Introduction
Periodontal disease refers to a group of conditions that affect the periodontium, the supporting structures of the teeth, including the gingiva, alveolar bone, cementum, and periodontal ligament. Early detection and timely management are essential to prevent long-term complications.
Gingivitis is the mildest form of periodontal disease, affecting up to 90% of the population. Gingivitis is characterized by inflammation of the gingiva caused by the accumulation of bacteria and debris along the gum line, leading to the formation of dental plaque. This condition is reversible with improved oral hygiene. However, if left untreated, gingivitis can progress to periodontitis.
Periodontitis is a chronic inflammatory disease that progressively damages the periodontal tissues. The hallmark feature of the condition is the apical migration of the junctional epithelium, which leads to loss of attachment and the formation of periodontal pockets. As bacteria penetrate deeper into the tissues, the body activates an immune response to fight the infection. However, this defense mechanism unintentionally contributes to the destruction of the periodontium. As periodontitis progresses, it causes continued attachment loss, alveolar bone resorption, and, in severe cases, tooth loss.[1][2][3]
In 2017, the American Academy of Periodontology introduced an updated classification system for periodontal and peri-implant diseases in collaboration with the European Federation of Periodontology. This system categorizes periodontitis into 3 main forms:
- Periodontitis
- Periodontitis as a manifestation of systemic diseases
- Necrotizing periodontal disease
Necrotizing periodontal disease is a severe and rapidly progressing form of periodontitis that primarily affects immunocompromised individuals, such as those with HIV. This condition is characterized by severe gingival necrosis, interproximal tissue destruction, spontaneous bleeding, and intense pain. This form of periodontal disease requires urgent intervention due to its rapid progression and significant tissue damage.[4][5]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Periodontal disease arises from multiple factors, including patient-specific risk factors and inadequate oral hygiene. These risk factors are categorized as modifiable (such as smoking, poor oral hygiene, diabetes mellitus, and pregnancy) or non-modifiable (including age, heredity, and genetic disorders). Poor oral hygiene is a major contributor, as plaque buildup correlates with increased disease severity and prevalence. Anaerobic bacteria, including Aggregatibacter actinomycetemcomitans, Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia, colonize deep within periodontal pockets, thereby triggering inflammation through host immune responses.[1][6][7][8][9]
Smoking is the most significant modifiable risk factor for periodontal disease, increasing the risk by 5- to 20-fold and carrying an odds ratio of 5.4 for chronic periodontitis. Smokers exhibit greater bone and attachment loss, deeper periodontal pockets, and higher rates of tooth loss compared to nonsmokers. Furthermore, smoking diminishes the effectiveness of periodontal treatment.[10][11][12][13][14][15]
Diabetes mellitus also plays a significant role in exacerbating periodontal breakdown due to impaired wound healing. Severe periodontal disease in patients with diabetes is associated with a higher risk of mortality compared to those with mild or no disease.[7][16]
Hormonal fluctuations during pregnancy can enhance inflammatory responses, contributing to the development of gingivitis and periodontitis. Although the exact mechanisms are not fully understood, maternal hormone levels have been linked to P gingivalis, which is a key pathogen in periodontal disease progression. Both hypoestrogenism and hyperestrogenism.[17][18][19][20]
Age is a well-documented, non-modifiable risk factor for periodontal disease and other health conditions. Older individuals tend to exhibit an exacerbated inflammatory response to plaque accumulation, with increased aggregation of inflammatory cells accelerating periodontal tissue destruction. Additionally, reduced dexterity in this population can further compromise oral hygiene, leading to higher plaque levels. Research indicates that individuals aged 60 to 90 experience greater clinical attachment loss (CAL) compared to those aged 50 or younger.[7][21][22][23]
Several genetic disorders, including Down syndrome, Ehlers-Danlos syndrome (types IV and VIII), and Crohn disease, have periodontal manifestations, highlighting the complex interplay between systemic conditions and oral health.[24][25][26]
Epidemiology
Periodontal diseases affect up to 90% of the global population, making them the most common oral health condition worldwide. In the United States, cross-sectional studies indicate that approximately 50% of adults currently have some form of gingivitis, and up to 80% have experienced some form of periodontal disease at some point in their lives. Higher incidence rates of periodontal diseases are observed in certain populations, including older adults, males, and individuals with lower income and education levels.[1][6][7][27]
Pathophysiology
Commensal oral bacteria initiate and exacerbate periodontal disease through dysbiosis, a microbial imbalance that disrupts the normal composition of oral bacteria. The disease typically follows a cyclical pattern of activity and inactivity until treated, or it progresses to tissue and tooth destruction, potentially resulting in tooth loss.
As gingivitis advances to periodontitis, anaerobic bacteria such as A actinomycetemcomitans and P gingivalis colonize deeper periodontal pockets, triggering a host inflammatory response. This response includes the release of C-reactive protein (CRP), which is a key biomarker of inflammation, along with neutrophil- and macrophage-derived mediators such as tumor necrosis factor-alpha (TNF-α), matrix metalloproteinases (MMPs), and interleukins (IL-1 and IL-8). Elevated serum CRP levels indicate a potential link between periodontitis-associated inflammation and cardiovascular disease. Smoking further exacerbates this process by fostering a more conducive environment for pathogenic bacteria, thereby accelerating disease progression.[1][2][9][24][28][29]
Histopathology
Page and Schroeder were the first to describe the histopathology of periodontal disease, identifying 4 distinct stages based on clinical and histological tissue changes. These stages—initial, early, established, and advanced—illustrate the progressive nature of the disease, beginning with subclinical inflammation and potentially leading to irreversible tissue destruction and bone loss.
- Initial lesion: The initial lesion is characterized by plaque accumulation, which leads to vascular changes and intercellular gap formation, thereby increasing the flow of gingival crevicular fluid. Adhesion molecules attract polymorphonuclear neutrophils (PMNs) to the lesion, while T lymphocytes modulate fibroblast activity in the affected area. Clinically, this stage is asymptomatic.
