Introduction
Asthma is a chronic inflammatory airway disease marked by recurrent wheezing, dyspnea, chest pain, and coughing (see Image. Pathophysiology of Asthma). Recommended treatments for mild-to-moderate asthma exacerbations include β2-agonists and corticosteroids. Patients with status asthmaticus are typically refractory to these standard therapies and may require intravenous medications, continuous nebulizer treatments, and mechanical ventilation.
Status asthmaticus is a medical emergency characterized by hypoxemia, hypercarbia, and secondary respiratory failure. The condition may progress to acute respiratory failure, end-organ dysfunction, and death if not promptly recognized and treated aggressively.
No single clinical or diagnostic index reliably predicts the outcomes of severe asthma exacerbations. A multipronged approach combining thorough clinical evaluation, judicious diagnostic testing, and rapid symptom management optimizes outcomes in these patients.
Anatomy of the Normal Lung
Embryologically, the respiratory system arises as an outgrowth of the ventral foregut. The trachea develops at the midline and gives rise to the lung buds. The right lung bud divides into 3 main bronchi, while the left lung bud divides into 2. Both main bronchi branch into progressively smaller airways, forming bronchioles, lobules, terminal bronchioles, acini, respiratory bronchioles, alveolar ducts, alveolar sacs, and alveoli. Several terminal bronchioles constitute a pulmonary lobule. Pulmonary and bronchial arteries accompany the airway and lung parenchymal branching.
The proximal airways conduct air to the distal lung regions. Cartilaginous tissue supports these airways to maintain patency. Pseudostratified columnar ciliated epithelium lines the larynx, trachea, and bronchi. Neuroendocrine cells in the bronchial mucosa secrete serotonin, calcitonin, and bombesin. Tracheal and bronchial walls also contain submucosal mucous glands.
Distal airways have progressively smaller diameters. Cartilage and submucosal mucus glands disappear at the level of the bronchioles. Acini, composed of respiratory bronchioles, are spherical structures distal to the terminal bronchioles and measure approximately 7 mm in diameter. Alveolar ducts arise from the respiratory bronchioles and branch into alveolar sacs. Alveoli develop from respiratory bronchioles, alveolar ducts, or alveolar sacs.
The alveolar anatomy is specialized for gas exchange. The blood-air barrier consists of the capillary epithelium, basement membrane, interstitial tissue, alveolar epithelium, and alveolar macrophages. Type I pneumocytes are flat epithelial cells constituting 95% of the alveolar surface. The remaining epithelium comprises Type II pneumocytes, rounded cells that secrete pulmonary surfactant and repair the alveolar epithelium following Type I pneumocyte injury. Pores of Kohn in the alveolar walls connect adjacent alveoli.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Asthma is a chronic inflammatory disorder of the airways characterized by recurrent episodes of breathing difficulty resulting from airway inflammation and bronchospasm. The condition is associated with airway hyperreactivity to certain environmental stimuli and occurs more commonly in individuals with atopy.[1]
Two distinct patterns of asthma exacerbations can lead to status asthmaticus. Slow-onset asthma exacerbation involves a gradual worsening of peak expiratory flow rate (PEFR) over several days. Predisposing patient-related factors include inadequate inhaler regimens, suboptimal compliance, and psychological stressors. Sudden-onset asthma exacerbation presents as a rapid, severe decline within hours, often triggered by abrupt massive exposure to allergens, food particles, or sulfites.[2]
Approximately 80% to 85% of asthma-related fatalities result from slow-onset exacerbations, reflecting prolonged inadequate disease control. These patients typically exhibit extensive airway inflammation and mucus plugging, complicating acute management. In contrast, sudden-onset exacerbations generally involve clear airways with predominant bronchospasm.[3]
Epidemiology
Asthma prevalence varies by country, sex, ethnicity, and socioeconomic status. The overall worldwide prevalence of asthma is estimated at approximately 4%. According to the World Health Organization (WHO), the highest prevalence occurs in Australia, Sweden, the U.K., the Netherlands, and Brazil. China and Vietnam report some of the lowest asthma rates. Highly developed countries generally exhibit higher reported asthma rates, which may reflect better healthcare access and greater asthma awareness. Reported rates may also be artificially low in regions lacking robust reporting infrastructure.[4]
Centers for Disease Control and Prevention (CDC) data indicate that the prevalence of current asthma diagnosis in the U.S. ranges from 8% to 9% in adults and 6% to 7% in children younger than 18 years. (Sources: CDC Interactive Summary Health Statistics for Adults, 2025; CDC Interactive Summary Health Statistics for Children, 2025) Additionally, 10.4% of people with asthma in the U.S. live below the 100% poverty threshold. Black and American Indian (Alaska Native) populations have the highest prevalence rates, at 10.9% and 12.3%, respectively.
Certain populations face a higher risk of severe asthma and status asthmaticus. Multiple observational studies report increased incidence of status asthmaticus among women, African Americans, and individuals with asthma onset after 17 years of age.[5][6] Status asthmaticus is more prevalent in people with lower socioeconomic status, likely due to limited access to specialist care.[7] Individuals living alone are also disproportionately affected. An estimated 3% to 16% of hospitalized adult asthmatic patients develop respiratory failure requiring ventilatory support. Less than 1% of children with status asthmaticus in the U.S. require intubation and mechanical ventilation.
The mortality rate associated with status asthmaticus is decreasing in many regions worldwide. Advances in therapies, including tidal-volume ventilation standardization, avoidance of prolonged neuromuscular blockade, and implementation of assist-control mode ventilation, have contributed to reduced mortality. Older adults exhibit the highest mortality rates compared to other age groups.[8] Afessa et al reported approximately 10% mortality in patients with status asthmaticus admitted to intensive care units (ICUs).[9] Mortality rates for status asthmaticus in children remain below 1% in most regions.[10]
Pathophysiology
Asthma arises from chronic inflammation of the lower airways, which may be mediated by genetic, environmental, or microbial factors.[11] Inflammation can lead to expiratory collapse of small airways due to alterations in laminar flow and airway diameter. Premature airway closure during exhalation increases functional residual capacity and causes air trapping. The heterogeneous distribution of air trapping results in ventilation-perfusion mismatch, leading to hypoxemia, cellular anaerobic metabolism, and lactic acidosis. Respiratory alkalosis from tachypnea initially offsets the metabolic acidosis caused by cellular hypoxia. Respiratory acidosis may develop as the work of breathing decreases with fatigue.
Acute asthma consists of 2 physiological phases. The early bronchospastic phase occurs minutes after allergen or trigger exposure, involving mast cell degranulation and release of inflammatory mediators such as histamine, prostaglandin D2, and leukotriene C4. The latter inflammatory phase results in airway edema due to eosinophilic release of cationic and major basic proteins.[12] Type 2 inflammation appears to increase the risk of acute asthma exacerbation. This process involves the release of interleukins 4, 5, and 13 from T-helper type 2 cells and type 2 innate lymphoid cells. Non-type 2 inflammation, characterized by neutrophil involvement, also likely plays a significant role in asthma exacerbations.[13]
Cellular-level changes play a key role in the development of asthma symptoms. Destruction of cilia and epithelial denudation increase nerve ending irritability, resulting in hyperreactivity. Inflammation causes hypertrophy and hyperfunction of goblet cells and mucous glands, promoting mucus plugging within the airways. Dysregulated parasympathetic activity contributes to cellular pathology through pulmonary vagal branches and parasympathetic ganglia in small bronchi. Stimulation of postganglionic acetylcholine muscarinic receptors causes bronchoconstriction and hypersecretion. Dysfunction of inhibitory M2 receptors, commonly seen in individuals with atopy, sustained allergen exposure, viral infection, and chronic inflammation, further enhances parasympathetic activity.
