Introduction
Retinopathy of prematurity (ROP) is a disease of retinal vascular and capillary proliferation affecting premature infants undergoing oxygen therapy.[1] Oxygen treatment can lead to the pathologic growth of vessels in the developing retina, potentially resulting in permanent damage to the retina, as well as retinal detachment and macular folds.[2][3][4] Screening guidelines for this leading cause of childhood blindness are based on gestational age and birth weight, although numerous factors contribute to both the incidence and severity of disease development.[5] Early treatment of disease with cryotherapy, laser photocoagulation, and anti-vascular endothelial growth factor therapy has improved visual outcomes for patients. However, early recognition through screening is critical.[5] Prevention of ROP requires a multidisciplinary approach that begins before the infant is born and continues throughout the child's development.
ROP is a vasoproliferative disorder of the developing retina in preterm infants and remains a leading cause of preventable childhood blindness globally. As neonatal survival continues to improve due to advancements in perinatal and neonatal care, particularly in low- and middle-income countries, the incidence and recognition of ROP have risen significantly. ROP represents a unique interplay of systemic immaturity, disordered retinal vascular development, and exposure to environmental risk factors, primarily oxygen therapy. This condition highlights the importance of timely screening, diagnosis, and intervention to prevent lifelong visual impairment.[6]
ROP develops due to incomplete retinal vascularization at the time of premature birth. Normally, the retina becomes fully vascularized between 36 and 40 weeks of gestation. In preterm infants, however, vascular development is interrupted, and the subsequent abnormal neovascularization can result in fibrous proliferation, retinal traction, and ultimately retinal detachment if left untreated. The pathogenesis of ROP is classically divided into 2 phases. Phase 1 involves hyperoxia-induced vaso-obliteration; phase 2 is characterized by hypoxia-driven pathological neovascularization due to increased expression of vascular endothelial growth factor.[7]
Multiple risk factors contribute to the development of ROP. The most significant include low gestational age, low birth weight, and the need for supplemental oxygen. Additional contributors include prolonged mechanical ventilation, sepsis, intraventricular hemorrhage, blood transfusions, and poor postnatal weight gain. The advent of the STOP-ROP (Supplemental Therapeutic Oxygen for Prethreshold–Retinopathy of Prematurity), WINROP (Weight, Insulin-like Growth Factor, Neonatal Retinopathy of Prematurity), and e-ROP (Telemedicine Approaches to Evaluating Acute-Phase Retinopathy of Prematurity) models has clarified how systemic risk factors and early biomarkers can predict disease progression, aiding in individualized screening strategies.[8]
Clinically, ROP is classified based on the International Classification of Retinopathy of Prematurity (ICROP), which considers the zone of retinal involvement, the stage of disease (ranging from stage 1 to 5tage 5), and the presence or absence of "plus disease"—a marker of severe vascular activity. Notably, Type 1 ROP, defined as threshold or pre-threshold disease with plus disease, mandates prompt treatment to prevent progression. The 2021 ICROP3 revision further refined disease classification by incorporating posterior zone location and aggressive posterior retinopathy of prematurity (AP-ROP), thereby improving diagnostic consistency and treatment planning.[9]
ROP screening and timely intervention are crucial to avoid permanent vision loss. Screening guidelines vary slightly across countries but are generally based on birth weight (<1500 g) and gestational age (younger than 32 weeks). Dilated retinal examination using indirect ophthalmoscopy remains the gold standard; however, advances in teleophthalmology and wide-field digital imaging are increasingly being used, particularly in resource-limited settings. Programs such as KIDROP (Karnataka Internet-Assisted Diagnosis of Retinopathy of Prematurity) in India have revolutionized access to timely screening in rural regions through a hub-and-spoke model, featuring real-time image transmission and remote expert grading.[10]
Treatment modalities for ROP have undergone significant evolution over the past 2 decades. Traditionally, laser photocoagulation of the avascular retina has been the standard of care for threshold ROP. However, intravitreal anti–vascular endothelial growth factor (anti–VEGF) agents, particularly bevacizumab and ranibizumab, have gained widespread use, especially for posterior zone I disease and AP-ROP. The Bevacizumab Eliminates the Angiogenic Threat of Retinopathy of Prematurity (BEAT-ROP) and the Ranibizumab Compared With Laser Therapy for the Treatment of Infants Born Prematurely With Retinopathy of Prematurity (RAINBOW) trials demonstrated the efficacy of anti–VEGF therapy in regressing neovascularization and reducing myopia compared with laser treatment. Nevertheless, concerns remain about systemic VEGF suppression, long-term neurodevelopmental safety, and disease recurrence, necessitating prolonged follow-up.[11]
Surgical management is reserved for advanced stages (eg, 4 and 5) where tractional retinal detachment occurs. Vitreoretinal procedures, including lens-sparing vitrectomy or lensectomy with vitrectomy, have demonstrated variable success rates, depending on the extent of detachment and the timing of intervention. However, visual prognosis in these advanced cases remains guarded, emphasizing the importance of early identification and treatment.[12]
From a public health perspective, the "third epidemic" of ROP in developing countries is marked by high survival of preterm infants without adequate ROP screening infrastructure. National programs, such as the Rashtriya Bal Swasthya Karyakram (RBSK) in India, have prioritized ROP and are working to integrate neonatal care and ophthalmic services. The interprofessional collaboration among neonatologists, nurses, ophthalmologists, and public health workers plays a pivotal role in achieving universal ROP screening and management.[13]
Recent advances in artificial intelligence (AI), machine learning, and predictive analytics have shown promise in enhancing ROP care. AI-based image grading tools have achieved expert-level diagnostic accuracy, potentially reducing interobserver variability and optimizing resource allocation. Moreover, innovations in portable imaging, smartphone-based retinal photography, and AI-assisted screening may bridge care gaps in underserved regions.[14]
Despite the progress, several lacunae persist in ROP care. Challenges include inconsistent adherence to screening protocols, delayed referrals and follow-ups, a lack of trained pediatric retinal specialists, and inadequate parental education. Furthermore, the long-term outcomes of anti-VEGF therapy on systemic development and ocular growth remain under investigation. Research into predictive biomarkers, individualized treatment thresholds, and integration of AI-based screening platforms could significantly impact ROP outcomes.[15]
This activity aims to contribute to the growing literature on ROP by evaluating the real-world effectiveness of screening models and analyzing treatment trends in a tertiary care setting. By identifying gaps in implementation and variations in clinical outcomes, this research seeks to inform policy decisions and optimize neonatal ocular care. Furthermore, this activity examines the interprofessional dynamics that enhance ROP surveillance and management, underscoring the importance of cohesive teamwork in improving infant vision and quality of life.[16]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
In utero, the retina is in a state of physiological hypoxia. Elevated levels of VEGF facilitate retinal angiogenesis.[5][17][18] The 2 phases of normal vascular development are characterized as vasculogenesis, which occurs from the 14th week until the 21st week of gestation, and angiogenesis, which begins in the 22nd week and continues until the retina is fully vascularized after term.[19] Given that the nasal and temporal portions of the retina form late in pregnancy, 32 and 40 weeks, respectively, preterm infants are born with incomplete vascularization of these portions.
The physiologic hypoxia that was previously driving vessel development is replaced with a state of hyperoxia, as many premature infants are exposed to supplemental in addition to atmospheric oxygen. ROP is a multifactorial vasoproliferative disorder that affects the development of retinal vasculature in premature infants. Its etiology is primarily driven by a disruption in the normal vascularization of the retina due to premature birth and exposure to postnatal environmental factors—particularly oxygen. The disease process unfolds in 2 critical phases, each with distinct pathogenic mechanisms.[16]
Disrupted Retinal Vascular Development
During normal gestation, retinal vascularization begins at approximately 16 weeks and completes by 40 weeks of gestation. Premature birth interrupts this process, leaving the peripheral retina avascular. These avascular areas become hypoxic as the metabolic demands of the maturing retina increase, triggering an abnormal cascade of molecular events.[20]
Biphasic pathophysiology
- Phase I (vaso-obliteration and hypoxia suppression): After birth, preterm infants are often exposed to high concentrations of supplemental oxygen, especially in neonatal intensive care units (NICUs). This relative hyperoxia suppresses the expression of hypoxia-inducible factor (HIF-1α) and its downstream angiogenic growth factors, including VEGF and insulin-like growth factor 1 (IGF-1). The consequence is vaso-obliteration and halted vessel growth in the peripheral retina.
- Phase II (hypoxia-induced pathological neovascularization): As the infant matures and oxygen supplementation is tapered, the avascular retina becomes profoundly hypoxic. This deficit reactivates the production of VEGF and other angiogenic cytokines in a disorganized manner, leading to neovascularization at the junction between the vascular and avascular retina. These vessels are fragile and prone to leakage, which can lead to fibrovascular proliferation, tractional detachment, and ultimately, retinal detachment if left untreated.[21]
Oxygen dysregulation
Oxygen therapy, although lifesaving, is the single most important modifiable risk factor for ROP. Fluctuations in arterial oxygen tension (PaO2) are more harmful than sustained moderate hyperoxia, as they increase oxidative stress and endothelial injury. Strict oxygen saturation targeting and avoidance of extreme oxygen variability, particularly in ranges below 88% and above 95%, have significantly reduced the incidence of severe ROP.[22]
Systemic and Maternal Risk Factors
Several systemic and perinatal factors implicated in the etiology of ROP are as follows:
- Low birth weight and gestational age: The most critical predictors of ROP severity. The earlier the gestational age and the lower the birth weight, the higher the risk due to increased retinal immaturity.
- Sepsis and inflammation: Neonatal sepsis and systemic inflammation increase cytokine levels, which can amplify VEGF production and compromise vascular integrity.
- Intraventricular hemorrhage: Associated with altered cerebral and ocular blood flow, contributing to ischemia and VEGF dysregulation.
- Blood transfusions and anemia: Frequent transfusions increase free iron and oxidative stress, both of which can damage retinal vessels.
- Poor postnatal weight gain: Weight gain serves as a surrogate marker for overall metabolic and growth factor availability, including IGF-1, which is crucial for normal retinal vascularization.[23]
Genetic and Epigenetic Factors
While ROP is predominantly environmental, study results have suggested a genetic predisposition in some infants. Variants in genes regulating angiogenesis (eg, VEGF, IGF-1, HIF-1α), oxidative stress, and inflammatory pathways may influence disease susceptibility. Epigenetic regulation of angiogenic factors due to intrauterine and neonatal environmental exposures is an emerging area of research.[24]
Delayed or Absent IGF-1 Levels
IGF-1 plays a crucial role in the normal growth of vessels. In utero, IGF-1 is maintained through placental transfer, but after preterm birth, serum levels drop sharply. Without adequate IGF-1, VEGF cannot exert its angiogenic effect, contributing to the development of the first phase of ROP. Later, when VEGF levels rise again, IGF-1 may return to adequate levels, exacerbating uncontrolled neovascularization.[25]
Role of Artificial Ventilation and Mechanical Support
Prolonged mechanical ventilation and high levels of oxygen support (>30% FiO2 for prolonged durations) have a significant role in the pathogenesis of ROP. These interventions can lead to oxygen-induced vascular damage and fluctuations in oxygen saturation, thereby exacerbating disease progression.
In summary, the etiology of ROP is a complex interaction of developmental interruption, oxygen-mediated vascular injury, systemic inflammation, and metabolic immaturity. Prevention of ROP hinges on optimal neonatal care practices, including judicious use of oxygen therapy, effective infection control, proper nutrition, and early screening. Advances in understanding the molecular and environmental basis of ROP have not only clarified its pathogenesis but have also opened avenues for novel preventive and therapeutic strategies.[26]
Epidemiology
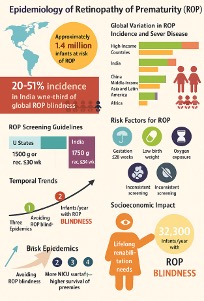
Many important epidemiological risk factors for ROP were established in the Early Treatment for Retinopathy of Prematurity (ETROP) study. This randomized, prospective, multicenter trial compared the safety of earlier versus conventionally timed ablation of the peripheral retina.[2] The incidence of any stage of ROP was 68% among infants weighing less than 1251 g. In the year 2010, a global number of 184,700 infants and 14.9 million preterm infants developed any stage ROP.[3] Of those afflicted, 20,000 became blind or severely visually impaired, and 12,300 developed mild to moderate visual impairment (see Image. Epidemiology of Retinopathy of Prematurity [ROP]).
The 2 strongest known risk factors for ROP are gestational age and birth weight. A multicenter study of over 4000 infants with a birth weight of 1251 g or less found that for each 100 g increase in birth weight, the odds of developing threshold ROP decreased by 27%, and for each extra week in gestational age, the odds of developing threshold ROP decreased by 19%.[27] Another important risk factor is oxygen. As mentioned above, the use of supplemental oxygen in combination with atmospheric oxygen results in the reversal of physiologic hypoxia, which then contributes to retinal ischemia and subsequent overgrowth of retinal vessels in ROP.
Additionally, the concentration of oxygen delivered is an independent risk factor for ROP, whereby increased oxygen concentrations increase the risk of ROP.[28] For every 12 hours with a transcutaneous PO2 of 80 mmHg or greater, the risk of ROP doubles.[18] Duration of oxygen therapy is a significant risk factor for severe ROP.[29] Possible risk factors include hypertensive disorders of pregnancy, maternal diabetes, medication use, age, smoking, assisted conception, birth outside of a study center hospital, and multiple gestations.[23] ROP is a leading cause of preventable childhood blindness globally, particularly affecting preterm infants with low birth weight. This condition's epidemiology varies significantly across regions, driven by disparities in neonatal care, availability of screening programs, and the survival rate of preterm infants.
Global Incidence and Burden
Global estimations say that around 15 million infants are born prematurely each year, and approximately 1.4 million are at risk of developing ROP. Among these, approximately 32,300 infants become blind or visually impaired from ROP annually. This burden is especially significant in middle-income countries where neonatal care facilities have improved survival rates but may still lack robust ROP screening and management infrastructure.[30]
In high-income countries (eg, the United States, the United Kingdom, Canada), ROP incidence ranges from 20% to 30% in infants born at a gestational age younger than 32 weeks or with a birth weight of less than 1500 g. However, due to early screening, strict oxygen protocols, and timely intervention, blindness due to ROP has drastically decreased. Conversely, in countries such as India, China, Latin America, and sub-Saharan Africa, the ROP incidence may exceed 35% to 40%, with a higher proportion of severe and untreated cases resulting in permanent vision loss.[31]
India: A significant global contributor
India bears a disproportionate burden of ROP-related blindness. With over 3.5 million preterm births annually, India accounts for a large share of at-risk infants. Study results have shown the following:
- The incidence of any stage of ROP in infants weighing <1750 g or born at younger than 34 weeks of gestation can range from 20% to 51%.
- Approximately 5% to 15% develop type 1 ROP, which requires treatment.
- A third epidemic of ROP blindness is underway in India due to increased neonatal survival without parallel development of widespread screening and follow-up systems.[32]
Major Indian studies, such as the KIDROP project, have shown that effective telemedicine screening programs can reduce the risk of blindness from ROP, even in rural and underserved areas.
Risk Factors Influencing Epidemiology
Several neonatal and institutional risk factors that influence the epidemiology of ROP are as follows:
- Gestational age and birth weight: These are the most consistent predictors. Infants born before 28 weeks or weighing <1000 g have the highest risk.
- Oxygen therapy practices: Inconsistent or excessive oxygen supplementation can significantly increase the incidence, especially in units without oxygen monitoring.
- Resource setting: In high-resource NICUs, ROP is more often restricted to extremely preterm infants, while in low-resource settings, even infants with birth weights up to 2000 g may develop ROP.
- NICU infrastructure and staffing: The availability of trained neonatologists, oxygen blenders, and nursing ratios influences outcomes.
- Screening policies: Countries with national ROP screening guidelines (eg, the United Kingdom, the United States, Brazil) report lower rates of blindness compared to countries with fragmented or absent screening protocols.[33]
Temporal Trends
ROP epidemiology has shifted over the decades through 3 distinct phases as follows:
- First epidemic (1940s-1950s): In high-income countries, unmonitored high-oxygen therapy led to a surge in ROP-related blindness.
- Second epidemic (1970s-1980s): Improved survival of extremely preterm infants in developed countries triggered a second rise in ROP cases.
- Third epidemic (2000s-present): Middle- and low-income countries, particularly in Asia and Africa, now face a new wave of ROP blindness due to increased NICU access without adequate screening infrastructure.[15]
Impact of Screening Guidelines
Implementation of ROP screening has directly impacted the incidence of blindness. Recommendations are as follows:
- The American Academy of Pediatrics recommends screening for infants with a birth weight of ≤1500 g or a gestational age of 30 weeks or younger.
- In India, ROP screening is advised for infants ≤1750 g and/or 34 weeks or younger, or larger infants with unstable clinical courses.
Timely screening, typically initiated between the postnatal ages of 3 to 4 weeks, plays a crucial role in early detection and treatment.[34]
Socioeconomic Impact
ROP-associated blindness is a significant burden on families and healthcare systems. Blind infants require lifelong rehabilitation, education, and support, and incur substantial economic costs. Moreover, a blind child may have a lifetime of over 70 disability-adjusted life years, making ROP blindness one of the most impactful pediatric preventable diseases globally. In summary, the epidemiology of ROP reflects a global health disparity—one where advancements in neonatal care have saved countless lives but have also introduced a new burden of avoidable blindness. Awareness, equitable access to screening, trained personnel, and integration of ROP services into neonatal care pathways are crucial to reducing the incidence of this disease and its long-term visual consequences.[35]
Pathophysiology
Events during the 2 phases of healthy vascular development, namely vasculogenesis and angiogenesis, underlie the pathologies seen in zones 1 and 2 of ROP.[19] During vasculogenesis, vascular precursor cells exit from the optic nerve to form the 4 major arcades of the posterior retina. Angiogenesis is characterized by the proliferation of endothelial cells, arising from the existing vasculature formed during vasculogenesis.[19]
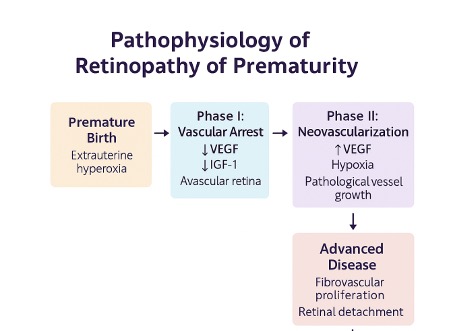
Vascular development in the retina is not completed in certain portions of the retina until after term. Specifically, the nasal and temporal portions do not complete development until 32 and 40 weeks, respectively. After birth, exposure to atmospheric oxygen and supplemental oxygen results in a rapid swing from relative hypoxia in utero to hyperoxia. The synthesis of IGF-1, a vital hormone that regulates VEGF-mediated vascular growth, is dependent on adequate supplies of amino acids and energy. A relative nutritional deficiency after birth in preterm infants results in depressed serum IGF-1 levels. Together, hyperoxia and low IGF-1 result in delayed retinal vascularization. Developing capillaries undergo vasoconstriction and eventual obliteration. These changes constitute phase 1 of ROP. Phase 2 of ROP consists of a decline in normal angiogenesis, with predominant pathologic angiogenesis (see Image. Pathophysiology of Retinopathy of Prematurity).
