Introduction
Spinal cord injuries (SCIs) are multidimensional disorders arising from direct or indirect spinal cord damage. The most common cause of SCI is acute trauma from motor vehicle collisions, although the condition may also arise from insidious etiologies such as malignancies and chronic tuberculous infection. Spinal cord lesions may lead to permanent disability, significant morbidity, and mortality. High spinal injuries often impair cardiorespiratory function and require emergent interventions.
Nerve axon disruption results in loss of motor and sensory function below the injury level.[1] SCIs disproportionately impact individuals younger than 30, resulting in substantial lifelong functional impairment and potential health, financial, and psychosocial complications.[2] One case of SCI is estimated to have a lifetime economic impact of $2 to $4 billion.[3][4] Interventions range from initial emergency stabilization to advanced treatments, such as stem cell therapy, which require a long-term commitment. As such, selecting the appropriate therapeutic approach is essential for clinicians across the continuum of care.
The spinal cord is a cylindrical structure that extends from the brain caudally through the vertebral column, typically beginning at the base of the brainstem at the medulla oblongata, passing through the foramen magnum, and continues within the spinal canal formed by the vertebrae, terminating at approximately the L1–L2 vertebrae in adults. The spinal cord serves as a critical conduit for communication between the brain and the rest of the body, transmitting sensory signals from the body to the brain through the afferent pathways and motor signals from the brain to the body through the efferent pathways; it also integrates certain reflexes independently of the brain. The spinal cord is divided into segments corresponding to the vertebral levels. Each segment gives rise to spinal nerves supplying specific body regions. The key structures within the spinal cord include the corticospinal (CST) and spinothalamic (STT) tracts, as well as the dorsal columns—nerve pathways that exchange information between the brain and the body.
The CST is a major motor pathway responsible for voluntary movement. This nerve tract originates from the motor cortex of the cerebrum and descends through the brainstem and spinal cord. Approximately 90% of the CST fibers travel on the lateral side of the spinal cord, forming the lateral CST (LCST). The LCST nerves travel throughout the cord. The remaining fibers transit ventrally or anteriorly, forming the ventral CST (VCST). However, VCST fibers do not reach levels below the superior thoracic spinal segments.
The STT is a sensory pathway that relays pain and temperature information from the body to the brain. The spinothalamic tract ascends through the anterolateral spinal cord, synapsing in the thalamus before projecting to the somatosensory cortex. Furthermore, the dorsal columns, also known as the posterior columns or dorsal funiculi, convey proprioceptive and tactile sensations (ie, touch, pressure, vibration) from the body to the brain. Dorsal column fibers ascend in the spinal cord's posterior area and consist of the fasciculus gracilis medially and the fasciculus cuneatus laterally.
The spinal cord comprises several distinct regions, each corresponding to a specific vertebral segment level. Cervical nerves (C1–C8) supply the neck, shoulders, arms, and hands. Thoracic nerves (T1–T12) innervate the ulnar side of the upper limbs, the trunk, and abdominal muscles. Lumbar nerves L1 to L5 supply the lower back, buttocks, and lower limbs. Sacral nerves (S1–S5) innervate the pelvic organs, buttocks, genitals, and lower limbs. The coccygeal nerve (C0) provides sensory innervation to the skin overlying the coccyx and surrounding areas and also contributes to the motor function of the pelvic floor muscles. Spinal nerve distributions are best represented by dermatomal maps (see Image. Dermatome Map).
The cauda equina consists of spinal nerve roots L2 to S5/C0, extending from the lower end of the spinal cord. The conus medullaris is the terminal portion of the spinal cord and is typically located at the L1 to L2 levels. The filum terminale extends from the conus medullaris and anchors the spinal cord and dural sac to the coccyx, providing structural support to the spinal cord. SCIs result in a wide range of clinical symptoms, each requiring individualized management strategies. Understanding the anatomy and organization of the spinal cord, including the locations of critical nerve pathways, is essential for diagnosing and managing neurological conditions that affect motor and sensory function.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
SCI may result from traumatic or nontraumatic mechanisms. Traumatic etiologies dominate globally, with motor vehicle collisions identified as the leading cause in the United States, responsible for approximately 38% of new SCI cases annually. Falls represent another significant cause, contributing to roughly 30% of SCI incidents, particularly among older adults. Violence accounts for approximately 13% of cases, sports-related injuries around 9%, and medical or surgical complications constitute about 5% of cases.
Nontraumatic SCI can arise from conditions such as tumors, infections, and degenerative spinal changes. Tumors, including primary spinal neoplasms or metastatic lesions, may compress or directly damage spinal cord tissue. Infections such as epidural abscesses or spinal osteomyelitis can also result in SCI through cord compression, vascular occlusion, and spinal instability. Degenerative spinal conditions, such as spinal stenosis and spondylosis, slowly narrow the spinal canal, making the spinal cord more susceptible to injury—even from minor trauma or everyday activities.
Older adults are at increased risk for SCI due to reduced bone density associated with osteoporosis and osteopenia. These conditions weaken the structural strength of the spine, making the spine vulnerable to fractures and SCI even from minimal trauma. Additionally, conditions such as ankylosing spondylitis and diffuse idiopathic skeletal hyperostosis predispose patients to highly unstable fractures.
Epidemiology
Globally, between 250,000 and 500,000 patients experience SCIs annually. Most SCI cases arise from preventable causes, such as violence and motor vehicle collisions. Approximately 17,000 new SCI cases in the United States (US) occur each year, and 282,000 persons are estimated to be living with SCIs. Most sports-related SCIs occur in men. In the US, the annual incidence rate is approximately 55 new cases per million population, excluding fatalities occurring before hospital transport.[5] Historically, SCI predominantly affected younger men. However, epidemiological trends show an increasing proportion of older adults experiencing SCI due to age-related vulnerabilities.
A growing proportion of individuals with SCI are older than 65. This increase is attributed to 2 factors: a higher average age at injury onset, referred to as "SCI with aging," and improved survival and longevity following the injury, known as "aging with SCI." Additionally, the growing number of older adults in society has led to an increased incidence and prevalence of both traumatic and nontraumatic SCI, predominantly resulting from falls and neoplasms.[6]
Pathophysiology
SCI Mechanisms
SCIs arise from complex mechanisms, resulting in varying neurologic deficits depending on the location and extent of the injury. The mechanisms underlying SCIs are classified as either primary or secondary (see Table. SCI Phases). Secondary SCI emerges from a series of biological phenomena that begin within minutes of a primary injury and continue for weeks to months. The acute secondary injury phase encompasses vascular damage, ionic imbalances, free radical formation, the initial inflammatory response, and neurotransmitter accumulation, known as excitotoxicity.[7][8]
Table. SCI Phases
| Phase | Timing and Duration After the Injury | Characteristic Features |
| Early acute phase | First 2 to 48 hours |
|
| Acute phase | First 2 hours to 2 weeks |
|
| Subacute phase | Day 2 to end of week 2 |
|
| Intermediate phase | Week 2 to month 6 |
|
| Chronic phase | Month 6 onward |
|
Post-SCI Immune Response
Post-SCI neuroinflammation exhibits a dual nature, potentially resulting in both beneficial and deleterious outcomes, depending on the timing and the type of immune cells present at the injury site. In the initial 3 days post-injury, blood-borne neutrophils, resident microglia, and astrocytes are recruited to the injury site, initiating the inflammatory response. A second phase ensues around 3 days postinjury, attracting macrophages and B and T lymphocytes. Antigen-presenting cells activate CD4+ helper T cells to release cytokines, which stimulate B cells to produce antibodies, thereby intensifying neuroinflammation and tissue destruction. Notably, neuroinflammation is most pronounced during the acute phase of SCI.
Inflammation is protracted in the subacute and chronic phases. The inflammatory cell composition and phenotype vary with the stage of inflammation and the signal molecules present within the injury microenvironment. T and B cells, microglia, and macrophages can adopt a proinflammatory, anti-inflammatory, or proregenerative phenotype. Spinal cord disruption results in deficits of motor and sensory function below the level of the injury. Disability patterns depend on the injury level and extent of spinal tract involvement.[9][10]
STT damage results in contralateral pain and loss of temperature sensation. CST disruption leads to ipsilateral weakness or paralysis. In the cervical spine, CST fibers supplying the upper extremities are proximal to the center of the spinal cord. In contrast, CST nerves to the lower extremities are located distally. Dorsal column injury leads to ipsilateral loss of tactile, proprioceptive, and vibratory sensations.
