Introduction
The kidneys are paired retroperitoneal organs located between the T12 and L3 vertebrae, positioned lateral to the spine and adjacent to multiple abdominal structures. Each kidney comprises the renal parenchyma—divided into cortex and medulla containing nephrons, the functional units—and the collecting system, which conveys urine to the ureter (see Image. Kidney Anatomy). The kidneys perform essential roles in excreting nitrogenous wastes, regulating electrolytes and acid-base balance, controlling blood pressure, reabsorbing vital substances, and producing hormones such as erythropoietin and calcitriol.
The kidneys hold clinical significance due to their roles in waste excretion, electrolyte and acid-base regulation, blood pressure control, and hormone production, making preservation of renal function essential in disease management. In surgery, the kidney’s end-artery vascular anatomy requires careful protection of accessory renal arteries and the Brodel line, while nephron-sparing techniques enable targeted parenchymal resection to preserve renal function and minimize ischemia. A thorough understanding of renal anatomy and physiology enables clinicians to optimize diagnostic, medical, and surgical interventions while minimizing complications.
Structure and Function
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Structure and Function
Gross Anatomy and Anatomical Relations of the Kidney
The kidneys are a pair of bean-shaped retroperitoneal organs, exhibiting medial concavity and lateral convexity, with an average weight of 160 to 162 g in men and 135 to 136 g in women.[1] The left kidney is generally slightly larger and approximately 10 g heavier than the right kidney. Typical dimensions include a length of 10 to 12 cm, a width of 5 to 7 cm, and a thickness of 3 to 5 cm, comparable in size to a closed fist. Both kidneys are normally positioned between the transverse processes of T12 and L3.
The upper renal poles are usually oriented slightly medial and posterior relative to the lower poles.[2] The right kidney typically occupies a slightly more inferior position than the left, reflecting displacement by the liver.[3] Kidney size correlates positively with total body surface area, height, and weight, and decreases with age due to progressive loss of renal parenchyma.[4][5]
Each kidney is positioned immediately inferior to its respective adrenal (suprarenal) gland. The upper pole of both kidneys lies anterior to the diaphragm, which covers the upper 1/3 of each renal unit, and is frequently crossed by the overlying 12th rib. The lower pole of both kidneys is located anterior to the psoas muscle and the lateral aspect of the quadratus lumborum.
The right kidney relates posteriorly to the ascending colon, medially to the 2nd part of the duodenum, and anteriorly to the liver, separated from the hepatic surface by the hepatorenal recess. The left kidney lies posterior to the descending colon, the tail of the pancreas (which may extend sufficiently to cover the renal hilum), the greater curvature of the stomach (which can overlie the superomedial renal surface), and the spleen, to which it is connected via the splenorenal ligament. The kidneys are aligned slightly obliquely, parallel to the ipsilateral psoas muscle. The colon is positioned anterior to both kidneys.[6][7][8]
The lower poles of the kidneys are more lateral, anterior (superficial), and farther apart than the upper poles.[9] These poles are more susceptible to traumatic injury due to limited protection from the lower rib cage.[10] In a 2010 Korean study, Joon et al measured the longitudinal axis of the kidneys and found a mean coronal angle of 16.8° (range, 15° to 20°) relative to the spine. This angle may be reduced in the presence of accessory renal arteries.
At the medial margin of each kidney lies the renal hilum, where the renal artery enters the kidney, and the renal vein and pelvis exit the renal sinus.[11][12] The renal vein is positioned anterior to the renal artery, with the renal pelvis located posterior to both.[13][14] The renal pelvis represents the superior end of the ureter and typically receives 2 or 3 major calyces, with each major calyx drained by 2 or 3 minor calyces, although considerable variation exists.[15] Minor calyces are indented by the renal papillae, which form the apices of the renal pyramids. A renal pyramid, together with its overlying cortical tissue, constitutes a renal lobe.[16]
Each kidney is enclosed by a 2-layered capsule and surrounded by perinephric fat, the Gerota fascia anteriorly, and the Zuckerkandl fascia posteriorly. The anterior renal fascia is termed "Gerota fascia," while the posterior renal fascia is called "Zuckerkandl fascia."[17] In common usage, the Gerota fascia frequently refers collectively to both fasciae. Perinephric adipose tissue is thickest along the borders of the kidneys and extends into the renal sinus at the renal hilum.
The region immediately surrounding the kidneys constitutes the retroperitoneum. This region encompasses the portion of the abdominal cavity and pelvis lying between the posterior parietal peritoneum anteriorly and the posterior abdominal wall. Structures contained within the retroperitoneum include the pancreas (excluding the tail embedded in the splenorenal ligament), ascending and descending colon, duodenum (except the 1st segment), adrenal glands, kidneys, ureters, renal vessels, abdominal aorta, inferior vena cava, and posterior bladder wall.[18] Further details are provided in the companion StatPearls reference “Anatomy, Abdomen and Pelvis, Retroperitoneum.”[19]
Internal Structure and Functional Anatomy of the Kidney
Each kidney comprises 2 primary regions: the renal parenchyma and the collecting system (pyelocalyceal system). The renal parenchyma is subdivided into 2 macroscopically and functionally distinct areas, the cortex and the medulla, each containing specific segments of the nephron, the functional unit of the kidney (see Image. Nephron and Vasculature.)
The renal cortex forms the outer portion of the renal parenchyma. This area contains most nephron components, including renal corpuscles, proximal and distal convoluted tubules, collecting tubules, and cortical collecting ducts. The cortex surrounds both the renal medulla and sinus and can be divided into 2 regions. The renal mantle denotes the peripheral layer of cortical tissue that covers the base of each renal pyramid. Renal columns (columns of Bertin), also referred to as "cortical septa," are extensions of cortical tissue that project inward from the mantle toward the renal sinus, flanking each renal pyramid.[20]
The renal medulla lies between the cortex and the renal sinus. This portion is organized into renal pyramids, which contain medullary rays that extend from adjacent cortical tissue into the medulla. Medullary rays consist of the descending and ascending limbs of the loop of Henle, as well as the medullary collecting ducts.[21] Each renal pyramid, together with its associated overlying cortex, constitutes a renal lobe, the anatomical unit of the kidney.[22] Urine from each lobe drains through a single renal papilla, located at the apex of the pyramid (area cribrosa), into a minor calyx. On average, each kidney contains approximately 9 renal lobes.[23]
The renal collecting system receives urine from the renal parenchyma via the collecting ducts and conveys it to the ureter. The system begins with a variable number of minor calyces, each typically enclosing a single renal papilla. Most kidneys (70%) contain 7 to 9 minor calyces, with 2 or 3 converging to form a major calyx.[24]
A minor calyx may drain a single papilla (simple calyx) or multiple papillae (compound calyx). Compound calyces, more frequently observed in the upper renal poles, form when 2 to 3 adjacent minor calyces merge, producing a major calyx. Major calyces subsequently unite to form the renal pelvis, a funnel-shaped structure that marks the transition to the proximal ureter.[25] Variability in intrarenal anatomy influences the selection and success of kidney procedures, including ureteroscopy, extracorporeal shockwave lithotripsy, and percutaneous nephrolithotomy.[26]
Nephrons are the functional units of the kidney, with each adult kidney containing approximately 1 to 1.5 million nephrons, occasionally exceeding 2.5 million.[27][28][29] Each nephron begins with an afferent arteriole that supplies a capillary tuft, the glomerulus.[30] The glomerulus is enclosed by the Bowman capsule, a double-layered epithelial structure. Together, these structures constitute the renal corpuscle. The efferent arteriole exits the glomerulus and gives rise to a secondary capillary network, including peritubular capillaries and vasa recta, which supply the renal tubules (see Image. Filtration Apparatus of the Glomerulus).[31]
Distal to the Bowman capsule, the nephron progresses through the following segments: proximal convoluted tubule, proximal straight tubule (thick descending limb of the loop of Henle), thin descending limb, thin ascending limb, distal straight tubule (thick ascending limb), distal convoluted tubule, and collecting duct. Multiple collecting tubules from distinct nephrons unite to form the collecting duct, which continues as the medullary collecting duct and ultimately as the papillary duct. Papillary ducts open at the apex of the renal pyramids, forming the area cribrosa, through which urine drains into the renal pelvis and the pyelocalyceal system.[32][33]
Kidney Functions
The kidneys perform several essential functions, including the excretion of nitrogenous waste products such as ammonia and urea; the regulation of electrolytes, including sodium, potassium, and calcium; and the maintenance of acid-base balance via hydrogen ion excretion and bicarbonate reabsorption.[34][35] These organs contribute to the regulation of blood pressure and intravascular volume through the renin-angiotensin-aldosterone system.[36] The kidneys also reabsorb vital substances, including glucose, amino acids, phosphate, calcium, and water.[37][38] In addition, these paired structures secrete critical hormones such as erythropoietin, which stimulates red blood cell production, and calcitriol, the active form of vitamin D, which regulates calcium and phosphate metabolism.[39][40]
Embryology
The human kidney develops from the intermediate mesoderm. Nephrogenesis occurs in 3 successive phases: the pronephros, mesonephros, and metanephros.
The pronephros, located in the cervical region of the developing embryo, comprises vestigial excretory units (nephrotomes) that are nonfunctional in humans and appear transiently during the 4th week of development.[41] As the pronephros regresses, the mesonephros arises from the intermediate mesoderm, extending from the upper thoracic to the upper lumbar regions. This transient embryonic kidney consists of excretory units that elongate, form loops, and develop capillary tufts surrounded by the Bowman capsule, forming primitive renal corpuscles. Each excretory tubule drains into a longitudinal collecting duct, the mesonephric (Wolffian) duct, which empties into the early cloaca. Approximately 40 pairs of functional excretory units develop and can drain small amounts of fluid into the early amniotic cavity.[42]
By the 6th week of development, paired mesonephric organs are fully formed, but regression occurs between the 8th and 10th weeks. In the male body, the mesonephric duct is retained and differentiates into components of the reproductive tract, including the epididymis, vas deferens, seminal vesicles, and ejaculatory ducts.[43]
The metanephros, which gives rise to the permanent kidney, appears during the 5th week of development and comprises excretory units derived from the metanephric mesoderm. Metanephric development depends on reciprocal interactions between the metanephric blastema and the ureteric bud. As the distal segment of the mesonephric duct fuses with the cloaca, surrounding mesoderm forms the metanephric blastema. This blastema secretes signaling factors that induce the formation of the ureteric bud as an evagination of the mesonephric duct.[44]
WT1, a transcription factor, regulates signal production. WT1 mutations are associated with nephroblastoma (Wilms tumor) and other syndromes, including Denys-Drash, Frasier, and WAGR (Wilms tumor, aniridia, genitourinary anomalies, and developmental delay).[45][46] This interaction drives ureteric bud dilation, which forms the renal pelvis and ureter, and subsequently splits into major calyces by the end of the 6th week.
