Introduction
A massive transfusion involves the administration of 10 units or more of whole blood or packed red blood cells (PRBCs) within 24 hours.[1] During an ultra-massive transfusion, clinicians administer more than 20 units of PRBCs within a 24- to 48-hour period.[2] The traditional definition of massive transfusion is arbitrary and originally chosen because 10 units approximates the total blood volume of an average adult. However, this does not account for differences in patient size or the rate of blood loss. In clinical practice, some patients may require fewer units given rapidly and still face a life-threatening hemorrhage. In contrast, others may receive more than 10 units in a controlled setting without the same urgency. Relying solely on this definition risks delayed recognition of critical bleeding. For this reason, other approaches favor more dynamic criteria, such as transfusing more than 3 to 4 units within 1 hour with ongoing bleeding, replacing more than 50% of blood volume within 3 hours, or activating massive transfusion protocols guided by tools like the Assessment of Blood Consumption (ABC) score. These updated definitions better reflect the urgency and variability of massive hemorrhage and improve timely intervention.
The primary objective of a massive transfusion is to prevent fatal outcomes resulting from critical hypoperfusion-related complications while striving to attain hemostasis.[3] Patients require massive transfusions across multiple medical specialties. While cardiac and vascular surgeries represent the most common indications, gastrointestinal bleeding, obstetric hemorrhage, liver transplantation, and trauma are also frequent causes. In trauma care, only a small portion of patients need massive transfusion—about 3% to 5% of those treated after civilian injuries and up to 10% of those injured in military settings.[4] Although uncommon, these situations carry a high risk of death.
Because massive transfusions are unpredictable and require large volumes of blood products over extended periods, coordination in advance among the emergency department, trauma service, surgical team, blood bank, and delivery personnel is essential. The ABC score can help predict the need for massive transfusion. Continuous monitoring of volume status, tissue oxygenation, bleeding, coagulation, and acid–base balance is critical during these events.[5] Establishing and implementing massive transfusion protocols reduces mortality and optimizes blood product use.
Transfusing with typed and crossmatched blood is ideal. However, this is often not possible. Type O RhD-negative blood is the universal donor, and all individuals can receive this type. Clinicians should ensure that patients with childbearing potential receive O-negative blood. If necessary, patients without childbearing potential can receive O-positive blood. Because blood may be scarce, some sites may utilize low-titer group O whole blood (LTOWB).[6][7][8] LTOWB is a leukocyte-reduced whole blood product containing packed RBCs, platelets, and plasma from a single blood group O donor with notably low levels of anti-A and anti-B antibodies. LTOWB reduces the risk of transfusion reactions due to the low levels of antibodies.
Protocols should also include clear criteria, such as hemodynamic stability, control of active bleeding, and normalization of laboratory values, for deactivating the massive transfusion process to avoid unnecessary transfusion and conserve resources. Ultimately, the success of a massive transfusion depends not only on timely access to blood products but also on structured protocols, interprofessional teamwork, and vigilant monitoring for adverse effects. By coordinating across disciplines and employing evidence-based strategies, healthcare teams can improve survival, reduce complications, and ensure the best possible outcomes for patients facing life-threatening hemorrhage.
Indications
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Indications
Any situation resulting in acute blood loss and hemodynamic instability is a potential indication for a massive transfusion. Some scenarios that may lead to a massive transfusion include trauma-related bleeding, obstetrical hemorrhage, surgery, and gastrointestinal bleeding.[9][10] Efforts to mitigate the uncertainty surrounding the timing and necessity of massive transfusions, such as the Shock Index, offer limited value.[11]
However, the ABC score is a validated clinical tool based on 4 variables: heart rate higher than 120 bpm, systolic blood pressure below 90 mm Hg, a positive FAST exam, and penetrating torso injury. Each variable scores 1 point. A score of 2 or higher suggests the patient is at high risk for requiring massive transfusion and should prompt activation of a massive transfusion protocol.
The ABC score has a positive predictive value of approximately 48% to 58%, meaning that nearly half of patients meeting criteria may not ultimately need massive transfusion. However, it demonstrates high sensitivity and a negative predictive value between 85% and 96%, making it particularly reliable at identifying patients who are unlikely to require massive transfusion.[12][13]
According to the American College of Surgeons, at least 1 of the following criteria is necessary to activate a massive transfusion protocol:
- An ABC score of 2 or more
- Persistent hemodynamic instability
- Active bleeding requiring surgery or angioembolization
- Blood transfusion in the trauma bay
Contraindications
There are no absolute contraindications for a massive transfusion when clinically indicated because, by definition, transfusion is a life-saving intervention for patients with life-threatening hemorrhage. If a patient is bleeding to the point of hemodynamic collapse, withholding transfusion would almost certainly be fatal.
Equipment
The availability of blood products and the establishment of reliable intravenous or intraosseous access are critical components of massive transfusion. According to the Hagen-Poiseuille equation, flow rate is directly proportional to the fourth power of the catheter radius and inversely proportional to its length and fluid viscosity. Thus, large-bore, short catheters, 14 to 18 gauge, provide the highest flow rates, making them the preferred choice for rapid transfusion. Peripheral IVs can be effective, but central venous access may provide more stable, long-term access. Standard triple-lumen central lines are not ideal for rapid transfusion because their narrow, long lumens significantly reduce flow rates. Alternatives include introducer sheaths (Cordis catheters), large-bore multilumen central lines, or hemodialysis catheters, which permit higher flow. For peripheral administration, rapid infusion catheters, large-bore devices inserted using a needle, guidewire, and catheter to insert (Seldinger technique), can also provide excellent flow in emergent settings.
