Stereotactic Radiosurgery (SRS) and Stereotactic Body Radiotherapy (SBRT)
Stereotactic Radiosurgery (SRS) and Stereotactic Body Radiotherapy (SBRT)
Introduction
Radiation therapy is intended to damage abnormal tissue, eg, neoplastic tumor cells, while causing minimal injury to adjacent normal tissue. Lars Leksell introduced stereotactic radiosurgery (SRS) in 1951 as an alternative treatment option to conventional whole-brain radiotherapy (WBRT).[1][2][3] SRS is typically used for tumors in the brain and spine, often in a single session, while stereotactic body radiotherapy (SBRT) is used for tumors outside the brain and spine, often in multiple sessions.
SRS/SBRT utilizes multiple, converging beams of high-energy x-rays, gamma rays, or protons, delivered to a discrete, radiographically defined treatment target volume.[4] The delivery of radiation therapy is highly conformal.[5] By using multiple, intersecting beams of radiation, the intended treatment volume receives a high, therapeutic prescription dose, while the surrounding normal brain tissue receives a relatively low therapeutic dose.[5] This treatment can be tailored precisely to the borders of the target volume, allowing for a rapid dissipation of energy beyond the margins that border normal tissue.[5] The steep radiation fall-off into adjacent tissue decreases potential toxicity and adverse effects, providing treatment safety.
SRS/SBRT causes radiation-induced DNA damage in the form of double-stranded DNA breakage and free radical formation. The vascular endothelium is a primary target that results in endothelial cell apoptosis, microvascular dysfunction, and a T-cell inflammatory response.[6] Histologically, a brisk inflammatory response and severe vasculopathy develop in lesions that are responsive to SRS/SBRT.[7]
Indications
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Indications
SRS/SBRT is appropriate for patients with brain metastases and certain primary brain tumors, eg, meningiomas, vestibular schwannomas, pituitary tumors, and hemangioblastomas, as well as those tumors considered resistant to conventional fractionated or whole-brain radiation therapy (WBRT).[6][8][9][10][11]
Brain metastases are the most common intracranial tumors in adults and can develop in up to 30% of patients with cancer, although this incidence is at present increasing.[12] SRS/SBRT can be used to treat single or multiple lesions, often deep-seated and surgically inaccessible due to their location.[4] SRS/SBRT can be used as an adjunct to surgery for postoperative residual disease and in cases of tumor recurrence. This treatment is ideal for patients with a limited number of cerebral metastases that would not be amenable to complete surgical resection.[13] Multiple, sequential SRS/SBRT treatment sessions can also be performed, with a median overall length of survival of 5 to 6 months for those undergoing 1, 2, 3, or more than 4 SRS/SBRT treatments.[14] Uncertainty exists regarding the use of SRS/SBRT combined with WBRT for brain metastasis, where no clear clinical consensus has been established. Trials for brain metastasis treatment have shown advantages in local control with combination therapy; however, conflicting outcomes have been reported for length of survival and the effect on cognition. Although most studies indicate that less cognitive deterioration occurs when SRS/SBRT is used alone, the effect on cognition remains unclear.[15][16][17][18][19][20]
Meningiomas and vestibular schwannomas are extra-axial tumors with clear, defined borders that are well-suited for SRS/SBRT treatment. Meningiomas are commonly treated with 12 to 13 Gy in a single radiation fraction, achieving growth control rates of 90% to 98% with a potential for toxicity of less than 10%.[21] SRS/SBRT provides a tumor control equivalent for Simpson grade 1 resection for tumors <3.5 cm in diameter over long-term follow-up.[21] Adjuvant SRS/SBRT following subtotal resection has a superior 15-year progression-free survival rate compared to patients with gross total resection who do not receive SRS/SBRT.[21]
Vestibular schwannomas are commonly treated with 12 to 13 Gy in a single fraction.[22] The 5-year local control rates for vestibular schwannomas range from 92% to 100%, with hearing preservation rates being superior to those achieved with surgery (32% to 71%), and cranial nerve V and VII preservation rates exceeding 95%.[23][24] Hearing preservation rates of 63% to 94% have been reported with fractionated SRS/SBRT; however, there is a lack of long-term follow-up, and no randomized controlled trials have been conducted comparing the 2 modalities.[23]
For pituitary adenomas with expansion of the intrasellar region or invasion into the cavernous sinus, surgery may only achieve a subtotal resection. In such cases, SRS or fractionated SBRT is an appropriate treatment option.[25] The reported rate of tumor control ranges from 91% to 99%. Endocrine disturbances occur in 1% to 22% of cases, and optic neuropathy results in 1% to 2% of cases, depending on the tumor volume.[26] The highest remission rates for endocrinopathies are observed in patients with Cushing disease, and the lowest are in those with prolactinomas.[27]
For primary gliomas, studies have evaluated the impact of SRS combined with conventional fractionated SBRT as a means of dose escalation and to decrease treatment time in patients with limited life expectancy.[22] Prospective and retrospective studies reveal a median survival of 6 to 18 months following salvage SRS for recurrent glioblastoma multiforme (GBM) and 6 to 14 months with fractionated SBRT.[22] SRS delivered to previously irradiated brain tissue increases the risk of future radionecrosis; therefore, some studies have evaluated the use of bevacizumab for treating GBM and have found positive results.[28][29]
SRS can be used to treat vascular pathologies, eg, arteriovenous malformations (AVMs), dural arteriovenous fistulas, and cavernomas.[30][31][32] Rates of complete obliteration for AVMs have been determined to be 76% for low-grade Spetzler-Martin I and II, 69% for grade III, and for high-grade IV-V AVMs, obliteration rates range from 0 to 61%.[33][34][35] Volume-staged SRS, where distinct geometrical parts of AVM are treated over time, has higher obliteration rates and similar complication rates compared to dose-staged fractionated SBRT or repeat SRS for large AVMs that are not amenable to single-session SRS.[36] SRS may have a role in patients with dural arteriovenous fistulas that are not amenable to surgery or endovascular embolization with an acceptable success rate and adverse effect profile.[37]
SRS was originally developed to treat functional disorders that include intractable pain, epilepsy, and movement disorders. The first SRS procedure performed was a thalamotomy for a patient with trigeminal neuralgia.[1] SRS is a treatment option in movement disorders for patients who are not suitable for open neurosurgery or deep brain stimulation (DBS). SRS has achieved comparable rates of tremor control to those of DBS placement and radiofrequency lesion generation, with an 85% to 90% rate of clinical improvement.[38] SRS thalamotomy has focused on treating the nucleus ventralis intermedius.[38] For trigeminal neuralgia, 50% to 80% of patients achieve complete pain relief, although several patients have required repeat SRS treatment, with associated trigeminal dysfunction in the form of facial numbness.[39][40]
