Introduction
Abusive head trauma (AHT) has been defined by the Centers for Disease Control and Prevention (CDC) as injury to the intracranial contents or skull of a pediatric patient (0-5 years of age) resulting from blunt force, forceful shaking, or a combination of both.[1] AHT is the most devastating form of child abuse and remains the leading cause of traumatic brain injury (TBI) in infants and toddlers, associated with high mortality and morbidity.[2][3][4][5] The term "shaken baby syndrome" (SBS) was previously used to describe injuries caused primarily by violent shaking. However, the terminology has evolved to reflect a broader understanding of injury mechanisms, including shaking, blunt trauma, rotational forces, and direct cranial impact (see Image. Shaken Baby Syndrome on Magnetic Resonance Imaging, 3-Month-Old Patient).[6]
Clinical features frequently lack specificity and often resemble accidental trauma, complicating the differentiation between abusive and nonabusive injuries. Accurate identification requires a high index of suspicion and a thorough, developmentally informed history. Diagnostic errors frequently occur due to the subtle nature of presenting signs and the absence of external trauma.[7] Reported histories are often vague, inconsistent, or frequently revised. Caregivers rarely admit to intentional harm, and common explanations, such as falls from cribs, stairs, highchairs, or beds, or blame placed on siblings, may be incongruent with the child's developmental capabilities or the biomechanical forces required to produce observed injuries.
Neurological sequelae of AHT can manifest as apnea, seizures, and retinal hemorrhage, typically resulting from hypoxic-ischemic or axonal injury.[8] Outcomes range from complete recovery from TBI to profound neurological damage with long-term cognitive and developmental delay; death may occur in severe cases. Prevention depends on comprehensive caregiver education, early identification of psychosocial risk factors, and targeted interventions to improve caregiver mental health services. Enhanced clinician training in the recognition of abuse-related injuries supports earlier detection and improved clinical outcomes. Effective risk assessment should encompass the child, family, and environmental context to inform prevention strategies. Survivors of AHT frequently experience severe, lifelong medical, cognitive, and psychosocial impairments, with considerable financial and societal costs.[9]
Abusive Head Trauma
The American Academy of Pediatrics and the CDC define AHT as intentional injury to the intracranial contents or skull of a pediatric patient (0–5 years of age) resulting from blunt force, forceful shaking involving acceleration, deceleration, and rotational movements, or a combination of these forces. This definition emphasizes the nature of the injury rather than the specific causative mechanism. Additional terminology includes the following:
- SBS
- Battered child [10]
- Whiplash shaken infant syndrome [11]
- Nonaccidental trauma
- Intentional head trauma
- Inflicted head trauma
Brain injuries are the most common cause of traumatic death in children younger than 2 years and remain the leading cause of injury during infancy.[12]
Shaken Baby Syndrome
Key features of SBS include cerebral edema, retinal hemorrhage, and subdural hematoma (SDH). The presence of cerebral edema indicates the severity of injury. Retinal hemorrhages caused by accidental trauma are typically mild and require high-impact mechanisms, such as motor vehicle collisions. Results from a study conducted in France found that grade 3A and 3B hemorrhages were specific for SBS, with a high positive predictive value of 0.96. Hemorrhages extending beyond the posterior pole demonstrate high specificity for severe head injury, particularly AHT.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Various risk factors for AHT have been associated with characteristics of the child, the caregiver, and the broader social environment. Understanding these variables is critical for guiding prevention, screening, and early intervention efforts.
Child-related risk factors include the following:
- Inconsolable or excessive crying, particularly during the "purple crying" period at 6 to 8 weeks of age [13]
- Infantile colic
- Developmental delay or physical disabilities
- Chronic medical conditions or comorbidities
- History of prematurity or low birth weight
- Multiple births
- Unplanned pregnancy [14]
Caregiver and family characteristics also contribute to AHT risk, including the following:
- Young parental age with limited social support
- Low socioeconomic status or self-esteem
- Limited parental education
- Absence of prenatal care
- Lack of childcare experience
- Poor impulse control or low frustration tolerance
- Mental illness or substance use
- Single-caregiver households
- Prior involvement with child protective services
- Inaccurate understanding of child development, including unrealistic expectations and misinterpretation of normal behavior
Certain community-level factors further increase vulnerability:
- Social isolation with limited access to recreational spaces
- Poverty
- Unemployment
- Exposure to domestic violence, including partner violence
- Limited availability of supportive community services
- Presence of an unrelated adult male caregiver in the home [15]
The presence of these risk factors may increase vulnerability, but their absence does not rule out a diagnosis of AHT. Perpetrators vary; however, male caregivers, such as fathers, stepfathers, or the mother’s boyfriend, are most frequently involved, followed by female babysitters and biological mothers.[16][17]
Epidemiology
Incidence
Accurate determination of AHT incidence remains challenging due to the absence of a centralized reporting system, the variability of clinical presentations, and frequent underrecognition of abuse. AHT often results from repeated maltreatment rather than a single acute event. In the United States (US), the estimated incidence of AHT in children younger than 1 year ranges from 25 to 35 per 100,000 annually.[18] AHT is the leading cause of fatal physical abuse in children, with most deaths occurring during infancy.[19]
Age Variation
The incidence of AHT peaks between 6 and 8 weeks of age, aligning with the period of increased infant crying known as the “purple crying period.” Reported mortality rates range from 10% to 20%. After 1 year of age, incidence drops substantially to approximately 3.8 per 100,000 annually.[20]
Geographic Variation
Geographic variation has been observed within the US, with the highest incidence reported in the Midwest and the lowest in the Northeast. No definitive explanation has been established for these regional differences.
Sentinel Injuries
Sentinel injuries frequently precede a formal diagnosis of child maltreatment. Nearly 25% of children later diagnosed with abuse present with previously unrecognized injuries. Bruising and intraoral trauma are the most common findings, particularly in children younger than 3 years. Up to 20% of these events are missed during earlier clinical encounters.[21]
Variation in Clinical Practice
Clinical practice variation further affects diagnosis. Bias based on caregiver demographics, socioeconomic status, or ethnicity may influence screening decisions. Standardized screening algorithms have been shown to improve AHT detection and reduce disparities in evaluation and care.[22]
Pathophysiology
The biomechanical forces involved in AHT typically include combinations of rotational, translational, and impact forces. These injury mechanisms may result from violent shaking, blunt impact, or both. Experimental studies using computational models, human surrogates, animal research, and postmortem analyses have enhanced understanding of how such forces injure the pediatric brain. Infants are particularly susceptible due to several anatomical and physiological features: a relatively large head-to-body ratio, immature neck musculature, increased ligamentous laxity, incompletely ossified skull, and higher intracranial water content. These characteristics magnify the effects of acceleration-deceleration and rotational forces, increasing the risk for diffuse brain injury and more severe coup injury.
