Introduction
Poorly controlled hypertension affects multiple organ systems, including the cardiovascular, renal, and cerebrovascular systems.[1] The retina also sustains damage, with changes in ocular structures such as vessels and nerves detectable through various imaging modalities. Damage to these organs is collectively referred to as "target-organ damage" (TOD).[2]
Hypertension causes 3 types of ocular injury: choroidopathy, retinopathy, and optic neuropathy.[3] Hypertensive retinopathy results from damage to retinal vessels due to elevated blood pressure. Substantial evidence indicates that hypertensive retinopathy serves as a predictor of systemic morbidity and mortality related to TOD. A study by Erden et al demonstrated that the incidence of retinopathy correlates with both the severity and duration of hypertension.[4]
Key features of hypertensive retinopathy include arteriovenous crossing changes, cotton wool spots, flame-shaped peripapillary hemorrhages, optic disc swelling (papilledema), and macular star formation.[5] Effective management of systemic hypertension and its associated morbidities is critical to limiting TOD.[6]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
In 2017, the American College of Cardiology and American Heart Association Task Force on Clinical Practice Guidelines established blood pressure categories for adults, outlined in Table 1 below.
Table. Blood Pressure Classification for Adults According to the 2017 American College of Cardiology and American Heart Association Guidelines
| Category of blood pressure | Systolic blood pressure in mm Hg | Diastolic blood pressure in mm Hg | |
| Normal | <120 | and | <80 |
|
Elevated blood pressure |
120-129 | and | <80 |
|
Stage 1 hypertension |
130-139 | or | 80-89 |
|
Stage 2 hypertension |
≥140 | or | ≥90 |
Systolic (SBP) and diastolic (DBP) blood pressure measurements require careful technique, with the average of at least 2 readings taken on 2 separate occasions used for classification. When SBP and DBP values fall into different categories, the higher category determines the individual’s classification.[7]
Blood pressure thresholds for different categories vary slightly across guidelines.[8] For instance, SBP between 120 and 129 mm Hg or DBP between 80 and 84 mm Hg is classified as normal according to the European Society of Cardiology (ESC) and European Society of Hypertension (ESH) guidelines.[9]
Definitions of malignant hypertension differ among guidelines.[10] The 2020 International Society of Hypertension (ISH) and 2018 ESC/ESH guidelines define this condition as a severe elevation of blood pressure, typically above 200/120 mm Hg, accompanied by advanced bilateral retinopathy characterized by retinal hemorrhages, cotton wool spots, and papilledema.[11]
The ESC position document describes hypertensive emergency as the coexistence of markedly elevated blood pressure, often above 200/120 mm Hg, with advanced retinopathy, acute renal failure, or thrombotic microangiopathy.[12] The term "acute hypertensive microangiopathy" has been proposed for cases with acute microvascular damage to the brain and kidneys that may lack retinal lesions.[13]
The National Institute for Health and Care Excellence (NICE, United Kingdom, 2019) defines accelerated hypertension as a severe increase in blood pressure to 180/120 mm Hg or higher, often exceeding 220/120 mm Hg, accompanied by signs of retinal hemorrhage or papilledema.[14]
Systemic hypertension is classified primarily into 2 main types: primary hypertension and secondary hypertension. Primary hypertension, also known as essential hypertension, represents the most common form. Risk factors for this type include advancing age, certain ethnic backgrounds, a sedentary lifestyle, smoking, excessive alcohol consumption, obesity, and an unhealthy diet characterized by high salt intake, diets rich in trans and saturated fats, and low vegetable consumption. Genetic predisposition and family history also play significant roles, alongside coexisting systemic conditions such as diabetes and kidney disease, and chronic stress.[15]
Secondary hypertension arises from an identifiable underlying condition, such as kidney or heart disease, or the use of certain substances. Younger individuals may present with bilateral dimness of vision due to malignant hypertensive retinopathy. Investigation in these cases may uncover underlying causes, including kidney disease or pheochromocytoma.
The most common cause of secondary hypertension is renal parenchymal disease, with diabetic nephropathy and glomerulonephritis frequently implicated. Other causes include renovascular diseases such as renal artery stenosis, endocrine disorders like primary hyperaldosteronism, Cushing syndrome, pheochromocytoma, hyperthyroidism, hyperparathyroidism, and acromegaly, as well as vascular disorders, including coarctation of the aorta and vasculitis affecting large and medium-sized vessels. Pregnancy-induced hypertension (PIH), particularly preeclampsia and eclampsia, also contributes, along with obstructive sleep apnea, polycystic ovarian syndrome, and certain medications such as nonsteroidal anti-inflammatory drugs and antidepressants.[16]
Besides essential and secondary hypertension, several other factors influence the development of hypertensive retinopathy. The condition shows a higher prevalence among individuals of Afro-Caribbean descent compared to Europeans.[17] Genetic factors also contribute, with specific genotypes linked to increased risk. Pontremoli et al identified that deletion of the angiotensin-converting enzyme allele is associated with a greater likelihood of developing hypertensive retinopathy.[18] Additional genetic loci implicated in retinal venular caliber and potentially systemic hypertension include 5q14, 6q24, 12q24, and 19q13.[19]
Smoking demonstrates a strong association with severe or malignant hypertensive retinopathy, as shown in the study by Poulter et al.[20] Renal dysfunction, evidenced by persistent microalbuminuria and reduced creatinine clearance, serves as a marker for various forms of TOD, including hypertensive retinopathy.[21] Uckaya et al observed elevated plasma leptin levels in patients with hypertensive retinopathy, suggesting a role for leptin in vascular endothelial injury.[22] The duration of systemic hypertension remains one of the most significant risk factors in the development of arteriosclerotic hypertensive retinopathy.[23]
Epidemiology
An estimated 1.39 billion adults worldwide have systemic hypertension.[24] Data from the National Health and Nutrition Examination Survey in the U.S. from August 2021 to August 2023 indicate that approximately 47.7% of adults have systemic hypertension, with about 60% aware of their condition. Only around half of those diagnosed with hypertension are taking medications to control their blood pressure.[25]
Hypertensive retinopathy is a common complication of systemic hypertension, with prevalence rates ranging from 28.5% to 77.1% among affected individuals.[26] Approximately 2% to 17% of adults without diabetes show signs of retinopathy, such as microaneurysms and retinal hemorrhages, likely due to hypertension.[27] Chronic hypertension leads to arteriosclerosis, which contributes to the arteriovenous changes observed in hypertensive retinopathy.
The severity and duration of systemic hypertension directly correlate with the incidence of hypertensive retinopathy, as demonstrated by Erden et al, who reported a prevalence of 66.3%. Kabedi et al found an even higher prevalence (83.6%) among individuals with hypertension and identified chronic kidney disease as the strongest predictor of severe hypertensive retinopathy.
In the study by Del Brutto et al, grade 1 hypertensive retinopathy was observed in 37% and grade 2 in 17% of hypertensive individuals.[28] Hypertensive retinopathy represents a common microvascular complication, affecting approximately 3% to 14% of the general population, with increased prevalence among individuals with poorly controlled or longstanding hypertension.[29] Incidence rises with advancing age and comorbidities such as diabetes and chronic kidney disease, and is more frequently observed in populations with limited access to healthcare or higher rates of undiagnosed hypertension.
The incidence of hypertensive retinopathy increases with advancing age, male sex (up to midlife), and certain racial backgrounds. The prevalence of systemic hypertension rises with age, and hypertensive retinopathy is independently associated with older age.[30] The Framingham Heart Study reported a residual lifetime risk for developing hypertension of approximately 90% in individuals aged 55 and 65 years.[31]
Hypertension tends to be more prevalent in men than in women, particularly up to the age of 50, but this difference diminishes and can reverse in older age.[32][33][34] Studies from India indicate that men have a higher prevalence of hypertension until approximately age 50, after which the prevalence in women increases and surpasses that in men.[35][36] In terms of racial differences, individuals of Chinese or African American descent exhibit a higher prevalence of hypertensive retinopathy than Caucasians.[37]
Pathophysiology
Retinal blood vessels possess distinct characteristics that differentiate them from other vascular structures. These features include the absence of sympathetic nerve supply, the presence of autoregulation of blood flow, and the integrity of the blood-retinal barrier.[38] Consequently, an increase in blood pressure is transmitted directly to the retinal vessels, initially triggering vasoconstriction. However, when blood pressure continues to rise beyond this compensatory capacity, damage to the vascular smooth muscle and endothelium occurs. Systemic hypertension affects both the retinal arterioles and capillaries and may lead to retinal nonperfusion.[39][40]
Focal intraretinal periarteriolar transudates (FIPTs) are specific indicators of malignant hypertension. These lesions result from the breakdown of the blood-retinal barrier at the level of the precapillary retinal arterioles, secondary to a sudden and severe rise in blood pressure that overwhelms autoregulatory mechanisms. The affected arterioles dilate, leading to the characteristic appearance of FIPTs as small, round or oval, dull white lesions, typically ranging from pinpoint to pinhead size. These transudates are located in the deeper retinal layers near the main retinal arteries and their primary branches.
