Indications
Introduction
Fluid replacement therapy primarily involves 2 types of fluids—crystalloids and colloids. Crystalloids consist of water (H2O) and small electrolytes, whereas colloids contain water and larger molecules such as starches or proteins. Crystalloids are most often preferred due to their ease of administration and lower risk of allergic reactions. These fluids can be further classified based on their electrolyte composition, osmolarity, and metabolic effects.
This activity reviews the key components of the most commonly used fluids, with a focus on lactated Ringer solution and normal saline. The advantages and disadvantages of each will be discussed, with special emphasis on the components and metabolism of lactated Ringer solution, supported by growing evidence of its superiority as a replacement fluid.
Normal Plasma
Understanding the composition of normal plasma is essential for evaluating and managing fluid and electrolyte balance. Although reference ranges may vary slightly between laboratories, typical plasma values are mentioned below.
- Sodium: 135-145 mmol/L
- Potassium: 4.5-5.0 mmol/L
- Calcium: 2.2-2.6 mmol/L
- Magnesium: 0.8-1.0 mmol/L
- Chloride: 94-111 mmol/L
- Bicarbonate: 23-27 mmol/L
- Osmolarity: 275-295 mOsml/L [1][2]
Lactated Ringer Solution
Lactated Ringer solution (also known as Ringer lactate) is an isotonic crystalloid fluid classified as a balanced or buffered solution, commonly used for fluid resuscitation. The contents of Ringer lactate include sodium, chloride, potassium, calcium, and lactate (in the form of sodium lactate), with an approximate osmolarity of 273 mOsm/L and a pH of around 6.5. In contrast, normal saline has a higher osmolarity of approximately 308 mOsm/L.
Ringer lactate is widely used for volume resuscitation in various clinical situations, including blood loss, sepsis, and dehydration.[3] Compared to normal saline, lactated Ringer solution is less likely to cause hyperchloremic metabolic acidosis and renal vasoconstriction.[4][5] The composition of lactated Ringer solution includes sodium 130 mmol/L, chloride 109 mmol/L, potassium 4.0 mmol/L, calcium 1.5 mmol/L, lactate 28 mmol/L, and an osmolarity of 273 mOsm/L.[5]
Multiple clinical trials have demonstrated the superiority of more physiological, balanced crystalloid solutions, such as lactated Ringer or acetate-based fluids, over normal saline.[6][7][8] According to the Infectious Diseases Society of America (IDSA) guidelines, intravenous (IV) fluids, such as lactated Ringer or normal saline, should be administered in cases of infectious diarrhea accompanied by shock, severe dehydration, altered mental status, or ileus. In patients with severe dehydration, IV fluid therapy should be continued until vital signs, tissue perfusion, and mental status have normalized, the ileus has resolved, and the risk of aspiration has been eliminated.[9]
Normal Saline
Normal saline, or 0.9% saline, contains 154 mmol/L of both sodium and chloride, with an osmolarity of 308 mOsm/L, which is higher than that of normal plasma.[1][5] This elevated chloride concentration (compared to the normal plasma range of 94–111 mmol/L) is thought to increase chloride delivery to the distal renal tubule, stimulating tubuloglomerular feedback. This, in turn, triggers afferent arteriolar vasoconstriction, resulting in a reduction in the glomerular filtration rate (GFR). Additionally, saline infusion may contribute to greater intracapsular edema compared to more physiologic crystalloid solutions, which could potentially further impair renal perfusion. These effects have been observed in both hospitalized patients and healthy volunteers (see Image. Sequential Effects of Hyperchloremia on the Kidney).[10][11]
According to the American Urological Association, bipolar transurethral resection of the prostate utilizes 0.9% sodium chloride (NaCl) solution, which reduces the risk of acute dilutional hyponatremia. In contrast, monopolar resection requires non-electrolyte irrigation fluids, such as glycine or sorbitol, which increases the risk of fluid-related complications, including transurethral resection syndrome, cerebral edema, and seizures, especially during prolonged procedures.[12] Normal saline may be preferred over less hypertonic fluids for patients with brain injuries, as brain edema is a concerning consequence.[10][11] Please refer to the "Hypertonic Saline" section below for more information. Please see StatPearls' companion resource, "Normal Saline," for more information.
Acetate-Based Infusion Fluids
Acetate-containing fluids are relatively new and use acetate as the buffering anion instead of lactate. These fluids are not commonly used in clinical practice. A study reported significantly higher levels of lactate and chloride in patients receiving Ringer lactate compared to those receiving acetate-containing formulas.[13]
Ringer acetate has a sodium concentration of 145 mmol/L, a chloride concentration of 127 mmol/L, a potassium concentration of 4.0 mmol/L, a calcium concentration of 2.5 mmol/L, a magnesium concentration of 1.0 mmol/L, and an acetate concentration of 24 mmol/L, with an osmolarity of 309 mOsm/L.
Plasmalyte® is a proprietary fluid that is rarely used, and its composition includes sodium 140 mmol/L, potassium 5.0 mmol/L, calcium 0 mmol/L, magnesium 1.5 mmol/L, chloride 98 mmol/L, acetate 27 mmol/L, gluconate 23 mmol/L, with an osmolarity of 294 mOsm/L. As it lacks calcium, Plasmalyte® can be safely administered with blood products.[1]
In a study, Ringer acetate solution and normal saline solution showed similar rates of major adverse kidney events within 28 days. However, patients receiving only normal saline solution required longer durations of invasive mechanical ventilation and showed a trend toward increased serum chloride levels.[14]
Hartmann's® is another proprietary solution similar to Plasmalyte® but contains calcium in its formulation.[15]
See the Table below for a comparison of the benefits of acetate versus lactate.
Half Normal Saline
Half-normal saline, or 0.45% saline, contains 77 mmol/L of both sodium and chloride, with an osmolarity of 154 mOsm/L. This is commonly administered in combination with 5% dextrose (usually abbreviated as "D5W").
Hypertonic Saline
Hypertonic saline is most commonly available as a 3% solution, containing 3 grams of NaCl per 100 mL of water, with an osmolarity of approximately 1026 mOsm/L. Higher concentrations, such as 7.5%, have also been reported. This is used in the treatment of hyponatremia, severe hypovolemia, cerebral edema, and other forms of brain injury.
Although a meta-analysis found no significant long-term benefit of hypertonic saline over mannitol in patients with traumatic brain injury,[16][17] other studies have demonstrated improvement in cerebral perfusion pressure. As a result, hypertonic saline remains a key therapeutic option—alongside mannitol—for managing elevated intracranial pressure, particularly in traumatic brain injury.[18][19][20] The Neurocritical Care Society guidelines recommend using either hypertonic saline or mannitol for initial management of intracranial pressure (ICP) or cerebral edema in patients with acute ischemic stroke.[21]
Hypertonic saline must be administered cautiously through a central line, and the correction of hyponatremia should not exceed 0.5 mEq per hour to prevent osmotic demyelination, particularly in cases of chronic hyponatremia. Please refer to StatPearls' companion resource, "Hyponatremia," for additional information.