Histopathology
Histopathological examination of the airways reveals inflammation, smooth muscle contraction, and airway hyperresponsiveness. Interactions among mast cells, T lymphocytes, and epithelial cells trigger a surge of inflammatory cells and cytokines that accumulate in lung tissue. Increased concentrations of histamines, leukotrienes, and platelet-activating factors are observed both locally and systemically. Lymphocytic and eosinophilic submucosal infiltrates in tracheal and bronchial biopsy specimens are associated with poorer outcomes in adult patients with asthma.[14]
History and Physical
Patients with an asthma exacerbation most often present with shortness of breath, cough, or chest tightness. Some report increased inhaler or nebulizer use within the preceding hours to days. Fever, upper respiratory tract infection symptoms, and chest pain may also occur. Key historical information includes comorbidities, current medications, known allergic or environmental triggers, and illicit substance use. Clinicians should determine whether the patient has ever required hospitalization or intubation for an asthma exacerbation.
Several risk factors predispose patients to status asthmaticus.[15][16] Poor perception of dyspnea and hypercapnia may delay treatment initiation, as may a blunted hypoxic ventilatory response from longstanding pulmonary disease or psychiatric illness.[17][18] Recurrent hospitalizations despite chronic oral steroid use, delayed presentation after symptom onset, altered mental status, and sleep deprivation during an exacerbation also increase the risk.
On physical examination, patients are typically tachycardic and tachypneic with increased work of breathing. Wheezing on pulmonary auscultation is often considered an indicator of bronchospasm but is unreliable in severe asthma exacerbations, as it may be absent when alveolar airflow is significantly impaired. The absence of wheezing should not be relied upon diagnostically, particularly in patients in extremis with a history of asthma.[19][20]
Tachycardia in the setting of asthma exacerbations may result from respiratory distress or β2-agonist administration. Sinus tachycardia is the most common rhythm, although supraventricular and ventricular arrhythmias have also been reported.
Brenner et al demonstrated that patients presenting with an asthma exacerbation who assumed an upright rather than supine position had significantly higher heart and respiratory rates than those who did not. Other findings in these patients included pulsus paradoxus, significantly lower arterial oxygen partial pressure (PaO2), and a lower PEFR. After initial treatment, diaphoresis, preference to sit upright, inability to speak in complete sentences, and accessory muscle use all suggest progression to status asthmaticus, which may result in progressive respiratory and mental status decline that eventually causes patients to rest supine. Patient positioning should not be relied upon alone for diagnostic or prognostic purposes.
Status asthmaticus causes marked respiratory-phase variation in pleural pressure that contributes to hemodynamic compromise. Increased inspiratory effort against an obstructed airway augments negative intrathoracic pressure, reducing left ventricular filling and cardiac output through the following mechanisms:
- Septal deviation to the left from right ventricular enlargement
- Increased left ventricular afterload
- Increased right ventricular afterload from elevated pulmonary arterial pressure
Obstructive shock may develop from bronchospasm and air trapping, which decrease venous return secondary to increased intrathoracic pressure. In addition to respiratory distress, affected patients may have altered mental status, cool skin with poor capillary refill, diminished peripheral pulses, and hypotension. Pulsus paradoxus, defined as a decrease in systolic blood pressure of at least 10 mm Hg during inspiration, may be present. In advanced stages, the magnitude of pulsus paradoxus may decline due to reduced respiratory effort.[21] Patients with preexisting cardiac disease may be unable to compensate for the hemodynamic dysfunction of status asthmaticus and are more likely to develop shock.
Evaluation
Patients should undergo continuous cardiac monitoring, pulse oximetry, and blood pressure monitoring. Vital signs should be recorded at least every 15 min during stabilization, as patients with status asthmaticus may rapidly deteriorate. Mental status should be monitored closely, since neurological decline indicates inadequate oxygenation and ventilation that may require mechanical support.
Airflow obstruction is best quantified by measuring PEFR and forced expiratory volume in 1 second (FEV1).[22] PEFR may be measured at the bedside in patients without altered mental status. A reduction in both values by 50% or greater from the patient's personal best indicates severe airway obstruction. An absolute PEFR less than 120 LPM and an absolute FEV1 less than 1 L indicate severe disease.[23]
Laboratory tests and imaging help identify treatable asthma triggers, assess disease severity, and evaluate alternative diagnoses. Initial blood tests may include a complete blood count (CBC), basic metabolic panel (BMP), and arterial blood gas (ABG) analysis. In status asthmaticus, CBC may show leukocytosis from inflammation with neutrophil demargination or superimposed infection. Marked neutrophilia is often associated with bacterial infection. Viral infections may present with leukocytosis or leukopenia, accompanied by lymphocytosis or mild lymphopenia. Infection may be confirmed with a nasopharyngeal swab, sputum test, or blood culture, depending on the suspected organism.
BMP evaluates electrolyte balance and kidney function. Dyspnea-related insensible fluid losses and poor oral intake can cause electrolyte abnormalities, dehydration, and prerenal acute kidney injury. Cardiac enzymes and electrocardiography (ECG) assess for primary or secondary acute cardiac pathology. Pulmonary obstruction may produce transient ECG signs of right heart strain, such as peaked P waves or right axis deviation. ECG may also show sinus tachycardia or dysrhythmias related to the underlying pathology or β2-agonist therapy.
Chest radiography may be normal or show lung hyperinflation with airway wall thickening in poorly controlled asthma or severe exacerbations (see Image. Asthma Radiography). X-rays may also reveal other causes of symptoms, including bronchiectasis, pneumonia, pleural effusion, pulmonary edema, or pneumothorax. Radiography should be considered in patients with poor treatment response or abnormal lung sounds such as rhonchi, rales, or absent breath sounds, which suggest alternative diagnoses.
Point-of-care ultrasound provides rapid bedside assessment of critically ill patients in respiratory distress. Severe asthma exacerbations may show reduced lung sliding. Lung and cardiac ultrasound can also identify pulmonary edema, consolidations, pleural or pericardial effusions, and abnormal cardiac wall motion, indicating other pathology. Ultrasound can assess fluid responsiveness and hemodynamic status in shock.[24]
ABG analysis yields essential information regarding oxygenation and ventilation, but should be interpreted alongside clinical appearance. Mild or moderate exacerbations typically cause respiratory alkalosis from tachypnea. Hypoxemia develops as severity increases. Carbon dioxide partial pressure (pCO2) may falsely normalize as fatigue reduces respiratory effort (see Table. Arterial Blood Gas Findings in Acute Asthma). Hypercarbia and hypoxemia indicate impending respiratory failure, and such patients may require mechanical ventilation.[25]
Table. Arterial Blood Gas Findings in Acute Asthma
|
Clinical State |
pH Status |
Partial Pressure of Carbon Dioxide (pCO2) |
Partial Pressure of Oxygen (pO2) |
|
Early or mild asthma exacerbation |
Respiratory alkalosis |
Decreased |
Normal |
|
Moderate exacerbation |
Respiratory alkalosis |
Moderately decreased |
Decreased |
|
Impending respiratory failure (status asthmaticus) |
Normal |
Normal |
Moderately decreased |
|
Impending respiratory arrest |
Respiratory acidosis |
Increased |
Severely decreased |
Outpatient testing for patients with severe chronic asthma may include pulmonary function and allergen testing. Immunoglobulin E levels, skin tests, and radioallergosorbent tests are useful in evaluating patients with suspected allergen triggers. Identifying and avoiding allergens may improve long-term asthma control.
Treatment / Management
Pharmacological Interventions for Bronchoconstriction
Reducing bronchospasm and improving oxygenation and ventilation are the goals of asthma exacerbation treatment. Several classes of pharmacological agents are used to reduce lower airway obstruction.
β2-agonists
Short-acting inhaled β2-agonists are the 1st-line treatment for acute asthma exacerbations. Albuterol's β2 selectivity and duration of action make it the preferred agent in this class. Patient factors such as preexisting bronchoconstriction, airway inflammation, mucus plugging, and poor effort may reduce efficacy and duration of effect, particularly if patients cannot coordinate breaths with inhaler deployment. Larger and more frequent dosing may be necessary in the presence of these factors. Administration via nebulization or the use of a spacer with a metered-dose inhaler (MDI) can enhance medication delivery to the lungs.
