Introduction
Sleep-disordered breathing (SDB) encompasses a spectrum of chronic conditions, including snoring, upper airway resistance syndrome (UARS), obstructive sleep apnea (OSA), and central sleep apnea (CSA), along with their subtypes.[1] These disorders have historically been described as abnormal breathing patterns during sleep, with definitions shaped by the recording technologies and clinical understanding available at the time.[2] Despite decades of research, no clear consensus has been established regarding the diagnostic criteria for UARS or whether it constitutes a distinct syndrome separate from OSA.[3]
OSA and CSA are typically defined by the number of apneic and hypopneic episodes per hour of sleep, measured as the apnea-hypopnea index (AHI). In contrast, UARS is generally characterized by increased upper airway resistance that causes arousals from sleep due to respiratory effort without significant oxygen desaturation—commonly referred to as respiratory effort–related arousals (RERAs)—and associated daytime symptoms.[4] A more specific definition includes an AHI of less than 5 events per hour, oxygen saturation at or above 92%, and a RERA index of at least 5 events per hour.[5] Another study defines UARS as having an AHI of less than 5 events per hour, a minimum peripheral capillary oxygen saturation (SpO2) of 92%, the presence of airflow limitation during at least 5% of total sleep time, and symptoms of daytime sleepiness or fatigue.[6]
This activity provides a comprehensive overview of the etiology, epidemiology, clinical presentation, diagnostic evaluation, management strategies, differential diagnosis, and potential complications of UARS. OSA and CSA are addressed in separate discussions.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Factors contributing to upper airway narrowing during sleep include disadvantageous anatomic features and impaired neuromuscular compensatory mechanisms that maintain airway patency. UARS occurs in the upper airway, most commonly due to partial narrowing and increased resistance in the retropalatal region (between the hard palate and the uvula) and the retroglossal region (between the uvula and the epiglottis).[7] Increased respiratory effort associated with inspiratory flow limitation can lead to repeated arousals from sleep, impacting both cortical and autonomic processes, which disrupt normal sleep architecture.[8]
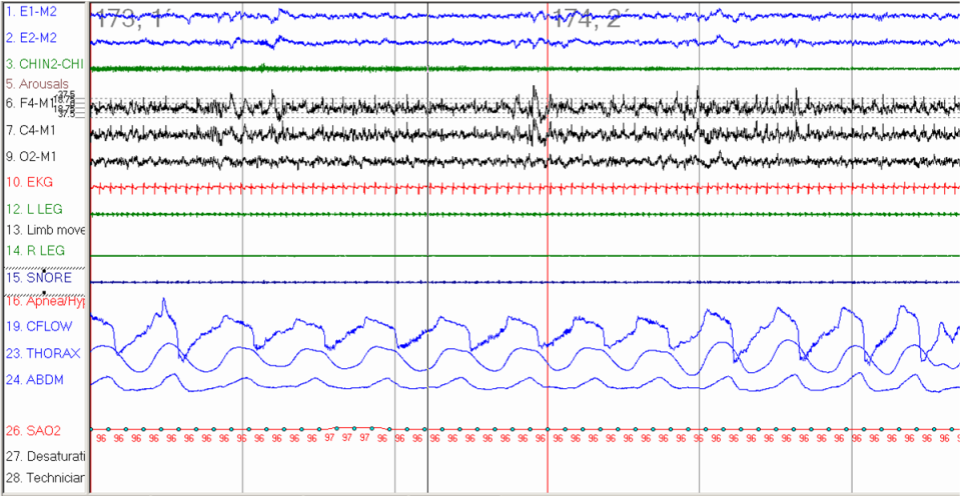
Frequent RERAs lead to nonrefreshing sleep, excessive daytime sleepiness, or unexplained daytime fatigue. A key parameter of UARS involves flow limitation during sleep that does not cause significant oxygen desaturation or meet the definition of hypopnea (see Image. Inspiratory Flow Limitation Consistent With Upper Airway Resistance Syndrome).[9]
UARS may represent an early stage in the development of OSA and offer valuable insight into the natural history of SDB. However, epidemiologic data remain limited regarding the role of nonapneic respiratory events, which are primarily associated with prolonged flow limitation and RERAs. In susceptible individuals with unfavorable upper airway anatomy, worsening airflow during sleep can lead to progressive upper airway narrowing. A low arousal threshold may further contribute to sleep fragmentation triggered by repeated episodes of flow limitation and SDB.[10]
Flow limitation can occur during inspiration, expiration, or both phases of the respiratory cycle.[11] The collapsibility of the upper airway also plays a critical role in maintaining airway patency. Upper airway collapsibility may be assessed by applying progressively negative pressure to the airway until the intraluminal pressure reaches the critical closing pressure (Pcrit). In healthy individuals, Pcrit is typically a negative value. In contrast, patients with UARS, obstructive hypopnea, or apnea demonstrate progressively increasing Pcrit values that may reach the positive range (Pcrit >0 cm H2O; see Image. Determination of Critical Closing Pressure).[12]
Airflow and upper airway resistance (Rua) are governed by Poiseuille law, commonly described in this context using the Starling resistor model of the upper airway.[13] Airway resistance is inversely proportional to the radius of the airway and directly related to the pressure gradient between the upstream pressure (Pus) and Pcrit. This relationship is expressed as Rua = (Pus − Pcrit)/Vmax, where Vmax represents maximal airflow. The collapsible segment of the upper airway narrows when Pus, typically measured as nasal pressure, approaches Pcrit, resulting in reduced airflow or complete obstruction, as observed in apnea.