- Early lesion: The early lesion manifests clinically as redness at the site. PMNs infiltrate the area, clearing apoptotic fibroblasts and causing collagen fiber breakdown, which creates space for further infiltration. The marginal connective tissue matrix also begins to degrade.
- Established lesion: The established lesion is characterized by the predominance of leukocytes and B cells, which leads to the transformation of the junctional and sulcular epithelium into pocket epithelium, which becomes highly permeable and vulnerable to further damage. Clinically, this stage is evidenced by gingival bleeding upon gentle probing.
- Advanced lesion: The advanced lesion signifies the progression from gingivitis to periodontitis. Bacterial biofilm extends into the periodontal pockets, creating an optimal environment for anaerobic bacteria to proliferate. At this stage, irreversible loss of attachment of the junctional epithelium and alveolar bone loss occur. The loss of gingival fibers and alveolar bone defines this stage, which can vary based on microbial and host factors.[30][31]
History and Physical
Gingivitis and periodontitis commonly present with gum inflammation, including redness, swelling, and changes in texture. The gums typically bleed when probed (see Image. Gingivitis with Calculus Buildup). In periodontitis, periodontal pockets develop due to damage to the supporting structures. As the disease progresses, gum recession exposes the tooth roots, leading to increased tooth mobility, shifting of teeth, and, eventually, tooth loss. The disease is generally painless, with pain occurring during acute flare-ups, often due to periodontal abscess formation or weakened tooth support.[2] As a result, the condition often goes unnoticed until it reaches an advanced stage, delaying treatment (see Image. Periodontitis).
Periodontitis can affect individuals of any age, but is more commonly seen in middle-aged and older adults. CAL is used to determine disease severity—mild (1–2 mm), moderate (3–4 mm), or severe (>5 mm).[32] Routine dental screenings are essential for early detection and timely intervention. Reviewing the patient’s medical history can help identify underlying risk factors. Probing depths greater than 3 mm indicate periodontal disease, while depths exceeding 5 mm often necessitate more intensive treatment, as these areas are more difficult to manage mechanically. Dental x-rays are also used to evaluate the extent of alveolar bone loss.[1] Please see StatPearls' companion resource, "Periodontitis," for more information.
Evaluation
Diagnosis is based on comparing clinical findings with a healthy periodontium, which is characterized by stippled, pale pink gingiva, a 1 to 3 mm sulcus, and no bleeding (see Image. Healthy Periodontium). Signs of periodontal disease include bleeding, pain, bad taste or odor, pocketing, radiographic bone loss, and tooth mobility. Deep periodontal pockets suggest active microbial-host interactions. Without intervention, progressive bone loss can result in increased tooth mobility and eventual tooth loss.[2][3]
Treatment / Management
Management of periodontal disease follows a structured, stepwise approach. Initial therapy includes professional cleaning, scaling, and root planing, as well as educating patients on proper oral hygiene practices. A follow-up evaluation determines the extent of disease resolution. As periodontitis is a chronic condition, ongoing maintenance is critical to prevent recurrence.[1][2][6]
Addressing modifiable risk factors is essential. Poor oral hygiene is a major contributor, and prevention depends on consistent brushing, flossing, rinsing, and routine professional cleanings. Tobacco use significantly increases the risk and severity of periodontal disease, while smoking cessation has been shown to improve treatment outcomes.[10][11][33][34] Patients must be informed about the detrimental effects of smoking on periodontal health and encouraged to quit. Diabetes mellitus exacerbates periodontitis, with poor glycemic control accelerating disease progression. Effective management of diabetes improves the outcomes of periodontal treatment.[7][16][35](B2)
Antibiotics may be administered locally or systemically in cases that do not respond to mechanical therapy. Chlorhexidine gluconate is a commonly used antimicrobial agent that complements mechanical periodontal treatment. This agent is typically administered as a mouth rinse, but can also be applied as a gel, varnish, or subgingival chip. When used alongside regular toothbrushing, chlorhexidine helps reduce plaque buildup, making it highly effective in treating chronic periodontitis. A relatively new advancement in pharmacotherapy for periodontal disease involves the use of a chlorhexidine gluconate chip, which is inserted into the periodontal pocket after cleaning. This chip provides a long-term, sustained release of chlorhexidine gluconate into the affected area, enhancing treatment effectiveness.[36][37][38] (A1)
Another adjunctive antimicrobial treatment involves minocycline hydrochloride microspheres placed into the periodontal pockets after mechanical debridement. Similar to the chlorhexidine chip, minocycline microspheres are effective in reducing dental plaque buildup.[39](A1)
Although rare, systemic antibiotics may be prescribed for persistent deep pockets. Commonly used agents include tetracyclines, penicillins, macrolides, quinolones, cephalosporins, and nitroimidazoles, either alone or in combination.[40][41][42] In severe cases, referral to a periodontist may be necessary for surgical intervention to clean periodontal pockets and restore lost bone and attachment.(B3)
After nonsurgical periodontal treatment, probing depths reduce as healing occurs with the formation of a long junctional epithelium. This healing process involves tissue development that mirrors the histology of the original junctional epithelium. During periodontitis, connective tissue is destroyed, causing the junctional epithelium to elongate until it reaches unaffected connective tissue. This marks the cessation of migration and the formation of the long junctional epithelium.
