Introduction
The sense of taste, or gustation, contributes significantly to quality of life by enabling differentiation among flavors and facilitating the enjoyment of food. Taste buds are located within the fungiform, foliate, and circumvallate papillae of the tongue but are absent from the filiform papillae, which serve only a tactile function. These neuroepithelial structures act as chemoreceptors within the oral cavity, distinguishing harmful from pleasurable stimuli, thereby influencing feeding behavior and digestion.[1]
Five basic taste modalities have been identified. Sweet taste permits recognition of sugars and carbohydrates. Salty taste regulates sodium intake and contributes to water balance. Sour taste results from acidic foods and functions as a protective mechanism against the ingestion of spoiled food. Bitter taste is generally associated with unpleasant flavor and signals potentially harmful substances. Umami taste is linked to protein content and conveys the savory flavor.[2]
Disorders of gustation can impair nutrition and serve as early indicators of systemic disease. Diagnostic evaluation of taste function aids in distinguishing peripheral from central pathology and can provide guidance in surgical planning when procedures involve the tongue or adjacent structures. Understanding the anatomy and physiology of taste buds allows clinicians to identify the origin of dysfunction, correlate symptoms with underlying pathology, and select appropriate therapeutic strategies.
Structure and Function
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Structure and Function
Taste is mediated by multicellular taste buds, which are concentrated on the tongue and are less numerous in the soft palate, epiglottis, and upper esophagus. Each taste bud consists of neuroepithelial receptor cells that undergo rapid turnover, with an average life span of 8 to 12 days, although some cells can persist for up to 3 weeks. Molecular characteristics of taste buds vary among individuals.
Three types of tongue papillae contain taste buds (see Image. Tongue Papillae and Taste Bud Distribution). The fungiform papillae are mushroom-shaped and located in the anterior 2/3 of the tongue. The circumvallate papillae are arranged in an inverted V-shape in the posterior 1/3 of the tongue and are larger and more complex than the fungiform papillae. The foliate papillae are positioned along the lateral aspects of the tongue.[3]
Filiform papillae, in contrast, lack taste receptors and provide a rigid surface that constitutes the majority of tongue papillae. Although the traditional “tongue map” proposed discrete regions for sweet, salty, sour, bitter, and umami perception, subsequent studies have demonstrated that receptor cells for all taste modalities are distributed across the tongue and oral mucosa.[4]
Taste buds are composed of specialized epithelial cells with distinct structural and functional characteristics. Four cell types have been identified.
Type I cells are generally considered supporting and structural elements, functioning in a manner analogous to glial cells in the nervous system. These cells express enzymes involved in the uptake and degradation of neurotransmitters, including adenosine triphosphate (ATP) and glutamate. Emerging evidence indicates additional roles in clearing degenerating taste cells and maintaining ionic balance within the taste bud.
Type II cells, also known as receptor cells, utilize G protein-coupled receptors to detect umami, sweet, and bitter modalities. These cells release ATP as a neurotransmitter through a nonvesicular mechanism mediated by the large ion channels calcium homeostasis modulator 1 (CALHM1) and 3 (CALHM3), thereby activating purinergic receptors on afferent nerve fibers.[5][6]
Type III cells mediate responses to sour and salty stimuli. These cells release the neurotransmitters serotonin (5-hydroxytryptamine, 5-HT), γ-aminobutyric acid (GABA), and norepinephrine through vesicular exocytosis to activate afferent fibers, although the specific receptors involved remain incompletely defined.[7] Neurotransmitters released from type II and type III cells also modulate the activity of neighboring taste cells in paracrine and autocrine fashions, thereby influencing overall taste bud output.[8][9]
Basal cells function as progenitors that sustain the continuous regeneration of taste buds by dividing and differentiating into the various taste cell types. Expression of markers, such as leucine-rich repeat-containing G protein-coupled receptor 5 (LGR5) and 6 (LGR6), has been identified in these progenitor cells, providing insight into the cellular mechanisms of taste bud renewal.