During sleep in UARS, flow limitation may affect both inspiratory and expiratory phases due to the altered pressure gradient. Expiratory snoring and flow limitation may also occur in chronic obstructive lung diseases such as asthma and chronic obstructive pulmonary disease. A study reported that expiratory snoring alone predicted lower airflow obstruction, defined as a forced expiratory volume in the first second of expiration to forced vital capacity (FEV1/FVC) ratio of less than 70%.[14]
Epidemiology
Using a specific definition of UARS (AHI <5 events per hour, SpO2 ≥92%, airflow limitation during at least 5% of total sleep time, and daytime sleepiness or fatigue), a recent study reported a prevalence of UARS of 3.1% to 4.4% in women and 1.5% in men. In contrast, the prevalence of at least mild OSA, defined by an AHI greater than 5 events per hour, is 24% in men and 9% in women, with recent international studies estimating approximately 1 billion individuals affected worldwide.[15][16]
The exact prevalence of UARS is uncertain. However, approximately 5.3% of all obstructive nonapneic events in patients with OSA are attributed to RERAs.[17] UARS appears to be more common in premenopausal and perimenopausal women compared to men or postmenopausal women.[18] Additionally, women with UARS have been reported to require approximately 30 minutes more sleep compared to men with the same condition.
History and Physical
Patients with UARS commonly present with snoring, fatigue, daytime tiredness, morning headaches, depressive symptoms, and excessive daytime sleepiness, typically without witnessed apneas or gasping episodes.[19] Sleep disruption and unexplained awakenings, often occurring 2 to 3 hours after sleep onset, are frequently reported. These arousals are associated with increased respiratory effort and contribute to sleep fragmentation, which, in turn, leads to persistent fatigue and excessive daytime sleepiness. Individuals with UARS and OSA have also demonstrated significantly reduced quality of life, reported to be 5 to 6 times lower compared to that of the general population.
Evaluation
A full polysomnography (PSG) is recommended for the adequate evaluation of individuals suspected of having UARS (see Image. Flow Limitations With Snoring). Home sleep apnea testing may underestimate the severity of SDB, whereas PSG enables detection of inspiratory flow limitation and associated arousals, which are essential for diagnosis. Compared to patients with OSA, those with UARS often demonstrate greater slow-wave sleep activity and fewer awakenings on PSG.[20]
Although patients with UARS may exhibit apneas or hypopneas, episodes of oxygen desaturation are less common. Instead, PSG typically reveals episodes of inspiratory flow limitation associated with arousals. UARS is frequently overlooked because RERAs are often underrecognized or inconsistently scored during PSG interpretation. Consequently, findings may be misclassified as normal or as mild OSA. UARS can also be identified through measurement of esophageal pressure (Pes) or using a supraglottic catheter; however, these methods are not routinely used in most clinical sleep laboratories (see Image. Pressure-Flow Relationship During Inspiratory Flow Limitation).[21]
Untreated UARS is associated with reduced quality of life and may progress over time, potentially leading to cardiovascular and metabolic consequences, including hypertension. Early diagnosis and appropriate treatment are crucial in mitigating these long-term effects.
Treatment / Management
Treatment of UARS involves addressing the underlying anatomical contributors to upper airway narrowing, such as nasal allergies, dental malocclusion, and modifiable lifestyle factors. The mainstay of therapy is continuous positive airway pressure (CPAP), with multiple studies demonstrating subjective, clinical, and physiological improvement (see Image. Normalized Inspiratory Flow Following Optimal Continuous Positive Airway Pressure Titration). Recent investigations have shown that CPAP reduces upper airway resistance, decreases arousal frequency, and stabilizes respiratory-related heart rate variability. In-laboratory CPAP titration is preferred over auto-CPAP to eliminate all episodes of flow limitation and restore normal sleep architecture.
