Introduction
Red eyes are a common complaint in emergency departments and outpatient clinics.[1] One frequent cause is subconjunctival hemorrhage (SCH), which is generally benign but potentially indicative of serious underlying disease if persistent or recurrent. This ocular condition is generally painless but presents as a sharply demarcated red patch that may appear diffusely hyperemic (see Image. Subconjunctival Hemorrhage). Physicians, advanced practice providers, and ophthalmologists frequently encounter SCH in clinical practice.
The conjunctiva is divided into 2 sections: the bulbar conjunctiva, which covers the sclera, and the tarsal conjunctiva, which covers the inside of the eyelids. This thin, transparent, vascular membrane consists of stratified epithelial and stromal layers supported by a dense capillary network.
SCH arises from rupture of small superficial conjunctival vessels overlying the sclera rather than from deeper intraocular structures. Blood accumulates in the potential space between the bulbar conjunctiva and the Tenon capsule, producing a localized, well-defined red area. Superficial location and limited structural support render these vessels vulnerable to rupture. Thus, minor venous pressure elevations or mechanical strain can trigger hemorrhage. Older adults with hypertension or diabetes face the greatest risk, whereas younger patients more often experience traumatic or spontaneous causes.[2]
SCH usually does not require specific treatment.[3] The condition follows a benign, self-limiting course, with most cases resolving within 1 to 2 weeks. The rate of blood resorption depends on the size of the hemorrhage, patient age, systemic comorbidities, and local factors such as conjunctival lymphatic drainage. Discoloration progresses in a predictable sequence. The patch initially appears bright red, darkens to a deeper red, and eventually takes on a yellow-green hue as hemoglobin is metabolized into biliverdin and bilirubin.
SCH does not cause pain, photophobia, reduced visual acuity, or pupillary abnormalities. These absent features help differentiate the condition from more serious ocular disorders. The benign presentation often reassures clinicians and patients, although its dramatic appearance can prompt urgent evaluation. Cases with recurrence or accompanying symptoms require further investigation to exclude systemic disease or structural abnormalities.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
SCH may be categorized as traumatic or spontaneous. Traumatic SCH is more frequently observed in younger individuals and often results from direct ocular trauma, foreign body injuries, excessive eye rubbing, or improper contact lens use. In pediatric populations, trauma accounts for the majority of cases. A retrospective study found that 83% of children presenting with SCH had sustained an eye injury.[4]
Incidences of traumatic SCH have increased due to greater contact lens use and the rising number of ocular surgeries. Contact lens wearers are predisposed to conjunctivochalasis, pinguecula, and superficial punctate keratitis.[5] These conjunctival disorders contribute to inflammation by promoting ocular dryness, increasing friction between the lens and the conjunctiva, and altering tear film stability.
Defects in lens material or surface deposits, as well as irregularities at the rim of hard or disposable lenses, can further heighten the risk of SCH. Prolonged use of disposable lenses with these defects increases the likelihood of conjunctival vessel injury and subsequent hemorrhage.[6][7]
Additional iatrogenic factors can induce SCH, particularly those related to ophthalmic surgical procedures, intravitreal injections, and periocular anesthetics. These interventions compromise conjunctival vessels and predispose to hemorrhage. Mechanical increases in venous pressure from Valsalva maneuvers, including strenuous lifting, constipation, emesis, or coughing, are also recognized precipitants in the absence of direct trauma. Severe trauma may produce SCH as part of more devastating ocular injuries, including open globe injury or orbital fracture. SCH arising from the fornix in the absence of globe trauma should prompt consideration of a basilar skull fracture.[8]
Nonaccidental trauma (NAT) must be suspected in infants presenting with bilateral isolated SCH, particularly when associated with facial petechiae. Traumatic asphyxia syndrome, caused by prolonged compression of the upper abdomen and chest, can produce sudden and severe venous congestion, resulting in SCH.[9] SCH in newborns can occur after vaginal delivery, with an estimated incidence of 1% to 2%. The mechanism mirrors that of Valsalva-related cases, with uterine contractions generating sufficient venous compression to rupture conjunctival vessels.[10]
Nontraumatic, or spontaneous, SCH occurs more often in older individuals and is frequently associated with systemic comorbidities or medications that impair vascular integrity or coagulation.[11][12] Hypertension is the most consistently documented systemic risk factor. Spontaneous SCH may also serve as a predictor of hypertension when blood pressure is elevated at initial presentation and remains high on follow-up.[13]
A large population-based case-control study of more than 65,000 individuals in Taiwan demonstrated strong associations between SCH and comorbid hypertension, purpura, thrombocytopenia, as well as aspirin use.[14] The pathophysiologic mechanism likely involves persistent vascular fragility and impaired autoregulation, which promote spontaneous conjunctival vessel rupture during periods of physiologic stress. Other systemic disorders, including diabetes mellitus, dyslipidemia, and bleeding diatheses such as thrombocytopenia and purpura, have also been implicated. These findings underscore the multifactorial nature of spontaneous SCH and the contribution of unmeasured systemic factors.
Individuals with vascular disease are often prescribed anticoagulants such as warfarin or heparin. Antiplatelet agents, including aspirin and P2Y12 inhibitors such as clopidogrel, also increase the risk of SCH. These associations hold particular relevance for older adults with cardiovascular disease receiving long-term pharmacologic therapy. Risk persists even when the international normalized ratio (INR) remains within the therapeutic range.[15]
Spontaneous SCH may also result from transient elevations in venous pressure caused by coughing, vomiting, strenuous exertion, heavy lifting, or Valsalva maneuvers.[16][17] Acute hemorrhagic conjunctivitis, most commonly associated with enterovirus 70, may produce extensive SCH, although the prevalence of this infection has declined.[18][19] Menstruation has also been reported as a precipitating factor, likely through venous pressure changes or underlying blood dyscrasia.[20]
Additional conditions linked to SCH include Stevens-Johnson syndrome, hemochromatosis, lymphoma, and vascular dermatologic disorders such as Kaposi sarcoma, pyogenic granuloma, telangiectasias, and hemangiomas.[21][22][23] Despite these associations, nearly half of spontaneous SCH cases remain idiopathic in origin.
Epidemiology
SCH is a prevalent ocular condition with occurrence influenced by age, sex, and geographic factors. In general ophthalmic practice, SCH accounts for approximately 3% of emergency or outpatient eye consultations. Prevalence rises substantially in adults older than 65 years, in whom SCH represents up to 10% of clinic-reported eye complaints, reflecting both increased incidence and heightened awareness of ocular changes. The age distribution demonstrates a bimodal pattern. A 1st peak occurs in childhood and adolescence, most often related to trauma, while a 2nd, more prominent rise appears in adults aged 60 to 69, when spontaneous causes predominate. These age-related patterns underscore shifting etiologies, comorbid health conditions, and medication exposures across the lifespan.
Population-based studies reveal sex-specific differences in the incidence of SCH, although results vary by geography and study methodology. Overall, no major sex discrepancy has been established. Traumatic SCH occurs more frequently in younger male individuals, likely reflecting greater involvement in heavy labor and higher participation in aggressive physical activities.
