Introduction
The pericardium is a fibroelastic sac that surrounds the heart and is composed of 2 layers, visceral and parietal, that are separated by a "potential" space. Within this potential space, it is normal to have 15 to 50 mL of fluid to serve the purpose of lubrication. The term acute pericarditis refers to inflammation of this fibroelastic sac. The causes of pericarditis are wide-ranging, including infection, autoimmune processes, malignancy, and uremia. As noted by William Osler in 1892, pathologic pericarditis can also be present in the absence of clinical symptoms.[1] Please see StatPearls companion reference, "Pericarditis," for further information.
Uremic pericarditis is an uncommon but significant complication of end-stage renal disease (ESRD). Richard Bright first described this condition in 1836. Uremic pericarditis was more common in the early days of dialysis; however, more recently, it has become an uncommon complication of ESRD, likely due to closer monitoring of patients for uremic symptoms and more efficient hemodialysis therapy. Currently, uremic pericarditis has become increasingly observed in patients with ESRD who are either not yet on dialysis or are inadequately dialyzed.[2][3] In the past, it was thought that a viral illness could cause uremic pericarditis. However, the exact pathophysiology is not fully understood.[4] Current theories suggest that uremic toxins and proinflammatory states contribute to pericardial changes.
Uremic pericarditis typically occurs in patients with ESRD and patients with severe azotemia (elevated blood urea nitrogen), typically above 60 mg/dL. Clinical features of uremic pericarditis include chest pain, particularly in the recumbent position, a pericardial rub that is often audible, and, in severe cases, cardiac tamponade may be present. Initial investigations for uremic pericarditis include an echocardiogram and an electrocardiogram, which typically shows diffuse ST and T-wave elevations. Treating this condition involves lowering blood urea nitrogen through initiating or increasing dialysis.[4][5]
Some clinicians further distinguish between uremic and dialysis-related pericarditis. Uremic pericarditis can be defined as occurring prior to or within 8 weeks of initiation of renal replacement therapy (RRT). Dialysis-related pericarditis occurs with clinical symptoms appearing more than 8 weeks after RRT. Eight weeks is considered the time in which RRT should clear uremic toxins and excess fluid; however, many patients presenting with dialysis-related pericarditis have missed sessions or underdialysis prior to clinical symptoms. Up to 12% of patients with dialysis-related pericarditis may progress to constrictive pericarditis.[6][7]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Patients with ESRD typically have a fluid and electrolyte balance defect leading to an accumulation of toxic metabolites such as nitrogenous wastes. Study results have shown that the combination of toxic metabolite accumulation, uremic toxins, fluid overload, and electrolyte derangement contributes to the pathogenesis of uremic pericarditis.[8] Some study results have also demonstrated that an increase in nitrogen waste products has a proinflammatory effect, leading to pericarditis; other results have concluded that the changes in acid-base homeostasis, hypercalcemia, and hyperuricemia are all implicated in the development of uremic pericarditis.[5] However, most recent studies and the fact that increased dialysis improves most cases support the theory of uremic toxins causing the pericarditis. The levels of blood urea nitrogen and creatine are not definitively linked to the development of uremic pericarditis, so it is likely that an unidentified or a combination of uremic toxins causes this condition.
