Introduction
Peritoneal surface malignancies (PSMs) are a heterogeneous group of tumors that arise from or spread to the peritoneum, including primary peritoneal tumors, pseudomyxoma peritonei (often originating from the appendix), and peritoneal carcinomatosis from gastrointestinal or gynecologic primaries. Although exact incidence rates are challenging to define, PSMs can account for a substantial proportion of advanced intra-abdominal malignancies. These malignancies are often multifocal, manifesting as widespread nodules or mucinous deposits within the peritoneal cavity, and can lead to significant morbidity through complications, eg, malignant ascites, bowel obstruction, and malnutrition.[1]
PSMs can be broadly divided into 3 main categories—mucinous, nonmucinous, and primary peritoneal—each with distinct histopathologic features, clinical behavior, and metastatic potential. Mucinous tumors tend to have an indolent course. However, they can accumulate large volumes of intraperitoneal mucin. At the same time, high-grade peritoneal carcinomatosis can be more aggressive, and primary peritoneal mesothelioma remains relatively rare but may present a challenging clinical course.[2] Diagnosis of PSMs typically involves a combination of cross-sectional imaging, histopathologic evaluation of peritoneal implants, and tumor markers such as CEA or CA-125. Computed tomography (CT) and magnetic resonance imaging (MRI) help delineate the distribution of disease, while laparoscopic assessment can confirm the extent and allow for tissue sampling.
Treatment strategies depend on the tumor's origin, grade, and disease burden. For many patients with PSMs, complete cytoreductive surgery (CRS) combined with hyperthermic intraperitoneal chemotherapy (HIPEC) offers the best chance of prolonged survival. In cases of unresectable or recurrent peritoneal surface malignancies, systemic chemotherapy and targeted therapies are viable treatment options. Additionally, palliative measures may be crucial to manage complications like bowel obstruction and refractory ascites.[3] Given the heterogeneity of these malignancies, an interprofessional approach is imperative to optimize patient outcomes. This resource will aid in understanding the classification, pathogenesis, diagnostic tools, and therapeutic modalities in managing patients with PSMs.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Peritoneal surface malignancies form a heterogeneous group of neoplasms, and their etiology varies depending on the type of tumor. Primary peritoneal malignancies, such as malignant peritoneal mesothelioma, are closely associated with asbestos exposure and genetic alterations, including mutations in tumor suppressor genes like BAP1.[3] Secondary peritoneal malignancies result from the transcoelomic spread of tumor cells from a primary lesion. The etiology of primary tumors is varied, and a detailed discussion of each is beyond the scope of this chapter.
Epidemiology
Accurate population-based incidence data for PSMs remain elusive because these malignancies arise from a diverse range of primary tumors and typically present at advanced stages. Approximately 30% of patients with colorectal cancer develop peritoneal metastases during their disease course, and 5% to 10% present with synchronous peritoneal disease at the time of diagnosis. Moreover, 25% of patients with peritoneal carcinomatosis from colorectal cancer show the peritoneum as the sole site of metastatic spread, emphasizing the unique dissemination pattern of these tumors.[4]
Epidemiological studies indicate that the incidence of appendiceal neoplasms ranges from 0.12 to 2 cases per million people per year. When left untreated, a significant proportion of these neoplasms progress to pseudomyxoma peritonei, a condition characterized by the accumulation of mucinous ascites and the development of widespread peritoneal implants. Primary peritoneal mesothelioma is more common in men, and represents about 7% to 18% of all mesothelioma cases, with an annual incidence of 1 in 100,000.[5]
Although each subset of PSMs—whether arising from the gastrointestinal tract, ovaries, or primary peritoneal lining—remains individually rare, these malignancies collectively contribute to a substantial fraction of advanced intra-abdominal cancers. Advances in diagnostic imaging and cytoreductive surgical techniques have improved detection and management, yet the heterogeneous nature of these diseases continues to challenge both epidemiological assessments and treatment strategies.
Pathophysiology
Peritoneal Cavity Anatomy
The anatomy of the peritoneal cavity plays a crucial role in determining the distribution of disease and selecting treatment approaches. The peritoneum is a layer of simple squamous epithelium that lines the abdominal cavity's visceral and parietal surfaces. This lining secretes peritoneal fluid, which continuously circulates among the organs and through potential spaces created by mesenteric attachments and other supporting ligaments, eg, the gastrohepatic and coronary ligaments.[6][7]
The constant movement of peritoneal fluid, driven partly by diaphragmatic contractions and gravity, influences where malignant cells may implant within the abdomen (see Image. Pathways of Peritoneal Fluid Movement). This explains why certain areas, including the subphrenic spaces due to diaphragmatic "pull" forces, paracolic gutters, and the pelvis, which is gravity-dependent, are more commonly involved in peritoneal disease.[3]
Peritoneal Surface Malignancy Pathophysiology
PSMs primarily spread through transcoelomic dissemination, a process in which tumor cells detach from the primary tumor and migrate within the peritoneal cavity. Tumor cells actively breach the basement membrane and subsequently adhere to and infiltrate the mesothelial lining of the peritoneum. As the peritoneal cavity is a large, serous surface with constantly moving peritoneal fluid, malignant cells spread throughout the cavity, evading immune cells and utilizing local growth factors to multiply.