A nationwide cohort study in South Korea reported a higher incidence in female individuals, with a male-to-female ratio of 0.80.[24] This trend was most pronounced among older adults with spontaneous SCH. In contrast, studies from Congo described a slight male predominance, particularly in settings where trauma represents the primary cause of SCH.[25] These inconsistencies highlight the influence of trauma exposure, occupational hazards, healthcare-seeking behavior, and systemic disease patterns across populations.
Consistent findings indicate that traumatic SCH predominates in younger male individuals, while spontaneous SCH is more common in older women, usually in association with hypertension and vascular fragility. Spontaneous SCH also becomes more frequent with advancing age, particularly after 50 years, when comorbidities such as hypertension, hyperlipidemia, and diabetes mellitus are more prevalent. Seasonal, environmental, and biological factors, along with socioenvironmental influences, may play minor contributory roles.
Pathophysiology
SCH develops when fragile conjunctival capillaries rupture, leading to extravasation of blood into the subconjunctival space between the bulbar conjunctiva and the underlying episclera. The conjunctival vasculature consists of fine, unsupported capillaries that are particularly vulnerable to mechanical stress and hemodynamic fluctuations.
Loss of vascular integrity from abrupt pressure changes, trauma, or systemic vascular fragility allows blood to accumulate beneath the conjunctiva. Limited lymphatic drainage in this region prevents rapid clearance, producing the sharply demarcated red patch characteristic of SCH. Surrounding tissues lack firm structural attachments and adequate lymphatic channels, which explains the slow resorption process lasting several days to weeks. Advancing age and comorbid conditions further weaken elastic and connective tissues, increasing vessel fragility and facilitating more extensive bleeding in older adults.
Traumatic SCH occurs when mechanical stress disrupts the integrity of superficial conjunctival capillaries. Even minor forces, including vigorous eye rubbing, sneezing, or coughing, can cause rupture if transient intravascular pressure exceeds vascular tensile strength. The Valsalva maneuver, which elevates intrathoracic and venous pressure, is a well-recognized mechanism.
A retrospective review of more than 34,000 pediatric patients demonstrated that SCH is uncommon in children, with an incidence of 0.4% when excluding surgical cases. Trauma accounted for over 80% of presentations across all age groups, followed by ocular surface inflammation. These findings emphasize the importance of thorough assessment for potential abuse or injury, particularly in infants and young children with SCH. In newborns, vaginal delivery may induce SCH through increased venous pressure and mechanical compression. Hemorrhage is localized and not associated with systemic coagulopathy in these cases, distinguishing it from other ocular bleeding disorders.[26]
Traumatic SCH is usually more localized to the site of impact than spontaneous SCH. The hemorrhage most often appears on the temporal side of the eye, where the bulbar conjunctiva is larger than on the nasal side, possibly because blood gravitates downward after injury.[27] Additional explanations include the higher incidence of conjunctivochalasis, the protective effect of the nose on the nasal aspect, and greater difficulty detecting projectiles on the temporal side.
Despite its often dramatic appearance, SCH is confined to the conjunctival space due to the anatomical barrier of the Tenon capsule and the absence of deeper vascular involvement. Unlike retinal or choroidal hemorrhages, SCH does not cause vision loss, photophobia, or intraocular inflammation. Blood resorption follows the same process as dermal bruising, with hemoglobin breakdown products such as biliverdin and bilirubin producing the characteristic color changes over time. Recurrent SCH warrants systematic evaluation to exclude hematologic disorders such as thrombocytopenia, clotting factor deficiencies, or vascular dysplasias, although these abnormalities are uncommon. The underlying pathophysiology reflects an interplay between local capillary fragility and systemic conditions that heighten the risk of vascular rupture.
Histopathology
The histological features of SCH demonstrate focal vascular rupture with blood accumulation in the potential space beneath the bulbar conjunctiva. Microscopic evaluation shows fresh or organizing hemorrhage between the conjunctival epithelium and the Tenon capsule, without extension into deeper ocular structures. In the acute stage, erythrocytes occupy the substantia propria of the conjunctiva, accompanied by a mild inflammatory response. The conjunctival epithelium usually remains intact, without necrosis or significant epithelial disruption, which distinguishes SCH from infectious or necrotizing processes.
The absence of arterial channels in the episcleral region contributes to the strict extravascular confinement of the hemorrhage. Trauma or contact lens wear may cause mechanical alteration of the conjunctival stroma, occasionally with goblet cell loss or basement membrane irregularity. These histologic changes are generally modest and transient, leaving no lasting sequelae.
History and Physical
A careful history and physical examination are essential to determine whether an SCH is benign or a sign of a more serious condition. Many patients remain unaware of the hemorrhage until noticing it in a mirror or being informed by others.
A thorough history should establish the onset and duration of the hemorrhage, the presence or absence of trauma, and potential triggers such as coughing, sneezing, vomiting, or heavy lifting. Inquiry into visual loss, discharge, photophobia, foreign body sensation, or headache is important, as these features suggest alternative etiologies.
In younger patients, trauma is the most frequent cause, making it necessary to assess for recent injuries, prior ocular surgery, aggressive eye rubbing, or contact lens wear. SCH associated with blunt trauma warrants evaluation for globe rupture or retrobulbar hematoma.[28] Past medical history is equally important to identify systemic risk factors, including hypertension, hyperlipidemia, diabetes, anticoagulant therapy, coagulopathy, and blood dyscrasia.
On physical examination, SCH appears as a painless, acute, sharply demarcated collection of blood beneath the ocular surface. Bleeding is generally unilateral and does not affect visual acuity. Traumatic SCH tends to be more localized, whereas spontaneous SCH in older individuals is usually more diffuse. The inferotemporal conjunctiva is the most common site. A simple SCH should not present with chemosis, proptosis, purulent discharge, or ophthalmoplegia. Scleral rupture may cause intraocular blood to escape through a defect and accumulate in the subconjunctival space, creating an elevated, bullous-appearing hemorrhage.
A key aspect of the physical examination is distinguishing conjunctival from ciliary injection. Conjunctival hemorrhage results from dilation of the posterior and more superficial conjunctival vessels, creating a continuous red pattern across the sclera. In contrast, ciliary injection involves dilation of the anterior ciliary arteries and suggests intraocular inflammation of the iris, cornea, or ciliary body. Also called a "circumcorneal flush," ciliary injection appears as a halo of redness. This distinction is critical, as ciliary injection is associated with more serious conditions such as iritis, acute glaucoma, episcleritis, and scleritis.[29]
SCH may also be mistaken for viral or bacterial conjunctivitis. These conditions usually cause some degree of pain, and the redness is diffuse rather than a discrete, confluent hemorrhage. Bilateral involvement is common in viral conjunctivitis, contrasting with the mostly unilateral presentation of SCH.[30]
Evaluation
The diagnostic assessment of SCH is primarily clinical, based on patient history and external ocular examination. Laboratory or imaging studies are rarely required, particularly when the hemorrhage presents as a solitary, unilateral event without systemic symptoms or other ocular abnormalities. A thorough history should exclude recent trauma, Valsalva maneuvers, and contact lens use. The physical examination must confirm normal visual acuity and intraocular pressure (IOP), with no pain or photophobia.[31] The diagnosis can be made confidently without additional testing in routine cases, especially in younger individuals with clear traumatic or mechanical triggers. Clinicians should remain alert for red flags that warrant further evaluation, including recurrence, bilateral involvement, systemic bruising, or a history of anticoagulant therapy.