Oral appliances offer an effective alternative for patients with UARS who are intolerant of CPAP or decline surgical intervention (see Image. Custom Oral Appliance for Upper Airway Resistance Syndrome).[22] These devices advance the mandible and soft tissue anteriorly, increasing the airway space posterior to the tongue. Long-term use, such as for 1.5 years, has been associated with reductions in stress-related symptoms, as measured by the Inventory of Stress Symptoms.[23] Additional oral appliance options include tongue-retaining devices, soft palate lifters, and tongue stabilizers.
Surgical intervention is reserved for patients who do not tolerate CPAP or oral appliance therapy. Common procedures for managing UARS include palatal surgeries and other interventions targeting sites of upper airway obstruction.[24]
Pharmacological treatment for UARS has remained investigational until recently, and no medication has received approval from the United States Food and Drug Administration (FDA) for this indication. However, tirzepatide has now been FDA-approved for moderate-to-severe OSA in adults with obesity.[25] However, this indication does not apply to individuals with an AHI below 15 events per hour. The FDA also granted fast-track designation to AD109—a combination of aroxybutynin, a novel antimuscarinic agent, and atomoxetine, a norepinephrine reuptake inhibitor.[26] The study excluded patients with an AHI below 10 events per hour or above 45 events per hour based on 4% desaturation criteria.(A1)
Hypnotic agents are of interest due to their potential to reduce RERAs and improve sleep fragmentation. Studies evaluating nonbenzodiazepines such as zolpidem have produced mixed results. Some evidence suggests that zolpidem lowers the respiratory arousal threshold and reduces the severity of SDB, whereas others report no measurable effect on arousal threshold.[27][28][29] Due to the absence of large, definitive trials, pharmacological therapy remains investigational but may have a synergistic role when combined with other established treatments.(B3)
Differential Diagnosis
Several conditions may present with features similar to UARS, including OSA, sleep-related breathing disorder, respiratory sleep disorder, and SDB. Although these terms differ in nomenclature, they all describe abnormal respiratory patterns during sleep.
Prognosis
UARS occupies an intermediate position between normal respiratory patterns and those observed in patients with mild-to-moderate OSA. Optimal outcomes depend on early recognition and appropriate intervention by the treating clinician. Evidence suggests that untreated UARS may be associated with an increased risk of arterial hypertension. Progression to OSA may occur, particularly in the presence of weight gain, advancing age, or the development of other comorbidities.[30]
Complications
Repetitive episodes of flow limitation typically result in arousals triggered by increased respiratory effort, leading to sleep fragmentation. Sleep disturbance contributes to excessive daytime sleepiness in individuals with UARS. Prolonged periods of inspiratory flow limitation during sleep have been associated with elevated carbon dioxide levels—highest compared to those observed during eupneic breathing, hypopnea, or apnea—suggesting a possible role of UARS in the development of daytime symptoms and neurocognitive impairment.[31] When left undiagnosed and untreated, individuals with UARS often exhibit reduced quality of life and persistent daytime symptoms, including fatigue, insomnia, and depressive mood.[32]
A retrospective study from Peru compared patients with UARS (n = 93, AHI <5 events per hour, oxygen saturation of at least 92%, and RERA index of at least 5 events per hour) to those with OSA (n = 795, AHI >5 events per hour) and the general population (n = 641). The study found that individuals with UARS had poor health-related quality of life scores, similar to those with OSA but significantly lower than those in the general population. Quality of life was further reduced in patients with UARS and symptoms such as muscle pain, obesity, depression, or use of psychotropic medications. Hypertension and cardiovascular complications have also been reported in untreated UARS, even in the absence of significant hypoxemia.[33]
The primary characteristic of UARS related to esophageal pressure (Pes) is marked negativity, which can produce a diastolic leftward shift of the interventricular septum accompanied by pulsus paradoxus, particularly when peak end-inspiratory Pes becomes more negative than −35 cm H2O. These cardiovascular effects arise directly from prolonged episodes of inspiratory flow limitation during sleep. Such episodes may also cause a mild elevation in end-tidal carbon dioxide, which can stimulate sympathetic nervous system activity and increase the risk of developing arterial hypertension.[34]
Deterrence and Patient Education
Patient counseling for UARS should include a clear explanation of the underlying pathophysiology and potential consequences in simple terms to ensure patient understanding. Clinicians should review available treatment options, demonstrate the proper use of mechanical devices such as CPAP and oral appliances, and provide patients with an opportunity to ask questions and receive informed answers.
Pearls and Other Issues
Historically, sleep apnea and UARS have been diagnosed by sleep medicine clinicians. However, as discussed in this article, effective management requires an interprofessional approach. Primary care providers, otolaryngologists, and dentists play a critical role in screening for risk factors associated with OSA and UARS. These risk factors include retrognathia, a high-arched palate, chronically enlarged tonsils, and a tongue that is disproportionately large relative to the oral cavity.