Repetitive shaking may cause rapid rotation with flexion-extension of the head and neck, resulting in shearing of intracranial blood vessels and producing subdural hematoma, subarachnoid hemorrhage, and cerebral edema (see Image. Subdural Hematoma on Computed Tomography). Expanding hematomas can elevate intracranial pressure (ICP), compromising cerebral perfusion and leading to ischemic injury. Diffuse axonal injury disrupts the neuromuscular cascade in a manner similar to TBI. Direct impact against surfaces can generate sufficient force to cause skull fractures and concussions. In clinical settings, many head injuries involve both contact and inertial forces related to brain motion. The cumulative effects of repeated abusive episodes are often more severe than those of a single incident, as progressive cerebral injury and rising ICP may intensify neurologic damage over time.
History and Physical
Diagnosing AHT remains challenging, particularly in preverbal children who often present with nonspecific symptoms such as increased fussiness or poor feeding.[23] A critical evaluation component involves assessing whether the reported mechanism of injury aligns with the child’s developmental capabilities and observed clinical findings. Caregivers may provide vague or inconsistent accounts, often delaying medical care without a plausible explanation. Denial of trauma is common, even in the presence of overt injuries, and explanations may change substantially over time. A lack of an appropriate history should raise concerns about nonaccidental trauma.
History
Key historical red flags include poor feeding, vomiting, diminished interaction with caregivers, loss of a social smile, increased irritability, lethargy, altered mental status, apnea, seizures, and hypothermia. Indicators of neglect may also be present, such as failure-to-thrive, a decline in growth percentiles, persistent poor hygiene, unclean clothing, untreated diaper dermatitis, poor wound care, and severe dental caries. A prior missed opportunity for diagnosis may exist, as children often present with sentinel injuries that were not previously recognized.
Physical Examination
The physical examination may reveal signs that are subtle or absent externally, contributing to missed diagnoses and delayed intervention. Bruising in nonambulatory infants, commonly called “bruising before cruising,” warrants concern. Additional findings may include intraoral injuries such as frenulum tears or lingual lacerations, as well as bruising or injury across multiple organ systems without a unifying explanation. Injuries in various stages of healing, patterned bruises, and trauma in atypical locations, including the torso, neck, ears, and genitalia, should raise immediate suspicion.
Neurologic signs suggestive of AHT include vomiting, apnea, seizures, bradycardia, macrocephaly, bulging or tense anterior fontanelle, and scalp bruising. Skull fractures may be present and are often accompanied by overlying soft tissue injury. The TEN-4-FACESp rule is beneficial in identifying abusive bruising patterns: bruising in children younger than 4 years on the torso, ears, or neck (TEN), and bruising on the frenulum, angle of the jaw, cheeks, eyelids, subconjunctivae, or in a patterned configuration (FACESp).[24][25]
Inflicted burns may appear with sharply demarcated borders and symmetric patterns, particularly in immersion burns affecting the lower extremities and genital region.[26] Grab marks should prompt evaluation for possible underlying fractures. Thoracoabdominal injuries may include rib fractures resulting from compressive anteroposterior forces during violent squeezing. Signs such as irregular breathing, chest or abdominal bruising, guarding, and muscular rigidity suggest underlying trauma to the lungs or abdominal organs. Musculoskeletal injuries, especially fractures in nonambulatory infants without a clear history of trauma or known bone fragility, are strongly associated with physical abuse. Fractures are the second most common manifestation of inflicted injury in this population.[27]
Evaluation
When AHT is suspected, a thorough and systematic evaluation is essential to exclude other medical conditions, such as hematologic or musculoskeletal disorders, that may present with similar findings and to distinguish inflicted injury from accidental trauma. Many hospitals have adopted specialized clinical practice guidelines for the evaluation of children with suspected abuse or AHT. These protocols promote interprofessional collaboration with law enforcement and Child Protective Services, ensuring timely investigation, accurate diagnosis, and verification of a safe home environment before discharge.[28]
Laboratory Testing
A thorough laboratory evaluation should include a complete blood count and a comprehensive metabolic panel. Elevated transaminases (≥80 IU/L) may suggest blunt abdominal trauma and warrant further imaging, such as contrast-enhanced computed tomography (CT) of the abdomen and pelvis, to assess for solid organ or hollow viscus injury. Troponin I should be measured if thoracoabdominal injury is suspected, as levels may be elevated.[29]
A coagulation panel should also be obtained, including prothrombin time, partial thromboplastin time, and international normalized ratio. Although coagulopathies are rare and typically mild, testing for specific factors (II, VII, IX, X, XII, XIII) and hematology consultation may be considered based on clinical findings. Urinalysis should be performed to assess for hematuria, which may indicate renal injury and correlate with elevated creatinine and blood urea nitrogen levels.
Imaging
Neuroimaging is essential in suspected AHT. A noncontrast-enhanced CT of the head is the first-line modality in acute settings and is effective for detecting skull fractures and various types of intracranial hemorrhage, including subdural hematoma (SDH), subarachnoid hemorrhage, and epidural bleeds. Brain magnetic resonance imaging provides further detail, accurately assessing the age of SDHs, differentiating acute from chronic bleeding, and identifying brain edema, parenchymal ischemia, and ventricular compression (see Image. Shaken Baby Syndrome on Magnetic Resonance Imaging, 8-Month-Old Patient).
A skeletal survey is the standard imaging approach for identifying occult fractures in children younger than 2 years when abuse is suspected. The American College of Radiology guidelines recommend the inclusion of plain radiographs of the skull, spine, ribs, and long bones. Repeating the skeletal survey 2 to 3 weeks after the initial evaluation enhances diagnostic accuracy in detecting healing fractures in infants with suspected child abuse.[30][31][32][33]
Radiologic Findings Highly Suggestive of Abuse
Imaging clues to nonaccidental trauma (NAT) include multiple fractures at various stages of healing, indicating repeated injury over time. Rib fractures, particularly posterior or lateral, are often caused by anteroposterior chest compression and are rarely seen in accidental trauma. Metaphyseal fractures, also known as “bucket handle” fractures, are characterized by curvilinear lucencies at the metaphysis and result from shearing or twisting forces near the growth plate. These features are considered highly specific for inflicted injury.
