Introduction
In the early 1900s, Swedish physician Henrik Sjögren (SHOW-gren) first described a group of women whose chronic arthritis was accompanied by dry eyes and dry mouth. Today, Rheumatologists know more about the syndrome that is named for Sjögren and—most significantly for patients—can offer advice about how to live with it.
Primary Sjogren syndrome is a systemic autoimmune disorder most commonly presenting with sicca symptoms. Sicca refers to dryness most often involving the eyes and mouth due to inflammation and resultant pathology of the lacrimal and salivary glands. Up to one-half of affected individuals also develop extra-glandular involvement implying the occurrence of signs and symptoms in organs distinct from the salivary and lacrimal glands including the joints, skin, lungs, gastrointestinal (GI) tract, nervous system, and kidneys. Sjogren syndrome frequently occurs in conjunction with other autoimmune disorders including rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE). In this setting, authors will refer to it as secondary Sjogren or Sjogren-overlap syndrome. Therapies are directed toward replacing moisture at affected glandular sites and suppressing the autoimmune response locally as well as systemically.[1][2][3]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
The precise etiology of Sjögren syndrome remains unknown, as with most autoimmune disorders. A complex interplay of genetic, hormonal, and environmental factors likely contributes to disease onset. A genome-wide association study identified a strong correlation between Sjögren syndrome and the HLA-DQB1 haplotype.[1] These major histocompatibility complex variants appear to promote an abnormal immune response when combined with specific environmental triggers. Laboratory findings and indirect epidemiological data implicate viral infections, particularly Epstein-Barr virus (EBV), in disease development.[2][3] Exposure to solvents and inorganic chemicals has also been associated with the etiopathogenesis of Sjögren syndrome.[4]
The leading “pathogenic model” suggests that environmental insults in genetically predisposed individuals injure the salivary gland epithelium. This epithelial damage initiates inflammation and triggers an aberrant immune response, driving the chronic autoimmune process that defines the disease.[5]
Epidemiology
Sjögren syndrome affects approximately 0.5% to 1.0% of the population, with an incidence approaching half that of rheumatoid arthritis. Estimates suggest that between 400,000 and 3.1 million adults live with the condition. Although Sjögren syndrome can develop at any age, symptoms most commonly begin between the ages of 45 and 55. Approximately half of those diagnosed also have rheumatoid arthritis or another connective tissue disease (eg, lupus).
Sjögren syndrome occurs globally in adults and, less commonly, in children, without evidence of racial or geographic bias in incidence. The disorder shows a strong predilection for women, with a female-to-male ratio of approximately 9:1, similar to the pattern seen in SLE.[6][7]
The lack of an evidence-based, standardized screening tool to identify which dry eye patients require evaluation for Sjögren syndrome contributes to the underreferral of these individuals. As a result, many cases of Sjögren syndrome remain undiagnosed, perpetuating a pattern of underrecognition and delayed diagnosis.[8]
Pathophysiology
The characteristic lesion of Sjögren syndrome, focal lymphocytic sialadenitis (FLS), affects exocrine glands. In FLS, lymphocyte-rich mononuclear cell foci infiltrate glandular tissue near blood vessels and excretory ducts. These foci consist primarily of T lymphocytes, although B lymphocytes, plasma cells, and other immune cells are also present. As disease severity increases, foci may coalesce.
LAMP3 (lysosome-associated membrane protein 3) becomes overexpressed in Sjögren syndrome, driven by elevated type I interferon levels. LAMP3 promotes salivary gland epithelial damage and triggers innate immune responses. Persistent epithelial activation leads to apoptosis and escalating inflammation. Plasmacytoid dendritic cells—key producers of type I interferon—play a central role in early disease development. Monocytes and macrophages, frequently found in salivary gland biopsies, sustain inflammation by interacting with B and T lymphocytes.[5]
B cells generate autoantibodies characteristic of Sjögren syndrome, including rheumatoid factor, SSA/Ro, and SSB/La. These cells appear in salivary glands and may form ectopic lymphoid structures. B cells, capable of transforming into malignant clones, contribute to the heightened risk of mucosa-associated lymphoid tissue (MALT) B-cell lymphoma in affected patients. Alongside antigen-presenting cells, B cells also activate T lymphocytes.[5]
Exocrine gland dysfunction results from mononuclear cell infiltration, humoral factors such as cytokines and antibodies, or both. This dysfunction reduces tear and saliva production by the lacrimal and salivary glands, respectively. Other exocrine glands may also become involved, leading to dryness of the skin, tracheobronchial tree, and vagina, and contributing to impairment of the lungs and kidneys. In rare cases, B lymphocyte malignancy may give rise to non-Hodgkin lymphoma. Additionally, immune complex deposition in the skin, joints, and organs can cause systemic vasculitis.[9]
Histopathology
Tissues involved in Sjögren syndrome—including the minor and major salivary glands and lacrimal glands—undergo infiltration by both T and B lymphocytes. While a tissue diagnosis remains nonessential, it may support diagnostic confirmation when other objective criteria are absent. Minor salivary glands (MSGs) are the preferred biopsy site due to their lower risk of complications. Access requires a small incision on the inner lip to excise 4 to 5 MSGs.