There is an increase in VEGF release into the vitreous, a drop in IGF-1 levels, and peripheral avascular retinal neurons are damaged through hypoxic injury. High levels of VEGF in the vitreous result in the growth of pathologic vessels out of the retina.[36] ROP is a vasoproliferative retinal disorder affecting premature infants, primarily due to abnormal retinal vascular development.
Pathophysiology of ROP
Phase I: Vascular growth arrest (hyperoxic phase)
In utero, the fetal retina develops under relatively hypoxic conditions, promoting the normal growth of retinal vessels from the optic disc toward the periphery. However, when a premature infant is exposed to extrauterine life, particularly with supplemental oxygen therapy, this environment becomes relatively hyperoxic. This hyperoxia suppresses VEGF and IGF-1, which are essential for normal vasculogenesis and angiogenesis.
The effects include the following:
- Retinal vessel growth halts prematurely.
- Peripheral avascular retina persists.
- The developing retina becomes metabolically stressed due to inadequate vascular support.[37]
Phase II: Hypoxic proliferative phase
As the retina matures and its oxygen demands increase, the avascular regions become progressively hypoxic. This process stimulates a rebound increase in VEGF and other proangiogenic cytokines.
Consequences are as follows:
- Pathological neovascularization at the junction of vascular and avascular retina.
- These new vessels are fragile and leaky, potentially leading to the following:
- Retinal hemorrhage
- Fibrovascular proliferation
- Tractional retinal detachment in advanced stages (stage 4 or 5 ROP)[38]
Molecular mediators involved
- VEGF: Central to neovascularization
- Initially downregulated in hyperoxia and later upregulated during hypoxia
- IGF-1: Supports VEGF-mediated angiogenesis
- Premature infants often have low IGF-1 levels
- Hypoxia-inducible factor: Stabilizes in hypoxic conditions and increases VEGF transcription [39]
Risk Amplification by Exogenous Factors
- Prolonged oxygen supplementation and mechanical ventilation exacerbate the hyperoxic insult.
- Sepsis, anemia, and poor postnatal weight gain also increase susceptibility by influencing systemic inflammation and angiogenic regulation.[40]
Retinal Zones and Staging
ROP predominantly affects the peripheral retina (zone II or III), where vascularization is incomplete. The International Classification of Retinopathy of Prematurity stages the disease from mild (stage 1: demarcation line) to severe (stage 5: total retinal detachment).
Why Early Detection Matters
Due to the 2-phase model, a latent period (typically 4 to 6 weeks postnatal) exists between vascular arrest and abnormal proliferation, which is critical for timely screening and intervention, especially with laser therapy or anti-VEGF agents, to prevent irreversible blindness.[41]
Histopathology
ROP is a vaso-proliferative disorder of the developing retinal vasculature, typically occurring in premature infants. Its histopathology reflects a dynamic, biphasic process of vascular regression and disorganized neovascularization in response to fluctuating oxygen levels postnatally. The pathological features vary depending on the stage of the disease, with distinct cellular and tissue-level changes seen across different phases.[42]
Phase 1: Vascular Arrest and Regression (Hyperoxia-Induced Phase)
Following preterm birth, exposure to extrauterine hyperoxia—either ambient or therapeutic—disrupts the normal development of the retinal vasculature. This process initiates vaso-obliteration and growth arrest.
Histopathological features in this phase include the following:
- Capillary dropout in the peripheral retina
- Attenuation and loss of endothelial cells in developing capillaries
- Disruption of the astrocytic and Müller cell template, which normally guides vascular growth
- Reduced VEGF and IGF-1 expression, leading to cessation of normal angiogenesis
- Ischemic avascular retina, especially in the temporal periphery
- Inner retinal thinning and a hypoplastic outer retina may be observed
The vessels in the central retina may appear dilated and tortuous, but histologically maintain a relatively intact structure.[43]
Phase 2: Hypoxia-Induced Pathological Neovascularization
As the metabolically active retina matures, the oxygen demand increases. In previously avascular zones, hypoxia triggers overexpression of VEGF, placental growth factor (PlGF), and angiopoietin, leading to exuberant but disorganized vessel proliferation.
Histopathological findings in this proliferative phase are as follows:
- The presence of disorganized neovascular tufts arising from retinal capillaries, especially near the demarcation line between vascular and avascular retina.
- These tufts grow anteriorly through the internal limiting membrane (ILM) into the vitreous.
- Endothelial proliferation, pericyte hyperplasia, and recruitment of macrophages and glial cells are prominent.
- The fibrovascular ridge—seen clinically as stage 3 ROP—shows dense fibrovascular tissue with immature vessels surrounded by collagen and fibrous stroma.
- Retinal edema, microglial activation, and inflammatory infiltrates may also be seen.
These fragile vessels are prone to leakage and hemorrhage, potentially triggering further fibrous proliferation.[44]
Advanced and Cicatricial ROP: Fibrosis and Retinal Distortion
In cases where neovascularization fails to regress, a fibrotic response develops, resulting in contraction and mechanical retinal distortion.
Histopathology in advanced ROP reveals the following:
- The presence of fibrovascular membrane formation adherent to the retinal surface or extending into the vitreous.
- Tractional retinal detachment typically begins at the ridge and progresses posteriorly.
- Retinal folding and retinal dragging are due to the contraction of the preretinal membranes.
- The presence of disorganized retinal layers with loss of inner plexiform and nuclear layer integrity.
- Gliosis, characterized predominantly by reactive Müller cells and astrocytes, is present.
- Retrolental fibroplasia (in stage 5 ROP), where dense fibrous tissue may be seen behind the lens, causing leukocoria.
- In some cases, calcification, pigmentary changes, and retinal necrosis may occur.[45]
Histopathologic Variants and Adjunct Findings
- Choroidal involvement: Rare, but ischemic atrophy of the choroid may accompany severe ROP.
- Persistent fetal vasculature may be seen as a differential or coexisting feature in a few cases.
- Optic nerve head pallor and gliosis may be present in longstanding disease.[46]
Immunohistochemical and Molecular Correlates
- Increased expression of VEGF, IGF-1, HIF-1α, MMP-2, and MMP-9 in neovascular zones
- Downregulation of pigment epithelium-derived factor (PEDF), which normally counters VEGF
- Presence of activated microglia, macrophages, and TGF-β in fibrotic areas
- Upregulation of angiogenesis-related genes in the proliferative phase [47]
The histopathology of ROP reflects a complex, staged process driven by oxygen dysregulation, growth factor imbalance, and aberrant cellular responses. The transition from an avascular immature retina to disorganized neovascularization and, eventually, fibrous membrane formation encapsulates the critical window for intervention. Understanding these microscopic changes enhances the understanding of therapeutic timing, such as anti-VEGF injections or laser therapy, and helps predict outcomes in severe or regressed cases of ROP.[48]
Toxicokinetics
While ROP is primarily a vasoproliferative retinal disorder in premature infants caused by oxygen dysregulation, the concept of toxicokinetics is becoming increasingly relevant due to the administration of various pharmacological agents in neonatal care, especially oxygen, antibiotics, steroids, and VEGF inhibitors (see Image. Toxicokinetics of Retinopathy of Prematurity).
Oxygen as a Therapeutic Toxicant
- Absorption: Administered via inhalation in NICUs, supplemental oxygen rapidly diffuses across alveoli into systemic circulation.
- Distribution: Oxygen reaches retinal tissue, where excess oxygen suppresses VEGF, impairing retinal vessel development.
- Metabolism: Oxygen is metabolized in tissues; however, high concentrations can lead to the formation of reactive oxygen species (ROS).
- Excretion: Eliminated via exhalation, but ROS-induced oxidative stress lingers in retinal tissues.[49]
Implication: Oxygen exhibits a dose-dependent toxicity profile, where both hypoxia and hyperoxia are detrimental, underscoring the importance of oxygen toxicokinetics in mitigating the risk of ROP.
Anti-VEGF Agents (eg, Ranibizumab, Bevacizumab)
- Absorption: Administered intravitreally; systemic leakage is possible, especially in neonates with immature barriers.
- Distribution: Potential systemic absorption may suppress systemic VEGF, affecting developing organs.
- Metabolism and elimination: These large molecules are slowly degraded proteolytically and excreted over weeks.[50]
Implication: Understanding systemic pharmacokinetics and toxicokinetics of anti-VEGF agents is crucial in evaluating long-term developmental safety.
Antibiotics and Steroids
- Drugs like gentamicin or dexamethasone, used for infection or inflammation, may accumulate due to immature renal/hepatic function in preterm neonates.
- Toxicokinetic profiles must be carefully adjusted to prevent retinal or systemic toxicity.
Clinical Relevance
- Toxicokinetics guides dose optimization, toxicity monitoring, and drug safety profiling in ROP care.
- Further research into the toxicokinetics of emerging therapies, such as gene therapy and biosimilars, may enhance personalized treatment protocols.[51]
History and Physical
Screening for ROP is crucial for premature or low-birth-weight infants. Guidelines from the American Academy of Ophthalmology, the American Academy of Pediatrics, and the American Association for Pediatric Ophthalmology and Strabismus recommend that infants born at 30 weeks' gestational age or less, or with a birth weight of 1500 g or less, should be screened for ROP. Depending on the clinical course, larger infants may also benefit from screening.[52] On average, each infant requires 3.4 serial examinations.[53] ROP is a vasoproliferative retinal disorder that primarily affects premature infants with incomplete development of their retinal vasculature. Accurate history-taking and a meticulous ophthalmic examination are critical for early detection, timely intervention, and prevention of irreversible blindness.
History
A thorough history is crucial for assessing the infant's risk of ROP and guiding screening and management.
Perinatal History
- Gestational age: The most significant risk factor. Infants born before 32 weeks of gestation are at the highest risk.
- Birth weight: Infants with a birth weight <1500 g are universally screened. Lower weights are associated with a higher risk due to incomplete retinal vascularization.
- Small for gestational age: Infants classified as small for gestational age (SGA) exhibit altered growth patterns and vascular development.[23]
Neonatal Intensive Care Unit Course
- Oxygen therapy:
- Prolonged, unregulated oxygen supplementation contributes to the progression of ROP through both Phase I (vaso-obliteration) and Phase II (vasoproliferation).
- Hyperoxia suppresses VEGF, leading to capillary dropout.
- Subsequent hypoxia drives neovascularization and retinal damage.[22]
- Mechanical ventilation:
- Duration and need for high FiO2 levels are directly associated with severe ROP.
- Conditions like bronchopulmonary dysplasia exacerbate the risk of complications.
- Sepsis or systemic infections:
- Gram-negative and fungal infections elevate inflammatory cytokines, promoting retinal neovascularization.[54]
- Intraventricular hemorrhage:
- Associated with systemic instability and correlates with worse ROP outcomes.
- Necrotizing enterocolitis:
- Reflects systemic inflammation and poor nutritional status, indirectly contributing to poor retinal development.[55]
- Blood transfusions and anemia:
- Multiple transfusions introduce adult hemoglobin, which reduces oxygen affinity and impairs tissue oxygenation.
- Iron overload from transfusions can increase oxidative stress.
- Postnatal growth and weight gain:
- Suboptimal weight gain during early postnatal weeks strongly predicts severe ROP.
- Predictive models incorporate weight gain trends into risk stratification.[56]
Maternal History
- Preeclampsia or chorioamnionitis: These conditions may contribute to fetal stress and premature delivery.
- Antenatal steroid use: While beneficial for lung maturity, the role in ROP modulation remains unclear.
- Multiple Gestation Pregnancies: This is associated with earlier preterm delivery and low birth weights.[57]
Physical Examination
ROP is typically asymptomatic in early stages and requires targeted ophthalmologic screening for diagnosis. However, systemic examination may reveal associated signs of prematurity-related complications.
General physical examination
- Vital signs: Blood pressure, heart rate, and oxygen saturation trends (eg, clues to systemic instability)
- Head circumference and weight: Essential growth parameters for systemic and ocular development tracking
- Skin findings: Cyanosis, pallor, or signs of sepsis (eg, indicate systemic illness)[58]
Systemic Evaluation
- Neurological status: Important due to the association of ROP with intraventricular hemorrhage and periventricular leukomalacia
- Respiratory examination: Chronic lung disease increases risk and can modify the timing of retinal vascular changes
Ocular Examination
A comprehensive dilated fundus examination is the cornerstone of ROP diagnosis and staging.
Timing of screening
- This is performed at 4 weeks postnatal age or 31 weeks postmenstrual age (PMA), whichever is later.
- Guidelines (AIOS–ROP, AAP, RCPCH) recommend individualized follow-up based on zone and stage.[59]
Examination technique
- Performed by a retina specialist or pediatric ophthalmologist using the following:
- Indirect ophthalmoscopy with a 28D or 20D lens
- RetCam imaging or similar wide-field digital imaging for documentation and telemedicine [60]
Key Features Evaluated
- Extent of retinal vascularization
- Classified by zones (I–III)
- Zone I involves the optic disc and macula.
- Zone II is the ring-shaped section of the retina surrounding Zone I.
- Zone III is the far peripheral retina.
- ROP stage
- Stage 1: Demarcation line
- Stage 2: Ridge
- Stage 3: Extraretinal fibrovascular proliferation
- Stage 4: Partial retinal detachment (subdivided into 4A and 4B)
- Stage 5: Total retinal detachment
- Plus disease: This includes the presence of arteriolar tortuosity and venous dilation in the posterior pole, a marker of severity.
- Pre-plus disease: This includes vascular changes that are greater than typical but not severe enough to qualify as plus disease.
- Aggressive posterior ROP: This is a rapidly progressing variant seen in extremely preterm infants; it requires urgent treatment.[61]
Anterior segment and lens
- Pupillary dilation, iris neovascularization, and lens clarity are assessed.
- Leukocoria may be noted in advanced stages due to retinal detachment or fibrovascular mass.
Documentation and follow-up
- Accurate recording of zone, stage, presence of plus disease, and recommendation for follow-up or treatment is essential.
- Digital retinal imaging is encouraged to supplement findings and enable longitudinal assessment.
- Screening intervals depend on the most advanced disease seen in either eye and current guidelines.
A structured approach to history and physical examination in at-risk preterm infants allows for timely ROP detection and intervention. Given the asymptomatic nature of early ROP, proactive screening, precise staging, and regular follow-up based on a detailed history and examination remain crucial in preventing vision-threatening complications.[62]
Evaluation
A dilated fundus exam should be performed on all infants born at 30 weeks' gestational age or younger and infants with a birth weight of 1500 g or less.[5] Screening should begin at 4 weeks postnatal age or corrected gestational age of 30 to 31 weeks in the NICU or the special newborn care unit, but may also be performed on an outpatient basis.[5][17] Before evaluation, dilation of the pupils is achieved with 2.5% phenylephrine hydrochloride and 1% cyclopentolate or tropicamide, administered in 2 instillations 15 minutes apart.[17] Care should be taken to prevent systemic absorption. Pupils may fail to dilate in advanced ROP.[52] Because infants may experience apnea and bradycardia during the examination, a nurse should be present to monitor their vital signs.[5]
To limit the number of unnecessary screenings while capturing every case of ROP, the Postnatal Growth and Retinopathy of Prematurity Screening Criteria (G-ROP) was developed. In a validation study of 3981 infants, these guidelines were 100% sensitive and reduced the number of infants screened by 30%.[63] This screening tool differs from traditional guidelines in that it includes weight gain as a variable in decision-making. Although the results of the 2019 study demonstrated excellent sensitivity and improved specificity, all new prediction models should be tested and validated to compare their performance in clinical and low-resource settings.[64]
Classification is described in terms of zone and stage of the disease.[65] The ICORP is a consensus statement of the grading of ROP. Zone refers to the location of the leading edge of vascularization. The retina is divided into 3 zones: Zone I relates to a concentric area centered on the optic disc with a diameter twice the distance between the center of the optic nerve and the fovea, zone II is a circle centered on the optic disc ending at the ora serrata and zone III includes the temporal retina not included in the previous zones.[65]
There are 5 stages to describe the severity of ROP. Each stage refers to a specific retinal and vascular pattern at the border of the vascular and avascular retina. Stage 1 is a thin but clear demarcation or structure that separates avascular retina anteriorly from vascularized retina posteriorly. The demarcation line is typically flat and white, with abnormal branching of vessels leading up to it.[65] Stage 2 is characterized by the presence of a ridge in the region of the demarcation line that extends above the plane of the retina.[65] "Popcorn" tufts of neovascular tissue may be seen posterior to the ridge. Stage 3 features the growth of extraretinal fibrovascular proliferation or neovascularization extending from the ridge into the vitreous. This tissue may give the ridge a ragged appearance. Stage 4 classification is given when a partial retinal detachment develops. This partial retinal detachment may be extrafoveal, stage 4A, or foveal, stage 4 B. The most severe classification, stage 5, is the tractional total retinal detachment. These detachments are often funnel-shaped or concave in shape.