For patients on dialysis with stable ESRD, missed or insufficient treatments—often due to vascular access issues or increased catabolic burden from acute illness—can cause pericarditis. Factors associated with more severe pericardial effusion in chronic kidney disease and ESRD include hypoalbuminemia and leukocytosis, highlighting the roles of inflammation and malnutrition in the pathogenesis.[3][9][10] Patients with ESRD also have an increased risk of bleeding, and hemopericardium is associated with constrictive pericarditis.[3]
Epidemiology
The actual incidence of uremic pericarditis is difficult to ascertain due to the variability of symptoms and diagnostic criteria. Historically, the prevalence used to range between 3% and 41%. However, recent data confirm a substantial decline. Results from recent studies showed that the prevalence has been less than 5% in recent decades due to improvements in hemodialysis therapy, earlier initiation of renal replacement therapy, and more efficient dialyzers.[1][3][6][11][12]
Pathophysiology
The pathophysiology of uremic pericarditis is primarily related to the accumulation of toxic metabolites and nitrogenous waste in the blood, triggering a systemic and local pro-inflammatory state. Research results indicate that these toxic metabolites affect the pericardium by inducing the release of proinflammatory markers such as interleukin 1, interleukin 6, and tumor necrosis factor. These mediators promote pericardial inflammation, activate fibroblasts, and contribute to fibrosis, ultimately resulting in pericardial damage. In severe cases, effusions can also occur in conjunction with uremic pericarditis, which can be quantified further into serous versus hemorrhagic; the cause of these pericardial effusions is multifactorial and is partially related to platelet dysfunction in renal failure patients.[10][13][14][15][16]
The risk of cardiac abnormalities has been historically associated with renal impairment across several studies. The relationship between renal dysfunction and cardiovascular disease has been defined as reciprocal or bidirectional.[17] High albuminuria in chronic kidney disease is strongly associated with systemic inflammation, fibrinolysis, endothelial dysfunction, and dyslipidemia. Also, albuminuria correlates with elevated inflammatory markers such as C-reactive protein and the neutrophil-to-lymphocyte ratio.[18][19] Excess urinary albumin excretion, aggravating endothelial permeability, may have a role in the pathogenesis of pericarditis.[12] Patients on dialysis have high levels of free radicals, indicating uremia is a pro-oxidant state, but dialysis is not entirely effective in correcting oxidative stress.[13] The causal association between oxidative stress and cardiovascular disease in patients with ESRD has not been firmly established.[13]
Histopathology
Normal pericardium tissue is composed of 3 layers: 1) the serosa, which is made of a surface layer of mesothelial cells associated with a small submesothelial space, 2) the fibrosa, containing collagen and elastin fibrils, and 3) the epicardium, also called the serous or visceral pericardium, is the closest layer to the heart and is comprised of connective tissue containing large collagen bundles joining the pericardiosternal ligament. Uremia causes fibrinous pericardial disease, although studies have shown that it is likely not the blood urea nitrogen and creatinine that cause the pericarditis, but another uremic toxin. A normal amount of pericardial fluid is usually 15 to 50 mL of plasma ultrafiltrate. Pericardial fluid is generally increased in most patients with renal failure; however, this does not cause uremic pericarditis unless fibrinous changes occur.[1][20]
The exudative fibrinous changes have been described as having a "bread and butter" appearance, although this is not specific, as it is common with other forms of exudative pericarditis. The thickening of the pericardium characterizing uremic pericarditis can involve the visceral, serosal, or both layers. Adhesions may also be present between the 2 layers. Multiple adhesions can eliminate the pericardial space, leading to "obliterative pericarditis."[1]
History and Physical
The most common presentation of uremic pericarditis is chest pain in patients with advanced chronic kidney disease or ESRD on dialysis. The chest pain is typically pleuritic in nature, may improve when the patient leans forward, and is often accompanied by dyspnea. Fever and other systemic symptoms are less prominent in uremic pericarditis than in infectious or idiopathic cases. Dyspnea and fatigue are common, especially with moderate or severe pericardial effusion.
