Introduction
Parkinson disease (PD), also known as Idiopathic or primary parkinsonism, hypokinetic rigid syndrome, or paralysis agitans, is the second most common progressive neurodegenerative disorder, affecting 2% to 3% of people older than 65. The degeneration of dopaminergic neurons in the substantia nigra, accompanied by the intracellular accumulation of alpha-synuclein (α-synuclein) (Lewy body), represents the neuropathological hallmark of the disease.[1] PD usually presents in later life with the cardinal clinical motor features of bradykinesia, resting tremor, and rigidity, often in various combinations. Postural instability emerges later in the disease course as another defining feature (see Image. An Illustration of Parkinson Disease). The motor symptoms of PD are typically asymmetric, which helps differentiate it from other Parkinsonian syndromes. By the time a clinical diagnosis is made, more than 50% and up to 80% of the dopaminergic neurons have degenerated. Recognition is growing that the pathology of PD begins a decade or more before clinical diagnosis, with α-synuclein deposition in other neurons, including those of the gastrointestinal tract, olfactory structures, hypothalamus, and autonomic nervous system. These early changes contribute to premotor and nonmotor symptoms such as anosmia or hyposmia, constipation, and sleep dysfunction, especially rapid eye movement sleep behavior disorder. Additional nonmotor features include depression, orthostatic hypotension, dementia, and psychosis.[2][3][4][5][6]
Current estimates suggest that PD affects at least 1% of individuals over the age of 60, and the disorder is the fastest-growing neurodegenerative condition worldwide. Most cases are idiopathic, though approximately 10% have a genetic cause, with 3% to 5% of patients carrying inherited pathogenic variants in known PD genes.[7] Onset is generally insidious, but progression is inevitable (see Image. Progression of Symptoms in Parkinson Disease). Tremor is often the first clinical sign and may later be accompanied by bradykinesia and rigidity. Postural instability typically develops in the later stages, significantly impairing quality of life. Dopamine transporter single-photon emission computed tomography scans can aid in uncertain cases or help exclude other neurological disorders. Management focuses on dopaminergic therapies to address motor symptoms, while nonmotor manifestations often require additional targeted treatments. No disease-modifying therapy has yet been proven. However, stereotactic neurosurgical interventions, such as deep-brain stimulation, offer effective options for patients with advanced motor complications.[6]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Over the past century, our understanding of the etiology of PD has evolved immensely. In 1919, it was first recognized that loss of pigmentation in the substantia nigra of the midbrain is a characteristic feature of postmortem brain examinations of patients with PD. In the 1950s, it was further understood that the pigmented neurons lost in the substantia nigra are dopaminergic, and it is the loss of dopamine in subcortical motor circuits that is implicated in the mechanism of the movement disorder in PD.[8][9] PD is a disorder of the basal ganglia, which is composed of many other nuclei. The striatum receives excitatory and inhibitory input from several parts of the cortex. The key pathology of the classical motor symptoms is the loss of dopaminergic neurons.
The cause of PD has been linked to the use of pesticides and herbicides and its proximity to industrial plants. Mild to moderate head injury has been shown to have an increased risk. Some individuals have been found to develop Parkinsonian-like features after injection of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine MPTP. This chemical accumulates in the mitochondria. There is also research suggesting that oxidation and the generation of free radicals may cause damage to the thalamic nuclei.[10] Decreased risk of PD has been associated with cigarette smoking, caffeine intake, and physical activity.[11]
Genes are important, as the risk of PD in siblings is increased if one member of the family has the disorder. Genetic and familial cases are identified in nearly 20% of PD patients. These cases also tend to occur much earlier in life. Approximately 3% to 5% of PD cases are inherited due to mutations in known PD genes.[7] Autosomal dominant inherited PD genes identified include LRRK2, the most common, accounting for 1% to 2% of all PD cases and 40% of familial cases. Other identified genes are GBA1, VPS35, SNCAPRKN, PINK1, and DJ1 genes.[12]
The key motor and diagnostic symptoms of PD are caused by loss of dopaminergic neurons in the substantia nigra. PD is a multisystem neurodegenerative disorder affecting neurons in both the central and peripheral nervous systems. The most significant pathology is the abnormal accumulation of α-synuclein in all affected neurons, resulting in premotor symptoms that may appear up to 15 years before clinical diagnosis and nonmotor symptoms.[2] Current research is focused on preventing the propagation and aggregation of α-synuclein.