Early splitting of the ureteric bud can result in a bifid ureter. Buds then arise from the calyces and are stimulated to divide into collecting tubules at the 7th week, which are incorporated into the developing tubules at the periphery to become minor calyces. Successive generations of collecting tubules continue to develop and converge to form renal pyramids. Tubules induce the surrounding metanephric tissue via fibroblast growth factor 2 (FGF2) and bone morphogenetic protein 7 (BMP7) to form S-shaped tubules and capillaries that differentiate into glomeruli.
The distal glomerulus connects with the connecting tubules, while the Bowman capsule surrounds the proximal glomerulus, forming the renal corpuscle. The tubule extends into the proximal convoluted tubule, loop of Henle, and distal convoluted tubule. Collectively, these tubules and renal corpuscles constitute the nephron, the functional renal excretory unit. The majority of nephrons (60%) form during the latter half of gestation.[47] Nephrons become functional and urine-producing by the 12th week and continue to develop until approximately 1 million per kidney are present at birth (range, 250,000 to over 2 million).[48][49][50]
The causes of this wide variation in nephron number are not fully understood, but likely contributors include genetic factors, in utero environmental differences, maternal health and nutrition, developmental anomalies, and toxic exposures during gestation.[51][52] Individuals born with a low nephron count exhibit increased susceptibility to renal disease and kidney failure later in life.[53][54]
Blood Supply and Lymphatics
Approximately 20% of total cardiac output, or 1 to 1.1 L/min, is delivered to the kidneys.[55][56][57] With a normal glomerular filtration rate of 120 mL/min, 180 L of fluid per day is filtered from the blood into the renal nephrons. Average urine output is approximately 1.5 L daily, indicating that 178.5 L of fluid initially filtered by the glomeruli must be reabsorbed.[58] This process requires a highly specialized tubular reabsorption system supported by extensive peritubular microvascular networks for efficient fluid return to the systemic circulation.[59][60][61][62]
Renal perfusion is supplied via the renal arteries, which branch from the aorta just below the superior mesenteric artery and enter the hilum at the level of L2. While typically singular, renal arteries may be duplicated or accompanied by accessory arteries, all of which are end arteries that must be preserved during surgery or transplantation. The longer right renal artery passes posterior to the inferior vena cava. Each renal artery divides into anterior and posterior branches, described relative to the renal pelvis. The anterior branch receives 75% of renal blood flow, while the posterior branch supplies the posterior segment as the posterior segmental artery.
The 1st branch of the renal artery is typically the inferior suprarenal (adrenal) artery, although considerable variability exists.[63][64][65] The anterior branch further divides into 4 main segmental arteries:
- Superior segmental artery, also known as the apical segmental artery
- Anterosuperior segmental artery, also referred to as the superior segmental artery
- Anteroinferior segmental artery, also referred to as the middle segmental artery
- Inferior segmental artery
Each segmental artery subsequently divides into interlobar, arcuate, and interlobular arteries.[66][67][68] The interlobar arteries extend parallel between major calyces. These vessels then branch into arcuate arteries, which traverse the base of the renal pyramids within the cortex. Interlobular arteries arise from the arcuate arteries and extend into the renal cortex, eventually forming afferent arterioles, peritubular capillaries, and efferent arterioles that contribute to the vasa recta and peritubular capillary networks.[69] Some interlobular branches supply the renal capsule, renal pelvis, and proximal ureter (see Image. Kidney Blood Supply).
Renal veins mirror the path of the arteries anteriorly. The left renal vein is several centimeters longer than the right vein, crossing the midline to reach the IVC located to the right of the abdominal aorta at the L2 or L3 level.[70][71] This anatomical difference often favors the selection of the left kidney for transplantation over the right kidney.
The term "nutcracker syndrome" refers to entrapment of the left renal vein caused by compression between the superior mesenteric artery and the aorta. The left renal vein passes posterior to the superior mesenteric artery and anterior to the aorta en route to the IVC, placing it at risk of compression.[72] Clinical manifestations are generally nonspecific and may include abdominal pain, hypotension, intermittent flank pain, and microscopic hematuria. Management typically involves endovascular stenting, though left renal vein transposition may be required in selected cases.[73][74] Further details are available in the companion StatPearls reference, "Nutcracker Syndrome and Left Renal Vein Entrapment."[75]
The left testicular (gonadal) vein usually drains into the higher-pressure left renal vein, whereas the right testicular (gonadal) vein empties directly into the lower-pressure IVC.[76] These anatomical differences account for the higher prevalence of left-sided varicoceles and explain why an isolated right-sided varicocele may indicate IVC obstruction, potentially due to tumor thrombus extending from the right renal vein.[77]
Renal lymphatic drainage, and potentially that of the proximal ureters, occurs via lymphatic channels. These channels enter the aortic lymphatic chain on the left and the right lateral IVC lymphatics on the right.[78][79]
Nerves
Innervation of the kidneys, suprarenal glands, and a portion of the proximal ureters is provided by the renal nerve plexus, which contains both sympathetic and parasympathetic fibers.[80] The plexus receives input from the abdominopelvic splanchnic nerves.
Sympathetic efferents supply the nephrons and renal vasculature exclusively, with dense distribution around the afferent arterioles, thick ascending limbs, and distal convoluted tubules.[81][82][83][84] Preganglionic fibers arise from T8 to L1 and synapse in the inferior mesenteric ganglion.[85][86][87][88][89] Postganglionic fibers follow the renal artery and vein to the kidney, positioning them adjacent to major renal vasculature and rendering them accessible to transcatheter renal denervation procedures.[90][91][92][93] These procedures ablate both afferent and efferent fibers to control blood pressure in cases of otherwise intractable hypertension.[94]
Activation of these sympathetic fibers increases sodium retention, stimulates renin release, and induces renal vasoconstriction, contributing to the development of hypertension.[95][96][97] This mechanism underlies the therapeutic efficacy of transcatheter renal denervation in managing otherwise uncontrolled hypertension.[98][99][100][101]
The sensory renal afferent nerves, comprising both mechanoreceptors (stretch) and chemoreceptors, extend from the renal cortex and proximal ureter, with the highest density in the walls of the renal pelvis.[102][103][104] No sensory fibers are present in the renal medulla, although some are observed in the cortex and proximal ureters.[105][106] These fibers follow the renal artery primarily to the ipsilateral dorsal root ganglia of T12 to L3, then ascend the spinal cord to the brainstem and hypothalamus, where they modulate sympathetic outflow and contribute to blood pressure regulation.[107][108]
Transcatheter renal denervation is approved by the U.S. Food and Drug Administration for the treatment of both afferent and efferent renal nerve fibers using renal arterial ultrasound or radiofrequency ablation. This procedure improves blood pressure control in patients with otherwise difficult, resistant, or intractable hypertension.[109][110][111][112] The procedure preserves renal function, enables the reduction of antihypertensive medications, and demonstrates sustained efficacy in multiple trials.[113][114][115] Additional benefits have been observed in individuals with heart failure and certain arrhythmias. The procedure is contraindicated in patients with significant renal artery stenosis, kidney transplants, untreated secondary hypertension, severe renal failure (glomerular filtration rate < 40 mL/min), or solitary kidneys.[116][117][118][119]
Visceral afferent fibers convey pain signals via sympathetic pathways to the spinal ganglia and spinal cord segments of T11 to L2. Pain is often referred to the corresponding dermatome. Therefore, flank discomfort may originate from the kidney.[120] Renal pain typically results from stretching caused by obstruction and dilation, inflammation, infection, or ischemia.[121] Nonobstructing renal calculi generally do not produce flank pain, renal discomfort, or renal colic.
Physiologic Variants
Physiological anatomical variations of the kidney are relatively common and may have significant implications for diagnosis, surgical planning, and interventional procedures. Some of these variants are discussed below.
Horseshoe kidneys are one of the most recognized renal anomalies, characterized by fusion of the lower poles, typically anterior to the aorta (see Image. Horseshoe Kidney on Computed Tomography). Fusion of the lower poles restricts renal ascent due to obstruction by the superior mesenteric artery, resulting in an atypical lower midline position anterior to the abdominal aorta and anterior-facing renal pelves. Horseshoe kidneys frequently possess multiple accessory renal arteries. These anomalies occur in approximately 1 in 400 individuals and are associated with increased rates of ureteropelvic junction (UPJ) obstruction, nephrolithiasis, and urinary tract infection.
Severe malformations, predominantly affecting the urogenital system, are present in roughly 23% of cases.[122] The anomalous position and orientation of horseshoe kidneys complicate surgical procedures. Additional information is provided in the companion StatPearls reference “Horseshoe Kidney.”[123]
A fused pelvic (pancake) kidney is a rare congenital condition where both kidneys unite into a single pelvic mass. Unlike the more common horseshoe kidney, a pancake kidney is located in the pelvis rather than the abdomen and exhibits complete fusion of the renal units. Fused pelvic kidneys are more prevalent in male individuals and are usually identified in men aged 30 to 60.
Accessory renal arteries comprise a common anatomical variation of renal vascular supply.[124][125] These vessels may arise from the main renal artery, abdominal aorta, or aberrant arterial sources.[126][127] As end arteries, accessory vessels do not form anastomoses within the kidney and are therefore not supplemental.[128] These blood vessels may contribute to the development of UPJ obstruction.[129]
Accessory renal arteries are present in approximately 20% to 30% of the population and must be identified prior to procedures such as nephrectomy, partial nephrectomy, aortic aneurysm repair, renal transplantation, and percutaneous or other uroradiological interventions.[130] The most frequently observed accessory renal artery is an extrahilar branch supplying the superior renal pole.[131] Accessory arteries, particularly those supplying the lower pole, may influence renal angulation.
Other vascular anomalies include a retroaortic left renal vein, where venous drainage from the left kidney to the inferior vena cava passes posterior to the aorta.[132][133][134] The reported incidence ranges from 1.7% to 2.5%.[135][136]
Ectopic kidneys, where the organ remains in an abnormal location due to failed ascent during fetal development, are also observed. The most common form is the pelvic kidney, where the kidney remains in the pelvis rather than ascending to the lumbar region. This anomaly occurs in approximately 1 in 12,000 individuals and is frequently discovered incidentally.[137][138] The adrenal glands are typically in their normal position due to their distinct embryological origins.[139] Additional anomalies include crossed-fused ectopia, UPJ obstruction, and renal duplication.[140][141][142][143][144][145][146]
Surgical Considerations
All intrarenal arteries are generally considered end arteries, with minimal or no significant native collateral circulation under normal physiological conditions.[147] Accessory renal arteries must be preserved during renal surgery, as they supply discrete anatomical regions. Injury can result in localized ischemia and parenchymal loss. In contrast, renal veins freely anastomose and communicate with each other.
