Introduction
Rett syndrome (RTT) is a neurodevelopmental disorder in which regression of previously acquired skills follows a period of typical development due to mutations of methylated CpG binding protein 2 (MECP2) gene on the X chromosome. RTT is associated with a complex phenotype and classified into typical, atypical, and variant presentations. Approximately 90% of reported cases of RTT inherit mutations of the MECP2 gene. Some atypical cases of RTT may result from mutations in cyclin-dependent kinase-like 5 (CDKL5). Mutations in MECP2 have been associated with impacting the development of neurons and axodendritic connections. Jellinger and Seitelberger (1986) were the first neuropathologists to identify and describe the pathology behind RTT. They found that the brain in patients of RTT weighed less, and the neurons of the substantia nigra pars compacta contained less melanin in comparison to the age-matched controls. Although RTT primarily affects females, recent studies define males with the phenotype and MECP2 mutations.[1][2][3][4]
RTT presents with a multitude of symptoms but not limited to a deceleration in head growth, abnormal gait, loss of purposeful hand movements often replaced with repetitive stereotypical movement (hand-wringing), rigid movement of hands, loss of language function, breathing abnormalities, and mental retardation. Currently, RTT has no cure; therefore, symptomatic management comprises treatment. Clinical diagnosis, treatment, and genetic counseling of patients with RTT is guided by the revised 2010 third edition of the Diagnostic Standard of RTT and integrated global clinical research reports.[4]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
RTT is an X-linked dominant condition with lethal consequences in hemizygous males. Recent studies have mentioned that the majority of RTT-causing variants in MECP2 are de novo and commonly occur on the paternally inherited X chromosome. The pathogenic MECP2 variants are identified in more than 95% of individuals with typical RTT. However, recent studies have found that MeCP2, the protein product of the MECP2 gene, is known to regulate gene expression and is highly expressed in the brain.[5][6]
While paternal age has been reported to be associated with an increased risk of genetic disorders, current studies revealed no significant difference in parental ages of RTT probands compared to the general population. A minimal increase in parental age has been noted with missense variants compared to those with nonsense variants. Additionally, no evident association was demonstrated between clinical severity and parental age. Therefore, advanced parental ages did not appear to be a risk factor for RTT and did not contribute to the clinical severity in individuals with RTT.[6]
Furthermore, 2 additional genes, cyclin-dependent kinase-like 5 (CDKL5) and forkhead box G1 (FOXG1), are involved in the pathogenesis of RTT. The spectrum of mutation types includes missense, nonsense, and frameshift mutations.[7][8] Mutations affecting the NLS region of MECP2 or early truncating mutations are associated with a more severe phenotype than missense mutations, whereas C-terminal deletions are linked to milder phenotypes. The R133C mutation is generally associated with a milder variant of RTT, often with preserved speech. The MeCP2 protein is responsible for 2 main functional roles: the methyl-binding domain, which specifically binds to the methylated CpG dinucleotides, and the transcription repression domain, which recruits repressor proteins that inhibit gene transcription. Mutations in an X-linked gene, CDKL5, have been reported in patients with a seizure variant of RTT. Notably, a wide spectrum of mutations associated with disease severity has been identified, and close to 30 different types of mutations can cause RTT.[9]
A recent study on the genetic mutational spectrum of MECP2 mutations was conducted among Indian patients with RTT, revealing associations with respiratory dysfunction, scoliosis, and sleep disturbances. The significant results of this study also suggest that clinicians screen for abnormal cholesterol, calcium, and TSH levels associated with MECP2 gene mutations to facilitate early prognosis of disease severity.[10]
Current literature also suggests 4 distinct social profiles in individuals with RTT, hinting at shared disrupted circuits between sensorimotor functioning and sleep-related neuronal pathways. This study found varying degrees of social impairments and yielded the following 4 social profiles:
- Interactive motricity
- Mood change
- Anxiety/agitation
- Gazing
Furthermore, longer sleep onset latency was linked with increased sociobehavioral impairments, particularly in interactive motricity reduction. On the other hand, higher rapid eye movement sleep was connected with fewer interactive sociomotor behaviors. No significant differences were found in social profiles concerning sleep-disordered breathing or daytime sleepiness.[11]
Epidemiology
RTT is a severe, progressive, neurodevelopmental disorder that predominantly affects females and is also one of the most frequent causes of mental disability in females, with an incidence of 1 in 10,000 to 20,000 live birth cases.[5][12] A systematic review of literature described RTT as the second most frequent cause of genetic intellectual disability that can lead to neurological regression between 6 and 18 months of life and could be linked with a variety of neurological impairments along with nonneurological organs.[13]
Current data on the involvement of RTT and the endocrine system have presented growth disorders, bone health issues, thyroid abnormalities, puberty onset delays, and weight abnormalities. Another study reported the following main endocrinopathies: malnutrition, short stature, menstrual cycle abnormalities, weight disorders, low bone mineral density, hyperprolactinemia, and thyroid disorders. Furthermore, endocrinopathies were significantly more common in patients with RTT with MECP2 mutations, and epilepsy was more frequent in CDKL5 deletions.[13][14]
A population-based registry in Texas reported a prevalence of classic RTT as 1 per 22,800 females (0.44 per 10,000) from ages 2 to 18. RTT was initially thought to only occur in females, and males with RTT were not considered to be viable. Further investigations are needed on the incidence and prevalence of RTT in males. A systematic review conducted in Texas in 2015 reported 57 cases of RTT in males, and a population study showed that the incidence of RTT in males was not more prevalent in any particular racial group.[15]
Another study among females in the United States highlighted the considerable burden of RTT based on disease subtype and age. Despite a reliance on supportive therapies and healthcare encounters, patients with RTT still have increasing disease severity and motor-behavioral dysfunction in childhood and adolescence, while not meeting the needs of these patients and the value of early intervention to their long-term management.[16]
A demographic study found that Irish patients with RTT have the same clinical presentation as those in other countries, but seizures, involuntary movements, and regression are more frequent. On the other hand, they have reported fewer skeletal deformities but with better responses to antiseizure drugs.[17]
Pathophysiology
RTT is a neurodevelopmental disorder caused by mutations in MECP2 in approximately 95% of RTT cases. MeCP2 is a non-cell-type-specific DNA-binding protein, and its mutation affects both neural and nonneural cells in the brain, including vasculature associated with endothelial cells.