Detailed history-taking, focusing on sleep quality, sleep position, obesity, hypertension, morning headaches, and orofacial pain, is essential. Most importantly, as previously emphasized, the identification of upper airway abnormalities on physical examination is a key component of the evaluation. Once suspicion is established, the clinician must refer the patient to an appropriate specialist based on individual findings.
Ongoing monitoring is crucial. Patients who are not comfortable with their treatment regimen are unlikely to show adherence, resulting in therapeutic failure and increased risk of cardiovascular and metabolic complications. Oral appliance therapy must be carefully selected based on the patient's specific anatomical and clinical needs, requiring expertise across disciplines. The effectiveness of oral appliances plays a central role in managing OSA associated with UARS.
Enhancing Healthcare Team Outcomes
Effective management of SDB and UARS depends on identifying relevant risk factors and implementing individualized therapy for patients with diurnal or nocturnal symptoms, supported by sustained interprofessional collaboration across the diagnostic and treatment process. Interprofessional communication may begin with a dental professional recognizing anatomical risk factors during a routine dental examination. Risk identification may also occur during visits with a primary care clinician or a relevant specialist. Follow-up history and symptom evaluation should be conducted by the healthcare provider, with subsequent referral to a sleep medicine specialist.
The diagnosis of UARS may be considered if PSG reveals no significant apneas or oxygen desaturation despite clinical symptoms. Treatment options may include CPAP or a custom oral appliance fabricated by a trained dentist.
Nursing professionals play a crucial role in patient education, demonstrating the correct use of prescribed devices and facilitating effective communication among team members. Any clinician who observes signs of therapeutic failure should document the findings and communicate with the broader care team to ensure timely intervention and effective management. Improved patient outcomes depend on coordinated, team-based care that is supported by open, interprofessional communication.
Media
(Click Image to Enlarge)
Inspiratory Flow Limitation Consistent With Upper Airway Resistance Syndrome. In-laboratory polysomnography during N2 sleep demonstrates flattening of the inspiratory phase on the flow signal, indicating inspiratory flow limitation without associated desaturation or definitive hypopnea. This pattern is characteristic of upper airway resistance syndrome, particularly when associated with arousals.
StatPearls
(Click Image to Enlarge)
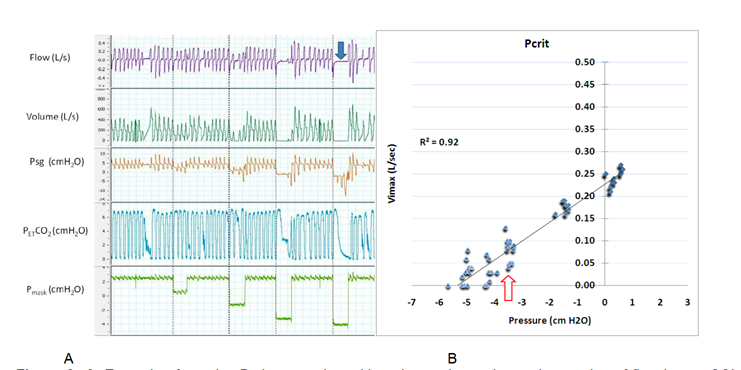
Determination of Critical Closing Pressure. Polygraph tracing during sleep (Image A) shows progressive reduction of mask pressure, leading to decreased airflow and complete airway closure (blue arrow), as indicated by flow and supraglottic pressure (Psg) signals. Pcrit is calculated from the linear relationship between maximal inspiratory flow (Vmax) and mask pressure (Image B). The critical closing pressure is determined at the point where flow falls below 0.05 L/s (red arrow), here approximately −3 cm H₂O, indicating increased upper airway collapsibility consistent with upper airway resistance syndrome.
Contributed by A Sankari, MD, PhD
(Click Image to Enlarge)
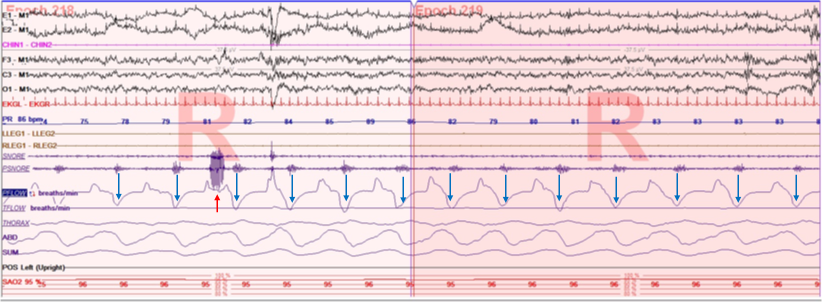
Flow Limitations With Snoring. A polygraph depicting snoring and flow limitation during rapid eye movement sleep in the inspiratory (red arrow) and expiratory (blue arrows) phases, respectively. Upward deflections represent the inspiratory phase, whereas the expiratory phase is shown as downward deflections. These flow limitations are characteristic of increased upper airway resistance during sleep.
