Introduction
The earliest known documentation of nasopharyngeal angiofibroma dates to Hippocrates in the 5th century B.C.[1] The condition is also known as juvenile nasopharyngeal angiofibroma (JNA), juvenile angiofibroma, or fibromatous or angiofibromatous hamartoma of the nasal cavity.[2]
The term "nasopharyngeal" may not be entirely accurate. Some sources state that the tumor arises from the sphenopalatine foramen and posterior nasal cavity, while others suggest a more choanal and nasopharyngeal origin.[3][4] Research consistently agrees, however, that JNA is a benign, highly vascular lesion comprising approximately 0.05% to 0.5% of all head and neck masses.[5][6]
Though histologically benign, JNA often demonstrates aggressive features with local invasion into the nasal turbinates, nasal septum, and medial pterygoid lamina. The tumor commonly extends into the nasal cavity, nasopharynx, and pterygopalatine fossa, with larger lesions involving the sphenoid, maxillary, and ethmoid sinuses. Growth may also extend through the inferior orbital fissure and into the masticator space via the infratemporal fossa. Severe disease may involve the orbit or intracranial structures, reported in approximately 10% to 37% of cases.[7]
JNA is a highly vascular lesion, typically with 1 or more feeding arterial pedicles. The most common primary arterial supply is the internal maxillary artery, a branch of the external carotid artery (ECA).[8] Larger lesions may recruit multiple feeding arteries, sometimes bilaterally. The ascending pharyngeal artery is the 2nd most common supplying branch, followed by the middle meningeal, accessory meningeal, and facial arteries. Internal carotid artery (ICA) branches may also be recruited, most commonly the vidian artery and, less frequently, the ophthalmic artery.[9]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
The etiology of JNA remains poorly understood and is frequently debated. Proposed origins include a vascular lesion, such as an arteriovenous malformation or a remnant of the 1st branchial arch.[10] Persistence of branchial arch tissue may explain the typical location of nasopharyngeal angiofibroma, as incomplete regression can leave remnants in or near the sphenopalatine foramen.[11] The expression of vascular growth receptors, particularly vascular endothelial growth factor receptor 2, may also account for the tumor’s marked vascularity.[12][13]
A hormonal influence has also been proposed, with proliferation of vascular erectile tissues following repeated microhemorrhages and repair.[14] Androgen, estrogen, and progesterone receptors have been identified in tumor tissue. The predominance in male adolescents may relate to increased androgen production at puberty, which can stimulate tumor growth and vascular expansion.[15] Tumor growth has been reported after testosterone administration, even posttreatment.[16][17] However, cases in older women, despite reduced estrogen and progesterone levels, and in pregnant individuals, suggest that the relationship between sex hormones and tumor development is complex. Some authors propose estrogen may have a protective effect, but overall, the role of hormonal influence remains unclear.[18][19]
Nasopharyngeal angiofibroma has been correlated with genetic anomalies and other disorders. Chromosome 17 deletions have been reported, drawing particular interest because of their association with the TP53 tumor suppressor gene and the human epidermal growth factor receptor 2 (HER2), involving the HER2/NEU oncogene, both well recognized in tumor growth and malignancy.[20][21][22] Other reported associations include familial adenomatous polyposis and Gardner syndrome, where altered APC gene expression has been observed in a subset of nasopharyngeal angiofibroma.[23][24]
Recent documentation suggests a potential association between human papillomavirus (HPV) infection and JNA. While HPV is well-established in its link to head and neck squamous cell carcinoma and recognized for its tumorigenic effects, similar to Epstein-Barr virus, its connection to JNA remains under investigation. A small study found a strong association between JNA and HPV, detecting HPV-specific proteins and DNA within nasopharyngeal angiofibroma tissue, with no corresponding infection in the control group’s adenoidal tissue.[25] Given the global rise in HPV incidence, this raises concerns about a possible increase in JNA cases and underscores the potential for developing preventative strategies. However, further research is necessary due to limited current evidence, and HPV is not currently accepted as a causative factor in JNA.[26]
Epidemiology
Nasopharyngeal angiofibroma occurs almost exclusively in adolescent boys and accounts for approximately 0.05% to 0.5% of head and neck tumors, with a reported incidence ranging from 1 in 150,000 to 1 in 1,500,000.[27] Reports suggest a higher incidence among individuals from India and the Middle East compared with those of European descent. The typical age range is 9 to 25 years, although diagnoses in older men are extremely rare.[28] Rare cases in female individuals have been reported. Further immunohistochemical and genetic testing is warranted in such instances, as genetic mosaicism may represent a potential predisposing factor.
Pathophysiology
Nasopharyngeal angiofibroma is characterized by angiogenesis and vascular proliferation within the posterior nasal cavity, sphenopalatine foramen, and nasopharynx. Hormonal influences, chromosomal abnormalities, and overexpression of vascular growth factor receptors have been proposed as possible contributing factors, although the exact mechanism remains uncertain. The tumor’s extensive vascularity facilitates recruitment of adjacent arterial supply, and its aggressive growth can cause osseous erosion with extension into the orbits, skull base, frontal and middle cranial fossae, and adjacent structures, complicating surgical resection.
Histopathology
Nasopharyngeal angiofibromas are nonencapsulated, well-circumscribed polypoid masses composed of vascular and fibrous stromal tissue. The intervening vessels vary in size, ranging from slit-like to ectatic with a staghorn configuration (see Image. Nasopharyngeal Angiofibroma Histopathology). Poorly developed myoid-type cells surround the endothelial-lined vascular channels, creating the appearance of a smooth muscle layer. A true muscular coat or elastic lamina is absent, which contributes to the tendency for profuse bleeding with minor trauma or manipulation.
The fibrous stroma is typically collagenous and contains fibroblasts of various morphologies, including spindle, plump, stellate, and angular forms. Most cells contain a single small nucleolus, although multinucleated cells have been documented. Cell morphology can vary between tumors and within different regions of the same tumor.[29] Despite this variability, atypia is uncommon, and mitotic activity is rare. Although nonencapsulated, the mass is covered by an epithelial layer, often respiratory epithelium. This layer can show reactive changes, such as inflammatory cell infiltration, granulation tissue formation, ulceration, and squamous metaplasia. Nervous and glandular tissue may be present within the examined section, likely secondary to entrapment during tumor growth.
