Introduction
The brain is a highly perfused organ, receiving up to 20% of the resting cardiac output. Blood reaches the brain through the internal carotid and vertebral arteries and is ultimately drained by the internal jugular veins. An intricate network of venous sinuses enables this drainage, collecting blood from the brain parenchyma, meninges, eyes, and cerebrospinal fluid (CSF). Drainage pathways can be categorized teleologically as either superficial, draining into the dural sinuses, or deep, draining through medullary veins into the cerebral vein of Galen. These vessels are uniformly valveless, allowing free communication between channels and facilitating the potential spread of infectious pathogens.
The cerebral venous system is particularly delicate. Since the brain is suspended in CSF, even minor trauma or abrupt deceleration can rupture cortical bridging veins and result in a subdural hematoma (SDH).
Diagnosing and managing conditions such as venous sinus thrombosis, SDH, and intracranial infections requires a thorough understanding of cerebral venous anatomy. During neurosurgical procedures, preserving venous drainage pathways helps prevent venous infarction and secondary brain injury. Mastery of cerebral venous architecture supports sound decision-making across diagnostic and operative settings.
Structure and Function
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Structure and Function
The cerebral veins drain the capillary network that supplies the brain, removing carbon dioxide and other metabolic waste products while allowing fresh blood to circulate. These veins collect blood from the entire brain, as well as from the eyes, meninges, and part of the face via the pterygoid plexus. The dural sinuses, particularly the superior sagittal sinus, also drain CSF through the arachnoid granulations, returning it to the bloodstream. Unlike systemic veins, cerebral veins lack valves and a muscular layer, allowing for substantial expansion. The only exception is the larger pial veins, which contain a circumferential smooth muscle layer. Superficial dural sinuses are further supported by layers of dura mater, making them resistant to compression during episodes of elevated intracranial pressure (ICP).
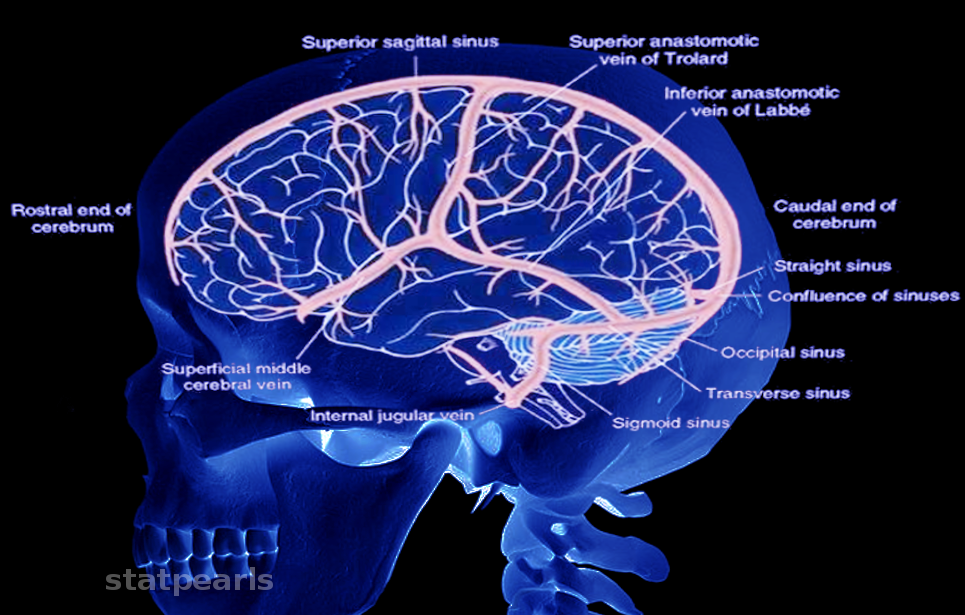
The venous anatomy of the brain is complex (see Image. Cerebral Venous System). The 1st major investigation was conducted by Okudera et al in 1999. Cerebral venous drainage is divided into 2 systems: the superficial medullary (or subcortical) and the deep medullary venous systems. Classification depends on whether blood from a given venule drains into superficial cortical veins or the deep cerebral veins and, ultimately, the vein of Galen.