Plus disease is a classification that may be present at any stage of the disease. This condition is described as increased venous dilation and arteriolar tortuosity of the posterior pole retinal vessels. Plus disease indicates vascular shunting and severe ROP.[65] Threshold ROP is diagnosed when there are at least 5 contiguous or 8 cumulative clock hours of stage 3 ROP, in zone 1 or 2, with plus disease.[66]
Treatment / Management
While early detection of ROP is crucial, not every case will require treatment. Based on findings from the Early Treatment for Retinopathy of Prematurity study (ETROP), the decision to treat depends on the type of ROP. Type I ROP, including any stage zone I ROP with plus disease, zone I stage 3 with or without plus disease, or zone II stage 2 or 3 with plus disease, should be treated. Observation is recommended for Type II ROP, including zone I, stage 1 or 2 without plus disease, or zone II, stage 3 without plus disease.[2](A1)
Treatment of ROP is primarily surgical. The first surgical treatment considered both safe and effective for ROP was cryotherapy to the avascular retina (CRYO-ROP study). In cryotherapy, the sclera, choroid, and full-thickness of the avascular retina are frozen from the surface of the eye.[17] While this treatment resulted in a 50% reduction in retinal detachments in threshold eyes, it was considered time-consuming and required general anesthesia as well as surgical displacement of the conjunctiva.[17] Argon and diode laser photocoagulation treatments for the avascular retina have further reduced unfavorable outcomes and have become the standard of treatment for ROP.[2](A1)
Due to the role of VEGF in the development of ROP, the use of anti-VEGF agents is a possible treatment strategy. The Bevacizumab Eliminated the Angiogenic Threat of Retinopathy of Prematurity (BEAT-ROP) study randomly assigned infants with stage 3 ROP in zone I or posterior zone II to receive either intravitreal bevacizumab (IVB) or conventional laser therapy. The primary outcome in this study was the recurrence of ROP requiring treatment before 54 weeks postmenstrual age. There was a statistically significant difference in recurrence, with 19 cases in the laser-treated group and 4 in the bevacizumab group. The treatment effect was significant in patients in zone I but not in zone II. Macular dragging and retinal detachment were seen more frequently after laser treatment for zone 1 disease.[67] Though this study did not observe any systemic or toxic effects of IVB therapy, some concerns about its use persist. First, peripheral retinal vessel development continued after treatment with IVB, and subsequent case reports have reported late reactivation of ROP, up to 3 years after birth.[68][69] (B2)
The beneficial effects of VEGF, a hormone that is both neurotrophic and neuroprotective, should not be overlooked, as it helps maintain the blood-brain barrier, generates surfactant in the lungs, facilitates the formation of glomeruli in the kidneys, and stimulates skeletal growth.[17] Results from a 2019 study demonstrated higher mortality and poor cognitive outcomes in infants treated with bevacizumab than with other treatment modalities.[70] Retinal detachment carries a significantly higher risk of a poor visual outcome.[71][72][73][74][75] For this reason, in advanced ROP, surgery is indicated for stages 4A, 4B, and 5. Modalities for surgical intervention in stage 4 ROP include scleral buckling or lens-sparing vitrectomy.(A1)
In stage 5 disease, scleral buckling plays a limited role, and the most common approaches include lensectomy with vitrectomy or open sky vitrectomy. Surgical outcomes are evaluated based on anatomical success, the attachment of the posterior retina, and functional success, as measured by visual acuity. Functional success is slow and challenging to measure. Rates of anatomical success range between 13% and 45.5%.[76]
ROP evaluation is a critical process designed to identify premature infants at risk of developing retinal vascular abnormalities that can lead to vision loss. Evaluation includes both systematic screening protocols and comprehensive ophthalmologic assessments, guided by international standards such as those established by the ICROP and the American Academy of Pediatrics (see Table. Summary of Retinopathy of Prematurity Evaluation).
Screening Criteria
ROP screening typically targets those who are as follows:
- Infants with birth weight ≤1500 g
- Gestational age ≤30 weeks
- Infants between 1500 g and 2000 g or >30 weeks of gestation with an unstable clinical course, at the discretion of the neonatologist (see Flowchart. Retinopathy of Prematurity Screening) [48]
India-specific guidelines include screening those as follows:
- Birth weight <1750 g
- Gestational age <34 weeks
- Sicker babies outside these criteria, especially if oxygen supplementation was prolonged [77] (B2)
Timing of initial screening
- The first screening is typically scheduled at 4 weeks postnatal age, or 31 weeks postmenstrual age, whichever is later.
- Earlier screening (as early as 2 to 3 weeks of age) is recommended for extremely low-birth-weight infants.
Method of Examination
- Performed using indirect ophthalmoscopy with scleral indentation after pharmacologic dilation
- Conducted by a trained pediatric ophthalmologist or retina specialist
- Wide-field digital imaging systems (eg, RetCam and 3nethra Neo) are increasingly used for documentation, tele-screening, and second opinions.[78]
Classification Based on the ICROP 3rd Edition (2021)
- Zones of involvement:
- Zone I: Posterior pole (most severe)
- Zone II: From the zone I edge to the nasal ora serrata
- Zone III: Temporal crescent
- Stages of disease:
- Stage 1: Demarcation line
- Stage 2: Ridge
- Stage 3: Extraretinal fibrovascular proliferation
- Stage 4: Partial retinal detachment
- Stage 5: Total retinal detachment
- Plus disease: Arteriolar tortuosity and venous dilatation in the posterior pole
- Aggressive ROP: Rapid progression, flat neovascularization, usually in zone I or posterior zone II [41]
Documentation
- Document zone, stage, and presence or absence of plus disease
- Image documentation using RetCam can be handy for serial follow-up and interdisciplinary communication
Follow-Up protocols
- Frequency of follow-up depends on zone and stage:
- Stage 1 or 2, Zone II without plus: Weekly or biweekly
- Stage 3, Zone II with plus: Requires urgent treatment within 48 to 72 hours
- Zone I disease: More frequent follow-up or immediate intervention is recommended.[79]
Multidisciplinary Involvement
Evaluation often involves:
- Neonatologists, who initiate referrals
- Ophthalmologists, who conduct the evaluation
- Nurses and technicians, for dilation and imaging
- Caregivers, for counseling, follow-up, compliance, and education
Role of Artificial Intelligence and Telemedicine
- AI-based image grading systems (eg, i-ROP DL) are under investigation to assist in ROP detection
- Tele-ROP screening using wide-field cameras has expanded access to rural and underserved areas
Prognostic Implications
- Timely detection is critical to prevent progression to blinding stages
- Delay in screening or missed follow-ups are major contributors to severe ROP outcomes in low- and middle-income countries.[80]
Table 1. Summary of Retinopathy of Prematurity Evaluation
|
Parameter |
Details |
|
Target Population |
Birth weight ≤1500 g, gestational age ≤30 weeks, or unstable clinical course |
|
India-Specific Criteria |
Birth weight <1750 g or gestational age <34 weeks or clinical instability |
|
Initial Screening Timing |
4 weeks postnatal age or 31 weeks postmenstrual age (whichever is later) |
|
Exam Technique |
Indirect ophthalmoscopy with scleral indentation; wide-field imaging (RetCam/Neo) |
|
Zones of Retina (ICROP) |
Zone I: Posterior pole; Zone II: Mid-retina; Zone III: Temporal periphery |
|
ROP Stages |
Stage 1: Demarcation line; Stage 2: Ridge; Stage 3: Extraretinal tissue; Stage 4/5: Retinal detachment |
|
Plus Disease |
Vascular dilatation and tortuosity in the posterior pole |
|
Aggressive ROP |
Rapid progression, posterior zone, flat neovascularization |
|
Follow-up Frequency |
Weekly/biweekly, depending on zone/stage; urgent review if stage 3+ disease |
|
Documentation |
Zone, stage, plus disease status; image-based if a digital camera is available |
|
Adjunct Tools |
RetCam, 3nethra Neo, telemedicine, AI (eg, i-ROP DL) |
Flowchart. Retinopathy of Prematurity Screening
Start Screening
¦
¦ Identify Eligible Infants ¦
¦ (Birth weight ≤1500 g, Gestational age ≤30 wks, ¦
¦ or unstable clinical course) ¦
¦
Schedule Initial Screening
(At 4 weeks postnatal or 31 weeks PMA)
¦
Dilated Eye Exam (IO/RetCam)
¦
Classify ROP Stage, Zone, Plus/A-ROP Status
¦
+---------------------------------------------------+
¦ No ROP ¦ ROP Stage 1-2 ¦ Stage 3 or Plus ¦
¦ (Immature) ¦ Zone II ¦ or A-ROP ¦
? ? ?
Follow up in 2–3 weeks. Weekly follow-up, Urgent Treatment
(Laser/Anti-VEGF)
¦
Repeat until retinal vascularization is complete.
¦
No further screening is required
Differential Diagnosis
ROP can mimic or overlap with other neonatal retinal or ocular conditions (see Table. Differential Diagnosis for Retinopathy of Prematurity). Differentiating these entities is crucial for appropriate management, as treatment protocols differ significantly.
Differential Diagnosis of Retinopathy of Prematurity
Familial exudative vitreoretinopathy
- Key features: Peripheral retinal avascularity, neovascularization, exudation, falciform folds
- Distinguishing factors:
- Full-term infants
- Family history
- Genetic testing (NDP, FZD4, LRP5) [81]
Norrie disease
- Key features: Bilateral leukocoria, total retinal detachment at birth, progressive blindness, hearing loss, cognitive impairment
- Distinguishing factors:
- X-linked inheritance
- Male predominance
- Confirmatory genetic testing [82]
Coats disease (early-onset)
- Key features: Retinal telangiectasia, lipid exudates, unilateral presentation
- Distinguishing factors:
- Unilateral
- No avascular retina
- Seen in older infants or children [83]
Persistent fetal vasculature
- Key features: Microphthalmia, leukocoria, and a fibrovascular stalk extending from the optic disc to the posterior lens
- Distinguishing factors:
- Unilateral
- No plus disease
- Absence of peripheral neovascularization [84]
Incontinentia Pigmenti
- Key features: Retinal neovascularization, retinal detachment, and pigmentary changes, accompanied by cutaneous findings
- Distinguishing factors:
- Skin lesions in Blaschko lines
- Female infants
- X-linked dominant [85]
Toxocariasis
- Key features: Retinal granuloma, vitreous inflammation, leukocoria
- Distinguishing factors:
- Unilateral
- Positive serology (eg, enzyme-linked immunosorbent assay)
- Systemic eosinophilia is possible [86]
Congenital cytomegalovirus retinitis
- Key features: Retinitis, chorioretinal scars, periventricular calcifications (on neuroimaging)
- Distinguishing factors:
- Cytomegalovirus polymerase chain reaction positive
- Sensorineural hearing loss
- Neurodevelopmental delay [87]
Retinoblastoma
- Key features: Leukocoria, strabismus, intraocular mass with calcification
- Distinguishing factors:
- Ultrasonography/computed tomography (CT), eg, calcified mass
- Requires urgent oncology referral [88]
Syphilitic retinopathy
- Key features: Chorioretinitis, salt-and-pepper fundus, interstitial keratitis
- Distinguishing factors:
- Maternal syphilis history
- Positive Venereal Disease Research Laboratory test and fluorescent treponemal antibody absorption test [89]
Walker-Warburg syndrome
- Key features: Congenital muscular dystrophy, brain malformations (eg, lissencephaly), and ocular abnormalities (retinal dysplasia)
- Distinguishing points: Systemic features dominate; genetic testing confirms diagnosis [90]
Albinism (oculocutaneous and ocular)
- Key features: Nystagmus, foveal hypoplasia, depigmented fundus
- Distinguishing points: Absence of neovascularization, generalized hyperpigmentation [91]
Leber congenital amaurosis
- Key features: Severe vision loss from birth, extinguished electroretinogram (ERG), oculodigital sign
- Distinguishing points: No plus disease, no avascular retina; ERG abnormal from early infancy [92]
Retinal dysplasia (nonsyndromic)
- Key features: Disorganized retina, rosettes, leukocoria, retinal detachment
- Distinguishing points: Static, nonprogressive; diagnosed via imaging and histopathology if available [93]
ROP mimicker in intrauterine growth restriction
- Key features: Vascular developmental delay without classic plus or stage signs
- Distinguishing points: Borderline birthweight, vascular tortuosity without classic ROP staging [94]
Combined hamartoma of retina and retinal pigment epithelium
- Key features: Epiretinal membrane, retinal dragging, macular thickening
- Distinguishing points: Usually unilateral; optical coherence tomography (OCT) is helpful in diagnosis [95]
Congenital zika syndrome retinopathy
- Key features: Macular atrophy, pigmentary changes, optic nerve hypoplasia
- Distinguishing points: History of maternal infection, confirmed with serology/polymerase chain reaction [96]
Table. Differential Diagnosis for Retinopathy of Prematurity
|
Condition |
Key Features |
How to Differentiate From ROP |
|
Familial exudative vitreoretinopathy |
Peripheral avascular retina, family history |
Genetic testing, full-term infants, and bilateral asymmetry |
|
Norrie disease |
Total retinal detachment at birth, male infants, and hearing loss |
X-linked, systemic associations, early blindness |
|
Coats disease |
Telangiectasia, exudation, unilateral |
Older age group, no plus disease |
|
Persistent fetal vasculature |
Microphthalmia, retrolental fibrovascular stalk |
Unilateral, no ROP staging, confirmed on ultrasound |
|
Incontinentia pigmenti |
Blaschko-line rash, neovascularization |
Female infants, X-linked dominant |
|
Toxocariasis |
Granuloma, vitritis, unilateral |
ELISA serology, eosinophilia, exposure history |
|
CMV retinitis (Congenital) |
Scars, retinitis, periventricular calcifications |
CMV IgM/IgG, CNS involvement |
|
Retinoblastoma |
Calcified intraocular tumor |
CT/USG, leukocoria, positive RB1 mutation |
|
Syphilitic chorioretinitis |
Salt-and-pepper fundus, interstitial keratitis |
Maternal syphilis history, VDRL positive |
|
Walker-warburg syndrome |
Ocular + brain + muscular abnormalities |
Genetic testing, brain MRI findings |
|
Albinism |
Nystagmus, iris transillumination, hypopigmented fundus |
Skin/hair findings, ERG intact, no retinal neovascularization |
|
Leber congenital amaurosis |
Early-onset vision loss, oculodigital sign |
Absent ERG, no plus disease |
|
Retinal dysplasia |
Rosettes, disorganized retina |
OCT, nonprogressive appearance |
|
IUGR with immature retina |
Delayed vascularization |
Lacks classic ROP staging or plus disease |
|
CHRRPE |
Macular dragging, gliotic membrane |
OCT confirms hamartoma, usually unilateral |
|
Congenital Zika syndrome |
Macular atrophy, pigment clumps, microcephaly |
History of maternal infection, Zika serology |
CHRRPE, combined hamartoma of the retina and retinal pigment epithelium; CT, computed tomography; ELISA, enzyme-linked immunosorbent assay; ERG, electroretinography; IgG, immunoglobulin G; IgM, immunoglobulin M; IUGR, intrauterine growth restriction; MRI, magnetic resonance imaging; OCT, optical coherence tomography; ROP, retinopathy of prematurity; USG, ultrasonography; VDRL, Venereal Disease Research Laboratory.
Pertinent Studies and Ongoing Trials
Pertinent studies and ongoing clinical trials continue to shape the evolving understanding and management of ROP (see Table. Pertinent Studies on Retinopathy of Prematurity). These investigations provide critical insights into disease progression, treatment efficacy, and long-term outcomes, guiding evidence-based clinical practice.
Table 3. Pertinent Studies on Retinopathy of Prematurity
|
Study Name |
Key Focus |
Findings/Significance |
|
ETROP study [97] |
Evaluated early laser treatment in high-risk prethreshold ROP |
Showed better outcomes with early treatment in type 1 ROP |
|
BEAT-ROP trial [98] |
Anti-VEGF (bevacizumab) vs laser for Zone I and posterior Zone II ROP |
Bevacizumab is superior in Zone I disease; reduced recurrence compared to laser |
|
RAINBOW study [99] |
Phase 3 RCT comparing ranibizumab 0.2 mg vs laser in infants with ROP |
Ranibizumab was noninferior to laser, with a favorable safety profile |
|
STOP-ROP study [100] |
Effect of supplemental oxygen to reduce progression in prethreshold ROP |
No significant benefit; high oxygen levels increased the risk of adverse events |
|
FIREFLEYE study [101] |
Safety and efficacy of Abicipar Pegol (anti-VEGF) in infants with ROP |
Ongoing phase 2 trial; exploring long-acting anti-VEGF for fewer retreatments |
|
BUTTERFLEYE study [102] |
Abicipar Pegol in Zone I and II ROP |
Companion study to FIREFLEYE for broader disease spectrum |
|
NEOLEV study [103] |
Levetiracetam for neuroprotection in preterms with ROP |
Investigating whether early treatment can prevent long-term neurodevelopmental delays |
|
INTREPID study [104] |
Real-world data on intravitreal anti-VEGF use in ROP across multiple centers |
Aims to establish post-market safety and recurrence rates with ranibizumab and bevacizumab |
|
DRIVE study [105] |
Artificial intelligence for automated ROP screening |
Validated deep learning model with comparable sensitivity to expert examiners |
|
NEST-ROP study [106] |
Investigate sleep, circadian rhythm, and their link with ROP progression |
Exploring if altered light cycles affect retinal vascular growth |
BEAT-ROP, Bevacizumab Eliminates the Angiogenic Threat of Retinopathy of Prematurity; BUTTERFLEYE, Baby Using Thermal Imaging and Tele-ophthalmology for Evaluation of ROP and Follow-up Eye Exams; DRIVE, Deep ROP Imaging with Vascular Extraction; ETROP, Early Treatment for Retinopathy of Prematurity; FIREFLEYE, Fundus Imaging in ROP Using a Handheld Camera With AI for Rapid Evaluation; INTREPID, Indian Twin-City Retinopathy of Prematurity Imaging and Data Repository; NEOLEV, Neonatal Evaluation and Outcomes of Low Birth Weight Infants With Elevated VEGF; NEST-ROP, Neurodevelopment and Structural Outcomes of ROP; RAINBOW, RAnibizumab Compared With Laser Therapy for the Treatment of Infants Born With Retinopathy of Prematurity; RCT, randomized controlled trial; STOP-ROP, Supplemental Therapeutic Oxygen for Prethreshold Retinopathy of Prematurity; TROP, Tele-ROP; VEGF, vascular endothelial growth factor.
Highlights from Recent Literature
- AI and telemedicine: Rapid developments in AI models for ROP screening (eg, i-ROP deep learning) are enhancing detection in low-resource settings.[107]
- VEGF suppression and systemic safety: Studies have highlighted systemic VEGF suppression after anti-VEGF therapy in neonates, prompting the need for careful monitoring.[108]
- Long-term follow-up: Recent research emphasizes the importance of a 3- to 5-year follow-up after anti-VEGF injections, considering the risks of late recurrence.[109]
Treatment Planning
Effective ROP management hinges on timely screening, precise staging, and individualized intervention strategies, all coordinated by an interprofessional team comprising neonatologists, ophthalmologists, nursing staff, and, when needed, pediatric anesthesiologists (see Table. Retinopathy of Prematurity Treatment Modalities). Once an infant meets screening criteria (eg, birth weight ≤1500 g or gestational age ≤30 weeks, or as per local guidelines), dilated indirect ophthalmoscopy should occur at 4 to 6 weeks’ postnatal age or 31 to 33 weeks’ postmenstrual age—whichever is later. Disease is classified by zone (I–III), stage (1–5), and the presence of “plus” disease, as per the ICROP.[31]
Observation vs Treatment
- Stage 1–2 without plus disease: Continue weekly to biweekly examinations until retinal vascularization reaches zone III or disease regresses spontaneously.
- Type 1 ROP (treatment-requiring: any stage 3 in zone I; stage 2 or 3 plus disease in zone II): Initiate treatment within 48 hours to reduce progression risk.
- Aggressive posterior ROP (AP-ROP): This is often located in zone I and is rapidly progressive; it should be treated emergently.[110]
First-Line Therapies
- Laser photocoagulation
- Ablation of the avascular peripheral retina to decrease VEGF production.
- High anatomical success (>90%) when applied according to ETROP guidelines.
- Intravitreal anti-VEGF (bevacizumab, ranibizumab, aflibercept)
- Consider for AP-ROP, zone I disease, or when laser is contraindicated (eg, poor pupil dilation, media opacity).
