Introduction
Degenerative disc disease (DDD) of the cervical spine typically develops equally in the aging population regardless of patient sex. Patients most commonly present with pain; when isolated or in combination with other neurological symptoms, pain may necessitate surgical intervention. Treatment options range from nonoperative measures to surgical options, such as decompression, arthroplasty, and instrumented fusion. This section examines the anatomy, natural history, etiology, pathophysiology, evaluation, and treatment options for patients with cervical DDD.
The cervical spine, spanning from C1 to C7, offers remarkable function and mobility. The upper cervical segments—C1 (the atlas, which articulates with the occiput) and C2 (the axis)—are uniquely structured to allow extensive motion, with primary rotation occurring at the C1–C2 joint and flexion-extension at the occiput–C1 joint. Surrounding or contained within these vertebrae are critical structures, including the spinal cord, nerve roots, major blood vessels, the trachea, and esophagus.
The intervertebral disc (IVD) is located from the C2–C3 level down, aiding in cervical spine mobility and stabilization. In contrast to the thoracic and lumbar vertebrae, the cervical vertebrae have a unique bony prominence called the uncinate process, which articulates with the adjacent level to form the joint of Luschka or uncovertebral joint. This joint helps to reinforce the IVD and provides additional stability and motion.[1] The IVD is an intricate structure composed mainly of 2 parts, the peripherally located annulus fibrosus and the centrally located nucleus pulposus, which are responsible for its load distribution function. The anterior and posterior longitudinal ligaments reinforce the IVD.
The annulus fibrosus of the intervertebral discs is mostly of type I collagen in layers (lamellae), proteoglycans, glycoproteins, elastic fibers, and extracellular matrix (ECM) secreting cells. These collagen layers are uniquely positioned to form a strong shell for the inner contents, the nucleus pulposus. The nucleus pulposus has a gel-like consistency composed mainly of water, which decreases with age (90% at birth and 70% by the age of 60). The remaining minority of the nucleus pulposus contents comprises type II collagen and proteoglycans. Aggrecan is a critical proteoglycan in the nucleus pulposus, which, when bound to hyaluronic acid, helps retain water within the nucleus pulposus, allowing for load resistance.
After the first year of life, the IVD becomes the largest avascular structure in the body. Most of the nutrition is delivered via metabolite diffusion from the vertebral endplates. Over time, the IVD loses its water content and proteoglycan supply, resulting in a more fibrotic consistency of the nucleus pulposus and subsequent fissuring as the vertebral endplates calcify with age.
There are different types of nucleus pulposus herniations. If the nucleus pulposus herniates but remains contained by the annulus, it is called disc protrusion. However, the nucleus pulposus can also penetrate through injured annular fibers, and nucleus pulposus contents can extrude through a defect in the annulus, referred to as a disc extrusion. Furthermore, nucleus pulposus fragments can be separated from the extruded disc material, yielding disc sequestration.[2]
The degenerative process of the cervical spine is classified into 3 distinct stages: dysfunction, instability, and stabilization. Dysfunction occurs between the ages of 15 and 45. During this stage, radial and circumferential tears can occur in the annulus, accompanied by facet joint localized synovitis. Instability can occur in individuals between the ages of 35 and 70. This stage is characterized by inner disc disruption, progressive resorption, and degeneration of the facet joints. This condition leads to the final stage of the process, stabilization, which typically occurs after age 60. Here, hypertrophic bone develops around the facet joints as well as the disc, promoting a stiff and possibly ankylosed spine.
Interestingly, each spine segment may be at a different stage of degeneration. One level could complete the dysfunction stage, while another could begin the stabilization phase. Disc herniations appear to occur due to the dysfunction and instability phase; spinal stenosis occurs due to the late instability stage and early stabilization stage due to the bony overgrowth and disc space narrowing. Consequently, patients may present with a combination of disc herniations and spinal stenosis affecting different levels of the cervical spine. The C5–6 segment is most commonly affected because of the cervical spine’s biomechanical stress and mobility patterns.
When discussing the natural history of cervical DDD and treatment options, symptomatic individuals can experience an array of symptoms, from intermittent or constant pain, along with possible neurological symptoms, without pain. Patients generally receive nonoperative treatment when experiencing nonprogressive pain and/or minimal neurological issues. If surgery is indicated, it is typically elective and can be delayed to achieve symptomatic improvement. However, an exception is patients diagnosed with cervical myelopathy, who should have more urgent surgical treatment to avoid neurological deterioration.
The proper diagnosis and treatment for spondylolytic cervical myelopathy can be extremely challenging, especially in patients with or even without ongoing axial neck pain with possible radiculopathy. One must also be aware that 20% of patients with cervical stenosis may also have lumbar stenosis. While many patients may have a straightforward diagnosis with a thorough history and physical examination accompanied by confirmatory imaging modalities, there is a significant subset of patients who have pain without experiencing neurological findings, aside from possible sensory changes, and whose imaging may not easily correlate with physical exam findings. In such cases, additional diagnostic modalities should complement a thorough history and physical examination to ensure an accurate diagnosis. Therefore, a systematic assessment is essential for effectively diagnosing and treating these patients.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Since IVD depends on intervertebral endplate diffusion for nutrition, cervical degeneration is a normal part of aging, which typically begins early in the second decade of life. As the discs lose water content, they can become less compliant and develop fissures, resulting in a decrease in disc height and potential collapse. This change in alignment can further put stress on the posterior aspect of the cervical spine and lead to spondylotic changes as well (ie, facet hypertrophy). Degeneration dehydrates the nucleus pulposus, redirecting cervical spinal loads to the annulus fibrosus; this added stress promotes fissuring and progressive weakening of the annulus fibrosus. This further causes the IVD to lose height, as the annulus fibrosus starts to bulge and increase in diameter, and then fissure.
In addition to aging, some environmental and genetic factors can predispose individuals to the development of cervical DDD. Notably, with the increasing use of electronics (such as handheld devices like smartphones) while sitting, chronic flexion at the neck can lead to increased stress on the discs. In the neutral position, the head weighs approximately 12 lbs (5.4 kg). With increasing flexion, the relative weight seen on the discs increases to 27 lbs (12.2 kg) at 15°, 40 lbs (18.1 kg) at 30°, 49 lbs (22.2 kg) at 45°, and 60 lbs (27.2 kg) at 60° of flexion. Chronic increased loading leads to increased stress on the IVD, particularly on the nucleus pulposus, resulting in poor diffusion and nutrition, and subsequent degeneration.[3]
Other possible risk factors and causes have been the subject of research, including smoking, occupation, genetics, atherosclerosis, contact sports, and prior surgeries. However, study results have found contradictory evidence on the contribution of body mass index, sex, sports, smoking, and alcohol consumption.[4] Gore et al showed no evidence suggesting cigarette smoking is a causative agent in cervical spine degeneration. Sports such as weightlifting were also not found to increase the risk of degeneration.[5]
Epidemiology
Like the lumbar spine, cervical disc degeneration is a naturally occurring age-related phenomenon. The prevalence of cervical DDD increases with age, regardless of the patient's symptomatology or lack thereof.[6][7] Literature has demonstrated that in a select population, a quarter of patients younger than 40 showed evidence of disc degeneration or narrowing at 1 level. This evidence was found in approximately 60% of patients 40 and older.[8] Lehto et al's study results demonstrated similar findings in asymptomatic individuals older than 40, in which 62% showed abnormalities on magnetic resonance imaging.[9]
According to results from a Japanese study of 497 individuals conducted by Matsumoto et al, patients older than 60 had abnormal cervical spine findings in nearly 90% of cases. In contrast, 17% and 12% of men and women in their 20s, respectively, demonstrated abnormalities.[10] Results from several studies have acknowledged that the most common disc level involved in degeneration was C5–6, and the second most common was C6–7. Study results have also demonstrated that cervical DDD was associated with lumbar degenerative changes in both men and women, but did appear later in life. An association has also been found in the pain distribution in the neck-shoulder-brachial region in patients with moderate to severe cervical DDD.[9][10][11]
Pathophysiology
The pathophysiology of cervical DDD is similar to that of the thoracic and lumbar spine. Typically, physiologic changes occur within the nucleus pulposus first, followed by progressive degeneration of the annulus. This normal degenerative process may lead to the extrusion of the nuclear components. The segments become hypermobile, leading to additional degenerative arthritic changes and instability. Unlike the lumbar spine, these hypertrophic changes primarily occur at the uncinate process, which forms the ventral wall of the foramen (the uncovertebral joint). The facet joints and vertebral bodies also eventually undergo these hypertrophic changes due to altered loads. As mentioned earlier, these changes result in increased stiffness and decreased cervical spine motion.
