Periprocedural Care for Patients Undergoing Bariatric Device Placement and Endoscopic Procedures
Periprocedural Care for Patients Undergoing Bariatric Device Placement and Endoscopic Procedures
Introduction
The obesity epidemic affects 42.4% of adults in the United States and generates more than $147 billion in annual healthcare costs.[1] Endoscopic bariatric and metabolic therapies (EBMTs) address the gap between lifestyle modification and bariatric surgery, targeting the 60 million Americans with a body mass index of 30 to 40 who need intermediate options.[2] These therapies typically achieve 5% to 20% total body weight loss with substantially lower morbidity than surgery, offering reversible, anatomy-preserving alternatives supported by favorable safety profiles.
Terminology clarification: "Endoscopic bariatric therapies" refers to minimally invasive procedures for weight loss, while "metabolic therapies" encompass treatments targeting metabolic dysfunction (diabetes, dyslipidemia) that are often associated with obesity. This article focuses on bariatric devices with metabolic benefits.
Pediatric considerations: All devices discussed are FDA-approved for adults only (≥18 years). No endoscopic bariatric devices currently have pediatric indications. ABOM certification includes pediatric obesity management, but these specific interventions are outside the pediatric scope. As detailed in the StatPearls article on Intragastric Balloon therapy, these devices serve as space-occupying mechanisms promoting satiation through gastric distension and delayed emptying. The growing evidence base supports their role in comprehensive obesity management when integrated with multidisciplinary care protocols.
Clinical Significance
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Clinical Significance
Device Overview and Mechanisms
Intragastric balloons (IGBs) function through multiple mechanisms, including occupying the gastric space, delaying gastric emptying, and enhancing satiety signaling (see Table 1). As described in the StatPearls comprehensive review, Intragastric Balloon, balloon volumes of 400 mL or greater induce satiation in patients whose gastric capacity can stretch 3 times compared to normal. See Image. Gastric Balloon.
Table 1. Gastric Balloons
|
Device |
Manufacturer |
Type |
Volume |
Placement |
Duration |
FDA Status |
Clinical Use |
|
Orbera |
Apollo Endosurgery |
Liquid-filled silicone |
450–700 mL saline |
Endoscopic under sedation |
6 months |
Active (2015) |
Clinically available |
|
Obalon |
ReShape Lifesciences |
Gas-filled, swallowable |
Up to 3 balloons |
Swallowable capsule |
6 months |
FDA approved (2016) |
Effectively discontinued* |
|
ReShape Duo |
Apollo Endosurgery |
Dual liquid-filled |
900 mL total |
Endoscopic under sedation |
6 months |
Discontinued 2019 |
Historical reference only |
FDA, United States Food and Drug Administration
*Note: Obalon retains FDA approval but is unavailable due to a company merger and has ceased commercial operations. Current clinical use is negligible.
High-Yield American Board of Obesity Medicine Examination Content: Patient Selection Criteria
Evidence-based IGB candidacy (American Board of Obesity Medicine[ABOM] core competencies)
- Primary inclusion criteria
- BMI 30–40 kg/m² (class I–II obesity with expanded European criteria)
- Age 18–65 with documented weight loss attempts
- Failed lifestyle modification within the preceding 5 years
- Commitment to a 12-month behavioral program with multidisciplinary support
- Psychological readiness confirmed by validated assessment tools
- Absolute contraindications (memory aid: "HUGE GAPS")
- Hiatal hernia >5cm
- Ulcerative/erosive gastric disease
- Gastric surgery history
- Eating disorders (bulimia, binge eating disorder)
- Graviditas (pregnancy/planning)
- Active substance abuse
- Psychiatric instability
- Severe cardiopulmonary disease
- Relative contraindications (memory aid: "ACID")
- Anticoagulation therapy requiring management
- Chronic steroid use or immunosuppression
- Inability to comply with follow-up requirements
- Diabetes with poor control (HbA1c >9%)
Orbera balloon: clinical evidence and outcomes
The Orbera balloon represents the most extensively studied IGB system with robust clinical data supporting its efficacy and safety profile. FDA pivotal trial results demonstrate a 10.2% total body weight loss (TBWL) at 6 months, compared to 3.3% in the control group, with an excess weight loss of 38.4%. Additionally, 65% of patients achieved a weight loss of 5% or more.[3]
High-yield Clinical data for the ABOM examination
- Efficacy endpoint: 10.2% vs 3.3% TBWL (treatment vs control)
- Safety profile: 5.6% serious adverse events vs 1.1% controls
- Early removal rate: 9.4% due to intolerance
- Weight maintenance: 89.5% maintained at 6 months post-removal
Critical safety update (FDA postmarket surveillance)
FDA postapproval studies have reported 18 deaths worldwide since Orbera's approval in 2015 (including 5 in the United States), along with a 2.3% incidence of balloon hyperinflation.[FDA in Brief: Postmarket Update on Liquid-Filled Intragastric Balloons. 2022] While these serious adverse events require careful patient counseling, the 9.4% early removal rate falls within acceptable safety benchmarks (<15% target) when proper selection criteria and protocols are followed (see Table 2). The overall benefit-risk profile remains favorable for patients who are appropriately selected and receive comprehensive periprocedural care.