The Brodel line is a relatively avascular zone between the anterior and posterior renal end arteries. This landmark extends longitudinally from superior to inferior, located just posterior to the lateral convex border of each kidney. This line represents the optimal site for nephrostomy placement or renal incision to minimize blood loss.[148]
The retroperitoneum is an enclosed space distinct from the peritoneal cavity. Leaving the retroperitoneum intact in cases of renal trauma maintains the tamponade effect and may prevent unnecessary nephrectomy.[149]
Nephron-sparing surgery includes conservative techniques aimed at preserving healthy renal parenchyma as an alternative to total nephrectomy.[150][151] Methods include partial nephrectomy and percutaneous ablation procedures.[152][153] Nephron-sparing surgery is the first-line treatment for small renal masses, with expanding indications.[154] The approach may involve resecting renal tissue supplied by a specific segmental artery, identified intraoperatively by arterial clipping. Both polar and midzone kidney regions can be suitable for nephron-sparing resection of lesions such as small tumors.[155]
Clinical Significance
Intimate knowledge of renal anatomy and its physiological variants is essential for kidney surgery to prevent inadvertent injury, preserve maximal renal function, and optimize outcomes. Renal disease encompasses any structural or functional abnormality of the kidneys. Although etiologies are diverse, progressive loss of renal function is a common endpoint.
Histologically, renal pathologies are classified into 4 major compartments.[156][157] Glomerular diseases include inflammatory glomerulonephritis, often presenting with nephritic syndrome, presenting with hematuria, hypertension, and reduced glomerular filtration rate, as well as proteinuria below 3.5 g/day.[158] Examples of conditions presenting with inflammatory glomerulonephritis include immunoglobulin A nephropathy, postinfectious glomerulonephritis, and lupus nephritis.[159][160][161] Noninflammatory glomerulopathies present with nephrotic syndrome, characterized by proteinuria exceeding 3.5 g/day, hypoalbuminemia, and edema.[162] Examples include minimal change disease, focal segmental glomerulosclerosis, and membranous nephropathy.[163][164][165]
Tubular diseases are commonly associated with acute kidney injury and electrolyte imbalances.[166][167] Examples include acute tubular necrosis, hereditary tubulopathies, and renal tubular acidosis.[168][169][170] Interstitial diseases encompass disorders such as acute or chronic interstitial nephritis, granulomatous conditions (tuberculosis, sarcoidosis), and amyloidosis, all of which primarily involve the renal interstitial tissue.[171][172][173] Vascular diseases affect the renal vasculature and include vasculitis, systemic lupus erythematosus, systemic sclerosis, and atherosclerotic cardiovascular disease.[174][175][176][177]
Other Issues
Nephrology is the medical specialty dedicated to the study, diagnosis, and nonsurgical management of renal disorders. Such conditions comprise chronic kidney disease, renal failure, diabetic nephropathy, lupus nephritis, hypertensive nephrosclerosis, acute kidney injury, glomerulonephritis, dialysis, medical management of kidney transplantation, and electrolyte disturbances.[178]
Urology, as defined by the American Urological Association, is the medical and surgical discipline that provides care for adults and children with conditions of the genitourinary tract and adrenal glands. The specialty addresses diseases, dysfunctions, infections, and neoplasms of the genitourinary system and male reproductive organs (eg, benign prostatic hyperplasia, prostatitis, bladder and prostate cancer, erectile dysfunction, incontinence, hematuria), as well as renal and adrenal conditions requiring surgical intervention, including kidney stones, tumors, transplant surgery, and congenital renal anomalies.
Media
(Click Image to Enlarge)
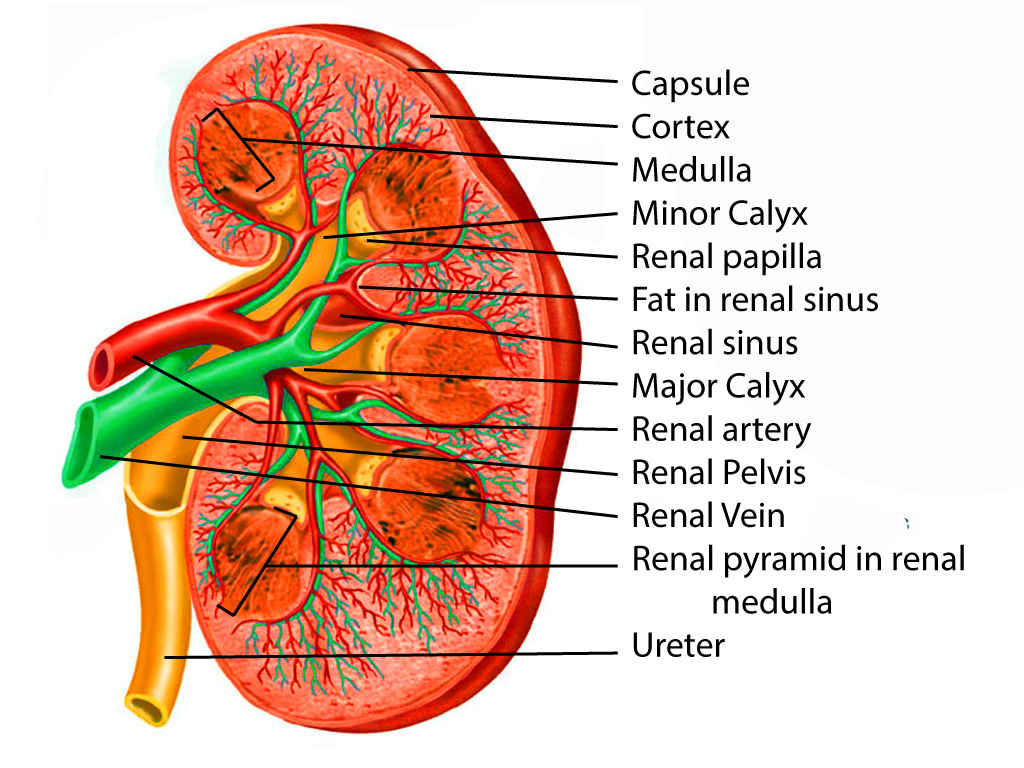
Kidney Anatomy. This illustration shows the internal structure of the kidney, highlighting key features such as the capsule, cortex, medulla, and renal pyramids. Other important labeled components include the minor and major calyces, the renal papilla, artery, vein, and pelvis, fat in the sinus, and the ureter. A clear view of the blood vessels and urine-collecting structures within the kidney is provided.
Contributed by Scott Dulebohn, MD
(Click Image to Enlarge)
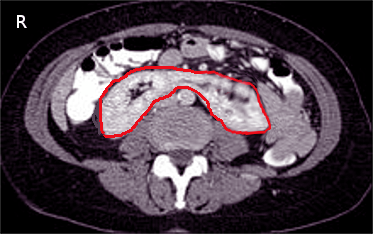
Horseshoe Kidney on Computed Tomography. This axial computed tomography image shows a horseshoe kidney, a congenital anomaly where the lower poles of both kidneys are fused, forming a U-shaped structure. The red outline highlights the fused renal tissue crossing the midline anterior to the vertebral column.
Contributed by S Bhimji, MD
(Click Image to Enlarge)
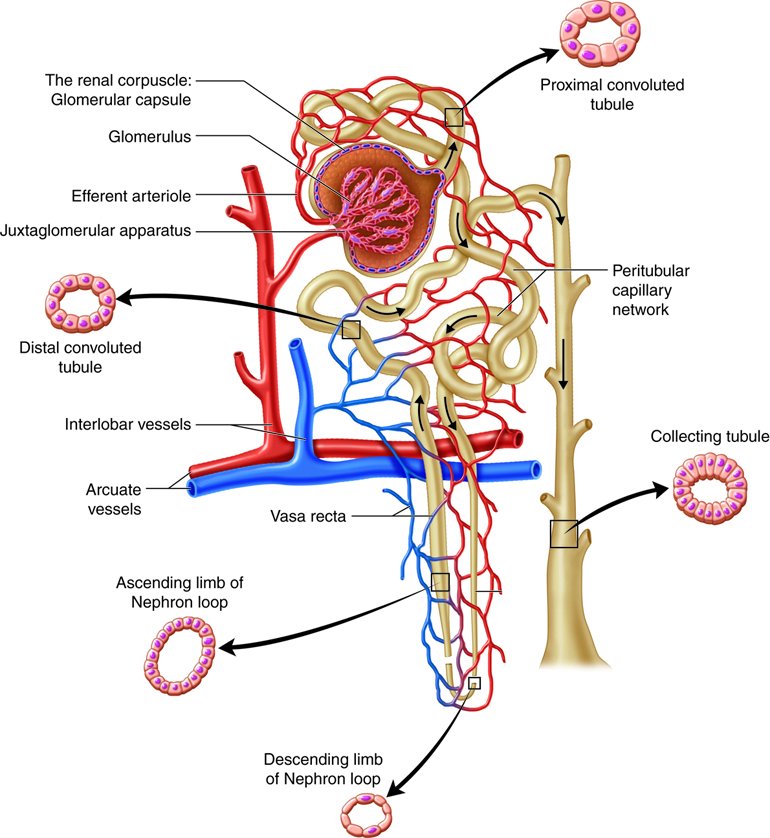
Nephron and Vasculature. The illustration shows the renal corpuscle with the Bowman capsule and glomerulus, the proximal convoluted tubule, nephron loop (descending and ascending limbs), distal convoluted tubule, and the collecting duct. The associated vasculature includes the afferent arteriole, efferent arteriole, peritubular capillaries, and vasa recta.
(Click Image to Enlarge)
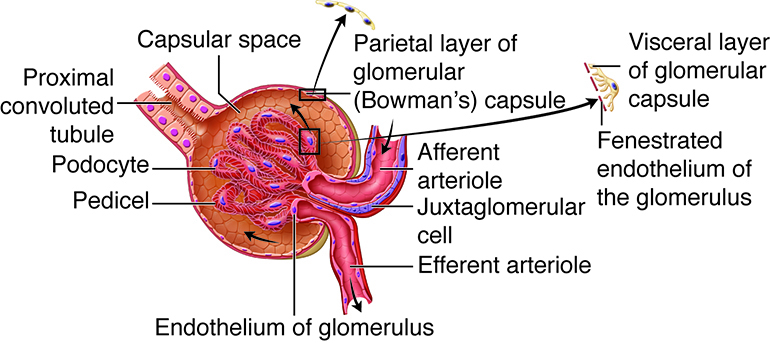
Filtration Apparatus of the Glomerulus. The illustration labels the glomerular capillary tuft (fenestrated endothelium), afferent and efferent arterioles, and the juxtaglomerular cell, along with the Bowman capsule’s parietal and visceral layers formed by podocytes and their pedicels. Filtrate accumulates in the capsular space and exits into the proximal convoluted tubule.
