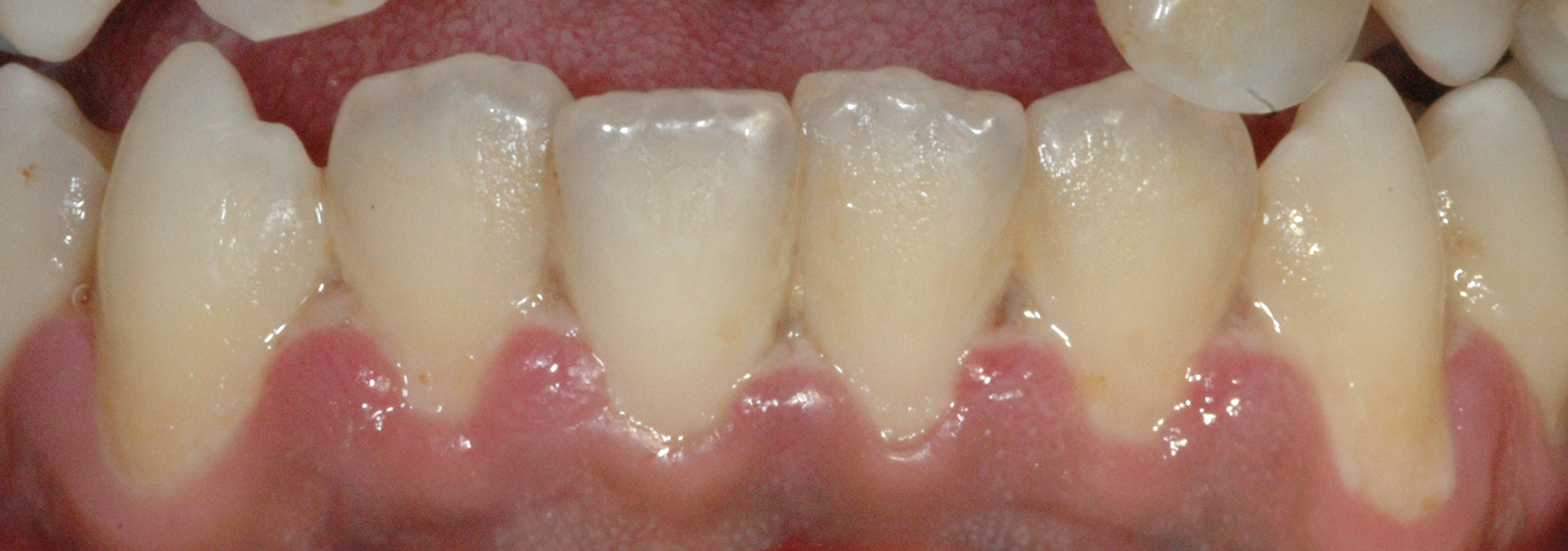
Introduction
Necrotizing periodontal diseases (NPDs) encompass necrotizing gingivitis, necrotizing periodontitis, and necrotizing stomatitis. In severe cases, NPDs may progress to noma (cancrum oris). These conditions represent stages of a single pathological process, sharing a common etiology and clinical profile.[1][2] Characteristic features include pain, gingival necrosis, interdental ulceration, and, in advanced stages, osteonecrosis.