The optimal delivery device of inhaled β2-agonists in patients without severe distress or altered mental status has, at times, been controversial. McIntyre and colleagues demonstrated that only 2.9% of a radioactive aerosol was deposited in the lungs when delivered by a small-volume nebulizer. The group advocated for the use of MDI via an adapter attached to the inspiratory limb of the ventilator circuit.
However, a subsequent study by Manthous and colleagues refuted these findings, demonstrating MDI's lesser effect on inspiratory flow-resistive pressure compared with nebulized albuterol.[26] Patients, nurses, and providers often perceive nebulizer treatments as more effective than MDIs despite evidence suggesting that they confer similar therapeutic benefits with proper use.[27]
Nebulization is often favored in emergency settings because it requires less supervision, patient breath coordination, and repeated instruction. This modality is also preferred in young children or other patients who cannot take deep breaths on command. Patients requiring invasive or noninvasive mechanical ventilation also typically receive albuterol via nebulization in line with the device circuit.
Initial nebulized albuterol treatment consists of 2.5 mg of albuterol (0.5 mL of a 0.5% solution in 2.5 mL normal saline) by nebulization every 20 min for 3 doses, followed by hourly treatments during the first several hours of therapy. This intervention may also be administered as hour-long or continuous nebulization treatments in severe exacerbations. Even in patients with severe disease, 4 puffs of albuterol (0.36 mg) delivered with an MDI and spacer may be as effective as a 2.5 mg dose by nebulization.
Some studies have shown a correlation between asthma mortality and inhaled β2-agonist use. This finding may reflect a paradoxical increase in airway hyperresponsiveness or the severity of underlying disease rather than adverse effects of the agents themselves.[28][29] Short-acting inhaled β2-agonists should not be withheld or underdosed during acute attacks, regardless of concerns about their long-term use. These agents remain the drugs of choice during acute asthma exacerbations.(A1)
Subcutaneous epinephrine (an α- and β-receptor agonist) and terbutaline (a selective β2-agonist) have fallen out of favor for routine use due to potential adverse effects and the efficacy of alternative treatments. Intramuscular or intravenous epinephrine may be considered when aerosolized albuterol is ineffective or cannot be administered, or when a severe allergen-triggered asthma attack occurs.[30][31] In such cases, 0.3 to 0.5 mg may be given intramuscularly, or 5 to 20 mcg intravenously every 2 to 5 min.
Patients with hypotension or symptoms refractory to other treatments may require an epinephrine infusion at 0.1 to 0.5 mcg/kg/min. Routine use of endotracheal epinephrine instillation is considered ineffective and unnecessary, given the availability of alternative routes of administration. Intravenous β2-agonists are not routinely recommended but have been tried with some success in younger patients with status asthmaticus unresponsive to inhaled therapy.
Methylxanthines
Aminophylline and theophylline are methylxanthine bronchodilators with immunomodulatory properties, acting through phosphodiesterase inhibition and adenosine receptor antagonism. Although these mechanisms may provide benefit, methylxanthines are associated with more adverse effects than β2-agonists and are rarely used in current practice. The narrow therapeutic index of these agents increases the risk of gastrointestinal distress, headaches, insomnia, cardiac dysrhythmias, seizures, and cardiac arrest. Use is limited to patients refractory to standard therapy, and those receiving these agents should be monitored closely for signs of toxicity.[32]
Corticosteroids
Evidence supports corticosteroid use in status asthmaticus in emergency settings. Expert recommendations favor initiating corticosteroid therapy concurrently with short-acting inhaled β2-agonists.[33] Corticosteroids reduce airway inflammation and mucus production, potentiate β2-agonist activity in smooth muscle, and mitigate β2-agonist tachyphylaxis in severe asthma. Optimal dosing in status asthmaticus is not well established, and oral and intravenous routes have similar efficacy.[34] Some patients may require a prolonged course after hospital discharge.(A1)
Anticholinergics
Anticholinergic agents produce variable responses in acute asthma exacerbations and exert bronchodilatory effects through antagonism of muscarinic smooth muscle receptors in the airways. These drugs also decrease airway secretions via antimuscarinic activity. The most commonly used agent is ipratropium bromide.
Anticholinergics may be useful in patients with β-blockade-induced bronchospasm or severe underlying obstructive disease with FEV1 less than 25% of the predicted value. Peak action may be delayed for 60 to 90 min. Therefore, these medications should be administered concurrently with the initiation of short-acting β2-agonists. Bryant and Rogers demonstrated that nebulization with 0.25 mg of ipratropium bromide plus 5 mg of albuterol produced greater FEV1 improvement than albuterol alone. The response time was also much faster than with corticosteroids, with a detectable FEV1 change within 19 min.
Nebulized glycopyrrolate is an alternative anticholinergic agent. However, this drug is rarely prescribed in the U.S. for this indication.
Magnesium sulfate
Magnesium inhibits calcium-mediated smooth muscle constriction, decreases acetylcholine release in the neuromuscular junction, and increases respiratory muscle force generation. Intravenous magnesium sulfate is a useful adjunct in cases of β2-agonist-refractory acute status asthmaticus.[35] The benefit does not appear limited to patients with low serum magnesium levels, although up to 50% of patients with acute asthma may have incidental hypomagnesemia.
Despite widespread use in emergency department settings, few studies have demonstrated statistically significant lung function improvement with magnesium in severe asthma exacerbations. The presumed benefits are largely extrapolated from experience with chronic obstructive pulmonary disease (COPD) exacerbations. However, magnesium is relatively inexpensive and safe when given as a 2-g intravenous infusion over 20 min in the setting of reactive airway disease.
Increased symptom responsiveness to magnesium among female patients may be due to estrogen's ability to potentiate magnesium's bronchodilator effects. Hypotension and hyporeflexia may occur at higher doses, and patients should be monitored for these adverse effects.
Supportive and Adjunctive Interventions
Managing severe asthma exacerbations often requires a multifaceted approach that extends beyond medication alone. The interventions discussed below target respiratory support and adjunctive care strategies to improve patient outcomes.
Heliox
Heliox is a 70:30 or 60:40 helium-oxygen mixture that decreases airway resistance and turbulence, work of breathing, and inspiratory muscle fatigue by enhancing laminar airflow. Routine use is limited by prohibitive cost, infrequent indication, and the need to recalibrate gas blenders and flow meters when used with mechanical ventilation.[36] In most areas, this modality is available only in specialized centers.(B3)
Noninvasive ventilation
Noninvasive positive-pressure ventilation (NIPPV) with continuous (CPAP) or bilevel (BiPAP) positive airway pressure may be used for ventilatory support in patients without significant encephalopathy or excessive secretions. The use of this intervention has increased despite stronger evidence for benefit in COPD exacerbations than in status asthmaticus. NIPPV may help keep smaller airways open that would otherwise collapse dynamically during expiration in asthma exacerbations. This treatment may also reduce the work of breathing by decreasing respiratory muscle use and preventing fatigue.
Prolonged NIPPV increases the risk of aspiration and facial pressure necrosis. Endotracheal intubation should be considered for patients requiring greater or prolonged ventilatory support.[37][38]
Evidence suggests that NIPPV may reduce bronchodilator requirements and shorten ICU and hospital stays in reactive airway disease exacerbations.[39][40] Studies have shown conflicting results regarding NIPPV's impact on the need for endotracheal intubation and mortality rates.[41][42] Other reports indicate that this therapy is safe, well-tolerated, and should be considered early if a patient presents in extremis but does not otherwise meet criteria for endotracheal intubation.[43](A1)
Mechanical ventilation and sedation
The decision to intubate a patient with status asthmaticus is clinical. Immediate indications for intubation include the following:
- Acute respiratory or cardiopulmonary arrest
- Severe obtundation or coma
- Frank evidence of respiratory fatigue with gasping or inability to speak
- Persistent hypoxia despite multimodal treatment
- Worsening respiratory acidosis despite aggressive management
Individuals who do not meet these criteria but continue to deteriorate despite initial pharmacologic treatment require close bedside monitoring for possible intubation.[44] Clinical findings that favor intubation include the following:(B3)
- Increasing lethargy
- Increasing accessory muscle use
- Posture or speech changes
- Decreasing respiratory rate and depth
The choice of induction agent is critical for patients with status asthmaticus who require intubation. Ketamine is currently preferred for its sedative, analgesic, and bronchodilatory properties. The recommended dose is 1 to 2 mg/kg given intravenously at a rate of 0.5 mg/kg/min to provide 10 to 15 min of general anesthesia without significant respiratory depression. Bolus dosing yields inconsistent systemic effects and is not recommended. Adverse effects include hypertension and tachycardia from sympathetic stimulation, increased airway secretions, and a lowered seizure threshold. Ketamine should be avoided in patients with uncontrolled hypertension, preeclampsia, raised intracranial pressure, epilepsy, or liver dysfunction.