(Click Image to Enlarge)
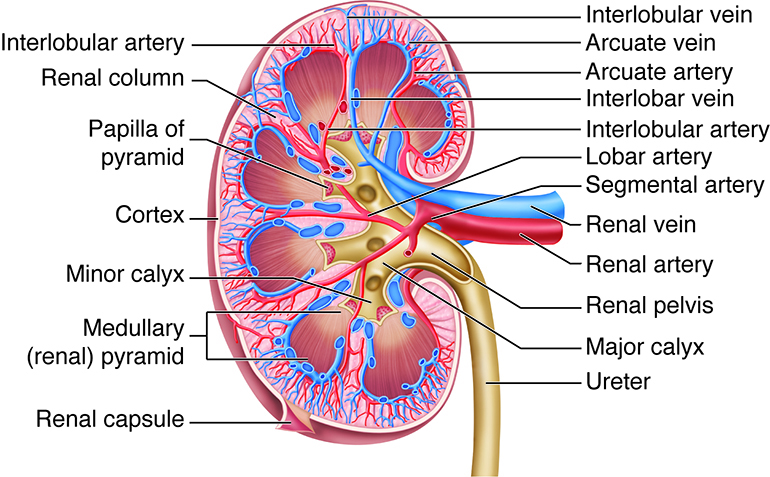
Kidney Blood Supply. The renal artery enters at the hilum and divides into segmental, lobar, interlobar, arcuate, and interlobular arteries. Interlobular arteries radiate into the cortex, while interlobar arteries course between renal pyramids. Venous return follows interlobular, arcuate, and interlobar veins, draining into the renal vein at the hilum. The renal capsule forms the outer covering. The cortex contains glomeruli and extends inward as renal columns between pyramids. Medullary pyramids terminate in papillae projecting into minor calyces, which merge into major calyces. Major calyces unite to form the renal pelvis, a funnel-shaped cavity continuous with the ureter, which conducts urine to the bladder.
References
de la Grandmaison GL, Clairand I, Durigon M. Organ weight in 684 adult autopsies: new tables for a Caucasoid population. Forensic science international. 2001 Jun 15:119(2):149-54 [PubMed PMID: 11376980]
Klatte T, Ficarra V, Gratzke C, Kaouk J, Kutikov A, Macchi V, Mottrie A, Porpiglia F, Porter J, Rogers CG, Russo P, Thompson RH, Uzzo RG, Wood CG, Gill IS. A Literature Review of Renal Surgical Anatomy and Surgical Strategies for Partial Nephrectomy. European urology. 2015 Dec:68(6):980-92. doi: 10.1016/j.eururo.2015.04.010. Epub 2015 Apr 22 [PubMed PMID: 25911061]
El-Reshaid W, Abdul-Fattah H. Sonographic assessment of renal size in healthy adults. Medical principles and practice : international journal of the Kuwait University, Health Science Centre. 2014:23(5):432-6. doi: 10.1159/000364876. Epub 2014 Jul 24 [PubMed PMID: 25060323]
Emamian SA, Nielsen MB, Pedersen JF, Ytte L. Kidney dimensions at sonography: correlation with age, sex, and habitus in 665 adult volunteers. AJR. American journal of roentgenology. 1993 Jan:160(1):83-6 [PubMed PMID: 8416654]
Zhang M, Ni H, Lin Y, Wang K, He T, Yuan L, Han Z, Zuo X. Renal aging and its consequences: navigating the challenges of an aging population. Frontiers in pharmacology. 2025:16():1615681. doi: 10.3389/fphar.2025.1615681. Epub 2025 Jul 24 [PubMed PMID: 40777989]
Coffin A, Boulay-Coletta I, Sebbag-Sfez D, Zins M. Radioanatomy of the retroperitoneal space. Diagnostic and interventional imaging. 2015 Feb:96(2):171-86. doi: 10.1016/j.diii.2014.06.015. Epub 2014 Dec 26 [PubMed PMID: 25547251]
Tirkes T, Sandrasegaran K, Patel AA, Hollar MA, Tejada JG, Tann M, Akisik FM, Lappas JC. Peritoneal and retroperitoneal anatomy and its relevance for cross-sectional imaging. Radiographics : a review publication of the Radiological Society of North America, Inc. 2012 Mar-Apr:32(2):437-51. doi: 10.1148/rg.322115032. Epub [PubMed PMID: 22411941]
Level 2 (mid-level) evidenceMegha R, Wehrle CJ, Kashyap S, Leslie SW. Anatomy, Abdomen and Pelvis: Adrenal Glands (Suprarenal Glands). StatPearls. 2025 Jan:(): [PubMed PMID: 29489211]
Benn M, Southgate SJ, Leslie SW. Ultrasound of the Urinary Tract. StatPearls. 2025 Jan:(): [PubMed PMID: 30571002]
Singh S, Sookraj K. Kidney Trauma. StatPearls. 2025 Jan:(): [PubMed PMID: 30422491]
Bowdino CS, Owens J, Shaw PM. Anatomy, Abdomen and Pelvis, Renal Veins. StatPearls. 2025 Jan:(): [PubMed PMID: 30855882]
Leslie SW, Sajjad H. Anatomy, Abdomen and Pelvis, Renal Artery. StatPearls. 2025 Jan:(): [PubMed PMID: 29083626]
Wu EH, De Cicco FL. Anatomy, Abdomen and Pelvis, Male Genitourinary Tract. StatPearls. 2025 Jan:(): [PubMed PMID: 32965962]
Trivedi S, Sharma U, Rathore M, John MR. Comprehensive Study of Arrangement of Renal Hilar Structures and Branching Pattern of Segmental Renal Arteries: An Anatomical Study. Cureus. 2023 Jul:15(7):e42165. doi: 10.7759/cureus.42165. Epub 2023 Jul 19 [PubMed PMID: 37602117]
Morais ARM, Favorito LA, Sampaio FJB. Kidney collecting system anatomy applied to endourology - a narrative review. International braz j urol : official journal of the Brazilian Society of Urology. 2024 Mar-Apr:50(2):164-177. doi: 10.1590/S1677-5538.IBJU.2024.9901. Epub [PubMed PMID: 38386787]
Level 3 (low-level) evidenceZhang JL, Rusinek H, Chandarana H, Lee VS. Functional MRI of the kidneys. Journal of magnetic resonance imaging : JMRI. 2013 Feb:37(2):282-93. doi: 10.1002/jmri.23717. Epub [PubMed PMID: 23355431]
Chesbrough RM, Burkhard TK, Martinez AJ, Burks DD. Gerota versus Zuckerkandl: the renal fascia revisited. Radiology. 1989 Dec:173(3):845-6 [PubMed PMID: 2682777]
Selçuk İ, Ersak B, Tatar İ, Güngör T, Huri E. Basic clinical retroperitoneal anatomy for pelvic surgeons. Turkish journal of obstetrics and gynecology. 2018 Dec:15(4):259-269. doi: 10.4274/tjod.88614. Epub 2019 Jan 9 [PubMed PMID: 30693143]
Lambert G, Samra NS. Anatomy, Abdomen and Pelvis, Retroperitoneum. StatPearls. 2025 Jan:(): [PubMed PMID: 31751047]
Fine H, Keen EN. Some observations on the medulla of the kidney. British journal of urology. 1976 Jun:48(3):161-9 [PubMed PMID: 938863]
Falkson SR, Bordoni B. Anatomy, Abdomen and Pelvis: Bowman Capsule. StatPearls. 2025 Jan:(): [PubMed PMID: 32119361]
Bonsib SM. Renal Hypoplasia, From Grossly Insufficient to Not Quite Enough: Consideration for Expanded Concepts Based Upon the Author's Perspective With Historical Review. Advances in anatomic pathology. 2020 Sep:27(5):311-330. doi: 10.1097/PAP.0000000000000269. Epub [PubMed PMID: 32520748]
Level 3 (low-level) evidenceMcMahon RS, Penfold D, Bashir K. Anatomy, Abdomen and Pelvis: Kidney Collecting Ducts. StatPearls. 2025 Jan:(): [PubMed PMID: 31747173]
Kaye KW, Goldberg ME. Applied anatomy of the kidney and ureter. The Urologic clinics of North America. 1982 Feb:9(1):3-13 [PubMed PMID: 7080291]
Gandhi KR, Chavan S. Revisiting the morphology of pelvicalyceal system in human cadaveric kidneys with a systematic review of literature. Asian journal of urology. 2019 Jul:6(3):249-255. doi: 10.1016/j.ajur.2018.12.006. Epub 2018 Dec 21 [PubMed PMID: 31297316]
Level 1 (high-level) evidenceSampaio FJ. Renal collecting system anatomy: its possible role in the effectiveness of renal stone treatment. Current opinion in urology. 2001 Jul:11(4):359-66 [PubMed PMID: 11429494]
Level 3 (low-level) evidenceHoy WE, Rees M, Kile E, Mathews JD, Wang Z. A new dimension to the Barker hypothesis: low birthweight and susceptibility to renal disease. Kidney international. 1999 Sep:56(3):1072-7 [PubMed PMID: 10469376]
Kanzaki G, Tsuboi N, Shimizu A, Yokoo T. Human nephron number, hypertension, and renal pathology. Anatomical record (Hoboken, N.J. : 2007). 2020 Oct:303(10):2537-2543. doi: 10.1002/ar.24302. Epub 2019 Nov 15 [PubMed PMID: 31729838]
Bertram JF, Douglas-Denton RN, Diouf B, Hughson MD, Hoy WE. Human nephron number: implications for health and disease. Pediatric nephrology (Berlin, Germany). 2011 Sep:26(9):1529-33. doi: 10.1007/s00467-011-1843-8. Epub 2011 May 22 [PubMed PMID: 21604189]
Pollak MR, Quaggin SE, Hoenig MP, Dworkin LD. The glomerulus: the sphere of influence. Clinical journal of the American Society of Nephrology : CJASN. 2014 Aug 7:9(8):1461-9. doi: 10.2215/CJN.09400913. Epub 2014 May 29 [PubMed PMID: 24875196]
Level 3 (low-level) evidenceMurray IV, Paolini MA. Histology, Kidney and Glomerulus. StatPearls. 2025 Jan:(): [PubMed PMID: 32119431]
Madrazo-Ibarra A, Vaitla P. Histology, Nephron. StatPearls. 2025 Jan:(): [PubMed PMID: 32119298]
Scott RP, Quaggin SE. Review series: The cell biology of renal filtration. The Journal of cell biology. 2015 Apr 27:209(2):199-210. doi: 10.1083/jcb.201410017. Epub [PubMed PMID: 25918223]
Yu E, Sharma S. Physiology, Calcium. StatPearls. 2025 Jan:(): [PubMed PMID: 29489276]
Hopkins E, Sanvictores T, Sharma S. Physiology, Acid Base Balance. StatPearls. 2025 Jan:(): [PubMed PMID: 29939584]
Fountain JH, Kaur J, Lappin SL. Physiology, Renin Angiotensin System. StatPearls. 2025 Jan:(): [PubMed PMID: 29261862]
Ogobuiro I, Tuma F. Physiology, Renal. StatPearls. 2025 Jan:(): [PubMed PMID: 30855923]
Qadeer HA, Bashir K. Physiology, Phosphate. StatPearls. 2025 Jan:(): [PubMed PMID: 32809760]
Lung BE, Komatsu DEE. Calcitriol. StatPearls. 2025 Jan:(): [PubMed PMID: 30252281]