Dextrose 5%
Dextrose 5% in water (also known as D5W) consists of 5 grams of dextrose per 100 mL of water, with an osmolality of 252 mOsm/L. However, because the body rapidly metabolizes the dextrose, it functions as a very hypotonic fluid in vivo. D5W provides approximately 170 kcal/L. A study in ICU patients found that using D5W as a diluent for medications reduced the incidence of hyperchloremia and hypernatremia without increasing the risk of hyperglycemia.[22]
Sodium Bicarbonate
Sodium bicarbonate can be administered either as an IV "push" or continuous infusion. This is frequently used in the treatment of acidemia, cardiac arrest, hyperkalemia, and certain ingestions, among other clinical indications.[23] However, in patients with diabetic ketoacidosis, IV sodium bicarbonate has not been shown to improve clinical outcomes.[24]
Sodium bicarbonate is commonly supplied in 50 mL ampules. The most frequently used concentration is 8.4%, which contains 1 mEq/mL, resulting in 50 mEq per ampule. This compound is also available in 7.5% solutions, providing 0.892 mEq/mL, and in 4.2% solutions (typically used for pediatric patients), containing 0.5 mEq/mL. These formulations are highly hyperosmolar—the 8.4% solution has an osmolarity of approximately 2000 mOsm/L, whereas the 7.5% solution has an osmolarity of about 1786 mOsm/L. For infusions, sodium bicarbonate is most commonly prepared as a 150 mEq/L solution diluted in 1 liter of either D5W (osmolarity ~578 mOsm/L) or sterile water (osmolarity ~300 mOsm/L).[23]
Table. Commonly Used Intravenous Fluids and Their Components
| Fluid Type | Na | Cl | K | HCO3 | Ca | Mg | Lactate | Acetate | Glucose | Osmolarity |
|
Normal plasma |
135-145 | 94-111 | 4.5-5.0 | 22-28 | 2.2-2.6 | 0.8-1.0 | 1-2 | 0.02-0.2 | 3.9-7.8 | 275-295 |
|
Lactated Ringer |
130 | 109 | 4.0 | 0 | 2.7 | 0 | 28 | 0 | 0 | 273 |
|
Normal saline |
154 | 154 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 308 |
|
Normal saline |
77 | 77 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 154 |
|
Hypertonic saline (3%-7.5%) |
513-1283 | 513-1283 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1026-2564 |
| Dextrose 5% (D5W) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 278 | 252 |
|
Sodium bicarbonate |
150 | 0 | 0 | 150 | 0 | 0 | 0 | 0 | 0 | 300 |
|
Ringer acetate |
145 | 127 | 4.0 | 0 | 2.5 | 1.0 | 0 | 24 | 0 | 309 |
All values are in mmol/L except osmolarity, which is in mOsm/L. Table reference.[2]
Mechanism of Action
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Mechanism of Action
Although volume kinetics is a complex topic, it can be better understood by considering the following 2 fluid characteristics—osmolarity and tonicity.
According to the literature review, total body water constitutes approximately 60% of body weight in adult males and about 50% in adult females, with variations based on age, gender, and body composition. Of this total body water, roughly 67% is contained within the intracellular fluid compartment, while the remaining 33% resides in the extracellular fluid compartment. The extracellular fluid is further divided into interstitial fluid, which comprises approximately 75% to 80% of the extracellular fluid, and intravascular fluid, which accounts for about 20% to 25% of the extracellular fluid. Osmolarity across all fluid compartments remains equal or rapidly equalizes, as water moves freely between compartments to maintain osmotic balance.
In contrast, tonicity refers to the osmotic gradient between 2 fluids across a semipermeable membrane and is a relative measure of the concentration of those 2 fluids. When describing a fluid’s tonicity—as hypotonic, isotonic, or hypertonic—it specifically refers to the osmotic gradient between the intracellular and extracellular compartments. Therefore, an isotonic fluid administered intravenously does not cross into the intracellular space but distributes evenly throughout the entire extracellular compartment, which includes the interstitial fluid (approximately 75%–80%) and the intravascular fluid (approximately 20%–25%), usually in proportion to the compartment volumes.[17][25]
Understanding lactate metabolism—and briefly reviewing its biochemistry and physiology—is essential for recognizing the specific benefits of Ringer lactate. Lactate is the conjugate (or compensatory) base of lactic acid. Under normal aerobic conditions, glucose metabolism leads to the production of pyruvate through cellular respiration. However, a small amount of anaerobic metabolism occurs continuously, during which pyruvate undergoes an oxidation-reduction reaction with NADH. This reaction, catalyzed by the enzyme lactate dehydrogenase (LDH), converts NADH to NAD+ and forms lactate. This process is crucial for maintaining sufficient NAD+ levels during anaerobic metabolism, allowing glycolysis to continue even in the absence of oxygen. Normally, through cellular respiration, the NADH/NAD+ ratio remains balanced as protons and electrons are transferred to generate ATP, with H2O and carbon dioxide (CO2) as the end products. However, when aerobic metabolism is impaired, these protons accumulate.
Lactate is produced and transported out of the cells to maintain the NADH/NAD+ ratio. The increased lactate production also functions as a buffer by consuming hydrogen ions (H+) to form lactic acid. Additionally, lactate can be converted back into pyruvate via LDH and enter cellular respiration, producing CO2 and H2O. These products combine to form carbonic acid (H2CO3) through the action of carbonic anhydrase, which rapidly dissociates into bicarbonate (HCO3−). Thus, lactate metabolism contributes to bicarbonate formation and has a role in maintaining acid-base balance.[26]
Administering 1 liter of Ringer lactate provides 2 important benefits, as mentioned below.
- Volume resuscitation: This process expands the intravascular volume, thereby increasing preload and improving tissue perfusion.
- Sodium lactate supply: Sodium lactate acts as a bioenergetic fuel that the body is adapted to metabolize during ischemic conditions, helping to reduce cellular injury and death caused by ischemia.[27]
Pharmacokinetics
Absorption: Crystalloids exhibit 100% bioavailability when administered intravenously, with immediate distribution into the intravascular compartment.
Distribution: Fluid distribution depends on the solution’s tonicity. Isotonic fluids (eg, 0.9% NaCl, lactated Ringer) primarily expand the extracellular fluid compartment. Hypotonic solutions (eg, 0.45% NaCl, dextrose 5%) allow water to shift into the intracellular space. Hypertonic saline draws water from the intracellular compartment into the extracellular space. After metabolism, dextrose-containing solutions act as free water.