Biopsy typically reveals lymphocytic aggregates exceeding 50 cells within a 4 mm² area, known as the "focus score" [10], along with macrophages and plasma cells. CD4+ T cells dominate the infiltrate, and approximately 10% of lymphocytes are CD5-positive B cells that produce IgG and IgM antibodies. Up to 25% of patients with primary Sjögren syndrome also exhibit germinal center-like structures in biopsy samples, a finding associated with more severe disease.[11]
History and Physical
Clinical History
Dryness of the eyes (xerophthalmia) and mouth (xerostomia) almost always occurs in patients with Sjögren syndrome, though symptom severity ranges from very mild to debilitating. Diagnosing Sjögren syndrome can be challenging due to the high prevalence of dry eyes and mouth, especially in older adults, among whom only a small subset actually has the disease. Clinicians should suspect Sjögren syndrome in patients with persistent dryness, abnormal serologic findings, or signs suggestive of organ involvement.
Extraglandular manifestations may include Raynaud syndrome, synovitis, interstitial lung disease, autoimmune cytopenias, hypergammaglobulinemia, cutaneous vasculitis, renal disease, myositis, lymphadenopathy, neuropathy, and central nervous system (CNS) disease. Many patients also experience systemic symptoms, eg, fatigue, arthralgia, and cognitive difficulties commonly described as brain fog. Additionally, non-Hodgkin lymphoma risk increases significantly in individuals diagnosed with Sjögren syndrome.
Physical Examination
An eye examination may reveal absent tear pooling in the inferior conjunctival sac and injected (reddened) eyes. Referral to an ophthalmologist remains essential for a slit-lamp evaluation. When performed with vital dye staining, this exam can confirm keratoconjunctivitis sicca, the hallmark ophthalmologic finding in Sjögren syndrome. A Schirmer test can further support the diagnosis by quantifying ocular dryness.
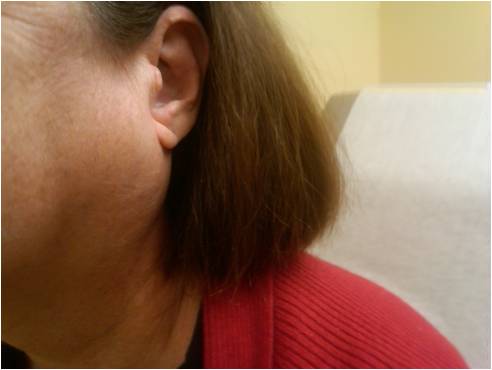
Oral findings often include a dry mouth, smooth tongue, and thinned mucosa. Around 50% of patients experience parotid or submandibular gland enlargement at some stage (see Image. Sjogrens Parotid). Extraglandular involvement frequently presents with polyarthritis, pulmonary abnormalities, lower extremity purpura, or signs of peripheral neuropathy.
Diagnostic Considerations
Diagnosis has evolved alongside updates to classification criteria from the American College of Rheumatology (ACR) and the European League Against Rheumatism (EULAR). The most recent classification criteria, published in 2016, should not be used as a stand-alone diagnostic tool, but rather may provide a structured framework for evaluating patients.
These criteria apply to individuals reporting at least 1 symptom of ocular or oral dryness, defined by a positive response to at least 1 of the following:
-
Daily, persistent, troublesome dry eyes
-
Recurrent sensation of sand or gravel in the eyes
-
Regular use of tear substitutes
-
Daily feeling of dry mouth
-
Frequent need to drink liquids to help swallow dry food [12]
Patients may also qualify based on a positive domain from the EULAR Sjögren Syndrome Disease Activity Index (ESSDAI).[13] This index assesses systemic involvement, including systemic symptoms (eg, fever and weight loss), lymphadenopathy, salivary or lacrimal gland swelling, inflammatory arthritis, cutaneous findings (eg, urticarial vasculitis and palpable purpura), interstitial lung disease, renal disease (eg, proteinuria, renal tubular acidosis, and glomerulonephritis), myositis, peripheral neuropathy, central nervous system syndromes (eg, optic neuritis, multiple sclerosis-like syndromes, vasculitis), and autoimmune cytopenias.[14]
Objective findings used for classification include:
-
Labial salivary gland biopsy showing focal lymphocytic sialadenitis with a focus score ≥1 focus per 4 mm² (3 points)
-
Positive anti-Ro/SSA or anti-La/SSB antibodies (3 points)
-
Ocular staining score ≥5 or van Bijsterveld score ≥4 in at least 1 eye (1 point)
-
Schirmer’s test result ≤5 mm/5 minutes in at least 1 eye (1 point)
-
Unstimulated whole salivary flow rate ≤0.1 mL/minute (1 point) [15]
A total score of ≥4 based on objective criteria confirms classification as Sjögren syndrome in patients meeting inclusion criteria.
Exclusion criteria include a history of head and neck irradiation, active hepatitis C infection, HIV/AIDS, sarcoidosis, amyloidosis, graft-versus-host disease, and IgG4-related disease.
Evaluation
Sjögren Syndrome Diagnostic Tools
Evaluation of a patient suspected of Sjögren syndrome should include a thorough assessment of oral and ocular dryness and glandular function.
In addition to clinical history, diagnostic tools may involve the Schirmer test, slit-lamp examination with vital dye staining, measurement of salivary flow rate, and evaluation of salivary gland function.
