 Anatomy, Head and Neck, Larynx Recurrent Laryngeal Nerve
Anatomy, Head and Neck, Larynx Recurrent Laryngeal Nerve
Introduction
The larynx is a flexible structure with a cartilaginous core, interconnecting membranes, and associated musculature. The larynx is positioned at the midline between the digestive and respiratory tracts. This structure houses the vocal cords and produces phonation. The larynx also facilitates airway protection and regulates intrathoracic and intraabdominal pressures. The anatomical position, composition, musculature, and innervation of the larynx contribute to its functions.[1][2][3]
The larynx may be affected by inflammatory, infectious, neoplastic, neurologic, and traumatic conditions, impacting phonation and airway function. The role of this structure in airway protection and vocalization makes it clinically significant in anesthesia, otolaryngology, and emergency medicine, especially for intubation and surgical interventions like laryngectomy. Understanding laryngeal anatomy and physiology is essential for diagnosing nerve injuries, managing airway complications, and optimizing voice rehabilitation. Clinicians rely on this knowledge for surgical planning, airway management, and treatment of vocal cord dysfunction.
Structure and Function
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Structure and Function
Laryngeal Position
The anatomical position of the larynx changes from birth to adulthood. In newborns and during the first few years of life, the larynx sits higher in the neck than in adults. This elevated position allows direct contact between the soft palate and epiglottis, enabling inspired air to pass directly from the nose to the trachea.[4] Consequently, infants can swallow liquids and breathe almost simultaneously.
By adulthood, the larynx descends to its final position within the visceral compartment of the neck, forming the “floor” of the anterior triangle.[5] As the uppermost portion of the respiratory tract, the larynx is vertically aligned with the trachea, which lies directly below and connects via the cricotracheal ligament. Anterosuperiorly, the larynx articulates with the hyoid bone through the thyrohyoid membrane, while posteriorly, it attaches to the muscular walls of the pharynx.
Laryngeal Skeleton
The larynx consists of 9 cartilages: 3 unpaired and 3 paired, all interconnected and linked to the hyoid bone superiorly and the trachea inferiorly. The epiglottic, thyroid, and cricoid cartilages form the 3 unpaired structures, arranged from superior to inferior. The thyroid cartilage, with the epiglottic cartilage above it, dominates the anterior aspect and forms the laryngeal prominence (Adam’s Apple), while the cricoid cartilage, positioned inferior to the thyroid cartilage, is the primary dorsal structure.
The paired cartilages include the arytenoid, corniculate, and cuneiform cartilages. The arytenoid cartilages are located on the posterior larynx, attaching superiorly to the cricoid cartilage. Each arytenoid cartilage extends laterally (muscular process) and anteriorly (vocal process) to support the vocal ligaments. The corniculate and cuneiform cartilages, which are smaller paired structures, reinforce the laryngeal vestibule. The corniculate cartilages sit at the apex of the arytenoid cartilages, while the cuneiform cartilages lie anterior and lateral to the arytenoids. These cartilages are interconnected by multiple membranes, ligaments, and synovial joints.
Two essential synovial joints facilitate laryngeal movement. The cricothyroid joints, located between the thyroid and cricoid cartilages, enable the thyroid cartilage to rotate relative to the cricoid and adjust its anterior positioning. The cricoarytenoid joints, situated between the cricoid and arytenoid cartilages, allow the arytenoid cartilages to translate along both anterior-posterior and lateral-medial axes and rotate along a cranial-caudal axis.
Laryngeal Folds and Membranes
The aryepiglottic folds extend over the lateral aspects of the epiglottic, cuneiform, corniculate, and arytenoid cartilages, marking the entrance to the laryngeal lumen. The piriform sinuses lie just lateral to these folds, which form their medial borders and are sometimes called the "lateral food channels." The aryepiglottic folds, along with their associated cartilages, create a protective ring that helps prevent food from entering the laryngeal aditus. This ring varies in height, with a lower dorsal region known as the interarytenoid notch, which increases susceptibility to food or liquid incursions.