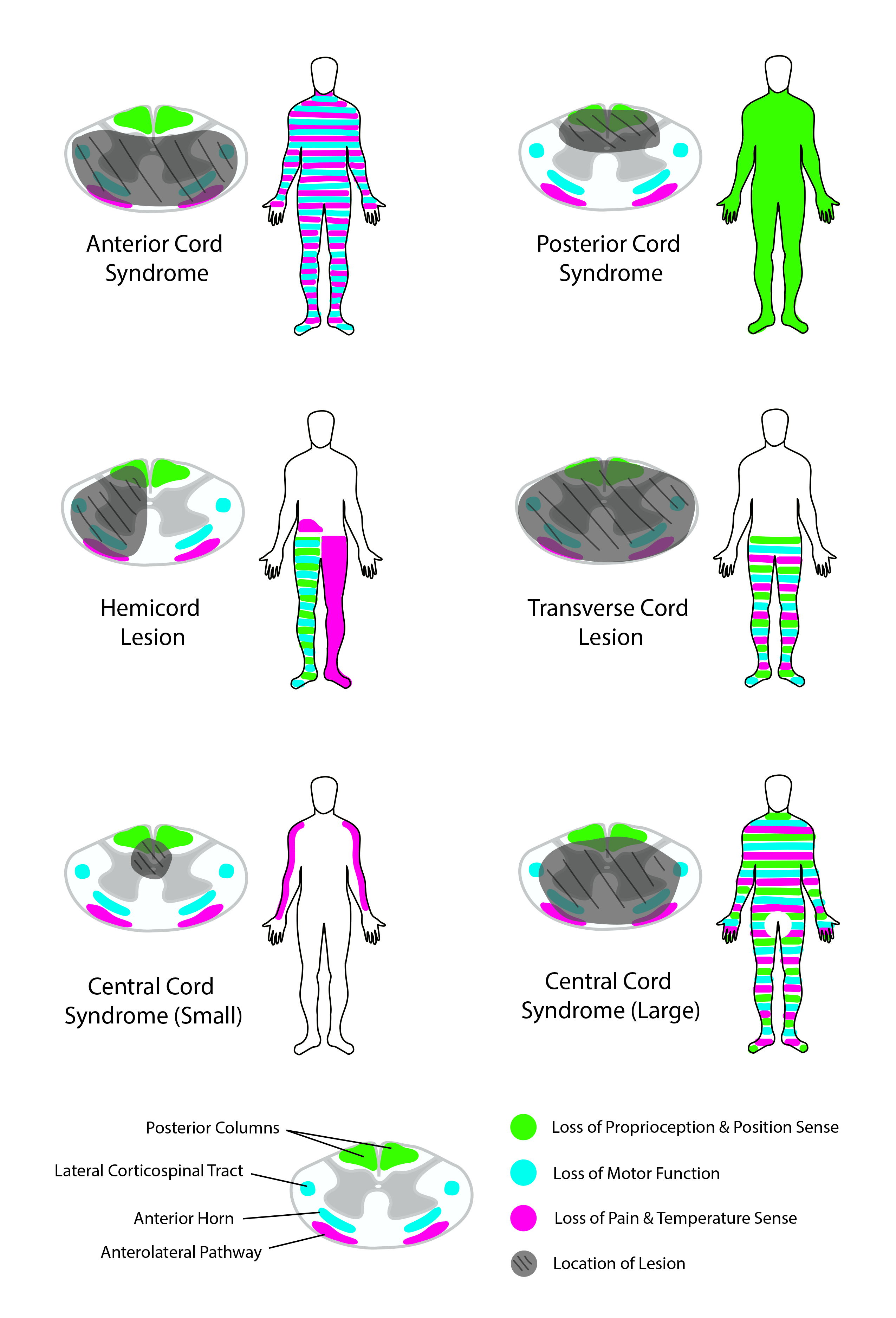
Spinal Cord Syndromes
Several SCI patterns are well described (see Image. Spinal Cord Syndromes). The origins and manifestations of these different spinal cord syndromes are explained below.[9][10][11]
Complete spinal cord transection
- Complete spinal cord transections typically demonstrate total bilateral loss of motor function and pain, temperature, proprioceptive, vibratory, and tactile perception below the injury level.
- Lumbosacral injuries present with lower extremity sensorimotor deficits and bowel, bladder, and sexual dysfunction.
- Thoracic injuries lead to the same deficits as lumbosacral injuries and, in addition, may result in trunk motor weakness, which can produce postural difficulties.
- Cervical injuries lead to the same deficits as thoracic injuries but with added upper limb function loss, leading to tetraplegia.
- High-spinal injuries above C5 may also cause respiratory compromise due to loss of diaphragm innervation.[12]
Central cord syndrome
- Central cord syndrome is the most common form of incomplete SCI. Hyperextension of the neck in the presence of pre-existing spinal stenosis typically causes compression of the cervical spinal cord. Clinically, this syndrome usually produces greater weakness in the upper extremities compared to the lower extremities. One proposed explanation for this pattern is that during injury, the central region of the spinal cord experiences greater mechanical stress.
- Due to the arrangement of the CST, axons controlling the upper limbs lie closer to the spinal cord's center, while those innervating the lower limbs are located more peripherally. Consequently, injuries primarily affecting the central region produce greater deficits in upper extremity function.
- An alternative hypothesis suggests that gray matter, predominantly controlling upper extremity function at cervical levels, is more vulnerable to injury than white matter. Additionally, loss of pain and temperature sensation may occur below the level of the lesion.
Anterior cord syndrome
- This condition is typically caused by compromised blood flow in the anterior spinal artery.
- Bilateral STT injuries lead to bilateral pain and temperature perception loss below the level of injury.
- Bilateral CST injuries paralyze the muscles below the injury level.
- Vibration sensations, proprioception, and, to some degree, tactile sensation remain intact, as the dorsal columns are unaffected.
Posterior cord syndrome
- This injury pattern occurs more frequently from infectious, toxic, or metabolic than traumatic causes.
- Dorsal column damage weakens tactile, vibratory, and proprioceptive perception.
- Pain and temperature sensory and motor function are preserved due to the lack of STT and CST involvement.
Brown-Séquard syndrome
- This condition results from right- or left-sided spinal cord hemisection.
- CST and dorsal column transection lead to ipsilateral loss of motor function, proprioception, and tactile and vibratory sensations below the injury level.
- STT disruption produces contralateral deficits in pain and temperature perception below the level of injury.[13]
Conus medullaris syndrome
- This injury pattern develops from terminal spinal cord damage at an area proximal to the cauda equina.
- Conus medullaris syndrome characteristically presents with sacral nerve dysfunction, manifesting as the loss of Achilles tendon reflexes, bowel, bladder, and sexual function.
Neurogenic shock
- Spinal cord injuries above the mid-thoracic level lead to a loss of sympathetic tone, producing neurogenic shock—a state characterized by hypotension and bradycardia.[14]
History and Physical
Patients with an SCI, especially in the setting of acute polytrauma or high spinal segment involvement, may present in shock or cardiorespiratory arrest. A quick primary survey must assess the airway, breathing, circulation, disability, and exposure. Resuscitative measures should be initiated immediately. A more detailed investigation may be conducted once the patient has stabilized.
Individuals with a suspected spinal cord injury frequently describe various trauma mechanisms, including motor vehicle collisions, falls, sports injuries, or penetrating trauma. Neurological symptoms commonly reported by these patients include weakness, tingling, or sensory loss in specific areas of the body below the level of injury. Pain, ranging from localized discomfort to radiating pain, may also be reported, depending on the severity and location of the injury. Additionally, patients may experience motor weakness, which manifests as difficulty moving the limbs or performing previously routine activities. Complaints of urinary or fecal incontinence, difficulty voiding, or loss of bowel control are frequently encountered following an SCI. Respiratory symptoms, such as tachypnea, difficulty coughing, or impaired breathing, may occur due to involvement of the cervical or thoracic spinal segments.