SRS/SBRT are useful for seizure control in patients with mass lesions, including tumors, AVMs (with a mean seizure remission rate of 70%), and cavernous malformations (with a mean seizure remission rate of 50%).[41] The results of SRS/SBRT for treating mesial temporal lobe epilepsy show a wide range of efficacies in the literature, ranging from 0% to 86%.[41] Possible mechanisms include neuromodulation and ablation.[42] The recent ROSE trial suggests that open surgery has an advantage over SRS/SBRT for seizure remission, but that SRS/SBRT is a safe and appropriate alternative.[43]
SRS is an option for spinal and paraspinal lesions due to its noninvasive and targeted approach, including intramedullary and intradural spinal tumors.[44][45] The most common indication is vertebral metastasis, causing cancer-related pain.[46] Other indications include primary treatment for metastases, radioresistant pathologies, failure of radiotherapy, and as adjuvant treatment for residual or recurrent disease.[46] SRS can yield high rates of pain relief (85% to 92% within a few days to weeks) and tumor control (77% to 94%) and is an indicated therapy option for patients who cannot tolerate surgery, have residual disease, or as a palliative treatment.[46][47]
SBRT has been in use since the 1990s and includes treating pulmonary and hepatic tumors.[44][48][49]
Contraindications
Contraindications to using SRS/SBRT include extensive target lesions or multiple lesions that are too numerous to be effectively treated. Single-fraction SRS is usually limited to small lesions that are typically <3 cm, although fractionated SBRT is being used increasingly for larger lesions.[50]
For newly diagnosed glioblastoma, a randomized trial comparing SRS followed by external beam radiotherapy and carmustine chemotherapy versus only SBRT and carmustine showed no difference in overall survival, improvement in local control, or quality of life.[51] Reports of late toxicity were 4%.[51] Further studies, trials, and systematic reviews support this data. Currently, insufficient evidence supports the use of SRS/SBRT.[52]
SRS pallidotomy yielded a high complication rate, including visual field defects, and is not routinely in use for this indication.[38]
Equipment
Radiation Therapy Types
Various devices support SRS/SBRT, each differing in the type of radiation delivered and the technique used to focus beams on the target.
Ionizing radiation includes both electromagnetic and particulate forms capable of producing ions as they pass through matter. A photon, defined as a discrete packet of electromagnetic energy, spans a range of wavelengths, including radio waves, infrared, visible light, ultraviolet, and x-rays, depending on its energy level. Among these, only x-rays possess sufficient energy to produce ions, making them suitable for use in SRS/SBRT. Photon beams originate either from radioisotope sources, eg, cobalt-60, or from x-ray generating machines like linear accelerators. Radioactive isotopes consist of atoms with unstable nuclei that emit ionizing radiation through radioactive decay as they transition to a more stable state.
Focused gamma beams
Focused gamma beams are the simplest and oldest SRS device, first used on in 1967.[2][44] This device is a cobalt-60 radioisotope-based device with 192 individual sources of radiation, arranged in a conical tungsten shell.[2][6] The radioactive decay of cobalt leads to the emission of ionizing radiation in the form of beta particles and 2 strong gamma radiations: one with 1.17 MeV of energy and one with 1.33 MeV of energy.[5] This approach means that the effective energy of these focused beams is 1.25 MeV. Treatment energy is measurable in MeV or 1 million electron-volts. One electron-volt is the energy gained by an electron that accelerates through a potential difference of 1 volt.
The cobalt sources are arranged in a cylindrical array via a collimator helmet to focus the beams to converge at a single target. Overlapping targets can generate "hot spots" within the treatment volume.[5] The cylindrical configuration limits its use to intracranial and some upper cervical spine level pathologies, and it is used mainly for the delivery of a single fraction.[5] This system uses an automated internal collimation system (with sizes of 4, 8, or 16 mm) to replace the helmet.[44] Cobalt-60 will decay to nickel, with a half-life of 5.26 years. This means that the cobalt sources need to be replaced after a period or time to avoid prohibitively long treatment times.
Linear accelerator radiosurgery
Linear accelerator SRS was introduced clinically in the early 1980s.[5] This technique utilizes a linear-accelerator-based device to generate high-energy x-rays, which are produced by a magnetron that accelerates electrons against a tungsten metal target. The electrons are produced from an electron gun by thermionic emission. X-rays are emitted as the electrons decelerate after striking a metal target. The photon output is a continuous spectrum of x-rays, with peaks at specific energies depending on the atoms in the target. The number of x-ray particles produced increases as the kinetic energy of the electron increases. A 6 MeV electron beam is often the choice of energy for SRS.[5] The photon output can take any energy from 0 to 6 MeV. Linear accelerator-produced energy is denoted as MV or megavolts, not MeV. This means that photon beams from linear accelerator radiosurgery have a continuous "bremsstrahlung" or "deceleration radiation" spectrum of energy that is fixed compared to radioisotope sources that decay over time. A collimator focuses these x-rays.[6] The accelerator is mounted on a gantry that can rotate with intersecting arcs of radiation focused at the isocenter. The accelerator can be used for extracranial targets and as fractionated treatments.
Another SRS/SBRT delivery system is intensity-modulated radiation therapy (IMRT), which utilizes a linear accelerator-based application to vary the shape and intensity of the beam, allowing for a nonuniform dose distribution with steep dose gradients between target volumes and normal brain tissue.[53] This system delivers helical tomotherapy plans using a 6 MV unflattened x-ray beam and a binary multileaf collimator.[54] Many small, elongated, and off-axis subfields create the intensity modulation.[53] This method is useful for extracranial and spinal SRS delivery.[46]
Protons are positively charged particles found in the nucleus of an atom and used for particulate radiosurgery. A synchrotron or cyclotron-based device can generate proton beams by ionizing hydrogen. The proton beam can have an energy range of 20 to 190 MeV, depending on the device.[44] When charged particles move through the material, they ionize atoms and deposit energy. Protons interact with matter by Coulomb collisions between electrons and nuclei (a binary elastic collision of charged particles interacting through their own electric field), by bremsstrahlung radiation loss, and by nuclear reactions.[55] As proton beams travel through tissue, they lose energy, and this energy loss is inversely proportional to the square of the velocity, meaning that the dose distribution pattern shows a slowly rising dose, followed by a sharp increase called the Bragg peak near the end of the range.[55] Most of the energy gets deposited at a discrete band of spatial depth, just before the particle stops (unmodulated Bragg peak). Several superimposed, intersecting beams produce a modulated Bragg peak, with sufficient energy for larger treatment volumes.[5] This can provide a moderate entrance dose, a uniformly high dose within the target tissue, and no doses beyond the target.[55] Proton beams can be precisely focused to control the depth of penetration while depositing most of the energy at the target.[6] Due to both the considerable cost of the equipment and the housing space considerations, proton SRS is an infrequent option.