Electron microscopy highlights the propensity for hemorrhage and supports classification as a vascular malformation. Discontinuous basement membrane, irregular smooth muscle layers, and absence of pericytes reinforce this interpretation. The single-layer endothelium of the vascular channels expresses vascular markers including CD34, CD31, von Willebrand factor, and endoglin.[30] Endoglin positivity has been proposed to correlate with recurrence and the overall vascular density of the mass.[31]
History and Physical
The most common presentation involves an adolescent boy with chronic unilateral nasal obstruction. Painless, unprovoked epistaxis is also frequent. Additional manifestations include headache and rhinorrhea. Large, invasive tumors can produce proptosis, visual disturbance, cranial nerve palsy, Eustachian tube dysfunction, and facial deformity. On physical examination, a mass is usually visible within the nasal cavity, although endoscopy may be required, depending on size. Cervical lymphadenopathy is usually absent.
Evaluation
JNA is diagnosed primarily through clinical assessment and imaging studies. Laboratory tests offer no significant value in the diagnosis of the condition. Nasal endoscopy typically reveals a firm, friable, reddish or reddish-purple mass within the nasal cavity, with erythema that may intensify during a Valsalva maneuver. Imaging is best performed with computed tomography (CT) or magnetic resonance imaging (MRI). Plain radiographs may demonstrate bowing or anterior displacement of the posterior maxillary sinus wall due to mass effect.
Contrast-enhanced CT typically demonstrates an avidly enhancing soft tissue mass within the posterior nasal cavity near the sphenopalatine foramen, often extending into the nasopharynx, pterygopalatine fossa, and adjacent sinuses.[32] Air cells may be evident, depending on tumor size, opacification of the sphenoid sinus, maxillary sinus, or ethmoid, either from obstruction or direct infiltration.
Bone kernel postprocessing aids in evaluating osseous remodeling or destruction. Expansion of the nasal cavity and pterygopalatine fossa, as well as anterior bowing of the posterior maxillary sinus wall (Holman-Miller sign), may be secondary to mass effect.[33] Osseous destruction is more common in aggressive tumors, particularly those involving the skull base with intracranial extension.
Further assessment with CT angiography may help define vascularity and arterial supply for preoperative planning (see Image. Nasopharyngeal Angiofibroma on Computed Tomography Angiography).[34] Findings such as unilateral enlargement of the ECA or internal maxillary artery can help identify the primary vascular source.
Traditional cerebral angiography remains useful, especially when multiple feeding arteries are present. Selective catheterization of the ICA and ECA, as well as subselecting individual branches, can guide preoperative decision-making.
Although CT provides superior spatial resolution and is better suited for assessing osseous involvement, MRI offers superior contrast resolution and serves as a valuable adjunct. On noncontrast sequences, nasopharyngeal angiofibroma typically appears heterogeneous, with intermediate signal intensity on T1-weighted images and intermediate to high signal intensity on T2-weighted images. Flow voids are present on both T1- and T2-weighted sequences. Areas of prior hemorrhage may be evident, particularly on susceptibility-weighted imaging. Diffusion-weighted imaging with apparent diffusion coefficient mapping generally demonstrates facilitated diffusion with elevated apparent diffusion coefficient values.[35]
As with CT, MRI demonstrates intense enhancement following gadolinium administration. MRI is also particularly useful for evaluating tumor extension into the cavernous or sphenoid sinus or identifying perineural spread through the skull base or into the orbit.[36] Magnetic resonance angiography, similar to CT angiography, can show enlargement of the ipsilateral ECA or internal maxillary artery, although it is less effective in defining the vascular source.
Due to the tumor’s marked vascularity, biopsy should not be performed as part of the diagnostic evaluation, especially in the outpatient setting. An unnecessary biopsy carries a risk of significant morbidity and potential mortality from hemorrhage. Diagnosis is instead established through clinical assessment combined with characteristic imaging findings.[37]
Treatment / Management
The treatment of choice and standard of care is surgical resection of the tumor. Hippocrates is credited with the oldest reported surgical treatment of JNA, recorded as a “longitudinal splitting of the ridge of the nose.”[38](B3)
The extensive vascularity of the tumor often necessitates preoperative embolization to reduce intraoperative hemorrhage. Embolization also facilitates identification of the vascular supply, particularly delineation of ECA and ICA feeders, and may be advantageous if a staged or segmented procedure is planned.
After establishing femoral artery access, digital subtraction angiography is typically performed with a 5F catheter of the common carotid artery, ICA, and ECA. The arterial feeders most clearly supplying the tumor are then selectively catheterized with a 3F microcatheter. Embolization materials include gelatin sponge, particles, and microcoils. Particle size varies, but 300 to 500 μm is preferred to lower the risk of nontarget embolization when ECA-ICA communications exist within the tumor.
Microcoils are useful when precise placement is required, such as in the presence of ECA-ICA communication or branch vessels arising from the ICA, where nontarget embolization carries more severe complications. Arterial branches located near cranial nerves are deliberately avoided to minimize the risk of iatrogenic cranial nerve injury. Preoperative embolization is most effective for higher-stage tumors with robust vascularity, with limited additional benefit in lower-stage disease.
Traditionally, lateral rhinotomy served as the main open surgical modality, with possible extensions to Weber-Ferguson or maxillary swing techniques to facilitate more comprehensive tumor resection.[39] Additional approaches exist, and multiple routes may be required to achieve safe resection. Options include transpalatal, transfacial, transnasal, sublabial/Le Fort I, transmaxillary, and infratemporal surgical corridors.
Early-stage cases with favorable anatomy may be treated endoscopically using an endonasal approach. This technique may be combined with an anterior maxillotomy or craniotomy, as required. Controlled hypotension under general anesthesia is typically employed, with topical epinephrine used to contract the nasal mucosa and improve visualization.
Partial removal of the inferior and middle turbinates and the nasal septum may be necessary during endonasal resection to expand the operative field. Depending on tumor size, the ethmoid air cells and maxillary ostium may also need to be opened for further access, particularly when removal of the posterior maxillary sinus wall is required to reach the pterygopalatine or infratemporal fossae.[40](B2)
Surgical strategy focuses on defining the periphery and extent of the tumor by dissecting around its margins with minimal direct manipulation. This method enables identification of key anatomic landmarks while preserving the tumor en bloc. Embolized and thrombosed feeding vessels may be isolated and divided once stripped from the periosteum, allowing delivery of the resected mass. Laser-assisted endonasal cautery and dissection have also been described, with the neodymium-doped yttrium aluminum garnet (Nd:YAG) laser offering more focused cautery.