StatPearls
(Click Image to Enlarge)
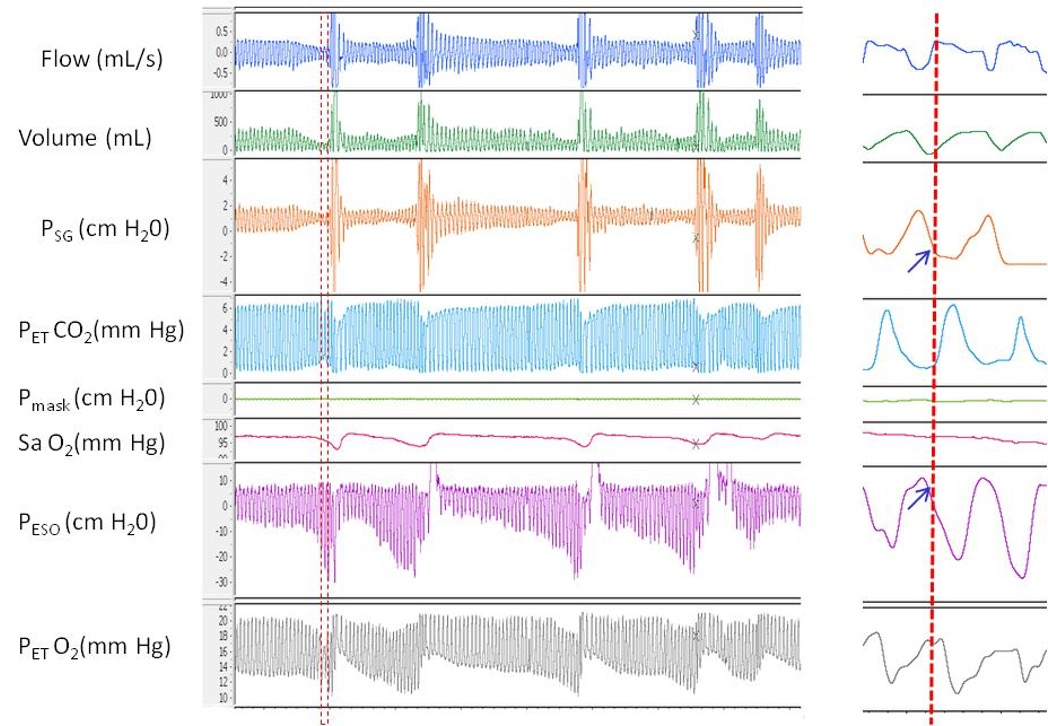
Pressure-Flow Relationship During Inspiratory Flow Limitation. Polygraph tracing demonstrates repetitive episodes of inspiratory flow limitation. Two pressure catheters were used—one at the supraglottic area (Psg) and another in the esophagus (Pes). In the magnified section, the onset of peak inspiratory flow shows a deceleration and flattening of the inspiratory limb, which coincides with a plateau in Psg and a marked decrease in Pes. This pattern indicates a significant increase in respiratory effort and elevated upper airway resistance distal to the supraglottic area.
Contributed by A Sankari, MD, PhD
(Click Image to Enlarge)
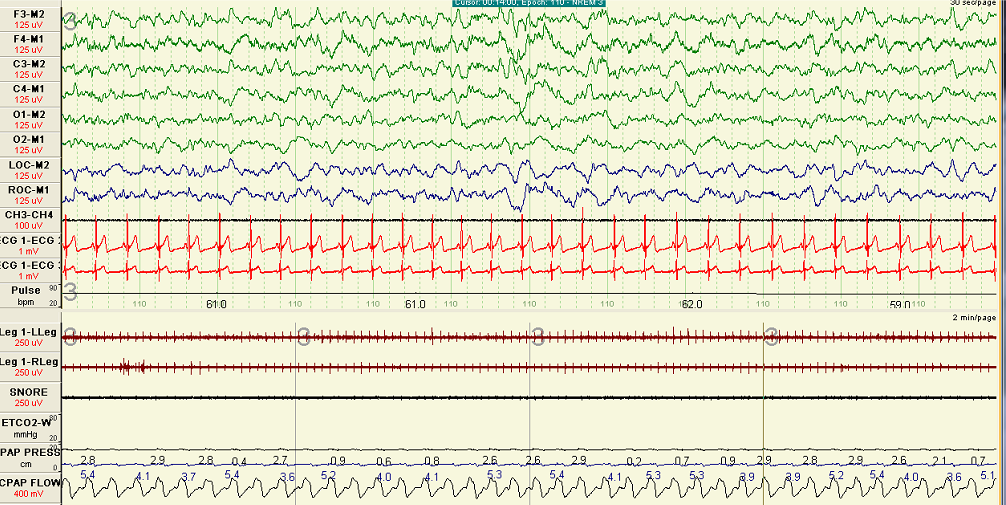
Normalized Inspiratory Flow Following Optimal Continuous Positive Airway Pressure Titration. Polygraph recording during non-rapid eye movement sleep shows inspiratory flow with a rounded contour, indicating the absence of flow limitation. These findings reflect effective airway stabilization after optimal continuous positive airway pressure titration.