Table 2. Preprocedure Optimization Protocol (ABOM Standards)
|
Category |
Test/Workup |
Target/Normal Values |
Clinical Goal |
|
Hematologic |
CBC, PT/INR, Type and screen |
Hgb ≥10 g/dL, normal coagulation |
Detect anemia, bleeding risk |
|
Metabolic |
CMP, HbA1c, lipids, TSH |
HbA1c <8%, euglycemia |
Optimize diabetes, prevent complications |
|
Infectious |
H pylori (breath test/stool Ag) |
Eradication, if positive |
87% eradication prevents ulceration |
|
Nutritional |
B12, folate, 25-OH vitamin D, and iron studies |
Correct deficiencies |
Baseline micronutrient optimization |
|
Cardiovascular |
ECG, echocardiogram if risk factors are present |
Normal rhythm, EF >40% |
Sedation risk stratification |
CBC, complete blood count; ECG, electrocardiogram; EF, ejection fraction; HbA1c, hemoglobin A1c; Hgb, hemoglobin; PT/INR, prothrombin time/international normalized ratio; stool Ag, stool antigen; 25-OH, 25-hydroxy vitamin D
Periprocedural Management Protocols
Preprocedure optimization (2–4 weeks)
Medical optimization includes individualized diabetes control (HbA1c targets typically <7% for most adults and <8% for older adults or those at risk of hypoglycemia, per ABOM guidelines), eradication of H pylori with triple therapy (proton pump inhibitor + amoxicillin + clarithromycin, achieving cure rates of approximately 87%), and mandatory smoking cessation for at least 4 weeks before the procedure.[4] Anticoagulation management requires coordination with cardiology to ensure the use of appropriate bridging protocols.
Intraprocedure standards
- Sedation protocol: Moderate conscious sedation is preferable, achieved with propofol under an anesthesiologist's supervision, or with a midazolam/fentanyl combination. Continuous monitoring should include pulse oximetry, capnography, and blood pressure measurement every 5 minutes, with emergency reversal agents readily available.
- Technical parameters: Orbera placement is performed via endoscopy under direct visualization, with optimal balloon positioning in the gastric fundus and body. The balloon is inflated with saline containing methylene blue to a volume of 450 to 700 mL. The procedure typically lasts 20 to 30 minutes and has a technical failure rate of less than 5% when performed by experienced clinicians.
Complications and evidence-based management
See Table 3 for IGB complications.