For large tumors, removal may be performed in a staged, segmented approach based on vascular territory. Some voluminous growths involve bilateral ECA branch vessels, and others may receive supply from the ICA, particularly when intracranial extension is present.
Staging the procedure to address each vascular territory separately can minimize blood loss by using a “one bleed at a time” approach.[41] The ECA branches are typically resected first, followed by the intracranial extensions and ICA-supplied segments. These steps are more complex and carry a higher risk of bleeding or stroke, particularly when a branch of the ICA is unsuitable for preoperative embolization.
Depending on the invasive extent and tumor course, resection or drilling of the skull base may be required, with expansion of foramina to achieve adequate access. An example is the expansion of the vidian, or pterygoid, canal to isolate the vidian artery. Even with these extensive endoscopic combination approaches, facial deformity occurs less frequently than with exclusively open techniques.
Surgical resection remains the primary treatment of choice, but adjuvant radiation therapy plays an important role in residual or recurrent disease. This modality is most often used for advanced tumors involving critical structures and cases with intracranial extension that cannot be completely resected.
Local control rates of 85% to 91% have been reported with adjuvant radiotherapy, with a low incidence of severe late complications in these series. Primary radiotherapy has also demonstrated effective control rates of approximately 80%, providing a viable alternative when complete surgical excision is not feasible or safe.[42] Some authors advocate definitive radiotherapy in inoperable cases, achieving long-term disease control rates of 80% to 88% with tumor regression. However, persistent tumor burden is common, and the risk of long-term radiation-related complications remains a concern.[43](B3)
Multiple external radiotherapy modalities are available, including stereotactic radiosurgery (γ knife surgery), intensity-modulated radiotherapy (IMRT) using a conformal technique, and conventional external beam radiotherapy. Reported success rates are similar, primarily in advanced-stage disease (Radkowski stage III). Among these techniques, conformal IMRT appears to balance disease control with reduced long-term morbidity.
Radiation therapy introduces additional concerns for both long-term and postoperative morbidity. Altered tissue quality and increased friability in the surgical bed after radiation can complicate subsequent surgery. Delayed cerebrospinal fluid leaks are an example, with cases of rhinorrhea following resection and stereotactic radiosurgery requiring multiple endoscopic repairs.[44] Although radiation therapy can induce tumor regression, preoperative radiotherapy may increase intraoperative morbidity and complication rates.(B3)
Hormonal therapy, such as androgen receptor blockers like flutamide, is an additional possible adjuvant therapy. These agents have been shown to reduce tumor size prior to surgical resection or upon recurrence, but they do not have curative results on their own.
Limited evidence is available regarding the use of chemotherapy or cytotoxic drugs in the treatment of JNA. Of the little data available, patient responses range from no recurrence to partial response, supporting the need for additional research into cytotoxic drugs as part of a therapeutic regimen.[45]
Differential Diagnosis
Olfactory neuroblastoma, also called esthesioneuroblastoma, is an avidly enhancing nasal cavity mass arising from the olfactory neuroepithelium that can present with symptoms and an age of onset similar to JNA.[46] These tumors may demonstrate a dumbbell appearance on imaging, with intracranial extension and the waist centered at the cribriform plate. Intracranial cysts, restricted diffusion, and areas of necrosis may also be present. Olfactory neuroblastoma occurs much more commonly in female individuals than in patients with JNA.
Rhabdomyosarcoma is a soft tissue sarcoma of striated muscle that, when occurring in the head and neck, most often arises within the orbit but may also involve parameningeal sites such as the nasopharynx, pterygopalatine fossa, middle ear, paranasal sinuses, or parapharyngeal space. This malignancy typically affects younger patients, with 70% of cases occurring before the age of 12 years and 40% before the age of 5 years.[47] Unlike JNA, these tumors generally demonstrate variable mild-to-moderate contrast enhancement, with avid enhancement being atypical.[48] These neoplasms also normally restrict diffusion, which can further help differentiate them from JNA.
Sinonasal polyps are inflammatory lesions that can become hypervascular after repeated injury but have less vascularity than JNA. The most common form is the antrochoanal polyp, which originates in the maxillary sinus and extends through the maxillary ostium into the nasal cavity. Sinonasal polyps may also arise from or extend into the nasopharynx, although they typically do not reach the sphenopalatine foramen or pterygopalatine fossa. Osseous remodeling in this condition is smooth rather than destructive. Imaging demonstrates peripheral enhancement without central enhancement, which differs from the avid enhancement pattern of nasopharyngeal angiofibroma. Similar to JNA, sinonasal polyps may occur in adolescents or young adults and present with nasal obstruction, but they rarely cause epistaxis.
Encephaloceles are meninges-covered outpouchings of brain parenchyma and CSF that protrude through a skull base defect. The nasoethmoidal or nasopharyngeal variants may present as nasal cavity masses. Compared with JNA, these lesions are generally positioned more anteriorly and are nonenhancing.
Nasopharyngeal carcinoma (NPC) is a mucosal tumor that arises in the superolateral aspect of the nasopharynx within the fossa of Rosenmuller. This malignancy predominantly affects adults, with peak incidence between 40 and 60 years, and is rare in pediatric and adolescent patients. Epstein-Barr virus infection has a strong association with the development of NPC. Unlike nasopharyngeal angiofibroma, NPC demonstrates mild homogeneous enhancement rather than avid enhancement. The neoplasm is destructive and tends to infiltrate the parapharyngeal fat and other deep facial soft tissues. Osseous destruction is often more extensive than in nasopharyngeal angiofibroma, with common involvement of the clivus and growth into the cavernous sinus.[49]
Radiation Oncology
As described above, radiotherapy is primarily used as an adjuvant treatment in cases of residual or recurrent disease. This intervention may serve as the primary treatment modality when tumors are unresectable due to the extent of invasion or involvement of critical structures. Reported local control rates with radiation alone range from 73% to 87.5%.
External beam radiation is typically delivered as IMRT using conformal techniques to minimize exposure to nearby structures such as the optic nerves, optic chiasm, lens, retina, brain, brainstem, spinal cord, and salivary glands. Case series describe prescribed dose ranges of 30 to 50 Gy, administered in daily fractions of 1.8 to 2 Gy.
