Secondary Causes of Obesity and Comprehensive Diagnostic Evaluation
Secondary Causes of Obesity and Comprehensive Diagnostic Evaluation
Introduction
Evaluating obesity requires a systematic approach beyond a simple body mass index calculation to identify secondary causes and characterize disease severity. Secondary causes of obesity affect approximately 5% to 10% of patients presenting with weight gain and may require specific therapeutic interventions distinct from lifestyle modification alone. This resource offers board-focused content that addresses American Board of Obesity Medicine Tasks A.11 and A.13, emphasizing evidence-based diagnostic approaches essential for clinical practice and success on the certification examination.
Function
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Function
Secondary Endocrine Causes of Obesity
Evaluating secondary causes of obesity is essential for accurate diagnosis and effective treatment planning, especially in patients who do not respond to standard lifestyle interventions.
Thyroid dysfunction
Thyroid disorders represent the most common secondary endocrine cause of weight gain, with hypothyroidism affecting up to 15% of obese patients (see Table 1). The European Society of Endocrinology recommends universal thyroid-stimulating hormone screening in obesity due to the high prevalence and therapeutic implications.[1] Clinical presentation includes fatigue, cold intolerance, constipation, and depression, though symptoms may be subtle in mild hypothyroidism.
Table 1. Thyroid Function Test Interpretation in Obesity
|
Condition |
TSH |
Free T4 |
Free T3 |
Clinical Significance |
|
Primary hypothyroidism |
↑ |
↓ |
↓ or normal |
Most common; requires levothyroxine treatment |
|
Subclinical hypothyroidism |
↑ |
Normal |
Normal |
Consider treatment if TSH >10 mIU/L |
|
Non-thyroidal illness |
Normal_/↓ |
↓ |
↓ |
Avoid treatment during acute illness |
|
Central hypothyroidism |
↓ or normal |
↓ |
↓ |
Rare; requires pituitary evaluation |
TSH, thyroid-stimulating hormone
Age-appropriate thyroid screening recommendations
The following age-based screening guidelines are recommended to identify thyroid dysfunction:
- Adults >35 years: Screen every 5 years
- Adults with obesity and metabolic complications or symptoms: Annual screening
- Adults with a family history of thyroid disease: Earlier and more frequent screening
- Pregnant women or individuals contemplating pregnancy: Screen using pregnancy-specific reference ranges
The bidirectional relationship between obesity and thyroid dysfunction complicates the interpretation of laboratory studies, as obesity can alter thyroid hormone metabolism and increase TSH levels.[2] TSH elevation between 2.5 and 10 mIU/L in obese individuals may reflect an adaptive response to obesity, rather than thyroid dysfunction. Please refer to StatPearls' companion resource, "Hypothyroidism," for additional information on thyroid physiology.
| Pause and Reflect |
Consider a 45-year-old woman who has recently gained 20 pounds despite adhering to a healthy diet.
|
Key considerations for clinicians include:
- TSH is the most sensitive indicator of thyroid dysfunction in obesity and serves as the best initial screening test.
- In patients with obesity, TSH levels may be mildly elevated (2.5-10 mIU/L) as an adaptive response without true hypothyroidism being present.
- Free T4 and anti-thyroid peroxidase (TPO) antibodies help distinguish true thyroid dysfunction from obesity-related TSH elevation. Anti-TPO antibodies aid in the identification of autoimmune thyroid disease.
- Borderline results can be repeated after weight stabilization.
- Treatment should be initiated for overt hypothyroidism, but should be individualized and may not be indicated for patients with subclinical disease.
Hypogonadism
Obesity-related hypogonadism represents a reversible condition affecting both sexes through distinct mechanisms. Female obesity-related secondary hypogonadism (FOSH) involves hypothalamic-pituitary-ovarian axis suppression, while male obesity-related secondary hypogonadism (MOSH) results from aromatase-mediated testosterone conversion to estradiol.[3]
Male obesity-related secondary hypogonadism pathophysiology
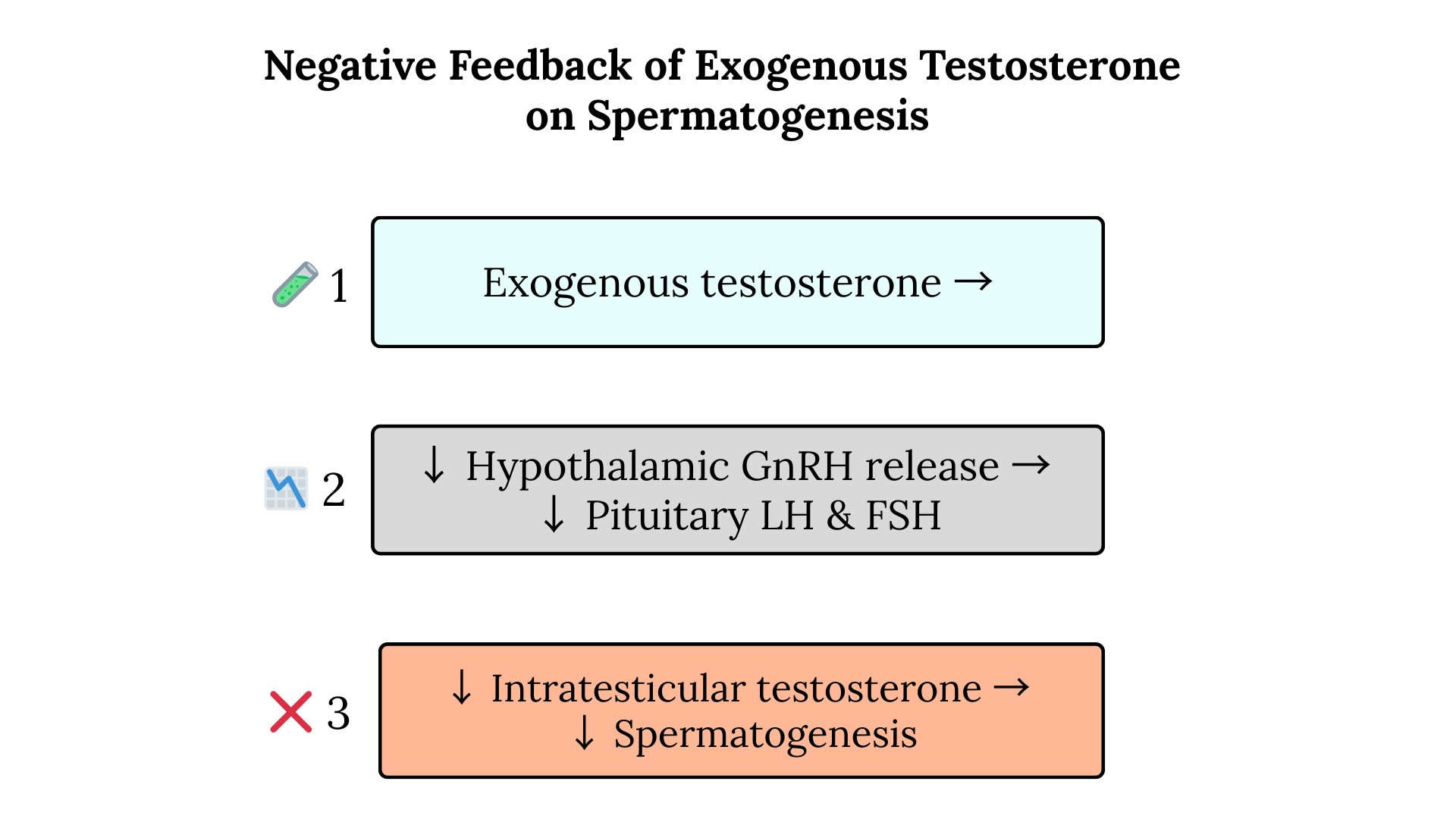
Obesity contributes to MOSH through several interrelated pathways. Increased adipose tissue elevates aromatase activity, increasing the conversion of testosterone to estradiol. Elevated estradiol suppresses the hypothalamic-pituitary-gonadal (HPG) axis via negative feedback, decreasing luteinizing hormone (LH), follicle-stimulating hormone (FSH), and testosterone. Inflammatory cytokines, eg, tumor necrosis factor (TNF)-α, interleukin (IL)-6, and IL-1β, suppress hypothalamic gonadotropin-releasing hormone (GnRH) neurons, leading to reduced secretion of LH and FSH.