Necrotizing gingivitis constitutes the most common and earliest manifestation, typically affecting immunosuppressed individuals. Necrosis is confined to the interdental papillae, which exhibit the classic punched-out morphology. The condition remains reversible with appropriate therapy while attachment loss has not yet occurred. However, without treatment, the disease may advance rapidly, causing periodontal attachment loss, pseudomembrane formation, and systemic signs such as fever and lymphadenopathy—features consistent with necrotizing periodontitis.[3]
Further extension into the alveolar bone with osteonecrosis and sequestration defines necrotizing stomatitis. The most severe presentation, noma, involves fulminant, noncontagious orofacial gangrene, primarily affecting severely malnourished individuals in low-resource settings. Noma rapidly destroys facial soft and hard tissues and carries a high risk of mortality (see Image. Noma).[4]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Although NPDs are infectious, immunosuppression is the most significant predisposing factor. Cases of necrotizing gingivitis and necrotizing periodontitis occur almost exclusively in individuals with immunodeficiency, most notably those with HIV infection. A CD4 count below 200 in an individual with HIV is more strongly associated with necrotizing periodontitis than any other known risk factor.[5] Additional conditions such as leukemia, neutropenia, diabetes mellitus, and prolonged immunosuppressive therapy have also been implicated.[6][7]
Tobacco smoking, severe psychological stress, malnutrition, inadequate sleep, and poor oral hygiene contribute to disease susceptibility. Many NPD cases occur in individuals who smoke tobacco. NPDs can also affect immunocompetent individuals. Malnutrition is a key factor, particularly in young children living in poverty with protein deficiency, and in populations with poor dietary habits, such as college students.[8] A history of necrotizing gingivitis is considered a risk factor for progression to necrotizing periodontitis.
Virulent bacteria, specifically Fusobacterium species, Prevotella intermedia, Porphyromonas gingivalis, and Treponema species, have been identified in necrotic lesions associated with NPDs.[9] Antibiotic therapy has been shown to alleviate symptoms during the acute phase, supporting a role for bacterial involvement in the disease process. Whether these organisms act as primary pathogens or represent secondary overgrowth in the context of immunosuppression is unclear. NPDs are not considered transmissible, suggesting that host-related factors, rather than exogenous sources, play a central role in disease initiation and progression.[10]
A recent study comparing microbial and inflammatory profiles in necrotizing gingivitis and chronic gingivitis identified a predominance of Bacteroidetes, particularly Prevotella species, and reduced microbial diversity in necrotizing gingivitis. Cases of necrotizing gingivitis also demonstrated elevated serum cytokine levels, consistent with a systemic inflammatory response. These findings support a dysbiosis model, in which synergistic microbial interactions, coupled with impaired host defenses, drive disease progression, characterizing necrotizing gingivitis as an opportunistic mixed microbial infection.[11]
The etiology of NPDs is incompletely understood. Current evidence suggests that preexisting periodontal conditions may undergo rapid and destructive exacerbation under permissive host or environmental conditions.[12]
Epidemiology
The global prevalence of NPDs is estimated to be less than 1%. Affected populations primarily include young adults aged 18 to 30, malnourished children, and individuals with immunocompromising conditions. NPDs occur more frequently in countries with lower socioeconomic status and are uncommon in developed nations.
Malnutrition is a major epidemiologic risk factor. The condition frequently affects severely malnourished children in low-income regions, particularly those with inadequate protein intake.[13] Additional contributing factors include tobacco smoking and poor oral hygiene.[14][15]
Pathophysiology
The pathogenesis of NPDs is incompletely understood. Necrotizing gingivitis is thought to result from commensal oral microorganisms that become pathogenic in the presence of impaired host defenses.[16] Fusobacterium species and spirochetes are frequently identified in NPD lesions. However, the role of these microbes as primary pathogens versus secondary or opportunistic organisms in immunocompromised individuals is uncertain.[17] These bacteria, along with other predominantly gram-negative species, produce destructive metabolites, including collagenases, endotoxins, hydrogen sulfide, and fibrinolysin, that contribute to the rapid degradation of the periodontium, gingiva, periodontal ligament, and alveolar bone.[18]
Histopathology
In 1965, Listgarten used electron microscopy to demonstrate spirochete invasion in what was then termed "necrotizing ulcerative gingivitis," identifying 4 distinct histological zones in order of increasing depth:
- Bacterial zone
- Neutrophil-rich zone
- Necrotic zone
- Zone of spirochete infiltration
The most superficial bacterial zone contains diverse bacterial species. The neutrophil-rich zone includes multiple leukocyte types, predominantly neutrophils. In the deeper necrotic zone, large numbers of dead cells and fragmented connective tissue are present. Spirochetes, along with other bacterial species, appear in all of the first 3 zones. However, only spirochetes are found in the zone of spirochete infiltration, penetrating the deeper, structurally intact tissue.[19]
History and Physical
NPDs are most commonly observed in patients with immunosuppression or significant psychological stress. A history of tobacco smoking is a major risk factor for disease onset.