Once a herniated nucleus pulposus (HNP) occurs, pain, which is the most common finding in cervical DDD, depends upon the annulus fibrosus injury level and the location of the herniation on the disc. HNPs may also be asymptomatic. Most herniations occur posterolaterally, given the annulus fibrosus's thinner and weaker structure in this area due to the posterior longitudinal ligament not covering this area as thickly. Given the posterolateral location of the nerve root, the HNP can produce neurological symptoms in 2 ways. One is if the dorsal root ganglion becomes compressed; the presenting symptoms typically follow a dermatomal pattern. However, if the HNP is directly posterior, the second way is that spinal cord compression can occur, with symptoms caudal to the level of cord compression.
DDD, cervical spondylosis, and disc herniation exist along a continuum of cervical spine degeneration: DDD refers specifically to the biochemical and structural deterioration of the intervertebral discs, whereas cervical spondylosis describes the broader osteoarthritic changes that follow, including remodeling of the vertebral bodies, hypertrophy of the facet joints, and thickening of ligaments.[12][13] Within this degenerative cascade, a disc herniation occurs when weakened annular fibers permit nucleus pulposus material to protrude or extrude into the spinal canal or neural foramina, representing a focal manifestation of the underlying disc pathology. Thus, DDD serves as the initial, disc-focused process; spondylosis encompasses the resultant multi-component remodeling of the entire motion segment; and disc herniation denotes the localized herniation event that may develop as discs progressively degenerate.
History and Physical
A careful history is critical to ensure that presenting symptoms are caused by degenerative changes and not more serious conditions. Discussion with the patient about possible constitutional symptoms (weight loss, fever, chills, and/or sweats) and infections can be crucial, along with oncologic history and trauma. If pain is a presenting symptom, a thorough pain history is necessary, including functional limitation, intensity, onset, alleviating and exacerbating factors, radiation, and related symptoms. A comprehensive history of neurologic complaints, including weakness, sensory loss, and gait disturbances, is imperative.
The physical exam should begin with the patient's vital signs (eg, fever). A thorough neurologic examination is necessary, which should include motor testing of the upper and lower extremities, cranial nerve testing, gait and balance assessment, sensation testing, reflex testing (including the Hoffman and Babinski reflexes), and any clonus. The range of motion of the cervical spine should be evaluated for both degrees of movement and any associated symptoms.
Most commonly, patients present with axial neck pain and difficulty with movement.[14] A small subset of patients may experience headaches, while others tend to report shoulder pain.[15] Unilateral radicular symptoms are also prevalent and most commonly result from posterolateral disc herniations and osteophytes at the neural foramen. Other signs and symptoms include changes in deep tendon reflexes, muscle atrophy, hypesthesia, paresthesia, or weakness, as specific nerve root signs demonstrate. A significant proportion of the flexion/extension motion of the cervical spine occurs between C4–C6. Interestingly, research indicates that the C5–C6 interspace exhibits the earliest and most significant degeneration.[16] The nerve roots most often affected by a disc protrusion are C6 and C7. The C6 nerve root exits the spinal canal between the C5 and C6 vertebrae.[17]
Provocative testing, such as the Spurling test and the shoulder abduction (relief) test, can help evaluate for any radicular symptoms, indicating radiculopathy.[18] The Lhermitte sign can also aid in diagnosing potential cervical myelopathy. Evaluation of the paraspinal musculature for spasm and trigger points is necessary. An assessment of the upper extremity would also be helpful in further distinguishing the presenting symptoms. For example, a patient may be complaining of a vague numbness and tingling in the hand, which may be secondary to carpal tunnel syndrome, double crush syndrome, or cervical radiculopathy.[19]
Cervical DDD may be asymptomatic, with degenerative changes only detected on imaging, such as a computed tomography scan, x-rays, or MRI. A posterolateral symptomatic HNP presents with dermatomal pain, myotomal weakness, or sensory change. The most common HNP is at C6–7, anterior to the C7 nerve root. When the C7 nerve root is inflamed or compressed, the patient complains of pain from the neck radiating to the lateral forearm and then palm, and possibly radiation to the middle finger. Muscle weakness may be present in the ipsilateral triceps muscle, making elbow extension difficult. The triceps reflex may also be depressed and asymmetrical.
C5 root compression can present with neck pain radiating to the shoulder and peri-scapula pain. The deltoid may be affected, and weakness may occur during shoulder abduction, external rotation, and elbow flexion. The biceps and brachioradialis reflexes may also be depressed and asymmetrical. When the C6 nerve is affected, neck pain radiates to the neck, shoulder, down to the thumb and index finger, in addition to periscapular pain. This nerve root innervates the biceps along with the wrist extensors, and weakness during elbow flexion and wrist extension may be present. The biceps and brachioradialis reflexes may also be depressed and asymmetrical. The C8 nerve root provides sensation from the neck to the medial forearm and medial hand. The muscle groups innervated are the finger extensors, and weakness during the thumb extension may occur. Similar to the C8 nerve root, the T1 nerve root, when involved, presents with pain in the neck radiating to the medial arm and forearm, but rarely goes to the hand. First dorsal interosseous muscle weakness may be present.
Cervical DDD can cause cervical spondylotic myelopathy.[20] This condition most commonly occurs in patients aged 50 and older. The signs and symptoms are variable and unique, describing their legs as stiff and/or wooden. Some may complain of numbness and tingling from the fingertips to the hands, as if they were wearing gloves.[21] Writing and other fine motor functions (dexterity) may also become altered. Hyperreflexia, a positive Hoffman sign, ankle clonus, and a Babinski reflex may also be present. Gait disturbances can occur. In severe cases, changes to the bladder and bowel can occur.[22]
Evaluation
Laboratory testing may aid in diagnosis, especially if an infection (ie, Lyme disease), autoimmune arthritis, or a metabolic disorder (eg, folate or vitamin B12 deficiency) is high on the differential diagnosis. A complete blood count with differential, an erythrocyte sedimentation rate, and C-reactive protein are also necessary; however, none of these tests lacks specificity for spinal infection. Blood cultures may also be indicated when an infection is suspected.
Imaging should begin with a radiographic evaluation, which may indicate a decrease in normal cervical lordosis, hypertrophic changes, and disc space narrowing. They are also helpful in ruling out additional problems, such as fractures or instability. Computed tomography may also be helpful for preoperative planning, especially in patients with dorsal osteophytes and ossification of the posterior longitudinal ligament. MRI is the gold standard for patients when considering degeneration of the cervical spine as a potential diagnosis because it provides excellent visualization of ligaments, discs, and neural structures. MRI enables the evaluation of soft tissue and bony structures, accurately measuring functionally relevant spinal canal and spinal cord dimensions in various planes.[23]
Reduced spinal canal width increases the risk of cervical cord compression and myelopathy.[24] The risk is higher at these levels because the space around the cord decreases relatively in the lower cervical spine segments.[25][26][27] On MRI, the inner disc spaces tend to have a moderately high signal intensity, and the surrounding rim, the annulus fibrosus, is of low signal intensity. A dorsal protrusion of disc material signifies a central or paracentral herniation into the spinal canal. Myelopathic individuals may not initially present with cord changes but may develop over time. These changes are demonstrated by hyperintensity at the level of the cord compression with cord edema or even myelomalacia. Notably, additional modalities can help rule out other potential diagnoses. For example, electromyelography may be beneficial to exclude compressive and/or peripheral neuropathies caused by occupational exposures, diabetes, or folate/vitamin B12 deficiencies.
