Perioperative Care for Metabolic and Bariatric Surgery
Perioperative Care for Metabolic and Bariatric Surgery
Introduction
Perioperative care for metabolic and bariatric surgery represents one of the most rapidly evolving fields in obesity medicine, with revolutionary changes in eligibility criteria and significant improvements in safety outcomes characterizing the current landscape. The 2022 American Society for Metabolic and Bariatric Surgery (ASMBS) and International Federation for the Surgery of Obesity and Metabolic Disorders (IFSO) guidelines marked a paradigm shift from the 1991 National Institutes of Health (NIH) criteria, expanding surgical eligibility to patients with a body mass index (BMI) of 35 kg/m² or greater, regardless of comorbidities and for those with a BMI of 30 to 34.9 kg/m² with coexisting metabolic disease.[1] This comprehensive approach to perioperative care encompasses patient selection, preoperative optimization, surgical technique selection, immediate postoperative management, and lifelong follow-up protocols that directly impact long-term success rates, with excess weight loss rates of 60% to 80% at 2 years after major procedures.
Modern bariatric surgery has achieved remarkable safety improvements, with overall mortality rates now 0.1% to 0.13% and major complication rates under 5% at accredited centers.[2] The integration of enhanced recovery protocols, standardized monitoring systems, and evidence-based nutritional management has significantly improved outcomes while highlighting persistent health equity challenges that affect access and outcomes across different populations. Understanding these comprehensive perioperative care protocols is essential for obesity medicine specialists who play crucial roles in patient selection, optimization, and long-term management of this increasingly important therapeutic intervention.
Function
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Function
Overview of Major Bariatric Procedures
Metabolic and bariatric surgery encompasses 4 primary procedures with distinct mechanisms, safety and efficacy profiles, and perioperative considerations (see Table 1).[3] Contemporary evidence suggests that sleeve gastrectomy and Roux-en-Y gastric bypass yield similar 5-year weight loss outcomes. In contrast, procedures with malabsorptive components achieve the most significant weight reduction and optimal resolution rates for diabetes.[4] The selection of specific procedures depends on patient factors, including BMI, comorbidities, anatomical considerations, and long-term compliance expectations.
Recent systematic reviews analyzing over 100,000 patients demonstrate clear effectiveness hierarchies among procedures. Biliopancreatic diversion with duodenal switch achieves the highest weight loss (70% to 80% excess weight loss), followed by Roux-en-Y (RYGB) gastric bypass (65% to 77% excess weight loss), sleeve gastrectomy (60% to 70% excess weight loss), and adjustable gastric banding (40% to 50% excess weight loss).[5] These effectiveness differences correlate directly with procedural complexity, complication rates, and nutritional monitoring requirements.
The emergence of newer procedures, particularly single anastomosis duodeno-ileostomy with sleeve gastrectomy (SADI-S), represents the most recent addition to the surgical armamentarium. SADI-S combines the restrictive benefits of sleeve gastrectomy with controlled malabsorption through a single anastomosis, offering a more straightforward surgical technique compared to traditional duodenal switch while maintaining superior metabolic outcomes compared to purely restrictive procedures.
Surgical procedure classification system
Restrictive procedures consist of sleeve gastrectomy and adjustable gastric band approaches. Combined restrictive-malabsorptive procedures include:
- RYGB
- Biliopancreatic diversion with duodenal switch (BPD-DS)
- SADI-S
Roux-en-Y Gastric Bypass
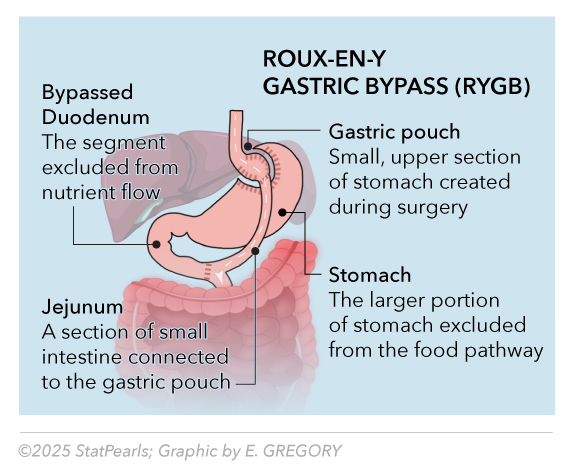
RYGB represents the historical "gold standard" for bariatric surgery, which combines restrictive and malabsorptive mechanisms through creation of a small gastric pouch and bypass of the duodenum and proximal jejunum. The procedure involves creating a 15- to 30-mL gastric pouch, performing a gastrojejunostomy with a 100- to 150-cm Roux limb, and then completing a jejunojejunostomy, thereby forming the "Y" configuration. See Image. Roux-en-Y Gastric Bypass. This anatomical reconstruction achieves multifactorial weight loss by reducing gastric capacity, altering gut hormone production, and decreasing caloric absorption.
Mechanistically, RYGB induces weight loss through multiple pathways, including mechanical restriction, malabsorption of approximately 20% to 30% of nutrients, and profound alterations in incretin hormone secretion.[6] The bypass of the duodenum and proximal jejunum results in the rapid delivery of nutrients to the distal small bowel, triggering the secretion of enhanced glucagon-like peptide-1 and peptide YY, while reducing ghrelin production. These hormonal changes contribute to sustained appetite suppression and improved insulin sensitivity independent of weight loss effects.
Sleeve Gastrectomy
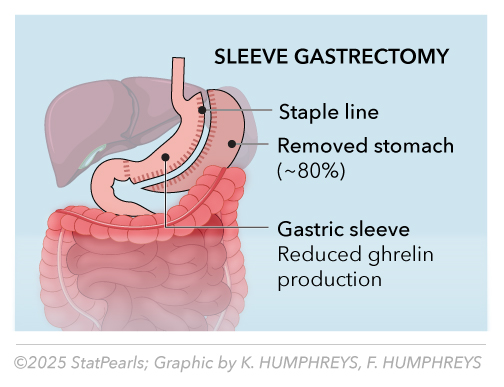
Sleeve gastrectomy is the most commonly performed bariatric procedure worldwide, accounting for approximately 65% of all bariatric surgeries.[7] The procedure involves removing 75% to 80% of the stomach along the greater curvature, creating a narrow gastric tube with a capacity of 60 to 100 mL (see Image. Sleeve Gastrectomy). This seemingly restrictive procedure produces complex metabolic effects through multiple mechanisms beyond simple volume restriction.
The primary mechanisms through which this procedure facilitates weight loss include a dramatic reduction in gastric capacity, accelerated gastric emptying, and a significant decrease in ghrelin production resulting from the removal of the gastric fundus.[8] Additional mechanisms involve altered gastric compliance, increased gastric pressure during eating, and modifications in the secretion of incretin hormones. Unlike RYGB, sleeve gastrectomy preserves normal intestinal anatomy while producing substantial metabolic benefits (see Table 2).
Biliopancreatic Diversion with Duodenal Switch
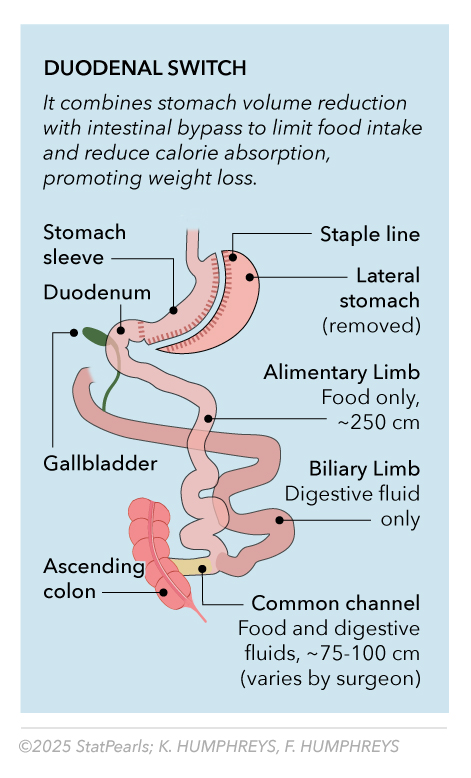
BPD-DS represents the most effective procedure for severe obesity and diabetes, particularly in patients with a BMI greater than 50 kg/m² or complex metabolic disease.[9] The procedure combines sleeve gastrectomy with extensive small bowel rearrangement, creating a 100 cm common channel and an alimentary limb of 150 to 200 cm in length. See Image. Duodenal Switch. This configuration produces the most significant caloric malabsorption of any commonly performed procedure.
