Introduction
Prader-Willi syndrome (PWS) is a rare, complex genetic condition that affects the metabolic, endocrine, and neurological systems; this disorder stands out as the predominant syndromic manifestation of obesity. Affected individuals exhibit behavioral, developmental, and intellectual difficulties characterized by severe hypotonia and feeding difficulties in the first years of life. Global developmental delay, hyperphagia, and the onset of obesity manifest around the age of 3. Children with PWS display distinctive facial features, strabismus, and musculoskeletal abnormalities. Hypothalamic dysfunction in PWS contributes to multiple endocrinopathies, including hypogonadism, hypothyroidism, central adrenal insufficiency, and growth hormone deficiency with resulting short stature and reduced bone mineral density.[1]
PWS results from the loss of expression of paternally inherited genes on chromosome 15q11.2–q13, most commonly due to paternal deletion, maternal uniparental disomy, or imprinting defects. In infancy, typical features include severe hypotonia and feeding difficulties. Older children exhibit hyperphagia, developmental delay, and excessive weight gain and benefit from multidisciplinary management to prevent severe obesity and related complications. This activity reviews the etiology, epidemiology, clinical manifestations, and current treatment recommendations, highlighting the role of the interprofessional team in improving long-term outcomes for individuals with PWS.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
PWS results from the absence of gene expression of the paternally inherited genes on the 15q11.2-q13 chromosome. Approximately 70% of cases result from errors in genomic imprinting due to a paternal deletion, while maternal uniparental disomy is responsible for about 25% of cases. Fewer cases stem from defects in the imprinting center, such as microdeletions or epimutations on chromosome 15.[1][2] While most occurrences of PWS are sporadic, familial instances may occur when paternal genes carry a microdeletion in the imprinting center, which is inherited from the paternal grandmother. PWS was the first genetic disorder identified to be caused by genomic imprinting, where the expression of a gene depends on the parental sex that donated the affected gene.
Epidemiology
PWS has a prevalence of 1 in every 20,000 to 30,000 births.[3]. Worldwide, around 400,000 individuals are affected, with 20,000 residing in the United States.[2] This condition stands as the most prevalent genetic cause of life-threatening obesity.[2] Both females and males are equally affected, with no discernible differences noted among racial and ethnic groups.[4]
Pathophysiology
The genetic abnormalities of PWS result in the absence of expression of several key genes necessary for normal hypothalamic function. This dysfunction underlies the major clinical features of PWS, which include neonatal hypotonia, poor feeding in infancy, followed by hyperphagia, impaired satiety, and severe obesity in later childhood and adulthood. Disruption of hypothalamic neuropeptide signaling impairs energy expenditure and multiple endocrine axes, leading to growth hormone deficiency, hypogonadism, hypothyroidism, and, rarely, central adrenal insufficiency.[5] Abnormalities in the oxytocin and ghrelin systems further contribute to hyperphagia. Cognitive impairment has been linked to brain structure and function alterations, particularly in the limbic and frontal pathways. Brain imaging studies have demonstrated significantly reduced thalamus, amygdala, and brainstem volumes, correlating with hyperphagia, behavioral symptoms, and poorer cognitive function.[6]
History and Physical
The clinical manifestations vary across different age groups, constituting a range of features. Symptoms may manifest at birth and become more apparent over time. Hypotonia is a common feature in nearly all infants with PWS and may be noted before birth. Prenatally, there may be reduced fetal movement, and the onset of "quickening," or perception of movement, occurs later.[2] At birth, affected infants often present with growth parameters—including weight, length, and body mass index—15% to 20% smaller than those of their unaffected siblings, reflecting decreased prenatal growth. Abnormal fetal positions may contribute to increased rates of assisted and cesarean deliveries.[7] Hypotonia is often accompanied by a poor sucking reflex, resulting in feeding difficulties, diminished muscle mass and strength, poor weight gain in early life, and a need for specialized feeding devices or prolonged enteral nutrition.