Vascular integrity is essential in brain homeostasis, and its modification may be related to neurodegenerative disease pathology. Current studies have revealed a disease-specific microvascular network (Rett-dMVNs) and observed higher permeability in the Rett-dMVNs compared to isogenic controls, indicating an alteration in barrier function resulting from MECP2 mutation. Furthermore, studies have also found that hyperpermeability is linked to the upregulation of miR-126-3p in RTT patient-derived endothelial cells, as determined by microRNA profiling and RNA sequencing, and that the rescue of miR-126-3p levels can recover their phenotype. Findings also revealed miR-126-3p-mediated vascular impairment in patients with RTT, suggesting the potential application of these findings in translational medicine.[18]
Recent studies also suggested that MECP2 has both repressor and activator transcription activities. The exact mechanism of how MECP2 mutations lead to RTT is unknown. A possible theory is that a deficiency of MECP2 causes a failure of appropriate synapse maturation in the cortex. Another hypothesis is that the lack of MECP2 disrupts brain cholesterol metabolism, resulting in abnormal neuronal development. One study found that MECP2 acts as a positive cofactor for RNA Pol II gene expression at many neuronal genes that harbor CpG islands in promoter-proximal regions, and that RTT is partly due to the loss of gene activity in these genes in neurons.[19]
Recent studies have highlighted that the clinical manifestations of RTT involve an imbalance of redox homeostasis and exacerbated inflammatory responses, which underscore the role of the p75 neurotrophin receptor (p75NTR) in regulating oxidative stress and inflammation. Findings revealed that p75NTR modulation by LM11A-31 repairs protein glutathionylation and lessens the expression of the pro-oxidant enzyme NOX4. Furthermore, LM11A-31 significantly reduces the expression of the pro-inflammatory mediators interleukin-6 and interleukin-8 and regulates the expression levels of transcription factors involved in controlling antioxidant response and inflammation.[20]
Studies have also suggested that the failure of dendritic arborization in the cortex has led to abnormal neuronal signaling, resulting in the lack of maturation of the autonomic nervous system and the motor and cortical regions. Recent evidence suggests that MECP2 is also expressed in glial cells, and glial cell dysfunction resulting from changes in DNA methylation may also contribute to the pathogenesis of RTT. The MECP2 mutation affects mitochondrial and metabolic pathways in astrocytes, thereby affecting neuronal health. The smaller mitochondria, particularly in glia versus neurons, resulted in decreased mitochondrial respiration and altered key proteins in the tricarboxylic acid cycle and electron transport chain in patients with RTT. Moreover, RTT astrocytes exhibited increased cytosolic amino acid levels under basal conditions, which decreased when energy demands were high. The mitochondria isolated from astrocytes of patients with RTT exhibited increased reactive oxygen species and influenced neuronal activity when transferred to cortical neurons.[21]
Recordings of sensory-perceptual functioning using event-related potential (ERP) approaches were identified as potentially objective tools for measuring neural function in RTT, particularly in cases of highly anomalous auditory evoked potentials (AEPs). To accurately determine the degree of neural dysfunction using the ERP technique, response reliability at the single-trial level is highly advised based on previous studies. Nonneural noise sources lead to overestimating the degree of pathological processing in RTT, and denoising source separation techniques during signal processing substantially improve this concern. On the other hand, the current study aimed to infer the neurophysiological mechanisms of auditory processing in children with RTT through auditory steady-state response (ASSR), which is related to the fine temporal analysis of auditory input, and sustained wave, which is connected with the integral processing of the auditory signal. Both processes showed potential as noninvasive electrophysiological biomarkers of auditory processing with clinical relevance, enabling the establishment of links between genetic impairment and the RTT phenotype.[22][23]
Histopathology
On autopsy, an RTT brain does not show signs of inflammation or degeneration. However, an overall decrease in brain size and the size of individual neurons is noted. A 12% to 34% reduction in brain weight has been reported, most prominent in the prefrontal, posterior frontal, and anterior frontal regions. These findings suggest that RTT is a neurodevelopmental, rather than a neurodegenerative, process. The neuronal cell size is decreased, but an increase in neuronal cell packing in the hippocampus, along with delayed neuronal maturation and synaptogenesis in the cerebral cortex, is demonstrated.
A recent study on the developmental change of brain volume in RTT in Taiwan observed a significant decrease in brain volume among patients with RTT. Furthermore, their cortical gray matter volume continues to decrease in the bilateral parietal lobes and the left occipital lobes.[24]
History and Physical
RTT is a neurodevelopmental disorder characterized by an initial period of normal growth and development, followed by regression in motor and communication skills. RTT is a clinical diagnosis; therefore, recognizing the characteristic findings of clinical assessment is essential. Key clinical features include slowed development, loss of hand function with distinctive repetitive movements, slowed brain and head growth, gait abnormalities, seizures, and cognitive impairment. Additional symptoms include irregular breathing, digestive issues, and apraxia, which affects speech and eye movements. The severity and progression of RTT vary among individuals, with some exhibiting atypical variants.[25][5]
Since RTT is still considered a clinical diagnosis, clinical features are divided into the following typical and atypical presentations:
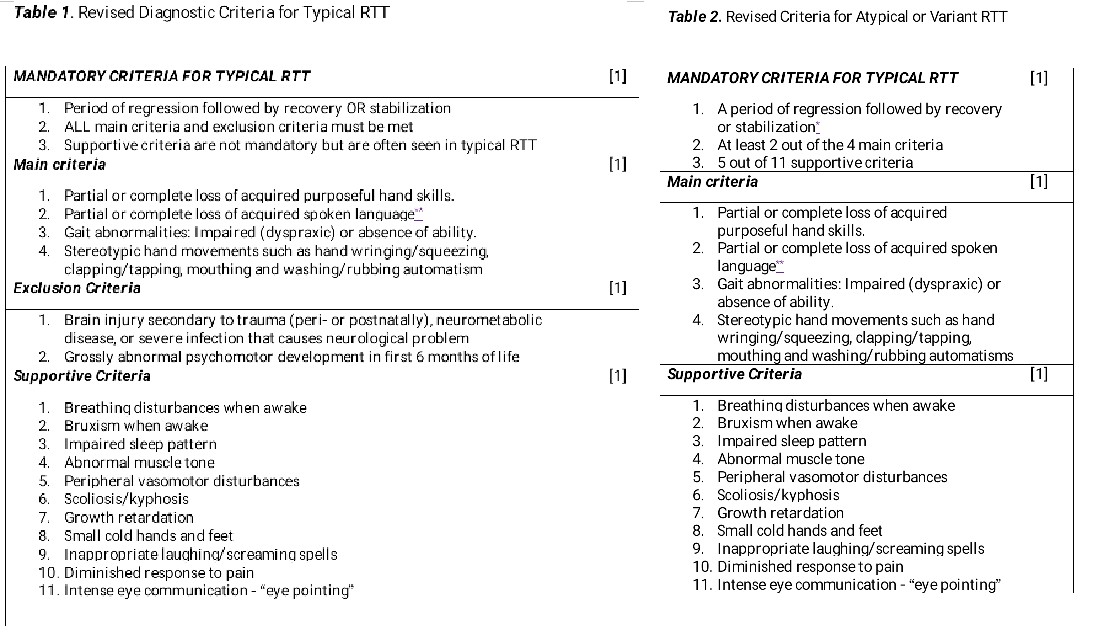
- Typical (classic) RTT: The diagnostic criteria for a typical presentation require the presence of regression, in addition to the main criteria, and the exclusion criteria must be met (see Image. Rett Syndrome Types).
- This category is an X-linked neurodevelopmental disorder characterized by a period of regression, partial or complete loss of purposeful hand use, and acquired speech, resulting in impairment of expressive language, hand-wringing movements, impaired gait, and ambulation, accompanied by stereotyped hand movements.[26][27]
- A stage of stabilization will often follow regression, and sometimes, improvement may occur. Not all individuals with typical RTT will have postnatal deceleration in head growth.