Degenerative Disc Disease Classifications
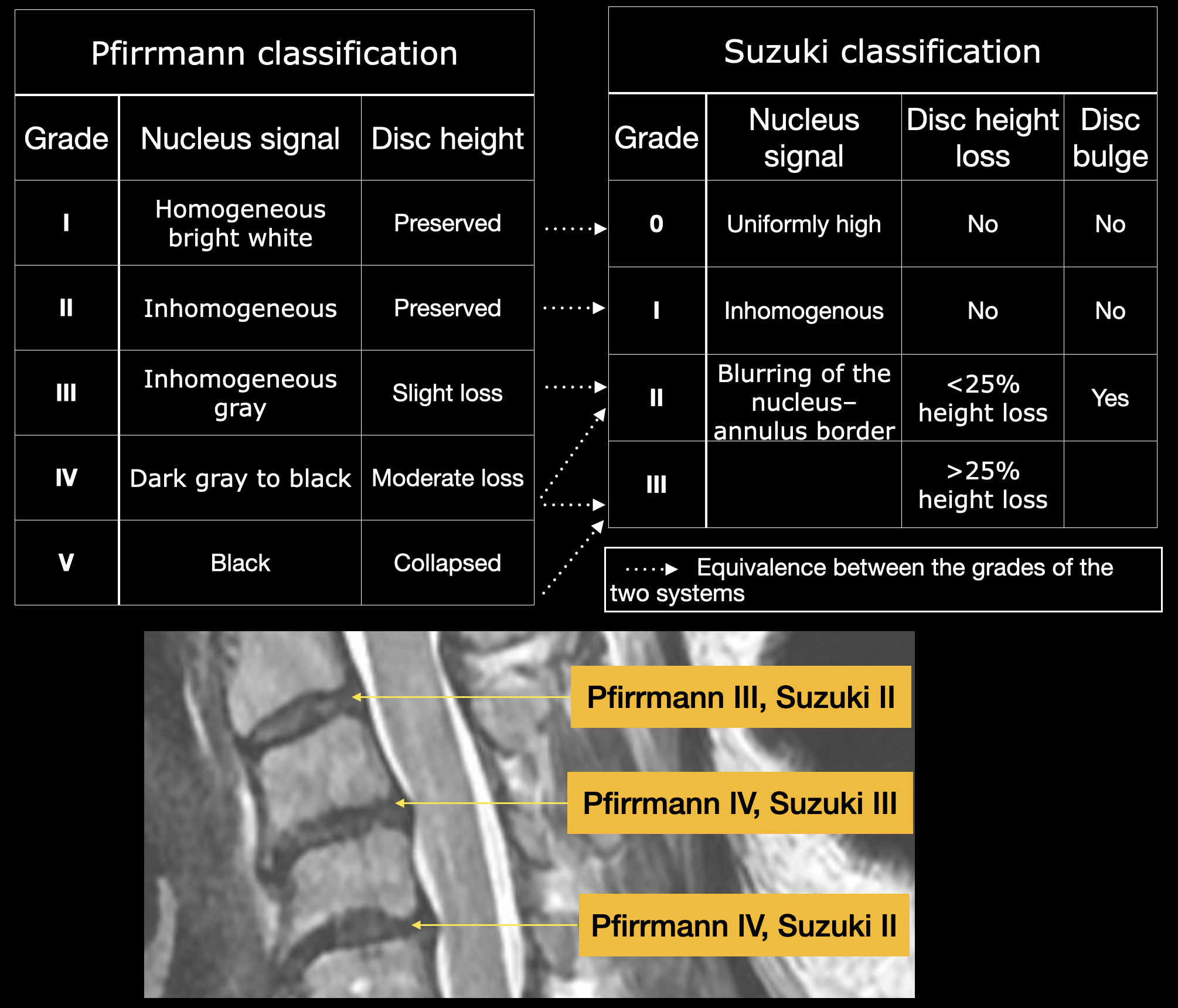
The Pfirrmann system, originally developed for lumbar discs, has nonetheless become the most commonly used method for grading cervical disc degeneration, ranging from I to V, based on T2-weighted signal intensity, clarity of the nucleus–annulus boundary, and disc height. In direct comparisons, Pfirrmann scores demonstrate only fair interobserver agreement but near-perfect intraobserver reliability, highlighting the practical robustness of this method despite its lack of tailoring to the cervical spine. The Suzuki scale, by contrast, was created specifically for cervical discs and condenses degeneration into 4 grades while explicitly documenting disc bulge. The interobserver agreement rivals that of Pfirrmann, and intraobserver consistency is substantial. By including bulging in its criteria, Suzuki offers a more direct index of morphological changes that may precipitate neural compression; however, it remains a largely qualitative tool susceptible to reader interpretation.[28]
The Pfirrmann classification:
- Grade I: Shows a homogeneous, bright-white disc on T2 imaging with a sharply defined nucleus–annulus distinction and preserved disc height
- Grade II: Reveals an inhomogeneous nucleus (sometimes with horizontal bands) but retains normal height
- Grade III: Presents a gray, heterogeneous nucleus with loss of the crisp nucleus–annulus boundary and initial signs of height reduction
- Grade IV: Depicts a dark gray to black disc lacking discernible internal architecture and demonstrating moderate height loss
- Grade V: Describes a collapsed, black disc space reflecting advanced structural failure
The Suzuki classification:
- Grade 0: A healthy disc with uniformly high T2 signal intensity and normal height
- Grade I: An inhomogeneous nucleus signal without bulging or height loss
- Grade II: Blurring of the nucleus–annulus border, a discrete disc bulge, and less than 25% height reduction
- Grade III: Over 25% loss of disc height, signifying advanced degeneration
The disc signal intensity index (DSI²) offers a quantitative metric by averaging T2 signal values from 3 distinct disc regions and normalizing this mean against the cerebrospinal fluid signal on the same midsagittal image. As degeneration progresses, the resulting continuous DSI² score decreases and correlates linearly with Pfirrmann grades (see Image. Pfirrmann and Suzuki Classification, Cervical Disc Degenerative Disease). Patient age, body mass index, and the presence of Modic end-plate changes each independently affect the DSI² value, underscoring its utility for longitudinal assessment and risk stratification.[29]
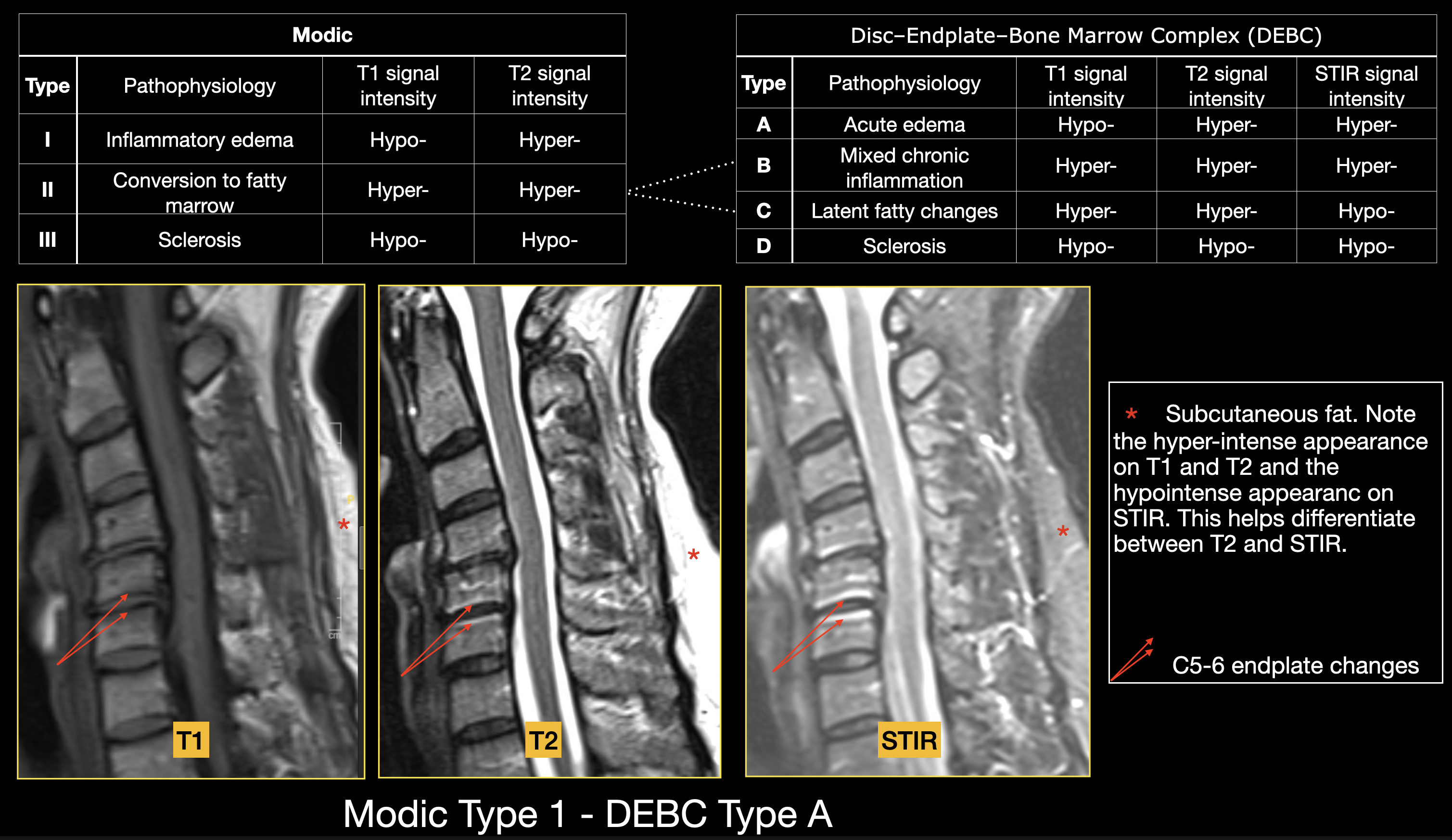
Modic changes are MRI signal alterations in vertebral endplates and adjacent marrow that reflect stages of degeneration. Type I appears dark on T1 and bright on T2 (inflammatory edema), type II is bright on both sequences (fatty marrow replacement), and type III is dark on both (sclerosis). Although some add a grade 0 for normal endplates or subdivide each type by extent, the 3-type classification remains standard. Modic changes occur in 5% to 40% of individuals in the cervical spine, most commonly at the C5–6 level, with type II being the most prevalent. Prevalence varies by population, lower in asymptomatic volunteers and higher in symptomatic cohorts. Segments with Modic changes are several times more likely to show high-grade disc degeneration or herniation, and patients with these changes report more chronic neck pain and disability. Type I lesions, indicating active inflammation, carry the strongest association with further disc collapse.[30]
The disc–endplate–bone marrow complex (DEBC) classification extends Modic by incorporating short tau inversion recover (STIR) sequences alongside T1- and T2-weighted imaging to grade the disc, endplate, and adjacent marrow as a single unit (see Image. Modic and Disc–Endplate–Bone Marrow Complex [DEBC] Classification Systems, Endplate Degenerative Changes). By suppressing the fat signal, STIR differentiates inflammatory edema from fatty replacement, yielding 4 phenotypes: type A (acute edema), type B (mixed chronic inflammation), type C (latent fatty change), and type D (sclerosis).