Patients typically report severe pain, fetid breath, and rapid symptom development. Necrotizing periodontitis can cause loss of periodontal attachment within days. Some individuals with NPDs demonstrate regional lymphadenopathy, and systemic symptoms such as fever and malaise occur in more advanced stages. Pain intensity distinguishes NPDs from other periodontal conditions, which are usually painless. Consequently, patients often suspend oral hygiene practices, worsening halitosis. Inadequate oral intake due to pain may further contribute to malaise.[20]
Tissue necrosis is the hallmark clinical feature of NPDs, most often seen as ulcerated, necrotic gingiva with a characteristic punched-out or cratered appearance (see Image. Necrotizing Gingivitis). A pseudomembrane composed of plasma proteins and white blood cells covers the necrotic tissue. Disease progression from necrotizing gingivitis to necrotizing periodontitis results in loss of attachment and alveolar bone (see Image. Necrotizing Gingivitis with Pseudomembrane Formation).
A linear erythematous band typically demarcates the ulcerated zone from the surrounding attached gingiva, alveolar mucosa, and free gingiva. Bleeding may occur spontaneously or on provocation. Although loss of attachment defines necrotizing periodontitis, deep periodontal pockets are uncommon due to necrosis of the junctional epithelium. Rapid tissue destruction limits the time available for pocket formation. In necrotizing stomatitis, mucosal destruction extends beyond the mucogingival junction, exposing bone and resulting in alveolar necrosis with potential sequestration.
Evaluation
NPDs are diagnosed clinically. Intraoral examination must be supplemented by extraoral assessment to identify lymphadenopathy or facial asymmetry. Biopsy provides limited diagnostic value, typically showing only nonspecific inflammation. Radiographs assist in evaluating the extent of alveolar bone loss.
Radiographic findings range from minimal to extensive changes. Osteonecrosis may appear as a moth-eaten, patchy radiolucency, similar to features seen in medication-related osteonecrosis of the jaw, osteoradionecrosis, or osteomyelitis. Radiopaque sequestra may also be present. Computed tomography offers greater detail and aids in distinguishing among osseous pathologies. A definitive diagnosis depends on clinical findings and patient history.
Blood tests are recommended to evaluate for underlying predisposing conditions such as leukemia, neutropenia, or agranulocytosis. Unexplained or recurrent NPD episodes may signal an undiagnosed systemic disorder requiring further investigation.
Treatment / Management
Necrotizing gingivitis and necrotizing periodontitis are primarily treated using local measures. Management of NPDs depends on disease severity and extent, but initial therapy typically includes the following interventions:
- Gentle mechanical debridement under local anesthesia
- Removal of the pseudomembrane using a cotton pellet soaked in 0.12% chlorhexidine
- Reinforcement of oral hygiene practices
- Use of either a 0.12% chlorhexidine mouthwash or a hydrogen peroxide rinse
Nonsteroidal anti-inflammatory drugs (NSAIDs) may be given as needed for pain relief. Systemic antibiotics are indicated as an adjunct to local therapy when systemic signs, such as fever, malaise, or regional lymphadenopathy, are present.[21] Metronidazole is the preferred agent due to its efficacy against anaerobic bacteria. Amoxicillin may be considered when metronidazole is contraindicated.
Modifying risk factors and addressing underlying systemic conditions are essential components of NPD management. Tobacco smoking, psychological stress, and malnutrition must be addressed to reduce the likelihood of recurrence. Patients with necrotizing periodontitis often require medical evaluation for potential immunocompromising conditions, such as HIV, which must be treated concurrently with dental therapy.
Supportive recommendations include vitamin supplementation, avoidance of spicy foods, adequate hydration, and improved sleep hygiene. Ongoing follow-up for further treatment and long-term maintenance is critical to optimizing outcomes.