In addition to the 4 epithelial cell types, gustatory sensory axons penetrate taste buds. These peripheral terminals of cranial nerves VII (facial), IX (glossopharyngeal), and X (vagus) establish synaptic contacts with type III cells, which respond to ATP released from type II cells to transmit gustatory information to the brainstem. Although essential for signal transmission, these neural elements are not classified as a distinct epithelial cell type within the taste bud.[10]
Embryology
Embryonic Development of the Taste Buds and Their Innervation
Taste bud development is closely linked to sensory innervation, although nerve fibers appear before the buds themselves. By approximately gestational week 7, gustatory axons from the facial and glossopharyngeal nerves extend into the tongue. Differentiation of the lingual papillae occurs between weeks 10 and 13, giving rise to morphologically distinct, innervated, and likely functional taste buds.[11]
During gestation, taste-active compounds from the maternal diet diffuse into the amniotic fluid, which is continuously swallowed by the fetus. After birth, breast milk conveys similar gustatory stimuli, contributing to early flavor learning and shaping dietary preferences.
Synaptic Organisation
High-resolution anatomical studies have advanced the understanding of how gustatory nerve fibers connect with taste cells. Techniques such as 3-dimensional reconstruction of circumvallate taste buds and serial block-face scanning electron microscopy have revealed complex branching patterns and synaptic arrangements within the buds. Two principal intragemmal axonal patterns have been identified. Some fibers remain entirely within the taste bud, forming synapses with specific taste cells before terminating, while others traverse the bud, occasionally establishing synapses at the basal region or extending along the exterior toward the apical region.
Not all fibers that contact taste cells form synaptic specializations. Most axons synapse with either type II or III cells, but not both, indicating modality-specific wiring. Complementary approaches, including immunohistochemistry and sparse labeling, produced similar results, although a higher proportion of fibers were observed to contact multiple taste cell types.[12]
Nerves
The innervation of taste buds varies according to papillary type. The fungiform papillae receive sensory input from the chorda tympani branch of the facial nerve. The glossopharyngeal nerve innervates the circumvallate papillae, while the foliate papillae receive contributions from both the facial and glossopharyngeal nerves.[13] Within the soft palate, taste buds are supplied by the greater superficial petrosal branch of the facial nerve. In contrast, the filiform papillae, which lack gustatory receptors, are innervated by the trigeminal nerve (cranial nerve V) and serve a purely somatosensory function.
Physiologic Variants
Although the 5 basic tastes are generally recognized, gustatory perception is likely more nuanced. Subtle sensory distinctions can be overlooked, and emerging evidence suggests the existence of additional taste modalities. Oleogustus, a fat-based taste, has been linked to elevated expression of CD36 receptors in human taste buds, correlating with heightened oral sensitivity to fatty acids and stronger fat-taste perception.[14] Kokumi, often described as a “heartiness” flavor enhancer, appears to act through calcium-sensing receptors, modulating the perception of other tastes.[15] A distinct “starchy” taste has also been proposed. A study found that glucose oligomers were perceived as “starchy” even when sweet taste receptors were blocked, suggesting a separate sensory pathway for complex carbohydrates.[16]
Surgical Considerations
Surgical procedures can disrupt taste through direct injury to taste buds or their afferent innervation. The chorda tympani nerve is particularly vulnerable during middle ear surgery, mandibular procedures, and tongue resections, leading to loss or alteration of taste perception in the anterior tongue. Injury to the glossopharyngeal nerve impairs taste in the posterior tongue, while damage to the greater petrosal nerve affects palatal taste buds.[17]
Preoperative counseling regarding the potential for altered taste perception is essential, as such changes may impair nutrition, appetite, and overall quality of life. When complications arise, management should involve an interprofessional approach, with contributions from speech and language therapists as well as dietitians.[18]
Clinical Significance
Taste Disorders Classification
Taste disorders are classified as either quantitative, characterized by changes in the intensity of taste perception, or qualitative, defined by abnormal taste experiences in the absence of an appropriate stimulus. Quantitative disorders include ageusia, the complete loss of taste sensation, which is rare because of the overlapping innervation of taste buds by multiple cranial nerves.[19] "Hypogeusia" refers to reduced taste intensity, whereas "hypergeusia" denotes heightened sensitivity to taste stimuli. Qualitative disorders include phantogeusia, in which a persistent, often unpleasant taste is perceived despite the absence of a stimulus in the oral cavity.[20][21] These conditions may occur independently, coexist, or overlap within the same individual.