In a 2025 cohort of 301 individuals with neck pain and 200 trauma controls, STIR reclassified about 25% of endplates previously labeled Modic type II, revealing that mixed edema–fat lesions are frequently missed without STIR. Importantly, combined DEBC lesions and disc herniations increased the odds of surgery nearly 7-fold compared with herniation alone, whereas isolated DEBC or endplate erosions had minimal impact. This evidence supports viewing the disc, endplate, and marrow as a unified biomechanical-biological complex, with combined pathology signaling segments at high risk for clinical failure.[31]
Treatment / Management
Treatment for cervical DDD is centered around decreasing pain, improving function, and minimizing the recurrence and duration of symptoms. Management generally begins with conservative measures and progresses to surgical intervention when warranted.
Nonoperative Treatment
Conservative modalities include rest, modification of activity, pharmacological agents, physical therapy, manipulation, injections, and acupuncture. Initial short-term immobilization may be beneficial. Medications may provide symptomatic relief. These may include nonsteroidal anti-inflammatory drugs (NSAIDs), steroids, and/or muscle relaxants. NSAIDs are commonly used and provide relief via interfering with prostaglandin synthesis, leading to the inhibition of the inflammatory cascade.
Steroids may be beneficial in initial management, but only for short-term usage, as they are associated with deleterious side effects. Muscle relaxants are an option when patients are experiencing muscle spasms. Gross et al demonstrated that cervical mobilization and manipulation might provide immediate or short-term relief for neck pain.[32] Gamma-aminobutyric acid class drugs, such as gabapentin, and serotonin reuptake inhibitors may also be considered, and tricyclic antidepressants may also play a role; should avoid using opioids. A cervical collar may be recommended in patients who present with intractable neck pain. Controversy exists over the efficacy of cervical traction for the treatment of HNP, as there is no evidence to suggest a reduction in the degree of disc herniation.[33] However, it may allow for some neuroforaminal decompression.(A1)
Physical therapy should start early in the treatment algorithm. Passive modalities should be used, including, but not limited to: heat, mechanical traction, massage, and a soft cervical collar.[34] Heat has been shown to decrease pain and reduce muscle spasms.[35][36] Evidence suggests cryotherapy can help decrease inflammation and reduce muscle guarding.[37][38] Using soft tissue mobilization/massage techniques on pertinent muscles affecting the pain allows for mechanical stimulation, which leads to increased circulation and promotes muscle relaxation.[39] Cervical traction may allow for joint distraction and potentially relieve pressure off nerve roots/discs; this may improve epidural blood flow and reduce pain, inflammation, and spasms.(A1)
Active modalities should also be part of rehabilitation, including aerobic conditioning, dynamic muscle training, isometric, and range of motion exercises. Postural training may help. Isometric exercises enable the strengthening of paravertebral muscles while avoiding certain motions that can cause pain. Recent reports suggest that no strong evidence supports the use of neck strengthening and stretching exercises for patients with chronic neck pain.[40](A1)
As stated previously, the diagnosis and treatment of cervical myelopathy may be difficult and unpredictable. Symptom trajectories vary with some patients deteriorating swiftly, whereas others plateau, but most eventually show progressive decline over variable timeframes.[41] Unfortunately, spontaneous improvement rarely occurs. Sometimes, close observation may be a consideration in patients with mild symptoms that appear not to be progressing.
Operative Treatment
Pain management procedures may also be beneficial. Nerve root injections or epidural steroid injections can not only be used as a diagnostic tool but can also be therapeutic. However, they are not without undesirable complications.[42][43] Historically, trigger point injection has also been used, but no evidence has shown its long-term effectiveness.(B3)
Patients who fail to respond to nonoperative treatment, especially those experiencing intractable pain, progressive or significant neurological compromise, may require surgical intervention. One surgical option is decompression. Decompression procedures may include fusion, usually performed with instrumentation. If the decompression surgery involves a laminectomy, typically it includes a fusion to minimize the potential for postsurgical deformity.