- More than 95% of typical RTT cases have a pathogenic variant in the MECP2 gene. However, a recent case of clinically diagnosed typical RTT syndrome in an individual who lacked a genetic diagnosis despite 20 years of investigation and multiple rounds of sequencing the MECP2 gene was documented. Additional genetic testing using next-generation sequencing revealed a partial insertion of the BCL11A gene within exon 4 of MECP2, caused by a small deletion in MECP2, which may result in a possible disruption of MeCP2 function due to a frameshift.[27]
- Atypical (variant) RTT: The diagnostic criteria for atypical RTT require the presence of regression plus 2 out of the 4 main criteria in addition to 5 out of the 11 supportive criteria (see Image. Rett Syndrome Types). The above-mentioned criteria for typical RTT and atypical RTT apply to both males and females.[28][29][30]
Current studies reported the prevalence of orthopedic conditions in RTT, wherein the majority of the cases develop foot deformities, followed by scoliosis, hip displacement, and knee deformities. One study identified that age at inclusion, Cobb angle at baseline, and epilepsy have the highest discriminative ability for the rapid progression of scoliosis in RTT. In both studies, these findings can provide clinical guidelines, surveillance protocols, and management strategies for patients with RTT.[31][32][33]
Movement disorders beyond hand stereotypies are also common, especially tremors. Hypertonia becomes more prevalent with age, accompanied by apparent changes in nomenclature over time, eg, early epoch spasticity and late epoch dystonia.[34]
Patients with RTT also have autonomic nervous system dysfunction, predominantly of the sympathetic nervous system, together with exercise fatigue and an increased risk of sudden death. A recent study demonstrated the feasibility of continuous 24-hour non-invasive home monitoring of biological vitals (biovitals) using an innovative wearable sensor device in pediatric, adolescent, and adult patients with RTT. Furthermore, the maximum heart rate and the heart rate-low-frequency ratio, indicating sympathetic nervous system activation during dynamic exercise, were identified as potential objective markers of fatigue, illness severity, and disease progression.[35]
Evaluation
The diagnosis of RTT is based on clinical observation of developmental regression, loss of hand function, characteristic repetitive movements, and other neurological and systemic symptoms, as clinicians assess early growth patterns and conduct ongoing physical and neurological evaluations. Genetic testing for MECP2 mutations supports clinical diagnosis, as over 95% of classical RTT cases and approximately 75% of atypical cases have a pathogenic MECP2 variant. Atypical RTT is diagnosed when an individual does not meet all the classical RTT criteria and may involve other genes, eg, CDKL5 and FOXG1.[5]
Therefore, MECP2 mutations alone are insufficient to diagnose RTT, and a wide variety of clinical presentations may be noted. Because of its unique characteristics, several evaluation tools of clinical severity, behavior, and functional motor abilities have been studied for RTT, including:
- Rett Assessment Rating Scale
- Rett Syndrome Gross Motor Scale
- Rett Syndrome Functional Scale
- Functional Mobility Scale-Rett Syndrome
- Two-Minute Walking Test modified for Rett syndrome
- Rett Syndrome Hand Function Scale
- StepWatch Activity Monitor
- ActivPALTM
- Modified Bouchard Activity Record
- Rett Syndrome Behavioral Questionnaire
- Rett Syndrome Fear of Movement Scale [36]
Treatment / Management
Pharmacologic Treatment
Trofinetide is the first pharmacologic treatment approved by the US Food and Drug Administration for RTT in adult and pediatric patients aged 2 years and older. Trofinetide is a synthetic analog of glycine-proline-glutamate, the tripeptide normally split from insulin-like growth factor 1 upon degradation. Due to the presence of glutamate and glycine in its structure, trofinetide is thought to act by modulating the NMDA receptor, thereby normalizing neuronal activity and promoting survival.[37][38] (A1)
Recent clinical studies have shown that trofinetide treatment, administered at a dosage of 200 mg for 40 weeks or longer, continues to improve symptoms of RTT, offering potentially effective and safe therapeutic opportunities for patients with RTT, who currently have limited treatment options. Furthermore, the LAVENDER and LILAC trials revealed the long-term safety and efficacy of trofinetide. Additionally, no severe adverse effects were reported on epilepsy, heart, or bone-related symptoms.[39][40][41]
Reviews revealed that trofinetide is associated with adverse gastrointestinal events, eg, diarrhea and vomiting, and consequent weight loss. Mild or moderate diarrhea was frequently associated with trofinetide and was responsible for the majority of discontinuations due to TEAEs; however, diarrhea was self-limited and resolved soon after withdrawal of trofinetide.[42] Other limitations include the limited duration of drug supplies, as 1 bottle may last only 3 days, depending on weight, and the relatively high cost per bottle. On the other hand, trofinetide has no direct competitors: single symptoms of the RTT, eg, seizures or aggressive behaviors, are currently treated with drugs that have been developed for patients without the RTT.[43][44][45](A1)
Supportive Therapies
Currently, RTT has no cure, and supportive management aims to provide symptomatic relief through an interprofessional approach. Some of the medical concerns that need to be addressed in patients with RTT include seizure disorders, behavioral alterations, sleep disorders, breathing irregularities, cardiac dysfunction (prolonged QT interval), gastrointestinal dysfunction, and bone fractures.
Gastrointestinal symptoms
Probiotics were studied in patients with RTT who experienced gastrointestinal symptoms and gut microbiota imbalances. The results showed that probiotic L. plantarum PS128 was well tolerated and feasible, with a withdrawal rate of 0% and a retention rate of 100%. Minimal adverse effects, including loose stool, were noted, with significant improvement in dystonia.[46] Digestive problems, eg, gastroesophageal reflux disease (GERD) and constipation, are common in patients with RTT and can be managed with calcium carbonate, histamine H2 receptor blockers (avoiding cimetidine), and increased fiber intake.(A1)
Seizure disorders
Approximately 60% of patients with RTT have some form of seizure disorder. Treatment options to alleviate seizures include valproate, lamotrigine, levetiracetam, carbamazepine, and AEDs. A multicenter study described that generalized tonic-clonic and myoclonic epilepsy were the most typical seizure semiologies, whereas absence and focal epilepsy were less prevalent. Antiseizure medications significantly alter seizure characteristics. The most commonly used antiseizure drugs affecting the severity and frequency of seizures are valproate, levetiracetam, lamotrigine, and clobazam. Reduction of seizures and improvement of cognitive functions were also observed in the ketogenic diet, vagal nerve stimulation, and steroid treatment.[47](B2)
A longitudinal observational study assessed the efficacy and tolerance of cannabidiol (CBD) as adjunctive therapy for RTT patients with epilepsy. Findings revealed a decrease in the incidence of seizures in the majority of the patients. No aggravation of symptoms or adverse effects was noted, except for a case of transient drooling and somnolence episodes at the initiation of CBD. Reduced agitation and anxiety attacks and improved spasticity were also reported. The study concluded that CBD had potential therapeutic value for the treatment of drug-resistant epilepsy, was well tolerated, and, when combined with clobazam, may increase the effectiveness of clobazam alone.[48]
Behavioral and sleep disorders
Behavioral alterations, most often anxiety, can be best addressed with serotonin reuptake inhibitors (SSRIs). Good cariprazine response emphasized the impact of dopamine dysfunction in the complex psychiatric symptoms of RTT.[49]
Patients with RTT often have difficulty initiating sleep and frequent nighttime awakenings, which can be managed with proper sleep hygiene and trazodone once airway obstructions and other causes are ruled out. Breathing irregularities can include apnea, hyperventilation, and breath-holding. Since dysfunction of the autonomic nervous system is a key feature of RTT, heart rate variability has been used to investigate autonomic nervous system dysfunction in RTT during wakefulness. A recent study revealed that the sympathovagal balance shifted towards sympathetic predominance and vagal withdrawal during both wakefulness and sleep in RTT, even when cardiac autonomic dynamics were intact during sleep.[50]
Respiratory and cardiac disorders
Respiratory issues should be managed at an early stage, regardless of epilepsy onset. Recent literature recommended that noninvasive ventilation is a suitable alternative for patients with RTT, especially those with sleep respiratory disorders and those with hypotonia associated with hypercapnia. Chest physiotherapy should be considered for individuals with difficulty managing the accumulation of respiratory secretions, utilizing airway clearance techniques and devices (eg, PEP mask, AMBU bag, or cough machine), which are more appropriate and tolerated by the patient. The study also emphasized the need for individualized programs to manage scoliosis and the importance of considering gastrostomy in patients at increased risk of aspiration pneumonia.[51][52] Successful management of these irregularities can be complex. Precautions should be taken to avoid medications that alter breathing patterns (eg, opioid medications).(B3)
Cardiac irregularities include a prolonged QT interval, which can be more challenging to manage in patients with RTT than in the general population. Studies in mice with MECP2 mutations suggest that beta-blockers may not be sufficient. Precautions should be taken to avoid medications that further prolong the QT interval (eg, macrolides).
Musculoskeletal disorders
Bone fractures are 4 times more common in patients with RTT compared to the general population, and vitamin D levels should be closely monitored and supplemented as required. Surgical treatment for RTT-associated scoliosis is rare but has increased over 19 years. However, there were reports of higher costs, longer hospital stays, more complications (particularly respiratory), and a higher rate of nonroutine discharge dispositions compared to other patients with neuromuscular scoliosis (NMS).[53]
Another study demonstrated that a 12-week course of low-dose extracorporeal shock wave therapy (ESWT) administered weekly benefits children with RTT by reducing spasticity and enhancing the gross motor function of the lower limbs. Acoustic radiation force impulse sonoelastography demonstrated deterioration in muscle stiffness. This diverse therapeutic response to ESWT may be due to the mutation of MECP2 in patients with RTT, which continuously impacts and drives the pathophysiology differently.[54]
Rehabilitative Therapies
A recent study highlighted that game-based cognitive stimulation elicits substantial EEG changes in patients with RTT, thereby enhancing brain connectivity and information flow, which contribute to improved cognitive functions and increased attention. This is observed during the shift towards higher frequency bands and increased spectral entropy, which is aligned with enhanced brain activation during cognitive sessions. Therefore, these findings serve as markers for evaluating cognitive interventions and propose gaming-related activities as an effective medium for enhancing learning outcomes.[55][56]
Other studies suggest that physical therapy and rehabilitation, including walking training with a treadmill combined with virtual reality, may represent a new strategy for Rett syndrome rehabilitation.[57] On the other hand, studies on speech therapy using alternative and augmentative communication (AAC) have shown that these procedures are effective in teaching various component skills required for expressive communication in patients with complex communication needs. The results of these studies showed that individualized procedures, including behavior chaining, differential reinforcement, and delayed prompting, were effective in teaching page-linking with both high-tech and low-tech AAC devices.[58](B3)
For psychosocial support, a recent study demonstrated an increase in social interaction among patients with RTT when interacting with classmates in a school setting through the use of a low-tech tool, called Click4all, integrated into cognitive and motor training. This is a vital finding, as patients with RTT are typically restricted and isolated.[59] Other treatment options include occupational therapy and psychosocial support for families. Management of these conditions can substantially improve the quality of life of patients with RTT and should not be overlooked.[60][61](B2)
Investigational Therapies
Recently, the CRISPR-Cas9 technology has the potential to impact stem cell therapies for various genetic diseases, eg, RTT, by enabling the correction of genes or mutations in human patient cells. The Magnetic Nanoparticle-Assisted Genome Editing (MAGE) platform significantly improved the transfection efficiency, biocompatibility, and genome-editing accuracy of CRISPR-Cas9 technology.