However, BPD-DS carries the highest nutritional monitoring requirements and complication rates, including protein malnutrition risk, fat-soluble vitamin deficiencies, and higher surgical morbidity.[10] The procedure requires lifelong intensive nutritional monitoring and aggressive supplementation protocols every 3 to 6 months. Patient selection criteria emphasize high BMI (>50 kg/m²), complex diabetes, and demonstrated ability to maintain long-term follow-up.
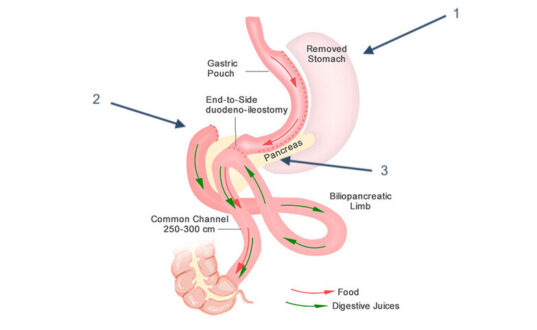
Single Anastomosis Duodeno-Ileostomy with Sleeve Gastrectomy
SADI-S represents the newest procedure endorsed by ASMBS, offering a simplified approach to malabsorptive surgery with a single anastomosis between the duodenum and the ileum after sleeve gastrectomy creation (see Image. Single Anastomosis Duodenal-Ileal Bypass With Sleeve Gastrectomy [SADI-S]). This procedure maintains the metabolic benefits of duodenal switch while reducing surgical complexity and operative time compared to traditional BPD-DS. Please refer to StatPearls' companion resource, "Obesity Surgery Indications and Contraindications," for additional information.
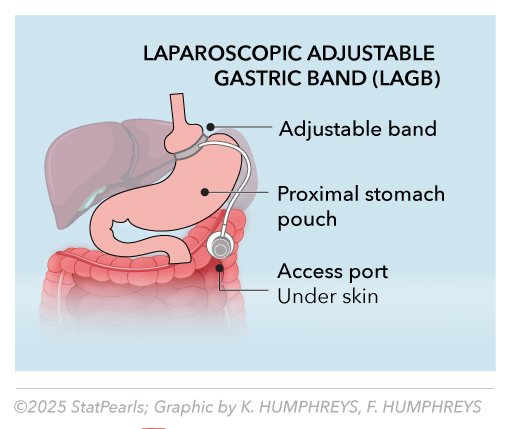
Laparoscopic Adjustable Gastric Band
The laparoscopic adjustable gastric band (LAGB) is a restrictive bariatric surgery technique that involves placing an inflatable silicone band around the proximal portion of the stomach, creating a small pouch that limits food intake and promotes early satiety. See Image. Laparoscopic Adjustable Gastric Band. The band's tightness can be adjusted via a subcutaneous access port connected by tubing, allowing for individualized control of gastric restriction over time. This minimally invasive approach preserves normal gastrointestinal anatomy, is reversible, and avoids intestinal bypass, but its long-term success depends heavily on patient adherence to dietary and lifestyle modifications. While LAGB offers lower perioperative complication rates compared to other bariatric procedures, potential drawbacks include band slippage, erosion, and the need for reoperation in some cases.
Table 1. Comparative Effectiveness and Characteristics of Major Bariatric Procedures
|
Procedure |
1-Year Estimated Weight Loss (%) |
5-Year Estimated Weight Loss (%) |
Diabetes Remission |
Mortality Rate |
Major Complications |
Malabsorption Level |
Ideal Candidate |
|
Sleeve Gastrectomy |
60%–70% |
50%–60% |
65%–71% |
0.1% |
5.8% |
None |
BMI 35–50, no severe gastroesophageal reflux disease (GERD) |
|
RYGB |
65%–77% |
50%–70% |
80%–98% |
0.13% |
8.0% |
+ (mild) |
BMI 35-55, diabetes, GERD |
|
BPD-DS |
70%–80% |
60%–70% |
90%–95% |
0.15%–0.2% |
12%–15% |
+++ (severe) |
BMI >50, severe diabetes |
|
SADI-S |
65%–75% |
55%–65% |
85%–90% |
0.1% |
8%–10% |
++ (moderate) |
BMI >45, high surgical risk |
|
Adjustable Gastric Band |
40%–50% |
35%–45% |
25% |
<0.1% |
15%–20% |
None |
BMI 30–40, low surgical risk |
Table 2. Malabsorptive Procedures Detailed Comparison
|
Feature |
RYGB |
BPD-DS |
SADI-S |
|
Malabsorption Level |
+ (mild) |
+++ (severe) |
++ (moderate) |
|
Restrictive Component |
Small pouch (15-30 mL) |
Sleeve gastrectomy |
Sleeve gastrectomy |
|
Common Channel |
None (Roux limb 100–150 cm) |
100 cm |
250–300 cm |
|
Intestine Bypassed (%) |
5%–10% |
75%–80% |
50%–60% |
|
Anastomoses |
2 (gastrojejunal, jejunojejunal) |
2 (gastroduodenal, duodenoileal) |
1 (duodenoileal) |
|
Operative Complexity |
Moderate |
High |
Moderate |
|
Nutritional Monitoring |
Every 6 months |
Every 3–6 months |
Every 3–6 months |
|
Protein Malnutrition Risk |
Low |
High |
Moderate |
|
Fat Malabsorption |
Minimal |
Severe |
Moderate |
|
Internal Hernia Risk |
3%–6% |
5%–10% |
Lower (single anastomosis) |
|
Revisional Options |
Good |
Limited |
Limited |
|
Pause and Reflect |
|
Issues of Concern
Patient Eligibility Criteria and Selection for Bariatric Surgery
Updated thresholds and indications
The 2022 ASMBS/IFSO guidelines revolutionized bariatric surgery eligibility, replacing the restrictive 1991 NIH criteria with evidence-based recommendations that expand access to surgery for BMI 35 kg/m² or greater, regardless of comorbidities.[1] This paradigm shift acknowledges that obesity constitutes a disease that warrants surgical intervention, irrespective of traditional comorbidity requirements. For patients with a BMI of 30 to 34.9 kg/m², surgery should be considered when metabolically focused medical treatment fails to achieve adequate disease control. This consideration is appropriate after failure of intensive medical therapy in patients with metabolic syndrome or type 2 diabetes (see Table 3).
Asian populations require adjusted BMI thresholds reflecting different obesity-related health risk profiles. Clinical obesity is recognized at a BMI of 25 kg/m² or higher, and surgical indication typically begins at a BMI of 27.5 kg/m² or greater, accompanied by metabolic disease. These adjustments acknowledge ethnic variations in visceral adiposity distribution and metabolic disease susceptibility at lower BMI levels in Asian populations. Therefore, surgery is not automatically offered at BMI 27.5 kg/m² or more without comorbidities in Asian populations.
Comorbidity considerations and metabolic surgery criteria
Type 2 diabetes represents a specific indication for metabolic surgery in patients with a BMI of 30 kg/m² or more (≥27.5 kg/m² in Asian populations) who demonstrate inadequate glycemic control despite optimal medical management.[11] The 2016 Diabetes Surgery Summit consensus established that metabolic surgery should be considered a standard treatment option for diabetes management, not merely a last-resort intervention.
Table 3. Current Eligibility Criteria by Organization
|
Organization |
BMI Threshold |
Comorbidity Requirements |
Special Populations |
|
American Society for Metabolic and Bariatric Surgery (ASMBS)/International Federation for the Surgery of Obesity and Metabolic Disorders (IFSO) 2022 |
≥35 kg/m² (any) |
None required |
Asian: ≥27.5 kg/m² |
| ASMBS/IFSO 2022 |
30–34.9 kg/m² |
Failed medical treatment |
|
|
American Diabetes Association (ADA) 2025 |
≥30 kg/m² |
Type 2 diabetes |
Asian: ≥27.5 kg/m² |
|
Medicare |
≥35 kg/m² |
Obesity-related comorbidity |
Facility certification required |
|
Endocrine Society |
≥35 kg/m² |
Comorbidities present |
Algorithm-based selection |
Age considerations and special populations
Pediatric and adolescent bariatric surgery requires specialized evaluation protocols that follow the 2018 ASMBS pediatric guidelines.[12] Eligibility includes a BMI equal to or greater than 140% of the 95th percentile at any age, or a BMI of 120% or more of the 95th percentile with a significant comorbidity. A comprehensive evaluation must assess physical and psychological maturity, personal motivation, family support systems, and the availability of specialized pediatric bariatric care teams.
