Introduction
Spontaneous pneumothorax is the presence of air within the pleural space that occurs without trauma or other iatrogenic causes. This condition is broadly classified into 2 types: primary spontaneous pneumothorax (PSP), which occurs in individuals without known underlying lung disease, and secondary spontaneous pneumothorax (SSP), which develops in patients with existing pulmonary pathology such as chronic obstructive pulmonary disease, cystic fibrosis, or interstitial lung disease. Patients typically present with the acute onset of pleuritic chest pain, dyspnea, and tachycardia. In severe cases, spontaneous pneumothorax can progress to a life-threatening tension pneumothorax, requiring immediate intervention. Diagnosis is primarily clinical, supported by imaging, most often chest radiography or point-of-care ultrasound.
Management strategies for spontaneous pneumothorax are evolving and increasingly emphasize individualized care based on clinical stability, size, recurrence status, and whether the pneumothorax is primary or secondary.[1][2] Recent updates, such as the 2023 British Thoracic Society guidelines, support a conservative approach for small, stable PSPs, highlighted by trials demonstrating spontaneous resolution in up to 94% of cases within 8 weeks without procedural intervention.[3][4] Conversely, surgical intervention—typically via video-assisted thoracoscopic surgery or thoracotomy with pleurodesis—is now recommended for recurrent or complicated pneumothoraces, particularly in patients with SSP or high-risk features, in line with both British and European guidance.[3] This review provides a comprehensive overview of the etiology, epidemiology, diagnosis, and updated management strategies for spontaneous pneumothorax.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Spontaneous pneumothorax is categorized into 2 primary types: PSP and SSP, based on the presence or absence of underlying lung pathology. PSP occurs in individuals without clinically apparent lung disease, most commonly affecting young adults between the ages of 15 and 40 years. This condition is frequently associated with subpleural bleb rupture, often in tall, thin men. The annual incidence of PSP ranges from 7.4 to 24 per 100,000 in men and 1.2 to 6 per 100,000 in women.[5]
SSP, by contrast, occurs in patients with established pulmonary disease. Common causes include coronary obstructive pulmonary disease, asthma, cystic fibrosis, necrotizing pneumonia, Pneumocystis jirovecii infection, pulmonary abscess, tuberculosis, malignancy, and various forms of interstitial lung disease (eg, idiopathic pulmonary fibrosis, sarcoidosis, lymphangioleiomyomatosis). Additional etiologies include connective tissue disorders (eg, Marfan syndrome, Ehlers-Danlos syndrome, rheumatoid arthritis), pulmonary infarction, foreign body aspiration, and rare conditions such as Birt-Hogg-Dubé syndrome and catamenial pneumothorax secondary to thoracic endometriosis. SSP tends to affect older adults and carries a higher risk of morbidity due to underlying pulmonary compromise. Epidemiologic studies report an annual incidence of SSP of 6.3 per 100,000 in men and 2.0 per 100,000 in women.[6]
Epidemiology
Spontaneous pneumothorax occurs more frequently in adults than in children and is significantly more common in men than women across all age groups. In the United States, the estimated annual incidence of PSP in adults is 7.4 to 18 per 100,000 population in men and 1.2 to 6.0 per 100,000 in women.[7][8] SSP, which occurs in the context of underlying pulmonary disease, has a reported annual incidence of 6.3 per 100,000 men and 2.0 per 100,000 women. Spontaneous pneumothorax is relatively rare in the pediatric population, with an estimated annual incidence of 4.0 per 100,000 in boys and 1.1 per 100,000 in girls.[8] Risk factors for PSP include smoking, which significantly increases risk across age groups, and a tall, thin body habitus, especially in adolescent and young adult men.[7]
Epidemiologic trends underscore the clinical and healthcare impact of this condition. Results from a large-scale study in the United Kingdom (2017–2023) reported approximately 72,000 hospital admissions for spontaneous pneumothorax, highlighting its substantial burden on health systems.[9] Recurrence is a major concern, particularly in PSP, where rates reach approximately 32%, with 21% to 54% recurring within 1 to 2 years after the initial event. SSP tends to recur less frequently (13%–39%), but its association with significant underlying disease often results in more complex management and higher morbidity.[9]