A variety of surgical approaches are available for managing cervical spine conditions. Commonly, the neural structures become compressed anteriorly; therefore, an anterior approach is preferable to directly remove the pathology compressing the neural structures, thus performing a decompression. The compressing pathology is typically herniated disc material and/or a disc/osteophyte complex, and is approached through the disc space. If a fusion follows, it is called an anterior cervical discectomy and fusion (ACDF). The approach of choice in patients with normal to kyphotic alignment is the anterior approach, as a laminectomy in these patients may further cause kyphosis secondary to the destabilization that occurs.[44] (B3)
During an ACDF, compressive and degenerative structures are removed, and a fusion is performed across the adjacent segments to decompress. Furthermore, several discs can be removed with multi-level fusions (with or without strut graft or cages of various materials, commonly titanium). A corpectomy with strut grafting or cage placement may be necessary for multi-level decompressions. An anterior cervical plate can be inserted to increase stability and allow for earlier mobilization. Distraction across the disc space with an interbody implant can lead to further indirect decompression of the neural foramen.
Some study results demonstrate a fusion rate of up to 94% after an ACDF.[45] However, for fusion to be successful, appropriate patient selection and precise operative techniques are key. The current literature indicates support for significant symptomatic improvement in most patients.[46][47][48] However, recurrent symptoms, even worsening, may still occur and are likely secondary to adjacent-level degeneration resulting from the fusion.(B2)
Furthermore, neurological compromise is rare.[49] Other potential causes of complications include soft tissue dissection when using the anterior approach, grafting, and plating. During dissection, the recurrent laryngeal nerve may suffer injury, causing a palsy, potentially leading to hoarseness. Additionally, the esophagus and/or trachea may be injured, possibly perforated, and the graft may become dislodged or subside into the vertebral bodies. Hoarseness and/or dysphasia can be noted postoperatively, especially with higher levels operated on or with multi-level cases.
The posterior approach is mainly for patients with neutral or lordotic cervical spine alignment requiring multi-level decompression or predominantly dorsal compressive pathology. Furthermore, the posterior approach is particularly helpful for patients with congenital stenosis, which often involves multiple levels. The muscles attaching to the second cervical segment are protected to avoid progressive kyphosis and postoperative neck pain. The posterior approach can be effective even in ventrally located compressive pathology. The posterior decompression provides the spinal cord additional space, moving away from the disc/bony ridges that compress anteriorly. Options include laminectomy (either with or without fusion) or open-door laminoplasty; both can achieve the same goal—to increase the diameter of the canal.
Highsmith et al showed similar neurological outcomes between the 2, but patients who underwent a laminectomy with fusion had less neck pain compared to laminoplasty.[50] If neck pain is present, fusion should be performed to address this, as just performing a decompression may allow for neurological recovery but not alter pain from DDD. Laminoplasty is typically reserved for patients with cord compression, whether accompanied by or without minimal neck pain. Laminoplasty may minimize the risk of postoperative kyphosis in patients with a neutral to lordotic spine.
Currently, laminectomies are typically accompanied by instrumented fusion, which enhances stability and can help maintain or restore lordosis. Lees et al demonstrated satisfactory results in 70% to 80% of patients who underwent a laminectomy.[51] Hirabayashi et al described the expansive open-door laminoplasty, which has shown good results in 66% of patients.[52] However, these techniques are not without their complications, which include hematoma, dural injury, paralysis, postoperative C5 palsy, postlaminectomy kyphosis, and neck pain. Moreover, a foraminotomy is an option for direct decompression of nerve roots in patients with radiculopathy alone.(B3)
Recently, cervical disc arthroplasty has gained popularity. Some study results show no significant difference between fusion and arthroplasty regarding revision rates 2 years postoperatively.[53] However, this procedure is not indicated for DDD but rather for herniated discs. Spinal instability is a contraindication for arthroplasty and laminoplasty. A combined approach (anterior and posterior) may be needed when a multi-level corpectomy is indicated or when there is diminished bone quality secondary to the patient’s metabolic state.
Differential Diagnosis
A structured differential diagnosis begins by identifying the source of the problem, which may originate from the spinal column, peripheral nerves, surrounding musculoskeletal structures, rheumatologic disorders, or systemic diseases. Within the spinal column, conditions that can resemble DDD include acute cervical disc herniation, ossification of the posterior longitudinal ligament, facet joint arthritis, and advancing cervical spondylosis. Other possibilities include traumatic or inflammatory instability, infections like spondylodiscitis, and metastatic lesions affecting the vertebrae.
Peripheral nerve disorders, such as brachial plexopathy, thoracic outlet syndrome, radiation-induced plexopathy, and diabetic plexopathy, can all cause neck-to-arm pain. Distal nerve entrapment syndromes—including carpal tunnel, cubital tunnel, and ulnar tunnel syndromes—may produce symptoms in the arm and hand that patients mistakenly attribute to cervical spine disease. Musculoskeletal conditions in the shoulder region, like rotator cuff tears, subacromial impingement, adhesive capsulitis, and acromioclavicular arthritis, often refer to lateral neck and upper arm pain. Myofascial trigger points, cervical sprains, facet joint strains, and ligament injuries can cause localized pain and stiffness.
Rheumatologic conditions such as rheumatoid arthritis, ankylosing spondylitis, polymyalgia rheumatica, and fibromyalgia may present with chronic neck stiffness or widespread pain, sometimes accompanied by systemic symptoms. Finally, some visceral or systemic conditions can mimic cervical DDD. These include Pancoast tumors, apical pneumonia, thyroid disorders, and even cardiac ischemia, all of which can refer pain to the neck or shoulder and create the impression of a spinal problem.
Prognosis
The prognosis and disease progression are difficult to predict and vary significantly from patient to patient. In an article published in 1956, Clark and associates followed 120 patients with cervical spondylosis. They found that in 75% of patients, the disease progressed in an episodic manner, in 20% of patients, symptoms gradually progressed, and in 5%, patients became symptomatic rapidly.[54]
Several years later, Lees et al further demonstrated the unpredictability of the disease.[51] In their study of 37 patients, Nurick et al found that there was an initial phase of deterioration, followed by a nonprogressive phase, which, in some cases, lasted for years. Older patients were more prone to further deterioration.[55] In another study, 26% of patients’ conditions worsened, 38% remained stable, and over one-third improved.[56]
Complications
Patients with cervical DDD can experience a significant impact from pain and disability that is often not correlated to the level of pathologic changes in the discs themselves. This disability can result in lost productive days at work, worsening of roles in personal life, and overall poor health due to a more sedentary lifestyle. Neurologic complications can occur as outlined above, with loss of lower extremity function and incontinence being a very catastrophic, albeit rare, complication with myelopathy.
However, the treatment of cervical DDD can also be associated with complications. Nonsteroidal anti-inflammatory drugs can precipitate gastrointestinal bleeding and renal impairment, and prolonged opioid therapy often leads to dependence and delayed return to work. Evidence supporting gabapentin, pregabalin, and tricyclic antidepressants remains limited; dizziness, imbalance, and sedation are frequent and can undermine rehabilitation. Brief oral corticosteroid tapers may sometimes reduce pain duration; however, they can also destabilize glycemic control and mood. Physical therapy and exercise carry little systemic risk, though temporary neck soreness is common, whereas overly forceful cervical manipulation can aggravate neurological symptoms.
Epidural injections deliver anti-inflammatory medication directly next to the irritated nerve root. Major complications are infrequent but documented, including epidural hematoma, abscess, chemical or bacterial meningitis, spinal cord or brainstem infarction from inadvertent intra-arterial injection of particulate steroid, and direct cord injury. Minor adverse events such as transient flushing and postdural puncture headache are also reported.[57]
ACDF, a common operation for cervical DDD, has 16% overall morbidity, with the prevertebral or neck swelling being the most common at 11.3%, followed by pseudarthrosis (10%) and dysphagia (9.5%).[58] Explainable models using Shapley additive values highlight key predictors of complications after surgeries commonly performed for cervical DDD: single- versus multilevel procedures, advanced age, higher body mass index, low preoperative hematocrit, and elevated American Society of Anesthesiologists physical status.[59][60][61][62]
Deterrence and Patient Education
Cervical DDD can significantly impact quality of life, sometimes leading to disability. Patient education plays a central role in prevention and management. Individuals should understand their diagnosis and the importance of early recognition to reduce the risk of functional decline. Primary care clinicians must remain vigilant for common physical exam findings and have a clear referral pathway when concerns arise.