Older adult patient evaluation focuses on individual assessment of operative risk versus potential benefit, with no specific upper age limit established in current guidelines.[13] Factors guiding management decisions include life expectancy, functional status, cognitive function, and the availability of social support. Enhanced preoperative cardiac and pulmonary evaluation becomes increasingly important with advancing age, though chronological age alone should not preclude surgical consideration.
Psychological evaluation requirements
A comprehensive psychological evaluation is a mandatory component of preoperative assessment, encompassing a clinical interview, objective testing, and behavioral assessment (see Table 4).[14] Standard evaluation components include assessment of eating behaviors and disorders, mental health conditions, substance use disorders, social support systems, and understanding of required lifestyle changes.
Table 4. Psychological Evaluation Components
| Clinical Interview Assessment |
|
| Objective Testing Instruments |
|
| Absolute Contraindications |
|
BDI-II, Beck Depression Inventory–II; BEDS, Binge Eating Disorder Screener; EDE-Q, Eating Disorder Examination Questionnaire; MMPI-2-RF, Minnesota Multiphasic Personality Inventory–2–Restructured Form; MMPI-3, Minnesota Multiphasic Personality Inventory–3; PHQ-9, Patient Health Questionnaire–9
Clinical Significance
Preoperative Optimization Protocols
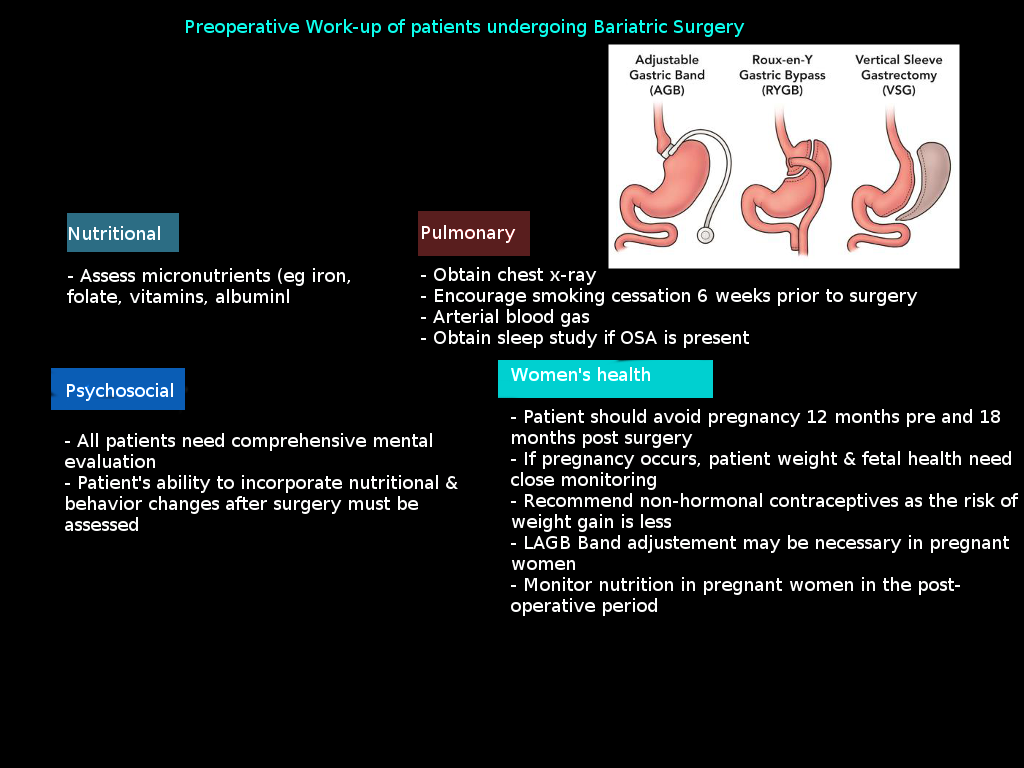
Nutritional assessment and deficiency correction
A comprehensive preoperative nutritional evaluation serves the dual purpose of identifying deficiencies and establishing baseline values for postoperative monitoring.[14] Standard assessments include vitamin D (25-hydroxyvitamin D), calcium with parathyroid hormone, iron studies (eg, ferritin, iron, total iron-binding capacity), folate, and vitamin B12 with methylmalonic acid (see Image: Preoperative Workup for Bariatric Surgery). Extended assessment for patients considering malabsorptive procedures should include vitamin B1 (thiamine diphosphate), as well as fat-soluble vitamins A, E, and K, and trace elements copper, zinc, and selenium. Preoperative deficiency rates demonstrate significant prevalence with vitamin D deficiency in 27% to 30% of patients, iron deficiency in 13% to 22%, and selenium deficiency in up to 39% of gastric bypass candidates.[15] Correction of identified deficiencies, when possible, improves postoperative outcomes and reduces early complications, although complete normalization may not always be achievable before surgery.
Medical optimization of comorbidities
Diabetes management optimization aims to achieve an HbA1c level of less than 8.5% whenever possible, with adjustments to hypoglycemic medications to prevent perioperative hypoglycemia.[16] An endocrinology consultation helps in complex cases involving insulin therapy, a recent diabetes diagnosis, or multiple complications. Patients should understand the expected improvement in diabetes management and potential adjustments to their medication that may be necessary following surgery. Cardiovascular optimization involves maintaining blood pressure control below 140/90 mmHg, performing an echocardiogram evaluation for patients with a history of heart disease, and conducting stress testing when clinically indicated. Beta-blocker therapy should be initiated when appropriate, and existing cardiac medications should be optimized to achieve the best possible outcomes.
Instructing patients to achieve smoking cessation 6 weeks or more before surgery represents a nonnegotiable requirement before bariatric surgery that is supported by significant evidence due to reduced wound complications, respiratory issues, and improved healing (see Table 5). Nicotine testing protocols ensure compliance, with positive tests typically requiring surgery postponement. Notably, the ASMBS position emphasizes that insurance-mandated preoperative weight loss is discriminatory and scientifically unfounded, with no evidence supporting mandatory weight loss for improved outcomes.[1] Such requirements may delay life-saving treatment and worsen existing comorbidities.
Table 5. Preoperative Optimization Timeline
|
6–12 weeks |
Smoking cessation |
Micronutrient correction |
Weight loss counseling |
|
4–8 weeks |
Cardiac/pulmonary evaluation |
Liver preparation diet |
Psychological evaluation |
|
2–4 weeks |
Medication adjustments |
Very low-calorie diet |
Support group initiation |
|
1 week |
Final medical clearance |
Clear liquid preparation |
Lifestyle education |
Perioperative Management and Care Coordination
Enhanced recovery protocol implementation
Enhanced recovery after surgery (ERAS) protocols have revolutionized perioperative bariatric care, incorporating evidence-based interventions that reduce complications, shorten hospital stays, and improve patient satisfaction (see Table 6).[17] These standardized protocols address preoperative preparation, intraoperative management, and postoperative care pathways through coordinated interprofessional approaches.
Table 6. ERAS Protocol Summary
|
ERAS Component |
Traditional Care | ERAS Protocol | Outcome Improvement |
|
Preoperative Fasting |
Nothing by mouth after midnight |
Only clear fluids until 2 hours before surgery |
Reduced insulin resistance, improved comfort |
|
Carbohydrate Loading |
Standard fasting |
Carbohydrate drink 2–3 hours preoperatively |
Reduced catabolism, faster recovery |
|
Mechanical Bowel Prep |
Routine use |
Avoided |
Reduced dehydration, electrolyte imbalance |
|
Prophylactic Antibiotics |
Variable timing |
Within 60 minutes of incision |
Reduced surgical site infections |
|
VTE Prophylaxis |
Postoperative start |
Preoperative initiation |
Reduced thromboembolism |
|
Anesthesia Approach |
High-dose opioids |
Multimodal, opioid-sparing |
Reduced postoperative nausea and vomiting, faster awakening |
|
Fluid Management |
Liberal fluid administration |
Goal-directed therapy |
Reduced edema, faster mobilization |
|
Postoperative Diet |
Clear liquids day 2–3 |
Clear liquids day 1 |
Faster gastrointestinal recovery, shorter length of stay |
|
Early Mobilization |
Bed rest for 24 hours |
Mobilization within 6 hours |
Reduced complications, faster recovery |
|
Pain Management |
Opioid-based |
Multimodal approach |
Reduced opioid adverse effects |
ERAS, enhanced recovery after surgery; VTE, venous thromboembolism
ERAS has been found to result in the following outcome improvements:
- Length of stay: Reduced by 0.5 to 1.5 days
- Complications: Reduced by 20% to 30%
- Patient satisfaction: Improved by 15% to 25%
- Readmission rates: Reduced by 10% to 20%
- Healthcare costs: Reduced by 10% to 15%
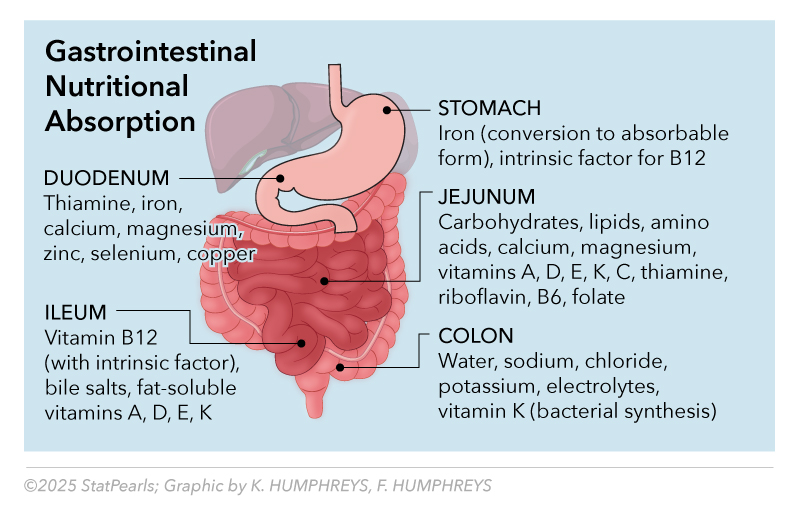
See Image. Gastrointestinal Nutritional Absorption.