Pathophysiology
The development of spontaneous pneumothorax arises from the accumulation of gas within the pleural space, a process driven by complex and multifactorial pathophysiological mechanisms. Central to this is the disruption of the alveolar-pleural barrier, typically caused by acute increases in transpulmonary pressure that exceed the tensile strength of the alveolar walls. These sudden pressure surges—commonly triggered by coughing, straining, or Valsalva maneuvers—can lead to alveolar rupture and air leakage into the pleural cavity.[10]
Both primary and secondary spontaneous pneumothoraces are linked to elevated alveolar pressure exceeding pulmonary interstitial pressure, facilitating alveolar rupture and subsequent pleural air leakage. Structural weaknesses in the visceral pleura, including subpleural blebs, bullae, lung necrosis, and connective tissue abnormalities, predispose alveoli to rupture, although the precise mechanisms remain incompletely understood. Notably, intact bullae without overt visceral pleural defects have been associated with spontaneous pneumothorax. Histopathological and scanning electron microscopic analyses suggest that sloughing of pleural mesothelial cells may significantly contribute to pneumothorax pathogenesis.[11][12]
In PSP, rupture typically occurs at pre-existing sites of weakness such as subpleural blebs and bullae—lesions present in up to 77% of cases on imaging and histology. These lesions may develop secondary to alveolar overdistension, microvascular ischemia, or inflammatory remodeling, often linked to tobacco exposure.[10] Conversely, SSP occurs in the context of underlying lung disease, including COPD, interstitial lung disease, or necrotizing infections, which cause architectural distortion, alveolar wall thinning, and focal necrosis, compromising the alveolar-pleural interface.[6]
Additionally, the formation of alveolar–pleural fistulae or bronchopleural fistulae has been implicated in acute pneumothorax and persistent air leaks. These fistulous tracts represent direct communications between the alveolar or bronchial spaces and the pleural cavity. They are more frequently seen in SSP but can occur in advanced cases of PSP as well. The current understanding of spontaneous pneumothorax emphasizes not only mechanical rupture due to pressure gradients but also structural and microscopic defects in the pleura, airway remodeling, and inflammatory damage as key contributors to its onset and recurrence.
History and Physical
Spontaneous pneumothorax most commonly occurs at rest and without an exertional trigger. Patients typically present with sudden-onset, sharp, pleuritic chest pain on the affected side and acute dyspnea. Increased work of breathing is especially pronounced in those with SSP due to underlying lung disease. Tachycardia is one of the most frequent physical exam findings, although in smaller pneumothoraces (less than 15% of the hemithorax), the exam may be unremarkable. In larger cases (over 15%), clinicians may observe reduced chest wall movement, ipsilateral decreased or absent breath sounds, hyperresonance to percussion, decreased tactile fremitus, jugular venous distension, and pulsus paradoxus. A tension pneumothorax, though rare, represents a life-threatening complication characterized by hypoxemia, hypotension, and tracheal deviation away from the affected side.[13]
Evaluation
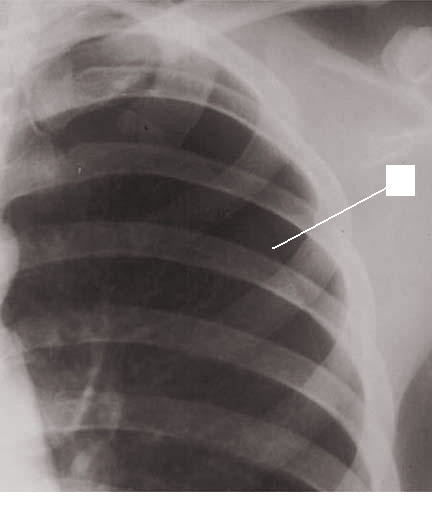
The diagnosis of spontaneous pneumothorax is often suggested by the patient’s history and physical exam findings, which can be confirmed by imaging. Chest radiography characteristically shows the displacement of the visceral pleural line with a space devoid of lung markings in between (see Image. Pneumothorax on Chest Radiography). While upright films are preferred, there is evidence that expiration does not necessarily increase the diagnostic yield. Ultrasound has also shown diagnostic potential. There is evidence that ultrasound has greater sensitivity than chest radiography; however, both modalities are limited in how well they estimate the size of a pneumothorax.