Stereotactic radiotherapy or radiosurgery has also been employed, particularly for well-defined residual or recurrent disease. This modality carries a proposed higher risk of late complications because of the single high-dose delivery over a tight distribution area. Successful use of γ knife radiosurgery has been reported, including a case involving delivery of 17 Gy to the 50% isodense line at the tumor margin for a recurrent lesion measuring 6.8 cm³. However, data on the efficacy and long-term outcomes of stereotactic radiosurgery are limited.
Staging
Several methods have been proposed for staging and classifying nasopharyngeal angiofibroma to guide surgical decision-making and adjunctive treatment. The Radkowski and Andrews-Fisch systems are the most widely used, as both correlate well with surgical approach, recurrence, and outcome. The University of Pittsburgh Medical Center classification was introduced more recently, emphasizing endoscopic staging in line with contemporary surgical techniques. All systems primarily determine stage based on anatomic extent.
The Radkowski system is divided into 3 stages, each with subcategories. Stage Ia disease is confined to the nasal cavity and nasopharyngeal vault, whereas extension into the paranasal sinuses defines stage Ib. Stage II disease is characterized by extension into the pterygopalatine fossa. Stage IIa is marked by minimal extension, stage IIb comprises complete fossa involvement, and stage IIc is characterized by involvement of the infratemporal fossa or posterior to the pterygoid plates. Stage III disease reflects advanced growth affecting the skull base and intracranial structures. Stage IIIa is defined by minimal extension into the middle cranial fossa or pterygoid plates, while stage IIIb indicates intracranial extension with or without cavernous sinus invasion.[50]
The Andrews-Fisch system is similar, with an extra stage delineating the intracranial extent. Stage I is limited to the nasal cavity and nasopharyngeal vault. Stage II includes pterygopalatine fossa or any sinus invasion. Stage IIIa involves extension into the infratemporal fossa or orbital invasion, whereas stage IIIb describes intracranial, extradural extension into the parasellar region. Intradural extension denotes a more advanced stage IV disease, with cavernous sinus, pituitary fossa, or optic chiasm invasion separating stage IVa from stage IVb.[51]
Other proposed classifications are less commonly used or remain too new to have their effectiveness relative to established systems assessed. Examples include the Sessions, Chandler, and Instituto Nacional de Cancerología (INCan) staging methods. The most recent classification was introduced in 2010 by a group from the University of Pittsburgh Medical Center, based on endoscopic staging.[52] This system classifies JNA into 5 stages, with greater emphasis on residual vascularity, primarily from the ICA after preoperative embolization. Tumor size and paranasal sinus invasion are deemphasized, as they are considered limited predictors of outcome after resection.
Stage I disease is confined to the nasal cavity and nasopharynx. Stage II involves the paranasal sinuses and lateral pterygopalatine fossa without residual vascularity. Stage III and IV disease are defined by skull base erosion with involvement of the orbit and infratemporal fossa, distinguished by the presence of ICA vascular supply in stage IV. Stage V disease is subdivided according to laterality, with medial [stage V(M)] versus lateral [stage V(L)] intracranial extension and associated residual vascularity.
Prognosis
Nasopharyngeal angiofibroma is a benign tumor and generally carries a favorable prognosis. However, advanced disease that precludes complete resection or leads to recurrence is the primary concern. Literature reports that up to 33% of advanced (Radkowski stage III) cases are unresectable. Recurrence affects 30% to 38% of patients undergoing resection, contributing to additional morbidity from residual or recurrent tumor growth and local invasion. Adjuvant radiotherapy is occasionally employed for residual or recurrent disease.
Rare reports describe secondary malignancies, such as basal cell or squamous cell carcinoma, within the radiation field. Malignant transformation of JNA is uncommon but has been documented, primarily as well-differentiated tumors after radiotherapy. Undifferentiated sarcomatous transformation has also been reported, resulting in a poorer prognosis.
Complications
The most significant complication of JNA is perioperative blood loss, which can be fatal without appropriate precautions. Orbital invasion by the tumor can result in exophthalmos, facial or orbital deformity, vision loss, and impaired extraocular movements. Vision loss may also occur as a complication of nontarget embolization when ICA branches are involved. Other serious risks of preoperative embolization include arterial vasospasm, facial palsy, infarction, and cranial nerve injury. Less severe, generally self-limiting complications include facial swelling, pain or altered sensation, headache, and nausea or vomiting. Surgical complications overlap with these embolization-related adverse events and may additionally include scarring and facial deformity. Hormonal therapy, when used, carries the risk of feminization in adolescent boys, which may be considered undesirable.
Postoperative and Rehabilitation Care
Imaging and clinical surveillance are essential to distinguish residual from recurrent disease. Assessing for residual disease is challenging, as the key imaging feature—tumor or tissue enhancement—may also be present in postoperative granulation tissue, particularly on early studies. Therefore, clinical examination and symptoms play a central role, serving as the primary means of evaluation or an adjunct to imaging. Most recurrences are reported within 6 to 36 months after surgery, warranting annual or biannual follow-up imaging for at least 4 years.
Consultations
When a pediatrician, family physician, or other primary care provider suspects nasopharyngeal angiofibroma, imaging should be obtained along with an otolaryngology referral. Further evaluation by otolaryngology with nasal endoscopy and imaging is warranted. Consultation with interventional radiology or neuroendovascular surgery is also recommended during preoperative planning, particularly if preoperative embolization is anticipated.
Deterrence and Patient Education
Patients should be informed that while nosebleeds are common, frequent, recurrent, and unprovoked episodes warrant further evaluation. Although nasopharyngeal angiofibroma is a benign tumor, treatment requires surgical resection and, in some cases, adjuvant radiation therapy. A thorough clinical and imaging evaluation by an otolaryngologist is the essential first step in management.
Pearls and Other Issues
JNA is diagnosed based on clinical and imaging findings. Recognizing demographic factors and characteristic presentations can facilitate earlier diagnosis and treatment. Since the tumor grows slowly, it may remain asymptomatic for extended periods, and patients often present with more advanced disease. The indolent course also explains the chronicity of symptoms, which typically persist for 6 months or longer.