Metabolism: Normal saline, half-normal saline, and hypertonic saline are not metabolized by the body. Lactate, present in lactated Ringer solution, is metabolized primarily in the liver to bicarbonate. Acetate, found in Ringer acetate, undergoes extrahepatic metabolism to bicarbonate. Dextrose is metabolized to CO2 and H2O, providing caloric energy. Bicarbonate acts as a buffer by binding hydrogen ions to form H2CO3, which then dissociates into CO2 and H2O.
Excretion: Sodium and chloride are eliminated primarily through renal glomerular filtration and tubular reabsorption or secretion. Excess chloride can impair renal perfusion. Metabolites of dextrose are cleared via both pulmonary exhalation and renal excretion. Excess bicarbonate is removed by the kidneys, while CO2 is expelled through respiration.
Administration
Fluids are usually administered via the IV route, but may also be safely administered intraosseously. The goal of administering IV fluids is to replenish the intravascular volume to permit adequate organ perfusion.[28] Please see StatPearls' companion resource, "Fluid Management," for recommendations on fluid administration rates.
Intravenous Fluid Therapy Principles
Intravenous fluid therapy should be tailored to the clinical context and guided by the "5Rs": resuscitation, routine maintenance, replacement, redistribution, and reassessment. Key considerations include the patient’s volume status, electrolyte balance, acid–base status, and underlying comorbidities.
The Surviving Sepsis Guidelines recommend using crystalloids as the initial choice for fluid resuscitation for adults with sepsis or septic shock. Among crystalloids, balanced solutions, such as lactated Ringer or PlasmaLyte, are preferred over normal saline.[4][29] For fluid resuscitation in cases of hypovolemia, shock, or sepsis, crystalloids remain the first-line therapy. The Surviving Sepsis Campaign specifically recommends administering 30 mL/kg of crystalloids, with a preference for balanced solutions over normal saline.[30][31]
Although normal saline is effective, large volumes may lead to hyperchloremic metabolic acidosis. In contrast, PlasmaLyte—formulated with acetate and gluconate buffers and a near-plasma pH—is associated with less acidosis and reduced risk of renal injury than normal saline.[32] For maintenance therapy, fluids should provide approximately 25 to 30 mL/kg/d of water along with 50 to 100 grams of glucose daily to prevent ketosis.[33]
Replacement fluids should be selected based on the composition of the fluid that has been lost. Normal saline is suitable for gastric losses, whereas lactated Ringer solution is preferred for biliary and pancreatic losses, which are typically alkaline in nature. D5W with potassium chloride is suitable for replacing diarrheal and insensible losses.
Severe symptomatic hyponatremia requires the administration of hypertonic 3% NaCl through a large vein, with careful monitoring and strict limits on the rate of sodium correction to prevent osmotic demyelination syndrome.
Specific Patient Populations and Cautions
Certain patient populations require special consideration when administering IV fluids due to the risk of volume overload or electrolyte disturbances.
Heart failure: Patients with chronic congestive heart failure (CHF) often have limited cardiac reserve. Excessive IV fluid administration can lead to pulmonary edema and worsening heart failure. The American Heart Association recommends fluid restriction, typically around 1.5 to 2 liters per day, especially in cases of advanced heart failure. Although evidence is mixed, studies suggest that liberal fluid administration can increase B-type natriuretic peptide (BNP) levels and markers of kidney injury. In patients with heart failure who require IV fluids (for example, in sepsis), smaller fluid boluses and slower infusion rates should be used, with consideration of loop diuretics as needed. Continuous hemodynamic monitoring is strongly advised to guide therapy.
Chronic kidney disease: Patients with chronic kidney disease (CKD) often have impaired fluid excretion and are prone to hyperkalemia. Saline overload should be avoided, as it may worsen metabolic acidosis and contribute to hypertension.
Cirrhosis: Individuals with cirrhosis typically have a reduced effective arterial blood volume and elevated levels of antidiuretic hormone, both of which contribute to dilutional hyponatremia. Despite decreased intravascular volume, these patients often develop ascites due to altered fluid distribution. In such cases, liberal administration of IV fluids can worsen both ascites and hyponatremia. Management of hyponatremia primarily involves fluid restriction, generally limited to 1 to 1.5 liters per day. Large-volume infusions of normal saline should be avoided, as they may further aggravate ascites and fluid overload.
Adverse Effects
As with any IV fluid administration, there is a possibility of swelling and edema. Especially at-risk patients are those with CHF, CKD, liver cirrhosis, and hypoalbuminemia. These individuals should be closely monitored during IV fluid therapy, including serial physical examinations, to assess for signs of clinical hypervolemia.
Brain Injury
In the acute setting, patients with cerebral edema requiring osmotic therapy should avoid hypotonic and isotonic fluids. The primary treatment goal is to draw free water out of the brain parenchyma through the administration of hypertonic fluids. Although evidence on the long-term benefits of hypertonic fluids in brain injury is mixed, conventional therapy typically involves their use in the acute phase.[34]
Ringer Lactate and Ringer Acetate
Concerns exist that Ringer lactate may cause hyperkalemia and worsen lactic acidosis. To put this into perspective, Ringer lactate contains a potassium concentration of 4 mEq/L. While it seems logical that administering potassium to a hyperkalemic patient would exacerbate hyperkalemia, this is not the case. The body's potassium volume of distribution extends beyond the extracellular compartment, equilibrating between intracellular and extracellular spaces. Even in patients with renal failure, administration of 4 mEq/L of potassium does not typically lead to hyperkalemia.[35] Multiple studies have shown that administering lactated Ringer to patients does not worsen hyperkalemia.[36][37][38]
Administering Ringer lactate to a patient with hyperkalemia may help normalize serum potassium levels toward 4 mEq/L.[39][40] Furthermore, hyperkalemia can worsen in the presence of metabolic acidosis.[17] Consequently, large-volume IV administration of normal saline can cause hyperchloremic non-anion gap metabolic acidosis, which can further worsen hyperkalemia. In contrast, Ringer lactate does not produce this effect.
Ringer lactate is often avoided in septic patients due to concerns about worsening lactic acidosis. However, this concern is unfounded. Ringer lactate contains sodium lactate, not lactic acid. Administration of Ringer lactate introduces exogenous lactate, which the body efficiently metabolizes and uses as an energy source. However, modern clinical practice often places significant emphasis on blood lactate levels,[27] which can lead to misinterpretation and confusion. Importantly, Ringer lactate does not cause a pathological rise in lactic acid levels or contribute to lactic acidosis.
Ringer lactate contains a low calcium concentration, which prohibits its use as a diluent for blood transfusions. Many blood products use citrate as an anticoagulant, and when citrate mixes with calcium, it can form calcium citrate precipitates, which can potentially cause clotting and obstruction of the IV line. While patients can receive blood products and Ringer lactate simultaneously, these fluids should not be administered through the same IV line.