Necessary Equipment
- Posted clear activation and deactivation criteria
- Large-bore peripheral IVs
- Rapid infusion catheter
- Cordis catheter or dialysis catheter
- Intraosseous needle or catheter
- Rapid infuser with blood warmer
- In-line fluid warmers and forced-air warming blanket
- Blood refrigerator with a minimum of 8 units of O-negative or O-positive PRBCs or LTOWB
- 8 units of thawed group AB or low-titer anti-B group A plasma
- In-line blood filters, Y-sets, high-flow tubing, and pressure bags
- IV poles and multiple infusion pumps
- Arterial line kit for continuous blood pressure monitoring
- Cell salvage/autotransfusion setup, especially in the operating room (See Image: Autotransfusion Set Up. ATS bags and atrium)
- Pelvic binder, tourniquets, hemostatic dressings
- Ultrasound for FAST examination and line placement
- Foley catheter
- Intubation kit
- Personal protective equipment
- Suction
- Continuous core temperature, blood pressure, heart rate, respiratory rate, and blood oxygen monitors
- NG tube as indicated
- A Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA) kit as necessary
- Uterine balloon for postpartum hemorrhage, uterine atony, or placenta accreta
Point of Care and Laboratory Testing
- Arterial blood gas;
- Lactate;
- Hemoglobin and hematocrit;
- Ionized calcium;
- Magnesium;
- Glucose;
- Potassium;
- PT/INR;
- aPTT;
- Fibrinogen;
- Thromboelastography (TEG) and rotational thromboelastometry (ROTEM) clotting time, when available, to detect fibrinolysis and potential need for tranexamic acid, as well as the later appearance of hypercoagulability; and
- Type and crossmatch with an antibody screen.[14][15]
Clinicians should monitor coagulation studies, fibrinogen, TEG, ROTEM, lactate, hemoglobin, and hematocrit every 30 to 60 minutes or per the hospital protocol. Please see StatPearls' companion topic, "Thromboelastography," for an in-depth discussion regarding TEG.
Medications
- Tranexamic acid or aminocaproic acid;
- Lactated Ringer solution;
- Cryoprecipitate or fibrinogen concentrate;
- Vasopressors such as norepinephrine and vasopressin;
- Idarucizumab, a reversal agent for dabigatran;
- Andexanet α, for life-threatening bleeding associated with factor Xa inhibitors;
- 4-factor or 3-factor unactivated prothrombin complex concentrate (PCC);
- Vitamin K for warfarin reversal;
- Insulin and glucose to treat hyperkalemia;
- Magnesium sulfate in the event of magnesium depletion;
- Oxytocin, carboprost, methylergonovine, and RhIG for Rh-negative patients with fetomaternal hemorrhage;
- Calcium chloride or calcium gluconate to treat hypocalcemia due to citrate in blood products; and
- Bicarbonate for severe metabolic acidosis.[14][15][16]
Personnel
Massive transfusions necessitate the coordination of the emergency department, trauma and surgical teams, laboratory personnel, hospitalists, and blood banks. Many institutions use an alert system similar to those used in trauma care, which notifies the requisite personnel that a massive transfusion is imminent or in progress. Essential personnel include the following:
- A designated team leader and time keeper.
- A transfusion medicine specialist available for consultation.
- A pharmacist available for consultation regarding medications and reversal agents.
- Adequate bedside staff for patient monitoring, blood product administration, and procedural assistance.
- Blood product runner and blood bank technologist.
- Blood bank personnel trained in antibody investigation for patients who test antibody positive.
- Respiratory therapy, operating room, and interventional radiology on stand-by
- Obstetrical specialist for pregnant patients.
- A gastroenterology specialist for gastrointestinal bleeding.
- Appropriate surgical specialty for patients requiring surgery.
Preparation
The most effective preparation involves having a major transfusion protocol in place, with immediate notification of the blood bank being of utmost importance. Swift activation of the protocol enables the blood bank to proactively prepare and deliver the necessary products before they are required. Additionally, healthcare professionals must ensure proper respiratory and cardiac monitoring for patients, as well as adequate IV or intraosseous access for blood product delivery.
Technique or Treatment
During massive transfusion, the primary goals are to maintain cardiac output and ensure adequate oxygen-carrying capacity. Institution-specific protocols help standardize the rapid ordering and delivery of blood products, though processes may vary. Major transfusion protocols should prioritize PRBCs given in combination with platelets and plasma.[16][17][18]
Under normal conditions, the body delivers 4 times more oxygen than tissues actually use at rest. This reserve means tissues can still get enough oxygen even when hemoglobin, and therefore oxygen-carrying capacity, drops, as long as the body maintains blood volume and cardiac function. To compensate for low hemoglobin, the body increases cardiac output and extracts more oxygen from the blood. If the hemoglobin drops too low, the heart has to work harder to pump blood. This increased cardiac work itself raises oxygen demand, eventually reaching a critical point where oxygen delivery is no longer enough, and tissue hypoxia occurs. The American Society of Anesthesiologists recommends that healthy individuals do not have a hemoglobin level below 6 g/dL, and older individuals or those with cardiovascular disease maintain a hemoglobin level of 8 g/dL or more.[19]
Crystalloid solutions can help restore intravascular volume, which helps maintain cardiac output and blood pressure after bleeding. However, large volume resuscitation with crystalloid solution risks dilutional coagulopathy. Experience in trauma care has shown that resuscitation with balanced blood products, whether as fresh whole blood or a 1:1:1 ratio of PRBCs, plasma, and platelets, improves outcomes compared with crystalloid-heavy strategies. Although the optimal ratio remains debated, balanced transfusion reduces the risks of dilutional coagulopathy, tissue edema, delayed healing, prolonged ventilation, and extended hospital stays. Warming blood products helps prevent hypothermia.
Severe trauma causes dysregulation of fibrinolysis. Evidence supports the use of tranexamic acid (TXA), particularly when administered within 3 hours of trauma. TXA competitively inhibits plasminogen activation, reducing fibrinolysis and hemorrhage, and has been shown to improve survival in victims of both military and civilian trauma.[20][21] Initial resuscitation typically begins with O-negative PRBCs and AB plasma or low-titer group A plasma if AB is unavailable, followed by type-specific products once crossmatching is complete.
