Introduction
Otto Ullrich, a German pediatrician, presented the first case of an 8-year-old girl exhibiting the complete clinical picture of Turner syndrome in 1930. In 1938, Henri Turner, an Oklahoma physician, independently described similar features.[1] The condition later became known as Turner syndrome, also referred to as Ullrich-Turner syndrome.
Turner syndrome is a sex chromosome disorder affecting individuals with a female phenotype who possess an intact X chromosome and a completely or partially absent second sex chromosome. This disorder is associated with 1 or more clinical manifestations. Turner syndrome ranks as the most common sex chromosome abnormality observed in individuals with a female phenotype. Traditional diagnosis relies on the identification of specific phenotypic features, such as characteristic facial appearance, neck webbing, and peripheral lymphedema (see Image. Girl with Turner Syndrome).
More recently, the recognized clinical manifestations of Turner syndrome have expanded to include, either alone or in combination, short stature, premature ovarian insufficiency (including delayed puberty), early sensorineural hearing loss, congenital cardiovascular, skeletal, and renal anomalies, a distinctive neurodevelopmental profile, and a higher prevalence of conditions, such as hypothyroidism and celiac disease.[2][3]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
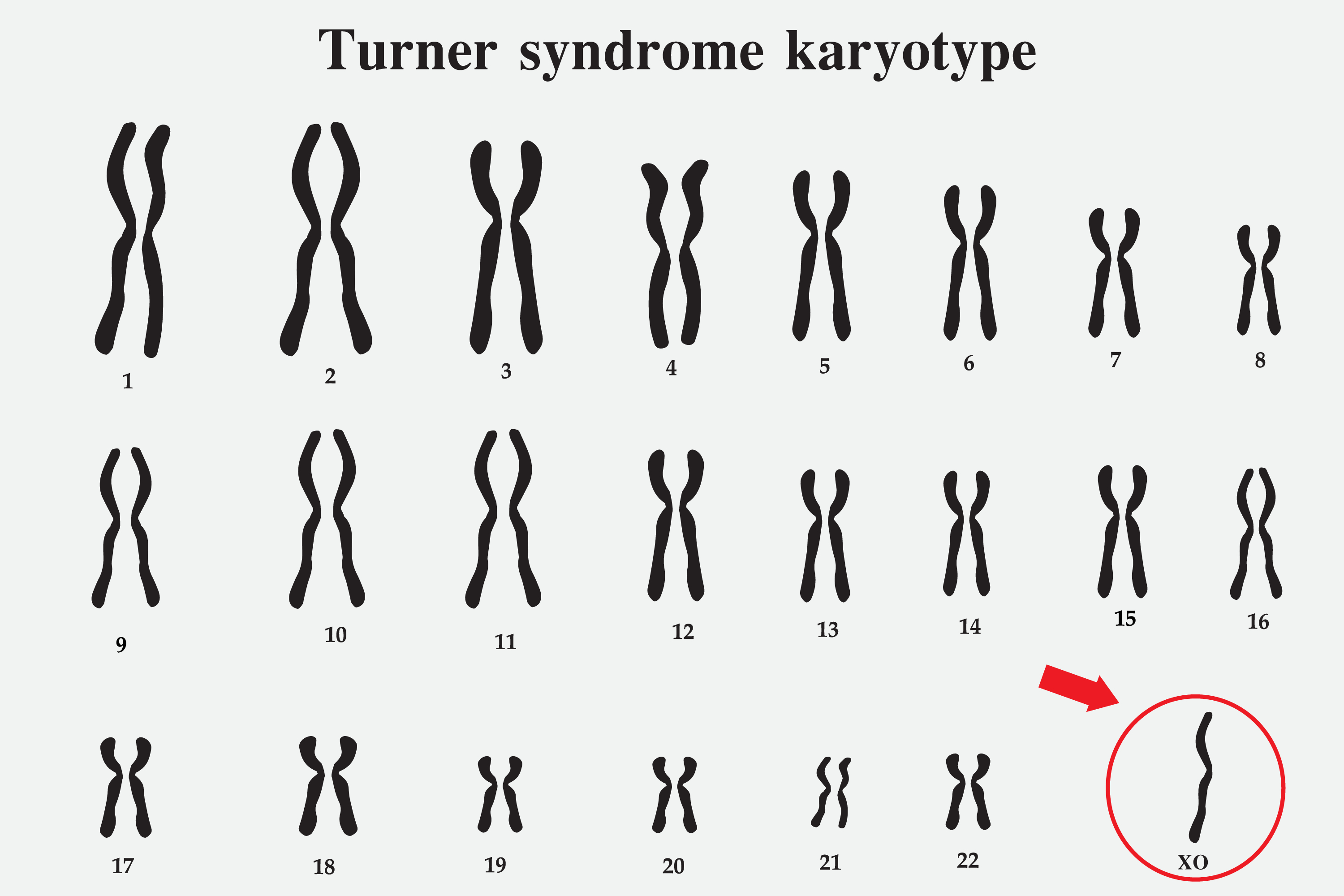
Turner syndrome is caused by the presence of an intact X chromosome alongside the complete or partial absence of the second sex chromosome (see Image. Turner Syndrome Karyotype). This chromosomal abnormality leads to 1 or more characteristic clinical manifestations (please refer to the History and Physical section for more information). The types and frequencies of karyotypes associated with Turner syndrome are summarized in Table 1 (see Table. Types and Frequencies of Karyotypes Associated with Turner Syndrome).
Table 1. Types and Frequencies of Karyotypes Associated with Turner Syndrome
|
Frequency |
Description |
|
40%-50% |
Monosomy X (45,X) |
|
15%-25% |
Mosaicism with 46,XX (45,X/46,XX) |
|
3% |
Mosaicism with 47,XXX (45,X/47,XXX; 45,X/46,XX/47,XXX) |
|
10%-12% |
Mosaicism with 46,XY (45,X/46,XY) |
|
15% |
Isochromosome Xq (46,X,i(Xq)) and isodicentric Xp (46,X,idic(Xp)) (two copies of the long arm connected head-to-head) |
|
Rare |
Ring X chromosome (45,X/46,X,r(X)), proximal deletion of Xp (46,XX,del(p11)), and unbalanced X-autosome translocation (a part of the ends of short and long arms of the X chromosome is missing) |
The diagnosis of Turner syndrome should be excluded in certain cases. First, individuals with small distal deletions of the short arm of the X chromosome (Xp22.33), which contains the SHOX (short stature homeobox) gene, often exhibit short stature and other skeletal anomalies associated with SHOX deletion. However, these patients generally do not present with hallmark features of Turner syndrome, such as increased risk for cardiac anomalies, neurocognitive issues, or ovarian insufficiency.