Epidemiology
PD affects 1 to 2 people per 1000 at any time; the prevalence increases with age to affect 1% of the population 60 years and older, and 2% to 3% older the age of 65.5% to 10% of patients have a genetic predisposition. The incidence and prevalence of PD do increase with advancing age; the condition is more common in men than women, with a ratio of approximately 2:1.[2]
Pathophysiology
PD is a progressive neurodegenerative disorder with the most important pathology being the deposition of aggregated α-synuclein. Various studies of cell and animal models, biochemical studies, and neurons of PD patients indicate that there is abnormal accumulation of α-synuclein in the myenteric plexus of the gut, followed by spreading of the pathology between the gut, the brainstem, the olfactory apparatus, and other brain regions, which underlie the development and progression of PD. Dysfunction of lysosomes, endosomes, and mitochondria has been identified at a cellular or subcellular level. Recent study results have also indicated that abnormal immune and inflammatory responses originating from the gut contribute to the final pathology of PD.[13][14]
The accumulation of α-synuclein in various parts of the brain, primarily the substantia nigra, leads to degeneration and subsequent loss of dopamine in the basal ganglia that control muscle tone and movement (see Image. Basal Ganglia Function in Extrapyramidal Motor Disorders). The accumulation of α-synuclein in the gut and other areas also addresses why patients with PD typically suffer from gastrointestinal motility issues with constipation and rapid eye movement sleep disorder, both of which can precede the motor manifestations of PD for many years. Therefore, as our understanding of the etiology of PD evolves, it is insufficient to regard this disorder as primarily caused by a deficiency of dopamine in the substantia nigra. There appears to be a more complex and extensive pathophysiology underlying this, and the nongenetic causes of α-synuclein deposition in the brain are a subject of active research.[15]
Histopathology
Loss of pigmented neurons in the substantia nigra is visible to the naked eye in cases of PD. Loss of neuromelanin secondary to loss of dopaminergic neurons in the substantia nigra pars compacta results in the pale appearing substantia nigra when compared with the normal "black substance"—the Latin word for substantia nigra. Histologically, PD is characterized by neuronal inclusions of α-synuclein in neuronal cell bodies (Lewy bodies) and within neuronal cell processes (Lewy neurites) (see Image. Alpha-Synuclein Deposits in the Substantia Nigra in Parkinson Disease). The combination of Lewy bodies and Lewy neurites is sometimes referred to as Lewy-related pathology.[16] The hallmark of any neurodegenerative disease is selective neuronal loss, and loss in PD is most marked in the substantia nigra pars compacta. However, it has been known for many years that Lewy bodies in PD extend well beyond the substantia nigra.
Based on the distribution of α-synuclein pathology, Braak and coworkers have proposed a staging scheme for PD. In this scheme, neuronal pathology occurs early in the myenteric plexus of the gut, followed by the dorsal motor nucleus of the vagus in the medulla and the anterior olfactory nucleus in the olfactory bulb. As the disease progresses, locus ceruleus neurons in the pons and then dopaminergic neurons in the substantia nigra are affected. Pathology extends to the basal forebrain, amygdala, and medial temporal lobe structures in later stages, with convexity cortical areas affected in the last stages. Pathologically, the peripheral nervous system is involved, preceding the central nervous system (CNS), which has prompted the recognition that PD is a multiorgan disease process, not merely a disorder of the CNS. Moreover, it has fed the debate on cell-to-cell transmission of transmissible agents from the gut to the brain through retrograde transmission in the vagus nerve.[17]
History and Physical
An earlier feature of PD is tremor, typically unilateral and present at rest, which is usually the reason for seeking help at a neurology clinic. After using the hands, such as to pick up a book, the tremor may subside for some minutes, only to return when the patient is distracted and at rest once again. This is the so-called reemerging tremor that is typical of PD. Although tremor is a prominent and early symptom of PD, it is not always present and is not a necessary feature for diagnosis.
Slowness, or bradykinesia, is a core feature of PD. Patients will notice it takes them longer to do simple tasks, their walking is slower, and their ability to respond to threats is compromised. In the clinic, patients cannot tap their index finger and thumb rapidly, tap their foot rhythmically on the floor, or walk steadily. Rigidity is the third prominent feature of the examination. Patients appear stiff and struggle to rise out of a chair without support. While walking, there is reduced arm swing, more so on 1 side than the other, as PD typically is asymmetric at the onset. Checking muscle tone, lead pipe, and/or cogwheel rigidity can be appreciated. A combination of bradykinesia and rigidity leads to some other characteristic features of PD, such as micrographia.
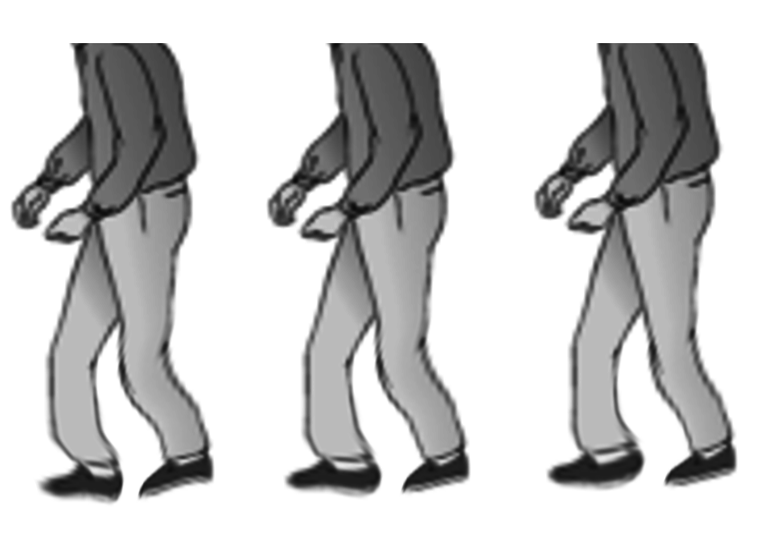
The fourth prominent feature of PD is gait instability, although this typically manifests late. Flexed posture, reduced arm swing, festination, march-a-petits-pas, camptocormia, retropulsion, and turning en bloc are common terms used to describe the gait in PD. In advanced PD, patients will have trouble rising from a chair without support, they take small, slow steps, they are unable to stop themselves from falling if pushed lightly, they cannot turn around without taking several small steps, and they tend to freeze when faced with certain stimuli such as a doorframe or a passerby (see Image. Shuffling Gait Seen in Parkinson Disease). With experience, a neurologist can diagnose a long-suffering PD patient based on their progress from the waiting room to the office chair. However, it is worth noting that other neurological conditions, such as normal pressure hydrocephalus, can also produce a similar gait. Gait disorder is not an early feature of PD, but is frequently described as it is easy to recognize and cinches the diagnosis in later stages.