Metabolic dysfunction further contributes to this process through insulin resistance, where elevated insulin levels directly suppress testicular testosterone production. Leptin resistance, a common feature of obesity, disrupts hypothalamic GnRH pulsatility and reduces levels of LH and FSH. Obesity also frequently leads to the development of sleep apnea and chronic stress, raising cortisol levels, activating the hypothalamic-pituitary-adrenal axis, and further suppressing the HPG axis. These mechanisms culminate in functional hypogonadotropic hypogonadism, creating a vicious cycle in which declining testosterone levels increase fat mass and decrease muscle mass, exacerbating obesity. See Image. Negative Feedback of Exogenous Testosterone.
Female obesity-related secondary hypogonadism pathophysiology
In women, obesity-related secondary hypogonadism also involves multiple physiological disruptions. Elevated aromatase activity increases estrone production, thereby enhancing peripheral estrogen levels and suppressing the hypothalamic-pituitary-ovarian axis, which results in reduced levels of LH, FSH, estradiol, and progesterone, ultimately leading to anovulation. Inflammatory mediators, such as TNF-α, IL-6, and C-reactive protein, impair the function of hypothalamic GnRH neurons, thereby decreasing gonadotropin release and ovarian responsiveness. Insulin resistance increases insulin levels, stimulating theca cells to overproduce androgens, resulting in hyperandrogenism and follicular arrest.
Central leptin resistance, a common feature of obesity, disrupts kisspeptin signaling and reduces GnRH pulsatility, ultimately leading to ovarian dysfunction. Lower adiponectin levels in obesity worsen insulin resistance and inflammation, further impairing ovarian estradiol production. Reduced sex hormone-binding globulin raises free androgen levels, reinforcing a hyperandrogenic state that suppresses normal ovarian function. These pathways collectively result in functional hypogonadotropic hypogonadism, often presenting as oligomenorrhea, amenorrhea, decreased fertility, and metabolic dysfunction. Reduced estradiol levels contribute to increased central fat accumulation, diminished bone density, and worsening metabolic health, perpetuating the cycle of obesity.
Clinical features of hypogonadism
- Male hypogonadism: Decreased libido, erectile dysfunction, fatigue, depression, and loss of muscle mass
- Female hypogonadism: Irregular menstruation, anovulation, decreased fertility, and mood changes
Management of Obesity-related Secondary Hypogonadism
Weight loss of 5% to 10% can significantly improve gonadal function, making lifestyle intervention the primary therapeutic approach. Due to potential increased risks, testosterone replacement therapy should be considered cautiously in obese men, and clinicians must evaluate cardiovascular risk factors before initiating treatment. Please refer to the StatPearls' companion resources, "Male Hypogonadism" and "Polycystic Ovarian Syndrome," for additional information.
| Pause and Reflect |
A 35-year-old man with a BMI of 38 kg/m² presents with fatigue and decreased libido.
|
Key considerations include:
- In obesity-related secondary hypogonadism, testosterone levels are low with low or inappropriately normal LH and FSH, whereas primary testicular failure presents with low testosterone and elevated LH and FSH.
- The timing of obesity onset versus symptom onset should be considered; secondary hypogonadism typically develops after obesity onset.
- American Association of Clinical Endocrinology guidelines recommend confirmation with repeat morning total testosterone, free testosterone, and SHBG levels, depending on the body mass index.
- A weight loss trial (not delaying necessary care) can confirm reversibility; secondary hypogonadism often improves with a 5% to 10% weight reduction.
- A morning testosterone <300 ng/dL warrants further evaluation.
- Consider cardiovascular risks before testosterone replacement in obese men.
- Female patterns include irregular menstruation and anovulation with metabolic dysfunction.
Menopause-Related Weight Gain
The menopausal transition contributes to weight gain through decreased estrogen levels, increased central adiposity, and reduced energy expenditure. The average weight gain during menopause is 2 to 5 kg, with a preferential fat accumulation in the abdominal region.[3]
Hormonal changes that occur during the menopausal transition include:
- Decreased estradiol production
- Increased FSH
- Altered leptin sensitivity
- Reduced growth hormone secretion
Distinguishing menopausal weight gain from other secondary causes requires a comprehensive hormonal assessment and consideration of timing relative to the cessation of menses. Hormone replacement therapy is not Food and Drug Administration-approved for weight loss, but may mitigate metabolic changes when used for other appropriate indications. Please refer to the StatPearls' companion resource, "Menopause," for additional information.
| Pause and Reflect |
A 52-year-old perimenopausal woman presents with complaints of gaining 15 kg over 2 years.
|
Key considerations include:
- Normal menopausal weight gain is typically 2 to 5 kg with a gradual onset.
- Red flags include rapid weight gain (>10 kg), associated symptoms (eg, cold intolerance, easy bruising, or purple striae), or weight gain despite appropriate lifestyle measures.
- Screening laboratory tests include TSH, morning cortisol, and a comprehensive metabolic panel.
