Introduction
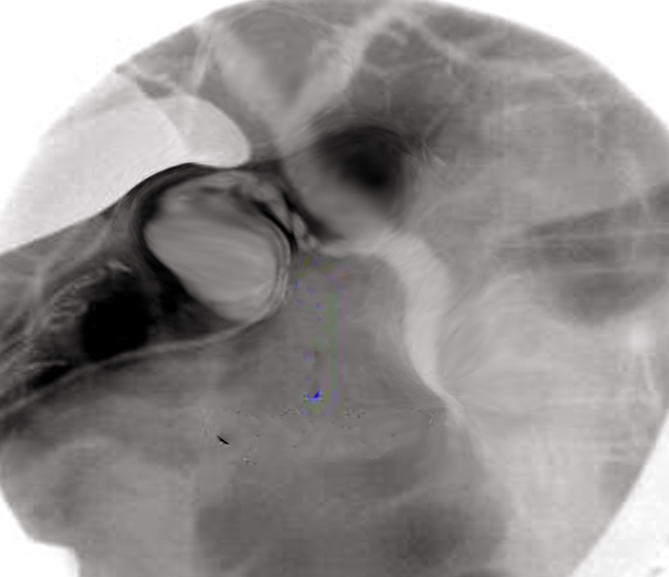
Initially reported in 1937, percutaneous transhepatic cholangiography (PTC) is an invasive procedure to visualize and subsequently access the biliary tract (see Image. Percutaneous Transhepatic Cholangiography). PTC involves inserting a needle through the skin, body wall, and liver parenchyma to inject contrast dye directly into the bile ducts. PTC can become important for clinical decision-making or as a precursor to therapy when endoscopic access to the bile ducts is not possible, or other imaging techniques offer inadequate information. PTC provides higher-resolution images of bile duct obstructions, strictures, and leaks than ultrasound, nuclear imaging, computed tomography, and magnetic resonance imaging, including magnetic resonance cholangiopancreatography.
After performing PTC, percutaneous interventions of the bile ducts can be attempted, including bile duct stone removal, biopsy, stenting, and drainage. Percutaneous transhepatic biliary drainage (PTBD) was introduced in 1981. For patients with contraindications to bile duct access via endoscopic guidance, such as endoscopic retrograde cholangiopancreatography or choledochoduodenoscopy, PTC and PTBD remain less invasive than surgical access. This activity describes therapies performed in conjunction with PTC to illustrate when PTC is indicated or contraindicated. However, a discussion of these therapies' technical performance and therapeutic outcomes is beyond this topic's scope.
Anatomy and Physiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Anatomy and Physiology
Successful PTC performance and interpretation require an understanding of choledochal anatomy and its variants. The extrahepatic portion of the biliary system consists of the common hepatic, cystic, and common bile ducts.
- The common hepatic duct (CHD) emerges from the liver and travels caudally.
- The cystic duct allows bidirectional flow between the gallbladder and the other 2 ducts.
- The common bile duct (CBD) is simply the continuation of the CHD toward the duodenum beyond the CHD-cystic duct union. The CBD usually enters the pancreas and receives the main pancreatic duct before entering the duodenal wall at the major duodenal papilla.
The sphincter of Oddi, under neurologic and endocrine stimulation, controls bile release from the CBD into the duodenum. Extrahepatic choledochal anatomy is much less variable than intrahepatic choledochal anatomy. Based on its blood supply, the liver can be divided into 8 segments within 2 lobes. The intrahepatic biliary system arises from microscopic bile sinuses that drain into small canals (canaliculi) between hepatocytes. Canaliculi converge to form progressively larger bile ducts, which subsequently develop into 8 segmental ducts, several sectoral ducts, and typically 2 lobar ducts. The right anterior sectoral duct drains segments 5 and 8 and usually courses in a somewhat craniocaudal orientation, whereas the right posterior sectoral duct drains segments 6 and 7 and usually courses more lateromedially. The location where the lobar or sectoral ducts join to form the CHD is called the porta hepatis. The most common configuration near the porta hepatis, seen in about 50% of people, consists of:
- The right lobar duct is formed by the union of an anterior and posterior sectoral duct.
- Both lobes drain into a single duct, which empties into the CHD.
Congenital variants and anomalies may affect the performance and interpretation of PTC. The most common variant features a right anterior sectoral duct that drains separately into the CHD. Common anomalies include choledochal cysts and diverticula.
Indications
Because less traumatic alternatives are often available for the diagnosis and treatment of biliary disease, PTC generally is not a first-line procedure. The Cardiovascular and Interventional Radiological Society of Europe, the Society of Interventional Radiology, the Japanese Society of Hepato-Biliary-Pancreatic Surgery, and the Indian College of Radiology and Imaging have published society guidelines specifying when PTC and PTBD are indicated.[1][2][3][4][5][6]
These include:
- Assessing biliary anatomy
- PTC can inform a surgeon on the operative approach to take when other imaging exams do not provide sufficient anatomic detail.
- Treating biliary leaks
- If endoscopic retrograde cholangiopancreatography (ERCP)-guided diagnosis and treatment are not feasible or have failed, then an approach using PTC is the next least invasive option.[7]
- Treating obstructive jaundice from benign or malignant biliary strictures
- PTC with PTBD can be used to perform cholangioplasty (bile duct dilation) for strictures and obstructions and to place stents or catheters that drain bile internally or externally.