On fluorescein angiography, FIPTs exhibit multiple small areas of dye leakage originating from the dilated precapillary arterioles, without evidence of focal capillary obliteration. These lesions usually persist for 2 to 3 weeks and resolve without leaving any detectable changes on ophthalmoscopy, angiography, or microvascular imaging.[41]
Additional retinal findings in hypertensive retinopathy include cotton wool spots (also referred to as "soft exudates"), hard exudates (lipid deposits), copper wiring of the retinal arterioles, and changes at arteriovenous crossings. Hemorrhages may be present in both the superficial and deep retinal layers, appearing as flame-shaped and blot hemorrhages, respectively.[42] Microaneurysms and optic disc edema can also occur. In rare cases, intraretinal microvascular abnormalities (IRMAs), venous beading, and retinal neovascularization may be observed.[43][44]
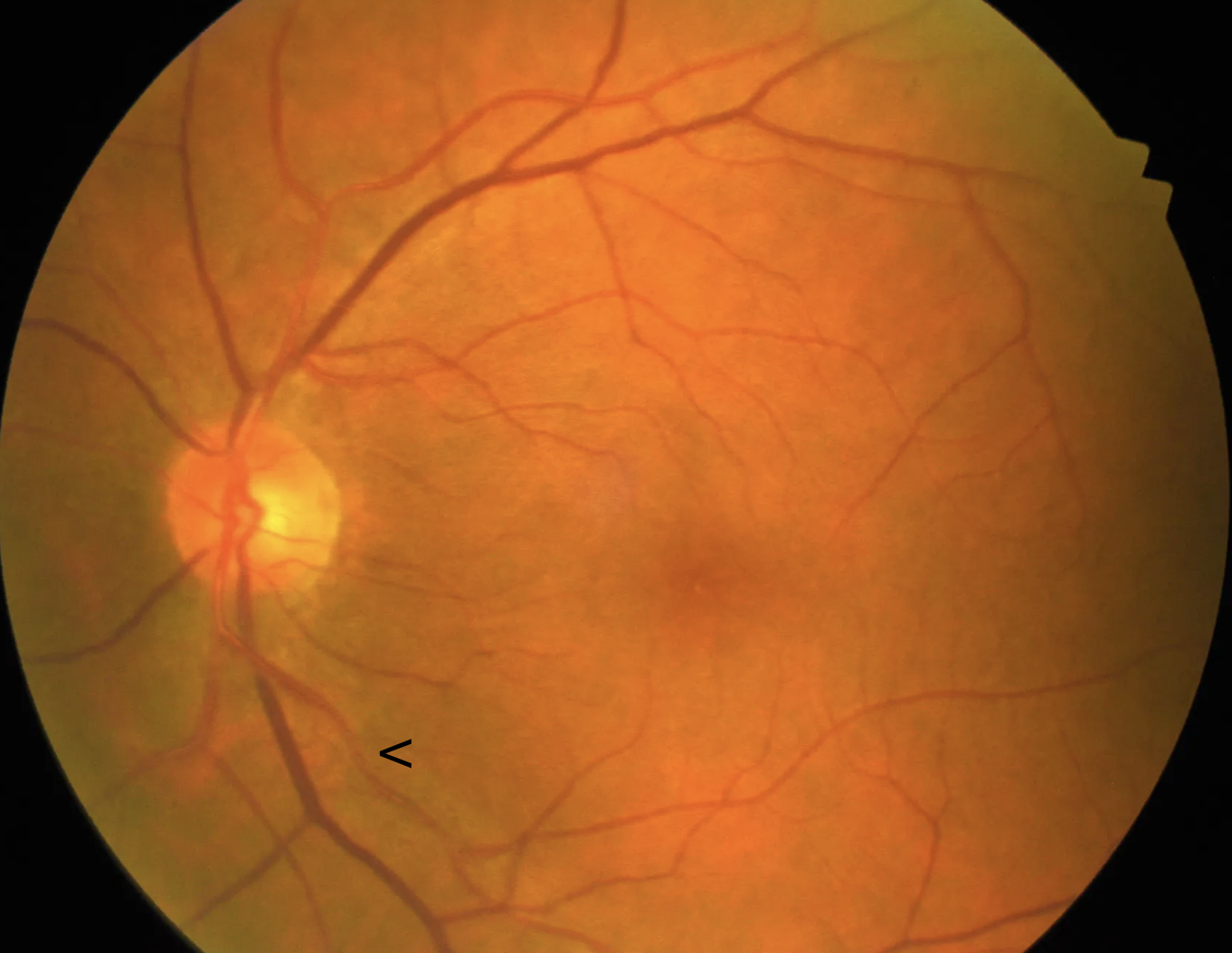
However, distinguishing whether certain vascular changes, such as arteriovenous crossing alterations and focal arteriolar narrowing, are caused solely by systemic hypertension remains a challenge (see Image. Focal Arteriolar Narrowing). These changes may have limited predictive value in assessing the severity of hypertension. The development of hypertensive and arteriosclerotic retinal vascular changes is multifactorial and depends on several factors. These factors include the patient’s age, the duration and severity of systemic hypertension, the presence and severity of other systemic conditions such as diabetes mellitus, kidney disease, and dyslipidemia, the extent of coexisting diabetic retinopathy, and smoking history.
Phases of Hypertensive Retinopathy
Hypertensive retinopathy progresses through 3 distinct phases: vasoconstrictive, sclerotic, and exudative. In the vasoconstrictive phase, local autoregulatory mechanisms attempt to reduce blood flow in response to elevated blood pressure. This compensatory response leads to vasospasm, an increase in vasomotor tone, and generalized or diffuse narrowing of the retinal arterioles. Clinically, this vasoconstriction is observed as a decreased arteriole-to-venule diameter ratio (normal value is 2:3). In older individuals, the narrowing may be less apparent due to age-related arteriosclerosis, which renders the vessel walls more rigid and less responsive to vasomotor stimuli.
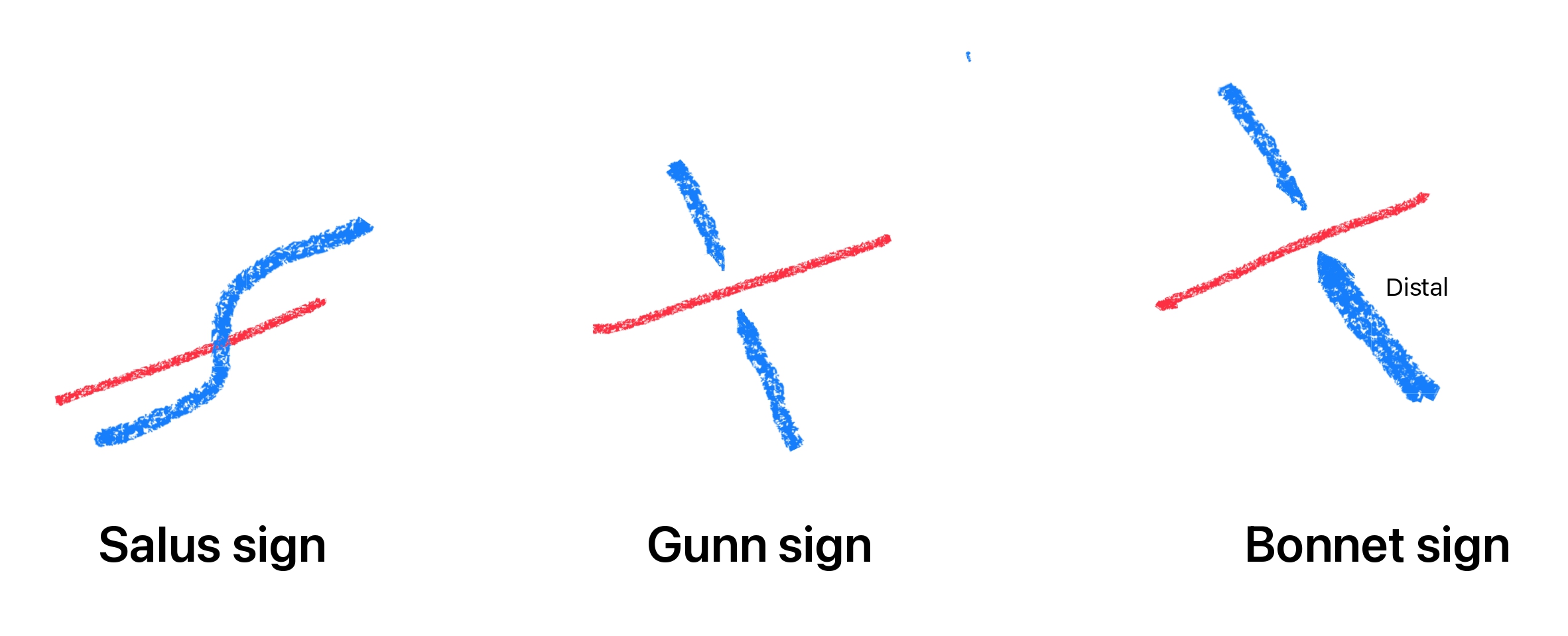
The sclerotic phase follows when elevated blood pressure persists, producing structural changes in the vessel walls. The intimal layer thickens, the medial layer undergoes hyperplasia, and the arteriolar wall shows signs of hyaline degeneration. These changes result in severe arteriolar narrowing, both diffuse and focal, along with arteriovenous crossing changes, commonly referred to as "arteriovenous nipping" or "nicking," and arteriolar wall opacification, seen as copper or silver wiring. Arteriovenous crossing changes occur when a thickened arteriole compresses a venule at a shared adventitial sheath. This compression leads to dilation and tortuosity of the vein distal to the crossing, often described as the Bonnet sign or retinal venous banking.
In the exudative phase, a marked elevation in blood pressure disrupts the blood-retinal barrier, allowing leakage of blood, lipids, and fluid from the compromised vessel walls. Autoregulatory mechanisms fail at this stage, and visible retinal changes become evident. These changes include microaneurysms, flame-shaped and dot-blot retinal hemorrhages, hard exudates due to lipid accumulation, necrosis of smooth muscle and endothelial cells, subretinal fluid (SRF), and signs of retinal ischemia, such as cotton wool spots.
Malignant Hypertension
Severe systemic hypertension causes optic nerve ischemia and edema (papilledema), often indicating elevated intracranial pressure and potentially presenting as hypertensive encephalopathy. The progression of retinal findings does not always follow a strict sequence. Additional factors such as inflammation, ischemia, platelet activation, oxidative stress, dysregulated angiogenesis, renin-angiotensin-aldosterone system (RAAS) activity, and endothelial dysfunction also contribute to the pathogenesis of hypertensive retinal disease.
Pathogenesis of Various Manifestations of Hypertensive Retinopathy
Hypertensive retinopathy manifests through a variety of retinal changes resulting from chronically elevated blood pressure. The underlying pathogenesis involves vascular injury, ischemia, and breakdown of protective barriers within the retina, producing distinct clinical features.
Retinal hemorrhages occur due to the breakdown of the blood-retinal barrier caused by endothelial damage and necrosis from prolonged or severe hypertension. This damage thickens the vessel walls and results in bleeding into the retina, producing flame-shaped or splinter hemorrhages in the nerve fiber layer, or dot-blot hemorrhages in the deeper retinal layers.
Cotton wool spots in hypertensive retinopathy result from ischemia in the nerve fiber layer of the retina. This ischemia arises due to narrowing and occlusion of retinal arterioles, particularly the precapillary arterioles, causing a deficiency of oxygen and nutrients. The resulting hypoxia disrupts orthograde and retrograde axoplasmic transport within ganglion cell axons, leading to the accumulation of cytoplasmic debris and the formation of cotton wool spots.[45]
McLeod proposed renaming cotton wool spots as "cotton wool sentinels."[46] These spots often mark the boundary of ischemic areas, such as in branch retinal arterial occlusion. These structures also act as sentinels of ischemia affecting the entire retinal midperiphery in preproliferative diabetic retinopathy, represent an ischemic penumbra in acute panretinal ischemia, or indicate neuronal damage from transient venous hyperdistension that overwhelms the protection provided by peripapillary axonal decompartmentalization in Purtscher retinopathy.[47][48]
Hypertensive Choroidopathy
Hypertensive choroidopathy results from fibrinoid necrosis of choroidal arterioles, leading to segmental infarction of the choriocapillaris. This process gives rise to several characteristic findings, described below.
Elschnig spots appear as areas where the overlying retinal pigment epithelium (RPE) looks yellow due to lobular nonperfusion of the choriocapillaris, which represents focal choroidal infarcts. Acute lesions are tan-colored and have a lobular or round shape, but over time, they become hyperpigmented with hypopigmented borders.
