Introduction
In 1836, Charles Dickens published his serialized book, The Posthumous Papers of the Pickwick Club, in which he described obesity-hypoventilation syndrome (OHS), defined as alveolar hypoventilation during wakefulness in an individual with obesity that cannot be attributed to other causes of hypercapnia, such as chronic obstructive pulmonary disease (COPD).[1][2]
OHS is defined as the presence of awake alveolar hypoventilation characterized by daytime hypercapnia (partial pressure of arterial CO2 [PaCO2] >45 mmHg [5.9 kPa]), which occurs as a result of diminished ventilatory drive and capacity related to obesity (BMI >30 kg/m2), without an alternative respiratory, neuromuscular, or metabolic cause for hypoventilation.[3] The mechanism of OHS involves a decreased central respiratory drive, a diminished hypercapnic ventilatory response, and a restrictive pattern of lung disease.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
OHS results from diminished ventilatory drive and capacity associated with obesity. An increased load on respiratory mechanics and a blunted ventilatory response to carbon dioxide (CO2) in individuals with a body mass index (BMI) greater than 30 kg/m2 contribute to the development of daytime hypercapnia. The etiology of OHS is multifactorial, with obesity and obstructive sleep apnea (OSA) being the primary contributing factors.
Additional contributing factors of OHS include ventilatory control defects, which lead to decreased responsiveness in the hypoxic and hypercapnic ventilatory drive (see Image. Key Contributing Factors in Obesity Hypoventilation Syndrome and refer to the Pathophysiology section for further details). OHS is considered a diagnosis of exclusion, established only after ruling out alternative neuromuscular, mechanical, or metabolic causes of hypoventilation.[4]
Apnea is defined as the cessation of airflow at the nose and mouth lasting at least 10 seconds. The American Academy of Sleep Medicine defines hypopnea as a reduction in airflow by at least 30% for a minimum of 10 seconds, accompanied by a 3% or greater drop in oxygen saturation or an arousal from sleep. Apnea and hypopnea are considered equivalent in clinical scoring. While several metrics are used to assess sleep-disordered breathing, the apnea-hypopnea index (AHI) remains the most widely used and validated index.[5]
OHS is commonly associated with OSA, which is defined by an AHI of 5 or more events per hour. However, not all patients with OHS have OSA; approximately 10% have OHS without comorbid OSA (also called nonobstructive sleep hypoventilation), suggesting a distinct phenotype. The majority of patients with OHS (around 70%) have severe OSA, with an AHI of 30 or more events per hour.[6]
Epidemiology
More than one-third of the current population in the United States is considered "obese." As obesity rates continue to rise, the prevalence of OHS is also expected to increase. The prevalence of morbid obesity (BMI ≥40 kg/m2) is 8% among adults in the United States.[7][8] Likewise, the prevalence of extreme obesity (BMI >50 kg/m2) has increased 10-fold between 2000 and 2005 and continues to rise.[9]
The prevalence of OHS among individuals with OSA is estimated to be between 20% and 30%.[10] In a study of hospitalized patients with a BMI over 35 kg/m2, the prevalence of OHS was found to be 31%.[11] The prevalence of obesity varies by gender, ethnicity, education level, and age, with the highest prevalence rates observed among women, non-Hispanic Black individuals, those with lower educational background, and adults aged 40 to 59.[12] In addition, OHS is known to occur at a lower BMI threshold in Asian populations.[13] Although earlier reports suggested that OHS is more common in men than women, clinical studies have shown that women referred to sleep clinics may be more frequently affected than men.[14]
Pathophysiology
OHS results from an increased mechanical load on the respiratory system, leading to reduced tidal volumes and a blunted chemoreflex response to CO2. This impaired response results in inadequate central respiratory drive in individuals with obesity. The condition arises from a complex interaction of multiple contributing mechanisms (see Image. Airway Resistance and End-Tidal O2).
Sleep-Disordered Breathing
Obesity-related factors such as altered upper airway mechanics and impaired ventilatory control significantly contribute to hypoventilation during both wakefulness and sleep. In individuals with obesity, narrowed airways and increased upper airway resistance are associated with elevated end-tidal CO2 (PCO2), as illustrated in the image. In patients with OSA alone, the hyperventilation phase following apneic episodes typically clears the retained CO2. However, when CO2 accumulation exceeds the ventilatory capacity for clearance, the kidneys begin to retain bicarbonate (HCO3) to compensate for the resulting respiratory acidosis. Over time, this leads to chronic hypercapnia and compensated respiratory acidosis.[15]
Impaired Pulmonary Mechanics
Patients with OHS exhibit higher upper airway resistance in both sitting and supine positions compared to eucapnic individuals with OSA.[16][17] Excess fat deposition impairs diaphragmatic descent and limits outward chest wall expansion, leading to increased intra-abdominal and pleural pressures (which are typically negative). These changes lead to reductions in functional residual capacity (FRC), expiratory reserve volume (ERV), and total lung capacity (TLC).
Spirometry in patients with OHS typically reveals a predominantly restrictive defect, characterized by reduced forced vital capacity (FVC) and forced expiratory volume in 1 second (FEV1), with a preserved FEV1/FVC ratio. This pattern is likely due to the inertial load of excess adipose tissue around the chest wall and abdomen, further exacerbated by gravitational effects during sleep.[16][17][18][19] The restrictive breathing pattern may also increase dead space ventilation, resulting from a reduced tidal volume and a compensatory increase in respiratory rate. Due to limited data on diaphragmatic performance and trans-diaphragmatic pressures, it remains uncertain whether respiratory muscle weakness contributes to OHS.
Blunted Respiratory Drive
In individuals with OSA without hypercapnia, PaCO2 levels do not increase due to ventilatory compensation, which facilitates the clearance of excess CO2. In contrast, patients with OHS have an impaired compensatory response, leading to the development of hypercapnia. Studies have shown that individuals with OHS fail to appropriately increase their minute ventilation when exposed to hypoxic ambient air or during CO2 rebreathing challenges.[20][21] However, they are capable of voluntarily hyperventilating to achieve eucapnia, and hypercapnia can also be reversed with the use of positive airway pressure (PAP) therapy.[22]
Leptin Resistance
Leptin is a 16-kilodalton satiety hormone produced by adipose tissue that promotes feelings of fullness and stimulates hyperventilation.[23] In individuals with obesity, leptin levels are typically elevated as a compensatory response to the increased CO2 load.[24][25] Leptin is encoded by the Ob gene.[26] Patients with OHS have been found to exhibit higher leptin levels compared to eucapnic obese individuals, suggesting the presence of leptin resistance.[27] These elevated leptin levels decrease following treatment with PAP.[28]
History and Physical
Patients with OHS often present in the medical intensive care unit (ICU) during an acute exacerbation of chronic hypoxemic and hypercapnic respiratory failure, requiring ventilatory support via noninvasive or invasive positive pressure ventilation. They may also be seen in outpatient settings by sleep specialists or pulmonologists. Typically, these patients have obesity with a BMI over 35 kg/m2 and are at high risk, often exhibiting hypersomnolence and daytime sleepiness. Other classic signs of OSA, including loud snoring, nocturnal choking, witnessed apneas, early morning headaches, daytime fatigue, impaired concentration and memory, and dyspnea, are frequently reported.