Interprofessional team coordination
The core bariatric team comprises fellowship-trained surgeons, registered dietitians with expertise in bariatric care, mental health professionals, nurse coordinators, and medical specialists familiar with bariatric care.[18] Extended team members include anesthesiologists experienced in bariatric anesthesia, pharmacists knowledgeable about absorption changes, exercise physiologists, and social workers addressing insurance and social barriers.
Anesthesia considerations
Bariatric anesthesia requires specialized expertise addressing the unique physiological challenges of severe obesity, including complex airway management, positioning considerations, altered pharmacokinetics, and increased aspiration risk.[19] Airway management planning becomes critical given the increased incidence of difficult intubation in patients with obesity, particularly those with a BMI greater than 40 kg/m² or significant sleep apnea.
Immediate postoperative monitoring
Early postoperative surveillance focuses on recognition of significant complications, including anastomotic leak, bleeding, and pulmonary embolism.[20] Clinical signs of anastomotic leak may be subtle, with tachycardia, fever, and leukocytosis often preceding the development of obvious abdominal findings. Clinicians should maintain a low threshold for reexploration when sepsis signs develop, as 44% of leaks may not be detectable on initial computed tomography imaging.
The following critical signs may be early indications of patients who require emergent clinical evaluation:
- Anastomotic leak (0.2% incidence)
- Tachycardia (often the first sign)
- Fever and leukocytosis
- Abdominal pain (may be minimal)
- Anxiety or a feeling of impending doom
- Bleeding (0.7% incidence)
- Tachycardia and hypotension
- Decreased hematocrit
- Abdominal distension
- Hematemesis or melena
- Pulmonary embolism (1.17% incidence)
- Dyspnea and tachypnea
- Chest pain
- Tachycardia
- Oxygen desaturation
Long-term Postoperative Care and Monitoring
Standardized long-term monitoring is the cornerstone of successful bariatric surgery outcomes, with evidence-based protocols varying by procedure type based on the risk of malabsorption and nutritional complexity. Current guidelines recommend minimum follow-up at 3, 6, and 12 months during the first year, followed by annual visits thereafter. However, patients experiencing complications, weight regain, or nutritional deficiencies require more frequent monitoring.
Nutritional monitoring protocols
Comprehensive nutritional monitoring varies by procedure type and individual risk factors, with malabsorptive procedures requiring more extensive and frequent assessment (see Tables 8 and 9).[21] Standard monitoring includes complete blood count, comprehensive metabolic panel, vitamin B12/methylmalonic acid, folate, 25-hydroxyvitamin D, calcium, parathyroid hormone, iron studies, and albumin (see Table 7).
Table 7. Nutrient Absorption Sites and Clinical Significance
|
Nutrient |
Primary Absorption Site |
Secondary Sites |
Clinical Significance Post-surgery |
|
Iron (heme) |
Duodenum, proximal jejunum |
Throughout the small intestine |
Bypassed in RYGB, reduced in sleeve |
|
Iron (nonheme) |
Duodenum, proximal jejunum |
Ileum |
Requires gastric acid, bypassed in RYGB |
|
Calcium |
Duodenum, jejunum |
Throughout the small intestine |
Requires vitamin D, reduced absorption |
|
Vitamin B12 |
Terminal ileum |
None |
Requires an intrinsic factor, long body stores |
|
Folate |
Proximal jejunum |
Duodenum |
Reduced gastric acid affects absorption |
|
Fat-soluble Vitamins (A, D, E, K) |
Jejunum, ileum |
Throughout the small intestine |
Requires bile acids, fat malabsorption |
|
Thiamine |
Duodenum, jejunum |
Throughout the small intestine |
Bypassed in RYGB |
|
Zinc |
Duodenum, jejunum |
Throughout the small intestine |
Competes with copper, iron |
|
Copper |
Duodenum, jejunum |
Stomach |
Reduced gastric acid affects absorption |
RYGB, Roux-en-Y gastric bypass
Table 8. Procedure-Specific Nutritional Monitoring Schedule
|
Micronutrient |
Sleeve Gastrectomy |
RYGB |
BPD-DS |
Deficiency RisK |
|
Vitamin B12/MMA |
6–12 months |
3–6 months |
3–6 months |
19%–35% at 5 years |
|
25(OH) Vitamin D |
6–12 months |
3–6 months |
3–6 months |
11.5%–20.3% |
|
Iron/Ferritin |
3–6 months |
3–6 months |
3–6 months |
>30% at 5 years |
|
Folate |
6–12 months |
6 months |
3–6 months |
9%–39% average |
|
Thiamine |
If symptomatic |
6–12 months |
3–6 months |
18.3% first year |
|
Calcium/PTH |
6–12 months |
3–6 months |
3–6 months |
Variable |
|
Vitamin A |
If symptomatic |
12 months |
6 months |
9.4%–15.9% |
|
Copper/Zinc |
If symptomatic |
If indicated |
6–12 months |
Rare, but serious |
MMA, methylmalonic acid; OH, hydroxy; PTH, parathyroid hormone;
Note: To detect subclinical deficiencies, consider monitoring vitamin A and copper levels annually in patients who have undergone malabsorptive procedures, even if they are asymptomatic.
Table 9. Minimum Daily Vitamin and Mineral Intake After Bariatric Surgery
|
Nutrient |
Sleeve Gastrectomy |
RYGB |
BPD-DS |
General Population RDA |
Special Considerations |
|
Vitamin B12 |
350–500 µg |
1000 µg |
1000–3000 µg |
2.4 µg |
Injectable may be required |
|
Folate |
400–800 µg |
800–1000 µg |
800–1000 µg |
400 µg |
Higher needs in pregnancy |
|
Thiamine (B1) |
12 mg |
12–100 mg |
50–100 mg |
1.2 mg |
Higher needs with inadequate intake |
|
Iron |
18-27 mg |
45–60 mg |
60–100 mg |
18 mg (women) |
Do not consume at the same time as calcium supplements |
|
Calcium |
1200–1500 mg |
1200–2000 mg |
1800–2400 mg |
1000 mg |
Take with vitamin D |
|
Vitamin D |
3000–5000 IU |
3000–5000 IU |
2400–50,000 IU |
600–800 IU |
Monitor 25(OH)D levels, typical dose ranges from 3000-5000 IU/day depending on serum levels |
|
Vitamin A |
5000–10,000 IU |
5000–10,000 IU |
10,000 IU |
700–900 µg |
Consider annual monitoring in malabsorptive procedures even if asymptomatic |
|
Vitamin E |
15 mg |
15 mg |
400 IU |
15 mg |
Higher needs with BPD-DS |
|
Vitamin K |
90–120 µg |
90–120 µg |
300 µg |
90–120 µg |
Monitor PT/INR |
|
Zinc |
8–11 mg |
8–22 mg |
16–22 mg |
8–11 mg |
Separate from iron and calcium |
|
Copper |
2 mg |
2 mg |
2 mg |
900 µg |
Consider annual monitoring in malabsorptive procedures, monitor with high zinc intake |
PT/INR, prothrombin time/international normalized ratio; RDA, recommended dietary allowance
Weight regain management strategies
Weight regain affects 20% to 30% of patients and typically begins 12 to 18 months post-surgery, with significant regain defined as more than 15% of the total weight lost from the nadir.[22] Early recognition and intervention provide the best outcomes, requiring systematic monitoring of weight trends and implementation of structured intervention programs when regain exceeds 10% from nadir (see Table 10)
Table 10. Weight Regain Management Algorithm
|
Regain Level From Nadir |
Timeline |
Intervention Strategy |
Monitoring Frequency |
|
<10% |
Ongoing |
Lifestyle counseling, diet review |
Every 6 months |
|
10%–15% |
Intensive program |
Behavioral therapy, nutrition counseling |
Every 3 months |
|
>15% |
Consider pharmacotherapy |
GLP-1 agonists, endoscopic options |
Monthly initially |
|
>25% |
Evaluate for revision |
Surgical consultation, comprehensive evaluation |
Monthly |
GLP-1, glucagon-like peptide-1
Pregnancy planning and management
Pregnancy timing recommendations suggest waiting 12 to 18 months post-surgery for weight stabilization and nutritional optimization.[23][24] Enhanced nutritional monitoring during pregnancy includes a first-trimester baseline assessment, a second-trimester comprehensive evaluation, and third-trimester continued monitoring, with an emphasis on iron, B12, folate, and vitamin D status.