Spiral fractures of long bones, produced by rotational mechanisms, are also strongly associated with NAT. Fractures involving the scapula, vertebrae, or sternum, as well as classic metaphyseal lesions, are uncommon in accidental injury and suggest possible abuse. Any fracture pattern that does not align with the reported mechanism of injury warrants further investigation.
Ophthalmologic Evaluation
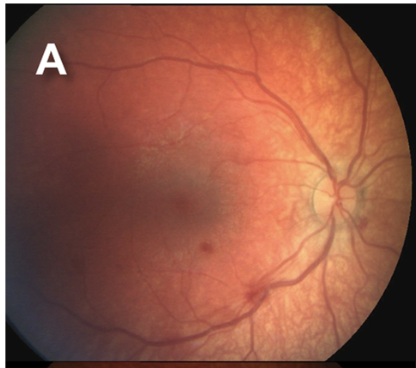
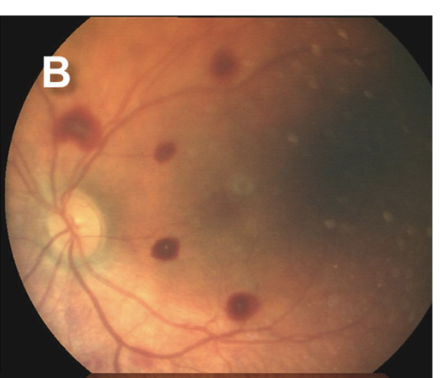
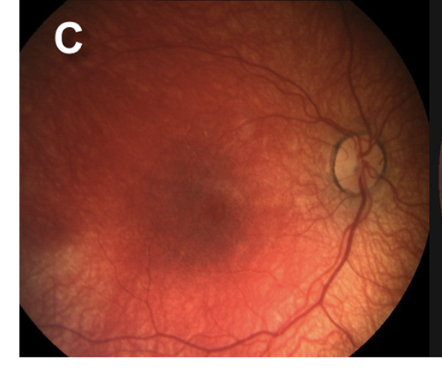
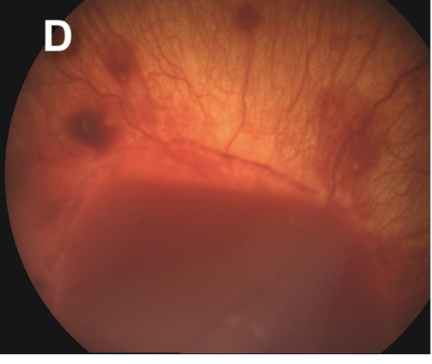
Retinal hemorrhages are a key diagnostic feature of AHT, particularly when multilayered and extending to the ora serrata. However, retinal hemorrhages may also occur in nontraumatic conditions, such as subarachnoid hemorrhage resulting from aneurysmal rupture. The location, depth, and distribution of the hemorrhages are critical in distinguishing abusive from nonabusive causes (see Images. Grades 1 to 3b Retinal Hemorrhages).
Since retinal hemorrhages may resolve within 7 to 10 days, prompt and repeated fundoscopic examinations are essential. Assessments should be performed by an ophthalmologist with pediatric expertise and documented with high-quality fundus photography. The absence of retinal hemorrhages does not exclude AHT or child abuse.
Documentation
Thorough documentation, including written notes, digital photographs, and annotated body diagrams, is essential for clinical care and legal proceedings. These records support diagnostic accuracy and serve as critical evidence in forensic investigations.
Treatment / Management
Initial Stabilization and Resuscitation
The American College of Surgeons advocates a standardized protocol for the early assessment and stabilization of children with suspected trauma, including AHT. This approach is detailed in the Advanced Trauma Life Support (ATLS) Program.[34] According to ATLS guidelines, initial trauma evaluation should involve a structured primary survey that includes the "ABCDE," outlined below:
- Airway with cervical spine protection
- Breathing
- Circulation with hemorrhage control
- Disability or neurologic status using the Glasgow Coma Scale (GCS)
- Exposure with full undressing to visualize injuries while maintaining temperature regulation
An age-adjusted pediatric GCS may be applied in preverbal or young children.[35] Neurologic assessment includes the evaluation of eye (scored from 1 to 4), verbal (scored from 1 to 5), and motor (scored from 1 to 6) responses.[36] The secondary survey consists of a systematic, head-to-toe inspection and palpation. The assessment begins with the head, neck, and maxillofacial region, while ensuring cervical spine stabilization, then proceeds to the chest, abdomen, and pelvis to identify signs of blunt or penetrating trauma. A musculoskeletal evaluation and comprehensive neurologic examination follow. Additional medical history may be obtained at this stage. The primary ABCDE assessment must be repeated whenever the patient’s clinical condition changes.
Management of Intracranial Hypertension in Pediatric Abusive Head Trauma
The management of severe TBI is complex, and the implementation of evidence-based recommendations at the bedside remains a significant challenge.[37] In 2019, the Brain Trauma Foundation published management strategies to reduce intracranial hypertension (ICH), maintain adequate cerebral perfusion, and improve neurological outcomes in children with severe TBI.[38][39] In the absence of reliable imaging or biomarkers for ICH, direct intracranial pressure (ICP) monitoring is supported by current evidence. Treatment targets include maintaining ICP below 20 mm Hg and cerebral perfusion pressure between 40 and 50 mm Hg to optimize survival and neurological outcomes in pediatric individuals.(B3)
Medical approach
Medical management of ICH includes hyperosmolar therapy, seizure prophylaxis, sedation, ventilation strategies, temperature control, nutrition, and adjunctive therapies. Hypertonic saline (3%) may be administered as a bolus of 2 to 5 mL/kg over 10 to 20 minutes or as a continuous infusion of 1 mL/kg/hour, titrated to ICP goals. A 4% hypertonic saline bolus (0.5 mL/kg, maximum 30 mL) may be used for refractory ICH.
Antiepileptic drugs are recommended during the first 7 days, with no clear superiority between levetiracetam and phenytoin. Bolus dosing of fentanyl or midazolam for pain control and sedation should be avoided to prevent cerebral hypoperfusion. Hyperventilation may be considered beyond 48 hours postinjury with advanced neuromonitoring. Moderate hypothermia (32 °C–33 °C/89.6 °F–91.4 °F) may aid in refractory cases.
Early enteral nutrition, preferably within 72 hours, is encouraged, though immune-modulating diets are not advised. Corticosteroids are not recommended. High-dose barbiturates may be used for refractory ICH in hemodynamically stable individuals.