- This preserves the peripheral retina but requires extended, scheduled follow-up for late reactivation.[111]
Adjunct and Advanced Surgical Options
- Cryotherapy: This is reserved for settings lacking laser access, with higher risks of inflammation and refractive errors.
- Scleral Buckling or Vitrectomy: This is indicated for stage 4A/B (macula-sparing/macular-involving) or stage 5 detachments; success correlates inversely with detachment severity.[112]
Follow-Up and Long-Term Care After intervention, infants require close monitoring, with weekly exams until regression is achieved, followed by exams at 6- to 12-month intervals to detect late sequelae, such as refractive errors and amblyopia. An interprofessional ROP clinic facilitates streamlined appointments, ensures adherence to imaging protocols (RetCam or wide-field), and integrates neurodevelopmental support services for vision rehabilitation. This comprehensive, team-based approach—grounded in evidence from ETROP, BEAT-ROP, and more recent trials—ensures that each infant receives the optimal blend of surveillance and timely intervention, minimizing ROP-related vision loss while safeguarding overall health.[113]
Table 4. Retinopathy of Prematurity Treatment Modalities
|
Treatment |
Indication |
Key Points |
|
Observation |
Stage 1–2 without plus disease |
Serial exams; the disease often regresses spontaneously |
|
Laser photocoagulation |
Type 1 ROP (Stage 3+) in Zone I/II with plus disease |
Gold standard; ablates avascular retina to reduce VEGF stimulus; high success (>90%) |
|
Intravitreal anti-VEGF |
Aggressive posterior ROP or contraindication to laser |
Bevacizumab, ranibizumab, aflibercept; preserves peripheral retina; requires long-term monitoring |
|
Cryotherapy |
When laser contact is not possible or in resource-limited settings |
More inflammation and myopia risk than laser; now largely replaced by laser |
|
Scleral Buckling/vitrectomy |
Stage 4A/B or Stage 5 retinal detachment |
Surgical for advanced ROP; anatomical success depends on timely referral and adjunctive medical care |
ROP, retinopathy of prematurity; VEGF, vascular endothelial growth factor.
Toxicity and Adverse Effect Management
Effective management of ROP extends beyond timely intervention to include careful consideration of treatment-related toxicities and adverse events. Understanding the potential ocular and systemic complications associated with therapies such as laser photocoagulation and anti-VEGF agents is essential for optimizing long-term outcomes in this vulnerable population.
Laser Photocoagulation
- Ocular surface injury: Corneal epithelial defects or punctate keratopathy may occur from prolonged eyelid speculum use and bright illumination.
- Management: Frequent preservative-free lubricants, therapeutic soft contact lenses for persistent epithelial defects, and reduction of light exposure duration
- Anterior segment inflammation: Iris synechiae, uveitis, or pupillary membrane formation can arise from thermal injury.
- Management: Topical corticosteroids (eg, prednisolone acetate 1% 4 times a day taper) and cycloplegics (eg, cyclopentolate 0.5% 2 times a day) for 1 to 2 weeks.
- Myopia and refractive change: Peripheral retina scarring often induces a myopic shift.
- Management: Early refraction at 6 to 9 months corrected age; prescription of spectacles and surveillance for amblyopia [114]
Intravitreal Anti-VEGF Agents
- Ocular complications: These include endophthalmitis (<0.1%), vitreous hemorrhage, lens touch, or retinal detachment.
- Management: Adhere to aseptic injection protocols and observe for signs of infection; administer prompt intravitreal antibiotics if infection is suspected.
- Systemic VEGF suppression: The potential effects on neurodevelopment, renal maturation, and organogenesis remain theoretical but warrant cautious dosing.
- Management: Use the lowest effective dose (eg, 0.2 mg bevacizumab or 0.2 mg ranibizumab, as per the BEAT-ROP and RAINBOW trials). Monitor growth parameters and coordinate with neonatology to monitor blood pressure and renal function.
- Late Reactivation: Up to 20% may exhibit ROP reactivation 6 to 12 weeks post-injection.
- Management: Scheduled dilated exams every 1 to 2 weeks through postmenstrual 60 weeks or until complete vascularization; retreat laser or repeat anti-VEGF as indicated.[115]
Cryotherapy (Historical/Resource-Limited Settings)
- Management: Use aggressive topical corticosteroids and systemic analgesics; consider nonsteroidal anti-inflammatory drugs, if safe.
- Intense inflammation and pain lead to lid swelling, conjunctival chemosis, and an increased risk of sympathetic ophthalmia.[116]
Surgical Interventions (Vitrectomy/Scleral Buckle)
- Management: Maintain low infusion pressures, use diathermy judiciously, and apply perfluorocarbon liquids to stabilize the retina.
- Management: Close intraocular pressure monitoring; topical steroids for inflammation, topical aqueous suppressants (eg, dorzolamide) for elevated intraocular pressure, and suturing/leak repair for hypotony.
- Intraoperative bleeding: Retinal or choroidal hemorrhage can complicate dissection.
- Postoperative hypotony/hypertension: Wound leaks or inflammatory reactions may alter intraocular pressure.[117]
General Supportive Care
- Management: These include oral sucrose, swaddling, topical anesthetic drops, and pacifier use during exams.
- Management: Collaborate with neonatology to maintain SpO2 targets (90–95%), ensure adequate caloric intake, and supplement omega-3 fatty acids as needed.
- Pain and stress minimization: Infants may experience discomfort during examinations and procedures.
- Nutrition and growth monitoring: Achieving optimal weight gain and maintaining oxygen saturation control minimizes the severity of ROP.[118]
Key Takeaway: The proactive identification and prompt management of treatment-related toxicities, through meticulous technique, interprofessional coordination, and vigilant follow-up, are crucial for safeguarding both ocular and systemic health in infants undergoing ROP therapy.
Staging
Staging of ROP is essential for assessing disease severity and guiding appropriate management. The ICROP provides a standardized framework based on retinal zone, disease stage, and vascular activity, enabling consistent diagnosis and treatment decisions (see Table. International Classification of Retinopathy of Prematurity [ICROP] Staging).
Table 5. International Classification of Retinopathy of Prematurity (ICROP) Staging
|
Stage |
Description |
Key Clinical Features |
|
1 |
Demarcation line |
Flat, thin, white line at junction between vascularized and avascular retina |
|
2 |
Ridge |
Thickened, elevated ridge with width and height at the junction |
|
3 |
Extraretinal fibrovascular proliferation |
Neovascular tufts “pop” into the vitreous from the ridge |
|
4A |
Partial retinal detachment, extrafoveal |
Shallow detachment not involving the fovea; traction by a ridge |
|
4B |
Partial retinal detachment, foveal-involving |
Shallow detachment, including the fovea |
|
5 |
Total retinal detachment |
Funnel-shaped, often closed-door detachment of the entire retina |
Additional ICROP descriptors (often recorded alongside the stage) include the following:
- Zone
- Zone I: Posterior circle, centred on optic disc (radius is twice the disc–fovea distance)
- Zone II: Extends centrifugally to the nasal ora serrata
- Zone III: Remaining temporal crescent
- Plus disease: Includes ≥ 2-quadrant venous dilation and arteriolar tortuosity in the posterior pole
- “Pre-plus” disease: Vascular changes insufficient for full plus
Note: In clinical practice, stage, zone, and plus-status are often combined (eg, “Stage 3 in Zone II with plus disease”) to inform screening intervals and treatment decisions.[41]
Prognosis
Advances have improved outcomes in treatment; nonetheless, many treated individuals experience adverse events on visual acuity. One of the goals of treatment is to avoid unfavorable retinal structural outcomes, as defined by the ETROP study, which include a posterior retinal fold involving the macula, a retinal detachment involving the macula, retrolental tissue or mass obscuring the view of the posterior pole, or the need for a vitrectomy or scleral buckling procedure. Results from a follow-up to the ETROP study found that at 2 years, unfavorable structural outcomes had been reduced to 9.1% in high-risk pre-threshold eyes treated early.[97] The 6-year follow-up of the same sample found that 34.6% of children had a visual acuity of 20/40 or better, 40.3% had an acuity greater than 20/40 and less than 20/200, and 23.7% had an acuity worse than 20/200, including light perception and blindness.[119]
The visual prognosis in infants with ROP varies widely depending on disease severity, timing of treatment, and associated comorbidities as follows:
Mild (Stage 1–2, no plus disease)
- Spontaneous regression occurs in more than 90% of cases, especially in zone II–III, at the early stage, without intervention.
- Final visual acuity is typically normal or near-normal.
Threshold and type 1 ROP (Stage 3 + disease, in zone I or II)
- Without timely treatment (laser or anti-VEGF), > 50% progress to retinal detachment and severe vision loss.
- With prompt laser photocoagulation (ET-ROP criteria) or intravitreal anti-VEGF therapy:
- Structural retinal outcome stabilizes in approximately 85% to 95% of eyes.
- Long-term refractive error: There is a high risk of myopia (approximately 30–50%).
- Peripheral visual field constriction is a common adverse effect of laser treatment.[120]
Advanced ROP (Stages 4–5 retinal detachment)
- Even with surgical repair (vitrectomy, scleral buckle), anatomical success (reattachment) is achieved in approximately 50% to 70% of eyes.
- Functional (useful) vision is achieved in only about 20% to 40% of cases; many remain legally blind.[121]
Anti-VEGF vs laser long-term considerations
- Anti-VEGF agents (eg, bevacizumab) show lower rates of peripheral visual field loss but carry uncertain systemic and ocular late-recurrence risks, necessitating extended follow-up.
- Laser treatment is associated with more substantial peripheral field loss and higher rates of myopia, but it has well-established durability.
Neurodevelopmental and refractive outcomes
- Eyes treated for ROP—regardless of modality—are at increased risk of strabismus (approximately 30%), amblyopia (about 20%), and refractive errors (myopia, astigmatism).
- Even regressed ROP may be associated with subtle deficits in contrast sensitivity and visual-motor integration.[122]
Follow-up and rehabilitation
- Lifelong ophthalmic surveillance, including annual exams, is recommended to monitor for late sequelae, such as retinal tears and refractive changes.
- Early low-vision services and amblyopia therapy can maximize functional vision in children with residual deficits.
Key prognostic factors • ROP zone (zone I carries the worst prognosis) • Presence of plus disease • Speed of progression to stage 3 • Timing and type of intervention [123]
Complications
Retinal detachment is the most frequent complication of ROP and is strongly associated with a poor visual outcome.[2][3] Macular folds are another common complication.[4] Threats to visual acuity persist through childhood; the most common sequelae are myopia.[5] Other late complications include glaucoma, amblyopia, cataract, and strabismus.[5]
Low vision secondary to ROP is associated with higher rates of developmental, educational, and social challenges. Results from a follow-up study to the CRYO-ROP study evaluated a cohort of children born at a weight of less than 1251 g and with threshold ROP at 5 and 8 years of age. Children in this cohort with unfavorable visual status, classified as vision limited to light perception or no light perception, had significantly higher rates of developmental disability, epilepsy, special education, and below-grade-level academic performance.[124]
Complications
-
Retinal detachment
-
Tractional rhegmatogenous detachment: Abnormal fibrovascular proliferation at the junction of vascularized and avascular retina can contract, pulling the neurosensory retina away from the retinal pigment epithelium. If left untreated, this can lead to irreversible vision loss.
-
Exudative detachment: In late-stage disease, fluid may leak under the retina, exacerbating structural damage.[125]
-
-
Foveal hypoplasia and amblyopia
-
Premature arrest of foveal development results in an underdeveloped foveal pit, reduced cone packing, and consequent diminished visual acuity even after successful ROP regression.
-
Strabismus and anisometropia, resulting from uneven macular development, further predispose individuals to amblyopia.[126]
-
-
High refractive errors
-
Myopia: Due to excessive growth in the anterior segment or changes in the posterior pole, it can exceed –10 D in severe cases.
-
Astigmatism and hyperopia: Abnormal corneal curvature from prior laser scars or zonular disruption leads to mixed refractive errors requiring early optical correction.[127]
-
-
Strabismus and nystagmus
-
Disrupted retinal development and sensory input imbalance commonly lead to esotropia or exotropia.
-
Sensory nystagmus may arise from poor fixation stability in eyes with central ROP damage.[128]
-
-
Glaucoma
-
Angle abnormalities resulting from peripheral anterior synechiae or fibrovascular membrane ingrowth can cause secondary open- or closed-angle glaucoma, characterized by increased intraocular pressure, corneal enlargement, and optic nerve damage.[129]
-
-
Band keratopathy and corneal scarring
-
Elevated serum calcium and phosphate levels, as well as chronic inflammation, can lead to calcium deposition in the superficial cornea, resulting in impaired transparency.
-
Prior cryotherapy or laser treatment may induce peripheral corneal scarring.[130]
-
-
Cataract formation
-
Intravitreal anti-VEGF injections and surgical interventions (laser or vitrectomy) carry a risk of lens opacity via direct trauma or altered anterior segment physiology.[131]
-
-
Vitreous hemorrhage
-
Fragile new vessels within cicatricial tissue can bleed spontaneously or during surgery, leading to sudden vision loss and complicating subsequent management.[132]
-
-
Phthisis bulbi
-
End-stage severe ROP with chronic retinal detachment and inflammation may progress to a non-functional, shrunken eye, necessitating enucleation for pain control.[133]
-
- Blindness and low-vision
- A significant minority of infants with aggressive ROP ultimately sustain bilateral blindness, requiring lifelong vocational and educational support services.[134]
Early detection, timely intervention, and meticulous long-term follow-up are crucial for minimizing these complications and optimizing visual and functional outcomes for ROP survivors.
Postoperative and Rehabilitation Care
Postoperatively, premature infants require mechanical or positive-pressure ventilation, parenteral nutrition, and antibiotic treatment. Patients should be monitored via pulse oximetry, neurologic screening, ultrasound screening, and assessment for bronchopulmonary dysplasia and patent ductus arteriosus.[135] Long-term therapy for amblyopia includes the use of glasses, patching for 6 to 12 hours a day, or pharmacologic penalization, wherein a miotic agent such as atropine is used to “blur” vision in the sound eye.[136][137]
Postoperative and Rehabilitation Care
- Immediate post-treatment monitoring
- Vital signs and systemic status: Continuously monitor oxygen saturation, heart rate, and blood pressure. Preterm infants often have labile cardiorespiratory function, and procedures (laser, intravitreal injection, or surgery) can precipitate apnea or bradycardia.
- Ocular examination: Within 24 to 48 hours, inspect for:
- Anterior segment: Check for corneal clarity, pupillary reaction, and signs of inflammation (hyaline membrane, anterior chamber cells/flare).
- Posterior segment: Perform indirect ophthalmoscopy to confirm regression of plus disease, adequacy of laser scars, and absence of new hemorrhage or retinal detachment.[138]
- Pain management and comfort measures
- Analgesia: Administer age-appropriate analgesics (eg, acetaminophen or small-dose opioids under NICU protocols) to minimize discomfort without depressing respiration.
- Nonpharmacologic comfort:
- Swaddling, facilitated tucking, or the use of sucrose pacifiers can reduce stress during postoperative examinations.
- Maintain a thermoneutral environment; preterm infants have a limited ability to regulate their temperature after procedures.[139]
- Infection prophylaxis
- Topical antibiotics: After intravitreal injection or surgery, apply broad-spectrum antibiotic drops (eg, moxifloxacin or tobramycin) every 4 hours for 5 to 7 days to prevent endophthalmitis.
- Aseptic handling: Ensure all follow-up examinations use sterile specula and instruments.[140]
- Nutrition and growth support
- Optimal nutrition: Ensure adequate caloric intake (breast milk fortified if needed) to support healing and neurodevelopment.
- Weight gain monitoring: Track daily weight gain; poor growth can delay ocular vascular maturation and complicate systemic recovery.[141]
- Visual rehabilitation
- Visual stimulation: Encourage gentle light exposure and age-appropriate visual interactions (eg, high-contrast patterns) once the child is medically stable to promote cortical visual development.
- Refractive correction: At 6 to 12 weeks post-regression, perform cycloplegic refraction. Prescribe corrective lenses for significant myopia, hyperopia, or astigmatism to reduce the risk of amblyopia.[142]
- Ongoing ophthalmic follow-up
- Schedule:
- Weekly to biweekly, until complete vascularization of zone III is achieved, or as directed by disease severity.
- Monthly thereafter until at least 6 months corrected age.
- Assess for late sequelae: Monitor for retinal folds, traction, or late-onset detachment.[143]
- Developmental and multidisciplinary rehabilitation
- Early intervention referral: Coordinate with developmental pediatrics for vision-specific early intervention services, such as orientation and mobility training and vision therapy.
- Physical and occupational therapy: Address motor delays common in preterm infants, supporting hand-eye coordination and postural stability.[144]
- Family education and support
- Caregiver training: Teach parents how to administer eye drops, recognize warning signs (eg, increased redness, discharge, poor feeding), and maintain follow-up schedules.
- Psychosocial support: Offer counseling resources and connect families with parent support groups for children who are visually impaired.[145]
- Transition to long-term care
- Pediatric ophthalmology to low vision services: As the child approaches 1 to 2 years of age, transition to low-vision specialists for assessment of functional vision aids, such as magnifiers and electronic devices.
- Educational planning: Collaborate with early childhood educators to integrate vision accommodations in preschool settings.[146]
- Documentation and quality improvement
- Detailed records: Document all postoperative findings, interventions, and parental instructions.
- Audit outcomes: Participate in unit-based ROP outcome reviews to refine postoperative protocols and identify areas for improvement.
Timely, comprehensive postoperative care coupled with early rehabilitative interventions and family engagement is pivotal to maximizing visual potential and developmental trajectories in infants treated for ROP.[147]
Consultations
Effective care of patients with ROP relies on timely consultations with key specialists to ensure comprehensive evaluation and management. Collaborative input is essential for accurate diagnosis, coordinated treatment, and optimal visual outcomes.
Consultations
-
Neonatology/perinatology:
-
Role: Optimize systemic care (oxygen therapy, nutrition, fluid balance) and schedule timely ROP screenings.
-
Consultation: Weekly rounds to adjust ventilator/oxygen settings and ensure stable weight gain, minimizing risk factors for ROP progression.[148]
-
-
Pediatric ophthalmology:
-
Role: Perform indirect ophthalmoscopic examinations, zone/stage classification, and decision-making for laser or anti-VEGF therapy.
-
Consultation: Initial exam by 31 to 32 weeks postmenstrual age, with follow-up intervals determined by findings (typically every 1–2 weeks).[149]
-
-
Retinal surgery/intervention team:
-
Role: Provide laser photocoagulation, intravitreal injections, or surgical intervention (eg, vitrectomy) for threshold or aggressive posterior ROP.
-
Consultation: Urgent notification and coordination within 24 to 48 hours when treatment criteria are met.[150]
-
-
Neonatal anesthesia/pediatric anesthesia:
-
Role: Safely anesthetize preterm infants for laser or surgical procedures, balancing hemodynamic stability and minimizing risks.