Siegrist streaks develop from RPE hyperplasia overlying choroidal infarcts and appear as linear hyperpigmentation that follows the meridional course of the choroidal arteries. Angiographic studies reveal early focal choroidal hypoperfusion followed by late multiple subretinal pinpoint leakages. This pattern reflects fibrinoid necrosis of the choroid. Other manifestations of hypertensive choroidopathy include neurosensory RPE detachments and exudative retinal detachments (see Image. Exudative Retinal Detachment and Elschnig Spots in Hypertensive Choroidopathy).
These signs typically occur in young patients who experience a sudden onset of severe systemic hypertension, often described as malignant hypertension, accelerated hypertension, or acute hypertensive crisis. This condition is commonly associated with underlying disorders such as kidney disease, PIH, or pheochromocytoma.
History and Physical
History
Patients with malignant hypertension may present with headaches or bilateral visual decline. In some cases, the visual decline results from malignant hypertensive retinopathy accompanied by macular SRF, which can provide an important clue to an underlying diagnosis of hypertension. However, hypertensive retinopathy is more often discovered incidentally during routine ophthalmic examinations. A thorough history should include the duration of hypertension, current medications, adherence to treatment, and the presence of systemic comorbidities such as diabetes mellitus. Additionally, a history of hypertension-related complications, including cerebrovascular accidents and myocardial infarction, is important to assess.
Physical Examination
The physical examination should include vital signs and systemic examination, including cardiovascular, respiratory, and neurological systems. Careful assessment of blood pressure is essential, as hypertensive retinopathy often correlates with the severity and duration of systemic hypertension. Additionally, evaluation for signs of TOD, such as heart failure or stroke, can provide important clinical context.
Ophthalmoscopic Features
Hypertensive retinopathy is primarily diagnosed through characteristic findings observed during ophthalmoscopic examination. These retinal signs reflect the severity and duration of systemic hypertension and help guide clinical assessment and management. These funduscopic features are discussed in detail below.
Arteriovenous crossing changes
These features represent some of the earliest ophthalmoscopic signs of hypertensive retinopathy and indicate vascular remodeling due to chronic hypertension. Arteriovenous crossing changes occur where retinal arterioles and veins share a common adventitial sheath, leading to mechanical effects on the veins caused by thickened, sclerotic arterioles.
The Salus sign is characterized by the deflection of the retinal vein as it crosses over or under the arteriole. When the retinal vein crosses obliquely over the arteriole, it shows a vertical hump or arch over the arteriole. More commonly, lateral deflections create an S-shaped appearance when the vein crosses the arteriole at right angles.[49] The Gunn sign denotes the tapering of the retinal vein on either side of the arteriovenous crossing, which reflects venous narrowing caused by arterial compression. The Bonnet sign describes the banking or dilatation of the retinal vein distal to the crossing (see Image. Arteriovenous Crossing Changes). This venous banking may precede branch retinal vein occlusion (RVO), a complication arising from the compression of the vein by the arteriole due to their shared adventitial sheath.[50]
Arterial changes
Arteriolar narrowing in hypertensive retinopathy is characterized by a decrease in the arteriovenous ratio, which can decrease to as low as 1:3 from the normal ratio of 2:3. This narrowing may be generalized, affecting large segments of the retinal arterioles, or focal, where irregular calibers result from localized spasm of the retinal arteriole.
Chronic hypertension also leads to retinal arteriolar sclerosis, marked by thickening and stiffening of the arteriolar walls. Normally, retinal arterioles exhibit a central light reflex that is broader and brighter than that of retinal veins, caused by light reflecting off the convex cylindrical surfaces of the transparent blood column and vessel walls. With sclerosis, the vascular wall thickens and its refractive index increases, resulting in a widening and increased brightness of the light streak.
Changes in the arteriolar light reflex appear as copper or silver wiring, which are hallmark signs of arteriolosclerosis. The earliest change is the widening and accentuation of the central light reflex, known as copper wiring, where the arteriolar light streak covers most of the vessel surface, giving it a burnished copper appearance. In more advanced cases, silver wiring occurs when the vessel wall becomes so opaque that it obscures the blood column, causing the arteriole to look like a white cord despite continued blood flow through it.
Retinal changes
Retinal changes in hypertensive retinopathy include various types of hemorrhages and exudates. Dot-blot hemorrhages occur due to bleeding in the deeper retinal layers, whereas flame-shaped hemorrhages result from bleeding in the superficial retinal layer, specifically the nerve fiber layer. Retinal exudates also manifest, with hard exudates representing lipid deposits within the retina. Soft exudates, also called cotton wool spots, arise due to ischemia affecting the nerve fibers.
Macular Changes
Macular star formation results from the deposition of hard exudates around the macula, specifically in the Henle layer. This star pattern becomes more prominent following the resolution of macular SRF.
Optic Nerve Changes
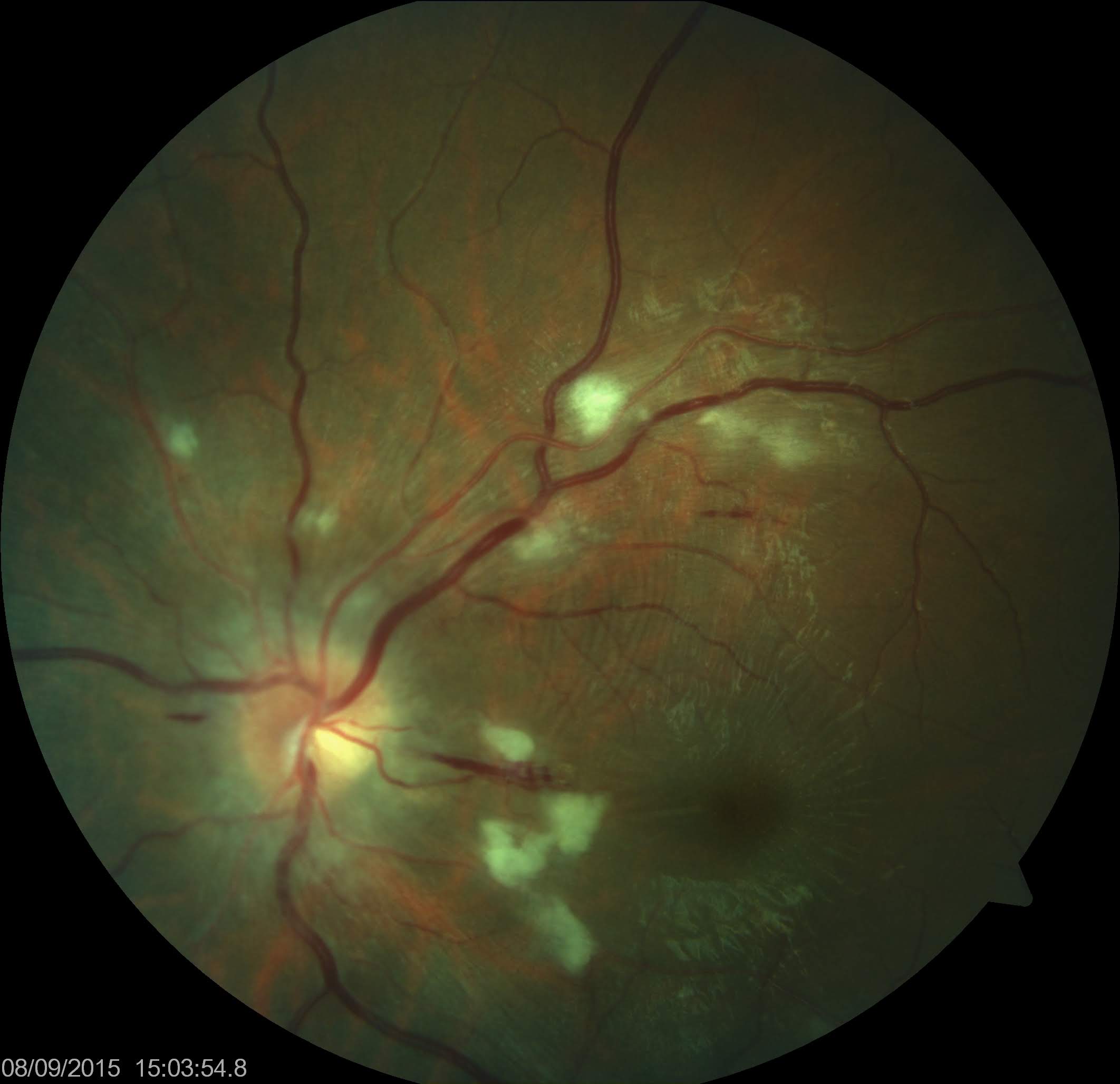
Hypertensive optic neuropathy is characterized by papilledema, which presents as blurring of the optic disc margins, radial peripapillary flame-shaped hemorrhages, severe disc swelling with retinal venous stasis, and the appearance of a macular fan or star caused by hard exudate deposition in the Henle layer. This finding indicates malignant or accelerated hypertension and defines malignant hypertensive retinopathy (see Image. Malignant Hypertensive Retinopathy).
In the acute phase, SRF accumulates at the fovea, predominantly on its nasal side, and is usually continuous with edema around the optic disc, as seen on optical coherence tomography (OCT). As the edema resolves over time, hard exudates organize in a radial pattern around the fovea, producing the characteristic macular fan or star. These patients require urgent but carefully controlled management of hypertension to prevent ischemic injury or hypoperfusion to vital organs such as the brain (ischemic stroke), heart (myocardial infarction), and kidneys.[51]
Wong et al identified several retinal signs associated with an increased risk of stroke. These signs include arteriovenous nicking, focal arteriolar narrowing (linked to arteriosclerosis), microaneurysms, cotton wool spots, retinal hemorrhages (both dot-blot and flame-shaped), and a decreased arteriovenous ratio.[52] The presence of arteriovenous nicking may indicate longstanding hypertension even when current blood pressure readings are normal. However, mild arteriovenous changes may be nonspecific and may occur in the absence of hypertension.
Wong and Mitchell proposed a classification system for hypertensive retinopathy based on population-level data, linking specific retinal findings to systemic vascular risks. This grading system, outlined in Table 2, helps stratify patients not only by ocular findings but also by their risk for cardiovascular and cerebrovascular complications.
Table 2. Classification of Hypertensive Retinopathy by Wong and Mitchell Based on Population-Based Studies
|
Grade of Hypertensive Retinopathy |
Retinal Features |
Systemic Associations |
|
None |
No detectable retinal signs |
No systemic associations |
|
Mild |
Generalized or focal arteriolar narrowing, arteriovenous nicking, or increased arteriolar wall reflectivity (eg, copper wiring), or a combination of these features |
Modest association with clinical stroke, subclinical stroke, coronary heart disease, and death (odds ratio >1 but <2) [53][54][55] |
|
Moderate |
Retinal hemorrhages (dot, blot, or flame-shaped), microaneurysms, cotton wool spots, hard exudates, or any combination of these findings |
Strong association with clinical and subclinical stroke, cognitive decline, and cardiovascular death (odds ratio >2) [56] |
|
Malignant |
Features of moderate retinopathy plus optic disc swelling (after excluding anterior ischemic optic neuropathy) |
Strong association with increased risk of mortality |
Evaluation
Classification
The following classification systems are based on clinical fundus findings observed using indirect ophthalmoscopy or a +90 diopter lens. These systems help stratify the severity of hypertensive retinopathy and guide systemic risk assessment and management.
