Introduction
The pulmonary veins are the exclusive conduits for returning oxygenated blood from the lungs to the left atrium, a function essential for systemic oxygen delivery. The clinical significance of these blood vessels extends beyond this role due to their complex and highly variable anatomy, which directly influences diagnostic imaging, interventional cardiology, and thoracic surgery. Variations in pulmonary vein number, ostial size, and drainage patterns are common and can substantially affect procedural planning and outcomes, particularly in lung resection, transplantation, and catheter ablation for atrial fibrillation.[1][2]
Pulmonary veins are central to the pathophysiology and treatment of atrial fibrillation. Myocardial sleeves extending from the left atrium into the proximal veins serve as frequent sources of ectopic electrical activity. The Heart Rhythm Society underscores the need for detailed anatomical knowledge of the pulmonary veins to enable safe and effective catheter and surgical ablation, given the high prevalence of anatomic variants and the arrhythmogenic potential of these structures.
Pulmonary veins are also vulnerable to congenital and acquired diseases, including anomalous pulmonary venous return, stenosis, and thrombosis, each with significant hemodynamic and clinical consequences.[3] Imaging with multidetector computed tomography (CT) and magnetic resonance imaging (MRI) is critical for identifying normal anatomy, anatomic variants, and pathology, and for guiding interventional procedures. The close relationship of the pulmonary veins to adjacent thoracic structures makes them relevant in assessing tumor invasion and mediastinal disease (see Image. Lungs and Mediastinum, Posterior View).[4] Comprehensive knowledge of pulmonary vein anatomy is indispensable for clinicians involved in cardiovascular and thoracic care.
Structure and Function
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Structure and Function
Most individuals have 4 pulmonary veins: right superior, right inferior, left superior, and left inferior, each draining a corresponding lung lobe and entering the left atrium through separate ostia (see Image. Anatomical Relationships of the Pulmonary Veins Within the Pericardium). These veins lack valves at the atrial junctions. The walls consist of an inner endothelial layer, a middle smooth muscle layer, and, proximally, a sleeve of atrial myocardium that extends variably into the veins and contributes to arrhythmogenic potential. The primary role of the pulmonary veins is to transport oxygenated blood from the pulmonary capillary bed to the systemic circulation.
Pulmonary veins originate within the alveolar capillary networks (see Image. Pulmonary Artery and Vein Distribution in a Primary Lobule). These capillaries coalesce into progressively larger vessels that form the interlobar pulmonary veins. Further confluence produces the subsegmental veins, which often drain multiple segments within a single lobe. Limited literature describes the total segmental drainage patterns, although some evidence indicates that 33% to 55% of individuals have middle lung segments drained by a single subsegmental vein. Another 45% to 67% exhibit drainage of the same segments through 2 or more subsegmental veins.[5] Subsegmental veins eventually converge into the major pulmonary veins. Additional peripheral drainage includes contributions from deoxygenated blood in the bronchial venous system.
Humans typically have 4 pulmonary veins, with 2 veins emerging from each lung hilum. The left superior pulmonary vein drains the left upper lobe and lingula. The left inferior pulmonary vein drains the left lower lobe. The right superior pulmonary vein drains both the right upper and middle lobes. The right inferior pulmonary vein drains the right lower lobe.
Superior pulmonary veins course anterior and caudal to the pulmonary arteries. Inferior pulmonary veins lie below the pulmonary arteries and are usually the most inferior structures visible at each hilum (see Image. Anatomy of the Heart, Left View). The pulmonary ligament is situated medial and inferior to the inferior pulmonary vein on both sides. All 4 veins course medially toward the left atrium, draining into separate ostial openings on its posterior wall.
The anatomical relationship at the junction between the pulmonary veins and the epicardium of the left atrium exhibits interindividual variability. A fibrous-adipose tissue plane typically separates the adventitia of the veins from the epicardium. This plane reflects the continuity of epicardial tissue invaginating into the venous adventitia. In some cases, the epicardium directly covers the vessel before it enters the atrial chamber.