The laryngeal ventricle is the space between the vocal and vestibular folds on each side. The vocal folds, commonly called "vocal cords," and the vestibular folds, or "false vocal cords," are separated by this ventricle. This space also marks the division between the quadrangular membrane superiorly and the cricovocal membrane inferiorly. Together, these membranes line the laryngeal interior from the epiglottic and arytenoid cartilages superiorly to the cricoid cartilage inferiorly.
The quadrangular membrane supports the aryepiglottic folds superiorly and transitions inferiorly into the vestibular folds. These folds contain the vestibular ligament, which extends between the arytenoid and thyroid cartilages. The vestibular folds are relatively immobile and do not contribute to phonation.
The laryngeal ventricle begins just below the free edge of the vestibular fold and extends laterally. This bilateral structure secretes mucus onto the superior surface of the vocal folds, forming a protective layer.
The lateral cricothyroid ligament lies within the cricovocal membrane and, like the vestibular ligament, extends from the arytenoid cartilage to the thyroid cartilage. However, this structure also follows the cricoid cartilage as it extends inferiorly. As the ligament thickens superiorly, it gives rise to the vocal ligament, which spans from the luminal surface of the thyroid cartilage to the vocal process of the arytenoid cartilage. The conus elasticus collectively refers to the cricovocal membrane and its contained ligaments. The medial convergence of these ligaments provides structural support to the vocal folds.
The vocal folds, or true vocal cords, are medial projections of the laryngeal walls that can approximate at the midline, fully obstructing the laryngeal lumen. These folds define the plane known as the glottis. The vocalis muscle runs alongside the vocal ligament within the folds. The ligament and the absence of blood vessels on the fold surfaces contribute to their characteristic white appearance, contrasting with the pink hue of the vestibular folds. The space between the vocal folds is termed the rima glottidis.
Laryngeal Cavity
The laryngeal inlet, also known as the aditus, refers to the entrance of the laryngeal cavity. Positioned superior to this inlet is the laryngopharynx, which serves as the passageway connecting the pharynx to the larynx.
The laryngeal cavity is divided into 3 distinct regions. The supraglottic space is located at the level of the vestibular folds and is bordered anteriorly by the epiglottis, laterally by the aryepiglottic folds, and posteriorly by the interarytenoid mucosa. The middle region consists of the paired laryngeal ventricles, which are situated between the vestibular and vocal folds. These ventricles play a role in the resonance and lubrication of the vocal folds. The subglottic space, also called the "infraglottic space," extends inferiorly to the junction between the cricoid cartilage and the trachea. This region serves as the transition between the larynx and the lower respiratory tract.
Embryology
The larynx is a complex structure of the respiratory tract, composed of both unpaired and paired cartilages. Embryologically, the larynx originates from both endoderm and mesoderm.[6]
The respiratory diverticulum, also referred to as the "lung bud," emerges from the foregut during the 4th week of development. The formation of this structure is driven by an increase in retinoic acid produced by the adjacent mesoderm, which subsequently upregulates transcription factors in the foregut endoderm at the site of the respiratory diverticulum. Thus, the inner lining of the respiratory tract, including the larynx, is derived from endoderm.
The connective tissue, muscle, and cartilage of the larynx originate from the 4th and 6th pharyngeal arches. The cartilaginous components of these arches give rise to the cricoid, thyroid, cuneiform, corniculate, and arytenoid cartilages. The intrinsic muscles of the larynx, along with the cricothyroid muscle, are also derived from these pharyngeal arches.
Unlike the rest of the laryngeal structures, the epiglottis and its associated cartilage do not originate from these same pharyngeal arches. Instead, the epiglottis develops later in mammals and does not appear to have a clear pharyngeal arch origin.
Blood Supply and Lymphatics
The vascular supply of the larynx is derived from the superior and inferior thyroid arteries. The superior thyroid artery originates from the external carotid artery, while the inferior thyroid artery arises from the thyrocervical trunk, which branches from the anterosuperior surface of the subclavian artery.[7]
Venous drainage occurs through the inferior, middle, and superior thyroid veins. The inferior thyroid veins drain into the subclavian or left brachiocephalic vein, whereas the middle and superior thyroid veins empty into the internal jugular vein. Lymphatic drainage is directed medially to the deep cervical and paratracheal nodes and further through the pretracheal and prelaryngeal nodes.