- Unless surgery is indicated, PTBD becomes the preferred rescue strategy when a peroral method (ie, ERCP) fails or is not feasible,[8][9] such as after a prior bile duct reconstruction like a Kasai portoenterostomy.[10][11]
- Bile duct stone removal and lithotripsy [12]
- Tissue collection via biopsy, aspiration, or brushing
- Foreign body retrieval [13]
- Assisting an endoscopist or surgeon who is having or expects difficulty accessing the bile ducts in retrograde fashion (a rendezvous approach)
Other guidelines for PTC and PTBD have been suggested.[14][15]
Contraindications
Due to its potential risks, PTC can be contraindicated, including when there is:
- The possibility of instead performing ERCP:
- ERCP should be the first-line therapy for stenoses or obstructions that are reachable via this method, given data showing that ERCP-guided procedures are associated with decreased adverse events, improved survival, and enhanced quality of life.[16]
- The National Comprehensive Cancer Network recommends ERCP over PTC for extrahepatic duct lesions.[17]
- A nondilated choledochal system:
- Attempting PTC when the ducts are not dilated, such as for a planned diversion of bile away from a site of traumatic bile leakage, significantly increases the chance of failed duct access and complications.[18]
- PTC should be deferred if there is a possibility that nondilated ducts will expand before significant interval clinical worsening.
- An allergy to contrast material:
- Patients with a known contrast allergy should receive preprocedural corticosteroids and antihistamines before undergoing percutaneous transhepatic cholangiography.
- A contraindication to sedation:
- PTC can be performed with local anesthesia alone. However, most people would be unable to tolerate therapeutic transhepatic procedures if administered local anesthesia only.
- A patient who cannot lie still:
- PTC requires a patient to lie still for usually 30 to 60 minutes.
- An anatomical limitation:
- Certain anatomical variations or abnormalities (eg, extreme obesity) may make PTC technically challenging or impossible to perform.
- Early pregnancy:
- PTC involves radiation exposure, which can injure a developing fetus.
- Low life expectancy:
- The Cardiovascular and Interventional Radiological Society of Europe guidelines advise against performing PTC in persons with a life expectancy of fewer than 30 days, as the procedure risks are likely to outweigh the benefits.
- Any of the following specific diseases/conditions:
- Ascites, because of the lack of the tamponade effect of solid tissue against the liver capsule, can prevent hepatic puncture site coagulation and result in death.
- Unstable hemodynamics can worsen PTC.
- Extensive hepatic disease occurs when bile duct drainage alone will not improve morbidity or mortality, such that urgent liver transplant should be pursued instead.
- Multifocal intrahepatic segmental stenoses would prevent successful PTBD.
- Uncontrolled infection, particularly in the area surrounding the liver or biliary tract, can result in sepsis or abscess formation.
- This includes coagulopathy or a bleeding disorder.
- Regarding hemorrhage risk, the Society of Interventional Radiology (SIR) categorizes PTC as a level 2 procedure.
- For patients undergoing PTC, the SIR recommends maintaining an international normalized ratio of no 1.5 or less and a platelet count of at least 50,000 cells/μL.
The activity's section on procedure preparation addresses additional issues that could be considered relative contraindications.
Equipment
Essential equipment for PTC includes the following:
- Image guidance machine:
- The traditional method for percutaneous needle guidance is x-ray fluoroscopy, which produces real-time x-ray video images. Because x-ray fluoroscopy does not show bile duct locations until after a duct has been punctured and filled with contrast, it necessitates a "pin cushion" guesswork approach to the bile duct puncture described below.
- Ultrasound (US) can often enable real-time visualization of bile ducts and hepatic arteries, making it a superior method for guiding a needle into a duct and away from arteries, thereby reducing the number of puncture attempts.[19][20]
- Computed tomography (CT—particularly CT fluoroscopy) has some data to support its use, as evidenced by an observational head-to-head comparison.[21]
- Magnetic resonance imaging and magnetic-guided US access techniques have been performed, but there is a lack of published head-to-head comparison data against other approaches.
- Contrast media and syringes:
- Usually, water-soluble iodinated contrast is used.
- A 25% contrast by volume is usually sufficient to visualize the ducts under fluoroscopy, while not obscuring tools placed into the ducts or unnecessarily increasing viscosity in the bile system, which can lead to cholangitis.
- Chlorhexidine, sterile drapes, gowns, and gloves:
- These items help maintain aseptic conditions.
- PTC is considered a clean-contaminated procedure because the bile may already contain enteric bacteria. The goal is not to introduce additional organisms into the body.
- Monitoring equipment:
- The patient's vital signs should be monitored during the procedure.
- Local anesthetic, sedation, and sedation monitoring equipment:
- At a minimum, local anesthetic is necessary to enable the patient to tolerate PTC.
- Transhepatic interventions often are too painful to tolerate without intravenous sedation.
- Needles:
- Certain needle types, such as 21- to 22-gauge Chiba or Seldinger needles, are suitable for accessing the bile ducts.
- A shorter 18-gauge coaxial needle can expedite bile duct access by preventing the thinner, more flexible access needle from inadvertent bending and misdirection.
- Guidewires, catheters, and sheaths:
- Navigating bile ducts is often best performed using a hydrophilic guidewire.
- 5 Fr angiographic catheters having short angles (eg, Bern, Berenstein, Kumpe, or reverse internal mammary catheters) may be necessary to navigate the ducts.
- Such catheters are typically placed through a percutaneous access sheath.
- Most other necessary equipment pertains to therapies performed in conjunction with PTC.