Embryology
Development of the pulmonary veins begins during the 4th week of embryogenesis, marked by the emergence of a midpharyngeal endothelial strand from the splanchnic mesoderm. This structure forms the anlage of the common pulmonary vein and appears as an endothelial invagination within the dorsal mesocardium, posterior to the developing left atrium. The pulmonary venous plexus drains into the systemic circulation via the cardinal and vitelline veins in the earliest stages. Pulmonary venous drainage shifts from the systemic venous system to the heart as the common pulmonary vein canalizes and connects to the left atrium. Regression of the splanchnic venous connections establishes the common pulmonary vein as the sole conduit for pulmonary venous return.[6]
The common pulmonary vein becomes identifiable by Carnegie stages 17 to 18 (approximately 5-6 weeks), and incorporation into the posterior wall of the left atrium begins. This incorporation is progressive: the common pulmonary vein and its proximal branches are gradually absorbed into the atrial wall, leading to the formation of 4 discrete pulmonary veins. Pericardial reflections and the dorsal mesocardial connection provide key anatomical landmarks throughout this process. Left and right pulmonary veins develop in parallel, although the right-sided veins enter the atrium at a steeper angle. The vertical distance between superior and inferior veins is less than the horizontal distance between right and left veins. The left superior pulmonary vein is typically the smallest and most compressed in shape.
Absorption of the pulmonary veins into the left atrium occurs in a nonuniform manner. During early development, a trunk-like structure, the spatium pulmonalis, forms and exceeds the size of the initial left atrium. As the atrium enlarges, the pulmonary vein openings become more widely separated, and the spatium elongates.
Left and right pulmonary veins open into distinct recesses (spatia) of the left atrium. The left spatium expands and opens broadly, while the right spatium becomes displaced anteriorly due to the growth of the right atrium. The typical 4-ostia configuration is established near term, corresponding with increased pulmonary blood flow and progressive expansion of the left atrium.[7][8]
At the molecular level, the 2nd heart field mesoderm contributes to the development of the pulmonary vein and the adjacent atrial myocardium. Local signaling pathways regulate the differentiation of the myocardial and smooth muscle components of the pulmonary veins. Myocardial sleeves that extend into the proximal pulmonary veins are derived from the left atrial myocardium.
Disruption of these morphogenetic processes can result in congenital anomalies. Failure of the common pulmonary vein to establish a connection with the left atrium, or persistence of early splanchnic-systemic venous channels, results in total (TAPVR) or partial (PAPVR) anomalous pulmonary venous return. In these conditions, pulmonary veins drain into the right atrium or systemic venous circulation. Incomplete absorption or abnormal incorporation may lead to sinus venosus defects, which feature interatrial communication and anomalous drainage of the right pulmonary veins. Stenosis or atresia of the pulmonary veins can also result from aberrant incorporation.[9][10][11]
Thus, pulmonary vein embryology involves a tightly regulated sequence of morphogenetic events: formation of the common pulmonary vein, the vein's incorporation into the left atrium, and regression of systemic venous connections. Errors in these steps are responsible for clinically significant malformations of pulmonary venous return.
Blood Supply and Lymphatics
The pulmonary veins transport oxygenated blood from the alveoli to the left atrium. Four separate ostia on the posterior wall of the left atrium receive blood from the right and left superior and inferior pulmonary veins. Additional venous structures exist within the pulmonary system. Bronchial arteries and veins supply and drain the conducting airways and supporting lung tissues. Right bronchial veins typically drain into the azygos vein, while left bronchial veins drain into the hemiazygos vein. In some individuals, bronchial veins partially drain into the pulmonary veins. This anatomical variation introduces a small volume of deoxygenated blood into the pulmonary venous return, slightly reducing the oxygen saturation of blood entering the left atrium below 100%.[12]
The pulmonary veins receive a limited arterial supply from small branches of adjacent bronchial arteries. Venous drainage occurs via both bronchial and pulmonary venous pathways. Lymphatic drainage follows bronchovascular bundles toward hilar and mediastinal lymph nodes, closely paralleling the course of the pulmonary arteries and veins.[13]
Nerves
Autonomic innervation of the pulmonary veins is particularly dense at the atrial junction, where parasympathetic and sympathetic fibers form an extensive neural plexus. This autonomic network contributes to the pathogenesis of atrial fibrillation, as the myocardial sleeves extending into the pulmonary veins exhibit high sensitivity to autonomic modulation.