Nerves
The larynx receives innervation from the superior laryngeal nerve (SLN), recurrent laryngeal nerve (RLN), and sympathetic fibers (see Image. Laryngeal Nerves).[8][9][10][11] The SLN branches from the vagus nerve approximately 2.5 cm below the base of the skull and divides into internal and external branches. The internal laryngeal nerve provides sensory and autonomic innervation to the mucosa superior to the glottis, including the superior portion of the laryngeal cavity, the epiglottis, and the superior surface of the vocal folds. Visceral afferents from the epiglottis also contribute to taste perception. Preganglionic parasympathetic fibers travel via the internal laryngeal nerve as well. The external laryngeal nerve supplies motor innervation and visceral efferents to the cricothyroid muscle.
The RLN is another branch of the vagus nerve. The left RLN loops inferior to the aortic arch and courses posterior to the ligamentum arteriosum. The right RLN arises from the vagus nerve as it passes posterior to the root of the right lung, looping around the right subclavian artery before ascending. Both RLNs travel superiorly along the lateral surfaces of the trachea and esophagus within the tracheoesophageal groove, passing posterior to the lobes of the thyroid gland. As these nerves enter the larynx, they course posterior to the cricothyroid joint and pass through fibers of the inferior constrictor muscles of the pharynx. At this point, the RLN transitions into the inferior laryngeal nerve.
The inferior laryngeal branch of the RLN innervates all intrinsic muscles of the larynx except for the cricothyroid muscle, which is supplied by the SLN. Additionally, the RLN carries general visceral sensory fibers from the region inferior to the glottis and sends branches to the inferior constrictor and cricopharyngeus muscles before entering the larynx.
Muscles
The muscles of the larynx are categorized as intrinsic or extrinsic. Intrinsic muscles function in phonation, while extrinsic muscles produce gross movements of the larynx.
Intrinsic Muscles
The intrinsic muscles of the larynx are responsible for sound production and the movements of the laryngeal cartilages and folds.[12] These muscles attach between laryngeal cartilages and, with the exception of the transverse arytenoid muscle, are paired bilaterally.
The oblique and transverse arytenoid muscles span the dorsal aspect of one arytenoid cartilage to the other on the opposite side, functioning to adduct the arytenoid cartilages. The aryepiglottic muscle aligns with the oblique arytenoid muscles and continues with the aryepiglottic fold, assisting in the adduction of the aryepiglottic folds. The thyroepiglottic muscle extends from the epiglottis to the thyroid cartilage, widening the inlet and depressing the epiglottis upon contraction.
The posterior cricoarytenoid muscle extends from the cricoid cartilage to the muscular process of each arytenoid, serving as the only muscle pair that abducts the vocal folds. The lateral cricoarytenoid muscle extends from the cricoid cartilage to the muscular process of the arytenoid cartilage, functioning in vocal fold adduction. The thyroarytenoid muscle extends from the angle of the thyroid cartilage to the arytenoid cartilage, pulling the arytenoid anteriorly to relax and approximate the vocal folds. The vocalis muscle runs along the lateral aspect of the vocal ligament, shortening the vocal folds.
Unlike the other intrinsic muscles, the cricothyroid muscle does not attach to the arytenoid cartilage. Instead, this muscle runs along the lateral cricoid cartilage, with 2 bellies oriented superior-inferior. The superior belly attaches to the inferior portion of the thyroid lamina, while the inferior belly attaches to the inferior horn of the thyroid cartilage. These muscles function to lengthen the vocal folds.
Extrinsic Muscles
The extrinsic muscles of the larynx are found in bilateral pairs and contribute to the gross movement of the larynx. The innervation of these muscles varies, involving the ansa cervicalis, trigeminal nerve, facial nerve, glossopharyngeal nerve, and hypoglossal nerve. The pharyngeal constrictors and palatopharyngeus receive innervation from the glossopharyngeal, vagus, and spinal accessory nerves through the pharyngeal plexus.
Several extrinsic muscles attach directly to the larynx. The sternothyroid muscle connects the anterolateral aspect of the thyroid cartilage to the sternum, contributing to laryngeal depression. The thyrohyoid muscle inserts superior and medial to the sternothyroid muscle on the thyroid cartilage, causing laryngeal elevation. The inferior pharyngeal constrictor muscle attaches anteriorly along the lateral regions of the thyroid and cricoid cartilages, extending superiorly and posteriorly to meet with opposing fibers at the posterior median raphe of the pharynx. This muscle elevates the larynx and provides the only direct connection between the larynx and the skull.