PWS is characterized by dysmorphic facial features, including a narrow frontal diameter, almond-shaped palpebral fissures, strabismus, a slender nasal bridge, a thin upper vermillion border with downturned mouth corners, and enamel hypoplasia. During physical examination, small hands and feet are frequently noted, although these characteristics may not be evident at birth but develop later.[2][7] Developmental delays and behavioral issues are common. Motor developmental delay is typical, with the attainment of early milestones occurring at about half the usual pace.[7] The median age of independent walking is 27 months.[8] Language impairment is commonly encountered, and intellectual and learning disabilities typically manifest by the time children reach school age.[9] Behavioral problems, such as anxiety, obsessive-compulsive disorder, temper outbursts, and self-inflicted injuries, are prevalent in almost all individuals with PWS.[4]
Growth hormone deficiency is the most frequently reported endocrinopathy. Short stature typically presents in early childhood and is evident by the second decade of life, often accompanied by accelerated weight gain and truncal obesity (see Image. Prader-Willi Syndrome Phenotype). Although the pathogenesis is unclear, insulin-like growth factor 1 is deficient or subnormal in nearly all patients with this condition. The growth hormone secretion test and the 24-hour spontaneous secretion of growth hormone show impairment.[1][7] Patients display short stature and growth deceleration, failing to achieve the typical puberty growth spurt.[10] The progression leading to the hallmark hyperphagia and obesity occurs in 4 phases, outlined in Table. Phases of Prader-Willi Syndrome.
Table. Phases of Prader-Willi Syndrome
| Phase | Presentation | Age |
| 1a | Infants are hypotonic and feed poorly, with slow weight gain | Birth to 9 to 15 months |
| 1b | Steady growth with a typical rate of weight gain | 9 to 24 months |
| 2a | Weight gain without significantly increasing caloric intake or appetite | 2 to 4.5 years |
| 2b | Hyperphagia and heightened interest in food | 4.5 years to 8 years [11] |
| 3 | Increased hyperphagia, food-seeking behaviors, and lack of satiety | 8 years to adulthood |
| 4 | Insatiable appetite resolves | Adulthood [2][7] |
Central obesity is typical, leading to significant complications with increased morbidity and mortality.[7] In adults, hyperphagia can result in choking, which can even cause sudden death.[12] Food-seeking behaviors include binge eating and consuming nonfood items, such as garbage. The biological mechanism for this impaired satiety is unclear. In some studies, ghrelin levels are elevated compared to those of weight- and age-matched controls.[13][14]
Hypogonadism is prevalent. Almost all affected males experience cryptorchidism, often necessitating orchiopexy. Male infants also display scrotal hypoplasia, while females may exhibit hypoplasia or absence of the labia minora and clitoral hypoplasia.[15] Penile size, usually average at birth, later falls 2 standard deviations below the normal curve. As a result of a smaller penile size and increased fat pad over the pubic area, standing urination can pose challenges, and a short course of testosterone may aid in potty training. Upon reaching puberty at the typical age, there is an increase in testosterone levels, but they remain abnormally low. Puberty arrest is evident at Tanner stage 3 in sexual maturity rating, characterized by testicular failure and a small testicular size persisting into adulthood.
Girls are born with hypoplasia of the external genitalia. Puberty usually begins at the typical age, but breast development often lags; spontaneous menarche is rare, typically occurring around age 20. Estrogen and luteinizing hormone levels are low to normal, while follicle-stimulating hormone levels are normal to high, indicating central and primary defects. Both males and females are considered infertile, and there is a notable incidence of premature pubarche in both sexes, apparently independent of obesity and insulin resistance.[16] Approximately 14% to 30% of cases are associated with advanced bone age.[1]
Additional manifestations include sleep disorders, thick, viscous saliva, high pain threshold, decreased vomiting, epilepsy, temperature instability, scoliosis/kyphosis, and osteoporosis. Relative to the expected family background, skin, iris, and hair hypopigmentation occur in 30% to 50% of patients.[17] Associated obesity contributes to comorbid conditions, including cardiovascular disease, metabolic dysfunction-associated steatotic liver disease, dyslipidemia, diabetes, sleep apnea, and respiratory failure.