Patients benefit from learning how lifestyle and occupational factors contribute to disease progression. Activities such as repetitive overhead work, heavy lifting, prolonged driving, and vibrating tools can strain the cervical spine. Smoking accelerates degeneration and slows recovery; discussing smoking cessation, ergonomic adjustments, mechanical aids, and scheduled breaks helps lower cumulative cervical stress.
Good posture is essential: keeping your ears aligned over your shoulders, setting screens at eye level, and turning your trunk rather than twisting your neck while lifting. Supportive seating with armrests can reduce neck strain. Regular aerobic exercise and neck and shoulder strengthening improve muscular endurance and stability, making long-term activity more effective than occasional therapy.
Safe medication use should also be discussed. Short-term use of NSAIDs or steroids may help alleviate acute inflammation but carries risks to the gastrointestinal system, kidneys, blood sugar levels, and mood. Opioids should be reserved for severe, persistent pain that limits rehabilitation, and medications like gabapentin or tricyclic antidepressants offer limited evidence and may cause dizziness or sedation.
Patients should learn safe self-care strategies, such as using a contoured cervical pillow and gentle physiotherapy while avoiding forceful cervical manipulation, which could worsen neurological symptoms. Most acute radicular episodes improve without surgery, and a period of nonoperative care is appropriate for motivated patients. Setting realistic expectations for recovery timelines and stressing adherence to exercise and lifestyle modifications are key.
Patients should be aware of red flags that require immediate reassessment, including new or worsening limb weakness, changes in walking balance, bladder or bowel dysfunction, or persistent, unrelenting pain. Early imaging and specialist evaluation can help prevent permanent neurological injury. Ultimately, encouraging patients to take an active role in their care—through consistent exercise, tobacco avoidance, ergonomic awareness, and regular follow-up—forms the foundation for maintaining function and quality of life.
Enhancing Healthcare Team Outcomes
Optimal management of cervical degenerative disc disease requires an interprofessional approach that begins at the initial evaluation and continues through ongoing follow-up. Each care team member brings unique expertise, and coordinating these contributions enhances patient safety, functional outcomes, and overall satisfaction. Primary care clinicians screen for red-flag symptoms, initiate imaging, and activate referral pathways that link patients to physiatrists, neurologists, spine surgeons, and pain specialists within defined time frames. Nurses reinforce screening protocols, coordinate appointments, and monitor treatment adherence through structured phone or digital follow-ups.
Physical therapists design personalized exercise programs and recommend ergonomic adjustments in the workplace. They also teach patients posture and neck-sparing movement strategies to improve their overall well-being. They play an important role in managing acute flare-ups and preventing chronic problems from becoming longstanding. Occupational therapists refine job-specific tasks and recommend adaptive devices that lower cervical load. Pharmacists assess medication regimens for polypharmacy, counsel patients on the safe use of NSAIDs, and identify potential drug interactions that may increase bleeding or kidney complications.
Pain management clinicians perform image-guided epidural, facet, and nerve root injections. High procedural expertise helps reduce the risk of rare but potentially devastating complications, such as hematoma or spinal cord infarction. In the cervical spine, these procedures should be reserved for clinicians with substantial experience, given the severe consequences of any complication.[63] Considering the potential for disability and reduced quality of life, building effective coping strategies is essential. Mental health clinicians play a crucial role in helping patients develop these skills through cognitive behavioral therapy and by utilizing biofeedback techniques to reduce pain perception.[64][65]
Effective team coordination depends on shared electronic health records and clear communication channels. Standardized prehabilitation and postoperative care plans help ensure smooth transitions across hospital care, outpatient rehabilitation, and home support. By clarifying roles, fostering open dialogue, and monitoring patient-centered outcomes, the team delivers integrated, evidence-based care that reduces complication rates, accelerates recovery, and supports sustained functional improvement.
Media
(Click Image to Enlarge)
Pfirrmann and Suzuki Classification, Cervical Disc Degenerative Disease. The Pfirrmann system is the most commonly used method for grading cervical disc degeneration, ranging from I to V, based on T2-weighted signal intensity, clarity of the nucleus–annulus boundary, and disc height.
Contributed by K Margetis, MD, PhD
(Click Image to Enlarge)
Modic and Disc–Endplate–Bone Marrow Complex (DEBC) Classification Systems, Endplate Degenerative Changes. The DEBC classification extends Modic by incorporating short tau inversion recovery sequences alongside T1- and T2-weighted imaging to grade the disc, endplate, and adjacent marrow as a single unit.
Contributed by K Margetis, MD, PhD
References
Shedid D, Benzel EC. Cervical spondylosis anatomy: pathophysiology and biomechanics. Neurosurgery. 2007 Jan:60(1 Supp1 1):S7-13 [PubMed PMID: 17204889]
Fardon DF, Williams AL, Dohring EJ, Murtagh FR, Gabriel Rothman SL, Sze GK. Lumbar disc nomenclature: version 2.0: Recommendations of the combined task forces of the North American Spine Society, the American Society of Spine Radiology and the American Society of Neuroradiology. The spine journal : official journal of the North American Spine Society. 2014 Nov 1:14(11):2525-45. doi: 10.1016/j.spinee.2014.04.022. Epub 2014 Apr 24 [PubMed PMID: 24768732]
Frost BA, Camarero-Espinosa S, Foster EJ. Materials for the Spine: Anatomy, Problems, and Solutions. Materials (Basel, Switzerland). 2019 Jan 14:12(2):. doi: 10.3390/ma12020253. Epub 2019 Jan 14 [PubMed PMID: 30646556]
Battié MC, Videman T. Lumbar disc degeneration: epidemiology and genetics. The Journal of bone and joint surgery. American volume. 2006 Apr:88 Suppl 2():3-9 [PubMed PMID: 16595435]
Gore DR, Carrera GF, Glaeser ST. Smoking and degenerative changes of the cervical spine: a roentgenographic study. The spine journal : official journal of the North American Spine Society. 2006 Sep-Oct:6(5):557-60 [PubMed PMID: 16934727]
Gore DR. Roentgenographic findings in the cervical spine in asymptomatic persons: a ten-year follow-up. Spine. 2001 Nov 15:26(22):2463-6 [PubMed PMID: 11707711]
Okada E, Matsumoto M, Ichihara D, Chiba K, Toyama Y, Fujiwara H, Momoshima S, Nishiwaki Y, Hashimoto T, Ogawa J, Watanabe M, Takahata T. Aging of the cervical spine in healthy volunteers: a 10-year longitudinal magnetic resonance imaging study. Spine. 2009 Apr 1:34(7):706-12. doi: 10.1097/BRS.0b013e31819c2003. Epub [PubMed PMID: 19333104]