Second, individuals with deletions distal to Xq24 frequently experience primary or secondary amenorrhea but lack short stature and other typical features of Turner syndrome. These cases should be classified as premature ovarian insufficiency rather than Turner syndrome. Finally, women older than 50 with less than 5% mosaicism for the 45,X karyotype should also not be considered to have Turner syndrome.[4][5] Turner syndrome is typically not inherited but results from a random event occurring during reproduction.
Epidemiology
Turner syndrome occurs in approximately 1 in 2000 to 1 in 2500 live female births.[6] A recent nationwide database estimated the prevalence at 3.2 per 10,000 female live births (95% confidence interval = 3.0–3.3) across all pregnancy outcomes, and 1.9 per 10,000 live births when only live births and stillbirths were considered.[7] Prevalence during the antenatal period is substantially higher, as most fetuses with Turner syndrome abort spontaneously.
Increased awareness and use of prenatal ultrasound scans have contributed to a decline in prevalence at birth, largely because some mothers carrying fetuses diagnosed with Turner syndrome elect to terminate the pregnancy. However, the true prevalence is unknown since many patients with a mild phenotype remain undiagnosed or receive a diagnosis later in adulthood.[8] A nationwide study identified 3 peaks in age at diagnosis—younger than 1 (14.9%), adolescence between the ages of 10 and 17 (33.2%), and adulthood (38.5%). The median age at diagnosis remained approximately 15 years, reflecting diagnostic delays when neonatal opportunities are missed.[9]
Pathophysiology
Most instances of Turner syndrome are not inherited. Monosomy X (45,X), the most common underlying karyotype, characterized by the complete absence of one X chromosome in all cells, typically arises as a random event during the formation of reproductive cells in one parent. This error in cell division, known as nondisjunction, can produce reproductive cells with an abnormal number of chromosomes. For example, nondisjunction can cause the loss of a sex chromosome from an egg or sperm cell. If an atypical reproductive cell contributes to a child's genetic makeup, every cell in the child possesses a single X chromosome, with the other sex chromosome missing (see Image. Turner Syndrome Karyotype).
Other chromosomal variations may lead to Turner syndrome besides the 45,X karyotype. Mosaic Turner syndrome, where some cells contain 45,X while others have 46,XX, 46,XY, or other rare karyotypes, is a relatively common variant (please refer to the Etiology section for more information). Isochromosome and ring chromosome variants, involving loss of critical portions of the X chromosome, are less common causes of Turner syndrome. Mosaic Turner syndrome arises from a random event during early fetal cell division. Consequently, some cells contain the usual 2 sex chromosomes, whereas others carry only 1 copy of the X chromosome.
The clinical features of Turner syndrome arise from the loss of function of genes located in the missing portion of the X chromosome, specifically the pseudoautosomal region. Among these genes, the SHOX gene has been most extensively studied in relation to the Turner syndrome phenotype. Haploinsufficiency of SHOX contributes to growth deficits, scoliosis, micrognathia, a high-arched palate, Madelung deformity, and reduced leg length commonly observed in affected individuals. Additionally, haploinsufficiency of TIMP1 and the presence of the risk-associated phenotype of TIMP3 have been linked to bicuspid aortic valve and aortic dysfunction. Other genes, including KDM6A, KDM5C, RPS4X, and CSF2RA, have been implicated in the phenotype but require further investigation to clarify their roles.
Karyotype-phenotype correlations reveal variations in clinical severity and comorbidity risk. Individuals with the 45,X karyotype exhibit a higher frequency of comorbidities and increased mortality, reflecting the extent of chromosome material loss. Individuals with mosaicism involving 45,X/46,XX typically present with a milder phenotype, characterized by fewer left-sided congenital heart defects, lower rates of obesity and hypertension, and a near-normal age at menarche. This group is also more likely to experience spontaneous menarche and pregnancies. Similarly, individuals with the 45,X/47,XXX karyotype tend to show milder external and cardiovascular features but face a higher risk of neurodevelopmental disabilities.
The isochromosome Xq variant generally presents an intermediate phenotype with reduced cardiac morbidity and mortality. Mosaicism of 45,X/46,XY is associated with the lowest risk of autoimmune thyroid disease and a reduced risk of coarctation of the aorta. Individuals with a ring X chromosome that retains the XIST gene face an increased risk of metabolic syndrome compared to 45,X individuals. In contrast, the loss of XIST in ring X chromosome carriers corresponds to a more severe cognitive phenotype.
History and Physical
Prenatal identification of Turner syndrome relies on abnormal ultrasound findings such as increased nuchal translucency, nuchal cystic hygroma, coarctation of the aorta or other left-sided cardiac anomalies, brachycephaly, horseshoe kidney, polyhydramnios, oligohydramnios, or nonimmune fetal hydrops. In newborns with a female phenotype, presenting features may include congenital lymphedema of the hands and feet, webbed neck, nail dysplasia, a narrow and high-arched palate, and shortened fourth metacarpals or metatarsals. During childhood, affected girls often develop short stature, a broad chest with widely spaced nipples, webbed neck, low posterior hairline, cubitus valgus, and Madelung deformity of the forearm and wrist.[10]
Most individuals with Turner syndrome possess normal intelligence, though many experience specific neurocognitive challenges such as deficits in visuospatial organization. These challenges increase the risk of learning difficulties, particularly in areas involving calculation, memory, and attention. Delayed puberty or primary amenorrhea frequently occurs during adolescence as a result of premature ovarian failure. Ovarian tissue in affected individuals, referred to as streak gonads, primarily consists of connective tissue with either no follicles or only a few atretic follicles.
Patients with Turner syndrome face an elevated risk of cardiovascular malformations, contributing to increased mortality. Common cardiac anomalies include bicuspid aortic valve, coarctation of the aorta, elongated transverse aortic arch, and pulmonary venous abnormalities. Aortic dissection poses an additional threat, further elevating the risk of death. Hearing loss frequently occurs due to recurrent otitis media, which causes conductive hearing loss, or due to defects in the outer hair cells of the cochlea, which result in sensorineural hearing loss.[11]
Renal anomalies are frequently observed and include collecting system malformations, positional abnormalities, and horseshoe kidneys. Ocular abnormalities may also occur, including nearsightedness, farsightedness, strabismus, amblyopia, epicanthic folds, ptosis, hypertelorism, and red-green color blindness.[12] Individuals with Turner syndrome are more likely to develop autoimmune conditions such as hypothyroidism, celiac disease, and inflammatory bowel disease.[13] The presence of dysgenic gonads, particularly those containing Y chromosome material, increases the risk of gonadoblastoma in affected individuals.