Personnel
The healthcare professionals working together to enable PTC usually include:
- A clinician trained in interventional radiology or hepatic surgery
- An imaging technologist
- An anesthesiologist, nurse anesthetist, or nurse dedicated to administering sedation
- A "circulator" who is free to move about to obtain supplies on an as-needed basis
Preparation
Imaging before PTC enables an informed approach to the procedure, such as whether a right- or left-lobe approach should be pursued initially. Cross-sectional imaging (CT, MRI, or US) can reveal the patient's duct anatomy and dilation distribution, the location of an obstruction, and possibly the obstructive cause. Imaging confirmation of a bile duct leak is often best performed using radionuclide scintigraphy (nuclear imaging), such as an iminodiacetic acid scan.[22]
The operator should plan an upper limit for hepatic punctures (transgressing the liver capsule) and passes (moving the needle deeper into the parenchyma without necessarily re-entering the liver itself), as well as a systemic approach for redirecting the needle after each unsuccessful puncture. Prospective observational data collected by the same investigator in different patient groups found a required average number of punctures to vary between 3 and 6.[23][24] When there is no ability to visualize a target duct with US, sometimes the planned upper limit of punctures is reached with no successful duct access, even using a limit of 50.[24] The same investigator concluded that greater than 5 punctures or 10 passes are thresholds where the odds of hemorrhagic events start to double; a puncture number to clinical complication relationship curve from a controlled trial has not yet been published. The patient's autonomy should be preserved via informed consent.[25]
Antibiotic prophylaxis, regardless of whether the patient is symptomatic, should cover gram-negative bacteria. A reduced rate of sepsis after PTC has been found with prophylactic gram-negative bacterial therapy, even in persons for whom there is no evidence of biliary tract retrograde contamination with enteric flora.[26] Some clinicians also cover gram-positive and anaerobic bacteria. For PTC only, a single dose of a third-generation cephalosporin may be considered. When therapy is also planned, the Society of Interventional Radiology recommends intravenous administration of 1 g of ceftriaxone or 1.5 g of ampicillin-sulbactam.[27]
Steps should be taken to correct modifiable risk factors, such as coagulopathy and sedation-related risks. Patients referred for PTBD are often dehydrated, posing a risk of procedure-induced hepatorenal failure; hydration should be provided even when hemodynamic instability findings are absent. The patient should be placed in a comfortable position that still allows access to the intended region of the liver selected for puncture. A sterile field should be created around that site.
Technique or Treatment
Mastery of the PTC technique is essential for ensuring diagnostic accuracy and procedural safety. The following section outlines the step-by-step approach to performing PTC.
Needle Insertion and Duct Cannulation
There is no prospective randomized data regarding access location and image guidance technique. The access location and image guidance technique should be determined based on anatomy identified on pre-PTC cross-sectional images, the expected goal of PTC, and any planned adjunct procedures. When only x-ray fluoroscopy is used for initial image guidance, such as in a nondilated intrahepatic duct system, the most common approach is to access a right lobe bile duct by inserting a needle slightly ventral to the midaxillary line caudal to the tenth rib, with the needled angled craniad to the expected location of the porta hepatis and toward the midclavicular line. This approach can: keep the needle out of the thoracic cavity, create an angle conducive to passing instruments into the bile ducts, and keep the operator's body out of the x-ray beam. Options for bile duct access using fluoroscopic guidance also include the left lobe and the gallbladder.
Ultrasound guidance should be used when a bile duct is large enough to be visualized with this technology, as it can yield faster and more accurate duct targeting and reduce radiation exposure.[19][28] Results from a retrospective, noncontrolled study supported the use of ultrasonographic guidance via a left lobe approach. Still, they found no advantage compared with conventional fluoroscopic guidance for a right lobe approach.[20] Further, a longstanding technique involves aspirating bile and injecting contrast during needle withdrawal, indicating that the needle has traversed and re-entered a duct—a method referred to as the double-wall puncture technique. However, results from a randomized, noncontrolled trial found no difference in outcomes between single-wall and double-wall puncturing techniques.[29]
Sufficient contrast must be injected to diagnose blockages and strictures, but excessive injection should be avoided. PTC can, in some cases, be performed with a needle only. When injection through a needle alone is insufficient to visualize enough of the biliary tract for diagnostic purposes, the Seldinger technique is used to exchange the needle for a catheter to navigate the ducts.
Bile Sampling
Aspirated bile should be assessed for its color and odor to guide decisions prior to ending the procedure. If doing so reveals evidence of infectious cholangitis, then a sample should be tested for microbes.
Image Documentation
Images should be recorded to show key portions of the procedure and the information needed for the next steps in management. True lateral views are often not possible using a conventional fluoroscope, but images can still be taken in multiple planes, such as supine oblique views. Newer x-ray fluoroscopes can also create CT images when needed.
Procedure Conclusion
A hemostatic patch can be placed along the liver capsule via the needle or sheath; however, there are currently no studies with results demonstrating that this approach reduces complications. Once the tools (needle, catheter, and/or sheath) are removed, dermal pressure and a bandage should be applied to promote superficial hemostasis and prevent skin contact with bile, a chemical irritant. The patient should be monitored for a period to manage any complications or until it is reasonable to assume that no severe complications have occurred.
Complications
The Society of Interventional Radiology in 2020 reported complication rates for PTC and PTBD of between 2% and 10% based largely on retrospective selected case series.[3] However, in more recent large prospective cohorts, PTC and PTBD were reported as having higher complication rates than those reported by the societies that have published guidelines for these procedures.[26][30] Hemobilia and/or melena occur in approximately 10% of cases and are generally considered expected outcomes rather than true complications. Similarly, postprocedural pain requiring analgesia is a common adverse effect and not classified as a complication in itself. The most common complications associated with PTC are listed below in descending order of frequency.[1][14][31]
- Infections:
- Bacteremia/sepsis, cholangitis, liver abscess, and/or infection may occur at the body wall puncture site.
- A review of different studies' results found varying overall infection rates: 67 of 343 patients (19.5%), 46 of 152 patients (30%), and 83 of 193 patients (43%).[26]
- Bile-related inflammation:
- Perforation of the bile ducts and the liver capsule can leak bile into the peritoneal space, resulting in sterile or chemical peritonitis and abscess formation; leaks can also occur in the pleural space, resulting in sterile/chemical pleuritis and empyema.