The Keith-Wagener-Barker system categorizes hypertensive retinopathy into 4 groups, progressing from mild vascular changes to severe retinal and optic nerve involvement. This classification is commonly used to correlate retinal changes with the severity and chronicity of systemic hypertension.
- Group 1: Mild generalized narrowing or increased tone of the retinal arterioles
- Group 2: Moderate to marked arteriolar sclerosis, which may be chronic (exaggerated arteriolar light reflex and arteriovenous compression) or postangiospastic (generalized or focal irregular narrowing). RVO may be observed.
- Group 3: Retinal edema, flame-shaped hemorrhages, and cotton-wool spots superimposed on sclerotic and spastic arteriolar changes
- Group 4: All Group 3 findings plus papilledema [57]
The Scheie system offers 2 separate grading schemes, one for hypertensive retinopathy and another for arteriolar sclerosis. This approach allows more precise documentation of both acute and chronic retinal vascular changes. Grading for hypertensive retinopathy focuses on acute retinal findings related to elevated blood pressure.
- None: No visible abnormalities
- Grade 1: Mild, diffuse narrowing of arterioles, especially in secondary branches
- Grade 2: Marked arteriolar narrowing with focal areas of vasospasm
- Grade 3: Grade 2 findings plus retinal hemorrhages or exudates
- Grade 4: Grade 3 findings accompanied by papilledema
Grading for arteriolar sclerosis describes the extent of chronic arteriolar wall thickening and associated vascular changes.
- Grade 0: Normal retinal arterioles
- Grade 1: Slight widening of the central light reflex with minimal arteriovenous crossing changes
- Grade 2: More pronounced arteriovenous crossing changes with further widening of the arteriolar light reflex
- Grade 3: Copper wiring of the arterioles with marked arteriovenous changes
- Grade 4: Silver wiring of the arterioles, indicating the most severe form of arteriolosclerosis
Systemic Evaluation
Systemic evaluation includes repeated measurement of blood pressure. In younger individuals, secondary causes such as renal disease should be ruled out. These patients often present with associated cardiovascular, renal, and cerebrovascular conditions, requiring coordinated care among physicians, ophthalmologists, cardiologists, nephrologists, and neurologists. Coexisting anemia and diabetes mellitus can aggravate the retinal findings and should be investigated.[58]
Ophthalmic Evaluation
A comprehensive eye examination should be performed. Fundus photography, using either standard or wide-field imaging, is useful for documenting retinal findings and monitoring treatment response.
In cases of malignant hypertensive retinopathy, OCT may reveal macular edema with SRF, with or without intraretinal fluid. This fluid accumulation is typically more prominent on the nasal side of the fovea and often connects with peripapillary SRF. A macular star may develop following the resolution of macular edema.
Given the potential for severe renal involvement, nephrology consultation is essential. These patients may also require a fundus fluorescein angiogram (FFA), which can demonstrate microaneurysms, capillary nonperfusion, intraretinal microvascular abnormalities, venous beading, optic disc leakage, and occasionally neovascularization. In early FFA phases, a dendritic pattern of choroidal filling defects may be seen, followed by diffuse subretinal leakage in the late phase. Multiple FIPTs and acute Elschnig spots can cause multiple pinpoint leakages.[59] The relationship between Elschnig spots and FIPTs may require further investigation. Indocyanine green angiography may show a moth-eaten appearance of the choriocapillaris in malignant hypertension, which can appear as flow voids on OCT angiography.[60]
In 2014, Ahn and colleagues proposed a classification of hypertensive retinopathy based on ophthalmoscopic findings, which showed a significant correlation with final best-corrected visual acuity.[61] The classification includes the following categories:
- Mild to moderate retinopathy, with or without SRF
- Malignant retinopathy without SRF
- Malignant retinopathy with SRF
Treatment / Management
Retinal vessels are the only blood vessels that can be directly seen during a routine eye exam, making them important for screening hypertensive retinopathy. Retinal and choroidal changes reflect the systemic vascular damage from chronically elevated blood pressure. Ophthalmologists and general physicians should collaborate to ensure timely screening and appropriate management to reduce the risk of ocular and systemic complications.[62] Henderson et al noted that hypertensive retinopathy remains associated with an increased risk of stroke, even after control of blood pressure and other vascular risk factors. Management includes both systemic regulation and ocular evaluation.
Systemic Control
Hypertensive retinopathy, including papilledema and macular subfoveal fluid, often improves with adequate systemic control, particularly blood pressure regulation and management of renal dysfunction. Treatment depends on disease severity. In mild cases, management consists of blood pressure control with regular monitoring. In moderate cases, referral to a physician is essential to evaluate for coexisting conditions such as diabetes mellitus and cardiovascular abnormalities. Ongoing care requires strict blood pressure regulation and close follow-up. Severe cases demand urgent intervention and referral due to the strong association with mortality. Evaluation for TOD involving the kidneys, cardiovascular system, and brain is critical.
Blood pressure should be lowered in a controlled manner in severe cases. Most guidelines recommend reducing the mean arterial pressure (MAP) by 10% to 15% in the first hour and no more than 25% of baseline within the first 24 hours during a hypertensive crisis.[63] MAP is calculated using the following formula:
MAP = [(2 × DBP) + SBP] ÷ 3
Controlled reduction is essential to prevent ischemic injury to critical end organs such as the optic nerve, kidneys, and brain. Management typically begins with parenteral medications, followed by a gradual transition to oral agents. Common intravenous drugs used in hypertensive emergencies include labetalol, nicardipine, clevidipine, fenoldopam, esmolol, and sodium nitroprusside.[64] Primary drug classes for long-term blood pressure control include angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, thiazide diuretics, and calcium channel blockers. Additional options include β-blockers, α-blockers, and vasodilators.[65]
Ophthalmic Management
Most cases of malignant hypertensive retinopathy respond well to systemic blood pressure control. However, foveal SRF may persist on OCT despite adequate systemic management and a reasonable observation period. In a case series, intravitreal bevacizumab led to improvement in macular edema and fluorescein leakage in 4 eyes from 2 patients. Visual acuity improved in all but 1 eye, which showed no improvement due to foveal atrophy.[66] Other reports have documented similarly encouraging outcomes.[67][68][69] Systemic control should always be prioritized before considering intravitreal therapy, given the associated risks, including endophthalmitis and potential blood pressure dysregulation in individuals with hypertension.[70][71][72][73] The role of anti-vascular endothelial growth factor agents requires further investigation.(B3)
Differential Diagnosis
Other conditions that often present with optic disc swelling include the following:
- Idiopathic intracranial hypertension
- Anterior ischemic optic neuropathy
- Optic neuritis
- Central RVO
- Diabetic papillopathy
- Neuroretinitis
- Radiation papillopathy
- Retrobulbar tumor
Conditions which mimic chronic hypertensive retinopathy include the following:
- Diabetic retinopathy
- Retinal venous obstruction
- Hyperviscosity syndrome
- Ocular ischemic syndrome
- Radiation retinopathy
- Anemia and other hematological disorders
Differentiating hypertensive retinopathy from other conditions with similar retinal or optic nerve findings is critical for guiding appropriate treatment. These overlapping features highlight the importance of a detailed history, systemic evaluation, and ocular imaging.
Prognosis
Chronic hypertensive retinopathy rarely leads to significant visual loss. Retinal changes can stabilize with effective treatment of hypertension, although arteriolar narrowing and vessel crossing changes often remain. In untreated malignant cases, mortality reaches up to 50% within 2 months and nearly 90% within 1 year of diagnosis. Vision loss results from secondary optic atrophy following prolonged papilledema or from pigmentary changes after exudative retinal detachment. Malignant hypertensive retinopathy with papilledema shows a strong association with increased cardiovascular risk and mortality.[74][75] These patients are also more prone to retinal vascular occlusions and retinal arterial macroaneurysms.[76]
Complications
Systemic hypertension contributes to a broad spectrum of ocular pathologies. Retinal artery occlusion may occur as a direct consequence of elevated vascular resistance. RVO often results from chronic vascular stress and may be complicated by macular edema, epiretinal membrane, vitreomacular traction, vitreous hemorrhage, and tractional retinal detachment.[77][78][79][80] Retinal arteriole macroaneurysms may develop due to chronic arterial wall damage.
When hypertensive retinopathy coexists with diabetic retinopathy, the condition is referred to as "mixed retinopathy." Hypertension plays a critical role in the progression and increased prevalence of diabetic retinopathy.[81] Anterior ischemic optic neuropathy may result from impaired optic nerve perfusion.[82] Age-related macular degeneration and glaucoma also show established associations with elevated systemic blood pressure.[83][84][85]
Retinal arteriolar emboli may originate from systemic vascular pathology and reflect the burden of embolic disease.[86] Subconjunctival hemorrhage is another ocular finding linked with elevated vascular fragility in hypertensive states.[87] Suprachoroidal hemorrhage, a sight-threatening event during intraocular surgeries, has been strongly associated with hypertension.[88] This complication may also occur spontaneously in patients with severe hypertension, particularly in cases of PIH.[89] Additional risk factors include antiplatelet therapy, coagulopathies, hematologic disorders, and chronic renal failure in elderly patients with exudative macular degeneration.[90]
Bilateral exudative retinal detachment may be seen in poorly controlled hypertension, especially among younger individuals.[91][92] These cases often exhibit signs of hypertensive choroidopathy in the attached retina.[93] Prolonged papilledema secondary to uncontrolled systemic hypertension may lead to optic atrophy, resulting in irreversible vision loss.[94] Persistent subfoveal fluid may cause long-term foveal thinning and RPE changes.[95]
Proliferative hypertensive retinopathy is a rare but severe manifestation of uncontrolled hypertension. This condition mimics proliferative diabetic retinopathy, with features including retinal ischemia, neovascularization, vitreous hemorrhage, and tractional detachment.[96][97] A diagnosis requires the exclusion of other potential causes of retinal neovascularization, such as proliferative diabetic retinopathy, RVO, ocular ischemic syndrome, sickle cell retinopathy, hyperviscosity syndromes, sarcoidosis, and systemic lupus erythematosus.
