Introduction
Paget-Schroetter syndrome (PSS) refers to effort-induced thrombosis of the axillary and subclavian veins caused by mechanical compression of the subclavian vein at the thoracic outlet. As the venous form of thoracic outlet syndrome, PSS involves subclavian vein obstruction, distinct from arterial or neurogenic compression involving the subclavian artery or brachial plexus. First described in 1875 by Sir James Paget, the condition was initially characterized as spontaneous subclavian vein thrombosis. In 1884, von Schroetter proposed that repetitive musculoskeletal activity contributed to endothelial injury and thrombosis. This historical trajectory underscores the transition from early descriptive accounts to a more refined understanding of the condition's mechanical and hemodynamic pathophysiology.[1][2][3][4][5]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
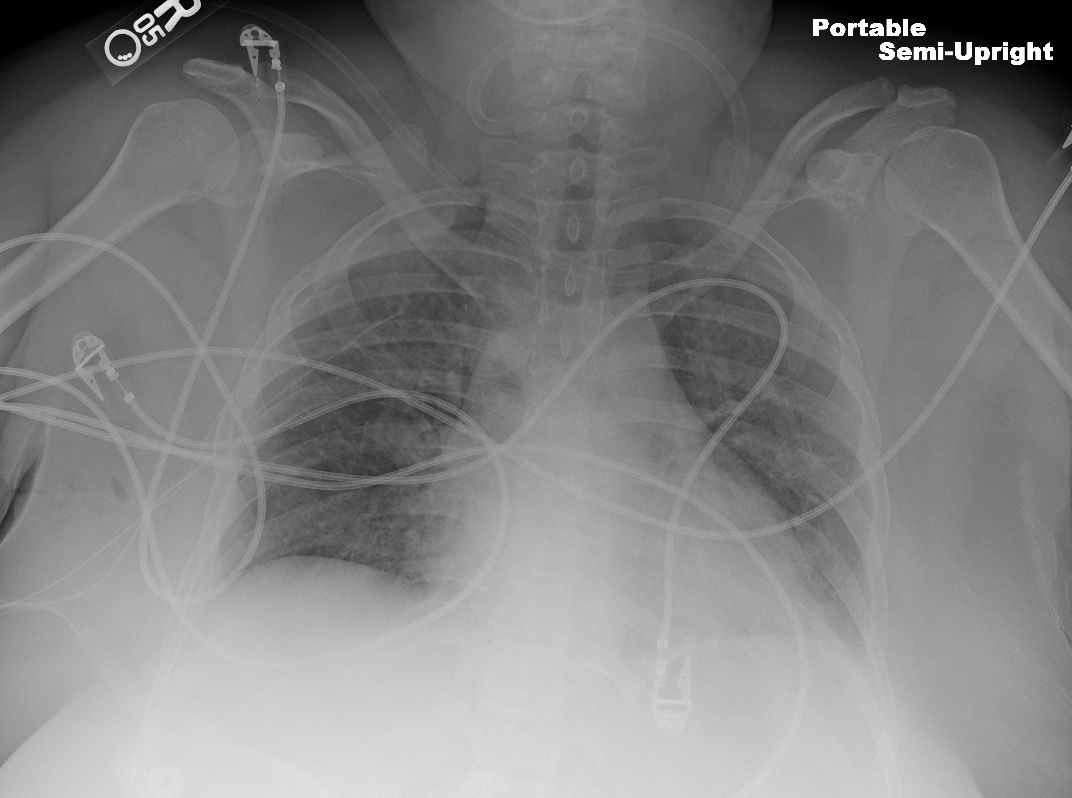
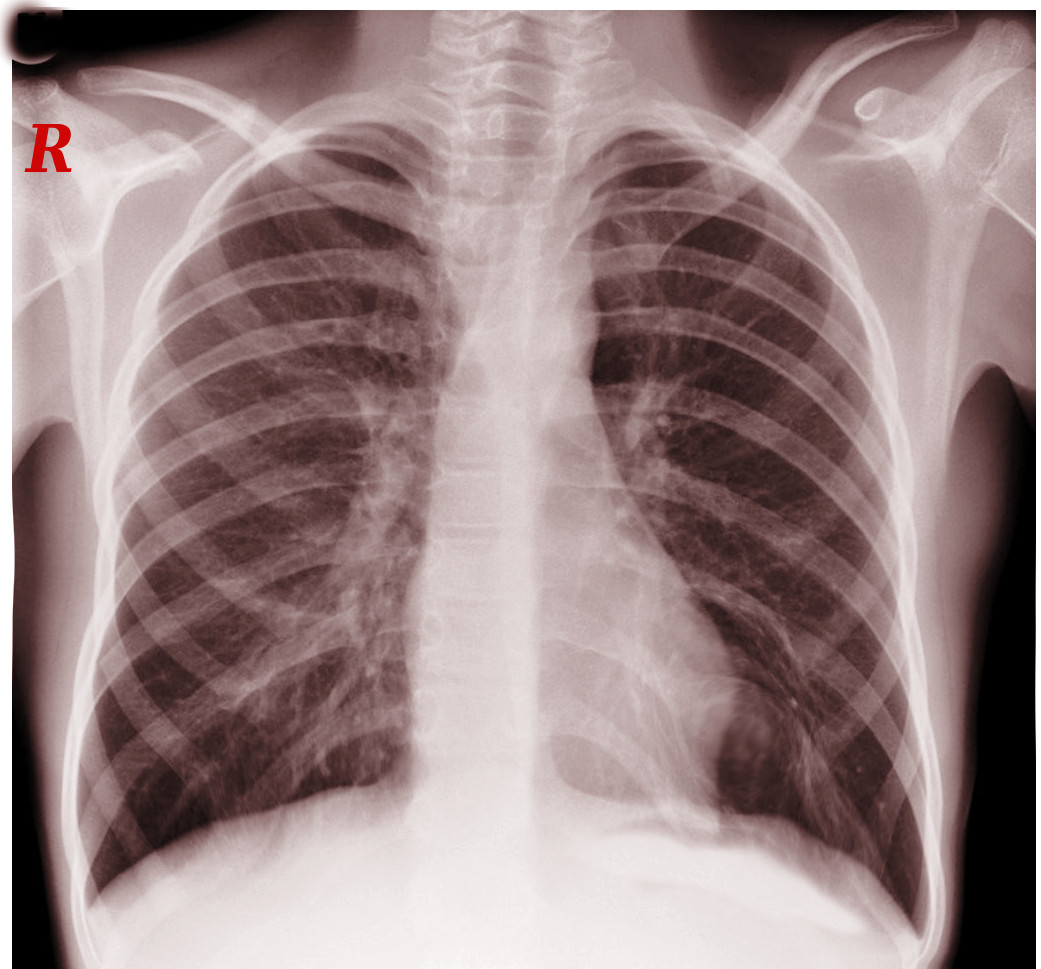
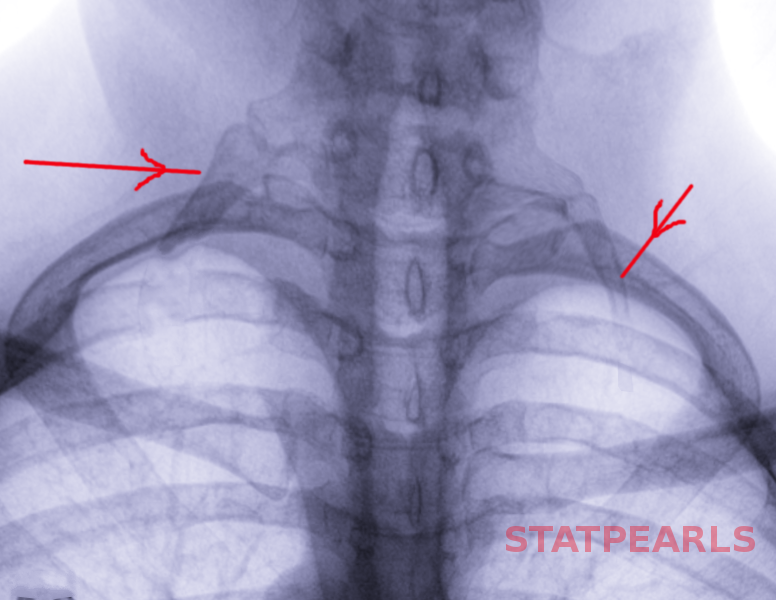
The subclavian vein courses between the clavicle and the first rib, adjacent to the anterior scalene and subclavius muscles (see Image. Anatomical Relationships at the Right Thoracic Outlet). Compression by these anatomical structures contributes to endothelial injury and venous stasis, predisposing to thrombosis. PSS arises from this mechanical obstruction of the subclavian vein (see Images. Left Cervical Rib on Chest X-ray and Radiographic Appearance of Bilateral Cervical Ribs).