The physical examination may reveal an individual with obesity and a short and wide neck, a crowded oropharynx, and a low-lying uvula. Findings suggestive of right heart failure secondary to pulmonary hypertension may also be present, including elevated jugular venous pressure, a prominent pulmonic component of the second heart sound, hepatomegaly, and lower extremity edema.
Evaluation
Clinical suspicion for OHS should be high in patients with a BMI greater than 30 kg/m2 who present with unexplained dyspnea on exertion and hypersomnolence.[33] Hence, screening for OHS is recommended in patients with severe obesity and those diagnosed with OSA. Additionally, patients exhibiting wakefulness hypoxia on room air, reduced pulse oximetry (SpO2) below 94%, or elevated serum HCO3 levels greater than 27 mEq/L should be evaluated for OHS.[10]
An elevated serum HCO3 level, usually reflecting metabolic compensation for respiratory acidosis, indicates the chronic nature of hypercapnia. Serum HCO3 can serve as a sensitive screening test for chronic hypercapnia. In patients with serum HCO3 levels above 27 mEq/L, OHS is present in approximately 50% of cases. Conversely, a serum HCO3 level below 27 mEq/L effectively excludes OHS when the pretest probability is low (<20%).[34]
Ultimately, arterial blood gas (ABG) analysis should demonstrate hypoventilation during complete wakefulness. The percentage of total sleep time with SpO2 below 90% is a valuable polysomnographic measure for evaluating patients with OHS. Additionally, patients with very severe OSA (AHI >100 events per hour) or profound hypoxia (nadir SpO2 <60%) during sleep have an increased prevalence of OHS exceeding 75%.[10]
The American Academy of Sleep Medicine has established the following diagnostic criteria for OHS:
- Presence of hypoventilation during wakefulness, which is defined as a PaCO2 greater than 45 mmHg, is measured by arterial PCO2, end-tidal PCO2, or transcutaneous PCO2.[35]
- Presence of obesity, which is defined as a BMI of more than 30 kg/m2 in adults or greater than the 95th percentile for age and sex in children.[35]
- Hypoventilation is not primarily attributable to other causes, such as lung parenchymal or airway disease, pulmonary vascular pathology, chest wall disorders (other than mass loading from obesity), medication use, neurological conditions, muscle weakness, or known congenital or idiopathic central alveolar hypoventilation syndromes.[35]
The recommended diagnostic approach for OHS involves confirming daytime hypoventilation. ABG analysis is the gold standard for detecting alveolar hypoventilation. However, ABG testing is often challenging to perform in outpatient settings. Fortunately, most patients with OHS initially present in a hospital setting due to acute exacerbations, where ABG and basic metabolic panel testing can be readily performed. Polysomnography is not required for diagnosis, but it can help differentiate patients with coexisting OSA from those with true sleep hypoventilation. Sleep hypoventilation is defined as a rise in PaCO2 of at least 10 mmHg above the awake baseline, not attributable to obstructive apneas or hypopneas.
OHS is a diagnosis of exclusion and must be distinguished from other disorders associated with hypoventilation. Once hypercapnia is confirmed by ABG analysis, pulmonary function testing (PFT) should be performed to rule out alternative causes. In patients with OHS, PFTs are typically normal or demonstrate a restrictive ventilatory defect, without significant evidence of airflow obstruction. Thus, in clinical practice, it is advisable to conduct the below-mentioned tests for suspected cases of OHS.
- Arterial blood gas analysis: An elevated serum HCO3 level (>27 mEq/L) can serve as a sensitive screening test for chronic hypercapnia. However, this finding is not specific, as HCO3 levels may also rise due to other conditions, such as vomiting, dehydration, or the effects of medication. ABG analysis is a more definitive test for alveolar hypoventilation, with hypercapnia defined as PaCO2 greater than 45 mmHg. PaCO2 measurement is preferred over SpO2 or HCO3 levels.[29] Hypoxemia during wakefulness is uncommon in OSA alone and requires confirmation by an ABG showing PaO2 less than 70 mmHg. Hypoxia can be measured noninvasively through pulse oximetry. Polysomnography is another valuable tool used in the evaluation of both OSA and OHS.
- Complete blood count: Polycythemia may be present as a result of chronic hypoventilation and hypoxia. Blood tests can help rule out secondary causes of erythrocytosis and other conditions that may mimic OHS, such as hypothyroidism.
- Pulmonary function testing and imaging: Once hypercapnia is confirmed, other potential causes should be excluded using PFTs, chest x-ray, or computed tomography (CT) scan, as clinically indicated. In patients with OHS, PFTs may show a moderate restrictive defect without evidence of airway obstruction, although results can also be normal.
- Sleep study: Polysomnography with continuous nocturnal CO2 monitoring is the gold standard for evaluating OHS. Additionally, the oxygen nadir and the percentage of time spent with SpO2 below 90% are important indicators suggestive of OHS.
- Cardiac studies: Electrocardiogram (ECG) and echocardiography are useful for evaluating right heart enlargement and right heart failure secondary to pulmonary hypertension. This complication typically occurs in the later stages of OHS.
Treatment / Management
New guidelines from leading societies outline the recommended approach to managing OHS.[29][30][31] Individual treatment modalities should address the underlying mechanisms and may include management of sleep-disordered breathing, weight loss, lifestyle modifications, surgical interventions, and pharmacological therapy.(A1)
Positive Airway Pressure Therapy
PAP therapy, particularly continuous PAP (CPAP), is the first-line treatment for OHS.[30][32] Alternative noninvasive ventilation methods, such as bilevel PAP (BPAP) or pressure support, are not recommended as initial therapy.[29] Treatment should not be delayed while the patient tries to lose weight. Supplemental oxygen may be required and should be regularly reassessed. As most patients with OHS (approximately 90%) have coexisting OSA, CPAP is typically the preferred initial modality.[33] For individuals with predominant sleep-related hypoventilation and fewer obstructive events, BPAP is the treatment of choice.[34](A1)
CPAP delivers constant pressure throughout the entire respiratory cycle, helping maintain upper airway patency and reducing obstructive events. In patients who do not show improvement in hypercapnia despite adequate CPAP adherence, BPAP is preferred. BPAP should also be considered if the patient is intolerant of CPAP or requires higher CPAP pressures (greater than 15 cm H2O).[35] Although comparative trials are limited, BPAP is generally considered to be a more effective option for enhancing ventilation when CPAP fails or is not tolerated. To initiate BPAP, an inspiratory PAP (IPAP) and expiratory PAP (EPAP) are independently titrated and set. Please see StatPearls' companion resource, "Noninvasive Ventilation," for more information.