A more thorough assessment may be warranted when spontaneous SCH occurs, particularly in older adults or individuals with comorbidities. Blood pressure measurement is essential for all patients with spontaneous or recurrent hemorrhage, given the strong association between spontaneous cerebral hemorrhage and uncontrolled hypertension.[32] Clinicians should assess adherence, recent dosage changes, and the presence of bleeding in nonocular sites in patients receiving anticoagulant or antiplatelet therapy.
Basic laboratory studies, including total blood count, prothrombin time, activated partial thromboplastin time, and INR, can help identify underlying coagulopathies or iatrogenic factors related to excessive anticoagulation. These assessments are particularly important in older or medically complex individuals, where hemorrhage may be the first indication of systemic disease.
A slit-lamp examination is indicated to exclude a retained foreign body. Slit-lamp biomicroscopy helps evaluate conjunctival anatomy and identify subtle disorders such as conjunctivochalasis or pinguecula. This assessment may reveal structural alterations that predispose to recurrent hemorrhage, particularly in older adults or long-term contact lens users. Additional evaluations, such as Seidel testing for globe rupture, fluorescein staining for corneal abrasions, and orbital imaging for suspected fractures, may be necessary in cases of SCH following trauma. When posterior segment involvement is suspected due to reduced visual acuity or an afferent pupillary defect, a dilated fundus examination and optical coherence tomography may be required to exclude retinal or vitreous hemorrhage, which carries different prognoses and therapeutic implications.
No international or national guidelines have been established specifically for SCH evaluation, likely owing to its predominantly benign nature. Broader clinical guidelines emphasize the exclusion of secondary causes based on age, systemic comorbidities, and recurrence patterns.
A Korean population-based study reported that patients with subclinical hypothyroidism were generally older and exhibited higher rates of systemic vascular disease, including hypertension and diabetes, supporting routine blood pressure measurement and cardiovascular risk assessment in older adults. In children, limited evaluation is appropriate unless concerns for abuse, systemic disease, or bilateral involvement arise, making laboratory testing and imaging warranted.[33] Overall, the evaluation of SCH is pragmatic, relying on clinical assessment while reserving additional studies for atypical or recurrent cases.[34][35]
Treatment / Management
The management of SCH is primarily conservative, as the condition is self-resolving in most cases. No specific medical or surgical intervention is required. Hemorrhages typically resolve within 7 to 14 days, depending on the volume of bleeding and the patient’s absorptive capacity. During this period, patients should be reassured of the benign nature of the condition and monitored as appropriate. Artificial tears may be recommended for symptomatic relief in cases of irritation, although they do not affect resolution. Patients should be advised to avoid eye rubbing, strenuous activity, or Valsalva maneuvers that may exacerbate bleeding or trigger recurrence.
In severe SCH, management requires careful evaluation to exclude concomitant ocular injury. No further intervention is needed beyond lubrication and comfort measures when the bleeding is localized and the globe is intact. Clinicians should assess for corneal abrasions, anterior chamber involvement, or orbital fractures if spontaneous hyphema occurs after trauma. In selected cases, fluorescein staining and orbital imaging may be warranted. With preserved vision and no associated injury, conservative therapy remains appropriate. Protective eyewear is advised for athletes or individuals at increased risk of ocular trauma. Treatment is generally unnecessary in children unless secondary complications, such as infection or retained foreign material, are identified.
In SCH, particularly among older adults or patients receiving anticoagulant therapy, management should address both ocular manifestations and systemic contributors. Blood pressure control is essential for recurrent or extensive hemorrhage, and adjustments to aspirin, warfarin, or direct oral anticoagulants may be considered in collaboration with a primary care physician or cardiologist. Evidence indicates a strong association between spontaneous SCH and systemic hypertension, and antihypertensive therapy may reduce recurrence. Antithrombotic therapy in patients with isolated SCH may generally be maintained, given the minimal ocular risk compared with the systemic benefits of anticoagulation. Coagulation profiles, including INR, may be assessed to confirm therapeutic levels.
Topical steroids or antibiotics are not recommended in uncomplicated SCH, as they are unnecessary and potentially harmful. Antibiotic drops may be prescribed in rare cases with conjunctival inflammation or concurrent conjunctivitis, although these medications do not influence hemorrhage resolution. Surgical intervention is reserved for cases involving suspected malignancy, foreign body retention, or scleral rupture. Emergency ophthalmology referral is indicated when SCH results from trauma with suspected intraocular or retinal injury. Dilute brimonidine and oxymetazoline have been shown to improve patient comfort and decrease the incidence of SCH following ocular surgery and intravitreal injection.[36][37](A1)
Differential Diagnosis
SCH generally manifests as a distinct red lesion on the sclera and is readily identifiable in most cases. Other conditions may mimic or be mistaken for SCH, particularly in atypical presentations. A key distinction exists between SCH and episcleritis or scleritis.[38] Episcleritis presents with localized redness and mild irritation but lacks the sharply demarcated appearance of SCH.[39] The condition often causes slight discomfort and responds to topical phenylephrine, unlike SCH. Scleritis represents a severe, often bilateral inflammatory disorder associated with systemic autoimmune disease, marked by violaceous discoloration and significant tenderness on palpation. Unlike SCH, both conditions demand urgent recognition and thorough evaluation to guide appropriate therapy.
Globe rupture and retrobulbar hematoma must be excluded if orbital trauma is suspected, as both are sight-threatening and require an emergent ophthalmology consult. In traumatic settings, additional considerations include corneal abrasion, conjunctival laceration, ocular foreign body, traumatic iritis, and traumatic hyphema. Patients with a globe rupture, orbital fracture, or retrobulbar hematoma may present with proptosis, chemosis, decreased visual acuity, or a tear-drop-shaped pupil.[40]
The physical examination may also distinguish other ocular disorders, such as an afferent pupillary defect in optic neuropathy or consensual photophobia in iritis. Slit-lamp examination with fluorescein staining is a valuable adjunct for identifying erosions, ulcers, and dendritic lesions. Many patients report grittiness or a foreign body sensation, which should not occur in a simple SCH. Differential diagnoses for nontraumatic cases include conjunctivitis, episcleritis, inflamed pterygium or pinguecula, corneal erosions, keratitis, and anterior uveitis. Acute angle-closure glaucoma, corneal ulcer, endophthalmitis, and scleritis must also be ruled out, as these represent ophthalmologic emergencies.