Extrinsic muscles that do not attach directly to the larynx can influence its position by altering the placement of the hyoid bone or by connecting it to the walls of the pharynx. Some of these muscles contribute to laryngeal elevation, while others facilitate laryngeal depression.
Several muscles elevate the pharyngeal walls, including the middle pharyngeal constrictor, stylopharyngeus, and palatopharyngeus. Muscles that contribute to laryngeal elevation include the stylohyoid, hyoglossus, geniohyoid, mylohyoid, and digastric muscles. The omohyoid and sternohyoid muscles are responsible for laryngeal depression.
Physiologic Variants
Laryngeal Skeleton
Sex differences influence laryngeal dimensions, including thyroid angles, with men averaging 95° and women 115°. Despite these differences, the larynx maintains symmetry when comparing one side to the other. Angles increase as diameters and dimensions decrease in a cranial-to-caudal direction.
The Broyle tendon, the connective tissue between the thyroid skeleton and the noduli elastici anterior, also exhibits sex-related differences, with an average length of 2.9 mm in men and 1.8 mm in women. While other absolute differences exist between male and female populations, relative dimensions remain largely consistent. These differences result from the anterior-posterior growth of the larynx during puberty, which primarily occurs in the sagittal plane. Additionally, the thyroid cartilage tends to be thicker in men than in women.
Besides size variations, the laryngeal skeleton's small accessory cartilages exhibit differences in number or location among individuals. For example, tiny cartilages may occasionally develop within the vocal ligament, interarytenoid region, and cricothyroid membrane. These structures, also referred to as the "cartilages of Luschka," represent normal anatomical variations.
Laryngeal Cavities
Physiologic variations also occur in the laryngeal ventricle. In addition to extending laterally, the ventricle may sometimes continue superiorly and anteriorly, forming a saccule beneath the fold.
Nerve Variation
Innervation of the laryngeal structures can vary, as seen in the phenomenon of laryngeal synkinesis.[13] This condition involves abnormal laryngeal innervation that becomes evident following trauma to the RLN. During nerve regeneration, motor axons from the RLN may exit their original pathway and follow branching nerves to establish new motor connections. Nearby nerve fibers can also sprout toward and reinnervate previously paralyzed intrinsic laryngeal muscles. These fibers may originate from the internal branch of the SLN, vagal branches of the pharyngeal constrictor muscles, or parasympathetic, sympathetic, and intralaryngeal branches. Laryngeal synkinesis is classified based on specific laryngeal and phonatory movement patterns.
Variations may also occur in the branching pattern of the RLN.[14] In approximately 40% of cases, the RLN divides into 2 or more branches before entering the larynx through the inferior constrictor muscle. The anterior branch may pass either anterior or posterior to the cricothyroid joint before innervating all intrinsic laryngeal muscles except the cricothyroid muscle. The posterior branch typically supplies the arytenoid muscles and the posterior cricoarytenoid muscle.
The location of RLN bifurcation or trifurcation varies, with documented distances ranging from 0.6 to 4.0 cm from the inferior border of the cricoid cartilage. Although extralaryngeal branching typically occurs above the level of the inferior thyroid artery, branching can take place at any point along the nerve’s course.
The course of the RLN may also be altered due to anatomic distortion from masses, inflammation, or vascular anomalies. In approximately 0.6% of cases, the RLN bypasses its usual course and travels directly from the vagus nerve to the larynx in the neck. This variation, known as a "nonrecurrent" inferior laryngeal nerve, is associated with an atypical right subclavian artery that arises after the left subclavian artery from the aortic arch.[15] This anomaly is exceedingly rare on the left side, with only a few documented cases.