StatPearls
(Click Image to Enlarge)
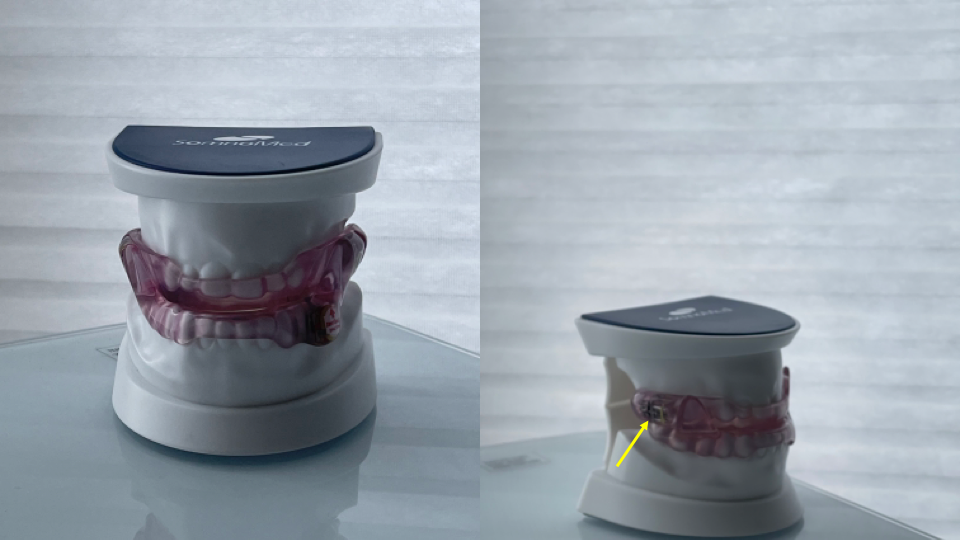
Custom Oral Appliance for Upper Airway Resistance Syndrome. An example of a dentist-fabricated oral appliance designed to treat upper airway resistance syndrome. The adjustable spring (yellow arrow) allows forward advancement of the mandible, increasing the posterior airway space behind the tongue.
Contributed by A Sankari, MD, PhD
References
Sankri-Tarbichi AG. Obstructive sleep apnea-hypopnea syndrome: Etiology and diagnosis. Avicenna journal of medicine. 2012 Jan:2(1):3-8. doi: 10.4103/2231-0770.94803. Epub [PubMed PMID: 23210013]
Arnold WC, Guilleminault C. Upper airway resistance syndrome 2018: non-hypoxic sleep-disordered breathing. Expert review of respiratory medicine. 2019 Apr:13(4):317-326. doi: 10.1080/17476348.2019.1575731. Epub 2019 Feb 6 [PubMed PMID: 30689957]
Chervin RD, Guilleminault C. Obstructive sleep apnea and related disorders. Neurologic clinics. 1996 Aug:14(3):583-609 [PubMed PMID: 8871978]
Ogna A, Tobback N, Andries D, Preisig M, Vollenweider P, Waeber G, Marques-Vidal P, Haba-Rubio J, Heinzer R. Prevalence and Clinical Significance of Respiratory Effort-Related Arousals in the General Population. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine. 2018 Aug 15:14(8):1339-1345. doi: 10.5664/jcsm.7268. Epub 2018 Aug 15 [PubMed PMID: 30092888]
Vizcarra-Escobar D, Duque KR, Barbagelata-Agüero F, Vizcarra JA. Quality of life in upper airway resistance syndrome. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine. 2022 May 1:18(5):1263-1270. doi: 10.5664/jcsm.9838. Epub [PubMed PMID: 34931609]
Level 2 (mid-level) evidenceTufik SB, Pires GN, Palombini L, Andersen ML, Tufik S. Prevalence of upper airway resistance syndrome in the São Paulo Epidemiologic Sleep Study. Sleep medicine. 2022 Mar:91():43-50. doi: 10.1016/j.sleep.2022.02.004. Epub 2022 Feb 14 [PubMed PMID: 35255282]
Trudo FJ, Gefter WB, Welch KC, Gupta KB, Maislin G, Schwab RJ. State-related changes in upper airway caliber and surrounding soft-tissue structures in normal subjects. American journal of respiratory and critical care medicine. 1998 Oct:158(4):1259-70 [PubMed PMID: 9769290]
Level 2 (mid-level) evidenceSankari A, Pranathiageswaran S, Maresh S, Hosni AM, Badr MS. Characteristics and Consequences of Non-apneic Respiratory Events During Sleep. Sleep. 2017 Jan 1:40(1):. doi: 10.1093/sleep/zsw024. Epub [PubMed PMID: 28364453]
Berry RB, Brooks R, Gamaldo C, Harding SM, Lloyd RM, Quan SF, Troester MT, Vaughn BV. AASM Scoring Manual Updates for 2017 (Version 2.4). Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine. 2017 May 15:13(5):665-666. doi: 10.5664/jcsm.6576. Epub 2017 May 15 [PubMed PMID: 28416048]
Rizwan A, Sankari A, Bascom AT, Vaughan S, Badr MS. Nocturnal swallowing and arousal threshold in individuals with chronic spinal cord injury. Journal of applied physiology (Bethesda, Md. : 1985). 2018 Aug 1:125(2):445-452. doi: 10.1152/japplphysiol.00641.2017. Epub 2018 Apr 19 [PubMed PMID: 29672224]
Alchakaki A, Riehani A, Shikh-Hamdon M, Mina N, Badr MS, Sankari A. Expiratory Snoring Predicts Obstructive Pulmonary Disease in Patients with Sleep-disordered Breathing. Annals of the American Thoracic Society. 2016 Jan:13(1):86-92. doi: 10.1513/AnnalsATS.201507-413OC. Epub [PubMed PMID: 26630563]