Proper postintubation sedation is critical to ensure patient comfort and avoid complications of mechanical ventilation. Overbreathing of ventilator settings may cause breath stacking and air trapping. Propofol is preferred in intubated patients with status asthmaticus for its rapid onset, titrability, sedative properties, and mild bronchodilatory effects. However, prolonged administration increases the risk of carbon dioxide retention by weakening the respiratory drive and reducing patient–ventilator synchrony. Propofol lacks analgesic properties. Thus, concurrent opioid administration may be necessary for pain control. Ketamine may also be considered for its combined bronchodilatory, sedative, and analgesic effects.
Outside of the initial intubation period, paralysis may be required to manage persistent ventilator asynchrony and minimize auto–positive end-expiratory pressure (auto-PEEP) or barotrauma. Commonly used agents in the U.S. include vecuronium, cisatracurium, and rocuronium. Vecuronium has less bronchoconstrictive potential than some alternatives and may be preferred. Atracurium can induce bronchoconstriction via histamine release and should be avoided. Prolonged paralytic infusions may cause critical illness myopathy and should be avoided when possible.[45] Paralytics should never be administered without sedation.(B3)
Ventilator settings for intubated patients with status asthmaticus require careful adjustment to minimize air trapping and prevent increases in intrathoracic pressure. Both pressure and volume control strategies may be used. Low-minute ventilation strategies with an inspiratory-to-expiratory (I:E) ratio of 1:4 or 1:5 reduce air trapping and lower the risk of hypotension and barotrauma.
Extracorporeal membrane oxygenation
Extracorporeal membrane oxygenation (ECMO) may be considered when hypoxia and acidosis persist despite mechanical ventilation.[46][47] ECMO is a highly specialized intervention that requires significant resources and support and is unavailable in many centers. This modality carries risks of major hemorrhage, limb ischemia, and mechanical complications related to the circuit. Potential ECMO candidates should be discussed with appropriate specialists.[48][49](B2)
Antibiotics
Graham et al's randomized, double-blinded trial demonstrated that routine antibiotic use in status asthmaticus did not improve symptom scores, spirometry, or length of hospitalization. Antibiotics should be reserved for patients who show clinical signs of infection. Respiratory culture specimens should be obtained early if infection is suspected and antibiotics are administered.[50][51](A1)
Indications for Hospitalization and Intensive Care
Serial PEFR measurement is a practical and reliable predictor of exacerbation severity and the need for hospitalization. Stein and Cole found that the extent of PEFR improvement 2 hours after treatment predicted hospitalization, whereas initial PEFR on presentation did not. PEFR increased from 250 to 330 LPM in successfully treated patients. Rodrigo and Rodrigo demonstrated a similar pattern in FEV1 with treatment response, although FEV1 is challenging to measure at the bedside.[52][53][54] Overall, FEV1 or PEFR between 40% and 70% of the predicted value after initial emergency treatment is considered an inadequate response, indicating the need for further stabilization.(B3)
Favorable initial responses in status asthmaticus include visible symptom improvement sustained for at least 30 min after the last bronchodilator treatment and a PEFR greater than 70% of the predicted value. Less than 10% PEFR improvement or a PEFR below 40% of the predicted value indicates ongoing significant airway obstruction and warrants consideration for ICU admission. Intensive care should also be considered in the presence of respiratory fatigue, altered mental status, new or unstable arrhythmias, hypotension, need for NIPPV, and cardiac or respiratory arrest.
Patients who present with severe exacerbations and respond quickly to initial treatments may benefit from longer observation before final disposition. Kelsen and colleagues found that patients treated for 2 hours or less in a facility had a 50% symptom relapse rate and required additional treatment. In contrast, only a 4% relapse rate occurred in individuals observed for an additional 2 to 4 hours. While precise observation period recommendations vary, patients who initially present with respiratory distress should be closely monitored for several hours even after favorable initial treatment responses.
The ultimate disposition depends on both clinical and psychosocial factors. Hospitalization may be considered for patients with poor access to outpatient follow-up who might otherwise be candidates for discharge.
Differential Diagnosis
The differential diagnosis of status asthmaticus is broad. Many conditions can cause respiratory distress with or without wheezing. The following are important differential diagnoses with their key differentiating features:
- Pneumothorax may present with absent or asymmetric breath sounds. Tension pneumothorax may cause tracheal deviation and signs of obstructive shock.
- Pneumomediastinum usually presents with a mediastinal crunch or crepitus around the neck or chest on examination.
- Tracheal obstruction, tracheal stenosis, or angioedema may cause dyspnea and inspiratory stridor.
- Foreign body inhalation, mucous plugging, or focal atelectasis may present with localized rather than diffuse wheezing on auscultation.[55]
- Excessive dynamic airway collapse during positive pressure ventilation may cause wheezing.
- Pneumonia often presents with other adventitious sounds such as rhonchi or lobar crackles.
- Patients with COPD often have chronic dyspnea with or without productive cough, frequently accompanied by a history of excessive cigarette smoking.
- Acute heart failure may be differentiated by elevated cardiac enzymes, peripheral edema, and pulmonary edema on ultrasound or chest radiography.
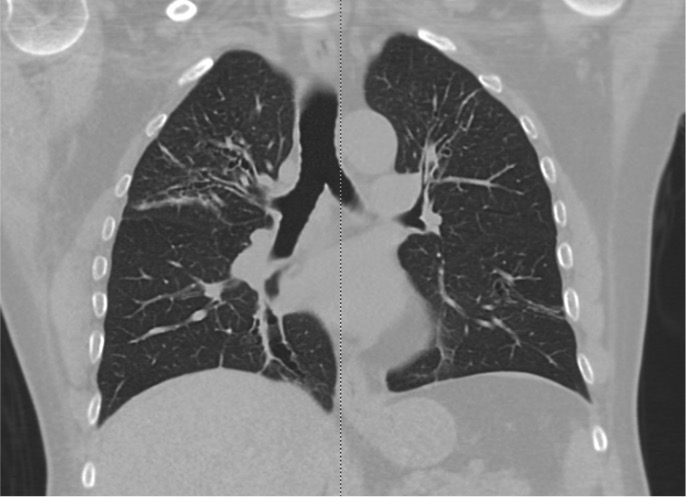
- Allergic bronchopulmonary aspergillosis may be distinguished by a positive Aspergillus skin prick test, elevated immunoglobulin E levels, and characteristic imaging findings (see Image. Allergic Bronchopulmonary Aspergillosis on Computed Tomography).
- Vocal cord dysfunction may cause inspiratory stridor and wheezing.
- Inhalational injury may precipitate or mimic an asthma exacerbation due to airway inflammation.
- In neuromuscular diseases such as myasthenia gravis, a thorough neurological examination may demonstrate systemic neuromuscular weakness with shortness of breath due to diaphragm or respiratory muscle involvement.
A comprehensive history, physical examination, and judicious use of diagnostic tests can help distinguish these conditions from status asthmaticus.
