini F, Granata A, Gunelli R, Liguori G, Palazzo S, Pavan N, Scattoni V, Virgili G, Imaging Working Group-Societa Italiana Urologia (SIU), Società Italiana Ecografia Urologica Andrologica Nefrologica (SIEUN). Practical recommendations for performing ultrasound scanning in the urological and andrological fields. Archivio italiano di urologia, andrologia : organo ufficiale [di] Societa italiana di ecografia urologica e nefrologica. 2014 Mar 28:86(1):56-78. doi: 10.4081/aiua.2014.1.56. Epub 2014 Mar 28 [PubMed PMID: 24704936]
Steel R, Poepping TL, Thompson RS, Macaskill C. Origins of the edge shadowing artefact in medical ultrasound imaging. Ultrasound in medicine & biology. 2004 Sep:30(9):1153-62 [PubMed PMID: 15550319]
Kremkau FW, Taylor KJ. Artifacts in ultrasound imaging. Journal of ultrasound in medicine : official journal of the American Institute of Ultrasound in Medicine. 1986 Apr:5(4):227-37 [PubMed PMID: 3514956]
Rubin JM, Adler RS, Fowlkes JB, Carson PL. Phase cancellation: a cause of acoustical shadowing at the edges of curved surfaces in B-mode ultrasound images. Ultrasound in medicine & biology. 1991:17(1):85-95 [PubMed PMID: 2021017]
Sapozhnikov O, Lu W, Bailey MR, Kaczkowski P, Crum LA. 2aBA6. Bubbles trapped on the surface of kidney stones as a cause of the twinkling artifact in ultrasound imaging. Proceedings of meetings on acoustics. Acoustical Society of America. 2013 Jun 2:19():. pii: 075033. Epub [PubMed PMID: 26290679]
Wood BG, Urban MW. Detecting Kidney Stones Using Twinkling Artifacts: Survey of Kidney Stones with Varying Composition and Size. Ultrasound in medicine & biology. 2020 Jan:46(1):156-166. doi: 10.1016/j.ultrasmedbio.2019.09.008. Epub 2019 Oct 18 [PubMed PMID: 31635759]
Level 3 (low-level) evidenceRokni E, Zinck S, Simon JC. Evaluation of Stone Features That Cause the Color Doppler Ultrasound Twinkling Artifact. Ultrasound in medicine & biology. 2021 May:47(5):1310-1318. doi: 10.1016/j.ultrasmedbio.2021.01.016. Epub 2021 Feb 16 [PubMed PMID: 33602553]
Laher AE, McDowall J, Gerber L, Aigbodion SJ, Enyuma COA, Buchanan S, Adam A. The ultrasound 'twinkling artefact' in the diagnosis of urolithiasis: hocus or valuable point-of-care-ultrasound? A systematic review and meta-analysis. European journal of emergency medicine : official journal of the European Society for Emergency Medicine. 2020 Feb:27(1):13-20. doi: 10.1097/MEJ.0000000000000601. Epub [PubMed PMID: 30829686]
Level 1 (high-level) evidenceAl-Balushi A, Al-Shibli A, Al-Reesi A, Ullah QZ, Al-Shukaili W, Baawain S, Al-Dhuhli H, Al-Shamsi M, Al-Hubaishi A, Al-Atbi AYH. The Accuracy of Point-of-Care Ultrasound Performed by Emergency Physicians in Detecting Hydronephrosis in Patients with Renal Colic. Sultan Qaboos University medical journal. 2022 Aug:22(3):351-356. doi: 10.18295/squmj.9.2021.130. Epub 2022 Aug 25 [PubMed PMID: 36072079]
Sibley S, Roth N, Scott C, Rang L, White H, Sivilotti MLA, Bruder E. Point-of-care ultrasound for the detection of hydronephrosis in emergency department patients with suspected renal colic. The ultrasound journal. 2020 Jun 8:12(1):31. doi: 10.1186/s13089-020-00178-3. Epub 2020 Jun 8 [PubMed PMID: 32507905]