Schirmer test
This standard method measures aqueous tear production by placing a sterile strip inside the lower eyelid and recording the amount of tear wetting in millimeters over a 5-minute period. The test offers an inexpensive and simple approach, though it can cause discomfort and demonstrates some variability on repeat testing. Wetting of ≤5 mm indicates abnormal tear production.
Lissamine green and fluorescein staining
These dyes detect areas of dryness or damage on the conjunctiva and cornea. Fluorescein also measures tear film break-up time, with ≤10 seconds considered abnormal, suggesting Meibomian gland dysfunction.
Salivary scintigraphy
This nuclear medicine technique assesses salivary gland dysfunction by measuring the uptake of radiolabeled technetium by the major salivary glands. While specific, the test shows limited sensitivity.
Sialometry
This test quantifies unstimulated salivary flow by collecting saliva over 5 to 15 minutes into a preweighed cup. The flow rate in grams per minute is calculated by reweighing the cup, with a rate of ≤0.1 mL/minute considered abnormal.
Laboratory Studies
Autoantibody testing, including ANA, rheumatoid factor, SSA, and SSB, plays an important role. SSA antibodies demonstrate the highest sensitivity and specificity for Sjögren syndrome, but they do not alone confirm a diagnosis, as they may also appear in other autoimmune diseases or be absent in up to one-third of Sjögren cases.
Baseline laboratory tests for patients suspected of having Sjögren syndrome should also include a complete blood count (CBC), erythrocyte sedimentation rate (ESR), a chemistry panel, and urinalysis to identify disease complications or concurrent conditions. For ongoing disease activity monitoring in established Sjögren syndrome, routine bloodwork may include complement levels (C3 and C4), rheumatoid factor (RF), serum protein electrophoresis (SPEP), and cryoglobulin testing. Abnormal results often correlate with more severe disease and increased lymphoma risk.
Sjögren Syndrome Histologic and Imaging Studies
The minor salivary gland (lip) biopsy remains the most specific single test, demonstrating focal lymphocytic sialadenitis (FLS) in positive cases.[16][17][18] Debate continues regarding the added diagnostic value of this biopsy in seropositive patients.[19] However, biopsy findings provide important prognostic information, helping to stratify disease severity and predict systemic manifestations, including the risk of lymphoma.[20]
Salivary gland ultrasonography (SGUS) has emerged as a promising, noninvasive, safe, and cost-effective tool for assessing structural changes in salivary glands. B-mode SGUS can distinguish primary Sjögren syndrome changes from non-Sjögren alterations.[21] Incorporating SGUS into ACR/EULAR classification criteria causes a moderate sensitivity decrease when substituting for minor salivary gland biopsy or SSA antibody testing, with a mild specificity decrease. Sensitivity and specificity remain comparable when SGUS replaces ocular staining score, Schirmer test, or unstimulated whole saliva flow rate.[22]
Treatment / Management
Treatment of Sjögren-Associated Dry Eye and Dry Mouth
First-line therapies
Management of Sjögren-associated dry eye typically begins with tear substitutes, followed by anti-inflammatory eye drops when needed. Preservative-free artificial tears are preferred due to lower ocular toxicity. These may include hydroxypropyl methylcellulose, carboxymethylcellulose, polyvinyl alcohol, hyaluronic acid, or liquid polyols. At night, preservative-free gels or ointments can offer additional relief.
Autologous serum eye drops, prepared from the patient’s blood, may improve symptoms and protect the ocular surface in some cases. However, further research is necessary to confirm its efficacy.[23] Use may be limited by access to approved kits and the need for safe preparation.(A1)
Anti-inflammatory drops and topical corticosteroids can help reduce inflammation; however, infrequent use is recommended due to the unclear long-term benefits and potential risks, including glaucoma, cataracts, and infections. Immunosuppressive eye drops such as cyclosporine or tacrolimus inhibit T-cell activity in the lacrimal glands. These agents require several weeks of consistent use to achieve effects and may be limited by cost or tolerability.
Additional supportive therapies
Punctal occlusion using plugs or cauterization is often needed in more severe cases. Cauterization should follow a successful trial of plugs to avoid excessive tearing.
For dry mouth, drinking water, chewing sugar-free gum, and using saliva substitutes may provide symptom relief. Prescription medications like pilocarpine (Salagen) or cevimeline (Evoxac) stimulate salivary flow. A meta-analysis of 383 patients across 8 randomized controlled trials showed that pilocarpine improved certain measures of saliva production and dry eye symptoms.[24](A1)
Antifungal therapies may be required when yeast infections develop. Additional measures, eg, humidifiers and nasal saline irrigation, may alleviate nasal dryness. Medications that reduce gastric acid, including proton-pump inhibitors and H2 blockers, can ease reflux-related symptoms.