- Bilomas are noninfected bile collections. Because they typically resolve independently, percutaneous drainage is reserved for cases where a collection remains unbearably painful despite medication or exhibits additional signs of infection.
- Damage to adjacent organs and spaces:
- Of these, the risk of pneumothorax is highest, especially in cases where the abdominal puncture site is close to the diaphragm. According to a review, transpleural punctures were associated with pneumothoraces at rates ranging from 8% to 22%.[32]
- The needle may inadvertently puncture the lung, gallbladder, or bowel. Catheter manipulation to the level of the pancreatic duct can cause pancreatitis.
- Bleeding:
- In addition to hemobilia and melena, bleeding may occur in the form of subcapsular hepatic hematoma, hemothorax, hemoperitoneum, pseudoaneurysm, fistula, and/or hemorrhage from the body wall at the catheter/drain site. Hemorrhage following PTC/PTBD may result from arterial, portal, and/or hepatic venous injury.[33]
- Hepatic artery bleeding is marked by bright red (occasionally pulsatile) blood, a large drop in hematocrit levels, or hemodynamic instability. Portal vein bleeding should be suspected when dark blood is present, without significant hemodynamic instability or a drop in hematocrit.
- Nonoperative treatment options may include PTBD drain repositioning, stent graft placement, and embolization. Stent graft placement across the injury site is feasible only with a central injury and favorable anatomy. Arterial embolization is more common, typically performed using coils via femoral or radial artery access.
- The Society of Interventional Radiology recommends performing a practice review if more than 5% of patients have experienced bleeding significant enough to warrant a second invasive procedure.[3]
- Death:
- As discussed in the sections above, PTC with PTBD is often performed on those who have significant comorbidities with little reserve for additional hemodynamic instability or infection.
- In the setting of malignant obstructions, one retrospective study's results showed a statistically significant greater risk of death from PTC and attempted PTBD compared to endoscopic retrograde cholangiopancreatography and attempted endoscopic drainage.[34]
Clinical Significance
PTC is most commonly used during the management of the following conditions:
- Bile duct obstruction due to malignant disease:
- Pancreatic cancer
- Cholangiocellular carcinoma
- Hepatocellular carcinoma
- Metastatic disease
- Bile duct obstruction due to benign disease:
- Cholangiolithiasis
- Iatrogenic injury to the biliary tract
- Primary sclerosing cholangitis
- Cholangitis due to ischemic or infectious causes
Technical Success of PTC and PTBD
The Cardiovascular and Interventional Radiological Society of Europe (CIRSE) has defined technical success as follows:
- For PTC: Diagnostic opacification of bile ducts.
- For PTBD: Placement of a drainage catheter.
Although success rates are likely to vary by practice, CIRSE guidelines reported that technical success for these procedures based on published retrospective reviews is:
- Rates of 90% or higher when there are dilated biliary ducts
- Rates of 80% or higher when there are nondilated biliary ducts [1]
As with all procedures, clinical success rates also depend on the patient's overall clinical condition and operator skill.
PTC versus Other Diagnostic and Therapeutic Options
For disease diagnosis, PTC has been largely replaced by magnetic resonance cholangiopancreatography, endoscopic ultrasound, and endoscopic retrograde cholangiopancreatography because PTC has higher morbidity and lacks comparative cost-effectiveness.[5][35][36] For tissue sampling, percutaneous or endoscopic biopsy is generally performed instead of PTC-guided biopsy. However, cholangiographic-guided biopsy may improve the accuracy of specimen diagnosis in unusual circumstances.[37][38]
For relief of bile duct obstruction, PTC is the first step for PTBD or percutaneous stenting, which is the second most common approach for treating obstructive jaundice after endoscopic retrograde cholangiopancreatography (ERCP)-guided drainage or stenting.[16][39] The success rates of both procedures are similar for obstructions near or caudal to the porta hepatis. However, PTBD has a higher overall success rate for isolated intrahepatic obstructions.[40]
Results from a prospective noncontrolled study of 30 patients showed that the only advantage of PTC over ERCP was the treatment of bile fistulas.[41] In the West, only data from nonrandomized studies is available. A randomized trial comparing percutaneous versus endoscopic bile duct interventions was attempted in the United States but failed to recruit enough patients over a 3-year period; future attempts in Europe have been proposed.[28][42] A prospective randomized study from China (not published in English) reported that PTBD is more cost-effective than an endoscopic approach when performed for end-of-life palliation.[43]
Enhancing Healthcare Team Outcomes
Patients undergoing PTC require the collaboration of several healthcare professionals to ensure patient-centered care that prioritizes safety and outcomes in line with the patient's goals. Interventional radiologists or hepatobiliary surgeons are the physicians who most commonly perform PTC and PTBD. Collaboration with hepatologists, transplant surgeons, hospitalists, and other clinical specialists is important for addressing contraindications and alternatives before proceeding with PTC/PTBD. Imaging technologists help with PTC/PTBD technical performance. Advanced care clinicians, nurses, and pharmacists assist with patient monitoring, medication administration, and education.
Creating an interprofessional team that meets regularly to review previous cases and discuss performance feedback can help identify areas for improvement and implement strategies to enhance team performance.[44] Healthcare teams making an effort to conduct shared decision-making with patients have been shown in 2 meta-analyses of randomized trials, both in nonsurgical and surgical settings, to:
- Reduce patient decision regret (0.29, 95% CI 0.07 to 0.51; 1 study; N= 326), and
- Reduce consultation length (0.51, 95% CI 0.21 to 0.81; 1 study, N= 175).[45]
- Reduce decision conflict (0.4, 95% CI 0.33 to 0.46; 24 studies),
- Reduce patient decision regret (0.2, 95% CI significant but not reported; 6 studies),
- Reduce patient decision anxiety (0.4, 95% CI 0.55 to 0.46; 4 studies), and
- Increase patient decision satisfaction (0.7, 95% CI significant but not reported; 9 studies).[46]
Note: These values are reported as: odds ratio, 95% confidence interval; number of studies +/- number of patients.
