Introduction
Tracheostomy is one of the oldest known surgical procedures, with depictions dating to 3,600 B.C. in ancient Egypt. The procedure involves creating an opening in the anterior tracheal wall to facilitate breathing and airway access. Although "tracheostomy" and "tracheotomy" have slightly different technical meanings, the terms are often used interchangeably. For clarity, the term "tracheostomy" will be used in this activity.
Historically, tracheostomy was the only intervention available for upper airway obstruction. While the procedure is lifesaving in that context, its indications have expanded. Today, tracheostomy is most often performed electively to facilitate prolonged mechanical ventilation, assist with ventilator weaning, improve pulmonary hygiene, or support patients with chronic neuromuscular or airway conditions.
Traditionally performed as an open surgical procedure in the operating room, tracheostomy may now also be performed percutaneously in other appropriately equipped hospital units. Minimally invasive percutaneous techniques are safe, effective, and widely used, particularly in critically ill patients, allowing bedside placement and reducing the need for transport and operative exposure.[1]
Anatomy and Physiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Anatomy and Physiology
The trachea is a tubular structure composed of 16 to 20 C-shaped cartilaginous rings that are open posteriorly. The 1st ring, the cricoid cartilage, is complete and marks the junction between the larynx and trachea. The trachea begins just below the subglottic larynx and descends to the carina, where it bifurcates into the mainstem bronchi.
The trachea's posterior wall is membranous and shared with the anterior wall of the esophagus. Anteriorly, the cervical trachea is overlain by the strap muscles—the sternohyoid and sternothyroid—and the thyroid isthmus, typically spanning the 2nd to 4th tracheal rings.
Laterally, the trachea is adjacent to the recurrent laryngeal nerves, peritracheal lympho-fatty tissue, and the common carotid arteries, enclosed within the carotid sheath, which is part of the deep cervical fascia. The pretracheal fascia, a component of the middle layer of deep cervical fascia, further encases these structures.
In the thorax, the trachea passes posterior to the thymus and anterior to the heart. The innominate artery crosses anteriorly as it arises from the aortic arch—an important consideration during tracheostomy.
Anatomic Landmarks for Tracheostomy
Several anatomic landmarks guide safe tracheostomy placement. The thyroid notch, a palpable midline prominence, marks the superior aspect of the larynx and serves as an initial reference point for identifying deeper airway structures. The cricothyroid membrane, a palpable depression between the thyroid and cricoid cartilages, represents the preferred site for emergent cricothyrotomy due to its superficial location and minimal vascular structures.
The cricoid cartilage, the only complete cartilaginous ring in the airway, is a key landmark for locating the junction between the larynx and trachea, with the tracheostomy incision typically made 1 to 2 cm inferior to this structure (see Image. Superficial Surface Anatomy of the Neck). The sternal notch identifies the thoracic inlet, and palpation at this level helps assess for a high-riding innominate artery, which may cross the trachea and increase the risk of hemorrhage during tracheostomy (see Image. Vascular Structures of the Anterior Neck).
Indications
Tracheostomy may be performed emergently or electively, depending on the clinical context. Emergent tracheostomy is warranted when less invasive airway access fails. Common scenarios include acute upper airway obstruction from foreign body aspiration, angioedema, deep neck infection such as Ludwig angina, and anaphylaxis. Other indications include formalization of a cricothyrotomy after airway stabilization, penetrating laryngeal trauma, and severe facial injuries such as LeFort III fractures.
Panfacial trauma or craniofacial dislocation may contraindicate nasal intubation. Although alternative airway strategies should be attempted when feasible, the surgical team must be prepared to perform an emergent tracheostomy immediately in life-threatening cases, including laryngeal disruption or other acute airway compromise.[2] Elective tracheostomy is indicated in patients with prolonged mechanical ventilation, anticipated airway obstruction due to extensive head and neck surgery or edema (eg, from cancer, trauma, or radiation), obstructive sleep apnea refractory to other therapies, recurrent aspiration from impaired airway protection, neuromuscular disease with respiratory failure, and subglottic stenosis.