Innervation of the pulmonary veins arises from the pulmonary plexus, which includes both parasympathetic and sympathetic fibers. Parasympathetic fibers, originating from the vagus nerve, mediate vasoconstriction. These fibers course anterior to the pulmonary bronchi and superior to the pulmonary veins. Sympathetic fibers, which promote vasodilation, originate from the thoracic sympathetic trunk. The sympathetic chain runs lateral to the vertebral column and enters the hilum medially, traveling primarily posterior to the pulmonary bronchi. The pulmonary sympathetic contribution derives from the 2nd to 5th thoracic ganglia (T2 to T5), which constitute the pulmonary branch of the sympathetic trunk.[14]
Muscles
Myocardial sleeves derived from the left atrium invest the proximal portions of the pulmonary veins and may extend several centimeters distally. These sleeves consist of atrium-type myocardium and serve as a substrate for ectopic electrical activity. Their arrhythmogenic potential makes them a principal target in catheter ablation procedures for atrial fibrillation.
Physiologic Variants
Multiple physiologically normal pulmonary vein configurations have been documented (see Image. Pulmonary Vein Variants on Multiplanar Computed Tomography Reconstruction). The typical pattern—4 well-differentiated veins, 2 from each lung—is present in approximately 60% to 70% of individuals. Anatomically atypical but clinically normal patterns occur in 30% to 38% of the population.[15] On the left side, 2 common variants have been described. A single left common trunk, classified as either long or short, represents the most frequent left-sided variation. The short left common trunk is observed in approximately 15% of individuals. Right-sided variants are more complex and less frequent. The most common right-sided anomaly is the presence of an accessory right pulmonary vein, often described as a 3rd vein. These accessory veins are further classified based on their anatomical relationship to the typical right superior and inferior pulmonary veins.
Surgical Considerations
Pulmonary veins are highly vascular structures. Any thoracic surgical procedure must be performed with caution to avoid injury to the veins and surrounding anatomy. Several congenital conditions are associated with left-sided pulmonary vein malformation. Many of these anomalies require surgical correction when medical management proves insufficient.[16]
Careful consideration of pulmonary venous anatomy is essential during lung surgeries such as segmentectomy, lobectomy, or transplantation. Common indications for these procedures include cystic fibrosis, tuberculosis, emphysema, benign neoplasms, and primary or metastatic malignancy. Historically, these interventions were performed via open posterolateral thoracotomy. Video-assisted thoracoscopic surgery, a more recent minimally invasive approach, has demonstrated a lower complication rate compared to traditional open techniques.[17]
Clinical Significance
Pulmonary veins are clinically relevant in 2 major contexts: congestion and anatomical malformation. Congestion most commonly results from left-sided heart failure. Pulmonary edema secondary to venous congestion accounts for the respiratory symptoms seen in most patients with congestive heart failure.[18] In the setting of heart failure, reduced cardiac output leads to elevated pressures within the pulmonary venous system. This venous congestion increases hydrostatic pressure in the pulmonary capillaries, resulting in transudation of fluid into the alveolar spaces. Pulmonary edema develops as a consequence, particularly in decompensated heart failure.[19] Management in such cases focuses on relieving volume overload to improve respiratory function and reduce pulmonary vascular pressures.