Patil SP, Schneider H, Marx JJ, Gladmon E, Schwartz AR, Smith PL. Neuromechanical control of upper airway patency during sleep. Journal of applied physiology (Bethesda, Md. : 1985). 2007 Feb:102(2):547-56 [PubMed PMID: 17008440]
Level 2 (mid-level) evidenceSankri-Tarbichi AG, Rowley JA, Badr MS. Expiratory pharyngeal narrowing during central hypocapnic hypopnea. American journal of respiratory and critical care medicine. 2009 Feb 15:179(4):313-9. doi: 10.1164/rccm.200805-741OC. Epub 2008 Nov 21 [PubMed PMID: 19201929]
Sankari A, Badr MS. Sleep-Disordered Breathing in Patients with Chronic Obstructive Pulmonary Disease. Annals of the American Thoracic Society. 2015 Sep:12(9):1419-20. doi: 10.1513/AnnalsATS.201506-313LE. Epub [PubMed PMID: 26372809]
Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. The New England journal of medicine. 1993 Apr 29:328(17):1230-5 [PubMed PMID: 8464434]
Benjafield AV, Ayas NT, Eastwood PR, Heinzer R, Ip MSM, Morrell MJ, Nunez CM, Patel SR, Penzel T, Pépin JL, Peppard PE, Sinha S, Tufik S, Valentine K, Malhotra A. Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. The Lancet. Respiratory medicine. 2019 Aug:7(8):687-698. doi: 10.1016/S2213-2600(19)30198-5. Epub 2019 Jul 9 [PubMed PMID: 31300334]
Cracowski C, Pépin JL, Wuyam B, Lévy P. Characterization of obstructive nonapneic respiratory events in moderate sleep apnea syndrome. American journal of respiratory and critical care medicine. 2001 Sep 15:164(6):944-8 [PubMed PMID: 11587975]
Stoohs R, Janicki J, Hohenhorst W. [Obstructive sleep apnea syndrome and upper airway resistance syndrome. Gender-related differences]. HNO. 2007 Oct:55(10):792-7 [PubMed PMID: 17287938]
Anttalainen U, Tenhunen M, Rimpilä V, Polo O, Rauhala E, Himanen SL, Saaresranta T. Prolonged partial upper airway obstruction during sleep - an underdiagnosed phenotype of sleep-disordered breathing. European clinical respiratory journal. 2016:3():31806. doi: 10.3402/ecrj.v3.31806. Epub 2016 Sep 6 [PubMed PMID: 27608271]
Guilleminault C, Do Kim Y, Chowdhuri S, Horita M, Ohayon M, Kushida C. Sleep and daytime sleepiness in upper airway resistance syndrome compared to obstructive sleep apnoea syndrome. The European respiratory journal. 2001 May:17(5):838-47 [PubMed PMID: 11488314]
Badr MS, Zahn BR. Images in clinical medicine. Upper-airway resistance syndrome. The New England journal of medicine. 2000 May 11:342(19):1408 [PubMed PMID: 10805826]
Level 3 (low-level) evidenceNerfeldt P, Friberg D. Effectiveness of Oral Appliances in Obstructive Sleep Apnea with Respiratory Arousals. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine. 2016 Aug 15:12(8):1159-65. doi: 10.5664/jcsm.6058. Epub 2016 Aug 15 [PubMed PMID: 27397661]
de Godoy LBM, Sousa KMM, Palombini LO, Poyares D, Dal-Fabbro C, Guimarães TM, Tufik S, Togeiro SM. Long term oral appliance therapy decreases stress symptoms in patients with upper airway resistance syndrome. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine. 2020 Nov 15:16(11):1857-1862. doi: 10.5664/jcsm.8698. Epub [PubMed PMID: 32686643]