Differential Diagnosis
When evaluating periodontal disease, it is essential to consider other conditions that may present with similar clinical signs. A thorough differential diagnosis ensures accurate assessment and appropriate management of the underlying cause. Potential differential diagnoses include:
- Periodontal abscesses, which are localized purulent infections found in the periodontium surrounding the tooth.[43][44][45]
- Endodontic lesions, which are localized infections originating from the tooth pulp. These infections can spread through the root tissue into the surrounding periodontium, potentially mimicking periodontal disease.[43][44][45]
- Leukemia may present as gingival enlargement accompanied by bleeding gums, often mimicking periodontal diseases such as gingivitis.[46]
- Certain medications, including calcium channel blockers, immunosuppressants, and anticonvulsants, can cause gingival hyperplasia, which may clinically resemble gingivitis.[47][48]
- Squamous cell carcinoma can cause extensive bone loss and mimic periodontal disease that is refractory to mechanical and pharmacological treatments.[49][50]
Staging
The most recent periodontitis staging guidelines, published in 2017, classify the disease by staging and grading. Staging indicates the severity and extent of the disease, while grading offers insight into the rate of progression and potential risk factors that could impact long-term outcomes.
Staging
Staging reflects the severity of periodontitis and the complexity of its management. This is determined by factors such as CAL, radiographic bone loss, and tooth loss, with the classification based on the site with the most severe bone loss. The stages include:
- Stage I—Initial periodontitis: Bone loss is limited to the coronal third (≤15%).
- Stage II—Moderate periodontitis: Bone loss extends beyond 15% but remains less than 30%.
- Stage III—Severe periodontitis with potential for additional tooth loss: Bone loss reaches the mid-third of the root (30%–60%).
- Stage IV—Severe periodontitis with potential for loss of dentition: Bone loss exceeds 60% and extends to the apical third.
Grading
Grading assesses the risk of disease progression and future tooth loss. This is determined by dividing the percentage of bone loss by the patient’s age.
- Grade A—Low risk of progression: <0.5
- Grade B—Moderate risk of progression: 0.5–1
- Grade C—High risk of progression: >1
A simplified approach to determining grading is as follows:
- Grade C: Bone loss exceeds the patient’s age
- Grade B: Bone loss falls between these ranges
- Grade A: Bone loss is less than half the patient’s age
Periodontal Status
Periodontal status is assessed based on bleeding on probing (BOP), reported as the percentage of bleeding sites, and the presence of BOP in pockets ≥4 mm deep.
- Stable: Probing depths are ≤4 mm, no BOP at 4 mm sites, and <10% of sites exhibit BOP.
- In remission: Probing depths remain ≤4 mm with no BOP at 4 mm sites, but >10% of sites show BOP.
- Unstable: Probing depths are ≥5 mm, or BOP is present in sites measuring 4 mm or deeper.
Prognosis
The prognosis is influenced by the stage of the disease, rate of progression, risk factors, and adherence to treatment. Severe stages and higher grades typically result in worse outcomes. Smoking and uncontrolled diabetes can significantly worsen the prognosis.
McGuire's classification system predicts individual tooth survival by categorizing the prognosis as good, fair, poor, questionable, or hopeless. This classification is based on factors such as patient age, medical status, oral hygiene, socioeconomic factors, bone loss distribution, and the overall periodontal condition.[51][52]
Complications
Tooth loss is one of the most serious consequences of periodontal disease, which occurs as the disease progresses and damages the periodontium that anchors the teeth. Beyond the oral tissues, periodontal disease is also associated with systemic conditions, including diabetes mellitus, cardiovascular disease, and pregnancy complications, such as preterm birth and low-birth-weight infants.[53] Therefore, integrating systemic health considerations into periodontal treatment is essential for optimal patient care.[54]
Patients with diabetes mellitus are at an increased risk of developing periodontal disease. Studies have estimated that patients with type 2 diabetes and severe periodontal disease have a 3.2 times higher mortality risk than those with diabetes, but without periodontal disease. Diabetes impairs wound healing and host responses, while enhanced collagenolytic activity results in an increased breakdown of the periodontium. Additionally, poorly controlled diabetes is associated with an increased severity of these effects. Research indicates that periodontal disease may act as a precursor to insulin resistance, as it is linked to hyperglycemia, impaired glucose tolerance, and poor glycemic control. Additionally, the hyperglycemic state in diabetic individuals may promote the proliferation of bacteria that contribute to periodontal disease.[7][16][24][55][56][57]
Periodontal disease is associated with an increased risk of cardiovascular disease. As mentioned earlier, C-reactive protein (CRP), which is a key biomarker of inflammation, is elevated in patients with periodontal disease. CRP is also linked to cardiovascular disease and related events. Research suggests a direct connection between the bacterial presence in periodontal disease and the development of atherosclerosis. However, a causal relationship has not yet been definitively established.[24][58][59][60][61] Maintaining good oral hygiene may be a preventive measure to reduce the risk of cardiovascular disease.[62]
Chronic periodontal inflammation can exacerbate components of metabolic syndrome, such as insulin resistance, obesity, and hypertension, by promoting systemic inflammation and oxidative stress. Conversely, the presence of metabolic syndrome can contribute to the progression and severity of periodontal disease.[63]
Periodontal disease may contribute to Alzheimer disease through both behavioral and biological mechanisms. While poor oral hygiene in Alzheimer patients is often due to cognitive decline and neglect, emerging evidence suggests that periodontal disease may actively play a role in the development and progression of Alzheimer disease. This is primarily through systemic inflammation, where elevated levels of proinflammatory cytokines (such as CRP, IL-6, IL-1β, and TNF-α) may promote changes in the brain, including β-amyloid accumulation and protein abnormalities.[64]
Additionally, periodontal pathogens such as P gingivalis and Treponema spp. have been detected in brain autopsies of patients with Alzheimer disease, suggesting bacterial migration to the brain, where they may trigger or exacerbate neurodegenerative processes.[64] Animal studies have confirmed these effects, linking oral infections to increased brain inflammation and amyloid buildup.[64]
Another significant complication associated with periodontal disease is the increased risk of preterm low birth weight infants. Research has shown a strong correlation between maternal periodontal disease and reduced infant birth weight. As the severity of periodontal disease increases, from none to severe, infant birth weight tends to decrease, according to prior studies.[65][66][67][68][69]
Deterrence and Patient Education
Deterrence and patient education are essential components in the prevention and management of periodontal disease. Educating patients about the importance of proper oral hygiene, including regular brushing, flossing, and professional cleanings, can significantly reduce the risk of developing gingivitis and periodontitis. Patients should also be informed about modifiable risk factors, including smoking and poor nutrition, to help them understand how lifestyle choices affect periodontal health.