Many patients will present similarly to a myocardial infarction patient, so it is essential to rule out an ischemic event.[9][21] Hypertension may be present related to renal disease and volume overload, in contrast to the usual hypotension observed with other pericarditis etiologies. One study of hemodynamic parameters results showed that patients with uremic pericarditis had higher blood pressure, cardiac output, total peripheral resistance, and plasma volume compared to those with signs of cardiac congestion.[6] Hypervolemia and hypotension despite adequate dialysis may also indicate constrictive pericarditis, although this is rare.[3]
Physical examination often reveals a pericardial friction rub, which is typically scratchy and squeaky, and is best appreciated on the left sternal border when the patient leans forward and holds their breath. According to results from several studies, pericardial friction rub is the most consistent physical finding in uremic pericarditis; however, it can sometimes be transient.[22] The rub has 3 components:
- Atrial systole
- Ventricular systole
- Rapid ventricular filling during diastole
Patients may also have other signs and symptoms of uremia, including the following:
- Neurologic: Encephalopathy, confusion, fatigue, memory deficiency, seizure, weakness, myoclonic jerks, muscle cramps, or asterixis
- Pulmonary: Cough, dyspnea, shortness of breath, decreased breath sounds at the bases, rales
- Gastrointestinal: Anorexia, nausea, vomiting
- Urinary: Urinary hesitation, decreased urination
- Hematologic: Easy bruising, petechiae, pallor
- Dermatologic: Uremic frost, dry skin, hair/nail changes [23]
Evaluation
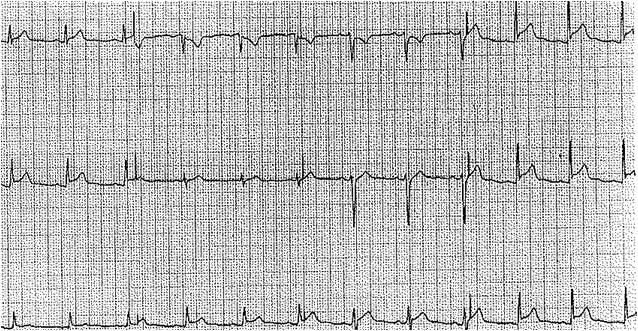
An electrocardiogram (ECG) is one of the most important diagnostic tools to evaluate a patient with suspected uremic pericarditis. The most characteristic ECG findings in acute pericarditis are widespread concave ST-segment elevation and PR-segment depression. Compared to an ST-segment elevation myocardial infarction, the ST and T-wave findings in pericarditis are typically diffuse and not localized to a coronary artery territory. Normalization of the ECG findings may be observed in the later stage of the disease course. Notably, fewer than 60% of patients will present with the classic ECG findings, and some patients may show no changes on ECG.[21][24][25] See Image. Acute Pericarditis Findings, Electrocardiograph. Further, cardiac biomarkers may also be elevated, ie, troponins, but are not necessary to diagnose. This can be complicated by the fact that tropins are often mildly elevated with advanced renal failure.[9]
A chest x-ray can reveal cardiomegaly, blunting of the costophrenic angle, and decreased retrocardial space on the lateral image.[20] See Image. Uremic Effusion, Radiograph. Other imaging options include cardiac magnetic resonance imaging and computed tomography (CT), which can be used to diagnose pericarditis.[26] However, gadolinium should be avoided in patients with advanced renal disease due to the risk of nephrogenic systemic fibrosis. A high CT attenuation indicates an exudative pericardial effusion and correlates with the pericardial fluid's protein, albumin, white blood cell (WBC) count, lactate dehydrogenase, protein-to-albumin ratio, and albumin gradient.[27]
An echocardiogram plays a key role in confirming or further evaluating the severity of uremic pericarditis. Typical findings include a pericardial effusion, often with evidence of atrial and ventricular restriction. Although laboratory tests and imaging studies provide supportive information, the diagnosis of pericarditis remains primarily clinical. These diagnostic tools may aid in assessment, but are not always required to establish the diagnosis.[6]
Routine blood work includes WBC count with differential count, inflammatory markers, renal and liver function tests, and cardiac enzymes.[28] Elevated erythrocyte sedimentation rate and C-reactive protein levels are commonly observed in patients with pericarditis.[29] One review found leukocytosis present in 40% to 60% of patients with uremic pericarditis.[20] A pericardial biopsy is not routinely performed; however, when indicated, the biopsy may reveal non-necrotizing or necrotizing fibrinous pericarditis in cases of constrictive pericarditis, or it may demonstrate normal histologic findings.[30] If fluid is retrieved from the pericardial effusion, it is exudative with high protein and mononuclear cells.[7][22]
Treatment / Management
Initial treatment for uremic pericarditis is to start hemodialysis in patients who are not on renal replacement therapy or intensify dialysis for patients already on dialysis. Hemodialysis removes uremic toxins faster than peritoneal dialysis. Recent literature supports this approach, which emphasizes that uremic pericarditis is a direct indication for urgent hemodialysis. The American College of Cardiology and the American Heart Association also recognize pericarditis as a formal indication for urgent dialysis initiation in advanced azotemia.[31][32][33] One regimen for intensified hemodialysis is to perform 4 hours of hemodialysis for 4 hours 6 to 7 times per week for 7 to 14 days. Monitoring progress through echocardiography and clinical symptoms can help guide the dialysis prescription.[7](A1)
The guidelines recommend anti-inflammatory agents and steroids for patients who fail to respond adequately to hemodialysis, but colchicine is often avoided in severe renal impairment.[28] Anti-inflammatories such as indomethacin may provide some relief for pain but are not successful in eliminating uremic pericarditis. The use of steroids has been controversial, as they can decrease inflammation. Still, they can also increase the risk of recurrence along with potential adverse effects such as hyperglycemia, the risk of osteoporosis, and neurologic effects in older adults.