The use of chest computed tomography (CT) for diagnosing spontaneous pneumothorax has been debated. The high sensitivity and specificity of CT can be of value when there is a high index of suspicion for spontaneous pneumothorax, and initial imaging is negative or equivocal. While arterial blood gas measurements are not necessary for diagnosing spontaneous pneumothorax, they can be useful in assessing acute respiratory alkalosis and increases in the alveolar-arterial oxygen gradient when tension physiology is present.[14]
Treatment / Management
The main goal for the treatment of spontaneous pneumothorax is to evacuate the gas from the pleural space and prevent recurrences. The guidelines from the American College of Chest Physicians (ACCP) and the British Thoracic Society are focused mainly on managing pneumothorax in adults, but not specifically pediatric cases. Nevertheless, it is appropriate to initiate 100% oxygen via a nonrebreather mask and continuous cardiopulmonary monitoring for patients with spontaneous pneumothorax. Oxygen increases the rate of absorption of the gas from the pleural space up to 4-fold compared to the absorption of 1% to 2% of the volume per day without oxygen. Clinically unstable individuals with severe symptoms or symptoms suggestive of tension pneumothorax can be treated with emergent needle decompression as a bridge to tube thoracostomy placement.[15][16][17]
For stable patients presenting with a small PSP for the first time, conservative management with supplemental oxygen and observation of at least 6 hours is recommended. Suppose a repeat chest radiograph shows evidence of a stable pneumothorax and the patient has access to adequate follow-up. In that case, the patient can be discharged with strict return precautions for a 24-hour recheck. The British Thoracic Society suggests that certain asymptomatic individuals with large PSP may be considered for observation without active intervention. The ACCP recommends aspiration for large or symptomatic PSP with a small-bore catheter (14F or smaller) or admission with a chest tube (16F to 22F) if the initial aspiration fails. Larger PSP can be further managed with video-assisted thoracoscopic surgery (VATS) or thoracotomy to perform bullectomy, pleurectomy, and mechanical pleurodesis (ie, dry gauze abrasion). VATS is less invasive than thoracotomy and is an effective measure in the treatment and prevention of spontaneous pneumothorax recurrence.
Patients with recurrent PSP should be admitted with thoracostomy tube placement as a bridge to VATS. In patients who are unwilling to undergo VATS, are poor surgical candidates, or are being managed in an institution where VATS is not readily available, chemical pleurodesis can be performed with the introduction of irritants such as tetracyclines (ie, doxycycline, minocycline) or talc via the thoracostomy tube. The inflammatory processes associated with chemical pleurodesis lead to the formation of pleural adhesions that effectively obliterate the pleural space.
In adults presenting with SSP, both the ACCP and the British Thoracic Society recommend admission with supplemental oxygen and a repeat chest radiograph. The organizations also recommend placement of a pleural catheter or thoracostomy tube if the SSP is large, the patient is symptomatic, or the SSP is bilateral. Observation alone is not recommended, as there is an increased risk of mortality in SSP. Referral to a thoracic specialist is recommended, but not until the patient is stabilized with a chest drain.[3]
Differential Diagnosis
The differential diagnoses for spontaneous pneumothorax include:
- Myocardial infarction
- Aortic dissection
- Acute pericarditis
- Pulmonary embolism
- Rib and chest trauma
Prognosis
PSP generally has an excellent survival prognosis, with low associated mortality and prompt recovery in most individuals. However, recurrence is a substantial clinical concern. Recent epidemiological data from South Korea found a 5-year recurrence rate of about 20.3%, with significantly higher rates in men (eg, 20.8% in men vs 10.9% in women); intriguingly, those treated with conservative therapy had lower recurrence (7.9%) compared to those undergoing early surgical intervention (23.7%)—a finding that contrasts with traditional expectations and highlights the importance of personalized treatment decisions. Broad systematic reviews confirm a 32% overall recurrence rate, with most recurrences occurring within the first year following the initial episode. Significantly, recurrence likelihood is influenced by age (ie, younger than 20), presence of blebs/bullae, male sex, and conservative management without intervention.[18] Surgical approaches—especially VATS combined with pleurodesis—can substantially reduce the long-term risk, often lowering recurrence to under 10%.
