Indications
Food and Drug Administration–Approved Indications
Currently, 3 cannabinoids are available on the pharmaceutical market. Dronabinol and nabilone are synthetic forms of tetrahydrocannabinol approved by the Food and Drug Administration (FDA) for the treatment of chemotherapy-induced nausea and vomiting after the failure of a trial of first-line antiemetics.[1][2] Both agents are also approved by the FDA to treat anorexia associated with AIDS. Recently, a cannabidiol product has also been approved by the FDA to treat seizures associated with Lennox-Gastaut syndrome and Dravet syndrome in pediatric patients. However, there is no FDA-approved indication for its use as an antiemetic. According to the American Society of Clinical Oncology (ASCO), adult patients with cancer receiving moderately or highly emetogenic chemotherapy who continue to experience refractory nausea or vomiting despite guideline-directed antiemetic prophylaxis may benefit from an augmented regimen. This augmented regimen may include the addition of dronabinol, nabilone, or a pharmaceutical-grade oral formulation containing a 1:1 ratio of cannabidiol to tetrahydrocannabinol.[3][4][5][6][7]
Off-Label Uses
A cannabidiol product has been approved by the FDA for the treatment of seizures associated with Lennox-Gastaut syndrome and Dravet syndrome in pediatric patients.[8][9] However, there is no FDA-approved indication for its use as an antiemetic. Independently produced cannabidiol extracts are being used increasingly in the general population for many non–FDA-approved indications, including nausea and emesis. In states where marijuana has been decriminalized for medical or recreational use, products with varying ratios of cannabidiol and tetrahydrocannabinol are also used for their antiemetic properties.[5]
The American College of Physicians (ACP) supports the decriminalization of the possession of limited amounts of cannabis for personal use. The ACP urges policymakers to adopt an evidence-based approach when deliberating amendments to the legal status of cannabis.[10] The ACP guidelines (2025) recommend that clinicians have informed discussions with patients considering cannabinoids for chronic noncancer pain, clearly outlining potential benefits and known risks. Special caution is advised when counseling high-risk groups, such as adolescents and young adults, individuals with current or past substance use disorders, patients with severe mental illness, frail individuals, and those at increased risk of falls, as the potential harms in these populations likely outweigh any therapeutic benefits. Additionally, the use of cannabis or cannabinoids should be discouraged in pregnant women, those who are breastfeeding, or individuals actively trying to conceive due to potential risks to reproductive and fetal health. Inhaled cannabis products are also not recommended for patients with chronic noncancer pain, given associated health concerns and insufficient evidence supporting their safety and effectiveness.[11]
Mechanism of Action
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Mechanism of Action
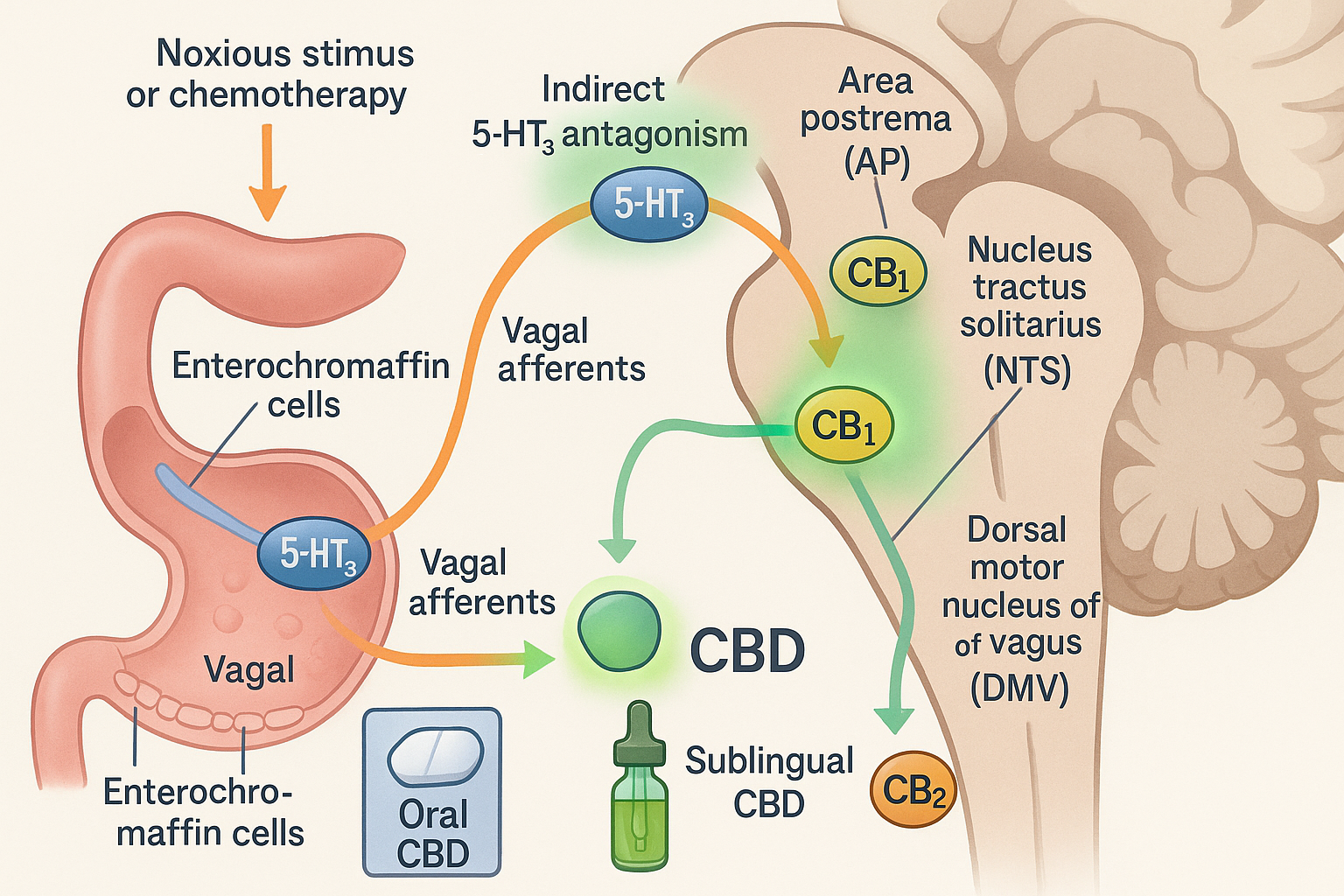
Cannabinoids and their antiemetic potential are still being researched, and many of the intricacies behind their mechanisms are still unknown or lack universal consensus. Cannabinoids exert their antiemetic properties through interactions with the centrally located CB1 receptors and 5-HT3 receptors in the dorsal vagal complex, which mediates emesis. A significant contributor to emesis appears to be through the activation of 5-HT3 receptors in the dorsal vagal complex, specifically in the area postrema. Studies in animal models have shown that anandamide, an endogenous cannabinoid, tetrahydrocannabinol, and several synthetic cannabinoids have demonstrated allosteric inhibitor effects on the 5-HT3 receptors in the dorsal vagal complex, providing a mechanism through which emesis control occurs. Animal models have also demonstrated that cannabinoids may act on presynaptic CB1 receptors to reduce the release of serotonin into the synapse, thereby inhibiting nausea and the emetic response.