- The timing of weight gain should correlate with menstrual irregularities or cessation if associated with menopause.
- Central fat redistribution is characteristic of menopausal changes.
- Hormone replacement therapy may aid in weight management for suitable candidates.
Hypercortisolemia and Cushing Syndrome
Cushing syndrome should be considered in patients with rapid weight gain and specific clinical features. Recent evidence suggests that screening should focus on metabolically unhealthy obesity phenotypes rather than universal screening (see Table 2).[4] For additional information, please refer to StatPearls' companion resource, "Hypercortisolism (Cushing Syndrome)."
Characteristic clinical features of Cushing syndrome include:
- Central obesity with relative limb sparing
- Purple striae >1 cm width
- Easy bruising without trauma
- Proximal muscle weakness
- Hypertension and diabetes mellitus
- Facial plethora and supraclavicular fat pads
Table 2. Hypercortisolemia Screening Tests
|
Test |
Method |
Normal Range |
Advantages |
Limitations |
|
24-hour urine cortisol |
Urine collection |
<100 μg/24h |
Gold standard |
Collection errors common |
|
Late-night salivary cortisol |
Saliva sample |
<0.09 μg/dL |
Convenient, reflects free cortisol |
Contamination possible |
|
Overnight dexamethasone suppression |
1 mg dexamethasone at 11 PM |
Cortisol <1.8 μg/dL |
Simple outpatient test |
False positives in obesity |
| Pause and Reflect |
A 42-year-old perimenopausal woman presents with complaints of having gained 35 kg over 2 years. She is noted to have central obesity with limb sparing, purple striae >1 cm, and hypertension onset within the last year.
|
Key considerations include:
- Highly suspicious features of Cushing syndrome include central obesity with limb sparing, purple striae >1 cm, easy bruising, proximal muscle weakness, and the onset of diabetes/hypertension accompanying the weight gain.
- Psychiatric symptoms and glucose intolerance may also occur.
- Late-night salivary cortisol is the preferred initial test (convenient, reflects free cortisol, although it has higher false positives in obesity). Some individuals prefer an overnight dexamethasone suppression test as a first-line study, although this test has a high false-positive rate in obesity.
- A 24-hour urine cortisol test may be performed when collection compliance can be reliably ensured.
- Screen selectively based on clinical features rather than universal screening.
- Consider plasma adrenocorticotropic hormone if the initial screening is positive, to differentiate potential alternative etiologies.
Hypothalamic Obesity
Hypothalamic obesity often develops following injury to the hypothalamus caused by conditions such as craniopharyngioma, brain surgery, traumatic brain injury, central nervous system infection, hypothalamic tumors, or radiation therapy. Patients typically present with rapid weight gain exceeding 10 kg within a few months, accompanied by hyperphagia and impaired satiety regulation. Unlike primary obesity, hypothalamic obesity involves dysregulation of appetite centers, resulting in persistent hunger and reduced responsiveness to internal satiety cues.[5] Additional clinical features of hypothalamic obesity include:
- Autonomic dysfunction
- Sleep disturbances
- Temperature regulation abnormalities
Management strategies differ significantly from those used in conventional obesity management. Standard diet and exercise approaches usually yield limited benefit. Treatment focuses on pharmacologic support with agents that enhance satiety, eg, glucagon-like peptide-1 receptor agonists, which demonstrate the most consistent promise. Structured meal timing and behavioral interventions also support energy balance. Additional therapies, including octreotide and diazoxide, have shown variable effectiveness. Many patients require hormone replacement to address coexisting endocrine deficiencies resulting from hypothalamic-pituitary disruption and common comorbidities, eg, sleep disturbances. Please refer to StatPearls' companion resource, "Craniopharyngioma," for additional information.
| Pause and Reflect |
A 17-year-old girl presents with a 14 kg weight gain over 4 months, accompanied by constant hunger and poor response to diet and exercise. She has a history of craniopharyngioma resection and radiation therapy 1 year ago, and currently receives hormone replacement for panhypopituitarism. Her symptoms include hyperphagia, irregular sleep, and infrequent menstruation.
|
Growth Hormone Deficiency
Adult growth hormone deficiency (GHD) contributes to increased visceral adiposity, reduced lean body mass, and metabolic dysfunction. Diagnosis requires stimulation testing with body mass index (BMI)-adjusted cut-offs due to obesity-related alterations in growth hormone response.[6]
Clinical findings associated with GHD include:
- Increased visceral fat
- Decreased muscle mass and strength
- Reduced exercise capacity
- Impaired quality of life
- Metabolic abnormalities
Diagnostic criteria used in the evaluation of GHD include:
- Clinical features (eg, increased visceral fat, decreased muscle mass and strength, reduced exercise capacity, impaired quality of life, metabolic abnormalities)
- Biochemical testing:
- Insulin-like growth factor-1 levels <-2 standard deviations for age and sex
- Growth hormone stimulation test with peak growth hormone <3 ng/mL (standard cut-off)
- BMI-adjusted cut-offs: <4 ng/mL for BMI <25 kg/m², <8 ng/mL for BMI 25 to 30 kg/m², <11 ng/mL for BMI >30 kg/m²
- Stimulation tests include the insulin tolerance, glucagon stimulation, and arginine-growth hormone-releasing hormone tests. Testing should be avoided in individuals with active malignancy, uncontrolled diabetes, or significant cardiovascular disease, due to increased risk.[7]
Insulinoma and Hypoglycemic Disorders
Insulinoma presents with the Whipple triad (symptoms of hypoglycemia, documented low glucose levels, and symptom relief with glucose administration), which is present in more than 95% of patients with insulinoma. Weight gain occurs via increased caloric intake to prevent hypoglycemic episodes. Diagnosis requires a supervised in-hospital fasting test.
Diagnostic Approach
The diagnostic process begins with a 72-hour supervised fast, during which plasma glucose, insulin, C-peptide, and proinsulin levels are monitored during a symptomatic episode. The presence of the Whipple triad remains central to the diagnosis:
- Symptoms of hypoglycemia, including neuroglycopenic or autonomic features
- Documented plasma glucose below 50 mg/dL (2.8 mmol/L)
- Symptom relief after glucose administration
Biochemical 72-hour supervised fast results commonly include:
- Plasma glucose <45 mg/dL (2.5 mmol/L)
- Insulin ≥3 μU/mL (18 pmol/L)
- C-peptide ≥0.6 ng/mL (0.2 nmol/L)
- Proinsulin ≥5 pmol/L
- β-hydroxybutyrate ≤2.7 mmol/L
- Absence of sulfonylurea metabolites
Additional diagnostic criteria:
- Inappropriate insulin secretion relative to glucose level
- Negative screening for exogenous insulin/sulfonylurea
- Positive response to glucagon (glucose rise >25 mg/dL)
For additional information on diagnostic evaluation, please refer to StatPearls' companion resources, "Insulinoma" and "Hypoglycemia."