Subacute SCI may arise from untreated, unstable spinal column injuries or etiologies like malignancy and infection. Patients with insidious SCI causes may report persistent back pain that worsens at night or with movement and weight-bearing. Constitutional symptoms may be reported, such as unexplained weight loss, intermittent fever, anorexia, and generalized weakness. The back may feel stiff and have limited mobility. Sensorimotor weakness progresses as the lesion grows. Patients may initially feel paresthesias in the distribution area of the involved nerve and develop motor weakness later. Loss of grasp, ambulation, and bladder and bowel function may emerge in late stages. Cardiorespiratory compromise may result from a high spinal injury. Risk factors that may be elicited include chronic smoking, previous cancer treatment, migration from a place where tuberculosis is endemic, recent surgery, and immunosuppression.
In clinical history taking, it is crucial to maintain awareness of degenerative cervical myelopathy (DCM), a prevalent yet frequently overlooked cause of nontraumatic spinal cord injury. Often characterized as a "slow-motion" spinal cord injury, DCM symptoms develop insidiously, with subtle neck pain, sensory disturbances, and gradually progressive motor impairment frequently mistaken for age-related changes. Heightened clinician awareness and early recognition of DCM can facilitate timely intervention, potentially preventing severe neurological deterioration and chronic disability.[15]
On physical examination, clinicians typically observe neurological deficits below the injury level, including decreased motor strength, altered sensation (hypoesthesia or anesthesia), and abnormal reflexes. Some individuals may develop the classic signs of a spinal cord syndrome, depending on the lesion's location and severity. Patients with acute trauma may have signs of multiple injuries, such as craniofacial and limb fractures, abrasions, bruises, bleeding, altered sensorium, thoracoabdominal tenderness, hypotension, and respiratory distress. If the clinical condition allows, then the International Standards for Neurological Classification of Spinal Cord Injury exam is performed.
On the other hand, patients with chronic SCI may exhibit muscle wasting or atrophy in areas affected by paralysis or disuse, alongside signs of spinal deformity such as abnormal curvature, tenderness, or palpable bony abnormalities. Skin changes indicative of pressure ulcers, such as redness, blistering, or breakdown over bony prominences, may be noted due to immobility. The cord injury pattern depends on the site and extent of the lesion.
The International Standards for Neurological Classification of Spinal Cord Injury exam
The International Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI), developed by the American Spinal Injury Association (ASIA) and the International Spinal Cord Society (ISCoS), are essential in evaluating SCI.[16] It systematically documents the severity and neurological level of SCI, providing a standardized approach to classify injuries for clinical management, prognostication, and research. Employing ISNCSCI facilitates consistent communication among healthcare professionals, guides treatment decisions, and helps track neurological recovery over time.
To perform ISNCSCI, clinicians systematically assess sensory and motor functions. The sensory examination involves bilateral testing of 28 dermatomes using both light touch and pinprick stimuli. The sensation is graded from 0 (absent) to 2 (normal), with a comparison to the facial sensation as a reference. The most caudal dermatome demonstrating normal sensation bilaterally identifies the sensory level of injury. The motor examination evaluates 5 key muscle groups in both upper and lower limbs bilaterally. Muscle strength is graded on a scale of 0 (total paralysis) to 5 (normal strength against resistance). The motor level of injury is the lowest spinal level with at least antigravity muscle strength (score ≥3), provided all higher muscle groups are normal (score of 5).
Assessment of sacral sparing involves evaluating sensory and motor function at the S4–S5 segments through deep anal pressure and voluntary anal contraction, thereby determining the completeness of the injury. Injury completeness or incompleteness is then classified using the ASIA Impairment Scale (AIS). The ISNCSCI examination is practical, requiring minimal equipment, and can be performed with the patient in a supine position, making it applicable across various clinical settings and phases of patient care. While comprehensive, ISNCSCI does not include reflex or proprioception testing and thus should be complemented by additional neurologic evaluations when clinically indicated.
Evaluation
Imaging Studies for SCI Assessment
SCIs most often occur in the context of significant trauma. Thus, a comprehensive clinical assessment for concurrent injuries is necessary at the time of presentation. Recognition of the above injury patterns can help localize the location and type of injury. A thorough clinical examination, including a precise assessment of motor and sensory function, is imperative for accurate classification. Spinal cord injuries are graded using the ASIA Impairment Scale. The grading system varies based on injury severity from letters A to E (see Image. American Spinal Injury Association [ASIA] Spinal Cord Injury Scoring Sheet).
- ASIA A: Complete injury with loss of motor and sensory function
- ASIA B: Incomplete injury with preserved sensory function but complete loss of motor function
- ASIA C: Incomplete injury with preserved motor function below the injury level
- Less than half of these muscles have Medical Research Council (MRC) grade 3 strength.
- ASIA D: Incomplete injury with preserved motor function below the injury level
- At least half of these muscles have MRC grade 3 strength.
- ASIA E: Normal motor and sensory examination; the neurological deficits have resolved [17]
Imaging is vital in accurately identifying injuries. Traditionally, plain radiographs were utilized to evaluate spinal trauma. However, due to their limited sensitivity, the American College of Surgeons Trauma Quality Programs' best practice guidelines no longer recommend them for initial screening. Noncontrast computed tomography (CT) is the primary imaging modality, providing rapid, high-resolution visualization of vertebral fractures and structural damage. CT provides sensitivity greater than 98% for cervical spine injuries and approaches 100% for thoracolumbar injuries, effectively identifying fractures, dislocations, and bony pathology.[18][19] Imaging should employ slice thicknesses of 3 mm or smaller in the cervical spine and 5 mm or smaller in the thoracolumbar spine, as per the American College of Surgeons Trauma Quality Programs' best practice guidelines. Routine reformatted CT images in coronal and sagittal planes enhance diagnostic accuracy and facilitate surgical planning. However, CT has inferior sensitivity for soft tissue injuries.
Magnetic resonance imaging (MRI) is more reliable than CT when looking for soft tissue pathology, including SCI; this modality can accurately locate the SCI level.[20] MRI can likewise help with prognostication. Several scoring systems can predict SCI prognosis using MRI findings (see Image. Traumatic Thoracic Spondyloptosis, Magnetic Resonance Image [MRI]).[21] Early SCI findings on MRI include spinal cord compression, contusion, edema, transection, hemorrhage, and ligamentum flavum bulging.[22] Subacute findings include spinal cord edema, subacute progressive ascending myelopathy, and syrinx.[23]
MRI is recommended in cases of neurological deficits inconsistent with CT findings, significant pain despite normal CT results, or suspected spinal instability (eg, ligamentous injuries detectable on short TI inversion recovery sequences). If possible, MRI should be conducted before surgery in adult patients with acute spinal cord injury to support clinical decision-making. Performing MRI in adults during the acute SCI phase, either pre- or post-operatively, is suggested to enhance prognostication of neurological recovery.[24] Traumatic disc herniation is typically seen in vertebral dislocations and hyperextension injuries. Associated MRI findings may include epidural hematoma, pseudomeningoceles, extradural fluid collections, damage to the craniocervical arteries (eg, carotid and vertebral arteries), and vertebral fractures.[25]
Vascular Evaluation
Patients with cervical spine trauma are at elevated risk for blunt cerebrovascular injuries (BCVI). CT angiography screening for BCVI is recommended for high-risk individuals, especially those with fractures involving the transverse foramen, injuries at the upper cervical spine (C1–C3), or cervical subluxations. Early detection of BCVI significantly reduces the risks of ischemic complications by enabling the timely initiation of anticoagulant or antiplatelet therapy.