The pressure difference between IPAP and EPAP—known as the delta or driving pressure—is the primary determinant of ventilation and CO2 elimination. High levels of positive pressure are often necessary due to reduced chest wall compliance resulting from excess adiposity, decreased lung compliance caused by atelectasis, and cephalad displacement of the diaphragm associated with central adiposity during sleep. ABGs should be monitored closely to assess clinical response to PAP therapy. In patients hospitalized with acute exacerbation of chronic hypoxic hypercapnic respiratory failure, the choice of ventilation mode should be guided by the severity of respiratory illness. A trial of noninvasive positive pressure ventilation may be considered as the initial approach for an arousable patient with intact gag and cough reflexes.
However, early intubation should be considered for patients who cannot protect their airways, do not tolerate BPAP, or fail to show rapid improvement. Many patients hospitalized for acute or chronic hypercapnia respiratory failure often do not have a formal diagnosis of OSA or known PAP settings (based on official titration). Therefore, empirical treatment is necessary. In these cases that require empirical therapy with noninvasive ventilation, the choice of IPAP and EPAP depends on the severity of respiratory acidosis and body weight, aiming to maintain upper airway patency while providing adequate pressure support for ventilation. After hospitalization for OHS, patients should be discharged with noninvasive ventilation and subsequently undergo outpatient diagnostic evaluations and PAP titration in a sleep laboratory.[29] Please see StatPearls' companion resource, "Noninvasive Ventilation," for more information.(A1)
Adherence to PAP therapy, measured by average daily usage over the past 30 days, is one of the most challenging aspects of managing OHS. Difficulties with the device and masks, patient noncompliance, inadequate education, and financial constraints can all contribute to poor adherence to treatment. A meta-analysis of 25 studies showed that PAP therapy improves OHS symptoms and reduces mortality. Continued PAP use also enhances gas exchange, reduces daytime sleepiness, improves sleep quality and overall quality of life, and decreases the frequency of emergency department visits.[36] Offering a variety of mask types and sizes can enhance comfort and compliance. Therefore, thorough patient education about the disease, mask options, and the importance of PAP therapy in preventing complications and morbidity is essential to ensure satisfactory adherence.
Supplemental Oxygen Therapy
This therapy is necessary for patients with OHS who remain hypoxemic despite appropriate PAP use—a situation reported in up to 50% of cases.[37] Over time, PAP may gradually improve hypoxemia to acceptable levels. This cohort of patients receiving both PAP and supplemental oxygen requires regular monitoring to minimize long-term costs and potential oxygen toxicity. Oxygen therapy alone, without PAP, is strongly discouraged, as it does not improve ventilation and may have poor outcomes with worsening CO2 retention.[32][38] A recent randomized crossover study demonstrated that 100% supplemental oxygen increased hypercapnia (PaCO2 rose by 5.0 mmHg) and decreased minute ventilation (by 1.4 L/min) in stable patients with obesity-related hypoventilation.[32][39](A1)
Weight loss and lifestyle
All patients with OHS should be encouraged to adopt dietary and lifestyle modifications to achieve weight loss. This process should be controlled and ideally supervised through a structured weight management program. Weight loss has been shown to reduce the risk of complications, such as pulmonary hypertension, in various other cardiac and respiratory pathologies. Weight loss improves nocturnal oxygen saturation, decreases the frequency of respiratory apneas and hypopneas, and improves pulmonary function.[40] The target weight reduction of 25% to 30% of actual body weight is recommended to effectively manage hypoventilation.[29] Newer pharmacological therapies for weight loss show promise. Glucagon-like peptide-1 (GLP-1) receptor agonists—such as tirzepatide—have been approved by the US Food and Drug Administration (FDA) for the treatment of OSA, although not yet for OHS.[41] Additional medications are currently under investigation. (A1)
Given that lifestyle and dietary modifications are often not sustainable for the majority of patients in the long term, surgical weight-loss interventions—such as bariatric surgery—are considered. Referral for surgery is appropriate when conservative measures fail, when there is low tolerance to high PAP pressures, or when OHS symptoms and hypercapnia worsen. Although studies specifically focused on patients with OHS are limited, available evidence suggests that these surgical interventions have shown mixed efficacy in achieving long-term improvements in OSA symptoms, AHI, and sustained weight loss. A 2009 meta-analysis of 12 studies found that patients who underwent sleep studies before and after achieving maximal weight loss through bariatric surgery experienced a 71% reduction in AHI. However, only 38% achieved a “cure,” defined as an AHI of less than 5 events per hour. Nearly two-thirds had residual disease, with most experiencing persistent moderate OSA (AHI ≥15 events per hour).[42] Given the mixed outcomes, bariatric surgery still carries notable risks and complications. Perioperative mortality is high, particularly among patients with coexisting OSA and OHS.[43] As a result, PAP therapy is typically initiated immediately after extubation, especially as there is no strong evidence linking PAP use to anastomotic complications.[44][45] (A1)
Tracheostomy
This is a surgical modality used to treat sleep-disordered breathing and is generally only for those intolerant of or consistently non-adherent to PAP therapy, or those with complications such as cor pulmonale. However, most individuals with OHS who undergo tracheostomy still require PAP therapy, as the procedure primarily addresses upper airway obstruction and does not modify underlying pulmonary mechanics, ventilatory drive, or neurohumoral milieu.[46] This procedure carries inherent surgical risks and challenges, especially in individuals with obesity.(A1)
Pharmacological treatments
The role of pharmacological therapies in managing OHS is limited. Respiratory stimulants such as acetazolamide, medroxyprogesterone, and theophylline offer theoretical benefits for patients with chronic hypercapnia or depressed respiratory drive, but clinical evidence supporting their use is sparse in a practical setting.[47] These agents are sometimes considered adjunctive therapies of last resort for patients who continue to experience hypoventilation despite PAP therapy and weight loss. By inhibiting the conversion of CO22 to HCO3, acetazolamide lowers brain pH, which may theoretically stimulate central ventilatory drive and increase minute ventilation.
Medroxyprogesterone acts as a respiratory stimulant at the hypothalamic level; however, study results have been limited and contradictory. The use of medroxyprogesterone carries increased risks of hypercoagulability and venous thromboembolism, along with adverse effects such as decreased libido and erectile dysfunction in men and uterine bleeding in women.[48] Theophylline, a bronchodilator and direct respiratory stimulant, has not been studied in OHS and is currently not recommended for this condition. (B3)
Recombinant human leptin (metreleptin), administered via subcutaneous injection, is FDA-approved for treating metabolic complications in patients with congenital or acquired generalized lipodystrophy related to leptin deficiency. However, no studies have yet evaluated its use in patients with OHS.[49]
Differential Diagnosis
Central Sleep Apnea
Central sleep apnea (CSA) is characterized by intermittent reductions in the central respiratory drive. CSA differs from OHS, as patients with CSA typically hyperventilate. Blood gas analysis in CSA patients generally shows normocapnia or mild hypocapnia.