To date, no randomized controlled trial exists to compare the various devices, so physician expertise and machine availability generally guide clinical practice. Comparative studies are inconclusive, although they commonly demonstrate equivalence.[40][56]
Dosimetry
Dosimetry is a vital but difficult component of SRS/SBRT. With SRS/SBRT, small fields are used that are typically 0.3 x 0.3 cm up to 4 x 4 cm in size. By definition, a small field is when at least 1 of 3 conditions are satisfied: (1) loss of lateral charged particle equilibrium on the beam axis; (2) partial occlusion of the photon source by the collimator; and (3) the size of the detector is similar or larger than the beam dimensions.[54][57] Challenges due to these small fields include lack of charged particle equilibrium, partial blocking of the beam source, changes in stopping power ratios, and the availability of detectors with comparable sizes to the field dimensions.[57][58] With lateral electronic disequilibrium, there is a lack of lower energy electrons, so that the average energy spectrum at the central axis will increase, and the stopping power ratio of water to air will decrease.[57] This results in an overlap between the field penumbra and the detector volume. The detector itself produces a perturbation that is hard to quantify, and a correction factor is necessary.[58]
The use of the IAEA/AAPM (International Atomic Energy Agency/American Association of Physicists in Medicine) protocol for reference and relative dosimetry based on Alfonso et al (2008) is recommended.[54] This uses a correction factor dependent on beam energy, type of detector, type of machine, and focal spot size.[54] Reference dosimetry is based on a 10 x 10 cm field. For SRS machines, where the conventional 10 x 10 cm reference field cannot be established, specific reference conditions related to the machine-specific reference fields are used.[54][58] Calculations are indirect, and significant deviations in the dosimetry result.[54][58] Monte Carlo simulation is extensively used to improve the accuracy of small-field dosimetry under nonequilibrium radiation conditions.[57][58]
The dose is in gray (Gy), which is the absorption of 1 joule of radiation energy per kilogram of matter. The SRS dose is biologically equivalent to 5 to 6 weeks of daily conventional radiation therapy.[6] The dose is deliverable in either a single session or 2 to 5 sessions of fractionated therapy over several days known as fractionated SBRT (or FSR).[5] The advent of nonfixed SRS immobilization systems, onboard imaging to verify accuracy, and patient monitoring systems during radiation allows reproducible, precise patient positions, making fractionation possible.[22]
SBRT can be used in eloquent areas, for larger lesions, and for lesions close to critical structures, eg, the optic chiasm, ventral cochlear nucleus, and the brain stem, which have a lower tolerance to radiation therapy.[5] The result of SBRT is that the biologically effective dose is increased, with decreased toxicity.[22][59] By using fractions, the total treatment dose can be kept below the radiation tolerance of critical structures, while still achieving therapeutic tumor control.[5] For example, an optic apparatus maximum dose associated with a clinically reasonable risk of radiation-induced necrosis of the optic nerve is 10 Gy in 1 fraction, 20 Gy in 3 fractions, and 25 Gy in 5 fractions.[60] The Radiation Therapy Oncology Group Trial, which looked at patients receiving SRS for brain metastases following WBRT or recurrent gliomas postradiation, developed dose limits for SRS of 24 Gy for lesions <2 cm, 18 Gy for lesions 2 to 3 cm, and 15 Gy for tumors 3 to 4 cm.[50] This meant that tumors with larger volumes received lower doses, resulting in poorer rates of local tumor control.[22] FSR may provide an improved balance of tumor control and toxicity in these instances. Meta-analysis has shown that for large brain metastases, SBRT can reduce the rate of radionecrosis while maintaining or improving the rates of 1-year local control versus single-fraction treatment.[22][59]
Deciding on using SRS or SBRT for brain metastases is multifactorial. Physical factors (eg, tumor size, margins, optimal dose), biological factors (histology of the metastases, use of systemic treatment agents), and clinical factors (life expectancy, comorbidities, concurrent treatments required) all play a role in this decision-making.[22] Currently, trials are ongoing to determine the role of FSR for brain metastases, glioblastomas, meningiomas, and vestibular schwannomas, with the primary outcome goal of determining the maximum tolerated dose for these lesions.[22]
Personnel
Healthcare team members who may be involved with SRS and SBRT include:
- Neurosurgeons
- Radiation oncologists
- Neuroradiologists
- Medical physicists
- Dosimetrist
- Radiation therapist
- Nurse specialists (oncology nurses)
Technique or Treatment
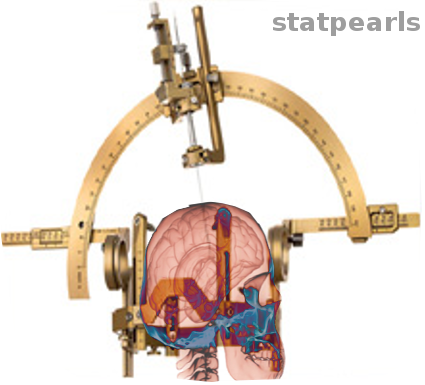
SRS/SBRT is a noninvasive, outpatient procedure that does not require a general anesthetic (see Image. Stereotaxic Surgery). The first step of the treatment procedure is to localize the treatment target. Focused gamma rays will be directed to the target using a specialized head frame that is physically attached to the skull using moderate sedation and a local anesthetic. The patient is immobilized during treatment by a head frame that is secured to the skull by sharp pins that pierce the scalp. A frameless head fixation device is also available. A fiducial reference box is secured to the head frame to provide target coordinates, and magnetic resonance imaging (MRI), computed tomography (CT), or angiography is performed to define the target in 3 dimensions. With the focused gamma ray treatment, the patient and the machine remain stationary throughout the procedure.