Media
(Click Image to Enlarge)
References
Das M, van der Leij C, Katoh M, Benten D, Hendriks BMF, Hatzidakis A. CIRSE Standards of Practice on Percutaneous Transhepatic Cholangiography, Biliary Drainage and Stenting. Cardiovascular and interventional radiology. 2021 Oct:44(10):1499-1509. doi: 10.1007/s00270-021-02903-4. Epub 2021 Jul 29 [PubMed PMID: 34327586]
Saad WE, Wallace MJ, Wojak JC, Kundu S, Cardella JF. Quality improvement guidelines for percutaneous transhepatic cholangiography, biliary drainage, and percutaneous cholecystostomy. Journal of vascular and interventional radiology : JVIR. 2010 Jun:21(6):789-95. doi: 10.1016/j.jvir.2010.01.012. Epub 2010 Mar 21 [PubMed PMID: 20307987]
Level 2 (mid-level) evidenceDevane AM, Annam A, Brody L, Gunn AJ, Himes EA, Patel S, Tam AL, Dariushnia SR. Society of Interventional Radiology Quality Improvement Standards for Percutaneous Cholecystostomy and Percutaneous Transhepatic Biliary Interventions. Journal of vascular and interventional radiology : JVIR. 2020 Nov:31(11):1849-1856. doi: 10.1016/j.jvir.2020.07.015. Epub 2020 Oct 1 [PubMed PMID: 33011014]
Level 2 (mid-level) evidenceMiura F, Okamoto K, Takada T, Strasberg SM, Asbun HJ, Pitt HA, Gomi H, Solomkin JS, Schlossberg D, Han HS, Kim MH, Hwang TL, Chen MF, Huang WS, Kiriyama S, Itoi T, Garden OJ, Liau KH, Horiguchi A, Liu KH, Su CH, Gouma DJ, Belli G, Dervenis C, Jagannath P, Chan ACW, Lau WY, Endo I, Suzuki K, Yoon YS, de Santibañes E, Giménez ME, Jonas E, Singh H, Honda G, Asai K, Mori Y, Wada K, Higuchi R, Watanabe M, Rikiyama T, Sata N, Kano N, Umezawa A, Mukai S, Tokumura H, Hata J, Kozaka K, Iwashita Y, Hibi T, Yokoe M, Kimura T, Kitano S, Inomata M, Hirata K, Sumiyama Y, Inui K, Yamamoto M. Tokyo Guidelines 2018: initial management of acute biliary infection and flowchart for acute cholangitis. Journal of hepato-biliary-pancreatic sciences. 2018 Jan:25(1):31-40. doi: 10.1002/jhbp.509. Epub 2018 Jan 8 [PubMed PMID: 28941329]
Kiriyama S, Kozaka K, Takada T, Strasberg SM, Pitt HA, Gabata T, Hata J, Liau KH, Miura F, Horiguchi A, Liu KH, Su CH, Wada K, Jagannath P, Itoi T, Gouma DJ, Mori Y, Mukai S, Giménez ME, Huang WS, Kim MH, Okamoto K, Belli G, Dervenis C, Chan ACW, Lau WY, Endo I, Gomi H, Yoshida M, Mayumi T, Baron TH, de Santibañes E, Teoh AYB, Hwang TL, Ker CG, Chen MF, Han HS, Yoon YS, Choi IS, Yoon DS, Higuchi R, Kitano S, Inomata M, Deziel DJ, Jonas E, Hirata K, Sumiyama Y, Inui K, Yamamoto M. Tokyo Guidelines 2018: diagnostic criteria and severity grading of acute cholangitis (with videos). Journal of hepato-biliary-pancreatic sciences. 2018 Jan:25(1):17-30. doi: 10.1002/jhbp.512. Epub 2018 Jan 5 [PubMed PMID: 29032610]
Madhusudhan KS, Jineesh V, Keshava SN. Indian College of Radiology and Imaging Evidence-Based Guidelines for Percutaneous Image-Guided Biliary Procedures. The Indian journal of radiology & imaging. 2021 Apr:31(2):421-440. doi: 10.1055/s-0041-1734222. Epub 2021 Jul 28 [PubMed PMID: 34556927]
Level 1 (high-level) evidenceJeong CY, Choi JW, Kim JR, Jang JY, Cho JK. Successful treatment through staged laparoscopic transgastric endoscopic retrograde cholangiopancreatography for postoperative bile leakage: A case report. Medicine. 2022 Sep 2:101(35):e30312. doi: 10.1097/MD.0000000000030312. Epub [PubMed PMID: 36107600]
Level 3 (low-level) evidenceSpadaccini M, Binda C, Fugazza A, Repici A, Tarantino I, Fabbri C, Cugia L, Anderloni A, On Behalf Of The Interventional Endoscopy Amp Ultra Sound I-Eus Group. Informed Consent for Endoscopic Biliary Drainage: Time for a New Paradigm. Medicina (Kaunas, Lithuania). 2022 Feb 22:58(3):. doi: 10.3390/medicina58030331. Epub 2022 Feb 22 [PubMed PMID: 35334507]
Ulvund Solstad T, Thorsteinsson M, Schultz N, Larsen PN, Taudorf M, Achiam M. Cholangioscopy with Spyglass DS using percutaneous transhepatic cholangiography access: a retrospective cohort study. Annals of medicine and surgery (2012). 2024 Apr:86(4):1867-1872. doi: 10.1097/MS9.0000000000001840. Epub 2024 Feb 16 [PubMed PMID: 38576952]
Level 2 (mid-level) evidenceAndo H, Inomata Y, Iwanaka T, Kuroda T, Nio M, Matsui A, Yoshida M, Japanese Biliary Atresia Society. Clinical practice guidelines for biliary atresia in Japan: A secondary publication of the abbreviated version translated into English. Journal of hepato-biliary-pancreatic sciences. 2021 Jan:28(1):55-61. doi: 10.1002/jhbp.816. Epub 2020 Oct 4 [PubMed PMID: 32780928]