Chronic Obstructive Pulmonary Disease
Patients with COPD and obesity who are also hypercapnic commonly experience sleep-disordered breathing.[50] Therefore, comprehensive PFT and ABG analysis are essential for establishing the correct diagnosis. The presence of an obstructive ventilatory defect excludes the diagnosis of OHS, as discussed in the Introduction and Evaluation sections.
Extrapulmonary Restriction
Certain conditions that impair lung expansion can lead to acute hypercapnic respiratory failure. These include extrapulmonary chest wall restrictions—such as pectus deformities, scoliosis, and kyphosis—which compromise respiratory mechanics. Additionally, conditions such as ascites or severe bowel distension can impair ventilation by exerting significant cephalad pressure on the diaphragm, thereby reducing its ability to function effectively. While extrapulmonary chest wall restriction often results in reduced ventilatory reserve, it does not typically cause overt respiratory failure.
Neuromuscular Disease
Neuromuscular disorders that impact respiratory function should be considered in the differential diagnosis of hypoventilation syndromes. Amyotrophic lateral sclerosis (ALS), for example, frequently results in hypercapnic respiratory failure.[51] Patients typically present with neurologic signs consistent with ALS, such as muscle weakness, fasciculations, and hyperactive deep tendon reflexes.
Additionally, individuals with spinal cord injuries (SCIs) may experience sleep-disordered breathing and chronic hypercapnia during both sleep and wakefulness.[52][53] These patients are generally not living with obesity and often have a history of acute injury or trauma resulting in neurological impairment. However, patients with SCI frequently exhibit sleep-disordered breathing and restrictive ventilatory patterns that can mimic OHS.[54][55]
Muscular Dystrophies
Conditions such as Duchenne and Becker muscular dystrophies can lead to hypercapnic respiratory failure but are typically accompanied by other distinguishing features, including generalized muscle weakness, growth delays, cardiomyopathies, and elevated creatine kinase levels. These characteristics usually make the diagnosis apparent in the pediatric population. While Becker muscular dystrophy tends to follow a more variable and benign course than Duchenne, it shares similar overall clinical features.
Guillain-Barré Syndrome
This condition generally presents with a rapid onset of ascending, symmetric paralysis and areflexia, progressing over 2 to 4 weeks. Dysautonomia is a common condition that may lead to hemodynamic instability or cardiac arrhythmias.
Myasthenia Gravis
This condition is characterized by muscle fatigability and presents with hallmark features, including diplopia, ptosis, dysarthria, limb weakness, and a weak cough.
Poliomyelitis and Post-Polio Syndrome
These conditions are characterized by acute flaccid paralysis or the development of new weakness and fatigability later in life. However, widespread vaccination efforts have largely eradicated them in the developed world.
Myxedema
Severely low levels of circulating free thyroid hormones can lead to respiratory insufficiency and hypercapnic respiratory failure. Affected individuals typically present with additional features such as hypothermia, bradycardia, and delayed tendon reflexes. In severe cases, patients may exhibit hemodynamic instability and neurological deficits, including coma.
Prognosis
OHS is commonly misdiagnosed, even in patients with morbid obesity, leading to repeated hospitalizations due to hypercapnic respiratory failure.[56] The clinical course of OHS is typically progressive and is associated with significant cardiovascular complications, including pulmonary hypertension and right heart failure, ultimately contributing to high morbidity and mortality rates.[57]
The impact of therapy, particularly noninvasive approaches such as PAP, on complications and mortality is generally positive. However, even with PAP treatment, patients with severe OHS experience significantly higher mortality rates compared to those with OSA alone. Additionally, patients with OHS have increased rates of hospitalization, intensive care unit admissions, and long-term post-discharge complications compared to patients with OSA only.[58]
Complications
In progressive or untreated OHS, biventricular heart failure, pulmonary hypertension, and volume overload are common complications.[59] Patients with OHS experience a lower quality of life with a higher overall symptom course, continued daytime sleepiness, and increased healthcare expenses. They are also at a higher risk for pulmonary and right-sided pressure overload, which significantly contributes to increased morbidity and earlier mortality compared to non-hypercapnic patients with sleep-disordered breathing alone.
A post-hoc analysis of the Pickwick trial found that 122 of the 246 participants with OHS had elevated systolic pulmonary artery pressures (≥40 mmHg).[59] In the non-severe OSA group, obesity and the early-to-late diastolic peak flow ratio were predictors of pH. In patients with OHS and severe OSA, low wake PaO2 levels and a higher BMI were risk factors for low pH.
Consultations
In patients suspected of having OHS, early referral to a sleep specialist for polysomnography and ABG testing is recommended.
Deterrence and Patient Education
As outlined in the Treatment/Management section, patient education should focus primarily on the natural history of OHS and its relationship with sleep-disordered breathing. Physicians should initiate early counseling on the extreme importance of weight loss and lifestyle modification. Additionally, educating patients on the importance of adhering to PAP therapy and its role in reducing long-term complications is essential.
Pearls and Other Issues
Key facts to keep in mind regarding "obesity-hypoventilation syndrome" are mentioned below.
- Obesity is associated with multiple medical complications, and OHS is among the most significant respiratory consequences related to obesity.
- The diagnosis of OHS is confirmed by the presence of hypoventilation during wakefulness, indicated by a PaCO2 greater than 45 mmHg, in the context of obesity (BMI >30 kg/m2), provided that hypoventilation is not attributable to lung parenchymal or airway disease, pulmonary vascular pathology, or neuromuscular or chest wall disorders.
- Serum HCO3 levels can serve as a sensitive screening test for chronic hypercapnia, but PCO2 from an ABG is considered more specific and sensitive.
- The percentage of total sleep time with SpO2 below 90% is a useful polysomnographic parameter for evaluating patients with OHS.
- PAP therapy, typically initiated with CPAP, is the first-line treatment for patients with OHS and coexisting OSA. PAP effectively reduces nocturnal PaCO2 accumulation and improves daytime sleepiness.
Enhancing Healthcare Team Outcomes
Patients with OHS are at high risk for cardiac-related mortality and increased overall morbidity. Early identification and prompt management are essential to reducing these risks. The care of patients with OHS requires a collaborative, multidisciplinary approach to ensure patient-centered care and improved outcomes.