Apart from the above 4 prominent features of PD in the clinic, patients are usually asked about constipation, drooling, mood disorder and depression, rapid eye movement sleep disorder, and anosmia. These subtle symptoms frequently accompany the tremor/rigidity paradox of patients with PD and reflect the underlying α-synuclein deposition-mediated neurodegeneration in parts of the brain other than the substantia nigra.[2] Autonomic symptoms are common in PD. In addition to orthostatic hypotension, constipation, difficulty swallowing, urinary retention, and erectile dysfunction frequently occur. Often, these symptoms do not improve with treatment. Depression is also very common in PD. As the disease progresses, dementia with significant loss of cognitive function often develops.
Evaluation
Evaluation of patients with PD typically begins with a comprehensive history and physical examination, focusing on identifying the signs described above. Movement disorders clinics may employ the Unified Parkinson Disease Rating Scale to quantify patient mentation, behavior, mood, activities of daily living, tremors, motor examination, and complications of therapy.[18][19][20] There is no specific laboratory or imaging study that can definitively diagnose PD. An essential part of the evaluation of PD is to exclude the effect of medications that can lead to extrapyramidal side effects and motor manifestations that are, at times, indistinguishable from classical PD. Typically, it is the traditional antipsychotics that are implied. In these cases, the nonmotor manifestations of PD, such as anosmia, are not found.
Notably, other neurodegenerative conditions must be excluded. Other α-synucleinopathies, such as Lewy body disease and multiple system atrophy, can present with PD but have subtle differences that distinguish them as separate disease entities. Similarly, tauopathies such as progressive supranuclear palsy can present with bradykinesia, rigidity, and gait disturbance.
For example, all the diseases below may have the same motor manifestations of PD, but in addition, they will have the following:
- Progressive supranuclear palsy patients will have vertical gaze paralysis on examination.
- Lewy body dementia will have the added features of earlier onset of dementia and hallucinations, especially vivid, formed visual hallucinations. These patients are also susceptible to the extrapyramidal side effects of neuroleptics.
- Multisystem atrophy typically presents with early-onset autonomic dysfunction and pyramidal and cerebellar signs.
Regarding investigations, PD remains 1 of the few medical conditions for which a clinical examination is sufficient for a formal diagnosis. Magnetic resonance imaging helps narrow the differential and exclude other conditions that may present with a similar exam, such as normal pressure hydrocephalus or subcortical stroke. In certain situations, such as research, a dopamine transporter scan may be used to identify the loss of dopaminergic uptake in the basal ganglia. However, interpreting this test can be challenging, and its routine use is discouraged.
Practically, 1 of the best ways of establishing a diagnosis of PD in a patient with suggestive symptoms is a clear response to levodopa treatment. This is either short-lived or poorly understood due to the overlap of syndromes.[19][21][22] Awareness of the high rate of diagnostic errors between PD and essential tremor in community practice is critical, as misdiagnosis can delay appropriate treatment and worsen patient outcomes. Imaging studies are helpful to exclude bleeding, stroke, hydrocephalus, mass lesions, and Wilson disease. Lumbar puncture is done to exclude normal pressure hydrocephalus.
Treatment / Management
Currently, there is no available disease-modifying or slowing therapy. Any treatment for those with PD is symptomatic and aimed at improving the patients' function. PD management should be done using a multidisciplinary approach early in the illness. Healthy lifestyles, including a balanced diet, regular physical activity, and adequate sleep, have been linked to improved outcomes and reduced mortality risk.[23]
Pharmacotherapy of PD Motor Symptoms
Pharmacotherapy for PD focuses on restoring dopaminergic activity in the basal ganglia to alleviate motor symptoms, with treatment strategies tailored to disease stage, symptom burden, and patient-specific factors (see Image. Treatment for Motor Symptoms in Parkinson Disease). Oral levodopa is the most effective treatment for the motor symptoms of PD. Levodopa (L-dopa), absorbed from the gut, is easily converted to dopamine in the periphery. Carbidopa, a dopa decarboxylase inhibitor, is added to prevent this conversion, allowing levodopa to enter the brain and be converted to dopamine. This combination, carbidopa-levodopa, is the primary treatment for PD. Tremor is generally less responsive than bradykinesia and rigidity.