-
Consultation: Preprocedure evaluation to plan sedation, airway management, and postoperative monitoring.[151]
-
-
Pediatric cardiology/pulmonology (as needed):
-
Role: Assess and manage comorbidities (eg, patent ductus arteriosus, bronchopulmonary dysplasia) that may influence perioperative risk and systemic oxygenation.
-
Consultation: Referral when significant cardiopulmonary disease is present to optimize cardiorespiratory status before ocular interventions.[152]
-
-
Developmental pediatrics/neonatal follow-up clinic:
-
Role: Monitor long-term visual development, refractive errors, strabismus, and neurodevelopmental milestones.
-
Consultation: Routine assessments at corrected ages of 6, 12, and 24 months to detect amblyopia, motor delays, or sensory impairments.[153]
-
-
Genetics/neonatal nutrition:
-
Role: Evaluate for underlying metabolic or syndromic associations; ensure adequate postnatal growth with fortified breast milk or preterm formula.
-
Consultation: As indicated by familial history or poor weight gain, to tailor nutritional strategies that reduce ROP risk.[154]
-
-
Social work/case management:
-
Role: Facilitate parent education on the importance of ROP screening, coordinate follow-up appointments, and ensure access to low-vision resources.
-
Consultation: Before discharge, engage families in scheduling outpatient visits and support services.[155]
-
By integrating these specialties—neonatology, ophthalmology, anesthesia, subspecialty medicine, developmental services, and social support—care teams can optimize both acute treatment and long-term outcomes for infants at risk of vision loss from ROP.
Deterrence and Patient Education
The role of oxygen therapy in preventing ROP is of crucial importance. There is a history of targeting SpO2 to reduce rates of ROP. In 2001, an observational study suggested that ROP rates could be decreased by using a lower SpO2 target without increasing the risk of mortality.[156] With the results of this study and others suggesting lower SpO2 targets for neonates, the Neonatal Oxygenation Prospective Meta-analysis (NeOProM) collaboration was formed. NeOProM consisted of an international group of neonatologists aimed at determining the optimal SpO2 level in premature infants.[157] Study authors sought to compare SpO2 targets of 85% to 89% in infants born at less than 28 weeks' gestation with the primary outcome of death or major disability at 18 to 24 months of age.[157] A total of 5 studies were funded. Analysis of the data revealed that insufficient oxygen levels increase the risk of death, and maintaining a SpO2 target of 90% to 94% is recommended.[158]
With such strong data suggesting that lowering SpO2 is an unacceptable solution to the problem of increasing rates of ROP, alternative solutions have been proposed. International data networks monitoring neonatal outcomes have shown that severe ROP is associated with financial investment within and between countries. Best practices in neonatal care encompass improved nutrition, infection control, pain management, temperature regulation, and supportive developmental care. These basic neonatal best care practices were outlined by Darlow et al in 2019.[158]
Optimal Oxygen Management
- Target saturation ranges: Maintain preterm infants’ arterial oxygen saturation between 90% and 95% to minimize both hypoxia-induced angiogenesis and hyperoxia-driven vessel obliteration.
- Automated titration systems: Use closed-loop oxygen delivery devices when available, with alarm thresholds set to prompt staff intervention for sustained out-of-range values.
- Gradual weaning protocols: Reduce supplemental oxygen gradually rather than abruptly, especially during the first 4 weeks of life, to prevent sudden oxidative stress on the developing retina.[159]
Nutritional Optimization
- Breast milk fortification: Provide human milk fortified with additional protein and calories to support steady somatic and retinal growth.
- Docosahexaenoic acid supplementation: Supplement with docosahexaenoic acid to promote normal retinal vascular development and neuroprotection.
- Mineral and vitamin support: Ensure adequate intake of zinc, as well as vitamins A and E, to bolster antioxidant defenses.[160]
Minimizing Other Modifiable Risks
- Infection Control: Adhere to strict hand hygiene practices, line-care bundles, and early removal of central lines to reduce sepsis episodes, which can exacerbate the risk of ROP.
- Avoiding Fluctuating Perfusion: Maintain stable blood pressure and avoid rapid volume shifts in circulatory management to prevent ischemia–reperfusion injury.[161]
Patient and Caregiver Education
Understanding ROP and its risks
- Explain that ROP is a “wiring” problem of the eye’s developing blood vessels that oxygen levels, nutrition, and overall health can influence.
- Stress that small preterm infants (<1500 g or before 32 weeks gestation) are at highest risk and require specialized eye care.[162]
Importance of timely screening and follow-up
- Emphasize adherence to the recommended screening schedule, which includes the first exam by 31 to 32 weeks postmenstrual age, followed by examinations every 1 to 2 weeks until complete retinal vascularization is achieved.
- Provide appointment reminders and coordinate transportation assistance to reduce missed visits.[163]
Signs to watch for
- Teach caregivers to recognize warning signs, such as abnormal eye movements, the “white reflex” visible in photographs, or eye redness. Although these signs are often late indicators, they should not be used as a substitute for scheduled exams.[164]
Preparing for treatment
- Describe what laser photocoagulation or intravitreal injection entails, including typical duration (<30 min), light sedation needs, and potential adverse events.
- Reassure parents about the safety measures in place, including pediatric anesthesia, continuous monitoring, and the expertise of experienced neonatal ophthalmologists.[165]
Home care and developmental support
- Instruct staff on maintaining a smoke-free environment and minimizing exposure to bright light in the nursery.
- Encourage “kangaroo care” (skin-to-skin contact) and developmental positioning to support infant stability, which may indirectly benefit retinal health.[166]
Long-term vision care
- Inform families that ROP survivors require ongoing vision assessments throughout their school years, including refraction, strabismus screening, and visual developmental milestones.
- Provide resources for early intervention services, low-vision aids, and support groups if sequelae (eg, refractive errors, amblyopia) arise.
By combining proactive neonatal care protocols with clear, culturally sensitive education for caregivers, healthcare teams can significantly reduce the incidence of ROP and ensure early detection and treatment, thereby optimizing visual outcomes for these vulnerable infants.[3]
Pearls and Other Issues
Understanding key clinical pearls of ROP can enhance early detection, streamline decision-making, and improve patient outcomes. These practical insights highlight critical aspects of screening, diagnosis, and treatment that may not be immediately evident in standard guidelines.
Pearls and Other Issues
- Timing is critical
- Screen at 4 to 6 weeks postnatal age or 31 to 33 weeks postmenstrual age, whichever is later. Early detection of “type 1 ROP” (zone I, any plus; zone I, stage 3 without plus; zone II, stage 2–3 with plus) enables prompt treatment.[167]
- Zone I disease warrants vigilance
- ROP in zone I progresses more rapidly and carries a worse prognosis. Consider conducting earlier re-examinations (weekly or more frequently) and setting a low threshold for intervention.[168]
- Plus disease is dynamic
- Defined by dilation and tortuosity of posterior vessels; even subtle “pre-plus” changes can herald rapid progression. Always compare serial RetCam or indirect ophthalmoscopy images.[169]
- Anti-VEGF therapy: Pros and cons
- Intravitreal bevacizumab or ranibizumab spares the peripheral retina and reduces myopia but may require extended monitoring for late reactivation, sometimes up to 70 weeks postmenstrual age.
- Laser photocoagulation remains the gold standard
- Durable effect after 1 session; peripheral field loss and higher myopia risk are trade-offs. Optimal confluent burns just anterior to the ridge minimize overtreatment.[7]
- Reactivation is possible
- Even after regression, continue to monitor for late-onset reactivation (especially post-anti-VEGF) until the retinal vessels reach zone III; return to weekly examinations if any vascular dragging or new plus disease develops.[8]
- Systemic health impacts outcomes
- Poor weight gain, sepsis, and unstable oxygenation are strong risk factors. Multidisciplinary coordination with neonatology to minimize fluctuations in oxygen saturation (target 90–95%) reduces the severity of ROP.[9]
- Family counseling and follow-up
- Educate families about the importance of long-term ophthalmic care (beyond infancy) to monitor refractive errors, strabismus, and late retinal complications (eg, retinal tears, detachments).[10]
- Documentation aids consistency
- Use standardized ROP diagrams or digital imaging to record zone, stage, presence of plus disease, and treatment areas. Consistency between providers ensures timely decisions.[170]
- Emerging technologies
- Tele-ROP screening, deep-learning-based image analysis, and wide-field OCT may enhance detection in resource-limited settings and improve interobserver agreement.[8]
- Ethical and resource considerations
- In low- and middle-income countries, balancing limited laser/anti-VEGF availability against a high disease burden is crucial; regional ROP programs and training can help expand capacity.[168]
- Neurodevelopmental correlates
- ROP severity and its treatment modalities may correlate with neurodevelopmental outcomes; coordinate with pediatric neurology for early intervention services when indicated.[171]
Enhancing Healthcare Team Outcomes
Antenatal, delivery suite, and NICU personnel have a role in preventing ROP. Antenatal prevention includes the use of corticosteroids before preterm delivery, a treatment shown to reduce morbidity and mortality.[57] The World Health Organization recommends that antenatal corticosteroids be used at less than 35 weeks’ gestation if preterm birth is imminent and obstetric care is available.[172] Delivery suite interventions such as delayed cord clamping may reduce the risk of anemia and the need for blood transfusions, 2 potential risk factors for the development of ROP.[173] Mechanisms to prevent hypothermia, such as plastic occlusive wrapping, should be utilized, as it was found that infants under 33 weeks with a NICU admission temperature of 36.5 °C to 37.2 °C ( 97.7?°F to 99.0?°F) had the lowest rate of ROP.[174]
Enhancing Healthcare Team Outcomes in ROP Management
- Interdisciplinary screening protocols
- Neonatologists and nurses: Implement standardized oxygen targeting (90–95%), monitor weight gain, and flag at-risk infants for referral to ophthalmology.
- ROP coordinators: Track screening schedules and ensure timely examinations; maintain real-time logs to prevent missed follow-ups.[167]
- Shared decision-making conferences
- Ophthalmologists, neonatologists, and parents: Hold weekly briefings to review each infant’s ROP status, systemic stability, and treatment readiness. This practice fosters consensus on the timing of interventions (laser vs anti-VEGF) and allows caregivers to express their concerns.[168]
- Telemedicine integration
- Imaging technicians and remote specialists: Deploy wide-field RetCam captures in the NICU, transmitting images to centralized ROP experts for rapid triage—expanding access in resource-limited settings and reducing examiner variability.[169]
- Standardized protocol checklists
- All team members: Use point-of-care digital checklists that detail screening criteria, zone/stage documentation, plus-disease evaluation, and treatment parameters to ensure consistency and minimize errors.[8]
- Regular interprofessional training
- Ophthalmology, nursing, and neonatal staff: Conduct quarterly workshops on ROP grading (ICROP3), laser technique, intravitreal injection safety, and post-treatment monitoring—bolstering skill proficiency and team cohesion.[9]
- Family-centered care coordination
- Social workers and counselors: Provide standardized educational materials, arrange tele-visits for rural families, and coordinate transportation/logistics for follow-up, thereby improving adherence to long-term surveillance.[170]
- Data-driven quality improvement
- Clinical informatics and quality teams: Analyze outcome metrics (treatment success rates, reactivation rates, and adverse events), share dashboards with the care team, and implement Plan-Do-Study-Act cycles to refine protocols.[11]
- Psychosocial support and burnout prevention
- Leadership and human resources: Offer debriefing sessions after critical ROP interventions, ensure adequate staffing to allow micro-breaks, and provide access to mental health resources, sustaining team well-being and vigilance.[171]
By synchronizing efforts across disciplines, leveraging technology, and fostering continuous learning, interprofessional healthcare teams can deliver timely and effective ROP care, ultimately preserving vision and optimizing developmental outcomes for premature infants.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
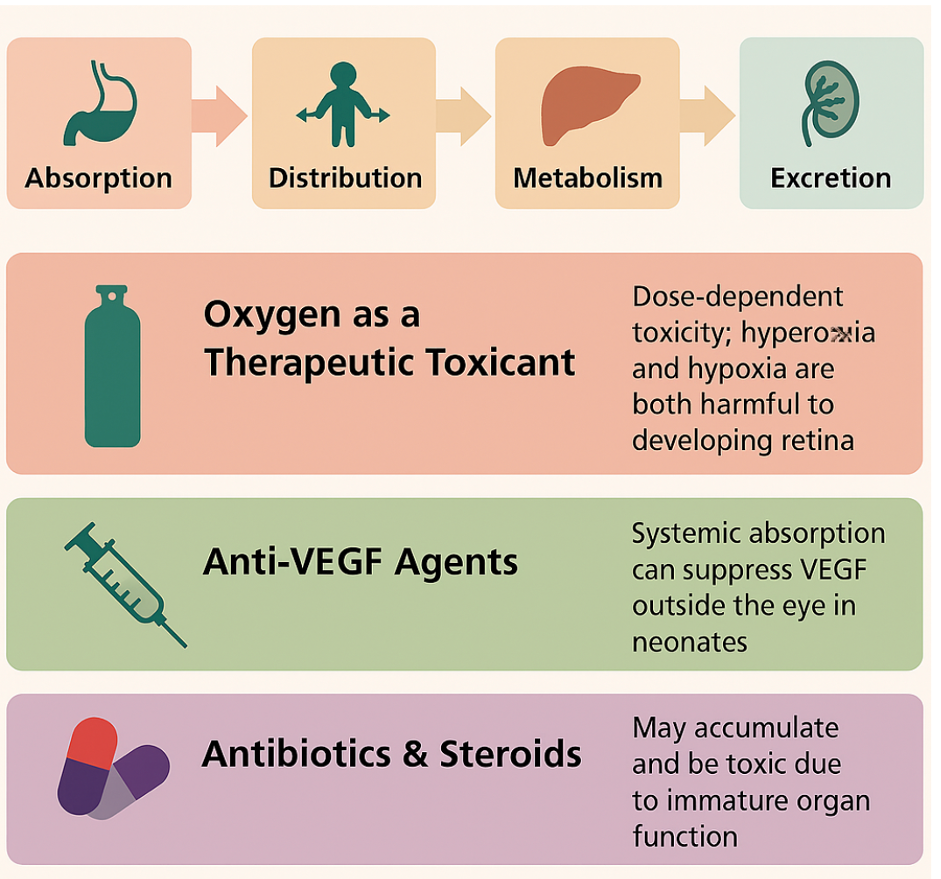
Toxicokinetics of Retinopathy of Prematurity. The concept of toxicokinetics is becoming increasingly relevant due to the administration of various pharmacological agents in neonatal care, especially oxygen, antibiotics, steroids, and vascular endothelial growth factor inhibitors
Contributed by K Kaur, MD
References
BEDROSSIAN RH, CARMICHAEL P, RITTER J. Retinopathy of prematurity (retrolental fibroplasia) and oxygen. I. Clinical study. II. Further observations on the disease. American journal of ophthalmology. 1954 Jan:37(1):78-86 [PubMed PMID: 13114325]
Hardy RJ, Good WV, Dobson V, Palmer EA, Phelps DL, Quintos M, Tung B, Early Treatment for Retinopathy of Prematurity Cooperative Group. Multicenter trial of early treatment for retinopathy of prematurity: study design. Controlled clinical trials. 2004 Jun:25(3):311-25 [PubMed PMID: 15157731]
Level 1 (high-level) evidenceBlencowe H, Lawn JE, Vazquez T, Fielder A, Gilbert C. Preterm-associated visual impairment and estimates of retinopathy of prematurity at regional and global levels for 2010. Pediatric research. 2013 Dec:74 Suppl 1(Suppl 1):35-49. doi: 10.1038/pr.2013.205. Epub [PubMed PMID: 24366462]
Level 3 (low-level) evidenceEssex RW, Carden SM, Elder JE. Two-year results of laser treatment for retinopathy of prematurity at a single neonatal intensive care unit. Clinical & experimental ophthalmology. 2005 Aug:33(4):390-4 [PubMed PMID: 16033352]
Broxterman EC, Hug DA. Retinopathy of Prematurity: A Review of Current Screening Guidelines and Treatment Options. Missouri medicine. 2016 May-Jun:113(3):187-90 [PubMed PMID: 27443043]
Kaur K, Gurnani B, Kannusamy V, Yadalla D. A tale of orbital cellulitis and retinopathy of prematurity in an infant: First case report. European journal of ophthalmology. 2022 Nov:32(6):NP20-NP23. doi: 10.1177/11206721211026098. Epub 2021 Jun 17 [PubMed PMID: 34137305]
Level 3 (low-level) evidenceDu Y, Dang Y. Recent Advances in Understanding the Role of PEST Sequence-Containing Proteins in Retinal Neovascularization. Current drug targets. 2025 Apr 18:():. doi: 10.2174/0113894501383009250410081726. Epub 2025 Apr 18 [PubMed PMID: 40257031]