Genetic testing for Turner syndrome should be considered in individuals of any age who exhibit clinical signs consistent with the disorder. Testing is warranted when even a single feature, such as fetal cystic hygroma or hydrops, left-sided outflow congenital heart defects (excluding isolated bicuspid aortic valve), delayed puberty or menarche, arrested pubertal progression, unexplained secondary amenorrhea or infertility, or characteristic physical features, is present. These features may include down-slanted palpebral fissures, epicanthal folds, low-set or anomalous ears, micrognathia, narrow or high-arched palate, short neck, or webbing of the neck. Unexplained short stature alone also merits investigation.
Genetic testing is likewise indicated when at least 2 features from the following group coexist—renal anomalies such as horseshoe kidney, renal agenesis, or hypoplasia; Madelung deformity; neuropsychological or psychiatric challenges; multiple typical nevi; dysplastic or hyperconvex nails; congenital heart defects beyond those previously mentioned; or early-onset sensorineural hearing loss (before 40 years) occurring alongside short stature. In such cases, karyotype analysis should be pursued to confirm or exclude a diagnosis of Turner syndrome.
Evaluation
Diagnostic Modalities for Turner Syndrome
Prenatal suspicion of Turner syndrome may arise when first-trimester screening reveals findings such as low pregnancy-associated plasma protein-A, increased nuchal translucency, or abnormal ductus venosus flow. Diagnostic confirmation may be obtained through chorionic villus sampling or amniocentesis. However, the exact karyotype, especially in cases involving mosaicism, often remains uncertain if based solely on prenatal testing. For this reason, standard chromosome analysis using a peripheral blood sample after birth should be performed regardless of prenatal findings. In cases where mosaicism is present, a normal karyotype may occasionally be observed. If clinical suspicion is strong, further testing or evaluation of a greater number of metaphases is recommended.
Noninvasive prenatal testing, which analyzes cell-free fetal DNA in maternal blood, may also indicate a high risk for Turner syndrome. In such cases, nondirective genetic counseling should be provided, and invasive fetal karyotyping should be offered for confirmation. Detailed fetal ultrasonography and echocardiography are crucial for assessing structural anomalies. Preimplantation genetic testing should be considered in women planning pregnancy with their own oocytes.
Indications for testing to confirm Turner syndrome vary depending on the stage of life at the time of presentation. Karyotype analysis using peripheral blood mononuclear cells is the gold standard and is considered the first diagnostic step. A minimum of 30 metaphases should be analyzed to detect mosaicism at a level of approximately 10% with 95% confidence limits.[14] Other molecular genetic techniques, including microarray, exome sequencing, and genome sequencing, may also be used. However, microarray analysis is less effective than sequencing methods in detecting low-level mosaicism.
When karyotype results are normal in a patient with clinical features suggestive of Turner syndrome, a second analysis using a different tissue source, such as skin fibroblasts, buccal mucosa cells, or bladder epithelial cells, should be considered. In individuals with a 45,X karyotype who show signs of virilization, molecular studies should be performed to detect the presence of Y chromosome material, which may be identified through karyotyping, fluorescence in situ hybridization with Y-chromosome probes, polymerase chain reaction using Y-specific primers, or array-comparative genomic hybridization. Approximately 10% to 12% of affected individuals may carry a normal or structurally abnormal Y chromosome.
During adolescence, patients may present with delayed onset of puberty or primary amenorrhea. Elevated serum follicle-stimulating hormone (FSH) levels support the diagnosis of Turner syndrome, whereas anti-Müllerian hormone (AMH) may serve as a more sensitive marker for predicting ovarian insufficiency.[15]
Once the diagnosis of Turner syndrome is established, a comprehensive evaluation should be conducted to identify associated abnormalities, including cardiac and renal anomalies and neurocognitive impairments. Baseline screening should be performed at the time of diagnosis, followed by periodic assessments throughout life (see Table. Evaluation of Turner Syndrome Comorbidities by Developmental Stage and Organ System).
Table 2. Evaluation of Turner Syndrome Comorbidities by Developmental Stage and Organ System
|
Life Stage |
Organ System |
Mode of Evaluation |
Onset of Evaluation |
Frequency |
|
Newborn |
Metabolic |
Pre-feed blood glucose |
First 48 hours |
Once, before discharge (or later if indicated) |
|
Hip stability, lymphedema |
Clinical ± imaging |
Newborn period |
Once, before discharge |
|
|
Cardiovascular |
Transthoracic echocardiogram |
2-3 days of life or at diagnosis |
Later in childhood, as indicated (refer to "Adverse Effects of Organophosphates" for more information) |
|
|
Renal |
Renal ultrasound |
Newborn period |
Once, before discharge; repeat if with recurrent urinary tract infections or new hypertension diagnosis |
|
|
Childhood |
Growth and nutrition |
Weight, height, weight for height, or body mass index |
At diagnosis |
Every 6 months |
|
Ophthalmologic |
Comprehensive ophthalmologic evaluation |
6-12 months or at diagnosis |
As needed |
|
|
Otologic |
Otoscopy and tympanometry Audiometry |
Otoscopy and tympanometry: During infancy Audiometry: At diagnosis |
Otoscopy, tympanometry: Annually or earlier if symptomatic Audiometry: Every 3 years |
|
|
Dental |
Clinical dental examination |
At diagnosis |
Annually |
|
|
Thyroid and celiac diseases |
Thyroid-stimulating hormone and anti-tissue transglutaminase antibodies |
≥2 years |
Thyroid-stimulating hormone: Every 1-2 years or if symptomatic Anti-tissue transglutaminase antibodies: Every 2-5 years or if symptomatic |
|
|
Skeletal |
Physical examination |
Early childhood |
Annually until linear growth is complete |
|
|
Blood pressure |
Ambulatory blood pressure preferred |
At diagnosis and from the second year onward |
At least annually |
|
|
Renal |
Renal ultrasound |
At diagnosis |
Repeat if with recurrent urinary tract infections or new hypertension diagnosis |
|
|
Neuropsychology |
Cognitive and behavioral assessment |
At diagnosis |
Periodically at key developmental stages or as clinically indicated |
|
|
Adolescence |
Metabolic and hepatic |
Fasting blood glucose, glycated hemoglobin, alanine aminotransferase, aspartate aminotransferase, γ-glutamyl transferase, alkaline phosphatase, and complete blood count |
9-11 years |
Every 1-2 years |
|
Islet antibodies |
At the diagnosis of diabetes mellitus |
|||
|