Another important differential diagnosis includes conjunctival lesions that present as hemorrhagic or vascularized. Conjunctival hemangiomas and arteriovenous malformations can cause localized vascular congestion that mimics SCH, particularly when thrombosed or irritated.[41][42][41] Kaposi sarcoma, a vascular neoplasm affecting mostly immunocompromised patients, may also manifest as conjunctival vascular lesions that resemble recurrent SCH.[43] These lesions typically persist beyond the resorption period of a simple SCH and may appear nodular or elevated on slit-lamp examination. Conjunctival melanoma and lymphoproliferative tumors can also produce bleeding lesions, especially in the context of secondary hemorrhage.[44] Any persistent conjunctival mass or blood accumulation requires further evaluation with anterior segment imaging or biopsy.
Vitreous or anterior chamber hemorrhages should be considered in the differential, particularly when subhyaloid hemorrhage follows trauma. Hyphema, defined as blood within the anterior chamber, appears as a crimson fluid level on slit-lamp examination and may present with discomfort, photophobia, and elevated IOP.[45] Vitreous hemorrhage, in contrast, produces floaters, vision loss, and obscuration of the fundus, often secondary to proliferative diabetic retinopathy, trauma, or retinal tears.
Unlike SCH, these hemorrhages involve intraocular compartments and carry a substantial risk of visual impairment. Careful assessment of visual acuity, pupillary response, and anterior segment findings is essential for distinguishing these conditions. When diagnostic uncertainty persists, ocular ultrasonography or optical coherence tomography can help differentiate subhyaloid hemorrhage from posterior segment hemorrhage.
In pediatric populations, the differential diagnosis should include NAT (child abuse), coagulopathies such as idiopathic thrombocytopenic purpura or leukemia, and infectious conjunctivitis.[46][47][48][49] SCH due to child abuse is often bilateral or associated with retinal hemorrhages and other signs of trauma, such as contusions or fractures.[50] Bilateral SCH may also occur with traumatic asphyxia syndromes, including strangulation.
Recurrent SCH in children requires evaluation for systemic bleeding disorders, particularly when accompanied by petechiae, epistaxis, or mucosal bleeding.[51] Infectious conjunctivitis produces a red eye typically associated with purulent discharge, pruritus, or a recent upper respiratory infection. In contrast, SCH is usually asymptomatic, with only bright red staining and no discharge or conjunctival chemosis. Clinicians must therefore rely on detailed history, physical examination, and, when appropriate, laboratory testing to distinguish SCH from its mimics in both pediatric and adult populations.
Staging
SCH is a benign ocular disorder without a formal staging system recognized by international ophthalmologic or hematologic organizations. Clinicians often describe a predictable clinical trajectory that can be theoretically categorized into stages according to onset, progression, and resolution. These stages are valuable for patient counseling, monitoring, and distinguishing uncomplicated SCH from ocular conditions that lack a similar resolution pattern. In practice, SCH follows distinct phases: acute presentation, intermediate transformation, and eventual resolution. This progression parallels cutaneous ecchymosis, reflecting hemoglobin breakdown within conjunctival tissues.[52]
The acute phase presents as a bright red or crimson lesion localized to the bulbar conjunctiva. This manifestation corresponds to recent vascular rupture and accumulation of intact erythrocytes in the subconjunctival space. The affected region is sharply demarcated, flat or slightly raised, and generally painless. This stage lasts 1 to 5 days, during which the intense coloration reflects high oxygen content and hemoglobin concentration of freshly extravasated blood. The lesion does not blanch under pressure and remains immobile on palpation due to its confinement within the conjunctival stroma. Patients may report foreign body sensation or mild irritation, but visual disturbance, photophobia, and systemic symptoms are absent.
During the subacute or metamorphosis phase, the hemorrhage changes in color as hemoglobin undergoes degradation. The affected area may appear purple, brown, or greenish-yellow as hemoglobin breaks down into biliverdin and bilirubin. Macrophage infiltration and erythrocyte phagocytosis dominate this phase, signifying histological and immunological clearance of the hemorrhagic material. This stage typically occurs between days 5 and 10 and serves as a clinical marker that resolution is underway. The lesion may fragment or adopt a patchy distribution, and the color gradient may shift inferonasally due to gravitational pooling. The absence of pain or new symptoms during this period corroborates the benign course.
The resolution phase is characterized by progressive clearing of discoloration and restoration of the normal conjunctival appearance, usually between 10 and 21 days after onset. More extensive hemorrhages, or those in older or anticoagulated patients, may persist longer. No scarring, fibrosis, or pigmentation develops, and recurrence in the same location does not indicate structural damage. Persistence beyond 3 weeks or frequent recurrence warrants reassessment for systemic or local risk factors, including hypertension, vascular fragility, and conjunctivochalasis. Although SCH is not formally classified in the literature, a practical understanding of its temporal stages enhances diagnostic accuracy, patient education, and clinical management.
Prognosis
The prognosis for SCH is highly favorable. Vision is unaffected, and most cases are self-limiting, resolving spontaneously within 7 to 14 days. Reabsorption of extravasated blood occurs efficiently due to the superficial vascular network and intact conjunctival tissue. Recurrence occurs in about 10% of cases without risk factors and is more frequent with anticoagulant or antiplatelet use. Recurrent episodes are commonly linked to uncontrolled hypertension, chronic anticoagulation, or anatomical variants such as conjunctivochalasis.
Patients with vascular or anatomic predispositions generally have a favorable outlook, although recurrence is more likely. Conjunctivochalasis, pinguecula, and improper contact lens use increase risk by introducing frictional stress and microvascular fragility in the bulbar conjunctiva.[53][54] In such cases, resolution may be slower and recurrence triggered by minimal stimuli. Permanent visual loss, scarring, or tissue damage does not occur. The prognosis of SCH is excellent across all causes and patient groups when systemic risk factors are identified and addressed.
Complications
SCH is considered a benign ocular condition, and complications are rare in otherwise healthy individuals. Most cases resolve within 2 weeks without intervention, as the hemorrhage is confined to the subconjunctival space and does not affect vision, IOP, or ocular motility. Infection, scarring, or permanent sequelae do not occur. The principal concern is cosmetic, as the striking appearance often causes disproportionate distress despite the absence of functional impairment. Delayed resolution or recurrence may occur in patients receiving anticoagulant therapy or those with systemic vascular disease, although these outcomes remain clinically insignificant.
Recurrent SCH may rarely indicate an unrecognized systemic disorder. Hematologic conditions such as thrombocytopenia, leukemia, or von Willebrand disease may first present with ocular hemorrhage.[55] In some cases, SCH is bilateral or associated with bleeding at other mucosal sites, including the gums or nasal cavities. These systemic disorders carry significant morbidity, underscoring the need for comprehensive evaluation in recurrent or unexplained presentations. Population-based data also suggest an association between SCH and subclinical hypothyroidism, in which higher baseline rates of hypertension, diabetes, and dyslipidemia contribute to vascular fragility and systemic morbidity.