Emphasizing the connection between periodontal disease and systemic conditions, such as cardiovascular disease and diabetes, can further motivate patients to prioritize oral and preventive care. Interdisciplinary healthcare collaboration is crucial in managing conditions such as diabetes mellitus, which are closely linked to periodontal health. By fostering awareness and promoting proactive oral hygiene practices, clinicians can contribute to reducing the prevalence and progression of periodontal disease.
Enhancing Healthcare Team Outcomes
Periodontal disease is the most common oral condition, and many of the risk factors influencing its onset and progression are modifiable. With appropriate guidance and intervention, the interprofessional healthcare team can significantly reduce the risk of developing the disease. Clinicians must understand the impact of these risk factors when assessing patients. General dentists are responsible for screening and providing initial treatment, while referrals to periodontal specialists are essential for managing deep pockets, periodontal damage, and bone defects that require surgical intervention. Effective periodontal treatment can significantly improve patients' perceived quality of life, highlighting the importance of effective periodontal care.[70] Practical risk assessment, timely referrals, and interdisciplinary collaboration among dental hygienists, general dentists, and periodontists are crucial to achieving optimal patient outcomes.
Media
(Click Image to Enlarge)
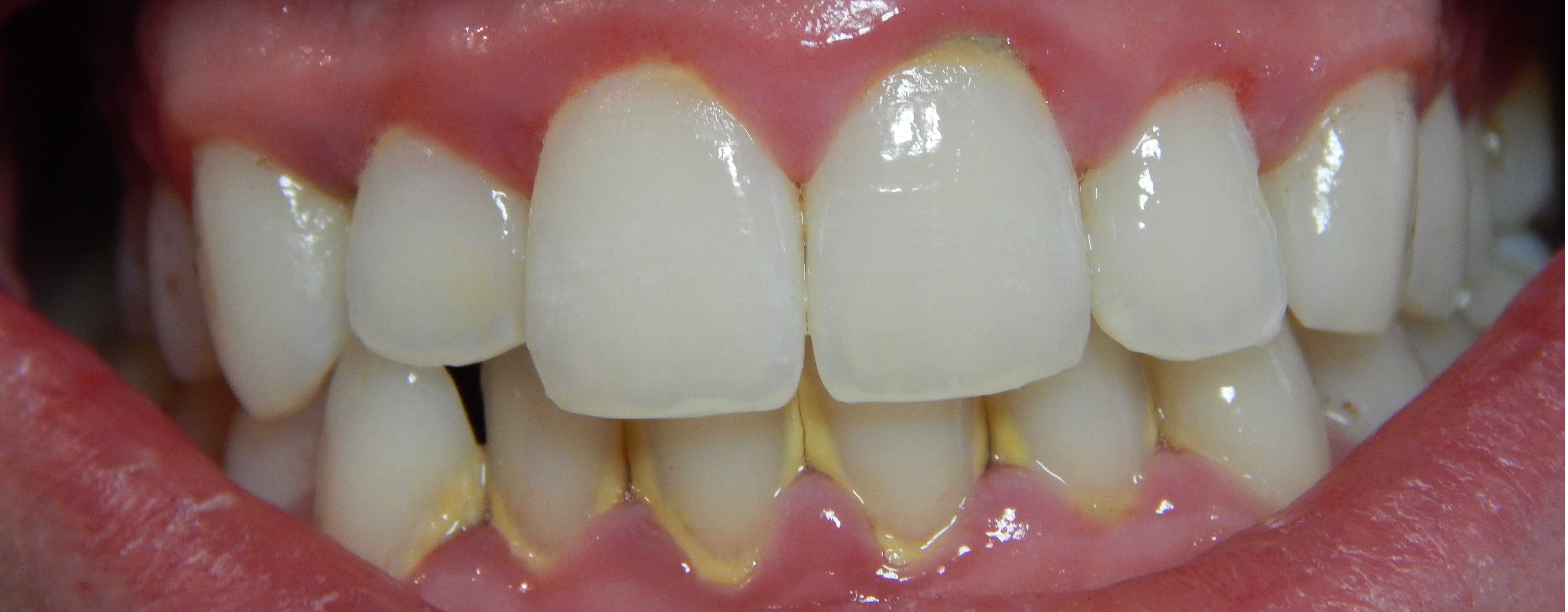
Gingivitis with Calculus Buildup. The image shows calculus accumulation along the gum line, which leads to erythema and inflammation in the marginal gingiva and interdental papillae.