Emerging Modalities
Quantitative MRI techniques, including magnetization transfer, magnetic resonance relaxation mapping, and diffusion imaging, aid in assessing the microstructural neuronal characteristics associated with myelination state and axonal degeneration or regeneration. A diffusion tensor tractography (DTT) reconstructs significant nerve bundles in 3-dimensional environments based on anisotropy features. DTT may be created from diffusion tensor imaging data using a computational technique. This modality can distinguish between damaged and deformed nerve fibers and healthy tissue. DTT provides information on the extent of SCI and may be used to plan surgical interventions. Implanting an epidural or intrathecal pressure-monitoring device may help clinicians better understand spinal cord perfusion pressure parameters following an injury.[8]
Biomarkers
Recent research highlights the potential utility of fluid biomarkers in evaluating acute SCI. Neurofilament light chain (NF-L) and glial fibrillary acidic protein (GFAP), measured in serum and cerebrospinal fluid, have emerged as promising biomarkers. These biomarkers correlate closely with injury severity and neurological outcomes, providing objective, quantitative assessments of SCI. Higher serum and cerebrospinal fluid levels of NF-L and GFAP within the first few days after injury significantly correlate with greater injury severity, as defined by the ASIA Impairment Scale (AIS). Importantly, elevated biomarker levels at 72 hours post-injury reliably predict poorer neurological recovery at 6 months. Therefore, incorporating NF-L and GFAP assessments could enhance early risk stratification, guide therapeutic interventions, and inform patient counseling regarding expected recovery trajectories.[26]
Treatment / Management
Acute SCI Treatment
Management of SCI begins immediately at the trauma scene. Emergency medical services personnel perform spinal motion restriction (SMR) to reduce further neurologic damage during patient transfer. SMR involves limiting undesired spine movement using devices such as backboards or scoop stretchers. The term "spinal motion restriction" is preferred over "immobilization," acknowledging that complete immobilization is unattainable. Per the American College of Surgeons/Trauma Quality Programs' best practice guidelines, adult patients with blunt trauma require SMR under certain circumstances: altered consciousness, midline spinal pain, focal neurologic deficits, spinal deformities, or distracting injuries preventing reliable assessment. Adequately trained personnel in safe transfer techniques are essential to minimize the risk of exacerbating potential spinal instability.
Concurrent life-threatening conditions, including hypotension and respiratory compromise, require immediate stabilization. Hypotension and shock aggravate spinal cord injury severity and worsen neurologic outcomes; prompt hemodynamic resuscitation is critical. Shock in SCI is typically neurogenic, resulting from disrupted sympathetic outflow; thus, management commonly involves vasopressor therapy to maintain hemodynamic stability.
Early surgical decompression—ideally within 24 hours, when indicated and feasible—may improve neurologic outcomes by relieving spinal cord compression.[27] Additionally, surgery can stabilize the spine structurally, preventing further deformity or neurological deficits. The 2024 guidelines recommend augmenting mean arterial pressure (MAP) to a minimum range of 75 to 80 mm Hg to enhance spinal cord perfusion following acute traumatic SCI, avoiding active elevation beyond an upper threshold of 90 to 95 mm Hg. Additionally, the guidelines suggested maintaining MAP within this range for approximately 3 to 7 days.[28](A1)
The use of steroids in the treatment of spinal cord injuries remains a topic of ongoing debate. Steroids were initially thought to enhance anti-inflammatory mechanisms, reduce secondary SCI, and increase cell viability. The results of initial studies suggested potential benefits from steroid administration, yet subsequent research has failed to substantiate any such advantage. The latest guidelines, published in 2017, recommend considering a 24-hour high-dose methylprednisolone infusion for adult patients within 8 hours following acute spinal cord injury.[29] (A1)
Indications for early intubation include higher SCI levels (above C5), total paralysis, low lung volumes on chest radiographs, and the presence of concomitant injuries, especially chest wall or intrathoracic lesions. Tracheostomy may benefit patients requiring mechanical ventilation longer than 2 weeks following injury.[1] Patients with acute urinary retention should have a urinary or suprapubic catheter inserted to relieve lower tract discomfort caused by a full bladder. The urethral catheter, which should be 16 to 18 French in size, may be inserted as a first-line treatment. Clean intermittent catheterization results in fewer problems, a higher spontaneous voiding rate, and a lower incidence of urinary tract infections. Patients can develop greater tolerance to clean intermittent catheterization if the nursing team focuses on thorough training and precise catheter placement. Additional outpatient support services may be useful; patients typically welcome education.[30]
The Neurocritical Care Society recommends initiating deep vein thrombosis (DVT) prophylaxis as soon as possible, but no later than 72 hours after the SCI. The Consortium of Spinal Cord Injury does not define a specific timing but proposes using low-molecular-weight heparin in the acute care period to avoid DVT. Daily bleeding risk assessment must be performed to avoid delays due to bleeding concerns. Enoxaparin is superior to unfractionated heparin in preventing pulmonary embolism in patients with SCIs.
Individuals with SCIs receive optimal care in neurological intensive care units, where staff are proficient in SCI treatment. Dedicated trauma units must be established to facilitate smooth patient transfer and care transition. Patients achieve optimal outcomes with intensive rehabilitation therapy under the guidance of physiatrists, physical therapists, and occupational therapists. Rehabilitation is continued on an outpatient basis after hospital discharge. Trials involving the administration of nimodipine, gacyclidine, thyrotropin-releasing hormone, riluzole, gangliosides, minocycline, magnesium, and acidic fibroblast growth factor in patients with spinal cord injuries have not shown significant benefits, though further studies are ongoing.[31][32][33] (A1)
Stem cell therapy
Stem cell treatments for spinal cord injuries can be categorized into 2 main types: supportive and regenerative therapies. Supportive stem cell therapy employs nonneural stem cells, such as mesenchymal stem cells (MSCs) derived from bone marrow, umbilical cord, or adipose tissue. These cells are administered intravenously or intrathecally due to limited migration to the target area and differentiation into neural cells. MSCs release neurotrophic factors that can repair the injured area; however, their ability to replenish the nervous system is limited.
Most clinical trials focus on this therapy due to the easier MSC preparation and regulatory compliance. In contrast, loading therapy employs stem cells capable of producing neural cells, such as olfactory ensheathing cells and neural progenitor cells, and stem cells derived from embryonic stem cells. Engraftment of these cells has a greater likelihood of replacing lost nerve cells in terms of functionality, though the procedure requires invasive transplantation methods and complex cell preparation processes.[34]
Chronic SCI Treatment
Chronic SCI management depends on the underlying etiology. The treatment should address the neurologic deficits and primary disorder. Complications such as pressure ulcers, secondary bacterial infection, and urinary dysfunction must also be addressed. Rehabilitation and supportive care are essential in optimizing quality of life and functional outcomes for people with chronic SCI, regardless of the underlying etiology. Combined modalities and seamless interprofessional collaboration are often necessary for treating patients with chronic SCI.
Stimulation techniques
Several stimulation methods have emerged as viable therapeutic strategies for SCI treatment, notably epidural spinal cord stimulation and noninvasive approaches like transcutaneous spinal cord stimulation. Epidural spinal cord stimulation involves the implantation of electrodes directly in the dorsal epidural space to deliver electrical impulses that modulate spinal neural networks, enhancing locomotor and autonomic function. Clinical evidence demonstrates significant motor recovery, with up to 44% of patients achieving assisted or independent stepping, 87% showing enhanced muscle activity, and substantial improvements in autonomic functions such as cardiovascular regulation and bladder and sexual control.
Although infrequent, complications like device migration, infections, and autonomic dysreflexia have been reported.[35] Conversely, transcutaneous spinal cord stimulation represents a less invasive alternative, as it applies electrodes externally to the skin overlying the spinal column. This method enhances spinal excitability and has demonstrated promising effects on motor function and reduction of spasticity.[36] Both techniques emphasize the importance of neuroplasticity and targeted neural stimulation, highlighting their role in enhancing rehabilitative outcomes in patients with SCI.(A1)
Differential Diagnosis
The diagnosis of SCIs is based on the patient’s presentation, with most cases emerging from a traumatic event. However, a broader differential must be considered for sensorimotor weakness when the onset and preceding events are unclear. Thus, the following conditions must be included in the differential diagnosis of SCI:
Central nervous system pathologies:
- Cerebrovascular accident
- Postictal (Todd) paralysis
- Hemiplegic migraine
- Multiple sclerosis
Peripheral nerve pathologies:
- Guillain-Barré syndrome
- Transverse myelitis
- Tick paralysis
Neuromuscular junction pathologies:
- Myasthenia gravis
- Organophosphate toxicity
- Botulism
Other pathologies:
- Hypoglycemia
- Hypokalemic periodic paralysis
- Hypocalcemia
- Diabetic neuropathy
- Conversion disorder
A thorough clinical investigation and prudent use of diagnostic examinations can help differentiate SCI from these disorders.