Continuous monitoring is essential, with regular evaluation for coagulopathy, hypothermia, acidosis, and electrolyte derangements. After 5 units of PRBCs, testing should include CBC with platelets, PT, aPTT, and fibrinogen. Clinicians must keep in mind that hemoglobin concentration does not fall immediately with acute blood loss, making it an unreliable early marker. Additional monitoring every 30 to 60 minutes should assess pH, blood gases, electrolytes, blood glucose, and lactate levels. TEG, when available, provides functional insight into platelet activity, clot stability, and fibrinolysis to guide targeted administration of plasma, platelets, cryoprecipitate, or antifibrinolytics.
The resuscitation targets during massive transfusion include the following parameters:
- MAP of 60 to 65 mm Hg or systolic blood pressure of 80 to 90 mmHg for patients with penetrating trauma
- MAP above 85 mm Hg or a systolic blood pressure above 120 mm Hg for patients with blunt trauma, especially those with suspected traumatic brain injury or spinal cord injury
- Fibrinogen of 1.5 to 2 g/L
- Platelet count higher than 50,000/µL
- pH 7.35 to 7.45
- Core temperature higher than 35 °C (95 °F)
- Maintain heart rate between 60 and 100 bpm
- Maintain oxygen saturation above 94%
- Maintain urine output above 0.5 mL/kg/h
- Serum lactate <2 mmol/L
Criteria for stopping a major transfusion protocol differ by site. According to the American College of Surgeons' practice guidelines, clinicians can stop a massive transfusion protocol or switch to goal-directed transfusion for patients based on the following lab parameters if they are no longer actively bleeding:
- Stop RBC transfusions for a hemoglobin of 10 g/dL or higher.
- Stop plasma transfusions for a PT less than 18 seconds and aPTT less than 35 seconds.
- Stop platelet transfusions for platelet counts higher than 150 x109/µL.
- Stop cryoprecipitate or fibrinogen concentrate for fibrinogen levels higher than 180 g/L.
Complications
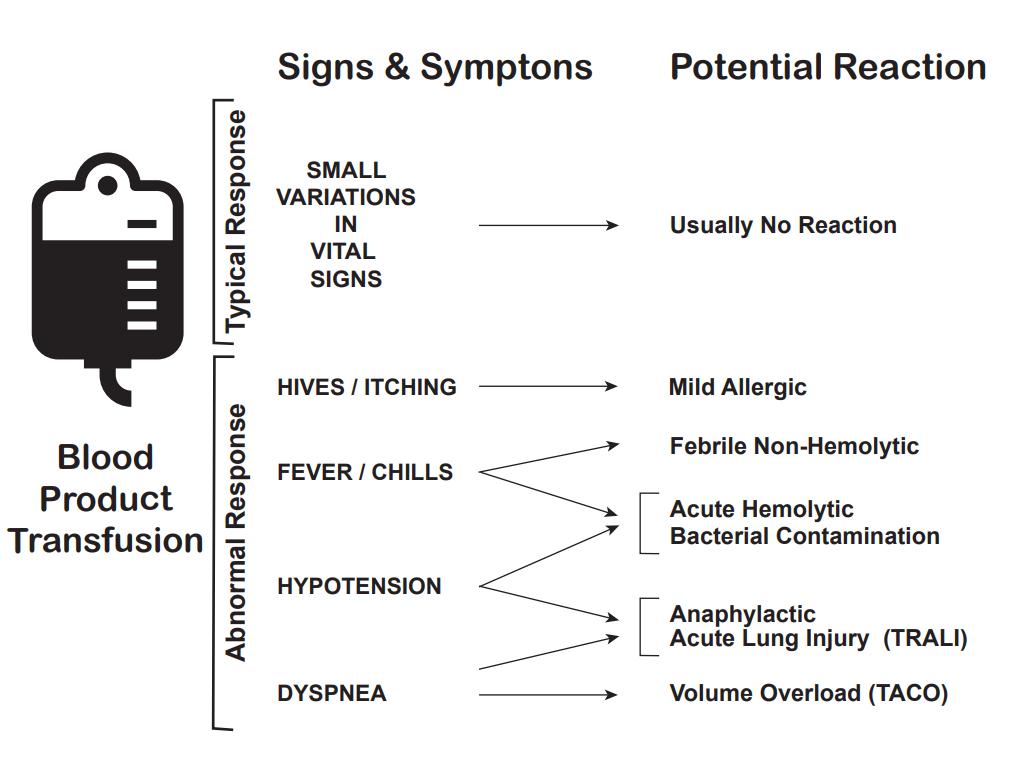
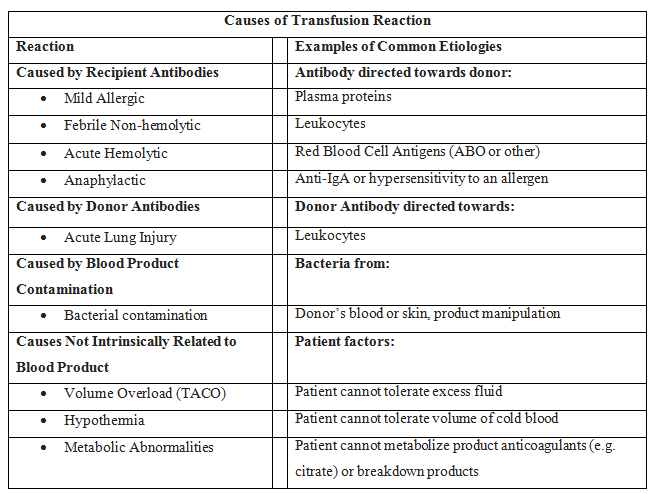
In addition to the mortality associated with the patient's injury or illness, massive transfusions have numerous potential complications (See Images: Transfusion Reaction Signs and Symptoms and Transfusion Reaction Causes). Vigilant monitoring of acid-base status, electrolytes, body temperature, volume status, tissue oxygenation, and coagulation parameters is essential. Non-fatal complications are observed in over 50% of patients when transfusing more than 5 units of blood products.[22][23][24] During blood transfusions, healthcare team members must exercise extreme caution as complications can be fatal.[25][26]
Coagulopathy
Coagulopathy is a frequent occurrence in patients requiring massive transfusion and results from both the underlying injury and the resuscitation process. Consumption and activation of clotting factors from tissue trauma, dilution from crystalloid and PRBC administration, and impaired factor function caused by shock, hypoxia, acidosis, and hypothermia all contribute.[16] To counter dilutional coagulopathy, the provision of plasma, fibrinogen, and platelets is essential.[27] Many patients present with acidosis before transfusion due to hypoperfusion, which further disrupts coagulation by interfering with the assembly and function of clotting factors. As pH declines, activity of the coagulation cascade decreases, resulting in delayed and weakened fibrin clot formation.[28]
Metabolic Abnormalities
The use of sodium citrate and citric acid in PRBCs, FFP, and platelets during storage to prevent coagulation can result in metabolic alkalosis and hypocalcemia.[29][30][31] Citrate metabolism generates bicarbonate and causes metabolic alkalosis when the kidneys are unable to excrete the excess. Citrate also binds to ionized calcium, potentially causing hypocalcemia, whereas calcium bound to albumin is unaffected. Severe hypocalcemia can lead to paresthesias and cardiac dysrhythmias. Regular monitoring of blood pressure and the corrected QT interval is crucial to prevent complications. Hypocalcemia results in more pronounced coagulopathy, necessitating additional transfusions, extended stays in the ICU, prolonged ventilator use, and heightened mortality rates. The majority of patients do not require calcium supplementation, and the administration of calcium does not demonstrate any benefit in mitigating these effects. However, symptomatic patients warrant treatment.