The diagnosis will likely be wrong if a patient does not have a good motor response to levodopa treatment. The duration of action of levodopa is relatively short, about 3 to 4 hours. The duration during which the patient experiences the beneficial effects of levodopa is referred to as the "on" time, and the duration when there is no effect is referred to as the "off" time. When the disease is mild, the "on" time is significantly longer, primarily because the remaining dopaminergic neurons act as reservoirs for dopamine. As the disease progresses, the "on" time becomes shorter due to the progressive degeneration of dopaminergic neurons and the short half-life of L-dopa. There will also be more motor fluctuations. The common side effects of L-dopa include GI upsets, dyskinesia, and psychosis with hallucinations. There are different formulations of L-dopa to prolong the duration of action and to be used at different stages of the illness. These formulations can be classified as follows:
- Carbidopa/levodopa immediate-release: Sinemet
- This is the original and most commonly used formulation.
- Carbidopa/levodopa extended-release: Sinemet-CR
- This form lasts longer than the immediate-release.
- Carbidopa/levodopa/entacapone: Stalevo
- This combination includes an entacapone, a catechol-O-methyltransferase inhibitor, to prolong the duration of action.
- Carbidopa/levodopa extended-release capsules: Rytary
- This formulation contains varying types of beads packaged within a capsule. The different beads release the levodopa at different rates, allowing for a more prolonged release of the medication from the stomach.
- Carbidopa/levodopa extended-release capsules: Crexont
- This formulation was approved by the United States Food and Drug Administration in 2024. This medication contains beads of different types within a capsule, with the immediate-release pellets dissolving first and the extended-release granules dissolving more slowly. The difference between Rytary and Crexont is that Crexont beads have additional features that allow a longer time for the medication to be released and absorbed.
- Carbidopa/levodopa enteral suspension: Duopa
- This is carbidopa/levodopa in gel form infused directly into the small intestine through a jejunostomy tube. This system is indicated and effective for those with advancing PD who have motor fluctuations that are no longer controlled by oral medications alone; this can also be beneficial for individuals with gastroparesis, a condition characterized by delayed gastric emptying.
- Continuous subcutaneous carbidopa/levodopa infusion: Vyalev
- This was approved in 2024. This form is a 24-hour continuous subcutaneous infusion for advanced PD with motor fluctuations.
- Carbidopa/levodopa formulations intended for use as rescue medications or as needed during periods of "off" symptoms include:
- Orally disintegrating tablets: Parcopa
- Inhalation powder: Inbrija
Most anti-Parkinsonian medications provide good symptom control for 3 to 6 years. After this period, the disease progresses and is often unresponsive to medications. Generally, younger patients should be treated more aggressively than older individuals.
Pharmacotherapy of Nonmotor Symptoms
The nonmotor symptoms, like psychiatric, autonomic, and sensory, are much harder to manage. Simply adding more medications to the patient's regimen is not the answer because of potential adverse reactions and drug-drug interactions. Current recommendations include:
- Sildenafil can be used to treat erectile dysfunction.
- Modafinil is used for daytime somnolence.
- Polyethylene glycol is given for constipation.
- Levodopa is helpful for periodic limb movements during sleep.
- Methylphenidate is used for fatigue.
- Dementia is managed with cholinesterase inhibitors.
- Depression is managed with selective serotonin reuptake inhibitors (SSRIs).
- Psychotic symptoms are managed with antipsychotics and pimavanserin.
- Anxiety is managed with SSRIs.
- Impulse behaviors are managed by cognitive behavior therapy.
Surgical Approaches
For some patients who do not respond to all measures, deep-brain stimulation (DBS) techniques are increasingly available to stabilize the balance of excitatory and inhibitory signals to the subthalamic nucleus or the globus pallidus. DBS involves stimulation of the subthalamic nucleus, globus pallidus interna, and the thalamus. The mechanism of action of DBS is not well understood, but is considered to be due in part to the interruption of aberrantly functioning basal ganglia circuits. DBS is typically implanted and managed in specialized centers.[24][25][26][27][28]
DBS ameliorates motor fluctuations and improves the overall quality of life. This treatment increases "on" time by at least 3 to 4 hours a day without adding any medication, and allows for a reduction in medication with an average dose reduction of 50%. DBS is indicated when there are motor fluctuations, with its benefits lasting up to 15 years.
Differential Diagnosis
The differential diagnosis of PD includes several conditions that can mimic its motor and nonmotor symptoms. Essential tremor is the most common differential, especially in tremor-predominant PD. Unlike the resting tremor of PD, essential tremor is typically an action or kinetic tremor, often bilateral, and best differentiated using dopamine transporter imaging. Multiple system atrophy can resemble PD but is marked by prominent autonomic dysfunction, particularly early orthostatic hypotension.
Dementia with Lewy bodies also shares overlapping features, but early psychosis and visual hallucinations are distinctive, and patients are notably sensitive to antipsychotics. Progressive supranuclear palsy is differentiated by impaired vertical gaze and other oculomotor abnormalities. Finally, normal pressure hydrocephalus can present with Parkinsonian features but is classically associated with the triad of urinary incontinence, gait apraxia, and cognitive decline, with MRI playing a key role in diagnosis.