The RLN may also vary in its relationship with the inferior thyroid artery as it approaches the inferior pole of the thyroid gland. In most cases (approximately 61%), the RLN ascends posterior to the inferior thyroid artery. However, this nerve may also ascend anteriorly in about 32.5% of cases or course between the branches of the inferior thyroid artery in approximately 6.5% of cases.[16]
As the RLN ascends, it typically follows the tracheoesophageal groove, though this pattern can vary. The RLN enters the groove more frequently on the left side (77%) compared to the right (65%). Conversely, the RLN is more often found ascending lateral to the trachea on the right side (33%) than on the left (22%). In rare cases, the RLN ascends anterolateral to the trachea, increasing its exposure and risk of surgical injury.
Surgical Considerations
Gross Anatomic Considerations
Although male and female larynges differ in absolute dimensions, no significant relative differences exist. Surgical approaches should be based on relative size and the use of anatomic landmarks rather than absolute measurements. For airway access, a cricothyrotomy involves making an incision through the cricothyroid membrane. This technique is faster and associated with fewer complications compared to a tracheotomy.
Nerve Considerations
Monitoring the RLN and SLN is essential during neck procedures, such as thyroid lobectomy and thyroidectomy.[17] An indirect laryngoscopy serves as a crucial tool for assessing RLN integrity both before and after surgery. In cases of vocal fold dysfunction, reinnervation techniques may be applied to the abductors and tensors using a nerve-muscle pedicle. However, this approach is only viable if the vocal fold is not fixed.
Clinical Significance
Due to its anatomical relationship with several critical structures, the RLN may be affected by various conditions. Thyroid masses, mediastinal and lung tumors, and cardiovascular lesions can compress or otherwise impact the RLN, with the left RLN being more commonly affected.[18][19] The close relationship between the thyroid gland, RLN, and SLN makes these nerves vulnerable during thyroid surgery. Surgical resection of the thyroid gland can result in direct or indirect injury to one or both nerves. Damage to the RLN leads to paralysis of all intrinsic laryngeal muscles except the cricothyroid, resulting in vocal cord paralysis. Injury to the external branch of the SLN causes paralysis of the cricothyroid muscle, leading to dysphonia and altered pitch. Beyond phonation and pitch disturbances, laryngeal weakness increases the risk of aspiration.[20]
Media
(Click Image to Enlarge)
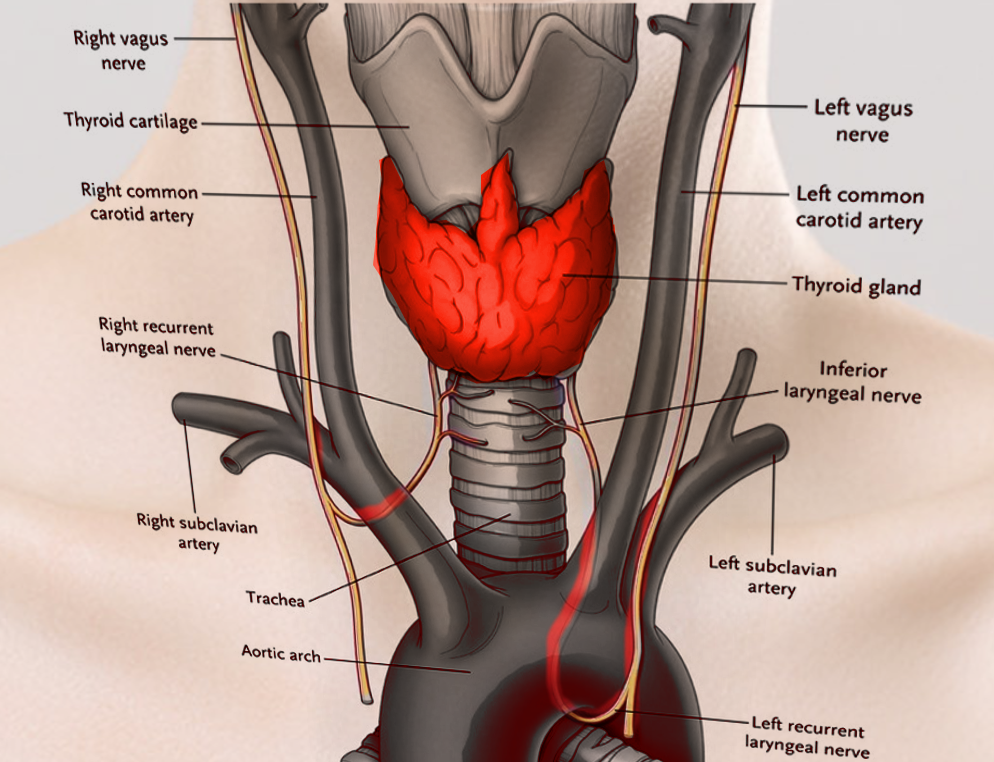
Laryngeal Nerves. This illustration shows the right and left vagus, right and left recurrent laryngeal, and inferior laryngeal nerves. Other structures included in this image are the right and left common carotid and right and left subclavian arteries, thyroid cartilage and gland, trachea, and aortic arch.