Citrate itself blunts coagulation dynamics beyond just lowering Ca²+. In vitro work shows that blood exposed to citrate has less and slower thrombin generation and worse platelet aggregation, even after careful recalcification to the same ionized calcium as controls. On TEG, this appears as a prolonged K-time (slower clot kinetics).[29] Citrate can also bind magnesium ions in the blood, reducing ionized magnesium levels. However, the effect is typically milder than with calcium.
Metabolic alkalosis can lead to hypokalemia as hydrogen ions exit cells to offset the alkalosis via an H+/K+ transporter.[29] Alternatively, stored RBCs may contain elevated extracellular potassium, and rapid infusion of older units, especially through central lines, high-volume transfusions, or blood exchange procedures involving heart-lung machines, extracorporeal membrane oxygenation (ECMO) devices, and apheresis machines that use older, cold-stored blood as priming solutions, can precipitate hyperkalemia and arrhythmias; using fresher or washed RBCs mitigates this risk.[5]
Though the physiologic process of shock and massive transfusions suggests the expected development of metabolic alkalosis, some patients can develop metabolic acidosis due to failure of H+/K+ and Na-K-ATP pumps as anaerobic metabolism works to meet ATP demands. These changes force excess potassium from transfused blood products into cells, followed by an efflux of hydrogen ions. These changes, in combination with the excess production of lactate, cause an acidosis. Excess lactate can also bind calcium, worsening hypocalcemia.[29]
Hypothermia
Many patients with acute blood loss are susceptible to hypothermia, which can lead to coagulopathy. Lower ambient temperatures and decreased blood volume can further predispose patients to hypothermia. Hypothermia reduces the efficacy of the coagulation cascade by reducing the enzymatic activity of coagulation proteins and platelet plug formation. Coagulation effects commence at 34 °C (93.2 °F). At 30 °C (86 °F), an approximately 50% reduction in platelet activation occurs. Because rapid infusion of cold blood, stored at 4 °C (39.2 °F), can induce a drop in core body temperature, many rapid infusers are equipped with warmers to mitigate the risk of hypothermia during massive transfusion. Additionally, hypothermia alone can raise peak citrate levels and slow metabolism by approximately 40%, prolonging hypocalcemia and compounding coagulopathy.[29]
Transfusion-Related Acute Lung Injury
Massive transfusion can cause transfusion-related acute lung injury (TRALI), a rapid-onset lung injury leading to non-cardiogenic pulmonary edema.[32][33] TRALI type I manifests in patients without additional risk factors for acute respiratory distress syndrome (ARDS). However, TRALI type II occurs in patients with ARDS risk factors or those who already have ARDS but experience acute decompensation due to a transfusion. Symptoms of TRALI typically present within 1 to 2 hours of the transfusion, but can potentially be delayed up to 6 hours. Approximately 85% of cases result from the infusion of antibodies targeting human leukocyte antigen (HLA) or antineutrophil antibodies present in the donor blood products. The remaining 15% likely originates from biological response modifiers, such as soluble CD40 ligand, within the stored blood product.
TRALI occurs when the recipient's neutrophils become primed to activate due to the patient's underlying condition. These primed neutrophils subsequently become activated by the antibodies targeting HLA or the biological response modifiers. Once activated, these neutrophils release cytokines, reactive oxygen species, oxidases, and proteases that damage the pulmonary capillary endothelium. The incidence of TRALI rises in direct proportion to the quantity of blood products administered.
Transfusion-Associated Circulatory Overload
Transfusion-associated circulatory overload (TACO) refers to respiratory distress and pulmonary edema resulting from excess volume or circulatory overload. Unlike TRALI, TACO may present with visible jugular venous distension, an S3 heart sound, and hypertension. This condition also responds well to diuresis and exhibits a pulmonary artery occlusion pressure exceeding 18 mm Hg. TACO develops when there is an overly aggressive transfusion or the administration of an excessive volume within a short time frame.[34] The symptoms of TACO typically occur within 6 hours of a transfusion, but can be delayed up to 12 hours. Patients with TRALI clinically resemble those with ARDS, presenting with a PaO2/FiO2 ratio of less than 300 mm Hg but greater than 200 mm Hg, bilateral infiltrates on chest radiograph, and no signs of systolic heart failure. If symptoms occur in the 12 hours before the transfusion or more than 6 hours after a transfusion, then ARDS is usually the appropriate diagnosis.