Necrotizing periodontitis may result in soft and hard tissue defects that complicate oral hygiene practices. After resolution of the acute phase, surgical intervention may be necessary. Gingivoplasty can correct minor soft tissue defects, while osteoplasty may be indicated for more extensive bony involvement.
Differential Diagnosis
NPDs should be differentiated from the following conditions:
- Oral mucositis
- Linear gingival erythema
- Herpes simplex virus
- Scurvy
- Gingivostomatitis
- Desquamative gingivitis
- Invasive fungal disease
- Illicit-drug-related gingival disease
- Agranulocytosis
- Leukemia
- Chronic periodontitis
Appropriate testing and history taking must be performed for an accurate diagnosis.[22]
Staging
According to the 2017 classification system proposed by the American Academy of Periodontology, NPD is a category of conditions that affect the periodontium through the degree of ulceration and tissue necrosis. This group includes 3 major subsets: necrotizing gingivitis, necrotizing periodontitis, and necrotizing stomatitis.
The differences between these subsets are based on the scope and location of damage. Necrotizing gingivitis refers to necrosis and ulceration confined to the gingiva. Progression to necrotizing periodontitis results in destruction and loss of the periodontium adjacent to each tooth, affecting the gingival tissues, alveolar bone, and periodontal ligament. Necrotizing stomatitis involves destruction of the mucous membranes in the mouth and extends beyond the mucogingival junction to areas such as the cheek, tongue, and palate.[23]
Prognosis
NPD recurrence is common, making regular recall visits essential for monitoring signs of reemergence. Prognosis largely depends on effective risk factor control. Improved outcomes are associated with better oral hygiene, adequate nutrition, stress reduction, smoking cessation, and appropriate treatment of underlying conditions, such as antiretroviral therapy in patients with HIV.
Complications
Complications of NPDs stem from their aggressive and destructive progression. Notable sequelae include the following:
- Tooth loss
- Progressive loss of attachment
- Extensive soft tissue necrosis
- Alveolar bone exposure
- Sequestration of bone fragments
- Bacteremia
- Weight loss and dehydration [24]
The risk of complications underscores the need for clinician vigilance and patient adherence. Preventive care and interprofessional collaboration play a central role in reducing disease burden.
Deterrence and Patient Education
Preventing NPDs hinges on addressing modifiable risk factors and encouraging early intervention. Patient education should emphasize the importance of meticulous oral hygiene, balanced nutrition with adequate protein intake, and effective stress management. Smoking cessation is essential, given the strong association between tobacco use and NPDs. Immunocompromised individuals, including those with HIV, leukemia, or undergoing immunosuppressive therapy, must be made aware of their heightened susceptibility.
Early recognition of signs such as acute gingival pain, bleeding, malodor, and the characteristic punched-out interdental papillae can help prevent progression to more destructive forms like necrotizing periodontitis. A history of NPD increases the risk of recurrence, necessitating regular monitoring. Timely dental care at the first sign of disease significantly improves outcomes and reduces the likelihood of complications.
Enhancing Healthcare Team Outcomes
NPDs are diagnosed clinically and distinguished by their rapid onset, severe pain, gingival necrosis, interdental ulceration, and, in advanced stages, osteonecrosis. General dentists must be proficient in identifying and managing NPDs and associated risk factors, including immunocompromised states, psychological stress, tobacco use, poor oral hygiene, and malnutrition. In addition to treatment, these providers play a key role in counseling patients on smoking cessation, nutrition, and stress management to reduce the risk of recurrence.
Although general dentists provide initial management, referral to a periodontal specialist or oral surgeon is often required for complex cases and long-term reconstruction. Effective treatment extends beyond the oral cavity and requires an interprofessional approach. Coordination with medical providers ensures that both oral and systemic factors are addressed, improving patient outcomes.