Evaluation
When clinical features suggest PWS, the recommended diagnostic approach involves molecular testing with DNA methylation analysis, which detects over 99% of cases.[18] A positive result prompts further testing to identify the presence of a deletion, maternal disomy, or imprinting defect. Chromosome analysis using fluorescence in situ hybridization can identify the 15q11-q13 deletion, while chromosomal microarray can reveal maternal disomy.[2][19] In resource-rich countries, diagnosis often occurs early, around 2 months of age. However, confirmation may be delayed until around 4 years of age, when more distinctive clinical features become evident.[9]
Additional studies tailored to individual patient needs include:
- Thyroid function tests to determine the presence of hypothyroidism, especially when considering growth hormone treatment [2]
- Liver function tests, serum insulin-like growth factor 1, and insulin-like growth factor binding protein-3 to evaluate possible growth hormone deficiency [2]
- Fasting glucose level, hemoglobin A1c, and oral glucose tolerance test if diabetes is suspected
- Polysomnography (sleep study) to diagnose sleep disorders
- Dual x-ray absorptiometry to assess bone mineralization and composition [2]
Treatment / Management
Caring for patients with PWS depends upon the affected individual's age and involves evaluating and monitoring various organ systems. Infants often exhibit muscular hypotonia, difficulty feeding, and inadequate weight gain, which may necessitate a feeding team evaluation to incorporate specialized feeding techniques and high-calorie supplements or formulas. As hyperphagia emerges in childhood, restricting food intake is critical. Most patients with PWS have reduced energy needs, requiring approximately 70% fewer calories than their age-matched peers. Because a calorie-restricted diet may lack sufficient vitamins and minerals, supplements should be prescribed to meet daily requirements. However, caution should be exercised when using gummy vitamins due to their high caloric content and the risk of overdose, given their candy-like taste.
Caregivers often implement physical barriers, such as locked food cupboards and refrigerators, to enforce food restrictions. Individuals with hyperphagia may not chew food thoroughly, risking choking and thus emphasizing the need for caregiver training in the Heimlich maneuver. In cases of severe obesity in older children and adults, treatment with weight loss medications and bariatric surgery may be helpful.
Physical and occupational therapy can address hypotonia and motor delays. Early treatment with recombinant human growth hormone (rhGH) improves strength, physical function, and muscle development. Formal growth hormone testing is not a prerequisite, and treatment with rhGH in children can be initiated upon diagnosis, preferably before the first birthday.[9][20] Continuous treatment with rhGH during adolescence is recommended to maximize linear growth.
Adults, particularly those with persistent growth hormone deficiency, may also benefit from rhGH treatment. Close monitoring of stature is essential for all children and teenagers with PWS. While the disadvantage of rhGH is the need for daily or weekly injections, the treatment is associated with improved linear growth, reduced obesity, and enhanced bone density and motor function. Patients treated with rhGH during childhood are likelier to approach their predicted final adult height.[10]
Cryptorchidism is observed in approximately 50% of young boys with PWS. While human chorionic gonadotropin treatment may be effective in some cases, the majority require orchiopexy.[1] Consultation with an endocrinologist is recommended at puberty since hormonal replacement may be indicated for delayed puberty. Treatment with testosterone is indicated for boys with delayed puberty, while girls receive estrogen replacement in the form of transdermal patches until the onset of menarche.[1][7]
Most affected individuals exhibit behavioral and psychiatric manifestations, including temper outbursts, obsessive–compulsive features, skin picking, rigidity, and, in some cases, mood or psychotic disorders, with about a quarter of children meeting the diagnostic criteria for autism spectrum disorder.[21] Adolescents and adults with mood disorders or psychoses may benefit from medication and therapy. Behavioral strategies help patients understand rules, schedules, expectations, and verbal cues, thereby minimizing symptoms of compulsion and aggression.[9] Skin picking is a frequent behavior, and a study has demonstrated the effectiveness of treatment with oral guanfacine.[22] Cognitive impairment affects many individuals, and adults may experience accelerated cognitive decline with age. Schoolchildren should have an individualized educational plan, while adults often benefit from a supervised vocational setting.(A1)
Reduced bone mineral density is typical, and patients should undergo evaluation through dual x-ray absorptiometry scans every 2 to 3 years, starting from the age of 5 years. Scoliosis is also common, and clinicians should monitor pediatric patients during routine visits, referring them for orthopedic evaluation when indicated. Routine testing for hypothyroidism should commence within the first year.[23] Type 2 diabetes can emerge as a complication of severe obesity, and laboratory evaluation should be conducted when indicated. Sleep-disordered breathing, a common complication of obesity, affects most children and young adults with PWS. Individuals experiencing daytime sleepiness and behavioral problems should be evaluated for obstructive sleep apnea. In some cases, surgical intervention, such as tonsillectomy or adenotonsillectomy, may be necessary.