Onetimeuseaccount, Public Domain, via Wikimedia Commons
(Click Image to Enlarge)
(Click Image to Enlarge)
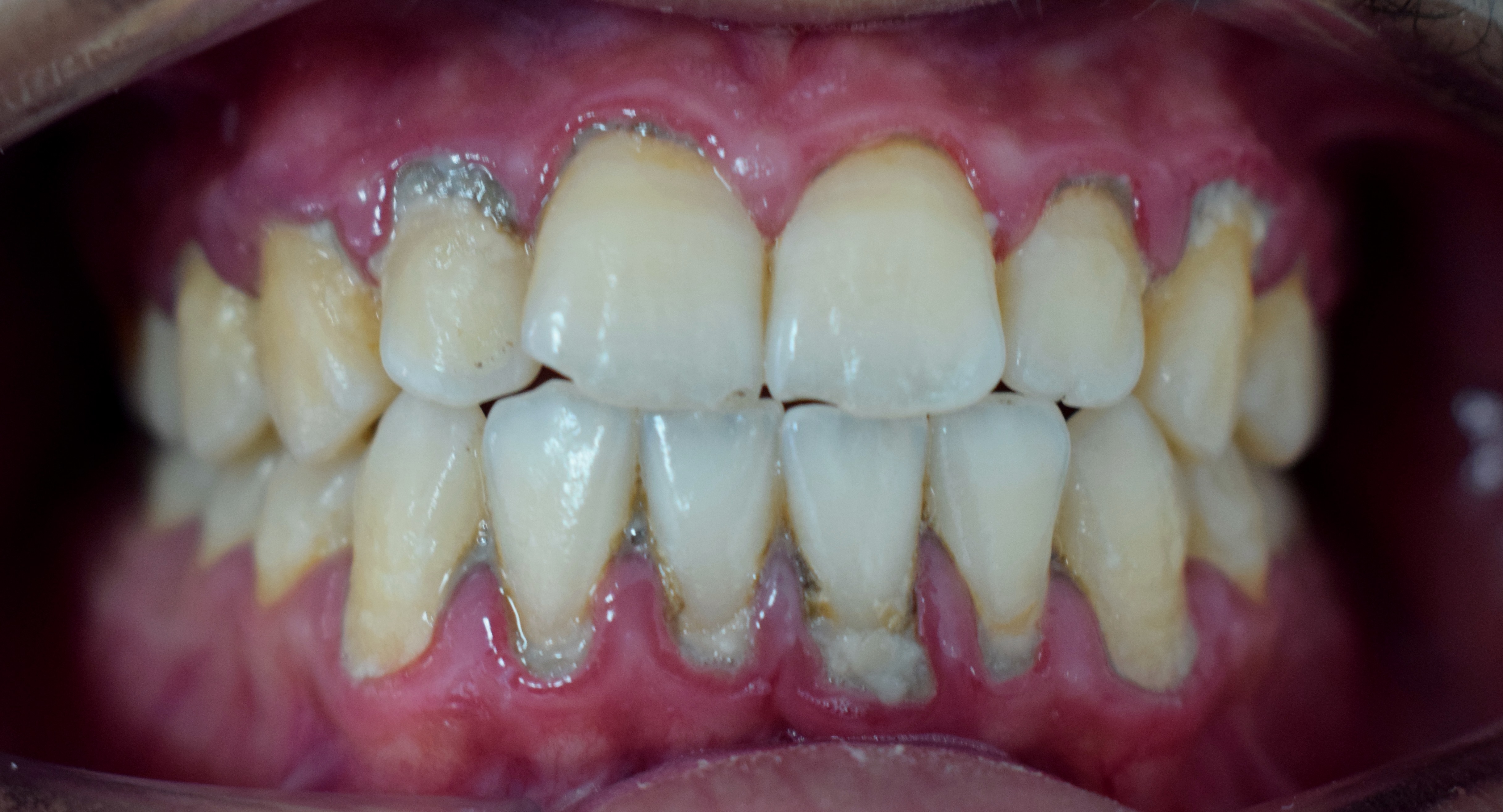
Periodontitis. The image demonstrates loss of attachment and gingival recession, characteristic features of periodontitis.
Shaimaa Abdellatif, Public Domain, via Wikimedia Commons
References
Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet (London, England). 2005 Nov 19:366(9499):1809-20 [PubMed PMID: 16298220]
Kinane DF, Stathopoulou PG, Papapanou PN. Periodontal diseases. Nature reviews. Disease primers. 2017 Jun 22:3():17038. doi: 10.1038/nrdp.2017.38. Epub 2017 Jun 22 [PubMed PMID: 28805207]
Highfield J. Diagnosis and classification of periodontal disease. Australian dental journal. 2009 Sep:54 Suppl 1():S11-26. doi: 10.1111/j.1834-7819.2009.01140.x. Epub [PubMed PMID: 19737262]
Babay N, Alshehri F, Al Rowis R. Majors highlights of the new 2017 classification of periodontal and peri-implant diseases and conditions. The Saudi dental journal. 2019 Jul:31(3):303-305. doi: 10.1016/j.sdentj.2019.04.006. Epub 2019 Apr 23 [PubMed PMID: 31337931]
Todescan S, Nizar R. Managing patients with necrotizing ulcerative periodontitis. Journal (Canadian Dental Association). 2013:79():d44 [PubMed PMID: 23763731]
Ridgeway EE. Periodontal disease: diagnosis and management. Journal of the American Academy of Nurse Practitioners. 2000 Mar:12(3):79-84 [PubMed PMID: 11033686]
Nazir MA. Prevalence of periodontal disease, its association with systemic diseases and prevention. International journal of health sciences. 2017 Apr-Jun:11(2):72-80 [PubMed PMID: 28539867]
Albandar JM. Global risk factors and risk indicators for periodontal diseases. Periodontology 2000. 2002:29():177-206 [PubMed PMID: 12102708]
Zee KY. Smoking and periodontal disease. Australian dental journal. 2009 Sep:54 Suppl 1():S44-50. doi: 10.1111/j.1834-7819.2009.01142.x. Epub [PubMed PMID: 19737267]
Bergström J. Tobacco smoking and chronic destructive periodontal disease. Odontology. 2004 Sep:92(1):1-8 [PubMed PMID: 15490298]
Hilgers KK, Kinane DF. Smoking, periodontal disease and the role of the dental profession. International journal of dental hygiene. 2004 May:2(2):56-63 [PubMed PMID: 16451463]
Brothwell DJ. Should the use of smoking cessation products be promoted by dental offices? An evidence-based report. Journal (Canadian Dental Association). 2001 Mar:67(3):149-55 [PubMed PMID: 11282035]
Grossi SG, Skrepcinski FB, DeCaro T, Zambon JJ, Cummins D, Genco RJ. Response to periodontal therapy in diabetics and smokers. Journal of periodontology. 1996 Oct:67(10 Suppl):1094-102 [PubMed PMID: 8910828]
Feldman RS, Bravacos JS, Rose CL. Association between smoking different tobacco products and periodontal disease indexes. Journal of periodontology. 1983 Aug:54(8):481-7 [PubMed PMID: 6578319]
Grossi SG, Zambon JJ, Ho AW, Koch G, Dunford RG, Machtei EE, Norderyd OM, Genco RJ. Assessment of risk for periodontal disease. I. Risk indicators for attachment loss. Journal of periodontology. 1994 Mar:65(3):260-7 [PubMed PMID: 8164120]
Level 2 (mid-level) evidenceDouglass CW. Risk assessment and management of periodontal disease. Journal of the American Dental Association (1939). 2006 Nov:137 Suppl():27S-32S [PubMed PMID: 17035673]
Daalderop LA, Wieland BV, Tomsin K, Reyes L, Kramer BW, Vanterpool SF, Been JV. Periodontal Disease and Pregnancy Outcomes: Overview of Systematic Reviews. JDR clinical and translational research. 2018 Jan:3(1):10-27. doi: 10.1177/2380084417731097. Epub 2017 Sep 25 [PubMed PMID: 30370334]
Level 3 (low-level) evidenceUwitonze AM, Uwambaye P, Isyagi M, Mumena CH, Hudder A, Haq A, Nessa K, Razzaque MS. Periodontal diseases and adverse pregnancy outcomes: Is there a role for vitamin D? The Journal of steroid biochemistry and molecular biology. 2018 Jun:180():65-72. doi: 10.1016/j.jsbmb.2018.01.010. Epub 2018 Jan 16 [PubMed PMID: 29341890]
Carrillo-de-Albornoz A, Figuero E, Herrera D, Bascones-Martínez A. Gingival changes during pregnancy: II. Influence of hormonal variations on the subgingival biofilm. Journal of clinical periodontology. 2010 Mar:37(3):230-40. doi: 10.1111/j.1600-051X.2009.01514.x. Epub 2010 Jan 19 [PubMed PMID: 20088983]