Prognosis
The time course for PD progression is variable. Individuals with just tremors as the initial presentation tend to have a protracted benign course. The early motor symptoms of tremor, rigidity, and bradykinesia tend to be asymmetric. These eventually become bilateral, characterized by postural instability, functional impairment, and loss of independence. Cognitive decline develops in about 10% of PD patients per year. [2] Premotor symptoms, including constipation, hyposmia, autonomic dysfunction, and REM sleep behavioral disorder, precede motor problems often by decades. The disorder leads to disability in most patients within 10 years. The mortality rate of patients with PD is 3 times that of the normal population. While treatment can improve symptoms, the quality of life for most patients with PD is poor.[29][30][31]
Complications
Complications of PD include:
- Depression
- Dementia
- Laryngeal dysfunction with dysphagia and aspiration
- Autonomic dysfunction
- Death
Postoperative and Rehabilitation Care
All patients diagnosed with PD need long-term follow-up because of the progressive behavior and motor abnormalities. Slight adjustments in medications are needed in most patients. In addition, many patients develop erratic behavior like impulsivity, psychosis, paranoia, and somnolence.
Consultations
Consultations for a patient with Parkinson disease may include:
- Psychiatrist
- Neurosurgeon
- Speech therapist
- Urologist
- Dietitian
- Gastroenterologist
- Otolaryngologist
- Physical therapist
- Occupational therapist
Deterrence and Patient Education
PD is a progressive neurodegenerative disorder with no known disease-modifying therapy. Any treatment for PD patients is symptomatic and aimed at improving the patients' function. The patients need to be well educated about this important fact. Good medication compliance by patients is necessary to improve their overall health and function. They also need to be made aware that their best management should be a multidisciplinary approach from the early stages of their illness. Healthy lifestyles, including a balanced diet, regular exercise, and adequate sleep, have been linked to improved outcomes and reduced mortality.
Pearls and Other Issues
Acknowledging the nonmotor manifestations of PD is essential, as these symptoms can profoundly affect quality of life and require targeted management. Sildenafil may be prescribed for erectile dysfunction, polyethylene glycol for constipation, modafinil for excessive daytime somnolence, and methylphenidate for persistent fatigue. Managing these symptoms in conjunction with motor features promotes comprehensive, patient-centered care.
Enhancing Healthcare Team Outcomes
Optimal management of PD requires a multidisciplinary approach that leverages the unique skills of physicians, advanced practitioners, nurses, pharmacists, and allied health professionals. Physicians and advanced practitioners must develop expertise in early recognition, accurate diagnosis, and individualized treatment planning, including titration of dopaminergic and adjunctive therapies. Nurses play a vital role in patient education, monitoring for medication side effects, and supporting adherence to complex treatment regimens. Pharmacists contribute by evaluating drug interactions, optimizing polypharmacy, and reinforcing safe medication use, particularly in elderly patients with multiple comorbidities. Rehabilitation specialists, including physical, occupational, and speech therapists, enhance function, mobility, and communication. Meanwhile, social workers and mental health providers address psychosocial needs and support caregivers. Patients should be provided with information on end-of-life or hospice care, financial planning, disability application processes, and referrals to nursing homes.[31][32]
Effective interprofessional communication and care coordination are central to improving outcomes for PD patients. Structured communication strategies like care conferences and shared decision-making allow the healthcare team to align treatment goals with patient values while minimizing complications. Coordinated care ensures continuity across inpatient, outpatient, and community settings, reducing hospitalizations, medication errors, and caregiver strain. By working collaboratively, healthcare teams can deliver patient-centered care that maximizes quality of life, preserves independence, enhances safety, and supports patients and their families throughout the disease trajectory.
Media
(Click Image to Enlarge)
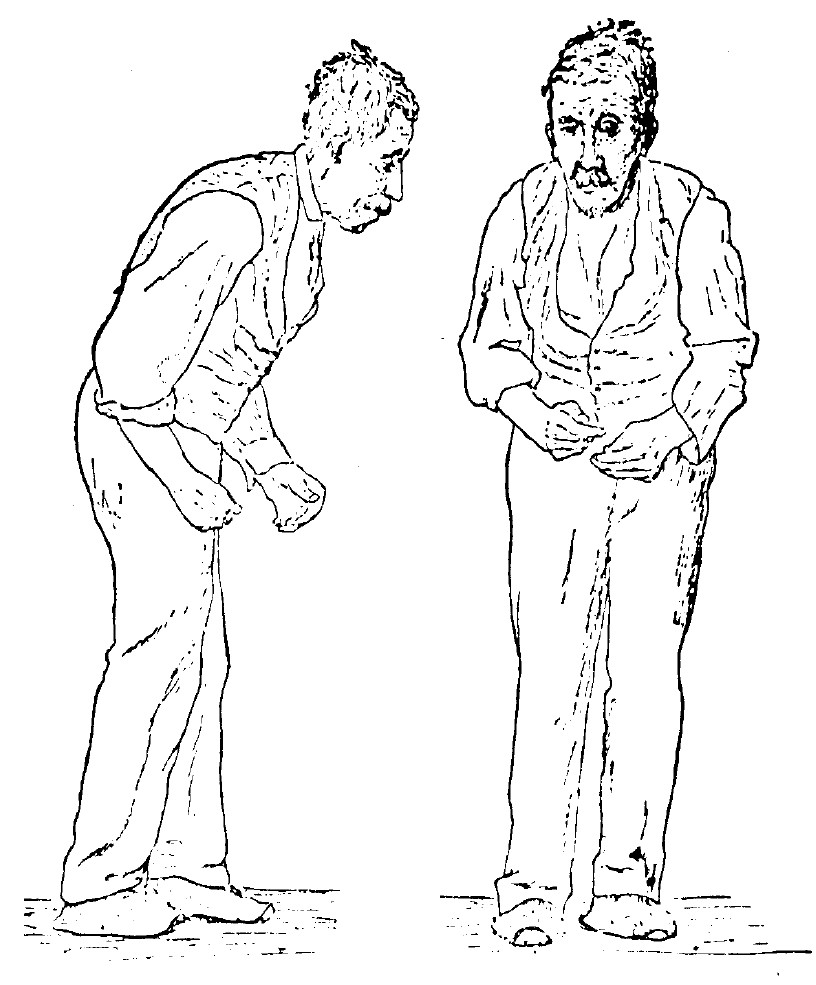
An Illustration of Parkinson Disease. Sir William Richard Gowers, neurologist, researcher, and artist, drew this illustration in 1886 as part of his documentation of Parkinson Disease. The image appeared in his book, A Manual of Diseases of the Nervous System, still used today by medical professionals as a primary reference for this disease.