In one study, the MAGE was applied to correct the mutated MECP2 gene in induced pluripotent stem cell-derived neural progenitor cells (iPSC-NPCs) from patients with RTT. When magnetofection and magnetic-activated cell sorting were combined, MAGE demonstrated higher multiplasmid delivery and repair efficiencies with significantly shorter incubation times than conventional transfection agents, with no size limitations on plasmids. When differentiated into neurons, the repaired iPSC-NPCs revealed the same characteristics as wild-type neurons, further confirming MAGE and its potential for future clinical applications.[62]
Differential Diagnosis
RTT can often be misdiagnosed as other neurological and developmental disorders because it has similar connections with the CNS and other organ systems, particularly in the gut region. Recent reviews have highlighted the connection between the central nervous system (CNS) and gut microbiota, with evidence suggesting bidirectional communication between the CNS and gut through the microbiota-gut-brain axis (MGBA). The gut microbiota affects the CNS by regulating neurogenesis, myelination, glial cell function, synaptic pruning, and blood-brain barrier permeability, with implications in various neurological diseases. Furthermore, the mood of delivery, exercise, psychotropic agents, stress, and neurologic drugs can influence the MGBA. Therefore, understanding the MGBA may be used as a springboard for possible research into microbial-based interventions and proper diagnosis, early detection, prompt intervention, and innovative therapeutic strategies for the following neurological diseases in both children and adults:
- Autism spectrum disorder
- Attention-deficit hyperactivity disorder
- Alzheimer's disease
- Non-Alzheimer's neurodegeneration and dementia
- Frontotemporal lobe dementia
- Wilson-Konovalov disease
- Multisystem atrophy
- Huntington's chorea
- Parkinson's disease
- Multiple sclerosis
- Amyotrophic lateral sclerosis
- Temporal lobe epilepsy
- Depression
- Schizophrenia
- Cerebral palsy
- Tourette syndrome
- Fetal alcohol spectrum disorders
- Genetic neurodevelopmental disorders (eg, Down, Angelman, and Turner syndromes)
- Nonspecific developmental delay [63][64][65]
Recent studies have also discussed how brain-derived neurotrophic factor (BDNF), expressed during brain development, plays crucial roles in regulating the development and function of the GABAergic system in the cortex and the striatum. The presence of BDNF single-nucleotide polymorphisms and disruptions in BDNF levels alter the excitatory/inhibitory balance in the brain. This imbalance has different effects on the pathogenesis of RTT as compared to other neurodevelopmental diseases like autism spectrum disorder and schizophrenia.[66]
RTT and Fragile X syndrome are 2 monogenetic neurodevelopmental disorders with complex clinical presentations. However, the latter is due to the silencing of the FMR1 gene, which encodes the Fragile X Mental Retardation Protein (FMRP), an RNA-binding protein that affects different steps of RNA metabolism and modulates the translation of numerous proteins, eg, a large set of synaptic proteins. Even if they have distinct genetic etiologies, overlapping features are present in RTT and Fragile X syndrome since both MeCP2 and FMRP affect brain activity.[67]
Additionally, patients with RTT may manifest with periodic episodes of gastrointestinal problems, hypoplasia, early-onset osteoporosis, bruxism, and screaming episodes, especially noted in males exhibiting a unique and variable phenotype. Recent case reports described the atypical presentation of RTT that remarkably mimics the clinical features of a genetic metabolic disorder called Bartter syndrome, which includes loss of previously acquired language and motor skills, repetitive hand movements, breathing irregularities, seizures, sporadic episodes of gastrointestinal distress, hypoplasia, early-onset osteoporosis, bruxism, and episodes of screaming. The main difference between Bartter and RTT is an unusual and atypical presentation of RTT in a young male child with a de novo c.806delG hemizygous mutation.[68]
Staging
Staging
Before the RTT criteria revisions and the discovery of MECP2, a staging system was implemented to guide clinicians to track the clinical course of RTT. Its progression has multiple stages, although most phenotypes are related to the nervous system, particularly the brain. Generally, approximately 95% of RTT cases are due to MECP2 gene mutations, an X-linked gene that encodes for MeCP2, a regulator of gene expression.[12] Notably, the following staging system does not predict life expectancy and should not be used for diagnostic purposes:
- Stage 1: This stage begins around 6 to 18 months and involves developmental arrest. Some signs include limited eye contact, deceleration of head growth, nonspecific hand-wringing, and gross motor delays.
- Stage 2: The onset occurs between the ages of 1 and 4, characterized by regression and rapid deterioration. Stage 2 is characterized by stereotypic hand movement (hand-wringing or washing), loss of speech, irritability, and disturbed sleep.
- Stage 3: The onset is between the ages of 2 and 10 and is characterized by improved behavior, communication skills, and hand use.
- Stage 4: The onset of this stage begins after the age of 10 years and is characterized by dystonia, reduced mobility, and bradykinesia.
Prognosis
The life expectancy of an individual with RTT can vary depending on the type of MECP2 mutation inherited.[27] Typically, individuals with RTT survive into middle age, and recent studies suggest they may survive longer.
Recent literature has compared the distribution of MECP2 variants and clinical severity between younger individuals with Classic RTT (those younger than 30 years old) and older individuals (those older than 30 years old). Unexpectedly, the overall severity of clinical features increased from the first to the last visit in the younger cohort, but not in the older cohort. Some specific clinical features in the older cohort remained unchanged from the first to the previous visit, while others showed improvement or worsening. These data demonstrated that clinical features change with increasing age among adults with RTT.[69]
Recent reviews mentioned that pneumonia is the most frequent cause of death for patients with RTT, with a 77.8% survival rate at the age of 25. Typically, patients with RTT survive until their fifth decade, with cardiorespiratory compromise as the leading cause of death.[12] Another study mentioned pneumonia as the leading cause of death due to gastrointestinal dysfunctions, including feeding abnormalities, swallowing dysfunction, and gastrointestinal issues leading to aspiration, which can also cause poor weight gain, oral motor dysfunction, and air swallowing among children with RTT. In this regard, a fiberoptic endoscopic evaluation of swallowing was performed among RTT patients with tongue dyskinesis and a prolonged oral stage. This revealed the following: liquid entering the airway without coughing, with the majority doing well with pureed meals. Age was not correlated with pneumonia episodes, but pureed material was related to pneumonia, whereas liquids were not. All aspiration/penetration was observed before the pharyngeal phase. No patients younger than 7 who experienced pneumonia episodes were identified. Moreover, silent aspiration can occur early in infancy, although pneumonia episodes can occur later.[70]
Another study characterized the long-term nutritional and gastrointestinal course of RTT and found that anthropometric parameters deteriorate with age, regardless of the specific genetic mutation underlying the condition. The study identified common symptoms in patients with RTT, eg, chewing and feeding difficulties, constipation, and gastroesophageal reflux.[71] Furthermore, RTT has a significant negative impact on family functioning. The quality of life in children, as perceived by their parents, is also affected. Counseling families and planning clinical and support management strategies should be considered.[72][73]
Complications
RTT is associated with multiple complications affecting various organ systems, requiring lifelong interprofessional care. Neurological issues, eg, seizures, cognitive impairment, and motor dysfunction, significantly impact daily living. Breathing irregularities, eg, breath-holding, hyperventilation, and swallowing air, can contribute to discomfort and further complications. Gastrointestinal problems, eg, feeding difficulties, growth restriction, and difficulty chewing, may lead to nutritional deficiencies. Orthopedic issues, eg, scoliosis and abnormal gait patterns, can cause mobility challenges. Sleep disturbances, teeth grinding (bruxism), and autonomic dysfunction further add to the disease burden. Due to its multisystem involvement, RTT management focuses on symptomatic and supportive care. (Please refer to the Treatment section for more information on the complications of RTT).