Specialized Adipose Tissue Disorders
Lipodystrophy
Lipodystrophy syndromes encompass a group of rare disorders characterized by partial or complete loss of subcutaneous adipose tissue. This adipose tissue deficiency leads to abnormal fat redistribution and ectopic fat deposition, often resulting in severe metabolic complications, including insulin resistance, hypertriglyceridemia, and hepatic steatosis. Prompt recognition of these syndromes remains essential for initiating appropriate treatment and providing genetic counseling, especially in congenital forms.[8]
Lipodystrophy may be congenital or acquired. Congenital forms include Berardinelli-Seip syndrome and familial partial lipodystrophy, while acquired variants may result from HIV infection, autoimmune conditions, or drug exposure. Clinical hallmarks include a visible loss of subcutaneous fat, particularly in characteristic distribution patterns, accompanied by muscular prominence. Affected individuals frequently exhibit severe insulin resistance, elevated triglycerides, and fatty liver disease. Diagnostic evaluation also requires attention to hormonal and metabolic markers.
Lipodystrophy Diagnostic Criteria
The types of lipodystrophy are associated with the following clinical features:
- Congenital generalized lipodystrophy (Berardinelli-Seip syndrome): Affected individuals have a complete absence of subcutaneous and visceral adipose tissue from birth. Prominent musculature and visible veins often accompany this presentation. Additional features include acanthosis nigricans, hepatomegaly, and early-onset diabetes driven by profound insulin resistance.
- Familial partial lipodystrophy (Dunnigan type): Patients experience progressive fat loss from the limbs and trunk, typically sparing the face and neck. This form commonly presents at puberty and occurs more frequently in females. Associated metabolic disturbances include diabetes, hypertriglyceridemia, and features of polycystic ovary syndrome.
- Acquired generalized lipodystrophy: This disorder is characterized by a gradual and widespread loss of subcutaneous fat, often occurring in the context of autoimmune diseases. Laboratory testing may reveal complement abnormalities, eg, low C4 or the presence of C3 nephritic factor.
Biochemical markers often reveal severe insulin resistance with fasting insulin levels exceeding 30 μU/mL. Triglyceride levels typically surpass 500 mg/dL. Serum leptin concentrations fall below 4 ng/mL, but must be interpreted in conjunction with overall fat mass and metabolic status. Elevated liver enzymes often signal hepatic steatosis. Imaging techniques, such as magnetic resonance imaging (MRI) or dual-energy x-ray absorptiometry (DEXA) scanning, help quantify and map fat distribution, supporting diagnosis and disease monitoring. Please refer to the StatPearls' companion resource, "Lipodystrophy," for additional information.
Lipedema
Lipedema is a chronic disorder characterized by the symmetrical enlargement of the legs and, occasionally, the arms due to the deposition of subcutaneous fat. Unlike lymphedema, lipedema spares the feet and hands, creating a characteristic "cuff" appearance (see Table 6). The estimated prevalence in women ranges from 1% to 10%, although the actual prevalence remains unknown. In men, the condition is rare.[9]
Diagnostic clinical features include:
- Bilateral, symmetrical fat deposition in legs/arms
- Sparing of the feet and hands
- Pain and tenderness to touch
- Easy bruising
- Family history (genetic predisposition)
- A negative Stemmer sign. (A positive Stemmer sign occurs in lymphedema, when the skin at the base of the second toe cannot be pinched and lifted because it is thickened from lymphatic fluid accumulation.)
Table 6. Differential Diagnosis of Lipedema Versus Lymphedema
|
Feature |
Lipedema |
Lymphedema |
|
Distribution |
Bilateral legs/arms, sparing feet/hands |
Can involve feet/hands |
|
Stemmer sign |
Negative |
Positive |
|
Pitting edema |
Minimal or absent |
Present |
|
Pain/tenderness |
Common, especially to touch |
Usually painless |
|
Skin texture |
Soft, nodular fat |
Firm, fibrotic |
|
Family history |
Often positive |
Usually absent |
|
Response to elevation |
Minimal improvement |
Significant improvement |
|
Onset |
Puberty, pregnancy, and menopause |
Post-surgical, infection, congenital |
Other differential diagnoses include:
- Venous insufficiency: unilateral, skin changes
- Obesity: generalized fat distribution
Diagnostic Evaluation
Recognizing lipedema early facilitates timely management and helps differentiate it from other causes of limb swelling, eg, obesity or lymphedema. A confirmed diagnosis requires the presence of all the following primary criteria:
- Bilateral symmetrical enlargement of legs with or without arm involvement
- Minimal involvement of feet and hands (creating a "cuff" or "bracelet" sign)
- A positive family history of similarly affected relatives (genetic predisposition)
Several supportive features often accompany the primary findings, including:
- Pain and tenderness disproportionate to the degree of swelling
- Easy bruising with minimal trauma
- Nodular, lumpy texture of subcutaneous tissue
- Negative Stemmer sign
- Minimal pitting edema (unlike lymphedema)
- Poor response to diet and exercise
Although lipedema and lymphedema are distinct conditions, they may coexist in a combined form known as lipolymphedema. Several key features aid in clinical recognition, including a negative Stemmer sign in lipedema, the typical cuff-like distribution of fat, and disproportionate pain and tenderness in the affected regions. Lipedema often responds poorly to diet and exercise in terms of reducing localized fat. Hormonal changes—particularly those occurring during puberty, pregnancy, or menopause—frequently trigger symptom onset or progression, underscoring the condition's endocrine sensitivity.
Clinical Staging
Lipedema progresses through the following defined clinical stages:
- Stage 1: Smooth skin surface, homogeneous subcutaneous tissue
- Stage 2: Uneven skin surface, nodular subcutaneous tissue
- Stage 3: Large tissue folds, lobular deformations
- Stage 4: Lipedema with lymphedema (lipolymphedema)
Exclusion Criteria
Certain features, including unilateral limb enlargement, significant involvement of the feet or hands, a positive Stemmer sign, and painless, pitting edema, suggest that a lipedema diagnosis is unlikely. Exclusion of these findings ensures accurate differentiation from other disorders with similar clinical manifestations. Please refer to StatPearls' companion resource, "Lipedema," for additional information.
| Pause and Reflect |
A 40-year-old woman presents with bilateral leg swelling.
|
Other Adipose Tissue Disorders
Several rare adipose tissue disorders require clinical recognition and exclusion of more common conditions. These disorders often present with characteristic fat distribution patterns, pain, or other systemic features and are generally diagnosed based on clinical criteria due to the absence of definitive biomarkers.