Sir William Richard Gowers, Public Domain, via Wikimedia Commons
(Click Image to Enlarge)
(Click Image to Enlarge)
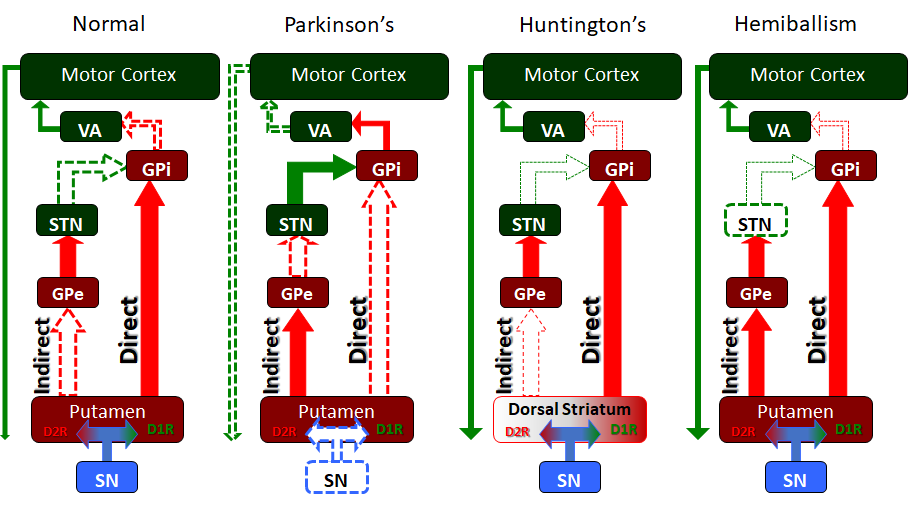
Basal Ganglia Function in Extrapyramidal Motor Disorders. The basal ganglia serve many functions, but especially in the releasing or "disinhibiting" of motor movement. In these simplified circuit maps, we see how the different nuclei and pathways of the basal ganglia interact in the normal individual to release motor function, as well as in several extrapyramidal motor disorders, including Parkinson disease, Huntington disease, and hemiballism.
Contributed by JWH Sonne, PhD
(Click Image to Enlarge)
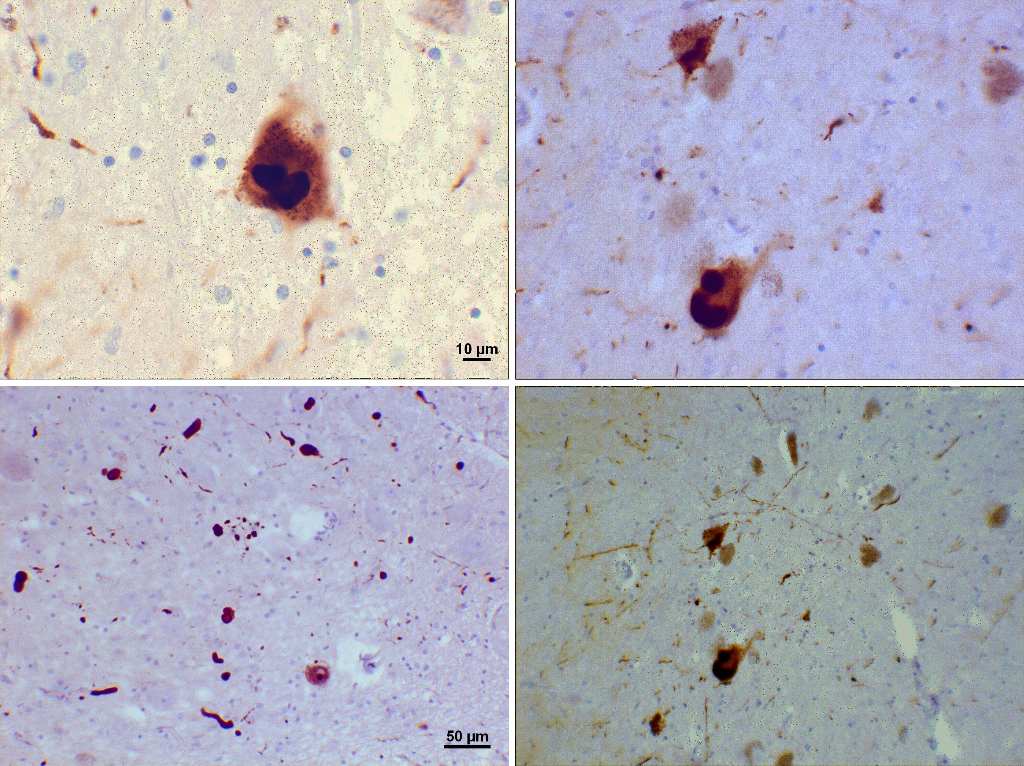
Alpha-Synuclein Deposits in the Substantia Nigra in Parkinson Disease. These histological images demonstrate alpha-synuclein deposits (Lewy bodies) in the substantia nigra as seen in Parkinson disease.