Deterrence and Patient Education
Deterrence and patient education in RTT focus on early recognition, genetic counseling, and comprehensive management strategies. Since RTT is a genetic disorder, families benefit from genetic testing and counseling to understand inheritance patterns and recurrence risks. Educating caregivers about disease progression, potential complications, and available therapies helps improve quality of life. Interprofessional care is essential, with specialists guiding symptom management, including physical therapy for mobility, speech therapy for communication, and dietary support for feeding difficulties. Emerging treatments, eg, trofinetide and gene therapy research, offer hope, emphasizing the importance of staying informed about ongoing clinical trials and advancements.
Enhancing Healthcare Team Outcomes
The management of RTT requires a coordinated interprofessional approach to address its complex and multisystemic nature. Physicians, including pediatric neurologists and developmental pediatricians, play a crucial role in diagnosing RTT, monitoring disease progression, and managing comorbidities such as seizures, breathing irregularities, and cardiac dysfunction. Advanced practitioners, eg, nurse practitioners and physician assistants, assist in routine monitoring, medication management, and caregiver education to improve symptom control and enhance quality of life. Nurses are vital in providing direct patient care, supporting feeding and mobility, and educating families on symptom management and daily care strategies. Pharmacists contribute by ensuring the safe use of medications, including antiseizure drugs and newly approved treatments like trofinetide, counseling caregivers on medication adherence, potential adverse effects, and drug interactions.
Effective interprofessional communication and care coordination are essential in RTT management to optimize patient-centered care, safety, and team performance. Physical and occupational therapists help improve motor function and prevent complications, eg, scoliosis and contractures, while speech therapists address communication challenges using augmentative and AAC devices. Dietitians support nutritional needs, especially for patients with feeding difficulties and gastrointestinal dysfunction. Psychologists and social workers assist families in coping with behavioral challenges, sleep disturbances, and emotional strain, recognizing the significant burden RTT places on caregivers. Since sleep disturbances are common in RTT and negatively impact patients and caregivers, a collaborative approach to sleep management can significantly improve overall well-being. Regular team meetings, shared electronic health records, and family-centered care models ensure that all clinicians are aligned in treatment goals, ultimately improving patient outcomes and enhancing the quality of life for individuals with RTT and their families.
Media
(Click Image to Enlarge)
References
Operto FF, Mazza R, Pastorino GMG, Verrotti A, Coppola G. Epilepsy and genetic in Rett syndrome: A review. Brain and behavior. 2019 May:9(5):e01250. doi: 10.1002/brb3.1250. Epub 2019 Mar 30 [PubMed PMID: 30929312]
Martínez-Rodríguez E, Martín-Sánchez A, Coviello S, Foiani C, Kul E, Stork O, Martínez-García F, Nacher J, Lanuza E, Santos M, Agustín-Pavón C. Lack of MeCP2 leads to region-specific increase of doublecortin in the olfactory system. Brain structure & function. 2019 May:224(4):1647-1658. doi: 10.1007/s00429-019-01860-6. Epub 2019 Mar 28 [PubMed PMID: 30923887]
Ehrhart F, Coort SL, Eijssen L, Cirillo E, Smeets EE, Bahram Sangani N, Evelo CT, Curfs LMG. Integrated analysis of human transcriptome data for Rett syndrome finds a network of involved genes. The world journal of biological psychiatry : the official journal of the World Federation of Societies of Biological Psychiatry. 2020 Dec:21(10):712-725. doi: 10.1080/15622975.2019.1593501. Epub 2019 Apr 29 [PubMed PMID: 30907210]
Writing Group For Practice Guidelines For Diagnosis And Treatment Of Genetic Diseases Medical Genetics Branch Of Chinese Medical Association, Guan R, Li Q, Fu S. [Clinical practice guidelines for Rett syndrome]. Zhonghua yi xue yi chuan xue za zhi = Zhonghua yixue yichuanxue zazhi = Chinese journal of medical genetics. 2020 Mar 10:37(3):308-312. doi: 10.3760/cma.j.issn.1003-9406.2020.03.014. Epub [PubMed PMID: 32128749]
Level 1 (high-level) evidenceGold WA, Percy AK, Neul JL, Cobb SR, Pozzo-Miller L, Issar JK, Ben-Zeev B, Vignoli A, Kaufmann WE. Rett syndrome. Nature reviews. Disease primers. 2024 Nov 7:10(1):84. doi: 10.1038/s41572-024-00568-0. Epub 2024 Nov 7 [PubMed PMID: 39511247]
Fang X, Baggett LM, Caylor RC, Percy AK, Neul JL, Lane JB, Glaze DG, Benke TA, Marsh ED, Motil KJ, Barrish JO, Annese FE, Skinner SA. Parental age effects and Rett syndrome. American journal of medical genetics. Part A. 2024 Feb:194(2):160-173. doi: 10.1002/ajmg.a.63396. Epub 2023 Sep 28 [PubMed PMID: 37768187]
Einspieler C, Marschik PB. Regression in Rett syndrome: Developmental pathways to its onset. Neuroscience and biobehavioral reviews. 2019 Mar:98():320-332. doi: 10.1016/j.neubiorev.2019.01.028. Epub [PubMed PMID: 30832924]
Banerjee A, Miller MT, Li K, Sur M, Kaufmann WE. Towards a better diagnosis and treatment of Rett syndrome: a model synaptic disorder. Brain : a journal of neurology. 2019 Feb 1:142(2):239-248. doi: 10.1093/brain/awy323. Epub [PubMed PMID: 30649225]
Vashi N, Justice MJ. Treating Rett syndrome: from mouse models to human therapies. Mammalian genome : official journal of the International Mammalian Genome Society. 2019 Jun:30(5-6):90-110. doi: 10.1007/s00335-019-09793-5. Epub 2019 Feb 28 [PubMed PMID: 30820643]
Gomathi M, Dhivya V, Padmavathi V, Pradeepkumar M, Robert Wilson S, Kumar NS, Balachandar V. Genetic Instability and Disease Progression of Indian Rett Syndrome Patients. Molecular neurobiology. 2024 Jul:61(7):4868-4878. doi: 10.1007/s12035-023-03882-y. Epub 2023 Dec 26 [PubMed PMID: 38147229]
Zhang X, Smits M, Curfs L, Spruyt K. Sleep and the Social Profiles of Individuals With Rett Syndrome. Pediatric neurology. 2024 Mar:152():153-161. doi: 10.1016/j.pediatrneurol.2024.01.004. Epub 2024 Jan 8 [PubMed PMID: 38290182]
Lopes AG, Loganathan SK, Caliaperumal J. Rett Syndrome and the Role of MECP2: Signaling to Clinical Trials. Brain sciences. 2024 Jan 24:14(2):. doi: 10.3390/brainsci14020120. Epub 2024 Jan 24 [PubMed PMID: 38391695]
Pepe G, Coco R, Corica D, Luppino G, Morabito LA, Lugarà C, Abbate T, Zirilli G, Aversa T, Stagi S, Wasniewska M. Endocrine disorders in Rett syndrome: a systematic review of the literature. Frontiers in endocrinology. 2024:15():1477227. doi: 10.3389/fendo.2024.1477227. Epub 2024 Oct 31 [PubMed PMID: 39544232]