Tumor cells utilize various strategies to facilitate their spread, including the secretion of proteolytic enzymes, such as matrix metalloproteinases, that degrade components of the extracellular matrix. This degradation not only enables tumor cells to penetrate tissue barriers but also prepares peritoneal surfaces for subsequent implantation. In addition, malignant cells overexpress adhesion molecules, including integrins and CD44, which enhance their ability to attach firmly to the mesothelial lining and increase the likelihood of successful implantation and subsequent growth on the peritoneal surface.[6][8]
Moreover, the peritoneal cavity itself actively participates in the metastatic process. The peritoneal fluid circulates continuously within the cavity, disseminating tumor cells throughout the abdomen. The mesothelial cells lining the peritoneum release cytokines and growth factors in response to the presence of tumor cells, which may inadvertently create a supportive microenvironment that promotes the survival and proliferation of tumor cells.[6][9]
In some cases, tumor cells can also exploit the lymphatic channels present in the peritoneal lining. These channels facilitate the absorption of peritoneal fluid, and when occupied by malignant cells, they provide an additional pathway for the spread of disease. Although hematogenous dissemination plays a lesser role in PSMs than other metastatic routes, it can still contribute to the disease's complexity by allowing isolated tumor cells to reach distant sites beyond the peritoneum.[6]
History and Physical
Clinical Presentations
Peritoneal carcinomatosis can present either synchronously or metachronously, with the timing often influenced by the primary tumor type and overall disease burden. In a retrospective series of patients with nongynecologic peritoneal carcinomatosis, 57% presented with synchronous disease. The most common symptoms at presentation were ascites (34%) and bowel obstruction (19%). In contrast, patients with a low-volume disease may remain asymptomatic or experience only vague symptoms, such as mild abdominal discomfort or early satiety.
When peritoneal carcinomatosis arises synchronously, patients often present with symptoms attributable to the underlying primary tumor (eg, hematochezia in colorectal cancer or pelvic pain in ovarian cancer) after which further staging investigations reveal peritoneal involvement. If imaging fails to capture small-volume disease, intraoperative findings may provide the first indication of peritoneal carcinomatosis. In a metachronous setting, patients typically have a known history of malignancy, including colorectal, appendiceal, or ovarian cancers, and they are diagnosed with peritoneal carcinomatosis either during routine surveillance or when new, often vague symptoms, such as fatigue, unintended weight loss, or altered bowel habits, warrant additional investigation. In advanced disease or when the tumor burden is high, progressive ascites, bowel obstruction, and significant weight changes commonly occur, reflecting extensive intraperitoneal seeding.[1][10]
Physical Examination
On examination, abdominal distension is often observed, and fluid waves or shifting dullness may indicate the presence of ascites. Ovarian cancer, which commonly presents at stage III when involving the peritoneum, can cause pelvic or low back pain, urinary frequency, and an increase in abdominal girth. Patients with pseudomyxoma peritonei, often resulting from appendiceal mucinous neoplasms, may experience a gradual increase in abdominal size, sometimes accompanied by hernias. Approximately 23% report progressive girth and 14% develop new hernias.
Peritoneal mesothelioma, which accounts for 10% to 30% of mesothelioma cases, may present with diffuse abdominal pain, distension, and early satiety. Approximately 30% to 50% of patients experience these symptoms at their initial evaluation. Physical examination may reveal palpable masses, localized tenderness, or signs of malnutrition and cachexia, all of which are attributable to chronic intra-abdominal pressure and diminished oral intake.[10][1]
Evaluation
The evaluation of peritoneal surface malignancies involves determining the tissue of origin, staging to assess for extraperitoneal spread, and quantifying the extent and distribution of peritoneal disease.
Laboratory Studies
Tumor markers
Tumor markers can be of value and depend on the site of tumor origin. They are not diagnostic, but provide prognostic information and help in surveillance, including:
- Carcinoembryonic antigen (CEA) is elevated in colorectal and gastric cancer-related peritoneal carcinomatosis.
- Cancer antigen 125 (CA-125) is particularly useful in ovarian and primary peritoneal carcinomas, with high sensitivity but low specificity.
- Cancer antigen 19-9 (CA 19-9) is suggestive of peritoneal involvement in pancreatic and gastrointestinal malignancies.
- Alpha-fetoprotein (AFP) is elevated in hepatocellular carcinoma and yolk sac tumors with peritoneal spread.
- Human epididymis protein 4 (HE4) is often elevated in peritoneal carcinomatosis of ovarian origin.[11][12]
Cytokine and inflammatory biomarkers
Inflammatory biomarkers help in assessing tumor burden and the peritoneal inflammatory response, including:
- C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) are elevated in advanced-stage peritoneal carcinomatosis.
- Interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α) correlate with disease progression, particularly in ovarian cancer-related PSM.[13]
- Neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) are predictive of prognosis in peritoneal carcinomatosis [13][12][13]
Peritoneal fluid cytology and biomarker analysis
The following peritoneal fluid analysis tests can provide direct evidence of malignancy in cases with ascites:
- Cytology: This study is the gold standard for detecting malignant cells in peritoneal carcinomatosis; however, its sensitivity varies based on the tumor type and burden.
- Lactate dehydrogenase (LDH): Increased levels of LDH in peritoneal fluid are associated with malignancy.
- CEA and CA-125 in peritoneal fluid: Elevated levels of these markers suggest malignancy over benign etiologies, eg, cirrhosis-related ascites.[13]
Molecular and Genetic Testing
Molecular testing is increasingly used to help assess prognosis and determine suitability for targeted treatment. Tests that may be utilized for PSMs include:
- KRAS, BRAF, and MSI testing are essential in colorectal cancer-related peritoneal carcinomatosis for therapy selection.