The optimal timing of tracheostomy in patients with prolonged ventilator dependence remains debated. Traditional practice favors placement at 5 to 7 days postintubation to reduce complications such as subglottic stenosis. With modern low-pressure endotracheal tube (ETT) cuffs (≤20 cm H2O), this interval may be safely extended when extubation remains likely. Early tracheostomy, however, has been associated with reduced sedation requirements, improved patient comfort, and potentially shorter intensive care unit (ICU) stays.[3][4]
Physiologically, tracheostomy reduces airway resistance, which may enhance ventilator weaning. According to Poiseuille’s law, resistance increases with tube length and decreases with internal diameter, making tracheostomy more efficient than prolonged endotracheal intubation.[5]
Professional bodies, such as the Eastern Association for the Surgery of Trauma, recommend early tracheostomy, within 3 to 7 days, for patients with severe closed head injury or anticipated prolonged mechanical ventilation.[6][7] Prophylactic tracheostomy may also be appropriate before extensive head and neck procedures when postoperative airway obstruction is expected.
Tracheostomy may provide the only effective airway management option for patients with refractory obstructive sleep apnea, particularly those with severe obesity. Elective tracheostomy also benefits individuals with chronic neurologic impairment and poor secretion clearance by reducing the risk of aspiration pneumonia. In neuromuscular disorders such as amyotrophic lateral sclerosis, tracheostomy supports long-term mechanical ventilation as respiratory muscle strength declines.
Contraindications
Tracheostomy is generally a safe and effective procedure. Although no condition constitutes an absolute contraindication in a true airway emergency, several factors may increase the risk of complications and should be addressed or optimized when feasible. Relative contraindications include active anterior neck cellulitis or infection, cervical spine instability, uncorrected coagulopathy, severe respiratory failure that precludes brief interruption of positive pressure ventilation, and difficult access due to distorted neck anatomy or prior surgical interventions.
In individuals with terminal illness, decisions regarding tracheostomy should be guided by discussions of care goals, prognosis, and quality of life. Shared decision-making with the patient, their surrogate, or both is essential to ensure that the procedure aligns with patient values and preferences.[8]
Equipment
Standard equipment for tracheostomy includes a dedicated tracheostomy tray with sterile instruments, a cuffed tracheostomy tube (typically nonfenestrated for initial placement), and appropriate personal protective equipment (PPE) for all team members. Enhanced PPE and negative-pressure environments may be indicated in situations with a high risk of aerosolization.
Percutaneous tracheostomy requires additional equipment, including a flexible bronchoscope for real-time airway visualization, a percutaneous tracheostomy kit (such as Blue Rhino®), and suction and oxygen delivery systems. Adjunct tools, such as fiberoptic laryngoscopy or bronchoscopy equipment, may be necessary in patients with challenging anatomy, prior tracheostomy, neck irradiation, or altered landmarks. Ultrasound can further assist by identifying vascular structures and confirming midline access before incision or needle entry, particularly in patients with difficult anatomy.
Personnel
Open tracheostomy typically involves an interprofessional team consisting of a surgeon, a surgical assistant, an anesthesiologist, a scrub technologist, and a circulating nurse. Percutaneous tracheostomy generally requires 2 trained physicians—one to perform percutaneous access and dilation and another to manage the bronchoscope for real-time visualization of wire and tube placement.
Bedside percutaneous tracheostomy may be performed by a critical care physician or pulmonologist with appropriate training and support. Additional personnel, including respiratory therapists and nursing staff, are often involved in the ICU. Clearly defining roles in advance is essential to ensure procedural safety and allow rapid response to potential complications.
Preparation
Open Tracheostomy
Open tracheostomy is typically performed in a controlled operating room environment by an interprofessional team. Most patients undergo the procedure under general anesthesia with endotracheal intubation. In select cases, such as in upper airway compromise due to conditions like Ludwig angina, the procedure may be performed under local anesthesia while the patient remains awake and breathes spontaneously.
Initial placement generally uses a cuffed, nonfenestrated tracheostomy tube to secure the airway and reduce aspiration risk (see Image. Cannula for Tracheostomy). Preoperative planning includes confirming the availability of appropriately sized tubes, suction, oxygen delivery systems, and emergency airway equipment. Anatomic landmarks should be carefully reviewed and palpated, particularly in patients with challenging neck anatomy or a history of prior surgery.