The superficial medullary veins begin with subcortical veins that drain the outer cortex. Blood from these vessels flows into the pial veins located on the cortical surface. Pial veins converge and drain into the larger cerebral veins. These vessels are numerous and lie on the surface of the brain.
The 3 largest superficial cerebral veins include the superficial middle cerebral vein, which courses along the Sylvian fissure; the vein of Trolard, which ascends to the superior sagittal sinus; and the vein of Labbe, which empties into the transverse sinus. All cerebral veins typically drain to the nearest dural sinus based on location. Superodorsal veins empty into the superior sagittal sinus. Posterior veins and cerebellar veins drain to the transverse sinus. Blood from the anterior veins may flow into the superior sagittal sinus, cavernous sinus, or superficial middle cerebral vein.
At the level of the dural sinuses, cerebral veins cross the arachnoid and dura mater as bridging veins to empty into the sinus lumen. Dural sinuses also receive blood from minor meningeal veins and the skull and scalp through emissary veins.
The superior sagittal sinus runs caudally until it joins the occipital sinus over the cerebellum, forming the confluence of sinuses. From this point, blood continues into the transverse sinuses.
The deep medullary venous system drains the deep cerebrum. This system is more extensive than the superficial medullary venous system, and the 2 communicate only rarely via transcerebral veins, the relatively large vessels that traverse the entire cerebral parenchyma. Deep medullary veins consist of venules located in the white matter, oriented roughly perpendicular to the lateral ventricles. These veins run in parallel and meet at 3 zones of convergence: the hat-rack, candelabra, and palmate formations. A final convergence occurs along the inner surface of the lateral ventricles, where the vessels form the subependymal veins.
This venous arrangement contributes to the characteristic periventricular and subcortical lesions observed in multiple sclerosis, where demyelinating leukocytes extravasate from these vessels. The subependymal veins then join 2 additional venous groups: the choroidal venous plexus and the thalamostriate veins. These systems drain the choroid plexus and much of the deep gray nuclei. These vessels form the internal cerebral veins upon merging with the subependymal veins. The left and right internal cerebral veins unite beneath the splenium of the corpus callosum to form the great vein of Galen, which serves as the principal drainage pathway for cerebral white matter and deep gray nuclei.
The inferior anterior surface of the brain is drained primarily by the cavernous sinuses. These dural sinuses lie on either side of the sella turcica and directly posterior to the orbits. Venous input arises from the brain via local cerebral veins, the eyes via the superior and inferior ophthalmic veins, and the skull and meninges via the sphenoparietal sinus and middle meningeal vein. Since it receives the middle meningeal vein, the cavernous sinus anastomoses with the maxillary vein. The cavernous sinus also drains the pterygoid plexus, which lies inferior to the cavernous sinus and anterior to the temporomandibular joint. The pterygoid plexus communicates with the facial vein, providing venous drainage for the nose and upper lip. Segments of the internal carotid artery, the sympathetic plexus, and cranial nerves III, IV, V1, V2, and VI also course through the cavernous sinuses.
The cavernous sinuses drain in 3 directions. Superior drainage may occur via the superficial middle cerebral vein. Lateral drainage proceeds through the superior and inferior petrosal sinuses into the sigmoid sinus. Medial drainage passes into the basilar plexus. The basilar plexus runs along the clivus, collecting blood from the anterior brainstem, then bifurcates around the foramen magnum and joins the vein of Galen.
The great cerebral vein of Galen lies inferior to the splenium of the corpus callosum. The inferior sagittal sinus courses along the inferior edge of the falx cerebri, just superior to the corpus callosum. These 2 vessels unite posterior to the splenium to form the straight sinus, which travels caudally until reaching the confluence of sinuses.
The confluence of sinuses lies at the midline along the border of the cerebellum and occipital lobes. Venous input arises from the superior sagittal sinus superiorly, the occipital sinus inferiorly, and the straight sinus anteriorly. From this junction, the venous flow divides laterally into the transverse sinuses. Each transverse sinus descends and continues as a sigmoid sinus. The sigmoid sinuses exit the skull through the jugular foramina, becoming the internal jugular veins.[1][2][3][4][5][6]
Embryology
The mature venous system of the brain originates from a precursor venous network that develops and increases in complexity alongside the fetal brain. These primitive vessels receive limited coverage in medical education texts. The embryologic origin of these vessels was first described in detail by Dr. George Linus Streeter in 1915.