Early imaging and referral to a specialist allow timely treatment, primarily through surgical resection. Preoperative embolization is frequently employed to reduce blood loss and surgical morbidity. Radiation, hormonal therapy, and chemotherapy serve largely adjuvant roles in cases of recurrence or residual disease. These modalities are also utilized occasionally to reduce tumor burden before surgery. Hormonal and cytotoxic therapies remain investigational, as data on their efficacy are limited.
Enhancing Healthcare Team Outcomes
The diagnosis of JNA is usually straightforward but is best managed within an interprofessional team. This team may include the primary care provider (pediatrician, family medicine physician, internist, physician assistant, or nurse practitioner), radiologist, otolaryngologist, neurosurgeon, interventional radiologist, radiation oncologist, anesthesiologist, pathologist, and oncology-trained nurses.
Patient and family education is essential. Families should be aware of the classic signs and symptoms of nasopharyngeal angiofibroma. Recurrent unprovoked epistaxis or persistent nasal obstruction unresponsive to allergy therapy in an adolescent boy warrants prompt evaluation by a primary care provider.
Patients should also be counseled on available treatment options, potential complications, and the significant bleeding risk associated with surgery. Education should emphasize that this tumor is benign and carries a high cure rate and long-term survival when treated appropriately. Nursing staff with appropriate training play a key role in providing education, supporting families, and coordinating follow-up care.
The primary treatment for nasopharyngeal angiofibroma is surgical resection. Depending on stage and tumor characteristics, preoperative embolization or adjuvant radiotherapy may be required. Treatment failure is defined by recurrence, which affects up to 37% of patients. Most recurrences develop within the first 3 years after surgery, necessitating annual or biannual clinical and imaging surveillance for at least 4 years. Recurrence may prompt additional surgery or radiotherapy, which may improve local control but increases the risk of treatment-related complications. An interprofessional team approach, involving physicians, subspecialists, and specialty-trained nurses, is essential to optimize patient safety, treatment outcomes, and long-term care.
Media
(Click Image to Enlarge)
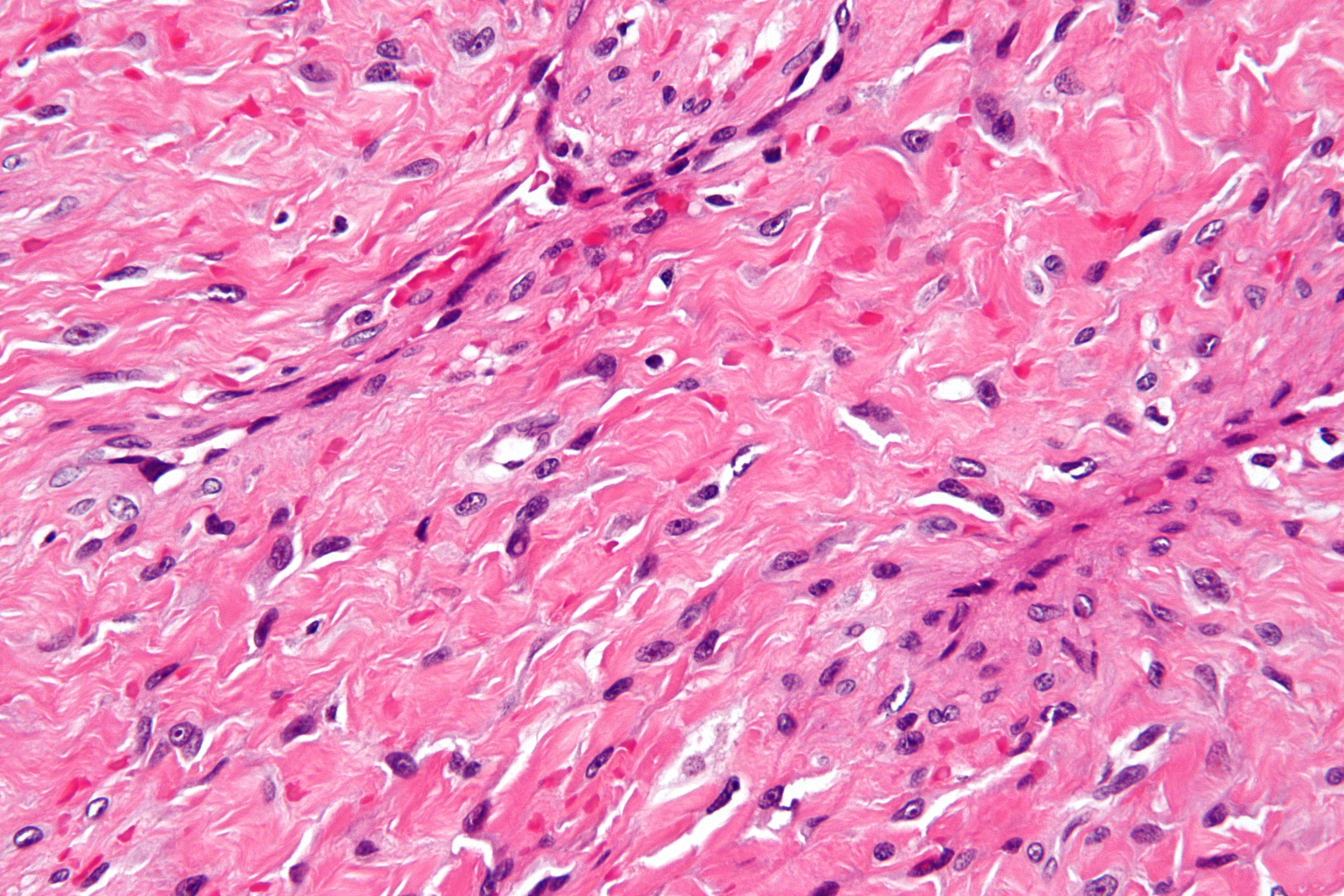
Nasopharyngeal Angiofibroma Histopathology. The image highlights the distinctive staghorn-shaped blood vessels and abundant fibrous stroma typical of this highly vascular tumor.
Nephron, Public Domain, via Wikimedia Commons
(Click Image to Enlarge)
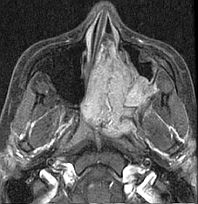
Nasopharyngeal Angiofibroma on Computed Tomography Angiography. The scan provided a detailed visualization of the tumor’s extensive arterial supply, aiding in the evaluation of tumor extent and presurgical vascular control.