Until 2025, no disease-specific treatment existed for people with PWS. In March 2025, the Food and Drug Administration (FDA) approved VYKAT XR (diazoxide choline) extended-release tablets for the treatment of hyperphagia in both adult and pediatric patients aged 4 and older.[24] Multiple clinical trials provided evidence to support the efficacy and safety of this medication before the FDA approved it.[25] (A1)
Differential Diagnosis
Several disorders exhibit similar clinical features and should be considered in the differential diagnosis. Neonatal hypotonia can result from neuropathies and myopathies such as spinal myotonic dystrophy, characterized by poor respiratory effort early in life.[7] Patients with Prader-Willi-like syndrome present with features of PWS such as hypotonia, short stature, obesity, and developmental delays, but differ genetically.[26] Children with craniopharyngioma, a rare tumor, may exhibit signs and symptoms that overlap with PWS caused by the impact of their treatment on the hypothalamus.[7] No other condition shares the distinct presentation of PWS, which involves a gradual progression from hypotonia with poor feeding and slow weight gain to hyperphagia, followed by severe obesity.
Prognosis
The prognosis for patients with PWS varies, influenced by the timing of diagnosis and the extent of complications. Initiating treatment early and preventing severe obesity increases the likelihood of patients achieving a normal lifespan. However, due to intellectual disabilities, most affected individuals require supportive services and are unlikely to lead fully independent lives as adults.
Complications such as obesity, diabetes, and heart failure shorten life expectancy. Death frequently occurs in the fourth decade due to poorly controlled medical comorbidities. However, individuals who maintain a healthy weight have the potential to reach their seventh decade or beyond.[2][27]
Complications
Obesity and its complications are the major contributors to morbidity and mortality, affecting multiple organ systems.[5] Other complications reflect underlying musculoskeletal, neurologic, behavioral, and endocrine dysfunction.
- Respiratory: Obstructive sleep apnea, hypoventilation, aspiration pneumonia, and obesity-related respiratory failure [28]
- Cardiovascular: Hypertension and right-sided heart failure, often related to chronic hypoxia and obesity
- Gastrointestinal: Gastroparesis, delayed gastric emptying, metabolic dysfunction-associated steatotic liver disease, and binge eating-related gastric perforation or obstruction
- Musculoskeletal: Osteoporosis, scoliosis, kyphosis, hip dysplasia, and increased fracture risk [29]
- Skin: Stasis ulcers and recurrent cellulitis caused by poor circulation and immobility
- Behavioral and Cognitive: Self-injurious behavior with risk of physical injury, family stress, reduced quality of life, and inability to live independently
- Neurologic: Seizures, temperature dysregulation, and persistent hypotonia [2]
- Endocrine: Type 2 diabetes and adrenal insufficiency [30]
Deterrence and Patient Education
Clinicians caring for children should maintain a high index of suspicion and assess infants with hypotonia, poor sucking, slow weight gain, or cryptorchidism for PWS. Early diagnosis and intervention can effectively address issues during infancy, and family caregivers should receive education about the developmental course of children with the condition. Affected individuals require lifelong dietary intervention to prevent severe obesity, along with comprehensive medical and educational services.