Deterrence and Patient Education
SCH generally resolves within 2 weeks. Patients noticing recurrence or persistent SCH, or bruising elsewhere, particularly if taking anticoagulant or antiplatelet therapy, should consult their general practitioner or cardiologist, who may arrange further evaluation. Artificial tears can provide symptomatic relief for dryness or a gritty sensation. Immediate medical attention is warranted if vision loss, ophthalmoplegia, or increasing pain and swelling develop.
Prevention focuses on addressing modifiable risk factors and educating patients regarding the benign nature and natural course of SCH. Regular monitoring and control of blood pressure are essential for individuals with recurrent episodes, especially older adults. Cardiovascular management plays a central role in reducing recurrence risk. Clinicians should review anticoagulant or antiplatelet therapy, confirming appropriate dosing and therapeutic range. While anticoagulation is not contraindicated in SCH, inadequate management may increase recurrence. Patient education on medication adherence and regular monitoring, such as INR assessment for warfarin users, constitutes a key preventive strategy.
Patient education should begin with a clear explanation of the anatomy and pathophysiology of SCH. Emphasizing that the hemorrhage is superficial, located beneath the conjunctiva, and does not threaten vision can alleviate concern. Patients should understand that color changes over time, from red to yellowish-green, are normal and reflect hemoglobin breakdown.
In cases of traumatic SCH, particularly among contact lens users, education should highlight refraining from lens use until resolution, safe handling techniques, proper hygiene, and gentle insertion and removal methods to prevent mechanical injury. Preventive strategies may also target triggers such as coughing, vomiting, constipation, or Valsalva maneuvers.
Patients must be informed about warning signs that distinguish uncomplicated SCH from more serious ocular or systemic conditions, including pain, photophobia, impaired vision, discharge, and recurrent bilateral hemorrhage. The presence of systemic bruising, gum bleeding, or a personal or family history of coagulopathies may require laboratory evaluation and hematology consultation.
Pearls and Other Issues
A crucial clinical insight in SCH is recognizing its distinctive presentation, which reassures patients about its benign nature. SCH is one of the few ocular conditions that appear severe yet are rarely serious. The absence of pain, photophobia, discharge, or visual changes helps differentiate SCH from other ocular emergencies. Clinicians should maintain heightened suspicion only when unusual features arise, such as recurrent hemorrhage, bilateral involvement, or systemic bleeding manifestations. Timely and accurate diagnosis prevents unnecessary referrals, imaging, or treatments, reducing patient anxiety and healthcare costs.
Physicians also play a key educational role in normalizing SCH for patients. Many individuals experience anxiety due to the abrupt, intense redness of the eye, fearing serious conditions such as stroke or retinal hemorrhage. Clear explanation of SCH anatomy and natural history reassures patients and equips them to recognize and respond to future occurrences appropriately. In pediatric cases, education focuses on caregivers, emphasizing the identification of patterns suggestive of trauma or bleeding disorders. Clinician confidence and clear communication are essential in these often benign but alarming conditions. Training students and residents to differentiate SCH from other causes of red eye through careful history-taking and physical examination is a critical component of medical education.
Enhancing Healthcare Team Outcomes
SCH is a frequently encountered complaint in medical practice. Most patients do not experience any symptoms aside from the visible red appearance of the hemorrhage. The cause of SCH is often idiopathic, and most cases are benign, arising from factors such as increased strain, Valsalva maneuvers, or contact lens use. However, some instances are associated with systemic predisposing conditions, warranting further evaluation to prevent potential morbidity or mortality.
Various medical professionals may encounter SCH. Primary care providers, emergency physicians, and ocular specialists are typically involved in patient assessment and management. Interprofessional collaboration is essential, as nurses and pharmacists play key roles in monitoring vital signs and adjusting medications. Ophthalmologists are central to follow-up care and coordination. Patients on anticoagulation therapy may require consultation with their cardiologist or vascular specialist. Neonatologists, pediatricians, and pediatric emergency physicians may be involved in pediatric cases. Clinicians must remain vigilant for SCH as a possible sign of NAT, particularly in children, and assess accordingly.
The prognosis also depends on ongoing education and quality improvement initiatives. Regular interprofessional training for emergency and primary care teams can enhance diagnostic accuracy and reduce unnecessary interventions. Integrating SCH evaluation protocols into electronic medical record systems can systematically prompt clinicians to assess blood pressure, medication history, and ocular findings. Thorough documentation, effective communication, and collaborative decision-making contribute to safe, efficient, and patient-centered care. SCH is generally benign and self-limiting, though its sudden appearance can cause considerable anxiety. A well-organized healthcare team ensures that patients receive not only appropriate clinical management but also the reassurance and education necessary for optimal outcomes.
Media
(Click Image to Enlarge)
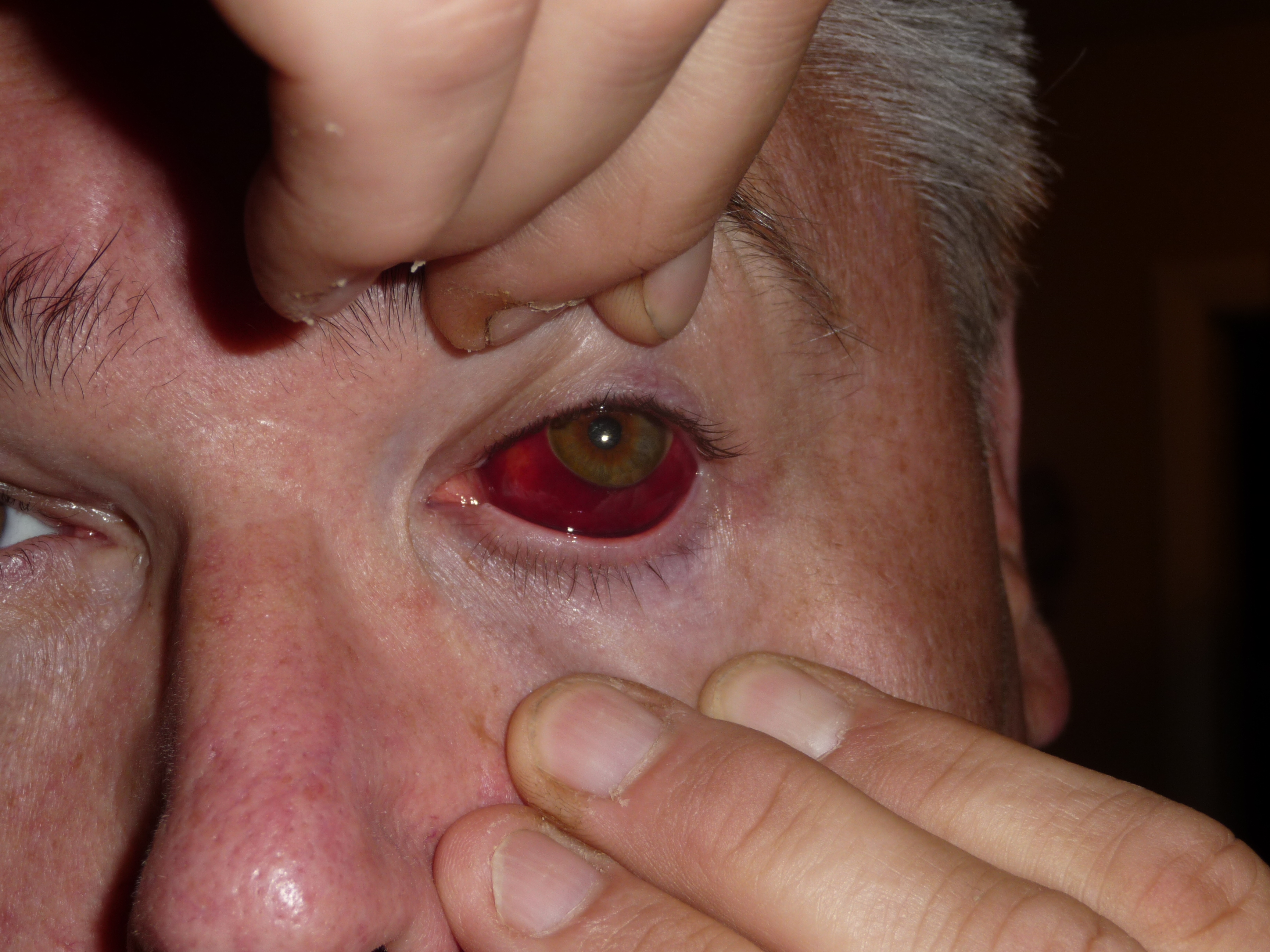
Subconjunctival Hemorrhage. This image displays a well-circumscribed, bright red patch beneath the conjunctiva of the eye, sharply demarcated from the surrounding sclera. The hemorrhage is nontender, does not cross the limbus, and is limited anteriorly. No signs of swelling, discharge, or involvement of the cornea are visible. The adjacent skin and periorbital tissues appear normal. The patient is on anticoagulant medication.