Jelkmann W. Physiology and pharmacology of erythropoietin. Transfusion medicine and hemotherapy : offizielles Organ der Deutschen Gesellschaft fur Transfusionsmedizin und Immunhamatologie. 2013 Oct:40(5):302-9. doi: 10.1159/000356193. Epub 2013 Jul 19 [PubMed PMID: 24273483]
Rehman S, Ahmed D. Embryology, Kidney, Bladder, and Ureter. StatPearls. 2025 Jan:(): [PubMed PMID: 31613527]
Ludwig KS, Landmann L. Early development of the human mesonephros. Anatomy and embryology. 2005 Jul:209(6):439-47 [PubMed PMID: 15915348]
dos Santos Junior AC, de Miranda DM, Simões e Silva AC. Congenital anomalies of the kidney and urinary tract: an embryogenetic review. Birth defects research. Part C, Embryo today : reviews. 2014 Dec:102(4):374-81. doi: 10.1002/bdrc.21084. Epub 2014 Nov 25 [PubMed PMID: 25420794]
Dressler GR. The cellular basis of kidney development. Annual review of cell and developmental biology. 2006:22():509-29 [PubMed PMID: 16822174]
Level 3 (low-level) evidenceLechner MS, Dressler GR. The molecular basis of embryonic kidney development. Mechanisms of development. 1997 Mar:62(2):105-20 [PubMed PMID: 9152004]
Level 3 (low-level) evidenceLopez-Gonzalez M, Ariceta G. WT1-related disorders: more than Denys-Drash syndrome. Pediatric nephrology (Berlin, Germany). 2024 Sep:39(9):2601-2609. doi: 10.1007/s00467-024-06302-y. Epub 2024 Feb 7 [PubMed PMID: 38326647]
Ryan D, Sutherland MR, Flores TJ, Kent AL, Dahlstrom JE, Puelles VG, Bertram JF, McMahon AP, Little MH, Moore L, Black MJ. Development of the Human Fetal Kidney from Mid to Late Gestation in Male and Female Infants. EBioMedicine. 2018 Jan:27():275-283. doi: 10.1016/j.ebiom.2017.12.016. Epub 2017 Dec 20 [PubMed PMID: 29329932]
Upadhyay KK, Silverstein DM. Renal development: a complex process dependent on inductive interaction. Current pediatric reviews. 2014:10(2):107-14 [PubMed PMID: 25088264]
Bagby SP. Developmental origins of renal disease: should nephron protection begin at birth? Clinical journal of the American Society of Nephrology : CJASN. 2009 Jan:4(1):10-3. doi: 10.2215/CJN.06101108. Epub 2009 Jan 7 [PubMed PMID: 19129313]
Puelles VG, Hoy WE, Hughson MD, Diouf B, Douglas-Denton RN, Bertram JF. Glomerular number and size variability and risk for kidney disease. Current opinion in nephrology and hypertension. 2011 Jan:20(1):7-15. doi: 10.1097/MNH.0b013e3283410a7d. Epub [PubMed PMID: 21099687]
Level 3 (low-level) evidenceCzekalski S, Oko A, Pawlaczyk K. [Inherited reduced number of nephrons versus primary arterial hypertension]. Polski merkuriusz lekarski : organ Polskiego Towarzystwa Lekarskiego. 2006 Aug:21(122):120-2; discussion 123-4 [PubMed PMID: 17144093]
Puddu M, Fanos V, Podda F, Zaffanello M. The kidney from prenatal to adult life: perinatal programming and reduction of number of nephrons during development. American journal of nephrology. 2009:30(2):162-70. doi: 10.1159/000211324. Epub 2009 Apr 2 [PubMed PMID: 19339773]
Hoy WE, Hughson MD, Bertram JF, Douglas-Denton R, Amann K. Nephron number, hypertension, renal disease, and renal failure. Journal of the American Society of Nephrology : JASN. 2005 Sep:16(9):2557-64 [PubMed PMID: 16049069]
Benz K, Amann K. Maternal nutrition, low nephron number and arterial hypertension in later life. Biochimica et biophysica acta. 2010 Dec:1802(12):1309-17. doi: 10.1016/j.bbadis.2010.03.002. Epub 2010 Mar 10 [PubMed PMID: 20226855]
Dalal R, Bruss ZS, Sehdev JS. Physiology, Renal Blood Flow and Filtration. StatPearls. 2025 Jan:(): [PubMed PMID: 29489242]
Mullens W, Nijst P. Cardiac Output and Renal Dysfunction: Definitely More Than Impaired Flow. Journal of the American College of Cardiology. 2016 May 17:67(19):2209-2212. doi: 10.1016/j.jacc.2016.03.537. Epub [PubMed PMID: 27173031]
Navar LG. Physiology: hemodynamics, endothelial function, renin-angiotensin-aldosterone system, sympathetic nervous system. Journal of the American Society of Hypertension : JASH. 2014 Jul:8(7):519-24. doi: 10.1016/j.jash.2014.05.014. Epub 2014 Jun 2 [PubMed PMID: 25064774]
Kaufman DP, Basit H, Knohl SJ. Physiology, Glomerular Filtration Rate. StatPearls. 2025 Jan:(): [PubMed PMID: 29763208]
Jen KY, Haragsim L, Laszik ZG. Kidney microvasculature in health and disease. Contributions to nephrology. 2011:169():51-72. doi: 10.1159/000313945. Epub 2011 Jan 20 [PubMed PMID: 21252511]
Fattah H, Layton A, Vallon V. How Do Kidneys Adapt to a Deficit or Loss in Nephron Number? Physiology (Bethesda, Md.). 2019 May 1:34(3):189-197. doi: 10.1152/physiol.00052.2018. Epub [PubMed PMID: 30968755]
Asmar A, Cramon PK, Simonsen L, Asmar M, Sorensen CM, Madsbad S, Moro C, Hartmann B, Jensen BL, Holst JJ, Bülow J. Extracellular Fluid Volume Expansion Uncovers a Natriuretic Action of GLP-1: A Functional GLP-1-Renal Axis in Man. The Journal of clinical endocrinology and metabolism. 2019 Jul 1:104(7):2509-2519. doi: 10.1210/jc.2019-00004. Epub [PubMed PMID: 30835273]
Yavuz YC, Altınkaynak K, Sevinc C, Ozbek Sebin S, Baydar I. The cartonectin levels at different stages of chronic kidney disease and related factors. Renal failure. 2019:41(1):42-46. doi: 10.1080/0886022X.2018.1561373. Epub 2019 Feb 7 [PubMed PMID: 30732504]
Bordei P, St Antohe D, Sapte E, Iliescu D. Morphological aspects of the inferior suprarenal artery. Surgical and radiologic anatomy : SRA. 2003 Jul-Aug:25(3-4):247-51 [PubMed PMID: 14504822]
Priya A, Narayan RK, Ghosh SK. Prevalence and clinical relevance of the anatomical variations of suprarenal arteries: a review. Anatomy & cell biology. 2022 Mar 31:55(1):28-39. doi: 10.5115/acb.21.211. Epub [PubMed PMID: 35046145]
Manso JC, DiDio LJ. Anatomical variations of the human suprarenal arteries. Annals of anatomy = Anatomischer Anzeiger : official organ of the Anatomische Gesellschaft. 2000 Sep:182(5):483-8 [PubMed PMID: 11035646]
Jamkar AA, Khan B, Joshi DS. Anatomical study of renal and accessory renal arteries. Saudi journal of kidney diseases and transplantation : an official publication of the Saudi Center for Organ Transplantation, Saudi Arabia. 2017 Mar-Apr:28(2):292-297. doi: 10.4103/1319-2442.202760. Epub [PubMed PMID: 28352010]
Lung K, Lui F. Anatomy, Abdomen and Pelvis: Arteries. StatPearls. 2025 Jan:(): [PubMed PMID: 30247834]
Wright N, Burns B. Anatomy, Abdomen and Pelvis, Posterior Abdominal Wall Arteries. StatPearls. 2025 Jan:(): [PubMed PMID: 30422567]
Rahmani S, Jafree DJ, Lee PD, Tafforeau P, Brunet J, Nandanwar S, Jacob J, Bellier A, Ackermann M, Jonigk DD, Shipley RJ, Long DA, Walsh CL. Mapping the blood vasculature in an intact human kidney using hierarchical phase-contrast tomography. bioRxiv : the preprint server for biology. 2024 Jul 5:():. pii: 2023.03.28.534566. doi: 10.1101/2023.03.28.534566. Epub 2024 Jul 5 [PubMed PMID: 37034801]
Tucker WD, Shrestha R, Burns B. Anatomy, Abdomen and Pelvis: Inferior Vena Cava. StatPearls. 2025 Jan:(): [PubMed PMID: 29493975]
Tran CT, Wu CY, Bordes SJ, Lui F. Anatomy, Abdomen and Pelvis: Abdominal Aorta. StatPearls. 2025 Jan:(): [PubMed PMID: 30247839]
Wu WC, Hsu WH, Chang TC, Huang LW. Pelvic congestion syndrome due to central venous outflow obstruction: A single-center experience with may-Thurner and nutcracker syndromes. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics. 2025 Jun 13:():. doi: 10.1002/ijgo.70268. Epub 2025 Jun 13 [PubMed PMID: 40512129]
Berthelot JM, Douane F, Maugars Y, Frampas E. Nutcracker syndrome: A rare cause of left flank pain that can also manifest as unexplained pelvic pain. Joint bone spine. 2017 Oct:84(5):557-562. doi: 10.1016/j.jbspin.2016.10.006. Epub 2016 Dec 5 [PubMed PMID: 27932281]
Sarikaya S, Hancer H, Karasu H, Kırali K. [MSB-31] Treatment of Nutcracker Syndrome: Outcomes with Left Renal Vein Transposition and Stenting. Turk gogus kalp damar cerrahisi dergisi. 2024 Nov:32(4 Suppl 2):054-55. doi: 10.5606/tgkdc.dergisi.2024.msb-31. Epub 2024 Dec 31 [PubMed PMID: 40322112]
Penfold D, Leslie SW, Lotfollahzadeh S. Nutcracker Syndrome and Left Renal Vein Entrapment. StatPearls. 2025 Jan:(): [PubMed PMID: 32644615]