Level 2 (mid-level) evidenceWu M, Chen SW, Jiang SY. Relationship between gingival inflammation and pregnancy. Mediators of inflammation. 2015:2015():623427. doi: 10.1155/2015/623427. Epub 2015 Mar 22 [PubMed PMID: 25873767]
Persson GR. Periodontal complications with age. Periodontology 2000. 2018 Oct:78(1):185-194. doi: 10.1111/prd.12227. Epub [PubMed PMID: 30198125]
Grodstein F, Colditz GA, Stampfer MJ. Post-menopausal hormone use and tooth loss: a prospective study. Journal of the American Dental Association (1939). 1996 Mar:127(3):370-7, quiz 392 [PubMed PMID: 8819784]
Level 2 (mid-level) evidenceRheu GB, Ji S, Ryu JJ, Lee JB, Shin C, Lee JY, Huh JB, Shin SW. Risk assessment for clinical attachment loss of periodontal tissue in Korean adults. The journal of advanced prosthodontics. 2011 Mar:3(1):25-32. doi: 10.4047/jap.2011.3.1.25. Epub 2011 Mar 31 [PubMed PMID: 21503190]
Borgnakke WS. Does Treatment of Periodontal Disease Influence Systemic Disease? Dental clinics of North America. 2015 Oct:59(4):885-917. doi: 10.1016/j.cden.2015.06.007. Epub [PubMed PMID: 26427573]
Kim J, Amar S. Periodontal disease and systemic conditions: a bidirectional relationship. Odontology. 2006 Sep:94(1):10-21 [PubMed PMID: 16998613]
Nualart Grollmus ZC, Morales Chávez MC, Silvestre Donat FJ. Periodontal disease associated to systemic genetic disorders. Medicina oral, patologia oral y cirugia bucal. 2007 May 1:12(3):E211-5 [PubMed PMID: 17468717]
Borrell LN, Beck JD, Heiss G. Socioeconomic disadvantage and periodontal disease: the Dental Atherosclerosis Risk in Communities study. American journal of public health. 2006 Feb:96(2):332-9 [PubMed PMID: 16380570]
Level 2 (mid-level) evidenceSmalley JW. Pathogenic mechanisms in periodontal disease. Advances in dental research. 1994 Jul:8(2):320-8 [PubMed PMID: 7865093]
Level 3 (low-level) evidenceEggert FM, McLeod MH, Flowerdew G. Effects of smoking and treatment status on periodontal bacteria: evidence that smoking influences control of periodontal bacteria at the mucosal surface of the gingival crevice. Journal of periodontology. 2001 Sep:72(9):1210-20 [PubMed PMID: 11577953]
Cekici A, Kantarci A, Hasturk H, Van Dyke TE. Inflammatory and immune pathways in the pathogenesis of periodontal disease. Periodontology 2000. 2014 Feb:64(1):57-80. doi: 10.1111/prd.12002. Epub [PubMed PMID: 24320956]
Kang W, Hu Z, Ge S. Healthy and Inflamed Gingival Fibroblasts Differ in Their Inflammatory Response to Porphyromonas gingivalis Lipopolysaccharide. Inflammation. 2016 Oct:39(5):1842-52. doi: 10.1007/s10753-016-0421-4. Epub [PubMed PMID: 27525424]
Wiebe CB, Putnins EE. The periodontal disease classification system of the American Academy of Periodontology--an update. Journal (Canadian Dental Association). 2000 Dec:66(11):594-7 [PubMed PMID: 11253351]
Haber J, Kent RL. Cigarette smoking in a periodontal practice. Journal of periodontology. 1992 Feb:63(2):100-6 [PubMed PMID: 1552463]
Level 2 (mid-level) evidenceHaber J. Cigarette smoking: a major risk factor for periodontitis. Compendium (Newtown, Pa.). 1994 Aug:15(8):1002, 1004-8 passim; quiz 1014 [PubMed PMID: 7987894]
Costa FO, Miranda Cota LO, Pereira Lages EJ, Soares Dutra Oliveira AM, Dutra Oliveira PA, Cyrino RM, Medeiros Lorentz TC, Cortelli SC, Cortelli JR. Progression of periodontitis and tooth loss associated with glycemic control in individuals undergoing periodontal maintenance therapy: a 5-year follow-up study. Journal of periodontology. 2013 May:84(5):595-605. doi: 10.1902/jop.2012.120255. Epub 2012 Jul 6 [PubMed PMID: 22769441]
Level 2 (mid-level) evidencePietruska M, Paniczko A, Waszkiel D, Pietruski J, Bernaczyk A. Efficacy of local treatment with chlorhexidine gluconate drugs on the clinical status of periodontium in chronic periodontitis patients. Advances in medical sciences. 2006:51 Suppl 1():162-5 [PubMed PMID: 17458083]