Media
(Click Image to Enlarge)
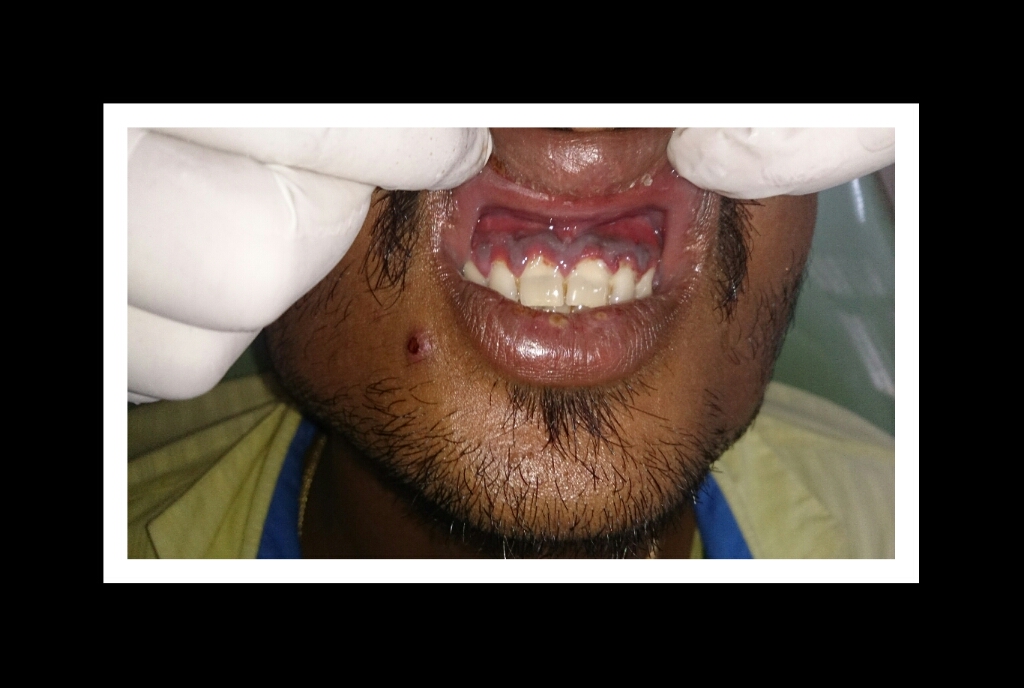
Necrotizing Gingivitis with Pseudomembrane Formation. The image shows necrotic interdental papillae covered by a grayish pseudomembrane, characteristic of early-stage necrotizing periodontal disease.
Contributed by Dr. Mohan M. Muthal, CC BY-SA 4.0 (https///creativecommons.org/licenses/by-sa/4.0), via Wikimedia Commons
(Click Image to Enlarge)
(Click Image to Enlarge)
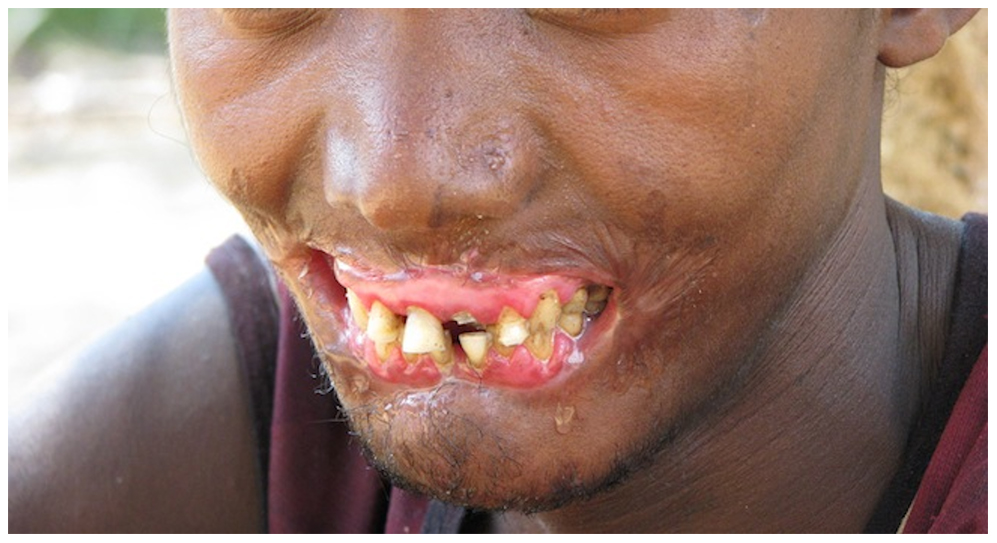
Noma. Also known as cancrum oris or mouth gangrene, this condition involves rapidly progressing gangrenous destruction of orofacial tissues, often beginning as necrotizing stomatitis.