In contrast, SSP carries a more guarded prognosis, reflecting higher morbidity, mortality, recurrence, and healthcare resource use. One large retrospective cohort reported a 2.3% in-hospital mortality rate and a 9% overall recurrence rate, with both adverse events strongly associated with poor functional status, comorbidities, and larger pneumothorax size.[19] Data also show that up to 41% of those with SSP experience complications, including persistent air leaks in 13.5%. Compared to PSP, patients with SSP endure more extended hospital stays, more frequent persistent leaks, and less favorable recovery trajectories. Surgical repair via VATS is associated with a mortality rate of approximately 2% to 4% and significantly reduced recurrence rates (0%–15.8%). However, patients with interstitial lung disease have higher postoperative mortality, reaching up to 15%, compared with those who have chronic obstructive pulmonary disease.
Complications
Spontaneous pneumothorax, while often manageable in otherwise healthy individuals, can lead to a range of complications that significantly impact morbidity and clinical outcomes, particularly in cases of SSP. One of the most common and well-documented complications is recurrence, with PSP showing a 5-year recurrence rate of approximately 30% and SSP recurrence rates approaching 43%. The risk of recurrence is higher in younger male patients and those treated with nonsurgical approaches. Another significant complication is the development of a persistent air leak (PAL), defined as continued air passage through a chest tube beyond 5 to 7 days, or 48 hours in patients with SSP. PALs are reported in approximately 16% of PSP and over 30% of SSP patients managed with tube thoracostomy.[20] Persistent leaks can be caused by bronchopleural or alveolopleural fistula, often necessitating prolonged hospital stays or advanced interventions such as surgical repair or endobronchial valve placement.
Although rare, tension pneumothorax remains the most life-threatening complication. This condition presents with hypotension, hypoxemia, tracheal deviation, and mediastinal shift, requiring immediate needle decompression followed by definitive chest tube placement. Reexpansion pulmonary edema is another serious but infrequent complication, occurring when a large or longstanding pneumothorax is rapidly evacuated, particularly if the lung has been collapsed for over 72 hours or if more than 2 liters of air are removed quickly. This complication can manifest as sudden hypoxia and respiratory distress, and has been reported in up to 46% of delayed cases.
Infectious complications can also arise, particularly in patients with indwelling chest tubes. These may include empyema, pleuritis, or even pyopneumothorax, especially if sterile technique is compromised or drainage is prolonged. In addition, bronchopleural and alveolopleural fistulae—the underlying mechanism behind many PALs—are reported in nearly 35% of spontaneous pneumothorax cases, particularly in those with structural lung disease or necrosis.
Finally, complications related to thoracostomy procedures themselves include bleeding, hemothorax, intercostal nerve injury, subcutaneous emphysema, and localized infection. Although rare, these can add significant morbidity, especially in elderly or comorbid populations. As newer strategies like ambulatory catheter systems and surgical pleurodesis gain traction, complication profiles may shift. Still, the core risks, especially persistent air leak and recurrence, remain pivotal in determining long-term outcomes.