Percutaneous Tracheostomy
Percutaneous tracheostomy may be performed at the bedside or in the operating room, most commonly in ICU settings. Patients are typically intubated and sedated, with or without neuromuscular blockade. Preprocedural ultrasound may be used to identify the tracheal midline, locate vessels, and select a safe puncture site.
A flexible bronchoscope is introduced through the ETT to facilitate visualization of needle and guidewire placement and confirm proper tube positioning. Bronchoscopic guidance should be coordinated between the proceduralist and the airway operator. Neck extension using a shoulder roll or chest bump improves access to the anterior trachea, and gentle manual retraction may enhance exposure in patients with prominent breast tissue or challenging anatomy.
Infection Control
Enhanced airborne precautions are essential when aerosolization of respiratory secretions is a concern, such as during outbreaks of airborne infectious diseases, including COVID-19 and tuberculosis. Recommended measures include full PPE and performing the procedure in a negative-pressure room when available. Appropriate suction systems and viral filters should be used to minimize aerosol dispersion during airway manipulation.
Technique or Treatment
Open Tracheostomy
After appropriate preparation, anatomic landmarks, including the thyroid notch, cricoid cartilage, and sternal notch, are palpated and marked. Special attention is given to the sternal notch to assess for a high-riding innominate artery. A midline skin incision is made approximately 1 to 2 cm inferior to the cricoid cartilage, using either a horizontal or vertical approach based on surgeon preference or patient anatomy.
The incision is carried through the platysma, and the sternohyoid and sternothyroid are separated along the midline raphe and retracted laterally. The cricoid cartilage and trachea are exposed. An overlying thyroid isthmus may be ligated or divided to enhance exposure of the target area. Hemostasis is maintained, often by cross-clamping the isthmus with hemostats and oversewing the stumps.
A cricoid hook may be used to elevate the trachea into the surgical field. The 2nd and 3rd tracheal rings are identified, and a tracheal incision is made between these cartilaginous structures. Stay sutures may be placed in the lateral tracheal wall to provide retraction and secure tube position postoperatively. Variations include removal of an anterior cartilage window, vertical tracheal incisions (often in pediatric cases), or creation of a Björk flap, a cartilage-based flap sutured to the skin to maintain a patent stoma.
The 1st tracheal ring is generally avoided to reduce the risk of stenosis. The tracheostomy tube should be connected to the anesthesia circuit once it is placed. End-tidal carbon dioxide confirms proper placement. The cricoid hook is then released. The tube is secured with sutures to the skin or tracheal stay sutures and supported with a soft neck tie. The initial tracheostomy tube is typically left in place until the 1st tube change, usually on postoperative day 5.[9]
Percutaneous Tracheostomy
The percutaneous technique involves a modified Seldinger approach, usually performed at the bedside in the ICU under bronchoscopic guidance. First described in 1957 and later standardized by Ciaglia in 1985, this method offers advantages over open tracheostomy, including reduced operative time, blood loss, and infection risk.[10] Numerous studies have compared outcomes between the 2 approaches, highlighting unique benefits and limitations.[11][12] This modality is preferred in many institutions and is now the most common method in the U.S., with over 100,000 procedures performed annually.[13]
After patient positioning and sedation, a bronchoscope is introduced through the ETT to identify the carina and monitor needle entry. A vertical midline skin incision is made below the cricoid cartilage, and the pretracheal tissue is bluntly dissected. Ultrasound may assist in assessing midline and vascular anatomy, although visualization of the posterior tracheal wall is limited.
Bronchoscopy facilitates early detection of airway injuries and confirms guidewire placement, even though it has not consistently reduced procedural complications. The original Ciaglia technique, which used serial dilators, has been streamlined with the Blue Rhino® single-step dilator, incorporating a protective sheath to minimize posterior wall injury.
The ETT is withdrawn under bronchoscopic guidance to just below the vocal cords. The introducer needle is inserted through the anterior tracheal wall with the bevel down, allowing the guidewire to advance caudally while avoiding puncture of the ETT cuff. The introducer needle and sheath are removed once the guidewire is visualized within the trachea.
Progressive dilation is performed over the guidewire, either with serial dilators or the Blue Rhino® single-step dilator. The tracheostomy tube with obturator is then advanced over the wire, after which the wire and obturator are removed and the cuff inflated. Tube placement is confirmed with end-tidal carbon dioxide and direct bronchoscopic visualization. The inner cannula is inserted, and the flange is secured to the neck skin with sutures or a neck tie. Proper placement and ventilation are reconfirmed at the procedure's conclusion.