Contributed By Brian L. Fisher, California Academy of Sciences. - Tonna JE, Lewin MR, Mensh B (2010) A Case and Review of Noma. PLoS Negl Trop Dis 4(12): e869. doi:10.1371/journal.pntd.0000869 (direct link to the image), CC BY 2.5, https://commons.wikimedia.org/w/index.php?curid=12651196
References
Herrera D, Alonso B, de Arriba L, Santa Cruz I, Serrano C, Sanz M. Acute periodontal lesions. Periodontology 2000. 2014 Jun:65(1):149-77. doi: 10.1111/prd.12022. Epub [PubMed PMID: 24738591]
Ogunleye R, Ukoha O, Nasterska W, McColl E, Dantata F, Adetula I. Necrotising periodontal diseases: an update on classification and management. British dental journal. 2022 Nov:233(10):855-858. doi: 10.1038/s41415-022-5201-y. Epub 2022 Nov 25 [PubMed PMID: 36434225]
Tkacz K, Gill J, McLernon M. Necrotising periodontal diseases and alcohol misuse - a cause of osteonecrosis? British dental journal. 2021 Aug:231(4):225-231. doi: 10.1038/s41415-021-3272-9. Epub 2021 Aug 27 [PubMed PMID: 34446893]
Srour ML, Marck K, Baratti-Mayer D. Noma: Overview of a Neglected Disease and Human Rights Violation. The American journal of tropical medicine and hygiene. 2017 Feb 8:96(2):268-274. doi: 10.4269/ajtmh.16-0718. Epub 2017 Jan 16 [PubMed PMID: 28093536]
Level 3 (low-level) evidenceJepsen S, Caton JG, Albandar JM, Bissada NF, Bouchard P, Cortellini P, Demirel K, de Sanctis M, Ercoli C, Fan J, Geurs NC, Hughes FJ, Jin L, Kantarci A, Lalla E, Madianos PN, Matthews D, McGuire MK, Mills MP, Preshaw PM, Reynolds MA, Sculean A, Susin C, West NX, Yamazaki K. Periodontal manifestations of systemic diseases and developmental and acquired conditions: Consensus report of workgroup 3 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. Journal of periodontology. 2018 Jun:89 Suppl 1():S237-S248. doi: 10.1002/JPER.17-0733. Epub [PubMed PMID: 29926943]
Level 3 (low-level) evidenceGowdey G, Alijanian A. Necrotizing ulcerative periodontitis in an HIV patient. Journal of the California Dental Association. 1995 Jan:23(1):57-9 [PubMed PMID: 9051993]
Level 3 (low-level) evidencePhiri R, Feller L, Blignaut E. The severity, extent and recurrence of necrotizing periodontal disease in relation to HIV status and CD4+ T cell count. Journal of the International Academy of Periodontology. 2010 Oct:12(4):98-103 [PubMed PMID: 21128527]
Level 2 (mid-level) evidenceHorning GM, Cohen ME. Necrotizing ulcerative gingivitis, periodontitis, and stomatitis: clinical staging and predisposing factors. Journal of periodontology. 1995 Nov:66(11):990-8 [PubMed PMID: 8558402]
Level 2 (mid-level) evidenceTodescan S, Nizar R. Managing patients with necrotizing ulcerative periodontitis. Journal (Canadian Dental Association). 2013:79():d44 [PubMed PMID: 23763731]
Bermejo-Fenoll A, Sánchez-Pérez A. Necrotising periodontal diseases. Medicina oral, patologia oral y cirugia bucal. 2004:9 Suppl():114-9; 108-14 [PubMed PMID: 15580128]