Primary care physicians, pulmonologists, sleep medicine specialists, critical care physicians, bariatric surgeons, advanced practitioners, nurses, pharmacists, and other healthcare professionals involved in treating these patients should possess the necessary clinical skills and knowledge to accurately diagnose and treat OHS. This includes a clear understanding of the overlap and distinctions between OHS and OSA. Educating patients and caregivers about the importance of weight loss, the potential benefits of bariatric surgery, and adherence to PAP therapy is also crucial for successful long-term management.
A strategic, evidence-based approach to treatment planning is crucial for optimizing outcomes and minimizing adverse effects. Ethical considerations must underpin all clinical decisions, ensuring informed consent and respecting patient autonomy in treatment choices. Each healthcare professional should understand their specific responsibilities and contribute their unique expertise to the care plan, promoting a truly multidisciplinary approach.
Effective interprofessional communication is critical for seamless information exchange and collaborative decision-making among healthcare team members. Care coordination is equally vital, ensuring the patient’s journey from diagnosis through treatment and follow-up is well-managed, reducing errors and enhancing safety. By integrating clinical expertise, ethical practice, clear communication, defined roles, and coordinated care, healthcare teams can deliver patient-centered management of OHS, ultimately improving patient outcomes and overall team performance.
Media
(Click Image to Enlarge)
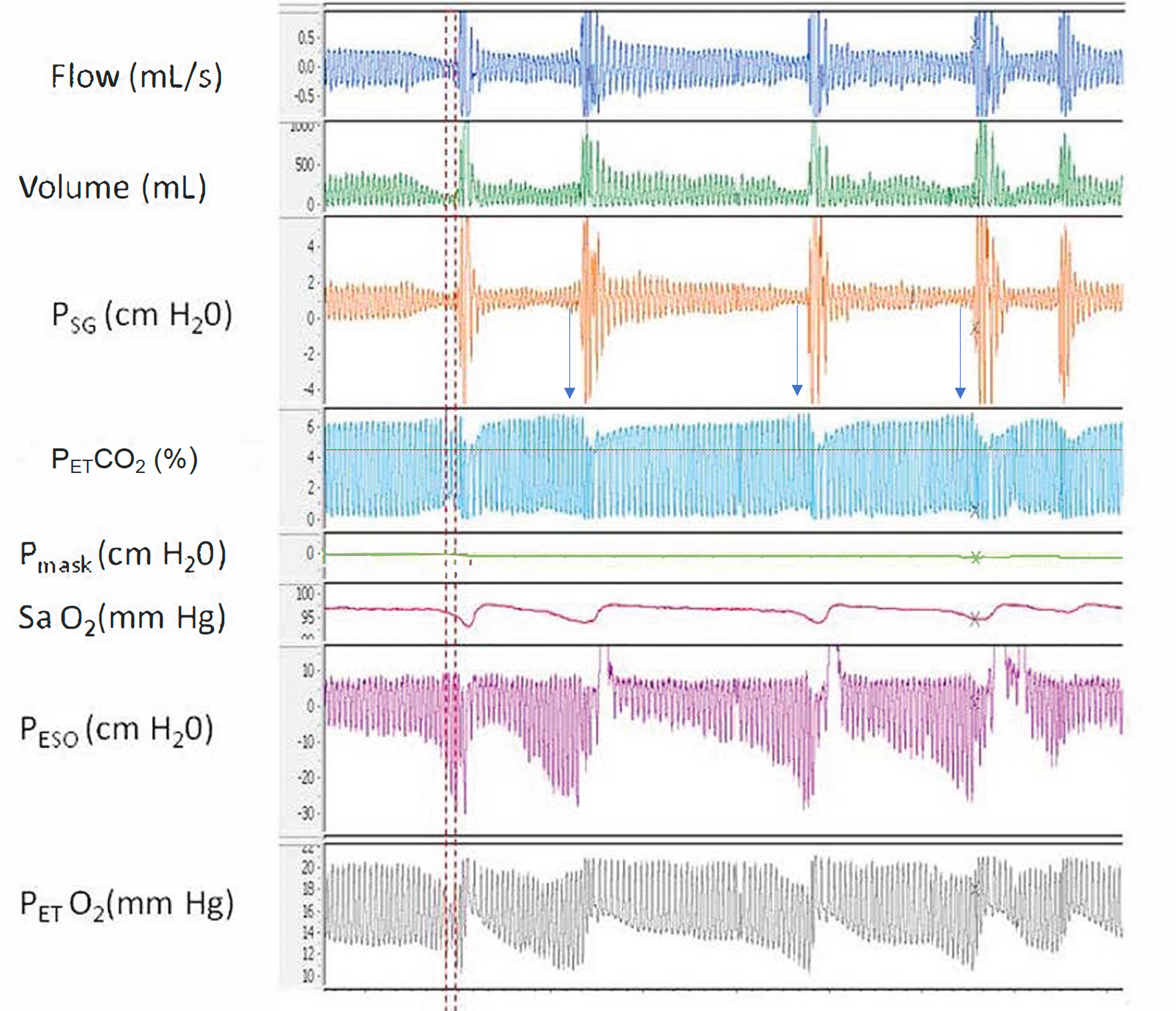
Airway Resistance and End-Tidal O2. This polygraph illustrates the relationship between increased airway resistance during respiratory events and elevated end-tidal CO2 (PETCO2), along with alternating periods of hypoventilation and hyperventilation corresponding to hypopnea and hyperpnea, respectively, throughout sleep.
Abbreviations: Psg, supraglottic pharyngeal pressure; Pmask, mask pressure; PETCO2, end-tidal CO2; Peso, esophageal pressure; PETO2, end-tidal O2; SaO2, oxygen saturation using pulse oximetry.
Contributed by A Sankari, MD, PhD
Courtesy to M Safwan Badr, MD
(Click Image to Enlarge)
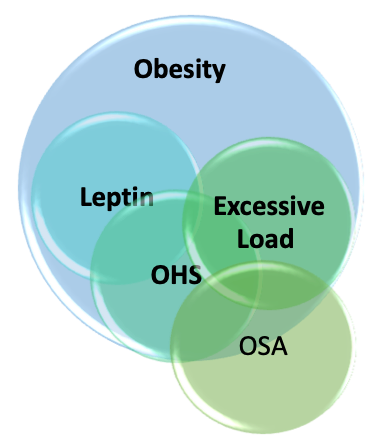
Key Contributing Factors in Obesity Hypoventilation Syndrome. This illustration depicts the major factors responsible for obesity hypoventilation syndrome (OHS) and their interrelationships. The image highlights the excessive mechanical and ventilatory load, specifically affecting the respiratory system.