Dercum Disease (Adiposis Dolorosa)
Dercum disease is a clinical diagnosis of exclusion, primarily affecting postmenopausal women. The major diagnostic features include multiple painful subcutaneous lipomas and chronic pain localized to adipose tissue. Minor features may accompany the condition, such as obesity with a body mass index greater than 30 kg/m², generalized fatigue, weakness, cognitive dysfunction ("brain fog"), sleep disturbances, and joint stiffness.
Classification divides Dercum disease into the following 4 types:
- Type I: Multiple painful lipomas
- Type II: Diffuse painful adipose tissue without discrete nodules
- Type III: Nodular painful adipose tissue
- Type IV: Mixed presentation with both nodular and diffuse patterns
Madelung disease (Multiple Symmetric Lipomatosis)
Madelung disease, another clinical diagnosis of exclusion, features symmetrical, nonencapsulated lipomatous masses, most commonly in the neck and shoulder regions. The condition predominantly affects middle-aged men and is strongly associated with chronic alcohol use, present in more than 80% of cases. In some patients, extensive lipomatous growth may lead to complications, eg, airway compression. See StatPearls' companion reference, Madelung Deformity.
The following clinical variants have been described:
- Type I: Involvement of the neck, shoulders, and upper back
- Type II: Prominent accumulation in the deltoid regions
Familial Multiple Lipomatosis
Familial multiple lipomatosis follows an autosomal dominant inheritance pattern and typically manifests with numerous encapsulated, nonpainful lipomas. Unlike Dercum disease, pain is not a characteristic feature of the condition. This condition affects both sexes equally and is typically characterized by its genetic basis and the absence of associated systemic symptoms.
Obesity Diagnostic Evaluation
Anthropometric assessment
BMI remains the primary diagnostic tool for obesity, but has significant limitations in assessing body composition and metabolic risk. BMI underestimates adiposity in older individuals and overestimates adiposity in those with a muscular build (see Table 7). Recent evidence emphasizes the importance of complementary anthropometric measurements.[10][11]
Table 7. Body Mass Index Classifications and Limitations
|
BMI Category |
BMI Range (kg/m²) |
Limitations |
Clinical Implications |
|
Normal weight |
18.5–24.9 |
May miss sarcopenic obesity |
Consider body composition analysis |
|
Overweight |
25.0–29.9 |
Variable metabolic risk |
Assess waist circumference |
|
Obesity Class I |
30.0–34.9 |
May include metabolically healthy |
Comprehensive metabolic evaluation |
|
Obesity Class II |
35.0–39.9 |
Increased comorbidity risk |
Consider pharmacotherapy or bariatric referral |
|
Obesity Class III |
≥40.0 |
Severe health risks |
Intensive medical management |
Waist Circumference Criteria
Waist circumference is a key indicator of central adiposity and associated cardiometabolic risk. The standard thresholds for increased risk vary by ethnicity, reflecting differences in body composition and fat distribution. For White individuals, a waist circumference greater than 102 cm (40 inches) in men and greater than 88 cm (35 inches) in women signifies an elevated risk for metabolic complications. These cut-offs correlate with increased visceral fat and a higher likelihood of developing conditions such as type 2 diabetes, hypertension, and cardiovascular disease.
Asian-specific criteria adopt lower thresholds due to a greater predisposition to visceral adiposity at lower BMI values. In Asian populations, increased metabolic risk occurs at a lower waist circumference, 90 cm (35.4 inches) in men and 80 cm (31.5 inches) in women. This population experiences an earlier onset of metabolic disorders, including diabetes and cardiovascular disease, due to differing patterns of fat accumulation and heightened metabolic sensitivity at lower anthropometric measurements. Please refer to StatPearls' companion resources, "Anthropometric Measurement" and "Physiology, Body Mass Index," for additional information.
| Pause and Reflect |
A female patient with normal BMI (23 kg/m²) but elevated waist circumference (92 cm) presents for a well-woman examination.
|
The "metabolically unhealthy normal weight" phenotype describes individuals with a normal BMI but increased visceral adiposity and elevated cardiometabolic risk. This condition often carries a greater cardiovascular risk than metabolically healthy obesity. In women, a waist circumference exceeding 88 cm indicates an increased risk, regardless of BMI. This phenotype often escapes clinical detection due to a patient's normal weight and BMI, but it warrants careful evaluation and targeted intervention.
Metabolic screening, which includes assessments of glucose levels, lipid profiles, and blood pressure, is crucial for the early identification of risk factors. Management strategies should emphasize lifestyle modifications that reduce central adiposity through regular physical activity and dietary adjustments, improving insulin sensitivity and lipid metabolism. Visceral fat is more metabolically active than subcutaneous fat and contributes significantly to adverse outcomes. In Asian populations, lower waist circumference thresholds are recommended because elevated health risks occur at lower levels of adiposity. Recognizing and addressing the "metabolically unhealthy normal weight" phenotype is critical in preventing long-term cardiovascular disease and diabetes.
Obesity Staging System
The Edmonton Obesity Staging System provides superior prognostic ability compared to BMI alone by incorporating metabolic, physical, and psychological parameters (see Table 8). This staging system better predicts healthcare utilization and mortality risk.[12]
Table 8. Edmonton Obesity Staging System
|
Stage |
Metabolic |
Physical |
Psychological |
Management Approach |
|
0 |
No risk factors |
No limitations |
No issues |
Lifestyle counseling |
|
1 |
Subclinical risk factors |
Mild limitations |
Mild concerns |
Risk factor modification |
|
2 |
Established disease |
Moderate limitations |
Moderate impairment |
Medical management May warrant bariatric referral |
|
3 |
End-organ damage |
Significant limitations |
Significant impairment |
Intensive treatment |
|
4 |
Severe complications |
Severe limitations |
Severe impairment |
Palliative care |
Body Composition Analysis
Body composition assessment provides valuable information beyond BMI, particularly for identifying sarcopenic obesity, monitoring treatment response, and risk stratification.[13] The following modalities may be used for body composition analysis:
- Dual-energy x-ray absorptiometry
- Is the gold standard for body composition analysis, providing precise measurements of bone mineral density, fat mass, and lean body mass
- Uses 2 low-dose x-ray beams with different energy levels to differentiate between bone, fat, and lean tissue
- Is excellent for detecting sarcopenic obesity (normal BMI with low muscle mass and high fat percentage) and monitoring treatment response
- Has limitations, including the inability to distinguish between visceral and subcutaneous fat and high equipment costs
- Bioelectrical impedance analysis
- Is a practical, portable method for measuring electrical impedance through body tissues to estimate body composition
- Is based on the principle that lean tissue conducts electricity better than fat tissue due to higher water content
- Provides rapid assessment of body fat percentage, muscle mass, and total body water in clinical settings
- Can be used in obesity, but its precision is reduced
- Accuracy is affected by hydration status, recent exercise, and food intake; this requires population-specific prediction equations
- Computed tomography and MRI
- The most accurate methods for quantifying visceral adipose tissue typically measure it at the L4–L5 vertebral level.