The cerebral veins begin development as undifferentiated mesenchyme, known as the primary meninx. This tissue differentiates into a capillary network, which then coalesces into several vessels lined by a thin layer of endothelium. These early vessels are believed to share a heterogeneous embryologic origin with the dura mater, arising from both neural crest and mesoderm.
The initial major vessels, arranged from rostral to caudal, include the anterior cerebral vein, pro-otic vein, vena capitis medialis, vena capitis lateralis, supraoptic anastomosis, and posterior rhombencephalic veins. These vessels ultimately converge to form the anterior cardinal vein, which drains into the primitive atrium.
These early venous structures undergo remodeling and differentiation to form the mature cerebral venous anatomy. The anterior cerebral vein develops into the transverse sinuses. The pro-otic vein and vena capitis medialis give rise to the cavernous sinus, which drains the middle meningeal and ophthalmic veins. The vena capitis lateralis regresses. The posterior rhombencephalic vein and supraoptic anastomosis contribute to the formation of the sigmoid sinuses. The anterior cardinal vein develops into the internal jugular vein. Additionally, the superior sagittal sinus forms within the space between the falx and dural layers along the midline.[7][8][9]
Physiologic Variants
Considerable anatomic variation characterizes the venous system of the body, including the cerebral veins. Variation affects both the structure and courses of the medullary veins in the parenchyma and the superficial cortical veins. In the deep medullary veins, variability is evident in the location of subependymal venous connections as the drainage system consolidates and enlarges. Significant variation also occurs in the superficial veins and dural sinuses. The most common variation of the major dural sinuses is a hypoplastic left transverse sinus, reported in up to 21% of cases in a study. That same study documented atresia of the anterior segment of the superior sagittal sinus in just under 1% of patients?.
The superficial anastomotic veins, including the superficial middle cerebral vein, the vein of Trolard, and the vein of Labbe, show substantial variation in dominance, connections, and development. The most frequent pattern involved codominance of the superficial middle cerebral vein and the vein of Labbe, although isolated dominance of any 1 of the 3, or codominance of all 3 vessels, was also observed. Dominant hemispheric laterality did not appear to influence these patterns. The primary drainage of the superficial middle cerebral vein was to the sphenoparietal sinus in 57% of cases and the cavernous sinus in 19%. The vein of Labbe drained into the transverse sinus in 80% of cases. Despite frequently exhibiting dominance, both veins were noted to be underdeveloped in 16% of cases.[10][11][12][13]
Surgical Considerations
Intracranial veins are generally considered less clinically significant than their arterial counterparts. Severe conditions, including cavernous sinus syndrome, can involve venous structures and necessitate surgical intervention. The cavernous sinus poses a particular challenge due to its location, requiring various surgical approaches based on the target structure. Common approaches include the transsphenoidal-transnasal route, superior access via frontotemporal craniotomy, lateral approaches beneath the temporal lobe, and the transorbital route.
In the deep cerebral venous system, surgical sacrifice has led to severe complications. A reported case involved extensive cerebellar venous infarction following ligation of the superior petrosal vein. Despite these risks, a 2013 review demonstrated that selective venous sacrifice can be performed safely in certain cases.[14][15][16][17]
Clinical Significance
Cavernous Sinus Syndrome
Cavernous sinus syndrome results from obstruction, inflammation, or increased pressure within the cavernous sinus. Common causes include thrombophlebitis, which can result from bacterial or fungal spread originating in the nasal mucosa, paranasal sinuses, or a skull fracture. Thrombus formation can also occur in individuals with a hypercoagulable state, such as protein C or protein S deficiency, factor V Leiden mutation, or combined oral contraceptive use. Tumors, such as a locally invasive pituitary adenoma, may also give rise to this syndrome.
An internal carotid artery aneurysm can cause similar signs and symptoms. A ruptured aneurysm may lead to a carotid-cavernous fistula, increasing pressure within the sinus. Symptoms of cavernous sinus syndrome reflect compression or injury to surrounding structures and the cranial and sympathetic nerves that traverse the sinus. Involvement of the sympathetic plexus can result in partial Horner syndrome. Cranial nerves III, IV, and VI are typically affected, producing ophthalmoplegia. Notably, cranial nerve VI is often the first involved in carotid artery aneurysm, as it lies closest to the vessel.