Surgical treatment
A significant proportion of children with AHT require neurosurgical intervention, although standardized, evidence-based guidelines are currently limited. In cases of elevated ICP, an external ventricular drain may be inserted to facilitate cerebrospinal fluid drainage. Decompressive craniectomy may be indicated for intractable ICH unresponsive to medical management.
Management of skull fractures depends on type and severity. Simple linear, isolated, nondepressed skull fractures may be treated nonoperatively, while compound or depressed fractures that exceed cranial thickness often necessitate surgical repair to reduce infection risk.[40] Epidural hemorrhages may require evacuation based on the size of the bleed and the patient's neurological status.[41] In contrast, no universal protocol exists for managing subdural collections in AHT. Clinical status guides the approach, which may include observation, transfontanelle tapping, open or closed irrigation, craniotomy, or placement of a subdural-peritoneal shunt.
Differential Diagnosis
A broad differential diagnosis should be considered when evaluating children with signs suggestive of AHT. Several nonabusive medical conditions may produce similar clinical findings, including the following:
- Accidental head injury
- Birth trauma, including epidural, subdural, subarachnoid, cerebellar, and parenchymal hemorrhage
- Arteriovenous malformations
- Coagulation or bleeding abnormalities
- Hematologic conditions, including blood dyscrasias
- Cerebellar hemorrhage
- Connective tissue disorders
- Infectious causes, such as subdural effusion, subdural empyema, meningitis, and encephalitis
- Inherited metabolic diseases, such as glutaric aciduria type 1, may cause retinal bleeding
- Vitamin K deficiency
- Cerebrovascular events, including strokes
Ruling out these conditions is essential before attributing findings to abuse. Diagnostic certainty is critical to ensure appropriate intervention and prevent misdiagnosis, which may produce serious consequences for families and caregivers.
Prognosis
AHT is associated with substantial morbidity and mortality. Morbidity may range from mild learning disabilities to severe cognitive or physical impairment and death. Common manifestations include blindness, attention-deficit disorder, developmental delay, intellectual disability, sensory and hearing deficits, motor dysfunction, failure to thrive, feeding difficulties, seizures, and behavioral or educational difficulties.[42][43]
Additional complications may include hemiplegia, quadriplegia, hydrocephaly, and microcephaly. Prognosis correlates closely with the extent of injury observed on CT and magnetic resonance imaging. Long-term survivors of severe AHT frequently experience significant reductions in quality of life. Even children with mild injuries may face lifelong impairments.
Study results comparing neurodevelopmental outcomes in children with AHT versus accidental head injuries have shown that infants younger than 36 months with AHT more often sustain noncontact injuries that lead to cardiorespiratory compromise, deeper brain damage, diffuse cerebral hypoxia-ischemia, and poorer outcomes. Children diagnosed with AHT are more likely to die than those with accidental head injuries.
More than 50% of children aged 0 to 4 years who have experienced AHT will die before reaching adulthood. Children with severe injuries experience, on average, a 55% reduction in health-related quality of life. Long-term sequelae are common. More than half of the affected children experience partial or complete blindness. Approximately 5% require eye surgery, and more than 20% require long-term use of a feeding tube.
Complications
AHT carries significant risks of both morbidity and mortality. Outcomes vary widely, ranging from complete recovery to profound, lifelong disability or death. Mortality rates are estimated between 10% and 20%, with the highest risk occurring in infants.
Among survivors, common long-term complications include the following:
- Neurologic deficits: Motor impairments, spasticity, seizures
- Cognitive impairments: Developmental delays, learning disabilities
- Sensory impairments: Visual deficits, including blindness
- Behavioral and emotional disorders: Attention-deficit/hyperactivity disorder, conduct problems, mood disorders [44]
The severity of injury observed on initial neuroimaging strongly correlates with long-term outcomes. Findings such as diffuse axonal injury, extensive cerebral edema, or large subdural collections are associated with a poorer prognosis. Severe AHT is frequently linked to significant reductions in quality of life and imposes long-term financial and emotional burdens on families and healthcare systems. Many affected children require prolonged rehabilitation along with specialized educational and therapeutic services.
Consultations
Collaboration with trauma surgery, pediatric neurosurgery, and child abuse pediatrics has been shown to improve patient outcomes. A coordinated, interprofessional approach ensures comprehensive care and timely intervention.
Deterrence and Patient Education
AHT is a preventable form of child maltreatment, though it remains a significant public health issue. In the US, the annual healthcare cost associated with AHT is estimated to exceed $70 million.[45] Prevention strategies focus on improving caregiver knowledge of normal infant behaviors and the severe consequences of shaking or striking a child’s head, promoting early recognition of AHT by healthcare professionals, and implementing supportive interventions for at-risk families.
Two major national, evidence-based initiatives have been developed to prevent AHT by increasing caregiver education and awareness. These initiatives emphasize early intervention and empower caregivers with tools to manage challenging infant behaviors safely. The first is the Period of PURPLE Crying Program (see below). Developed at British Columbia's Children’s Hospital, this hospital-based initiative educates caregivers about normal infant crying patterns, particularly the peak period around 6 to 8 weeks of age. The acronym "PURPLE" describes the typical features of this developmental phase:
- Peak of crying
- Unpredictable
- Resists soothing
- Pain-like expression
- Long-lasting
- Evening clustering
Implementing the PURPLE program in Canada was associated with a 35% reduction in AHT-related hospital admissions among children younger than 2 years.[42] The second is the National Center on Shaken Baby Syndrome Initiatives. These programs are designed to educate new and expectant parents and enhance their confidence and caregiving skills. The overarching mission is to prevent SBS and promote infant well-being. The program has demonstrated consistent, replicable outcomes in reducing AHT when implemented internationally.[46]
In clinical practice, healthcare professionals must maintain a high index of suspicion for caregiver stress, unsafe coping behaviors, and psychosocial risk factors during all patient encounters. Early referral to preventive mental health services, parenting support, and community-based resources is essential. Caregivers should be educated and equipped with effective coping strategies to manage infant crying safely. Counseling must also address normal developmental expectations and emphasize when and how to seek appropriate assistance.
Enhancing Healthcare Team Outcomes
AHT and other forms of child abuse are serious public health issues with lasting physical and psychological consequences. AHT survivors may experience neurological deficits, developmental delays, cerebral palsy, and permanent disability. Psychologically, victims of child abuse face elevated risks of depression, conduct disorder, and substance misuse. Academically, these patients often show decreased cognitive function and poor school performance.