Animal models have also indicated that cannabidiol has an allosteric inhibitory effect on the 5-HT3 receptor. However, this effect may occur through activation of the 5-HT1A receptor. Activating the 5-HT1A receptor ultimately reduces the amount of serotonin released, thus lowering the potential to trigger emesis. Cannabidiol is also believed to activate CB1 receptors in the gastrointestinal tract through its inhibitory effect on G-protein-coupled receptors, leading to decreased gastrointestinal motility. Tetrahydrocannabinol and cannabidiol were previously believed to inhibit fatty acid amide hydrolase, thereby increasing anandamide concentrations and enhancing its antiemetic effects; however, recent studies investigating this mechanism have been equivocal.[12][13]
Pharmacokinetics
Absorption: Cannabidiol, nabilone, and dronabinol are all well-absorbed when taken orally; however, their actual bioavailability is limited by significant first-pass metabolism. Cannabidiol exhibits variable absorption, which is enhanced by high-fat meals; its peak plasma concentration (Tmax) typically occurs 2 to 5 h after administration. Nabilone has consistent, dose-proportional absorption that is not noticeably affected by food. Dronabinol is almost completely absorbed (approximately 95%), but only 10% to 20% enters the systemic circulation due to first-pass metabolism. A high-fat meal delays Tmax by approximately 4 h and increases overall exposure (area under the curve) nearly 3-fold, while the peak concentration (Cmax) remains relatively unchanged.
Distribution: All 3 compounds—cannabidiol, nabilone, and dronabinol—are lipophilic, allowing them to distribute widely throughout body tissues. The volume of distribution (Vd) of cannabidiol exceeds 30 L/kg, indicating broad tissue penetration. Nabilone also spreads widely throughout the body and remains detectable in plasma alongside longer-lasting metabolites. Dronabinol has a Vd of approximately 10 L/kg and is approximately 97% bound to plasma proteins. Similarly, cannabidiol exhibits plasma protein binding of greater than 90%. Both compounds tend to accumulate in adipose tissue, resulting in a prolonged systemic presence as they gradually redistribute back into the bloodstream over time.
Metabolism: Each cannabinoid is extensively metabolized in the liver, mainly by cytochrome P450 enzymes.[14] Cannabidiol is primarily metabolized through CYP3A4 and CYP2C19, resulting in the formation of hydroxylated and carboxylated metabolites. Nabilone undergoes both reduction and oxidation through enzymes such as CYP2E1, CYP3A4, CYP2C8, and CYP2C9, producing several metabolites, including carbinols and diols; however, the pharmacological roles of these metabolites are not fully understood. Dronabinol is primarily metabolized by CYP2C9 and CYP3A4 to form the active metabolite 11-hydroxy-tetrahydrocannabinol.[15] Interestingly, both cannabidiol and nabilone may act as mild inhibitors of CYP enzymes, raising the possibility of drug interactions with other medications.
Elimination: Cannabidiol and nabilone are eliminated primarily through feces, with only small amounts excreted in the urine. Nabilone's metabolites are cleared through bile; some may accumulate with repeated dosing. Dronabinol is primarily excreted in feces (approximately 50%) and urine (10%-15%), with less than 5% remaining unchanged. Due to their high lipid solubility, all 3 compounds tend to remain in the body for extended periods, with metabolites detectable in bodily waste for several days or even weeks after use.
Administration
Available Dosage Forms and Strengths
Cannabidiol is available as a 100 mg/mL solution, nabilone in 1 mg capsules, and dronabinol in 2.5 mg, 5 mg, and 10 mg strengths, as well as an oral solution at a 5 mg/mL concentration.