Pearls and Other Issues
Key facts include:
- PWS is a genetic disorder caused by the lack of expression of paternally inherited genes on chromosome 15q11-13, often due to a deletion in this region, maternal uniparental disomy (where both copies are from the mother), or imprinting defects.
- PWS is characterized by neonatal hypotonia, feeding difficulties, developmental delays, and the later onset of hyperphagia, which can lead to obesity. Distinct facial features, such as almond-shaped eyes and a thin upper lip, may be present.
- DNA methylation analysis is a key diagnostic test that detects abnormalities in over 99% of cases.
- Hypotonia and poor feeding occur in infancy, followed by excessive eating and obesity starting around age 3. Behavioral issues, including food-seeking behaviors, are common.
- Individuals with PWS are typically infertile.
- Central obesity is a characteristic feature that compromises health and increases morbidity and mortality.
Enhancing Healthcare Team Outcomes
PWS is a complex genetic disorder resulting from a defect on chromosome 15. Healthcare professionals should suspect this diagnosis when an infant demonstrates significant hypotonia, difficulty feeding, and slow weight gain, followed by hyperphagia that results in severe obesity. Upon diagnosis, it is crucial to incorporate an interprofessional team, including consultations with genetics, endocrinology, and developmental medicine, to evaluate and treat symptoms, as well as prevent and manage complications. Parents should undergo genetic counseling to estimate the recurrence risk in future pregnancies.
A nutritionist or registered dietitian can provide essential dietary advice for infants to facilitate appropriate weight gain and mitigate the risk of severe obesity and its complications. Infants may require evaluation by a feeding team to enable appropriate weight gain during the first year. Children younger than 3 should participate in early intervention programs to assess and treat global developmental delays. Older children require an individualized education program at school that may include speech, physical, and occupational therapy.
Clinical pharmacists assist with dosing hormonal replacement medication when indicated. Nursing staff monitor and document the response to treatment at each visit, charting growth and laboratory results to allow the clinical team to modify the regimen as needed. Nurses support families and coordinate care for individuals with Prader-Willi syndrome, who have specialized nutritional, behavioral, and educational needs. A mental health nurse is a valuable member of the interprofessional team, assisting individuals affected by mental health issues and their caregivers. Psychiatrists and psychiatric nurse practitioners prescribe medication for mood disorders and psychoses. Visiting nurses conduct home visits and help families with practical techniques to manage food-seeking behaviors safely. Patients with orthopedic problems often benefit from physical and occupational therapy, enabling them to perform daily living activities. This interprofessional team approach is necessary to optimize clinical outcomes, reduce morbidity, and improve the quality of life and longevity of patients with PWS.
Media
(Click Image to Enlarge)
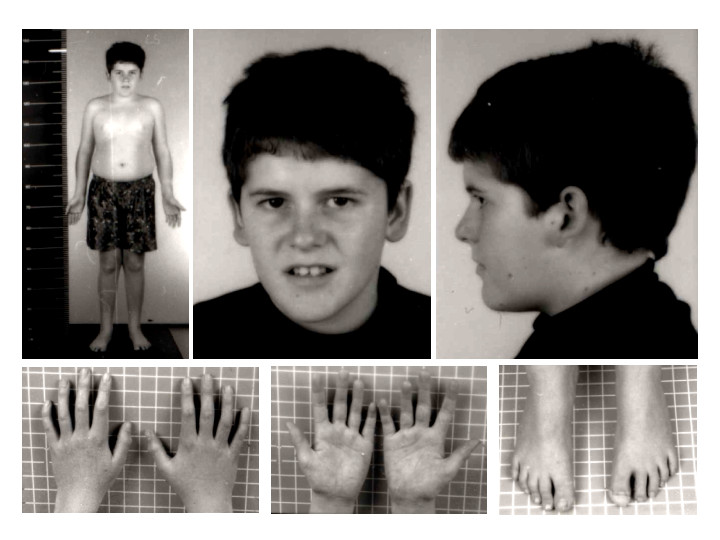
Prader-Willi Syndrome Phenotype. The child is 15 years of age with the Prader-Willi phenotype. Note the absence of typical Prader-Willi syndrome facial features. Mild truncal obesity can be noted.