Complications Recognition and Management
Early postoperative complications
Early postoperative complications that occur within 30 days develop in 5.8% of sleeve gastrectomy patients and 8% of patients undergoing RYGB, with overall mortality rates of 0.1 to 0.13% representing significant improvements from historical rates.[7] Recognition and rapid intervention for major complications directly impact outcomes, requiring systematic surveillance protocols and low thresholds for diagnostic evaluation when concerning signs develop (see Tables 11 and 16).
Late postoperative complications
A late postoperative complication, occurring 30 days or more postoperatively, includes anastomotic stricture, which develops in 4.7% to 16% of patients undergoing RYGB at the gastrojejunal anastomosis. Strictures typically present around 8 weeks postoperatively with progressive intolerance to solid foods, vomiting, and signs of dehydration.[25] Management involves serial endoscopic balloon dilation, with surgical revision considered if 3 to 4 dilation attempts fail to achieve sustained improvement.
Table 11. Ulcer Versus Stricture
|
Feature |
Marginal Ulcer |
Anastomotic Stricture |
|
Timing |
3–24 months postoperatively |
6–12 weeks postoperatively |
|
Primary Symptoms |
Epigastric pain, food intolerance |
Progressive dysphagia, vomiting |
|
Pain Character |
Burning, gnawing pain |
Cramping, postprandial |
|
Food Tolerance |
Variable, worse with spicy foods |
Progressive solid intolerance |
|
Endoscopic Findings |
Mucosal break, inflammation |
Narrowed anastomosis, scarring |
|
Treatment |
Proton pump inhibitors, smoking cessation, Helicobacter pylori eradication |
Serial balloon dilation |
|
Recurrence |
High, if risk factors persist |
10%–30% after dilation |
Table 12. Causes of Hypoglycemia After RYGB
|
Cause |
Mechanism |
Timing |
Symptoms |
Diagnostic Tests |
Treatment |
|
Late Dumping Syndrome |
Reactive hyperinsulinemia |
1–3 hours postmeal |
Diaphoresis, palpitations, confusion |
Glucose <70 mg/dL, high insulin |
Dietary modification, acarbose |
|
Nesidioblastosis |
Beta-cell hyperplasia |
1–3 hours postmeal |
Severe hypoglycemia, neuroglycopenia |
Glucose <50 mg/dL, high insulin, high C-peptide |
Medical management, rarely pancreatectomy |
|
Insulinoma |
Insulin-secreting tumor |
Random timing |
Whipple triad of symptoms |
Glucose <50 mg/dL, high insulin, high C-peptide |
Surgical resection |
|
Alimentary Hypoglycemia |
Rapid glucose absorption |
30–90 minutes post-meal |
Mild to moderate symptoms |
Post-meal glucose curve |
Dietary modification |
Nutritional emergency recognition
Thiamine deficiency represents the most urgent nutritional emergency, capable of developing within 9 to 18 days during periods of poor intake or persistent vomiting (see Tables 12 and 13).[26] Wernicke-Korsakoff syndrome presents with confusion, ataxia, eye movement abnormalities, and memory impairment, with potential for irreversible neurological damage if treatment is delayed. Several other conditions may occur due to nutritional deficiencies (see Tables 14 and 15).
Table 13. B Vitamin Deficiencies
|
Vitamin |
Deficiency Name |
Early Symptoms |
Advanced Symptoms |
Neurological Findings |
Post-surgery Timeline |
|
B1 (Thiamine) |
Beriberi/Wernicke-Korsakoff |
Fatigue, irritability, poor concentration |
Confusion, ataxia, ophthalmoplegia |
Memory loss, confabulation, peripheral neuropathy |
9–18 days with poor intake |
|
B2 (Riboflavin) |
Ariboflavinosis |
Angular cheilitis, glossitis |
Seborrheic dermatitis, corneal vascularization |
Peripheral neuropathy (rare) |
6–12 months |
|
B6 (Pyridoxine) |
Pyridoxine deficiency |
Seborrheic dermatitis, glossitis |
Peripheral neuropathy, seizures |
Distal sensory neuropathy, cognitive impairment |
6–24 months |
|
B9 (Folate) |
Folate deficiency |
Fatigue, weakness, pale skin |
Megaloblastic anemia, glossitis |
Depression, cognitive impairment |
6–12 months |
|
B12 (Cobalamin) |
Pernicious anemia |
Fatigue, weakness, pale skin |
Megaloblastic anemia, glossitis |
Subacute combined degeneration, peripheral neuropathy |
2–5 years (with adequate pre-surgery body stores) |
Table 14. Micronutrient Deficiencies Clinical Features
|
Micronutrient |
Early Symptoms |
Advanced Symptoms |
Neurological Examination |
Physical Examination |
Laboratory Findings |
|
Iron |
|
|
|
|
|
|
Calcium |
|
|
|
|
|
|
Vitamin D |
|
|
|
|
|
|
Zinc |
|
|
|
|
|
|
Copper |
|
|
|
|
|
|
Selenium |
|
|
|
|
|
|
Vitamin A |
|
|
|
|
|
|
Vitamin E |
|
|
|
|
|
|
Vitamin K |
|
|
Intracranial bleeding (rare) |
|
|
Hgb/Hct, hemoglobin/hematocrit; PT/INR, prothrombin time/international normalized ratio
Table 15. Neurological Findings in Nutritional Deficiencies
|
Condition |
Primary Deficiency |
Neurological Presentation |
Diagnostic Tests |
Treatment |
Reversibility |
|
Wernicke-Korsakoff Syndrome |
Thiamine (B1) |
|
|
High-dose thiamine 200-300 mg daily |
Partially reversible if caught early |
|
Subacute Combined Degeneration |
Vitamin B12 |
|
|
B12 injections 1000 µg weekly |
Partially reversible |
|
Peripheral Neuropathy |
B1, B6, B12, Copper |
|
|
Specific vitamin replacement |
Variable reversibility |
|
Myelopathy |
Copper, B12 |
|
|
Copper/B12 supplementation |
Limited reversibility |
|
Optic Neuropathy |
B12, Folate |
|
|
|
Partially reversible |
|
Seizures |
Thiamine, B6, Magnesium |
Generalized or focal seizures |
|
|
Usually reversible |
|
Cognitive Impairment |
B12, Folate, Thiamine |
|
|
Vitamin replacement |
Variable reversibility |
EEG, electroencephalogram; MMA, methylmalonic acid; MRI, magnetic resonance imaging
Table 16. Complication Rates by Procedure Type
|
Complication |
Sleeve Gastrectomy |
RYGB |
BPD-DS |
Management Priority |
|
Leaking/Bleeding |
1.21%/0.7% |
1.14%/0.7% |
2%–3% /1% |
Immediate surgical evaluation |
|
Stricture |
≤0.5% |
4.7%–16% |
5%–8% |
Endoscopic dilation |
|
GERD/Reflux |
15%–20% |
Improved |
Rare |
Medical management |
|
Dumping Syndrome |
Rare |
70%–80% (symptoms) |
90% (symptoms) |
Dietary modification |
|
Internal Hernia |
None |
3%–16% |
5%–10% |
Surgical repair |
|
Reoperation Rate |
9.1% |
3%–20% |
15%–20% |
Procedure-specific |
Emergency presentation algorithms
Acute abdominal pain in postbariatric patients requires systematic evaluation considering procedure-specific complications.[27] For patients undergoing RYGB, internal hernia represents a primary concern requiring urgent surgical consultation even with negative initial imaging. Computed tomography (CT) findings may be subtle in the early stages of internal hernia, and clinical suspicion should drive management decisions.