Low-dose corticosteroids can be used in patients who cannot take nonsteroidal anti-inflammatories.[34] Intrapericardial steroid injections have also been studied, but this approach is rarely used due to the risk of hemothorax, infection, pneumothorax, cardiac arrhythmia, and pneumopericardium. A recent clinical trial demonstrated the effectiveness of interleukin-1 blockers (anakinra, rilonacept) in treating colchicine-resistant or corticosteroid-dependent recurrent pericarditis.[35] If dialysis treatment fails, pericardiocentesis is recommended within 7 to 14 days in patients with uremic pericarditis with effusion. In patients with severe uremic pericarditis and effusion leading to cardiac tamponade, emergent pericardiocentesis is recommended.(B2)
Pericardiectomy is typically not the first-line management option and is only used for recurrent pericarditis with pericardial effusions. The recommendation is to undergo an echocardiogram every 3 to 5 days during the acute event to monitor the resolution of pericarditis and effusion.[36] Rarely, a pericardial window is needed for recurrent effusions, but this is increasingly uncommon in areas with adequate access to dialysis.
Differential Diagnosis
The differential diagnosis for uremic pericarditis is broad. Pericarditis has multiple etiologies, which must be ruled out before a diagnosis can be made. Although uremic pericarditis is the most common form of pericarditis in end-stage renal disease and dialysis patients, others causes must be considered.
Other causes include the following:
- Infectious (viral, bacterial, fungal)
- Inflammatory (systemic lupus erythematosus, scleroderma, vasculitis)
- Metabolic (hypothyroidism)
- Neoplastic (primary or metastatic)
- Trauma (blunt or penetrating)
- Cardiac (postpericardiotomy syndrome, myocardial infarction, aortic dissection)
- Medication-induced (ie, hydralazine, methyldopa, procainamide, minoxidil) [3]
Prognosis
Overall, the resolution of uremic pericarditis is excellent. Recent literature confirms that uremic pericarditis is not that common due to widespread dialysis, but when it occurs, the prognosis is generally favorable with appropriate management. Resolution of the disease is achieved in the majority of cases with a success rate approaching 100% following intensified dialysis and, when indicated, pericardial drainage for large or symptomatic effusions. Even with advanced treatment, mortality and morbidity are not negligible. Recent data showed that one-year survival in uremic pericarditis is approximately 86% especially in patients with advanced kidney disease and other comorbidities. Deaths are primarily related to underlying renal or cardiovascular disease rather than pericarditis itself.[37]
Complications
The major life-threatening complication of uremic pericarditis is the development of cardiac tamponade. If a rapid pericardial effusion accompanies uremic pericarditis, this has the potential to prevent the heart from functioning in pumping blood to the rest of the body. A typical presentation of cardiac tamponade includes hypotension, increased jugular venous distension, pulsus paradoxus, and distant heart sounds. An electrocardiogram may show electrical alternans, a voltage discrepancy caused by the heart floating within the pericardium due to the effusion. The treatment for this is emergent pericardiocentesis.[4][7][4][36]
Deterrence and Patient Education
Uremic pericarditis is thought to result primarily from toxin accumulation leading to pericarditis. As such, there is not much the patient can do beyond receiving dialysis and exercising medication compliance. However, patients with chronic kidney disease should know that uremic pericarditis is one of the complications, and in case of chest pain, they should seek medical help. Clinicians should counsel patients receiving dialysis that nonadherence to renal replacement therapy can result in multiple complications, including the development of uremic pericarditis.