Level 3 (low-level) evidenceGao J, Fang N, Xu Y. Application of Artificial Intelligence in Retinopathy of Prematurity From 2010 to 2023: A Bibliometric Analysis. Health science reports. 2025 Apr:8(4):e70718. doi: 10.1002/hsr2.70718. Epub 2025 Apr 18 [PubMed PMID: 40256143]
Ahmedhussain HK, Raffa LH, Alosaimif AM, Alessa SK, Alharbi SY, Almarzouki H, AlQurashi MA. Validation of DIGIROP- Birth and DIGIROP- Screen for the discovery of retinopathy of prematurity requiring treatment in preterm births in Saudi Arabia. Saudi medical journal. 2025 Apr:46(4):345-351. doi: 10.15537/smj.2025.46.4.20240773. Epub [PubMed PMID: 40254328]
Level 1 (high-level) evidenceBisen S, Gogoi P, Sharma A, Mukhopadhyay CS, Singh NK. A Disintegrin and Metalloproteinase 10 Regulates Ephrin B2-Mediated Endothelial Cell Sprouting and Ischemic Retinopathy. The American journal of pathology. 2025 Apr 17:():. pii: S0002-9440(25)00114-2. doi: 10.1016/j.ajpath.2025.03.007. Epub 2025 Apr 17 [PubMed PMID: 40252970]
Hosny R, Gouda J, Macky TA, Khattab A, Mekkawy H, Abdullatif AM. OCT macular changes in type 1 ROP following Ranibizumab injections. International journal of retina and vitreous. 2025 Apr 17:11(1):47. doi: 10.1186/s40942-025-00664-7. Epub 2025 Apr 17 [PubMed PMID: 40247366]
Abdelmassih Y, El-Khoury S, Mikhail M. Fluorescein Angiography for Retinopathy of Prematurity with High Oxygen Exposure in a Tertiary Referral Center in Rwanda. Ophthalmology. Retina. 2025 Apr 17:():. pii: S2468-6530(25)00129-0. doi: 10.1016/j.oret.2025.03.020. Epub 2025 Apr 17 [PubMed PMID: 40243969]
Schmitz AM, Bumbaru SM, Fakhouri LS, Zhang DQ. Long-Term Impairment of Retinal Ganglion Cell Function After Oxygen-Induced Retinopathy. Cells. 2025 Mar 29:14(7):. doi: 10.3390/cells14070512. Epub 2025 Mar 29 [PubMed PMID: 40214465]
Gensure RH, Chiang MF, Campbell JP. Artificial intelligence for retinopathy of prematurity. Current opinion in ophthalmology. 2020 Sep:31(5):312-317. doi: 10.1097/ICU.0000000000000680. Epub [PubMed PMID: 32694266]
Level 3 (low-level) evidenceHong EH, Shin YU, Cho H. Retinopathy of prematurity: a review of epidemiology and current treatment strategies. Clinical and experimental pediatrics. 2022 Mar:65(3):115-126. doi: 10.3345/cep.2021.00773. Epub 2021 Oct 12 [PubMed PMID: 34645255]
Kaya Güner E, Inci Bozbiyik D. Evaluation of the update for screening for retinopathy of prematurity in a tertiary care center in Türkiye with retrospective cohorts. Turkish journal of medical sciences. 2024:54(6):1295-1301. doi: 10.55730/1300-0144.5912. Epub 2024 Oct 20 [PubMed PMID: 39734336]
Level 2 (mid-level) evidenceDogra MR, Katoch D, Dogra M. An Update on Retinopathy of Prematurity (ROP). Indian journal of pediatrics. 2017 Dec:84(12):930-936. doi: 10.1007/s12098-017-2404-3. Epub 2017 Jul 4 [PubMed PMID: 28674824]
Flynn JT, Bancalari E, Snyder ES, Goldberg RN, Feuer W, Cassady J, Schiffman J, Feldman HI, Bachynski B, Buckley E. A cohort study of transcutaneous oxygen tension and the incidence and severity of retinopathy of prematurity. The New England journal of medicine. 1992 Apr 16:326(16):1050-4 [PubMed PMID: 1549150]
Level 2 (mid-level) evidenceFlynn JT, Chan-Ling T. Retinopathy of prematurity: two distinct mechanisms that underlie zone 1 and zone 2 disease. American journal of ophthalmology. 2006 Jul:142(1):46-59 [PubMed PMID: 16815250]
Level 2 (mid-level) evidenceHartnett ME. Studies on the pathogenesis of avascular retina and neovascularization into the vitreous in peripheral severe retinopathy of prematurity (an american ophthalmological society thesis). Transactions of the American Ophthalmological Society. 2010 Dec:108():96-119 [PubMed PMID: 21212851]
Sotiropoulos JX, Saugstad OD, Oei JL. Aspects on Oxygenation in Preterm Infants before, Immediately after Birth, and Beyond. Neonatology. 2024:121(5):562-569. doi: 10.1159/000540481. Epub 2024 Aug 1 [PubMed PMID: 39089224]
Hartnett ME, Lane RH. Effects of oxygen on the development and severity of retinopathy of prematurity. Journal of AAPOS : the official publication of the American Association for Pediatric Ophthalmology and Strabismus. 2013 Jun:17(3):229-34. doi: 10.1016/j.jaapos.2012.12.155. Epub [PubMed PMID: 23791404]
Kim SJ, Port AD, Swan R, Campbell JP, Chan RVP, Chiang MF. Retinopathy of prematurity: a review of risk factors and their clinical significance. Survey of ophthalmology. 2018 Sep-Oct:63(5):618-637. doi: 10.1016/j.survophthal.2018.04.002. Epub 2018 Apr 19 [PubMed PMID: 29679617]
Level 3 (low-level) evidenceJi H, Khurana Hershey GK. Genetic and epigenetic influence on the response to environmental particulate matter. The Journal of allergy and clinical immunology. 2012 Jan:129(1):33-41. doi: 10.1016/j.jaci.2011.11.008. Epub [PubMed PMID: 22196522]
Hellström A, Ley D, Hansen-Pupp I, Hallberg B, Ramenghi LA, Löfqvist C, Smith LE, Hård AL. Role of Insulinlike Growth Factor 1 in Fetal Development and in the Early Postnatal Life of Premature Infants. American journal of perinatology. 2016 Sep:33(11):1067-71. doi: 10.1055/s-0036-1586109. Epub 2016 Sep 7 [PubMed PMID: 27603537]
Huang HY, Huang CY, Li LF. Prolonged Mechanical Ventilation: Outcomes and Management. Journal of clinical medicine. 2022 Apr 27:11(9):. doi: 10.3390/jcm11092451. Epub 2022 Apr 27 [PubMed PMID: 35566577]
Schaffer DB, Palmer EA, Plotsky DF, Metz HS, Flynn JT, Tung B, Hardy RJ. Prognostic factors in the natural course of retinopathy of prematurity. The Cryotherapy for Retinopathy of Prematurity Cooperative Group. Ophthalmology. 1993 Feb:100(2):230-7 [PubMed PMID: 8437832]
Pastro J, Toso BRGO. Influence of oxygen in the development of retinopathy of prematurity. Revista brasileira de enfermagem. 2019 Jun 27:72(3):592-599. doi: 10.1590/0034-7167-2018-0361. Epub 2019 Jun 27 [PubMed PMID: 31269121]
Zhu Z, Hua X, Yu Y, Zhu P, Hong K, Ke Y. Effect of red blood cell transfusion on the development of retinopathy of prematurity: A systematic review and meta-analysis. PloS one. 2020:15(6):e0234266. doi: 10.1371/journal.pone.0234266. Epub 2020 Jun 8 [PubMed PMID: 32512582]
Level 1 (high-level) evidenceWang S, Liu J, Zhang X, Liu Y, Li J, Wang H, Luo X, Liu S, Liu L, Zhang J. Global, regional and national burden of retinopathy of prematurity among childhood and adolescent: a spatiotemporal analysis based on the Global Burden of Disease Study 2019. BMJ paediatrics open. 2024 Jan 6:8(1):. doi: 10.1136/bmjpo-2023-002267. Epub 2024 Jan 6 [PubMed PMID: 38184302]
Blazon MN, Rezar-Dreindl S, Wassermann L, Neumayer T, Berger A, Stifter E. Retinopathy of Prematurity: Incidence, Risk Factors, and Treatment Outcomes in a Tertiary Care Center. Journal of clinical medicine. 2024 Nov 17:13(22):. doi: 10.3390/jcm13226926. Epub 2024 Nov 17 [PubMed PMID: 39598070]
Senjam SS, Chandra P. Retinopathy of prematurity: Addressing the emerging burden in developing countries. Journal of family medicine and primary care. 2020 Jun:9(6):2600-2605. doi: 10.4103/jfmpc.jfmpc_110_20. Epub 2020 Jun 30 [PubMed PMID: 32984093]
Athikarisamy SE, Vinekar A, Patole S. Retinopathy of prematurity in India - what can we learn from the polio legacy? The Lancet regional health. Southeast Asia. 2023 Jul:14():100210. doi: 10.1016/j.lansea.2023.100210. Epub 2023 May 10 [PubMed PMID: 37492414]
Berrocal AM, Fan KC, Al-Khersan H, Negron CI, Murray T. Retinopathy of Prematurity: Advances in the Screening and Treatment of Retinopathy of Prematurity Using a Single Center Approach. American journal of ophthalmology. 2022 Jan:233():189-215. doi: 10.1016/j.ajo.2021.07.016. Epub 2021 Jul 21 [PubMed PMID: 34298009]
Level 3 (low-level) evidenceKöberlein J, Beifus K, Schaffert C, Finger RP. The economic burden of visual impairment and blindness: a systematic review. BMJ open. 2013 Nov 7:3(11):e003471. doi: 10.1136/bmjopen-2013-003471. Epub 2013 Nov 7 [PubMed PMID: 24202057]
Level 1 (high-level) evidenceHård AL, Smith LE, Hellström A. Nutrition, insulin-like growth factor-1 and retinopathy of prematurity. Seminars in fetal & neonatal medicine. 2013 Jun:18(3):136-142. doi: 10.1016/j.siny.2013.01.006. Epub 2013 Feb 18 [PubMed PMID: 23428885]
Zhang L, Buonfiglio F, Fieß A, Pfeiffer N, Gericke A. Retinopathy of Prematurity-Targeting Hypoxic and Redox Signaling Pathways. Antioxidants (Basel, Switzerland). 2024 Jan 25:13(2):. doi: 10.3390/antiox13020148. Epub 2024 Jan 25 [PubMed PMID: 38397746]
Aiello LP, Northrup JM, Keyt BA, Takagi H, Iwamoto MA. Hypoxic regulation of vascular endothelial growth factor in retinal cells. Archives of ophthalmology (Chicago, Ill. : 1960). 1995 Dec:113(12):1538-44 [PubMed PMID: 7487623]
Krock BL, Skuli N, Simon MC. Hypoxia-induced angiogenesis: good and evil. Genes & cancer. 2011 Dec:2(12):1117-33. doi: 10.1177/1947601911423654. Epub [PubMed PMID: 22866203]
Balcarcel DR, Coates BM, Chong G, Sanchez-Pinto LN. Excessive Oxygen Supplementation in the First Day of Mechanical Ventilation Is Associated With Multiple Organ Dysfunction and Death in Critically Ill Children. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies. 2022 Feb 1:23(2):89-98. doi: 10.1097/PCC.0000000000002861. Epub [PubMed PMID: 35119429]
Chiang MF, Quinn GE, Fielder AR, Ostmo SR, Paul Chan RV, Berrocal A, Binenbaum G, Blair M, Peter Campbell J, Capone A Jr, Chen Y, Dai S, Ells A, Fleck BW, Good WV, Elizabeth Hartnett M, Holmstrom G, Kusaka S, Kychenthal A, Lepore D, Lorenz B, Martinez-Castellanos MA, Özdek Ş, Ademola-Popoola D, Reynolds JD, Shah PK, Shapiro M, Stahl A, Toth C, Vinekar A, Visser L, Wallace DK, Wu WC, Zhao P, Zin A. International Classification of Retinopathy of Prematurity, Third Edition. Ophthalmology. 2021 Oct:128(10):e51-e68. doi: 10.1016/j.ophtha.2021.05.031. Epub 2021 Jul 8 [PubMed PMID: 34247850]
Strube YNJ, Wright KW. Pathophysiology of retinopathy of prematurity. Saudi journal of ophthalmology : official journal of the Saudi Ophthalmological Society. 2022 Jul-Sep:36(3):239-242. doi: 10.4103/sjopt.sjopt_18_22. Epub 2022 Oct 14 [PubMed PMID: 36276256]
Li R, Yang X, Wang Y, Chu Z, Liu T, Zhu T, Gao X, Ma Z. Effect(s) of preterm birth on normal retinal vascular development and oxygen-induced retinopathy in the neonatal rat. Current eye research. 2013 Dec:38(12):1266-73. doi: 10.3109/02713683.2013.813556. Epub 2013 Jul 25 [PubMed PMID: 23885967]
Pe'er J, Shweiki D, Itin A, Hemo I, Gnessin H, Keshet E. Hypoxia-induced expression of vascular endothelial growth factor by retinal cells is a common factor in neovascularizing ocular diseases. Laboratory investigation; a journal of technical methods and pathology. 1995 Jun:72(6):638-45 [PubMed PMID: 7540233]
Friedlander M. Fibrosis and diseases of the eye. The Journal of clinical investigation. 2007 Mar:117(3):576-86 [PubMed PMID: 17332885]
Weiter J, Fine BS. A histologic study of regional choroidal dystrophy. American journal of ophthalmology. 1977 May:83(5):741-50 [PubMed PMID: 868973]
Penn JS, Madan A, Caldwell RB, Bartoli M, Caldwell RW, Hartnett ME. Vascular endothelial growth factor in eye disease. Progress in retinal and eye research. 2008 Jul:27(4):331-71. doi: 10.1016/j.preteyeres.2008.05.001. Epub 2008 May 28 [PubMed PMID: 18653375]
Hartnett ME. Pathophysiology and mechanisms of severe retinopathy of prematurity. Ophthalmology. 2015 Jan:122(1):200-10. doi: 10.1016/j.ophtha.2014.07.050. Epub 2014 Oct 14 [PubMed PMID: 25444347]
Woods J, Biswas S. Retinopathy of prematurity: from oxygen management to molecular manipulation. Molecular and cellular pediatrics. 2023 Sep 15:10(1):12. doi: 10.1186/s40348-023-00163-5. Epub 2023 Sep 15 [PubMed PMID: 37712996]
Burtscher J, Mallet RT, Pialoux V, Millet GP, Burtscher M. Adaptive Responses to Hypoxia and/or Hyperoxia in Humans. Antioxidants & redox signaling. 2022 Nov:37(13-15):887-912. doi: 10.1089/ars.2021.0280. Epub 2022 Apr 22 [PubMed PMID: 35102747]
Campbell RE, Chen CH, Edelstein CL. Overview of Antibiotic-Induced Nephrotoxicity. Kidney international reports. 2023 Nov:8(11):2211-2225. doi: 10.1016/j.ekir.2023.08.031. Epub 2023 Aug 25 [PubMed PMID: 38025228]
Level 3 (low-level) evidenceFierson WM, AMERICAN ACADEMY OF PEDIATRICS Section on Ophthalmology, AMERICAN ACADEMY OF OPHTHALMOLOGY, AMERICAN ASSOCIATION FOR PEDIATRIC OPHTHALMOLOGY AND STRABISMUS, AMERICAN ASSOCIATION OF CERTIFIED ORTHOPTISTS. Screening Examination of Premature Infants for Retinopathy of Prematurity. Pediatrics. 2018 Dec:142(6):. pii: e20183061. doi: 10.1542/peds.2018-3061. Epub [PubMed PMID: 30478242]
Dunbar JA, Hsu V, Christensen M, Black B, Williams P, Beauchamp G. Cost-utility analysis of screening and laser treatment of retinopathy of prematurity. Journal of AAPOS : the official publication of the American Association for Pediatric Ophthalmology and Strabismus. 2009 Apr:13(2):186-90. doi: 10.1016/j.jaapos.2008.10.014. Epub [PubMed PMID: 19393519]
Level 2 (mid-level) evidenceEscobar V, Soares DS, Kreling J, Ferrari LSL, Felcar JM, Camillo CAM, Probst VS. Influence of time under mechanical ventilation on bronchopulmonary dysplasia severity in extremely preterm infants: a pilot study. BMC pediatrics. 2020 May 21:20(1):241. doi: 10.1186/s12887-020-02129-2. Epub 2020 May 21 [PubMed PMID: 32438923]
Level 3 (low-level) evidenceJen HC, Graber JJ, Hill JL, Alaish SM, Voigt RW, Strauch ED. Surgical necrotizing enterocolitis and intraventricular hemorrhage in premature infants below 1000 g. Journal of pediatric surgery. 2006 Aug:41(8):1425-30 [PubMed PMID: 16863849]
İnce C. Blood Transfusions Correct Anemia and Improve Tissue Oxygenation in Surgical and Critically ill Patients. Turkish journal of anaesthesiology and reanimation. 2017 Jun:45(3):119-121. doi: 10.5152/TJAR.2017.08051. Epub 2017 Feb 1 [PubMed PMID: 28751998]
Roberts D, Brown J, Medley N, Dalziel SR. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. The Cochrane database of systematic reviews. 2017 Mar 21:3(3):CD004454. doi: 10.1002/14651858.CD004454.pub3. Epub 2017 Mar 21 [PubMed PMID: 28321847]
Level 1 (high-level) evidenceMitchell AJ, Green A, Jeffs DA, Roberson PK. Physiologic effects of retinopathy of prematurity screening examinations. Advances in neonatal care : official journal of the National Association of Neonatal Nurses. 2011 Aug:11(4):291-7. doi: 10.1097/ANC.0b013e318225a332. Epub [PubMed PMID: 22123352]
Level 3 (low-level) evidenceNakazawa Y, Yasukawa T, Goto H, Kobayashi S, Yokoi K. The Role of Acute Phase Reactants, Including α1-Acid Glycoprotein, in Predicting Onset and Severity of Retinopathy of Prematurity. Diagnostics (Basel, Switzerland). 2025 Feb 27:15(5):. doi: 10.3390/diagnostics15050571. Epub 2025 Feb 27 [PubMed PMID: 40075818]
Dhaliwal C, Wright E, Graham C, McIntosh N, Fleck BW. Wide-field digital retinal imaging versus binocular indirect ophthalmoscopy for retinopathy of prematurity screening: a two-observer prospective, randomised comparison. The British journal of ophthalmology. 2009 Mar:93(3):355-9. doi: 10.1136/bjo.2008.148908. Epub 2008 Nov 21 [PubMed PMID: 19028742]
Level 1 (high-level) evidenceMolinari A, Weaver D, Jalali S. Classifying retinopathy of prematurity. Community eye health. 2017:30(99):55-56 [PubMed PMID: 29434438]
Canzano JC, Handa JT. Utility of pupillary dilation for detecting leukocoria in patients with retinoblastoma. Pediatrics. 1999 Oct:104(4):e44 [PubMed PMID: 10506269]
Binenbaum G, Tomlinson LA, de Alba Campomanes AG, Bell EF, Donohue P, Morrison D, Quinn GE, Repka MX, Rogers D, Yang MB, Yu Y, Ying GS, Postnatal Growth and Retinopathy of Prematurity (G-ROP) Study Group. Validation of the Postnatal Growth and Retinopathy of Prematurity Screening Criteria. JAMA ophthalmology. 2020 Jan 1:138(1):31-37. doi: 10.1001/jamaophthalmol.2019.4517. Epub [PubMed PMID: 31725856]