Contributed by Wikimedia Commons, Schüle B et al (CC by 2.0) https://creativecommons.org/licenses/by/2.0/
References
Heksch R, Kamboj M, Anglin K, Obrynba K. Review of Prader-Willi syndrome: the endocrine approach. Translational pediatrics. 2017 Oct:6(4):274-285. doi: 10.21037/tp.2017.09.04. Epub [PubMed PMID: 29184809]
Butler MG, Manzardo AM, Forster JL. Prader-Willi Syndrome: Clinical Genetics and Diagnostic Aspects with Treatment Approaches. Current pediatric reviews. 2016:12(2):136-66 [PubMed PMID: 26592417]
Pacoricona Alfaro DL, Lemoine P, Ehlinger V, Molinas C, Diene G, Valette M, Pinto G, Coupaye M, Poitou-Bernert C, Thuilleaux D, Arnaud C, Tauber M. Causes of death in Prader-Willi syndrome: lessons from 11 years' experience of a national reference center. Orphanet journal of rare diseases. 2019 Nov 4:14(1):238. doi: 10.1186/s13023-019-1214-2. Epub 2019 Nov 4 [PubMed PMID: 31684997]
Bohonowych J, Miller J, McCandless SE, Strong TV. The Global Prader-Willi Syndrome Registry: Development, Launch, and Early Demographics. Genes. 2019 Sep 14:10(9):. doi: 10.3390/genes10090713. Epub 2019 Sep 14 [PubMed PMID: 31540108]
Höybye C, Tauber M. Approach to the Patient With Prader-Willi Syndrome. The Journal of clinical endocrinology and metabolism. 2022 May 17:107(6):1698-1705. doi: 10.1210/clinem/dgac082. Epub [PubMed PMID: 35150573]
Yamada K, Watanabe M, Suzuki K. Differential volume reductions in the subcortical, limbic, and brainstem structures associated with behavior in Prader-Willi syndrome. Scientific reports. 2022 Mar 23:12(1):4978. doi: 10.1038/s41598-022-08898-3. Epub 2022 Mar 23 [PubMed PMID: 35322075]
Cassidy SB, Schwartz S, Miller JL, Driscoll DJ. Prader-Willi syndrome. Genetics in medicine : official journal of the American College of Medical Genetics. 2012 Jan:14(1):10-26. doi: 10.1038/gim.0b013e31822bead0. Epub 2011 Sep 26 [PubMed PMID: 22237428]
Butler MG. Prader-Willi syndrome: current understanding of cause and diagnosis. American journal of medical genetics. 1990 Mar:35(3):319-32 [PubMed PMID: 2309779]
Level 3 (low-level) evidencePassone CBG, Pasqualucci PL, Franco RR, Ito SS, Mattar LBF, Koiffmann CP, Soster LA, Carneiro JDA, Cabral Menezes-Filho H, Damiani D. PRADER-WILLI SYNDROME: WHAT IS THE GENERAL PEDIATRICIAN SUPPOSED TO DO? - A REVIEW. Revista paulista de pediatria : orgao oficial da Sociedade de Pediatria de Sao Paulo. 2018 Jul-Sep:36(3):345-352. doi: 10.1590/1984-0462/;2018;36;3;00003. Epub [PubMed PMID: 30365815]
Angulo MA, Butler MG, Cataletto ME. Prader-Willi syndrome: a review of clinical, genetic, and endocrine findings. Journal of endocrinological investigation. 2015 Dec:38(12):1249-63. doi: 10.1007/s40618-015-0312-9. Epub 2015 Jun 11 [PubMed PMID: 26062517]
Miller JL, Lynn CH, Driscoll DC, Goldstone AP, Gold JA, Kimonis V, Dykens E, Butler MG, Shuster JJ, Driscoll DJ. Nutritional phases in Prader-Willi syndrome. American journal of medical genetics. Part A. 2011 May:155A(5):1040-9. doi: 10.1002/ajmg.a.33951. Epub 2011 Apr 4 [PubMed PMID: 21465655]