Level 1 (high-level) evidencePepe G, Coco R, Corica D, Di Rosa G, Bossowski F, Skorupska M, Aversa T, Stagi S, Wasniewska M. Prevalence of Endocrinopathies in a Cohort of Patients with Rett Syndrome: A Two-Center Observational Study. Genes. 2024 Feb 24:15(3):. doi: 10.3390/genes15030287. Epub 2024 Feb 24 [PubMed PMID: 38540345]
Level 2 (mid-level) evidenceGold WA, Krishnarajy R, Ellaway C, Christodoulou J. Rett Syndrome: A Genetic Update and Clinical Review Focusing on Comorbidities. ACS chemical neuroscience. 2018 Feb 21:9(2):167-176. doi: 10.1021/acschemneuro.7b00346. Epub 2017 Dec 15 [PubMed PMID: 29185709]
May D, Kponee-Shovein K, Neul JL, Percy AK, Mahendran M, Downes N, Chen G, Watson T, Pichard DC, Kennedy M, Lefebvre P. Characterizing the journey of Rett syndrome among females in the United States: a real-world evidence study using the Rett syndrome natural history study database. Journal of neurodevelopmental disorders. 2024 Jul 26:16(1):42. doi: 10.1186/s11689-024-09557-6. Epub 2024 Jul 26 [PubMed PMID: 39061009]
Zade K, Campbell C, Bach S, Fernandes H, Tropea D. Rett syndrome in Ireland: a demographic study. Orphanet journal of rare diseases. 2024 Jan 31:19(1):34. doi: 10.1186/s13023-024-03046-8. Epub 2024 Jan 31 [PubMed PMID: 38291497]
Osaki T, Wan Z, Haratani K, Jin Y, Campisi M, Barbie DA, Kamm R, Sur M. miR126-mediated impaired vascular integrity in Rett syndrome. bioRxiv : the preprint server for biology. 2024 Oct 13:():. pii: 2024.10.11.617929. doi: 10.1101/2024.10.11.617929. Epub 2024 Oct 13 [PubMed PMID: 39415995]
Liu Y, Flamier A, Bell GW, Diao AJ, Whitfield TW, Wang HC, Wu Y, Schulte F, Friesen M, Guo R, Mitalipova M, Liu XS, Vos SM, Young RA, Jaenisch R. MECP2 directly interacts with RNA polymerase II to modulate transcription in human neurons. Neuron. 2024 Jun 19:112(12):1943-1958.e10. doi: 10.1016/j.neuron.2024.04.007. Epub 2024 May 1 [PubMed PMID: 38697112]
Varone M, Scavo G, Colardo M, Martella N, Pensabene D, Bisesto E, Del Busso A, Segatto M. p75NTR Modulation Reduces Oxidative Stress and the Expression of Pro-Inflammatory Mediators in a Cell Model of Rett Syndrome. Biomedicines. 2024 Nov 16:12(11):. doi: 10.3390/biomedicines12112624. Epub 2024 Nov 16 [PubMed PMID: 39595188]
Tomasello DL, Barrasa MI, Mankus D, Alarcon KI, Lytton-Jean AKR, Liu XS, Jaenisch R. Mitochondrial dysfunction and increased reactive oxygen species production in MECP2 mutant astrocytes and their impact on neurons. Scientific reports. 2024 Sep 4:14(1):20565. doi: 10.1038/s41598-024-71040-y. Epub 2024 Sep 4 [PubMed PMID: 39232000]
Neklyudova A, Kuramagomedova R, Voinova V, Sysoeva O. Atypical brain responses to 40-Hz click trains in girls with Rett syndrome: Auditory steady-state response and sustained wave. Psychiatry and clinical neurosciences. 2024 May:78(5):282-290. doi: 10.1111/pcn.13638. Epub 2024 Feb 6 [PubMed PMID: 38321640]
Brima T, Beker S, Prinsloo KD, Butler JS, Djukic A, Freedman EG, Molholm S, Foxe JJ. Probing a neural unreliability account of auditory sensory processing atypicalities in Rett Syndrome. medRxiv : the preprint server for health sciences. 2024 Jan 26:():. pii: 2024.01.25.24301723. doi: 10.1101/2024.01.25.24301723. Epub 2024 Jan 26 [PubMed PMID: 38343802]
Jan TY, Wong LC, Hsu CJ, Huang CJ, Peng SS, Tseng WI, Lee WT. Developmental change of brain volume in Rett syndrome in Taiwan. Journal of neurodevelopmental disorders. 2024 Jul 3:16(1):36. doi: 10.1186/s11689-024-09549-6. Epub 2024 Jul 3 [PubMed PMID: 38961335]
Vilvarajan S, McDonald M, Douglas L, Newham J, Kirkland R, Tzannes G, Tay D, Christodoulou J, Thompson S, Ellaway C. Multidisciplinary Management of Rett Syndrome: Twenty Years' Experience. Genes. 2023 Aug 11:14(8):. doi: 10.3390/genes14081607. Epub 2023 Aug 11 [PubMed PMID: 37628658]
Cabal-Herrera AM, Beatty CW. [Rett syndrome: from pathophysiology to developments in treatment]. Medicina. 2024 Sep:84 Suppl 3():45-49 [PubMed PMID: 39331775]
Abbott M, Angione K, Forbes E, Stoecker M, Saenz M, Neul JL, Marsh ED, Skinner SA, Percy AK, Benke TA. Rett syndrome diagnostic odyssey: Limitations of NextGen sequencing. American journal of medical genetics. Part A. 2024 Oct:194(10):e63725. doi: 10.1002/ajmg.a.63725. Epub 2024 May 22 [PubMed PMID: 38775384]
Neul JL, Benke TA, Marsh ED, Skinner SA, Merritt J, Lieberman DN, Standridge S, Feyma T, Heydemann P, Peters S, Ryther R, Jones M, Suter B, Kaufmann WE, Glaze DG, Percy AK. The array of clinical phenotypes of males with mutations in Methyl-CpG binding protein 2. American journal of medical genetics. Part B, Neuropsychiatric genetics : the official publication of the International Society of Psychiatric Genetics. 2019 Jan:180(1):55-67. doi: 10.1002/ajmg.b.32707. Epub 2018 Dec 7 [PubMed PMID: 30536762]
Zengin-Akkuş P, Taşkıran EZ, Kabaçam S, Şimşek-Kiper PÖ, Haliloğlu G, Boduroğlu K, Utine GE. Clinical and molecular evaluation of 16 patients with Rett syndrome. The Turkish journal of pediatrics. 2018:60(1):1-9. doi: 10.24953/turkjped.2018.01.001. Epub [PubMed PMID: 30102473]
Schönewolf-Greulich B, Bisgaard AM, Møller RS, Dunø M, Brøndum-Nielsen K, Kaur S, Van Bergen NJ, Lunke S, Eggers S, Jespersgaard C, Christodoulou J, Tümer Z. Clinician's guide to genes associated with Rett-like phenotypes-Investigation of a Danish cohort and review of the literature. Clinical genetics. 2019 Feb:95(2):221-230. doi: 10.1111/cge.13153. Epub 2018 Jan 25 [PubMed PMID: 29023665]
Galán-Olleros M, González-Alguacil E, Soto-Insuga V, Vara-Arias MT, Ortiz-Cabrera NV, Egea-Gámez RM, García-Peñas JJ, Martínez-Caballero I, RTT‐HNJ, Rett Syndrome Multidisciplinary Group of Hospital Infantil Universitario Niño Jesús. Prevalence of orthopaedic conditions in Rett syndrome: a systematic review and meta-analysis. Journal of intellectual disability research : JIDR. 2024 Dec:68(12):1331-1343. doi: 10.1111/jir.13193. Epub 2024 Oct 21 [PubMed PMID: 39429113]
Level 1 (high-level) evidenceWeeda JE, van Kuijk SMJ, van den Berg MP, Bastiaenen CHG, Borst HE, van Rhijn LW, de Bie RA. Identification of Predictors for Progression of Scoliosis in Rett Syndrome. Developmental neurorehabilitation. 2024 Apr-May:27(3-4):126-133. doi: 10.1080/17518423.2024.2365794. Epub 2024 Jun 22 [PubMed PMID: 38907992]
Galán-Olleros M, González-Alguacil E, Soto-Insuga V, Vara-Arias MT, Ortiz-Cabrera NV, Serrano JI, Egea-Gámez RM, García-Peñas JJ, Martínez-Caballero I, RTT-HNJ, Rett Syndrome Multidisciplinary Group of Hospital Infantil Universitario Niño Jesús. Orthopedic Conditions and Interplay with Functional Abilities and MECP2 Variant Subtype in Rett Syndrome Patients. Journal of autism and developmental disorders. 2025 Aug:55(8):2873-2883. doi: 10.1007/s10803-024-06399-y. Epub 2024 May 25 [PubMed PMID: 38795288]