For linear accelerator-based SRS, either the patient or the gantry will move in space to change the radiation delivery point. In the past, a stereotactic frame was used to restrict head movement. However, newer frameless approaches have been developed, with improved patient comfort.[44] One of these technologies utilizes a small 6 MV linear accelerator mounted on a robotic arm with 6 degrees of freedom and 2 orthogonal x-ray cameras to create a dynamically manipulated, real-time therapy beam.[44] The treatment couch features electronic controls for 5 degrees of freedom (x, y, z, head tilt, and left-right rotation), as well as an additional manual rotation (clockwise or counterclockwise) based on the patient's position.[61]
An individualized treatment plan is developed to focus the radiation treatment as accurately as possible. A computerized treatment planning system is used and requires depth doses, tissue maximum ratio (the ratio of the dose at a given point in a phantom to the dose at the same point at the reference depth of maximum dose), off-axis factor, and collimator output factor for each stereotactic collimator.[57] Individual beams can be developed to conform to irregular shapes, minimizing radiation delivery and potential damage to normal brain tissue. Dose adjustment is according to the maximum dose that is deliverable to surrounding structures. Multiple lesions can be treated sequentially or simultaneously, depending on the type of device used.
Once quality assurance is guaranteed and an independent output check has been performed, the patient is positioned on the treatment couch, and the radiation treatment is delivered.[62] The treatment time varies from 30 minutes to 3 hours. On occasion, intravenous dexamethasone is administered to prevent complications caused by swelling from the radiation treatment that can be tapered posttreatment. Most patients are discharged within an hour after treatment and can resume normal activities within a few days.
Complications
SRS/SBRT causes vascular endothelial damage and demyelination of the white matter, leading to tissue necrosis.[63] Acute effects, which occur within weeks of treatment, are often due to cerebral edema resulting from disruption of the blood-brain barrier. These effects can also be caused by white matter tract damage following an injury to vascular endothelial cells, axonal demyelination, and coagulation necrosis, which can be permanent and may include progressive memory loss.[4] Symptoms include headache, nausea, and vomiting.[4] Subacute effects are due to diffuse demyelination and resolve within 6 months of treatment. Symptoms include somnolence and fatigue.[4] Late effects that occur 6 months after treatment are the result of damage to white matter tracts following an injury to vascular endothelial cells, axonal demyelination, and coagulation necrosis, which can be permanent and may include progressive memory loss.[4]
SRS has fewer adverse effects than WBRT.[64] An acute adverse effect commonly experienced after treatment is headache, with less frequent complications that include pin-site infections, seizures, and short-term exacerbations of neurological symptoms, affecting less than 5% of patients.[6][65] Late effects that occur in <5% of patients include radiation necrosis, brain edema, and new or worsening neurological deficits.[65][6] Systematic reviews suggest that the development of brain edema depends on the size of the tumor treatment margin, maximum treatment dose, tumor size/volume, lesion location (particularly parasagittal, parafalcine, or convexity), first surgical resection, and preexisting cerebral edema.[66] SRS can cause delayed cranial neuropathies, especially of the optic nerve and visual pathways.[67] Factors associated with the injury include the type of nerve involved, the maximum dose, and the length of the irradiated nerve.[67]
SBRT is emerging as a valuable treatment option. This technique has a lower rate of radiation necrosis than SRS and a reduced risk of radiation-induced optic nerve neuropathy.[59][60]
Clinical Significance
SRS is an invaluable treatment option for brain and spine metastases, primary brain tumors, vascular malformations, and functional disorders. As the techniques continue to develop, the clinical indications for treatment are expanding.
SRS is a superior treatment option to WBRT, which is associated with significant neurological complications, including neurocognitive impairment, which limits its use.[4] The advantage of SRS over surgery is its minimally invasive nature. The procedure is performed as an outpatient and does not require general anesthesia; multiple lesions can be treated in the same session. Lesions located in deep, surgically inaccessible areas or those in eloquent areas can be safely and successfully treated.
Enhancing Healthcare Team Outcomes
The successful delivery of SRS/SBRT depends on a coordinated interprofessional team approach involving physicians, advanced practitioners, nurses, pharmacists, radiation therapists, medical physicists, and dosimetrists. Each professional contributes specialized expertise throughout the care continuum, from pretreatment assessment to postprocedural follow-up. Physicians—particularly neurosurgeons and radiation oncologists—jointly evaluate clinical indications, determine treatment plans, and ensure alignment with surgical or systemic therapies. Advanced practitioners support clinical assessments and ongoing monitoring, often serving as critical liaisons between the patient and specialists. Neuroradiologists assist in precise imaging and target delineation, while dosimetrists and medical physicists collaborate to design and verify highly conformal treatment plans, ensuring both safety and efficacy. Effective team communication, real-time collaboration, and shared decision-making are essential for accurate execution and optimal patient-centered outcomes.
Radiation oncology nurses play a pivotal role in facilitating procedural and supportive care. Their responsibilities extend beyond technical assistance to include patient education, emotional support, and coordination of interprofessional efforts. Before treatment, nurses conduct comprehensive assessments, provide patients and their families with vital information, and serve as key communicators. During treatment, they assist with setup and immobilization, monitor patient status, and ensure comfort and safety throughout the session. Postprocedure, they manage discharge planning and address any patient concerns or complications.[68] This level of coordination requires seamless communication across all involved professionals, reinforcing safety, improving outcomes, and ensuring a high standard of care delivery. The success of SRS/SBRT rests not only on advanced technology but also on the cohesion and performance of the interprofessional team.