Deterrence and Patient Education
Generalized retinal arteriolar attenuation correlates with elevated blood pressure and an increased risk of systemic hypertension.[98][99] In some cases, this vascular change may precede the clinical onset of hypertension.[100][101] Even in early childhood, between ages 4 and 8 years, generalized arteriolar narrowing may reflect the early vascular impact of elevated blood pressure.[102][103][104] Arteriolar narrowing and arteriovenous nicking typically indicate cumulative vascular stress from longstanding hypertension and serve as markers of chronic disease. In contrast, microaneurysms, retinal hemorrhages, and focal arteriolar attenuation tend to reflect short-term blood pressure fluctuations.[105] Dilated retinal veins also appear to be associated with elevated blood pressure levels.[106][107][108]
Given the shared anatomy, physiology, and embryology between retinal and cerebral small vessels, hypertensive changes in the retina may predict the risk of subclinical or clinical cerebrovascular accidents.[109] Hypertensive retinopathy serves as a recognized indicator of TOD, including renal dysfunction, microalbuminuria, and left ventricular hypertrophy.[110] Associations have also been documented between hypertensive retinopathy and conditions such as congestive cardiac failure, clinical coronary artery disease, aortic stiffness, coronary artery calcification, and left ventricular hypertrophy.[111][112]
Hypertensive retinopathy is linked to higher risks of cardiovascular disease mortality, coronary heart disease mortality, and stroke-related death.[113] Retinal vascular changes and hypertensive retinopathy may also be associated with cognitive impairment, dementia, and Alzheimer disease, though further research is needed in this area.[114][115]
Pearls and Other Issues
Blood pressure measurement is recommended in patients presenting with diffusely reduced retinal arteriolar caliber in the absence of significant ocular disease. Most global hypertension management guidelines recognize hypertensive retinopathy, along with renal dysfunction and left ventricular hypertrophy, as evidence of TOD. These findings indicate the need for more intensive blood pressure control.
Enhancing Healthcare Team Outcomes
Hypertension involves multiple organ systems, requiring coordinated care among physicians, physician assistants, nurse practitioners, ophthalmologists, nephrologists, and cardiologists to support early detection and effective management. Patients must be advised to attend regular follow-up appointments with an ophthalmologist for comprehensive eye evaluations and receive clear education on the importance of adhering to antihypertensive therapy.[116]
Vision is generally preserved in patients with hypertensive retinopathy if blood pressure is controlled. Uncontrolled hypertension, however, may result in irreversible vision loss within a short timeframe. The underlying causes include retinal pigmentary changes and secondary optic atrophy, both of which are permanent.[117]
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
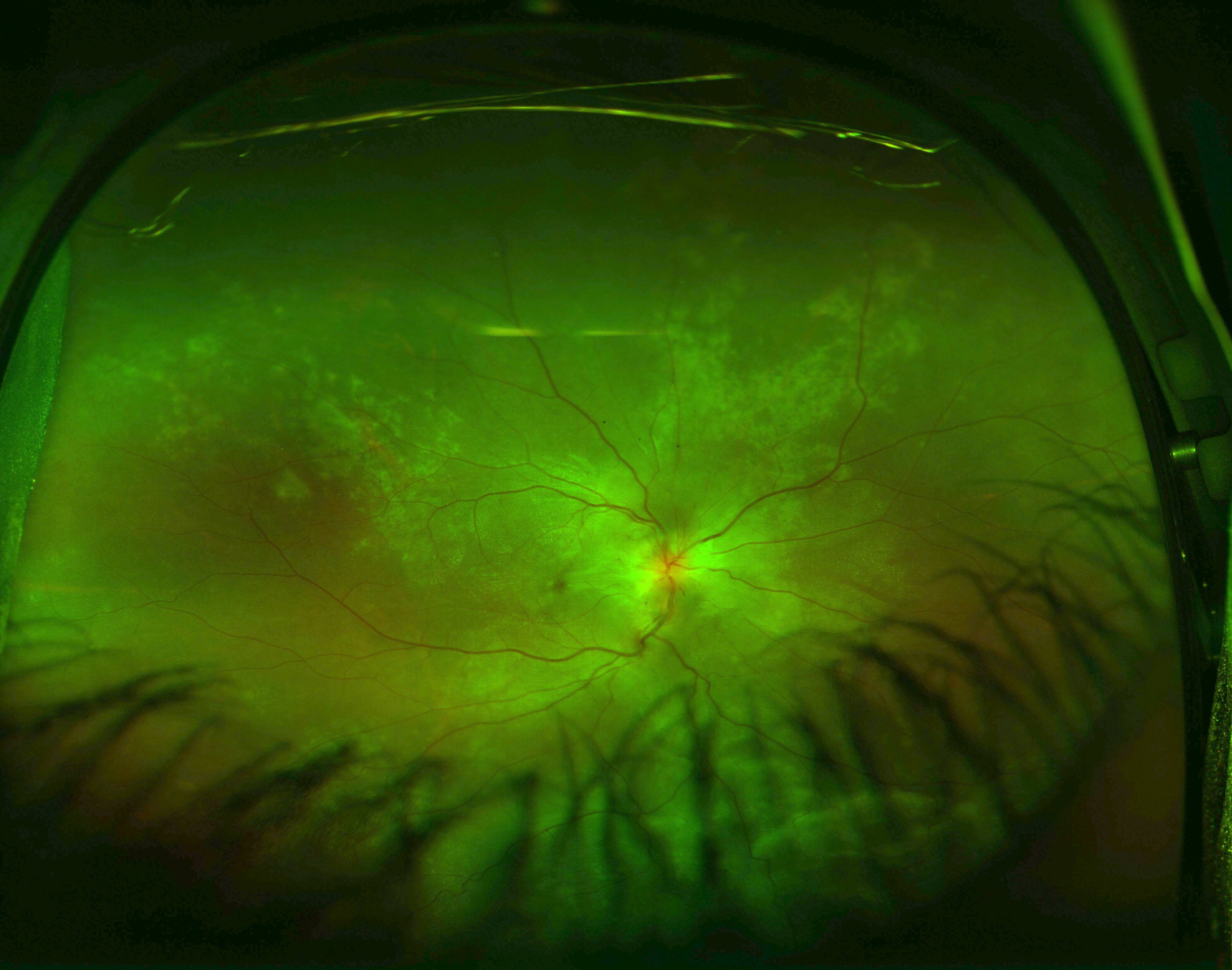
Exudative Retinal Detachment and Elschnig Spots in Hypertensive Choroidopathy. This ultrawide field image reveals an inferior exudative retinal detachment, visible between eyelash artifacts at the lower part of the image. The superotemporal and superonasal retina display signs of hypertensive choroidopathy, including multiple Elschnig spots. Similar findings were present in the patient’s other eye.
Contributed by Koushik Tripathy, MD
References
Prakash D. Target organ damage in newly detected hypertensive patients. Journal of family medicine and primary care. 2019 Jun:8(6):2042-2046. doi: 10.4103/jfmpc.jfmpc_231_19. Epub [PubMed PMID: 31334177]
Kabedi NN, Mwanza JC, Lepira FB, Kayembe TK, Kayembe DL. Hypertensive retinopathy and its association with cardiovascular, renal and cerebrovascular morbidity in Congolese patients. Cardiovascular journal of Africa. 2014 Sep-Oct:25(5):228-32. doi: 10.5830/CVJA-2014-045. Epub [PubMed PMID: 25629539]
Henderson AD, Bruce BB, Newman NJ, Biousse V. Hypertension-related eye abnormalities and the risk of stroke. Reviews in neurological diseases. 2011:8(1-2):1-9 [PubMed PMID: 21769065]
Erden S, Bicakci E. Hypertensive retinopathy: incidence, risk factors, and comorbidities. Clinical and experimental hypertension (New York, N.Y. : 1993). 2012:34(6):397-401. doi: 10.3109/10641963.2012.663028. Epub 2012 Apr 2 [PubMed PMID: 22468968]
Tsukikawa M, Stacey AW. A Review of Hypertensive Retinopathy and Chorioretinopathy. Clinical optometry. 2020:12():67-73. doi: 10.2147/OPTO.S183492. Epub 2020 May 5 [PubMed PMID: 32440245]
Carey RM, Wright JT Jr, Taler SJ, Whelton PK. Guideline-Driven Management of Hypertension: An Evidence-Based Update. Circulation research. 2021 Apr 2:128(7):827-846. doi: 10.1161/CIRCRESAHA.121.318083. Epub 2021 Apr 1 [PubMed PMID: 33793326]
Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr, Williamson JD, Wright JT Jr. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension (Dallas, Tex. : 1979). 2018 Jun:71(6):1269-1324. doi: 10.1161/HYP.0000000000000066. Epub 2017 Nov 13 [PubMed PMID: 29133354]
Level 1 (high-level) evidenceUnger T, Borghi C, Charchar F, Khan NA, Poulter NR, Prabhakaran D, Ramirez A, Schlaich M, Stergiou GS, Tomaszewski M, Wainford RD, Williams B, Schutte AE. 2020 International Society of Hypertension global hypertension practice guidelines. Journal of hypertension. 2020 Jun:38(6):982-1004. doi: 10.1097/HJH.0000000000002453. Epub [PubMed PMID: 32371787]
Level 1 (high-level) evidenceWilliams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, Kahan T, Mahfoud F, Redon J, Ruilope L, Zanchetti A, Kerins M, Kjeldsen SE, Kreutz R, Laurent S, Lip GYH, McManus R, Narkiewicz K, Ruschitzka F, Schmieder RE, Shlyakhto E, Tsioufis C, Aboyans V, Desormais I, ESC Scientific Document Group. 2018 ESC/ESH Guidelines for the management of arterial hypertension. European heart journal. 2018 Sep 1:39(33):3021-3104. doi: 10.1093/eurheartj/ehy339. Epub [PubMed PMID: 30165516]
Boulestreau R, van den Born BH, Lip GYH, Gupta A. Malignant Hypertension: Current Perspectives and Challenges. Journal of the American Heart Association. 2022 Apr 5:11(7):e023397. doi: 10.1161/JAHA.121.023397. Epub 2022 Mar 15 [PubMed PMID: 35289189]
Level 3 (low-level) evidenceUnger T, Borghi C, Charchar F, Khan NA, Poulter NR, Prabhakaran D, Ramirez A, Schlaich M, Stergiou GS, Tomaszewski M, Wainford RD, Williams B, Schutte AE. 2020 International Society of Hypertension Global Hypertension Practice Guidelines. Hypertension (Dallas, Tex. : 1979). 2020 Jun:75(6):1334-1357. doi: 10.1161/HYPERTENSIONAHA.120.15026. Epub 2020 May 6 [PubMed PMID: 32370572]
Level 1 (high-level) evidencevan den Born BH, Lip GYH, Brguljan-Hitij J, Cremer A, Segura J, Morales E, Mahfoud F, Amraoui F, Persu A, Kahan T, Agabiti Rosei E, de Simone G, Gosse P, Williams B. ESC Council on hypertension position document on the management of hypertensive emergencies. European heart journal. Cardiovascular pharmacotherapy. 2019 Jan 1:5(1):37-46. doi: 10.1093/ehjcvp/pvy032. Epub [PubMed PMID: 30165588]
Amraoui F, van Montfrans GA, van den Born BJ. Value of retinal examination in hypertensive encephalopathy. Journal of human hypertension. 2010 Apr:24(4):274-9. doi: 10.1038/jhh.2009.82. Epub 2009 Oct 29 [PubMed PMID: 19865107]
. Hypertension in adults: diagnosis and management. 2023 Nov 21:(): [PubMed PMID: 31577399]
Iqbal AM, Jamal SF. Essential Hypertension. StatPearls. 2025 Jan:(): [PubMed PMID: 30969681]
Hegde S, Ahmed I, Aeddula NR. Secondary Hypertension. StatPearls. 2025 Jan:(): [PubMed PMID: 31335025]
Sharp PS, Chaturvedi N, Wormald R, McKeigue PM, Marmot MG, Young SM. Hypertensive retinopathy in Afro-Caribbeans and Europeans. Prevalence and risk factor relationships. Hypertension (Dallas, Tex. : 1979). 1995 Jun:25(6):1322-5 [PubMed PMID: 7768581]