Table 3. IGB Complications: Timeline, Recognition, and Management
|
Timeline |
Complication |
Incidence (%) |
Symptoms/Presentation |
Management/Treatment |
Notes/Outcomes |
|
Early (0–7 days) |
Nausea/vomiting |
80%–90% |
Universal, peaks day 2–3 |
Ondansetron 8 mg every 8 hours, clear liquids |
Usually resolves by day 5 |
|
Gastric pain |
60%–70% |
Cramping, distension |
Acetaminophen 1 g 6 hours, avoid NSAIDs |
Heat application helpful |
|
|
GERD symptoms |
25%–30% |
Heartburn, regurgitation |
PPI therapy BID, elevate HOB |
May require H2 blockers |
|
|
Intermediate (1–4 weeks) |
Dehydration |
15%–20% |
Poor PO intake, tachycardia |
IV hydration, electrolyte monitoring |
Primary readmission cause |
|
Gastric ulceration |
5%–8% |
Epigastric pain, GI bleeding |
Intensive PPI therapy, EGD evaluation |
Exclude H pylori |
|
|
Late (>1 month) |
Balloon deflation |
2%–3% (49% device issues) |
Green urine, loss of satiety |
URGENT endoscopic removal |
Methylene blue indicator |
|
Hyperinflation |
2.3% (postapproval) |
Severe pain, vomiting |
Emergency deflation ± ICU care |
Life-threatening event |
BID, twice daily; EGD, esophagogastroduodenoscopy; GERD, gastroesophageal reflux disease; GI, gastrointestinal; H2, histamine-2 receptor antagonist; HOB, head of bed; ICU, intensive care unit; IV, intravenous; NSAIDs, nonsteroidal anti-inflammatory drugs; PO, by mouth; PPI, proton pump inhibitor
Emergency recognition protocol, "BALLOON ALERT"
- Balloon deflation (green urine: methylene blue leak)
- Acute severe pain with peritoneal signs
- Loss of satiety with food intolerance
- Liquid intolerance >12 hours
- Obstruction symptoms (early satiety, vomiting)
- Onset of hemodynamic instability
- Nausea/vomiting refractory to treatment
Diagnostic approach
- Abdomen/pelvis computed tomography with contrast (first-line)
- Upper endoscopy for therapeutic intervention, laboratory assessment (complete blood count, comprehensive metabolic panel, lactate, lipase)
- Surgical consultation for perforation, massive bleeding, or complete obstruction
Long-term management and quality metrics
Structured follow-up schedule (memory aid, "WM3M6P")
- Week 1: Telephone triage for tolerance and hydration
- Month 1: Weight assessment, dietary counseling, lab review
- Month 3: Comprehensive evaluation, comorbidity assessment
- Month 6: Preremoval planning and transition preparation
- Postremoval: 6-month and 12-month weight maintenance support
ABOM quality metrics and benchmarks
- Primary efficacy targets:
- ≥5% total body weight loss: Clinically significant threshold (target >80% patients)
- ≥10% total body weight loss: Optimal response (target >50% patients)
- Sustained weight loss: Maintenance at 12 months post-removal
- Safety benchmarks:
- Serious adverse events: <10% target rate
- 30-day readmissions: <5% for device-related complications
- Early removal rate: <15% due to intolerance
- Patient satisfaction: >80% recommend the procedure
Clinical Vignette
Case presentation
A 45-year-old female accountant with a BMI of 32 kg/m², type 2 diabetes (HbA1c 7.8%), and hypertension presents for IGB evaluation. She takes metformin 1000 mg twice a day and lisinopril 10 mg daily. She has tried multiple commercial diet programs over the past 3 years without sustained weight loss. The initial workup revealed H pylori positivity in stool antigen testing.
Clinical decision-making memory aid, "MESH"
- Medical optimization (diabetes, H pylori, cardiac clearance)
- Eligibility assessment (BMI, contraindications, readiness)
- Safety protocols (preprocedure, intraprocedure, post-procedure)
- Handoff planning (follow-up, complications, team coordination)
| Pause and Reflect |
|
Endoscopic Sleeve Gastroplasty
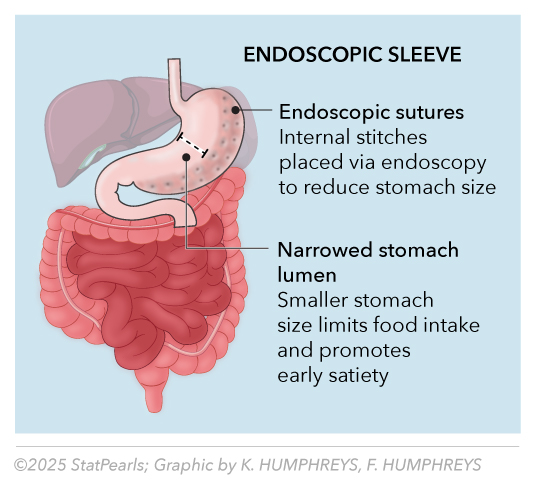
Endoscopic sleeve gastroplasty (ESG) creates a tubular gastric restriction through endoscopic suturing, reducing gastric volume by approximately 70% without permanent anatomical alteration. Recent data demonstrate a 13.6% total body weight loss at 1 year, with 80% of the weight loss maintained at 5 years.[5] The safety profile shows a serious complication rate of 1.5% to 2.3%, with bleeding (1%–2%) and perforation (<0.5%) managed endoscopically in more than 90% of cases. Endoscopic sleeve gastroplasty in patients with obesity and metabolic dysfunction–associated steatotic liver disease led to early, weight-independent improvements in insulin resistance and sustained reductions in estimated hepatic steatosis and fibrosis over 2 years.[6] See Image. Endoscopic Sleeve.