Prognosis
SCI typically carries a guarded prognosis, with limited potential for full recovery, despite advances in medical and surgical management. The underlying cause influences the long-term prognosis of SCI, the extent of neurological damage, and the timeliness and appropriateness of medical interventions. The proportion of patients experiencing improvement by at least 1 AIS/Frankel grade (AIS/Frankel conversion rate) varies according to initial injury severity: 19% for patients initially classified as grade A, 73% for grade B, 87% for grade C, and 46% for grade D. The neurological level of injury significantly influences recovery likelihood, with lumbar injuries demonstrating the highest recovery rates, followed by cervical and thoracolumbar, and lastly, thoracic injuries.
Thoracic and penetrating spinal cord injuries are significantly associated with complete injuries, and penetrating injuries exhibit notably lower rates of recovery compared to blunt trauma.[37] The degree of disability directly correlates with the injury level, with higher-level injuries resulting in more significant disability and higher complication rates. Advanced modeling using machine learning algorithms has demonstrated good predictive abilities for in-hospital mortality and discharge disposition. Still, these models currently have only fair predictive capacity for major complications and prolonged hospitalization.[38][39]
The neuroprognostication guidelines suggest the Dutch clinical prediction rule in forecasting independent ambulation at 1 year.[40][41] Clinicians must maintain a comprehensive approach to neuroprognostication, considering multiple predictive variables rather than relying solely on isolated clinical parameters. Clear communication of prognosis with patients and caregivers should openly cover expected functional limitations, the potential for neurological recovery, and approaches to enhance the quality of life despite lasting impairments.
The prognosis paradigm may shift significantly with the accelerated advancements in medical science and technology. Emerging treatments, potentially involving neuroprotective strategies, regenerative therapies, brain-computer interface technologies, spinal cord electrical stimulation, and novel rehabilitation technologies, hold promise for altering the current limitations of SCI recovery. Ongoing research and innovation in these areas are crucial for driving a potential breakthrough in the prognosis of spinal cord injury.
Complications
SCI is linked to numerous complications that can greatly affect patient morbidity, mortality, and quality of life. Common complications include neurogenic bladder, bowel dysfunction, urinary tract infections (UTIs), pressure injuries, venous thromboembolism, autonomic dysreflexia, blood pressure instability, spasticity, chronic pain, impaired thermoregulation, and psychological distress. Significant indirect SCI costs include lost mobility, inability to work, and heavy caregiver burden.[42]
Neurogenic bowel dysfunction following SCI typically presents in 2 primary patterns based on the level of injury. Lesions above the T12 level generally result in a reflexic bowel, characterized by preserved spinal reflexes and increased anal sphincter tone, which can hinder effective evacuation of stool. Conversely, injuries at lumbar or sacral levels often result in areflexic bowel characterized by flaccid sphincters and impaired reflex coordination. Both patterns require structured bowel management programs that incorporate dietary adjustments, stool softeners, and manual evacuation techniques.
Similarly, neurogenic bladder dysfunction, whether from upper or lower motor neuron lesions, predisposes patients to incomplete bladder emptying, bladder spasms, overflow incontinence, and chronic retention. Incomplete bladder emptying significantly elevates the risk of recurrent UTIs, hydronephrosis, and potential renal dysfunction. Management typically involves early indwelling catheterization, transitioning to intermittent catheterization, timed voiding protocols, or surgical intervention to preserve renal function.
Pressure injuries constitute a significant source of morbidity and mortality post-SCI due to altered sensation, immobility, and impaired circulation. Preventive measures involve regular repositioning, specialized support surfaces, and vigilant skin inspections. Severe ulcers necessitate extensive medical or surgical interventions. Patients with SCI are at heightened risk for venous thromboembolism, including deep vein thrombosis and pulmonary embolism, due to immobility, endothelial injury, and hypercoagulability. Prophylactic anticoagulation with low-molecular-weight heparin is strongly advised within 72 hours post-injury, typically continuing for at least 8 weeks, based on patient-specific factors.[43][44]
Autonomic dysreflexia, occurring primarily in lesions at or above T6, is characterized by severe hypertension from uncontrolled sympathetic activation. Common triggers include bladder distention, bowel impaction, or skin irritation. Immediate identification and elimination of the stimulus, patient repositioning, and administration of antihypertensive medications are essential to prevent severe complications like hypertensive encephalopathy or stroke. Blood pressure dysregulation, including orthostatic hypotension, results from disrupted autonomic pathways. Management strategies include fluid optimization, abdominal binders, elastic stockings, salt supplementation, and medications such as midodrine and fludrocortisone.
Spasticity, characterized by involuntary muscle stiffness and spasms, occurs due to the hyperexcitability of spinal reflexes following SCI; this significantly restricts patient mobility and causes discomfort. Spasticity often complicates rehabilitation efforts, increasing the risk of contractures and joint deformities. Management typically involves physical therapy, oral antispasmodics, intrathecal baclofen therapy, or localized treatments such as botulinum toxin injections.
Chronic neuropathic pain significantly impacts quality of life post-SCI, with nearly half of the patients reporting persistent symptoms. Pain management strategies include pharmacologic therapies, targeted injections, and interdisciplinary approaches. Spinal cord stimulation is also an option. Impaired thermoregulation is frequent, especially in cervical and high thoracic injuries, making patients vulnerable to hyperthermia or hypothermia.
Finally, psychological complications, including depression and increased suicide risk, necessitate regular mental health screenings, counseling, peer support programs, and appropriate pharmacologic interventions. Suicide is notably prevalent among younger patients with SCI. Pneumonia and sepsis remain the leading causes of mortality in those with SCI, underscoring the importance of proactive multidisciplinary management throughout the patient's lifetime.
Deterrence and Patient Education
Primary preventive measures for SCI focus on raising awareness of risk factors, such as motor vehicle collisions and falls, promoting safety measures like seatbelt use and helmet wearing, and advocating for legislation enforcement to uphold safety standards. Environmental modifications, such as installing handrails and improving lighting, further reduce the risk of accidents in public spaces, workplaces, and homes. Prevention centers can help mitigate factors leading to traumatic injuries, like improvement in motor vehicle safety and programs aimed at violence prevention. Smoking cessation, occupational safety, and regular health check-ups can reduce the risk of chronic SCI.
Secondary prevention strategies include immediate medical attention and proper spine immobilization to prevent SCI exacerbation. Patient education is an integral part of clinical management. Counseling on prognosis, complications, and outcomes must be provided. Early initiation of rehabilitation programs, such as physical and occupational therapy, supports functional recovery and minimizes disability.
Careful monitoring and proactive management of complications—such as pressure ulcers and urinary tract infections—along with access to assistive devices and strong support networks, are crucial in improving mobility, independence, and overall well-being for individuals with SCI. Support groups help address emotional and social challenges like anxiety, frustration, loneliness, and depression, ensuring patients receive well-rounded care. By prioritizing these preventive strategies, healthcare professionals and communities can collaborate to reduce the incidence and impact of SCI, ultimately improving the safety and quality of life for those affected.
Pearls and Other Issues
SCIs occur due to damage to the spinal cord, resulting in motor, sensory, and autonomic dysfunction below the level of injury. Timely diagnosis using neurological exams and imaging—such as MRI or CT scans—is essential for accurate classification. Effective treatment focuses on acute stabilization to prevent secondary damage and may include surgical intervention to decompress the spinal cord or stabilize fractured vertebrae.
Following diagnosis, long-term care focuses on rehabilitation to optimize functional recovery and quality of life. This includes physical and occupational therapy, alongside assistive devices, to enhance mobility and independence. Proactive management of complications like pressure ulcers and UTIs is essential to prevent further harm and improve outcomes for patients with SCI. Timely intervention and comprehensive care are vital in mitigating the impact of SCI and promoting better patient outcomes.
Enhancing Healthcare Team Outcomes
Optimizing care for patients with SCI necessitates a coordinated interprofessional team approach. Clinicians are responsible for timely recognition, evaluation, and acute management of SCI to minimize complications. Neurosurgeons or orthopedic spine surgeons provide essential surgical interventions to stabilize injuries and prevent neurological deterioration. Nurses play a critical role in monitoring patients closely, identifying early signs of complications such as pressure ulcers, infections, or autonomic dysreflexia. Nursing responsibilities include educating patients and caregivers on skin care, catheterization techniques, and bowel management programs to promote patient independence. Pharmacists provide guidance regarding medication management, particularly for blood pressure control, pain relief, anticoagulation, and the avoidance of adverse drug interactions. Physical and occupational therapists collaborate to maximize function, mobility, and independence restoration through tailored rehabilitation programs.