Enhancing Healthcare Team Outcomes
As part of an interprofessional team, all healthcare professionals must be aware of the potential differential diagnoses in a patient complaining of chest pain. Patients with renal disease are at high risk of cardiac events. Uremic pericarditis must be especially considered in those with advanced chronic kidney disease or missed/inadequate dialysis. Early identification and management of uremic pericarditis are imperative in reducing long-term pericardial damage. The care of patients with uremic pericarditis necessitates a collaborative approach among healthcare professionals to ensure patient-centered care and improve overall outcomes. Nephrologists, cardiologists, emergency medicine and critical care clinicians, advanced clinicians, nurses, pharmacists, dialysis staff, and other healthcare professionals involved in the care of these patients should possess the essential clinical skills and knowledge to diagnose and manage this condition accurately. This includes expertise in recognizing the varied clinical presentations and understanding the nuances of diagnostic techniques such as ECG interpretation and echocardiography.
A strategic approach is equally crucial, involving evidence-based strategies to optimize treatment plans and minimize adverse effects. Ethical considerations must guide decision-making, ensuring informed consent and respecting patient autonomy in treatment choices. Each healthcare professional must be aware of their responsibilities and contribute their unique expertise to the patient's care plan, fostering a multidisciplinary approach. Effective interprofessional communication is paramount, allowing seamless information exchange and collaborative decision-making among the team members. Care coordination plays a pivotal role in ensuring that the patient's journey from diagnosis to treatment and follow-up is well-managed, minimizing errors and enhancing patient safety. By embracing these principles of skill, strategy, ethics, responsibilities, interprofessional communication, and care coordination, healthcare professionals can deliver patient-centered care, ultimately improving patient outcomes and enhancing team performance in the management of uremic pericarditis.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Roberts WC. Pericardial heart disease: its morphologic features and its causes. Proceedings (Baylor University. Medical Center). 2005 Jan:18(1):38-55 [PubMed PMID: 16200146]
Thind AK, Blackburn S, Corbett RW, Ashby DR. Diagnostic value of pericardial aspiration in dialysis patients: Case report with management informed by a retrospective case series. Clinical nephrology. 2022 Jun:97(6):361-366. doi: 10.5414/CN110690. Epub [PubMed PMID: 35343434]
Level 2 (mid-level) evidenceRestrepo D, Vaduganathan M, Fenves AZ. Uremic Pericarditis: Distinguishing Features in a Now-Uncommon Clinical Syndrome. Southern medical journal. 2018 Dec:111(12):754-757. doi: 10.14423/SMJ.0000000000000899. Epub [PubMed PMID: 30512129]
Dad T, Sarnak MJ. Pericarditis and Pericardial Effusions in End-Stage Renal Disease. Seminars in dialysis. 2016 Sep:29(5):366-73. doi: 10.1111/sdi.12517. Epub 2016 May 26 [PubMed PMID: 27228946]