All healthcare professionals hold both legal and ethical responsibilities in the recognition and reporting of suspected child abuse.[47][48][49] A high index of suspicion must be maintained, as early identification may be lifesaving. Although concerns about the legal implications of a preliminary diagnosis are common, mandatory reporting laws in all US states require healthcare providers to notify Child Protective Services when abuse is suspected.
Thorough documentation is essential because many AHT cases lead to legal proceedings. Early recognition can significantly improve outcomes. Study results suggest that up to 80% of deaths related to AHT might have been prevented with earlier intervention. While most cases involve children younger than 2 years who exhibit classic signs, children aged 2 to 7 years may present with similar findings, including bilateral retinal hemorrhage, diffuse axonal injury, and subdural hematoma.
Given the frequency, severe consequences, and economic burden of AHT, clinicians must develop expertise in recognizing its signs and symptoms. All healthcare professionals evaluating infants and children should remain vigilant for injury patterns consistent with AHT, conduct objective and thorough assessments, and collaborate with ophthalmologists, radiologists, and neurosurgeons to interpret clinical findings and establish a diagnosis. An accurate and comprehensive medical evaluation is essential.
Clinicians should consistently use the term "abusive head trauma" rather than "shaken baby syndrome" or other terminology that implies a single mechanism of injury. Parental education regarding the risks of shaking or striking a child’s head must be incorporated into routine care. Clinicians are also encouraged to engage in community-based prevention initiatives, emphasizing the importance of entrusting children only to caregivers capable of ensuring their safety.
Recognizing, reporting, and appropriately responding to suspected abuse is a legal and ethical obligation. Although some cases of AHT may be immediately apparent, others may elude recognition, particularly when clinicians lack experience or fail to maintain suspicion. In ambiguous situations, the diagnostic process must proceed with care and completeness. The optimal management of AHT involves an interprofessional team, including nurses, primary care and emergency clinicians, radiologists, intensivists, and neurosurgeons.
Media
(Click Image to Enlarge)
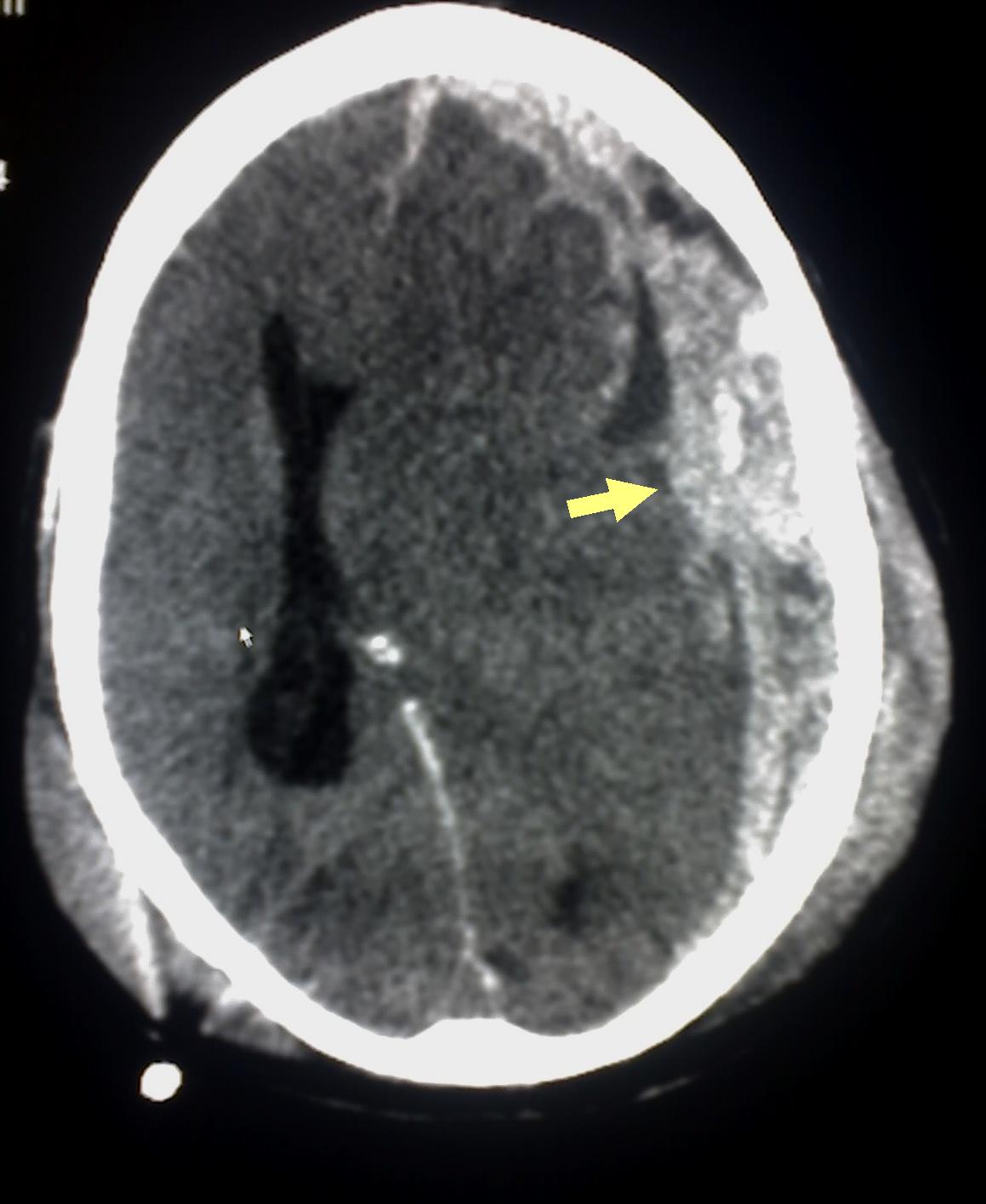
Subdural Hematoma on Computed Tomography. The image shows a subdural hematoma (yellow arrow), indicating bleeding between the dura mater and the brain. This finding is consistent with abusive head trauma in a pediatric patient.
Glitzy queen00, (Public Domain), via Wikimedia Commons.