Systemic Diseases
Taste dysfunction arises in association with a wide range of systemic conditions. Infectious causes include viral upper respiratory tract infections, oral infections, and HIV. Autoimmune conditions such as Sjögren syndrome, systemic lupus erythematosus, rheumatoid arthritis, and inflammatory bowel disease are also implicated, along with iatrogenic causes such as chemotherapy and radiotherapy.
A systematic review estimated that between 1/2 and 2/3 of patients undergoing chemotherapy or radiotherapy develop taste disturbances. The consequences may be profound, as illustrated by a reported case of severe chemotherapy-induced dysgeusia that produced an extreme preference for sweet foods and precipitated life-threatening decompensation of diabetes mellitus.[22]
Neurological Causes
Both central and peripheral nervous system disorders contribute to gustatory dysfunction. Neurodegenerative diseases, including Parkinson disease and Alzheimer disease, are associated with impaired taste perception. Gustatory dysfunction has also been reported in approximately 16% of patients with multiple sclerosis, underscoring the broad neurological influences on taste.[23]
Neuropsychiatric Causes
Neuropsychiatric disorders, including temporal lobe epilepsy, schizophrenia, depression, and anxiety, can alter taste perception through disruptions in central processing rather than peripheral taste bud dysfunction. Manifestations may include gustatory hallucinations, phantogeusia, or diminished taste sensitivity. Recognition of these associations is clinically important, as unexplained taste disturbances can occasionally serve as early indicators of underlying neurological or psychiatric disease.[24]
Aging
Taste perception declines with aging, with salty and bitter qualities most affected. Abnormalities in taste are common in older adults but often underreported.[25] The underlying mechanisms are multifactorial and include loss of taste buds, reduced salivary secretion, and polypharmacy.[26]
Dysgeusia
Dysgeusia is a taste disorder characterized by altered taste interpretation or the perception of taste in the absence of a stimulus. The condition is the most frequently reported taste abnormality. Etiologies include local factors such as oral infections, medication effects, systemic disease, and central or peripheral nervous system disorders. Sweet dysgeusia may, in rare cases, represent the first presenting sign of lung tumors. Dysgeusia has also been reported as an initial manifestation of vestibular schwannomas, although this presentation is uncommon.[27] In many patients, no identifiable cause is found, leading to a diagnosis of idiopathic oral dysgeusia.
Obesity and Taste
Taste plays a critical role in obesity by influencing food preference, caloric intake, and weight regulation. Reduced taste sensitivity has been documented in some individuals with obesity.[28] Emerging evidence suggests that obesity alters the tongue, the primary organ of taste, indicating a bidirectional relationship in which altered taste may both contribute to and result from obesity.[29] Sweet-sensitive taste cells rely on the adipokine leptin to detect sweetness.[30] Importantly, obesity has been associated with a 25% reduction in taste bud number, along with altered signaling mechanisms, which may reinforce maladaptive eating behaviors.[31] These findings suggest that targeting taste function and taste bud biology may provide novel strategies for obesity prevention and treatment.
Coronavirus Disease 2019 and Taste
Taste impairment, which is a more common and severe symptom of coronavirus disease 2019 (COVID-19) than respiratory disorders unrelated to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, was reported by an average of 45% of people worldwide. About 5% of COVID-19 patients experience persistent taste abnormalities. Sustained taste loss created difficulties for patients, as clinicians were unprepared for the extent of this complication. The exact mechanism by which COVID-19 causes dysgeusia remains unclear.[32] However, the mechanisms may include direct viral effects on taste buds via angiotensin-converting enzyme 2 (ACE2) entry and local inflammatory damage to the gustatory epithelium.[33]
Patient Examination
The assessment of patients with suspected taste disorders should begin with a comprehensive examination of the oral cavity and ears. Oral health, including dental hygiene, mucosal integrity, and salivary flow, must be evaluated because local abnormalities can contribute to dysgeusia. A detailed history should document pain, swelling, alterations in chewing habits, recent or recurrent infections, prior surgical procedures, and systemic conditions known to affect taste. Careful attention to these factors facilitates differentiation between local, systemic, and neurological causes of taste disturbances.