Brunetti S, Lumsden DE. Rett Syndrome as a movement and motor disorder - A narrative review. European journal of paediatric neurology : EJPN : official journal of the European Paediatric Neurology Society. 2020 Sep:28():29-37. doi: 10.1016/j.ejpn.2020.06.020. Epub 2020 Jul 28 [PubMed PMID: 32807681]
Level 3 (low-level) evidenceLeoncini S, Boasiako L, Di Lucia S, Beker A, Scandurra V, Vignoli A, Canevini MP, Prato G, Nobili L, Nicotera AG, Di Rosa G, Chiarini MBT, Cutrera R, Grosso S, Lazzeri G, Tongiorgi E, Morano P, Botteghi M, Barducci A, De Felice C. 24-h continuous non-invasive multiparameter home monitoring of vitals in patients with Rett syndrome by an innovative wearable technology: evidence of an overlooked chronic fatigue status. Frontiers in neurology. 2024:15():1388506. doi: 10.3389/fneur.2024.1388506. Epub 2024 Jun 17 [PubMed PMID: 38952469]
Lotan M, Downs J, Stahlhut M, Romano A. Evaluation Tools Developed for Rett Syndrome. Diagnostics (Basel, Switzerland). 2023 May 11:13(10):. doi: 10.3390/diagnostics13101708. Epub 2023 May 11 [PubMed PMID: 37238191]
Motil KJ, Beisang A, Smith-Hicks C, Lembo A, Standridge SM, Liu E. Recommendations for the management of gastrointestinal comorbidities with or without trofinetide use in Rett syndrome. Expert review of gastroenterology & hepatology. 2024 Jun:18(6):227-237. doi: 10.1080/17474124.2024.2368014. Epub 2024 Jun 18 [PubMed PMID: 38869952]
Abo Zeid M, Elrosasy A, Mohamed RG, Ghazou A, Goufa E, Hassan N, Abuzaid Y. A meta-analysis of the efficacy and safety of trofinetide in patients with rett syndrome. Neurological sciences : official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology. 2024 Oct:45(10):4767-4778. doi: 10.1007/s10072-024-07584-8. Epub 2024 May 21 [PubMed PMID: 38771525]
Level 1 (high-level) evidencePercy AK, Neul JL, Benke TA, Berry-Kravis EM, Glaze DG, Marsh ED, An D, Bishop KM, Youakim JM. Trofinetide for the treatment of Rett syndrome: Results from the open-label extension LILAC study. Med (New York, N.Y.). 2024 Sep 13:5(9):1178-1189.e3. doi: 10.1016/j.medj.2024.05.018. Epub 2024 Jun 24 [PubMed PMID: 38917793]
Kennedy M, Glass L, Glaze DG, Kaminsky S, Percy AK, Neul JL, Jones NE, Tropea D, Horrigan JP, Nues P, Bishop KM, Youakim JM. Development of trofinetide for the treatment of Rett syndrome: from bench to bedside. Frontiers in pharmacology. 2023:14():1341746. doi: 10.3389/fphar.2023.1341746. Epub 2024 Jan 22 [PubMed PMID: 38318312]
Neul JL, Percy AK, Benke TA, Berry-Kravis EM, Glaze DG, Peters SU, Marsh ED, An D, Bishop KM, Youakim JM. Trofinetide Treatment Demonstrates a Benefit Over Placebo for the Ability to Communicate in Rett Syndrome. Pediatric neurology. 2024 Mar:152():63-72. doi: 10.1016/j.pediatrneurol.2023.11.005. Epub 2023 Nov 23 [PubMed PMID: 38232652]
Neul JL, Percy AK, Benke TA, Berry-Kravis EM, Glaze DG, Marsh ED, Lin T, Stankovic S, Bishop KM, Youakim JM. Trofinetide for the treatment of Rett syndrome: a randomized phase 3 study. Nature medicine. 2023 Jun:29(6):1468-1475. doi: 10.1038/s41591-023-02398-1. Epub 2023 Jun 8 [PubMed PMID: 37291210]
Level 1 (high-level) evidenceAbbas A, Fayoud AM, El Din Moawad MH, Hamad AA, Hamouda H, Fouad EA. Safety and efficacy of trofinetide in Rett syndrome: a systematic review and meta-analysis of randomized controlled trials. BMC pediatrics. 2024 Mar 23:24(1):206. doi: 10.1186/s12887-024-04526-3. Epub 2024 Mar 23 [PubMed PMID: 38521908]
Level 1 (high-level) evidenceTropea D. Trofinetide treatment for Rett syndrome: Lessons to learn. Med (New York, N.Y.). 2024 Oct 11:5(10):1194-1196. doi: 10.1016/j.medj.2024.07.028. Epub [PubMed PMID: 39395401]
Camillo L, Pozzi M, Bernardo P, Pisano S, Nobile M. Profile of Trofinetide in the Treatment of Rett Syndrome: Design, Development and Potential Place in Therapy. Drug design, development and therapy. 2024:18():5023-5040. doi: 10.2147/DDDT.S383133. Epub 2024 Nov 6 [PubMed PMID: 39525048]
Wong LC, Hsu CJ, Wu YT, Chu HF, Lin JH, Wang HP, Hu SC, Tsai YC, Tsai WC, Lee WT. Investigating the impact of probiotic on neurological outcomes in Rett syndrome: A randomized, double-blind, and placebo-controlled pilot study. Autism : the international journal of research and practice. 2024 Sep:28(9):2267-2281. doi: 10.1177/13623613231225899. Epub 2024 Feb 15 [PubMed PMID: 38361371]
Level 1 (high-level) evidenceYıldız N, Serdaroğlu E, Kart PÖ, Besen S, Kanmaz S, Toprak DE, Kilic B, Ersoy O, Gencpinar P, Dundar NO, Okuyaz C, Serdaroglu A, Carman KB, Yarar C, Ekici B, Tatlı B, Erol İ, Aydın K, Tekgül H, Cansu A. Evaluation of seizure semiology, genetics, magnetic resonance imaging, and electroencephalogram findings in children with Rett syndrome: A multicenter retrospective study. Epilepsy research. 2024 Sep:205():107399. doi: 10.1016/j.eplepsyres.2024.107399. Epub 2024 Jul 6 [PubMed PMID: 39003968]
Level 2 (mid-level) evidenceDesnous B, Beretti T, Muller N, Neveu J, Villeneuve N, Lépine A, Daquin G, Milh M. Efficacy and tolerance of cannabidiol in the treatment of epilepsy in patients with Rett syndrome. Epilepsia open. 2024 Feb:9(1):397-403. doi: 10.1002/epi4.12796. Epub 2023 Nov 15 [PubMed PMID: 37485779]
Balicza P, Gezsi A, Fedor M, Sagi JC, Gal A, Varga NA, Molnar MJ. Multilevel evidence of MECP2-associated mitochondrial dysfunction and its therapeutic implications. Frontiers in psychiatry. 2023:14():1301272. doi: 10.3389/fpsyt.2023.1301272. Epub 2024 Jan 5 [PubMed PMID: 38250256]
Rodrigues GD, Cordani R, Veneruso M, Chiarella L, Prato G, Ferri R, Carandina A, Tobaldini E, Nobili L, Montano N. Predominant cardiac sympathetic modulation during wake and sleep in patients with Rett syndrome. Sleep medicine. 2024 Jul:119():188-191. doi: 10.1016/j.sleep.2024.04.036. Epub 2024 Apr 28 [PubMed PMID: 38692221]
Cherchi C, Chiappini E, Amaddeo A, Chiarini Testa MB, Banfi P, Veneselli E, Cutrera R, panel for the Problems in Patients with Rett Syndrome. Management of respiratory issues in patients with Rett syndrome: Italian experts' consensus using a Delphi approach. Pediatric pulmonology. 2024 Jul:59(7):1970-1978. doi: 10.1002/ppul.27030. Epub 2024 May 9 [PubMed PMID: 38721909]
Level 3 (low-level) evidencePeri F, Cherchi C, Chiarini Testa MB, Pavone M, Verrillo E, Cutrera R. The Efficacy of Noninvasive Ventilation in Patients Affected by Rett Syndrome With Hypoventilation. Pediatric neurology. 2024 Sep:158():81-85. doi: 10.1016/j.pediatrneurol.2024.05.005. Epub 2024 May 9 [PubMed PMID: 39002354]