Contributed by Stephan Moll, MD
References
Cronau H, Kankanala RR, Mauger T. Diagnosis and management of red eye in primary care. American family physician. 2010 Jan 15:81(2):137-44 [PubMed PMID: 20082509]
Tarlan B, Kiratli H. Subconjunctival hemorrhage: risk factors and potential indicators. Clinical ophthalmology (Auckland, N.Z.). 2013:7():1163-70. doi: 10.2147/OPTH.S35062. Epub 2013 Jun 12 [PubMed PMID: 23843690]
Sahinoglu-Keskek N, Cevher S, Ergin A. Analysis of subconjunctival hemorrhage. Pakistan journal of medical sciences. 2013 Jan:29(1):132-4. doi: 10.12669/pjms.291.2802. Epub [PubMed PMID: 24353524]
Parikh AO, Christian CW, Forbes BJ, Binenbaum G. Prevalence and Causes of Subconjunctival Hemorrhage in Children. Pediatric emergency care. 2022 Aug 1:38(8):e1428-e1432. doi: 10.1097/PEC.0000000000002795. Epub 2022 Jun 13 [PubMed PMID: 35696303]
Mimura T, Usui T, Yamagami S, Funatsu H, Noma H, Honda N, Amano S. Recent causes of subconjunctival hemorrhage. Ophthalmologica. Journal international d'ophtalmologie. International journal of ophthalmology. Zeitschrift fur Augenheilkunde. 2010:224(3):133-7. doi: 10.1159/000236038. Epub 2009 Sep 9 [PubMed PMID: 19738393]
Mimura T, Yamagami S, Mori M, Funatsu H, Usui T, Noma H, Amano S. Contact lens-induced subconjunctival hemorrhage. American journal of ophthalmology. 2010 Nov:150(5):656-665.e1. doi: 10.1016/j.ajo.2010.05.028. Epub 2010 Aug 14 [PubMed PMID: 20709310]
Level 2 (mid-level) evidenceLiu W, Li H, Qiao J, Tian T, An L, Xing X, Liu A, Ji J. The tear film characteristics of spontaneous subconjunctival hemorrhage patients detected by Schirmer test I and tear interferometry. Molecular vision. 2012:18():1952-4 [PubMed PMID: 22876120]
Level 2 (mid-level) evidenceKING AB, WALSH FB. Trauma to the head with particular reference to the ocular signs; injuries involving the hemispheres and brain stem; miscellaneous conditions; diagnostic principles; treatment. American journal of ophthalmology. 1949 Mar:32(3):379-98 [PubMed PMID: 18112994]
Spitzer SG, Luorno J, Noël LP. Isolated subconjunctival hemorrhages in nonaccidental trauma. Journal of AAPOS : the official publication of the American Association for Pediatric Ophthalmology and Strabismus. 2005 Feb:9(1):53-6 [PubMed PMID: 15729281]
Level 3 (low-level) evidenceKatzman GH. Pathophysiology of neonatal subconjunctival hemorrhage. Clinical pediatrics. 1992 Mar:31(3):149-52 [PubMed PMID: 1547586]
Kittisupamongkol W. Blood pressure in subconjunctival hemorrhage. Ophthalmologica. Journal international d'ophtalmologie. International journal of ophthalmology. Zeitschrift fur Augenheilkunde. 2010:224(5):332; author reply 332. doi: 10.1159/000313835. Epub 2010 Apr 30 [PubMed PMID: 20431313]
Level 3 (low-level) evidencePitts JF, Jardine AG, Murray SB, Barker NH. Spontaneous subconjunctival haemorrhage--a sign of hypertension? The British journal of ophthalmology. 1992 May:76(5):297-9 [PubMed PMID: 1390514]
Çalış Karanfil F, Karanfil M, Toklu Y. Association of nocturnal subconjunctival hemorrhage and non-dipper blood pressure pattern: A benign clue for serious diseases. European journal of ophthalmology. 2022 Sep:32(5):3043-3049. doi: 10.1177/11206721211070939. Epub 2021 Dec 29 [PubMed PMID: 34964388]
Hu DN, Mou CH, Chao SC, Lin CY, Nien CW, Kuan PT, Jonas JB, Sung FC. Incidence of Non-Traumatic Subconjunctival Hemorrhage in a Nationwide Study in Taiwan from 2000 to 2011. PloS one. 2015:10(7):e0132762. doi: 10.1371/journal.pone.0132762. Epub 2015 Jul 16 [PubMed PMID: 26181776]
Bodack MI. A warfarin-induced subconjunctival hemorrhage. Optometry (St. Louis, Mo.). 2007 Mar:78(3):113-8 [PubMed PMID: 17321459]
Level 3 (low-level) evidenceKoti AS, Brown ECB, Campbell KA. Subconjunctival Hemorrhages Are Rare Among Infants With Cough and Gastrointestinal Conditions. Pediatric emergency care. 2025 Feb 1:41(2):127-130. doi: 10.1097/PEC.0000000000003293. Epub 2024 Oct 30 [PubMed PMID: 39475329]
Kougou Ntoutoume AR, Madbouhi K, Mekyna S, Matsanga RO, Tachfouti S, Cherkaoui O. [Glasses-shaped bruise, bilateral subconjunctival and retinal hemorrhages following Valsalva maneuver as presenting sign of acute myeloid leukemia]. Journal francais d'ophtalmologie. 2020 Dec:43(10):1117-1119. doi: 10.1016/j.jfo.2019.12.028. Epub 2020 Aug 20 [PubMed PMID: 32829940]
Chavan NA, Shinde P, Tikute S, Vishwanathan R, Deoshatwar AR, Gurav YK, Waghchaure R, Ahmed NH, Rani VS, Khan V, Kelkar A, Jain HH, Jain A, Lavania M, Tandale BV. Acute hemorrhagic conjunctivitis outbreaks associated with Coxsackievirus A-24 in India, 2023. Journal of infection and public health. 2025 Feb:18(2):102626. doi: 10.1016/j.jiph.2024.102626. Epub 2024 Dec 17 [PubMed PMID: 39740338]