Systemic treatment
Systemic treatment is generally unnecessary for isolated dry eye (xerophthalmia) or dry mouth (xerostomia). However, moderate to severe extraglandular manifestations call for systemic therapy. In such cases, glucocorticoids, antimalarial medication (eg, hydroxychloroquine), and steroid-sparing immunosuppressants, including methotrexate, azathioprine, mycophenolate mofetil, or leflunomide, may be used. Rituximab or cyclophosphamide may be considered occasionally. No clear consensus exists regarding the superiority of one therapeutic approach over another.[25][26][27](A1)
Differential Diagnosis
Differential diagnoses that should also be considered in the evaluation of Sjögren syndrome include:
- Sarcoidosis
- Hashimoto thyroiditis
- Celiac disease
- Rosacea
- Mumps
- Dehydration
- Medications (eg, antidepressants and anticholinergics)
- Mouth breathing
- Lymphoma
- Advanced age with atrophy of exocrine tissues
- sclerosing sialadenitis
- Parkinson disease
- Scleroderma
- Rheumatoid arthritis
- AIDS
- Lupus
- IgG4-related disease
- Sjögren syndrome/sicca syndrome associated with cancer immunotherapy
Prognosis
Mild Sjögren syndrome generally carries a favorable prognosis, while moderate to severe disease often results in diminished quality of life. Persistent symptoms such as dry mouth and dry eyes commonly lead to chronic pain, dental decay, and tooth loss.
Among patients with seropositive disease (positive SSA or SSB antibodies), approximately 7% developed ANA-associated rheumatologic conditions, eg, systemic lupus erythematosus, polymyositis, and systemic sclerosis, over a follow-up period of 5 to 6 years.[28] Constitutional symptoms, cutaneous manifestations, renal abnormalities, and hematological findings occur more frequently in patients with positive ANA or SSA serologies.[29]
Ongoing long-term studies aim to identify which patients face the greatest risk of complications. A recent French study revealed three distinct clinical subsets based on disease activity scores (ESSDAI), patient-reported symptom burden (ESSPRI), and markers of B-cell activation.[30]
One group consists of younger individuals with low systemic disease activity but a high symptom burden, typically including fatigue, dryness, and pain. These patients often exhibit increased B-cell activation, eg, hypergammaglobulinemia, reduced C4 levels, and higher seropositivity for SSA or SSB.[30] A second group presents with both high systemic disease activity and a high symptom burden. These individuals frequently possess additional autoantibodies, including anti-RNP or anti-centromere antibodies.
The third subset includes patients with a high symptom burden but low systemic disease activity. This cohort demonstrated minimal disease progression over a 5-year period and no lymphoma development during a 15-year follow-up. Patients with both high symptom burden and high systemic disease activity demonstrated the greatest risk for developing lymphoma.[30]
Complications
Complications from Sjögren syndrome may be glandular or extraglandular.[31]
Glandular complications include:
- Keratoconjuntivitis sicca: corneal erosions, vision loss
- Xerostomia: accelerated dental caries, dental decay, and tooth loss
Extraglandular complications include:
- Musculoskeletal: Arthritis, arthralgia, and myalgias occur in most patients with Sjögren syndrome. The estimated prevalence of arthralgia is 96%, while arthritis is about 16%, and myalgia is approximately 70%.[32] Patients may also develop myositis, though low in incidence.
- Dermatologic: The skin is affected in up to 72% of patients with Sjögren syndrome. Complications range from the more common, eg, xeroderma, eyelid dermatitis, and Raynaud phenomenon, to the less common, including cutaneous vasculitis (palpable purpura), cutaneous amyloidosis, and annular erythema.[32]
- Neurologic: Both the central and peripheral nervous systems may be involved in Sjögren syndrome. CNS complications occur in approximately 5% of patients and can vary from mild cognitive dysfunction to transverse myelitis, demyelinating diseases such as neuromyelitis optica, lymphocytic meningitis, or cerebral vasculitis. Cranial neuropathies are uncommon but tend to affect the trigeminal, facial, or cochlear nerves.[32] Peripheral nervous system complications occur in up to 16% of patients with Sjögren syndrome and can involve sensory and sensorimotor neuropathies, small fiber neuropathy, sensory ataxic neuronopathy, and mononeuritis multiplex. Autonomic dysfunction is also reported in patients with Sjögren syndrome.
- Renal: Disorders of the kidney are rare in Sjögren syndrome and are estimated to occur in up to 5% of patients.[32] These complications may include tubulointerstitial nephritis, distal renal tubular acidosis (RTA), and rarely, membranoproliferative glomerulonephritis.
- Hematologic: Cytopenias, hypergammaglobulinemia, hypogammaglobulinemia, monoclonal gammopathy, and cryoglobulins are the most common manifestations. Leukopenia can occur in up to 20% of patients with Sjögren syndrome, anemia in up to 24%, and thrombocytopenia in up to 30% of patients.[32] Hypergammaglobulinemia may occur in up to 40% of patients.
- Pulmonary: Lung involvement has a wide range of prevalence from 9% to 75%.[33] Manifestations can include respiratory symptoms, eg, cough and dyspnea related to dryness, airway disease (eg, bronchiectasis and bronchiolitis), interstitial lung disease (including nonspecific interstitial pneumonia, lymphocytic interstitial pneumonia, organizing pneumonia), cystic lung disease, pulmonary hypertension, and more rare complications.[33]
- Non-Hodgkin lymphoma: A higher risk for lymphoma has been noted compared to the general population. A 2024 meta-analysis of studies examining the relationship between Sjögren syndrome and lymphoma showed an 8.7-fold increased risk in patients with Sjögren syndrome.[34] The risk was also highest in the 40 to 49 age range and for females compared to males.