Nursing, Allied Health, and Interprofessional Team Monitoring
- Monitor the vital signs
- Assess post-procedural pain and communicate the pain score to the clinician
- Watch for any immediate adverse events after the procedure and get back to the clinician
- Communicate discharge orders to the patient and make sure they have the necessary contact information
- Communicate with the interprofessional team if any untoward changes in vital signs or any other issues develop
Media
References
Ganz JC. The journey from proton to gamma knife. Progress in brain research. 2014:215():67-75. doi: 10.1016/B978-0-444-63520-4.00007-7. Epub [PubMed PMID: 25376570]
Monaco EA, Grandhi R, Niranjan A, Lunsford LD. The past, present and future of Gamma Knife radiosurgery for brain tumors: the Pittsburgh experience. Expert review of neurotherapeutics. 2012 Apr:12(4):437-45. doi: 10.1586/ern.12.16. Epub [PubMed PMID: 22449215]
Trifiletti DM, Ruiz-Garcia H, Quinones-Hinojosa A, Ramakrishna R, Sheehan JP. The evolution of stereotactic radiosurgery in neurosurgical practice. Journal of neuro-oncology. 2021 Feb:151(3):451-459. doi: 10.1007/s11060-020-03392-0. Epub 2021 Feb 21 [PubMed PMID: 33611711]
Baschnagel A, Wolters PL, Camphausen K. Neuropsychological testing and biomarkers in the management of brain metastases. Radiation oncology (London, England). 2008 Sep 17:3():26. doi: 10.1186/1748-717X-3-26. Epub 2008 Sep 17 [PubMed PMID: 18798997]
Chen JC, Girvigian MR. Stereotactic radiosurgery: instrumentation and theoretical aspects-part 1. The Permanente journal. 2005 Fall:9(4):23-6 [PubMed PMID: 22811641]
Suh JH. Stereotactic radiosurgery for the management of brain metastases. The New England journal of medicine. 2010 Mar 25:362(12):1119-27. doi: 10.1056/NEJMct0806951. Epub [PubMed PMID: 20335588]
Szeifert GT, Atteberry DS, Kondziolka D, Levivier M, Lunsford LD. Cerebral metastases pathology after radiosurgery: a multicenter study. Cancer. 2006 Jun 15:106(12):2672-81 [PubMed PMID: 16700040]
Level 2 (mid-level) evidenceChang EL, Selek U, Hassenbusch SJ 3rd, Maor MH, Allen PK, Mahajan A, Sawaya R, Woo SY. Outcome variation among "radioresistant" brain metastases treated with stereotactic radiosurgery. Neurosurgery. 2005 May:56(5):936-45; discussion 936-45 [PubMed PMID: 15854241]
Level 2 (mid-level) evidenceBuss EJ, Wang TJC, Sisti MB. Stereotactic radiosurgery for management of vestibular schwannoma: a short review. Neurosurgical review. 2021 Apr:44(2):901-904. doi: 10.1007/s10143-020-01279-2. Epub 2020 Mar 13 [PubMed PMID: 32170501]
Gendreau JL, Sheaffer K, Macdonald N, Craft-Hacherl C, Abraham M, Patel NV, Herschman Y, Lindley JG. Stereotactic radiosurgery for cerebellopontine meningiomas: a systematic review and meta-analysis. British journal of neurosurgery. 2023 Apr:37(2):199-205. doi: 10.1080/02688697.2022.2064425. Epub 2022 Apr 27 [PubMed PMID: 35475408]
Level 1 (high-level) evidenceQiu J, Cai D, Yang F, Zhou J, Gong Y, Cai L, Gong K. Stereotactic radiosurgery for central nervous system hemangioblastoma in von Hippel-Lindau disease: A systematic review and meta-analysis. Clinical neurology and neurosurgery. 2020 Aug:195():105912. doi: 10.1016/j.clineuro.2020.105912. Epub 2020 May 15 [PubMed PMID: 32474257]
Level 1 (high-level) evidencePatchell RA. The management of brain metastases. Cancer treatment reviews. 2003 Dec:29(6):533-40 [PubMed PMID: 14585263]
Suh JH, Videtic GM, Aref AM, Germano I, Goldsmith BJ, Imperato JP, Marcus KJ, McDermott MW, McDonald MW, Patchell RA, Robins HI, Rogers CL, Wolfson AH, Wippold FJ 2nd, Gaspar LE. ACR Appropriateness Criteria: single brain metastasis. Current problems in cancer. 2010 May-Jun:34(3):162-74. doi: 10.1016/j.currproblcancer.2010.04.003. Epub [PubMed PMID: 20541055]
Marshall DC, Marcus LP, Kim TE, McCutcheon BA, Goetsch SJ, Koiso T, Alksne JF, Ott K, Carter BS, Hattangadi-Gluth JA, Yamamoto M, Chen CC. Management patterns of patients with cerebral metastases who underwent multiple stereotactic radiosurgeries. Journal of neuro-oncology. 2016 May:128(1):119-128. doi: 10.1007/s11060-016-2084-2. Epub 2016 Mar 7 [PubMed PMID: 26948673]
Aoyama H, Shirato H, Tago M, Nakagawa K, Toyoda T, Hatano K, Kenjyo M, Oya N, Hirota S, Shioura H, Kunieda E, Inomata T, Hayakawa K, Katoh N, Kobashi G. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA. 2006 Jun 7:295(21):2483-91 [PubMed PMID: 16757720]
Level 1 (high-level) evidenceManon R, O'Neill A, Knisely J, Werner-Wasik M, Lazarus HM, Wagner H, Gilbert M, Mehta M, Eastern Cooperative Oncology Group. Phase II trial of radiosurgery for one to three newly diagnosed brain metastases from renal cell carcinoma, melanoma, and sarcoma: an Eastern Cooperative Oncology Group study (E 6397). Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005 Dec 1:23(34):8870-6 [PubMed PMID: 16314647]
Chang EL, Wefel JS, Hess KR, Allen PK, Lang FF, Kornguth DG, Arbuckle RB, Swint JM, Shiu AS, Maor MH, Meyers CA. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. The Lancet. Oncology. 2009 Nov:10(11):1037-44. doi: 10.1016/S1470-2045(09)70263-3. Epub 2009 Oct 2 [PubMed PMID: 19801201]
Level 1 (high-level) evidenceKhan M, Lin J, Liao G, Tian Y, Liang Y, Li R, Liu M, Yuan Y. Whole Brain Radiation Therapy Plus Stereotactic Radiosurgery in the Treatment of Brain Metastases Leading to Improved Survival in Patients With Favorable Prognostic Factors. Frontiers in oncology. 2019:9():205. doi: 10.3389/fonc.2019.00205. Epub 2019 Mar 29 [PubMed PMID: 30984624]