- BRCA1/2 and homologous recombination deficiency (HRD) testing guide the use of PARP inhibitors in ovarian cancer with peritoneal spread.
- Next-generation sequencing (NGS) enables identifying actionable mutations for targeted therapies in peritoneal mesothelioma and other PSMs.
Liquid Biopsy
Circulating tumor DNA and circulating tumor cells (ctDNA and CTCs) are increasingly used in certain tumors, such as colorectal cancer, and are likely to be more widely used in the coming years.
Imaging Studies
Several imaging studies may be utilized to evaluate patients with suspected or known PSMs.[14]
Computed tomography
A contrast-enhanced computed tomography (CT) scan of the abdomen and pelvis is the primary imaging modality for evaluating patients with suspected or known PSMs.[15] It provides detailed anatomic information, suggesting tumor deposits along the peritoneal reflections, the greater and lesser omenta, mesentery, and serosal surfaces of abdominal organs. Features that raise suspicion for PSMs include soft-tissue nodules on the peritoneal surfaces, focal or diffuse peritoneal thickening, omental caking, and ascites. CT can also help identify mucinous components by revealing low-attenuation masses, characteristic of pseudomyxoma peritonei (see Image. Pseudomyxoma Peritonei) secondary to appendiceal or ovarian neoplasms. However, sensitivity may be limited for detecting smaller lesions (<5 mm), and the disease burden in the small bowel or mesentery is frequently underestimated due to overlapping loops of bowel and variable bowel distension.[16]
Several radiologic signs can predict a high likelihood of nonresectability, eg, a large solitary mass in the upper abdomen (often >5 cm), diffuse small bowel wall thickening, or the “smudge” sign where small bowel loops appear matted together, findings sometimes referred to collectively as “Sugarbaker’s Criteria.” Although CT is routinely used to approximate the peritoneal cancer index (PCI), studies suggest it can either underestimate or overestimate the true extent of disease by up to 20% to 30%.[17]
Magnetic resonance imaging
Magnetic resonance imaging (MRI) plays an increasingly vital role in evaluating PSMs, offering superior soft-tissue contrast and multi-planar capabilities without the risks associated with ionizing radiation. In particular, diffusion-weighted imaging enhances the detection of small peritoneal deposits and mucinous lesions, which are critical for assessing conditions, such as pseudomyxoma peritonei. When combined with contrast-enhanced sequences, MRI further refines staging by clearly delineating the extent of disease, a key factor in planning cytoreductive surgery and intraperitoneal chemotherapy.[18]
Recent studies have demonstrated that MRI can achieve sensitivity values of approximately 90% to 95% and specificity values of around 85% to 92% for detecting peritoneal metastases, particularly for lesions larger than 5 mm.[19]
Positron emission tomography
Fluorodeoxyglucose positron emission tomography (FDG-PET), typically combined with computed tomography (PET/CT), can reveal hypermetabolic foci within peritoneal surface malignancies and detect extraperitoneal metastases that may alter therapeutic decision-making. Its sensitivity, however, is limited in low-cellularity mucinous neoplasms, including pseudomyxoma peritonei, where radiotracer uptake can be insufficient for accurate detection. Moreover, inflammatory changes or surgical manipulation can yield false-positive signals. Thus, PET/CT is generally regarded as an adjunct to contrast-enhanced CT or MRI, most valuable for ruling out distant metastatic disease or clarifying equivocal findings rather than serving as the principal staging modality.[20][21]
Diagnostic Laparoscopy
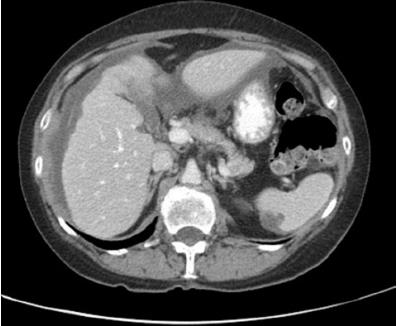
Diagnostic laparoscopy provides the most accurate assessment of peritoneal disease burden and distribution, while also allowing for the collection of biopsies. It should be performed in all patients for whom cytoreduction and intraperitoneal chemotherapy are considered, and is often also performed in cases where imaging is equivocal. By permitting real-time assessment of tumor distribution and burden, particularly in areas prone to underestimation on CT or MRI, laparoscopy enables a more precise estimation of the PCI and aids in evaluating resectability (see Image. Peritoneal Cancer Index).[22][23][24]
PCI is a standardized quantitative tool for estimating the extent and distribution of PSMs. It partitions the abdominal cavity into 13 discrete anatomical regions (0 through 12) and assigns each region a lesion size (LS) score ranging from 0 to 3. The PCI is then calculated by summing the lesion size scores across all areas, with a maximum possible value of 39 reflecting extensive disease. Notably, lesion size 3 (>5 cm or confluence of disease) is associated with a poor prognosis.[25][26]
In clinical practice, the PCI is a robust surrogate for tumor burden, correlates strongly with both resectability and survival outcomes, and often dictates eligibility for cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC). While radiologic estimates of the PCI using CT or MRI can guide patient selection for surgery, definitive scoring typically occurs intraoperatively via either laparotomy or, in selected centers, laparoscopic exploration. Accurate scoring is paramount, as patients with a PCI exceeding a certain threshold—commonly 20 for high-grade appendiceal or colorectal cancer, for example—are more likely to have incomplete cytoreduction and diminished survival benefits from aggressive surgical intervention. Consequently, the PCI is integral to risk stratification, surgical planning, and counseling regarding prognosis in the interprofessional management of PSMs.[25]
Treatment / Management
Peritoneal Surface Malignancy Management
The management of peritoneal surface malignancies is predicated on disease extent, tumor biology, and patient fitness. In general, complete cytoreductive surgery followed by hyperthermic intraperitoneal chemotherapy (HIPEC) offers a chance of long-term survival in well-selected patients, particularly those with low- to moderate-volume disease and favorable histologies. Adjunctive systemic chemotherapy, early postoperative intraperitoneal chemotherapy (EPIC), or emerging techniques like pressurized intraperitoneal aerosol chemotherapy (PIPAC) may further refine treatment outcomes, while targeted therapies and immunotherapies hold promise in specific molecular subsets. Given the complexity of care, patients benefit from coordinated, interprofessional care at high-volume institutions.