Epidemiology
PSS more commonly affects younger individuals in their 20s and 30s, with a male-to-female ratio of 2:1. Most affected individuals have no significant medical comorbidities. Reported incidence ranges from 1 to 2 per 100,000 individuals annually. Right-sided involvement occurs more frequently. Venous thoracic outlet syndrome (TOS) is less prevalent than neurogenic TOS but more common than arterial TOS, accounting for approximately 3% of all TOS cases. Many individuals with PSS are athletes engaged in repetitive overhead arm movements, including baseball or softball pitchers, swimmers, rowers, and weightlifters.
Pathophysiology
PSS typically arises in the context of anatomical predispositions, particularly among individuals with a narrowed costoclavicular space or extrinsic compression of the subclavian vein. Structural contributors include medially positioned insertions of the costoclavicular ligament relative to the vein, hypertrophy or aberrant insertion of the subclavius muscle or tendon, and reduced clearance between the clavicle and first rib, each of which may compromise venous patency.
Repetitive overhead or externally rotated upper limb movements, especially in the presence of hypercoagulable states, further contribute to endothelial injury and thrombus formation. Compared to neurogenic TOS, PSS occurs more frequently in male individuals. Sustained upper extremity exertion may provoke perivenous inflammation and fibrosis, resulting in progressive luminal narrowing. Chronic microtrauma and subsequent fibrotic remodeling promote perivenous scarring and the development of extensive collateral venous networks. These changes impair venous return from the affected extremity and produce clinically evident edema.[6][7][8][9]
History and Physical
Patients often present with upper extremity swelling and pain, with symptom onset classified as acute, subacute, or chronic. A history of recent strenuous or repetitive upper limb activity is commonly elicited. Clinical examination reveals unilateral upper extremity edema, typically with increased limb girth compared to the contralateral side. The affected limb may appear tense and cyanotic. Prominent superficial veins over the chest and shoulder, known as the Urschel sign, suggest the presence of collateral venous drainage.
Evaluation
Provocative maneuvers commonly employed in the evaluation of TOS lack diagnostic reliability in the assessment of PSS due to a high false-positive rate and limited specificity for vascular compression. These tests primarily detect neurogenic compression and have reduced utility in venous presentations. In contrast, duplex ultrasonography is endorsed by the American College of Radiology as the initial imaging modality of choice, offering a reported sensitivity of 78% to 100% and specificity between 82% and 100%.
Detailed clinical history remains essential, particularly in identifying predisposing factors such as inherited or acquired thrombophilic states. Due to potential overlap with other hypercoagulable conditions, diagnostic workup should include evaluation for concurrent thrombophilic disorders. PSS most often presents acutely in 60% to 85% of cases, typically within 24 hours of vigorous upper extremity activity. Hallmark symptoms include sudden pain, swelling, limb discoloration, and a sensation of heaviness.
The Urschel sign may be evident, characterized by engorged superficial veins over the upper arm, anterior shoulder, neck base, and chest wall. Comprehensive physical examination should include inspection and palpation of the affected extremity, spinal axis, shoulder girdle, chest wall, and supraclavicular region. Auscultation over the supraclavicular fossa may reveal a bruit, suggestive of venous obstruction or turbulent flow at the site of compression.