Level 1 (high-level) evidenceOnishi Y, Shimizu H, Ohno T, Furuta A, Isoda H, Okamoto T, Okajima H, Nakamoto Y. Percutaneous Transhepatic Biliary Intervention in Adult Biliary Atresia Patients After Kasai Portoenterostomy. JPGN reports. 2022 May:3(2):e206. doi: 10.1097/PG9.0000000000000206. Epub 2022 May 9 [PubMed PMID: 37168905]
Alabraba E, Travis S, Beckingham I. Percutaneous transhepatic cholangioscopy and lithotripsy in treating difficult biliary ductal stones: Two case reports. World journal of gastrointestinal endoscopy. 2019 Apr 16:11(4):298-307. doi: 10.4253/wjge.v11.i4.298. Epub [PubMed PMID: 31040891]
Level 3 (low-level) evidenceMisbahuddin-Leis M, Ankolvi M, Mishra M, Dubasz K, Marinov A, Müller T, Graeb C, Radeleff B. Unlocking the enigma: Combined percutaneous-transhepatic and endoscopic strategies for retrieval of severed Dormia basket in choledocholithiasis. A case report and literature review. Radiology case reports. 2024 Jul:19(7):2745-2750. doi: 10.1016/j.radcr.2024.03.074. Epub 2024 Apr 19 [PubMed PMID: 38680740]
Level 3 (low-level) evidenceChen L, Wu Z, Guo C, Wang G, Tu K, Jiang J. Evaluation of Clinical Indications of Three Treatments for Choledocholithiasis with Acute Cholangitis. International journal of general medicine. 2023:16():4669-4680. doi: 10.2147/IJGM.S429781. Epub 2023 Oct 16 [PubMed PMID: 37868815]
Lindor KD, Bowlus CL, Boyer J, Levy C, Mayo M. Primary Biliary Cholangitis: 2018 Practice Guidance from the American Association for the Study of Liver Diseases. Hepatology (Baltimore, Md.). 2019 Jan:69(1):394-419. doi: 10.1002/hep.30145. Epub 2018 Nov 6 [PubMed PMID: 30070375]
Tavakkoli A, Beauchamp A, Prasad T, Zhu H, Singal AG, Elmunzer BJ, Kubiliun NM, Kwon RS, Hughes AE, Pruitt SL. Accessibility to ERCP-performing hospitals among patients with pancreatic cancer living in SEER regions. Cancer medicine. 2024 Feb:13(3):e7020. doi: 10.1002/cam4.7020. Epub [PubMed PMID: 38400670]
Benson AB, D'Angelica MI, Abrams T, Abbott DE, Ahmed A, Anaya DA, Anders R, Are C, Bachini M, Binder D, Borad M, Bowlus C, Brown D, Burgoyne A, Castellanos J, Chahal P, Cloyd J, Covey AM, Glazer ES, Hawkins WG, Iyer R, Jacob R, Jennings L, Kelley RK, Kim R, Levine M, Palta M, Park JO, Raman S, Reddy S, Ronnekleiv-Kelly S, Sahai V, Singh G, Stein S, Turk A, Vauthey JN, Venook AP, Yopp A, McMillian N, Schonfeld R, Hochstetler C. NCCN Guidelines® Insights: Biliary Tract Cancers, Version 2.2023. Journal of the National Comprehensive Cancer Network : JNCCN. 2023 Jul:21(7):694-704. doi: 10.6004/jnccn.2023.0035. Epub [PubMed PMID: 37433432]
Pedersoli F, Schröder A, Zimmermann M, Schulze-Hagen M, Keil S, Ulmer TF, Neumann UP, Kuhl CK, Bruners P, Isfort P. Percutaneous transhepatic biliary drainage (PTBD) in patients with dilated vs. nondilated bile ducts: technical considerations and complications. European radiology. 2021 May:31(5):3035-3041. doi: 10.1007/s00330-020-07368-6. Epub 2020 Oct 13 [PubMed PMID: 33051733]
Wagner A, Mayr C, Kiesslich T, Berr F, Friesenbichler P, Wolkersdörfer GW. Reduced complication rates of percutaneous transhepatic biliary drainage with ultrasound guidance. Journal of clinical ultrasound : JCU. 2017 Sep:45(7):400-407. doi: 10.1002/jcu.22461. Epub 2017 Mar 2 [PubMed PMID: 28251661]
Nennstiel S, Treiber M, Faber A, Haller B, von Delius S, Schmid RM, Neu B. Comparison of Ultrasound and Fluoroscopically Guided Percutaneous Transhepatic Biliary Drainage. Digestive diseases (Basel, Switzerland). 2019:37(1):77-86. doi: 10.1159/000493120. Epub 2018 Sep 25 [PubMed PMID: 30253406]
Laufer U, Kirchner J, Kickuth R, Adams S, Jendreck M, Liermann D. A comparative study of CT fluoroscopy combined with fluoroscopy versus fluoroscopy alone for percutaneous transhepatic biliary drainage. Cardiovascular and interventional radiology. 2001 Jul-Aug:24(4):240-4 [PubMed PMID: 11779013]
Level 2 (mid-level) evidenceGawlik C, Carneval M. A Review of the Management of Bile Leaks. Cureus. 2021 May 10:13(5):e14937. doi: 10.7759/cureus.14937. Epub 2021 May 10 [PubMed PMID: 34123634]