Complications
Complications
Tracheostomy complications are best categorized based on when they occur, whether during the intraoperative, early postoperative, or late postoperative period. Each category encompasses specific risks that clinicians must anticipate and manage to ensure patient safety.
Operative Period
The most common intraoperative complication of tracheostomy is bleeding. Many patients are critically ill and may have underlying coagulopathy, which should be corrected when possible. Thrombocytopenic patients may require platelet transfusion to achieve a count exceeding 50,000 before airway surgery. Even minimal bleeding can obscure the surgical field or hinder bronchoscopy.
Anatomic considerations include anterior jugular veins, which are usually retractable but may present aberrantly. Approximately 5% of patients have a thyroidea ima artery running along the anterior trachea, which may retract and bleed if divided improperly.[14] Meticulous technique is essential, particularly when ligating the thyroid isthmus. In percutaneous tracheostomy, ultrasound can help identify high-riding vascular structures such as the innominate artery.[15]
Airway fire is a rare but catastrophic event caused by high oxygen concentrations and electrocautery ignition. Prevention requires close coordination between anesthesia and surgical teams. If a fire occurs, the circuit must be removed immediately, the patient ventilated via mask, and the airway inspected with laryngoscopy, bronchoscopy, and esophagoscopy for thermal injury.[16]
Pneumothorax and pneumomediastinum may result from false passage creation, apical injury, or posterior wall disruption. Bronchoscopic visualization often prevents posterior wall injuries. Wire kinking during percutaneous placement may indicate improper trajectory. Postoperative chest radiographs may be used to screen for pneumothorax, though this assessment is rarely required in routine cases.
Early Postoperative Complications
Infection following tracheostomy is uncommon and typically mild, often managed with local wound care. Deep infections or abscesses may develop in immunocompromised patients and require culture-directed antibiotic therapy. Acute tube obstruction may result from blood, mucus, or dislodgement. Preventive measures include regular suctioning, humidified oxygen, and daily cannula cleaning. Dislodgement into soft tissue or a false passage may lead to airway compromise. Stay sutures placed during surgery can facilitate reentry. Reintubation, preferably under flexible laryngoscopic guidance, may be necessary to reestablish a secure airway.
Late Complications
Late complications most often relate to prolonged cuff pressure, which can cause ischemia, tracheal wall necrosis, and stenosis. The risk has decreased with low-pressure cuffs, and cuff pressures should be maintained below 20 cm H2O. Stenosis may also result from excessive dilation or manipulation, with rates comparable between open and percutaneous techniques.[17] Risk factors include high tracheostomy placement, steroid use, gastroesophageal reflux, prior tracheostomy, and neck irradiation. Management may require endoscopic or open surgical intervention.[18]
Tracheoesophageal fistula
Tracheoesophageal fistula is a rare (<1%) but serious complication, typically arising from prolonged posterior tracheal wall pressure caused by overinflated cuffs, malpositioned tubes, or the presence of a nasogastric tube alongside a rigid tracheostomy. Clinical manifestations may include bilious tracheal secretions, gastric distension, recurrent pneumonia, or suppuration. Fistulae generally measure 1 to 4 cm in length.[19][20] Definitive management requires surgical resection with primary esophageal closure, tracheal anastomosis, and interposition of a muscle flap. Further procedural and postoperative details are discussed in the dedicated StatPearls activity on tracheoesophageal fistula.[21]
Tracheoinnominate fistula
Tracheoinnominate fistula is rare (<1%) but carries a high mortality rate, approaching 80%.[22] A sentinel bleed from the stoma may precede catastrophic hemorrhage. Risk factors include low tracheostomy placement (below the 3rd tracheal ring), a high-riding innominate artery, and oversized tracheostomy tubes.