Fabio Hared, Public Domain, via Wikimedia Commons
References
Makhasana JA, Kulkarni MA, Vaze S, Shroff AS. Juvenile nasopharyngeal angiofibroma. Journal of oral and maxillofacial pathology : JOMFP. 2016 May-Aug:20(2):330. doi: 10.4103/0973-029X.185908. Epub [PubMed PMID: 27601836]
López F, Triantafyllou A, Snyderman CH, Hunt JL, Suárez C, Lund VJ, Strojan P, Saba NF, Nixon IJ, Devaney KO, Alobid I, Bernal-Sprekelsen M, Hanna EY, Rinaldo A, Ferlito A. Nasal juvenile angiofibroma: Current perspectives with emphasis on management. Head & neck. 2017 May:39(5):1033-1045. doi: 10.1002/hed.24696. Epub 2017 Feb 15 [PubMed PMID: 28199045]
Level 3 (low-level) evidenceSzymańska A, Szymański M, Czekajska-Chehab E, Szczerbo-Trojanowska M. Invasive growth patterns of juvenile nasopharyngeal angiofibroma: radiological imaging and clinical implications. Acta radiologica (Stockholm, Sweden : 1987). 2014 Jul:55(6):725-31. doi: 10.1177/0284185113506189. Epub 2013 Oct 16 [PubMed PMID: 24132768]
McKnight CD, Parmar HA, Watcharotone K, Mukherji SK. Reassessing the Anatomic Origin of the Juvenile Nasopharyngeal Angiofibroma. Journal of computer assisted tomography. 2017 Jul/Aug:41(4):559-564. doi: 10.1097/RCT.0000000000000566. Epub [PubMed PMID: 28632604]
Allensworth JJ, Troob SH, Lanciault C, Andersen PE. High-grade malignant transformation of a radiation-naïve nasopharyngeal angiofibroma. Head & neck. 2016 Apr:38 Suppl 1():E2425-7. doi: 10.1002/hed.24378. Epub 2016 Feb 3 [PubMed PMID: 26841332]
Park CK, Kim DG, Paek SH, Chung HT, Jung HW. Recurrent juvenile nasopharyngeal angiofibroma treated with gamma knife surgery. Journal of Korean medical science. 2006 Aug:21(4):773-7 [PubMed PMID: 16891831]
Level 3 (low-level) evidenceMallick S, Benson R, Bhasker S, Mohanti BK. Long-term treatment outcomes of juvenile nasopharyngeal angiofibroma treated with radiotherapy. Acta otorhinolaryngologica Italica : organo ufficiale della Societa italiana di otorinolaringologia e chirurgia cervico-facciale. 2015 Apr:35(2):75-9 [PubMed PMID: 26019389]
Overdevest JB, Amans MR, Zaki P, Pletcher SD, El-Sayed IH. Patterns of vascularization and surgical morbidity in juvenile nasopharyngeal angiofibroma: A case series, systematic review, and meta-analysis. Head & neck. 2018 Feb:40(2):428-443. doi: 10.1002/hed.24987. Epub 2017 Nov 11 [PubMed PMID: 29130560]
Level 2 (mid-level) evidenceMehan R, Rupa V, Lukka VK, Ahmed M, Moses V, Shyam Kumar NK. Association between vascular supply, stage and tumour size of juvenile nasopharyngeal angiofibroma. European archives of oto-rhino-laryngology : official journal of the European Federation of Oto-Rhino-Laryngological Societies (EUFOS) : affiliated with the German Society for Oto-Rhino-Laryngology - Head and Neck Surgery. 2016 Dec:273(12):4295-4303. doi: 10.1007/s00405-016-4136-9. Epub 2016 Jun 11 [PubMed PMID: 27289235]
Alshaikh NA, Eleftheriadou A. Juvenile nasopharyngeal angiofibroma staging: An overview. Ear, nose, & throat journal. 2015 Jun:94(6):E12-22 [PubMed PMID: 26053985]
Level 3 (low-level) evidenceEl-Shaarawy EAA, Hassan SS. The sphenopalatine foramen in man: anatomical, radiological and endoscopic study. Folia morphologica. 2018:77(2):345-355. doi: 10.5603/FM.a2017.0104. Epub 2017 Nov 13 [PubMed PMID: 29131280]
Mishra A, Jaiswal R, Amita P, Mishra SC. Molecular interactions in juvenile nasopharyngeal angiofibroma: preliminary signature and relevant review. European archives of oto-rhino-laryngology : official journal of the European Federation of Oto-Rhino-Laryngological Societies (EUFOS) : affiliated with the German Society for Oto-Rhino-Laryngology - Head and Neck Surgery. 2019 Jan:276(1):93-100. doi: 10.1007/s00405-018-5178-y. Epub 2018 Nov 1 [PubMed PMID: 30387011]
Pandey P, Mishra A, Tripathi AM, Verma V, Trivedi R, Singh HP, Kumar S, Patel B, Singh V, Pandey S, Pandey A, Mishra SC. Current molecular profile of juvenile nasopharyngeal angiofibroma: First comprehensive study from India. The Laryngoscope. 2017 Mar:127(3):E100-E106. doi: 10.1002/lary.26250. Epub 2016 Aug 31 [PubMed PMID: 27577998]
Marshall AH, Bradley PJ. Management dilemmas in the treatment and follow-up of advanced juvenile nasopharyngeal angiofibroma. ORL; journal for oto-rhino-laryngology and its related specialties. 2006:68(5):273-8 [PubMed PMID: 16682808]
Hwang HC, Mills SE, Patterson K, Gown AM. Expression of androgen receptors in nasopharyngeal angiofibroma: an immunohistochemical study of 24 cases. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 1998 Nov:11(11):1122-6 [PubMed PMID: 9831211]
Level 3 (low-level) evidenceBubley JA, Yeung H, Cole E, Amin M, Parker D, Arbiser JL. Angiofibroma stimulation in a transgender person receiving gender-affirming testosterone. JAAD case reports. 2020 Oct:6(10):1101-1103. doi: 10.1016/j.jdcr.2020.06.016. Epub 2020 Jun 17 [PubMed PMID: 33005714]