ABOM examination focus: ESG received FDA approval in 2023 with expanded indications for BMI ≥30 kg/m² (class I obesity) with metabolic comorbidities, BMI ≥35 kg/m² (class II obesity), and traditional BMI ≥40 kg/m² (class III obesity) criteria. Key contraindications include a large hiatal hernia (>5 cm), previous gastric surgery, and active gastric pathology.
ESG follow-up protocol and quality metrics
- Structured follow-up schedule (memory aid, "ESG-PACE"):
- Early (1 week): Telephone assessment, diet progression
- Short-term (1 month): Weight/symptom evaluation, dietary counseling
- Goal assessment (3 months): Comprehensive evaluation, lab studies
- Progress review (6 months): Weight loss trajectory, comorbidity improvement
- Annual (12+ months): Long-term maintenance, potential re-intervention assessment
- Continuous: Ongoing nutritional and behavioral support
- Emergency: 24/7 access for complications
- ESG quality benchmarks:
- Primary efficacy: >80% achieve ≥10% TBWL at 12 months
- Safety target: <3% serious complications requiring intervention
- Technical success: >95% successful suture placement
- Patient satisfaction: >85% would recommend the procedure
Endoscopic Suturing Devices: Gastric Volume Reduction Device Overview and Clinical Applications
Endoscopic suturing devices enable gastric volume reduction by placing full-thickness sutures, creating a restrictive anatomy without requiring permanent surgical alteration. The most established procedure is ESG, which reduces gastric volume by approximately 70% through systematic suturing along the greater curvature of the stomach.[7]
Current FDA-approved endoscopic suturing systems
- OverStitch System (Apollo Endosurgery): Primary platform for ESG procedures
- Endomina System (Endo Tools): Alternative suturing platform with European Conformité Européenne mark
- g-Prox System (United States Gastrointestinal [USGI] Medical): Historical reference, limited current use
Endoscopic sleeve gastroplasty specifications and outcomes
ESG utilizes systematic suturing patterns to create a tubular gastric configuration, reducing capacity from approximately 1500 mL to 450 mL (see Table 4). The procedure typically requires 45 to 90 minutes, with 15 to 20 sutures placed in a standardized pattern along the greater curvature, from the antrum to the fundus.
Table 4. Endoscopic Sleeve Gastroplasty Clinical Outcomes and Quality Metrics
| Endpoint | 12 Months | 36 Months | 60 Months | Clinical Significance |
| Total body weight loss (TBWL) | 15.6% | 14.9% | 15.9% | Weight loss sustained with minor fluctuations over 5 years |
| Excess weight loss | 47.9% | 45.1% | 45.3% | Clinically meaningful |
| Patients achieving ≥5% TBWL | 89% | 85% | 90% | FDA efficacy threshold |
| Patients achieving ≥10% TBWL | 77% | 63% | 61% | Optimal response rate |
FDA, United States Food and Drug Administration; TBWL, total body weight loss
ESG complications recognition and management
Major complications (incidence 1.5%–2.3%)
- Bleeding: 1% to 2% incidence, managed endoscopically in >90% cases
- Perforation: <0.5% incidence, requires immediate surgical consultation
- Suture-line complications: Rare, conservative management protocols
Emergency recognition protocol, "SUTURE ALERT"
- Severe abdominal pain with peritoneal signs