Proptosis and globe tenderness can also occur due to elevated pressure behind the eye. Imaging of the head typically confirms the diagnosis, often demonstrating sinus occlusion, thickening, or swelling, along with engorgement of the ophthalmic veins. Management focuses on addressing the underlying infection or decompressing the sinus surgically.[18]
Venous Sinus Thrombosis
Thrombi occluding the dural sinuses are rare and typically associated with local trauma or a hypercoagulable state. Occlusion impairs drainage from tributary veins, leading to ischemic injury and increased ICP. Symptoms vary by vessel size and region involved, but can include focal neurologic deficits, progressive headache, nausea, vomiting, visual disturbances, seizures, and signs of elevated ICP such as papilledema. Computed tomography findings are often negative, though an empty triangle or “empty delta sign” in the posterior superior sagittal sinus may indicate absent perfusion. Magnetic resonance venography is preferred and demonstrates absent venous flow, confirming the diagnosis. Treatment consists of anticoagulation.[19][20]
Subdural Hematoma
An SDH forms when blood collects beneath the dura mater in the skull. This condition most commonly results from rupture of the bridging veins that connect the superficial cerebral veins to the major dural sinuses, especially the superior sagittal sinus. Venous blood leaks out and compresses the brain parenchyma. Since dural folds do not confine the blood, the lesion can extend beyond suture lines, producing the classic crescent-shaped collection between the dura and brain.
This expanding hematoma can exert pressure on the brain, leading to midline shift or herniation. Common causes include head trauma or a rapid change in velocity, such as a fall without direct head impact. This injury mechanism occurs most often in adults older than 80.
SDAs frequently present in older individuals and others with cerebral atrophy, such as people with chronic alcohol use. In these populations, the bridging veins are already stretched. SDAs are also seen in abused infants with fragile vessels (shaken baby syndrome) and occur as recognized complications of antiplatelet therapy, anticoagulation, and long-term hemodialysis.
Clinical presentation varies widely, from complete loss of consciousness after massive trauma to a nearly asymptomatic course. An asymptomatic hematoma can delay diagnosis. In sufficiently large bleeds, symptoms may include progressive memory loss, weakness, gait impairment, headache, nausea, and vomiting. A chronic hematoma can predispose to further falls and may be complicated by recurrent acute bleeding layered over the existing chronic collection.
Chronic SDH is primarily a disease of older adults. Imaging typically reveals a crescent-shaped collection of blood that extends around suture lines but spares the cisterns and ventricles. Midline shift and obliteration of the ventricles may accompany the hemorrhage. Treatment ranges from conservative observation to burr hole evacuation or craniotomy with drainage. Management decisions depend on patient factors and the size and extent of the hematoma.[21]
Developmental Venous Anomalies
Developmental venous malformations (DVAs), also known as cerebral venous angiomas, medullary venous malformations, or cerebral venous malformations, are aberrations of venous structure in the cerebrum. The architecture of a DVA typically includes a single dilated cerebral vein with multiple surrounding tributary veins draining into it, rather than several small, separate veins. In simple terms, a DVA is a vein responsible for draining a disproportionately large region of cerebral parenchyma.
DVAs affect approximately 3% of the population and are believed to arise from the aberrant development of a cerebral venous segment in utero. Compensatory changes allow the malformed vein to maintain appropriate drainage of its watershed territory. Most DVAs are asymptomatic and clinically insignificant. However, developmental venous malformations have been associated with a range of cerebral vascular abnormalities, including regional brain atrophy, white matter lesions, adjacent hemorrhage, and calcifications.
A study of 11 patients with Sturge-Weber syndrome found cerebral venous abnormalities, including developmental venous malformations, in all cases. This association aligns with the known pathophysiology of Sturge-Weber syndrome, a neurocutaneous disorder of vascular development defined by leptomeningeal angiomatosis and an absence of subcortical veins. The resulting venous insufficiency may necessitate compensatory dilation of deep medullary veins.