Anatomical malformations of the pulmonary veins include PAPVR and the more severe form, TAPVR.[20] In PAPVR, anomalous drainage may occur as an isolated finding or in association with other congenital anomalies such as atrial septal defect or scimitar syndrome.[21] Scimitar syndrome is characterized by a triad of right lung hypoplasia, anomalous pulmonary venous drainage to the inferior vena cava, and systemic arterial supply to the right lower lobe.[22] The term "scimitar" refers to the curved radiographic appearance of the anomalous vein, resembling a Turkish sword on chest x-ray. PAPVR has also been reported in association with Turner syndrome (45,XO).[23]
TAPVR involves drainage of all 4 pulmonary veins into the right atrium, bypassing the left atrium entirely. The resultant mixing of oxygenated and deoxygenated blood causes cyanosis, classifying TAPVR among the cyanotic congenital heart diseases. TAPVR is categorized into 4 types based on the site of anomalous venous drainage: supracardiac, infracardiac, cardiac, and mixed, listed in order of decreasing prevalence. All forms of TAPVR historically required surgical correction due to a poor natural prognosis, with mortality approaching 80% within the 1st year of life.[24]
Alveolar-capillary dysplasia with misaligned pulmonary veins represents another disease associated with abnormal pulmonary venous anatomy. This rare and uniformly fatal condition, with nearly 100% mortality, is attributed to an unidentified teratogenic insult that disrupts fetal pulmonary vascular development. Marked reduction in alveolar capillary density results in direct drainage of blood from the pulmonary arterioles into misaligned veins, bypassing the capillary bed.[25]
Multiple imaging modalities are available for visualizing the pulmonary veins. Options include chest radiography, echocardiography, MRI, and CT. Among these tools, CT venography or CT angiography provides the most detailed evaluation of pulmonary venous anatomy.
Right pulmonary vein agenesis is an exceptionally rare congenital anomaly. Collateral venous channels may develop, but oxygenation remains impaired due to the absence of normal venous return from the right lung.
Pulmonary veins are central to the pathophysiology of atrial fibrillation, owing to the arrhythmogenic potential of the myocardial sleeves within their proximal segments. Clinically significant disorders involving the pulmonary veins include stenosis, thrombosis, and congenital anomalies such as PAPVR and TAPVR. Pulmonary vein stenosis may develop as a complication of catheter ablation or as a result of external compression. This condition can lead to pulmonary congestion and pulmonary hypertension. Precise imaging and understanding of both normal and variant pulmonary venous anatomy are essential for accurate diagnosis and effective intervention.[26]
Other Issues
Chronic obstructive pulmonary disease and idiopathic pulmonary arterial hypertension are associated with adverse remodeling of the pulmonary veins. This process is characterized by intimal fibrosis and structural thickening. Impaired vasorelaxation in chronic obstructive pulmonary disease results from reduced availability of nitric oxide and endothelium-derived hyperpolarizing factor, both critical regulators of vascular tone.[27]
Media
(Click Image to Enlarge)
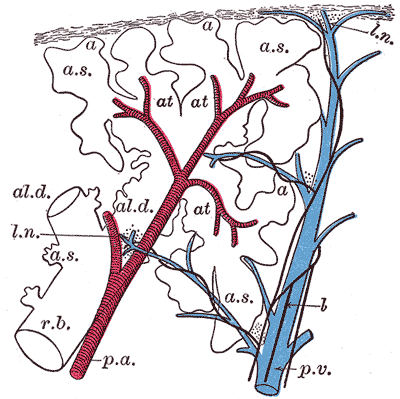
Pulmonary Artery and Vein Distribution in a Primary Lobule. This schematic longitudinal section illustrates the branching of the pulmonary artery (red) and pulmonary vein (blue) within a primary lobule of the lung. Pulmonary arteries follow the bronchioles and supply alveolar capillaries, while pulmonary veins run independently through interlobular septa and drain oxygenated blood into larger tributaries.