Doppler ultrasonography of the upper extremities is a convenient, noninvasive first-line imaging modality. In cases of acute thrombosis, ultrasonography reveals a noncompressible, echogenic vein with distention and absent spectral or color Doppler flow. Chronic thrombus is a collapsed, irregular vein, often accompanied by collateral formation. Limitations of ultrasonography include incomplete visualization of central veins. Computed tomography (CT) and magnetic resonance venography provide a more comprehensive assessment of the deep venous system. Contrast venography, though invasive, is the definitive method for evaluating central venous patency.
Laboratory evaluation includes a thrombophilia panel, typically assessing protein C and S, antithrombin, factor V Leiden, prothrombin gene mutation status, and D-dimer levels. Elevated D-dimer values support the presence of deep venous thrombosis but lack specificity, as elevations may occur in inflammatory, infectious, or physiologic states. A CT pulmonary angiogram (CT thoracic angiography using a pulmonary embolism protocol) should be obtained in individuals with symptoms suggestive of pulmonary embolism. A ventilation-perfusion scan may also detect pulmonary embolism, but is generally considered a secondary option. CT angiography is the preferred diagnostic study.
Treatment / Management
PSS is essentially a deep vein thrombosis in the upper extremity. Thus, the first management step is to initiate anticoagulation therapy, typically via an intravenous heparin drip. Patients are given an initial bolus of intravenous heparin and then kept on a maintenance intravenous heparin drip. The dose of the bolus and maintenance heparin drip is weight-based. The intravenous heparin drip is titrated according to activated partial thromboplastin time or factor X levels. The affected extremity should be elevated to mitigate edema.
Presentation within 2 weeks of symptom onset warrants consideration of a venogram and catheter-directed thrombolysis (CDT) to reduce clot burden. A lysis catheter is placed under fluoroscopic guidance to drip a thrombolytic agent, such as alteplase, for 24 to 48 hours. Alternatively, a pharmacomechanical thrombectomy system may be used. When using alteplase, the fibrinogen levels should be monitored closely to direct the dosing and duration of the catheter-directed lysis. When using pharmacomechanical thrombectomy, one should be mindful of the risk of acute kidney injury associated with hemolysis.
Suppose the presentation is more than 2 to 4 weeks from the onset of symptoms. In that case, heparinization alone may be initiated without thrombolytic therapy, as the success of thrombolytic therapy is low beyond that window of time.[10][11][12] CDT serves as the primary intervention for thrombus dissolution in PSS. This technique directly infuses thrombolytic agents such as alteplase or urokinase into the thrombus through a percutaneously placed catheter. Reported venous recanalization rates range from 75% to 84%. However, the risk of bleeding necessitates careful patient selection and vigilant monitoring.(B3)
Mechanical thrombectomy employs endovascular devices to extract thrombus physically and may be used alone or in conjunction with CDT to improve clot clearance. When combined with CDT, mechanical thrombectomy may reduce thrombolytic dose and infusion time, thereby minimizing hemorrhagic risk.[13] Decompression of the thoracic outlet is the definitive management of PSS (see Image. Postoperative Thoracic Outlet Decompression). This intervention usually includes a first rib resection with some or all of the following procedures: anterior scalenectomy, resection of the subclavius muscle, resection of the costoclavicular ligament, and lysis of scar tissue or adhesions surrounding the subclavian vein.
Venoplasty or stenting without decompression may produce favorable results, but rib resection is the definitive treatment. The surgical approach can either be transaxillary, supraclavicular, infraclavicular, or paraclavicular. The timing of decompression after thrombolytic therapy is widely debated. The role of long-term anticoagulation in these patients after adequate decompression is unclear. Some clinicians perform a venogram, often with intravascular ultrasound, 2 weeks postoperatively, and decide about the duration of anticoagulation at that time.
In this setting, venoplasty may be used for any residual stenosis since decompression has been achieved surgically. Stents are discouraged in this anatomic location due to the repetitive motion and risk of stent fracture and thrombosis. The duration of anticoagulation in patients with underlying hypercoagulability disorders after decompression is also unclear. In most patients, anticoagulation for 3 to 6 months following an episode of DVT, such as PSS, is reasonable. This therapy may be given using either warfarin or direct oral anticoagulants.