Prognosis
Status asthmaticus has a good prognosis if recognized and treated promptly. Concurrent exacerbations of comorbidities such as congestive heart failure and COPD are associated with a worse prognosis. Patients with greater acidemia and carbon dioxide retention, as well as those requiring mechanical ventilation, have a poorer prognosis.[56] A study by Adnet et al found that neuromuscular blockade increases the risk of postintubation myopathy, ventilator-associated pneumonia, and prolonged ICU stay in patients with status asthmaticus.
Severe exacerbations and status asthmaticus can be fatal. Tens of thousands of people die from severe asthma each year worldwide. Mortality rates are higher among male patients, people with underlying cardiac or pulmonary pathology, older adults, and smokers. A history of near-fatal asthma requiring endotracheal intubation is the single greatest predictor of death from asthma. All-cause mortality in patients with asthma following a severe exacerbation peaks within the first month after the episode.[57]
Complications
Complications of status asthmaticus may be related to the underlying pathology or treatments rendered. Refractory exacerbations and sustained tachypnea, hypoxemia, or hypercapnia may result in electrolyte abnormalities, hypotension, dysrhythmias, and end-organ dysfunction.[58] Severe hypotension and respiratory acidosis may cause myocardial infarction, hypoxic encephalopathy, respiratory or cardiac arrest, and death.
Treatment side effects include tachycardia, arrhythmias, hypokalemia, and lactic acidosis from β2-agonists. Long-term corticosteroid use may cause weight gain, hyperglycemia, fluid retention, and adrenal insufficiency, particularly in critically ill patients with comorbidities. Chronic use of both short- and long-acting β2-agonists may be associated with increased airway hyperresponsiveness and mortality, possibly mediated by concurrent use of inhaled corticosteroids.
Dynamic hyperinflation from air trapping and auto-PEEP increases the risk of pulmonary barotrauma and obstructive shock due to elevated intrathoracic pressure and reduced venous return. This effect is worsened by invasive or noninvasive positive-pressure ventilation. Barotrauma may result in pneumothorax, tension pneumothorax, or pneumomediastinum. Patients receiving positive-pressure ventilation should be closely monitored for shock and barotrauma. Pneumothorax management in patients on NIPPV or mechanical ventilation requires tube thoracostomy to prevent tension physiology.
Patients manifesting with auto-PEEP should be transiently removed from mechanical ventilation circuits and have their chest walls compressed to empty the lungs of trapped air.[59] Ventilator exhalation time should be increased by reducing tidal volume or respiratory rate. Some patients may require deeper sedation or paralysis if over-breathing preset ventilator settings is contributing to air trapping. Ventilator-applied PEEP should be minimized to reduce the risk of barotrauma and obstructive shock. High peak pressure with stable plateau pressure should prompt airway and endotracheal tube clearance, as secretions may be thick and tenacious in severe asthma. A larger-lumen endotracheal tube (French 7.5 or 8) is preferred to reduce airflow resistance and facilitate bronchoscopy if needed.
Purposeful hypoventilation and permissive hypercapnia may be useful in status asthmaticus and seldom cause increased intracranial pressure or depressed myocardial function. Moderate hypercapnia reduces dynamic hyperinflation and ventilation-perfusion heterogeneity by preventing increases in alveolar dead space. Serum pH should be maintained above 7.15 rather than targeting a specific pCO2 value.
Consultations
Most patients with status asthmaticus will require intensive care. Critical care and pulmonology consultations are often necessary, with additional subspecialty involvement for exacerbations of comorbidities as indicated. Social services should be engaged to address modifiable social determinants of health, such as medication affordability and access, to reduce the risk of future severe exacerbations. Physical and occupational therapy may be required before discharge.
Deterrence and Patient Education
Exacerbation prevention is the cornerstone of long-term asthma management. Patients should be educated on trigger avoidance and provided with asthma action plans to reduce the risk of severe exacerbations and status asthmaticus. Individuals at higher risk, such as those at extremes of age or with limited access to medications, should be identified and managed proactively. Inpatient education by trained laypeople has been shown to improve inhaler technique compliance and postdischarge care.[60]
Pearls and Other Issues
The key points to remember when evaluating and managing status asthmaticus are the following:
- Exacerbation prevention is the best approach to long-term asthma management. Patient education should emphasize trigger avoidance and treatment adherence. Factors predisposing patients to exacerbations should be investigated in those with frequent emergency department visits or hospitalizations.
- Recognizing early signs and symptoms of an asthma attack is crucial. Acute dyspnea, cough, and chest pain in a patient with a prior asthma diagnosis may indicate an exacerbation. Refractoriness to the usual treatments may signal status asthmaticus and should prompt patients to seek emergency department care.
- Inhaled β2-agonists are the 1st-line agents for asthma exacerbations. Other pharmacologic options include corticosteroids, methylxanthines, anticholinergics, and magnesium sulfate. Oxygen supplementation should be initiated for hypoxemic patients.
- Infections, allergies, and inhalational injuries may trigger asthma exacerbations and should be treated accordingly. Bacterial infections require antibiotics. Mitigation of environmental triggers may reduce exacerbation frequency and severity.
- Patients with status asthmaticus often require ventilatory support. Noninvasive modalities should be considered for patients without significant encephalopathy or excess secretions. Patients with poor work of breathing, altered mental status, or refractory respiratory distress may require intubation and mechanical ventilation.
- Ketamine has bronchodilatory properties and should be considered for asthmatic patients requiring sedation or intubation.
- ECMO may be an option for patients who do not improve with mechanical ventilation after all other potential causes of respiratory failure have been addressed.
Management decisions should be individualized for each patient. Evidence-based guidelines provide a framework, but flexibility is needed to address unique patient circumstances.
Enhancing Healthcare Team Outcomes
Status asthmaticus management requires an interprofessional approach. Emergency medical services or emergency medicine physicians often perform the initial evaluation and stabilization. Nursing staff and technicians assist with establishing intravenous access, administering medications, and continuously monitoring the patient’s vital signs and clinical status.
Patients requiring mechanical ventilation or intensive care may be treated by physicians from internal medicine, critical care, and pulmonary medicine, along with advanced practice providers, respiratory therapists, and critical care nurses. Other specialists, including infectious disease physicians and cardiologists, may be consulted when comorbid conditions require targeted management. Occupational and physical therapists assist patients in regaining functional independence before discharge. Social workers help patients and their families navigate the healthcare system to facilitate appropriate outpatient care. Pharmacists provide guidance on medication management, particularly when multiple agents are necessary for symptom control.[61][62]
Primary care providers play a crucial role in exacerbation prevention through medication management and the development of asthma action plans. Vaccination against preventable diseases, including COVID-19 and influenza, should be administered. Patients should be able to recognize early warning signs of an exacerbation and be prepared for self-treatment or hospital transport according to their action plans. A 20% drop in PEFR from the predicted value or personal best is an objective indicator of exacerbation that can be measured at home. Patients with a history of anaphylaxis or sudden asphyxic asthma should also be equipped with an EpiPen® for immediate subcutaneous administration during a severe exacerbation. Some outpatient pharmacies in the U.S. can monitor medication compliance and detect increasing disease severity based on prescription refill patterns.
Understanding asthma prevalence, pathophysiology, and management is essential for all members of the healthcare team to minimize morbidity from severe asthma exacerbations. Shared knowledge supports early recognition, timely intervention, and appropriate long-term care.
Media
(Click Image to Enlarge)
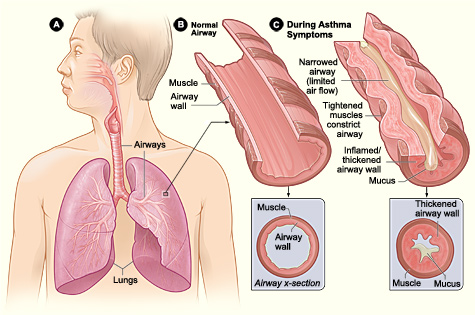
Pathophysiology of Asthma. Figure A displays the location of the lungs and airways in the body. Figure B shows a cross section of a normal airway. Figure C illustrates a cross section of an airway during asthma symptoms.