The condition results from pressure necrosis of the anterior tracheal wall at the point of contact with the innominate artery.[23] Maintaining cuff pressures below 20 cm H2O may reduce risk.[24] Immediate management includes removing the tracheostomy tube, orally intubating with the cuff positioned distal to the bleeding site, and applying digital pressure through the stoma to tamponade the artery against the manubrium. Definitive treatment typically involves median sternotomy with ligation of the innominate artery, occasionally supplemented by interpositional flap placement. Endovascular stenting or embolization may be considered in selected cases.[25]
Persistent tracheocutaneous fistula
The tracheostomy stoma closes spontaneously within 24 to 48 hours after decannulation in most patients. Persistent granulation tissue may respond to topical silver nitrate, though surgical closure may be required in some cases. Surgical management typically involves multilayer closure with advancement of the strap muscles to ensure durable repair.
Patient Care Implications
Tracheostomy provides reliable airway access for prolonged mechanical ventilation and facilitates effective secretion clearance. Decisions regarding tracheostomy should consider patient prognosis and align with individualized goals of care, particularly in older adults or patients with terminal illness. Tracheostomy increases the risk of aerosolization. Consequently, airborne precautions are recommended for patients with transmissible respiratory pathogens, including tuberculosis and COVID-19.
Clinical Significance
Tracheostomy offers meaningful benefits for patients requiring long-term airway support, including enhanced comfort, improved secretion management, and facilitation of sedation weaning and ventilator liberation. The procedure also introduces important ethical and clinical considerations. Preoperative discussions should address prognosis, goals of care, and potential impacts on quality of life, particularly in individuals with advanced illness or limited life expectancy. These conversations should actively involve the patient or their designated surrogates.
Tracheostomy generates aerosols, increasing the risk of airborne transmission of pathogens such as COVID-19 or tuberculosis. In these circumstances, strict airborne precautions and appropriate personal protective equipment are essential to protect healthcare personnel.[26]
Enhancing Healthcare Team Outcomes
Posttracheostomy Care
Tracheostomy tube cuff pressures should be monitored and maintained between 20 and 25 mm Hg to prevent tracheal injury.[27] Delivery of humidified gas reduces the risk of mucous plugging. Elevating the head of the bed helps minimize aspiration, and early swallowing evaluation by a speech-language pathologist is recommended before initiating oral intake.
Suctioning should be performed at least hourly during the first 24 hours postprocedure, decreasing to every 4 hours as tolerated. The initial tracheostomy tube change typically occurs on postoperative days 5 to 7, coinciding with the removal of skin and stay sutures.
Early tube changes are discouraged unless absolutely necessary, as the immature tract increases the risk of false passage and airway compromise. Urgent replacement within this period should be performed under optimal conditions, with emergency airway equipment, adequate lighting, and bronchoscopic guidance when available. Backup supplies should include smaller tracheostomy tubes, ETTs, and exchange catheters.[28]
Nursing, Allied Health, and Interprofessional Team Interventions
Successful tracheostomy care requires collaboration within an interprofessional team. Nurses manage routine monitoring, suctioning, stoma care, and early recognition of complications such as bleeding, obstruction, or dislodgement. Respiratory therapists oversee ventilator settings, humidification, and maintenance of airway patency. Speech-language pathologists assess swallowing function and readiness for oral intake.
Complication risk exists throughout all procedural phases, with tracheoinnominate fistula representing one of the most catastrophic events. Percutaneous tracheostomy requires close collaboration between the proceduralist and the bronchoscopist, with respiratory therapists providing support for ventilation and suctioning. Bronchoscopic visualization enhances procedural safety by allowing direct confirmation of guidewire and dilator placement, though it may not consistently reduce overall complication rates.
Structured protocols, clear documentation, regular communication, and well-defined team roles optimize safety and care quality. Ready access to emergency equipment and ongoing interprofessional training further improve outcomes and reduce morbidity.