Additional Complications
Acute hemolytic transfusion reaction
Acute hemolytic transfusion reactions present with symptoms such as fever, chills, flank pain, and oozing from IV sites, due to acute intravascular hemolysis of transfused RBCs. The leading cause is typically ABO incompatibility or a reaction to other RBC antigens.[35]
Transfusion-Associated sepsis
Sepsis due to transfusion occurs when a patient receives blood containing a microorganism, causing symptoms such as fever, chills, and hypotension.[36][37]
Anaphylactic transfusion reaction
Anaphylaxis is a severe allergic reaction characterized by angioedema, wheezing, and hypotension.[38]
Allergic transfusion reaction
This allergic reaction commonly arises from antigen-antibody interactions between the patient and the transfused product, and typically presents with symptoms limited to hives and itching. In the absence of additional symptoms, the transfusion can proceed.[38]
Febrile non-hemolytic transfusion reaction
Febrile non-hemolytic transfusion reactions (FNHTR) occur due to the release of cytokines from WBCs in the transfused product. Fever is the sole symptom in this type of reaction.[39][40] FNHTRs occur in 10% to 15% of patients who receive blood products, and the clinical judgment of a healthcare professional guides the decision as to whether to continue or halt the transfusion.
Hypotensive transfusion reaction
Patients with hypotensive transfusion reactions experience a significant drop in systolic blood pressure, often 30 mm Hg or more. Most frequently associated with platelet transfusions, the underlying mechanism is likely related to vasoactive kinins. Patients taking an ACE inhibitor are at an elevated risk for hypotensive transfusion reactions.
To help mitigate complications, a designated healthcare team member is responsible for confirming that the administered blood matches the correct blood type. If an adverse reaction occurs, the first step is to immediately stop the transfusion and notify the patient's healthcare professional. The healthcare team should promptly return the blood and all associated tubing to the laboratory. Please see StatPearls' companion topics, "Noninfectious Complications of Blood Transfusions" and "Infectious Complications of Blood Transfusions," for further discussion on the potential complications related to blood transfusions.
Clinical Significance
Hemorrhage is the second leading cause of death within the first hour of trauma care, making massive transfusion a critical life-saving intervention. The use of massive transfusion protocols reduces mortality, particularly when plasma and platelets are incorporated early to limit coagulopathy.[41][42] While clinicians commonly activate massive transfusion protocols during the treatment of trauma, obstetrics, gastroenterology, and surgical settings, the principles of balanced resuscitation apply across specialties. Despite their benefits, massive transfusions carry significant risks. Protocol-driven activation and careful monitoring during transfusion improve survival, decrease complications, optimize blood product use, and reduce waste.[43][44][45]
Enhancing Healthcare Team Outcomes
Massive transfusion, commonly defined as transfusion of 10 units or more of PRBCs within 24 hours, is frequently required in trauma, obstetrics, gastrointestinal bleeding, and major surgery. It serves as a critical life-saving intervention for patients with severe hemorrhage. Implementing a massive transfusion protocol reduces early mortality, mitigates coagulopathy, and improves outcomes by ensuring timely and balanced delivery of PRBCs, plasma, and platelets. Due to the unpredictable nature of massive hemorrhage and the high risk of complications, including hypocalcemia, hyperkalemia, hypothermia, and dilutional coagulopathy, protocol-driven care, continuous monitoring of acid-base status, coagulation, electrolytes, and temperature, plus the availability of O-negative blood and thawed plasma, are vital. When available, TEG further guides targeted therapy. Familiarity with massive transfusion protocol activation criteria, awareness of potential complications, and adherence to evidence-based algorithms decrease morbidity and mortality while minimizing unnecessary blood product use and waste.
Massive transfusion requires seamless interprofessional coordination and pre-planning, as these scenarios involve unpredictable timing, significant personnel demands, and large volumes of blood products. Physicians and advanced practitioners must promptly identify candidates for massive transfusion protocol activation and direct resuscitation efforts. Nurses play a central role in monitoring vital signs, administering blood products, and ensuring rapid bedside response. Pharmacists provide expertise in adjunctive therapies such as tranexamic acid and reversal agents. Blood bank personnel and laboratory staff facilitate rapid product release and testing, while surgical, obstetrical, gastroenterology, anesthesia, trauma, and emergency medicine teams coordinate operative and critical care management. Regularly scheduled team drills enhance preparedness and efficiency. Effective communication, shared decision-making, and coordinated execution amongst all team members not only improve patient safety and outcomes but also strengthen team performance and patient-centered care.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Lier H, Hossfeld B. Massive transfusion in trauma. Current opinion in anaesthesiology. 2024 Apr 1:37(2):117-124. doi: 10.1097/ACO.0000000000001347. Epub 2024 Jan 19 [PubMed PMID: 38390985]