Level 1 (high-level) evidenceKumar AJ, Ramesh Reddy BV, Chava VK. Effect of chlorhexidine chip in the treatment of chronic periodontitis. Journal of natural science, biology, and medicine. 2014 Jul:5(2):268-72. doi: 10.4103/0976-9668.136159. Epub [PubMed PMID: 25097396]
Jhinger N, Kapoor D, Jain R. Comparison of Periochip (chlorhexidine gluconate 2.5 mg) and Arestin (Minocycline hydrochloride 1 mg) in the management of chronic periodontitis. Indian journal of dentistry. 2015 Jan-Mar:6(1):20-6. doi: 10.4103/0975-962X.151697. Epub [PubMed PMID: 25767356]
Lu HK, Chei CJ. Efficacy of subgingivally applied minocycline in the treatment of chronic periodontitis. Journal of periodontal research. 2005 Feb:40(1):20-7 [PubMed PMID: 15613075]
Level 1 (high-level) evidenceBlair FM, Chapple IL. Prescribing for periodontal disease. Primary dental journal. 2014 Nov:3(4):38-43. doi: 10.1308/205016814813877234. Epub [PubMed PMID: 25668374]
Leszczyńska A, Buczko P, Buczko W, Pietruska M. Periodontal pharmacotherapy - an updated review. Advances in medical sciences. 2011:56(2):123-31. doi: 10.2478/v10039-011-0044-9. Epub [PubMed PMID: 22112427]
Level 3 (low-level) evidenceBarca E, Cifcibasi E, Cintan S. Adjunctive use of antibiotics in periodontal therapy. Journal of Istanbul University Faculty of Dentistry. 2015:49(3):55-62. doi: 10.17096/jiufd.90144. Epub 2015 Jan 12 [PubMed PMID: 28955547]
Singh P. Endo-perio dilemma: a brief review. Dental research journal. 2011 Winter:8(1):39-47 [PubMed PMID: 22132014]
Al-Fouzan KS. A new classification of endodontic-periodontal lesions. International journal of dentistry. 2014:2014():919173. doi: 10.1155/2014/919173. Epub 2014 Apr 14 [PubMed PMID: 24829580]
Herrera D, Alonso B, de Arriba L, Santa Cruz I, Serrano C, Sanz M. Acute periodontal lesions. Periodontology 2000. 2014 Jun:65(1):149-77. doi: 10.1111/prd.12022. Epub [PubMed PMID: 24738591]
Lim HC, Kim CS. Oral signs of acute leukemia for early detection. Journal of periodontal & implant science. 2014 Dec:44(6):293-9. doi: 10.5051/jpis.2014.44.6.293. Epub 2014 Dec 31 [PubMed PMID: 25568810]
Level 3 (low-level) evidenceNyska A, Shemesh M, Tal H, Dayan D. Gingival hyperplasia induced by calcium channel blockers: mode of action. Medical hypotheses. 1994 Aug:43(2):115-8 [PubMed PMID: 7990738]
Level 3 (low-level) evidenceUmeizudike KA, Olawuyi AB, Umeizudike TI, Olusegun-Joseph AD, Bello BT. Effect of Calcium Channel Blockers on Gingival Tissues in Hypertensive Patients in Lagos, Nigeria: A Pilot Study. Contemporary clinical dentistry. 2017 Oct-Dec:8(4):565-570. doi: 10.4103/ccd.ccd_536_17. Epub [PubMed PMID: 29326507]
Level 3 (low-level) evidenceBornstein MM, Andreoni C, Meier T, Leung YY. Squamous Cell Carcinoma of the Gingiva Mimicking Periodontal Disease: A Diagnostic Challenge and Therapeutic Dilemma. The International journal of periodontics & restorative dentistry. 2018 Mar/Apr:38(2):253-259. doi: 10.11607/prd.3253. Epub [PubMed PMID: 29447319]
Gupta R, Debnath N, Nayak PA, Khandelwal V. Gingival squamous cell carcinoma presenting as periodontal lesion in the mandibular posterior region. BMJ case reports. 2014 Aug 19:2014():. doi: 10.1136/bcr-2013-202511. Epub 2014 Aug 19 [PubMed PMID: 25139914]
Level 3 (low-level) evidenceMartinez-Canut P, Llobell A. A comprehensive approach to assigning periodontal prognosis. Journal of clinical periodontology. 2018 Apr:45(4):431-439. doi: 10.1111/jcpe.12857. Epub 2018 Feb 28 [PubMed PMID: 29247450]
Ioannou AL, Kotsakis GA, Hinrichs JE. Prognostic factors in periodontal therapy and their association with treatment outcomes. World journal of clinical cases. 2014 Dec 16:2(12):822-7. doi: 10.12998/wjcc.v2.i12.822. Epub [PubMed PMID: 25516855]
Level 3 (low-level) evidenceMawardi HH, Elbadawi LS, Sonis ST. Current understanding of the relationship between periodontal and systemic diseases. Saudi medical journal. 2015 Feb:36(2):150-8. doi: 10.15537/smj.2015.2.9424. Epub [PubMed PMID: 25719577]
Level 3 (low-level) evidenceKwon T, Lamster IB, Levin L. Current Concepts in the Management of Periodontitis. International dental journal. 2021 Dec:71(6):462-476. doi: 10.1111/idj.12630. Epub 2021 Feb 19 [PubMed PMID: 34839889]