Media
(Click Image to Enlarge)
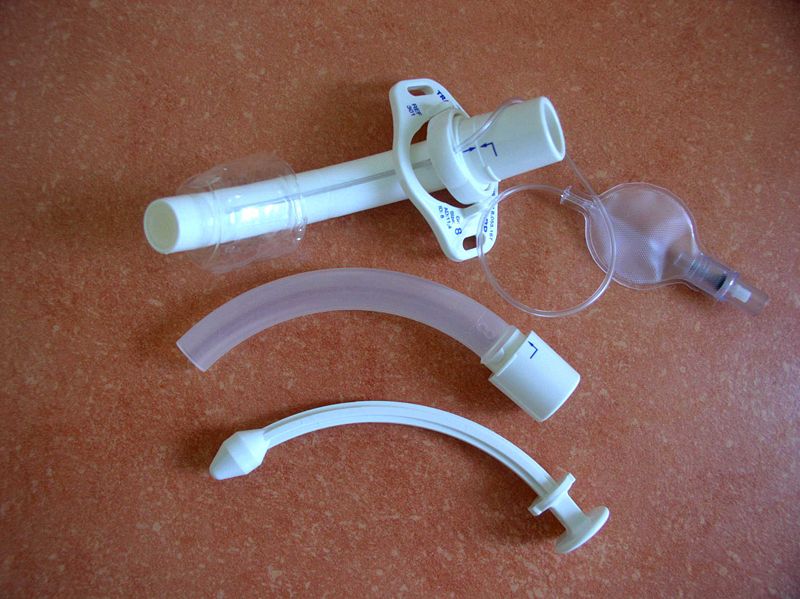
Cannula for Tracheostomy. The image displays the 3 main components of a tracheostomy tube system: the outer cannula (the top item with the inflatable cuff), the inner cannula (the middle object), and the obturator (the bottom piece).
Klaus D Peter, Public Domain, via Wikimedia Commons
(Click Image to Enlarge)
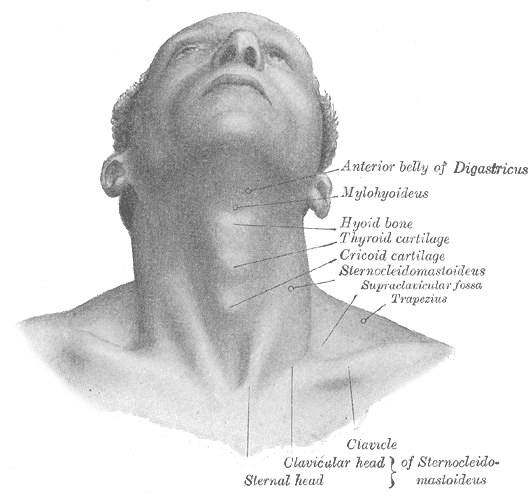
Superficial Surface Anatomy of the Neck. The image shows the surface anatomy of the anterior neck, highlighting key bony and cartilaginous landmarks. Labeled structures include the anterior belly of the digastric muscle, mylohyoid muscle, hyoid bone, thyroid cartilage, and cricoid cartilage. The sternocleidomastoid and trapezius muscles are also shown, along with the clavicle and the sternal head of the sternocleidomastoid muscle. The supraclavicular fossa is also shown.
Henry Vandyke Carter, Public Domain, via Wikimedia Commons
(Click Image to Enlarge)
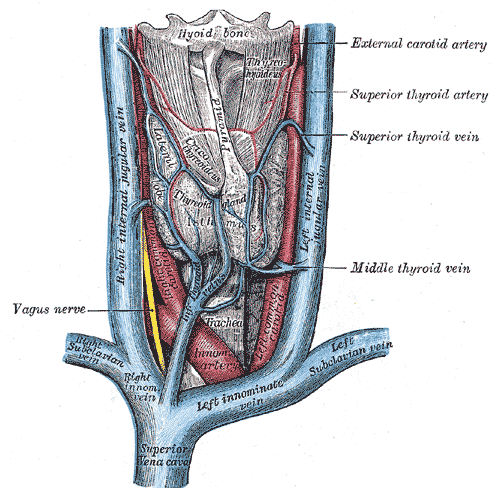
Vascular Structures of the Anterior Neck. The illustration depicts the major veins and arteries of the anterior neck, including the superior vena cava, right and left innominate veins, right and left subclavian veins, and right and left internal jugular veins. Arterial structures shown are the innominate artery and portions of the common carotid arteries. The image also indicates the relative position of the hyoid bone, thyroid gland, and trachea. Additionally, the vagus nerve and the superior, middle, and inferior thyroid veins, as well as the superior thyroid artery, are labeled.