Ringer acetate solutions may offer certain advantages over Ringer lactate solutions. Acetate corrects acidosis and hyperchloremia more quickly than lactate and is metabolized independently of liver function. Additionally, acetate does not interfere with glucose metabolism, whereas lactate has been associated with hyperglycemia through its role in gluconeogenesis.[15][41]
Sodium Bicarbonate
Excessive infusion of bicarbonate can cause hypoxia by shifting the oxygen dissociation curve to the left. Alkalosis may also increase calcium binding to albumin, resulting in hypotension and reduced cardiac contractility. Additionally, if minute ventilation is limited, bicarbonate administration can lead to hypercapnia.[23]
Interactions and Incompatibility
- Intravenous fluids are essential for hydration, drug dilution, and parenteral therapy. However, careful consideration of both solution and Y-site compatibility is essential, as certain combinations can lead to precipitation, denaturation, or inactivation of medications. Y-site incompatibility refers to the interaction that occurs when 2 IV solutions mix briefly at a Y-connector or manifold before entering the patient. These reactions may not always be visible to the naked eye but can result in clinically significant adverse effects.
- Lactated Ringer solution contains calcium and is incompatible with ceftriaxone, particularly in neonates, where calcium-ceftriaxone precipitation has led to fatal outcomes. Additionally, it is incompatible with sodium bicarbonate, as the combination can result in the immediate precipitation of calcium carbonate. Phenytoin has poor solubility in lactated Ringer and may precipitate at the Y-site. Additionally, biological agents such as infliximab and eculizumab are considered incompatible with calcium-containing fluids due to the risk of protein aggregation and degradation.
- Normal saline (0.9% NaCl) is widely considered the safest and most compatible fluid for drug dilution and line flushing. This is preferred for blood transfusions because it contains no calcium or dextrose. However, instability may occur with medications such as phenytoin and ampicillin if not administered promptly. Certain biologics, including palivizumab and alemtuzumab, may require specific diluents and should only be mixed with normal saline if explicitly indicated in the product labeling. While normal saline generally has favorable Y-site compatibility, drugs such as phenytoin still necessitate dedicated lines or thorough flushing to prevent precipitation.
- Hypertonic saline, typically ranging from 3% to 7.5% NaCl, is hyperosmolar and incompatible with most medications. This should not be coadministered with other drugs via a Y-site, especially at concentrations above 3%. Central venous administration is generally preferred. Exposure through admixture or Y-site contact can lead to precipitation, endothelial injury, or osmotic damage.
- Dextrose 5% in water (D5W) is incompatible with medications such as phenytoin, amphotericin B, diazepam, hydralazine, and several monoclonal antibodies, including rituximab and trastuzumab. Dextrose may destabilize protein-based drugs by altering pH and promoting glycation. Both admixture in IV bags and Y-site coadministration should be avoided unless explicitly confirmed as compatible.
- Sodium bicarbonate is highly alkaline and reacts with multiple agents. This is incompatible with calcium, magnesium, phosphate, dobutamine, catecholamines, and lactated solutions. Co-infusion at the Y-site often results in visible or microcrystalline precipitation. Sodium bicarbonate must be administered through a separate line and flushed thoroughly before and after use.
- PlasmaLyte, an acetate- and gluconate-buffered solution, has a composition similar to Lactated Ringer and shares similar incompatibility concerns. PlasmaLyte contains calcium and magnesium and is incompatible with ceftriaxone, sodium bicarbonate, phenytoin, and multiple biological products. Unless specifically indicated in the prescribing information, biologics should not be mixed with or administered via Y-site with PlasmaLyte. In summary, careful attention to both solution and Y-site compatibility is essential to prevent adverse drug events. Drugs such as phenytoin, amphotericin B, sodium bicarbonate, calcium salts, and monoclonal antibodies are commonly incompatible when administered via a Y-site. These medications should be delivered through separate lumens or with sufficient line flushing to minimize the risk of adverse interactions. Clinicians should consult institutional guidelines or manufacturer prescribing information when managing the administration of multiple IV therapies.[15]
Contraindications
Although not an absolute contraindication, caution is advised when administering Ringer lactate to patients with liver dysfunction. As most lactate is metabolized by the liver, hepatic impairment can lead to lactate accumulation and reduced conversion to bicarbonate, potentially complicating the interpretation of lactate levels. Rare cases of anaphylaxis to Ringer lactate have also been reported.[42]
Adverse effects associated with IV fluid administration may include allergic reactions ranging from mild local erythema and pruritus to more generalized symptoms. Local site infections and regional cellulitis may also occur, potentially progressing to systemic infection if not properly managed. These complications are often related to IV site access rather than the fluid contents themselves. Allergic responses are more commonly linked to adhesive dressings used to secure the IV rather than the contents of the fluid. IV infiltration—characterized by localized swelling, redness, and pain—is another potential complication, typically managed with conservative measures and replacement of the IV line. Infections may require treatment with local or systemic antibiotics, depending on the severity.
Warning and Precautions
Technique: Aseptic technique must be strictly followed during all single or multiple entries into containers to prevent contamination. When diluting or dissolving pharmaceuticals, ensure complete mixing and use the solution promptly. Reconstituted injectable drug solutions should not be stored unless specifically recommended by the manufacturer's instructions..
Hypertonic saline: Hypertonic saline can cause infusion and hypersensitivity reactions, including fever, chills, tremors, urticaria, and hypotension. The high sodium content increases the risk of hypernatremia, hypervolemia, and subsequent pulmonary edema or congestive complications, particularly in patients with heart failure, cirrhosis, kidney disease, hyperaldosteronism, or those taking sodium-retaining medications.
Fluid balance, serum electrolytes, and acid–base status should be closely monitored during rapid administration to prevent dangerous fluid shifts or dilutional imbalances. Hypertonic saline should be administered through a large peripheral or central vein to minimize venous irritation. Pressurizing containers before evacuating air should be avoided, and flexible plastic bags should not be connected in series or used with vented sets to reduce the risk of air embolism. Additionally, coadministration with blood products is contraindicated.
Medication administration: Certain medications are incompatible with specific IV fluids, and pharmacists must remain vigilant about potential interactions. For instance, ceftriaxone and cefepime can interact with lactated Ringer solution and 0.45% NaCl. Additionally, parenteral diazepam may precipitate in standard saline solutions, and amphotericin B is incompatible with normal saline and should not be infused with it.[43][44] All potential interactions must be thoroughly reviewed and verified for accuracy.[45]
Monitoring
Patients receiving any IV fluid replacement require monitoring for fluid overload. As noted earlier, crystalloid fluids distribute across the extracellular compartment in an approximate 3:1 ratio (interstitial to intravascular) under normal physiological conditions. This means that administering 1 liter of Ringer lactate results in only about 250 mL remaining within the intravascular compartment. Excessive administration of crystalloid fluids can lead to fluid overload, progressively worsening peripheral and pulmonary edema.[46]
Additionally, monitoring of electrolytes and kidney function may be warranted with any IV fluid administration.[47][48] The infusion site and IV access should be regularly assessed to confirm proper delivery of fluid into the vein. Signs of infiltration, redness, pain, swelling, or discomfort at the IV site require immediate cessation of the infusion and establishment of alternative IV access. Infusion-site monitoring is crucial for all IV access points during the administration of any drug or fluid.