Andrews DW, Scott CB, Sperduto PW, Flanders AE, Gaspar LE, Schell MC, Werner-Wasik M, Demas W, Ryu J, Bahary JP, Souhami L, Rotman M, Mehta MP, Curran WJ Jr. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet (London, England). 2004 May 22:363(9422):1665-72 [PubMed PMID: 15158627]
Level 1 (high-level) evidenceBrown PD, Jaeckle K, Ballman KV, Farace E, Cerhan JH, Anderson SK, Carrero XW, Barker FG 2nd, Deming R, Burri SH, Ménard C, Chung C, Stieber VW, Pollock BE, Galanis E, Buckner JC, Asher AL. Effect of Radiosurgery Alone vs Radiosurgery With Whole Brain Radiation Therapy on Cognitive Function in Patients With 1 to 3 Brain Metastases: A Randomized Clinical Trial. JAMA. 2016 Jul 26:316(4):401-409. doi: 10.1001/jama.2016.9839. Epub [PubMed PMID: 27458945]
Level 1 (high-level) evidenceMansouri A, Guha D, Klironomos G, Larjani S, Zadeh G, Kondziolka D. Stereotactic radiosurgery for intracranial meningiomas: current concepts and future perspectives. Neurosurgery. 2015 Apr:76(4):362-71. doi: 10.1227/NEU.0000000000000633. Epub [PubMed PMID: 25599213]
Level 3 (low-level) evidenceKirkpatrick JP, Soltys SG, Lo SS, Beal K, Shrieve DC, Brown PD. The radiosurgery fractionation quandary: single fraction or hypofractionation? Neuro-oncology. 2017 Apr 1:19(suppl_2):ii38-ii49. doi: 10.1093/neuonc/now301. Epub [PubMed PMID: 28380634]
Murphy ES, Suh JH. Radiotherapy for vestibular schwannomas: a critical review. International journal of radiation oncology, biology, physics. 2011 Mar 15:79(4):985-97. doi: 10.1016/j.ijrobp.2010.10.010. Epub [PubMed PMID: 21353158]
Persson O, Bartek J Jr, Shalom NB, Wangerid T, Jakola AS, Förander P. Stereotactic radiosurgery vs. fractionated radiotherapy for tumor control in vestibular schwannoma patients: a systematic review. Acta neurochirurgica. 2017 Jun:159(6):1013-1021. doi: 10.1007/s00701-017-3164-6. Epub 2017 Apr 13 [PubMed PMID: 28409393]
Level 1 (high-level) evidenceLi X, Li Y, Cao Y, Li P, Liang B, Sun J, Feng E. Safety and efficacy of fractionated stereotactic radiotherapy and stereotactic radiosurgery for treatment of pituitary adenomas: A systematic review and meta-analysis. Journal of the neurological sciences. 2017 Jan 15:372():110-116. doi: 10.1016/j.jns.2016.11.024. Epub 2016 Nov 15 [PubMed PMID: 28017195]
Level 1 (high-level) evidenceChen Y, Li ZF, Zhang FX, Li JX, Cai L, Zhuge QC, Wu ZB. Gamma knife surgery for patients with volumetric classification of nonfunctioning pituitary adenomas: a systematic review and meta-analysis. European journal of endocrinology. 2013 Oct:169(4):487-95. doi: 10.1530/EJE-13-0400. Epub 2013 Sep 14 [PubMed PMID: 23904281]
Level 1 (high-level) evidenceCohen-Inbar O. [RADIOSURGERY FOR PITUITARY ADENOMAS]. Harefuah. 2017 Jan:156(1):45-50 [PubMed PMID: 28530316]
Cuneo KC, Vredenburgh JJ, Sampson JH, Reardon DA, Desjardins A, Peters KB, Friedman HS, Willett CG, Kirkpatrick JP. Safety and efficacy of stereotactic radiosurgery and adjuvant bevacizumab in patients with recurrent malignant gliomas. International journal of radiation oncology, biology, physics. 2012 Apr 1:82(5):2018-24. doi: 10.1016/j.ijrobp.2010.12.074. Epub 2011 Apr 12 [PubMed PMID: 21489708]
Level 2 (mid-level) evidencePark KJ, Kano H, Iyer A, Liu X, Niranjan A, Flickinger JC, Lieberman FS, Lunsford LD, Kondziolka D. Salvage gamma knife stereotactic radiosurgery followed by bevacizumab for recurrent glioblastoma multiforme: a case-control study. Journal of neuro-oncology. 2012 Apr:107(2):323-33. doi: 10.1007/s11060-011-0744-9. Epub 2011 Nov 5 [PubMed PMID: 22057917]
Level 2 (mid-level) evidenceGraffeo CS, Kotecha R, Sahgal A, Fariselli L, Gorgulho A, Levivier M, Ma L, Paddick I, Regis J, Sheehan JP, Suh JH, Yomo S, Pollock BE. Stereotactic Radiosurgery for Intermediate (III) or High (IV-V) Spetzler-Martin Grade Arteriovenous Malformations: International Stereotactic Radiosurgery Society Practice Guideline. Neurosurgery. 2025 Feb 1:96(2):298-307. doi: 10.1227/neu.0000000000003102. Epub 2024 Jul 11 [PubMed PMID: 38989995]
Level 1 (high-level) evidenceTos SM, Shaaban A, Mantziaris G, Dumot C, Kotecha R, Fariselli L, Gorgulho A, Levivier M, Ma L, Paddick I, Pollock BE, Regis J, Suh JH, Yomo S, Sahgal A, Sheehan JP. Stereotactic Radiosurgery for Intracranial Cavernous Malformations: International Stereotactic Radiosurgery Society, Systematic Review, Meta-Analysis, and Practice Guidelines. World neurosurgery. 2024 Dec:192():e366-e401. doi: 10.1016/j.wneu.2024.09.106. Epub 2024 Oct 18 [PubMed PMID: 39341276]
Level 1 (high-level) evidenceTos SM, Mantziaris G, Shaaban A, Sheehan JP. Stereotactic radiosurgery for intracranial cavernous malformations of the deep-seated locations: systematic review and meta-analysis. Neurosurgical review. 2024 Apr 24:47(1):186. doi: 10.1007/s10143-024-02434-9. Epub 2024 Apr 24 [PubMed PMID: 38653844]
Level 1 (high-level) evidenceDing D, Yen CP, Xu Z, Starke RM, Sheehan JP. Radiosurgery for low-grade intracranial arteriovenous malformations. Journal of neurosurgery. 2014 Aug:121(2):457-67. doi: 10.3171/2014.1.JNS131713. Epub 2014 Mar 7 [PubMed PMID: 24605839]