Dosage
In chemotherapy-induced nausea and vomiting, patients should take dronabinol 1 to 3 h before starting their chemotherapy session, with a repeat dose available 2 to 4 h after the session begins, as needed. Nabilone doses can be increased or decreased at the practitioner's discretion. Patients should take one dose the night before starting their chemotherapy session, one dose twice a day during the entire chemotherapy course, and one dose twice a day after chemotherapy has concluded. According to ASCO guidelines, for refractory chemotherapy-induced nausea and vomiting due to moderate/highly emetogenic chemotherapy, synthetic cannabinoids such as dronabinol and nabilone may be used as salvage antiemetics. Dronabinol is typically initiated at 2.5 mg 3 times daily, with a maximum dosage of 10 mg 3 to 4 times daily. Nabilone is started at 1 mg twice daily, with a maximum dosage of 2 mg 4 times daily. Participants in some studies have self-titrated a regimen combining tetrahydrocannabinol and cannabidiol (10 mg:10 mg) capsules, taking 1 to 4 capsules per day as tolerated.[6] Cannabidiol is sold legally throughout the United States without a prescription by many private entities that are not regulated by the FDA; there are no concrete guidelines for dosing. However, doses generally range from 10 to 50 mg at a time, taken as needed or daily. Products containing high levels of tetrahydrocannabinol are sold legally by medicinal and recreational dispensaries in several states, depending on legal status. Doses for these generally range between 5 and 15 mg and are typically taken as needed, as opposed to daily, due to their psychotropic effects.[5][16]
Specific Patient Populations
Hepatic impairment: Dosage adjustments for cannabidiol are necessary for patients with moderate/severe hepatic impairment. Cannabidiol should be discontinued if symptoms of jaundice occur alongside alanine aminotransferase elevations or if alanine aminotransferase levels remain above 5 times the upper limit of normal.[14] Regarding nabilone, the product labeling does not specify any dosage adjustments; this medication should be administered cautiously to patients with hepatic impairment. Similarly, the manufacturer's labeling does not indicate that any dosage adjustments are necessary for dronabinol.
Renal impairment: No dosage adjustments are provided in the labeling for cannabidiol, nabilone, or dronabinol.
Pregnancy considerations: Cannabinoid use during pregnancy is unsafe and associated with adverse effects on the mother and fetus, including small-for-gestational-age infants, preterm birth, and possible neurodevelopmental consequences.[17] The American College of Obstetricians and Gynecologists (ACOG) states that cannabinoids primarily affect the central nervous system through cannabinoid receptor type 1 (CB1). These receptors are formed in the fetal brain by 14 weeks of gestation and increase with age, indicating a neurodevelopmental role. Animal studies have demonstrated that exposure to external cannabinoids during pregnancy can disrupt fetal brain development, leading to potential cognitive impairment. Human studies have linked prenatal marijuana exposure to attention, behavior, and visual-motor coordination deficits and a higher likelihood of adolescent marijuana use. Although structural congenital disabilities are not consistently associated with marijuana exposure, some studies have reported a possible increased risk of anencephaly with first-trimester use, potentially confounded by folic acid deficiency. Evidence regarding perinatal mortality is mixed; some studies show no significant differences, whereas others indicate a slight stillbirth risk, often complicated by tobacco use. Frequent marijuana use in the first and second trimesters has been associated with lower birth weight, shorter crown-heel length, and smaller head circumference. Additionally, weekly or more frequent use may also increase the risk of preterm birth, especially with tobacco. Due to potential fetal neurodevelopment and perinatal outcome risks, the ACOG advises against marijuana use during pregnancy and recommends routine, nonjudgmental screening and cessation counseling.[18] Pregnant women should be advised to avoid the use of cannabidiol, dronabinol, and nabilone.
Breastfeeding considerations: Cannabidiol, a component of cannabis, has been detected in breastmilk. However, data on its use as an antiepileptic during lactation are lacking. Due to the absence of safety data, an alternate drug is preferred, particularly for newborns or preterm infants. Dronabinol, a synthetic form of delta-9-tetrahydrocannabinol (Δ9-THC), has also been found in breastmilk. Although its presence does not necessitate stopping breastfeeding, an alternate antiemetic is recommended due to limited safety data during lactation. The Lactation and Cannabis (LAC) Study showed that delta-9-tetrahydrocannabinol peaks in milk a few hours after use and accumulates with repeated exposure. Cannabinoid concentrations in milk vary among individuals and correlate with the frequency of use. These findings highlight the need for cautious counseling about cannabis use during breastfeeding.[19][20][21]
Pediatric patients: Dronabinol is indicated for the treatment of chemotherapy-induced nausea and vomiting in pediatric patients; however, the use of nabilone in pediatrics should be avoided.[22] The American Academy of Pediatrics opposes the use of medical marijuana outside the regulatory framework of the FDA. However, the AAP recognizes that cannabinoid-based therapies may be considered for children with severe or life-limiting conditions when standard treatments have failed.[23]
Older patients: Older adults may be more sensitive to adverse effects such as postural hypotension and neuropsychiatric symptoms.[24] These patients should be initiated on a lower dose of nabilone, dronabinol, and cannabidiol.
Adverse Effects
Adverse effects depend on the cannabinoid administered. The most common adverse effects of formulas containing a tetrahydrocannabinol component are acute intoxication, tachycardia, abulia, and psychosis. Psychosis manifests most commonly as perceptual alterations but also frequently includes panic attacks, anxiety, paranoia, and depression.[25] Chronic, high-volume use of tetrahydrocannabinol-containing products increases the risk of developing cannabis hyperemesis syndrome, characterized by intractable nausea and vomiting.[26] Cannabinoid formulas containing only a cannabidiol component have less potential to cause behavioral adverse effects, although drowsiness occurs frequently. Cannabidiol is a CYP3A4 inhibitor, which may lead to drug interactions and toxicities in molecules metabolized by the CYP3A4 system.[27] In vitro studies have demonstrated an association between cannabidiol and reduced fertility, as well as alterations in cell viability. Dronabinol has reported adverse effects such as gastrointestinal upset, dizziness, paranoia, somnolence, and abnormal thoughts.[20] Reported adverse effects of nabilone include acute intoxication, ataxia, headache, drowsiness, and deficits in concentration.[1]
Drug-Drug Interactions
Cannabidiol
- CYP3A4 and CYP2C19 inducers: Co-administration of cannabidiol with potent cytochrome P450 3A4 and 2C19 inducers, such as rifampicin, significantly reduces plasma concentrations of cannabidiol and its active metabolites. A dose increase of up to 2-fold may be warranted, guided by clinical response and patient tolerability.[27]
- CYP1A2 inhibitors: Cannabidiol exhibits weak inhibitory activity on CYP1A2, which may lead to increased systemic exposure to CYP1A2 substrates, such as caffeine and theophylline. Monitoring for toxicity is recommended during co-administration.