Deterrence and Patient Education
Sjögren syndrome frequently presents with dry eye and dry mouth; however, not all individuals with these persistent symptoms meet the criteria for a diagnosis. When dry eye and dry mouth occur alongside swollen salivary glands or lymph nodes, accelerated dental cavities, or signs of extraglandular involvement, further evaluation for Sjögren syndrome becomes warranted. Extraglandular features may include dry skin, joint pain, fatigue, or symptoms affecting other organ systems. Patients should be encouraged to report such symptoms to healthcare professionals, including primary care clinicians, ophthalmologists, and dentists, for appropriate assessment.
Effective self-care remains essential for individuals living with Sjögren syndrome. Regular use of preservative-free hydrating eye drops can help manage ocular dryness. Oral care should include consistent brushing and flossing, fluoride-containing toothpaste, and sugar-free chewing gum or lozenges to stimulate saliva production. Avoiding cigarette smoking, alcohol, and other substances or medications that reduce salivary output supports better symptom control.
For patients with dry eye, proper use of artificial tears and gels, warm compresses, and limiting contact lens use may alleviate discomfort. Ongoing care by dental and ophthalmologic clinicians plays a critical role in monitoring disease impact and preventing complications. Those with a confirmed diagnosis of Sjögren syndrome should be informed of the increased risk for lymphoma. Any new or concerning symptoms, including persistent glandular swelling, visual disturbances, unexplained weight loss, fevers, chronic dry cough, new neurological complaints, or rashes, should prompt timely medical evaluation.
Pearls and Other Issues
Patients with primary Sjögren syndrome have an increased relative risk of developing B-cell non-Hodgkin lymphoma. Many of these lymphomas are extranodal and may involve the salivary glands. Risk factors include persistent salivary gland swelling, enlarging lymph nodes, leukopenia, palpable purpura, and low complement C4 at presentation. These patients require careful monitoring and referral for oncologic evaluation when appropriate. Biological therapies are used to treat severe cases of Sjögren's disease. Rituximab is an option for patients with keratoconjunctivitis sicca, vasculitis, xerostomia, and severe parotid gland swelling.
Enhancing Healthcare Team Outcomes
Effective management of Sjögren syndrome, a complex systemic autoimmune disorder, requires a coordinated interprofessional team committed to patient-centered care. Rheumatologists play a central role in confirming diagnoses, assessing systemic involvement, and guiding overall disease management. They often act as the hub for referrals to specialists based on organ-specific complications. Ophthalmologists manage ocular manifestations by diagnosing keratoconjunctivitis sicca, monitoring for long-term complications such as corneal ulceration, and prescribing topical therapies, including immunosuppressive agents. Dentists, likewise, are vital to managing the oral complications of the disease. Routine dental cleanings every 3 to 6 months, prevention of caries, and education in oral hygiene practices help mitigate long-term damage caused by chronic xerostomia.
Primary care clinicians maintain continuity of care and monitor for the development of new symptoms or comorbid conditions. They, along with pharmacists, evaluate medication regimens and identify drugs with anticholinergic properties that can worsen dryness, ensuring safer pharmacologic management. The involvement of additional specialists, eg, pulmonologists for interstitial lung disease, nephrologists for renal tubular acidosis, or neurologists for peripheral neuropathies, further enhances patient outcomes through tailored evaluation and treatment. Mental health professionals address fatigue, depression, or cognitive symptoms, which patients often report. Nurses, case managers, and allied health professionals facilitate ongoing care coordination, patient education, and monitoring of treatment adherence to ensure effective patient care. Clear, consistent interprofessional communication and shared decision-making not only promote patient safety and team efficiency but also optimize outcomes by addressing the multifaceted needs of individuals with Sjögren syndrome.[35][36][37]
Media
References