Cytoreductive surgery
Cytoreductive surgery (CRS) focuses on excising all macroscopically visible tumors, often involving peritonectomy and resection of involved visceral structures (eg, the omentum, spleen, segments of small or large bowel) and occasionally other organs (eg, liver wedge resections, hysterectomy, bilateral salpingo-oophorectomy in select cases). The goal is to achieve a completeness of cytoreduction (CC) score of 0 or 1 (ie, no visible disease or residual nodules <2.5 mm) because intraperitoneal chemotherapy penetrates only a few millimeters into tissue. Achieving a CC-0/1 resection is strongly associated with improved survival.[3][27][28]
Patient selection for CRS depends on tumor and patient-related factors. Tumor burden is classically quantified using the PCI. Most centers discourage CRS if the PCI substantially exceeds 20 for high-grade appendiceal or colorectal etiologies, due to the lower likelihood of complete cytoreduction and diminished survival benefits. Favorable histopathologic subtypes (eg, low-grade mucinous appendiceal neoplasms and epithelial mesothelioma) are typically more amenable to CRS than high-grade or signet ring cell carcinomas. Additional considerations include performance status (ECOG/WHO) and comorbidities, as well as prior response to systemic therapies.
Hyperthermic intraperitoneal chemotherapy
Immediately following CRS, HIPEC is administered to eliminate microscopic residual disease. Heat (40 °C to 43 °C) enhances the cytotoxicity of chemotherapy and improves tissue penetration, it may increase tumor destruction in regions inaccessible to surgical resection. Commonly used agents include mitomycin C, oxaliplatin, or cisplatin, which may be combined with doxorubicin.
Drug choice is guided by histology (eg, oxaliplatin and mitomycin C in colorectal PSM, cisplatin-based regimens in peritoneal mesothelioma).[29][30][31] CRS plus HIPEC has shown robust survival benefits for carefully selected patients, especially those with low- or intermediate-volume disease and favorable tumor biology. However, morbidity can be significant, including hematologic toxicity (neutropenia, thrombocytopenia), anastomotic leaks, abscesses, and organ-specific complications (eg, renal dysfunction with cisplatin).[29][30][31] Please see StatPearls' companion resource, "Cytoreduction CRS and Hyperthermic Intraperitoneal Chemotherapy HIPEC", for further information. (A1)
Systemic chemotherapy
Systemic therapy can be used preoperatively to stabilize or downstage disease and assess tumor biology in high-grade malignancies, eg, colorectal or gastric primary tumors. Postoperative therapy may be considered to eradicate microscopic disease, especially in high-risk features (eg, nodal involvement or high-grade histology). However, data on its precise efficacy in the CRS plus HIPEC setting remains evolving.
Standard regimens mirror those used in metastatic gastrointestinal cancers, eg, FOLFOX (5-FU, leucovorin, and oxaliplatin) or FOLFIRI (5-FU, leucovorin, and irinotecan), often combined with targeted agents (bevacizumab or anti-EGFR therapies) or immunotherapies in select settings. Pemetrexed-based and immunotherapy regimens, with cisplatin or carboplatin, remain the mainstay of treatment for peritoneal mesothelioma. For low-grade appendiceal tumors, some centers use capecitabine or 5-FU/mitomycin regimens. The optimal sequence, duration, and combination of systemic therapy with CRS plus HIPEC remain areas of active clinical investigation.[32][33][34](A1)
Palliative Care and Symptomatic Management
Noncurative surgical intervention, eg, diverting stomas or debulking without HIPEC, may be warranted to relieve obstructive symptoms, mitigate pain, and improve quality of life in individuals with advanced, unresectable PSMs. Repeated paracentesis can provide temporary symptomatic relief for refractory malignant ascites, and indwelling peritoneal catheters may improve outpatient symptom control. Nutritional assessments, often involving dietitians and supplements, are essential, as reduced oral intake and high metabolic demands frequently lead to malnutrition.[35][36]
Follow-Up and Surveillance
Patients who undergo CRS and HIPEC require regular imaging (CT or MRI) and clinical evaluation at intervals determined by the type of tumor, such as every 3 to 6 months for 2 years, then every 6 to 12 months. Tumor markers (eg, CEA, CA 19-9, or CA-125) may be monitored if they are initially elevated. Recurrence patterns can include new deposits or progression in unresected sites, and some centers consider repeat CRS plus HIPEC for highly selected patients with limited recurrence.