Level 1 (high-level) evidenceYing GS. A Prediction Model for Retinopathy of Prematurity-Is It Ready for Prime Time? JAMA ophthalmology. 2020 Jan 1:138(1):29-30. doi: 10.1001/jamaophthalmol.2019.4608. Epub [PubMed PMID: 31697322]
Quinn GE. The 'ideal' management of retinopathy of prematurity. Eye (London, England). 2005 Oct:19(10):1044-9 [PubMed PMID: 16304583]
Agarwal K, Jalali S. Classification of retinopathy of prematurity: from then till now. Community eye health. 2018:31(101):S4-S7 [PubMed PMID: 30275659]
Mintz-Hittner HA, Kuffel RR Jr. Intravitreal injection of bevacizumab (avastin) for treatment of stage 3 retinopathy of prematurity in zone I or posterior zone II. Retina (Philadelphia, Pa.). 2008 Jun:28(6):831-8. doi: 10.1097/IAE.0b013e318177f934. Epub [PubMed PMID: 18536599]
Level 2 (mid-level) evidenceHu J, Blair MP, Shapiro MJ, Lichtenstein SJ, Galasso JM, Kapur R. Reactivation of retinopathy of prematurity after bevacizumab injection. Archives of ophthalmology (Chicago, Ill. : 1960). 2012 Aug:130(8):1000-6 [PubMed PMID: 22491394]
Level 3 (low-level) evidenceSnyder LL, Garcia-Gonzalez JM, Shapiro MJ, Blair MP. Very Late Reactivation of Retinopathy of Prematurity After Monotherapy With Intravitreal Bevacizumab. Ophthalmic surgery, lasers & imaging retina. 2016 Mar:47(3):280-3. doi: 10.3928/23258160-20160229-12. Epub [PubMed PMID: 26985803]
Natarajan G, Shankaran S, Nolen TL, Sridhar A, Kennedy KA, Hintz SR, Phelps DL, DeMauro SB, Carlo WA, Gantz MG, Das A, Greenberg RG, Younge NE, Bliss JM, Seabrook R, Sánchez PJ, Wyckoff MH, Bell EF, Vohr BR, Higgins RD. Neurodevelopmental Outcomes of Preterm Infants With Retinopathy of Prematurity by Treatment. Pediatrics. 2019 Aug:144(2):. doi: 10.1542/peds.2018-3537. Epub 2019 Jul 23 [PubMed PMID: 31337693]
Quinn GE, Dobson V, Barr CC, Davis BR, Flynn JT, Palmer EA, Robertson J, Trese MT. Visual acuity in infants after vitrectomy for severe retinopathy of prematurity. Ophthalmology. 1991 Jan:98(1):5-13 [PubMed PMID: 2023732]
Level 1 (high-level) evidenceSeaber JH, Machemer R, Eliott D, Buckley EG, deJuan E, Martin DF. Long-term visual results of children after initially successful vitrectomy for stage V retinopathy of prematurity. Ophthalmology. 1995 Feb:102(2):199-204 [PubMed PMID: 7862407]
Trese MT, Droste PJ. Long-term postoperative results of a consecutive series of stages 4 and 5 retinopathy of prematurity. Ophthalmology. 1998 Jun:105(6):992-7 [PubMed PMID: 9627647]
Level 2 (mid-level) evidenceShah PK, Narendran V, Kalpana N, Tawansy KA. Anatomical and visual outcome of stages 4 and 5 retinopathy of prematurity. Eye (London, England). 2009 Jan:23(1):176-80 [PubMed PMID: 17676022]
Level 2 (mid-level) evidenceSingh R, Reddy DM, Barkmeier AJ, Holz ER, Ram R, Carvounis PE. Long-term visual outcomes following lens-sparing vitrectomy for retinopathy of prematurity. The British journal of ophthalmology. 2012 Nov:96(11):1395-8. doi: 10.1136/bjophthalmol-2011-301353. Epub 2012 Aug 24 [PubMed PMID: 22923456]
Level 2 (mid-level) evidenceSen P, Jain S, Bhende P. Stage 5 retinopathy of prematurity: An update. Taiwan journal of ophthalmology. 2018 Oct-Dec:8(4):205-215. doi: 10.4103/tjo.tjo_61_18. Epub [PubMed PMID: 30637192]
Aradhya AS, Kaur I, Gupta R, Kaur S, Shrimanth YS, Masih PD, Kumar P. Implementing a three-hourly feeding schedule in stable preterm infants to decrease maternal fatigue. BMJ open quality. 2021 Jul:10(Suppl 1):. doi: 10.1136/bmjoq-2021-001439. Epub [PubMed PMID: 34344736]
Level 2 (mid-level) evidenceYu Y, Tomlinson LA, Binenbaum G, Ying GS, G-Rop Study Group. Incidence, timing and risk factors of type 1 retinopathy of prematurity in a North American cohort. The British journal of ophthalmology. 2021 Dec:105(12):1724-1730. doi: 10.1136/bjophthalmol-2020-317467. Epub 2020 Sep 26 [PubMed PMID: 32980817]
Agrawal R, Kulkarni S, Walambe R, Kotecha K. Assistive Framework for Automatic Detection of All the Zones in Retinopathy of Prematurity Using Deep Learning. Journal of digital imaging. 2021 Aug:34(4):932-947. doi: 10.1007/s10278-021-00477-8. Epub 2021 Jul 8 [PubMed PMID: 34240273]
Gurnani B, Kaur K. Anti-infective therapies for Pythium insidiosum keratitis. Expert review of anti-infective therapy. 2024 Oct:22(10):805-817. doi: 10.1080/14787210.2024.2403146. Epub 2024 Sep 13 [PubMed PMID: 39268901]
John VJ, McClintic JI, Hess DJ, Berrocal AM. Retinopathy of Prematurity Versus Familial Exudative Vitreoretinopathy: Report on Clinical and Angiographic Findings. Ophthalmic surgery, lasers & imaging retina. 2016 Jan:47(1):14-9. doi: 10.3928/23258160-20151214-02. Epub [PubMed PMID: 26731204]
Zhou Y, Shapiro MJ, Burton BK, Mets MB, Kurup SP. Case report: A case of Norrie disease due to deletion of the entire coding region of NDP gene. American journal of ophthalmology case reports. 2021 Sep:23():101151. doi: 10.1016/j.ajoc.2021.101151. Epub 2021 Jun 17 [PubMed PMID: 34189345]
Level 3 (low-level) evidenceLaasri K, El Houss S, Halfi IM, Kettani NE, Fikri M, Jiddane M, Taoursa F. Coats' syndrome: A rare cause of infant leukocoria to keep in mind. Radiology case reports. 2024 Jan:19(1):7-11. doi: 10.1016/j.radcr.2023.09.046. Epub 2023 Oct 19 [PubMed PMID: 37881471]
Level 3 (low-level) evidenceShrivastava AK, Patial Y, Singh B, Bodhey NK. Myopic Posterior Persistent Fetal Vasculature: A Rare Presentation. Cureus. 2024 Apr:16(4):e58623. doi: 10.7759/cureus.58623. Epub 2024 Apr 20 [PubMed PMID: 38770505]
Dwiyana RF, Banjarnahor ID, Diana IA, Gondokaryono SP, Effendi RMRA, Feriza V. Retinal Neovascularization in Two Patients with Incontinentia Pigmenti. Clinical, cosmetic and investigational dermatology. 2022:15():803-808. doi: 10.2147/CCID.S363179. Epub 2022 Apr 29 [PubMed PMID: 35521560]
Ahn SJ, Ryoo NK, Woo SJ. Ocular toxocariasis: clinical features, diagnosis, treatment, and prevention. Asia Pacific allergy. 2014 Jul:4(3):134-41. doi: 10.5415/apallergy.2014.4.3.134. Epub 2014 Jul 29 [PubMed PMID: 25097848]
Gowda VK, Kulhalli P, Vamyanmane DK. Neurological Manifestations of Congenital Cytomegalovirus Infection at a Tertiary Care Centre from Southern India. Journal of neurosciences in rural practice. 2021 Jan:12(1):133-136. doi: 10.1055/s-0040-1721557. Epub 2021 Jan 29 [PubMed PMID: 33531772]
Balmer A, Munier F. Differential diagnosis of leukocoria and strabismus, first presenting signs of retinoblastoma. Clinical ophthalmology (Auckland, N.Z.). 2007 Dec:1(4):431-9 [PubMed PMID: 19668520]
Leslie SW, Vaidya R. Congenital and Maternal Syphilis. StatPearls. 2025 Jan:(): [PubMed PMID: 30725772]
Dobyns WB, Pagon RA, Armstrong D, Curry CJ, Greenberg F, Grix A, Holmes LB, Laxova R, Michels VV, Robinow M. Diagnostic criteria for Walker-Warburg syndrome. American journal of medical genetics. 1989 Feb:32(2):195-210 [PubMed PMID: 2494887]
Adam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, Amemiya A, Thomas MG, Zippin J, Brooks BP. Oculocutaneous Albinism and Ocular Albinism Overview. GeneReviews(®). 1993:(): [PubMed PMID: 37053367]
Level 3 (low-level) evidenceHuang CH, Yang CM, Yang CH, Hou YC, Chen TC. Leber's Congenital Amaurosis: Current Concepts of Genotype-Phenotype Correlations. Genes. 2021 Aug 19:12(8):. doi: 10.3390/genes12081261. Epub 2021 Aug 19 [PubMed PMID: 34440435]
Godel V, Nemet P, Lazar M. Retinal dysplasia. Documenta ophthalmologica. Advances in ophthalmology. 1981 Jul 15:51(3):277-88 [PubMed PMID: 7285774]
Level 3 (low-level) evidenceSharma D, Shastri S, Sharma P. Intrauterine Growth Restriction: Antenatal and Postnatal Aspects. Clinical medicine insights. Pediatrics. 2016:10():67-83. doi: 10.4137/CMPed.S40070. Epub 2016 Jul 14 [PubMed PMID: 27441006]
Abramowicz S, Delvaulx P, Delle Fave MM, Le Roux P, Buisseret D, Postolache L. Subtle Combined Hamartoma of the Retina and Retinal Pigment Epithelium Causing Recurrent Exodeviation. Case reports in ophthalmology. 2022 Jan-Apr:13(1):305-312. doi: 10.1159/000524074. Epub 2022 Apr 22 [PubMed PMID: 35702519]
Level 3 (low-level) evidenceTsui I, Moreira MEL, Rossetto JD, Vasconcelos Z, Gaw SL, Neves LM, Zin OA, Haefeli L, Silveira Filho JCB, Gomes SC Jr, Adachi K, Pone MVDS, Pone SM, Pereira JP Jr, Belfort R, Arumugaswami V, Brasil P, Nielsen-Saines K, Zin AA. Eye Findings in Infants With Suspected or Confirmed Antenatal Zika Virus Exposure. Pediatrics. 2018 Oct:142(4):. doi: 10.1542/peds.2018-1104. Epub 2018 Sep 13 [PubMed PMID: 30213843]
Good WV, Early Treatment for Retinopathy of Prematurity Cooperative Group. The Early Treatment for Retinopathy Of Prematurity Study: structural findings at age 2 years. The British journal of ophthalmology. 2006 Nov:90(11):1378-82 [PubMed PMID: 16914473]
Level 1 (high-level) evidenceMintz-Hittner HA, Kennedy KA, Chuang AZ, BEAT-ROP Cooperative Group. Efficacy of intravitreal bevacizumab for stage 3+ retinopathy of prematurity. The New England journal of medicine. 2011 Feb 17:364(7):603-15. doi: 10.1056/NEJMoa1007374. Epub [PubMed PMID: 21323540]
Stahl A, Lepore D, Fielder A, Fleck B, Reynolds JD, Chiang MF, Li J, Liew M, Maier R, Zhu Q, Marlow N. Ranibizumab versus laser therapy for the treatment of very low birthweight infants with retinopathy of prematurity (RAINBOW): an open-label randomised controlled trial. Lancet (London, England). 2019 Oct 26:394(10208):1551-1559. doi: 10.1016/S0140-6736(19)31344-3. Epub 2019 Sep 12 [PubMed PMID: 31522845]
Level 1 (high-level) evidence. Supplemental Therapeutic Oxygen for Prethreshold Retinopathy Of Prematurity (STOP-ROP), a randomized, controlled trial. I: primary outcomes. Pediatrics. 2000 Feb:105(2):295-310 [PubMed PMID: 10654946]
Level 1 (high-level) evidenceStahl A, Azuma N, Wu WC, Lepore D, Sukgen E, Nakanishi H, Mazela J, Leal S, Pieper A, Schlief S, Eissing T, Turner KC, Zhao A, Winkler J, Höchel J, Köfüncü E, Zimmermann T, FIREFLEYE Study Group. Systemic exposure to aflibercept after intravitreal injection in premature neonates with retinopathy of prematurity: results from the FIREFLEYE randomized phase 3 study. Eye (London, England). 2024 Jun:38(8):1444-1453. doi: 10.1038/s41433-023-02919-9. Epub 2024 Jan 10 [PubMed PMID: 38200320]
Level 1 (high-level) evidenceStahl A, Nakanishi H, Lepore D, Wu WC, Azuma N, Jacas C, Vitti R, Athanikar A, Chu K, Iveli P, Zhao F, Leal S, Schlief S, Schmelter T, Miller T, Köfüncü E, Fielder A, FIREFLEYE next Study Group. Intravitreal Aflibercept vs Laser Therapy for Retinopathy of Prematurity: Two-Year Efficacy and Safety Outcomes in the Nonrandomized Controlled Trial FIREFLEYE next. JAMA network open. 2024 Apr 1:7(4):e248383. doi: 10.1001/jamanetworkopen.2024.8383. Epub 2024 Apr 1 [PubMed PMID: 38687481]
Level 1 (high-level) evidenceSharpe C, Reiner GE, Davis SL, Nespeca M, Gold JJ, Rasmussen M, Kuperman R, Harbert MJ, Michelson D, Joe P, Wang S, Rismanchi N, Le NM, Mower A, Kim J, Battin MR, Lane B, Honold J, Knodel E, Arnell K, Bridge R, Lee L, Ernstrom K, Raman R, Haas RH, NEOLEV2 INVESTIGATORS. Levetiracetam Versus Phenobarbital for Neonatal Seizures: A Randomized Controlled Trial. Pediatrics. 2020 Jun:145(6):. doi: 10.1542/peds.2019-3182. Epub 2020 May 8 [PubMed PMID: 32385134]
Level 1 (high-level) evidenceGreer DM, Ritter J, Helbok R, Badjatia N, Ko SB, Guanci M, Sheth KN. Impact of Fever Prevention in Brain-Injured Patients (INTREPID): Study Protocol for a Randomized Controlled Trial. Neurocritical care. 2021 Oct:35(2):577-589. doi: 10.1007/s12028-021-01208-1. Epub 2021 Mar 24 [PubMed PMID: 33761119]
Level 1 (high-level) evidenceIvers RQ, Blows SJ, Stevenson MR, Norton RN, Williamson A, Eisenbruch M, Woodward M, Lam L, Palamara P, Wang J. A cohort study of 20,822 young drivers: the DRIVE study methods and population. Injury prevention : journal of the International Society for Child and Adolescent Injury Prevention. 2006 Dec:12(6):385-9 [PubMed PMID: 17170187]
Slevin M, Murphy JF, Daly L, O'Keefe M. Retinopathy of prematurity screening, stress related responses, the role of nesting. The British journal of ophthalmology. 1997 Sep:81(9):762-4 [PubMed PMID: 9422929]
Zhang-Nunes S. Light Therapy for Facial Rejuvenation and Dry Eyes, Blepharitis, and Styes. International ophthalmology clinics. 2024 Jul 1:64(3):9-12. doi: 10.1097/IIO.0000000000000527. Epub 2024 Jun 24 [PubMed PMID: 38910500]
Hang A, Feldman S, Amin AP, Ochoa JAR, Park SS. Intravitreal Anti-Vascular Endothelial Growth Factor Therapies for Retinal Disorders. Pharmaceuticals (Basel, Switzerland). 2023 Aug 11:16(8):. doi: 10.3390/ph16081140. Epub 2023 Aug 11 [PubMed PMID: 37631054]
Chujo S, Matsubara H, Mase Y, Kato K, Kondo M. Recurrence Rate during 5-Year Period after Suspension of Anti-Vascular Endothelial Growth Factor Treatment for Neovascular Age-Related Macular Degeneration. Journal of clinical medicine. 2024 Jul 24:13(15):. doi: 10.3390/jcm13154317. Epub 2024 Jul 24 [PubMed PMID: 39124583]
Hu Q, Bai Y, Chen X, Huang L, Chen Y, Li X. Recurrence of Retinopathy of Prematurity in Zone II Stage 3+ after Ranibizumab Treatment: A Retrospective Study. Journal of ophthalmology. 2017:2017():5078565. doi: 10.1155/2017/5078565. Epub 2017 Apr 9 [PubMed PMID: 28491468]
Level 2 (mid-level) evidenceLinghu D, Cheng Y, Zhu X, Deng X, Yin H, Jiang Y, Zhao M, Li X, Liang J. Comparison of Intravitreal Anti-VEGF Agents With Laser Photocoagulation for Retinopathy of Prematurity of 1,627 Eyes in China. Frontiers in medicine. 2022:9():911095. doi: 10.3389/fmed.2022.911095. Epub 2022 May 31 [PubMed PMID: 35712119]
Saeed OB, Siatkowski RM, Coussa RG. Primary scleral buckling vs. vitrectomy for stage 4A retinopathy of prematurity: a systematic review and meta-analysis of surgical outcomes. Eye (London, England). 2025 Feb:39(3):418-423. doi: 10.1038/s41433-024-03563-7. Epub 2025 Jan 2 [PubMed PMID: 39748110]
Level 1 (high-level) evidenceHolmström M, el Azazi M, Kugelberg U. Ophthalmological long-term follow up of preterm infants: a population based, prospective study of the refraction and its development. The British journal of ophthalmology. 1998 Nov:82(11):1265-71 [PubMed PMID: 9924330]
Vaidyanathan U, Hopping GC, Liu HY, Somani AN, Ronquillo YC, Hoopes PC, Moshirfar M. Persistent Corneal Epithelial Defects: A Review Article. Medical hypothesis, discovery & innovation ophthalmology journal. 2019 Fall:8(3):163-176 [PubMed PMID: 31598519]
Sachdeva MM, Moshiri A, Leder HA, Scott AW. Endophthalmitis following intravitreal injection of anti-VEGF agents: long-term outcomes and the identification of unusual micro-organisms. Journal of ophthalmic inflammation and infection. 2016 Dec:6(1):2. doi: 10.1186/s12348-015-0069-5. Epub 2016 Jan 12 [PubMed PMID: 26758203]
Schalnus R. Topical nonsteroidal anti-inflammatory therapy in ophthalmology. Ophthalmologica. Journal international d'ophtalmologie. International journal of ophthalmology. Zeitschrift fur Augenheilkunde. 2003 Mar-Apr:217(2):89-98 [PubMed PMID: 12592044]
Stirpe M, Orciuolo M. Vitrectomy, scleral buckling, and peripheral diathermy treatment for severe proliferative vitreoretinopathy. Retina (Philadelphia, Pa.). 1987:7(4):219-22 [PubMed PMID: 3432742]
Stevens B, Yamada J, Ohlsson A, Haliburton S, Shorkey A. Sucrose for analgesia in newborn infants undergoing painful procedures. The Cochrane database of systematic reviews. 2016 Jul 16:7(7):CD001069. doi: 10.1002/14651858.CD001069.pub5. Epub 2016 Jul 16 [PubMed PMID: 27420164]