Skeletal |
Vitamin D Dual-energy x-ray absorptiometry scan |
Vitamin D: 9-11 years Dual-energy x-ray absorptiometry: Growth completion (before 21 years) |
Vitamin D: Every 2-3 years Dual-energy x-ray absorptiometry: See adult recommendations |
|
|
Fertility |
Anti-Müllerian hormone |
8-9 years |
Annually until 11-12 years or if ovarian failure develops |
|
|
Adult |
Otologic |
Otoscopy, tympanoscopy, and audiometry |
Continued from childhood or at diagnosis |
Every 5 years |
|
Metabolic |
Fasting lipid panel |
At transition to adult care or as per local guidelines |
Every 3 years if normal |
|
|
Skeletal |
Dual-energy x-ray absorptiometry scan |
At growth completion (before 21 years) |
Every 5-10 years or at hormone replacement therapy cessation |
Cardiac Evaluation
Fetal two-dimensional echocardiography should be performed when Turner syndrome is diagnosed antenatally. Conversely, the presence of a left-sided cardiac lesion (excluding bicuspid aortic valve) in a female fetus should prompt evaluation for Turner syndrome. All infants or children diagnosed with Turner syndrome should undergo TTE on the second or third day of life, or at the time of diagnosis, even in the absence of clinical signs. TTE should assess for bicuspid aortic valve, coarctation of the aorta, partial anomalous pulmonary venous return, hypoplastic left heart syndrome, and coronary artery anomalies, including visualization of their origin and proximal course.
Cardiac magnetic resonance imaging (MRI) provides superior visualization of extracardiac vascular anomalies compared to TTE and should be included in the initial evaluation of all children and adults. In children with adequate TTE visualization and no abnormalities, cardiac MRI or computed tomography (CT) may be deferred until imaging can be performed without anesthesia. Electrocardiography (ECG) should be conducted at the initial evaluation to assess for rhythm abnormalities.
If the initial cardiac evaluation is normal, follow-up should be performed between the ages of 9 and 11, which should include an ECG, blood pressure measurement, and a TTE. In the absence of aortic dilation, the next examination may be performed upon completion of growth, incorporating ECG, blood pressure, and cardiac MRI or CT to establish baseline aortic imaging. Cardiac assessment is recommended again during the transition to adult care, accompanied by counseling on future surveillance, lipid monitoring, and pregnancy planning. In asymptomatic adults with Turner syndrome, cardiac evaluations should continue every 5 to 10 years.
Cardiology consultation is advised during pregnancy or fertility counseling, upon the onset of new cardiovascular symptoms, or following the diagnosis of a cardiac condition such as bicuspid aortic valve or aortic dilation. Lifelong monitoring is essential due to the substantially increased risk of aortic dilation and dissection—164 per 100,000 person-years compared to 6 per 100,000 person-years in the general population. For patients younger than 15, Turner syndrome–specific aortic Z-scores should be applied. For individuals older than 15, assessment may utilize the aortic height index, aortic size index, Turner syndrome–specific Z-scores, or general population Z-scores, depending on local protocols.[16]
Neuropsychological Evaluation and Counseling
Neuropsychological evaluation and counseling should be initiated at the time of diagnosis and continued throughout development. These services address anticipatory guidance, developmental surveillance, social cognition, learning and educational challenges, psychosexual concerns, and reproductive health.
Treatment / Management
Short Stature
Short stature frequently serves as the initial presenting concern in girls with Turner syndrome. Large studies have reported an untreated adult height deficit of approximately 20 cm compared to the general population, equivalent to a 3 SD reduction. Although growth hormone deficiency is not a characteristic feature of Turner syndrome, affected individuals often respond favorably to human growth hormone (hGH) therapy.
Initiation of hGH therapy is recommended as early as 2 years in individuals exhibiting a declining growth rate, established short stature, or a high likelihood of developing short stature. Early initiation is especially beneficial, as growth velocity declines rapidly during early childhood, particularly within the first 2 years. Greater height gains are achieved during the prepubertal years when treatment begins early.[17](A1)
Response to hGH varies among patients. However, an average annual height gain of 1 cm/year of therapy has been observed. The initial dosage of hGH is substantially higher than that used for growth hormone deficiency, typically 40 to 50 mcg/kg/d, and may be increased up to 68 mcg/kg/d in cases of suboptimal response. Please see StatPearls' companion resource, "Short Stature," for further information.
hGH therapy should be continued until a bone age of 14 or until height velocity falls below 2 cm/year. Treatment may be discontinued earlier if the patient expresses satisfaction with the attained height. HGH therapy can occasionally unmask preexisting scoliosis. Therefore, regular monitoring of spinal alignment is essential throughout the course of treatment. Development of scoliosis warrants referral to an orthopedic surgeon for evaluation and consideration of bracing or surgical correction. Other potential adverse effects, similar to those observed with hGH therapy in other contexts, include intracranial hypertension, slipped capital femoral epiphysis, and local site reactions.
When additional support for linear growth is necessary despite hGH therapy, adjunctive options such as oxandrolone (30-50 mcg/kg/d) or delayed initiation of puberty may be considered. The recent withdrawal of oxandrolone's approval by the United States Food and Drug Administration due to safety concerns should be carefully weighed in clinical decision-making. Local regulatory guidelines and approval status should direct its use in different regions.[Federal Register. Determination That Oxandrin (Oxandrolone) Tablets, 2.5 Milligrams and 10 Milligrams, Were Withdrawn From Sale for Reasons of Safety or Effectiveness]
HGH therapy has been generally considered safe. However, evidence regarding its effect on aortic diameter is conflicting. A cautious approach is warranted in individuals with aortic dilation or other cardiovascular risk factors. Serum insulin-like growth factor 1 should be measured at least annually, with values maintained within the normal range (up to +2 SD).
Preliminary data support the safety and efficacy of long-acting growth hormone in children diagnosed with Turner syndrome. However, additional studies are required before this agent can be adopted as a standard therapeutic option. Please see StatPearls' companion resource, "Short Stature," for further information.