Suraj Rajan, Public Domain, via Wikimedia Commons
(Click Image to Enlarge)
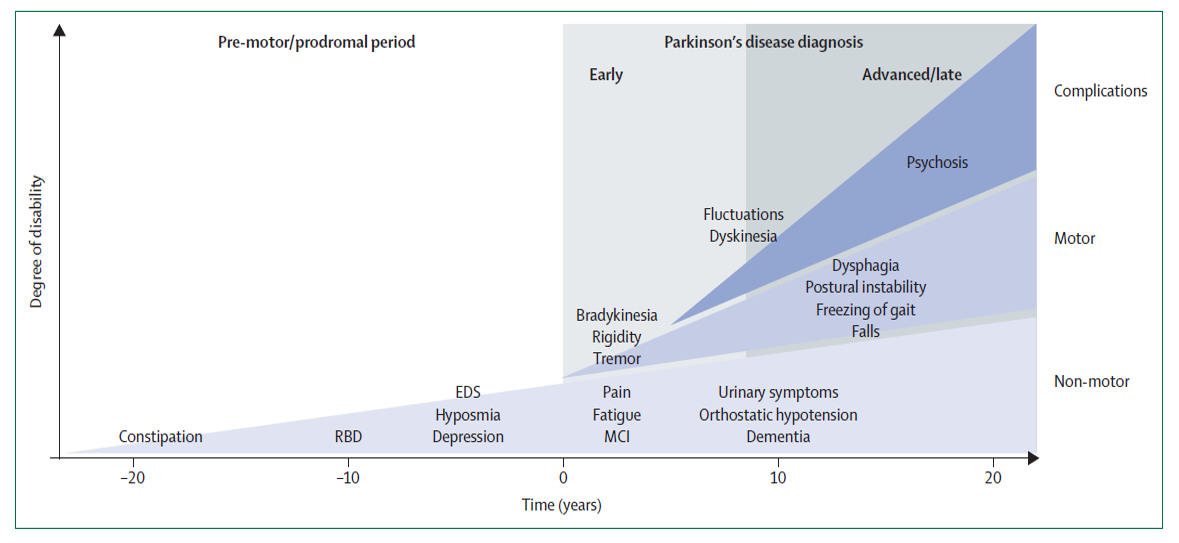
Progression of Symptoms in Parkinson Disease. This chart shows symptom progression in Parkinson disease from premotor/prodromal period (up to >20 years) to early and advanced motor and nonmotor presentations, as well as late complications.
Kalia LV, Lang AE. Lancet. 2015;386(9996):896-912. doi: 10.1016/S0140-6736(14)61393-3.
(Click Image to Enlarge)

Treatment for Motor Symptoms in Parkinson Disease. Oral levodopa is the most effective treatment for the motor symptoms of PD. Levodopa (L-dopa), absorbed from the gut, is easily converted to dopamine in the periphery. Carbidopa, a dopa decarboxylase inhibitor, is added to prevent this conversion, allowing levodopa to enter the brain and be converted to dopamine. This combination, carbidopa-levodopa, is the primary treatment for PD.
Wexler M, de Barros A. Treatment options for Parkinson's disease. Parkinson's News Today Web site. https://parkinsonsnewstoday.com/parkinsons-disease-treatments. Updated February 21, 2025. Accessed September 9, 2025.