Pontremoli R, Sofia A, Tirotta A, Ravera M, Nicolella C, Viazzi F, Bezante GP, Borgia L, Bobola N, Ravazzolo R, Sacchi G, Deferrari G. The deletion polymorphism of the angiotensin I-converting enzyme gene is associated with target organ damage in essential hypertension. Journal of the American Society of Nephrology : JASN. 1996 Dec:7(12):2550-8 [PubMed PMID: 8989733]
Ikram MK, Sim X, Jensen RA, Cotch MF, Hewitt AW, Ikram MA, Wang JJ, Klein R, Klein BE, Breteler MM, Cheung N, Liew G, Mitchell P, Uitterlinden AG, Rivadeneira F, Hofman A, de Jong PT, van Duijn CM, Kao L, Cheng CY, Smith AV, Glazer NL, Lumley T, McKnight B, Psaty BM, Jonasson F, Eiriksdottir G, Aspelund T, Global BPgen Consortium, Harris TB, Launer LJ, Taylor KD, Li X, Iyengar SK, Xi Q, Sivakumaran TA, Mackey DA, Macgregor S, Martin NG, Young TL, Bis JC, Wiggins KL, Heckbert SR, Hammond CJ, Andrew T, Fahy S, Attia J, Holliday EG, Scott RJ, Islam FM, Rotter JI, McAuley AK, Boerwinkle E, Tai ES, Gudnason V, Siscovick DS, Vingerling JR, Wong TY. Four novel Loci (19q13, 6q24, 12q24, and 5q14) influence the microcirculation in vivo. PLoS genetics. 2010 Oct 28:6(10):e1001184. doi: 10.1371/journal.pgen.1001184. Epub 2010 Oct 28 [PubMed PMID: 21060863]
Poulter NR. Independent effects of smoking on risk of hypertension: small, if present. Journal of hypertension. 2002 Feb:20(2):171-2 [PubMed PMID: 11821697]
Biesenbach G, Zazgornik J. High prevalence of hypertensive retinopathy and coronary heart disease in hypertensive patients with persistent microalbuminuria under short intensive antihypertensive therapy. Clinical nephrology. 1994 Apr:41(4):211-8 [PubMed PMID: 8026113]
Uckaya G, Ozata M, Sonmez A, Kinalp C, Eyileten T, Bingol N, Koc B, Kocabalkan F, Ozdemir IC. Is leptin associated with hypertensive retinopathy? The Journal of clinical endocrinology and metabolism. 2000 Feb:85(2):683-7 [PubMed PMID: 10690876]
Grosso A, Veglio F, Porta M, Grignolo FM, Wong TY. Hypertensive retinopathy revisited: some answers, more questions. The British journal of ophthalmology. 2005 Dec:89(12):1646-54 [PubMed PMID: 16299149]
Mills KT, Stefanescu A, He J. The global epidemiology of hypertension. Nature reviews. Nephrology. 2020 Apr:16(4):223-237. doi: 10.1038/s41581-019-0244-2. Epub 2020 Feb 5 [PubMed PMID: 32024986]
Fryar CD, Kit B, Carroll MD, Afful J. Hypertension Prevalence, Awareness, Treatment, and Control Among Adults Age 18 and Older: United States, August 2021-August 2023. NCHS data brief. 2024 Oct:(511):. doi: CS354233. Epub [PubMed PMID: 40085792]
Gudayneh YA, Shumye AF, Gelaye AT, Tegegn MT. Prevalence of hypertensive retinopathy and its associated factors among adult hypertensive patients attending at Comprehensive Specialized Hospitals in Northwest Ethiopia, 2024, a multicenter cross-sectional study. International journal of retina and vitreous. 2025 Feb 17:11(1):17. doi: 10.1186/s40942-025-00631-2. Epub 2025 Feb 17 [PubMed PMID: 39962536]
Level 2 (mid-level) evidenceOjaimi E, Nguyen TT, Klein R, Islam FM, Cotch MF, Klein BE, Wang JJ, Wong TY. Retinopathy signs in people without diabetes: the multi-ethnic study of atherosclerosis. Ophthalmology. 2011 Apr:118(4):656-62. doi: 10.1016/j.ophtha.2010.08.007. Epub 2010 Nov 4 [PubMed PMID: 21055817]
Del Brutto OH, Mera RM, Viteri EM, Pólit J, Ledesma EA, Cano JA, Plaza KJ, Zambrano M, Costa AF. Hypertensive retinopathy and cerebral small vessel disease in Amerindians living in rural Ecuador: The Atahualpa Project. International journal of cardiology. 2016 Sep 1:218():65-68. doi: 10.1016/j.ijcard.2016.05.020. Epub 2016 May 13 [PubMed PMID: 27232913]
Wong TY, Mitchell P. Hypertensive retinopathy. The New England journal of medicine. 2004 Nov 25:351(22):2310-7 [PubMed PMID: 15564546]
Sekkarie A, Fang J, Hayes D, Loustalot F. Prevalence of Self-Reported Hypertension and Antihypertensive Medication Use Among Adults - United States, 2017-2021. MMWR. Morbidity and mortality weekly report. 2024 Mar 7:73(9):191-198. doi: 10.15585/mmwr.mm7309a1. Epub 2024 Mar 7 [PubMed PMID: 38451865]
Vasan RS, Beiser A, Seshadri S, Larson MG, Kannel WB, D'Agostino RB, Levy D. Residual lifetime risk for developing hypertension in middle-aged women and men: The Framingham Heart Study. JAMA. 2002 Feb 27:287(8):1003-10 [PubMed PMID: 11866648]
Hanif AAM, Shamim AA, Hossain MM, Hasan M, Khan MSA, Hossaine M, Ullah MA, Sarker SK, Rahman SMM, Mitra DK, Mridha MK. Gender-specific prevalence and associated factors of hypertension among elderly Bangladeshi people: findings from a nationally representative cross-sectional survey. BMJ open. 2021 Jan 21:11(1):e038326. doi: 10.1136/bmjopen-2020-038326. Epub 2021 Jan 21 [PubMed PMID: 33478960]
Level 2 (mid-level) evidenceMohanty P, Patnaik L, Nayak G, Dutta A. Gender difference in prevalence of hypertension among Indians across various age-groups: a report from multiple nationally representative samples. BMC public health. 2022 Aug 10:22(1):1524. doi: 10.1186/s12889-022-13949-5. Epub 2022 Aug 10 [PubMed PMID: 35948916]
Connelly PJ, Currie G, Delles C. Sex Differences in the Prevalence, Outcomes and Management of Hypertension. Current hypertension reports. 2022 Jun:24(6):185-192. doi: 10.1007/s11906-022-01183-8. Epub 2022 Mar 7 [PubMed PMID: 35254589]
Harjasouliha A, Raiji V, Garcia Gonzalez JM. Review of hypertensive retinopathy. Disease-a-month : DM. 2017 Mar:63(3):63-69. doi: 10.1016/j.disamonth.2016.10.002. Epub 2016 Dec 5 [PubMed PMID: 27931746]
Nwankwo T, Yoon SS, Burt V, Gu Q. Hypertension among adults in the United States: National Health and Nutrition Examination Survey, 2011-2012. NCHS data brief. 2013 Oct:(133):1-8 [PubMed PMID: 24171916]
Level 3 (low-level) evidenceWong TY, Klein R, Duncan BB, Nieto FJ, Klein BE, Couper DJ, Hubbard LD, Sharrett AR. Racial differences in the prevalence of hypertensive retinopathy. Hypertension (Dallas, Tex. : 1979). 2003 May:41(5):1086-91 [PubMed PMID: 12654714]
Chaine G, Kohner EM. [Hypertensive retinopathy]. Journal francais d'ophtalmologie. 1983:6(12):995-1005 [PubMed PMID: 6674324]
Level 3 (low-level) evidenceChua J, Chin CWL, Hong J, Chee ML, Le TT, Ting DSW, Wong TY, Schmetterer L. Impact of hypertension on retinal capillary microvasculature using optical coherence tomographic angiography. Journal of hypertension. 2019 Mar:37(3):572-580. doi: 10.1097/HJH.0000000000001916. Epub [PubMed PMID: 30113530]
Dziedziak J, Zaleska-Żmijewska A, Szaflik JP, Cudnoch-Jędrzejewska A. Impact of Arterial Hypertension on the Eye: A Review of the Pathogenesis, Diagnostic Methods, and Treatment of Hypertensive Retinopathy. Medical science monitor : international medical journal of experimental and clinical research. 2022 Jan 20:28():e935135. doi: 10.12659/MSM.935135. Epub 2022 Jan 20 [PubMed PMID: 35046380]
Hayreh SS, Servais GE, Virdi PS. Fundus lesions in malignant hypertension. IV. Focal intraretinal periarteriolar transudates. Ophthalmology. 1986 Jan:93(1):60-73 [PubMed PMID: 3951817]
Shukla UV, Tripathy K. Diabetic Retinopathy. StatPearls. 2025 Jan:(): [PubMed PMID: 32809640]
Jung EE, Ameri H. Hypertension: A Cause of Bilateral Proliferative Retinopathy. Journal of current ophthalmology. 2022 Oct-Dec:34(4):478-482. doi: 10.4103/joco.joco_87_22. Epub 2023 Apr 29 [PubMed PMID: 37180527]
Georgiadis O, Kabanarou SA, Batsos G, Feretis E, Xirou T. Bilateral Hypertensive Retinopathy Complicated with Retinal Neovascularization: Panretinal Photocoagulation or Intravitreal Anti-VEGF Treatment? Case reports in ophthalmology. 2014 May:5(2):231-8. doi: 10.1159/000365865. Epub 2014 Jul 30 [PubMed PMID: 25232335]
Level 3 (low-level) evidenceMcLeod D, Marshall J, Kohner EM, Bird AC. The role of axoplasmic transport in the pathogenesis of retinal cotton-wool spots. The British journal of ophthalmology. 1977 Mar:61(3):177-91 [PubMed PMID: 66930]
Level 3 (low-level) evidenceMcLeod D. Why cotton wool spots should not be regarded as retinal nerve fibre layer infarcts. The British journal of ophthalmology. 2005 Feb:89(2):229-37 [PubMed PMID: 15665358]
Tripathy K, Patel BC. Purtscher Retinopathy. StatPearls. 2025 Jan:(): [PubMed PMID: 31194324]
Tripathy K, Shah SS, Waymack JR. Central Retinal Artery Occlusion. StatPearls. 2025 Jan:(): [PubMed PMID: 29262124]