Contributed by A Sankari, MD, PhD
References
Lavie P. Who was the first to use the term Pickwickian in connection with sleepy patients? History of sleep apnoea syndrome. Sleep medicine reviews. 2008 Feb:12(1):5-17 [PubMed PMID: 18037311]
Gastaut H, Tassinari CA, Duron B. Polygraphic study of the episodic diurnal and nocturnal (hypnic and respiratory) manifestations of the Pickwick syndrome. Brain research. 1966 Feb:1(2):167-86 [PubMed PMID: 5923125]
Mokhlesi B, Kryger MH, Grunstein RR. Assessment and management of patients with obesity hypoventilation syndrome. Proceedings of the American Thoracic Society. 2008 Feb 15:5(2):218-25. doi: 10.1513/pats.200708-122MG. Epub [PubMed PMID: 18250215]
Zwillich CW, Sutton FD, Pierson DJ, Greagh EM, Weil JV. Decreased hypoxic ventilatory drive in the obesity-hypoventilation syndrome. The American journal of medicine. 1975 Sep:59(3):343-8 [PubMed PMID: 1163544]
Malhotra A, Ayappa I, Ayas N, Collop N, Kirsch D, Mcardle N, Mehra R, Pack AI, Punjabi N, White DP, Gottlieb DJ. Metrics of sleep apnea severity: beyond the apnea-hypopnea index. Sleep. 2021 Jul 9:44(7):. doi: 10.1093/sleep/zsab030. Epub [PubMed PMID: 33693939]
Masa JF, Corral J, Alonso ML, Ordax E, Troncoso MF, Gonzalez M, Lopez-Martínez S, Marin JM, Marti S, Díaz-Cambriles T, Chiner E, Aizpuru F, Egea C, Spanish Sleep Network. Efficacy of Different Treatment Alternatives for Obesity Hypoventilation Syndrome. Pickwick Study. American journal of respiratory and critical care medicine. 2015 Jul 1:192(1):86-95. doi: 10.1164/rccm.201410-1900OC. Epub [PubMed PMID: 25915102]
Hales CM, Fryar CD, Carroll MD, Freedman DS, Aoki Y, Ogden CL. Differences in Obesity Prevalence by Demographic Characteristics and Urbanization Level Among Adults in the United States, 2013-2016. JAMA. 2018 Jun 19:319(23):2419-2429. doi: 10.1001/jama.2018.7270. Epub [PubMed PMID: 29922829]
Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in Obesity Among Adults in the United States, 2005 to 2014. JAMA. 2016 Jun 7:315(21):2284-91. doi: 10.1001/jama.2016.6458. Epub [PubMed PMID: 27272580]
Sturm R. Increases in morbid obesity in the USA: 2000-2005. Public health. 2007 Jul:121(7):492-6 [PubMed PMID: 17399752]
Mokhlesi B, Tulaimat A, Faibussowitsch I, Wang Y, Evans AT. Obesity hypoventilation syndrome: prevalence and predictors in patients with obstructive sleep apnea. Sleep & breathing = Schlaf & Atmung. 2007 Jun:11(2):117-24 [PubMed PMID: 17187265]
Level 2 (mid-level) evidenceNowbar S, Burkart KM, Gonzales R, Fedorowicz A, Gozansky WS, Gaudio JC, Taylor MR, Zwillich CW. Obesity-associated hypoventilation in hospitalized patients: prevalence, effects, and outcome. The American journal of medicine. 2004 Jan 1:116(1):1-7 [PubMed PMID: 14706658]
Kessler R, Chaouat A, Schinkewitch P, Faller M, Casel S, Krieger J, Weitzenblum E. The obesity-hypoventilation syndrome revisited: a prospective study of 34 consecutive cases. Chest. 2001 Aug:120(2):369-76 [PubMed PMID: 11502631]
Level 3 (low-level) evidenceIftikhar IH, Roland J. Obesity Hypoventilation Syndrome. Clinics in chest medicine. 2018 Jun:39(2):427-436. doi: 10.1016/j.ccm.2018.01.006. Epub [PubMed PMID: 29779600]
BaHammam AS, Pandi-Perumal SR, Piper A, Bahammam SA, Almeneessier AS, Olaish AH, Javaheri S. Gender differences in patients with obesity hypoventilation syndrome. Journal of sleep research. 2016 Aug:25(4):445-53. doi: 10.1111/jsr.12400. Epub 2016 Mar 18 [PubMed PMID: 26990045]
Norman RG, Goldring RM, Clain JM, Oppenheimer BW, Charney AN, Rapoport DM, Berger KI. Transition from acute to chronic hypercapnia in patients with periodic breathing: predictions from a computer model. Journal of applied physiology (Bethesda, Md. : 1985). 2006 May:100(5):1733-41 [PubMed PMID: 16384839]
Lin CC, Wu KM, Chou CS, Liaw SF. Oral airway resistance during wakefulness in eucapnic and hypercapnic sleep apnea syndrome. Respiratory physiology & neurobiology. 2004 Jan 15:139(2):215-24 [PubMed PMID: 15123004]
Level 2 (mid-level) evidenceDixon AE, Peters U. The effect of obesity on lung function. Expert review of respiratory medicine. 2018 Sep:12(9):755-767. doi: 10.1080/17476348.2018.1506331. Epub 2018 Aug 14 [PubMed PMID: 30056777]
Javaheri S, Colangelo G, Lacey W, Gartside PS. Chronic hypercapnia in obstructive sleep apnea-hypopnea syndrome. Sleep. 1994 Aug:17(5):416-23 [PubMed PMID: 7991952]
Lopata M, Freilich RA, Onal E, Pearle J, Lourenço RV. Ventilatory control and the obesity hypoventilation syndrome. The American review of respiratory disease. 1979 Feb:119(2 Pt 2):165-8 [PubMed PMID: 426345]
Lin CC. Effect of nasal CPAP on ventilatory drive in normocapnic and hypercapnic patients with obstructive sleep apnoea syndrome. The European respiratory journal. 1994 Nov:7(11):2005-10 [PubMed PMID: 7875273]
Sampson MG, Grassino K. Neuromechanical properties in obese patients during carbon dioxide rebreathing. The American journal of medicine. 1983 Jul:75(1):81-90 [PubMed PMID: 6407317]
Han F, Chen E, Wei H, He Q, Ding D, Strohl KP. Treatment effects on carbon dioxide retention in patients with obstructive sleep apnea-hypopnea syndrome. Chest. 2001 Jun:119(6):1814-9 [PubMed PMID: 11399709]
O'donnell CP, Schaub CD, Haines AS, Berkowitz DE, Tankersley CG, Schwartz AR, Smith PL. Leptin prevents respiratory depression in obesity. American journal of respiratory and critical care medicine. 1999 May:159(5 Pt 1):1477-84 [PubMed PMID: 10228114]