(Click Image to Enlarge)
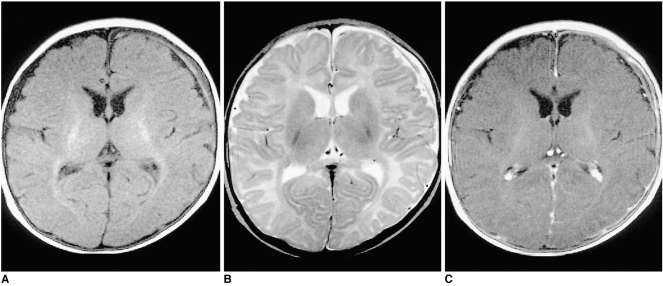
Shaken Baby Syndrome on Magnetic Resonance Imaging, 3-Month-Old Patient. Results from a 3-month-old girl with chronic subdural hematoma (SDH). (A) T1-weighted image shows predominantly low-signal SDH with a high-signal focus in the left frontal region. (B) T2-weighted image reveals mainly high-signal intensity with a focal area of low intensity. (C) Contrast-enhanced T1-weighted image demonstrates linear dural enhancement.
Contributed by The National Center for Biotechnology Information (MRI by The Korean Radiological Society; http://creativecommons.org/licenses/by-nc/3.0)
(Click Image to Enlarge)
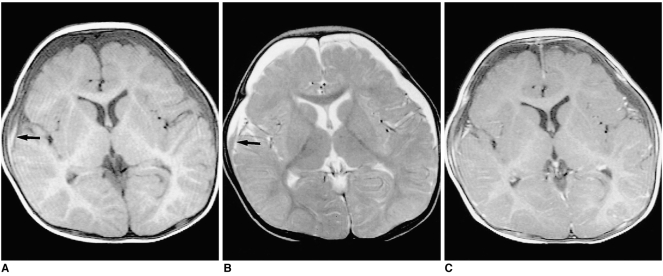
Shaken Baby Syndrome on Magnetic Resonance Imaging, 8-Month-Old Patient. Chronic subdural hematoma (SDH) in an 8-month-old boy. (A) T1-weighted image shows low-signal SDH in both frontal regions, with a high-signal focus in the right frontal area suggesting subacute hemorrhage (black arrow). (B) T2-weighted image reveals predominantly high-signal SDH with a focal area of low intensity (black arrow). (C) Contrast-enhanced T1-weighted image demonstrates diffuse linear dural enhancement.
Contributed by The National Center for Biotechnology Information (MRI by The Korean Radiological Society; http://creativecommons.org/licenses/by-nc/3.0)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Chaturvedi J, Goyal D, S R, Ruchika F, Mir MA. A Systematic Review of Pediatric Abusive Head Trauma: What a Surgeon Needs to Know Before Using a Knife. Cureus. 2025 Feb:17(2):e78419. doi: 10.7759/cureus.78419. Epub 2025 Feb 3 [PubMed PMID: 40046378]
Level 1 (high-level) evidenceElinder G, Eriksson A, Hallberg B, Lynøe N, Sundgren PM, Rosén M, Engström I, Erlandsson BE. Traumatic shaking: The role of the triad in medical investigations of suspected traumatic shaking. Acta paediatrica (Oslo, Norway : 1992). 2018 Sep:107 Suppl 472(Suppl Suppl 472):3-23. doi: 10.1111/apa.14473. Epub [PubMed PMID: 30146789]
Vinchon M. Shaken baby syndrome: what certainty do we have? Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2017 Oct:33(10):1727-1733. doi: 10.1007/s00381-017-3517-8. Epub 2017 Sep 6 [PubMed PMID: 29149395]
Rosén M, Lynøe N, Elinder G, Hallberg B, Sundgren P, Eriksson A. Shaken baby syndrome and the risk of losing scientific scrutiny. Acta paediatrica (Oslo, Norway : 1992). 2017 Dec:106(12):1905-1908. doi: 10.1111/apa.14056. Epub 2017 Oct 10 [PubMed PMID: 28871599]
McNamara CR, Menchaca CI, Abel TJ, Horvat CM, Berger RP, Fink EL, Kochanek PM, Simon DW. Effectiveness of Fosphenytoin and Levetiracetam to Prevent Posttraumatic Seizures in Young Children with Accidental or Abusive Traumatic Brain Injury. Neurocritical care. 2025 Apr:42(2):502-511. doi: 10.1007/s12028-024-02093-0. Epub 2024 Sep 5 [PubMed PMID: 39237847]
Hung KL. Pediatric abusive head trauma. Biomedical journal. 2020 Jun:43(3):240-250. doi: 10.1016/j.bj.2020.03.008. Epub 2020 Apr 21 [PubMed PMID: 32330675]
Hoehn EF, Wilson PM, Riney LC, Ngo V, Bennett B, Duma E. Identification and Evaluation of Physical Abuse in Children. Pediatric annals. 2018 Mar 1:47(3):e97-e101. doi: 10.3928/19382359-20180227-01. Epub [PubMed PMID: 29538781]
Burns J, Rohl S, Marth D, Proctor D, Amin R, Sekhon C. Which Clinical Features of Children on Initial Presentation to the Emergency Department With Head Injury Are Associated With Clinically Important Traumatic Brain Injury, Classification as Abuse, and Poor Prognosis? Pediatric emergency care. 2022 Jan 1:38(1):e254-e258. doi: 10.1097/PEC.0000000000002239. Epub [PubMed PMID: 32925700]
Miller TR, Steinbeigle R, Lawrence BA, Peterson C, Florence C, Barr M, Barr RG. Lifetime Cost of Abusive Head Trauma at Ages 0-4, USA. Prevention science : the official journal of the Society for Prevention Research. 2018 Aug:19(6):695-704. doi: 10.1007/s11121-017-0815-z. Epub [PubMed PMID: 28685210]
KEMPE CH, SILVERMAN FN, STEELE BF, DROEGEMUELLER W, SILVER HK. The battered-child syndrome. JAMA. 1962 Jul 7:181():17-24 [PubMed PMID: 14455086]
Caffey J. The whiplash shaken infant syndrome: manual shaking by the extremities with whiplash-induced intracranial and intraocular bleedings, linked with residual permanent brain damage and mental retardation. Pediatrics. 1974 Oct:54(4):396-403 [PubMed PMID: 4416579]
Narang SK, Haney S, Duhaime AC, Martin J, Binenbaum G, de Alba Campomanes AG, Barth R, Bertocci G, Care M, McGuone D, COUNCIL ON CHILD ABUSE AND NEGLECT, SECTION ON OPHTHALMOLOGY, SECTION ON RADIOLOGY, SECTION ON NEUROLOGICAL SURGERY, SOCIETY FOR PEDIATRIC RADIOLOGY, AMERICAN ASSOCIATION OF CERTIFIED ORTHOPTISTS, AMERICAN ASSOCIATION FOR PEDIATRIC OPHTHALMOLOGY AND STRABISMUS, AMERICAN ACADEMY OF OPHTHALMOLOGY. Abusive Head Trauma in Infants and Children: Technical Report. Pediatrics. 2025 Mar 1:155(3):. pii: e2024070457. doi: 10.1542/peds.2024-070457. Epub [PubMed PMID: 39992695]