- Unremitting nausea/vomiting >24 hours
- Tachycardia with hemodynamic instability
- Upper gastrointestinal bleeding (hematemesis, melena)
- Rigid abdomen or rebound tenderness
- Elevated lactate or leukocytosis
Current clinical use and ABOM examination focus
Clinical update, 2024–2025: ESG received FDA clearance in July 2022 and demonstrates growing clinical adoption among endoscopic bariatric therapies. The procedure is increasingly available in academic and private practice settings across the United States.[FDA Grants De Novo Marketing Authorization to Apollo Endosurgery. 2022]
ABOM examination high-yield facts:
- Mechanism: Gastric volume reduction via endoscopic suturing (70% volume reduction)
- FDA status: Cleared in July 2022 for obesity treatment
- Efficacy: 15%–16% TBWL sustained at 5 years
- Safety: 1.5%–2.3% serious complication rate
- Technical success: >95% successful procedure completion
- Current availability: Growing adoption in the United States clinical practice
Vagal Neuromodulation Therapy (vBloc)
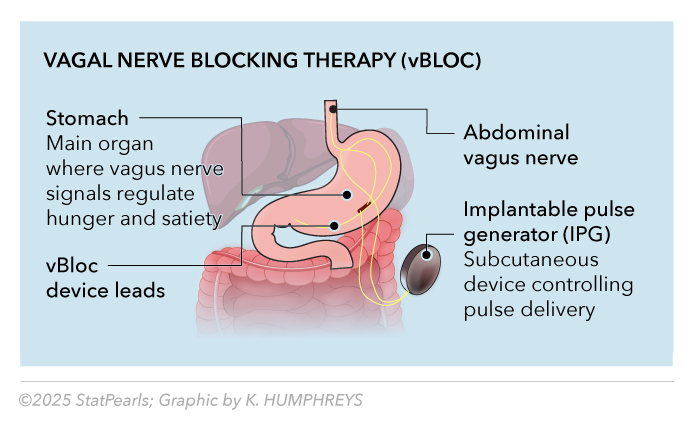
The Maestro Rechargeable System (vBloc device) delivers intermittent vagal nerve blocking through laparoscopically implanted electrodes at the gastroesophageal junction. The vBloc device received FDA approval in 2015 for patients with a BMI of 35 to 45 kg/m² or those with a BMI of 35 to 39.9 kg/m² with associated comorbidities.[8] See Image. Vagal Neuromodulation Therapy (vBLOC).
Clinical outcomes
Results from the ReCharge study demonstrated an 8.5% greater excess weight loss compared to sham controls at 12 months, with 52.5% of participants achieving an excess weight loss of 20% or more.[9] Meta-analysis confirms a weighted mean difference of 17.19% in excess weight loss and a 2.24 kg/m² reduction in BMI at 12 months.[10]
Critical ABOM update: Despite FDA approval, vBloc has extremely limited clinical availability due to:
- Insurance noncoverage and reimbursement challenges
- Company pivots to a direct-pay model in select markets only
- Minimal current clinical utilization despite regulatory approval
- Limited physician training programs and device access
ABOM examination relevance: Although historically important for understanding neuromodulation approaches, the current clinical relevance of vBloc is limited. Candidates should be familiar with the device's basic mechanism of action and its FDA approval status, while also recognizing its practical limitations in clinical practice.
vBloc high-yield facts for ABOM:
- Mechanism: Intermittent vagal nerve blocking via implanted electrodes
- FDA approval: 2015 for specific BMI/comorbidity criteria
- Efficacy: 8.5% greater excess weight loss vs sham at 12 months
- Current status: FDA approved but effectively withdrawn from widespread clinical use
- Clinical significance: Limited due to reimbursement and access barriers
Historical Device Update: AspireAssist System
Critical ABOM update: AspireAssist was withdrawn from the United States market due to economic reasons, not safety concerns. While retaining FDA approval (premarket approval P150024), it is no longer commercially available in the United States. Historical efficacy demonstrated a 12.1% TBWL compared to 3.5% in controls, as measured through percutaneous gastric aspiration therapy.