Level 3 (low-level) evidenceMajor FR, Pickering TA, Stefanescu K, Singh M, Clark DH, Inaba K, Nahmias JT, Tay-Lasso EL, Alvarez C, Chen JL, Ahmed F, Kaslow OY, Tong JL, Xiao J, Hall E, Elkhateb R, Bahgat Y, Tatum D, Simpson JT, Singh S, Klein NJ, Applegate RL 2nd, Kuza CM. A Retrospective Study of Ultramassive Transfusion in Trauma Patients: Is There a Value After Which Additional Transfusions Are Futile? Anesthesia and analgesia. 2025 May 30:():. doi: 10.1213/ANE.0000000000007569. Epub 2025 May 30 [PubMed PMID: 40445862]
Level 2 (mid-level) evidenceLin VS, Sun E, Yau S, Abeyakoon C, Seamer G, Bhopal S, Tucker H, Doree C, Brunskill SJ, McQuilten ZK, Stanworth SJ, Wood EM, Green L. Definitions of massive transfusion in adults with critical bleeding: a systematic review. Critical care (London, England). 2023 Jul 5:27(1):265. doi: 10.1186/s13054-023-04537-z. Epub 2023 Jul 5 [PubMed PMID: 37407998]
Level 1 (high-level) evidenceNunez TC, Young PP, Holcomb JB, Cotton BA. Creation, implementation, and maturation of a massive transfusion protocol for the exsanguinating trauma patient. The Journal of trauma. 2010 Jun:68(6):1498-505. doi: 10.1097/TA.0b013e3181d3cc25. Epub [PubMed PMID: 20539192]
Aubron C, Aries P, Le Niger C, Sparrow RL, Ozier Y. How clinicians can minimize transfusion-related adverse events? Transfusion clinique et biologique : journal de la Societe francaise de transfusion sanguine. 2018 Nov:25(4):257-261. doi: 10.1016/j.tracli.2018.08.158. Epub 2018 Aug 25 [PubMed PMID: 30197000]
Shea SM, Mihalko EP, Lu L, Thomas KA, Schuerer D, Brown JB, Bochicchio GV, Spinella PC. Doing more with less: low-titer group O whole blood resulted in less total transfusions and an independent association with survival in adults with severe traumatic hemorrhage. Journal of thrombosis and haemostasis : JTH. 2024 Jan:22(1):140-151. doi: 10.1016/j.jtha.2023.09.025. Epub 2023 Oct 4 [PubMed PMID: 37797692]
Feeney EV, Khalil EA, Gaines BA, Spinella PC, Leeper CM. Expanding beyond trauma: Characterizing low titer group O whole blood (LTOWB) use in children requiring massive transfusion protocol activation. Transfusion. 2025 May:65 Suppl 1(Suppl 1):S173-S180. doi: 10.1111/trf.18203. Epub [PubMed PMID: 40292836]
Jansen JO, Pedroza C, Novelo LL, Hao T, DeWildt GR, Coton CF, Mansoor K, Stephens SW, Marques MB, Stubbs JR, Richter JR, Wang HE, Holcomb JB, DeSantis SM. Trauma resuscitation with Low-Titer Group O Whole Blood Or Products: study protocol for a randomized clinical trial (the TROOP trial). Trials. 2025 Aug 2:26(1):266. doi: 10.1186/s13063-025-08971-y. Epub 2025 Aug 2 [PubMed PMID: 40753420]
Level 1 (high-level) evidenceAkkök ÇA, Seghatchian J. Pediatric red cell and platelet transfusions. Transfusion and apheresis science : official journal of the World Apheresis Association : official journal of the European Society for Haemapheresis. 2018 Jun:57(3):358-362. doi: 10.1016/j.transci.2018.05.019. Epub 2018 May 16 [PubMed PMID: 29804934]
Hess JR, Ramos PJ, Sen NE, Cruz-Cody VG, Tuott EE, Louzon MJ, Bulger EM, Arbabi S, Pagano MB, Metcalf RA. Quality management of a massive transfusion protocol. Transfusion. 2018 Feb:58(2):480-484. doi: 10.1111/trf.14443. Epub 2017 Dec 13 [PubMed PMID: 29238996]
Level 2 (mid-level) evidenceCarsetti A, Antolini R, Casarotta E, Damiani E, Gasparri F, Marini B, Adrario E, Donati A. Shock index as predictor of massive transfusion and mortality in patients with trauma: a systematic review and meta-analysis. Critical care (London, England). 2023 Mar 5:27(1):85. doi: 10.1186/s13054-023-04386-w. Epub 2023 Mar 5 [PubMed PMID: 36872322]
Level 1 (high-level) evidenceKalkwarf KJ, Goodman MD, Press GM, Wade CE, Cotton BA. Prehospital ABC Score Accurately Forecasts Patients Who Will Require Immediate Resource Utilization. Southern medical journal. 2021 Apr:114(4):193-198. doi: 10.14423/SMJ.0000000000001236. Epub [PubMed PMID: 33787930]
Komori A, Iriyama H, Aoki M, Deshpande GA, Saitoh D, Naito T, Abe T. Assessment of blood consumption score for pediatrics predicts transfusion requirements for children with trauma. Medicine. 2021 Mar 5:100(9):e25014. doi: 10.1097/MD.0000000000025014. Epub [PubMed PMID: 33655972]
Goodman MD, Makley AT, Hanseman DJ, Pritts TA, Robinson BR. All the bang without the bucks: Defining essential point-of-care testing for traumatic coagulopathy. The journal of trauma and acute care surgery. 2015 Jul:79(1):117-24; discussion 124. doi: 10.1097/TA.0000000000000691. Epub [PubMed PMID: 26091324]