Individuals with Turner syndrome have a higher baseline risk of metabolic complications compared to the general population. HGH therapy may further increase this risk.[19] Long-term follow-up studies are needed to determine the optimal timing and frequency of metabolic monitoring in patients receiving hGH treatment.
Cardiac Health
Both congenital and acquired cardiac conditions are common in Turner syndrome and represent a leading cause of mortality in adulthood. Congenital heart disease is present in approximately 50% of affected children and may include bicuspid aortic valve, coarctation of the aorta, hypoplastic left heart syndrome, and aortic arteriopathy. Over a lifetime, nearly 25% of individuals may develop an aortic aneurysm.
Cardiac management in individuals with Turner syndrome and congenital heart disease should follow established standards of care used for other patients with congenital heart disease across the lifespan.[20][21][22] Similarly, valvular diseases should be treated according to standard guidelines, with additional counseling regarding the increased cardiovascular risks associated with Turner syndrome.
In adults, aortic dilation poses a significant risk of dissection and requires active surveillance. Elective aortic surgery should be considered in the presence of moderate dilation, defined as an aortic height index over 23 mm/m, aortic size index above 2.3 cm/m², or Z-score greater than 3.5, when accompanied by at least 1 risk factor such as bicuspid aortic valve, coarctation of the aorta, hypertension, or a rapid increase in diameter (>3 mm/year). Severe dilation (aortic height index >25 mm/m, aortic size index >2.5 cm/m², or Z-score >4) also warrants surgical evaluation. In children younger than 15, Z-scores should guide clinical decision-making.
Prolonged QT interval necessitates the avoidance of QT-prolonging medications, including antiarrhythmics, macrolides, fluoroquinolones, and certain psychiatric drugs. Coarctation of the aorta, when present, requires surgical correction.
In patients with aortic dilation or an aortic Z-score over 2.5, antihypertensive therapy using β-blockers, angiotensin receptor blockers, or a combination of both is recommended, even in the absence of hypertension.[23] For individuals with normal aortic dimensions, blood pressure management should follow the general guidelines for pediatric or adult hypertension. In the presence of aortic dilation, limitations on exercise intensity should be observed to reduce cardiovascular risk.
Preconception and antenatal cardiac care should include cardiac MRI to evaluate aortic dimensions and aortic valve anatomy. If baseline findings are normal, at least 1 TTE assessment should be performed during pregnancy. Comprehensive cardiac risk assessment is essential before conception, with a clearly defined management plan outlining follow-up intervals and the mode of delivery. Pregnancy care should be coordinated at centers experienced in managing Turner syndrome during pregnancy.
Ovarian Failure
Girls with Turner syndrome typically present with primary amenorrhea or delayed puberty due to premature ovarian failure. To enable early diagnosis and timely referral for fertility preservation, serum FSH, luteinizing hormone, and AMH should be measured beginning at 8 to 9 years and monitored annually until 11 to 12 years. Approximately one-third of individuals with a 45,X karyotype may undergo spontaneous pubertal development, more frequently observed in those with mosaicism. However, most do not experience spontaneous menarche or achieve pregnancy. Consequently, hormone replacement therapy becomes necessary for the majority of individuals at some point.
Serum AMH is a useful marker for assessing ovarian function. Detectable AMH levels are associated with a higher likelihood of spontaneous pubertal onset, whereas levels below 4 pmol/L are strongly associated with absent pubertal development.[24] Estrogen replacement should be initiated if breast development has not occurred by approximately 11 to 12 years.
Hormone replacement therapy: The guiding principle of estrogen therapy in Turner syndrome is to replicate normal physiology as closely as possible in terms of timing, duration, and progression. Therapy is generally initiated between the ages of 11 and 12, after confirmation of elevated FSH on at least 2 separate occasions. For girls diagnosed later, low-dose estrogen may be introduced alongside hGH therapy.
The preferred estrogen for replacement is 17-β estradiol (E2), which is identical to the body's natural estrogen. The transdermal route is considered the most physiological and is preferred whenever feasible. Oral E2 serves as an effective alternative and is superior to other synthetic estrogen preparations.
Initial estrogen dosing is low, aimed at mimicking early pubertal progression. A typical starting dose consists of 7 mcg of transdermal E2 or 0.25 mg of oral E2, equivalent to one-eighth of an adult replacement dose. The dose is gradually escalated every 6 to 12 months with a target of reaching adult levels by the fourth year of treatment. This titration may be approximated using one-fourth of a 25 mcg E2 patch or a 1 mg estradiol valerate tablet initially, increasing to half and then a full patch or tablet in the second and third years, respectively. By the fourth year, a full adult dose, typically 50 mcg (up to 200 mcg) transdermally or 2 mg (up to 4 mg) orally, should be achieved.
Titration of estrogen doses should be guided by breast development and serum estradiol concentrations, with a goal of 100 to 150 pg/mL at full adult replacement. Additional parameters, such as uterine maturity assessed by pelvic ultrasound and bone mineral density evaluated using dual-energy x-ray absorptiometry, may also be used to determine the adequacy of therapy. In patients who begin puberty spontaneously but later experience gonadal failure, estrogen doses corresponding to the Tanner stage may be used, typically requiring higher initial doses than those used in girls without spontaneous onset.
Progesterone therapy: Cyclic progesterone should be introduced after 18 to 24 months of estradiol therapy, once withdrawal bleeding has commenced. The purpose is to induce and sustain regular uterine bleeding and to prevent endometrial hyperplasia. In cases where bleeding begins earlier in the replacement course, transabdominal or transvaginal ultrasound can be used to guide therapy, aiming to maintain endometrial thickness between 4 and 8 mm. If endometrial thickness remains below this threshold, or if stage 3 of breast development has not yet been attained, estradiol doses should be further increased to support maturation of both the endometrium and secondary sexual characteristics. Micronized progesterone at 200 mg daily for 12 days per month is considered the preferred option. Dydrogesterone at 10 mg daily for 12 days serves as a suitable alternative.
Hormone replacement therapy in adults with Turner syndrome: Hormone replacement therapy should be continued in adulthood using a cyclic combination of estrogen and progesterone, with a recommended duration extending to the average age of menopause, approximately 50 years. After this age, the necessity for continued hormone therapy should be reassessed, with the potential use of lower-dose regimens, depending on individual needs. In adult women, hormone therapy can be customized based on personal preference to improve adherence. Combined preparations are often preferred, in contrast to the sequential approach generally recommended for adolescents.