Level 2 (mid-level) evidenceDing D, Yen CP, Starke RM, Xu Z, Sun X, Sheehan JP. Radiosurgery for Spetzler-Martin Grade III arteriovenous malformations. Journal of neurosurgery. 2014 Apr:120(4):959-69. doi: 10.3171/2013.12.JNS131041. Epub 2014 Jan 24 [PubMed PMID: 24460487]
Patibandla MR, Ding D, Kano H, Xu Z, Lee JYK, Mathieu D, Whitesell J, Pierce JT, Huang PP, Kondziolka D, Feliciano C, Rodriguez-Mercado R, Almodovar L, Grills IS, Silva D, Abbassy M, Missios S, Barnett GH, Lunsford LD, Sheehan JP. Stereotactic radiosurgery for Spetzler-Martin Grade IV and V arteriovenous malformations: an international multicenter study. Journal of neurosurgery. 2018 Aug:129(2):498-507. doi: 10.3171/2017.3.JNS162635. Epub 2017 Sep 8 [PubMed PMID: 28885118]
Level 2 (mid-level) evidenceMoosa S, Chen CJ, Ding D, Lee CC, Chivukula S, Starke RM, Yen CP, Xu Z, Sheehan JP. Volume-staged versus dose-staged radiosurgery outcomes for large intracranial arteriovenous malformations. Neurosurgical focus. 2014 Sep:37(3):E18. doi: 10.3171/2014.5.FOCUS14205. Epub [PubMed PMID: 25175437]
Level 1 (high-level) evidenceDalyai RT, Ghobrial G, Chalouhi N, Dumont AS, Tjoumakaris S, Gonzalez LF, Rosenwasser R, Jabbour P. Radiosurgery for dural arterio-venous fistulas: a review. Clinical neurology and neurosurgery. 2013 May:115(5):512-6. doi: 10.1016/j.clineuro.2013.01.020. Epub 2013 Mar 5 [PubMed PMID: 23481896]
Frighetto L, Bizzi J, Annes RD, Silva Rdos S, Oppitz P. Stereotactic radiosurgery for movement disorders. Surgical neurology international. 2012:3(Suppl 1):S10-6. doi: 10.4103/2152-7806.91605. Epub 2012 Jan 14 [PubMed PMID: 22826805]
Pollock BE, Foote RL, Stafford SL, Link MJ, Gorman DA, Schomberg PJ. Results of repeated gamma knife radiosurgery for medically unresponsive trigeminal neuralgia. Journal of neurosurgery. 2000 Dec:93 Suppl 3():162-4 [PubMed PMID: 11143237]
Ma L, Kwok Y, Chin LS, Yu C, Regine WF. Comparative analyses of linac and Gamma Knife radiosurgery for trigeminal neuralgia treatments. Physics in medicine and biology. 2005 Nov 21:50(22):5217-27 [PubMed PMID: 16264249]
Level 2 (mid-level) evidenceQuigg M, Harden C. Minimally invasive techniques for epilepsy surgery: stereotactic radiosurgery and other technologies. Journal of neurosurgery. 2014 Dec:121 Suppl():232-40. doi: 10.3171/2014.8.GKS141608. Epub [PubMed PMID: 25434958]
Quigg M, Rolston J, Barbaro NM. Radiosurgery for epilepsy: clinical experience and potential antiepileptic mechanisms. Epilepsia. 2012 Jan:53(1):7-15. doi: 10.1111/j.1528-1167.2011.03339.x. Epub 2011 Dec 22 [PubMed PMID: 22191545]
Barbaro NM, Quigg M, Ward MM, Chang EF, Broshek DK, Langfitt JT, Yan G, Laxer KD, Cole AJ, Sneed PK, Hess CP, Yu W, Tripathi M, Heck CN, Miller JW, Garcia PA, McEvoy A, Fountain NB, Salanova V, Knowlton RC, Bagić A, Henry T, Kapoor S, McKhann G, Palade AE, Reuber M, Tecoma E. Radiosurgery versus open surgery for mesial temporal lobe epilepsy: The randomized, controlled ROSE trial. Epilepsia. 2018 Jun:59(6):1198-1207. doi: 10.1111/epi.14045. Epub 2018 Mar 30 [PubMed PMID: 29600809]
Level 1 (high-level) evidenceBenedict SH, Bova FJ, Clark B, Goetsch SJ, Hinson WH, Leavitt DD, Schlesinger DJ, Yenice KM. Anniversary Paper: the role of medical physicists in developing stereotactic radiosurgery. Medical physics. 2008 Sep:35(9):4262-77 [PubMed PMID: 18841876]
Ryu S, Deshmukh S, Timmerman RD, Movsas B, Gerszten P, Yin FF, Dicker A, Abraham CD, Zhong J, Shiao SL, Tuli R, Desai A, Mell LK, Iyengar P, Hitchcock YJ, Allen AM, Burton S, Brown D, Sharp HJ, Dunlap NE, Siddiqui MS, Chen TH, Pugh SL, Kachnic LA. Stereotactic Radiosurgery vs Conventional Radiotherapy for Localized Vertebral Metastases of the Spine: Phase 3 Results of NRG Oncology/RTOG 0631 Randomized Clinical Trial. JAMA oncology. 2023 Jun 1:9(6):800-807. doi: 10.1001/jamaoncol.2023.0356. Epub [PubMed PMID: 37079324]
Level 1 (high-level) evidenceHarel R, Zach L. Spine radiosurgery for spinal metastases: indications, technique and outcome. Neurological research. 2014 Jun:36(6):550-6. doi: 10.1179/1743132814Y.0000000364. Epub 2014 Apr 13 [PubMed PMID: 24725288]
Purvis TE, Goodwin CR, Lubelski D, Laufer I, Sciubba DM. Review of stereotactic radiosurgery for intradural spine tumors. CNS oncology. 2017 Apr:6(2):131-138. doi: 10.2217/cns-2016-0039. Epub [PubMed PMID: 28425771]
Franzese C, Louie AV, Kotecha R, Zhang Z, Guckenberger M, Kim MS, Tree AC, Slotman BJ, Sahgal A, Scorsetti M. Stereotactic Body Radiation therapy for Liver Metastases: Systematic Review and Meta-Analysis With International Stereotactic Radiosurgery Society (ISRS) Practice Guidelines. Practical radiation oncology. 2025 Mar-Apr:15(2):e172-e188. doi: 10.1016/j.prro.2024.09.011. Epub 2024 Oct 16 [PubMed PMID: 39419281]
Level 1 (high-level) evidenceChen H, Louie AV, Higginson DS, Palma DA, Colaco R, Sahgal A. Stereotactic Radiosurgery and Stereotactic Body Radiotherapy in the Management of Oligometastatic Disease. Clinical oncology (Royal College of Radiologists (Great Britain)). 2020 Nov:32(11):713-727. doi: 10.1016/j.clon.2020.06.018. Epub 2020 Jul 24 [PubMed PMID: 32718762]
Shaw E, Scott C, Souhami L, Dinapoli R, Kline R, Loeffler J, Farnan N. Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: final report of RTOG protocol 90-05. International journal of radiation oncology, biology, physics. 2000 May 1:47(2):291-8 [PubMed PMID: 10802351]