Baksaas-Aasen K, Gall LS, Stensballe J, Juffermans NP, Curry N, Maegele M, Brooks A, Rourke C, Gillespie S, Murphy J, Maroni R, Vulliamy P, Henriksen HH, Pedersen KH, Kolstadbraaten KM, Wirtz MR, Kleinveld DJB, Schäfer N, Chinna S, Davenport RA, Naess PA, Goslings JC, Eaglestone S, Stanworth S, Johansson PI, Gaarder C, Brohi K. Viscoelastic haemostatic assay augmented protocols for major trauma haemorrhage (ITACTIC): a randomized, controlled trial. Intensive care medicine. 2021 Jan:47(1):49-59. doi: 10.1007/s00134-020-06266-1. Epub 2020 Oct 13 [PubMed PMID: 33048195]
Level 1 (high-level) evidenceHarvey CJ. Evidence-Based Strategies for Maternal Stabilization and Rescue in Obstetric Hemorrhage. AACN advanced critical care. 2018 Fall:29(3):284-294. doi: 10.4037/aacnacc2018966. Epub [PubMed PMID: 30185495]
Tang J, Shi Z, Hu J, Wu H, Yang C, Le G, Zhao J. Optimal sequence of surgical procedures for hemodynamically unstable patients with pelvic fracture: A network meta-analysis. The American journal of emergency medicine. 2019 Apr:37(4):571-578. doi: 10.1016/j.ajem.2018.06.027. Epub 2018 Jun 20 [PubMed PMID: 29933894]
Level 1 (high-level) evidenceFlint AWJ, McQuilten ZK, Wood EM. Massive transfusions for critical bleeding: is everything old new again? Transfusion medicine (Oxford, England). 2018 Apr:28(2):140-149. doi: 10.1111/tme.12524. Epub 2018 Apr 1 [PubMed PMID: 29607593]
Ducrocq G, Gonzalez-Juanatey JR, Puymirat E, Lemesle G, Cachanado M, Durand-Zaleski I, Arnaiz JA, Martínez-Sellés M, Silvain J, Ariza-Solé A, Ferrari E, Calvo G, Danchin N, Avendaño-Solá C, Frenkiel J, Rousseau A, Vicaut E, Simon T, Steg PG, REALITY Investigators. Effect of a Restrictive vs Liberal Blood Transfusion Strategy on Major Cardiovascular Events Among Patients With Acute Myocardial Infarction and Anemia: The REALITY Randomized Clinical Trial. JAMA. 2021 Feb 9:325(6):552-560. doi: 10.1001/jama.2021.0135. Epub [PubMed PMID: 33560322]
Level 1 (high-level) evidenceLanglais ML, Dargère M, Le Niger C, Goetghebeur D. [Appropriate use of red blood cell transfusion in the emergency department before and after a specific protocol]. Transfusion clinique et biologique : journal de la Societe francaise de transfusion sanguine. 2019 Feb:26(1):38-47. doi: 10.1016/j.tracli.2018.03.003. Epub 2018 Apr 16 [PubMed PMID: 29673931]
Meza Monge K, Domene SS, Diaz Mendoza DL, Vidal-Gallardo A, Alfaro Llique AM, Rodriguez M, Premchandra P, Anwar Pandya S, Arruarana VS, Aleman Paredes K, Calderon Martinez E. Effectiveness of Tranexamic Acid in Trauma Patients: A Systematic Review. Cureus. 2024 Jan:16(1):e52111. doi: 10.7759/cureus.52111. Epub 2024 Jan 11 [PubMed PMID: 38213943]
Level 1 (high-level) evidenceKim HJ, Park HS, Jang MJ, Koh WU, Song JG, Lee CS, Yang HS, Ro YJ. Predicting massive transfusion in adolescent idiopathic scoliosis patients undergoing corrective surgery: Association of preoperative radiographic findings. Medicine. 2018 Jun:97(22):e10972. doi: 10.1097/MD.0000000000010972. Epub [PubMed PMID: 29851849]
Wegner Araya A. [Damage control resuscitation in pediatric severe trauma]. Revista chilena de pediatria. 2018 Feb:89(1):118-127. doi: 10.4067/S0370-41062018000100118. Epub [PubMed PMID: 29664514]
Scerbo MH, Holcomb JB, Taub E, Gates K, Love JD, Wade CE, Cotton BA. The trauma center is too late: Major limb trauma without a pre-hospital tourniquet has increased death from hemorrhagic shock. The journal of trauma and acute care surgery. 2017 Dec:83(6):1165-1172. doi: 10.1097/TA.0000000000001666. Epub [PubMed PMID: 29190257]
Maw G, Furyk C. Pediatric Massive Transfusion: A Systematic Review. Pediatric emergency care. 2018 Aug:34(8):594-598. doi: 10.1097/PEC.0000000000001570. Epub [PubMed PMID: 30080793]
Level 1 (high-level) evidenceHarris CT, Dudley BM, Davenport D, Higgins J, Fryman L, Bernard A. Use of Plasma-Based Trauma Transfusion Protocols at Level IV Trauma Centers. Journal of trauma nursing : the official journal of the Society of Trauma Nurses. 2018 Jul/Aug:25(4):213-217. doi: 10.1097/JTN.0000000000000375. Epub [PubMed PMID: 29985853]
Keltner NM, Cushing MM, Haas T, Spinella PC. Analyzing and modeling massive transfusion strategies and the role of fibrinogen-How much is the patient actually receiving? Transfusion. 2024 May:64 Suppl 2():S136-S145. doi: 10.1111/trf.17774. Epub 2024 Mar 3 [PubMed PMID: 38433522]
Cap AP, Pidcoke HF, Spinella P, Strandenes G, Borgman MA, Schreiber M, Holcomb J, Tien HC, Beckett AN, Doughty H, Woolley T, Rappold J, Ward K, Reade M, Prat N, Ausset S, Kheirabadi B, Benov A, Griffin EP, Corley JB, Simon CD, Fahie R, Jenkins D, Eastridge BJ, Stockinger Z. Damage Control Resuscitation. Military medicine. 2018 Sep 1:183(suppl_2):36-43. doi: 10.1093/milmed/usy112. Epub [PubMed PMID: 30189070]
Schriner JB, Van Gent JM, Meledeo MA, Olson SD, Cotton BA, Cox CS Jr, Gill BS. Impact of Transfused Citrate on Pathophysiology in Massive Transfusion. Critical care explorations. 2023 Jun:5(6):e0925. doi: 10.1097/CCE.0000000000000925. Epub 2023 May 31 [PubMed PMID: 37275654]