Level 1 (high-level) evidenceEarly Treatment for Retinopathy of Prematurity Cooperative Group, Good WV, Hardy RJ, Dobson V, Palmer EA, Phelps DL, Tung B, Redford M. Final visual acuity results in the early treatment for retinopathy of prematurity study. Archives of ophthalmology (Chicago, Ill. : 1960). 2010 Jun:128(6):663-71. doi: 10.1001/archophthalmol.2010.72. Epub 2010 Apr 12 [PubMed PMID: 20385926]
Level 1 (high-level) evidenceJu RH, Zhang JQ, Ke XY, Lu XH, Liang LF, Wang WJ. Spontaneous regression of retinopathy of prematurity: incidence and predictive factors. International journal of ophthalmology. 2013:6(4):475-80. doi: 10.3980/j.issn.2222-3959.2013.04.13. Epub 2013 Aug 18 [PubMed PMID: 23991382]
Roohipoor R, Karkhaneh R, Riazi-Esfahani M, Ghasemi F, Nili-Ahmadabadi M. Surgical management in advanced stages of retinopathy of prematurity; our experience. Journal of ophthalmic & vision research. 2009 Jul:4(3):185-90 [PubMed PMID: 23198072]
Wallsh JO, Gallemore RP. Anti-VEGF-Resistant Retinal Diseases: A Review of the Latest Treatment Options. Cells. 2021 Apr 29:10(5):. doi: 10.3390/cells10051049. Epub 2021 Apr 29 [PubMed PMID: 33946803]
Adams GG, Sloper JJ. Update on squint and amblyopia. Journal of the Royal Society of Medicine. 2003 Jan:96(1):3-6 [PubMed PMID: 12519794]
Msall ME, Phelps DL, Hardy RJ, Dobson V, Quinn GE, Summers CG, Tremont MR, Cryotherapy for Retinopathy of Prematurity Cooperative Group. Educational and social competencies at 8 years in children with threshold retinopathy of prematurity in the CRYO-ROP multicenter study. Pediatrics. 2004 Apr:113(4):790-9 [PubMed PMID: 15060229]
Level 2 (mid-level) evidenceGurnani B, Kaur K. Re: Dot et al.: Incidence of retinal detachment, macular edema, and ocular hypertension after neodymium:yttrium-aluminum-garnet capsulotomy: a population-based nationwide study-The French YAG 2 Study (Ophthalmology. 2022;130:478-487). Ophthalmology. 2023 Dec:130(12):e43. doi: 10.1016/j.ophtha.2023.08.012. Epub 2023 Sep 20 [PubMed PMID: 37737811]
Kaur K, Kannusamy V, Mouttapa F, Gurnani B, Venkatesh R, Khadia A. To assess the accuracy of Plusoptix S12-C photoscreener in detecting amblyogenic risk factors in children aged 6 months to 6 years in remote areas of South India. Indian journal of ophthalmology. 2020 Oct:68(10):2186-2189. doi: 10.4103/ijo.IJO_2046_19. Epub [PubMed PMID: 32971637]
Gurnani B, Kaur K. Recent Advances in Refractive Surgery: An Overview. Clinical ophthalmology (Auckland, N.Z.). 2024:18():2467-2472. doi: 10.2147/OPTH.S481421. Epub 2024 Sep 2 [PubMed PMID: 39246558]
Level 3 (low-level) evidenceGurnani B, Kaur K, Lalgudi VG, Kundu G, Mimouni M, Liu H, Jhanji V, Prakash G, Roy AS, Shetty R, Gurav JS. Role of artificial intelligence, machine learning and deep learning models in corneal disorders - A narrative review. Journal francais d'ophtalmologie. 2024 Sep:47(7):104242. doi: 10.1016/j.jfo.2024.104242. Epub 2024 Jul 15 [PubMed PMID: 39013268]
Level 3 (low-level) evidenceKaur K, Zeppieri M, Gurnani B. Primary Congenital Glaucoma. StatPearls. 2025 Jan:(): [PubMed PMID: 34662067]
Kaur K, Gurnani B. Viscoelastics. StatPearls. 2025 Jan:(): [PubMed PMID: 35201717]
Nizami AA, Gurnani B, Gulani AC. Cataract. StatPearls. 2025 Jan:(): [PubMed PMID: 30969521]
Shukla UV, Gurnani B, Kaufman EJ. Intraocular Hemorrhage. StatPearls. 2025 Jan:(): [PubMed PMID: 33620856]
Gurnani B, Christy J, Narayana S, Rajkumar P, Kaur K, Gubert J. Retrospective multifactorial analysis of Pythium keratitis and review of literature. Indian journal of ophthalmology. 2021 May:69(5):1095-1101. doi: 10.4103/ijo.IJO_1808_20. Epub [PubMed PMID: 33913840]
Level 2 (mid-level) evidenceLee SY, Gurnani B, Mesfin FB. Blindness. StatPearls. 2025 Jan:(): [PubMed PMID: 28846303]
Görbe E, Vámos R, Joó GJ, Jeager J, Molvarec A, Berecz B, Horváth A, Sulya B, Rigó J Jr. Perioperative analgesia of infants during the therapy for retinopathy of prematurity. Medical science monitor : international medical journal of experimental and clinical research. 2010 Apr:16(4):CR186-189 [PubMed PMID: 20357717]
Level 2 (mid-level) evidenceHolmes JM, Kraker RT, Beck RW, Birch EE, Cotter SA, Everett DF, Hertle RW, Quinn GE, Repka MX, Scheiman MM, Wallace DK, Pediatric Eye Disease Investigator Group. A randomized trial of prescribed patching regimens for treatment of severe amblyopia in children. Ophthalmology. 2003 Nov:110(11):2075-87 [PubMed PMID: 14597512]
Level 1 (high-level) evidencePediatric Eye Disease Investigator Group. The course of moderate amblyopia treated with patching in children: experience of the amblyopia treatment study. American journal of ophthalmology. 2003 Oct:136(4):620-9 [PubMed PMID: 14516801]
Level 1 (high-level) evidenceKumar N, Akangire G, Sullivan B, Fairchild K, Sampath V. Continuous vital sign analysis for predicting and preventing neonatal diseases in the twenty-first century: big data to the forefront. Pediatric research. 2020 Jan:87(2):210-220. doi: 10.1038/s41390-019-0527-0. Epub 2019 Aug 4 [PubMed PMID: 31377752]
Hall RW, Anand KJ. Pain management in newborns. Clinics in perinatology. 2014 Dec:41(4):895-924. doi: 10.1016/j.clp.2014.08.010. Epub 2014 Oct 7 [PubMed PMID: 25459780]
Bhatt SS, Stepien KE, Joshi K. Prophylactic antibiotic use after intravitreal injection: effect on endophthalmitis rate. Retina (Philadelphia, Pa.). 2011 Nov:31(10):2032-6. doi: 10.1097/IAE.0b013e31820f4b4f. Epub [PubMed PMID: 21659941]
Bakshi S, Paswan VK, Yadav SP, Bhinchhar BK, Kharkwal S, Rose H, Kanetkar P, Kumar V, Al-Zamani ZAS, Bunkar DS. A comprehensive review on infant formula: nutritional and functional constituents, recent trends in processing and its impact on infants' gut microbiota. Frontiers in nutrition. 2023:10():1194679. doi: 10.3389/fnut.2023.1194679. Epub 2023 Jun 21 [PubMed PMID: 37415910]
Sica A, Michieletto P, Pensiero S, Barbi E. Successful treatment of cortical visual impairment in children using anti-amblyopia treatment despite the absence of amblyopia: a case report. Italian journal of pediatrics. 2024 Jul 2:50(1):123. doi: 10.1186/s13052-024-01679-w. Epub 2024 Jul 2 [PubMed PMID: 38956699]
Level 3 (low-level) evidenceDikopf MS, Machen LA, Hallak JA, Chau FY, Kassem IS. Zone of retinal vascularization and refractive error in premature eyes with and without spontaneously regressed retinopathy of prematurity. Journal of AAPOS : the official publication of the American Association for Pediatric Ophthalmology and Strabismus. 2019 Aug:23(4):211.e1-211.e6. doi: 10.1016/j.jaapos.2019.03.006. Epub 2019 Jun 20 [PubMed PMID: 31229607]
Shin Y, Park EJ, Lee A. Early Intervention for Children With Developmental Disabilities and Their Families via Telehealth: Systematic Review. Journal of medical Internet research. 2025 Jan 17:27():e66442. doi: 10.2196/66442. Epub 2025 Jan 17 [PubMed PMID: 39819975]
Level 1 (high-level) evidenceLindauer A, Sexson K, Harvath TA. Teaching Caregivers to Administer Eye Drops, Transdermal Patches, and Suppositories. The American journal of nursing. 2017 May:117(5 Suppl 1):S11-S16. doi: 10.1097/01.NAJ.0000516388.97515.ca. Epub [PubMed PMID: 28452802]
Barker L, Thomas R, Rubin G, Dahlmann-Noor A. Optical reading aids for children and young people with low vision. The Cochrane database of systematic reviews. 2015 Mar 4:2015(3):CD010987. doi: 10.1002/14651858.CD010987.pub2. Epub 2015 Mar 4 [PubMed PMID: 25738963]
Level 1 (high-level) evidenceKhalil H, de Moel-Mandel C, Verma D, Kynoch K, Fernandez R, Ramis MA, Opie JE. Characteristics of Quality Improvement Projects in Health Services: A Systematic Scoping Review. Journal of evidence-based medicine. 2025 Mar:18(1):e12670. doi: 10.1111/jebm.12670. Epub 2025 Jan 22 [PubMed PMID: 39838939]
Level 1 (high-level) evidenceBishnoi K, Prasad R, Upadhyay T, Mathurkar S. A Narrative Review on Managing Retinopathy of Prematurity: Insights Into Pathogenesis, Screening, and Treatment Strategies. Cureus. 2024 Mar:16(3):e56168. doi: 10.7759/cureus.56168. Epub 2024 Mar 14 [PubMed PMID: 38618439]
Level 3 (low-level) evidenceLam M, Suh D. Screening, Diagnosis, and Treatment of Pediatric Ocular Diseases. Children (Basel, Switzerland). 2022 Dec 10:9(12):. doi: 10.3390/children9121939. Epub 2022 Dec 10 [PubMed PMID: 36553382]
Hubbard GB 3rd. Surgical management of retinopathy of prematurity. Current opinion in ophthalmology. 2008 Sep:19(5):384-90. doi: 10.1097/ICU.0b013e328309f1a5. Epub [PubMed PMID: 18772670]
Level 3 (low-level) evidenceBeaulieu FP, Zuckerberg G, Coletti K, Mapelli E, Flibotte J, Sampath S, Hwang M, Drum ET. Sedation and anesthesia for imaging of the infant and neonate-a brief review. Pediatric radiology. 2024 Sep:54(10):1579-1588. doi: 10.1007/s00247-024-05995-5. Epub 2024 Jul 26 [PubMed PMID: 39060413]
Nawaytou H, Hills NK, Clyman RI. Patent ductus arteriosus and the risk of bronchopulmonary dysplasia-associated pulmonary hypertension. Pediatric research. 2023 Aug:94(2):547-554. doi: 10.1038/s41390-023-02522-4. Epub 2023 Feb 17 [PubMed PMID: 36804505]
Bullaj R, Dyet L, Mitra S, Bunce C, Clarke CS, Saunders K, Dale N, Horwood A, Williams C, St Clair Tracy H, Marlow N, Bowman R. Effectiveness of early spectacle intervention on visual outcomes in babies at risk of cerebral visual impairment: a parallel group, open-label, randomised clinical feasibility trial protocol. BMJ open. 2022 Sep 21:12(9):e059946. doi: 10.1136/bmjopen-2021-059946. Epub 2022 Sep 21 [PubMed PMID: 36130761]
Level 1 (high-level) evidenceSkinner AM, Narchi H. Preterm nutrition and neurodevelopmental outcomes. World journal of methodology. 2021 Nov 20:11(6):278-293. doi: 10.5662/wjm.v11.i6.278. Epub 2021 Nov 20 [PubMed PMID: 34888181]
AlBathi L, Abouammoh N, AlSwaina N, AlBalawi HB, Al Qahtani AA, Talea M, AlSulaiman SM, Abouammoh MA. Pitfalls of Advanced Retinopathy of Prematurity Presentation: A Content Analysis of Medical Records. Risk management and healthcare policy. 2021:14():3873-3882. doi: 10.2147/RMHP.S326757. Epub 2021 Sep 16 [PubMed PMID: 34557046]
Tin W, Milligan DW, Pennefather P, Hey E. Pulse oximetry, severe retinopathy, and outcome at one year in babies of less than 28 weeks gestation. Archives of disease in childhood. Fetal and neonatal edition. 2001 Mar:84(2):F106-10 [PubMed PMID: 11207226]
Level 2 (mid-level) evidenceAskie LM, Brocklehurst P, Darlow BA, Finer N, Schmidt B, Tarnow-Mordi W, NeOProM Collaborative Group. NeOProM: Neonatal Oxygenation Prospective Meta-analysis Collaboration study protocol. BMC pediatrics. 2011 Jan 17:11():6. doi: 10.1186/1471-2431-11-6. Epub 2011 Jan 17 [PubMed PMID: 21235822]
Level 1 (high-level) evidenceDarlow BA, Husain S. Primary prevention of ROP and the oxygen saturation targeting trials. Seminars in perinatology. 2019 Oct:43(6):333-340. doi: 10.1053/j.semperi.2019.05.004. Epub 2019 May 10 [PubMed PMID: 31151776]
Manja V, Lakshminrusimha S, Cook DJ. Oxygen saturation target range for extremely preterm infants: a systematic review and meta-analysis. JAMA pediatrics. 2015 Apr:169(4):332-40. doi: 10.1001/jamapediatrics.2014.3307. Epub [PubMed PMID: 25664703]
Level 1 (high-level) evidenceBrown JV, Lin L, Embleton ND, Harding JE, McGuire W. Multi-nutrient fortification of human milk for preterm infants. The Cochrane database of systematic reviews. 2020 Jun 3:6(6):CD000343. doi: 10.1002/14651858.CD000343.pub4. Epub 2020 Jun 3 [PubMed PMID: 35658821]
Level 1 (high-level) evidenceHughes RG, Collins AS. Preventing Health Care–Associated Infections. Patient Safety and Quality: An Evidence-Based Handbook for Nurses. 2008 Apr:(): [PubMed PMID: 21328782]
Level 2 (mid-level) evidenceWu PY, Fu YK, Lien RI, Chiang MC, Lee CC, Chen HC, Hsueh YJ, Chen KJ, Wang NK, Liu L, Chen YP, Hwang YS, Lai CC, Wu WC. Systemic Cytokines in Retinopathy of Prematurity. Journal of personalized medicine. 2023 Feb 5:13(2):. doi: 10.3390/jpm13020291. Epub 2023 Feb 5 [PubMed PMID: 36836525]
Jefferies A. Retinopathy of prematurity: Recommendations for screening. Paediatrics & child health. 2010 Dec:15(10):667-74 [PubMed PMID: 22131866]
Saxena R, Sharma P, Pediatric Ophthalmology Expert Group. National consensus statement regarding pediatric eye examination, refraction, and amblyopia management. Indian journal of ophthalmology. 2020 Feb:68(2):325-332. doi: 10.4103/ijo.IJO_471_19. Epub [PubMed PMID: 31957721]
Level 3 (low-level) evidenceHuang Y, Zheng WW, Li YZ, Sun ZH, Lin B. Retinal laser photocoagulation and intravitreal injection of anti-VEGF for hemorrhagic retinal arterial microaneurysm. International journal of ophthalmology. 2023:16(12):2041-2048. doi: 10.18240/ijo.2023.12.17. Epub 2023 Dec 18 [PubMed PMID: 38111952]
McCall EM, Alderdice F, Halliday HL, Vohra S, Johnston L. Interventions to prevent hypothermia at birth in preterm and/or low birth weight infants. The Cochrane database of systematic reviews. 2018 Feb 12:2(2):CD004210. doi: 10.1002/14651858.CD004210.pub5. Epub 2018 Feb 12 [PubMed PMID: 29431872]
Level 1 (high-level) evidenceJang HJ, Sung MS, Park SW, Heo H. Delayed-Onset Glaucoma Following Intravitreal Bevacizumab in a Preterm Infant with Retinopathy of Prematurity: a case report. Korean journal of ophthalmology : KJO. 2025 Apr 23:():. doi: 10.3341/kjo.2025.0002. Epub 2025 Apr 23 [PubMed PMID: 40267991]
Level 3 (low-level) evidenceWu R, Zhang Y, Huang P, Xie Y, Wang J, Wang S, Lin Q, Bai Y, Feng S, Cai N, Lu X. Prediction of Reactivation After Antivascular Endothelial Growth Factor Monotherapy for Retinopathy of Prematurity: Multimodal Machine Learning Model Study. Journal of medical Internet research. 2025 Apr 23:27():e60367. doi: 10.2196/60367. Epub 2025 Apr 23 [PubMed PMID: 40267476]
Tomioka M, Murakami T, Okamoto F, Kinoshita T, Shinomiya K, Nishi T, Jujo T, Obata S, Tsukitome H, Ogura S, Ueda K, Ishii R, Oshika T. Five-year Visual Outcome of Treatment for Retinopathy of Prematurity in Infants Weighing {500 G at Birth: A Multicenter Cohort Study from J-CREST: Erratum. Retina (Philadelphia, Pa.). 2025 May 1:45(5):e40. doi: 10.1097/IAE.0000000000004400. Epub [PubMed PMID: 40262067]
Tai F, Jindani Y, Mukerji A, Sabri K. Validation of the SCREENROP Criteria for Retinopathy of Prematurity Screening: A Canadian Model for Consideration. American journal of ophthalmology. 2025 Apr 16:276():230-234. doi: 10.1016/j.ajo.2025.04.010. Epub 2025 Apr 16 [PubMed PMID: 40250745]
Level 1 (high-level) evidenceZhang X, Yan B, Jiang Z, Luo Y. Machine Learning Identification of Neutrophil Extracellular Trap-Related Genes as Potential Biomarkers and Therapeutic Targets for Bronchopulmonary Dysplasia. International journal of molecular sciences. 2025 Mar 31:26(7):. doi: 10.3390/ijms26073230. Epub 2025 Mar 31 [PubMed PMID: 40244055]
Rohwer AC, Oladapo OT, Hofmeyr GJ. Strategies for optimising antenatal corticosteroid administration for women with anticipated preterm birth. The Cochrane database of systematic reviews. 2020 May 26:5(5):CD013633. doi: 10.1002/14651858.CD013633. Epub 2020 May 26 [PubMed PMID: 32452555]
Level 1 (high-level) evidenceLundgren P, Hellgren G, Pivodic A, Sävman K, Smith LEH, Hellström A. Erythropoietin serum levels, versus anaemia as risk factors for severe retinopathy of prematurity. Pediatric research. 2019 Aug:86(2):276-282. doi: 10.1038/s41390-018-0186-6. Epub 2018 Sep 18 [PubMed PMID: 30297879]
Lyu Y, Shah PS, Ye XY, Warre R, Piedboeuf B, Deshpandey A, Dunn M, Lee SK, Canadian Neonatal Network. Association between admission temperature and mortality and major morbidity in preterm infants born at fewer than 33 weeks' gestation. JAMA pediatrics. 2015 Apr:169(4):e150277. doi: 10.1001/jamapediatrics.2015.0277. Epub 2015 Apr 6 [PubMed PMID: 25844990]