Level 3 (low-level) evidenceKalra SP. Central leptin gene therapy ameliorates diabetes type 1 and 2 through two independent hypothalamic relays; a benefit beyond weight and appetite regulation. Peptides. 2009 Oct:30(10):1957-63. doi: 10.1016/j.peptides.2009.07.021. Epub 2009 Aug 6 [PubMed PMID: 19647774]
Level 3 (low-level) evidenceShimura R, Tatsumi K, Nakamura A, Kasahara Y, Tanabe N, Takiguchi Y, Kuriyama T. Fat accumulation, leptin, and hypercapnia in obstructive sleep apnea-hypopnea syndrome. Chest. 2005 Feb:127(2):543-9 [PubMed PMID: 15705994]
Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994 Dec 1:372(6505):425-32 [PubMed PMID: 7984236]
Level 3 (low-level) evidenceAmorim MR, Aung O, Mokhlesi B, Polotsky VY. Leptin-mediated neural targets in obesity hypoventilation syndrome. Sleep. 2022 Sep 8:45(9):. doi: 10.1093/sleep/zsac153. Epub [PubMed PMID: 35778900]
Phipps PR, Starritt E, Caterson I, Grunstein RR. Association of serum leptin with hypoventilation in human obesity. Thorax. 2002 Jan:57(1):75-6 [PubMed PMID: 11809994]
Mokhlesi B, Masa JF, Brozek JL, Gurubhagavatula I, Murphy PB, Piper AJ, Tulaimat A, Afshar M, Balachandran JS, Dweik RA, Grunstein RR, Hart N, Kaw R, Lorenzi-Filho G, Pamidi S, Patel BK, Patil SP, Pépin JL, Soghier I, Tamae Kakazu M, Teodorescu M. Evaluation and Management of Obesity Hypoventilation Syndrome. An Official American Thoracic Society Clinical Practice Guideline. American journal of respiratory and critical care medicine. 2019 Aug 1:200(3):e6-e24. doi: 10.1164/rccm.201905-1071ST. Epub [PubMed PMID: 31368798]
Level 1 (high-level) evidenceSoghier I, Brożek JL, Afshar M, Tamae Kakazu M, Wilson KC, Masa JF, Mokhlesi B. Noninvasive Ventilation versus CPAP as Initial Treatment of Obesity Hypoventilation Syndrome. Annals of the American Thoracic Society. 2019 Oct:16(10):1295-1303. doi: 10.1513/AnnalsATS.201905-380OC. Epub [PubMed PMID: 31365842]
. Obstructive sleep apnoea/hypopnoea syndrome and obesity hypoventilation syndrome in over 16s: summary of NICE guidance. BMJ (Clinical research ed.). 2022 Mar 9:376():o619. doi: 10.1136/bmj.o619. Epub 2022 Mar 9 [PubMed PMID: 35264334]
Xu J, Wei Z, Li W, Wang W. Effect of different modes of positive airway pressure treatment on obesity hypoventilation syndrome: a systematic review and network meta-analysis. Sleep medicine. 2022 Mar:91():51-58. doi: 10.1016/j.sleep.2022.01.008. Epub 2022 Jan 13 [PubMed PMID: 35272117]
Level 1 (high-level) evidencePiper AJ, Wang D, Yee BJ, Barnes DJ, Grunstein RR. Randomised trial of CPAP vs bilevel support in the treatment of obesity hypoventilation syndrome without severe nocturnal desaturation. Thorax. 2008 May:63(5):395-401. doi: 10.1136/thx.2007.081315. Epub 2008 Jan 18 [PubMed PMID: 18203817]
Level 1 (high-level) evidenceMasa JF, Benítez I, Sánchez-Quiroga MÁ, Gomez de Terreros FJ, Corral J, Romero A, Caballero-Eraso C, Alonso-Álvarez ML, Ordax-Carbajo E, Gomez-Garcia T, González M, López-Martín S, Marin JM, Martí S, Díaz-Cambriles T, Chiner E, Egea C, Barca J, Vázquez-Polo FJ, Negrín MA, Martel-Escobar M, Barbé F, Mokhlesi B, Spanish Sleep Network. Long-term Noninvasive Ventilation in Obesity Hypoventilation Syndrome Without Severe OSA: The Pickwick Randomized Controlled Trial. Chest. 2020 Sep:158(3):1176-1186. doi: 10.1016/j.chest.2020.03.068. Epub 2020 Apr 25 [PubMed PMID: 32343963]
Level 1 (high-level) evidenceKushida CA, Chediak A, Berry RB, Brown LK, Gozal D, Iber C, Parthasarathy S, Quan SF, Rowley JA, Positive Airway Pressure Titration Task Force, American Academy of Sleep Medicine. Clinical guidelines for the manual titration of positive airway pressure in patients with obstructive sleep apnea. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine. 2008 Apr 15:4(2):157-71 [PubMed PMID: 18468315]
Wearn J, Akpa B, Mokhlesi B. Adherence to Positive Airway Pressure Therapy in Obesity Hypoventilation Syndrome. Sleep medicine clinics. 2021 Mar:16(1):43-59. doi: 10.1016/j.jsmc.2020.10.009. Epub 2020 Dec 9 [PubMed PMID: 33485531]
Banerjee D, Yee BJ, Piper AJ, Zwillich CW, Grunstein RR. Obesity hypoventilation syndrome: hypoxemia during continuous positive airway pressure. Chest. 2007 Jun:131(6):1678-84 [PubMed PMID: 17565018]
Kaw R, Doufas AG. Is regular oxygen supplementation safe for obese postoperative patients? Cleveland Clinic journal of medicine. 2020 Nov 23:87(12):723-727. doi: 10.3949/ccjm.87a.19051. Epub 2020 Nov 23 [PubMed PMID: 33229388]
Wijesinghe M, Williams M, Perrin K, Weatherall M, Beasley R. The effect of supplemental oxygen on hypercapnia in subjects with obesity-associated hypoventilation: a randomized, crossover, clinical study. Chest. 2011 May:139(5):1018-1024. doi: 10.1378/chest.10-1280. Epub 2010 Oct 14 [PubMed PMID: 20947648]
Level 1 (high-level) evidenceThomas PS, Cowen ER, Hulands G, Milledge JS. Respiratory function in the morbidly obese before and after weight loss. Thorax. 1989 May:44(5):382-6 [PubMed PMID: 2503905]
Malhotra A, Grunstein RR, Fietze I, Weaver TE, Redline S, Azarbarzin A, Sands SA, Schwab RJ, Dunn JP, Chakladar S, Bunck MC, Bednarik J, SURMOUNT-OSA Investigators. Tirzepatide for the Treatment of Obstructive Sleep Apnea and Obesity. The New England journal of medicine. 2024 Oct 3:391(13):1193-1205. doi: 10.1056/NEJMoa2404881. Epub 2024 Jun 21 [PubMed PMID: 38912654]
Greenburg DL, Lettieri CJ, Eliasson AH. Effects of surgical weight loss on measures of obstructive sleep apnea: a meta-analysis. The American journal of medicine. 2009 Jun:122(6):535-42. doi: 10.1016/j.amjmed.2008.10.037. Epub [PubMed PMID: 19486716]