Taste function evaluation may be undertaken using both bedside and specialized methods. Clinically, liquid stimuli or validated taste strips may be applied to different regions of the tongue to evaluate regional taste perception.[34] Electrogustometry provides an objective measure by delivering a mild electrical current to evoke taste sensations, whereas gustatory evoked potentials assess cortical responses to gustatory input.[35] Subjective evaluation through validated questionnaires, such as the Taste and Smell Survey, further quantifies the impact of taste disturbances on daily life.[36]
Diagnostic modalities such as magnetic resonance imaging and computed tomography may be indicated when structural or neurological causes are suspected. In cases where infection is a concern, oral swabs and cultures may be obtained to identify bacterial or fungal pathogens.
Treatment of Taste Disorders
Management of taste disorders centers on identifying and addressing the underlying etiology, such as infection, medication adverse effects, or nutritional deficiency. Pharmacologic interventions, including zinc supplementation, vitamin A, and corticosteroids, have been investigated, but outcomes remain inconsistent, and no standardized therapy exists.[37] Supportive approaches, such as dietary counseling and management of xerostomia, can enhance quality of life. Additional research is required to establish effective, evidence-based therapeutic strategies.
Media
(Click Image to Enlarge)
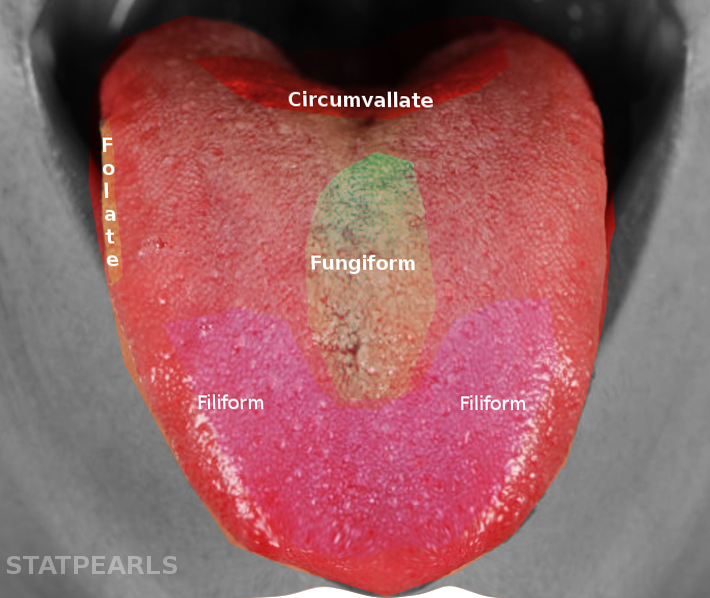
Tongue Papillae and Taste Bud Distribution. The tongue contains 4 papillae: the circumvallate papillae are arranged in a V-shape at the posterior tongue; the foliate papillae are located along the lateral margins; the fungiform papillae are scattered mainly on the anterior dorsal surface; and the filiform papillae are distributed across the anterior two-thirds, providing mechanical but not gustatory function.