Boro P, Gongo T, Ori K, Kamki Y, Ete N, Jini M, Jampa L, Patgiri SJ, Sarmah N, Siddique AI, Bhattacharjee CK, Bali NK, Borkakoty B. An outbreak of acute hemorrhagic conjunctivitis due to Coxsackievirus A24 in a residential school, Naharlagun, Arunachal Pradesh: July 2023. Indian journal of medical microbiology. 2024 Mar-Apr:48():100549. doi: 10.1016/j.ijmmb.2024.100549. Epub 2024 Mar 5 [PubMed PMID: 38395257]
Celebi ARC, Aygun EG. A rare cause of recurrent subconjunctival hemorrhage: ocular vicarious menstruation. GMS ophthalmology cases. 2023:13():Doc05. doi: 10.3205/oc000213. Epub 2023 Jan 30 [PubMed PMID: 36875630]
Level 3 (low-level) evidenceLootah S, Alshammari E, Alqanatish J. Complete Remission in a Child With Multisystem Inflammatory Syndrome and Stevens-Johnson Syndrome Treated With Infliximab. Cureus. 2023 Apr:15(4):e37076. doi: 10.7759/cureus.37076. Epub 2023 Apr 3 [PubMed PMID: 37153258]
Tong JW, Sawamura MH. Subconjunctival hemorrhages: presenting sign for hereditary hemochromatosis. Optometry and vision science : official publication of the American Academy of Optometry. 2011 Sep:88(9):1133-9. doi: 10.1097/OPX.0b013e3182223683. Epub [PubMed PMID: 21666526]
Jange RG, Koka K, Krishnakumar S, Jayaraman D, Scott JX, Mukherjee B. A rare case of precursor B-cell lymphoblastic lymphoma of the orbit in a child. Oman journal of ophthalmology. 2025 May-Aug:18(2):212-215. doi: 10.4103/ojo.ojo_346_24. Epub 2025 Jun 24 [PubMed PMID: 40666786]
Level 3 (low-level) evidenceHong IH, Cho BJ, Choi SH. Association between subconjunctival hemorrhage and hemorrhagic disorders: a nationwide population-based study. Scientific reports. 2023 Dec 14:13(1):22237. doi: 10.1038/s41598-023-49428-z. Epub 2023 Dec 14 [PubMed PMID: 38097669]
Kaimbo Wa Kaimbo D. Epidemiology of traumatic and spontaneous subconjunctival haemorrhages in Congo. Bulletin de la Societe belge d'ophtalmologie. 2009:(311):31-6 [PubMed PMID: 19621552]
Karademir Z, Semerci SY, Ozdemir FE, Bayramoglu SE. Subconjunctival hemorrhage in the newborn: Experience of a tertiary care hospital. Northern clinics of Istanbul. 2023:10(1):74-78. doi: 10.14744/nci.2021.19971. Epub 2023 Feb 13 [PubMed PMID: 36910442]
Mimura T, Yamagami S, Usui T, Funatsu H, Noma H, Honda N, Fukuoka S, Hotta H, Amano S. Location and extent of subconjunctival hemorrhage. Ophthalmologica. Journal international d'ophtalmologie. International journal of ophthalmology. Zeitschrift fur Augenheilkunde. 2010:224(2):90-5. doi: 10.1159/000235798. Epub 2009 Aug 28 [PubMed PMID: 19713719]
Dhillon J, Nassrallah G, Nithianandan H, Gaffar J, Kondoff M, Ross M, Deschênes J. Significance of subconjunctival hemorrhage in predicting ocular pathology for patients with orbital fracture. Canadian journal of ophthalmology. Journal canadien d'ophtalmologie. 2023 Aug:58(4):295-301. doi: 10.1016/j.jcjo.2022.02.003. Epub 2022 Mar 10 [PubMed PMID: 35278371]
Powdrill S. Ciliary injection: a differential diagnosis for the patient with acute red eye. JAAPA : official journal of the American Academy of Physician Assistants. 2010 Dec:23(12):50-4 [PubMed PMID: 21229837]
Level 3 (low-level) evidenceWinters S, Frazier W, Winters J. Conjunctivitis: Diagnosis and Management. American family physician. 2024 Aug:110(2):134-144 [PubMed PMID: 39172671]
Cagini C, Iannone A, Bartolini A, Fiore T, Fierro T, Gresele P. Reasons for visits to an emergency center and hemostatic alterations in patients with recurrent spontaneous subconjunctival hemorrhage. European journal of ophthalmology. 2016 Mar-Apr:26(2):188-92. doi: 10.5301/ejo.5000692. Epub 2015 Oct 14 [PubMed PMID: 26480948]
Chiang PH, Lai JN, Chiang YC, Hu KC, Hsu MY, Wei JC. Association Between Subconjunctival Hemorrhage and Acute Coronary Syndrome: A 14-Year Nationwide Population-Based Cohort Study. Frontiers in cardiovascular medicine. 2021:8():728570. doi: 10.3389/fcvm.2021.728570. Epub 2021 Oct 1 [PubMed PMID: 34660729]
Koti AS, Crichton KG, Liker K, Hashmi Z, Thackeray JD. Occult Injury Screening Among Infants With Subconjunctival Hemorrhage. Journal of pediatric ophthalmology and strabismus. 2021 Jul-Aug:58(4):213-217. doi: 10.3928/01913913-20210201-02. Epub 2021 Jul 1 [PubMed PMID: 34288770]
Cho N, Koti AS. Identifying inflicted injuries in infants and young children. Seminars in pediatric neurology. 2024 Jul:50():101138. doi: 10.1016/j.spen.2024.101138. Epub 2024 May 11 [PubMed PMID: 38964814]
Luo HR, Zhai X, Xie SM, Jin X. A retrospective study of 51 pediatric cases of traumatic asphyxia. Journal of cardiothoracic surgery. 2022 Mar 13:17(1):34. doi: 10.1186/s13019-022-01773-2. Epub 2022 Mar 13 [PubMed PMID: 35282839]
Level 2 (mid-level) evidenceGonzalez-Saldivar G, Pita-Ortiz IY, Flores-Villalobos EO, Jaurrieta-Hinojos JN, Espinosa-Soto I, Rios-Nequis G, Ramirez-Estudillo A, Jimenez-Rodriguez M. Oxymetazoline: reduction of subconjunctival hemorrhage incidence after intravitreal injections. Canadian journal of ophthalmology. Journal canadien d'ophtalmologie. 2019 Aug:54(4):513-516. doi: 10.1016/j.jcjo.2018.09.006. Epub 2018 Nov 23 [PubMed PMID: 31358153]