Level 2 (mid-level) evidenceBoden SD, McCowin PR, Davis DO, Dina TS, Mark AS, Wiesel S. Abnormal magnetic-resonance scans of the cervical spine in asymptomatic subjects. A prospective investigation. The Journal of bone and joint surgery. American volume. 1990 Sep:72(8):1178-84 [PubMed PMID: 2398088]
Lehto IJ, Tertti MO, Komu ME, Paajanen HE, Tuominen J, Kormano MJ. Age-related MRI changes at 0.1 T in cervical discs in asymptomatic subjects. Neuroradiology. 1994:36(1):49-53 [PubMed PMID: 8107998]
Matsumoto M, Fujimura Y, Suzuki N, Nishi Y, Nakamura M, Yabe Y, Shiga H. MRI of cervical intervertebral discs in asymptomatic subjects. The Journal of bone and joint surgery. British volume. 1998 Jan:80(1):19-24 [PubMed PMID: 9460946]
Lawrence JS. Disc degeneration. Its frequency and relationship to symptoms. Annals of the rheumatic diseases. 1969 Mar:28(2):121-38 [PubMed PMID: 4237972]
Kuo DT, Tadi P. Cervical Spondylosis. StatPearls. 2025 Jan:(): [PubMed PMID: 31855384]
Sharrak S, Al Khalili Y. Cervical Disc Herniation. StatPearls. 2025 Jan:(): [PubMed PMID: 31536225]
Heller JG. The syndromes of degenerative cervical disease. The Orthopedic clinics of North America. 1992 Jul:23(3):381-94 [PubMed PMID: 1620533]
Nilsson N. The prevalence of cervicogenic headache in a random population sample of 20-59 year olds. Spine. 1995 Sep 1:20(17):1884-8 [PubMed PMID: 8560336]
Level 2 (mid-level) evidenceHolmes A, Wang C, Han ZH, Dang GT. The range and nature of flexion-extension motion in the cervical spine. Spine. 1994 Nov 15:19(22):2505-10 [PubMed PMID: 7855673]
Montgomery DM, Brower RS. Cervical spondylotic myelopathy. Clinical syndrome and natural history. The Orthopedic clinics of North America. 1992 Jul:23(3):487-93 [PubMed PMID: 1620540]
Magnus W, Viswanath O, Viswanathan VK, Mesfin FB. Cervical Radiculopathy. StatPearls. 2025 Jan:(): [PubMed PMID: 28722858]
Malanga GA, Landes P, Nadler SF. Provocative tests in cervical spine examination: historical basis and scientific analyses. Pain physician. 2003 Apr:6(2):199-205 [PubMed PMID: 16883381]
Donnally III CJ, Hanna A, Odom CK. Cervical Myelopathy. StatPearls. 2025 Jan:(): [PubMed PMID: 29493937]
Voskuhl RR, Hinton RC. Sensory impairment in the hands secondary to spondylotic compression of the cervical spinal cord. Archives of neurology. 1990 Mar:47(3):309-11 [PubMed PMID: 2310314]
Iyer S, Kim HJ. Cervical radiculopathy. Current reviews in musculoskeletal medicine. 2016 Sep:9(3):272-80. doi: 10.1007/s12178-016-9349-4. Epub [PubMed PMID: 27250042]
Ulbrich EJ, Schraner C, Boesch C, Hodler J, Busato A, Anderson SE, Eigenheer S, Zimmermann H, Sturzenegger M. Normative MR cervical spinal canal dimensions. Radiology. 2014 Apr:271(1):172-82. doi: 10.1148/radiol.13120370. Epub 2013 Dec 12 [PubMed PMID: 24475792]
Yanase M, Matsuyama Y, Hirose K, Takagi H, Yamada M, Iwata H, Ishiguro N. Measurement of the cervical spinal cord volume on MRI. Journal of spinal disorders & techniques. 2006 Apr:19(2):125-9 [PubMed PMID: 16760787]
Tierney RT, Maldjian C, Mattacola CG, Straub SJ, Sitler MR. Cervical Spine Stenosis Measures in Normal Subjects. Journal of athletic training. 2002 Jun:37(2):190-193 [PubMed PMID: 12937434]
Torg JS, Naranja RJ Jr, Pavlov H, Galinat BJ, Warren R, Stine RA. The relationship of developmental narrowing of the cervical spinal canal to reversible and irreversible injury of the cervical spinal cord in football players. The Journal of bone and joint surgery. American volume. 1996 Sep:78(9):1308-14 [PubMed PMID: 8816644]
Level 2 (mid-level) evidenceMorishita Y, Naito M, Wang JC. Cervical spinal canal stenosis: the differences between stenosis at the lower cervical and multiple segment levels. International orthopaedics. 2011 Oct:35(10):1517-22. doi: 10.1007/s00264-010-1169-3. Epub 2010 Nov 27 [PubMed PMID: 21113592]
Urbanschitz L, Bensler S, Merat S, Lenz CG, Eid K. How Should We Grade Cervical Disk Degeneration? A Comparison of Two Popular Classification Systems. Spine surgery and related research. 2021:5(6):347-351. doi: 10.22603/ssrr.2021-0048. Epub 2021 Apr 28 [PubMed PMID: 34966859]
Tsuchiya K, Okano I, Guven AE, Verna B, Köhli P, Hambrecht J, Evangelisti G, Chiapparelli E, Burkhard MD, Tripathi V, Shue J, Girardi FP, Cammisa FP, Sama AA, Hughes AP. Quantitative assessment of cervical disc degeneration using disc signal intensity index. The spine journal : official journal of the North American Spine Society. 2025 May:25(5):903-910. doi: 10.1016/j.spinee.2024.11.017. Epub 2024 Dec 5 [PubMed PMID: 39645168]
Yang X, Karis DSA, Vleggeert-Lankamp CLA. Association between Modic changes, disc degeneration, and neck pain in the cervical spine: a systematic review of literature. The spine journal : official journal of the North American Spine Society. 2020 May:20(5):754-764. doi: 10.1016/j.spinee.2019.11.002. Epub 2019 Nov 13 [PubMed PMID: 31731008]
Level 1 (high-level) evidenceJagadish T, Murugan C, Ramachandran K, Thippeswamy PB, Anand K S SV, Kanna RM, Shetty AP, Rajasekaran S. The Association of Modic Changes and Disc-Endplate-Bone Marrow Complex Classification in Patients With Cervical Degenerative Disc Disease. Global spine journal. 2025 Feb 14:():21925682251320893. doi: 10.1177/21925682251320893. Epub 2025 Feb 14 [PubMed PMID: 39953676]
Gross A, Miller J, D'Sylva J, Burnie SJ, Goldsmith CH, Graham N, Haines T, Brønfort G, Hoving JL. Manipulation or mobilisation for neck pain. The Cochrane database of systematic reviews. 2010 Jan 20:(1):CD004249. doi: 10.1002/14651858.CD004249.pub3. Epub 2010 Jan 20 [PubMed PMID: 20091561]
Level 1 (high-level) evidenceHarris PR. Cervical traction. Review of literature and treatment guidelines. Physical therapy. 1977 Aug:57(8):910-4 [PubMed PMID: 877159]
Tan JC, Nordin M. Role of physical therapy in the treatment of cervical disk disease. The Orthopedic clinics of North America. 1992 Jul:23(3):435-49 [PubMed PMID: 1620537]
McCray RE, Patton NJ. Pain relief at trigger points: a comparison of moist heat and shortwave diathermy. The Journal of orthopaedic and sports physical therapy. 1984:5(4):175-8 [PubMed PMID: 18806417]
FOUNTAIN FP, GERSTEN JW, SENGIR O. Decrease in muscle spasm produced by ultrasound, hot packs, and infrared radiation. Archives of physical medicine and rehabilitation. 1960 Jul:41():293-8 [PubMed PMID: 13824160]
Pangarkar S, Lee PC. Conservative treatment for neck pain: medications, physical therapy, and exercise. Physical medicine and rehabilitation clinics of North America. 2011 Aug:22(3):503-20, ix. doi: 10.1016/j.pmr.2011.04.001. Epub 2011 Jun 14 [PubMed PMID: 21824590]
Garra G, Singer AJ, Leno R, Taira BR, Gupta N, Mathaikutty B, Thode HJ. Heat or cold packs for neck and back strain: a randomized controlled trial of efficacy. Academic emergency medicine : official journal of the Society for Academic Emergency Medicine. 2010 May:17(5):484-9. doi: 10.1111/j.1553-2712.2010.00735.x. Epub [PubMed PMID: 20536800]