Fluid management must be dynamic and closely monitored to ensure optimal outcomes. Patients receiving IV fluids require regular assessment of vital signs, intake and output, weight, and laboratory values. Daily reassessment of fluid status and laboratory results—including serum sodium, potassium, chloride, blood urea nitrogen (BUN)/creatinine, glucose, and acid–base balance—is recommended for the duration of IV fluid administration. The response to crystalloid therapy should be evaluated continuously by monitoring vital signs and laboratory parameters. In practice, this involves maintaining strict intake and output records, measuring daily weights, monitoring electrolytes and acid-base status, and adjusting fluid type and infusion rate as the clinical situation evolves.[49] Fluid therapy is not a set-and-forget intervention; it demands frequent reevaluation and modification.
Toxicity
The toxicity of crystalloid fluids is primarily related to volume overload from IV administration rather than the fluid’s composition. Patients may exhibit symptoms ranging from mild peripheral edema to respiratory distress caused by pulmonary edema. Symptomatic individuals should receive diuretic therapy and undergo close monitoring of serum electrolytes. In severe cases of respiratory distress, noninvasive positive pressure ventilation or intubation may be necessary.[50] Asymptomatic fluid overload can typically be managed conservatively with fluid restriction and continued monitoring.
Enhancing Healthcare Team Outcomes
All members of the interprofessional healthcare team should be knowledgeable about crystalloid fluids and their appropriate indications. Ringer lactate and acetate-based infusion fluids are versatile and more physiologically appropriate options for resuscitation, but may be underutilized compared to normal saline. Clinicians sometimes avoid these fluids due to misconceptions about their lactate content and limited familiarity. A thorough understanding of the composition and specific uses of available IV fluids enables clinicians to provide more effective and tailored care for hospitalized patients.
Responsibilities within the healthcare team are clearly defined. Nurses are responsible for administering IV fluids and closely monitoring patients for any adverse reactions that may occur. Physicians diagnose and initiate treatment, addressing life-threatening issues such as hypotension, volume overload, metabolic disturbances, and respiratory distress. Pharmacists play a critical role in ensuring the appropriate selection and administration of fluids.[51] Fluid stewardship was implemented in a community hospital ICU using the "4 rights" and the ROSE (rescue, optimization, stabilization, evacuation) model. This approach enhanced pharmacist-led fluid management by promoting the selection of appropriate patients and routes of administration—particularly during the stabilization phase—to support safer and more effective fluid therapy.[52]
Nephrology should be consulted when there are significant metabolic derangements.[53] Physicians, advanced practitioners, nurses, pharmacists, and other healthcare professionals must collaborate closely to streamline the patient’s care journey from diagnosis through treatment and follow-up. This coordinated, interprofessional approach helps minimize errors, reduce delays, and improve patient safety, ultimately leading to better outcomes. Additionally, it fosters patient-centered care focused on the well-being and satisfaction of those receiving crystalloid fluid therapy.
Media
(Click Image to Enlarge)
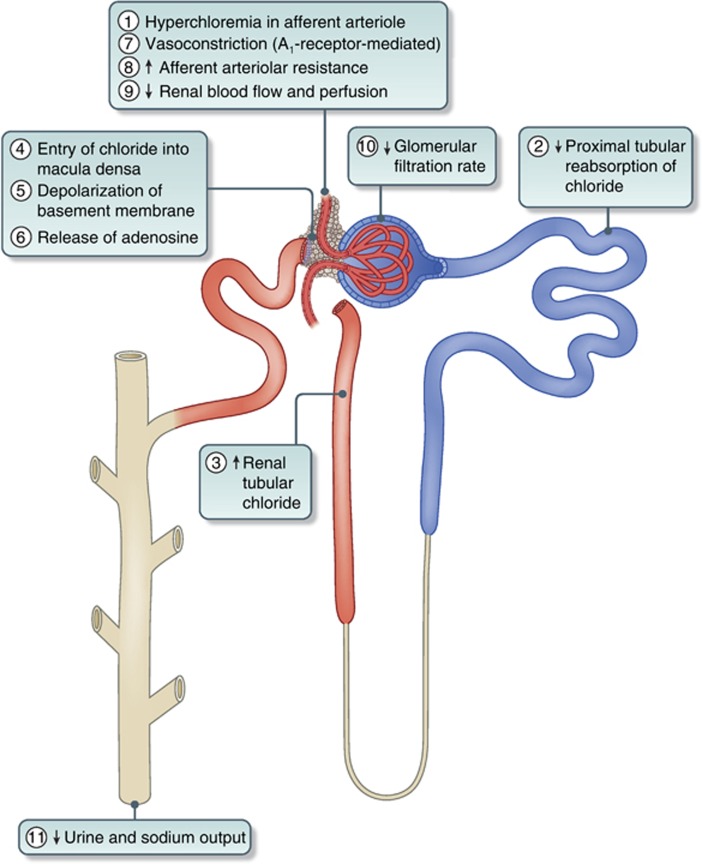
Sequential Effects of Hyperchloremia on the Kidney. Abbreviation: A1 receptor, adenosine1 receptor.
Lobo DN, Awad S. Should chloride-rich crystalloids remain the mainstay of fluid resuscitation to prevent ‘pre-renal' acute kidney injury? Kidney Int. 2014;86(6):1096-1105. doi: 10.1038/ki.2014.105.