Beyond its role in supporting pubertal development and menstrual function, several broader benefits of hormone replacement therapy have been suggested. These potential benefits include a reduced need for antihypertensive, antidiabetic, and thyroid medications, along with decreased rates of hospitalization for cerebrovascular events and osteoporotic fractures.[25]
Fertility considerations: Approximately 10% of individuals with Turner syndrome are capable of achieving spontaneous pregnancy. Factors associated with this possibility include spontaneous menarche, mosaic karyotypes with at least partial preservation of the second X chromosome, measurable serum AMH levels, and FSH concentrations below 10 IU/L. However, pregnancies in this population are frequently complicated by both maternal and fetal risks, such as a threefold increase in miscarriage rates, heightened likelihood of preeclampsia, increased cesarean delivery rates, small-for-gestational-age neonates, preterm births, and fetal anomalies.
Fertility preservation options may be explored in individuals who do not conceive spontaneously. Controlled ovarian stimulation followed by oocyte cryopreservation may be offered after menarche, with careful psychological counseling provided beforehand. Ovarian tissue cryopreservation remains investigational and should be pursued only within ethically approved clinical studies. In patients who cannot achieve pregnancy using their own gametes, options such as oocyte or embryo donation and the use of gestational carriers should be discussed, especially in light of the substantial cardiovascular and obstetric risks posed by pregnancy in this population.
All counseling and follow-up related to fertility and pregnancy should be conducted in specialized centers by an interprofessional team. This team should include endocrinologists, cardiologists, fertility experts, and clinicians with experience in the comprehensive care of patients with Turner syndrome.[26]
Gonadoblastoma
Patients who have marker chromosome elements on the karyotype or develop virilization should be screened for the presence of the Y chromosome. The presence of the Y chromosome increases the risk of gonadoblastoma. However, the reported rates of gonadoblastoma vary widely (0%-100%), whereas the risk of malignant transformation remains comparatively low (1%-22%). Gonadoblastoma typically occurs after the second decade of life and rarely metastasizes. Given the absence of reliable clinical markers or imaging for follow-up and the risk of loss to follow-up, individualized decisions regarding prophylactic gonadectomy should be made, balancing these risks carefully.[27]
Currently, standard care does not recommend general cancer surveillance in this population. Adherence to population screening guidelines is advised. Nevertheless, a recent study indicated an increased risk of colon cancer compared to the general population, highlighting the need for larger long-term studies to guide future recommendations.[28]
Bone Health
Low bone mineral density and increased fracture risk are common in individuals with Turner syndrome. Estrogen therapy combined with vitamin D and calcium supplementation can reduce this risk.[29] Turner syndrome also elevates the risk of scoliosis, necessitating annual screening (please refer to the Complications section for more information).
Neuropsychiatric Function
Despite normal intelligence, girls with Turner syndrome frequently experience learning disabilities that may require specialized education and assessment. Interventions should be tailored according to the diagnosed disability. Although earlier reports suggested a higher prevalence of psychiatric illnesses, including depression, a recent nationwide retrospective study from Sweden found a relatively lower prevalence of psychiatric conditions in individuals with Turner syndrome compared to the general population. This finding urges caution in overdiagnosing severe psychiatric disorders in this group.[30]
Hearing Loss
Early tympanostomy intervention is recommended for middle ear disease, following similar protocols to those used for other high-risk populations. Evaluation methods and frequency at different life stages are detailed in Table 2 (see Table. Evaluation of Turner Syndrome Comorbidities by Developmental Stage and Organ System).
Renal Conditions
Renal abnormalities are common in Turner syndrome, including collecting system malformations, positional or horseshoe kidneys, and malrotation. Obstruction caused by ureteropelvic junction anomalies may lead to hydronephrosis and increase the risk of pyelonephritis.[31] Patients with detected abnormalities should be referred promptly to nephrology and urology specialists as indicated.
Transition Care
Transition from adolescence to adulthood, typically between the ages of 14 and 25, supports improved adherence to follow-up care. This phase should be integrated into any comprehensive Turner syndrome care program to ensure continuity and holistic management.
Differential Diagnosis
The clinical features of Turner syndrome share similarities with other genetic and developmental disorders, necessitating thorough differential diagnosis. Identifying key distinctions at various stages aids in accurate diagnosis and treatment planning.
During the antenatal period, findings such as hygroma colli, fetal hydrops, coarctation of the aorta, and increased nuchal translucency may suggest Turner syndrome but are not exclusive to this condition. Similar prenatal markers may also be observed in trisomy 21, trisomy 18, and Noonan syndrome.[18][19]
In childhood, short stature accompanied by morphologic features resembling Turner syndrome can occur in Noonan syndrome and cases of haploinsufficiency of the SHOX gene. Noonan syndrome often presents with multiple characteristics overlapping with Turner syndrome, including webbed neck, short stature, and cardiac and renal anomalies. However, Noonan syndrome affects both boys and girls, whereas Turner syndrome is restricted to girls.[20] During adolescence, failure to initiate or progress through puberty raises suspicion for alternative diagnoses such as 46,XX gonadal dysgenesis, 46,XY complete gonadal dysgenesis, or premature ovarian failure due to genetic abnormalities including deletions distal to Xq24.
Recognition of similar presentations in other genetic conditions highlights the importance of targeted diagnostic testing in suspected Turner syndrome cases. This approach ensures appropriate treatment and surveillance tailored to the specific diagnosis.
Prognosis
Gaps remain in understanding the genetic basis of various comorbidities and the optimal treatment strategies. However, the use of hGH, hormone replacement therapy, structured cardiac monitoring and interventions, fertility assistance, enhanced psychosocial support, and improved management of other comorbidities have significantly improved the quality of life for individuals with Turner syndrome in recent decades (please refer to the Evaluation and Treatment / Management sections for more information).[21]
Mortality rates are increased among patients with Turner syndrome. A nationwide Danish study reported approximately 3 times greater mortality compared to the general population. Standardized mortality ratios were elevated for coronary diseases, congenital malformations, and other causes. Increased mortality occurred across all karyotypes but was highest among individuals with 45,X and isoXq karyotypes. Cardiovascular disease, particularly coronary heart disease and stroke in older patients, represents a major cause of death. Among congenital cardiovascular conditions, aortic aneurysm stands out as the leading contributor. Additional causes of increased mortality include pneumonia, diabetes mellitus, epilepsy, liver disease, and kidney disease.[22]
Complications
Some complications or comorbidities commonly associated with Turner syndrome include the following:
- Short stature
- Pubertal failure and infertility
- Aortic aneurysm, dissection, and coronary artery disease
- Skeletal abnormalities and impaired metabolic bone health
- Hearing loss, both conductive and sensorineural
- Increased incidence of autoimmune conditions, such as hypothyroidism and celiac disease
- Liver function abnormalities and renal malformations
- Neurocognitive deficits
- Increased risk of gonadoblastoma in individuals with Y chromosome material
- Metabolic disorders, including central obesity, insulin resistance, type 1 and type 2 diabetes, and dyslipidemia
Additional complications related to the treatment of these comorbidities may also occur in individuals with Turner syndrome, including those associated with hGH therapy, as mentioned.