Level 1 (high-level) evidencePatel KC, Gross A, Graham N, Goldsmith CH, Ezzo J, Morien A, Peloso PM. Massage for mechanical neck disorders. The Cochrane database of systematic reviews. 2012 Sep 12:(9):CD004871. doi: 10.1002/14651858.CD004871.pub4. Epub 2012 Sep 12 [PubMed PMID: 22972078]
Level 1 (high-level) evidenceKay TM, Gross A, Goldsmith CH, Rutherford S, Voth S, Hoving JL, Brønfort G, Santaguida PL. Exercises for mechanical neck disorders. The Cochrane database of systematic reviews. 2012 Aug 15:(8):CD004250. doi: 10.1002/14651858.CD004250.pub4. Epub 2012 Aug 15 [PubMed PMID: 22895940]
Level 1 (high-level) evidenceSuri A, Chabbra RP, Mehta VS, Gaikwad S, Pandey RM. Effect of intramedullary signal changes on the surgical outcome of patients with cervical spondylotic myelopathy. The spine journal : official journal of the North American Spine Society. 2003 Jan-Feb:3(1):33-45 [PubMed PMID: 14589243]
Hodges SD, Castleberg RL, Miller T, Ward R, Thornburg C. Cervical epidural steroid injection with intrinsic spinal cord damage. Two case reports. Spine. 1998 Oct 1:23(19):2137-42; discussion 2141-2 [PubMed PMID: 9794061]
Level 3 (low-level) evidenceMcLain RF, Fry M, Hecht ST. Transient paralysis associated with epidural steroid injection. Journal of spinal disorders. 1997 Oct:10(5):441-4 [PubMed PMID: 9355063]
Level 3 (low-level) evidenceRahme R, Boubez G, Bouthillier A, Moumdjian R. Acute swan-neck deformity and spinal cord compression after cervical laminectomy. The Canadian journal of neurological sciences. Le journal canadien des sciences neurologiques. 2009 Jul:36(4):504-6 [PubMed PMID: 19650366]
Level 3 (low-level) evidenceFountas KN, Kapsalaki EZ, Nikolakakos LG, Smisson HF, Johnston KW, Grigorian AA, Lee GP, Robinson JS Jr. Anterior cervical discectomy and fusion associated complications. Spine. 2007 Oct 1:32(21):2310-7 [PubMed PMID: 17906571]
Level 2 (mid-level) evidenceBernard TN Jr, Whitecloud TS 3rd. Cervical spondylotic myelopathy and myeloradiculopathy. Anterior decompression and stabilization with autogenous fibula strut graft. Clinical orthopaedics and related research. 1987 Aug:(221):149-60 [PubMed PMID: 3608294]
Emery SE, Bohlman HH, Bolesta MJ, Jones PK. Anterior cervical decompression and arthrodesis for the treatment of cervical spondylotic myelopathy. Two to seventeen-year follow-up. The Journal of bone and joint surgery. American volume. 1998 Jul:80(7):941-51 [PubMed PMID: 9697998]
Okada K, Shirasaki N, Hayashi H, Oka S, Hosoya T. Treatment of cervical spondylotic myelopathy by enlargement of the spinal canal anteriorly, followed by arthrodesis. The Journal of bone and joint surgery. American volume. 1991 Mar:73(3):352-64 [PubMed PMID: 2002073]
Level 3 (low-level) evidenceFlynn TB. Neurologic complications of anterior cervical interbody fusion. Spine. 1982 Nov-Dec:7(6):536-9 [PubMed PMID: 7167824]
Highsmith JM, Dhall SS, Haid RW Jr, Rodts GE Jr, Mummaneni PV. Treatment of cervical stenotic myelopathy: a cost and outcome comparison of laminoplasty versus laminectomy and lateral mass fusion. Journal of neurosurgery. Spine. 2011 May:14(5):619-25. doi: 10.3171/2011.1.SPINE10206. Epub [PubMed PMID: 21388285]
LEES F, TURNER JW. NATURAL HISTORY AND PROGNOSIS OF CERVICAL SPONDYLOSIS. British medical journal. 1963 Dec 28:2(5373):1607-10 [PubMed PMID: 14066179]
Hirabayashi K, Watanabe K, Wakano K, Suzuki N, Satomi K, Ishii Y. Expansive open-door laminoplasty for cervical spinal stenotic myelopathy. Spine. 1983 Oct:8(7):693-9 [PubMed PMID: 6420895]
Level 3 (low-level) evidenceRobertson JT, Papadopoulos SM, Traynelis VC. Assessment of adjacent-segment disease in patients treated with cervical fusion or arthroplasty: a prospective 2-year study. Journal of neurosurgery. Spine. 2005 Dec:3(6):417-23 [PubMed PMID: 16381202]
CLARKE E, ROBINSON PK. Cervical myelopathy: a complication of cervical spondylosis. Brain : a journal of neurology. 1956 Sep:79(3):483-510 [PubMed PMID: 13364095]
Nurick S. The pathogenesis of the spinal cord disorder associated with cervical spondylosis. Brain : a journal of neurology. 1972:95(1):87-100 [PubMed PMID: 5023093]
Epstein JA, Janin Y, Carras R, Lavine LS. A comparative study of the treatment of cervical spondylotic myeloradiculopathy. Experience with 50 cases treated by means of extensive laminectomy, foraminotomy, and excision of osteophytes during the past 10 years. Acta neurochirurgica. 1982:61(1-3):89-104 [PubMed PMID: 6280455]
Level 2 (mid-level) evidencePeene L, Cohen SP, Brouwer B, James R, Wolff A, Van Boxem K, Van Zundert J. 2. Cervical radicular pain. Pain practice : the official journal of World Institute of Pain. 2023 Sep:23(7):800-817. doi: 10.1111/papr.13252. Epub 2023 Jun 4 [PubMed PMID: 37272250]
Tavanaei R, Ansari A, Hatami A, Heidari MJ, Dehghani M, Hajiloo A, Khorasanizadeh M, Margetis K. Postoperative complications of anterior cervical discectomy and fusion: A comprehensive systematic review and meta-analysis. North American Spine Society journal. 2025 Mar:21():100596. doi: 10.1016/j.xnsj.2025.100596. Epub 2025 Feb 8 [PubMed PMID: 40145067]
Level 1 (high-level) evidenceKarabacak M, Schupper A, Carr M, Margetis K. A machine learning-based approach for individualized prediction of short-term outcomes after anterior cervical corpectomy. Asian spine journal. 2024 Aug:18(4):541-549. doi: 10.31616/asj.2024.0048. Epub 2024 Aug 8 [PubMed PMID: 39113482]
Karabacak M, Bhimani AD, Schupper AJ, Carr MT, Steinberger J, Margetis K. Machine learning models on a web application to predict short-term postoperative outcomes following anterior cervical discectomy and fusion. BMC musculoskeletal disorders. 2024 May 21:25(1):401. doi: 10.1186/s12891-024-07528-5. Epub 2024 May 21 [PubMed PMID: 38773464]
Karabacak M, Margetis K. Development of personalized machine learning-based prediction models for short-term postoperative outcomes in patients undergoing cervical laminoplasty. European spine journal : official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society. 2023 Nov:32(11):3857-3867. doi: 10.1007/s00586-023-07923-x. Epub 2023 Sep 12 [PubMed PMID: 37698693]
Karabacak M, Margetis K. Interpretable machine learning models to predict short-term postoperative outcomes following posterior cervical fusion. PloS one. 2023:18(7):e0288939. doi: 10.1371/journal.pone.0288939. Epub 2023 Jul 21 [PubMed PMID: 37478157]
Schreiber AL, McDonald BP, Kia F, Fried GW. Cervical epidural steroid injections and spinal cord injuries. The spine journal : official journal of the North American Spine Society. 2016 Oct:16(10):1163-1166. doi: 10.1016/j.spinee.2015.08.068. Epub 2015 Sep 14 [PubMed PMID: 26383496]
Urits I, Hubble A, Peterson E, Orhurhu V, Ernst CA, Kaye AD, Viswanath O. An Update on Cognitive Therapy for the Management of Chronic Pain: a Comprehensive Review. Current pain and headache reports. 2019 Jul 10:23(8):57. doi: 10.1007/s11916-019-0794-9. Epub 2019 Jul 10 [PubMed PMID: 31292747]
Kuo YL, Wang PS, Ko PY, Huang KY, Tsai YJ. Immediate effects of real-time postural biofeedback on spinal posture, muscle activity, and perceived pain severity in adults with neck pain. Gait & posture. 2019 Jan:67():187-193. doi: 10.1016/j.gaitpost.2018.10.021. Epub 2018 Oct 15 [PubMed PMID: 30359957]