Newman JP, Clerk AA, Moore M, Utley DS, Terris DJ. Recognition and surgical management of the upper airway resistance syndrome. The Laryngoscope. 1996 Sep:106(9 Pt 1):1089-93 [PubMed PMID: 8822711]
Malhotra A, Grunstein RR, Fietze I, Weaver TE, Redline S, Azarbarzin A, Sands SA, Schwab RJ, Dunn JP, Chakladar S, Bunck MC, Bednarik J, SURMOUNT-OSA Investigators. Tirzepatide for the Treatment of Obstructive Sleep Apnea and Obesity. The New England journal of medicine. 2024 Oct 3:391(13):1193-1205. doi: 10.1056/NEJMoa2404881. Epub 2024 Jun 21 [PubMed PMID: 38912654]
Schweitzer PK, Taranto-Montemurro L, Ojile JM, Thein SG, Drake CL, Rosenberg R, Corser B, Abaluck B, Sangal RB, Maynard J. The Combination of Aroxybutynin and Atomoxetine in the Treatment of Obstructive Sleep Apnea (MARIPOSA): A Randomized Controlled Trial. American journal of respiratory and critical care medicine. 2023 Dec 15:208(12):1316-1327. doi: 10.1164/rccm.202306-1036OC. Epub [PubMed PMID: 37812772]
Level 1 (high-level) evidenceMessineo L, Eckert DJ, Lim R, Chiang A, Azarbarzin A, Carter SG, Carberry JC. Zolpidem increases sleep efficiency and the respiratory arousal threshold without changing sleep apnoea severity and pharyngeal muscle activity. The Journal of physiology. 2020 Oct:598(20):4681-4692. doi: 10.1113/JP280173. Epub 2020 Aug 30 [PubMed PMID: 32864734]
Carberry JC, Grunstein RR, Eckert DJ. The effects of zolpidem in obstructive sleep apnea - An open-label pilot study. Journal of sleep research. 2019 Dec:28(6):e12853. doi: 10.1111/jsr.12853. Epub 2019 Apr 10 [PubMed PMID: 30968498]
Level 3 (low-level) evidenceSmith PR, Sheikh KL, Costan-Toth C, Forsthoefel D, Bridges E, Andrada TF, Holley AB. Eszopiclone and Zolpidem Do Not Affect the Prevalence of the Low Arousal Threshold Phenotype. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine. 2017 Jan 15:13(1):115-119. doi: 10.5664/jcsm.6402. Epub 2017 Jan 15 [PubMed PMID: 27784413]
M'saad S, Yangui I, Feki W, Abid N, Bahloul N, Marouen F, Chakroun A, Kammoun S. [The syndrome of increased upper airways resistance: What are the clinical features and diagnostic procedures?]. Revue des maladies respiratoires. 2015 Dec:32(10):1002-15. doi: 10.1016/j.rmr.2015.08.001. Epub 2015 Oct 30 [PubMed PMID: 26525135]
Rimpilä V, Hosokawa K, Huhtala H, Saaresranta T, Salminen AV, Polo O. Transcutaneous carbon dioxide during sleep-disordered breathing. Respiratory physiology & neurobiology. 2015 Dec:219():95-102. doi: 10.1016/j.resp.2015.10.002. Epub 2015 Oct 22 [PubMed PMID: 26474829]
de Godoy LB, Palombini LO, Guilleminault C, Poyares D, Tufik S, Togeiro SM. Treatment of upper airway resistance syndrome in adults: Where do we stand? Sleep science (Sao Paulo, Brazil). 2015 Jan-Mar:8(1):42-8. doi: 10.1016/j.slsci.2015.03.001. Epub 2015 Mar 20 [PubMed PMID: 26483942]
Guilleminault C, Stoohs R, Shiomi T, Kushida C, Schnittger I. Upper airway resistance syndrome, nocturnal blood pressure monitoring, and borderline hypertension. Chest. 1996 Apr:109(4):901-8 [PubMed PMID: 8635368]
Guilleminault C, Los Reyes VD. Upper-airway resistance syndrome. Handbook of clinical neurology. 2011:98():401-9. doi: 10.1016/B978-0-444-52006-7.00026-5. Epub [PubMed PMID: 21056201]