Level 3 (low-level) evidenceRiggs S, Orlandi RR. Juvenile nasopharyngeal angiofibroma recurrence associated with exogenous testosterone therapy. Head & neck. 2010 Jun:32(6):812-5. doi: 10.1002/hed.21152. Epub [PubMed PMID: 19626637]
Montag AG, Tretiakova M, Richardson M. Steroid hormone receptor expression in nasopharyngeal angiofibromas. Consistent expression of estrogen receptor beta. American journal of clinical pathology. 2006 Jun:125(6):832-7 [PubMed PMID: 16690481]
Ralli M, Fusconi M, Visconti IC, Martellucci S, de Vincentiis M, Greco A. Nasopharyngeal angiofibroma in an elderly female patient: A rare case report. Molecular and clinical oncology. 2018 Dec:9(6):702-704. doi: 10.3892/mco.2018.1735. Epub 2018 Oct 5 [PubMed PMID: 30546905]
Level 3 (low-level) evidenceSchick B, Veldung B, Wemmert S, Jung V, Montenarh M, Meese E, Urbschat S. p53 and Her-2/neu in juvenile angiofibromas. Oncology reports. 2005 Mar:13(3):453-7 [PubMed PMID: 15706416]
Kumari K, Afroj S, Madhry D, Verma Y, Kairo AK, Thakar A, Sikka K, Verma H, Verma B. Comprehensive Analysis of Juvenile Nasopharyngeal Angiofibromas via Whole-Exome Sequencing. Genes, chromosomes & cancer. 2024 Sep:63(9):e23265. doi: 10.1002/gcc.23265. Epub [PubMed PMID: 39297564]
Coutinho-Camillo CM, Brentani MM, Nagai MA. Genetic alterations in juvenile nasopharyngeal angiofibromas. Head & neck. 2008 Mar:30(3):390-400. doi: 10.1002/hed.20775. Epub [PubMed PMID: 18228521]
Guertl B, Beham A, Zechner R, Stammberger H, Hoefler G. Nasopharyngeal angiofibroma: an APC-gene-associated tumor? Human pathology. 2000 Nov:31(11):1411-3 [PubMed PMID: 11112217]
Pegues J, McCown ET, Buck LS, Carron JD. Juvenile Nasopharyngeal Angiofibroma and Familial Adenomatous Polyposis. Ear, nose, & throat journal. 2021 Dec:100(10_suppl):1027S-1028S. doi: 10.1177/0145561320934602. Epub 2020 Jun 16 [PubMed PMID: 32543227]
Mishra A, Sachadeva M, Jain A, Shukla NM, Pandey A. Human Papilloma virus in Juvenile Nasopharyngeal Angiofibroma: possible recent trend. American journal of otolaryngology. 2016 Jul-Aug:37(4):317-22. doi: 10.1016/j.amjoto.2016.03.001. Epub 2016 Mar 8 [PubMed PMID: 27157983]
Dickinson A, Xu M, Silén S, Wang Y, Fu Y, Sadeghi M, Toppinen M, Carpén T, Hedman K, Mäkitie A, Söderlund-Venermo M. Newly detected DNA viruses in juvenile nasopharyngeal angiofibroma (JNA) and oral and oropharyngeal squamous cell carcinoma (OSCC/OPSCC). European archives of oto-rhino-laryngology : official journal of the European Federation of Oto-Rhino-Laryngological Societies (EUFOS) : affiliated with the German Society for Oto-Rhino-Laryngology - Head and Neck Surgery. 2019 Feb:276(2):613-617. doi: 10.1007/s00405-018-5250-7. Epub 2018 Dec 21 [PubMed PMID: 30578435]
Tan G, Ma Z, Long W, Liu L, Zhang B, Chen W, Yang J, Li H. Efficacy of Preoperative Transcatheter Arterial Embolization for Nasopharyngeal Angiofibroma: A Comparative Study. Cardiovascular and interventional radiology. 2017 Jun:40(6):836-844. doi: 10.1007/s00270-017-1587-3. Epub 2017 Feb 7 [PubMed PMID: 28175976]
Level 2 (mid-level) evidenceMcGarey PO Jr, David AP, Payne SC. Nasopharyngeal angiofibroma in a 32-year-old man. BMJ case reports. 2018 Feb 8:2018():. pii: bcr-2017-222763. doi: 10.1136/bcr-2017-222763. Epub 2018 Feb 8 [PubMed PMID: 29437803]
Level 3 (low-level) evidenceYi Z, Fang Z, Lin G, Lin C, Xiao W, Li Z, Cheng J, Zhou A. Nasopharyngeal angiofibroma: a concise classification system and appropriate treatment options. American journal of otolaryngology. 2013 Mar-Apr:34(2):133-41. doi: 10.1016/j.amjoto.2012.10.004. Epub 2013 Jan 16 [PubMed PMID: 23332298]
Beham A, Regauer S, Beham-Schmid C, Kainz J, Stammberger H. Expression of CD34-antigen in nasopharyngeal angiofibromas. International journal of pediatric otorhinolaryngology. 1998 Aug 1:44(3):245-50 [PubMed PMID: 9780070]
Wang JJ, Sun XC, Hu L, Liu ZF, Yu HP, Li H, Wang SY, Wang DH. Endoglin (CD105) expression on microvessel endothelial cells in juvenile nasopharyngeal angiofibroma: tissue microarray analysis and association with prognostic significance. Head & neck. 2013 Dec:35(12):1719-25. doi: 10.1002/hed.23210. Epub 2013 Mar 8 [PubMed PMID: 23471755]
Lloyd G, Howard D, Phelps P, Cheesman A. Juvenile angiofibroma: the lessons of 20 years of modern imaging. The Journal of laryngology and otology. 1999 Feb:113(2):127-34 [PubMed PMID: 10396561]
Singh RK, Lakhkar BB, Patwa PA, Mishra GV. Juvenile nasopharyngeal angiofibroma. BMJ case reports. 2022 Mar 8:15(3):. doi: 10.1136/bcr-2021-248023. Epub 2022 Mar 8 [PubMed PMID: 35260405]
Level 3 (low-level) evidenceDillard DG, Cohen C, Muller S, Del Gaudio J, Reichman O, Parrish B, Rackley D, Gal AA. Immunolocalization of activated transforming growth factor beta1 in juvenile nasopharyngeal angiofibroma. Archives of otolaryngology--head & neck surgery. 2000 Jun:126(6):723-5 [PubMed PMID: 10864108]