Thrombolysis and venography are usually attempted again in cases of recurrent thrombosis after decompression surgery, followed by maintenance of long-term anticoagulation therapy. Chronic total occlusion of the subclavian vein may persist in some patients with PSS despite adequate decompression. Venous reconstruction should be considered, depending on symptom severity. This intervention may be performed using a bypass or a jugular vein turndown procedure, with or without medial claviculectomy.
Differential Diagnosis
Upper extremity swelling occurs in patients with lymphatic disorders or systemic conditions such as end-stage renal disease and congestive heart failure. Upper extremity DVT also develops in the setting of indwelling catheter use.
Prognosis
Combined thrombolysis and thoracic outlet decompression achieves success rates of up to 90%. Anticoagulation with decompression is less effective than thrombolysis with decompression but produces better outcomes than anticoagulation alone.
Complications
Complications of PSS include pulmonary embolism, recurrent thrombosis, and postthrombotic syndrome. These sequelae may lead to chronic venous insufficiency, persistent limb discomfort, and functional limitations.
Deterrence and Patient Education
Adherence to prescribed pharmacologic therapy and regular follow-up with healthcare professionals is essential. Activity and lifestyle modifications help reduce the risk of recurrent thrombosis. Patients with underlying hypercoagulable conditions require counseling on the potential need for long-term anticoagulation.
Enhancing Healthcare Team Outcomes
Optimal diagnosis and treatment of PSS require interprofessional collaboration among a primary care provider, sports medicine clinician, vascular surgeon, and radiologist. Venous obstruction must be addressed to prevent persistent pain and swelling of the affected limb. Anticoagulation is the initial intervention, followed in many cases by thoracic outlet decompression. Although most patients experience favorable outcomes with timely management, residual arm swelling may persist depending on the extent of subclavian vein damage. Selected cases may benefit from endovascular intervention.[14]
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
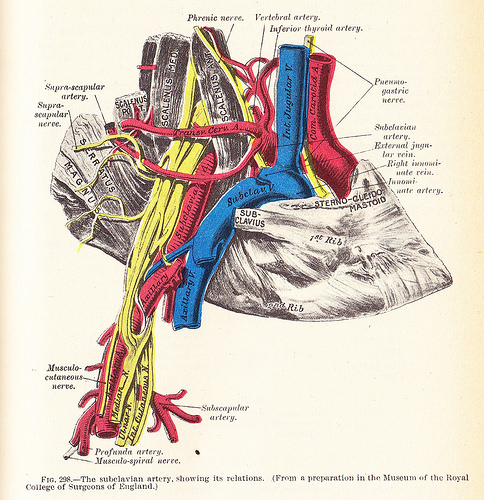
Anatomical Relationships at the Right Thoracic Outlet. The diagram depicts the right subclavian vein and artery, along with adjacent vessels, nerves, muscles, and bones involved in thoracic outlet compression.
Henry Van Dyke Carter, Public Domain, via Wikimedia Commons
(Click Image to Enlarge)
References
Sayın A, Güngör H, Bilgin M, Ertürk U. Paget-von Schrötter syndrome: upper extremity deep vein thrombosis after heavy exercise. Turk Kardiyoloji Dernegi arsivi : Turk Kardiyoloji Derneginin yayin organidir. 2012 Jun:40(4):354-7. doi: 10.5543/tkda.2012.02212. Epub [PubMed PMID: 22951853]
Level 3 (low-level) evidenceLewandowski A, Syska-Sumińska J, Dłuzniewski M. [Pulmonary embolism suspicion in a young female patient with the Paget-von Schrötter syndrome]. Kardiologia polska. 2008 Sep:66(9):969-71 [PubMed PMID: 18924025]
Level 3 (low-level) evidenceZell L, Sommerfeld A, Buchter A. [The Paget-von Schroetter syndrome. On the centenary of the death of Sir James Paget and on the 50th anniversary of the naming of the syndrome]. Deutsche medizinische Wochenschrift (1946). 1999 Aug 6:124(31-32):948-51 [PubMed PMID: 10480017]