Barr RG, Trent RB, Cross J. Age-related incidence curve of hospitalized Shaken Baby Syndrome cases: convergent evidence for crying as a trigger to shaking. Child abuse & neglect. 2006 Jan:30(1):7-16 [PubMed PMID: 16406023]
Level 3 (low-level) evidenceChristian CW, Committee on Child Abuse and Neglect, American Academy of Pediatrics. The evaluation of suspected child physical abuse. Pediatrics. 2015 May:135(5):e1337-54. doi: 10.1542/peds.2015-0356. Epub [PubMed PMID: 25917988]
Niederkrotenthaler T, Xu L, Parks SE, Sugerman DE. Descriptive factors of abusive head trauma in young children--United States, 2000-2009. Child abuse & neglect. 2013 Jul:37(7):446-55. doi: 10.1016/j.chiabu.2013.02.002. Epub 2013 Mar 25 [PubMed PMID: 23535075]
Bell E, Shouldice M, Levin AV. Abusive head trauma: A perpetrator confesses. Child abuse & neglect. 2011 Jan:35(1):74-7. doi: 10.1016/j.chiabu.2010.11.001. Epub [PubMed PMID: 21315450]
Esernio-Jenssen D, Tai J, Kodsi S. Abusive head trauma in children: a comparison of male and female perpetrators. Pediatrics. 2011 Apr:127(4):649-57. doi: 10.1542/peds.2010-1770. Epub 2011 Mar 7 [PubMed PMID: 21382943]
Shanahan ME, Zolotor AJ, Parrish JW, Barr RG, Runyan DK. National, regional, and state abusive head trauma: application of the CDC algorithm. Pediatrics. 2013 Dec:132(6):e1546-53. doi: 10.1542/peds.2013-2049. Epub 2013 Nov 25 [PubMed PMID: 24276842]
Piteau SJ, Ward MG, Barrowman NJ, Plint AC. Clinical and radiographic characteristics associated with abusive and nonabusive head trauma: a systematic review. Pediatrics. 2012 Aug:130(2):315-23. doi: 10.1542/peds.2011-1545. Epub 2012 Jul 9 [PubMed PMID: 22778309]
Level 1 (high-level) evidenceKeenan HT, Runyan DK, Marshall SW, Nocera MA, Merten DF, Sinal SH. A population-based study of inflicted traumatic brain injury in young children. JAMA. 2003 Aug 6:290(5):621-6 [PubMed PMID: 12902365]
Sheets LK, Leach ME, Koszewski IJ, Lessmeier AM, Nugent M, Simpson P. Sentinel injuries in infants evaluated for child physical abuse. Pediatrics. 2013 Apr:131(4):701-7. doi: 10.1542/peds.2012-2780. Epub 2013 Mar 11 [PubMed PMID: 23478861]
Rangel EL, Cook BS, Bennett BL, Shebesta K, Ying J, Falcone RA. Eliminating disparity in evaluation for abuse in infants with head injury: use of a screening guideline. Journal of pediatric surgery. 2009 Jun:44(6):1229-34; discussion 1234-5. doi: 10.1016/j.jpedsurg.2009.02.044. Epub [PubMed PMID: 19524746]
Jenny C, Hymel KP, Ritzen A, Reinert SE, Hay TC. Analysis of missed cases of abusive head trauma. JAMA. 1999 Feb 17:281(7):621-6 [PubMed PMID: 10029123]
Level 3 (low-level) evidencePierce MC, Kaczor K, Lorenz DJ, Bertocci G, Fingarson AK, Makoroff K, Berger RP, Bennett B, Magana J, Staley S, Ramaiah V, Fortin K, Currie M, Herman BE, Herr S, Hymel KP, Jenny C, Sheehan K, Zuckerbraun N, Hickey S, Meyers G, Leventhal JM. Validation of a Clinical Decision Rule to Predict Abuse in Young Children Based on Bruising Characteristics. JAMA network open. 2021 Apr 1:4(4):e215832. doi: 10.1001/jamanetworkopen.2021.5832. Epub 2021 Apr 1 [PubMed PMID: 33852003]
Level 1 (high-level) evidenceThackeray JD. Frena tears and abusive head injury: a cautionary tale. Pediatric emergency care. 2007 Oct:23(10):735-7 [PubMed PMID: 18090110]
Maguire S, Moynihan S, Mann M, Potokar T, Kemp AM. A systematic review of the features that indicate intentional scalds in children. Burns : journal of the International Society for Burn Injuries. 2008 Dec:34(8):1072-81. doi: 10.1016/j.burns.2008.02.011. Epub 2008 Jun 6 [PubMed PMID: 18538478]
Level 1 (high-level) evidenceRaynor E, Konala P, Freemont A. The detection of significant fractures in suspected infant abuse. Journal of forensic and legal medicine. 2018 Nov:60():9-14. doi: 10.1016/j.jflm.2018.09.002. Epub 2018 Sep 5 [PubMed PMID: 30196192]
Higginbotham N, Lawson KA, Gettig K, Roth J, Hopper E, Higginbotham E, George TM, Maxson T, Edwards G, Garcia NM. Utility of a child abuse screening guideline in an urban pediatric emergency department. The journal of trauma and acute care surgery. 2014 Mar:76(3):871-7. doi: 10.1097/TA.0000000000000135. Epub [PubMed PMID: 24553563]
Riney LC, Frey TM, Fain ET, Duma EM, Bennett BL, Murtagh Kurowski E. Standardizing the Evaluation of Nonaccidental Trauma in a Large Pediatric Emergency Department. Pediatrics. 2018 Jan:141(1):. pii: e20171994. doi: 10.1542/peds.2017-1994. Epub 2017 Dec 6 [PubMed PMID: 29212880]
Bradford R, Choudhary AK, Dias MS. Serial neuroimaging in infants with abusive head trauma: timing abusive injuries. Journal of neurosurgery. Pediatrics. 2013 Aug:12(2):110-9. doi: 10.3171/2013.4.PEDS12596. Epub 2013 Jun 25 [PubMed PMID: 23799250]
Kleinman PK, Nimkin K, Spevak MR, Rayder SM, Madansky DL, Shelton YA, Patterson MM. Follow-up skeletal surveys in suspected child abuse. AJR. American journal of roentgenology. 1996 Oct:167(4):893-6 [PubMed PMID: 8819377]