Bentata Y, Hamdi F, Chemlal A, Haddiya I, Ismaili N, El Ouafi N. Uremic pericarditis in patients with End Stage Renal Disease: Prevalence, symptoms and outcome in 2017. The American journal of emergency medicine. 2018 Mar:36(3):464-466. doi: 10.1016/j.ajem.2017.11.048. Epub 2017 Nov 21 [PubMed PMID: 29248269]
Rehman KA, Betancor J, Xu B, Kumar A, Rivas CG, Sato K, Wong LP, Asher CR, Klein AL. Uremic pericarditis, pericardial effusion, and constrictive pericarditis in end-stage renal disease: Insights and pathophysiology. Clinical cardiology. 2017 Oct:40(10):839-846. doi: 10.1002/clc.22770. Epub 2017 Sep 5 [PubMed PMID: 28873222]
Rosen RJ, Valeri AM. Management of Patients with Kidney Failure and Pericarditis. Clinical journal of the American Society of Nephrology : CJASN. 2023 Feb 1:18(2):270-272. doi: 10.2215/CJN.07470622. Epub [PubMed PMID: 36328398]
Rostand SG, Rutsky EA. Pericarditis in end-stage renal disease. Cardiology clinics. 1990 Nov:8(4):701-7 [PubMed PMID: 2249224]
Eslami V, Mousavi S, Irilouzadian R, Baghsheikhi H, Fesharaki MJ, Samavat S. Pericardial effusion in patients with chronic kidney disease: A two-center study. PloS one. 2024:19(6):e0302200. doi: 10.1371/journal.pone.0302200. Epub 2024 Jun 6 [PubMed PMID: 38843270]
Wang Y, Gao L. Inflammation and Cardiovascular Disease Associated With Hemodialysis for End-Stage Renal Disease. Frontiers in pharmacology. 2022:13():800950. doi: 10.3389/fphar.2022.800950. Epub 2022 Feb 10 [PubMed PMID: 35222026]
Reddy P, Kane GC, Oh JK, Luis SA. The Evolving Etiologic and Epidemiologic Portrait of Pericardial Disease. The Canadian journal of cardiology. 2023 Aug:39(8):1047-1058. doi: 10.1016/j.cjca.2023.05.011. Epub 2023 May 20 [PubMed PMID: 37217161]
Ashraf H, Lee H, Tran KH, Agasthi P, Keddis MT, Unzek S, Narayanasamy H, Wilansky S. Prevalence and Outcomes of Pericardial Effusion in Kidney Transplant Candidates. The American journal of cardiology. 2020 Oct 1:132():140-146. doi: 10.1016/j.amjcard.2020.07.009. Epub 2020 Jul 14 [PubMed PMID: 32773224]
Lim YJ, Sidor NA, Tonial NC, Che A, Urquhart BL. Uremic Toxins in the Progression of Chronic Kidney Disease and Cardiovascular Disease: Mechanisms and Therapeutic Targets. Toxins. 2021 Feb 13:13(2):. doi: 10.3390/toxins13020142. Epub 2021 Feb 13 [PubMed PMID: 33668632]
Curaj A, Vanholder R, Loscalzo J, Quach K, Wu Z, Jankowski V, Jankowski J. Cardiovascular Consequences of Uremic Metabolites: an Overview of the Involved Signaling Pathways. Circulation research. 2024 Mar:134(5):592-613. doi: 10.1161/CIRCRESAHA.123.324001. Epub 2024 Feb 29 [PubMed PMID: 38422175]
Level 3 (low-level) evidenceDel Buono MG, Bonaventura A, Vecchié A, Moroni F, Golino M, Bressi E, De Ponti R, Dentali F, Montone RA, Kron J, Lazzerini PE, Crea F, Abbate A. Pathogenic pathways and therapeutic targets of inflammation in heart diseases: A focus on Interleukin-1. European journal of clinical investigation. 2024 Feb:54(2):e14110. doi: 10.1111/eci.14110. Epub 2023 Oct 14 [PubMed PMID: 37837616]