TransPyloric Shuttle: Novel Gastric Emptying Modulation
Device overview and mechanisms
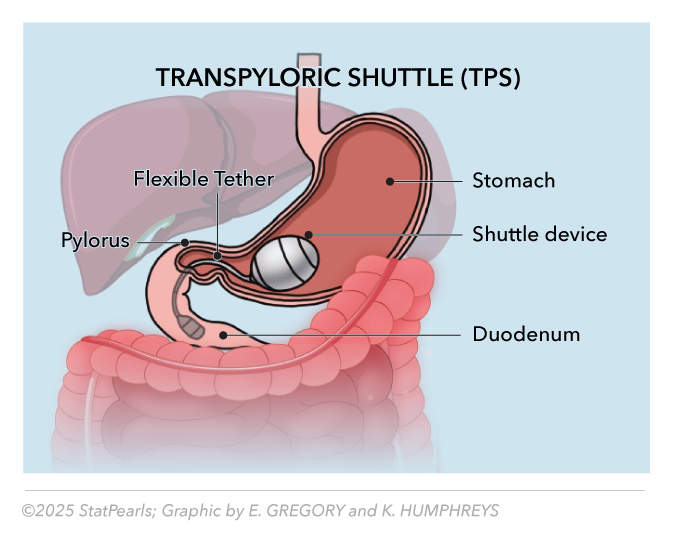
The TransPyloric Shuttle (TPS) offers a distinct approach to endoscopic bariatric therapy by inducing weight loss through delayed gastric emptying, rather than occupying space (see Table 5). FDA-approved in April 2019, the TPS device consists of a large, spherical silicone bulb connected to a smaller, cylindrical bulb by a flexible tether, designed to engage the pylorus and intermittently regulate gastric outflow.[11] See Image. Transpyloric Shuttle.
Table 5. TransPyloric Shuttle Device Specifications
|
Parameter |
Specification |
Clinical Significance |
|
Material |
Medical-grade silicone |
Noninflatable, maintains structural integrity |
|
Dimensions |
5.6 cm diameter (large bulb) |
Occupies ~85%–90% less gastric volume than traditional balloons |
|
Deployment |
Endoscopic via overtube |
Single-helical coil delivery system |
|
Duration |
12 months |
Longest FDA-approved intragastric device |
|
Mechanism |
Delayed gastric emptying |
Intermittent transpyloric seal delays gastric emptying |
Clinical evidence and ABOM examination relevance
Results from the pivotal ENDObesity II study (n = 302) demonstrated superior efficacy compared to sham controls, with statistical significance across multiple endpoints. Patients achieved a 9.5% total body weight loss, compared to 2.8% in the control group (P < .0001). Notably, 67% of patients achieved a clinically meaningful weight loss of 5% or more, compared to 22% in the control group.[ENDObesity® II Study: TransPyloric Shuttle® System for Weight Loss]
High-yield clinical data for ABOM examination
- Efficacy endpoint: 9.5% vs 2.8% TBWL (treatment vs control)
- Clinically significant response: 67% achieved ≥5% TBWL (vs 29.3% controls)
- Optimal response: 40% achieved ≥10% TBWL vs 14% controls²°
- Trial design: Randomized, double-blind, sham-controlled (n= 302)
- Device retention: 12-month placement duration with endoscopic retrieval
TPS-specific selection criteria (ABOM core competencies)"
Primary inclusion criteria:
- BMI 35.0–40.0 kg/m² or BMI 30.0–34.9 kg/m² with obesity-related comorbidities
- Age 18–65 with documented lifestyle modification failure
- Commitment to a supervised diet and behavior modification program
- Willingness to participate in a 12-month treatment protocol
Absolute contraindications (memory aid, "PYLORIC")
- Pyloric stenosis or gastric outlet obstruction
- Young age (<18, no pediatric indication)
- Large hiatal hernia (>5 cm)
- Obstruction history (gastric surgery, adhesions)
- Recent gastric pathology (ulcers, tumors)
- Inflammatory bowel disease affecting the upper gastrointestinal tract
- Coagulopathy or anticoagulation requiring management
Complications and management protocols
See Table 6 for TPS complications and management.
Table 6. TPS-Specific Complications and Management
|
Phase |
Complication |
Incidence |
Clinical Recognition |
Evidence-Based Management |
|
Early (0–14 days) |
Nausea/vomiting |
70%–85% |
Universal, mechanism-related |
Antiemetics, clear liquids, gradual diet progression |
|
Early (0–14 days) |
Abdominal pain |
60%–75% |
Cramping, gastric distension |
Acetaminophen, avoid nonsteroidal anti-inflammatories, reassurance |
|
Intermediate (2–8 weeks) |
Device migration |
2%–5% |
Loss of satiety, positional symptoms |
Endoscopic repositioning or removal |
|
Late (>2 months) |
Mechanical failure |
<2% |
Sudden symptom relief, device fragmentation |
Urgent endoscopic evaluation and removal |
Current clinical status and ABOM examination considerations
Critical update for ABOM candidates: Since FDA approval in April 2019, the TransPyloric Shuttle has achieved limited widespread clinical adoption. The device represents a novel mechanism of action, achieved through delayed gastric emptying rather than space occupation, which distinguishes it from traditional balloon therapies.