Schauer SG, Conte JM, Hudson IL, Mendez J, Sifuentes D, Mancha F, Martinez MA, Huaman RJ, Arana AA, Corley JB, Fisher AD, Meledeo MA, Kirkwood BJ, April MD. An assessment of laboratory changes during autologous whole blood transfusion training: A prospective, observational study. Transfusion. 2025 May:65 Suppl 1():S57-S62. doi: 10.1111/trf.18178. Epub 2025 Feb 28 [PubMed PMID: 40021807]
Level 2 (mid-level) evidenceAlghanem H, Liu NC, Gupta A, Liao C, Wool GD, Rubin DS, Carll T. Ratios of calcium to citrate administration in blood transfusion for traumatic hemorrhage: A retrospective cohort study. Transfusion. 2024 Nov:64(11):2104-2113. doi: 10.1111/trf.18029. Epub 2024 Oct 1 [PubMed PMID: 39351914]
Level 2 (mid-level) evidenceSemple JW, Rebetz J, Kapur R. Transfusion-associated circulatory overload and transfusion-related acute lung injury. Blood. 2019 Apr 25:133(17):1840-1853. doi: 10.1182/blood-2018-10-860809. Epub 2019 Feb 26 [PubMed PMID: 30808638]
Yu Y, Lian Z. Update on transfusion-related acute lung injury: an overview of its pathogenesis and management. Frontiers in immunology. 2023:14():1175387. doi: 10.3389/fimmu.2023.1175387. Epub 2023 May 12 [PubMed PMID: 37251400]
Level 3 (low-level) evidenceMeyer DE, Reynolds JW, Hobbs R, Bai Y, Hartwell B, Pommerening MJ, Fox EE, Wade CE, Holcomb JB, Cotton BA. The Incidence of Transfusion-Related Acute Lung Injury at a Large, Urban Tertiary Medical Center: A Decade's Experience. Anesthesia and analgesia. 2018 Aug:127(2):444-449. doi: 10.1213/ANE.0000000000003392. Epub [PubMed PMID: 29697510]
Rout P, Harewood J, Ramsey A, Master SR. Hemolytic Transfusion Reaction. StatPearls. 2025 Jan:(): [PubMed PMID: 28846280]
Gallagher LT, Cohen MJ, Wright FL, Winkle JM, Douin DJ, April MD, Fisher AD, Rizzo JA, Schauer SG. Risk of Severe Sepsis After Blood Product Administration for Traumatic Hemorrhage: A Trauma Quality Improvement Program Study. The Journal of surgical research. 2025 Mar:307():8-13. doi: 10.1016/j.jss.2024.12.009. Epub 2025 Feb 12 [PubMed PMID: 39946990]
Level 2 (mid-level) evidenceYin M, Wang T, Jiang Q, Qu X, Ma J, Xu J, Jin X, Chen X. The association of red blood cell transfusion with mortality in pediatric patients with sepsis, severe sepsis, and septic shock: A single-center retrospective cohort study. Transfusion clinique et biologique : journal de la Societe francaise de transfusion sanguine. 2025 Feb:32(1):62-68. doi: 10.1016/j.tracli.2024.12.002. Epub 2024 Dec 20 [PubMed PMID: 39710203]
Level 2 (mid-level) evidenceChen YJ, Lin CL, Er TK. Trends and Incidence of Transfusion Reactions: a Single-Center 5-Year Retrospective Analysis. Clinical laboratory. 2025 Apr 1:71(4):. doi: 10.7754/Clin.Lab.2024.241101. Epub [PubMed PMID: 40209778]
Level 2 (mid-level) evidenceYıldız A, Evren G, Zihar B, Yaman S. Non-hemolytic acute transfusion reactions: the impact of patient and blood product characteristics. Postgraduate medicine. 2024 Sep:136(7):726-730. doi: 10.1080/00325481.2024.2396797. Epub 2024 Aug 30 [PubMed PMID: 39192816]
McCormick M, Triulzi D. The use of premedications for platelet transfusions in pediatric patients. Hematology. American Society of Hematology. Education Program. 2020 Dec 4:2020(1):523-526. doi: 10.1182/hematology.2020000165. Epub [PubMed PMID: 33275693]
Consunji R, Elseed A, El-Menyar A, Sathian B, Rizoli S, Al-Thani H, Peralta R. The effect of massive transfusion protocol implementation on the survival of trauma patients: a systematic review and meta-analysis. Blood transfusion = Trasfusione del sangue. 2020 Nov:18(6):434-445. doi: 10.2450/2020.0065-20. Epub 2020 Sep 18 [PubMed PMID: 32955420]
Level 1 (high-level) evidenceDente CJ, Shaz BH, Nicholas JM, Harris RS, Wyrzykowski AD, Patel S, Shah A, Vercruysse GA, Feliciano DV, Rozycki GS, Salomone JP, Ingram WL. Improvements in early mortality and coagulopathy are sustained better in patients with blunt trauma after institution of a massive transfusion protocol in a civilian level I trauma center. The Journal of trauma. 2009 Jun:66(6):1616-24. doi: 10.1097/TA.0b013e3181a59ad5. Epub [PubMed PMID: 19509623]
Jebbia M, Nguyen J, Marty M, Carcamo R, Alvarez C, Tay Lasso E, Barrios C, Lugo B. Predictors of Mortality in Trauma patients Receiving massive Transfusion Protocol. The American surgeon. 2023 Oct:89(10):4089-4094. doi: 10.1177/00031348231175503. Epub 2023 May 16 [PubMed PMID: 37194204]
Parker MJ, Crowder EW, Miles MVP, Harrell KN, Maxwell RA. Hypofibrinogenemic Massive Transfusion Trauma Patients Experience Worse Outcomes. The American surgeon. 2023 Aug:89(8):3423-3428. doi: 10.1177/00031348231162711. Epub 2023 Mar 12 [PubMed PMID: 36908225]
Mains CW, Sercy E, Elder T, Salottolo K, DHuyvetter C, Bar-Or D. Predictors of Massive Transfusion Protocol Initiation Among Trauma Patients Transported From the Scene Via Flight Emergency Management Services. Air medical journal. 2023 Jan-Feb:42(1):19-23. doi: 10.1016/j.amj.2022.11.005. Epub 2022 Dec 22 [PubMed PMID: 36710030]