Leslie SW, Sajjad H, Siref LE. Varicocele. StatPearls. 2025 Jan:(): [PubMed PMID: 28846314]
Gat Y, Bachar GN, Zukerman Z, Belenky A, Gornish M. Varicocele: a bilateral disease. Fertility and sterility. 2004 Feb:81(2):424-9 [PubMed PMID: 14967384]
Russell PS, Hong J, Windsor JA, Itkin M, Phillips ARJ. Renal Lymphatics: Anatomy, Physiology, and Clinical Implications. Frontiers in physiology. 2019:10():251. doi: 10.3389/fphys.2019.00251. Epub 2019 Mar 14 [PubMed PMID: 30923503]
Lescay HA, Jiang J, Leslie SW, Tuma F. Anatomy, Abdomen and Pelvis Ureter. StatPearls. 2025 Jan:(): [PubMed PMID: 30422575]
Kirkpatrick JJ, Foutz S, Leslie SW. Anatomy, Abdomen and Pelvis: Kidney Nerves. StatPearls. 2025 Jan:(): [PubMed PMID: 29083631]
Barajas L, Powers K. Innervation of the renal proximal convoluted tubule of the rat. The American journal of anatomy. 1989 Dec:186(4):378-88 [PubMed PMID: 2589222]
Barajas L, Powers K. Monoaminergic innervation of the rat kidney: a quantitative study. The American journal of physiology. 1990 Sep:259(3 Pt 2):F503-11 [PubMed PMID: 2396676]
Barajas L, Powers K, Wang P. Innervation of the late distal nephron: an autoradiographic and ultrastructural study. Journal of ultrastructure research. 1985 Sep:92(3):146-57 [PubMed PMID: 3854360]
Barajas L, Powers K, Wang P. Innervation of the renal cortical tubules: a quantitative study. The American journal of physiology. 1984 Jul:247(1 Pt 2):F50-60 [PubMed PMID: 6742205]
LeBouef T, Yaker Z, Whited L. Physiology, Autonomic Nervous System. StatPearls. 2025 Jan:(): [PubMed PMID: 30860751]
N'Guetta PY, McLarnon SR, Tassou A, Geron M, Shirvan S, Hill RZ, Scherrer G, O'Brien LL. Comprehensive mapping of sensory and sympathetic innervation of the developing kidney. bioRxiv : the preprint server for biology. 2024 Mar 7:():. pii: 2023.11.15.567276. doi: 10.1101/2023.11.15.567276. Epub 2024 Mar 7 [PubMed PMID: 38496522]
Scott-Solomon E, Boehm E, Kuruvilla R. The sympathetic nervous system in development and disease. Nature reviews. Neuroscience. 2021 Nov:22(11):685-702. doi: 10.1038/s41583-021-00523-y. Epub 2021 Oct 1 [PubMed PMID: 34599308]
Glebova NO, Ginty DD. Growth and survival signals controlling sympathetic nervous system development. Annual review of neuroscience. 2005:28():191-222 [PubMed PMID: 16022594]
Huang J, Chowhdury SI, Weiss ML. Distribution of sympathetic preganglionic neurons innervating the kidney in the rat: PRV transneuronal tracing and serial reconstruction. Autonomic neuroscience : basic & clinical. 2002 Jan 10:95(1-2):57-70 [PubMed PMID: 11871786]
García-Touchard A, Maranillo E, Mompeo B, Sañudo JR. Microdissection of the Human Renal Nervous System: Implications for Performing Renal Denervation Procedures. Hypertension (Dallas, Tex. : 1979). 2020 Oct:76(4):1240-1246. doi: 10.1161/HYPERTENSIONAHA.120.15106. Epub 2020 Aug 24 [PubMed PMID: 32829660]
Choe WS, Song WH, Jeong CW, Choi EK, Oh S. Anatomic Conformation of Renal Sympathetic Nerve Fibers in Living Human Tissues. Scientific reports. 2019 Mar 18:9(1):4831. doi: 10.1038/s41598-019-41159-4. Epub 2019 Mar 18 [PubMed PMID: 30886195]
García-Touchard A, Sañudo JR. Renal denervation. Importance of knowledge of sympathetic nervous system anatomy in refining the technique. Revista espanola de cardiologia (English ed.). 2019 Jul:72(7):531-534. doi: 10.1016/j.rec.2019.01.016. Epub 2019 May 13 [PubMed PMID: 31097344]
Atherton DS, Deep NL, Mendelsohn FO. Micro-anatomy of the renal sympathetic nervous system: a human postmortem histologic study. Clinical anatomy (New York, N.Y.). 2012 Jul:25(5):628-33. doi: 10.1002/ca.21280. Epub 2011 Oct 4 [PubMed PMID: 21976355]
Guber K, Kirtane AJ. Renal Sympathetic Denervation for Hypertension. Kidney international reports. 2022 Oct:7(10):2129-2140. doi: 10.1016/j.ekir.2022.06.019. Epub 2022 Jul 14 [PubMed PMID: 36217529]
Esler MD, Osborn JW, Schlaich MP. Sympathetic Pathophysiology in Hypertension Origins: The Path to Renal Denervation. Hypertension (Dallas, Tex. : 1979). 2024 Jun:81(6):1194-1205. doi: 10.1161/HYPERTENSIONAHA.123.21715. Epub 2024 Apr 1 [PubMed PMID: 38557153]
Evans LC, Dayton A, Osborn JW. Renal nerves in physiology, pathophysiology and interoception. Nature reviews. Nephrology. 2025 Jan:21(1):57-69. doi: 10.1038/s41581-024-00893-3. Epub 2024 Oct 3 [PubMed PMID: 39363020]
Iyer A, Roh S, Muhammad H, Hammad A, Kumar V, Patel D, Sayanlar J. Renal denervation for resistant hypertension: A review of current literature. Cardiovascular revascularization medicine : including molecular interventions. 2025 Aug:77():122-127. doi: 10.1016/j.carrev.2025.05.014. Epub 2025 May 13 [PubMed PMID: 40450401]
Ye Y, Wang J, Peng Y. Biomarkers for Predicting Blood Pressure Response to Renal Denervation. Current hypertension reports. 2025 Jun 24:27(1):19. doi: 10.1007/s11906-025-01336-5. Epub 2025 Jun 24 [PubMed PMID: 40555927]
Giao DM, Poluha AM, Secemsky EA, Krawisz AK. Endovascular renal denervation for the treatment of hypertension. Vascular medicine (London, England). 2025 Aug:30(4):499-509. doi: 10.1177/1358863X251322179. Epub 2025 May 23 [PubMed PMID: 40405806]
Coppolino G, Pisano A, Rivoli L, Bolignano D. Renal denervation for resistant hypertension. The Cochrane database of systematic reviews. 2017 Feb 21:2(2):CD011499. doi: 10.1002/14651858.CD011499.pub2. Epub 2017 Feb 21 [PubMed PMID: 28220472]
Level 1 (high-level) evidenceKannan A, Medina RI, Nagajothi N, Balamuthusamy S. Renal sympathetic nervous system and the effects of denervation on renal arteries. World journal of cardiology. 2014 Aug 26:6(8):814-23. doi: 10.4330/wjc.v6.i8.814. Epub [PubMed PMID: 25228960]
Converse RL Jr, Jacobsen TN, Toto RD, Jost CM, Cosentino F, Fouad-Tarazi F, Victor RG. Sympathetic overactivity in patients with chronic renal failure. The New England journal of medicine. 1992 Dec 31:327(27):1912-8 [PubMed PMID: 1454086]
Recordati G, Moss NG, Genovesi S, Rogenes P. Renal chemoreceptors. Journal of the autonomic nervous system. 1981 Apr:3(2-4):237-51 [PubMed PMID: 7276433]
Iheanacho F, Vellipuram AR. Physiology, Mechanoreceptors. StatPearls. 2024 Jan:(): [PubMed PMID: 31082112]
Marfurt CF, Echtenkamp SF. Sensory innervation of the rat kidney and ureter as revealed by the anterograde transport of wheat germ agglutinin-horseradish peroxidase (WGA-HRP) from dorsal root ganglia. The Journal of comparative neurology. 1991 Sep 15:311(3):389-404 [PubMed PMID: 1720146]
Level 2 (mid-level) evidenceFrame AA, Carmichael CY, Wainford RD. Renal Afferents. Current hypertension reports. 2016 Sep:18(9):69. doi: 10.1007/s11906-016-0676-z. Epub [PubMed PMID: 27595156]
Kopp UC. Role of renal sensory nerves in physiological and pathophysiological conditions. American journal of physiology. Regulatory, integrative and comparative physiology. 2015 Jan 15:308(2):R79-95. doi: 10.1152/ajpregu.00351.2014. Epub 2014 Nov 19 [PubMed PMID: 25411364]
Level 3 (low-level) evidenceZheng H, Patel KP. Integration of renal sensory afferents at the level of the paraventricular nucleus dictating sympathetic outflow. Autonomic neuroscience : basic & clinical. 2017 May:204():57-64. doi: 10.1016/j.autneu.2016.08.008. Epub 2016 Aug 6 [PubMed PMID: 27527558]
Cluett JL, Blazek O, Brown AL, East C, Ferdinand KC, Fisher NDL, Ford CD, Griffin KA, Mena-Hurtado CI, Sarathy H, Vongpatanasin W, Townsend RR, American Heart Association Council on Hypertension; Council on Cardiovascular and Stroke Nursing; Council on the Kidney in Cardiovascular Disease; and Council on Peripheral Vascular Disease. Renal Denervation for the Treatment of Hypertension: A Scientific Statement From the American Heart Association. Hypertension (Dallas, Tex. : 1979). 2024 Oct:81(10):e135-e148. doi: 10.1161/HYP.0000000000000240. Epub 2024 Aug 5 [PubMed PMID: 39101202]
Sesa-Ashton G, Nolde JM, Muente I, Carnagarin R, Lee R, Macefield VG, Dawood T, Sata Y, Lambert EA, Lambert GW, Walton A, Kiuchi MG, Esler MD, Schlaich MP. Catheter-Based Renal Denervation: 9-Year Follow-Up Data on Safety and Blood Pressure Reduction in Patients With Resistant Hypertension. Hypertension (Dallas, Tex. : 1979). 2023 Apr:80(4):811-819. doi: 10.1161/HYPERTENSIONAHA.122.20853. Epub 2023 Feb 10 [PubMed PMID: 36762561]