- Inhibition of UGT and CYP isoenzymes: Cannabidiol may inhibit UDP-glucuronosyltransferases (UGT1A9 and UGT2B7) and cytochrome P450 isoforms (CYP2C8 and CYP2C9). In patients receiving concomitant substrates of these enzymes, such as lamotrigine, morphine, and phenytoin, dose adjustment may be required based on the adverse effect profile.
- CYP2C19 substrates: Cannabidiol increases systemic concentrations of CYP2C19 substrates, such as diazepam. When administered with clobazam, cannabidiol increases the levels of its active metabolite, N-desmethylclobazam, by approximately 3-fold. A dose reduction of clobazam should be considered in the event of sedation or other adverse central nervous system (CNS) effects.
- Stiripentol: Co-administration of cannabidiol increases stiripentol plasma exposure.[28] Clinical monitoring is advised to mitigate potential toxicity.
- Everolimus: Concomitant use with cannabidiol results in approximately 2.5-fold increases in peak concentration and area under the curve of everolimus. Therapeutic drug monitoring is recommended, with dose reduction based on pharmacokinetic parameters and clinical status.
- P-glycoprotein substrates: Cannabidiol may increase plasma concentrations of P-glycoprotein substrates, including tacrolimus and digoxin.[29] Therapeutic drug monitoring and individualized dose adjustments are advisable during co-administration.
- Valproic acid: Combined administration of cannabidiol and valproate has been associated with an increased incidence of elevated serum aminotransferases. If hepatotoxicity is suspected, dose reduction or discontinuation should be considered.
- Central nervous system depressants and ethanol: Concurrent use of cannabidiol with other central nervous system depressants or alcohol may enhance sedative effects, including somnolence. Close clinical observation is recommended, particularly during the initiation and titration phases of treatment.
Dronabinol
- CYP2C9 and CYP3A4 interactions: Dronabinol is metabolized by cytochrome P450 enzymes CYP2C9 and CYP3A4.[15] Co-administration with inhibitors of these enzymes may increase dronabinol plasma concentrations, increasing the risk of adverse effects. Conversely, inducers may lower systemic exposure, potentially reducing efficacy. Clinicians should monitor for changes in clinical response and adjust dosing as needed.
- Highly protein-bound drugs: Dronabinol is extensively bound to plasma proteins. Initiation or dose escalation may displace other highly protein-bound agents, increasing the free fraction of drugs with narrow therapeutic indices, such as warfarin, cyclosporine, or amphotericin B. Careful monitoring is warranted for signs of toxicity or reduced therapeutic effect of concomitant medications.
Nabilone
- Highly protein-bound drugs: Nabilone exhibits high plasma protein binding and may displace other protein-bound drugs. Clinicians should monitor for altered pharmacological effects when nabilone is co-administered with other highly protein-bound agents.
- Amphetamines, cocaine, and other sympathomimetic agents: Combined use may result in additive hypertensive and tachycardic effects, with a potential increase in cardiotoxic risk.
- Atropine, scopolamine, and other anticholinergic agents: Co-administration may enhance tachycardia and CNS effects, including sedation.
- Benzodiazepines, alcohol, buspirone, and tricyclic antidepressants: These combinations may enhance sedative effects and CNS depression.
- Theophylline: Cannabis smoking has been associated with increased theophylline metabolism, a pattern similar to that observed with tobacco smoke.
- Opioids: Evidence supports the presence of cross-tolerance and mutual potentiation between cannabinoids and opioids. Cannabinoids exhibit an opioid-sparing effect.[30]
Contraindications
Cannabinoids are contraindicated in individuals with a history of hypersensitivity reactions related to any form of cannabinoid use. Caution is advised in patients who have previously experienced adverse effects. Dronabinol is specifically contraindicated in patients with hypersensitivity to sesame oil.[20]
Warning and Precautions
- Substance use disorder: The ACP acknowledges cannabis use disorder and supports comprehensive insurance coverage for evidence-based management of this condition.[10] Dronabinol is classified as a Drug Enforcement Administration (DEA) Schedule CIII drug, whereas nabilone is a DEA Schedule CII drug.
- Allergic reactions: Sesame allergy commonly presents with anaphylaxis and dermatologic symptoms such as urticaria, whereas gastrointestinal symptoms are less frequently observed. IgE-mediated and non–IgE-mediated reactions to sesame have been reported, including food protein–induced enterocolitis syndrome and occupational inhalation sensitization. Diagnosis is challenging due to cross-reactivity with other allergens. Patients with sesame allergy should strictly avoid sesame-containing foods and be advised to carry and be proficient in using epinephrine autoinjectors. Oral immunotherapy has shown promise in inducing tolerance and may become a viable treatment option for select individuals.[31]
Cannabidiol
-
Hepatic injury: Cannabidiol can increase liver transaminases, especially at higher doses or when used with valproate. Regular monitoring of liver enzymes and bilirubin is advised.
-
Sedation: Patients should be monitored for signs of drowsiness. Caution is advised when engaging in activities that require alertness, such as driving or operating machinery.
-
Suicidal behavior and ideation: Patients should be evaluated for new or worsening suicidal thoughts or behaviors.