SCHEIE HG. Evaluation of ophthalmoscopic changes of hypertension and arteriolar sclerosis. A.M.A. archives of ophthalmology. 1953 Feb:49(2):117-38 [PubMed PMID: 13007237]
Cochran ML, Mahabadi N, Czyz CN. Branch Retinal Vein Occlusion. StatPearls. 2025 Jan:(): [PubMed PMID: 30570991]
Ahmed I, Chauhan S, Afzal M. Hypertensive Crisis. StatPearls. 2025 Jan:(): [PubMed PMID: 29939523]
Wong TY, Klein R, Couper DJ, Cooper LS, Shahar E, Hubbard LD, Wofford MR, Sharrett AR. Retinal microvascular abnormalities and incident stroke: the Atherosclerosis Risk in Communities Study. Lancet (London, England). 2001 Oct 6:358(9288):1134-40 [PubMed PMID: 11597667]
Wong TY, Klein R, Sharrett AR, Couper DJ, Klein BE, Liao DP, Hubbard LD, Mosley TH, ARIC Investigators. Atheroslerosis Risk in Communities Study. Cerebral white matter lesions, retinopathy, and incident clinical stroke. JAMA. 2002 Jul 3:288(1):67-74 [PubMed PMID: 12090864]
Duncan BB, Wong TY, Tyroler HA, Davis CE, Fuchs FD. Hypertensive retinopathy and incident coronary heart disease in high risk men. The British journal of ophthalmology. 2002 Sep:86(9):1002-6 [PubMed PMID: 12185127]
Wong TY, Klein R, Sharrett AR, Duncan BB, Couper DJ, Tielsch JM, Klein BE, Hubbard LD. Retinal arteriolar narrowing and risk of coronary heart disease in men and women. The Atherosclerosis Risk in Communities Study. JAMA. 2002 Mar 6:287(9):1153-9 [PubMed PMID: 11879113]
Wong TY, Klein R, Nieto FJ, Klein BE, Sharrett AR, Meuer SM, Hubbard LD, Tielsch JM. Retinal microvascular abnormalities and 10-year cardiovascular mortality: a population-based case-control study. Ophthalmology. 2003 May:110(5):933-40 [PubMed PMID: 12750093]
Level 2 (mid-level) evidenceKeith NM, Wagener HP, Barker NW. Some different types of essential hypertension: their course and prognosis. The American journal of the medical sciences. 1974 Dec:268(6):336-45 [PubMed PMID: 4616627]
Petrie JR, Guzik TJ, Touyz RM. Diabetes, Hypertension, and Cardiovascular Disease: Clinical Insights and Vascular Mechanisms. The Canadian journal of cardiology. 2018 May:34(5):575-584. doi: 10.1016/j.cjca.2017.12.005. Epub 2017 Dec 11 [PubMed PMID: 29459239]
Ruia S, Tripathy K. Fluorescein Angiography. StatPearls. 2025 Jan:(): [PubMed PMID: 35015403]
Rezkallah A, Kodjikian L, Abukhashabah A, Denis P, Mathis T. Hypertensive choroidopathy: Multimodal imaging and the contribution of wide-field swept-source oct-angiography. American journal of ophthalmology case reports. 2019 Mar:13():131-135. doi: 10.1016/j.ajoc.2019.01.001. Epub 2019 Jan 9 [PubMed PMID: 30705996]
Level 3 (low-level) evidenceAhn SJ, Woo SJ, Park KH. Retinal and choroidal changes with severe hypertension and their association with visual outcome. Investigative ophthalmology & visual science. 2014 Nov 13:55(12):7775-85. doi: 10.1167/iovs.14-14915. Epub 2014 Nov 13 [PubMed PMID: 25395485]
Fraser-Bell S, Symes R, Vaze A. Hypertensive eye disease: a review. Clinical & experimental ophthalmology. 2017 Jan:45(1):45-53. doi: 10.1111/ceo.12905. Epub 2017 Jan 21 [PubMed PMID: 27990740]
Kulkarni S, Glover M, Kapil V, Abrams SML, Partridge S, McCormack T, Sever P, Delles C, Wilkinson IB. Management of hypertensive crisis: British and Irish Hypertension Society Position document. Journal of human hypertension. 2023 Oct:37(10):863-879. doi: 10.1038/s41371-022-00776-9. Epub 2022 Nov 22 [PubMed PMID: 36418425]
Aronow WS. Treatment of hypertensive emergencies. Annals of translational medicine. 2017 May:5(Suppl 1):S5. doi: 10.21037/atm.2017.03.34. Epub [PubMed PMID: 28567387]
Khalil H, Zeltser R. Antihypertensive Medications. StatPearls. 2025 Jan:(): [PubMed PMID: 32119466]
Kim EY, Lew HM, Song JH. Effect of intravitreal bevacizumab (Avastin(®)) therapy in malignant hypertensive retinopathy: a report of two cases. Journal of ocular pharmacology and therapeutics : the official journal of the Association for Ocular Pharmacology and Therapeutics. 2012 Jun:28(3):318-22. doi: 10.1089/jop.2011.0113. Epub 2011 Dec 9 [PubMed PMID: 22149905]
Level 3 (low-level) evidenceAl-Halafi AM. Tremendous result of bevacizumab in malignant hypertensive retinopathy. Oman journal of ophthalmology. 2015 Jan-Apr:8(1):61-3. doi: 10.4103/0974-620X.149872. Epub [PubMed PMID: 25709280]
Padhy S, Kumar V. Dramatic response to intravitreal Bevacizumab in hypertensive retinopathy. Indian journal of ophthalmology. 2018 Oct:66(10):1494-1495. doi: 10.4103/ijo.IJO_214_18. Epub [PubMed PMID: 30249851]
Salman AG. Intravitreal bevacizumab in persistent retinopathy secondary to malignant hypertension. Saudi journal of ophthalmology : official journal of the Saudi Ophthalmological Society. 2013 Jan:27(1):25-9. doi: 10.1016/j.sjopt.2012.02.005. Epub 2012 Feb 16 [PubMed PMID: 23964183]
Samanta R. Comment on: Dramatic response to intravitreal bevacizumab in hypertensive retinopathy. Indian journal of ophthalmology. 2019 Jan:67(1):178-179. doi: 10.4103/ijo.IJO_1642_18. Epub [PubMed PMID: 30574944]
Level 3 (low-level) evidenceTripathy K, Chaudhuri A. Comment on: Dramatic response to intravitreal bevacizumab in hypertensive retinopathy. Indian journal of ophthalmology. 2019 Jan:67(1):180. doi: 10.4103/ijo.IJO_1654_18. Epub [PubMed PMID: 30574946]
Level 3 (low-level) evidenceRasier R, Artunay O, Yuzbasioglu E, Sengul A, Bahcecioglu H. The effect of intravitreal bevacizumab (avastin) administration on systemic hypertension. Eye (London, England). 2009 Aug:23(8):1714-8. doi: 10.1038/eye.2008.360. Epub 2008 Dec 12 [PubMed PMID: 19079149]
Kumar A, Tripathy K, Chawla R. Intraocular use of bevacizumab in India: An issue resolved? The National medical journal of India. 2017 Nov-Dec:30(6):345-347. doi: 10.4103/0970-258X.239079. Epub [PubMed PMID: 30117450]
Wong TY, Klein R, Klein BE, Tielsch JM, Hubbard L, Nieto FJ. Retinal microvascular abnormalities and their relationship with hypertension, cardiovascular disease, and mortality. Survey of ophthalmology. 2001 Jul-Aug:46(1):59-80 [PubMed PMID: 11525792]
Level 3 (low-level) evidenceWong TY, McIntosh R. Hypertensive retinopathy signs as risk indicators of cardiovascular morbidity and mortality. British medical bulletin. 2005:73-74():57-70 [PubMed PMID: 16148191]
Singh D, Tripathy K. Retinal Macroaneurysm. StatPearls. 2025 Jan:(): [PubMed PMID: 35015432]
Kim HR, Lee NK, Lee CS, Byeon SH, Kim SS, Lee SW, Kim YJ. Retinal Vascular Occlusion Risks in High Blood Pressure and the Benefits of Blood Pressure Control. American journal of ophthalmology. 2023 Jun:250():111-119. doi: 10.1016/j.ajo.2023.01.023. Epub 2023 Feb 1 [PubMed PMID: 36736752]
Blair K, Czyz CN. Central Retinal Vein Occlusion. StatPearls. 2025 Jan:(): [PubMed PMID: 30252241]
Jena S, Tripathy K. Vitreous Hemorrhage. StatPearls. 2025 Jan:(): [PubMed PMID: 32644557]
Mishra C, Tripathy K. Retinal Traction Detachment. StatPearls. 2025 Jan:(): [PubMed PMID: 32644378]
Zhang M, Wu J, Wang Y, Wu J, Hu W, Jia H, Sun X. Associations between blood pressure levels and diabetic retinopathy in patients with diabetes mellitus: A population-based study. Heliyon. 2023 Jun:9(6):e16830. doi: 10.1016/j.heliyon.2023.e16830. Epub 2023 Jun 1 [PubMed PMID: 37484372]
Raizada K, Margolin E. Non-Arteritic Anterior Ischemic Optic Neuropathy. StatPearls. 2025 Jan:(): [PubMed PMID: 32644471]
Wang T, Xia J, Yuan M, Wu X, Zhu Y, Chen C, Bergunder SJ, Liu Z, Chen W, Huang K, Lin H. Hypertension affects the treatment of wet age-related macular degeneration. Acta ophthalmologica. 2021 Dec:99(8):871-876. doi: 10.1111/aos.14791. Epub 2021 Mar 31 [PubMed PMID: 33787087]
Katsi VK, Marketou ME, Vrachatis DA, Manolis AJ, Nihoyannopoulos P, Tousoulis D, Vardas PE, Kallikazaros I. Essential hypertension in the pathogenesis of age-related macular degeneration: a review of the current evidence. Journal of hypertension. 2015 Dec:33(12):2382-8. doi: 10.1097/HJH.0000000000000766. Epub [PubMed PMID: 26536087]
Leeman M, Kestelyn P. Glaucoma and Blood Pressure. Hypertension (Dallas, Tex. : 1979). 2019 May:73(5):944-950. doi: 10.1161/HYPERTENSIONAHA.118.11507. Epub [PubMed PMID: 30852921]