Gerhard N, Thurnheer T, Kreutzer S, Gmür RD, Attin T, Russo G, Karygianni L. Necrotizing Gingivitis: Microbial Diversity and Quantification of Protein Secretion in Necrotizing Gingivitis. Antibiotics (Basel, Switzerland). 2021 Oct 1:10(10):. doi: 10.3390/antibiotics10101197. Epub 2021 Oct 1 [PubMed PMID: 34680779]
Novak MJ. Necrotizing ulcerative periodontitis. Annals of periodontology. 1999 Dec:4(1):74-8 [PubMed PMID: 10863377]
Jiménez LM, Duque FL, Baer PN, Jiménez SB. Necrotizing ulcerative periodontal diseases in children and young adults in Medellín, Colombia, 1965--2000. Journal of the International Academy of Periodontology. 2005 Apr:7(2):55-63 [PubMed PMID: 15912925]
Wade DN, Kerns DG. Acute necrotizing ulcerative gingivitis-periodontitis: a literature review. Military medicine. 1998 May:163(5):337-42 [PubMed PMID: 9597852]
Gollapudi M, Mohod S, Pankey N, Gatlewar P. Acute Necrotizing Ulcerative Gingivitis: A Case Report. Cureus. 2024 Jun:16(6):e63023. doi: 10.7759/cureus.63023. Epub 2024 Jun 24 [PubMed PMID: 39050307]
Level 3 (low-level) evidenceLoesche WJ, Syed SA, Laughon BE, Stoll J. The bacteriology of acute necrotizing ulcerative gingivitis. Journal of periodontology. 1982 Apr:53(4):223-30 [PubMed PMID: 6122728]
Brook I. Microbiology and management of periodontal infections. General dentistry. 2003 Sep-Oct:51(5):424-8 [PubMed PMID: 15055631]
Dufty J, Gkranias N, Donos N. Necrotising Ulcerative Gingivitis: A Literature Review. Oral health & preventive dentistry. 2017:15(4):321-327. doi: 10.3290/j.ohpd.a38766. Epub [PubMed PMID: 28761942]
LISTGARTEN MA. ELECTRON MICROSCOPIC OBSERVATIONS ON THE BACTERIAL FLORA OF ACUTE NECROTIZING ULCERATIVE GINGIVITIS. The Journal of periodontology. 1965 Jul-Aug:36():328-39 [PubMed PMID: 14326701]
Berres F, Marinello CP. [Necrotizing ulcerative periodontitis. Diagnosis, treatment and follow-up--a case report]. Schweizer Monatsschrift fur Zahnmedizin = Revue mensuelle suisse d'odonto-stomatologie = Rivista mensile svizzera di odontologia e stomatologia. 2004:114(5):479-95 [PubMed PMID: 15250177]
Level 3 (low-level) evidenceBlair FM, Chapple IL. Prescribing for periodontal disease. Primary dental journal. 2014 Nov:3(4):38-43. doi: 10.1308/205016814813877234. Epub [PubMed PMID: 25668374]
Hu J, Kent P, Lennon JM, Logan LK. Acute necrotising ulcerative gingivitis in an immunocompromised young adult. BMJ case reports. 2015 Sep 16:2015():. doi: 10.1136/bcr-2015-211092. Epub 2015 Sep 16 [PubMed PMID: 26376700]
Level 3 (low-level) evidenceCaton JG, Armitage G, Berglundh T, Chapple ILC, Jepsen S, Kornman KS, Mealey BL, Papapanou PN, Sanz M, Tonetti MS. A new classification scheme for periodontal and peri-implant diseases and conditions - Introduction and key changes from the 1999 classification. Journal of clinical periodontology. 2018 Jun:45 Suppl 20():S1-S8. doi: 10.1111/jcpe.12935. Epub [PubMed PMID: 29926489]
Atout RN, Todescan S. Managing patients with necrotizing ulcerative gingivitis. Journal (Canadian Dental Association). 2013:79():d46 [PubMed PMID: 23763733]