- Computed tomography provides excellent tissue contrast for distinguishing between visceral and subcutaneous fat compartments with minimal radiation exposure.
- MRI offers superior soft tissue resolution without radiation, ideal for research and pediatric populations
- Both methods are expensive and time-consuming, primarily used for research rather than routine clinical assessment.
Sarcopenic Obesity
Sarcopenic obesity represents the coexistence of excess adiposity with reduced muscle mass and function, creating a particularly high-risk phenotype. Normal or elevated BMI characterizes this condition with decreased muscle mass (typically <2 standard deviations below young adult reference values), reduced muscle strength, and impaired physical performance. According to the European Working Group on Sarcopenia in Older People criteria, muscle strength and function are more important than mass alone.
Sarcopenic obesity is associated with greater metabolic dysfunction, increased cardiovascular risk, and higher mortality compared to either sarcopenia or obesity alone. Diagnosis requires body composition analysis (preferably dual-energy x-ray absorptiometry [DEXA], although DEXA can underestimate visceral fat) combined with functional assessments, such as grip strength and gait speed. This condition is increasingly recognized in older adults and emphasizes the limitations of BMI as a sole diagnostic tool for obesity-related health risks.
Metabolic Phenotyping
Distinguishing between metabolically healthy and unhealthy obesity phenotypes has important prognostic and therapeutic implications. No universally accepted definition exists; criteria vary slightly across studies.[14][15]
Metabolically healthy obesity criteria include:
- Waist circumference <102 cm in men and <88 cm in women
- Normal blood pressure (<130/85 mm Hg)
- Normal glucose metabolism (HbA1c <5.7%)
- High-density lipoprotein cholesterol >40 mg/dL in men and >50 mg/dL in women
- Triglycerides <150 mg/dL
Metabolically unhealthy normal weight may be defined by the following parameters:
- BMI 18.5–24.9 kg/m² with metabolic abnormalities
- Increased visceral adiposity
- Insulin resistance
- Higher cardiovascular risk than metabolically healthy obesity
| Pause and Reflect |
A 45-year-old man with a BMI of 32 kg/m² presents for an annual examination.
|
Metabolically healthy obesity (MHO) affects approximately 25% to 30% of obese individuals. This phenotype reflects the presence of obesity without the typical metabolic disturbances such as insulin resistance, dyslipidemia, or hypertension. Waist circumference, given its correlation with visceral adiposity and cardiometabolic risk, is critical in distinguishing between metabolically healthy and unhealthy obesity.
The designation of MHO requires fulfillment of all 5 metabolic health criteria, emphasizing the need for comprehensive assessment. However, this state often remains transient, with many individuals progressing to metabolically unhealthy obesity over time. Regular monitoring of metabolic status enables the early detection of such changes, allowing for timely intervention and effective management. Inflammatory markers may offer additional prognostic value, aiding risk stratification. Clinicians may adjust treatment intensity based on an individual's metabolic profile, balancing intervention needs with the current risk level associated with the MHO phenotype.
Energy Expenditure Assessment
Accurate assessment of energy expenditure is essential for personalized treatment planning and monitoring metabolic adaptation to weight loss. Various methods can be used to estimate a patient's energy expenditure (see Table 9). Please refer to StatPearls' companion resources, "Biochemistry Heat and Calories" and Metabolic Syndrome, for further information on metabolic energy measurements.
Table 9. Energy Expenditure Estimation Methods
|
Method |
Accuracy |
Clinical Utility |
Limitations |
|
Indirect calorimetry |
Gold standard |
Research settings |
Equipment intensive |
|
Harris-Benedict equation |
Moderate |
Clinical practice |
Overestimates in patients with obesity |
|
Mifflin-St. Jeor equation |
Good |
Clinical practice |
Better accuracy in patients with obesity |
|
Predictive equations |
Variable |
Screening |
Population-specific |
Resting Metabolic Rate
Several physiological and clinical factors influence the resting metabolic rate (RMR). Age plays a significant role, with RMR gradually declining over time due to reductions in lean body mass. Sex also affects metabolic rate, as men generally exhibit higher RMRs than women, mainly due to differences in muscle mass and hormonal profiles. Body composition remains a key determinant, with individuals possessing greater lean mass demonstrating higher RMR.
Thyroid function significantly impacts metabolic rate, with hypothyroidism reducing and hyperthyroidism increasing energy expenditure. A history of weight loss may also reduce RMR through adaptive thermogenesis, a compensatory response that lowers energy expenditure and promotes weight regain. Additionally, various medications and medical conditions can modulate RMR, either enhancing or suppressing metabolic activity depending on their physiological effects. Understanding these factors supports individualized approaches to weight management and metabolic health.
Quality of Life Assessment
Health-related quality of life assessment provides important outcomes data for treatment planning and monitoring therapeutic response.[16]
Validated quality of life assessment tools include:
- Impact of Weight on Quality of Life-Lite
- Short Form-36 Health Survey
- Beck depression inventory
Clinical Significance
Clinical Integration and Board Examination Preparation
Mastery of secondary obesity evaluation and comprehensive diagnostic characterization represents essential competencies for the American Board of Obesity Medicine board certification. This systematic approach ensures thorough patient evaluation while preparing candidates for examination success through evidence-based clinical reasoning and diagnostic precision. Integrating anthropometric measurements, laboratory assessments, body composition analysis, and quality of life evaluation provides a comprehensive framework for obesity management that extends beyond simple BMI classification.
Understanding the importance of universal thyroid screening in individuals with obesity, recognizing reversible obesity-related hypogonadism, utilizing the Edmonton Obesity Staging System appropriately, and acknowledging the clinical significance of metabolic phenotyping are all essential. Early identification of secondary causes can lead to targeted interventions that may be more effective than lifestyle modification alone, emphasizing the critical role of systematic evaluation in obesity medicine practice.
Approach to Secondary Obesity Evaluation: A Diagnostic Algorithm
Step 1: Comprehensive assessment
A thorough initial assessment begins with a detailed history that includes the timing of weight gain, associated symptoms, current medications, and relevant family history. Physical examination should focus on anthropometric measurements and identifying clinical signs suggestive of secondary causes of obesity. Attention to red flag symptoms suggestive of endocrine disorders guides further evaluation and management.