Deterrence and Patient Education
Effective prevention of recurrent spontaneous pneumothorax hinges on comprehensive patient education and lifestyle modification. First and foremost, all patients should be strongly encouraged to quit smoking, as tobacco use remains the most significant modifiable risk factor; cessation efforts—including counseling, nicotine replacement therapy, bupropion, or varenicline—can significantly lower the likelihood of recurrence and improve overall respiratory health. Clinicians should assess a patient’s readiness to quit, provide pharmacotherapy, and incorporate behavioral support to bolster success. Air travel and scuba diving pose serious risks following a pneumothorax. Patients should be advised not to fly or dive until full resolution is confirmed via imaging—typically after at least 2 weeks of proven resolution—or until definitive surgical management, such as pleurodesis, is completed in recurrent cases.
High-risk professions (eg, pilots, divers) may require occupational restrictions or preflight surgical intervention to mitigate catastrophic decompression-related collapse. Patients should understand the importance of close follow-up postdischarge, including repeat chest radiography to ensure pneumothorax resolution and detect early recurrence. Education on recognizing warning symptoms—sudden pleuritic chest pain or dyspnea—is essential, enabling prompt return to care before complications arise.
For recurrent or persistent disease, discussions about definitive treatment options such as VATS with pleurodesis or chemical pleurodesis are necessary; guidelines for reducing recurrence risk support these interventions. Finally, lifestyle guidance should include avoiding rapid altitude changes and strenuous activities shortly after resolution, and emphasizing smoking and vaping avoidance, as emerging evidence links electronic cigarette use to recurrent pneumothorax in young adults. By providing patients with a clear understanding of risk factors, red flags, and safe behaviors, clinicians can meaningfully reduce recurrence and support long-term pulmonary health.
Pearls and Other Issues
The disposition of patients with spontaneous pneumothorax is multifactorial. For patients with a first-time episode of PSP that is small and asymptomatic, conservative measures can be a reasonable option. For patients with a first-time episode of PSP that is large and/or symptomatic, aspiration can be attempted as discussed in the treatment/management section; however, failure should prompt admission. The current recommendation is that all patients with SSP, regardless of the stability of the patient and the size of the pneumothorax, should be admitted. Even with proper management, the recurrence rate for spontaneous pneumothorax is relatively high. Some study results estimate a recurrence rate of over 50% with the highest risk within the first 30 days. However, the recurrence rate can be less than 5% after VATS with resection of blebs/bullae and pleurodesis.[7][21]
Special considerations should be made for patients with travel plans due to the risk of pneumothorax expansion. While some guidelines suggest that air travel should be delayed at least a week after radiographic evidence of pneumothorax resolution, thoracic surgeons in the United States do not have a definite consensus about the timeframe; further investigation of the topic is warranted. A consensus is that patients with a spontaneous pneumothorax should be urged to quit smoking and avoid air travel or travel to areas where medical care is not readily accessible. [22][23]
Enhancing Healthcare Team Outcomes
Effective management of spontaneous pneumothorax requires a coordinated, multidisciplinary approach to ensure timely diagnosis, appropriate intervention, and optimal patient outcomes. Clinicians must possess strong clinical assessment skills, rapidly interpret imaging studies, and implement evidence-based treatment strategies, whether that involves conservative observation, needle aspiration, or chest tube placement. Nurses are critical in continuous patient monitoring, especially for signs of respiratory distress or progression to tension pneumothorax, and in maintaining chest tube systems. Pharmacists contribute by reviewing medications that may affect respiratory status (eg, sedatives, bronchodilators), managing pain control regimens, and ensuring safe use of supplemental oxygen when needed.
Interprofessional communication and structured care coordination are essential to promoting patient safety and enhancing team performance. Rapid communication between the emergency department, radiology, pulmonology, and thoracic surgery teams ensures seamless escalation of care when necessary. Using standardized protocols and checklists can reduce variability and error, particularly in procedures like chest tube placement. Collaboration during discharge planning—including educating patients on symptom recognition, smoking cessation, and follow-up imaging—strengthens patient-centered care and reduces recurrence risk. High-functioning teams that share accountability and communicate clearly across disciplines significantly improve outcomes for patients presenting with spontaneous pneumothorax.