Lessard CJ, Li H, Adrianto I, Ice JA, Rasmussen A, Grundahl KM, Kelly JA, Dozmorov MG, Miceli-Richard C, Bowman S, Lester S, Eriksson P, Eloranta ML, Brun JG, Gøransson LG, Harboe E, Guthridge JM, Kaufman KM, Kvarnström M, Jazebi H, Cunninghame Graham DS, Grandits ME, Nazmul-Hossain AN, Patel K, Adler AJ, Maier-Moore JS, Farris AD, Brennan MT, Lessard JA, Chodosh J, Gopalakrishnan R, Hefner KS, Houston GD, Huang AJ, Hughes PJ, Lewis DM, Radfar L, Rohrer MD, Stone DU, Wren JD, Vyse TJ, Gaffney PM, James JA, Omdal R, Wahren-Herlenius M, Illei GG, Witte T, Jonsson R, Rischmueller M, Rönnblom L, Nordmark G, Ng WF, UK Primary Sjögren's Syndrome Registry, Mariette X, Anaya JM, Rhodus NL, Segal BM, Scofield RH, Montgomery CG, Harley JB, Sivils KL. Variants at multiple loci implicated in both innate and adaptive immune responses are associated with Sjögren's syndrome. Nature genetics. 2013 Nov:45(11):1284-92. doi: 10.1038/ng.2792. Epub 2013 Oct 6 [PubMed PMID: 24097067]
Baer AN, Akpek EK, Alevizos I, 18-21 April 2018, Washington, DC, USA. 14th International Symposium on Sjögren's Syndrome. Clinical and experimental rheumatology. 2018 May-Jun:36 Suppl 112(3):241-255 [PubMed PMID: 30156551]
Kroese FGM, Haacke EA, Bombardieri M. The role of salivary gland histopathology in primary Sjögren's syndrome: promises and pitfalls. Clinical and experimental rheumatology. 2018 May-Jun:36 Suppl 112(3):222-233 [PubMed PMID: 30156550]
Björk A, Mofors J, Wahren-Herlenius M. Environmental factors in the pathogenesis of primary Sjögren's syndrome. Journal of internal medicine. 2020 May:287(5):475-492. doi: 10.1111/joim.13032. Epub 2020 Feb 27 [PubMed PMID: 32107824]
Baldini C, Fulvio G, La Rocca G, Ferro F. Update on the pathophysiology and treatment of primary Sjögren syndrome. Nature reviews. Rheumatology. 2024 Aug:20(8):473-491. doi: 10.1038/s41584-024-01135-3. Epub 2024 Jul 9 [PubMed PMID: 38982205]
Kiadaliri AA, Mohammad AJ, Englund M. Hospitalizations due to systemic connective tissue diseases: Secular trends and regional disparities in Sweden, 1998-2016. International journal of rheumatic diseases. 2018 Nov:21(11):1900-1906. doi: 10.1111/1756-185X.13341. Epub 2018 Aug 30 [PubMed PMID: 30168267]
Alani H, Henty JR, Thompson NL, Jury E, Ciurtin C. Systematic review and meta-analysis of the epidemiology of polyautoimmunity in Sjögren's syndrome (secondary Sjögren's syndrome) focusing on autoimmune rheumatic diseases. Scandinavian journal of rheumatology. 2018 Mar:47(2):141-154. doi: 10.1080/03009742.2017.1324909. Epub 2017 Sep 20 [PubMed PMID: 28927315]
Level 1 (high-level) evidenceBunya VY, Fernandez KB, Ying GS, Massaro-Giordano M, Macchi I, Sulewski ME, Hammersmith KM, Nagra PK, Rapuano CJ, Orlin SE. Survey of Ophthalmologists Regarding Practice Patterns for Dry Eye and Sjogren Syndrome. Eye & contact lens. 2018 Nov:44 Suppl 2(Suppl 2):S196-S201. doi: 10.1097/ICL.0000000000000448. Epub [PubMed PMID: 29369232]
Level 3 (low-level) evidenceTzioufas AG, Goules AV. Limited efficacy of targeted treatments in Sjögren's syndrome: why? Clinical and experimental rheumatology. 2018 May-Jun:36 Suppl 112(3):27-28 [PubMed PMID: 29998826]
Tarpley TM Jr, Anderson LG, White CL. Minor salivary gland involvement in Sjögren's syndrome. Oral surgery, oral medicine, and oral pathology. 1974 Jan:37(1):64-74 [PubMed PMID: 4586901]
Jonsson MV, Skarstein K, Jonsson R, Brun JG. Serological implications of germinal center-like structures in primary Sjögren's syndrome. The Journal of rheumatology. 2007 Oct:34(10):2044-9 [PubMed PMID: 17787040]
Fox PC, Busch KA, Baum BJ. Subjective reports of xerostomia and objective measures of salivary gland performance. Journal of the American Dental Association (1939). 1987 Oct:115(4):581-4 [PubMed PMID: 3477595]
Seror R, Ravaud P, Bowman SJ, Baron G, Tzioufas A, Theander E, Gottenberg JE, Bootsma H, Mariette X, Vitali C, EULAR Sjögren's Task Force. EULAR Sjogren's syndrome disease activity index: development of a consensus systemic disease activity index for primary Sjogren's syndrome. Annals of the rheumatic diseases. 2010 Jun:69(6):1103-9. doi: 10.1136/ard.2009.110619. Epub 2009 Jun 28 [PubMed PMID: 19561361]