Das A, Bhalla AS, Sharma R, Kumar A, Thakar A, Vishnubhatla SM, Sharma MC, Sharma SC. Can Diffusion Weighted Imaging Aid in Differentiating Benign from Malignant Sinonasal Masses?: A Useful Adjunct. Polish journal of radiology. 2017:82():345-355. doi: 10.12659/PJR.900633. Epub 2017 Jun 28 [PubMed PMID: 28740564]
Alimli AG, Ucar M, Oztunali C, Akkan K, Boyunaga O, Damar C, Derinkuyu B, Tokgöz N. Juvenile Nasopharyngeal Angiofibroma: Magnetic Resonance Imaging Findings. Journal of the Belgian Society of Radiology. 2016 Jun 1:100(1):63. doi: 10.5334/jbr-btr.1090. Epub 2016 Jun 1 [PubMed PMID: 30038985]
Mishra S, Praveena NM, Panigrahi RG, Gupta YM. Imaging in the diagnosis of juvenile nasopharyngeal angiofibroma. Journal of clinical imaging science. 2013:3(Suppl 1):1. doi: 10.4103/2156-7514.109469. Epub 2013 Mar 22 [PubMed PMID: 23878770]
Mair EA, Battiata A, Casler JD. Endoscopic laser-assisted excision of juvenile nasopharyngeal angiofibromas. Archives of otolaryngology--head & neck surgery. 2003 Apr:129(4):454-9 [PubMed PMID: 12707194]
Level 3 (low-level) evidenceGołąbek W, Szymańska A, Morshed K. Transnasal microscopic approach for juvenile nasopharyngeal angiofibroma. Otolaryngologia polska = The Polish otolaryngology. 2018 Aug 6:72(5):31-36. doi: 10.5604/01.3001.0012.2303. Epub [PubMed PMID: 30460914]
Ye D, Shen Z, Wang G, Deng H, Qiu S, Zhang Y. Analysis of factors in successful nasal endoscopic resection of nasopharyngeal angiofibroma. Acta oto-laryngologica. 2016:136(2):205-13. doi: 10.3109/00016489.2015.1099734. Epub 2015 Oct 23 [PubMed PMID: 26492972]
Level 2 (mid-level) evidenceSnyderman CH, Pant H. Endoscopic Management of Vascular Sinonasal Tumors, Including Angiofibroma. Otolaryngologic clinics of North America. 2016 Jun:49(3):791-807. doi: 10.1016/j.otc.2016.02.009. Epub [PubMed PMID: 27267026]
Lu TX, Mai WY, Teh BS, Zhao C, Han F, Huang Y, Deng XW, Lu LX, Huang SM, Zeng ZF, Lin CG, Lu HH, Chiu JK, Carpenter LS, Grant WH 3rd, Woo SY, Cui NJ, Butler EB. Initial experience using intensity-modulated radiotherapy for recurrent nasopharyngeal carcinoma. International journal of radiation oncology, biology, physics. 2004 Mar 1:58(3):682-7 [PubMed PMID: 14967420]
Wiatrak BJ, Koopmann CF, Turrisi AT. Radiation therapy as an alternative to surgery in the management of intracranial juvenile nasopharyngeal angiofibroma. International journal of pediatric otorhinolaryngology. 1993 Dec:28(1):51-61 [PubMed PMID: 8300314]
Level 3 (low-level) evidenceMin HJ, Chung HJ, Kim CH. Delayed cerebrospinal fluid rhinorrhea four years after gamma knife surgery for juvenile angiofibroma. The Journal of craniofacial surgery. 2014 Nov:25(6):e565-7. doi: 10.1097/SCS.0000000000001164. Epub [PubMed PMID: 25377982]
Level 3 (low-level) evidenceScholfield DW, Brundler MA, McDermott AL, Mussai F, Kearns P. Adjunctive Treatment in Juvenile Nasopharyngeal Angiofibroma: How Should We Approach Recurrence? Journal of pediatric hematology/oncology. 2016 Apr:38(3):235-9. doi: 10.1097/MPH.0000000000000524. Epub [PubMed PMID: 26907644]
Fiani B, Quadri SA, Cathel A, Farooqui M, Ramachandran A, Siddiqi I, Ghanchi H, Zafar A, Berman BW, Siddiqi J. Esthesioneuroblastoma: A Comprehensive Review of Diagnosis, Management, and Current Treatment Options. World neurosurgery. 2019 Jun:126():194-211. doi: 10.1016/j.wneu.2019.03.014. Epub 2019 Mar 9 [PubMed PMID: 30862589]
Level 2 (mid-level) evidenceItamochi H, Ariga H, Shiga K, Uesugi N, Sugai T. Primary rhabdomyosarcoma of the ethmoid sinus with orbital extension and metastasis to the pancreatic body. Clinical case reports. 2022 Aug:10(8):e04149. doi: 10.1002/ccr3.4149. Epub 2021 May 4 [PubMed PMID: 36052027]
Level 3 (low-level) evidenceJawad N, McHugh K. The clinical and radiologic features of paediatric rhabdomyosarcoma. Pediatric radiology. 2019 Oct:49(11):1516-1523. doi: 10.1007/s00247-019-04386-5. Epub 2019 Oct 16 [PubMed PMID: 31620851]
King AD. MR Imaging of Nasopharyngeal Carcinoma. Magnetic resonance imaging clinics of North America. 2022 Feb:30(1):19-33. doi: 10.1016/j.mric.2021.06.015. Epub [PubMed PMID: 34802578]
Radkowski D, McGill T, Healy GB, Ohlms L, Jones DT. Angiofibroma. Changes in staging and treatment. Archives of otolaryngology--head & neck surgery. 1996 Feb:122(2):122-9 [PubMed PMID: 8630204]
Andrews JC, Fisch U, Valavanis A, Aeppli U, Makek MS. The surgical management of extensive nasopharyngeal angiofibromas with the infratemporal fossa approach. The Laryngoscope. 1989 Apr:99(4):429-37 [PubMed PMID: 2538688]
Snyderman CH, Pant H, Carrau RL, Gardner P. A new endoscopic staging system for angiofibromas. Archives of otolaryngology--head & neck surgery. 2010 Jun:136(6):588-94. doi: 10.1001/archoto.2010.83. Epub [PubMed PMID: 20566910]