Media
(Click Image to Enlarge)
References
Baig MA, Majeed MB, Attar BM, Khan Z, Demetria M, Gandhi SR. Efficacy and Safety of Indwelling Pleural Catheters in Management of Hepatic Hydrothorax: A Systematic Review of Literature. Cureus. 2018 Aug 6:10(8):e3110. doi: 10.7759/cureus.3110. Epub 2018 Aug 6 [PubMed PMID: 30338185]
Level 1 (high-level) evidenceOjeda Rodriguez JA, Hipskind JE. Iatrogenic Pneumothorax. StatPearls. 2025 Jan:(): [PubMed PMID: 30252313]
Cheng HS, Wong C, Chiu PH, Tong CW, Miu PF. Management of spontaneous pneumothorax: a mini-review on its latest evidence. Journal of thoracic disease. 2024 Jul 30:16(7):4756-4763. doi: 10.21037/jtd-24-415. Epub 2024 Jul 15 [PubMed PMID: 39144356]
Shorthose M, Barton E, Walker S. The contemporary management of spontaneous pneumothorax in adults. Breathe (Sheffield, England). 2023 Dec:19(4):230135. doi: 10.1183/20734735.0135-2023. Epub 2024 Jan 16 [PubMed PMID: 38229681]
Wang R, Chen X, Xu S, Jiang X, Liu J, Liu X, Ryu JH, Hu X. Prevalence and recurrence rates of spontaneous pneumothorax in patients with diffuse cystic lung diseases in China. Orphanet journal of rare diseases. 2025 Feb 11:20(1):69. doi: 10.1186/s13023-025-03587-6. Epub 2025 Feb 11 [PubMed PMID: 39934870]
Liao KM, Chiu CC, Lu HY. The risk of secondary spontaneous pneumothorax in patients with chronic obstructive pulmonary disease in Taiwan. Respiratory medicine. 2024 Jul:228():107672. doi: 10.1016/j.rmed.2024.107672. Epub 2024 May 17 [PubMed PMID: 38763446]
Hallifax RJ, Goldacre R, Landray MJ, Rahman NM, Goldacre MJ. Trends in the Incidence and Recurrence of Inpatient-Treated Spontaneous Pneumothorax, 1968-2016. JAMA. 2018 Oct 9:320(14):1471-1480. doi: 10.1001/jama.2018.14299. Epub [PubMed PMID: 30304427]
Savitsky E, Oh SS, Lee JM. The Evolving Epidemiology and Management of Spontaneous Pneumothorax. JAMA. 2018 Oct 9:320(14):1441-1443. doi: 10.1001/jama.2018.12878. Epub [PubMed PMID: 30304415]
Zhong X, Goldacre R, Morris EJA, Hallifax RJ. Trends in incidence of pneumothorax in England before, during and after the COVID-19 pandemic (2017-2023): a population-based observational study. The Lancet regional health. Europe. 2024 Sep:44():100994. doi: 10.1016/j.lanepe.2024.100994. Epub 2024 Jul 1 [PubMed PMID: 39049868]
Level 2 (mid-level) evidenceHallifax R. Aetiology of Primary Spontaneous Pneumothorax. Journal of clinical medicine. 2022 Jan 19:11(3):. doi: 10.3390/jcm11030490. Epub 2022 Jan 19 [PubMed PMID: 35159942]
Walker SP, Bibby AC, Halford P, Stadon L, White P, Maskell NA. Recurrence rates in primary spontaneous pneumothorax: a systematic review and meta-analysis. The European respiratory journal. 2018 Sep:52(3):. pii: 1800864. doi: 10.1183/13993003.00864-2018. Epub 2018 Sep 6 [PubMed PMID: 30002105]
Level 1 (high-level) evidenceBertolaccini L, Congedo MT, Bertani A, Solli P, Nosotti M. A project to assess the quality of the published guidelines for managing primary spontaneous pneumothorax from the Italian Society of Thoracic Surgeons. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2018 Nov 1:54(5):920-925. doi: 10.1093/ejcts/ezy199. Epub [PubMed PMID: 29788194]