Emergency response protocols include the following algorithms:
- Persistent vomiting protocol
- Assessment of hydration and electrolyte status
- Upper gastrointestinal series or endoscopy
- Nutritional assessment (e.g., thiamine is a priority.)
- Antiemetic therapy
- Consideration of intravenous (IV) nutrition for prolonged symptoms
- Acute abdominal pain algorithm
- Vital signs and hemodynamic assessment
- Complete blood count and basic metabolic panel
- CT abdomen/pelvis with contrast
- Immediate surgical consultation
- Nothing by mouth (NPO) status and IV hydration
- Consideration of exploratory surgery
- Neurological emergency response
- Immediate thiamine replacement with 200 to 300 mg IV or orally
- Vitamin B12, folate, and copper levels
- Neurological examination with documentation
- Consider a brain MRI
- High-dose vitamin supplementation
- Neurology consultation
|
Pause and Reflect |
|
Other Issues
Disparities in Surgical Access
Significant disparities exist in bariatric surgery access across racial, ethnic, and socioeconomic lines, with only 1% to 2% of eligible patients receiving surgery annually despite the availability of effective procedures.[28] White individuals comprise 54.3% of patients undergoing surgery despite higher obesity rates in minority populations, highlighting substantial access barriers that perpetuate health inequities. Medicare-imposed access restrictions to centers of excellence have been associated with decreased surgery rates among non-White individuals, suggesting that well-intentioned quality measures may inadvertently create access barriers for vulnerable populations.[29] Geographic variations in surgical availability particularly affect individuals residing in rural and underserved communities, where bariatric surgery centers may be hundreds of miles away.
Outcome Disparities by Demographics
Black patients experience significantly worse outcomes, including higher odds ratios for 30-day readmissions (eg, 1.39), grade 3 to 5 complications, postoperative pulmonary embolism, and renal complications.[13] Weight loss outcomes demonstrate 25% to 35% less weight loss in Black individuals compared to White individuals, suggesting complex interactions between genetic, social, and healthcare factors. Contributing factors are likely multifactorial, including differences in preoperative health status, socioeconomic factors, quality of healthcare access, and potential clinician bias in perioperative care delivery.[13] Quality improvement initiatives must address these disparities through targeted interventions, cultural competency training, and systematic monitoring of outcomes by demographic characteristics.
Healthcare System and Policy Interventions
Insurance coverage expansion represents the most impactful intervention, with state Medicaid expansion associated with improved access, particularly for minority and low-income populations.[30] Improving geographic access requires the use of telemedicine, mobile clinic programs, and partnerships between major medical centers and community hospitals (see Table 17).
Table 17. Health Equity Intervention Strategies
|
Insurance Coverage |
Medicaid expansion, coverage standardization |
High |
1–2 years |
|
Geographic Access |
Telemedicine, hub-and-spoke models |
Medium |
2–3 years |
|
Clinician Training |
Cultural competency, bias recognition |
Medium |
1–2 years |
|
Community Outreach |
Patient navigation, community partnerships |
High |
6–12 months |
|
Quality Monitoring |
Disparities surveillance, targeted improvement |
High |
6–12 months |
Future Directions and Emerging Evidence
Technological innovations and outcomes
Robotic-assisted bariatric surgery has demonstrated a sharp increase in utilization, reaching 15.2% of procedures by 2022, with improved safety profiles over time and comparable outcomes to those of laparoscopic approaches. The robotic platform offers potential advantages for patients with a high BMI, complex revision cases, and situations requiring extensive dissection; however, operative times remain longer, and cost considerations persist. Artificial intelligence and machine learning applications are emerging in patient selection, outcome prediction, and the prevention of complications.[31] The American College of Surgeons Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program (MBSAQIP) risk calculators now provide accurate 30-day risk prediction and 1-year outcome forecasting, enabling personalized informed consent and risk stratification for optimal patient selection and perioperative management.
Pharmacotherapy integration
GLP-1 receptor agonists represent a paradigm shift in the integration of bariatric surgery, with demonstrated effectiveness in managing postsurgical weight regain, achieving 5% to 15% additional weight loss when combined with lifestyle interventions.[32] Perioperative use guidance from the recent 2025 American Diabetes Association guidelines suggests potential roles in preoperative optimization and postoperative weight maintenance. Cost-effectiveness analyses favor surgery over long-term pharmacotherapy for most patients, though combination approaches may optimize outcomes for specific patient populations.[32] Future medication development focuses on multireceptor agonists, oral formulations, and longer-acting preparations that may complement surgical interventions or provide nonsurgical alternatives for appropriate patients.
Quality Improvement and Standardization
Patient-reported outcome Measures (PROMs) implementation began in 2019 through MBSAQIP, incorporating the PROMIS-10 Global Health Survey, the Obesity-Related Problems scale, and the Obesity and Weight-Loss Quality of Life Instrument.[33] These measures provide a standardized assessment of functional outcomes beyond traditional weight loss and complication metrics. Long-term outcome tracking, extending beyond 30 days, remains a challenge yet is essential for comprehensive quality assessment.[34] Registry development for extended follow-up and integration with electronic health records could improve long-term surveillance capabilities while reducing administrative burden on patients and clinicians.
Summary and Clinical Pearls
Perioperative care for metabolic and bariatric surgery has evolved into a sophisticated, evidence-based discipline combining expanded surgical eligibility, enhanced safety protocols, and comprehensive long-term management. The 2022 ASMBS/IFSO guidelines' paradigm shift toward a BMI of 35 kg/m² or greater for bariatric surgery, regardless of comorbidities, coupled with consideration of surgery for BMI 30 to 34.9 kg/m² with metabolic disease, represents a fundamental change in patient selection that acknowledges obesity as a disease warranting surgical intervention. Current evidence demonstrates remarkable safety improvements, with overall mortality rates ranging from 0.1% to 0.13% and major complication rates of less than 5% at accredited centers. Effectiveness outcomes show sustained 60% to 80% excess weight loss at 2 years across major procedures. Integrating enhanced recovery protocols, standardized monitoring systems, and evidence-based nutritional management has transformed perioperative care while highlighting the critical importance of lifelong follow-up and monitoring for optimal long-term outcomes.
Persistent health equity challenges require systematic attention, as significant disparities in access and outcomes across racial, ethnic, and socioeconomic lines undermine the potential population health impact of these effective interventions. The 25% to 35% difference in weight loss outcomes between racial groups, combined with substantial access barriers affecting eligible populations, demands targeted interventions, including insurance coverage expansion, improved geographic access, and quality monitoring that focuses on equity measures. Future directions emphasize the integration of technology, combination approaches to pharmacotherapy, and enhanced quality measurement through patient-reported outcomes, as well as the development of long-term registries.
The evolution toward personalized medicine approaches, incorporating artificial intelligence for risk prediction and outcome optimization, promises continued improvements in both safety and effectiveness while potentially addressing some current equity challenges through improved access and care coordination. Understanding these comprehensive perioperative care protocols provides essential knowledge for effective patient counseling, appropriate referral decision-making, and optimal long-term comanagement of bariatric surgery patients for obesity medicine specialists preparing for the American Board of Obesity Medicine examinations. The complexity of modern bariatric care requires interprofessional expertise, evidence-based protocols, and sustained commitment to individual patient care and population health improvement through continued advocacy for equitable access to these life-saving interventions.