Red flag symptoms
- Rapid weight gain (>5 kg in 6 months)
- Weight gain despite appropriate lifestyle interventions
- Associated endocrine symptoms (eg, fatigue, cold intolerance, and mood changes)
- Family history of endocrine disorders
- Onset of diabetes or hypertension with weight gain
Step 2: Universal screening
All patients should undergo universal screening, including thyroid-stimulating hormone testing, given that thyroid dysfunction is the most common secondary endocrine cause of obesity. Additional laboratory studies include a comprehensive metabolic panel, lipid profile, HbA1c, and complete blood count to assess metabolic status and identify potential comorbidities.
Step 3: Targeted evaluation based on clinical suspicion
Clinical suspicion should guide targeted evaluation for possible underlying causes of obesity. Symptoms such as fatigue and cold intolerance suggest thyroid dysfunction, while erectile dysfunction or irregular menstruation may indicate gonadal dysfunction. Features of adrenal disorders, including purple striae greater than 1 cm and easy bruising, raise concern for Cushing syndrome. Specialized conditions, eg, hypothalamic obesity, lipodystrophy, or lipedema, warrant focused clinical investigation based on a patient's presentation.
Step 4: Comprehensive obesity characterization
Assessing obesity involves tools such as the Edmonton Obesity Staging System to evaluate the functional and metabolic impact. Body composition analysis, preferably using DEXA, provides a more accurate measure of adiposity than the body mass index alone. Phenotyping, distinguishing between metabolically healthy and unhealthy obesity, supports risk stratification. Additional components, such as quality of life assessment and energy expenditure estimation, provide a comprehensive clinical picture.
Step 5: Integrated treatment planning
Effective treatment planning involves directly and effectively addressing confirmed secondary causes of obesity. Personalized lifestyle interventions should align with the individual's phenotype and clinical findings. Medical therapy must be tailored accordingly, and bariatric surgery evaluation may be appropriate for select patients. Ongoing monitoring and periodic reassessment remain essential to adjust interventions based on patient progress and evolving clinical needs.
Please refer to StatPearls' companion resource, "Obesity Evaluation and Management", for further information.
| Pause and Reflect |
A 45-year-old woman presented with a 15-kg weight gain over 18 months despite adherence to dietary recommendations. Additional symptoms include fatigue, irregular menstruation, and easy bruising.
|
Clinicians should remember that a focused history and physical examination are the foundation for guiding appropriate and targeted diagnostic testing in individuals with obesity. Universal screening for thyroid dysfunction remains a critical component of the initial evaluation, given the prevalence of subclinical hypothyroidism and its potential contribution to weight gain. Additionally, the presence of red flag symptoms, eg, rapid weight gain, muscle weakness, easy bruising, menstrual irregularities, or signs of adrenal dysfunction, requires further investigation to rule out secondary causes of obesity.
Furthermore, the Edmonton Obesity Staging System offers a more nuanced assessment of obesity-related risk beyond BMI, enabling comprehensive clinical characterization. Body composition analysis can reveal phenotypes, eg, sarcopenic obesity or increased visceral fat, that BMI alone may fail to detect. Metabolic phenotyping holds essential prognostic and therapeutic value by informing individualized management strategies. A systematic and structured diagnostic approach enhances clinical accuracy, reduces the likelihood of missed secondary diagnoses, and supports the development of effective, personalized treatment plans.
Other Issues
Quick Reference: Secondary Causes of Obesity
See Table 10 below, which outlines the key diagnostic features to observe when treating patients with obesity.
Table 10. Key Diagnostic Features
|
Condition |
Prevalence |
Key Clinical Features |
Primary Screening Test |
Confirmatory Studies |
|
Primary hypothyroidism |
10%–15% |
Fatigue, cold intolerance, constipation |
TSH |
Free T4, anti-TPO antibodies |
|
Male hypogonadism |
25%–40% |
Decreased libido, erectile dysfunction, fatigue |
Morning testosterone |
LH, FSH, repeat testosterone |
|
Female hypogonadism |
Variable |
Irregular menses, anovulation, and mood changes |
FSH, LH, estradiol |
Comprehensive hormone panel |
|
Cushing syndrome |
<1% |
Purple striae >1 cm, easy bruising, proximal weakness |
Late-night salivary cortisol |
24-hour urine cortisol, dexamethasone suppression |
|
Growth hormone deficiency |
1%–3%* |
Increased visceral fat, decreased muscle mass |
IGF-1 |
GH stimulation test (BMI-adjusted) |
|
Hypothalamic obesity |
<1% |
Rapid weight gain post-surgery/trauma, hyperphagia |
Clinical history |
MRI brain, comprehensive hormone panel |
|
Insulinoma |
<0.1% |
Whipple triad, reactive hypoglycemia |
72-hour fast |
Insulin, C-peptide, and proinsulin during a hypoglycemia event |
|
Lipodystrophy |
Rare |
Absence of subcutaneous fat, severe insulin resistance |
Clinical examination |
Leptin levels, body composition analysis |
|
Lipedema |
1%–10%** |
Bilateral leg swelling sparing feet, painful fat |
Clinical examination |
Negative Stemmer sign, family history |
BMI, body mass index; FSH, follicle-stimulating hormone; GH, growth hormone; IGF-1, insulin-like growth factor 1; LH, luteinizing hormone; MRI, magnetic resonance imaging; TPO, thyroid peroxidase antibody; TSH, thyroid-stimulating hormone
*In specific high-risk populations (post-craniopharyngioma or pituitary damage)
**Estimated prevalence in women; true prevalence unknown; rare in men
Enhancing Healthcare Team Outcomes
An interprofessional team approach ensures that all potential contributors to obesity are addressed comprehensively, with each discipline bringing unique expertise to optimize diagnosis, treatment, and patient outcomes. Identifying secondary causes of obesity is crucial for making accurate diagnoses and developing effective treatment plans. Conditions such as hypothyroidism, Cushing syndrome, and other endocrine or medication-related disorders can contribute to weight gain and often go unrecognized. A systematic evaluation, including a comprehensive history, physical examination, laboratory screening, and body composition analysis, allows clinicians to detect underlying etiologies and tailor interventions accordingly.
Physicians and advanced clinicians obtain detailed medical histories, recognize red flag symptoms, and order appropriate tests to diagnose secondary causes of obesity. Nurses play a vital role in patient education and follow-up monitoring, while pharmacists ensure medication regimens do not contribute to further weight gain. Collaboration across disciplines supports early diagnosis, timely referrals, and effective treatment planning. Coordinated communication among health professionals, especially during care transitions, promotes consistency and enhances outcomes. Using staging systems, such as the Edmonton Obesity Staging System and metabolic phenotyping, enhances risk stratification and personalizes care, improving patient safety and more effective management. By integrating the skills and perspectives of multiple disciplines, the healthcare team can more effectively identify secondary causes of obesity, implement targeted interventions, and improve immediate and long-term patient outcomes.