-
Withdrawal of antiepileptic drugs: Cannabidiol dosage should be reduced gradually to lower the risk of increased seizure activity and status epilepticus.
Dronabinol
- Neuropsychiatric adverse reactions: Dronabinol may cause psychiatric and cognitive effects, impairing mental or physical abilities.[32] Therefore, its use should be avoided in patients with a history of psychiatric disorders.
- Hemodynamic instability: Patients with cardiac conditions may experience hypotension, hypertension, syncope, or tachycardia. Clinicians should avoid using drugs with similar effects and monitor patients for changes in blood pressure and heart rate after initiating dronabinol or increasing the dosage.
- Paradoxical nausea, vomiting, or abdominal pain: If these symptoms worsen during treatment, dose reduction or discontinuation of dronabinol should be considered.
Nabilone
- Psychiatric adverse effects: The effects of nabilone are unpredictable after oral administration, with psychiatric reactions potentially lasting 48 to 72 h after treatment is stopped.
- Central nervous system effects: Nabilone may affect the central nervous system, causing dizziness, drowsiness, euphoria, ataxia, anxiety, disorientation, depression, hallucinations, and psychosis.
- Cardiovascular effects: Nabilone can cause tachycardia and orthostatic hypotension.[33]
- Precautions for hazardous activities: Patients using nabilone should be cautioned against driving, operating machinery, or engaging in hazardous activities while taking this medication.
Monitoring
Monitoring of patients receiving cannabinoids should be guided by clinical presentation, as there are no streamlined laboratory procedures that can measure cannabinoid serum levels. Clinical monitoring should include assessment for tachycardia, orthostatic hypotension, and behavioral changes.[34] Patients with a history of cannabis use disorder warrant close observation due to the high risk of relapse.[35] Precautions in patients with hepatic or renal dysfunction have not been investigated. Co-administration with CYP inhibitors may lead to toxicity, and concurrent administration with CYP inducers may result in lower efficacy, as discussed above.[36] Baseline and periodic monitoring of liver function is required, as cannabidiol has the potential for hepatotoxicity.[14] Clinicians should use the prescription drug monitoring program if there is suspicion of substance use disorder.[37]
Toxicity
Signs and Symptoms of Overdose
Data on cannabinoid toxicities remain limited in the literature. The lethal dose (LD50) has not been established, and studies on dog and monkey models have shown that doses up to 3000 mg/kg do not lead to fatality.[25] A recent study suggests the potential of electroencephalography for the detection of acute cannabis consumption. Electroencephalography displays alterations in θ-band oscillatory activity (4-7 Hz).[38] There is no antidote for cannabinoid overdose; only supportive therapy is available to help manage the symptoms. Chronic, prolonged, high-volume use of cannabinoids can lead to neuropsychiatric effects, with IQ and cognition being most impacted.[39] The estimated toxic dose for dronabinol is 30 mg/kg. Illicit synthetic cannabinoids are known to lead to toxicity at much lower doses.
Overdosage with dronabinol or nabilone can lead to a range of symptoms affecting the central nervous system, cardiovascular system, gastrointestinal tract, and autonomic functions. These symptoms may include drowsiness, euphoria, heightened sensory perception, altered time awareness, memory impairment, mood alteration, depersonalization, lethargy, slurred speech, and impaired motor coordination. Autonomic features include dry mouth, reddened conjunctivae, urinary retention, and reduced bowel motility. Tachycardia and postural hypotension are observed. Individuals with a history of anxiety or nervousness may experience panic reactions, and seizures can occur in patients with known seizure disorders. Psychotic features such as hallucinations, delusions, or paranoia are observed at very high doses.
Management of Overdosage
There is no antidote for cannabinoid overdose; only supportive therapy is available to help manage the symptoms. Discontinuation of the drug is essential. Vital signs must be monitored regularly, with particular attention to airway, breathing, and circulation. In patients with respiratory depression or significantly altered mental status, airway protection and ventilatory support are essential. Hypotension should be managed with intravenous fluids and, if necessary, vasopressors or inotropes. Activated charcoal is preferred for gastrointestinal decontamination and may be administered in repeated doses to enhance elimination. Gastric emptying should only be considered if the airway is protected. Reassurance and verbal support may be sufficient for managing mild-to-moderate psychiatric symptoms. In more severe cases, antipsychotic medications may be used with caution.[40]
Enhancing Healthcare Team Outcomes
Opinions and values regarding the use of cannabinoids in medicine vary widely. Some individuals see cannabinoids as having significant potential to reduce pain, whereas others believe cannabis-derived compounds have no place in modern clinical practice. Regardless of personal values, healthcare providers should recognize the existence and use of cannabinoids among their patient population and consider this when deciding treatment options. Patients should feel comfortable discussing their cannabinoid use, including the type, dosage, and a cannabidiol to tetrahydrocannabinol ratio.[41] Dronabinol and nabilone are both legal and are available by prescription throughout all 50 states of the United States.[5][42] The legal status of formulas containing only cannabidiol is more variable. Laws are much more variable between the states concerning restricting products with tetrahydrocannabinol components.[5] If a patient informs the medical staff about using a cannabinoid, that information should be communicated to the primary clinician overseeing their care.
The effective use of cannabinoids for emetic control is best achieved through an interprofessional healthcare team model. Dronabinol and nabilone are most commonly prescribed by oncologists, palliative care specialists, and infectious disease specialists. Oncologists frequently prescribe cannabinoids alongside chemotherapy regimens.[43] Palliative care and infectious disease specialists often prescribe cannabinoids to provide comfort in the setting of nausea or to benefit from their orexigenic components. In states where medical marijuana is legal, a team of healthcare professionals, including clinicians, nurse practitioners, and physician assistants, can authorize the use of cannabidiol- and tetrahydrocannabinol-containing formulations for patients with various conditions, including nausea and vomiting. Healthcare professionals from various specialties have also recommended over-the-counter cannabidiol-only products to their patients for numerous indications, including nausea and vomiting. Research into the potential of cannabinoids in medicine is still in its initial stages, and many of the biomedical applications being researched are based on recent animal models and anecdotal cases. Pharmacists play a crucial role in verifying dosing, checking for potential drug interactions, and providing additional counseling to patients as needed.[44] The entire clinical picture must be considered when deciding whether to initiate cannabinoid therapy. Therefore, an interprofessional healthcare team is crucial for achieving optimal patient outcomes.