Henry Vandyke Carter, Public Domain, via Wikimedia Commons
(Click Image to Enlarge)
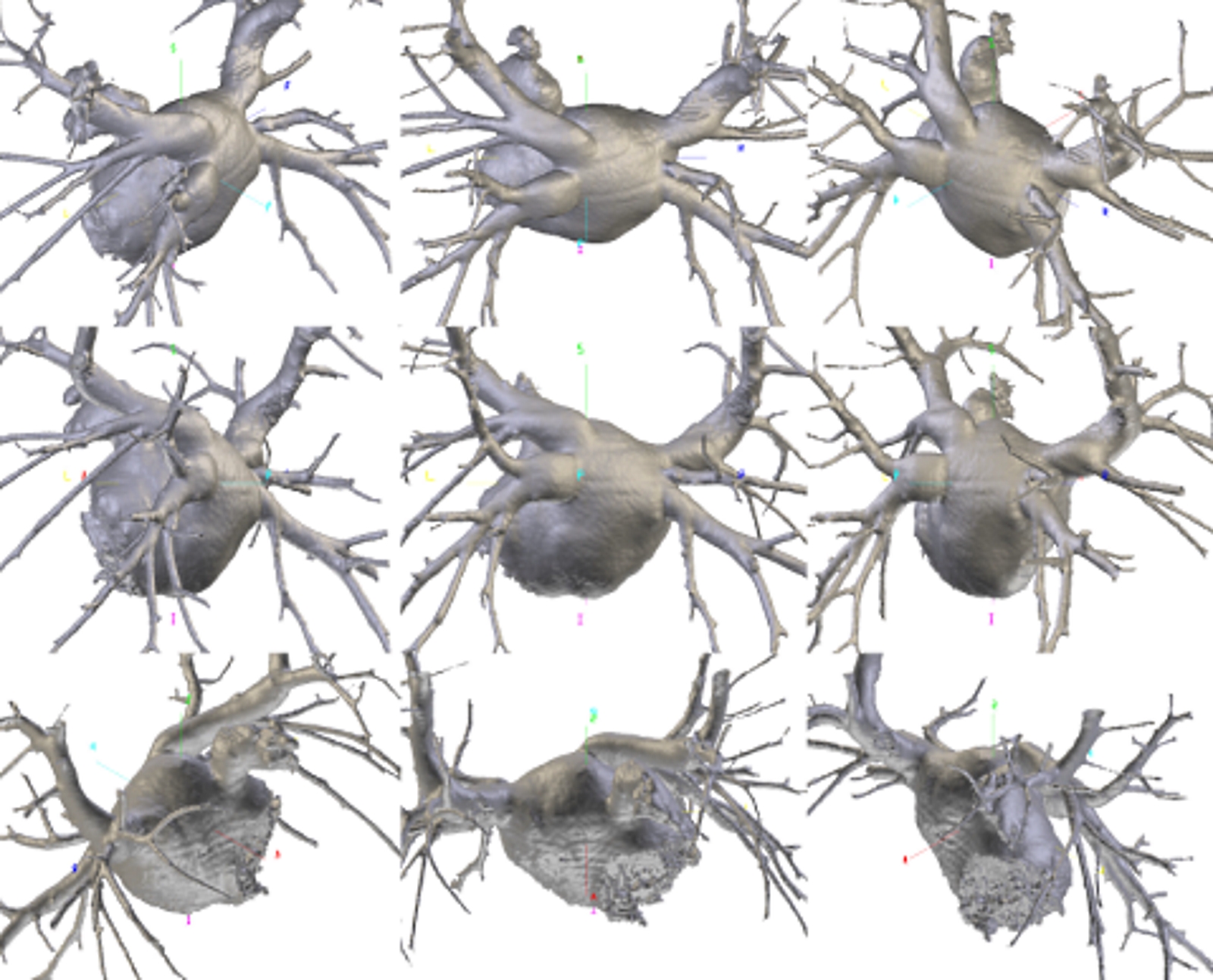
Pulmonary Vein Variants on Multiplanar Computed Tomography Reconstruction. Multiplanar volume-rendered reconstructions of the pulmonary veins and left atrium demonstrate individual anatomical variation in ostial number, caliber, course, and branching. Images were acquired using a maximum intensity projection algorithm along oblique coronal planes. Color-coded arrows mark the early branching of the lower right pulmonary vein.