Level 3 (low-level) evidenceZimmerman S, Makoroff K, Care M, Thomas A, Shapiro R. Utility of follow-up skeletal surveys in suspected child physical abuse evaluations. Child abuse & neglect. 2005 Oct:29(10):1075-83 [PubMed PMID: 16315349]
Level 3 (low-level) evidenceBennett BL, Chua MS, Care M, Kachelmeyer A, Mahabee-Gittens M. Retrospective review to determine the utility of follow-up skeletal surveys in child abuse evaluations when the initial skeletal survey is normal. BMC research notes. 2011 Sep 12:4():354. doi: 10.1186/1756-0500-4-354. Epub 2011 Sep 12 [PubMed PMID: 21910901]
Level 2 (mid-level) evidenceGalvagno SM Jr, Nahmias JT, Young DA. Advanced Trauma Life Support(®) Update 2019: Management and Applications for Adults and Special Populations. Anesthesiology clinics. 2019 Mar:37(1):13-32. doi: 10.1016/j.anclin.2018.09.009. Epub 2018 Dec 27 [PubMed PMID: 30711226]
Borgialli DA, Mahajan P, Hoyle JD Jr, Powell EC, Nadel FM, Tunik MG, Foerster A, Dong L, Miskin M, Dayan PS, Holmes JF, Kuppermann N, Pediatric Emergency Care Applied Research Network (PECARN). Performance of the Pediatric Glasgow Coma Scale Score in the Evaluation of Children With Blunt Head Trauma. Academic emergency medicine : official journal of the Society for Academic Emergency Medicine. 2016 Aug:23(8):878-84. doi: 10.1111/acem.13014. Epub 2016 Aug 1 [PubMed PMID: 27197686]
Jain S, Margetis K, Iverson LM. Glasgow Coma Scale. StatPearls. 2025 Jan:(): [PubMed PMID: 30020670]
Kjaergård H, Nordkild P. [Bilateral compartment syndrome in a drug addict]. Ugeskrift for laeger. 1985 Mar 11:147(11):959-60 [PubMed PMID: 3992695]
Kochanek PM, Tasker RC, Carney N, Totten AM, Adelson PD, Selden NR, Davis-O'Reilly C, Hart EL, Bell MJ, Bratton SL, Grant GA, Kissoon N, Reuter-Rice KE, Vavilala MS, Wainwright MS. Guidelines for the Management of Pediatric Severe Traumatic Brain Injury, Third Edition: Update of the Brain Trauma Foundation Guidelines, Executive Summary. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies. 2019 Mar:20(3):280-289. doi: 10.1097/PCC.0000000000001736. Epub [PubMed PMID: 30830016]
Kochanek PM, Tasker RC, Bell MJ, Adelson PD, Carney N, Vavilala MS, Selden NR, Bratton SL, Grant GA, Kissoon N, Reuter-Rice KE, Wainwright MS. Management of Pediatric Severe Traumatic Brain Injury: 2019 Consensus and Guidelines-Based Algorithm for First and Second Tier Therapies. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies. 2019 Mar:20(3):269-279. doi: 10.1097/PCC.0000000000001737. Epub [PubMed PMID: 30830015]
Level 3 (low-level) evidenceBullock MR, Chesnut R, Ghajar J, Gordon D, Hartl R, Newell DW, Servadei F, Walters BC, Wilberger J, Surgical Management of Traumatic Brain Injury Author Group. Surgical management of depressed cranial fractures. Neurosurgery. 2006 Mar:58(3 Suppl):S56-60; discussion Si-iv [PubMed PMID: 16540744]
Bullock MR, Chesnut R, Ghajar J, Gordon D, Hartl R, Newell DW, Servadei F, Walters BC, Wilberger JE, Surgical Management of Traumatic Brain Injury Author Group. Surgical management of acute epidural hematomas. Neurosurgery. 2006 Mar:58(3 Suppl):S7-15; discussion Si-iv [PubMed PMID: 16710967]
Barr RG, Barr M, Rajabali F, Humphreys C, Pike I, Brant R, Hlady J, Colbourne M, Fujiwara T, Singhal A. Eight-year outcome of implementation of abusive head trauma prevention. Child abuse & neglect. 2018 Oct:84():106-114. doi: 10.1016/j.chiabu.2018.07.004. Epub 2018 Aug 1 [PubMed PMID: 30077049]
Lind K, Toure H, Brugel D, Meyer P, Laurent-Vannier A, Chevignard M. Extended follow-up of neurological, cognitive, behavioral and academic outcomes after severe abusive head trauma. Child abuse & neglect. 2016 Jan:51():358-67. doi: 10.1016/j.chiabu.2015.08.001. Epub 2015 Aug 20 [PubMed PMID: 26299396]
Tilak GS, Pollock AN. Missed opportunities in fatal child abuse. Pediatric emergency care. 2013 May:29(5):685-7. doi: 10.1097/PEC.0b013e31828f3e39. Epub [PubMed PMID: 23640154]
Level 3 (low-level) evidenceKrishnaprasadh D, Joyce T, Huecker MR. Pediatric Abusive Head Trauma. StatPearls. 2025 Jan:(): [PubMed PMID: 29763011]
Daro D, Dodge KA. Creating community responsibility for child protection: possibilities and challenges. The Future of children. 2009 Fall:19(2):67-93 [PubMed PMID: 19719023]
Trossman S. PRACTICE Preventing tragedies New Mexico nurses lead initiative on shaken baby syndrome. The American nurse. 2016 Sep:48(4):13 [PubMed PMID: 29787658]
Rideout L. Nurses' Perceptions of Barriers and Facilitators Affecting the Shaken Baby Syndrome Education Initiative: An Exploratory Study of a Massachusetts Public Policy. Journal of trauma nursing : the official journal of the Society of Trauma Nurses. 2016 May-Jun:23(3):125-37. doi: 10.1097/JTN.0000000000000206. Epub [PubMed PMID: 27163220]
Nocera M, Shanahan M, Murphy RA, Sullivan KM, Barr M, Price J, Zolotor A. A statewide nurse training program for a hospital based infant abusive head trauma prevention program. Nurse education in practice. 2016 Jan:16(1):e1-6. doi: 10.1016/j.nepr.2015.07.013. Epub 2015 Aug 14 [PubMed PMID: 26341727]