Media
(Click Image to Enlarge)
References
Polito S, MacDonald T, Romanick M, Jupp J, Wiernikowski J, Vennettilli A, Khanna M, Patel P, Ning W, Sung L, Dupuis LL. Safety and efficacy of nabilone for acute chemotherapy-induced vomiting prophylaxis in pediatric patients: A multicenter, retrospective review. Pediatric blood & cancer. 2018 Dec:65(12):e27374. doi: 10.1002/pbc.27374. Epub 2018 Jul 26 [PubMed PMID: 30051617]
Level 2 (mid-level) evidenceChow R, Valdez C, Chow N, Zhang D, Im J, Sodhi E, Lock M. Oral cannabinoid for the prophylaxis of chemotherapy-induced nausea and vomiting-a systematic review and meta-analysis. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2020 May:28(5):2095-2103. doi: 10.1007/s00520-019-05280-4. Epub 2020 Jan 8 [PubMed PMID: 31916006]
Level 1 (high-level) evidenceHesketh PJ, Kris MG, Basch E, Bohlke K, Barbour SY, Clark-Snow RA, Danso MA, Dennis K, Dupuis LL, Dusetzina SB, Eng C, Feyer PC, Jordan K, Noonan K, Sparacio D, Lyman GH. Antiemetics: ASCO Guideline Update. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2020 Aug 20:38(24):2782-2797. doi: 10.1200/JCO.20.01296. Epub 2020 Jul 13 [PubMed PMID: 32658626]
Chow R, Basu A, Kaur J, Hui D, Im J, Prsic E, Boldt G, Lock M, Eng L, Ng TL, Zimmermann C, Scotte F. Efficacy of cannabinoids for the prophylaxis of chemotherapy-induced nausea and vomiting-a systematic review and meta-analysis. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2025 Feb 14:33(3):193. doi: 10.1007/s00520-025-09251-w. Epub 2025 Feb 14 [PubMed PMID: 39953210]
Level 1 (high-level) evidenceCorroon J, Kight R. Regulatory Status of Cannabidiol in the United States: A Perspective. Cannabis and cannabinoid research. 2018:3(1):190-194. doi: 10.1089/can.2018.0030. Epub 2018 Sep 27 [PubMed PMID: 30283822]
Level 3 (low-level) evidenceBraun IM, Bohlke K, Abrams DI, Anderson H, Balneaves LG, Bar-Sela G, Bowles DW, Chai PR, Damani A, Gupta A, Hallmeyer S, Subbiah IM, Twelves C, Wallace MS, Roeland EJ. Cannabis and Cannabinoids in Adults With Cancer: ASCO Guideline. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2024 May 1:42(13):1575-1593. doi: 10.1200/JCO.23.02596. Epub 2024 Mar 13 [PubMed PMID: 38478773]
Grimison P, Mersiades A, Kirby A, Tognela A, Olver I, Morton RL, Haber P, Walsh A, Lee Y, Abdi E, Della-Fiorentina S, Aghmesheh M, Fox P, Briscoe K, Sanmugarajah J, Marx G, Kichenadasse G, Wheeler H, Chan M, Shannon J, Gedye C, Begbie S, Simes RJ, Stockler MR. Oral Cannabis Extract for Secondary Prevention of Chemotherapy-Induced Nausea and Vomiting: Final Results of a Randomized, Placebo-Controlled, Phase II/III Trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2024 Dec:42(34):4040-4050. doi: 10.1200/JCO.23.01836. Epub 2024 Aug 16 [PubMed PMID: 39151115]
Level 1 (high-level) evidenceBonanni P, Ragona F, Fusco C, Gambardella A, Operto FF, Parmeggiani L, Sartori S, Specchio N. Cannabidiol use in patients with Dravet syndrome and Lennox-Gastaut syndrome: experts' opinions using a nominal group technique (NGT) approach. Expert opinion on pharmacotherapy. 2023 Apr:24(5):655-663. doi: 10.1080/14656566.2023.2187697. Epub 2023 Apr 6 [PubMed PMID: 37021712]
Level 3 (low-level) evidenceTalwar A, Estes E, Aparasu R, Reddy DS. Clinical efficacy and safety of cannabidiol for pediatric refractory epilepsy indications: A systematic review and meta-analysis. Experimental neurology. 2023 Jan:359():114238. doi: 10.1016/j.expneurol.2022.114238. Epub 2022 Oct 4 [PubMed PMID: 36206805]
Level 1 (high-level) evidenceCrowley R, Cline K, Hilden D, Beachy M, Health and Public Policy Committee of the American College of Physicians. Regulatory Framework for Cannabis: A Position Paper From the American College of Physicians. Annals of internal medicine. 2024 Aug:177(8):1104-1105. doi: 10.7326/M24-0638. Epub 2024 Jul 23 [PubMed PMID: 39038289]
Kansagara D, Hill KP, Yost J, Humphrey LL, Shaw B, Obley AJ, Haeme R, Akl EA, Qaseem A, Population Health and Medical Science Committee of the American College of Physicians, Dunn AS, Jackson CD, Jokela JA, Lee RA, Mackey K, Saini SD, Tschanz MP, Wilt TJ, Etxeandia-Ikobaltzeta I, Shamliyan T, Vigna C. Cannabis or Cannabinoids for the Management of Chronic Noncancer Pain: Best Practice Advice From the American College of Physicians. Annals of internal medicine. 2025 May:178(5):714-724. doi: 10.7326/ANNALS-24-03319. Epub 2025 Apr 4 [PubMed PMID: 40183677]