Treatment of DVAs is conservative due to their generally benign course. Surgical intervention carries a significant risk of venous infarction.[22][23][24][25]
Facial Venous Plexus Anastomosis
The facial venous plexus primarily drains the skin over the nose and upper lip. This valveless venous network empties into the facial vein and anastomoses with the pterygoid plexus, as well as with veins draining the nasal mucosa. The absence of valves permits free communication between the nasal mucosa, facial epidermis, cavernous sinus, and cerebral cortex. This anatomical arrangement underlies the so-called "danger triangle of the face," the region encompassing the nose and upper lip, where rupture or irritation of a pimple or skin lesion may allow pathogens to spread to the cavernous sinus or cerebral cortex, potentially resulting in infection or cavernous sinus syndrome.
Media
(Click Image to Enlarge)
References
Okudera T, Huang YP, Fukusumi A, Nakamura Y, Hatazawa J, Uemura K. Micro-angiographical studies of the medullary venous system of the cerebral hemisphere. Neuropathology : official journal of the Japanese Society of Neuropathology. 1999 Jan:19(1):93-111. doi: 10.1046/j.1440-1789.1999.00215.x. Epub [PubMed PMID: 19519653]
Kiliç T, Akakin A. Anatomy of cerebral veins and sinuses. Frontiers of neurology and neuroscience. 2008:23():4-15 [PubMed PMID: 18004050]
Kuijf HJ, Bouvy WH, Zwanenburg JJ, Razoux Schultz TB, Viergever MA, Vincken KL, Biessels GJ. Quantification of deep medullary veins at 7 T brain MRI. European radiology. 2016 Oct:26(10):3412-8. doi: 10.1007/s00330-016-4220-y. Epub 2016 Feb 16 [PubMed PMID: 26883328]
Taoka T, Fukusumi A, Miyasaka T, Kawai H, Nakane T, Kichikawa K, Naganawa S. Structure of the Medullary Veins of the Cerebral Hemisphere and Related Disorders. Radiographics : a review publication of the Radiological Society of North America, Inc. 2017 Jan-Feb:37(1):281-297. doi: 10.1148/rg.2017160061. Epub [PubMed PMID: 28076020]
Uddin MA, Haq TU, Rafique MZ. Cerebral venous system anatomy. JPMA. The Journal of the Pakistan Medical Association. 2006 Nov:56(11):516-9 [PubMed PMID: 17183980]
Schaller B. Physiology of cerebral venous blood flow: from experimental data in animals to normal function in humans. Brain research. Brain research reviews. 2004 Nov:46(3):243-60 [PubMed PMID: 15571768]
Level 3 (low-level) evidenceBUTLER H. The development of certain human dural venous sinuses. Journal of anatomy. 1957 Oct:91(4):510-26 [PubMed PMID: 13475149]
Mitsuhashi Y, Hayasaki K, Kawakami T, Nagata T, Kaneshiro Y, Umaba R, Ohata K. Dural Venous System in the Cavernous Sinus: A Literature Review and Embryological, Functional, and Endovascular Clinical Considerations. Neurologia medico-chirurgica. 2016 Jun 15:56(6):326-39. doi: 10.2176/nmc.ra.2015-0346. Epub 2016 Apr 11 [PubMed PMID: 27063146]
Tanaka M. Embryological Consideration of Dural AVFs in Relation to the Neural Crest and the Mesoderm. Neurointervention. 2019 Mar:14(1):9-16. doi: 10.5469/neuroint.2018.01095. Epub 2019 Feb 28 [PubMed PMID: 30827062]
Tanriverdi T, Al-Jehani H, Poulin N, Olivier A. Superficial anastomotic veins: neurosurgical view depending on 251 craniotomies. The Canadian journal of neurological sciences. Le journal canadien des sciences neurologiques. 2009 Jan:36(1):65-71 [PubMed PMID: 19294891]