Cires-Drouet R, Sharma J, McDonald T, Sorkin JD, Lal BK. Variability in the management of line-related upper extremity deep vein thrombosis. Phlebology. 2019 Sep:34(8):552-558. doi: 10.1177/0268355519827155. Epub 2019 Jan 31 [PubMed PMID: 30704347]
Weaver LA, Kanter CR, Costantino TG. Effort Thrombosis Provoked by Saxophone Performance. The Journal of emergency medicine. 2019 Mar:56(3):323-326. doi: 10.1016/j.jemermed.2018.12.003. Epub 2019 Jan 9 [PubMed PMID: 30638648]
Thiyagarajah K, Ellingwood L, Endres K, Hegazi A, Radford J, Iansavitchene A, Lazo-Langner A. Post-thrombotic syndrome and recurrent thromboembolism in patients with upper extremity deep vein thrombosis: A systematic review and meta-analysis. Thrombosis research. 2019 Feb:174():34-39. doi: 10.1016/j.thromres.2018.12.012. Epub 2018 Dec 8 [PubMed PMID: 30553163]
Level 1 (high-level) evidenceKumar R, Harsh K, Saini S, O'Brien SH, Stanek J, Warren P, Giver J, Go MR, Kerlin BA. Treatment-Related Outcomes in Paget-Schroetter Syndrome-A Cross-Sectional Investigation. The Journal of pediatrics. 2019 Apr:207():226-232.e1. doi: 10.1016/j.jpeds.2018.11.018. Epub 2018 Dec 7 [PubMed PMID: 30528572]
Level 2 (mid-level) evidenceChen AWY, Oraii Yazdani K, Candilio L. Upper Limb Deep Vein Thrombosis: A Case Report of an Increasingly Common Condition. The journal of Tehran Heart Center. 2018 Apr:13(2):73-75 [PubMed PMID: 30483316]
Level 3 (low-level) evidenceLim W, Le Gal G, Bates SM, Righini M, Haramati LB, Lang E, Kline JA, Chasteen S, Snyder M, Patel P, Bhatt M, Patel P, Braun C, Begum H, Wiercioch W, Schünemann HJ, Mustafa RA. American Society of Hematology 2018 guidelines for management of venous thromboembolism: diagnosis of venous thromboembolism. Blood advances. 2018 Nov 27:2(22):3226-3256. doi: 10.1182/bloodadvances.2018024828. Epub [PubMed PMID: 30482764]
Level 3 (low-level) evidenceWooster M, Fernandez B, Summers KL, Illig KA. Surgical and endovascular central venous reconstruction combined with thoracic outlet decompression in highly symptomatic patients. Journal of vascular surgery. Venous and lymphatic disorders. 2019 Jan:7(1):106-112.e3. doi: 10.1016/j.jvsv.2018.07.019. Epub 2018 Nov 12 [PubMed PMID: 30442583]
Fenando A, Mujer M, Rai MP, Alratroot A. Paget-Schroetter syndrome. BMJ case reports. 2018 Nov 8:2018():. pii: bcr-2018-227754. doi: 10.1136/bcr-2018-227754. Epub 2018 Nov 8 [PubMed PMID: 30413468]
Level 3 (low-level) evidenceGwozdz AM, Silickas J, Smith A, Saha P, Black SA. Endovascular Therapy for Central Venous Thrombosis. Methodist DeBakey cardiovascular journal. 2018 Jul-Sep:14(3):214-218. doi: 10.14797/mdcj-14-3-214. Epub [PubMed PMID: 30410652]
Kärkkäinen JM, Nuutinen H, Riekkinen T, Sihvo E, Turtiainen J, Saari P, Mäkinen K, Manninen H. Pharmacomechanical Thrombectomy in Paget-Schroetter Syndrome. Cardiovascular and interventional radiology. 2016 Sep:39(9):1272-9. doi: 10.1007/s00270-016-1376-4. Epub 2016 May 26 [PubMed PMID: 27230515]
Garg V, Poon G, Tan A, Poon KB. Paget-Schroetter syndrome as a result of 1st rib stress fracture due to gym activity presenting with Urschel's sign - A case report and review of literature. International journal of surgery case reports. 2018:49():81-86. doi: 10.1016/j.ijscr.2018.05.029. Epub 2018 Jun 20 [PubMed PMID: 29966955]
Level 3 (low-level) evidence