Contributed by S Bhimji, MD
References
Sun H, Wu CW, Zhang D, Makay Ö, Zhao Y, Carcofaro P, Kim HY, Dionigi G, Pino A, Caruso E, Pontin A, Pappalardo V. New Paradigms for Neural Monitoring in Thyroid Surgery. Surgical technology international. 2019 May 15:34():79-86 [PubMed PMID: 30664223]
Cirocchi R, Arezzo A, D'Andrea V, Abraha I, Popivanov GI, Avenia N, Gerardi C, Henry BM, Randolph J, Barczyñski M. Intraoperative neuromonitoring versus visual nerve identification for prevention of recurrent laryngeal nerve injury in adults undergoing thyroid surgery. The Cochrane database of systematic reviews. 2019 Jan 19:1(1):CD012483. doi: 10.1002/14651858.CD012483.pub2. Epub 2019 Jan 19 [PubMed PMID: 30659577]
Level 1 (high-level) evidenceBakalinis E, Makris I, Demesticha T, Tsakotos G, Skandalakis P, Filippou D. Non-Recurrent Laryngeal Nerve and Concurrent Vascular Variants: A Review. Acta medica academica. 2018 Nov:47(2):186-192. doi: 10.5644/ama2006-124.230. Epub [PubMed PMID: 30585070]
Matsuo K, Palmer JB. Anatomy and physiology of feeding and swallowing: normal and abnormal. Physical medicine and rehabilitation clinics of North America. 2008 Nov:19(4):691-707, vii. doi: 10.1016/j.pmr.2008.06.001. Epub [PubMed PMID: 18940636]
Kikuta S, Iwanaga J, Kusukawa J, Tubbs RS. Triangles of the neck: a review with clinical/surgical applications. Anatomy & cell biology. 2019 Jun:52(2):120-127. doi: 10.5115/acb.2019.52.2.120. Epub 2019 Jun 30 [PubMed PMID: 31338227]
Lungova V, Thibeault SL. Mechanisms of larynx and vocal fold development and pathogenesis. Cellular and molecular life sciences : CMLS. 2020 Oct:77(19):3781-3795. doi: 10.1007/s00018-020-03506-x. Epub 2020 Apr 6 [PubMed PMID: 32253462]
Branca JJV, Lascialfari Bruschi A, Pilia AM, Carrino D, Guarnieri G, Gulisano M, Pacini A, Paternostro F. The Thyroid Gland: A Revision Study on Its Vascularization and Surgical Implications. Medicina (Kaunas, Lithuania). 2022 Jan 17:58(1):. doi: 10.3390/medicina58010137. Epub 2022 Jan 17 [PubMed PMID: 35056445]
Williamson AJ, Shermetaro C. Unilateral Vocal Cord Paralysis. StatPearls. 2025 Jan:(): [PubMed PMID: 30571041]
Espinosa MC, Ongkasuwan J. Recurrent laryngeal nerve reinnervation: is this the standard of care for pediatric unilateral vocal cord paralysis? Current opinion in otolaryngology & head and neck surgery. 2018 Dec:26(6):431-436. doi: 10.1097/MOO.0000000000000499. Epub [PubMed PMID: 30300212]
Level 3 (low-level) evidenceMahabadi N, Goizueta AA, Bordoni B. Anatomy, Thorax, Lung Pleura And Mediastinum. StatPearls. 2025 Jan:(): [PubMed PMID: 30085590]
Daggumati S, Panossian M D H, Sataloff M D D M A F A C S RT. Vocal Fold Paresis: Incidence, and the Relationship between Voice Handicap Index and Laryngeal EMG Findings. Journal of voice : official journal of the Voice Foundation. 2019 Nov:33(6):940-944. doi: 10.1016/j.jvoice.2018.05.008. Epub 2018 Jul 17 [PubMed PMID: 30025622]