Consultations
Comprehensive care for Turner syndrome involves the timely involvement of multiple specialists to monitor and treat its diverse manifestations. Coordinated consultations with the following specialties help ensure optimal outcomes across developmental stages:
- Pediatric endocrinology or adult endocrinology for hormone management and growth monitoring
- Pediatric cardiology or cardiology for congenital and acquired cardiac evaluation and surveillance
- Medical genetics for diagnosis, counseling, and genetic risk assessment
- Gynecology and fertility specialists for reproductive health and fertility preservation
- Audiology for hearing assessment and management of middle and inner ear disorders
- Ophthalmology for vision screening and ocular abnormalities
- Orthopedic surgery for the evaluation and treatment of scoliosis or other musculoskeletal issues
- Nephrology and urology for the management of renal malformations and urinary tract abnormalities
Ongoing communication between specialties enhances care quality and addresses evolving needs over time. Such interprofessional collaboration remains crucial throughout the patient's lifespan.
Deterrence and Patient Education
Preimplantation genetic testing may be offered to individuals with Turner syndrome who plan to conceive using their own oocytes. Patients with mosaic karyotypes (45,X/46,XX) who conceive spontaneously should be offered prenatal diagnostic testing. Families with an antenatal diagnosis should receive nondirective genetic counseling from qualified counselors and clinicians experienced in the long-term management of Turner syndrome.
Turner syndrome is a lifelong condition that requires consistent follow-up. Numerous complications are associated with this diagnosis, including short stature, ovarian insufficiency, congenital heart and kidney anomalies, skeletal abnormalities and compromised bone health, hearing impairment, neurocognitive challenges, increased risk of gonadoblastoma, autoimmune thyroid and gastrointestinal disorders, obesity, diabetes, and hypertension. Proactive surveillance and timely intervention can reduce the impact of many of these comorbidities.
As discussed, the median age at diagnosis is approximately 15 years, highlighting a major diagnostic delay due to missed opportunities during infancy. Improving awareness of the clinical features of Turner syndrome and the indications for karyotype testing among primary care physicians and the general public may help facilitate earlier diagnosis and intervention.
Enhancing Healthcare Team Outcomes
Patients diagnosed with Turner syndrome commonly present with multiple comorbidities and require the involvement of several specialties functioning as an interprofessional team. Although primary care providers typically oversee screening and long-term follow-up, comprehensive care often necessitates collaboration with specialists in various fields, including cardiologists, endocrinologists, gynecologists and fertility specialists, ophthalmologists, audiologists, psychologists, nephrologists, and orthopedic surgeons.
Clear communication among these specialists, and particularly with the primary care team, is essential to address the full spectrum of potential complications. Nurses with specialized training in genetics play a key role in patient education, coordination of referrals, and communication among team members. Pharmacists contribute by reviewing hormone replacement regimens and supporting families with medication administration and adherence. An interprofessional team approach enhances continuity of care and is essential for achieving optimal outcomes in individuals with this condition.
Media
(Click Image to Enlarge)

Girl With Turner Syndrome. Photos showing the patient before and immediately after surgical correction of neck webbing.
Johannes Nielsen, Public Domain, via Wikimedia Commons
(Click Image to Enlarge)
References
. Classic pages in obstetrics and gynecology by Henry H. Turner. A syndrome of infantilism, congenital webbed neck, and cubitus valgus. Endocrinology, vol. 23, pp. 566-574, 1938. American journal of obstetrics and gynecology. 1972 May 15:113(2):279 [PubMed PMID: 4557013]
Gravholt CH, Andersen NH, Christin-Maitre S, Davis SM, Duijnhouwer A, Gawlik A, Maciel-Guerra AT, Gutmark-Little I, Fleischer K, Hong D, Klein KO, Prakash SK, Shankar RK, Sandberg DE, Sas TCJ, Skakkebæk A, Stochholm K, van der Velden JA, International Turner Syndrome Consensus Group, Backeljauw PF. Clinical practice guidelines for the care of girls and women with Turner syndrome. European journal of endocrinology. 2024 Jun 5:190(6):G53-G151. doi: 10.1093/ejendo/lvae050. Epub [PubMed PMID: 38748847]
Level 1 (high-level) evidenceGravholt CH, Andersen NH, Conway GS, Dekkers OM, Geffner ME, Klein KO, Lin AE, Mauras N, Quigley CA, Rubin K, Sandberg DE, Sas TCJ, Silberbach M, Söderström-Anttila V, Stochholm K, van Alfen-van derVelden JA, Woelfle J, Backeljauw PF, International Turner Syndrome Consensus Group. Clinical practice guidelines for the care of girls and women with Turner syndrome: proceedings from the 2016 Cincinnati International Turner Syndrome Meeting. European journal of endocrinology. 2017 Sep:177(3):G1-G70. doi: 10.1530/EJE-17-0430. Epub [PubMed PMID: 28705803]