Level 2 (mid-level) evidenceBellis SA, Kuhn I, Adams S, Mullarkey L, Holland A. The consequences of hyperphagia in people with Prader-Willi Syndrome: A systematic review of studies of morbidity and mortality. European journal of medical genetics. 2022 Jan:65(1):104379. doi: 10.1016/j.ejmg.2021.104379. Epub 2021 Nov 5 [PubMed PMID: 34748997]
Level 1 (high-level) evidenceCummings DE, Clement K, Purnell JQ, Vaisse C, Foster KE, Frayo RS, Schwartz MW, Basdevant A, Weigle DS. Elevated plasma ghrelin levels in Prader Willi syndrome. Nature medicine. 2002 Jul:8(7):643-4 [PubMed PMID: 12091883]
Haqq AM, Farooqi IS, O'Rahilly S, Stadler DD, Rosenfeld RG, Pratt KL, LaFranchi SH, Purnell JQ. Serum ghrelin levels are inversely correlated with body mass index, age, and insulin concentrations in normal children and are markedly increased in Prader-Willi syndrome. The Journal of clinical endocrinology and metabolism. 2003 Jan:88(1):174-8 [PubMed PMID: 12519848]
Level 2 (mid-level) evidenceCrinò A, Schiaffini R, Ciampalini P, Spera S, Beccaria L, Benzi F, Bosio L, Corrias A, Gargantini L, Salvatoni A, Tonini G, Trifirò G, Livieri C, Genetic Obesity Study Group of Italian Society of Pediatric endocrinology and diabetology (SIEDP). Hypogonadism and pubertal development in Prader-Willi syndrome. European journal of pediatrics. 2003 May:162(5):327-33 [PubMed PMID: 12692714]
Griffing E, Halpin K, Lee BR, Paprocki E. Premature pubarche in Prader-Willi syndrome: Risk factors and consequences. Clinical endocrinology. 2024 Aug:101(2):162-169. doi: 10.1111/cen.15108. Epub 2024 Jun 27 [PubMed PMID: 38935853]
Butler MG. Hypopigmentation: a common feature of Prader-Labhart-Willi syndrome. American journal of human genetics. 1989 Jul:45(1):140-6 [PubMed PMID: 2741944]
Adam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, Amemiya A, Driscoll DJ, Miller JL, Cassidy SB. Prader-Willi Syndrome. GeneReviews(®). 1993:(): [PubMed PMID: 20301505]
Strom SP, Hossain WA, Grigorian M, Li M, Fierro J, Scaringe W, Yen HY, Teguh M, Liu J, Gao H, Butler MG. A Streamlined Approach to Prader-Willi and Angelman Syndrome Molecular Diagnostics. Frontiers in genetics. 2021:12():608889. doi: 10.3389/fgene.2021.608889. Epub 2021 May 11 [PubMed PMID: 34046054]
Duis J, van Wattum PJ, Scheimann A, Salehi P, Brokamp E, Fairbrother L, Childers A, Shelton AR, Bingham NC, Shoemaker AH, Miller JL. A multidisciplinary approach to the clinical management of Prader-Willi syndrome. Molecular genetics & genomic medicine. 2019 Mar:7(3):e514. doi: 10.1002/mgg3.514. Epub 2019 Jan 29 [PubMed PMID: 30697974]
Bennett JA, Germani T, Haqq AM, Zwaigenbaum L. Autism spectrum disorder in Prader-Willi syndrome: A systematic review. American journal of medical genetics. Part A. 2015 Dec:167A(12):2936-44. doi: 10.1002/ajmg.a.37286. Epub 2015 Aug 29 [PubMed PMID: 26331980]