Azar SS, Chhetri DK. Phonation Threshold Pressure Revisited: Effects of Intrinsic Laryngeal Muscle Activation. The Laryngoscope. 2022 Jul:132(7):1427-1432. doi: 10.1002/lary.29944. Epub 2021 Nov 16 [PubMed PMID: 34784055]
Foerster G, Podema R, Guntinas-Lichius O, Crumley RL, Mueller AH. Crumley's Classification of Laryngeal Synkinesis: A Comparison of Laryngoscopy and Electromyography. The Laryngoscope. 2021 May:131(5):E1605-E1610. doi: 10.1002/lary.29275. Epub 2020 Nov 21 [PubMed PMID: 33220002]
Valenzuela-Fuenzalida JJ, Baeza-Garrido V, Navia-Ramírez MF, Cariseo-Ávila C, Bruna-Mejías A, Becerra-Farfan Á, Lopez E, Orellana Donoso M, Loyola-Sepulveda W. Systematic Review and Meta-Analysis: Recurrent Laryngeal Nerve Variants and Their Implication in Surgery and Neck Pathologies, Using the Anatomical Quality Assurance (AQUA) Checklist. Life (Basel, Switzerland). 2023 Apr 24:13(5):. doi: 10.3390/life13051077. Epub 2023 Apr 24 [PubMed PMID: 37240722]
Level 1 (high-level) evidenceDos Santos Menezes Siqueira GV, Dos Santos Rodrigues MH, Santos CNN, Gonçalves PE, Garção DC. Anatomical variations of recurrent laryngeal nerve: a systematic review and meta-analyses. Surgical and radiologic anatomy : SRA. 2024 Mar:46(3):353-362. doi: 10.1007/s00276-023-03293-7. Epub 2024 Feb 8 [PubMed PMID: 38329522]
Level 1 (high-level) evidenceHenry BM, Vikse J, Graves MJ, Sanna S, Sanna B, Tomaszewska IM, Hsieh WC, Tubbs RS, Tomaszewski KA. Variable relationship of the recurrent laryngeal nerve to the inferior thyroid artery: A meta-analysis and surgical implications. Head & neck. 2017 Jan:39(1):177-186. doi: 10.1002/hed.24582. Epub 2016 Sep 14 [PubMed PMID: 27627737]
Level 1 (high-level) evidenceGür EO, Haciyanli M, Karaisli S, Haciyanli S, Kamer E, Acar T, Kumkumoglu Y. Intraoperative nerve monitoring during thyroidectomy: evaluation of signal loss, prognostic value and surgical strategy. Annals of the Royal College of Surgeons of England. 2019 Nov:101(8):589-595. doi: 10.1308/rcsann.2019.0087. Epub 2019 Jun 20 [PubMed PMID: 31219340]
Demiryas S, Donmez T, Cekic E. Effect of nerve monitoring on complications of thyroid surgery. Northern clinics of Istanbul. 2018:5(1):14-19. doi: 10.14744/nci.2017.93764. Epub 2018 Jan 19 [PubMed PMID: 29607426]
Engeseth MS, Olsen NR, Maeland S, Halvorsen T, Goode A, Røksund OD. Left vocal cord paralysis after patent ductus arteriosus ligation: A systematic review. Paediatric respiratory reviews. 2018 Jun:27():74-85. doi: 10.1016/j.prrv.2017.11.001. Epub 2017 Nov 15 [PubMed PMID: 29336933]
Level 1 (high-level) evidenceSchneider M, Dahm V, Passler C, Sterrer E, Mancusi G, Repasi R, Gschwandtner E, Fertl E, Handgriff L, Hermann M. Complete and incomplete recurrent laryngeal nerve injury after thyroid and parathyroid surgery: Characterizing paralysis and paresis. Surgery. 2019 Sep:166(3):369-374. doi: 10.1016/j.surg.2019.05.019. Epub 2019 Jun 28 [PubMed PMID: 31262569]