National Institutes of Health, Public Domain, via Wikimedia Commons
(Click Image to Enlarge)
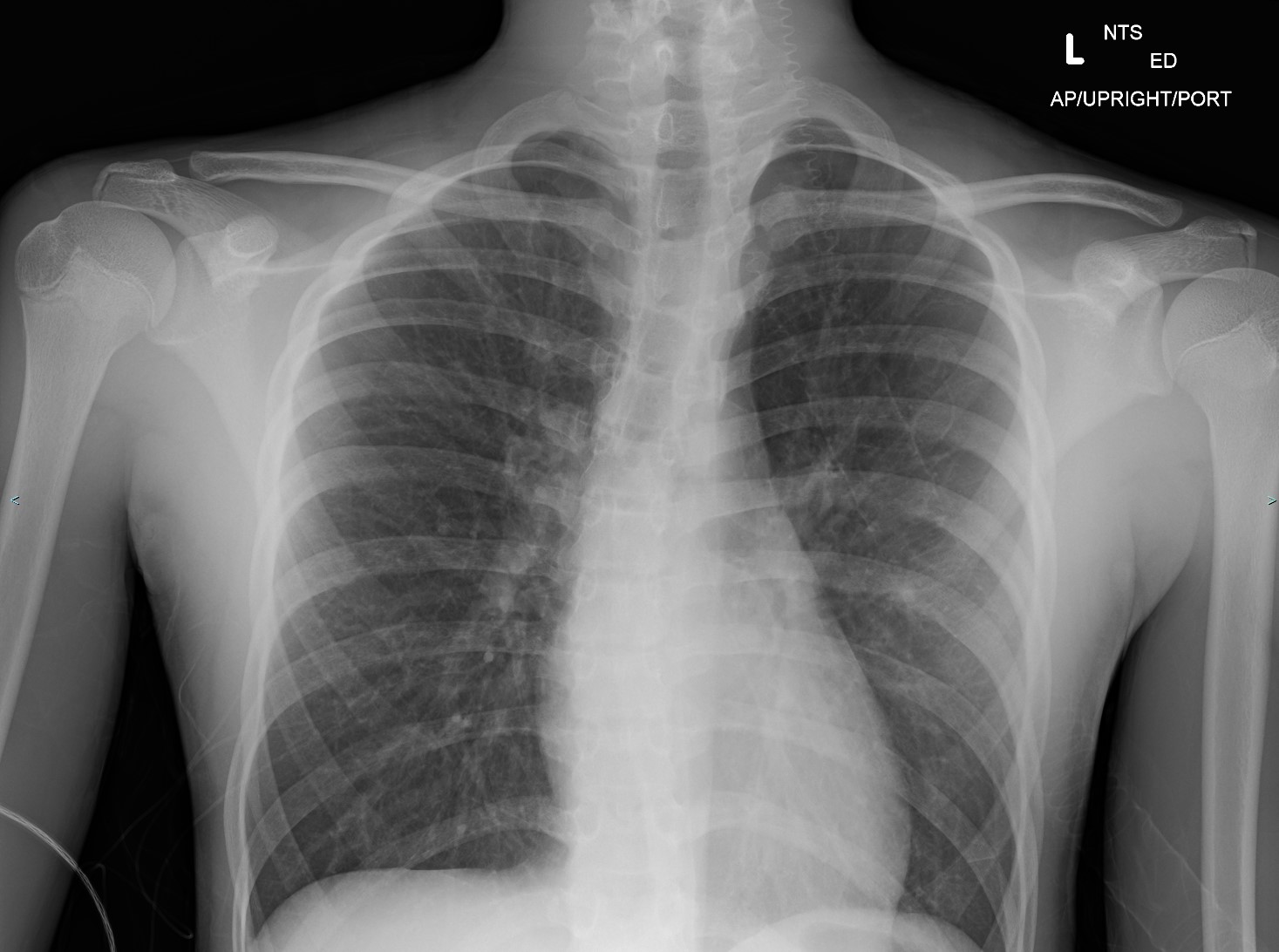
Asthma Radiography. This chest x-ray shows hyperinflation, indicated by a flattened diaphragm with 7 anterior ribs and 11 posterior ribs visible. Mild increases in interstitial markings are seen. These findings are consistent with airway obstruction typically seen in asthma exacerbation.
Contributed by Steve Lange, MD
(Click Image to Enlarge)
References
National Asthma Education and Prevention Program. Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. The Journal of allergy and clinical immunology. 2007 Nov:120(5 Suppl):S94-138 [PubMed PMID: 17983880]
Papiris S, Kotanidou A, Malagari K, Roussos C. Clinical review: severe asthma. Critical care (London, England). 2002 Feb:6(1):30-44 [PubMed PMID: 11940264]
Lamblin C, Gosset P, Tillie-Leblond I, Saulnier F, Marquette CH, Wallaert B, Tonnel AB. Bronchial neutrophilia in patients with noninfectious status asthmaticus. American journal of respiratory and critical care medicine. 1998 Feb:157(2):394-402 [PubMed PMID: 9476849]
Swed S, Sawaf B, Al-Obeidat F, Hafez W, Rakab A, Alibrahim H, Nasif MN, Alghalyini B, Zia Zaidi AR, Alshareef L, Alqatati F, Zamrath Zahir F, Ahmed AI, Alom M, Sultan A, AlMahmoud A, Bakkour A, Cherrez-Ojeda I. Asthma prevalence among United States population insights from NHANES data analysis. Scientific reports. 2024 Apr 5:14(1):8059. doi: 10.1038/s41598-024-58429-5. Epub 2024 Apr 5 [PubMed PMID: 38580691]
Braman SS, Kaemmerlen JT. Intensive care of status asthmaticus. A 10-year experience. JAMA. 1990 Jul 18:264(3):366-8 [PubMed PMID: 2362333]
Mauer Y, Taliercio RM. Managing adult asthma: The 2019 GINA guidelines. Cleveland Clinic journal of medicine. 2020 Aug 31:87(9):569-575. doi: 10.3949/ccjm.87a.19136. Epub 2020 Aug 31 [PubMed PMID: 32868307]
Hanania NA, David-Wang A, Kesten S, Chapman KR. Factors associated with emergency department dependence of patients with asthma. Chest. 1997 Feb:111(2):290-5 [PubMed PMID: 9041971]
GBD 2021 Asthma and Allergic Diseases Collaborators. Global, regional, and national burden of asthma and atopic dermatitis, 1990-2021, and projections to 2050: a systematic analysis of the Global Burden of Disease Study 2021. The Lancet. Respiratory medicine. 2025 May:13(5):425-446. doi: 10.1016/S2213-2600(25)00003-7. Epub 2025 Mar 24 [PubMed PMID: 40147466]
Level 1 (high-level) evidenceAfessa B, Morales I, Cury JD. Clinical course and outcome of patients admitted to an ICU for status asthmaticus. Chest. 2001 Nov:120(5):1616-21 [PubMed PMID: 11713143]
Joseph A, Ganatra H. Status Asthmaticus in the Pediatric ICU: A Comprehensive Review of Management and Challenges. Pediatric reports. 2024 Jul 31:16(3):644-656. doi: 10.3390/pediatric16030054. Epub 2024 Jul 31 [PubMed PMID: 39189288]
Bunyavanich S, Schadt EE. Systems biology of asthma and allergic diseases: a multiscale approach. The Journal of allergy and clinical immunology. 2015 Jan:135(1):31-42. doi: 10.1016/j.jaci.2014.10.015. Epub 2014 Nov 21 [PubMed PMID: 25468194]
Dunican EM, Fahy JV. The Role of Type 2 Inflammation in the Pathogenesis of Asthma Exacerbations. Annals of the American Thoracic Society. 2015 Nov:12 Suppl 2(Suppl 2):S144-9. doi: 10.1513/AnnalsATS.201506-377AW. Epub [PubMed PMID: 26595730]
Ramsahai JM, Hansbro PM, Wark PAB. Mechanisms and Management of Asthma Exacerbations. American journal of respiratory and critical care medicine. 2019 Feb 15:199(4):423-432. doi: 10.1164/rccm.201810-1931CI. Epub [PubMed PMID: 30562041]
Sur S, Crotty TB, Kephart GM, Hyma BA, Colby TV, Reed CE, Hunt LW, Gleich GJ. Sudden-onset fatal asthma. A distinct entity with few eosinophils and relatively more neutrophils in the airway submucosa? The American review of respiratory disease. 1993 Sep:148(3):713-9 [PubMed PMID: 8368644]
Level 3 (low-level) evidenceMcFadden ER Jr. Acute severe asthma. American journal of respiratory and critical care medicine. 2003 Oct 1:168(7):740-59 [PubMed PMID: 14522812]
Brenner BE, Abraham E, Simon RR. Position and diaphoresis in acute asthma. The American journal of medicine. 1983 Jun:74(6):1005-9 [PubMed PMID: 6407304]
Nowak RM, Tomlanovich MC, Sarkar DD, Kvale PA, Anderson JA. Arterial blood gases and pulmonary function testing in acute bronchial asthma. Predicting patient outcomes. JAMA. 1983 Apr 15:249(15):2043-6 [PubMed PMID: 6403719]
Mountain RD, Sahn SA. Clinical features and outcome in patients with acute asthma presenting with hypercapnia. The American review of respiratory disease. 1988 Sep:138(3):535-9 [PubMed PMID: 3202409]
Corbridge TC, Hall JB. The assessment and management of adults with status asthmaticus. American journal of respiratory and critical care medicine. 1995 May:151(5):1296-316 [PubMed PMID: 7735578]
Agnihotri NT, Saltoun C. Acute severe asthma (status asthmaticus). Allergy and asthma proceedings. 2019 Nov 1:40(6):406-409. doi: 10.2500/aap.2019.40.4258. Epub [PubMed PMID: 31690381]
Knowles GK, Clark TJ. Pulsus paradoxus as a valuable sign indicating severity of asthma. Lancet (London, England). 1973 Dec 15:2(7842):1356-9 [PubMed PMID: 4128055]
Rebuck AS, Read J. Assessment and management of severe asthma. The American journal of medicine. 1971 Dec:51(6):788-98 [PubMed PMID: 5129547]
Gunen H, Hacievliyagil SS, Kosar F, Gulbas G, Kizkin O, Sahin I. The role of arterial blood gases, exercise testing, and cardiac examination in asthma. Allergy and asthma proceedings. 2006 Jan-Feb:27(1):45-52 [PubMed PMID: 16598992]