Contributed by S Bhimji, MD
References
Witt M. Anatomy and development of the human taste system. Handbook of clinical neurology. 2019:164():147-171. doi: 10.1016/B978-0-444-63855-7.00010-1. Epub [PubMed PMID: 31604544]
Gravina SA, Yep GL, Khan M. Human biology of taste. Annals of Saudi medicine. 2013 May-Jun:33(3):217-22. doi: 10.5144/0256-4947.2013.217. Epub [PubMed PMID: 23793421]
Level 3 (low-level) evidenceBarlow LA, Klein OD. Developing and regenerating a sense of taste. Current topics in developmental biology. 2015:111():401-19. doi: 10.1016/bs.ctdb.2014.11.012. Epub 2015 Jan 20 [PubMed PMID: 25662267]
Level 3 (low-level) evidenceDoyle ME, Premathilake HU, Yao Q, Mazucanti CH, Egan JM. Physiology of the tongue with emphasis on taste transduction. Physiological reviews. 2023 Apr 1:103(2):1193-1246. doi: 10.1152/physrev.00012.2022. Epub 2022 Nov 24 [PubMed PMID: 36422992]
Taruno A, Vingtdeux V, Ohmoto M, Ma Z, Dvoryanchikov G, Li A, Adrien L, Zhao H, Leung S, Abernethy M, Koppel J, Davies P, Civan MM, Chaudhari N, Matsumoto I, Hellekant G, Tordoff MG, Marambaud P, Foskett JK. CALHM1 ion channel mediates purinergic neurotransmission of sweet, bitter and umami tastes. Nature. 2013 Mar 14:495(7440):223-6. doi: 10.1038/nature11906. Epub 2013 Mar 6 [PubMed PMID: 23467090]
Romanov RA, Rogachevskaja OA, Bystrova MF, Jiang P, Margolskee RF, Kolesnikov SS. Afferent neurotransmission mediated by hemichannels in mammalian taste cells. The EMBO journal. 2007 Feb 7:26(3):657-67 [PubMed PMID: 17235286]
Vandenbeuch A, Zorec R, Kinnamon SC. Capacitance measurements of regulated exocytosis in mouse taste cells. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010 Nov 3:30(44):14695-701. doi: 10.1523/JNEUROSCI.1570-10.2010. Epub [PubMed PMID: 21048127]
Huang YA, Dando R, Roper SD. Autocrine and paracrine roles for ATP and serotonin in mouse taste buds. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009 Nov 4:29(44):13909-18. doi: 10.1523/JNEUROSCI.2351-09.2009. Epub [PubMed PMID: 19890001]
Roper SD. Taste buds as peripheral chemosensory processors. Seminars in cell & developmental biology. 2013 Jan:24(1):71-9. doi: 10.1016/j.semcdb.2012.12.002. Epub 2012 Dec 20 [PubMed PMID: 23261954]
Level 3 (low-level) evidenceChaudhari N, Roper SD. The cell biology of taste. The Journal of cell biology. 2010 Aug 9:190(3):285-96. doi: 10.1083/jcb.201003144. Epub [PubMed PMID: 20696704]
Landon SM, Baker K, Macpherson LJ. Give-and-take of gustation: the interplay between gustatory neurons and taste buds. Chemical senses. 2024 Jan 1:49():. doi: 10.1093/chemse/bjae029. Epub [PubMed PMID: 39078723]
Huang T, Ohman LC, Clements AV, Whiddon ZD, Krimm RF. Variable Branching Characteristics of Peripheral Taste Neurons Indicates Differential Convergence. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2021 Jun 2:41(22):4850-4866. doi: 10.1523/JNEUROSCI.1935-20.2021. Epub 2021 Apr 19 [PubMed PMID: 33875572]
Barlow LA. Progress and renewal in gustation: new insights into taste bud development. Development (Cambridge, England). 2015 Nov 1:142(21):3620-9. doi: 10.1242/dev.120394. Epub [PubMed PMID: 26534983]
Degrace-Passilly P, Besnard P. CD36 and taste of fat. Current opinion in clinical nutrition and metabolic care. 2012 Mar:15(2):107-11. doi: 10.1097/MCO.0b013e32834ff19c. Epub [PubMed PMID: 22248592]