Souhami L, Seiferheld W, Brachman D, Podgorsak EB, Werner-Wasik M, Lustig R, Schultz CJ, Sause W, Okunieff P, Buckner J, Zamorano L, Mehta MP, Curran WJ Jr. Randomized comparison of stereotactic radiosurgery followed by conventional radiotherapy with carmustine to conventional radiotherapy with carmustine for patients with glioblastoma multiforme: report of Radiation Therapy Oncology Group 93-05 protocol. International journal of radiation oncology, biology, physics. 2004 Nov 1:60(3):853-60 [PubMed PMID: 15465203]
Level 1 (high-level) evidenceTsao MN, Mehta MP, Whelan TJ, Morris DE, Hayman JA, Flickinger JC, Mills M, Rogers CL, Souhami L. The American Society for Therapeutic Radiology and Oncology (ASTRO) evidence-based review of the role of radiosurgery for malignant glioma. International journal of radiation oncology, biology, physics. 2005 Sep 1:63(1):47-55 [PubMed PMID: 16111571]
Lee N, Puri DR, Blanco AI, Chao KS. Intensity-modulated radiation therapy in head and neck cancers: an update. Head & neck. 2007 Apr:29(4):387-400 [PubMed PMID: 16358297]
Alfonso R, Andreo P, Capote R, Huq MS, Kilby W, Kjäll P, Mackie TR, Palmans H, Rosser K, Seuntjens J, Ullrich W, Vatnitsky S. A new formalism for reference dosimetry of small and nonstandard fields. Medical physics. 2008 Nov:35(11):5179-86 [PubMed PMID: 19070252]
Miller DW. A review of proton beam radiation therapy. Medical physics. 1995 Nov:22(11 Pt 2):1943-54 [PubMed PMID: 8587548]
Perks JR, St George EJ, El Hamri K, Blackburn P, Plowman PN. Stereotactic radiosurgery XVI: Isodosimetric comparison of photon stereotactic radiosurgery techniques (gamma knife vs. micromultileaf collimator linear accelerator) for acoustic neuroma--and potential clinical importance. International journal of radiation oncology, biology, physics. 2003 Dec 1:57(5):1450-9 [PubMed PMID: 14630285]
Heydarian M, Hoban PW, Beddoe AH. A comparison of dosimetry techniques in stereotactic radiosurgery. Physics in medicine and biology. 1996 Jan:41(1):93-110 [PubMed PMID: 8685261]
Das IJ, Ding GX, Ahnesjö A. Small fields: nonequilibrium radiation dosimetry. Medical physics. 2008 Jan:35(1):206-15 [PubMed PMID: 18293576]
Lehrer EJ, Peterson JL, Zaorsky NG, Brown PD, Sahgal A, Chiang VL, Chao ST, Sheehan JP, Trifiletti DM. Single versus Multifraction Stereotactic Radiosurgery for Large Brain Metastases: An International Meta-analysis of 24 Trials. International journal of radiation oncology, biology, physics. 2019 Mar 1:103(3):618-630. doi: 10.1016/j.ijrobp.2018.10.038. Epub 2018 Nov 2 [PubMed PMID: 30395902]
Level 1 (high-level) evidenceMilano MT, Grimm J, Soltys SG, Yorke E, Moiseenko V, Tomé WA, Sahgal A, Xue J, Ma L, Solberg TD, Kirkpatrick JP, Constine LS, Flickinger JC, Marks LB, El Naqa I. Single- and Multi-Fraction Stereotactic Radiosurgery Dose Tolerances of the Optic Pathways. International journal of radiation oncology, biology, physics. 2021 May 1:110(1):87-99. doi: 10.1016/j.ijrobp.2018.01.053. Epub 2018 Jan 31 [PubMed PMID: 29534899]
Kuo JS, Yu C, Petrovich Z, Apuzzo ML. The CyberKnife stereotactic radiosurgery system: description, installation, and an initial evaluation of use and functionality. Neurosurgery. 2008 Feb:62 Suppl 2():785-9. doi: 10.1227/01.neu.0000316282.07124.31. Epub [PubMed PMID: 18596427]
Shaw E, Kline R, Gillin M, Souhami L, Hirschfeld A, Dinapoli R, Martin L. Radiation Therapy Oncology Group: radiosurgery quality assurance guidelines. International journal of radiation oncology, biology, physics. 1993 Dec 1:27(5):1231-9 [PubMed PMID: 8262852]
Level 1 (high-level) evidenceTofilon PJ, Fike JR. The radioresponse of the central nervous system: a dynamic process. Radiation research. 2000 Apr:153(4):357-70 [PubMed PMID: 10798963]
Schimmel WCM, Gehring K, Eekers DBP, Hanssens PEJ, Sitskoorn MM. Cognitive effects of stereotactic radiosurgery in adult patients with brain metastases: A systematic review. Advances in radiation oncology. 2018 Oct-Dec:3(4):568-581. doi: 10.1016/j.adro.2018.06.003. Epub 2018 Jul 11 [PubMed PMID: 30370357]
Level 3 (low-level) evidenceSolberg TD, Balter JM, Benedict SH, Fraass BA, Kavanagh B, Miyamoto C, Pawlicki T, Potters L, Yamada Y. Quality and safety considerations in stereotactic radiosurgery and stereotactic body radiation therapy: Executive summary. Practical radiation oncology. 2012 Jan-Mar:2(1):2-9. doi: 10.1016/j.prro.2011.06.014. Epub 2011 Sep 15 [PubMed PMID: 25740120]
Level 2 (mid-level) evidenceMilano MT, Sharma M, Soltys SG, Sahgal A, Usuki KY, Saenz JM, Grimm J, El Naqa I. Radiation-Induced Edema After Single-Fraction or Multifraction Stereotactic Radiosurgery for Meningioma: A Critical Review. International journal of radiation oncology, biology, physics. 2018 Jun 1:101(2):344-357. doi: 10.1016/j.ijrobp.2018.03.026. Epub 2018 Mar 29 [PubMed PMID: 29726362]
Stafford SL, Pollock BE, Leavitt JA, Foote RL, Brown PD, Link MJ, Gorman DA, Schomberg PJ. A study on the radiation tolerance of the optic nerves and chiasm after stereotactic radiosurgery. International journal of radiation oncology, biology, physics. 2003 Apr 1:55(5):1177-81 [PubMed PMID: 12654424]
Law E, Mangarin E, Kelvin JF. Nursing management of patients receiving stereotactic radiosurgery. Clinical journal of oncology nursing. 2003 Jul-Aug:7(4):387-92 [PubMed PMID: 12929271]