Bhatt DL, Vaduganathan M, Kandzari DE, Leon MB, Rocha-Singh K, Townsend RR, Katzen BT, Oparil S, Brar S, DeBruin V, Fahy M, Bakris GL, SYMPLICITY HTN-3 Steering Committee Investigators. Long-term outcomes after catheter-based renal artery denervation for resistant hypertension: final follow-up of the randomised SYMPLICITY HTN-3 Trial. Lancet (London, England). 2022 Oct 22:400(10361):1405-1416. doi: 10.1016/S0140-6736(22)01787-1. Epub 2022 Sep 18 [PubMed PMID: 36130612]
Level 1 (high-level) evidenceTung M, Kobayashi T, Swaminathan RV, Cohen DL, Feldman DN, Fulton B. Renal Denervation: A Review of Current Devices, Techniques, and Evidence. Interventional cardiology clinics. 2025 Apr:14(2):225-234. doi: 10.1016/j.iccl.2024.11.008. Epub 2025 Jan 24 [PubMed PMID: 40049849]
do Carmo LV, Pereira KD, Goulart MA, Laurinavicius AG, Souza J, Junior OP, Armaganijan L, Staico R, Amodeo C, Abizaid A, Cesena FY, Sousa MG, Consolim-Colombo F. Blood Pressure Control and Clinical Outcomes After Renal Denervation Through Irrigated Catheter Radiofrequency Ablation in Patients with Resistant Hypertension: A Case Series with Up to 10 Years of Follow-Up. High blood pressure & cardiovascular prevention : the official journal of the Italian Society of Hypertension. 2024 Nov:31(6):687-694. doi: 10.1007/s40292-024-00685-7. Epub 2024 Nov 18 [PubMed PMID: 39557771]
Level 2 (mid-level) evidenceFernandes A, David C, Pinto FJ, Costa J, Ferreira JJ, Caldeira D. The effect of catheter-based sham renal denervation in hypertension: systematic review and meta-analysis. BMC cardiovascular disorders. 2023 May 12:23(1):249. doi: 10.1186/s12872-023-03269-w. Epub 2023 May 12 [PubMed PMID: 37173636]
Level 1 (high-level) evidenceMufarrih SH, Qureshi NQ, Khan MS, Kazimuddin M, Secemsky E, Bloch MJ, Giri J, Cohen D, Swaminathan RV, Feldman DN, Alaswad K, Kirtane A, Kandzari D, Aronow HD. Randomized Trials of Renal Denervation for Uncontrolled Hypertension: An Updated Meta-Analysis. Journal of the American Heart Association. 2024 Aug 20:13(16):e034910. doi: 10.1161/JAHA.124.034910. Epub 2024 Aug 14 [PubMed PMID: 39140334]
Level 1 (high-level) evidenceSchmieder R, Burnier M, East C, Tsioufis K, Delaney S. Renal Denervation: A Practical Guide for Health Professionals Managing Hypertension. Interventional cardiology (London, England). 2023:18():e06. doi: 10.15420/icr.2022.38. Epub 2023 Mar 7 [PubMed PMID: 37601735]
Swaminathan RV, East CA, Feldman DN, Fisher ND, Garasic JM, Giri JS, Kandzari DE, Kirtane AJ, Klein A, Kobayashi T, Koenig G, Li J, Secemsky E, Townsend RR, Aronow HD. SCAI Position Statement on Renal Denervation for Hypertension: Patient Selection, Operator Competence, Training and Techniques, and Organizational Recommendations. Journal of the Society for Cardiovascular Angiography & Interventions. 2023 Nov-Dec:2(6Part A):101121. doi: 10.1016/j.jscai.2023.101121. Epub 2023 Aug 21 [PubMed PMID: 39129887]
Barbato E, Azizi M, Schmieder RE, Lauder L, Böhm M, Brouwers S, Bruno RM, Dudek D, Kahan T, Kandzari DE, Lüscher TF, Parati G, Pathak A, Ribichini FL, Schlaich MP, Sharp ASP, Sudano I, Volpe M, Tsioufis C, Wijns W, Mahfoud F. Renal denervation in the management of hypertension in adults. A clinical consensus statement of the ESC Council on Hypertension and the European Association of Percutaneous Cardiovascular Interventions (EAPCI). EuroIntervention : journal of EuroPCR in collaboration with the Working Group on Interventional Cardiology of the European Society of Cardiology. 2023 Mar 20:18(15):1227-1243. doi: 10.4244/EIJ-D-22-00723. Epub [PubMed PMID: 36789560]
Level 3 (low-level) evidenceVukadinović D, Lauder L, Kandzari DE, Bhatt DL, Kirtane AJ, Edelman ER, Schmieder RE, Azizi M, Böhm M, Mahfoud F. Effects of Catheter-Based Renal Denervation in Hypertension: A Systematic Review and Meta-Analysis. Circulation. 2024 Nov 12:150(20):1599-1611. doi: 10.1161/CIRCULATIONAHA.124.069709. Epub 2024 Oct 2 [PubMed PMID: 39355923]
Level 1 (high-level) evidenceGlazer K, Brea IJ, Leslie SW, Vaitla P. Ureterolithiasis. StatPearls. 2025 Jan:(): [PubMed PMID: 32809509]
Sikandar S, Dickenson AH. Visceral pain: the ins and outs, the ups and downs. Current opinion in supportive and palliative care. 2012 Mar:6(1):17-26. doi: 10.1097/SPC.0b013e32834f6ec9. Epub [PubMed PMID: 22246042]
Level 3 (low-level) evidenceGlodny B, Petersen J, Hofmann KJ, Schenk C, Herwig R, Trieb T, Koppelstaetter C, Steingruber I, Rehder P. Kidney fusion anomalies revisited: clinical and radiological analysis of 209 cases of crossed fused ectopia and horseshoe kidney. BJU international. 2009 Jan:103(2):224-35. doi: 10.1111/j.1464-410X.2008.07912.x. Epub 2008 Aug 14 [PubMed PMID: 18710445]
Level 3 (low-level) evidenceKirkpatrick JJ, Leslie SW. Horseshoe Kidney. StatPearls. 2025 Jan:(): [PubMed PMID: 28613757]
Pradhay G, Gopidas GS, Karumathil Pullara S, Mathew G, Mathew AJ, Sukumaran TT, Pavikuttan N, Sudhakaran R Sr. Prevalence and Relevance of Multiple Renal Arteries: A Radioanatomical Perspective. Cureus. 2021 Oct:13(10):e18957. doi: 10.7759/cureus.18957. Epub 2021 Oct 21 [PubMed PMID: 34815903]
Level 3 (low-level) evidenceTriantafyllou G, Paschopoulos I, Węgiel A, Olewnik Ł, Tsakotos G, Zielinska N, Piagkou M. The accessory renal arteries: A systematic review with meta-analysis. Clinical anatomy (New York, N.Y.). 2025 Sep:38(6):660-672. doi: 10.1002/ca.24255. Epub 2024 Dec 8 [PubMed PMID: 39648312]
Level 1 (high-level) evidenceGulas E, Wysiadecki G, Szymański J, Majos A, Stefańczyk L, Topol M, Polguj M. Morphological and clinical aspects of the occurrence of accessory (multiple) renal arteries. Archives of medical science : AMS. 2018 Mar:14(2):442-453. doi: 10.5114/aoms.2015.55203. Epub 2016 Mar 17 [PubMed PMID: 29593819]
MERKLIN RJ, MICHELS NA. The variant renal and suprarenal blood supply with data on the inferior phrenic, ureteral and gonadal arteries: a statistical analysis based on 185 dissections and review of the literature. The Journal of the International College of Surgeons. 1958 Jan:29(1 Pt 1):41-76 [PubMed PMID: 13502578]
Stephens FD. Ureterovascular hydronephrosis and the "aberrant" renal vessels. The Journal of urology. 1982 Nov:128(5):984-7 [PubMed PMID: 7176065]
Addonizio JC, Patel RC. Innocent aberrant renal vessels producing ureteropelvic junction obstruction. Urology. 1980 Aug:16(2):176-80 [PubMed PMID: 7404914]
Yufa A, Mikael A, Lara G, Nurick H, Andacheh I. Accessory renal arteries involved in atherosclerotic occlusive disease at the aortic bifurcation. Journal of vascular surgery cases and innovative techniques. 2020 Sep:6(3):425-429. doi: 10.1016/j.jvscit.2020.06.003. Epub 2020 Jun 26 [PubMed PMID: 33367190]
Level 3 (low-level) evidenceSampaio FJ, Passos MA. Renal arteries: anatomic study for surgical and radiological practice. Surgical and radiologic anatomy : SRA. 1992:14(2):113-7 [PubMed PMID: 1641734]
Hostiuc S, Rusu MC, Negoi I, Dorobanțu B, Grigoriu M. Anatomical variants of renal veins: A meta-analysis of prevalence. Scientific reports. 2019 Jul 25:9(1):10802. doi: 10.1038/s41598-019-47280-8. Epub 2019 Jul 25 [PubMed PMID: 31346244]
Level 1 (high-level) evidenceHuff WA Jr, Swan DD, Geller JE, Winn E, Chelf S. Retroaortic Left Renal Vein: A Case Report. Cureus. 2025 Jan:17(1):e78087. doi: 10.7759/cureus.78087. Epub 2025 Jan 27 [PubMed PMID: 40018483]
Level 3 (low-level) evidenceKaraman B, Koplay M, Ozturk E, Basekim CC, Ogul H, Mutlu H, Kizilkaya E, Kantarci M. Retroaortic left renal vein: multidetector computed tomography angiography findings and its clinical importance. Acta radiologica (Stockholm, Sweden : 1987). 2007 Apr:48(3):355-60 [PubMed PMID: 17453511]
Yi SQ, Ueno Y, Naito M, Ozaki N, Itoh M. The three most common variations of the left renal vein: a review and meta-analysis. Surgical and radiologic anatomy : SRA. 2012 Nov:34(9):799-804. doi: 10.1007/s00276-012-0968-1. Epub 2012 Apr 26 [PubMed PMID: 22535303]