Staub LJ, Mazzali Biscaro RR, Kaszubowski E, Maurici R. Lung Ultrasound for the Emergency Diagnosis of Pneumonia, Acute Heart Failure, and Exacerbations of Chronic Obstructive Pulmonary Disease/Asthma in Adults: A Systematic Review and Meta-analysis. The Journal of emergency medicine. 2019 Jan:56(1):53-69. doi: 10.1016/j.jemermed.2018.09.009. Epub 2018 Oct 9 [PubMed PMID: 30314929]
Level 1 (high-level) evidenceRodriguez-Roisin R. Acute severe asthma: pathophysiology and pathobiology of gas exchange abnormalities. The European respiratory journal. 1997 Jun:10(6):1359-71 [PubMed PMID: 9192945]
Frei SP. Cost comparison of bronchodilator delivery methods in Emergency Department treatment of asthma. The Journal of emergency medicine. 2000 Nov:19(4):323-6 [PubMed PMID: 11074323]
Alsuwaigh R, Cao Y, Puan Y, Yii A, Mohamed Noor SB, Ye H, Chen H, Li XL, Binte Mohd Noor N, Liew J, Tay TR. Nebulizer versus metered dose inhaler with space chamber (MDI spacer) for acute asthma and chronic obstructive pulmonary disease exacerbation: attitudes of patients and healthcare providers in the COVID-19 era. The Journal of asthma : official journal of the Association for the Care of Asthma. 2023 Mar:60(3):600-608. doi: 10.1080/02770903.2022.2082307. Epub 2022 Jun 7 [PubMed PMID: 35608065]
Pilcher J, Patel M, Pritchard A, Thayabaran D, Ebmeier S, Shaw D, Black P, Braithwaite I, Weatherall M, Beasley R. Beta-agonist overuse and delay in obtaining medical review in high risk asthma: a secondary analysis of data from a randomised controlled trial. NPJ primary care respiratory medicine. 2017 May 11:27(1):33. doi: 10.1038/s41533-017-0032-z. Epub 2017 May 11 [PubMed PMID: 28496190]
Level 1 (high-level) evidenceSuh DI, Johnston SL. The Wiser Strategy of Using Beta-Agonists in Asthma: Mechanisms and Rationales. Allergy, asthma & immunology research. 2024 May:16(3):217-234. doi: 10.4168/aair.2024.16.3.217. Epub [PubMed PMID: 38910281]
Long B, Lentz S, Koyfman A, Gottlieb M. Evaluation and management of the critically ill adult asthmatic in the emergency department setting. The American journal of emergency medicine. 2021 Jun:44():441-451. doi: 10.1016/j.ajem.2020.03.029. Epub 2020 Mar 19 [PubMed PMID: 32222313]
Smith D, Riel J, Tilles I, Kino R, Lis J, Hoffman JR. Intravenous epinephrine in life-threatening asthma. Annals of emergency medicine. 2003 May:41(5):706-11 [PubMed PMID: 12712039]
Maselli DJ, Peters JI. Medication Regimens for Managing Acute Asthma. Respiratory care. 2018 Jun:63(6):783-796. doi: 10.4187/respcare.05953. Epub [PubMed PMID: 29794211]
Edmonds ML, Milan SJ, Camargo CA Jr, Pollack CV, Rowe BH. Early use of inhaled corticosteroids in the emergency department treatment of acute asthma. The Cochrane database of systematic reviews. 2012 Dec 12:12(12):CD002308. doi: 10.1002/14651858.CD002308.pub2. Epub 2012 Dec 12 [PubMed PMID: 23235589]
Level 1 (high-level) evidenceGayen S, Dachert S, Lashari BH, Gordon M, Desai P, Criner GJ, Cardet JC, Shenoy K. Critical Care Management of Severe Asthma Exacerbations. Journal of clinical medicine. 2024 Feb 1:13(3):. doi: 10.3390/jcm13030859. Epub 2024 Feb 1 [PubMed PMID: 38337552]
Griffiths B, Kew KM, Normansell R. Intravenous magnesium sulfate for treating children with acute asthma in the emergency department. Paediatric respiratory reviews. 2016 Sep:20():45-47. doi: 10.1016/j.prrv.2016.07.001. Epub 2016 Jul 22 [PubMed PMID: 27522304]
Tobias JD, Garrett JS. Therapeutic options for severe, refractory status asthmaticus: inhalational anaesthetic agents, extracorporeal membrane oxygenation and helium/oxygen ventilation. Paediatric anaesthesia. 1997:7(1):47-57 [PubMed PMID: 9041574]
Level 3 (low-level) evidenceMeduri GU, Cook TR, Turner RE, Cohen M, Leeper KV. Noninvasive positive pressure ventilation in status asthmaticus. Chest. 1996 Sep:110(3):767-74 [PubMed PMID: 8797425]
Hess DR. Noninvasive ventilation for acute respiratory failure. Respiratory care. 2013 Jun:58(6):950-72. doi: 10.4187/respcare.02319. Epub [PubMed PMID: 23709194]
Gupta D, Nath A, Agarwal R, Behera D. A prospective randomized controlled trial on the efficacy of noninvasive ventilation in severe acute asthma. Respiratory care. 2010 May:55(5):536-43 [PubMed PMID: 20420722]
Level 1 (high-level) evidenceGreen E, Jain P, Bernoth M. Noninvasive ventilation for acute exacerbations of asthma: A systematic review of the literature. Australian critical care : official journal of the Confederation of Australian Critical Care Nurses. 2017 Nov:30(6):289-297. doi: 10.1016/j.aucc.2017.01.003. Epub 2017 Jan 27 [PubMed PMID: 28139368]
Level 1 (high-level) evidenceNanchal R, Kumar G, Majumdar T, Taneja A, Patel J, Dagar G, Jacobs ER, Whittle J. Utilization of mechanical ventilation for asthma exacerbations: analysis of a national database. Respiratory care. 2014 May:59(5):644-53. doi: 10.4187/respcare.02505. Epub 2013 Oct 8 [PubMed PMID: 24106317]
Stefan MS, Nathanson BH, Lagu T, Priya A, Pekow PS, Steingrub JS, Hill NS, Goldberg RJ, Kent DM, Lindenauer PK. Outcomes of Noninvasive and Invasive Ventilation in Patients Hospitalized with Asthma Exacerbation. Annals of the American Thoracic Society. 2016 Jul:13(7):1096-104. doi: 10.1513/AnnalsATS.201510-701OC. Epub [PubMed PMID: 27070493]
Althoff MD, Holguin F, Yang F, Grunwald GK, Moss M, Vandivier RW, Ho PM, Kiser TH, Burnham EL. Noninvasive Ventilation Use in Critically Ill Patients with Acute Asthma Exacerbations. American journal of respiratory and critical care medicine. 2020 Dec 1:202(11):1520-1530. doi: 10.1164/rccm.201910-2021OC. Epub [PubMed PMID: 32663410]