Stone LE, Kelly MP, Alexander M, Brandel M, Lam SK, Ravindra VM. Rett Syndrome-Associated Scoliosis: Analysis of National Trends and Treatment Patterns of a Rare Indication for Posterior Instrumented Fusion. Spine. 2023 Dec 15:48(24):E409-E416. doi: 10.1097/BRS.0000000000004802. Epub 2023 Aug 29 [PubMed PMID: 37642479]
Su TY, Huang YC, Ko JY, Hsin YJ, Yu MY, Hung PL. Therapeutic effects of extracorporeal shock wave therapy on patients with spastic cerebral palsy and Rett syndrome: clinical and ultrasonographic findings. Orphanet journal of rare diseases. 2024 Jan 3:19(1):6. doi: 10.1186/s13023-023-03010-y. Epub 2024 Jan 3 [PubMed PMID: 38172891]
Tost A, Bachiller A, Medina-Rivera I, Romero S, Serna LY, Rojas-Martínez M, García-Cazorla Á, Mañanas MÁ. Repetitive active and passive cognitive stimulations induce EEG changes in patients with Rett syndrome. Pediatric research. 2025 Feb:97(2):751-762. doi: 10.1038/s41390-024-03254-9. Epub 2024 Jul 16 [PubMed PMID: 39014240]
Tost A, Romero S, Alonso JF, Bachiller A, Serna LY, Medina-Rivera I, García-Cazorla Á, Mañanas MÁ. EEG connectivity patterns in response to gaming and learning-based cognitive stimulations in Rett syndrome. Research in developmental disabilities. 2024 Jul:150():104751. doi: 10.1016/j.ridd.2024.104751. Epub 2024 May 24 [PubMed PMID: 38795554]
Panzeri D, Perina M, Biffi E, Semino M, Diella E, Caprì T. Effects of Immersive Virtual Reality with Treadmill in Subjects with Rett Syndrome: A Pilot Study. Children (Basel, Switzerland). 2024 Sep 11:11(9):. doi: 10.3390/children11091110. Epub 2024 Sep 11 [PubMed PMID: 39334642]
Level 3 (low-level) evidenceGirtler SN, Unholz-Bowden EK, Shipchandler A, Kolb RL, McComas JJ. Use of Augmentative and Alternative Communication by Individuals with Rett Syndrome Part 1: Page-Linking. Journal of developmental and physical disabilities. 2024 Feb:36(1):125-145. doi: 10.1007/s10882-023-09903-x. Epub 2023 Apr 10 [PubMed PMID: 38449899]
Caprì T, Dovigo L, Semino M, Lotan M, Mohammadhasani N, Zamarra G, Fabio RA. Use of a low-tech tool in the improvement of social interaction of patients with Rett Syndrome: an observational study. Frontiers in public health. 2024:12():1353099. doi: 10.3389/fpubh.2024.1353099. Epub 2024 Apr 4 [PubMed PMID: 38645452]
Level 2 (mid-level) evidenceSadeghi S, Shevell M. Consideration of Genetic Diagnoses of Developmental Delay in Children of Consanguineous Families. Seminars in pediatric neurology. 2018 Jul:26():60-62. doi: 10.1016/j.spen.2017.03.007. Epub 2017 Apr 1 [PubMed PMID: 29961522]
Jefferson A, Leonard H, Siafarikas A, Woodhead H, Fyfe S, Ward LM, Munns C, Motil K, Tarquinio D, Shapiro JR, Brismar T, Ben-Zeev B, Bisgaard AM, Coppola G, Ellaway C, Freilinger M, Geerts S, Humphreys P, Jones M, Lane J, Larsson G, Lotan M, Percy A, Pineda M, Skinner S, Syhler B, Thompson S, Weiss B, Witt Engerström I, Downs J. Clinical Guidelines for Management of Bone Health in Rett Syndrome Based on Expert Consensus and Available Evidence. PloS one. 2016:11(2):e0146824. doi: 10.1371/journal.pone.0146824. Epub 2016 Feb 5 [PubMed PMID: 26849438]
Level 3 (low-level) evidenceCho HY, Yoo M, Pongkulapa T, Rabie H, Muotri AR, Yin PT, Choi JW, Lee KB. Magnetic Nanoparticle-Assisted Non-Viral CRISPR-Cas9 for Enhanced Genome Editing to Treat Rett Syndrome. Advanced science (Weinheim, Baden-Wurttemberg, Germany). 2024 Jun:11(24):e2306432. doi: 10.1002/advs.202306432. Epub 2024 Apr 22 [PubMed PMID: 38647391]
Gan Y, Chen Y, Zhong H, Liu Z, Geng J, Wang H, Wang W. Gut microbes in central nervous system development and related disorders. Frontiers in immunology. 2023:14():1288256. doi: 10.3389/fimmu.2023.1288256. Epub 2024 Jan 26 [PubMed PMID: 38343438]
Nakhal MM, Yassin LK, Alyaqoubi R, Saeed S, Alderei A, Alhammadi A, Alshehhi M, Almehairbi A, Al Houqani S, BaniYas S, Qanadilo H, Ali BR, Shehab S, Statsenko Y, Meribout S, Sadek B, Akour A, Hamad MIK. The Microbiota-Gut-Brain Axis and Neurological Disorders: A Comprehensive Review. Life (Basel, Switzerland). 2024 Sep 26:14(10):. doi: 10.3390/life14101234. Epub 2024 Sep 26 [PubMed PMID: 39459534]
Borrego-Ruiz A, Borrego JJ. Neurodevelopmental Disorders Associated with Gut Microbiome Dysbiosis in Children. Children (Basel, Switzerland). 2024 Jun 28:11(7):. doi: 10.3390/children11070796. Epub 2024 Jun 28 [PubMed PMID: 39062245]
Hernández-Del Caño C, Varela-Andrés N, Cebrián-León A, Deogracias R. Neurotrophins and Their Receptors: BDNF's Role in GABAergic Neurodevelopment and Disease. International journal of molecular sciences. 2024 Jul 30:25(15):. doi: 10.3390/ijms25158312. Epub 2024 Jul 30 [PubMed PMID: 39125882]
Bach S, Shovlin S, Moriarty M, Bardoni B, Tropea D. Rett Syndrome and Fragile X Syndrome: Different Etiology With Common Molecular Dysfunctions. Frontiers in cellular neuroscience. 2021:15():764761. doi: 10.3389/fncel.2021.764761. Epub 2021 Nov 19 [PubMed PMID: 34867203]
Wang W, Li H, Xiao M, Mu M, Xu H, Wang B. Clinical Research on Rett Syndrome: Central Hypoxemia and Hypokalemic Metabolic Alkalosis. Alternative therapies in health and medicine. 2024 Jan:30(1):167-171 [PubMed PMID: 37773669]
Neul JL, Benke TA, Marsh ED, Suter B, Fu C, Ryther RC, Skinner SA, Lieberman DN, Feyma T, Beisang A, Heydemann P, Peters SU, Ananth A, Percy AK. Clinical Features and Disease Progression in Older Individuals with Rett Syndrome. Genes. 2024 Aug 22:15(8):. doi: 10.3390/genes15081107. Epub 2024 Aug 22 [PubMed PMID: 39202466]
Sideris G, Panagoulis E, Grigoropoulos C, Mermiri D, Nikolopoulos T, Delides A. Fiberoptic Endoscopic Evaluation of Swallowing Findings in Children With Rett Syndrome. Clinical pediatrics. 2024 May:63(4):551-556. doi: 10.1177/00099228231184673. Epub 2023 Jul 10 [PubMed PMID: 37424375]
Berger TD, Fogel Berger C, Gara S, Ben-Zeev B, Weiss B. Nutritional and gastrointestinal manifestations in Rett syndrome: long-term follow-up. European journal of pediatrics. 2024 Sep:183(9):4085-4091. doi: 10.1007/s00431-024-05668-3. Epub 2024 Jul 3 [PubMed PMID: 38960904]
Rozensztrauch A, Sebzda A, Śmigiel R. Clinical presentation of Rett syndrome in relation to quality of life and family functioning. The Journal of international medical research. 2021 Apr:49(4):3000605211007714. doi: 10.1177/03000605211007714. Epub [PubMed PMID: 33906527]
Level 2 (mid-level) evidenceMendoza J, Downs J, Wong K, Leonard H. Determinants of quality of life in Rett syndrome: new findings on associations with genotype. Journal of medical genetics. 2021 Sep:58(9):637-644. doi: 10.1136/jmedgenet-2020-107120. Epub 2020 Aug 25 [PubMed PMID: 32843489]
Level 2 (mid-level) evidence