Level 2 (mid-level) evidenceJune C, Viscusi C, DeLange B. Simultaneous Spontaneous Bilateral Tension Pneumothorax Post COVID-19 Infection: A Case Study. Cureus. 2022 May:14(5):e25107. doi: 10.7759/cureus.25107. Epub 2022 May 18 [PubMed PMID: 35733472]
Level 3 (low-level) evidenceAguinagalde B, Aranda JL, Busca P, Martínez I, Royo I, Zabaleta J, Grupo de trabajo de la GPC para el Manejo de Pacientes con Neumotórax espontáneo. SECT Clinical practice guideline on the management of patients with spontaneous pneumothorax. Cirugia espanola. 2018 Jan:96(1):3-11. doi: 10.1016/j.ciresp.2017.11.005. Epub 2017 Dec 13 [PubMed PMID: 29248330]
Level 1 (high-level) evidenceSchnell J, Beer M, Eggeling S, Gesierich W, Gottlieb J, Herth FJF, Hofmann HS, Jany B, Kreuter M, Ley-Zaporozhan J, Scheubel R, Walles T, Wiesemann S, Worth H, Stoelben E. Management of Spontaneous Pneumothorax and Post-Interventional Pneumothorax: German S3 Guideline. Respiration; international review of thoracic diseases. 2019:97(4):370-402. doi: 10.1159/000490179. Epub 2018 Jul 24 [PubMed PMID: 30041191]
Wong A, Galiabovitch E, Bhagwat K. Management of primary spontaneous pneumothorax: a review. ANZ journal of surgery. 2019 Apr:89(4):303-308. doi: 10.1111/ans.14713. Epub 2018 Jul 5 [PubMed PMID: 29974615]
. . :(): [PubMed PMID: 28745853]
Yi E, Park JE, Chung JH, Ahn CB, Chung E, Noh OK, Lee S. Trends in recurrence of primary spontaneous pneumothorax in young population after treatment for first episode based on a nationwide population data. Scientific reports. 2023 Aug 18:13(1):13478. doi: 10.1038/s41598-023-39717-y. Epub 2023 Aug 18 [PubMed PMID: 37596298]
Farag GAI, Zineldin MAI, Al Awady RSAA, Abd El Salam AB, Elkahely MA. Comparative Analysis of Demographic Characteristics, Management, and Outcomes in Primary Versus Secondary Spontaneous Pneumothorax. Cureus. 2024 Jul:16(7):e65216. doi: 10.7759/cureus.65216. Epub 2024 Jul 23 [PubMed PMID: 39176370]
Level 2 (mid-level) evidenceDeğirmenci M. Morbidity, mortality, and surgical treatment of secondary spontaneous pneumothorax. Ulusal travma ve acil cerrahi dergisi = Turkish journal of trauma & emergency surgery : TJTES. 2023 Aug:29(8):909-919. doi: 10.14744/tjtes.2023.20566. Epub [PubMed PMID: 37563896]
Santos C, Gupta S, Baraket M, Collett PJ, Xuan W, Williamson JP. Outcomes of an initiative to improve inpatient safety of small bore thoracostomy tube insertion. Internal medicine journal. 2019 May:49(5):644-649. doi: 10.1111/imj.14110. Epub [PubMed PMID: 30230151]
Sano A. Multidisciplinary team approach for complicated pneumothorax. Journal of thoracic disease. 2018 Jul:10(Suppl 18):S2109-S2110. doi: 10.21037/jtd.2018.06.94. Epub [PubMed PMID: 30123534]
Li X, Su X, Chen B, Yao H, Yu Y, Leng X, Lu Q, Wang C, Lei J, Ruetzler K, Fernando HC, Gilbert S, Yeung C, Filosso PL, Shen J, Zhu C, Written, AME Thoracic Surgery Collaborative Group. Multidisciplinary team approach on a case of bilateral tension pneumothorax. Journal of thoracic disease. 2018 Apr:10(4):2528-2536. doi: 10.21037/jtd.2018.04.81. Epub [PubMed PMID: 29850161]
Level 3 (low-level) evidence