Level 3 (low-level) evidenceSeror R, Bowman SJ, Brito-Zeron P, Theander E, Bootsma H, Tzioufas A, Gottenberg JE, Ramos-Casals M, Dörner T, Ravaud P, Vitali C, Mariette X, Asmussen K, Jacobsen S, Bartoloni E, Gerli R, Bijlsma JW, Kruize AA, Bombardieri S, Bookman A, Kallenberg C, Meiners P, Brun JG, Jonsson R, Caporali R, Carsons S, De Vita S, Del Papa N, Devauchelle V, Saraux A, Fauchais AL, Sibilia J, Hachulla E, Illei G, Isenberg D, Jones A, Manoussakis M, Mandl T, Jacobsson L, Demoulins F, Montecucco C, Ng WF, Nishiyama S, Omdal R, Parke A, Praprotnik S, Tomsic M, Price E, Scofield H, L Sivils K, Smolen J, Laqué RS, Steinfeld S, Sutcliffe N, Sumida T, Valesini G, Valim V, Vivino FB, Vollenweider C. EULAR Sjögren's syndrome disease activity index (ESSDAI): a user guide. RMD open. 2015:1(1):e000022. doi: 10.1136/rmdopen-2014-000022. Epub 2015 Feb 20 [PubMed PMID: 26509054]
Shiboski CH, Shiboski SC, Seror R, Criswell LA, Labetoulle M, Lietman TM, Rasmussen A, Scofield H, Vitali C, Bowman SJ, Mariette X, International Sjögren's Syndrome Criteria Working Group. 2016 American College of Rheumatology/European League Against Rheumatism classification criteria for primary Sjögren's syndrome: A consensus and data-driven methodology involving three international patient cohorts. Annals of the rheumatic diseases. 2017 Jan:76(1):9-16. doi: 10.1136/annrheumdis-2016-210571. Epub 2016 Oct 26 [PubMed PMID: 27789466]
Level 3 (low-level) evidenceDe Vita S, Gandolfo S, Zandonella Callegher S, Zabotti A, Quartuccio L. The evaluation of disease activity in Sjögren's syndrome based on the degree of MALT involvement: glandular swelling and cryoglobulinaemia compared to ESSDAI in a cohort study. Clinical and experimental rheumatology. 2018 May-Jun:36 Suppl 112(3):150-156 [PubMed PMID: 30156548]
Martel A, Coiffier G, Bleuzen A, Goasguen J, de Bandt M, Deligny C, Magnant J, Ferreira N, Diot E, Perdriger A, Maillot F. What is the best salivary gland ultrasonography scoring methods for the diagnosis of primary or secondary Sjögren's syndromes? Joint bone spine. 2019 Mar:86(2):211-217. doi: 10.1016/j.jbspin.2018.06.014. Epub 2018 Jul 24 [PubMed PMID: 30053612]
Baer AN, Walitt B. Update on Sjögren Syndrome and Other Causes of Sicca in Older Adults. Rheumatic diseases clinics of North America. 2018 Aug:44(3):419-436. doi: 10.1016/j.rdc.2018.03.002. Epub [PubMed PMID: 30001784]
Clark M, Walsh H, Stephens-Laborde I, Khurram SA. The Value of Labial Gland Biopsies as a Diagnostic Test for Sjögren's Syndrome. Head and neck pathology. 2024 Jul 3:18(1):64. doi: 10.1007/s12105-024-01662-1. Epub 2024 Jul 3 [PubMed PMID: 38958850]
Dal Pozzolo R, Cafaro G, Perricone C, Calvacchi S, Bruno L, Colangelo A, Tromby F, Gerli R, Bartoloni E. Salivary gland biopsy as a prognostic tool in Sjögren's syndrome. Expert review of clinical immunology. 2024 Oct:20(10):1139-1147. doi: 10.1080/1744666X.2024.2368189. Epub 2024 Jun 24 [PubMed PMID: 38881375]
Mossel E, Arends S, van Nimwegen JF, Delli K, Stel AJ, Kroese FGM, Spijkervet FKL, Vissink A, Bootsma H, EULAR US-pSS study group. Scoring hypoechogenic areas in one parotid and one submandibular gland increases feasibility of ultrasound in primary Sjögren's syndrome. Annals of the rheumatic diseases. 2018 Apr:77(4):556-562. doi: 10.1136/annrheumdis-2017-211992. Epub 2017 Dec 12 [PubMed PMID: 29233833]
Level 2 (mid-level) evidencevan Nimwegen JF, Mossel E, Delli K, van Ginkel MS, Stel AJ, Kroese FGM, Spijkervet FKL, Vissink A, Arends S, Bootsma H. Incorporation of Salivary Gland Ultrasonography Into the American College of Rheumatology/European League Against Rheumatism Criteria for Primary Sjögren's Syndrome. Arthritis care & research. 2020 Apr:72(4):583-590. doi: 10.1002/acr.24017. Epub [PubMed PMID: 31254454]
Pan Q, Angelina A, Marrone M, Stark WJ, Akpek EK. Autologous serum eye drops for dry eye. The Cochrane database of systematic reviews. 2017 Feb 28:2(2):CD009327. doi: 10.1002/14651858.CD009327.pub3. Epub 2017 Feb 28 [PubMed PMID: 28245347]
Level 1 (high-level) evidenceWu J, Li D. Pilocarpine treatment of Sjögren's syndrome curative effect of meta-analysis of randomised controlled trials. Clinical and experimental rheumatology. 2024 Dec:42(12):2490-2498. doi: 10.55563/clinexprheumatol/lkk9qq. Epub 2024 Dec 19 [PubMed PMID: 39699871]
Level 1 (high-level) evidenceSumida T, Azuma N, Moriyama M, Takahashi H, Asashima H, Honda F, Abe S, Ono Y, Hirota T, Hirata S, Tanaka Y, Shimizu T, Nakamura H, Kawakami A, Sano H, Ogawa Y, Tsubota K, Ryo K, Saito I, Tanaka A, Nakamura S, Takamura E, Tanaka M, Suzuki K, Takeuchi T, Yamakawa N, Mimori T, Ohta A, Nishiyama S, Yoshihara T, Suzuki Y, Kawano M, Tomiita M, Tsuboi H. Clinical practice guideline for Sjögren's syndrome 2017. Modern rheumatology. 2018 May:28(3):383-408. doi: 10.1080/14397595.2018.1438093. Epub 2018 Mar 13 [PubMed PMID: 29409370]