References
Semler MW, Rice TW. Saline Is Not the First Choice for Crystalloid Resuscitation Fluids. Critical care medicine. 2016 Aug:44(8):1541-4. doi: 10.1097/CCM.0000000000001941. Epub [PubMed PMID: 27428117]
Semler MW, Kellum JA. Balanced Crystalloid Solutions. American journal of respiratory and critical care medicine. 2019 Apr 15:199(8):952-960. doi: 10.1164/rccm.201809-1677CI. Epub [PubMed PMID: 30407838]
Iqbal U, Anwar H, Scribani M. Ringer's lactate versus normal saline in acute pancreatitis: A systematic review and meta-analysis. Journal of digestive diseases. 2018 Jun:19(6):335-341. doi: 10.1111/1751-2980.12606. Epub 2018 Jun 10 [PubMed PMID: 29732686]
Level 1 (high-level) evidenceGelbenegger G, Shapiro NI, Zeitlinger M, Jilma B, Douglas IS, Jorda A. Lactated Ringer's or Normal Saline for Initial Fluid Resuscitation in Sepsis-Induced Hypotension. Critical care medicine. 2025 May 1:53(5):e1140-e1144. doi: 10.1097/CCM.0000000000006601. Epub 2025 Feb 19 [PubMed PMID: 39969246]
Self WH, Semler MW, Wanderer JP, Wang L, Byrne DW, Collins SP, Slovis CM, Lindsell CJ, Ehrenfeld JM, Siew ED, Shaw AD, Bernard GR, Rice TW, SALT-ED Investigators. Balanced Crystalloids versus Saline in Noncritically Ill Adults. The New England journal of medicine. 2018 Mar 1:378(9):819-828. doi: 10.1056/NEJMoa1711586. Epub 2018 Feb 27 [PubMed PMID: 29485926]
Semler MW, Self WH, Wanderer JP, Ehrenfeld JM, Wang L, Byrne DW, Stollings JL, Kumar AB, Hughes CG, Hernandez A, Guillamondegui OD, May AK, Weavind L, Casey JD, Siew ED, Shaw AD, Bernard GR, Rice TW, SMART Investigators and the Pragmatic Critical Care Research Group. Balanced Crystalloids versus Saline in Critically Ill Adults. The New England journal of medicine. 2018 Mar 1:378(9):829-839. doi: 10.1056/NEJMoa1711584. Epub 2018 Feb 27 [PubMed PMID: 29485925]
Level 3 (low-level) evidenceTinawi M. New Trends in the Utilization of Intravenous Fluids. Cureus. 2021 Apr 21:13(4):e14619. doi: 10.7759/cureus.14619. Epub 2021 Apr 21 [PubMed PMID: 34040918]
Carvalho Pereira L, Carvalho Pereira I, Dias Delfino Cabral T, Viana P, Mendonça Ribeiro A, Amaral S. Balanced Crystalloids Versus Normal Saline in Kidney Transplant Patients: An Updated Systematic Review, Meta-analysis, and Trial Sequential Analysis. Anesthesia and analgesia. 2024 Jul 1:139(1):58-67. doi: 10.1213/ANE.0000000000006932. Epub 2024 Apr 5 [PubMed PMID: 38578867]
Level 1 (high-level) evidenceShane AL, Mody RK, Crump JA, Tarr PI, Steiner TS, Kotloff K, Langley JM, Wanke C, Warren CA, Cheng AC, Cantey J, Pickering LK. 2017 Infectious Diseases Society of America Clinical Practice Guidelines for the Diagnosis and Management of Infectious Diarrhea. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2017 Nov 29:65(12):1963-1973. doi: 10.1093/cid/cix959. Epub [PubMed PMID: 29194529]
Level 1 (high-level) evidenceChowdhury AH, Cox EF, Francis ST, Lobo DN. A randomized, controlled, double-blind crossover study on the effects of 2-L infusions of 0.9% saline and plasma-lyte® 148 on renal blood flow velocity and renal cortical tissue perfusion in healthy volunteers. Annals of surgery. 2012 Jul:256(1):18-24. doi: 10.1097/SLA.0b013e318256be72. Epub [PubMed PMID: 22580944]
Level 1 (high-level) evidenceLobo DN, Awad S. Should chloride-rich crystalloids remain the mainstay of fluid resuscitation to prevent 'pre-renal' acute kidney injury?: con. Kidney international. 2014 Dec:86(6):1096-105. doi: 10.1038/ki.2014.105. Epub 2014 Apr 9 [PubMed PMID: 24717302]
Foster HE, Barry MJ, Dahm P, Gandhi MC, Kaplan SA, Kohler TS, Lerner LB, Lightner DJ, Parsons JK, Roehrborn CG, Welliver C, Wilt TJ, McVary KT. Surgical Management of Lower Urinary Tract Symptoms Attributed to Benign Prostatic Hyperplasia: AUA Guideline. The Journal of urology. 2018 Sep:200(3):612-619. doi: 10.1016/j.juro.2018.05.048. Epub 2018 Jun 11 [PubMed PMID: 29775639]
Hofmann-Kiefer KF, Chappell D, Kammerer T, Jacob M, Paptistella M, Conzen P, Rehm M. Influence of an acetate- and a lactate-based balanced infusion solution on acid base physiology and hemodynamics: an observational pilot study. European journal of medical research. 2012 Jul 6:17(1):21. doi: 10.1186/2047-783X-17-21. Epub 2012 Jul 6 [PubMed PMID: 22769740]
Level 3 (low-level) evidenceZhang J, Liu F, Wu Z, Jiang J, Wang B, Qian Y, Suo J, Li Y, Peng Z. ACETATE RINGER'S SOLUTION VERSUS NORMAL SALINE SOLUTION IN SEPSIS: A RANDOMIZED, CONTROLLED TRIAL. Shock (Augusta, Ga.). 2024 Apr 1:61(4):520-526. doi: 10.1097/SHK.0000000000002324. Epub 2024 Feb 1 [PubMed PMID: 38369528]
Level 1 (high-level) evidenceWeinberg L, Collins N, Van Mourik K, Tan C, Bellomo R. Plasma-Lyte 148: A clinical review. World journal of critical care medicine. 2016 Nov 4:5(4):235-250 [PubMed PMID: 27896148]
Chen H, Song Z, Dennis JA. Hypertonic saline versus other intracranial pressure-lowering agents for people with acute traumatic brain injury. The Cochrane database of systematic reviews. 2020 Jan 17:1(1):CD010904. doi: 10.1002/14651858.CD010904.pub3. Epub 2020 Jan 17 [PubMed PMID: 31978260]
Level 1 (high-level) evidenceHahn RG. The kinetics of isotonic and hypertonic resuscitation fluids is dependent on the sizes of the body fluid volumes. Journal of anaesthesiology, clinical pharmacology. 2023 Apr-Jun:39(2):264-272. doi: 10.4103/joacp.joacp_189_21. Epub 2022 Sep 14 [PubMed PMID: 37564831]
Froese L, Dian J, Batson C, Gomez A, Unger B, Zeiler FA. The impact of hypertonic saline on cerebrovascular reactivity and compensatory reserve in traumatic brain injury: an exploratory analysis. Acta neurochirurgica. 2020 Nov:162(11):2683-2693. doi: 10.1007/s00701-020-04579-0. Epub 2020 Sep 21 [PubMed PMID: 32959342]