Social workers and case managers facilitate care transitions, arrange community resources, and assist with insurance and reimbursement processes. Psychiatrists or psychologists address the significant mental health challenges commonly encountered by patients, including depression, anxiety, and emotional adjustment. Pain management specialists can help manage chronic pain. Regular interprofessional communication through structured team meetings ensures cohesive management, promoting patient safety and improving outcomes. Ethical care, emphasizing patient autonomy and informed decision-making, is paramount. Effective collaboration ensures comprehensive care delivery, enhancing patient satisfaction and clinical outcomes.[42][45]
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
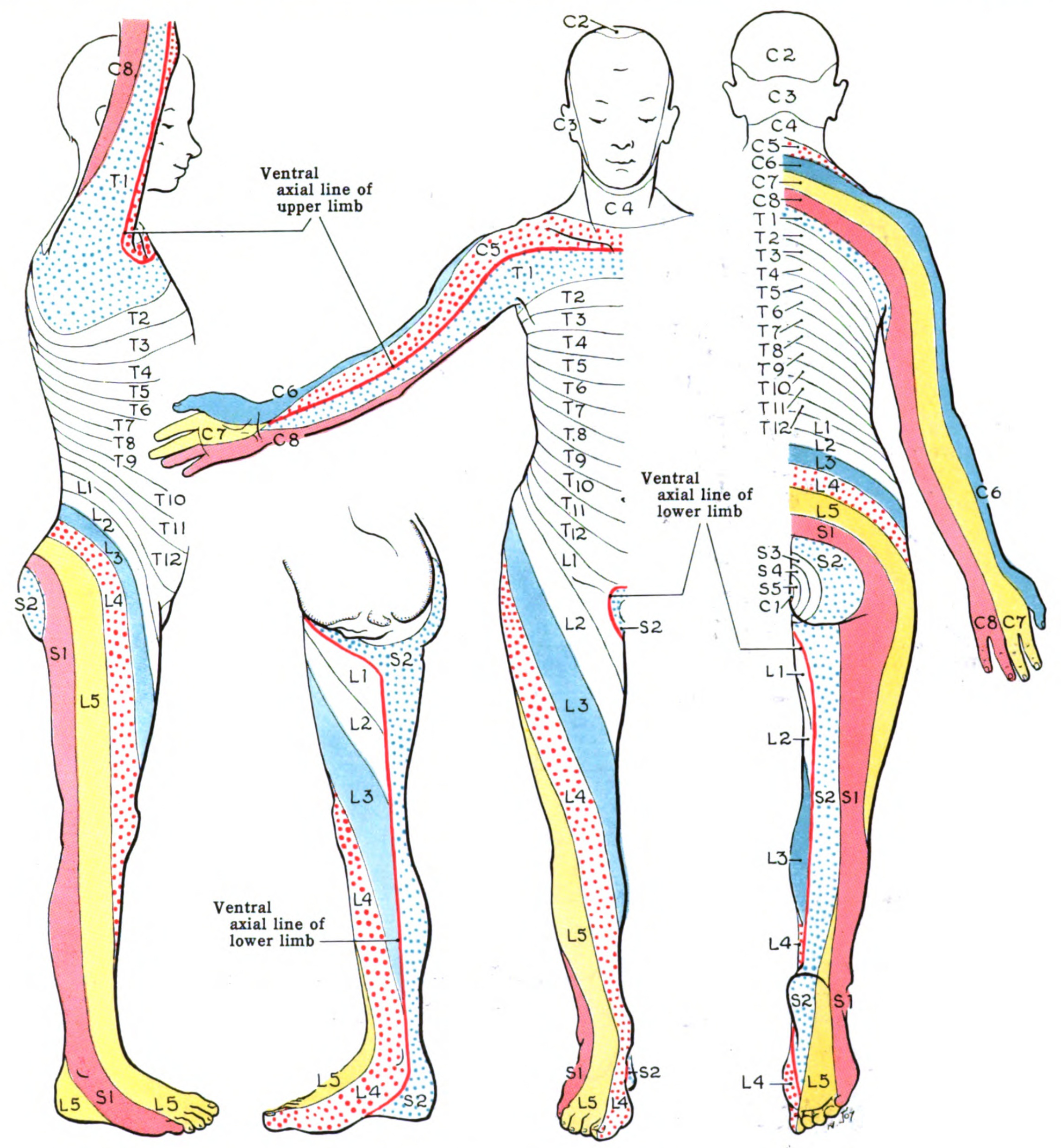
Dermatome Map. A dermatome is an area of skin receiving sensory innervation from a single spinal nerve dorsal root. Shown here is a map of the body's dermatomes.
Cmdrjameson, Public Domain, via Wikimedia Commons
References
Eckert MJ, Martin MJ. Trauma: Spinal Cord Injury. The Surgical clinics of North America. 2017 Oct:97(5):1031-1045. doi: 10.1016/j.suc.2017.06.008. Epub [PubMed PMID: 28958356]
. Spinal Cord Injury (SCI) 2016 Facts and Figures at a Glance. The journal of spinal cord medicine. 2016 Jul:39(4):493-4. doi: 10.1080/10790268.2016.1210925. Epub [PubMed PMID: 27471859]
Varma AK, Das A, Wallace G 4th, Barry J, Vertegel AA, Ray SK, Banik NL. Spinal cord injury: a review of current therapy, future treatments, and basic science frontiers. Neurochemical research. 2013 May:38(5):895-905. doi: 10.1007/s11064-013-0991-6. Epub 2013 Mar 6 [PubMed PMID: 23462880]
McDaid D, Park AL, Gall A, Purcell M, Bacon M. Understanding and modelling the economic impact of spinal cord injuries in the United Kingdom. Spinal cord. 2019 Sep:57(9):778-788. doi: 10.1038/s41393-019-0285-1. Epub 2019 May 13 [PubMed PMID: 31086273]
Level 3 (low-level) evidenceJain NB, Ayers GD, Peterson EN, Harris MB, Morse L, O'Connor KC, Garshick E. Traumatic spinal cord injury in the United States, 1993-2012. JAMA. 2015 Jun 9:313(22):2236-43. doi: 10.1001/jama.2015.6250. Epub [PubMed PMID: 26057284]
Seijas V, Schrepfer L, Posada AM, Spir MA, Machado B, Sigrist-Nix D, Scheel-Sailer A, Eriks-Hoogland I, Sabariego C. Evidence-based recommendations for the rehabilitation and management of the ageing population with spinal cord injury: a systematic review of clinical practice guidelines. European journal of physical and rehabilitation medicine. 2024 Jun:60(3):433-444. doi: 10.23736/S1973-9087.24.08244-3. Epub 2024 Mar 29 [PubMed PMID: 38551520]
Level 1 (high-level) evidenceAlizadeh A, Dyck SM, Karimi-Abdolrezaee S. Traumatic Spinal Cord Injury: An Overview of Pathophysiology, Models and Acute Injury Mechanisms. Frontiers in neurology. 2019:10():282. doi: 10.3389/fneur.2019.00282. Epub 2019 Mar 22 [PubMed PMID: 30967837]
Level 3 (low-level) evidenceEli I, Lerner DP, Ghogawala Z. Acute Traumatic Spinal Cord Injury. Neurologic clinics. 2021 May:39(2):471-488. doi: 10.1016/j.ncl.2021.02.004. Epub 2021 Mar 31 [PubMed PMID: 33896529]
Kirshblum SC, Biering-Sorensen F, Betz R, Burns S, Donovan W, Graves DE, Johansen M, Jones L, Mulcahey MJ, Rodriguez GM, Schmidt-Read M, Steeves JD, Tansey K, Waring W. International Standards for Neurological Classification of Spinal Cord Injury: cases with classification challenges. The journal of spinal cord medicine. 2014 Mar:37(2):120-7. doi: 10.1179/2045772314Y.0000000196. Epub [PubMed PMID: 24559416]
Level 3 (low-level) evidenceKirshblum SC, Burns SP, Biering-Sorensen F, Donovan W, Graves DE, Jha A, Johansen M, Jones L, Krassioukov A, Mulcahey MJ, Schmidt-Read M, Waring W. International standards for neurological classification of spinal cord injury (revised 2011). The journal of spinal cord medicine. 2011 Nov:34(6):535-46. doi: 10.1179/204577211X13207446293695. Epub [PubMed PMID: 22330108]
Waring WP 3rd, Biering-Sorensen F, Burns S, Donovan W, Graves D, Jha A, Jones L, Kirshblum S, Marino R, Mulcahey MJ, Reeves R, Scelza WM, Schmidt-Read M, Stein A. _ 2009 review and revisions of the international standards for the neurological classification of spinal cord injury. The journal of spinal cord medicine. 2010:33(4):346-52 [PubMed PMID: 21061894]
Waters RL, Adkins RH, Yakura JS. Definition of complete spinal cord injury. Paraplegia. 1991 Nov:29(9):573-81 [PubMed PMID: 1787981]
Roth EJ, Park T, Pang T, Yarkony GM, Lee MY. Traumatic cervical Brown-Sequard and Brown-Sequard-plus syndromes: the spectrum of presentations and outcomes. Paraplegia. 1991 Nov:29(9):582-9 [PubMed PMID: 1787982]
Dave S, Dahlstrom JJ, Weisbrod LJ. Neurogenic Shock. StatPearls. 2025 Jan:(): [PubMed PMID: 29083597]
Zipser CM, Margetis K, Pedro KM, Curt A, Fehlings M, Sadler I, Tetreault L, Davies BM, AO Spine RECODE DCM Steering Committee, Members of the Diagnostic Criteria Working Group. Increasing awareness of degenerative cervical myelopathy: a preventative cause of non-traumatic spinal cord injury. Spinal cord. 2021 Nov:59(11):1216-1218. doi: 10.1038/s41393-021-00711-8. Epub 2021 Oct 9 [PubMed PMID: 34628477]
Rupp R, Biering-Sørensen F, Burns SP, Graves DE, Guest J, Jones L, Read MS, Rodriguez GM, Schuld C, Tansey-Md KE, Walden K, Kirshblum S. International Standards for Neurological Classification of Spinal Cord Injury: Revised 2019. Topics in spinal cord injury rehabilitation. 2021 Spring:27(2):1-22. doi: 10.46292/sci2702-1. Epub [PubMed PMID: 34108832]