Contributed by Bruno Bordoni PhD
(Click Image to Enlarge)
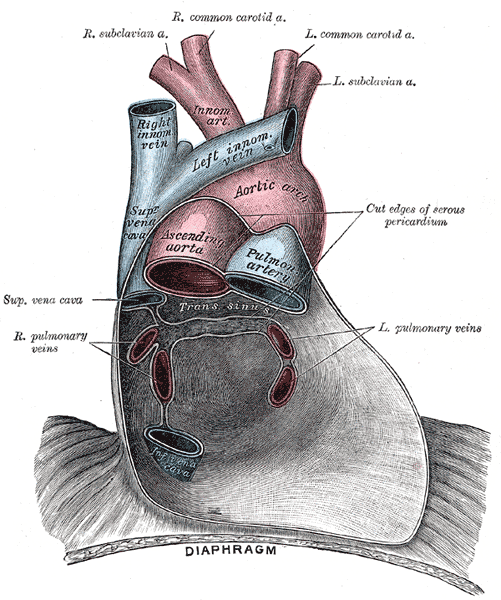
Anatomical Relationships of the Pulmonary Veins Within the Pericardium. This anterior view illustrates the major thoracic vessels enclosed by the serous pericardium, including the left and right pulmonary veins draining into the posterior wall of the left atrium. The ascending aorta, aortic arch, pulmonary artery, and superior vena cava are shown in relation to the pulmonary venous return. The image also depicts the great arterial branches and the anatomical continuity between the pericardium and diaphragm.
Henry Vandyke Carter, Public Domain, via Wikimedia Commons
(Click Image to Enlarge)
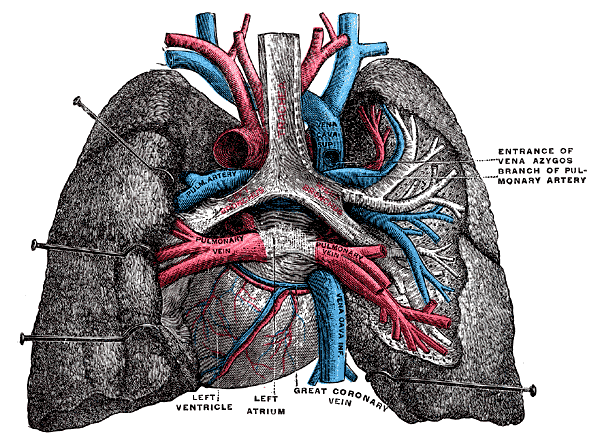
Lungs and Mediastinum, Posterior View. This illustration shows the anatomic relationships between the heart, right and left lungs, right and left pulmonary veins, aortic arch, superior and inferior vena cavae, pulmonary artery branches, and main bronchi. Cardiac structures visible from this region that are labeled include the left atrium and ventricle and the great coronary vein.
Henry Vandyke Carter, Public Domain, via Wikimedia Commons
(Click Image to Enlarge)
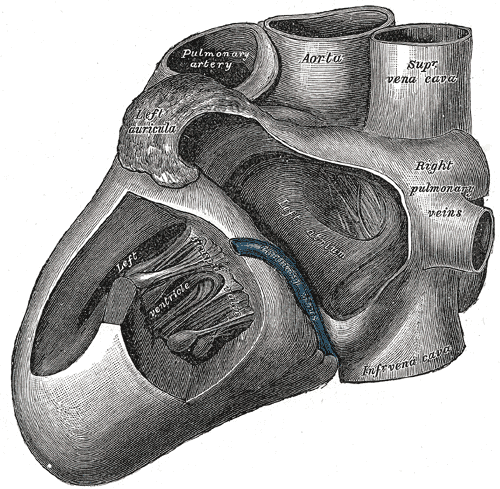
Anatomy of the Heart, Left View. This anatomical illustration shows the heart from the left side, with major structures labeled. Visible features include the left ventricle, right pulmonary veins, superior and inferior vena cava, aorta, pulmonary artery, and the left atrium.