Boone MD, Oren-Grinberg A, Robinson TM, Chen CC, Kasper EM. Mannitol or hypertonic saline in the setting of traumatic brain injury: What have we learned? Surgical neurology international. 2015:6():177. doi: 10.4103/2152-7806.170248. Epub 2015 Nov 23 [PubMed PMID: 26673517]
Gharizadeh N, Ghojazadeh M, Naseri A, Dolati S, Tarighat F, Soleimanpour H. Hypertonic saline for traumatic brain injury: a systematic review and meta-analysis. European journal of medical research. 2022 Nov 20:27(1):254. doi: 10.1186/s40001-022-00897-4. Epub 2022 Nov 20 [PubMed PMID: 36404350]
Level 1 (high-level) evidenceCook AM, Morgan Jones G, Hawryluk GWJ, Mailloux P, McLaughlin D, Papangelou A, Samuel S, Tokumaru S, Venkatasubramanian C, Zacko C, Zimmermann LL, Hirsch K, Shutter L. Guidelines for the Acute Treatment of Cerebral Edema in Neurocritical Care Patients. Neurocritical care. 2020 Jun:32(3):647-666. doi: 10.1007/s12028-020-00959-7. Epub [PubMed PMID: 32227294]
Aoyagi Y, Yoshida T, Uchino S, Takinami M, Uezono S. Saline versus 5% dextrose in water as a drug diluent for critically ill patients: a retrospective cohort study. Journal of intensive care. 2020:8():69. doi: 10.1186/s40560-020-00489-6. Epub 2020 Sep 11 [PubMed PMID: 32944250]
Level 2 (mid-level) evidenceWardi G, Holgren S, Gupta A, Sobel J, Birch A, Pearce A, Malhotra A, Tainter C. A Review of Bicarbonate Use in Common Clinical Scenarios. The Journal of emergency medicine. 2023 Aug:65(2):e71-e80. doi: 10.1016/j.jemermed.2023.04.012. Epub 2023 Apr 21 [PubMed PMID: 37442665]
Achanti A, Szerlip HM. Acid-Base Disorders in the Critically Ill Patient. Clinical journal of the American Society of Nephrology : CJASN. 2023 Jan 1:18(1):102-112. doi: 10.2215/CJN.04500422. Epub 2022 Aug 23 [PubMed PMID: 35998977]
Zhang N, Zhang J, Du S, He H, Yan X, Ma G. Association between the content of intracellular and extracellular fluid and the amount of water intake among Chinese college students. Nutrition & metabolism. 2019:16():67. doi: 10.1186/s12986-019-0397-9. Epub 2019 Sep 18 [PubMed PMID: 31548843]
Gladden LB. Lactate metabolism: a new paradigm for the third millennium. The Journal of physiology. 2004 Jul 1:558(Pt 1):5-30 [PubMed PMID: 15131240]
Level 3 (low-level) evidenceIchai C, Orban JC, Fontaine E. Sodium lactate for fluid resuscitation: the preferred solution for the coming decades? Critical care (London, England). 2014 Jul 7:18(4):163. doi: 10.1186/cc13973. Epub 2014 Jul 7 [PubMed PMID: 25043707]
Piper GL, Kaplan LJ. Fluid and electrolyte management for the surgical patient. The Surgical clinics of North America. 2012 Apr:92(2):189-205, vii. doi: 10.1016/j.suc.2012.01.004. Epub 2012 Feb 9 [PubMed PMID: 22414407]
Jamison A, Mohamed A, Chedester C, Klindworth K, Hamarshi M, Sembroski E. Lactated Ringer's versus normal saline in the management of acute diabetic ketoacidosis (RINSE-DKA). Pharmacotherapy. 2024 Aug:44(8):623-630. doi: 10.1002/phar.4600. Epub 2024 Jul 30 [PubMed PMID: 39077895]
Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith CM, French C, Machado FR, Mcintyre L, Ostermann M, Prescott HC, Schorr C, Simpson S, Wiersinga WJ, Alshamsi F, Angus DC, Arabi Y, Azevedo L, Beale R, Beilman G, Belley-Cote E, Burry L, Cecconi M, Centofanti J, Coz Yataco A, De Waele J, Dellinger RP, Doi K, Du B, Estenssoro E, Ferrer R, Gomersall C, Hodgson C, Hylander Møller M, Iwashyna T, Jacob S, Kleinpell R, Klompas M, Koh Y, Kumar A, Kwizera A, Lobo S, Masur H, McGloughlin S, Mehta S, Mehta Y, Mer M, Nunnally M, Oczkowski S, Osborn T, Papathanassoglou E, Perner A, Puskarich M, Roberts J, Schweickert W, Seckel M, Sevransky J, Sprung CL, Welte T, Zimmerman J, Levy M. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock 2021. Critical care medicine. 2021 Nov 1:49(11):e1063-e1143. doi: 10.1097/CCM.0000000000005337. Epub [PubMed PMID: 34605781]
Mekontso Dessap A, AlShamsi F, Belletti A, De Backer D, Delaney A, Møller MH, Gendreau S, Hernandez G, Machado FR, Mer M, Monge Garcia MI, Myatra SN, Peng Z, Perner A, Pinsky MR, Sharif S, Teboul JL, Vieillard-Baron A, Alhazzani W, European Society of Intensive Care Medicine. European Society of Intensive Care Medicine (ESICM) 2025 clinical practice guideline on fluid therapy in adult critically ill patients: part 2-the volume of resuscitation fluids. Intensive care medicine. 2025 Mar:51(3):461-477. doi: 10.1007/s00134-025-07840-1. Epub 2025 Mar 31 [PubMed PMID: 40163133]
Level 1 (high-level) evidenceOthman MI, Nashwan AJ, Alfayoumi M, Khatib M, Abujaber AA. Plasma-Lyte-148 Versus Normal Saline 0.9% in Diabetic Ketoacidosis Management: A Review. Cureus. 2023 Jun:15(6):e41079. doi: 10.7759/cureus.41079. Epub 2023 Jun 28 [PubMed PMID: 37519584]
. Intravenous fluid therapy in adults in hospital. 2017 May:(): [PubMed PMID: 32101393]
Cooper DJ, Myles PS, McDermott FT, Murray LJ, Laidlaw J, Cooper G, Tremayne AB, Bernard SS, Ponsford J, HTS Study Investigators. Prehospital hypertonic saline resuscitation of patients with hypotension and severe traumatic brain injury: a randomized controlled trial. JAMA. 2004 Mar 17:291(11):1350-7 [PubMed PMID: 15026402]
Level 1 (high-level) evidenceHUGGINS RA, BRECKENRIDGE CG, HOFF HE. Volume of distribution of potassium and its alteration by sympatholytic and antihistaminic drugs. The American journal of physiology. 1950 Oct:163(1):153-8 [PubMed PMID: 14771288]