Level 2 (mid-level) evidenceIkushima I, Korogi Y, Kitajima M, Yamura M, Yamashita Y. Evaluation of drainage patterns of the major anastomotic veins on the lateral surface of the cerebrum using three-dimensional contrast-enhanced MP-RAGE sequence. European journal of radiology. 2006 Apr:58(1):96-101 [PubMed PMID: 16387463]
Goyal G, Singh R, Bansal N, Paliwal VK. Anatomical Variations of Cerebral MR Venography: Is Gender Matter? Neurointervention. 2016 Sep:11(2):92-8. doi: 10.5469/neuroint.2016.11.2.92. Epub 2016 Sep 3 [PubMed PMID: 27621945]
Fujii S, Kanasaki Y, Matsusue E, Kakite S, Kminou T, Ogawa T. Demonstration of cerebral venous variations in the region of the third ventricle on phase-sensitive imaging. AJNR. American journal of neuroradiology. 2010 Jan:31(1):55-9. doi: 10.3174/ajnr.A1752. Epub 2009 Sep 3 [PubMed PMID: 19729543]
Level 2 (mid-level) evidenceDavidson L, McComb JG. The safety of the intraoperative sacrifice of the deep cerebral veins. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2013 Feb:29(2):199-207. doi: 10.1007/s00381-012-1958-7. Epub 2012 Nov 22 [PubMed PMID: 23180313]
Level 3 (low-level) evidenceMasuoka J, Matsushima T, Hikita T, Inoue E. Cerebellar swelling after sacrifice of the superior petrosal vein during microvascular decompression for trigeminal neuralgia. Journal of clinical neuroscience : official journal of the Neurosurgical Society of Australasia. 2009 Oct:16(10):1342-4. doi: 10.1016/j.jocn.2008.12.024. Epub 2009 Jul 2 [PubMed PMID: 19576780]
Level 3 (low-level) evidenceInoue T, Rhoton AL Jr, Theele D, Barry ME. Surgical approaches to the cavernous sinus: a microsurgical study. Neurosurgery. 1990 Jun:26(6):903-32 [PubMed PMID: 2362670]
Ngnitewe Massa R, Minutello K, Mesfin FB. Neuroanatomy, Cavernous Sinus. StatPearls. 2025 Jan:(): [PubMed PMID: 29083662]
Lee JH, Lee HK, Park JK, Choi CG, Suh DC. Cavernous sinus syndrome: clinical features and differential diagnosis with MR imaging. AJR. American journal of roentgenology. 2003 Aug:181(2):583-90 [PubMed PMID: 12876052]
Lee SK, Kim BS, Terbrugge KG. Clinical Presentation, Imaging and Treatment of Cerebral Venous Thrombosis (CVT). Interventional neuroradiology : journal of peritherapeutic neuroradiology, surgical procedures and related neurosciences. 2002 Mar 30:8(1):5-14 [PubMed PMID: 20594505]
Gupta RK, Jamjoom AA, Devkota UP. Superior sagittal sinus thrombosis presenting as a continuous headache: a case report and review of the literature. Cases journal. 2009 Dec 21:2():9361. doi: 10.1186/1757-1626-2-9361. Epub 2009 Dec 21 [PubMed PMID: 20062608]
Level 3 (low-level) evidenceUno M, Toi H, Hirai S. Chronic Subdural Hematoma in Elderly Patients: Is This Disease Benign? Neurologia medico-chirurgica. 2017 Aug 15:57(8):402-409. doi: 10.2176/nmc.ra.2016-0337. Epub 2017 Jun 26 [PubMed PMID: 28652561]
Mooney MA, Zabramski JM. Developmental venous anomalies. Handbook of clinical neurology. 2017:143():279-282. doi: 10.1016/B978-0-444-63640-9.00026-6. Epub [PubMed PMID: 28552150]
San Millán Ruíz D, Gailloud P. Cerebral developmental venous anomalies. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2010 Oct:26(10):1395-406. doi: 10.1007/s00381-010-1253-4. Epub 2010 Aug 12 [PubMed PMID: 20703485]
San Millán Ruíz D, Delavelle J, Yilmaz H, Gailloud P, Piovan E, Bertramello A, Pizzini F, Rüfenacht DA. Parenchymal abnormalities associated with developmental venous anomalies. Neuroradiology. 2007 Dec:49(12):987-95 [PubMed PMID: 17703296]
Bentson JR, Wilson GH, Newton TH. Cerebral venous drainage pattern of the Sturge-Weber syndrome. Radiology. 1971 Oct:101(1):111-8 [PubMed PMID: 5111963]