American Board of Obesity Medicine Certification Examination Strategy Tips
The following topics are the most commonly tested on the ABOM certification examination:
- BMI eligibility criteria (especially Asian populations)
- Thiamine deficiency recognition and emergency treatment
- Complication rates by procedure type
- Iron absorption and monitoring post-RYGB
- Weight regain management algorithms
- Pregnancy recommendations and monitoring
- Health equity disparities in access and outcomes
- MBSAQIP accreditation importance
The following are key points that clinicians should keep in mind for the ABOM examination:
- Updated eligibility criteria: BMI 35 kg/m² and higher, regardless of comorbidities; BMI 30 to 34.9 kg/m² with metabolic disease
- Safety improvements: Mortality rates 0.1% to 0.13%, major complications <5% at accredited centers
- Procedure selection: Based on BMI, comorbidities, malabsorption tolerance, and long-term compliance
- Nutritional monitoring: Varies by procedure type, with malabsorptive procedures requiring more intensive surveillance
- Complication recognition: Early detection of leaks, bleeding, and nutritional emergencies is critical for outcomes
- Long-term management: Weight regain affects 20% to 30%; combination medical and surgical approaches are emerging
- Health equity: Significant disparities require targeted interventions and systematic monitoring
- Future integration: Technology, pharmacotherapy, and personalized medicine approaches are advancing rapidly
Enhancing Healthcare Team Outcomes
Metabolic and bariatric surgery has emerged as a cornerstone in obesity medicine, with the 2022 ASMBS/IFSO guidelines expanding eligibility criteria and redefining standards of care. Advances in perioperative management, including preoperative optimization, surgical procedure selection, enhanced recovery protocols, and structured long-term follow-up, have transformed safety and outcomes. Modern bariatric surgery now achieves mortality rates as low as 0.1% to 0.13% and sustained excess weight loss of 60% to 80% at 2 years for major procedures, underscoring the importance of evidence-based protocols and interprofessional collaboration in optimizing patient outcomes.
Effective perioperative care requires the integration of diverse clinical skills and the coordination of effective strategies to ensure optimal patient outcomes. Physicians and advanced practitioners lead patient selection, risk assessment, and surgical planning, while nurses provide critical perioperative monitoring and patient education. Pharmacists contribute by managing medication adjustments and preventing nutritional deficiencies. Registered dietitians and nutritionists play a central role in nutritional assessment, individualized dietary planning, and long-term support for sustainable behavior change. General clinicians and allied health professionals extend care through long-term follow-up, lifestyle support, and the recognition of complications. Seamless interprofessional communication and collaborative care coordination enhance patient safety, promote equity in access, and improve long-term outcomes while strengthening team performance in the delivery of obesity care.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Eisenberg D, Shikora SA, Aarts E, Aminian A, Angrisani L, Cohen RV, De Luca M, Faria SL, Goodpaster KPS, Haddad A, Himpens JM, Kow L, Kurian M, Loi K, Mahawar K, Nimeri A, O'Kane M, Papasavas PK, Ponce J, Pratt JSA, Rogers AM, Steele KE, Suter M, Kothari SN. 2022 American Society for Metabolic and Bariatric Surgery (ASMBS) and International Federation for the Surgery of Obesity and Metabolic Disorders (IFSO): Indications for Metabolic and Bariatric Surgery. Surgery for obesity and related diseases : official journal of the American Society for Bariatric Surgery. 2022 Dec:18(12):1345-1356. doi: 10.1016/j.soard.2022.08.013. Epub 2022 Oct 21 [PubMed PMID: 36280539]
English WJ, DeMaria EJ, Brethauer SA, Mattar SG, Rosenthal RJ, Morton JM. American Society for Metabolic and Bariatric Surgery estimation of metabolic and bariatric procedures performed in the United States in 2016. Surgery for obesity and related diseases : official journal of the American Society for Bariatric Surgery. 2018 Mar:14(3):259-263. doi: 10.1016/j.soard.2017.12.013. Epub 2017 Dec 16 [PubMed PMID: 29370995]
Malik A, Malik MI, Javaid S, Qureshi S, Nadir A. Comparative effectiveness of metabolic and bariatric surgeries: a network meta-analysis. International journal of obesity (2005). 2025 Jan:49(1):54-62. doi: 10.1038/s41366-024-01648-7. Epub 2024 Oct 14 [PubMed PMID: 39397157]
Level 1 (high-level) evidenceArterburn DE, Telem DA, Kushner RF, Courcoulas AP. Benefits and Risks of Bariatric Surgery in Adults: A Review. JAMA. 2020 Sep 1:324(9):879-887. doi: 10.1001/jama.2020.12567. Epub [PubMed PMID: 32870301]
Syn NL, Cummings DE, Wang LZ, Lin DJ, Zhao JJ, Loh M, Koh ZJ, Chew CA, Loo YE, Tai BC, Kim G, So JB, Kaplan LM, Dixon JB, Shabbir A. Association of metabolic-bariatric surgery with long-term survival in adults with and without diabetes: a one-stage meta-analysis of matched cohort and prospective controlled studies with 174 772 participants. Lancet (London, England). 2021 May 15:397(10287):1830-1841. doi: 10.1016/S0140-6736(21)00591-2. Epub 2021 May 6 [PubMed PMID: 33965067]
Level 1 (high-level) evidenceMingrone G, Panunzi S, De Gaetano A, Guidone C, Iaconelli A, Nanni G, Castagneto M, Bornstein S, Rubino F. Bariatric-metabolic surgery versus conventional medical treatment in obese patients with type 2 diabetes: 5 year follow-up of an open-label, single-centre, randomised controlled trial. Lancet (London, England). 2015 Sep 5:386(9997):964-73. doi: 10.1016/S0140-6736(15)00075-6. Epub [PubMed PMID: 26369473]
Level 1 (high-level) evidenceChang SH, Stoll CR, Song J, Varela JE, Eagon CJ, Colditz GA. The effectiveness and risks of bariatric surgery: an updated systematic review and meta-analysis, 2003-2012. JAMA surgery. 2014 Mar:149(3):275-87. doi: 10.1001/jamasurg.2013.3654. Epub [PubMed PMID: 24352617]
Level 1 (high-level) evidenceO'Brien PE, Hindle A, Brennan L, Skinner S, Burton P, Smith A, Crosthwaite G, Brown W. Long-Term Outcomes After Bariatric Surgery: a Systematic Review and Meta-analysis of Weight Loss at 10 or More Years for All Bariatric Procedures and a Single-Centre Review of 20-Year Outcomes After Adjustable Gastric Banding. Obesity surgery. 2019 Jan:29(1):3-14. doi: 10.1007/s11695-018-3525-0. Epub [PubMed PMID: 30293134]
Level 1 (high-level) evidenceMagro DO, Geloneze B, Delfini R, Pareja BC, Callejas F, Pareja JC. Long-term weight regain after gastric bypass: a 5-year prospective study. Obesity surgery. 2008 Jun:18(6):648-51. doi: 10.1007/s11695-007-9265-1. Epub 2008 Apr 8 [PubMed PMID: 18392907]
Picot J, Jones J, Colquitt JL, Gospodarevskaya E, Loveman E, Baxter L, Clegg AJ. The clinical effectiveness and cost-effectiveness of bariatric (weight loss) surgery for obesity: a systematic review and economic evaluation. Health technology assessment (Winchester, England). 2009 Sep:13(41):1-190, 215-357, iii-iv. doi: 10.3310/hta13410. Epub [PubMed PMID: 19726018]
Level 1 (high-level) evidenceRubino F, Nathan DM, Eckel RH, Schauer PR, Alberti KG, Zimmet PZ, Del Prato S, Ji L, Sadikot SM, Herman WH, Amiel SA, Kaplan LM, Taroncher-Oldenburg G, Cummings DE, Delegates of the 2nd Diabetes Surgery Summit. Metabolic Surgery in the Treatment Algorithm for Type 2 Diabetes: A Joint Statement by International Diabetes Organizations. Diabetes care. 2016 Jun:39(6):861-77. doi: 10.2337/dc16-0236. Epub [PubMed PMID: 27222544]