Houghton EJ, Invernizzi E, Acquafresca P, Palermo M, Giménez ME. RISK OF BLEEDING COMPLICATIONS IN PERCUTANEOUS BILIARY DRAINAGE: THE PARADOX OF THE NORMAL HEMOSTASIS. Arquivos brasileiros de cirurgia digestiva : ABCD = Brazilian archives of digestive surgery. 2019:32(3):e1454. doi: 10.1590/0102-672020190001e1454. Epub 2019 Oct 21 [PubMed PMID: 31644674]
Houghton EJ,Uribe AK,De Battista JM,Finger C,Acquafresca P,Palermo M,Giménez ME, Risk Factors for Hemorrhagic Adverse Events in Percutaneous Transhepatic Biliary Drainage: A Prospective Multicenter Study. Journal of vascular and interventional radiology : JVIR. 2022 Aug; [PubMed PMID: 35504435]
Level 2 (mid-level) evidenceYoung M, Wagner A. Medical Ethics. StatPearls. 2025 Jan:(): [PubMed PMID: 30570982]
Araz H, Eren T, Kocagül-Çelikbaş A, Özdemir N. Evaluation of Blood Stream and Biliary Tract Infections Related to Percutaneous Transhepatic Cholangiography and Prophylaxis Given in Patients with Malignancy. Infectious diseases & clinical microbiology. 2022 Dec:4(4):274-279. doi: 10.36519/idcm.2022.176. Epub 2022 Dec 21 [PubMed PMID: 38633711]
Chehab MA, Thakor AS, Tulin-Silver S, Connolly BL, Cahill AM, Ward TJ, Padia SA, Kohi MP, Midia M, Chaudry G, Gemmete JJ, Mitchell JW, Brody L, Crowley JJ, Heran MKS, Weinstein JL, Nikolic B, Dariushnia SR, Tam AL, Venkatesan AM. Adult and Pediatric Antibiotic Prophylaxis during Vascular and IR Procedures: A Society of Interventional Radiology Practice Parameter Update Endorsed by the Cardiovascular and Interventional Radiological Society of Europe and the Canadian Association for Interventional Radiology. Journal of vascular and interventional radiology : JVIR. 2018 Nov:29(11):1483-1501.e2. doi: 10.1016/j.jvir.2018.06.007. Epub 2018 Sep 28 [PubMed PMID: 30274857]
Schmitz D, Valiente CT, Dollhopf M, Perez-Miranda M, Küllmer A, Gornals J, Vila J, Weigt J, Voigtländer T, Redondo-Cerezo E, von Hahn T, Albert J, Vom Dahl S, Beyna T, Hartmann D, Franck F, García-Alonso FJ, Schmidt A, Garcia-Sumalla A, Arrubla A, Joerdens M, Kleemann T, Tomo JRA, Grassmann F, Rudi J. Percutaneous transhepatic or endoscopic ultrasound-guided biliary drainage in malignant distal bile duct obstruction using a self-expanding metal stent: Study protocol for a prospective European multicenter trial (PUMa trial). PloS one. 2022:17(10):e0275029. doi: 10.1371/journal.pone.0275029. Epub 2022 Oct 27 [PubMed PMID: 36302047]
Level 1 (high-level) evidenceLee SH, Hahn ST, Hahn HJ, Cho KJ. Single-wall puncture: a new technique for percutaneous transhepatic biliary drainage. AJR. American journal of roentgenology. 2003 Sep:181(3):717-9 [PubMed PMID: 12933466]
Turan AS, Jenniskens S, Martens JM, Rutten MJCM, Yo LSF, van Strijen MJL, Drenth JPH, Siersema PD, van Geenen EJM. Complications of percutaneous transhepatic cholangiography and biliary drainage, a multicenter observational study. Abdominal radiology (New York). 2022 Sep:47(9):3338-3344. doi: 10.1007/s00261-021-03207-4. Epub 2021 Aug 6 [PubMed PMID: 34357434]
Level 2 (mid-level) evidenceMastier C, Valette PJ, Adham M, Mabrut JY, Glehen O, Ponchon T, Rousset P, Rode A. Complex Biliary Leaks: Effectiveness of Percutaneous Radiological Treatment Compared to Simple Leaks in 101 Patients. Cardiovascular and interventional radiology. 2018 Oct:41(10):1566-1572. doi: 10.1007/s00270-018-2005-1. Epub 2018 Jun 5 [PubMed PMID: 29872897]
Widyaningtiyas I, Sarastika HY, Utama HW. Bleeding after percutaneous transhepatic biliary drainage due to arterial injury: A case study in patient with stable hemodynamic. Radiology case reports. 2022 Dec:17(12):4868-4873. doi: 10.1016/j.radcr.2022.09.061. Epub 2022 Oct 12 [PubMed PMID: 36263331]
Level 3 (low-level) evidencePatel RK, Alagappan A, Tripathy T, Nayak HK, Pattnaik B, Dutta T, Gupta S, Mohakud S, Naik S, Deep Bag N. Bloody Bile and Rescue Intervention-A Case Series of Post-PTBD Hemorrhagic Complications With a Review of the Literature. Journal of clinical and experimental hepatology. 2024 Jul-Aug:14(4):101392. doi: 10.1016/j.jceh.2024.101392. Epub 2024 Mar 5 [PubMed PMID: 38558862]
Level 2 (mid-level) evidenceLubbe J, Lindemann J, Gondo W, Kolev N, Aclavio P, Hofmeyr S, Jonas E. Endoscopic versus percutaneous intervention for palliation in malignant hilar bile duct obstruction - A comparative cohort study. HPB : the official journal of the International Hepato Pancreato Biliary Association. 2022 Dec:24(12):2145-2156. doi: 10.1016/j.hpb.2022.09.005. Epub 2022 Sep 16 [PubMed PMID: 36253268]