Henry Vandyke Carter, Public Domain, via Wikimedia Commons
References
Klimek-Piotrowska W, Hołda MK, Piątek K, Koziej M, Hołda J. Normal distal pulmonary vein anatomy. PeerJ. 2016:4():e1579. doi: 10.7717/peerj.1579. Epub 2016 Jan 14 [PubMed PMID: 26793429]
Polaczek M, Szaro P, Baranska I, Burakowska B, Ciszek B. Morphology and morphometry of pulmonary veins and the left atrium in multi-slice computed tomography. Surgical and radiologic anatomy : SRA. 2019 Jul:41(7):721-730. doi: 10.1007/s00276-019-02210-1. Epub 2019 Mar 2 [PubMed PMID: 30826845]
Douglas YL, Jongbloed MR, Deruiter MC, Gittenberger-de Groot AC. Normal and abnormal development of pulmonary veins: state of the art and correlation with clinical entities. International journal of cardiology. 2011 Feb 17:147(1):13-24. doi: 10.1016/j.ijcard.2010.07.004. Epub 2010 Aug 2 [PubMed PMID: 20674049]
Hassani C, Saremi F. Comprehensive Cross-sectional Imaging of the Pulmonary Veins. Radiographics : a review publication of the Radiological Society of North America, Inc. 2017 Nov-Dec:37(7):1928-1954. doi: 10.1148/rg.2017170050. Epub [PubMed PMID: 29131765]
Level 2 (mid-level) evidenceRyba S, Topol M. Venous drainage of the middle lobe of the right lung in man. Folia morphologica. 2004 Aug:63(3):303-8 [PubMed PMID: 15478105]
Anderson RH, Brown NA, Moorman AF. Development and structures of the venous pole of the heart. Developmental dynamics : an official publication of the American Association of Anatomists. 2006 Jan:235(1):2-9 [PubMed PMID: 16193508]
Fukui N, Kanahashi T, Matsubayashi J, Imai H, Yoneyama A, Otani H, Yamada S, Takakuwa T. Morphogenesis of the pulmonary vein and left atrial appendage in human embryos and early fetuses. Journal of anatomy. 2024 Jan:244(1):142-158. doi: 10.1111/joa.13941. Epub 2023 Aug 9 [PubMed PMID: 37559438]
Cho KH, Hayashi S, Jin ZW, Kim JH, Murakami G, Rodríguez-Vázquez JF. The so-called absorption process of the pulmonary vein into the left atrium of the heart: a histological study using human embryos and fetuses. Surgical and radiologic anatomy : SRA. 2023 Apr:45(4):469-478. doi: 10.1007/s00276-023-03100-3. Epub 2023 Feb 14 [PubMed PMID: 36786933]
Poelmann RE, Jongbloed MRM, DeRuiter MC. TAPVR: Molecular Pathways and Animal Models. Advances in experimental medicine and biology. 2024:1441():599-614. doi: 10.1007/978-3-031-44087-8_34. Epub [PubMed PMID: 38884736]
Level 3 (low-level) evidenceBlom NA, Gittenberger-de Groot AC, Jongeneel TH, DeRuiter MC, Poelmann RE, Ottenkamp J. Normal development of the pulmonary veins in human embryos and formulation of a morphogenetic concept for sinus venosus defects. The American journal of cardiology. 2001 Feb 1:87(3):305-9 [PubMed PMID: 11165965]
van den Berg G, Moorman AF. Development of the pulmonary vein and the systemic venous sinus: an interactive 3D overview. PloS one. 2011:6(7):e22055. doi: 10.1371/journal.pone.0022055. Epub 2011 Jul 11 [PubMed PMID: 21779373]
Level 3 (low-level) evidenceMarini TJ, He K, Hobbs SK, Kaproth-Joslin K. Pictorial review of the pulmonary vasculature: from arteries to veins. Insights into imaging. 2018 Dec:9(6):971-987. doi: 10.1007/s13244-018-0659-5. Epub 2018 Oct 31 [PubMed PMID: 30382495]
Kelly B, Daley S. Pulmonary Lymphatics History, Anatomy, and Pathophysiology: Emerging Knowledge and a Look to the Future. Lymphology. 2024:57(1):19-26 [PubMed PMID: 39413758]
Belvisi MG. Overview of the innervation of the lung. Current opinion in pharmacology. 2002 Jun:2(3):211-5 [PubMed PMID: 12020459]