Roberts TT, Leonard GR, Cepela DJ. Classifications In Brief: American Spinal Injury Association (ASIA) Impairment Scale. Clinical orthopaedics and related research. 2017 May:475(5):1499-1504. doi: 10.1007/s11999-016-5133-4. Epub 2016 Nov 4 [PubMed PMID: 27815685]
Inaba K, Byerly S, Bush LD, Martin MJ, Martin DT, Peck KA, Barmparas G, Bradley MJ, Hazelton JP, Coimbra R, Choudhry AJ, Brown CV, Ball CG, Cherry-Bukowiec JR, Burlew CC, Joseph B, Dunn J, Minshall CT, Carrick MM, Berg GM, Demetriades D, WTA C-Spine Study Group. Cervical spinal clearance: A prospective Western Trauma Association Multi-institutional Trial. The journal of trauma and acute care surgery. 2016 Dec:81(6):1122-1130 [PubMed PMID: 27438681]
Sixta S, Moore FO, Ditillo MF, Fox AD, Garcia AJ, Holena D, Joseph B, Tyrie L, Cotton B, Eastern Association for the Surgery of Trauma. Screening for thoracolumbar spinal injuries in blunt trauma: an Eastern Association for the Surgery of Trauma practice management guideline. The journal of trauma and acute care surgery. 2012 Nov:73(5 Suppl 4):S326-32. doi: 10.1097/TA.0b013e31827559b8. Epub [PubMed PMID: 23114489]
Ellingson BM, Salamon N, Holly LT. Imaging techniques in spinal cord injury. World neurosurgery. 2014 Dec:82(6):1351-8. doi: 10.1016/j.wneu.2012.12.004. Epub 2012 Dec 12 [PubMed PMID: 23246741]
Wilson JR, Grossman RG, Frankowski RF, Kiss A, Davis AM, Kulkarni AV, Harrop JS, Aarabi B, Vaccaro A, Tator CH, Dvorak M, Shaffrey CI, Harkema S, Guest JD, Fehlings MG. A clinical prediction model for long-term functional outcome after traumatic spinal cord injury based on acute clinical and imaging factors. Journal of neurotrauma. 2012 Sep:29(13):2263-71. doi: 10.1089/neu.2012.2417. Epub 2012 Jul 31 [PubMed PMID: 22709268]
Liu Q, Liu Q, Zhao J, Yu H, Ma X, Wang L. Early MRI finding in adult spinal cord injury without radiologic abnormalities does not correlate with the neurological outcome: a retrospective study. Spinal cord. 2015 Oct:53(10):750-3. doi: 10.1038/sc.2015.45. Epub 2015 Mar 17 [PubMed PMID: 25777331]
Level 2 (mid-level) evidenceChandra J, Sheerin F, Lopez de Heredia L, Meagher T, King D, Belci M, Hughes RJ. MRI in acute and subacute post-traumatic spinal cord injury: pictorial review. Spinal cord. 2012 Jan:50(1):2-7. doi: 10.1038/sc.2011.107. Epub 2011 Nov 8 [PubMed PMID: 22064660]
Fehlings MG, Martin AR, Tetreault LA, Aarabi B, Anderson P, Arnold PM, Brodke D, Burns AS, Chiba K, Dettori JR, Furlan JC, Hawryluk G, Holly LT, Howley S, Jeji T, Kalsi-Ryan S, Kotter M, Kurpad S, Kwon BK, Marino RJ, Massicotte E, Merli G, Middleton JW, Nakashima H, Nagoshi N, Palmieri K, Singh A, Skelly AC, Tsai EC, Vaccaro A, Wilson JR, Yee A, Harrop JS. A Clinical Practice Guideline for the Management of Patients With Acute Spinal Cord Injury: Recommendations on the Role of Baseline Magnetic Resonance Imaging in Clinical Decision Making and Outcome Prediction. Global spine journal. 2017 Sep:7(3 Suppl):221S-230S. doi: 10.1177/2192568217703089. Epub 2017 Sep 5 [PubMed PMID: 29164028]
Level 1 (high-level) evidenceKumar Y, Hayashi D. Role of magnetic resonance imaging in acute spinal trauma: a pictorial review. BMC musculoskeletal disorders. 2016 Jul 22:17():310. doi: 10.1186/s12891-016-1169-6. Epub 2016 Jul 22 [PubMed PMID: 27448661]
Stukas S, Cooper J, Gill J, Fallah N, Skinnider MA, Belanger L, Ritchie L, Tsang A, Dong K, Streijger F, Street J, Paquette S, Ailon T, Dea N, Charest-Morin R, Fisher CG, Bailey CS, Dhall S, Mac-Thiong JM, Wilson JR, Christie S, Dvorak MF, Wellington CL, Kwon BK. Association of CSF and Serum Neurofilament Light and Glial Fibrillary Acidic Protein, Injury Severity, and Outcome in Spinal Cord Injury. Neurology. 2023 Mar 21:100(12):e1221-e1233. doi: 10.1212/WNL.0000000000206744. Epub 2023 Jan 4 [PubMed PMID: 36599698]
Fehlings MG, Tetreault LA, Hachem L, Evaniew N, Ganau M, McKenna SL, Neal CJ, Nagoshi N, Rahimi-Movaghar V, Aarabi B, Hofstetter CP, Wengel VT, Nakashima H, Martin AR, Kirshblum S, Rodrigues Pinto R, Marco RAW, Wilson JR, Kahn DE, Newcombe VFJ, Zipser CM, Douglas S, Kurpad SN, Lu Y, Saigal R, Samadani U, Arnold PM, Hawryluk GWJ, Skelly AC, Kwon BK. An Update of a Clinical Practice Guideline for the Management of Patients With Acute Spinal Cord Injury: Recommendations on the Role and Timing of Decompressive Surgery. Global spine journal. 2024 Mar:14(3_suppl):174S-186S. doi: 10.1177/21925682231181883. Epub [PubMed PMID: 38526922]
Level 1 (high-level) evidenceKwon BK, Tetreault LA, Martin AR, Arnold PM, Marco RAW, Newcombe VFJ, Zipser CM, McKenna SL, Korupolu R, Neal CJ, Saigal R, Glass NE, Douglas S, Ganau M, Rahimi-Movaghar V, Harrop JS, Aarabi B, Wilson JR, Evaniew N, Skelly AC, Fehlings MG. A Clinical Practice Guideline for the Management of Patients With Acute Spinal Cord Injury: Recommendations on Hemodynamic Management. Global spine journal. 2024 Mar:14(3_suppl):187S-211S. doi: 10.1177/21925682231202348. Epub [PubMed PMID: 38526923]
Level 1 (high-level) evidenceFehlings MG, Wilson JR, Tetreault LA, Aarabi B, Anderson P, Arnold PM, Brodke DS, Burns AS, Chiba K, Dettori JR, Furlan JC, Hawryluk G, Holly LT, Howley S, Jeji T, Kalsi-Ryan S, Kotter M, Kurpad S, Kwon BK, Marino RJ, Martin AR, Massicotte E, Merli G, Middleton JW, Nakashima H, Nagoshi N, Palmieri K, Skelly AC, Singh A, Tsai EC, Vaccaro A, Yee A, Harrop JS. A Clinical Practice Guideline for the Management of Patients With Acute Spinal Cord Injury: Recommendations on the Use of Methylprednisolone Sodium Succinate. Global spine journal. 2017 Sep:7(3 Suppl):203S-211S. doi: 10.1177/2192568217703085. Epub 2017 Sep 5 [PubMed PMID: 29164025]