Henry Vandyke Carter, Public Domain, via Wikimedia Commons
References
Al-Shathri Z, Susanto I. Percutaneous Tracheostomy. Seminars in respiratory and critical care medicine. 2018 Dec:39(6):720-730. doi: 10.1055/s-0038-1676573. Epub 2019 Jan 14 [PubMed PMID: 30641590]
Alidad A, Aghaz A, Hemmati E, Jadidi H, Aghazadeh K. Prevalence of Tracheostomy and Its Indications in Iran: A Systematic Review and Meta-Analysis. Tanaffos. 2019 Apr:18(4):285-293 [PubMed PMID: 32607109]
Level 1 (high-level) evidenceDavis K Jr, Campbell RS, Johannigman JA, Valente JF, Branson RD. Changes in respiratory mechanics after tracheostomy. Archives of surgery (Chicago, Ill. : 1960). 1999 Jan:134(1):59-62 [PubMed PMID: 9927132]
Szakmany T, Russell P, Wilkes AR, Hall JE. Effect of early tracheostomy on resource utilization and clinical outcomes in critically ill patients: meta-analysis of randomized controlled trials. British journal of anaesthesia. 2015 Mar:114(3):396-405. doi: 10.1093/bja/aeu440. Epub 2014 Dec 22 [PubMed PMID: 25534400]
Level 1 (high-level) evidenceGhattas C, Alsunaid S, Pickering EM, Holden VK. State of the art: percutaneous tracheostomy in the intensive care unit. Journal of thoracic disease. 2021 Aug:13(8):5261-5276. doi: 10.21037/jtd-19-4121. Epub [PubMed PMID: 34527365]
Holevar M, Dunham JC, Brautigan R, Clancy TV, Como JJ, Ebert JB, Griffen MM, Hoff WS, Kurek SJ Jr, Talbert SM, Tisherman SA. Practice management guidelines for timing of tracheostomy: the EAST Practice Management Guidelines Work Group. The Journal of trauma. 2009 Oct:67(4):870-4. doi: 10.1097/TA.0b013e3181b5a960. Epub [PubMed PMID: 19820599]
Adly A, Youssef TA, El-Begermy MM, Younis HM. Timing of tracheostomy in patients with prolonged endotracheal intubation: a systematic review. European archives of oto-rhino-laryngology : official journal of the European Federation of Oto-Rhino-Laryngological Societies (EUFOS) : affiliated with the German Society for Oto-Rhino-Laryngology - Head and Neck Surgery. 2018 Mar:275(3):679-690. doi: 10.1007/s00405-017-4838-7. Epub 2017 Dec 19 [PubMed PMID: 29255970]
Level 1 (high-level) evidenceHashimoto DA, Axtell AL, Auchincloss HG. Percutaneous Tracheostomy. The New England journal of medicine. 2020 Nov 12:383(20):e112. doi: 10.1056/NEJMvcm2014884. Epub 2020 Oct 28 [PubMed PMID: 33113296]
Kim SM, Kim HJ. Successful advancement of endotracheal tube with combined fiberoptic bronchoscopy and videolaryngoscopy in a patient with a huge goiter. SAGE open medical case reports. 2020:8():2050313X20923232. doi: 10.1177/2050313X20923232. Epub 2020 Jun 10 [PubMed PMID: 32577281]
Level 3 (low-level) evidenceCiaglia P, Firsching R, Syniec C. Elective percutaneous dilatational tracheostomy. A new simple bedside procedure; preliminary report. Chest. 1985 Jun:87(6):715-9 [PubMed PMID: 3996056]
Oliver ER, Gist A, Gillespie MB. Percutaneous versus surgical tracheotomy: an updated meta-analysis. The Laryngoscope. 2007 Sep:117(9):1570-5 [PubMed PMID: 17667139]
Level 1 (high-level) evidencePorter JM, Ivatury RR. Preferred route of tracheostomy--percutaneous versus open at the bedside: a randomized, prospective study in the surgical intensive care unit. The American surgeon. 1999 Feb:65(2):142-6 [PubMed PMID: 9926749]
Level 1 (high-level) evidenceZouk AN, Batra H. Managing complications of percutaneous tracheostomy and gastrostomy. Journal of thoracic disease. 2021 Aug:13(8):5314-5330. doi: 10.21037/jtd-19-3716. Epub [PubMed PMID: 34527368]