Level 1 (high-level) evidenceSingh D, Wakimoto Y, Filangieri C, Pinkhasov A, Angulo M. Guanfacine Extended Release for the Reduction of Aggression, Attention-Deficit/Hyperactivity Disorder Symptoms, and Self-Injurious Behavior in Prader-Willi Syndrome-A Retrospective Cohort Study. Journal of child and adolescent psychopharmacology. 2019 May:29(4):313-317. doi: 10.1089/cap.2018.0102. Epub 2019 Feb 6 [PubMed PMID: 30724590]
Level 2 (mid-level) evidenceAlves C, Franco RR. Prader-Willi syndrome: endocrine manifestations and management. Archives of endocrinology and metabolism. 2020 May-Jun:64(3):223-234. doi: 10.20945/2359-3997000000248. Epub [PubMed PMID: 32555988]
Butler MG. New drug approved for hyperphagia in Prader-Willi syndrome. The lancet. Diabetes & endocrinology. 2025 Jul:13(7):547-549. doi: 10.1016/S2213-8587(25)00161-5. Epub 2025 May 29 [PubMed PMID: 40451226]
Miller JL, Gevers E, Bridges N, Yanovski JA, Salehi P, Obrynba KS, Felner EI, Bird LM, Shoemaker AH, Angulo M, Butler MG, Stevenson D, Abuzzahab J, Barrett T, Lah M, Littlejohn E, Mathew V, Cowen NM, Bhatnagar A, DESTINY PWS Investigators. Diazoxide Choline Extended-Release Tablet in People With Prader-Willi Syndrome: A Double-Blind, Placebo-Controlled Trial. The Journal of clinical endocrinology and metabolism. 2023 Jun 16:108(7):1676-1685. doi: 10.1210/clinem/dgad014. Epub [PubMed PMID: 36639249]
Level 1 (high-level) evidenceCheon CK. Genetics of Prader-Willi syndrome and Prader-Will-Like syndrome. Annals of pediatric endocrinology & metabolism. 2016 Sep:21(3):126-135 [PubMed PMID: 27777904]
Butler MG, Manzardo AM, Heinemann J, Loker C, Loker J. Causes of death in Prader-Willi syndrome: Prader-Willi Syndrome Association (USA) 40-year mortality survey. Genetics in medicine : official journal of the American College of Medical Genetics. 2017 Jun:19(6):635-642. doi: 10.1038/gim.2016.178. Epub 2016 Nov 17 [PubMed PMID: 27854358]
Level 3 (low-level) evidenceDuis J, Pullen LC, Picone M, Friedman N, Hawkins S, Sannar E, Pfalzer AC, Shelton AR, Singh D, Zee PC, Glaze DG, Revana A. Diagnosis and management of sleep disorders in Prader-Willi syndrome. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine. 2022 Jun 1:18(6):1687-1696. doi: 10.5664/jcsm.9938. Epub [PubMed PMID: 35172921]
Greggi T, Martikos K, Lolli F, Bakaloudis G, Di Silvestre M, Cioni A, Bròdano GB, Giacomini S. Treatment of scoliosis in patients affected with Prader-Willi syndrome using various techniques. Scoliosis. 2010 Jun 15:5():11. doi: 10.1186/1748-7161-5-11. Epub 2010 Jun 15 [PubMed PMID: 20550681]
Yang A, Kim J, Cho SY, Jin DK. Prevalence and risk factors for type 2 diabetes mellitus with Prader-Willi syndrome: a single center experience. Orphanet journal of rare diseases. 2017 Aug 30:12(1):146. doi: 10.1186/s13023-017-0702-5. Epub 2017 Aug 30 [PubMed PMID: 28854950]