Level 3 (low-level) evidenceKato R, Lickfett L, Meininger G, Dickfeld T, Wu R, Juang G, Angkeow P, LaCorte J, Bluemke D, Berger R, Halperin HR, Calkins H. Pulmonary vein anatomy in patients undergoing catheter ablation of atrial fibrillation: lessons learned by use of magnetic resonance imaging. Circulation. 2003 Apr 22:107(15):2004-10 [PubMed PMID: 12681994]
Level 1 (high-level) evidencePorres DV, Morenza OP, Pallisa E, Roque A, Andreu J, Martínez M. Learning from the pulmonary veins. Radiographics : a review publication of the Radiological Society of North America, Inc. 2013 Jul-Aug:33(4):999-1022. doi: 10.1148/rg.334125043. Epub [PubMed PMID: 23842969]
Dinic VD, Stojanovic MD, Markovic D, Cvetanovic V, Vukovic AZ, Jankovic RJ. Enhanced Recovery in Thoracic Surgery: A Review. Frontiers in medicine. 2018:5():14. doi: 10.3389/fmed.2018.00014. Epub 2018 Feb 5 [PubMed PMID: 29459895]
Dupont M, Mullens W, Tang WH. Impact of systemic venous congestion in heart failure. Current heart failure reports. 2011 Dec:8(4):233-41. doi: 10.1007/s11897-011-0071-7. Epub [PubMed PMID: 21861070]
Wilk MM, Wilk J, Urban S, Gajewski P. Current Review of Heart Failure-Related Risk and Prognostic Factors. Biomedicines. 2024 Nov 8:12(11):. doi: 10.3390/biomedicines12112560. Epub 2024 Nov 8 [PubMed PMID: 39595125]
Dimas VV, Dillenbeck J, Josephs S. Congenital pulmonary vascular anomalies. Cardiovascular diagnosis and therapy. 2018 Jun:8(3):214-224. doi: 10.21037/cdt.2018.01.02. Epub [PubMed PMID: 30057871]
Najm HK, Williams WG, Coles JG, Rebeyka IM, Freedom RM. Scimitar syndrome: twenty years' experience and results of repair. The Journal of thoracic and cardiovascular surgery. 1996 Nov:112(5):1161-8; discussion 1168-9 [PubMed PMID: 8911312]
Level 2 (mid-level) evidenceTomás Alvarado E, Zaragoza Martínez MS, Ramirez Teran OA. Scimitar Syndrome: A Thorough Diagnosis in a Pediatric Patient. Cureus. 2024 Aug:16(8):e66302. doi: 10.7759/cureus.66302. Epub 2024 Aug 6 [PubMed PMID: 39238762]
Moore JW, Kirby WC, Rogers WM, Poth MA. Partial anomalous pulmonary venous drainage associated with 45,X Turner's syndrome. Pediatrics. 1990 Aug:86(2):273-6 [PubMed PMID: 2371102]
Shi G, Zhu Z, Chen J, Ou Y, Hong H, Nie Z, Zhang H, Liu X, Zheng J, Sun Q, Liu J, Chen H, Zhuang J. Total Anomalous Pulmonary Venous Connection: The Current Management Strategies in a Pediatric Cohort of 768 Patients. Circulation. 2017 Jan 3:135(1):48-58. doi: 10.1161/CIRCULATIONAHA.116.023889. Epub 2016 Nov 15 [PubMed PMID: 27881562]
Bishop NB, Stankiewicz P, Steinhorn RH. Alveolar capillary dysplasia. American journal of respiratory and critical care medicine. 2011 Jul 15:184(2):172-9. doi: 10.1164/rccm.201010-1697CI. Epub 2011 Mar 11 [PubMed PMID: 21471096]
Level 3 (low-level) evidenceSimard T, Sarma D, Miranda WR, Jain CC, Anderson JH, Collins JD, El Sabbagh A, Jhand A, Peikert T, Reeder GS, Munger TM, Packer DL, Holmes DR. Pathogenesis, Evaluation, and Management of Pulmonary Vein Stenosis: JACC Review Topic of the Week. Journal of the American College of Cardiology. 2023 Jun 20:81(24):2361-2373. doi: 10.1016/j.jacc.2023.04.016. Epub [PubMed PMID: 37316116]
Alqarni AA, Aldhahir AM, Alghamdi SA, Alqahtani JS, Siraj RA, Alwafi H, AlGarni AA, Majrshi MS, Alshehri SM, Pang L. Role of prostanoids, nitric oxide and endothelin pathways in pulmonary hypertension due to COPD. Frontiers in medicine. 2023:10():1275684. doi: 10.3389/fmed.2023.1275684. Epub 2023 Oct 10 [PubMed PMID: 37881627]