Level 3 (low-level) evidenceOhsu T, Amino Y, Nagasaki H, Yamanaka T, Takeshita S, Hatanaka T, Maruyama Y, Miyamura N, Eto Y. Involvement of the calcium-sensing receptor in human taste perception. The Journal of biological chemistry. 2010 Jan 8:285(2):1016-22. doi: 10.1074/jbc.M109.029165. Epub 2009 Nov 5 [PubMed PMID: 19892707]
Lapis TJ, Penner MH, Lim J. Humans Can Taste Glucose Oligomers Independent of the hT1R2/hT1R3 Sweet Taste Receptor. Chemical senses. 2016 Nov 1:41(9):755-762. doi: 10.1093/chemse/bjw088. Epub [PubMed PMID: 27553043]
Blijleven EE, Wegner I, Stokroos RJ, Thomeer HGXM. The impact of injury of the chorda tympani nerve during primary stapes surgery or cochlear implantation on taste function, quality of life and food preferences: A study protocol for a double-blind prospective prognostic association study. PloS one. 2023:18(5):e0284571. doi: 10.1371/journal.pone.0284571. Epub 2023 May 18 [PubMed PMID: 37200313]
Level 1 (high-level) evidenceLafargue B, D'Andréa G, Fabre R, Alshukry A, Vandersteen C, Guevara N. Taste Disorders After Middle Ear Surgery: Chorda Tympani Nerve Injury and Quality of Life. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2024 Dec:171(6):1834-1841. doi: 10.1002/ohn.920. Epub 2024 Aug 1 [PubMed PMID: 39087766]
Level 2 (mid-level) evidenceHummel T, Landis BN, Hüttenbrink KB. Smell and taste disorders. GMS current topics in otorhinolaryngology, head and neck surgery. 2011:10():Doc04. doi: 10.3205/cto000077. Epub 2012 Apr 26 [PubMed PMID: 22558054]
Mikhail C, Elgaaly K, Abd El Latif Abd El Hamid A, Shaker O, Ali S. Gustatory Dysfunction among a Sample of Depressed Egyptian Adults under Antidepressants Therapy: A Retrospective Cohort Study. International journal of dentistry. 2021:2021():5543840. doi: 10.1155/2021/5543840. Epub 2021 Mar 4 [PubMed PMID: 33747081]
Level 2 (mid-level) evidenceHeckmann JG, Heckmann SM, Lang CJ, Hummel T. Neurological aspects of taste disorders. Archives of neurology. 2003 May:60(5):667-71 [PubMed PMID: 12756129]
Pombo F, Seabra C, Sá AJ, Ferreira I. Chemotherapy-Induced Dysgeusia and Its Perverse Consequences: A Case Report. Cureus. 2022 Aug:14(8):e27908. doi: 10.7759/cureus.27908. Epub 2022 Aug 11 [PubMed PMID: 36120262]
Level 3 (low-level) evidenceRajai Firouzabadi S, Mohammadi I, Alinejadfard M, Yazdan Panah M, Vaheb S, Shaygannejad V, Mirmosayyeb O. Gustatory dysfunction in multiple sclerosis: a systematic review and meta-analysis. Chemical senses. 2025 Jan 22:50():. pii: bjae046. doi: 10.1093/chemse/bjae046. Epub [PubMed PMID: 39741469]
Level 1 (high-level) evidenceLiu J, Sun SJ, Lu Y, Ping X, Zhang W, Pei L. Taste dysfunction as a predictor of depression in schizophrenia: A systematic review and meta-analysis. PloS one. 2024:19(3):e0300935. doi: 10.1371/journal.pone.0300935. Epub 2024 Mar 22 [PubMed PMID: 38517844]
Level 1 (high-level) evidenceFeng P, Huang L, Wang H. Taste bud homeostasis in health, disease, and aging. Chemical senses. 2014 Jan:39(1):3-16. doi: 10.1093/chemse/bjt059. Epub 2013 Nov 28 [PubMed PMID: 24287552]
Level 3 (low-level) evidenceDoty RL. Age-Related Deficits in Taste and Smell. Otolaryngologic clinics of North America. 2018 Aug:51(4):815-825. doi: 10.1016/j.otc.2018.03.014. Epub [PubMed PMID: 30001793]