Level 1 (high-level) evidenceValenzuela Fuenzalida JJ, Vera-Tapia K, Urzúa-Márquez C, Yáñez-Castillo J, Trujillo-Riveros M, Koscina Z, Orellana-Donoso M, Nova-Baeza P, Suazo-Santibañez A, Sanchis-Gimeno J, Bruna-Mejias A, Gutiérrez Espinoza H. Anatomical Variants of the Renal Veins and Their Relationship with Morphofunctional Alterations of the Kidney: A Systematic Review and Meta-Analysis of Prevalence. Journal of clinical medicine. 2024 Jun 25:13(13):. doi: 10.3390/jcm13133689. Epub 2024 Jun 25 [PubMed PMID: 38999255]
Level 1 (high-level) evidenceMeizner I, Yitzhak M, Levi A, Barki Y, Barnhard Y, Glezerman M. Fetal pelvic kidney: a challenge in prenatal diagnosis? Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 1995 Jun:5(6):391-3 [PubMed PMID: 7552800]
Eid S, Iwanaga J, Loukas M, Oskouian RJ, Tubbs RS. Pelvic Kidney: A Review of the Literature. Cureus. 2018 Jun 9:10(6):e2775. doi: 10.7759/cureus.2775. Epub 2018 Jun 9 [PubMed PMID: 30109168]
Donovan MF, Cascella M. Embryology, Weeks 6-8. StatPearls. 2025 Jan:(): [PubMed PMID: 33085328]
Al Aaraj MS, Badreldin AM. Ureteropelvic Junction Obstruction. StatPearls. 2025 Jan:(): [PubMed PMID: 32809575]
Solanki S, Bhatnagar V, Gupta AK, Kumar R. Crossed fused renal ectopia: Challenges in diagnosis and management. Journal of Indian Association of Pediatric Surgeons. 2013 Jan:18(1):7-10. doi: 10.4103/0971-9261.107006. Epub [PubMed PMID: 23599575]
Loganathan AK, Bal HS. Crossed fused renal ectopia in children: a review of clinical profile, surgical challenges, and outcome. Journal of pediatric urology. 2019 Aug:15(4):315-321. doi: 10.1016/j.jpurol.2019.06.019. Epub 2019 Jun 26 [PubMed PMID: 31331806]
Tilahun SB, Ejeta MA. Crossed fused renal ectopia diagnosed in an adult: Case report. International journal of surgery case reports. 2024 Feb:115():109278. doi: 10.1016/j.ijscr.2024.109278. Epub 2024 Jan 22 [PubMed PMID: 38262217]
Level 3 (low-level) evidenceSociety for Maternal-Fetal Medicine (SMFM), Hopkins LM. Duplicated collecting system. American journal of obstetrics and gynecology. 2021 Nov:225(5):B12-B13. doi: 10.1016/j.ajog.2021.06.040. Epub 2021 Sep 8 [PubMed PMID: 34507788]
Yener S, Pehlivanoğlu C, Akis Yıldız Z, Ilce HT, Ilce Z. Duplex Kidney Anomalies and Associated Pathologies in Children: A Single-Center Retrospective Review. Cureus. 2022 Jun:14(6):e25777. doi: 10.7759/cureus.25777. Epub 2022 Jun 9 [PubMed PMID: 35812643]
Level 2 (mid-level) evidenceDecter RM. Renal duplication and fusion anomalies. Pediatric clinics of North America. 1997 Oct:44(5):1323-41 [PubMed PMID: 9326964]
Rani N, Singh S, Dhar P, Kumar R. Surgical importance of arterial segments of human kidneys: an angiography and corrosion cast study. Journal of clinical and diagnostic research : JCDR. 2014 Mar:8(3):1-3. doi: 10.7860/JCDR/2014/7396.4086. Epub 2014 Mar 15 [PubMed PMID: 24783063]
Macchi V, Picardi E, Inferrera A, Porzionato A, Crestani A, Novara G, De Caro R, Ficarra V. Anatomic and Radiologic Study of Renal Avascular Plane (Brödel's Line) and Its Potential Relevance on Percutaneous and Surgical Approaches to the Kidney. Journal of endourology. 2018 Feb:32(2):154-159. doi: 10.1089/end.2017.0689. Epub [PubMed PMID: 29160086]
Erlich T, Kitrey ND. Renal trauma: the current best practice. Therapeutic advances in urology. 2018 Oct:10(10):295-303. doi: 10.1177/1756287218785828. Epub 2018 Jul 10 [PubMed PMID: 30186367]
Level 3 (low-level) evidenceSilvestri A, Gavi F, Sighinolfi MC, Assumma S, Panio E, Fettucciari D, Pallotta G, Schubert O, Carerj C, Ragonese M, Russo P, Bientinesi R, Foschi N, Ciccarese C, Iacovelli R, Rocco B. Management of Small Renal Masses: Literature and Guidelines Review. International braz j urol : official journal of the Brazilian Society of Urology. 2025 Sep-Oct:51(5):. pii: e20250203. doi: 10.1590/S1677-5538.IBJU.2025.0203. Epub [PubMed PMID: 40339174]
Venkatramani V, Swain S, Satyanarayana R, Parekh DJ. Current Status of Nephron-Sparing Surgery (NSS) in the Management of Renal Tumours. Indian journal of surgical oncology. 2017 Jun:8(2):150-155. doi: 10.1007/s13193-016-0587-0. Epub 2017 Jan 30 [PubMed PMID: 28546710]
Nusrat NB, Walsh K, Darcy F, Durkan G, Aslam A, Imtiaz S. Evaluation of surgical outcomes of nephron-sparing surgery in a leading hospital of an advanced nation. JPMA. The Journal of the Pakistan Medical Association. 2025 Jan:75(1):56-60. doi: 10.47391/JPMA.11367. Epub [PubMed PMID: 39828828]
Ergün M, Sağır S, Akyüz O, Akman RY. Evolving Approach in Nephron-Sparing Surgery: Has Anything Changed from Open Surgery to Laparoscopy? Archivos espanoles de urologia. 2024 Aug:77(7):726-731. doi: 10.56434/j.arch.esp.urol.20247707.101. Epub [PubMed PMID: 39238295]
Wang Y, Butaney M, Wilder S, Ghani K, Rogers CG, Lane BR. The evolving management of small renal masses. Nature reviews. Urology. 2024 Jul:21(7):406-421. doi: 10.1038/s41585-023-00848-6. Epub 2024 Feb 16 [PubMed PMID: 38365895]
Sampaio FJ. Renal anatomy. Endourologic considerations. The Urologic clinics of North America. 2000 Nov:27(4):585-607, vii [PubMed PMID: 11098758]
Prasad M, Vora T, Agarwala S, Laskar S, Arora B, Bansal D, Kapoor G, Chinnaswamy G, Radhakrishnan V, Kaur T, Rath GK, Bakhshi S. Management of Wilms Tumor: ICMR Consensus Document. Indian journal of pediatrics. 2017 Jun:84(6):437-445. doi: 10.1007/s12098-017-2305-5. Epub 2017 Apr 3 [PubMed PMID: 28367612]
Level 3 (low-level) evidenceZuk A, Bonventre JV. Recent advances in acute kidney injury and its consequences and impact on chronic kidney disease. Current opinion in nephrology and hypertension. 2019 Jul:28(4):397-405. doi: 10.1097/MNH.0000000000000504. Epub [PubMed PMID: 30925515]
Level 3 (low-level) evidenceHashmi MS, Pandey J. Nephritic Syndrome. StatPearls. 2025 Jan:(): [PubMed PMID: 32965911]
Rout P, Limaiem F, Hashmi MF. IgA Nephropathy (Berger Disease). StatPearls. 2025 Jan:(): [PubMed PMID: 30855802]
Rawla P, Padala SA, Ludhwani D. Poststreptococcal Glomerulonephritis. StatPearls. 2025 Jan:(): [PubMed PMID: 30855843]
Musa R, Rout P, Qurie A. Lupus Nephritis. StatPearls. 2025 Jan:(): [PubMed PMID: 29762992]
Tapia C, Bashir K. Nephrotic Syndrome. StatPearls. 2025 Jan:(): [PubMed PMID: 29262216]
Zamora G, Pearson-Shaver AL. Minimal Change Disease. StatPearls. 2025 Jan:(): [PubMed PMID: 32809474]
Rout P, Hashmi MF, Baradhi KM. Focal Segmental Glomerulosclerosis. StatPearls. 2025 Jan:(): [PubMed PMID: 30335305]
Alok A, Yadav A. Membranous Nephropathy. StatPearls. 2025 Jan:(): [PubMed PMID: 32644595]
Goyal A, Daneshpajouhnejad P, Hashmi MF, Bashir K. Acute Kidney Injury. StatPearls. 2025 Jan:(): [PubMed PMID: 28722925]
Shrimanker I, Bhattarai S. Electrolytes. StatPearls. 2025 Jan:(): [PubMed PMID: 31082167]
Hanif MO, Rout P, Ramphul K. Acute Renal Tubular Necrosis. StatPearls. 2025 Jan:(): [PubMed PMID: 29939592]
Parmar MS, Muppidi V, Bashir K. Gitelman Syndrome. StatPearls. 2025 Jan:(): [PubMed PMID: 29083583]
Mustaqeem R, Arif A. Renal Tubular Acidosis. StatPearls. 2025 Jan:(): [PubMed PMID: 30085586]
Bhandari J, Thada PK, Rout P, Leslie SW, Arif H. Tubulointerstitial Nephritis. StatPearls. 2025 Jan:(): [PubMed PMID: 32491469]
Roddy K, Tobin EH, Leslie SW, Rathish B. Genitourinary Tuberculosis. StatPearls. 2025 Jan:(): [PubMed PMID: 32491490]
Bustamante JG, Zaidi SRH. Amyloidosis. StatPearls. 2025 Jan:(): [PubMed PMID: 29261990]
Tobe SW, Burgess E, Lebel M. Atherosclerotic renovascular disease. The Canadian journal of cardiology. 2006 May 15:22(7):623-8 [PubMed PMID: 16755319]
de Bhailis ÁM, Lake E, Chrysochou C, Green D, Chinnadurai R, Kalra PA. Improving outcomes in atherosclerotic renovascular disease: importance of clinical presentation and multi-disciplinary review. Journal of nephrology. 2024 May:37(4):1093-1105. doi: 10.1007/s40620-024-01902-1. Epub 2024 Apr 9 [PubMed PMID: 38594599]
Baradhi KM, Bream P. Fibromuscular Dysplasia. StatPearls. 2025 Jan:(): [PubMed PMID: 29630256]
Bokhari MR, Bokhari SRA. Renal Artery Stenosis. StatPearls. 2025 Jan:(): [PubMed PMID: 28613469]
Maxwell AP. So you want to be a Nephrologist. The Ulster medical journal. 2010 Sep:79(3):154-5 [PubMed PMID: 22375090]