Stanford FC, Jones DB, Schneider BE, Blackburn GL, Apovian CM, Hess DT, Chiodi S, Robert S, Bourland AC, Wee CC. Patient race and the likelihood of undergoing bariatric surgery among patients seeking surgery. Surgical endoscopy. 2015 Sep:29(9):2794-9. doi: 10.1007/s00464-014-4014-8. Epub 2014 Dec 10 [PubMed PMID: 25492453]
Arterburn DE, Olsen MK, Smith VA, Livingston EH, Van Scoyoc L, Yancy WS Jr, Eid G, Weidenbacher H, Maciejewski ML. Association between bariatric surgery and long-term survival. JAMA. 2015 Jan 6:313(1):62-70. doi: 10.1001/jama.2014.16968. Epub [PubMed PMID: 25562267]
Mechanick JI, Apovian C, Brethauer S, Timothy Garvey W, Joffe AM, Kim J, Kushner RF, Lindquist R, Pessah-Pollack R, Seger J, Urman RD, Adams S, Cleek JB, Correa R, Figaro MK, Flanders K, Grams J, Hurley DL, Kothari S, Seger MV, Still CD. Clinical Practice Guidelines for the Perioperative Nutrition, Metabolic, and Nonsurgical Support of Patients Undergoing Bariatric Procedures - 2019 Update: Cosponsored by American Association of Clinical Endocrinologists/American College of Endocrinology, The Obesity Society, American Society for Metabolic and Bariatric Surgery, Obesity Medicine Association, and American Society of Anesthesiologists. Obesity (Silver Spring, Md.). 2020 Apr:28(4):O1-O58. doi: 10.1002/oby.22719. Epub [PubMed PMID: 32202076]
Level 1 (high-level) evidenceBen-Porat T, Elazary R, Yuval JB, Wieder A, Khalaileh A, Weiss R. Nutritional deficiencies after sleeve gastrectomy: can they be predicted preoperatively? Surgery for obesity and related diseases : official journal of the American Society for Bariatric Surgery. 2015 Sep-Oct:11(5):1029-36. doi: 10.1016/j.soard.2015.02.018. Epub 2015 Feb 25 [PubMed PMID: 25857443]
Kheniser K, Saxon DR, Kashyap SR. Long-Term Weight Loss Strategies for Obesity. The Journal of clinical endocrinology and metabolism. 2021 Jun 16:106(7):1854-1866. doi: 10.1210/clinem/dgab091. Epub [PubMed PMID: 33595666]
De Luca M, Shikora S, Eisenberg D, Angrisani L, Parmar C, Alqahtani A, Aminian A, Aarts E, Brown W, Cohen RV, Di Lorenzo N, Faria SL, Goodpaster KPS, Haddad A, Herrera M, Rosenthal R, Himpens J, Iossa A, Kermansaravi M, Kow L, Kurian M, Chiappetta S, LaMasters T, Mahawar K, Merola G, Nimeri A, O'Kane M, Papasavas P, Piatto G, Ponce J, Prager G, Pratt JSA, Rogers AM, Salminen P, Steele KE, Suter M, Tolone S, Vitiello A, Zappa M, Kothari SN. Scientific Evidence for the Updated Guidelines on Indications for Metabolic and Bariatric Surgery (IFSO/ASMBS). Obesity surgery. 2024 Nov:34(11):3963-4096. doi: 10.1007/s11695-024-07370-7. Epub 2024 Sep 25 [PubMed PMID: 39320627]
Stenberg E, Szabo E, Agren G, Näslund E, Boman L, Bylund A, Hedenbro J, Laurenius A, Lundegårdh G, Lönroth H, Möller P, Sundbom M, Ottosson J, Näslund I, Scandinavian Obesity Surgery Registry Study Group. Early complications after laparoscopic gastric bypass surgery: results from the Scandinavian Obesity Surgery Registry. Annals of surgery. 2014 Dec:260(6):1040-7. doi: 10.1097/SLA.0000000000000431. Epub [PubMed PMID: 24374541]
Stenberg E, Cao Y, Marsk R, Sundbom M, Jernberg T, Näslund E. Association between metabolic surgery and cardiovascular outcome in patients with hypertension: A nationwide matched cohort study. PLoS medicine. 2020 Sep:17(9):e1003307. doi: 10.1371/journal.pmed.1003307. Epub 2020 Sep 15 [PubMed PMID: 32931494]
Cardoso L, Rodrigues D, Gomes L, Carrilho F. Short- and long-term mortality after bariatric surgery: A systematic review and meta-analysis. Diabetes, obesity & metabolism. 2017 Sep:19(9):1223-1232. doi: 10.1111/dom.12922. Epub 2017 May 31 [PubMed PMID: 28244626]
Level 1 (high-level) evidenceParrott J, Frank L, Rabena R, Craggs-Dino L, Isom KA, Greiman L. American Society for Metabolic and Bariatric Surgery Integrated Health Nutritional Guidelines for the Surgical Weight Loss Patient 2016 Update: Micronutrients. Surgery for obesity and related diseases : official journal of the American Society for Bariatric Surgery. 2017 May:13(5):727-741. doi: 10.1016/j.soard.2016.12.018. Epub 2017 Jan 19 [PubMed PMID: 28392254]
Cooper TC, Simmons EB, Webb K, Burns JL, Kushner RF. Trends in Weight Regain Following Roux-en-Y Gastric Bypass (RYGB) Bariatric Surgery. Obesity surgery. 2015 Aug:25(8):1474-81. doi: 10.1007/s11695-014-1560-z. Epub [PubMed PMID: 25595383]
Espinal M, Hairston JC, Kominiarek MA. Updates in Pregnancy in Individuals with Obesity and After Bariatric Surgery. Obstetrics and gynecology clinics of North America. 2025 Sep:52(3):413-430. doi: 10.1016/j.ogc.2025.05.001. Epub 2025 Jun 9 [PubMed PMID: 40769654]
Rottenstreich A, Graham Y. Implications of Metabolic Bariatric Surgery on Reproductive Health. Journal of clinical medicine. 2025 Aug 2:14(15):. doi: 10.3390/jcm14155446. Epub 2025 Aug 2 [PubMed PMID: 40807068]
Welbourn R, Pournaras D. Bariatric surgery: a cost-effective intervention for morbid obesity; functional and nutritional outcomes. The Proceedings of the Nutrition Society. 2010 Nov:69(4):528-35. doi: 10.1017/S0029665110001515. Epub 2010 May 4 [PubMed PMID: 20441667]
Ganipisetti VM, Naha S. Bariatric Surgery Malnutrition Complications. StatPearls. 2025 Jan:(): [PubMed PMID: 37276282]
Kassir R, Debs T, Blanc P, Gugenheim J, Ben Amor I, Boutet C, Tiffet O. Complications of bariatric surgery: Presentation and emergency management. International journal of surgery (London, England). 2016 Mar:27():77-81. doi: 10.1016/j.ijsu.2016.01.067. Epub 2016 Jan 22 [PubMed PMID: 26808323]
AbuHasan Q, Miller PM, Li WS, Burney CP, Yuce TK, Stefanidis D. Racial disparities in the utilization and outcomes of robotic bariatric surgery: an 8-year analysis of Metabolic and Bariatric Surgery Accreditation Quality Improvement Program data. Surgery for obesity and related diseases : official journal of the American Society for Bariatric Surgery. 2025 Feb:21(2):158-165. doi: 10.1016/j.soard.2024.09.002. Epub 2024 Sep 16 [PubMed PMID: 39395845]
Level 2 (mid-level) evidenceWee CC, Huskey KW, Bolcic-Jankovic D, Colten ME, Davis RB, Hamel M. Sex, race, and consideration of bariatric surgery among primary care patients with moderate to severe obesity. Journal of general internal medicine. 2014 Jan:29(1):68-75. doi: 10.1007/s11606-013-2603-1. Epub 2013 Sep 19 [PubMed PMID: 24048655]
Campos GM, Khoraki J, Browning MG, Pessoa BM, Mazzini GS, Wolfe L. Changes in Utilization of Bariatric Surgery in the United States From 1993 to 2016. Annals of surgery. 2020 Feb:271(2):201-209. doi: 10.1097/SLA.0000000000003554. Epub [PubMed PMID: 31425292]
Hsu JL, Ismail S, Hodges MM, Agala CB, Farrell TM. Bariatric surgery: trends in utilization, complications, conversions and revisions. Surgical endoscopy. 2024 Aug:38(8):4613-4623. doi: 10.1007/s00464-024-10985-7. Epub 2024 Jun 20 [PubMed PMID: 38902405]
Moll H, Frey E, Gerber P, Geidl B, Kaufmann M, Braun J, Beuschlein F, Puhan MA, Yebyo HG. GLP-1 receptor agonists for weight reduction in people living with obesity but without diabetes: a living benefit-harm modelling study. EClinicalMedicine. 2024 Jul:73():102661. doi: 10.1016/j.eclinm.2024.102661. Epub 2024 May 27 [PubMed PMID: 38846069]
Greene ME, Grieco A, Evans-Labok K, Ko CY, Hutter MM. First report of outcomes from the patient-reported outcome measures program in the Metabolic and Bariatric Surgery Accreditation Quality Improvement Program. Surgery for obesity and related diseases : official journal of the American Society for Bariatric Surgery. 2024 Feb:20(2):173-183. doi: 10.1016/j.soard.2023.09.010. Epub 2023 Sep 15 [PubMed PMID: 37949691]
Level 2 (mid-level) evidenceRam Sohan P, Mahakalkar C, Kshirsagar S, Bikkumalla S, Reddy S, Hatewar A, Dixit S. Long-Term Effectiveness and Outcomes of Bariatric Surgery: A Comprehensive Review of Current Evidence and Emerging Trends. Cureus. 2024 Aug:16(8):e66500. doi: 10.7759/cureus.66500. Epub 2024 Aug 9 [PubMed PMID: 39247032]