Media
(Click Image to Enlarge)
References
Pasquali R, Casanueva F, Haluzik M, van Hulsteijn L, Ledoux S, Monteiro MP, Salvador J, Santini F, Toplak H, Dekkers OM. European Society of Endocrinology Clinical Practice Guideline: Endocrine work-up in obesity. European journal of endocrinology. 2020 Jan:182(1):G1-G32. doi: 10.1530/EJE-19-0893. Epub [PubMed PMID: 31855556]
Level 1 (high-level) evidenceSong RH, Wang B, Yao QM, Li Q, Jia X, Zhang JA. The Impact of Obesity on Thyroid Autoimmunity and Dysfunction: A Systematic Review and Meta-Analysis. Frontiers in immunology. 2019:10():2349. doi: 10.3389/fimmu.2019.02349. Epub 2019 Oct 1 [PubMed PMID: 31681268]
Level 1 (high-level) evidenceKapoor E, Collazo-Clavell ML, Faubion SS. Weight Gain in Women at Midlife: A Concise Review of the Pathophysiology and Strategies for Management. Mayo Clinic proceedings. 2017 Oct:92(10):1552-1558. doi: 10.1016/j.mayocp.2017.08.004. Epub [PubMed PMID: 28982486]
Nieman LK. Diagnosis of Cushing's Syndrome in the Modern Era. Endocrinology and metabolism clinics of North America. 2018 Jun:47(2):259-273. doi: 10.1016/j.ecl.2018.02.001. Epub [PubMed PMID: 29754631]
van Iersel L, Brokke KE, Adan RAH, Bulthuis LCM, van den Akker ELT, van Santen HM. Pathophysiology and Individualized Treatment of Hypothalamic Obesity Following Craniopharyngioma and Other Suprasellar Tumors: A Systematic Review. Endocrine reviews. 2019 Feb 1:40(1):193-235. doi: 10.1210/er.2018-00017. Epub [PubMed PMID: 30247642]
Level 1 (high-level) evidenceYuen KCJ, Biller BMK, Radovick S, Carmichael JD, Jasim S, Pantalone KM, Hoffman AR. AMERICAN ASSOCIATION OF CLINICAL ENDOCRINOLOGISTS AND AMERICAN COLLEGE OF ENDOCRINOLOGY GUIDELINES FOR MANAGEMENT OF GROWTH HORMONE DEFICIENCY IN ADULTS AND PATIENTS TRANSITIONING FROM PEDIATRIC TO ADULT CARE. Endocrine practice : official journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists. 2019 Nov:25(11):1191-1232. doi: 10.4158/GL-2019-0405. Epub [PubMed PMID: 31760824]
Kim JH, Chae HW, Chin SO, Ku CR, Park KH, Lim DJ, Kim KJ, Lim JS, Kim G, Choi YM, Ahn SH, Jeon MJ, Hwangbo Y, Lee JH, Kim BK, Choi YJ, Lee KA, Moon SS, Ahn HY, Choi HS, Hong SM, Shin DY, Seo JA, Kim SH, Oh S, Yu SH, Kim BJ, Shin CH, Kim SW, Kim CH, Lee EJ. Diagnosis and Treatment of Growth Hormone Deficiency: A Position Statement from Korean Endocrine Society and Korean Society of Pediatric Endocrinology. Endocrinology and metabolism (Seoul, Korea). 2020 Jun:35(2):272-287. doi: 10.3803/EnM.2020.35.2.272. Epub 2020 Jun 24 [PubMed PMID: 32615711]
Araújo-Vilar D, Santini F. Diagnosis and treatment of lipodystrophy: a step-by-step approach. Journal of endocrinological investigation. 2019 Jan:42(1):61-73. doi: 10.1007/s40618-018-0887-z. Epub 2018 Apr 27 [PubMed PMID: 29704234]
Buso G, Depairon M, Tomson D, Raffoul W, Vettor R, Mazzolai L. Lipedema: A Call to Action! Obesity (Silver Spring, Md.). 2019 Oct:27(10):1567-1576. doi: 10.1002/oby.22597. Epub [PubMed PMID: 31544340]
Sweatt K, Garvey WT, Martins C. Strengths and Limitations of BMI in the Diagnosis of Obesity: What is the Path Forward? Current obesity reports. 2024 Sep:13(3):584-595. doi: 10.1007/s13679-024-00580-1. Epub 2024 Jul 3 [PubMed PMID: 38958869]
Ross R, Neeland IJ, Yamashita S, Shai I, Seidell J, Magni P, Santos RD, Arsenault B, Cuevas A, Hu FB, Griffin BA, Zambon A, Barter P, Fruchart JC, Eckel RH, Matsuzawa Y, Després JP. Waist circumference as a vital sign in clinical practice: a Consensus Statement from the IAS and ICCR Working Group on Visceral Obesity. Nature reviews. Endocrinology. 2020 Mar:16(3):177-189. doi: 10.1038/s41574-019-0310-7. Epub 2020 Feb 4 [PubMed PMID: 32020062]
Level 3 (low-level) evidenceAtlantis E, Fahey P, Williams K, Edwards S, Samaras K, Dugdale P, Shi Z, Sharma AM. Comparing the predictive ability of the Edmonton Obesity Staging System with the body mass index for use of health services and pharmacotherapies in Australian adults: A nationally representative cross-sectional study. Clinical obesity. 2020 Aug:10(4):e12368. doi: 10.1111/cob.12368. Epub 2020 May 18 [PubMed PMID: 32419298]
Level 2 (mid-level) evidenceKhattak ZE, Zahra F. Evaluation of Patients With Obesity. StatPearls. 2025 Jan:(): [PubMed PMID: 35015424]
Schulze MB, Stefan N. Metabolically healthy obesity: from epidemiology and mechanisms to clinical implications. Nature reviews. Endocrinology. 2024 Nov:20(11):633-646. doi: 10.1038/s41574-024-01008-5. Epub 2024 Jun 27 [PubMed PMID: 38937638]
Blüher M. Metabolically Healthy Obesity. Endocrine reviews. 2020 May 1:41(3):. doi: 10.1210/endrev/bnaa004. Epub [PubMed PMID: 32128581]
Kolotkin RL, Andersen JR. A systematic review of reviews: exploring the relationship between obesity, weight loss and health-related quality of life. Clinical obesity. 2017 Oct:7(5):273-289. doi: 10.1111/cob.12203. Epub 2017 Jul 10 [PubMed PMID: 28695722]
Level 1 (high-level) evidence