Level 1 (high-level) evidenceDougherty JM, Leslie SW, Aeddula NR. Male Urinary Retention: Acute and Chronic. StatPearls. 2025 Jan:(): [PubMed PMID: 30860734]
Wu JC, Huang WC, Chen YC, Tu TH, Tsai YA, Huang SF, Huang HC, Cheng H. Acidic fibroblast growth factor for repair of human spinal cord injury: a clinical trial. Journal of neurosurgery. Spine. 2011 Sep:15(3):216-27. doi: 10.3171/2011.4.SPINE10404. Epub 2011 Jun 10 [PubMed PMID: 21663406]
Freund P, Schmidlin E, Wannier T, Bloch J, Mir A, Schwab ME, Rouiller EM. Nogo-A-specific antibody treatment enhances sprouting and functional recovery after cervical lesion in adult primates. Nature medicine. 2006 Jul:12(7):790-2 [PubMed PMID: 16819551]
Level 3 (low-level) evidenceGeisler FH, Dorsey FC, Coleman WP. Recovery of motor function after spinal-cord injury--a randomized, placebo-controlled trial with GM-1 ganglioside. The New England journal of medicine. 1991 Jun 27:324(26):1829-38 [PubMed PMID: 2041549]
Level 1 (high-level) evidenceShinozaki M, Nagoshi N, Nakamura M, Okano H. Mechanisms of Stem Cell Therapy in Spinal Cord Injuries. Cells. 2021 Oct 6:10(10):. doi: 10.3390/cells10102676. Epub 2021 Oct 6 [PubMed PMID: 34685655]
Chalif JI, Chavarro VS, Mensah E, Johnston B, Fields DP, Chalif EJ, Chiang M, Sutton O, Yong R, Trumbower R, Lu Y. Epidural Spinal Cord Stimulation for Spinal Cord Injury in Humans: A Systematic Review. Journal of clinical medicine. 2024 Feb 14:13(4):. doi: 10.3390/jcm13041090. Epub 2024 Feb 14 [PubMed PMID: 38398403]
Level 1 (high-level) evidenceHernandez-Navarro A, Ros-Alsina A, Yurtseven M, Wright M, Kumru H. Non-invasive cerebral and spinal cord stimulation for motor and gait recovery in incomplete spinal cord injury: systematic review and meta-analysis. Journal of neuroengineering and rehabilitation. 2025 Mar 7:22(1):53. doi: 10.1186/s12984-025-01557-4. Epub 2025 Mar 7 [PubMed PMID: 40050875]
Level 1 (high-level) evidenceKhorasanizadeh M, Yousefifard M, Eskian M, Lu Y, Chalangari M, Harrop JS, Jazayeri SB, Seyedpour S, Khodaei B, Hosseini M, Rahimi-Movaghar V. Neurological recovery following traumatic spinal cord injury: a systematic review and meta-analysis. Journal of neurosurgery. Spine. 2019 May 1:30(5):683-699. doi: 10.3171/2018.10.SPINE18802. Epub 2019 Feb 15 [PubMed PMID: 30771786]
Level 1 (high-level) evidenceKarabacak M, Jagtiani P, Margetis K. The Predictive Abilities of Machine Learning Algorithms in Patients with Thoracolumbar Spinal Cord Injuries. World neurosurgery. 2024 Feb:182():e67-e90. doi: 10.1016/j.wneu.2023.11.043. Epub 2023 Nov 28 [PubMed PMID: 38030070]
Karabacak M, Margetis K. Precision medicine for traumatic cervical spinal cord injuries: accessible and interpretable machine learning models to predict individualized in-hospital outcomes. The spine journal : official journal of the North American Spine Society. 2023 Dec:23(12):1750-1763. doi: 10.1016/j.spinee.2023.08.009. Epub 2023 Aug 23 [PubMed PMID: 37619871]
Mahanes D, Muehlschlegel S, Wartenberg KE, Rajajee V, Alexander SA, Busl KM, Creutzfeldt CJ, Fontaine GV, Hocker SE, Hwang DY, Kim KS, Madzar D, Mainali S, Meixensberger J, Varelas PN, Weimar C, Westermaier T, Sakowitz OW. Guidelines for neuroprognostication in adults with traumatic spinal cord injury. Neurocritical care. 2024 Apr:40(2):415-437. doi: 10.1007/s12028-023-01845-8. Epub 2023 Nov 13 [PubMed PMID: 37957419]
van Middendorp JJ, Hosman AJ, Donders AR, Pouw MH, Ditunno JF Jr, Curt A, Geurts AC, Van de Meent H, EM-SCI Study Group. A clinical prediction rule for ambulation outcomes after traumatic spinal cord injury: a longitudinal cohort study. Lancet (London, England). 2011 Mar 19:377(9770):1004-10. doi: 10.1016/S0140-6736(10)62276-3. Epub 2011 Mar 4 [PubMed PMID: 21377202]
Tate DG, Kalpakjian CZ, Forchheimer MB. Quality of life issues in individuals with spinal cord injury. Archives of physical medicine and rehabilitation. 2002 Dec:83(12 Suppl 2):S18-25 [PubMed PMID: 12474168]
Level 2 (mid-level) evidenceFehlings MG, Tetreault LA, Aarabi B, Anderson P, Arnold PM, Brodke DS, Burns AS, Chiba K, Dettori JR, Furlan JC, Hawryluk G, Holly LT, Howley S, Jeji T, Kalsi-Ryan S, Kotter M, Kurpad S, Kwon BK, Marino RJ, Martin AR, Massicotte E, Merli G, Middleton JW, Nakashima H, Nagoshi N, Palmieri K, Singh A, Skelly AC, Tsai EC, Vaccaro A, Wilson JR, Yee A, Harrop JS. A Clinical Practice Guideline for the Management of Patients With Acute Spinal Cord Injury: Recommendations on the Type and Timing of Anticoagulant Thromboprophylaxis. Global spine journal. 2017 Sep:7(3 Suppl):212S-220S. doi: 10.1177/2192568217702107. Epub 2017 Sep 5 [PubMed PMID: 29164026]
Level 1 (high-level) evidence. Prevention of Venous Thromboembolism in Individuals with Spinal Cord Injury: Clinical Practice Guidelines for Health Care Providers, 3rd ed.: Consortium for Spinal Cord Medicine. Topics in spinal cord injury rehabilitation. 2016 Summer:22(3):209-240. doi: 10.1310/sci2203-209. Epub [PubMed PMID: 29339863]
Level 1 (high-level) evidenceHancock KM, Craig AR, Dickson HG, Chang E, Martin J. Anxiety and depression over the first year of spinal cord injury: a longitudinal study. Paraplegia. 1993 Jun:31(6):349-57 [PubMed PMID: 8336997]