Amador-Martínez I, Aparicio-Trejo OE, Bernabe-Yepes B, Aranda-Rivera AK, Cruz-Gregorio A, Sánchez-Lozada LG, Pedraza-Chaverri J, Tapia E. Mitochondrial Impairment: A Link for Inflammatory Responses Activation in the Cardiorenal Syndrome Type 4. International journal of molecular sciences. 2023 Nov 1:24(21):. doi: 10.3390/ijms242115875. Epub 2023 Nov 1 [PubMed PMID: 37958859]
Anavekar NS, Pfeffer MA. Cardiovascular risk in chronic kidney disease. Kidney international. Supplement. 2004 Nov:(92):S11-5 [PubMed PMID: 15485401]
Ma X, Qian Y, Qian C, Lin H, Sun Y. Association between inflammation indicators and albuminuria in US adults: a cross-sectional study. Scientific reports. 2025 Jul 1:15(1):21496. doi: 10.1038/s41598-025-06540-6. Epub 2025 Jul 1 [PubMed PMID: 40594878]
Level 2 (mid-level) evidenceHwang SW, Lee T, Uh Y, Lee JY. Urinary albumin creatinine ratio is associated with lipid profile. Scientific reports. 2024 Jun 27:14(1):14870. doi: 10.1038/s41598-024-65037-w. Epub 2024 Jun 27 [PubMed PMID: 38937496]
Patel M, Rao SJ, Chittal AR, Al-Talib K, Padmanabhan S. Uremic Pericarditis and Cardiac Tamponade Resolving With Intensive Hemodialysis. Journal of community hospital internal medicine perspectives. 2024:14(2):67-71. doi: 10.55729/2000-9666.1307. Epub 2024 Mar 4 [PubMed PMID: 38966517]
Level 3 (low-level) evidenceCremer PC, Klein AL, Imazio M. Diagnosis, Risk Stratification, and Treatment of Pericarditis: A Review. JAMA. 2024 Oct 1:332(13):1090-1100. doi: 10.1001/jama.2024.12935. Epub [PubMed PMID: 39235771]
Sadjadi SA, Mashahdian A. Uremic pericarditis: a report of 30 cases and review of the literature. The American journal of case reports. 2015 Mar 22:16():169-73. doi: 10.12659/AJCR.893140. Epub 2015 Mar 22 [PubMed PMID: 25796283]
Level 3 (low-level) evidenceRout P, Foris LA, Katta S, Bashir K. Uremia. StatPearls. 2025 Jan:(): [PubMed PMID: 28722889]
Saeed S, Mohamed Ali A, Wasim D, Saeed N, Lunde T, Solheim E, Vegsundvåg J, Imazio M. Natural Course of Electrocardiogram Changes and the Value of Multimodality Imaging in Acute Pericarditis. Cardiology. 2023:148(3):219-227. doi: 10.1159/000530207. Epub 2023 Mar 22 [PubMed PMID: 36948161]
Imazio M, Squarotti GB, Andreis A, Agosti A, Millesimo M, Frea S, Giustetto C, Deferrari GM. Diagnostic and prognostic role of the electrocardiogram in patients with pericarditis. Heart (British Cardiac Society). 2022 Aug 25:108(18):1474-1478. doi: 10.1136/heartjnl-2021-320443. Epub 2022 Aug 25 [PubMed PMID: 35523541]
Klein AL, Abbara S, Agler DA, Appleton CP, Asher CR, Hoit B, Hung J, Garcia MJ, Kronzon I, Oh JK, Rodriguez ER, Schaff HV, Schoenhagen P, Tan CD, White RD. American Society of Echocardiography clinical recommendations for multimodality cardiovascular imaging of patients with pericardial disease: endorsed by the Society for Cardiovascular Magnetic Resonance and Society of Cardiovascular Computed Tomography. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 2013 Sep:26(9):965-1012.e15. doi: 10.1016/j.echo.2013.06.023. Epub [PubMed PMID: 23998693]
Çetin MS, Özcan Çetin EH, Özdemir M, Topaloğlu S, Aras D, Temizhan A, Aydoğdu S. Effectiveness of computed tomography attenuation values in characterization of pericardial effusion. Anatolian journal of cardiology. 2017 Apr:17(4):322-327. doi: 10.14744/AnatolJCardiol.2016.7353. Epub 2017 Jan 17 [PubMed PMID: 28100899]