Level 2 (mid-level) evidenceScheiman JM, Carlos RC, Barnett JL, Elta GH, Nostrant TT, Chey WD, Francis IR, Nandi PS. Can endoscopic ultrasound or magnetic resonance cholangiopancreatography replace ERCP in patients with suspected biliary disease? A prospective trial and cost analysis. The American journal of gastroenterology. 2001 Oct:96(10):2900-4 [PubMed PMID: 11693324]
Artifon EL, Aparicio D, Paione JB, Lo SK, Bordini A, Rabello C, Otoch JP, Gupta K. Biliary drainage in patients with unresectable, malignant obstruction where ERCP fails: endoscopic ultrasonography-guided choledochoduodenostomy versus percutaneous drainage. Journal of clinical gastroenterology. 2012 Oct:46(9):768-74. doi: 10.1097/MCG.0b013e31825f264c. Epub [PubMed PMID: 22810111]
Sun N, Xu Q, Liu X, Liu W, Wang J. Comparison of preoperative evaluation of malignant low-level biliary obstruction using plain magnetic resonance and coronal liver acquisition with volume acceleration technique alone and in combination. European journal of medical research. 2015 Nov 25:20():92. doi: 10.1186/s40001-015-0188-3. Epub 2015 Nov 25 [PubMed PMID: 26607835]
Rösch T, Meining A, Frühmorgen S, Zillinger C, Schusdziarra V, Hellerhoff K, Classen M, Helmberger H. A prospective comparison of the diagnostic accuracy of ERCP, MRCP, CT, and EUS in biliary strictures. Gastrointestinal endoscopy. 2002 Jun:55(7):870-6 [PubMed PMID: 12024143]
Mocan T, Horhat A, Mois E, Graur F, Tefas C, Craciun R, Nenu I, Spârchez M, Sparchez Z. Endoscopic or percutaneous biliary drainage in hilar cholangiocarcinoma: When and how? World journal of gastrointestinal oncology. 2021 Dec 15:13(12):2050-2063. doi: 10.4251/wjgo.v13.i12.2050. Epub [PubMed PMID: 35070041]
Wang Y, Zhao X, She Y, Kang Q, Chen X. The clinical efficacy and safety of different biliary drainage in malignant obstructive jaundice: a meta-analysis. Frontiers in oncology. 2024:14():1370383. doi: 10.3389/fonc.2024.1370383. Epub 2024 Apr 9 [PubMed PMID: 38655140]
Level 1 (high-level) evidenceKurdia KC, Irrinki S, Siddharth B, Gupta V, Lal A, Yadav TD. Percutaneous transhepatic cholangiography in the era of magnetic resonance cholangiopancreatography: A prospective comparative analysis in preoperative evaluation of benign biliary stricture. JGH open : an open access journal of gastroenterology and hepatology. 2021 Jul:5(7):820-824. doi: 10.1002/jgh3.12594. Epub 2021 Jun 23 [PubMed PMID: 34263078]
Level 2 (mid-level) evidenceElmunzer BJ, Smith ZL, Tarnasky P, Wang AY, Yachimski P, Banovac F, Buscaglia JM, Buxbaum J, Chak A, Chong B, Coté GA, Draganov PV, Dua K, Durkalski V, Geller BS, Jamil LH, Keswani RN, Khashab MA, Law R, Lo SK, McCarthy S, Selby JB, Singh VK, Taylor JR, Willingham FF, Spitzer RL, Foster LD, INTERCPT study group and the United States Cooperative for Outcomes Research in Endoscopy (USCORE). An Unsuccessful Randomized Trial of Percutaneous vs Endoscopic Drainage of Suspected Malignant Hilar Obstruction. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2021 Jun:19(6):1282-1284. doi: 10.1016/j.cgh.2020.05.035. Epub 2020 May 23 [PubMed PMID: 32454259]
Level 1 (high-level) evidenceSun XR, Tang CW, Lu WM, Xu YQ, Feng WM, Bao Y, Zheng YY. Endoscopic Biliary Stenting Versus Percutaneous Transhepatic Biliary Stenting in Advanced Malignant Biliary Obstruction: Cost-effectiveness Analysis. Hepato-gastroenterology. 2014 May:61(131):563-6 [PubMed PMID: 26176036]
Young M, Smith MA. Standards and Evaluation of Healthcare Quality, Safety, and Person-Centered Care. StatPearls. 2025 Jan:(): [PubMed PMID: 35015457]
Level 2 (mid-level) evidenceLégaré F, Adekpedjou R, Stacey D, Turcotte S, Kryworuchko J, Graham ID, Lyddiatt A, Politi MC, Thomson R, Elwyn G, Donner-Banzhoff N. Interventions for increasing the use of shared decision making by healthcare professionals. The Cochrane database of systematic reviews. 2018 Jul 19:7(7):CD006732. doi: 10.1002/14651858.CD006732.pub4. Epub 2018 Jul 19 [PubMed PMID: 30025154]
Level 1 (high-level) evidenceNiburski K, Guadagno E, Abbasgholizadeh-Rahimi S, Poenaru D. Shared Decision Making in Surgery: A Meta-Analysis of Existing Literature. The patient. 2020 Dec:13(6):667-681. doi: 10.1007/s40271-020-00443-6. Epub [PubMed PMID: 32880820]
Level 1 (high-level) evidence