Wong TY, Klein R. Retinal arteriolar emboli: epidemiology and risk of stroke. Current opinion in ophthalmology. 2002 Jun:13(3):142-6 [PubMed PMID: 12011681]
Level 3 (low-level) evidenceTarlan B, Kiratli H. Subconjunctival hemorrhage: risk factors and potential indicators. Clinical ophthalmology (Auckland, N.Z.). 2013:7():1163-70. doi: 10.2147/OPTH.S35062. Epub 2013 Jun 12 [PubMed PMID: 23843690]
Mohan S, Sadeghi E, Mohan M, Iannetta D, Chhablani J. Suprachoroidal Hemorrhage. Ophthalmologica. Journal international d'ophtalmologie. International journal of ophthalmology. Zeitschrift fur Augenheilkunde. 2023:246(5-6):255-277. doi: 10.1159/000533937. Epub 2023 Sep 2 [PubMed PMID: 37660688]
Tripathy K, Chawla R, Mutha V, Selvan H. Spontaneous suprachoroidal haemorrhage with exudative retinal detachment in pregnancy-induced hypertension. BMJ case reports. 2018 Mar 9:2018():. pii: bcr-2017-223907. doi: 10.1136/bcr-2017-223907. Epub 2018 Mar 9 [PubMed PMID: 29523618]
Level 3 (low-level) evidenceHsiao SF, Shih MH, Huang FC. Spontaneous suprachoroidal hemorrhage: Case report and review of the literature. Taiwan journal of ophthalmology. 2016 Jan-Mar:6(1):36-41. doi: 10.1016/j.tjo.2014.10.008. Epub 2015 Jan 20 [PubMed PMID: 29018708]
Level 3 (low-level) evidenceTripathy K, Chawla R. Bilateral exudative retinal detachment with choroidopathy in malignant hypertension. The National medical journal of India. 2015 Sep-Oct:28(5):261 [PubMed PMID: 27132968]
Shukla UV, Gupta A, Tripathy K. Exudative Retinal Detachment. StatPearls. 2025 Jan:(): [PubMed PMID: 36944005]
Kapoor A, Kumar A, Chawla R. Bilateral exudative retinal detachment and choroidopathy as the presenting signs of malignant hypertension. BMJ case reports. 2021 Apr 15:14(4):. doi: 10.1136/bcr-2021-242413. Epub 2021 Apr 15 [PubMed PMID: 33858909]
Level 3 (low-level) evidenceAhmad SS, Blair K, Kanukollu VM. Optic Atrophy. StatPearls. 2025 Jan:(): [PubMed PMID: 32644556]
Kohli P, Tripathy K, Patel BC. Macular Edema. StatPearls. 2024 Jan:(): [PubMed PMID: 35015421]
Stryjewski TP, Papakostas TD, Vavvas D. Proliferative Hypertensive Retinopathy. JAMA ophthalmology. 2016 Mar:134(3):345-6. doi: 10.1001/jamaophthalmol.2015.5583. Epub [PubMed PMID: 26767543]
Brancato R, Menchini U, Bandello F. Proliferative retinopathy and toxemia of pregnancy. Annals of ophthalmology. 1987 May:19(5):182-3 [PubMed PMID: 2438970]
Smith W, Wang JJ, Wong TY, Rochtchina E, Klein R, Leeder SR, Mitchell P. Retinal arteriolar narrowing is associated with 5-year incident severe hypertension: the Blue Mountains Eye Study. Hypertension (Dallas, Tex. : 1979). 2004 Oct:44(4):442-7 [PubMed PMID: 15302843]
Wong TY, Klein R, Sharrett AR, Duncan BB, Couper DJ, Klein BE, Hubbard LD, Nieto FJ, Atherosclerosis Risk in Communities Study. Retinal arteriolar diameter and risk for hypertension. Annals of internal medicine. 2004 Feb 17:140(4):248-55 [PubMed PMID: 14970147]
Klein R, Klein BE, Moss SE, Wong TY. The relationship of retinopathy in persons without diabetes to the 15-year incidence of diabetes and hypertension: Beaver Dam Eye Study. Transactions of the American Ophthalmological Society. 2006:104():98-107 [PubMed PMID: 17471330]
Ikram MK, Witteman JC, Vingerling JR, Breteler MM, Hofman A, de Jong PT. Retinal vessel diameters and risk of hypertension: the Rotterdam Study. Hypertension (Dallas, Tex. : 1979). 2006 Feb:47(2):189-94 [PubMed PMID: 16380526]
Mitchell P, Cheung N, de Haseth K, Taylor B, Rochtchina E, Islam FM, Wang JJ, Saw SM, Wong TY. Blood pressure and retinal arteriolar narrowing in children. Hypertension (Dallas, Tex. : 1979). 2007 May:49(5):1156-62 [PubMed PMID: 17372033]
Gishti O, Jaddoe VW, Felix JF, Klaver CC, Hofman A, Wong TY, Ikram MK, Gaillard R. Retinal microvasculature and cardiovascular health in childhood. Pediatrics. 2015 Apr:135(4):678-85. doi: 10.1542/peds.2014-3341. Epub 2015 Mar 9 [PubMed PMID: 25755243]
Li LJ, Cheung CY, Liu Y, Chia A, Selvaraj P, Lin XY, Chan YM, Varma R, Mitchell P, Wong TY, Saw SM. Influence of blood pressure on retinal vascular caliber in young children. Ophthalmology. 2011 Jul:118(7):1459-65. doi: 10.1016/j.ophtha.2010.12.007. Epub 2011 Mar 27 [PubMed PMID: 21444115]
Wong TY, Hubbard LD, Klein R, Marino EK, Kronmal R, Sharrett AR, Siscovick DS, Burke G, Tielsch JM. Retinal microvascular abnormalities and blood pressure in older people: the Cardiovascular Health Study. The British journal of ophthalmology. 2002 Sep:86(9):1007-13 [PubMed PMID: 12185128]
Cheung CY, Tay WT, Mitchell P, Wang JJ, Hsu W, Lee ML, Lau QP, Zhu AL, Klein R, Saw SM, Wong TY. Quantitative and qualitative retinal microvascular characteristics and blood pressure. Journal of hypertension. 2011 Jul:29(7):1380-91. doi: 10.1097/HJH.0b013e328347266c. Epub [PubMed PMID: 21558958]
Level 2 (mid-level) evidenceDing J, Wai KL, McGeechan K, Ikram MK, Kawasaki R, Xie J, Klein R, Klein BB, Cotch MF, Wang JJ, Mitchell P, Shaw JE, Takamasa K, Sharrett AR, Wong TY, Meta-Eye Study Group. Retinal vascular caliber and the development of hypertension: a meta-analysis of individual participant data. Journal of hypertension. 2014 Feb:32(2):207-15. doi: 10.1097/HJH.0b013e32836586f4. Epub [PubMed PMID: 24322199]
Level 1 (high-level) evidenceSun C, Wang JJ, Mackey DA, Wong TY. Retinal vascular caliber: systemic, environmental, and genetic associations. Survey of ophthalmology. 2009 Jan-Feb:54(1):74-95. doi: 10.1016/j.survophthal.2008.10.003. Epub [PubMed PMID: 19171211]
Level 3 (low-level) evidenceChen X, Liu L, Liu M, Huang X, Meng Y, She H, Zhao L, Zhang J, Zhang Y, Gu X, Qin X, Zhang Y, Li J, Xu X, Wang B, Hou FF, Tang G, Liao R, Huo Y, Li J, Yang L. Hypertensive Retinopathy and the Risk of Stroke Among Hypertensive Adults in China. Investigative ophthalmology & visual science. 2021 Jul 1:62(9):28. doi: 10.1167/iovs.62.9.28. Epub [PubMed PMID: 34283210]
Level 2 (mid-level) evidenceYip W, Sabanayagam C, Teo BW, Tay WT, Ikram MK, Tai ES, Chow KY, Wong TY, Cheung CY. Retinal microvascular abnormalities and risk of renal failure in Asian populations. PloS one. 2015:10(2):e0118076. doi: 10.1371/journal.pone.0118076. Epub 2015 Feb 6 [PubMed PMID: 25658337]
Wong TY, Cheung N, Islam FM, Klein R, Criqui MH, Cotch MF, Carr JJ, Klein BE, Sharrett AR. Relation of retinopathy to coronary artery calcification: the multi-ethnic study of atherosclerosis. American journal of epidemiology. 2008 Jan 1:167(1):51-8 [PubMed PMID: 17893402]
Cheung N, Bluemke DA, Klein R, Sharrett AR, Islam FM, Cotch MF, Klein BE, Criqui MH, Wong TY. Retinal arteriolar narrowing and left ventricular remodeling: the multi-ethnic study of atherosclerosis. Journal of the American College of Cardiology. 2007 Jul 3:50(1):48-55 [PubMed PMID: 17601545]
Liew G, Wong TY, Mitchell P, Cheung N, Wang JJ. Retinopathy predicts coronary heart disease mortality. Heart (British Cardiac Society). 2009 Mar:95(5):391-4. doi: 10.1136/hrt.2008.146670. Epub 2008 Aug 12 [PubMed PMID: 18697802]
Cheung CY, Ong YT, Ikram MK, Chen C, Wong TY. Retinal microvasculature in Alzheimer's disease. Journal of Alzheimer's disease : JAD. 2014:42 Suppl 4():S339-52. doi: 10.3233/JAD-141596. Epub [PubMed PMID: 25351108]
Heringa SM, Bouvy WH, van den Berg E, Moll AC, Kappelle LJ, Biessels GJ. Associations between retinal microvascular changes and dementia, cognitive functioning, and brain imaging abnormalities: a systematic review. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2013 Jul:33(7):983-95. doi: 10.1038/jcbfm.2013.58. Epub 2013 Apr 17 [PubMed PMID: 23591648]
Level 1 (high-level) evidenceWolz J, Audebert H, Laumeier I, Ahmadi M, Steinicke M, Ferse C, Michelson G. Telemedical assessment of optic nerve head and retina in patients after recent minor stroke or TIA. International ophthalmology. 2017 Feb:37(1):39-46. doi: 10.1007/s10792-016-0222-7. Epub 2016 Mar 26 [PubMed PMID: 27016938]
Do DV, Wang X, Vedula SS, Marrone M, Sleilati G, Hawkins BS, Frank RN. Blood pressure control for diabetic retinopathy. The Cochrane database of systematic reviews. 2015 Jan 31:1():CD006127. doi: 10.1002/14651858.CD006127.pub2. Epub 2015 Jan 31 [PubMed PMID: 25637717]
Level 1 (high-level) evidence