Many PSM patients experience prolonged survival after successful CRS and HIPEC, especially those with low-grade appendiceal or mesothelioma histology. Nonetheless, vigilant surveillance for late recurrences, comorbidities, and complications of extensive surgery (eg, adhesions and incisional hernias) is vital to optimize outcomes.
Differential Diagnosis
The differential diagnoses for peritoneal metastasis include:
- Primary peritoneal malignancy
- Peritoneal tuberculosis
- Peritonitis
Prognosis
Quantitative prognostic indicators currently used for peritoneal carcinomatosis include:
- Tumor histology
- Intraoperative assessment of the extent of carcinomatosis at the time of surgical exploration, which can be determined by different scoring systems as described earlier. They include:
- Gilly staging
- PCI scoring system
- Staging by the Japanese Cancer Society for gastric cancer
- Dutch simplified peritoneal carcinomatosis index (SPCI)
- Completeness of cytoreduction (CC)
- Patient's clinical symptoms [37]
The primary outcome parameters, eg, overall survival, disease-free survival, and 5-year survival rate, depend on the type of primary cancer, achieving cytoreduction (based on CC scoring), HIPEC treatment, and/or the cancer's biological activity. Peritoneal metastasis from an unknown primary tumor has a poor prognosis, with survival as low as 3 months. Although specific histologic subtypes have shown favorable survival, efforts should focus on detecting and identifying the primary tumor, potentially increasing the prognostic benefits of treatment.[38]
Complications
Complications related to untreated or inoperable peritoneal carcinomatosis include:
- Refractory ascites
- Intestinal obstruction
- Gastrointestinal dysmotility
- Pulmonary thromboembolism
- Peritonitis
- Complications related to portal hypertension (eg, upper GI bleed related to esophageal varices, splenomegaly, hepatic encephalopathy, and ascites)
- Enteric fistula [36]
Postoperative complications related to CRS include bleeding, infection, bowel obstruction, hemorrhage, or peritonitis. Complications associated with HIPEC agents include oxaliplatin, which is used in conjunction with dextrose solutions, that can potentially contribute to postoperative acidosis and hyperglycemia, and mitomycin C, which can cause neutropenia in approximately one-third of patients and other gastrointestinal adverse effects.
Deterrence and Patient Education
PSMs represent a diverse group of tumors that may arise from the lining of the abdominal cavity or spread from intraabdominal organs. Their clinical presentation varies based on the site of origin, necessitating evaluation by a specialized interprofessional team. Management often involves a combination of surgery, chemotherapy, or targeted therapies tailored to the specific type and extent of disease.
The prognosis of PSMs is closely linked to the primary tumor origin and the burden of peritoneal involvement. Adherence to general cancer prevention strategies, such as maintaining a healthy lifestyle, avoiding tobacco, and participating in recommended cancer screenings, may reduce the risk of developing certain malignancies that can spread to the peritoneum.
Enhancing Healthcare Team Outcomes
Patients with PSM face complex clinical challenges due to the often advanced stage of disease at diagnosis. Early recognition and interprofessional management are critical in improving survival rates and quality of life. Managing patients with PSM requires a coordinated approach involving surgical oncologists, medical oncologists, radiologists, pathologists, advanced practitioners, nurses, pharmacists, and other allied health professionals to ensure comprehensive, patient-centered care. Clinicians treating these malignancies must possess specialized knowledge and skills to accurately diagnose and manage these conditions, including understanding peritoneal carcinomatosis indices, CRS, and HIPEC.
Interprofessional tumor boards can significantly improve treatment planning by optimizing surgical timing and tailoring systemic therapy regimens to individual patients. Educating patients and caregivers about treatment expectations, potential complications (eg, infections, bowel obstructions, or recurrence) and the importance of ongoing surveillance is also essential to encourage adherence and reduce morbidity.
A structured, evidence-based approach is vital to balancing aggressive treatment with quality-of-life considerations. Ethical decision-making, including discussions about care goals and informed consent for high-risk procedures, must be prioritized. By prioritizing clinical excellence, strategic planning, ethical practice, defined responsibilities, communication, and care coordination, the healthcare team can significantly impact patient outcomes and optimize the interprofessional management of peritoneal surface malignancies.
Media
(Click Image to Enlarge)
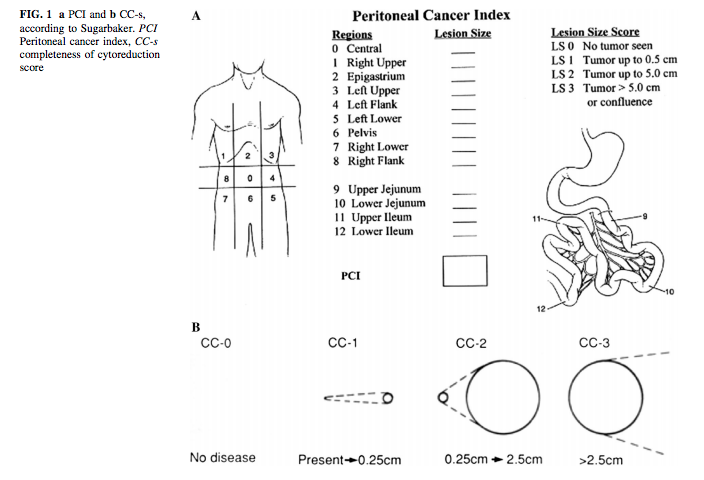
Peritoneal Cancer Index. The peritoneal cancer index (PCI) is a standardized quantitative tool for estimating the extent and distribution of peritoneal surface malignancies (PSMs).