Kidambi S, Patel SB. Diabetes mellitus: considerations for dentistry. Journal of the American Dental Association (1939). 2008 Oct:139 Suppl():8S-18S [PubMed PMID: 18809649]
Kumar M, Mishra L, Mohanty R, Nayak R. "Diabetes and gum disease: the diabolic duo". Diabetes & metabolic syndrome. 2014 Oct-Dec:8(4):255-8. doi: 10.1016/j.dsx.2014.09.022. Epub 2014 Oct 13 [PubMed PMID: 25450824]
Ship JA. Diabetes and oral health: an overview. Journal of the American Dental Association (1939). 2003 Oct:134 Spec No():4S-10S [PubMed PMID: 18196667]
Level 3 (low-level) evidenceKodovazenitis G, Pitsavos C, Papadimitriou L, Deliargyris EN, Vrotsos I, Stefanadis C, Madianos PN. Periodontal disease is associated with higher levels of C-reactive protein in non-diabetic, non-smoking acute myocardial infarction patients. Journal of dentistry. 2011 Dec:39(12):849-54. doi: 10.1016/j.jdent.2011.09.005. Epub 2011 Sep 17 [PubMed PMID: 21946158]
Desvarieux M, Demmer RT, Rundek T, Boden-Albala B, Jacobs DR Jr, Sacco RL, Papapanou PN. Periodontal microbiota and carotid intima-media thickness: the Oral Infections and Vascular Disease Epidemiology Study (INVEST). Circulation. 2005 Feb 8:111(5):576-82 [PubMed PMID: 15699278]
Level 2 (mid-level) evidenceJoshipura K, Ritchie C, Douglass C. Strength of evidence linking oral conditions and systemic disease. Compendium of continuing education in dentistry. (Jamesburg, N.J. : 1995). Supplement. 2000:(30):12-23; quiz 65 [PubMed PMID: 11908384]
Schure R, Costa KD, Rezaei R, Lee W, Laschinger C, Tenenbaum HC, McCulloch CA. Impact of matrix metalloproteinases on inhibition of mineralization by fetuin. Journal of periodontal research. 2013 Jun:48(3):357-66. doi: 10.1111/jre.12015. Epub 2012 Oct 12 [PubMed PMID: 23058002]
Etta I, Kambham S, Girigosavi KB, Panjiyar BK. Mouth-Heart Connection: A Systematic Review on the Impact of Periodontal Disease on Cardiovascular Health. Cureus. 2023 Oct:15(10):e46585. doi: 10.7759/cureus.46585. Epub 2023 Oct 6 [PubMed PMID: 37933364]
Level 1 (high-level) evidenceAizenbud I, Wilensky A, Almoznino G. Periodontal Disease and Its Association with Metabolic Syndrome-A Comprehensive Review. International journal of molecular sciences. 2023 Aug 21:24(16):. doi: 10.3390/ijms241613011. Epub 2023 Aug 21 [PubMed PMID: 37629193]
Sedghi LM, Bacino M, Kapila YL. Periodontal Disease: The Good, The Bad, and The Unknown. Frontiers in cellular and infection microbiology. 2021:11():766944. doi: 10.3389/fcimb.2021.766944. Epub 2021 Dec 7 [PubMed PMID: 34950607]
López NJ, Da Silva I, Ipinza J, Gutiérrez J. Periodontal Therapy Reduces the Rate of Preterm Low Birth Weight in Women With Pregnancy-Associated Gingivitis. Journal of periodontology. 2005 Nov:76 Suppl 11S():2144-2153. doi: 10.1902/jop.2005.76.11-S.2144. Epub [PubMed PMID: 29539053]
Offenbacher S, Lin D, Strauss R, McKaig R, Irving J, Barros SP, Moss K, Barrow DA, Hefti A, Beck JD. Effects of periodontal therapy during pregnancy on periodontal status, biologic parameters, and pregnancy outcomes: a pilot study. Journal of periodontology. 2006 Dec:77(12):2011-24 [PubMed PMID: 17209786]
Level 3 (low-level) evidencePiscoya MD, Ximenes RA, Silva GM, Jamelli SR, Coutinho SB. Periodontitis-associated risk factors in pregnant women. Clinics (Sao Paulo, Brazil). 2012:67(1):27-33 [PubMed PMID: 22249477]
Level 2 (mid-level) evidenceOffenbacher S, Boggess KA, Murtha AP, Jared HL, Lieff S, McKaig RG, Mauriello SM, Moss KL, Beck JD. Progressive periodontal disease and risk of very preterm delivery. Obstetrics and gynecology. 2006 Jan:107(1):29-36 [PubMed PMID: 16394036]
Level 2 (mid-level) evidenceTurton M, Africa CWJ. Further evidence for periodontal disease as a risk indicator for adverse pregnancy outcomes. International dental journal. 2017 Jun:67(3):148-156. doi: 10.1111/idj.12274. Epub 2016 Dec 17 [PubMed PMID: 27988930]
Wong LB, Yap AU, Allen PF. Periodontal disease and quality of life: Umbrella review of systematic reviews. Journal of periodontal research. 2021 Jan:56(1):1-17. doi: 10.1111/jre.12805. Epub 2020 Sep 23 [PubMed PMID: 32965050]
Level 1 (high-level) evidence