Level 1 (high-level) evidenceWolff DJ, Van Dyke DL, Powell CM, Working Group of the ACMG Laboratory Quality Assurance Committee. Laboratory guideline for Turner syndrome. Genetics in medicine : official journal of the American College of Medical Genetics. 2010 Jan:12(1):52-5. doi: 10.1097/GIM.0b013e3181c684b2. Epub [PubMed PMID: 20081420]
Level 2 (mid-level) evidenceGravholt CH, Viuff MH, Brun S, Stochholm K, Andersen NH. Turner syndrome: mechanisms and management. Nature reviews. Endocrinology. 2019 Oct:15(10):601-614. doi: 10.1038/s41574-019-0224-4. Epub 2019 Jun 18 [PubMed PMID: 31213699]
Cui X, Cui Y, Shi L, Luan J, Zhou X, Han J. A basic understanding of Turner syndrome: Incidence, complications, diagnosis, and treatment. Intractable & rare diseases research. 2018 Nov:7(4):223-228. doi: 10.5582/irdr.2017.01056. Epub [PubMed PMID: 30560013]
Level 3 (low-level) evidenceMartin-Giacalone BA, Lin AE, Rasmussen SA, Kirby RS, Nestoridi E, Liberman RF, Agopian AJ, Carey JC, Cragan JD, Forestieri N, Leedom V, Boyce A, Nembhard WN, Piccardi M, Sandidge T, Shan X, Shumate CJ, Stallings EB, Stevenson R, Lupo PJ. Prevalence and descriptive epidemiology of Turner syndrome in the United States, 2000-2017: A report from the National Birth Defects Prevention Network. American journal of medical genetics. Part A. 2023 May:191(5):1339-1349. doi: 10.1002/ajmg.a.63181. Epub 2023 Mar 15 [PubMed PMID: 36919524]
Gunther DF, Eugster E, Zagar AJ, Bryant CG, Davenport ML, Quigley CA. Ascertainment bias in Turner syndrome: new insights from girls who were diagnosed incidentally in prenatal life. Pediatrics. 2004 Sep:114(3):640-4 [PubMed PMID: 15342833]
Stochholm K, Juul S, Juel K, Naeraa RW, Gravholt CH. Prevalence, incidence, diagnostic delay, and mortality in Turner syndrome. The Journal of clinical endocrinology and metabolism. 2006 Oct:91(10):3897-902 [PubMed PMID: 16849410]
Sävendahl L, Davenport ML. Delayed diagnoses of Turner's syndrome: proposed guidelines for change. The Journal of pediatrics. 2000 Oct:137(4):455-9 [PubMed PMID: 11035820]
Barrenäs ML, Nylén O, Hanson C. The influence of karyotype on the auricle, otitis media and hearing in Turner syndrome. Hearing research. 1999 Dec:138(1-2):163-70 [PubMed PMID: 10575123]
Level 1 (high-level) evidenceAdhikary HP. Ocular manifestations of Turner's syndrome. Transactions of the ophthalmological societies of the United Kingdom. 1981:101 (Pt 4)():395-6 [PubMed PMID: 6964261]
Lowenstein EJ, Kim KH, Glick SA. Turner's syndrome in dermatology. Journal of the American Academy of Dermatology. 2004 May:50(5):767-76 [PubMed PMID: 15097963]
Silva M, de Leeuw N, Mann K, Schuring-Blom H, Morgan S, Giardino D, Rack K, Hastings R. European guidelines for constitutional cytogenomic analysis. European journal of human genetics : EJHG. 2019 Jan:27(1):1-16. doi: 10.1038/s41431-018-0244-x. Epub 2018 Oct 1 [PubMed PMID: 30275486]
Lunding SA, Aksglaede L, Anderson RA, Main KM, Juul A, Hagen CP, Pedersen AT. AMH as Predictor of Premature Ovarian Insufficiency: A Longitudinal Study of 120 Turner Syndrome Patients. The Journal of clinical endocrinology and metabolism. 2015 Jul:100(7):E1030-8. doi: 10.1210/jc.2015-1621. Epub 2015 May 15 [PubMed PMID: 25978111]
Mortensen KH, Young L, De Backer J, Silberbach M, Collins RT, Duijnhouwer AL, Pandya B, Gravholt CH, Lopez L, Roos-Hesselink JW. Cardiovascular imaging in Turner syndrome: state-of-the-art practice across the lifespan. Heart (British Cardiac Society). 2018 Nov:104(22):1823-1831. doi: 10.1136/heartjnl-2017-312658. Epub 2018 Sep 18 [PubMed PMID: 30228249]
Aversa T, Li Pomi A, Pepe G, Corica D, Messina MF, Coco R, Sippelli F, Ferraloro C, Luppino G, Valenzise M, Wasniewska MG. Growth Hormone Treatment to Final Height in Turner Syndrome: Systematic Review. Clinical therapeutics. 2024 Feb:46(2):146-153. doi: 10.1016/j.clinthera.2023.12.004. Epub 2023 Dec 26 [PubMed PMID: 38151406]
Level 1 (high-level) evidenceSchreurs L, Lannoo L, De Catte L, Van Schoubroeck D, Devriendt K, Richter J. First trimester cystic hygroma colli: Retrospective analysis in a tertiary center. European journal of obstetrics, gynecology, and reproductive biology. 2018 Dec:231():60-64. doi: 10.1016/j.ejogrb.2018.10.019. Epub 2018 Oct 9 [PubMed PMID: 30321790]
Level 2 (mid-level) evidenceSparks TN, Thao K, Lianoglou BR, Boe NM, Bruce KG, Datkhaeva I, Field NT, Fratto VM, Jolley J, Laurent LC, Mardy AH, Murphy AM, Ngan E, Rangwala N, Rottkamp CAM, Wilson L, Wu E, Uy CC, Valdez Lopez P, Norton ME, University of California Fetal–Maternal Consortium (UCfC). Nonimmune hydrops fetalis: identifying the underlying genetic etiology. Genetics in medicine : official journal of the American College of Medical Genetics. 2019 Jun:21(6):1339-1344. doi: 10.1038/s41436-018-0352-6. Epub 2018 Nov 9 [PubMed PMID: 30410095]
Roberts AE, Allanson JE, Tartaglia M, Gelb BD. Noonan syndrome. Lancet (London, England). 2013 Jan 26:381(9863):333-42. doi: 10.1016/S0140-6736(12)61023-X. Epub 2013 Jan 10 [PubMed PMID: 23312968]
Gravholt CH, Viuff M, Just J, Sandahl K, Brun S, van der Velden J, Andersen NH, Skakkebaek A. The Changing Face of Turner Syndrome. Endocrine reviews. 2023 Jan 12:44(1):33-69. doi: 10.1210/endrev/bnac016. Epub [PubMed PMID: 35695701]
Schoemaker MJ, Swerdlow AJ, Higgins CD, Wright AF, Jacobs PA, United Kingdom Clinical Cytogenetics Group. Mortality in women with turner syndrome in Great Britain: a national cohort study. The Journal of clinical endocrinology and metabolism. 2008 Dec:93(12):4735-42. doi: 10.1210/jc.2008-1049. Epub 2008 Sep 23 [PubMed PMID: 18812477]
Level 2 (mid-level) evidence