Key ABOM examination points:
- FDA approval status: Active since April 2019
- Unique mechanism: Delayed gastric emptying vs space occupation
- Clinical availability: FDA-approved but limited commercial use
- Duration advantage: 12-month placement versus 6-month balloon therapies
Enhancing Healthcare Team Outcomes
Evidence-Based Team Structure
Core team competencies require gastroenterologist expertise in device selection and complication management, coordination with obesity medicine clinicians for metabolic optimization, guidance from a registered dietitian for nutritional protocols, support from a behavioral health specialist for psychological readiness, and coordination with nursing staff for patient education and monitoring.
Team communication memory aid, "SHARP"
- Standardized electronic health records templates and documentation
- Huddles weekly for high-risk patient identification
- After-hours on-call protocols established
- Rapid response systems for emergencies
- Patient portal access for continuous monitoring
Quality improvement standards include standardized communication protocols, monthly team meetings for case review, patient safety reporting with root cause analysis, and continuous education on emerging evidence.
ABOM team-based care excellence metrics
- Successful programs demonstrate:
- Standardized protocols reduce care variation by more than 50%.
- Systematic follow-up decreases readmissions by 40%.
- Patient engagement improves satisfaction scores by more than 85%.
- Quality metrics enable continuous improvement through data-driven decisions.
Conclusion
Periprocedural care for bariatric devices requires evidence-based approaches that integrate rigorous patient selection, standardized protocols, systematic complication management, and coordinated interprofessional care. Successful programs achieve a 5% to 20% total body weight loss in patients, with a serious complication rate of less than 10% through comprehensive care delivery aligned with ABOM competency standards. The range of FDA-approved devices varies in commercial availability, reflecting the current state of therapies: Orbera balloon (active), ESG (expanding indications), vBloc (limited availability), while previously available options face market challenges. Healthcare professionals must maintain competency in device-specific protocols while prioritizing patient safety and realistic outcome expectations within comprehensive frameworks for obesity management.
Media
(Click Image to Enlarge)
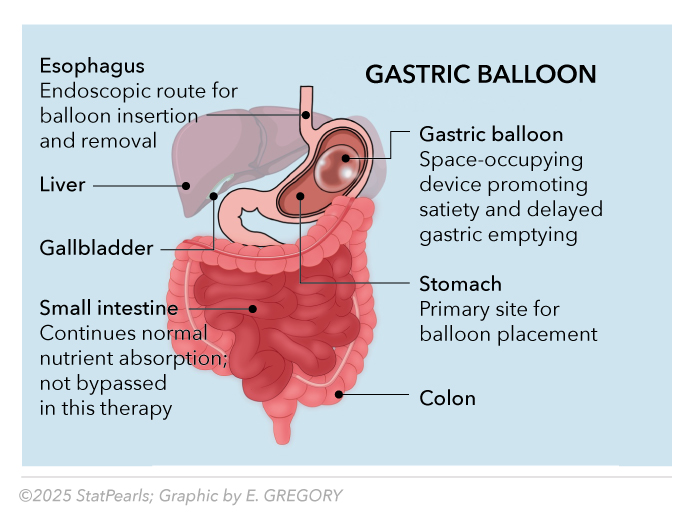
Gastric Balloon. Gastric balloons function through multiple mechanisms, including occupying the gastric space, delaying gastric emptying, and enhancing satiety signaling. Balloon volumes of 400 mL or higher induce satiation in patients whose gastric capacity can stretch 3-fold compared to normal capacity.
Contributed and Illustrated by E Gregory.