Level 1 (high-level) evidenceFernandez AZ Jr, Demaria EJ, Tichansky DS, Kellum JM, Wolfe LG, Meador J, Sugerman HJ. Multivariate analysis of risk factors for death following gastric bypass for treatment of morbid obesity. Annals of surgery. 2004 May:239(5):698-702; discussion 702-3 [PubMed PMID: 15082974]
Ebeo CT, Benotti PN, Byrd RP Jr, Elmaghraby Z, Lui J. The effect of bi-level positive airway pressure on postoperative pulmonary function following gastric surgery for obesity. Respiratory medicine. 2002 Sep:96(9):672-6 [PubMed PMID: 12243311]
Level 1 (high-level) evidenceHuerta S, DeShields S, Shpiner R, Li Z, Liu C, Sawicki M, Arteaga J, Livingston EH. Safety and efficacy of postoperative continuous positive airway pressure to prevent pulmonary complications after Roux-en-Y gastric bypass. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract. 2002 May-Jun:6(3):354-8 [PubMed PMID: 12022987]
Camacho M, Teixeira J, Abdullatif J, Acevedo JL, Certal V, Capasso R, Powell NB. Maxillomandibular advancement and tracheostomy for morbidly obese obstructive sleep apnea: a systematic review and meta-analysis. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2015 Apr:152(4):619-30. doi: 10.1177/0194599814568284. Epub 2015 Feb 2 [PubMed PMID: 25644497]
Level 1 (high-level) evidenceGinter G, Sankari A, Eshraghi M, Obiakor H, Yarandi H, Chowdhuri S, Salloum A, Badr MS. Effect of acetazolamide on susceptibility to central sleep apnea in chronic spinal cord injury. Journal of applied physiology (Bethesda, Md. : 1985). 2020 Apr 1:128(4):960-966. doi: 10.1152/japplphysiol.00532.2019. Epub 2020 Feb 20 [PubMed PMID: 32078469]
Poulter NR, Chang CL, Farley TM, Meirik O. Risk of cardiovascular diseases associated with oral progestagen preparations with therapeutic indications. Lancet (London, England). 1999 Nov 6:354(9190):1610 [PubMed PMID: 10560679]
Level 3 (low-level) evidenceMosbah H, Vantyghem MC, Nobécourt E, Andreelli F, Archambeaud F, Bismuth E, Briet C, Cartigny M, Chevalier B, Donadille B, Daguenel A, Fichet M, Gautier JF, Janmaat S, Jéru I, Legagneur C, Leguier L, Maitre J, Mongeois E, Poitou C, Renard E, Reznik Y, Spiteri A, Travert F, Vergès B, Zammouri J, Vigouroux C, Vatier C. Therapeutic indications and metabolic effects of metreleptin in patients with lipodystrophy syndromes: Real-life experience from a national reference network. Diabetes, obesity & metabolism. 2022 Aug:24(8):1565-1577. doi: 10.1111/dom.14726. Epub 2022 May 12 [PubMed PMID: 35445532]
Brennan M, McDonnell MJ, Walsh SM, Gargoum F, Rutherford R. Review of the prevalence, pathogenesis and management of OSA-COPD overlap. Sleep & breathing = Schlaf & Atmung. 2022 Dec:26(4):1551-1560. doi: 10.1007/s11325-021-02540-8. Epub 2022 Jan 16 [PubMed PMID: 35034250]
Dorst J, Behrendt G, Ludolph AC. Non-invasive ventilation and hypercapnia-associated symptoms in amyotrophic lateral sclerosis. Acta neurologica Scandinavica. 2019 Feb:139(2):128-134. doi: 10.1111/ane.13043. Epub 2018 Nov 19 [PubMed PMID: 30394534]
Bauman KA, Kurili A, Schotland HM, Rodriguez GM, Chiodo AE, Sitrin RG. Simplified Approach to Diagnosing Sleep-Disordered Breathing and Nocturnal Hypercapnia in Individuals With Spinal Cord Injury. Archives of physical medicine and rehabilitation. 2016 Mar:97(3):363-71. doi: 10.1016/j.apmr.2015.07.026. Epub 2015 Aug 20 [PubMed PMID: 26297810]
Sankari A, Badr MS. Diagnosis of Sleep Disordered Breathing in Patients With Chronic Spinal Cord Injury. Archives of physical medicine and rehabilitation. 2016 Jan:97(1):176-7. doi: 10.1016/j.apmr.2015.10.085. Epub [PubMed PMID: 26710861]
Sankari A, Vaughan S, Bascom A, Martin JL, Badr MS. Sleep-Disordered Breathing and Spinal Cord Injury: A State-of-the-Art Review. Chest. 2019 Feb:155(2):438-445. doi: 10.1016/j.chest.2018.10.002. Epub 2018 Oct 12 [PubMed PMID: 30321507]
Sankari A, Bascom A, Oomman S, Badr MS. Sleep disordered breathing in chronic spinal cord injury. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine. 2014 Jan 15:10(1):65-72. doi: 10.5664/jcsm.3362. Epub 2014 Jan 15 [PubMed PMID: 24426822]
Marik PE, Chen C. The clinical characteristics and hospital and post-hospital survival of patients with the obesity hypoventilation syndrome: analysis of a large cohort. Obesity science & practice. 2016 Mar:2(1):40-47 [PubMed PMID: 27812378]
Borel JC, Burel B, Tamisier R, Dias-Domingos S, Baguet JP, Levy P, Pepin JL. Comorbidities and mortality in hypercapnic obese under domiciliary noninvasive ventilation. PloS one. 2013:8(1):e52006. doi: 10.1371/journal.pone.0052006. Epub 2013 Jan 16 [PubMed PMID: 23341888]
Castro-Añón O, Pérez de Llano LA, De la Fuente Sánchez S, Golpe R, Méndez Marote L, Castro-Castro J, González Quintela A. Obesity-hypoventilation syndrome: increased risk of death over sleep apnea syndrome. PloS one. 2015:10(2):e0117808. doi: 10.1371/journal.pone.0117808. Epub 2015 Feb 11 [PubMed PMID: 25671545]
Level 2 (mid-level) evidenceMasa JF, Benítez ID, Javaheri S, Mogollon MV, Sánchez-Quiroga MÁ, de Terreros FJG, Corral J, Gallego R, Romero A, Caballero-Eraso C, Ordax-Carbajo E, Troncoso MF, González M, López-Martín S, Marin JM, Martí S, Díaz-Cambriles T, Chiner E, Egea C, Barca J, Barbé F, Mokhlesi B, Spanish Sleep Network. Risk factors associated with pulmonary hypertension in obesity hypoventilation syndrome. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine. 2022 Apr 1:18(4):983-992. doi: 10.5664/jcsm.9760. Epub [PubMed PMID: 34755598]