Level 1 (high-level) evidenceSeror R, Mariette X. Guidelines for treatment of primary Sjögren's syndrome: a first useful stone but still much to do. Rheumatology (Oxford, England). 2017 Oct 1:56(10):1641-1642. doi: 10.1093/rheumatology/kex320. Epub [PubMed PMID: 28957571]
Price EJ, Rauz S, Tappuni AR, Sutcliffe N, Hackett KL, Barone F, Granata G, Ng WF, Fisher BA, Bombardieri M, Astorri E, Empson B, Larkin G, Crampton B, Bowman SJ, British Society for Rheumatology Standards, Guideline and Audit Working Group. The British Society for Rheumatology guideline for the management of adults with primary Sjögren's Syndrome. Rheumatology (Oxford, England). 2017 Oct 1:56(10):e24-e48. doi: 10.1093/rheumatology/kex166. Epub [PubMed PMID: 28957550]
Fauchais AL, Martel C, Gondran G, Lambert M, Launay D, Jauberteau MO, Hachulla E, Vidal E, Hatron PY. Immunological profile in primary Sjögren syndrome: clinical significance, prognosis and long-term evolution to other auto-immune disease. Autoimmunity reviews. 2010 Jul:9(9):595-9. doi: 10.1016/j.autrev.2010.05.004. Epub 2010 May 10 [PubMed PMID: 20457283]
Yayla ME, Aksoy R, Uslu E, Karaman Z, Yilmaz R, Ilbay A, Göveç Giynaş N, Gaydan A, Usta A, Mahmutoğlu Y, Ateş A, Turgay M. Are there differences in the clinical and laboratory features of patients with seronegative primary Sjögren's syndrome? Turkish journal of medical sciences. 2024:54(5):956-962. doi: 10.55730/1300-0144.5873. Epub 2024 Oct 7 [PubMed PMID: 39473752]
Nguyen Y, Nocturne G, Henry J, Ng WF, Belkhir R, Desmoulins F, Bergé E, Morel J, Perdriger A, Dernis E, Devauchelle-Pensec V, Sène D, Dieudé P, Couderc M, Fauchais AL, Larroche C, Vittecoq O, Salliot C, Hachulla E, Le Guern V, Gottenberg JE, Mariette X, Seror R. Identification of distinct subgroups of Sjögren's disease by cluster analysis based on clinical and biological manifestations: data from the cross-sectional Paris-Saclay and the prospective ASSESS cohorts. The Lancet. Rheumatology. 2024 Apr:6(4):e216-e225. doi: 10.1016/S2665-9913(23)00340-5. Epub 2024 Mar 1 [PubMed PMID: 38437852]
Level 2 (mid-level) evidenceZhao T, Zhang R, Li Z, Qin D, Wang X. A comprehensive review of Sjögren's syndrome: Classification criteria, risk factors, and signaling pathways. Heliyon. 2024 Sep 15:10(17):e36220. doi: 10.1016/j.heliyon.2024.e36220. Epub 2024 Aug 15 [PubMed PMID: 39286095]
Level 2 (mid-level) evidenceMihai A, Caruntu C, Jurcut C, Blajut FC, Casian M, Opris-Belinski D, Ionescu R, Caruntu A. The Spectrum of Extraglandular Manifestations in Primary Sjögren's Syndrome. Journal of personalized medicine. 2023 Jun 7:13(6):. doi: 10.3390/jpm13060961. Epub 2023 Jun 7 [PubMed PMID: 37373950]
Natalini JG, Johr C, Kreider M. Pulmonary Involvement in Sjögren Syndrome. Clinics in chest medicine. 2019 Sep:40(3):531-544. doi: 10.1016/j.ccm.2019.05.002. Epub 2019 Jul 6 [PubMed PMID: 31376889]
Ansari N, Salesi M. The association between primary Sjogren's syndrome and non-Hodgkin's lymphoma: a systematic review and meta-analysis of cohort studies. Clinical rheumatology. 2024 Jul:43(7):2177-2186. doi: 10.1007/s10067-024-06993-6. Epub 2024 May 9 [PubMed PMID: 38722505]
Level 1 (high-level) evidenceLópez-Pintor RM, Fernández Castro M, Hernández G. Oral involvement in patients with primary Sjögren's syndrome. Multidisciplinary care by dentists and rheumatologists. Reumatologia clinica. 2015 Nov-Dec:11(6):387-94. doi: 10.1016/j.reuma.2015.03.010. Epub 2015 May 26 [PubMed PMID: 26022574]
Brito-Zerón P, Theander E, Baldini C, Seror R, Retamozo S, Quartuccio L, Bootsma H, Bowman SJ, Dörner T, Gottenberg JE, Mariette X, Bombardieri S, de Vita S, Mandl T, Ng WF, Kruize AA, Tzioufas A, Vitali C, Buyon J, Izmirly P, Fox R, Ramos-Casals M, Eular Sjögren Syndrome Task Force. Early diagnosis of primary Sjögren's syndrome: EULAR-SS task force clinical recommendations. Expert review of clinical immunology. 2016:12(2):137-56. doi: 10.1586/1744666X.2016.1109449. Epub 2015 Dec 22 [PubMed PMID: 26691952]
Milin M, Cornec D, Chastaing M, Griner V, Berrouiguet S, Nowak E, Marhadour T, Saraux A, Devauchelle-Pensec V. Sicca symptoms are associated with similar fatigue, anxiety, depression, and quality-of-life impairments in patients with and without primary Sjögren's syndrome. Joint bone spine. 2016 Dec:83(6):681-685. doi: 10.1016/j.jbspin.2015.10.005. Epub 2016 Jan 13 [PubMed PMID: 26774177]
Level 2 (mid-level) evidence