Brown E, Staines K. Vestibular Schwannoma Presenting as Oral Dysgeusia: An Easily Missed Diagnosis. Case reports in dentistry. 2016:2016():7081919. doi: 10.1155/2016/7081919. Epub 2016 Feb 18 [PubMed PMID: 27022490]
Level 3 (low-level) evidenceSkrandies W, Zschieschang R. Olfactory and gustatory functions and its relation to body weight. Physiology & behavior. 2015 Apr 1:142():1-4. doi: 10.1016/j.physbeh.2015.01.024. Epub 2015 Jan 22 [PubMed PMID: 25619950]
Kaufman A, Choo E, Koh A, Dando R. Inflammation arising from obesity reduces taste bud abundance and inhibits renewal. PLoS biology. 2018 Mar:16(3):e2001959. doi: 10.1371/journal.pbio.2001959. Epub 2018 Mar 20 [PubMed PMID: 29558472]
Kubasova N, Burdakov D, Domingos AI. Sweet and Low on Leptin: Hormonal Regulation of Sweet Taste Buds. Diabetes. 2015 Nov:64(11):3651-2. doi: 10.2337/dbi15-0004. Epub [PubMed PMID: 26494218]
Brondel L, Quilliot D, Mouillot T, Khan NA, Bastable P, Boggio V, Leloup C, Pénicaud L. Taste of Fat and Obesity: Different Hypotheses and Our Point of View. Nutrients. 2022 Jan 27:14(3):. doi: 10.3390/nu14030555. Epub 2022 Jan 27 [PubMed PMID: 35276921]
Tan BKJ, Han R, Zhao JJ, Tan NKW, Quah ESH, Tan CJ, Chan YH, Teo NWY, Charn TC, See A, Xu S, Chapurin N, Chandra RK, Chowdhury N, Butowt R, von Bartheld CS, Kumar BN, Hopkins C, Toh ST. Prognosis and persistence of smell and taste dysfunction in patients with covid-19: meta-analysis with parametric cure modelling of recovery curves. BMJ (Clinical research ed.). 2022 Jul 27:378():e069503. doi: 10.1136/bmj-2021-069503. Epub 2022 Jul 27 [PubMed PMID: 35896188]
Level 1 (high-level) evidenceLin W, Gao F, Wang X, Qin N, Chen X, Tam KY, Zhang C, Zhang M, Sha O. The oral manifestations and related mechanisms of COVID-19 caused by SARS-CoV-2 infection. Frontiers in cellular neuroscience. 2022:16():1006977. doi: 10.3389/fncel.2022.1006977. Epub 2023 Jan 4 [PubMed PMID: 36687524]
Level 2 (mid-level) evidenceLandis BN, Welge-Luessen A, Brämerson A, Bende M, Mueller CA, Nordin S, Hummel T. "Taste Strips" - a rapid, lateralized, gustatory bedside identification test based on impregnated filter papers. Journal of neurology. 2009 Feb:256(2):242-8. doi: 10.1007/s00415-009-0088-y. Epub 2009 Feb 7 [PubMed PMID: 19221845]
Matsuda T, Mysore Ganesh P, Brown R, Grosso V, Doty RL. Electrogustometry: validation of bipolar electrode stimulation. Chemical senses. 2023 Jan 1:48():. pii: bjad009. doi: 10.1093/chemse/bjad009. Epub [PubMed PMID: 36960972]
Level 1 (high-level) evidenceNiklassen AS, Christensen KB, Fjaeldstad AW, Ovesen T. Development and Psychometric Validation of the Taste And Smell Tool for Evaluation (TASTE) Questionnaire. JAMA otolaryngology-- head & neck surgery. 2022 Dec 1:148(12):1164-1172. doi: 10.1001/jamaoto.2022.3392. Epub [PubMed PMID: 36326741]
Level 1 (high-level) evidenceMozaffar B, Ardavani A, Muzafar H, Idris I. The Effectiveness of Zinc Supplementation in Taste Disorder Treatment: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Journal of nutrition and metabolism. 2023:2023():6711071. doi: 10.1155/2023/6711071. Epub 2023 Mar 8 [PubMed PMID: 36937245]
Level 1 (high-level) evidence