Toni R, Della Casa C, Mosca S, Malaguti A, Castorina S, Roti E. Anthropological variations in the anatomy of the human thyroid arteries. Thyroid : official journal of the American Thyroid Association. 2003 Feb:13(2):183-92 [PubMed PMID: 12699593]
Level 1 (high-level) evidenceKhaja M, Haider A, Alapati A, Qureshi ZA, Yapor L. Percutaneous Tracheostomy: A Bedside Procedure. Cureus. 2022 Apr:14(4):e24083. doi: 10.7759/cureus.24083. Epub 2022 Apr 12 [PubMed PMID: 35573523]
Dion GR, Pingree CS, Rico PJ, Christensen CL. Laryngeal Thermal Injury Model. Journal of burn care & research : official publication of the American Burn Association. 2020 May 2:41(3):626-632. doi: 10.1093/jbcr/iraa009. Epub [PubMed PMID: 32087018]
Keirns DL, Rajan AK, Wee SH, Govardhan IS, Eitan DN, Dilsaver DB, Ng I, Balters MW. Tracheal Stenosis in Open Versus Percutaneous Tracheostomy. Cureus. 2024 Mar:16(3):e57075. doi: 10.7759/cureus.57075. Epub 2024 Mar 27 [PubMed PMID: 38681475]
Raghuraman G, Rajan S, Marzouk JK, Mullhi D, Smith FG. Is tracheal stenosis caused by percutaneous tracheostomy different from that by surgical tracheostomy? Chest. 2005 Mar:127(3):879-85 [PubMed PMID: 15764771]
Macchiarini P, Verhoye JP, Chapelier A, Fadel E, Dartevelle P. Evaluation and outcome of different surgical techniques for postintubation tracheoesophageal fistulas. The Journal of thoracic and cardiovascular surgery. 2000 Feb:119(2):268-76 [PubMed PMID: 10649202]
Level 2 (mid-level) evidencevan den Bongard HJ, Boot H, Baas P, Taal BG. The role of parallel stent insertion in patients with esophagorespiratory fistulas. Gastrointestinal endoscopy. 2002 Jan:55(1):110-5 [PubMed PMID: 11756930]
Salik I, Paul M. Tracheoesophageal Fistula. StatPearls. 2025 Jan:(): [PubMed PMID: 30570997]
Donaldson L, Raper R. Successful emergency management of a bleeding tracheoinnominate fistula. BMJ case reports. 2019 Dec 17:12(12):. doi: 10.1136/bcr-2019-232257. Epub 2019 Dec 17 [PubMed PMID: 31852691]
Level 3 (low-level) evidenceOshinsky AE, Rubin JS, Gwozdz CS. The anatomical basis for post-tracheotomy innominate artery rupture. The Laryngoscope. 1988 Oct:98(10):1061-4 [PubMed PMID: 3050341]
Sultan P, Carvalho B, Rose BO, Cregg R. Endotracheal tube cuff pressure monitoring: a review of the evidence. Journal of perioperative practice. 2011 Nov:21(11):379-86 [PubMed PMID: 22165491]
Hamaguchi S, Nakajima Y. Two cases of tracheoinnominate artery fistula following tracheostomy treated successfully by endovascular embolization of the innominate artery. Journal of vascular surgery. 2012 Feb:55(2):545-7. doi: 10.1016/j.jvs.2011.08.006. Epub 2011 Sep 29 [PubMed PMID: 21958569]
Level 3 (low-level) evidenceYun HJ, Rhee SH, Park JY, Chae YS, Han JH, Ryoo SH, Seo KS, Kim HJ, Karm MH. A novel technique of submandibular intubation with a camera cable drape: a case report. Journal of dental anesthesia and pain medicine. 2020 Jun:20(3):155-160. doi: 10.17245/jdapm.2020.20.3.155. Epub 2020 Jun 24 [PubMed PMID: 32617410]
Level 3 (low-level) evidenceDe Leyn P, Bedert L, Delcroix M, Depuydt P, Lauwers G, Sokolov Y, Van Meerhaeghe A, Van Schil P, Belgian Association of Pneumology and Belgian Association of Cardiothoracic Surgery. Tracheotomy: clinical review and guidelines. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2007 Sep:32(3):412-21 [PubMed PMID: 17588767]
White AC, Kher S, O'Connor HH. When to change a tracheostomy tube. Respiratory care. 2010 Aug:55(8):1069-75 [PubMed PMID: 20667154]