Siddiqui S, Gonem S, Wardlaw AJ. Advances in the management of severe asthma. Seminars in respiratory and critical care medicine. 2012 Dec:33(6):666-84. doi: 10.1055/s-0032-1326964. Epub 2012 Oct 9 [PubMed PMID: 23047316]
Level 3 (low-level) evidenceGoh AY, Chan PW. Acute myopathy after status asthmaticus: steroids, myorelaxants or carbon dioxide? Respirology (Carlton, Vic.). 1999 Mar:4(1):97-9 [PubMed PMID: 10339738]
Level 3 (low-level) evidenceDi Lascio G, Prifti E, Messai E, Peris A, Harmelin G, Xhaxho R, Fico A, Sani G, Bonacchi M. Extracorporeal membrane oxygenation support for life-threatening acute severe status asthmaticus. Perfusion. 2017 Mar:32(2):157-163. doi: 10.1177/0267659116670481. Epub 2016 Oct 7 [PubMed PMID: 27758969]
Medar SS, Derespina KR, Jakobleff WA, Ushay MH, Peek GJ. A winter to remember! Extracorporeal membrane oxygenation for life-threatening asthma in children: A case series and review of literature. Pediatric pulmonology. 2020 Feb:55(2):E1-E4. doi: 10.1002/ppul.24616. Epub 2019 Dec 20 [PubMed PMID: 31860773]
Level 2 (mid-level) evidenceYeo HJ, Kim D, Jeon D, Kim YS, Rycus P, Cho WH. Extracorporeal membrane oxygenation for life-threatening asthma refractory to mechanical ventilation: analysis of the Extracorporeal Life Support Organization registry. Critical care (London, England). 2017 Dec 6:21(1):297. doi: 10.1186/s13054-017-1886-8. Epub 2017 Dec 6 [PubMed PMID: 29212551]
Wrisinger WC, Thompson SL. Basics of Extracorporeal Membrane Oxygenation. The Surgical clinics of North America. 2022 Feb:102(1):23-35. doi: 10.1016/j.suc.2021.09.001. Epub [PubMed PMID: 34800387]
Graham V, Lasserson T, Rowe BH. Antibiotics for acute asthma. The Cochrane database of systematic reviews. 2001:(3):CD002741 [PubMed PMID: 11687022]
Level 1 (high-level) evidenceJohnston SL, Szigeti M, Cross M, Brightling C, Chaudhuri R, Harrison T, Mansur A, Robison L, Sattar Z, Jackson D, Mallia P, Wong E, Corrigan C, Higgins B, Ind P, Singh D, Thomson NC, Ashby D, Chauhan A, AZALEA Trial Team. Azithromycin for Acute Exacerbations of Asthma : The AZALEA Randomized Clinical Trial. JAMA internal medicine. 2016 Nov 1:176(11):1630-1637. doi: 10.1001/jamainternmed.2016.5664. Epub [PubMed PMID: 27653939]
Level 1 (high-level) evidenceJudson MA, Sperl PL. Status asthmaticus with acute decompensation with therapy in a 27-year-old woman. Chest. 1995 Feb:107(2):563-5 [PubMed PMID: 7842796]
Level 3 (low-level) evidenceBerg KT, O'Connor MG, Lescallette RD, Arnold DH, Stack LB. AAIRS Score Overview: The Acute Asthma Intensity Research Score. Academic emergency medicine : official journal of the Society for Academic Emergency Medicine. 2015 Oct:22(10):E25-6. doi: 10.1111/acem.12760. Epub 2015 Sep 9 [PubMed PMID: 26352797]
Level 3 (low-level) evidenceSerrano-Pariente J, Rodrigo G, Fiz JA, Crespo A, Plaza V, High Risk Asthma Research Group. Identification and characterization of near-fatal asthma phenotypes by cluster analysis. Allergy. 2015 Sep:70(9):1139-47. doi: 10.1111/all.12654. Epub 2015 Jun 9 [PubMed PMID: 26011771]
Henke CA, Hertz M, Gustafson P. Combined bronchoscopy and mucolytic therapy for patients with severe refractory status asthmaticus on mechanical ventilation: a case report and review of the literature. Critical care medicine. 1994 Nov:22(11):1880-3 [PubMed PMID: 7956296]
Level 3 (low-level) evidenceWilliams TJ, Tuxen DV, Scheinkestel CD, Czarny D, Bowes G. Risk factors for morbidity in mechanically ventilated patients with acute severe asthma. The American review of respiratory disease. 1992 Sep:146(3):607-15 [PubMed PMID: 1519836]
Level 2 (mid-level) evidenceEngelkes M, de Ridder MA, Svensson E, Berencsi K, Prieto-Alhambra D, Lapi F, Giaquinto C, Picelli G, Boudiaf N, Albers FC, Cockle SM, Bradford ES, Suruki RY, Brusselle GG, Rijnbeek PR, Sturkenboom MC, Verhamme KM. Multinational cohort study of mortality in patients with asthma and severe asthma. Respiratory medicine. 2020 Apr-May:165():105919. doi: 10.1016/j.rmed.2020.105919. Epub 2020 Mar 2 [PubMed PMID: 32174450]
Grossman J. The occurrence of arrhythmias in hospitalized asthmatic patients. The Journal of allergy and clinical immunology. 1976 Apr:57(4):310-7 [PubMed PMID: 1262607]
Brooks R, Cohen-Cymberknoh M, Glicksman C, Eisenstein EM, Shoseyov D. Manual external chest compression reverses respiratory failure in children with severe air trapping. Pediatric pulmonology. 2021 Dec:56(12):3887-3890. doi: 10.1002/ppul.25689. Epub 2021 Sep 28 [PubMed PMID: 34583418]
Rice JL, Matlack KM, Simmons MD, Steinfeld J, Laws MA, Dovey ME, Cohen RT. LEAP: A randomized-controlled trial of a lay-educator inpatient asthma education program. Patient education and counseling. 2015 Jun 29:():. pii: S0738-3991(15)30006-9. doi: 10.1016/j.pec.2015.06.020. Epub 2015 Jun 29 [PubMed PMID: 26210342]
Level 1 (high-level) evidenceAl-Muhsen S, Horanieh N, Dulgom S, Aseri ZA, Vazquez-Tello A, Halwani R, Al-Jahdali H. Poor asthma education and medication compliance are associated with increased emergency department visits by asthmatic children. Annals of thoracic medicine. 2015 Apr-Jun:10(2):123-31. doi: 10.4103/1817-1737.150735. Epub [PubMed PMID: 25829964]
Pinnock H. Supported self-management for asthma. Breathe (Sheffield, England). 2015 Jun:11(2):98-109. doi: 10.1183/20734735.015614. Epub [PubMed PMID: 26306110]