Contributed by F Moustarah, MD, MPH, and J Desai, MBBS; adapted from Jacquet P, Sugarbaker PH. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat Res. 1996;82:359-374. doi: 10.1007/978-1-4613-1247-5_23.
(Click Image to Enlarge)
(Click Image to Enlarge)
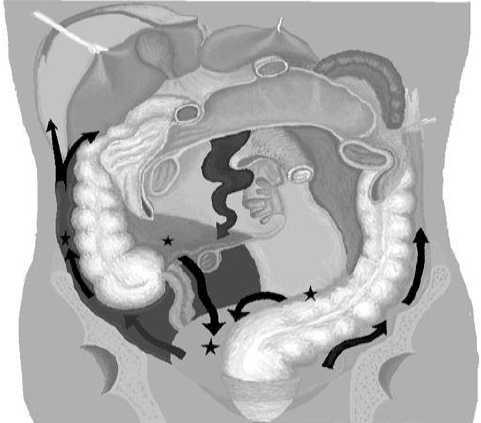
Pathways of Peritoneal Fluid Movement. The constant movement of peritoneal fluid, driven partly by diaphragmatic contractions and gravity, influences where malignant cells may implant within the abdomen.
Mikael Häggström, Public Domain, via Wikimedia Commons
References
Cortés-Guiral D, Hübner M, Alyami M, Bhatt A, Ceelen W, Glehen O, Lordick F, Ramsay R, Sgarbura O, Van Der Speeten K, Turaga KK, Chand M. Primary and metastatic peritoneal surface malignancies. Nature reviews. Disease primers. 2021 Dec 16:7(1):91. doi: 10.1038/s41572-021-00326-6. Epub 2021 Dec 16 [PubMed PMID: 34916522]
Vazzano J, Esnakula AK. Pathology and Classification of Peritoneal Surface Malignancies. Surgical oncology clinics of North America. 2025 Apr:34(2):155-171. doi: 10.1016/j.soc.2024.10.003. Epub 2024 Nov 29 [PubMed PMID: 40015797]
Stewart JH 4th, Blazer DG 3rd, Calderon MJG, Carter TM, Eckhoff A, Al Efishat MA, Fernando DG, Foster JM, Hayes-Jordan A, Johnston FM, Lautz TB, Levine EA, Maduekwe UN, Mangieri CW, Moaven O, Mogal H, Shen P, Votanopoulos KI. The Evolving Management of Peritoneal Surface Malignancies. Current problems in surgery. 2021 Apr:58(4):100860. doi: 10.1016/j.cpsurg.2020.100860. Epub 2020 Jul 14 [PubMed PMID: 33832580]
Lurvink RJ, Bakkers C, Rijken A, van Erning FN, Nienhuijs SW, Burger JW, Creemers GJ, Verhoef C, Lemmens VE, De Hingh IH. Increase in the incidence of synchronous and metachronous peritoneal metastases in patients with colorectal cancer: A nationwide study. European journal of surgical oncology : the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2021 May:47(5):1026-1033. doi: 10.1016/j.ejso.2020.11.135. Epub 2020 Nov 26 [PubMed PMID: 33272737]
Moolgavkar SH, Meza R, Turim J. Pleural and peritoneal mesotheliomas in SEER: age effects and temporal trends, 1973-2005. Cancer causes & control : CCC. 2009 Aug:20(6):935-44. doi: 10.1007/s10552-009-9328-9. Epub 2009 Mar 18 [PubMed PMID: 19294523]
Demuytere J, Ernst S, Ceelen W. Pathophysiology of Peritoneal Metastasis. Journal of surgical oncology. 2024 Nov:130(6):1299-1305. doi: 10.1002/jso.27890. Epub 2024 Oct 13 [PubMed PMID: 39400354]
Lemoine L, Sugarbaker P, Van der Speeten K. Pathophysiology of colorectal peritoneal carcinomatosis: Role of the peritoneum. World journal of gastroenterology. 2016 Sep 14:22(34):7692-707. doi: 10.3748/wjg.v22.i34.7692. Epub [PubMed PMID: 27678351]
Bootsma S, Bijlsma MF, Vermeulen L. The molecular biology of peritoneal metastatic disease. EMBO molecular medicine. 2023 Mar 8:15(3):e15914. doi: 10.15252/emmm.202215914. Epub 2023 Jan 26 [PubMed PMID: 36700339]
Ren K, Xie X, Min T, Sun T, Wang H, Zhang Y, Dang C, Zhang H. Development of the Peritoneal Metastasis: A Review of Back-Grounds, Mechanisms, Treatments and Prospects. Journal of clinical medicine. 2022 Dec 23:12(1):. doi: 10.3390/jcm12010103. Epub 2022 Dec 23 [PubMed PMID: 36614904]
Ikegami T, Ishiki H, Kadono T, Ito T, Yokomichi N. Narrative review of malignant ascites: epidemiology, pathophysiology, assessment, and treatment. Annals of palliative medicine. 2024 Jul:13(4):842-857. doi: 10.21037/apm-23-554. Epub 2024 Apr 18 [PubMed PMID: 38644553]
Level 3 (low-level) evidenceAn SL, Zhang K, Ji ZH, Li XB, Yu Y, Zhang YB, Liu G, Li B, Yan GJ, Li Y. [The effect of cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy on peritoneal carcinomatosis from colorectal cancer]. Zhonghua zhong liu za zhi [Chinese journal of oncology]. 2021 Dec 23:43(12):1298-1303. doi: 10.3760/cma.j.cn112152-20200305-00173. Epub [PubMed PMID: 34915640]
Enblad M, Cashin P, Ghanipour L, Graf W. Patterns of Preoperative Tumor Markers Can Predict Resectability and Prognosis of Peritoneal Metastases: A Clustering Analysis. Annals of surgical oncology. 2025 May:32(5):3638-3647. doi: 10.1245/s10434-024-16860-y. Epub 2025 Jan 22 [PubMed PMID: 39841338]