Adler Y, Charron P, Imazio M, Badano L, Barón-Esquivias G, Bogaert J, Brucato A, Gueret P, Klingel K, Lionis C, Maisch B, Mayosi B, Pavie A, Ristic AD, Sabaté Tenas M, Seferovic P, Swedberg K, Tomkowski W, ESC Scientific Document Group. 2015 ESC Guidelines for the diagnosis and management of pericardial diseases: The Task Force for the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology (ESC)Endorsed by: The European Association for Cardio-Thoracic Surgery (EACTS). European heart journal. 2015 Nov 7:36(42):2921-2964. doi: 10.1093/eurheartj/ehv318. Epub 2015 Aug 29 [PubMed PMID: 26320112]
Wood JE, Mahnensmith RL. Pericarditis associated with renal failure: evolution and management. Seminars in dialysis. 2001 Jan-Feb:14(1):61-6 [PubMed PMID: 11208042]
Level 3 (low-level) evidenceBataille S, Brunet P, Decourt A, Bonnet G, Loundou A, Berland Y, Habib G, Vacher-Coponat H. Pericarditis in uremic patients: serum albumin and size of pericardial effusion predict drainage necessity. Journal of nephrology. 2015 Feb:28(1):97-104. doi: 10.1007/s40620-014-0107-7. Epub 2014 May 20 [PubMed PMID: 24840780]
Level 2 (mid-level) evidenceChen TK, Knicely DH, Grams ME. Chronic Kidney Disease Diagnosis and Management: A Review. JAMA. 2019 Oct 1:322(13):1294-1304. doi: 10.1001/jama.2019.14745. Epub [PubMed PMID: 31573641]
Gaudry S, Palevsky PM, Dreyfuss D. Extracorporeal Kidney-Replacement Therapy for Acute Kidney Injury. The New England journal of medicine. 2022 Mar 10:386(10):964-975. doi: 10.1056/NEJMra2104090. Epub [PubMed PMID: 35263520]
Gulati M, Levy PD, Mukherjee D, Amsterdam E, Bhatt DL, Birtcher KK, Blankstein R, Boyd J, Bullock-Palmer RP, Conejo T, Diercks DB, Gentile F, Greenwood JP, Hess EP, Hollenberg SM, Jaber WA, Jneid H, Joglar JA, Morrow DA, O'Connor RE, Ross MA, Shaw LJ. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR Guideline for the Evaluation and Diagnosis of Chest Pain: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021 Nov 30:144(22):e368-e454. doi: 10.1161/CIR.0000000000001029. Epub 2021 Oct 28 [PubMed PMID: 34709879]
Level 1 (high-level) evidenceImazio M, Brucato A, Cumetti D, Brambilla G, Demichelis B, Ferro S, Maestroni S, Cecchi E, Belli R, Palmieri G, Trinchero R. Corticosteroids for recurrent pericarditis: high versus low doses: a nonrandomized observation. Circulation. 2008 Aug 5:118(6):667-71. doi: 10.1161/CIRCULATIONAHA.107.761064. Epub 2008 Jul 21 [PubMed PMID: 18645054]
Level 2 (mid-level) evidenceImazio M, Lazaros G, Gattorno M, LeWinter M, Abbate A, Brucato A, Klein A. Anti-interleukin-1 agents for pericarditis: a primer for cardiologists. European heart journal. 2022 Aug 14:43(31):2946-2957. doi: 10.1093/eurheartj/ehab452. Epub [PubMed PMID: 34528670]
Long B, Koyfman A, Lee CM. Emergency medicine evaluation and management of the end stage renal disease patient. The American journal of emergency medicine. 2017 Dec:35(12):1946-1955. doi: 10.1016/j.ajem.2017.09.002. Epub 2017 Sep 5 [PubMed PMID: 28893450]
Kim SB, Yang EH, Shin JH. Percutaneous pericardial catheter drainage for symptomatic uremic pericardial effusions with narrow safety margins. PloS one. 2022:17(10):e0276498. doi: 10.1371/journal.pone.0276498. Epub 2022 Oct 31 [PubMed PMID: 36315499]