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of Obesity and Severe Obesity Among Adults: United States, 2017-2018. NCHS data brief. 2020 Feb:(360):1-8 [PubMed PMID: 32487284]
Arterburn DE, Telem DA, Kushner RF, Courcoulas AP. Benefits and Risks of Bariatric Surgery in Adults: A Review. JAMA. 2020 Sep 1:324(9):879-887. doi: 10.1001/jama.2020.12567. Epub [PubMed PMID: 32870301]
Courcoulas A, Abu Dayyeh BK, Eaton L, Robinson J, Woodman G, Fusco M, Shayani V, Billy H, Pambianco D, Gostout C. Intragastric balloon as an adjunct to lifestyle intervention: a randomized controlled trial. International journal of obesity (2005). 2017 Mar:41(3):427-433. doi: 10.1038/ijo.2016.229. Epub 2016 Dec 23 [PubMed PMID: 28017964]
Level 1 (high-level) evidenceMalfertheiner P, Megraud F, O'Morain CA, Gisbert JP, Kuipers EJ, Axon AT, Bazzoli F, Gasbarrini A, Atherton J, Graham DY, Hunt R, Moayyedi P, Rokkas T, Rugge M, Selgrad M, Suerbaum S, Sugano K, El-Omar EM, European Helicobacter and Microbiota Study Group and Consensus panel. Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut. 2017 Jan:66(1):6-30. doi: 10.1136/gutjnl-2016-312288. Epub 2016 Oct 5 [PubMed PMID: 27707777]
Level 3 (low-level) evidenceAngrisani L, Santonicola A, Iovino P, Vitiello A, Zundel N, Buchwald H, Scopinaro N. Bariatric Surgery and Endoluminal Procedures: IFSO Worldwide Survey 2014. Obesity surgery. 2017 Sep:27(9):2279-2289. doi: 10.1007/s11695-017-2666-x. Epub [PubMed PMID: 28405878]
Level 3 (low-level) evidenceHajifathalian K, Mehta A, Ang B, Skaf D, Shah SL, Saumoy M, Dawod Q, Dawod E, Shukla A, Aronne L, Brown RS, Cohen DE, Dannenberg AJ, Fortune B, Kumar S, Sharaiha RZ. Improvement in insulin resistance and estimated hepatic steatosis and fibrosis after endoscopic sleeve gastroplasty. Gastrointestinal endoscopy. 2021 May:93(5):1110-1118. doi: 10.1016/j.gie.2020.08.023. Epub 2020 Aug 27 [PubMed PMID: 32861753]
Neto MG, Jerez J, Vásconez DC, Arau RT, de Quadros LG, Teixeira A, Falcón K, Fors M. Transforming obesity care: the impact of endoscopic sleeve gastroplasty on weight loss and metabolic health. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract. 2025 Jul 26:29(10):102166. doi: 10.1016/j.gassur.2025.102166. Epub 2025 Jul 26 [PubMed PMID: 40721021]
Johnston BC, Kanters S, Bandayrel K, Wu P, Naji F, Siemieniuk RA, Ball GD, Busse JW, Thorlund K, Guyatt G, Jansen JP, Mills EJ. Comparison of weight loss among named diet programs in overweight and obese adults: a meta-analysis. JAMA. 2014 Sep 3:312(9):923-33. doi: 10.1001/jama.2014.10397. Epub [PubMed PMID: 25182101]
Level 1 (high-level) evidenceApovian CM, Shah SN, Wolfe BM, Ikramuddin S, Miller CJ, Tweden KS, Billington CJ, Shikora SA. Two-Year Outcomes of Vagal Nerve Blocking (vBloc) for the Treatment of Obesity in the ReCharge Trial. Obesity surgery. 2017 Jan:27(1):169-176. doi: 10.1007/s11695-016-2325-7. Epub [PubMed PMID: 27506803]
Fadel MG, Fehervari M, Das B, Soleimani-Nouri P, Ashrafian H. Vagal Nerve Therapy in the Management of Obesity: A Systematic Review and Meta-Analysis. European surgical research. Europaische chirurgische Forschung. Recherches chirurgicales europeennes. 2023:64(4):365-375. doi: 10.1159/000533358. Epub 2023 Aug 4 [PubMed PMID: 37544303]
Level 1 (high-level) evidenceSullivan S, Edmundowicz SA, Thompson CC. Endoscopic Bariatric and Metabolic Therapies: New and Emerging Technologies. Gastroenterology. 2017 May:152(7):1791-1801. doi: 10.1053/j.gastro.2017.01.044. Epub 2017 Feb 10 [PubMed PMID: 28192103]