Vlaeminck-Guillem V, Bienvenu J, Isaac S, Grangier B, Golfier F, Passot G, Bakrin N, Rodriguez-Lafrasse C, Gilly FN, Glehen O. Intraperitoneal cytokine level in patients with peritoneal surface malignancies. A study of the RENAPE (French Network for Rare Peritoneal Malignancies). Annals of surgical oncology. 2013 Aug:20(8):2655-62. doi: 10.1245/s10434-013-2933-6. Epub 2013 Mar 22 [PubMed PMID: 23519518]
Miguez González J, Calaf Forn F, Pelegrí Martínez L, Lozano Arranz P, Oliveira Caiafa R, Català Forteza J, Palacio Arteaga LM, Losa Gaspà F, Ramos Bernadó I, Barrios Sánchez P, Ayuso Colella JR. Primary and secondary tumors of the peritoneum: key imaging features and differential diagnosis with surgical and pathological correlation. Insights into imaging. 2023 Jul 3:14(1):115. doi: 10.1186/s13244-023-01417-6. Epub 2023 Jul 3 [PubMed PMID: 37395913]
Patel CM, Sahdev A, Reznek RH. CT, MRI and PET imaging in peritoneal malignancy. Cancer imaging : the official publication of the International Cancer Imaging Society. 2011 Aug 24:11(1):123-39. doi: 10.1102/1470-7330.2011.0016. Epub 2011 Aug 24 [PubMed PMID: 21865109]
de Bree E, Koops W, Kröger R, van Ruth S, Witkamp AJ, Zoetmulder FA. Peritoneal carcinomatosis from colorectal or appendiceal origin: correlation of preoperative CT with intraoperative findings and evaluation of interobserver agreement. Journal of surgical oncology. 2004 May 1:86(2):64-73 [PubMed PMID: 15112247]
Rivard JD, Temple WJ, McConnell YJ, Sultan H, Mack LA. Preoperative computed tomography does not predict resectability in peritoneal carcinomatosis. American journal of surgery. 2014 May:207(5):760-4; discussion 764-5. doi: 10.1016/j.amjsurg.2013.12.024. Epub 2014 Mar 12 [PubMed PMID: 24791641]
Torkzad MR, Casta N, Bergman A, Ahlström H, Påhlman L, Mahteme H. Comparison between MRI and CT in prediction of peritoneal carcinomatosis index (PCI) in patients undergoing cytoreductive surgery in relation to the experience of the radiologist. Journal of surgical oncology. 2015 May:111(6):746-51. doi: 10.1002/jso.23878. Epub 2015 Jan 8 [PubMed PMID: 25580825]
Low RN, Sebrechts CP, Barone RM, Muller W. Diffusion-weighted MRI of peritoneal tumors: comparison with conventional MRI and surgical and histopathologic findings--a feasibility study. AJR. American journal of roentgenology. 2009 Aug:193(2):461-70. doi: 10.2214/AJR.08.1753. Epub [PubMed PMID: 19620444]
Level 2 (mid-level) evidenceLi J, Yan R, Lei J, Jiang C. Comparison of PET with PET/CT in detecting peritoneal carcinomatosis: a meta-analysis. Abdominal imaging. 2015 Oct:40(7):2660-6. doi: 10.1007/s00261-015-0418-8. Epub [PubMed PMID: 25893503]
Level 1 (high-level) evidenceVietti Violi N, Gavane S, Argiriadi P, Law A, Heiba S, Bekhor EY, Babb JS, Ghesani M, Labow DM, Taouli B. FDG-PET/MRI for the preoperative diagnosis and staging of peritoneal carcinomatosis: a prospective multireader pilot study. Abdominal radiology (New York). 2023 Dec:48(12):3634-3642. doi: 10.1007/s00261-022-03703-1. Epub 2022 Oct 29 [PubMed PMID: 36308554]
Level 3 (low-level) evidenceOliveira AA, Morais J, Pires O, Marques IL. Peritoneal carcinomatosis: the importance of laparoscopy. BMJ case reports. 2021 Oct 1:14(10):. doi: 10.1136/bcr-2021-243972. Epub 2021 Oct 1 [PubMed PMID: 34598962]
Level 3 (low-level) evidenceHanna DN, Ghani MO, Hermina A, Mina A, Bailey CE, Idrees K, Magge D. Diagnostic Laparoscopy in Patients With Peritoneal Carcinomatosis Is Safe and Does Not Delay Cytoreductive Surgery With Hyperthermic Intraperitoneal Chemotherapy. The American surgeon. 2022 Apr:88(4):698-703. doi: 10.1177/00031348211048819. Epub 2021 Nov 3 [PubMed PMID: 34732056]
Carboni F, Federici O, Giofrè M, Valle M. An 18-Year Experience in Diagnostic Laparoscopy of Peritoneal Carcinomatosis: Results from 744 Patients. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract. 2020 Sep:24(9):2096-2103. doi: 10.1007/s11605-019-04368-w. Epub 2019 Aug 20 [PubMed PMID: 31432327]