Mersiades AJ, Tognela A, Haber PS, Stockler M, Lintzeris N, Simes J, McGregor I, Olver I, Allsop DJ, Gedye C, Kirby AC, Morton RL, Fox P, Clarke S, Briscoe K, Aghmesheh M, Wong N, Walsh A, Hahn C, Grimison P. Oral cannabinoid-rich THC/CBD cannabis extract for secondary prevention of chemotherapy-induced nausea and vomiting: a study protocol for a pilot and definitive randomised double-blind placebo-controlled trial (CannabisCINV). BMJ open. 2018 Sep 12:8(9):e020745. doi: 10.1136/bmjopen-2017-020745. Epub 2018 Sep 12 [PubMed PMID: 30209152]
Level 3 (low-level) evidenceCouttas TA, Boost C, Pahlisch F, Sykorova EB, Mueller JK, Jieu B, Leweke JE, Dammann I, Hoffmann AE, Loeffler M, Grimm O, Enning F, Flor H, Meyer-Lindenberg A, Koethe D, Rohleder C, Leweke FM. Dose-dependent effects of oral cannabidiol and delta-9-tetrahydrocannabinol on serum anandamide and related N-acylethanolamines in healthy volunteers. BMJ mental health. 2024 Aug 25:27(1):. doi: 10.1136/bmjment-2024-301027. Epub 2024 Aug 25 [PubMed PMID: 39182921]
. Cannabidiol. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012:(): [PubMed PMID: 31644197]
Pratt VM, Scott SA, Pirmohamed M, Esquivel B, Kattman BL, Malheiro AJ, Dean L, Kane M. Dronabinol Therapy and CYP2C9 Genotype. Medical Genetics Summaries. 2012:(): [PubMed PMID: 33211456]
Drug Enforcement Administration, Department of Justice. Schedules of Controlled Substances: Placement in Schedule V of Certain FDA-Approved Drugs Containing Cannabidiol; Corresponding Change to Permit Requirements. Final order. Federal register. 2018 Sep 28:83(189):48950-3 [PubMed PMID: 30272400]
Metz TD, Borgelt LM. Marijuana Use in Pregnancy and While Breastfeeding. Obstetrics and gynecology. 2018 Nov:132(5):1198-1210. doi: 10.1097/AOG.0000000000002878. Epub [PubMed PMID: 30234728]
. Committee Opinion No. 722: Marijuana Use During Pregnancy and Lactation. Obstetrics and gynecology. 2017 Oct:130(4):e205-e209. doi: 10.1097/AOG.0000000000002354. Epub [PubMed PMID: 28937574]
Level 3 (low-level) evidenceHoldsworth EA, Berim A, Gang DR, Williams JE, Smith CB, Caffé B, Brooks O, Barbosa-Leiker C, McGuire MA, McGuire MK, Meehan CL. Human Milk Cannabinoid Concentrations and Associations with Maternal Factors: The Lactation and Cannabis (LAC) Study. Breastfeeding medicine : the official journal of the Academy of Breastfeeding Medicine. 2024 Jul:19(7):515-524. doi: 10.1089/bfm.2024.0021. Epub 2024 May 2 [PubMed PMID: 38695182]
. Dronabinol. Drugs and Lactation Database (LactMed®). 2006:(): [PubMed PMID: 30000656]
. Cannabidiol. Drugs and Lactation Database (LactMed®). 2006:(): [PubMed PMID: 30601607]
Chao YS, McCormack S. Medicinal and Synthetic Cannabinoids for Pediatric Patients: A Review of Clinical Effectiveness and Guidelines. 2019 Oct 11:(): [PubMed PMID: 31873990]
Committee on Substance Abuse, Committee on Adolescence, Committee on Substance Abuse Committee on Adolescence. The impact of marijuana policies on youth: clinical, research, and legal update. Pediatrics. 2015 Mar:135(3):584-7. doi: 10.1542/peds.2014-4146. Epub 2015 Jan 26 [PubMed PMID: 25624383]
Beedham W, Sbai M, Allison I, Coary R, Shipway D. Cannabinoids in the Older Person: A Literature Review. Geriatrics (Basel, Switzerland). 2020 Jan 13:5(1):. doi: 10.3390/geriatrics5010002. Epub 2020 Jan 13 [PubMed PMID: 31941020]
Brutlag A, Hommerding H. Toxicology of Marijuana, Synthetic Cannabinoids, and Cannabidiol in Dogs and Cats. The Veterinary clinics of North America. Small animal practice. 2018 Nov:48(6):1087-1102. doi: 10.1016/j.cvsm.2018.07.008. Epub [PubMed PMID: 30342565]
Level 3 (low-level) evidenceSharma U. Cannabis hyperemesis syndrome. BMJ case reports. 2018 Oct 14:2018():. pii: bcr-2018-226524. doi: 10.1136/bcr-2018-226524. Epub 2018 Oct 14 [PubMed PMID: 30323105]
Level 3 (low-level) evidenceStout SM, Cimino NM. Exogenous cannabinoids as substrates, inhibitors, and inducers of human drug metabolizing enzymes: a systematic review. Drug metabolism reviews. 2014 Feb:46(1):86-95. doi: 10.3109/03602532.2013.849268. Epub 2013 Oct 25 [PubMed PMID: 24160757]