Catahay JA, Polintan ET, Casimiro M, Notarte KI, Velasco JV, Ver AT, Pastrana A, Macaranas I, Patarroyo-Aponte G, Lo KB. Balanced electrolyte solutions versus isotonic saline in adult patients with diabetic ketoacidosis: A systematic review and meta-analysis. Heart & lung : the journal of critical care. 2022 Jul-Aug:54():74-79. doi: 10.1016/j.hrtlng.2022.03.014. Epub 2022 Mar 28 [PubMed PMID: 35358905]
Level 1 (high-level) evidenceCho YS, Lim H, Kim SH. Comparison of lactated Ringer's solution and 0.9% saline in the treatment of rhabdomyolysis induced by doxylamine intoxication. Emergency medicine journal : EMJ. 2007 Apr:24(4):276-80 [PubMed PMID: 17384382]
Rajasekaran A, Bade N, Cutter GR, Rizk DV, Zarjou A. Lactated Ringer's solution and risk of hyperkalemia in patients with reduced kidney function. The American journal of the medical sciences. 2022 Oct:364(4):433-443. doi: 10.1016/j.amjms.2022.04.024. Epub 2022 Apr 29 [PubMed PMID: 35490704]
O'Malley CMN, Frumento RJ, Hardy MA, Benvenisty AI, Brentjens TE, Mercer JS, Bennett-Guerrero E. A randomized, double-blind comparison of lactated Ringer's solution and 0.9% NaCl during renal transplantation. Anesthesia and analgesia. 2005 May:100(5):1518-1524. doi: 10.1213/01.ANE.0000150939.28904.81. Epub [PubMed PMID: 15845718]
Level 1 (high-level) evidenceModi MP, Vora KS, Parikh GP, Shah VR. A comparative study of impact of infusion of Ringer's Lactate solution versus normal saline on acid-base balance and serum electrolytes during live related renal transplantation. Saudi journal of kidney diseases and transplantation : an official publication of the Saudi Center for Organ Transplantation, Saudi Arabia. 2012 Jan:23(1):135-7 [PubMed PMID: 22237237]
Level 3 (low-level) evidenceAkanji AO, Hockaday TD. Acetate tolerance and the kinetics of acetate utilization in diabetic and nondiabetic subjects. The American journal of clinical nutrition. 1990 Jan:51(1):112-8 [PubMed PMID: 2153334]
Tiwari AK, Tayal S, Awasthi D, Valson G. Allergy to lactated ringer solution-an unusual case presentation. American journal of therapeutics. 2011 May:18(3):e86-8. doi: 10.1097/MJT.0b013e3181fafb30. Epub [PubMed PMID: 21326085]
Level 3 (low-level) evidenceStemple K, Schnoor J, Ahern J, Bunnell A. Utilization of an Order Panel to Encourage Safe Ordering and Administration of Amphotericin B. Hospital pharmacy. 2019 Aug:54(4):212-216. doi: 10.1177/0018578719829417. Epub 2019 Feb 9 [PubMed PMID: 31320767]
Onuki Y, Hasegawa N, Kida C, Ikegami-Kawai M, Tsubuki M, Shirozu S, Obata Y, Takayama K. Supersaturated state of diazepam injection following dilution with infusion fluid. Journal of pharmaceutical health care and sciences. 2015:1():9. doi: 10.1186/s40780-014-0009-9. Epub 2015 Mar 9 [PubMed PMID: 26819720]
Kelley M, Spooneybarger C, Howard M, Reinert J, Churchwell MD, Baki G. Physical compatibility of ceftriaxone and cefepime in 0.45% sodium chloride, Ringer's lactate solution, and Plasma-Lyte A. European journal of hospital pharmacy : science and practice. 2024 Dec 30:():. pii: ejhpharm-2024-004128. doi: 10.1136/ejhpharm-2024-004128. Epub 2024 Dec 30 [PubMed PMID: 38862193]
Pfortmueller CA, Faeh L, Müller M, Eberle B, Jenni H, Zante B, Prazak J, Englberger L, Takala J, Jakob SM. Fluid management in patients undergoing cardiac surgery: effects of an acetate- versus lactate-buffered balanced infusion solution on hemodynamic stability (HEMACETAT). Critical care (London, England). 2019 May 6:23(1):159. doi: 10.1186/s13054-019-2423-8. Epub 2019 May 6 [PubMed PMID: 31060591]
Liamis G, Filippatos TD, Elisaf MS. Correction of hypovolemia with crystalloid fluids: Individualizing infusion therapy. Postgraduate medicine. 2015 May:127(4):405-12. doi: 10.1080/00325481.2015.1029421. Epub 2015 Mar 26 [PubMed PMID: 25812486]
Zampieri FG, Ranzani OT, Azevedo LC, Martins ID, Kellum JA, Libório AB. Lactated Ringer Is Associated With Reduced Mortality and Less Acute Kidney Injury in Critically Ill Patients: A Retrospective Cohort Analysis. Critical care medicine. 2016 Dec:44(12):2163-2170 [PubMed PMID: 27495820]
Level 2 (mid-level) evidenceYang SH, Mu PF, Wu HL, Curia M. Fluid balance monitoring in congestive heart failure patients in hospital: a best practice implementation project. JBI database of systematic reviews and implementation reports. 2019 Oct:17(10):2202-2211. doi: 10.11124/JBISRIR-2017-004021. Epub [PubMed PMID: 31464851]
Level 1 (high-level) evidenceDe Jong A, Myatra SN, Roca O, Jaber S. How to improve intubation in the intensive care unit. Update on knowledge and devices. Intensive care medicine. 2022 Oct:48(10):1287-1298. doi: 10.1007/s00134-022-06849-0. Epub 2022 Aug 20 [PubMed PMID: 35986748]
Abbood SK, Assad HC, Al-Jumaili AA. Pharmacist intervention to enhance postoperative fluid prescribing practice in an Iraqi hospital through implementation of NICE guideline. Pharmacy practice. 2019 Jul-Sep:17(3):1552. doi: 10.18549/PharmPract.2019.3.1552. Epub 2019 Aug 29 [PubMed PMID: 31592296]
Level 2 (mid-level) evidenceHawkins WA, Butler SA, Poirier N, Wilson CS, Long MK, Smith SE. From theory to bedside: Implementation of fluid stewardship in a medical ICU pharmacy practice. American journal of health-system pharmacy : AJHP : official journal of the American Society of Health-System Pharmacists. 2022 Jun 7:79(12):984-992. doi: 10.1093/ajhp/zxab453. Epub [PubMed PMID: 34849544]
Askenazi DJ, Heung M, Connor MJ Jr, Basu RK, Cerdá J, Doi K, Koyner JL, Bihorac A, Golestaneh L, Vijayan A, Okusa MD, Faubel S, American Society of Nephrology Acute Kidney Injury Advisory Group. Optimal Role of the Nephrologist in the Intensive Care Unit. Blood purification. 2017:43(1-3):68-77. doi: 10.1159/000452317. Epub 2016 Dec 3 [PubMed PMID: 27923227]