Pasquali TA, Aufderheide A, Brinton JP, Avila MR, Stahl ED, Durrie DS. Dilute brimonidine to improve patient comfort and subconjunctival hemorrhage after LASIK. Journal of refractive surgery (Thorofare, N.J. : 1995). 2013 Jul:29(7):469-75. doi: 10.3928/1081597X-20130617-05. Epub [PubMed PMID: 23820229]
Level 1 (high-level) evidenceSinha S, Tarini S, Panchal B, Mishra DK. Subconjunctival Dirofilaria masquerading as nodular scleritis. BMJ case reports. 2024 May 22:17(5):. pii: e259519. doi: 10.1136/bcr-2023-259519. Epub 2024 May 22 [PubMed PMID: 38782433]
Level 3 (low-level) evidencePerray L, Ungerer L, Chazal T, Monnet D, Brézin A, Terrier B. [Scleritis and episcleritis]. La Revue de medecine interne. 2023 Dec:44(12):646-655. doi: 10.1016/j.revmed.2023.05.013. Epub 2023 Jun 19 [PubMed PMID: 37344292]
Elghannam M, Hassan BA, Shams N, Merbs SL, Manson PN, Grant MP. Retrobulbar Hematoma: Presentation, Management, and Visual Outcomes. The Journal of craniofacial surgery. 2024 Dec 20:():. doi: 10.1097/SCS.0000000000010924. Epub 2024 Dec 20 [PubMed PMID: 39704540]
Yadav G, Khanal S, Yadav R. Hemangioma of Conjunctiva and Eyelid. Ophthalmology. 2023 Nov:130(11):1230. doi: 10.1016/j.ophtha.2022.11.025. Epub 2023 Jan 5 [PubMed PMID: 36609022]
Tavallali A, Mousavi Z, Rezaei L. Isolated conjunctival cavernous hemangioma: a rare case report. BMC ophthalmology. 2024 Jul 25:24(1):311. doi: 10.1186/s12886-024-03572-w. Epub 2024 Jul 25 [PubMed PMID: 39054506]
Level 3 (low-level) evidenceGouveia-Moraes F, Campos N. Conjunctival Kaposi's Sarcoma. The New England journal of medicine. 2021 Sep 16:385(12):e36. doi: 10.1056/NEJMicm2105217. Epub [PubMed PMID: 34525288]
Butt K, Hussain R, Coupland SE, Krishna Y. Conjunctival Melanoma: A Clinical Review and Update. Cancers. 2024 Sep 10:16(18):. doi: 10.3390/cancers16183121. Epub 2024 Sep 10 [PubMed PMID: 39335093]
Chean CS, Lim CS, Kumar P, Kapoor B. An Atypical Presentation of Sympathetic Ophthalmia in an Intact Globe Following Mechanical Fall: A Case Report and Literature Review. Vision (Basel, Switzerland). 2021 Feb 21:5(1):. doi: 10.3390/vision5010011. Epub 2021 Feb 21 [PubMed PMID: 33669961]
Level 3 (low-level) evidenceWolford JE, Berger RP, Eichman AL, Lindberg DM, ExSTRA Investigators. Injuries Suggestive of Physical Abuse in Young Children With Subconjunctival Hemorrhages. Pediatric emergency care. 2022 Feb 1:38(2):e468-e471. doi: 10.1097/PEC.0000000000002436. Epub [PubMed PMID: 34009893]
Ahmed N, Asreb A, Chofor R, Melese A. Treatment of Severe Immune Thrombocytopenic Purpura Associated with COVID-19. The American journal of case reports. 2021 Oct 21:22():e932557. doi: 10.12659/AJCR.932557. Epub 2021 Oct 21 [PubMed PMID: 34671000]
Level 3 (low-level) evidenceLaimon DN, Sakr DH, Atef B, Shaaban Y. Highlights of ophthalmological manifestations in newly diagnosed acute leukemia: a correlation with hematological parameters. Annals of hematology. 2024 Sep:103(9):3519-3533. doi: 10.1007/s00277-024-05861-2. Epub 2024 Jul 10 [PubMed PMID: 38985179]
McGinley TC Jr. Adult Eye Conditions: Common Eye Conditions. FP essentials. 2022 Aug:519():11-18 [PubMed PMID: 35947131]
Shah SN, Fong HF, Haney SB, Harper NS, Pierce MC, Neuman MI. Has This Child Experienced Physical Abuse?: The Rational Clinical Examination Systematic Review. JAMA. 2025 Jul 8:334(2):160-170. doi: 10.1001/jama.2025.2216. Epub [PubMed PMID: 40257808]
Level 1 (high-level) evidenceCanty KW, Sharma S, McLain K, Young K. Petechiae and subconjunctival hemorrhages in a child after a visit to the dentist. Journal of forensic and legal medicine. 2022 Apr:87():102331. doi: 10.1016/j.jflm.2022.102331. Epub 2022 Feb 24 [PubMed PMID: 35240542]
Guda VA, Brown ME, Palacios M. Spontaneous ecchymoses. The Journal of family practice. 2022 Oct:71(8):E9-E11. doi: 10.12788/jfp.0492. Epub [PubMed PMID: 36508558]
Yamamoto Y, Yokoi N, Ogata M, Shiraishi A, Yamaguchi M, Uno T, Inagaki K, Hayashi K, Kinoshita S, Ohashi Y. Correlation Between Recurrent Subconjunctival Hemorrhages and Conjunctivochalasis by Clinical Profile and Successful Surgical Outcome. Eye & contact lens. 2015 Nov:41(6):367-72. doi: 10.1097/ICL.0000000000000139. Epub [PubMed PMID: 26269933]
Ahn SJ, Shin KH, Kim MK, Wee WR, Kwon JW. One-year outcome of argon laser photocoagulation of pinguecula. Cornea. 2013 Jul:32(7):971-5. doi: 10.1097/ICO.0b013e31828344c9. Epub [PubMed PMID: 23449487]
Level 2 (mid-level) evidenceRodriguez-Roisin R, Torres A, Agustí AG, Ussetti P, Agustí-Vidal A. Subconjunctival haemorrhage: a feature of acute severe asthma. Postgraduate medical journal. 1985 Jul:61(717):579-81 [PubMed PMID: 4022890]
Level 3 (low-level) evidence