Level 1 (high-level) evidenceBen-Menachem E, Gunning B, Arenas Cabrera CM, VanLandingham K, Crockett J, Critchley D, Wray L, Tayo B, Morrison G, Toledo M. A Phase II Randomized Trial to Explore the Potential for Pharmacokinetic Drug-Drug Interactions with Stiripentol or Valproate when Combined with Cannabidiol in Patients with Epilepsy. CNS drugs. 2020 Jun:34(6):661-672. doi: 10.1007/s40263-020-00726-4. Epub [PubMed PMID: 32350749]
Level 1 (high-level) evidenceZhu HJ, Wang JS, Markowitz JS, Donovan JL, Gibson BB, Gefroh HA, Devane CL. Characterization of P-glycoprotein inhibition by major cannabinoids from marijuana. The Journal of pharmacology and experimental therapeutics. 2006 May:317(2):850-7 [PubMed PMID: 16439618]
Tsang CC, Giudice MG. Nabilone for the Management of Pain. Pharmacotherapy. 2016 Mar:36(3):273-86. doi: 10.1002/phar.1709. Epub 2016 Feb 29 [PubMed PMID: 26923810]
Weiss S, Smith D. Open sesame: Shedding light on an emerging global allergen. Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology. 2023 Jan:130(1):40-45. doi: 10.1016/j.anai.2022.08.002. Epub 2022 Aug 13 [PubMed PMID: 35973653]
Allen D. Dronabinol Therapy: Central Nervous System Adverse Events in Adults With Primary Brain Tumors. Clinical journal of oncology nursing. 2019 Feb 1:23(1):23-26. doi: 10.1188/19.CJON.23-26. Epub [PubMed PMID: 30681988]
Bajtel Á, Kiss T, Tóth B, Kiss S, Hegyi P, Vörhendi N, Csupor-Löffler B, Gede N, Hohmann J, Csupor D. The Safety of Dronabinol and Nabilone: A Systematic Review and Meta-Analysis of Clinical Trials. Pharmaceuticals (Basel, Switzerland). 2022 Jan 14:15(1):. doi: 10.3390/ph15010100. Epub 2022 Jan 14 [PubMed PMID: 35056154]
Level 1 (high-level) evidenceRezkalla S, Kloner RA. Cardiovascular effects of marijuana. Trends in cardiovascular medicine. 2019 Oct:29(7):403-407. doi: 10.1016/j.tcm.2018.11.004. Epub 2018 Nov 10 [PubMed PMID: 30447899]
Lattanzi S, Brigo F, Trinka E, Zaccara G, Cagnetti C, Del Giovane C, Silvestrini M. Efficacy and Safety of Cannabidiol in Epilepsy: A Systematic Review and Meta-Analysis. Drugs. 2018 Nov:78(17):1791-1804. doi: 10.1007/s40265-018-0992-5. Epub [PubMed PMID: 30390221]
Level 1 (high-level) evidenceAnderson GD, Chan LN. Pharmacokinetic Drug Interactions with Tobacco, Cannabinoids and Smoking Cessation Products. Clinical pharmacokinetics. 2016 Nov:55(11):1353-1368 [PubMed PMID: 27106177]
Steuart SR. The addition of cannabis to prescription drug monitoring programs and medication fills in Medicaid. Health economics. 2025 Feb:34(2):283-296. doi: 10.1002/hec.4911. Epub 2024 Nov 4 [PubMed PMID: 39496576]
Schiemer C, Summers MJ, Stefanidis KB. Identifying EEG markers related to acute cannabis consumption: A systematic review. Neuroscience and biobehavioral reviews. 2025 May:172():106092. doi: 10.1016/j.neubiorev.2025.106092. Epub 2025 Mar 6 [PubMed PMID: 40057256]
Level 1 (high-level) evidenceBraidwood R, Mansell S, Waldron J, Rendell PG, Kamboj SK, Curran HV. Non-Dependent and Dependent Daily Cannabis Users Differ in Mental Health but Not Prospective Memory Ability. Frontiers in psychiatry. 2018:9():97. doi: 10.3389/fpsyt.2018.00097. Epub 2018 Mar 27 [PubMed PMID: 29636705]
Wilson RP, Bhattacharyya S. Antipsychotic efficacy in psychosis with co-morbid cannabis misuse: A systematic review. Journal of psychopharmacology (Oxford, England). 2016 Feb:30(2):99-111. doi: 10.1177/0269881115612237. Epub 2015 Oct 28 [PubMed PMID: 26510450]
Level 1 (high-level) evidenceLeen NA, Kowal MA, Batalla A, Bossong MG. The effects of standardized cannabis products in healthy volunteers and patients: a systematic literature review. Frontiers in pharmacology. 2024:15():1411631. doi: 10.3389/fphar.2024.1411631. Epub 2024 Oct 17 [PubMed PMID: 39484170]
Level 1 (high-level) evidenceRubin R. The Path to the First FDA-Approved Cannabis-Derived Treatment and What Comes Next. JAMA. 2018 Sep 25:320(12):1227-1229. doi: 10.1001/jama.2018.11914. Epub [PubMed PMID: 30193358]
Zylla D, Steele G, Eklund J, Mettner J, Arneson T. Oncology Clinicians and the Minnesota Medical Cannabis Program: A Survey on Medical Cannabis Practice Patterns, Barriers to Enrollment, and Educational Needs. Cannabis and cannabinoid research. 2018:3(1):195-202. doi: 10.1089/can.2018.0029. Epub 2018 Oct 1 [PubMed PMID: 30426072]
Level 3 (low-level) evidenceSchmitz N, Richert L. Pharmacists and the future of cannabis medicine. Journal of the American Pharmacists Association : JAPhA. 2020 Jan-Feb:60(1):207-211. doi: 10.1016/j.japh.2019.11.007. Epub 2019 Dec 20 [PubMed PMID: 31870860]