Ihemelandu C. The Landmark Series: Scoring Systems for Primary Peritoneal Surface Malignancy. Annals of surgical oncology. 2023 Mar:30(3):1832-1837. doi: 10.1245/s10434-022-12941-y. Epub 2022 Dec 22 [PubMed PMID: 36550329]
Dohan A, Hobeika C, Najah H, Pocard M, Rousset P, Eveno C. Preoperative assessment of peritoneal carcinomatosis of colorectal origin. Journal of visceral surgery. 2018 Sep:155(4):293-303. doi: 10.1016/j.jviscsurg.2018.01.002. Epub 2018 Mar 27 [PubMed PMID: 29602696]
Foster JM, Zhang C, Rehman S, Sharma P, Alexander HR. The contemporary management of peritoneal metastasis: A journey from the cold past of treatment futility to a warm present and a bright future. CA: a cancer journal for clinicians. 2023 Jan:73(1):49-71. doi: 10.3322/caac.21749. Epub 2022 Aug 15 [PubMed PMID: 35969103]
Clair KH, Wolford J, Zell JA, Bristow RE. Surgical Management of Gynecologic Cancers. Surgical oncology clinics of North America. 2021 Jan:30(1):69-88. doi: 10.1016/j.soc.2020.09.004. Epub [PubMed PMID: 33220810]
van Stein RM, Aalbers AGJ, Sonke GS, van Driel WJ. Hyperthermic Intraperitoneal Chemotherapy for Ovarian and Colorectal Cancer: A Review. JAMA oncology. 2021 Aug 1:7(8):1231-1238. doi: 10.1001/jamaoncol.2021.0580. Epub [PubMed PMID: 33956063]
Filis P, Mauri D, Markozannes G, Tolia M, Filis N, Tsilidis K. Hyperthermic intraperitoneal chemotherapy (HIPEC) for the management of primary advanced and recurrent ovarian cancer: a systematic review and meta-analysis of randomized trials. ESMO open. 2022 Oct:7(5):100586. doi: 10.1016/j.esmoop.2022.100586. Epub 2022 Sep 16 [PubMed PMID: 36116421]
Level 1 (high-level) evidenceKusamura S, Bhatt A, van Der Speeten K, Kepenekian V, Hübner M, Eveno C, de Hingh I, Delhorme JB, Taibi A, Villeneuve L, Dico RL, Moran B, Govaerts K, Zivanovic O, Brennan D, Nadeau C, Van Driel W, Bakrin N, Piso P, Verwaal VJ, González-Moreno S, Alyami M, Sgarbura O, Rau B, Deraco M, Glehen O. Review of 2022 PSOGI/RENAPE Consensus on HIPEC. Journal of surgical oncology. 2024 Nov:130(6):1290-1298. doi: 10.1002/jso.27885. Epub 2024 Sep 16 [PubMed PMID: 39285659]
Level 3 (low-level) evidencevan den Heuvel TBM, Lurvink RJ, Rovers KPB, van Hellemond IEG, de Hingh IHJT. Systemic chemotherapy in addition to CRS-HIPEC for colorectal peritoneal metastases: A critical systematic review on the impact on overall survival. Journal of surgical oncology. 2024 Nov:130(6):1378-1389. doi: 10.1002/jso.27849. Epub 2024 Sep 11 [PubMed PMID: 39257287]
Level 1 (high-level) evidenceAlexander HR. Perioperative Systemic Chemotherapy in Patients with Malignant Peritoneal Mesothelioma Undergoing Cytoreduction and HIPEC: Don't Put the Cart Before the Horse. Annals of surgical oncology. 2023 Oct:30(11):6301-6303. doi: 10.1245/s10434-023-13783-y. Epub 2023 Jun 26 [PubMed PMID: 37358682]
Verwaal VJ. Long-term results of cytoreduction and HIPEC followed by systemic chemotherapy. Cancer journal (Sudbury, Mass.). 2009 May-Jun:15(3):212-5. doi: 10.1097/PPO.0b013e3181a58d7c. Epub [PubMed PMID: 19556907]
Bleicher J, Lambert LA. A Palliative Approach to Management of Peritoneal Carcinomatosis and Malignant Ascites. Surgical oncology clinics of North America. 2021 Jul:30(3):475-490. doi: 10.1016/j.soc.2021.02.004. Epub [PubMed PMID: 34053663]
Lambert LA, Hendrix RJ. Palliative Management of Advanced Peritoneal Carcinomatosis. Surgical oncology clinics of North America. 2018 Jul:27(3):585-602. doi: 10.1016/j.soc.2018.02.008. Epub [PubMed PMID: 29935691]
Harmon RL, Sugarbaker PH. Prognostic indicators in peritoneal carcinomatosis from gastrointestinal cancer. International seminars in surgical oncology : ISSO. 2005 Feb 8:2(1):3 [PubMed PMID: 15701175]
Thomassen I, Verhoeven RH, van Gestel YR, van de Wouw AJ, Lemmens VE, de Hingh IH. Population-based incidence, treatment and survival of patients with peritoneal metastases of unknown origin. European journal of cancer (Oxford, England : 1990). 2014 Jan:50(1):50-6. doi: 10.1016/j.ejca.2013.08.009. Epub 2013 Sep 3 [PubMed PMID: 24011935]