References
Poewe W, Seppi K, Tanner CM, Halliday GM, Brundin P, Volkmann J, Schrag AE, Lang AE. Parkinson disease. Nature reviews. Disease primers. 2017 Mar 23:3():17013. doi: 10.1038/nrdp.2017.13. Epub 2017 Mar 23 [PubMed PMID: 28332488]
Tanner CM, Ostrem JL. Parkinson's Disease. The New England journal of medicine. 2024 Aug 1:391(5):442-452. doi: 10.1056/NEJMra2401857. Epub [PubMed PMID: 39083773]
Mirpour S, Turkbey EB, Marashdeh W, El Khouli R, Subramaniam RM. Impact of DAT-SPECT on Management of Patients Suspected of Parkinsonism. Clinical nuclear medicine. 2018 Oct:43(10):710-714. doi: 10.1097/RLU.0000000000002240. Epub [PubMed PMID: 30153144]
Alexoudi A, Alexoudi I, Gatzonis S. Parkinson's disease pathogenesis, evolution and alternative pathways: A review. Revue neurologique. 2018 Dec:174(10):699-704. doi: 10.1016/j.neurol.2017.12.003. Epub 2018 Aug 18 [PubMed PMID: 30131173]
Kabra A, Sharma R, Kabra R, Baghel US. Emerging and Alternative Therapies For Parkinson Disease: An Updated Review. Current pharmaceutical design. 2018:24(22):2573-2582. doi: 10.2174/1381612824666180820150150. Epub [PubMed PMID: 30124146]
Armstrong MJ, Okun MS. Diagnosis and Treatment of Parkinson Disease: A Review. JAMA. 2020 Feb 11:323(6):548-560. doi: 10.1001/jama.2019.22360. Epub [PubMed PMID: 32044947]
Funayama M, Nishioka K, Li Y, Hattori N. Molecular genetics of Parkinson's disease: Contributions and global trends. Journal of human genetics. 2023 Mar:68(3):125-130. doi: 10.1038/s10038-022-01058-5. Epub 2022 Jul 11 [PubMed PMID: 35821405]
Ehgoetz Martens KA, Shine JM, Walton CC, Georgiades MJ, Gilat M, Hall JM, Muller AJ, Szeto JYY, Lewis SJG. Evidence for subtypes of freezing of gait in Parkinson's disease. Movement disorders : official journal of the Movement Disorder Society. 2018 Jul:33(7):1174-1178. doi: 10.1002/mds.27417. Epub [PubMed PMID: 30153383]
Chung SJ, Yoo HS, Lee HS, Oh JS, Kim JS, Sohn YH, Lee PH. The Pattern of Striatal Dopamine Depletion as a Prognostic Marker in De Novo Parkinson Disease. Clinical nuclear medicine. 2018 Nov:43(11):787-792. doi: 10.1097/RLU.0000000000002251. Epub [PubMed PMID: 30153150]
Langston JW. The MPTP Story. Journal of Parkinson's disease. 2017:7(s1):S11-S19. doi: 10.3233/JPD-179006. Epub [PubMed PMID: 28282815]
Reynoso A, Torricelli R, Jacobs BM, Shi J, Aslibekyan S, Norcliffe-Kaufmann L, Noyce AJ, Heilbron K. Gene-Environment Interactions for Parkinson's Disease. Annals of neurology. 2024 Apr:95(4):677-687. doi: 10.1002/ana.26852. Epub 2024 Jan 12 [PubMed PMID: 38113326]
Blauwendraat C, Nalls MA, Singleton AB. The genetic architecture of Parkinson's disease. The Lancet. Neurology. 2020 Feb:19(2):170-178. doi: 10.1016/S1474-4422(19)30287-X. Epub 2019 Sep 11 [PubMed PMID: 31521533]
Morris HR, Spillantini MG, Sue CM, Williams-Gray CH. The pathogenesis of Parkinson's disease. Lancet (London, England). 2024 Jan 20:403(10423):293-304. doi: 10.1016/S0140-6736(23)01478-2. Epub [PubMed PMID: 38245249]
Zhou ZD, Yi LX, Wang DQ, Lim TM, Tan EK. Role of dopamine in the pathophysiology of Parkinson's disease. Translational neurodegeneration. 2023 Sep 18:12(1):44. doi: 10.1186/s40035-023-00378-6. Epub 2023 Sep 18 [PubMed PMID: 37718439]
Gratton C, Koller JM, Shannon W, Greene DJ, Maiti B, Snyder AZ, Petersen SE, Perlmutter JS, Campbell MC. Emergent Functional Network Effects in Parkinson Disease. Cerebral cortex (New York, N.Y. : 1991). 2019 Jun 1:29(6):2509-2523. doi: 10.1093/cercor/bhy121. Epub [PubMed PMID: 29878081]
Stoker TB, Greenland JC, Kouli A, Torsney KM, Kuan WL. Parkinson’s Disease: Etiology, Neuropathology, and Pathogenesis. Parkinson’s Disease: Pathogenesis and Clinical Aspects. 2018 Dec 21:(): [PubMed PMID: 30702842]
Bonaz B. The gut-brain axis in Parkinson's disease. Revue neurologique. 2024 Jan-Feb:180(1-2):65-78. doi: 10.1016/j.neurol.2023.11.004. Epub 2023 Dec 20 [PubMed PMID: 38129277]
Berg D, Adler CH, Bloem BR, Chan P, Gasser T, Goetz CG, Halliday G, Lang AE, Lewis S, Li Y, Liepelt-Scarfone I, Litvan I, Marek K, Maetzler C, Mi T, Obeso J, Oertel W, Olanow CW, Poewe W, Rios-Romenets S, Schäffer E, Seppi K, Heim B, Slow E, Stern M, Bledsoe IO, Deuschl G, Postuma RB. Movement disorder society criteria for clinically established early Parkinson's disease. Movement disorders : official journal of the Movement Disorder Society. 2018 Oct:33(10):1643-1646. doi: 10.1002/mds.27431. Epub 2018 Aug 25 [PubMed PMID: 30145841]
Creaby MW, Cole MH. Gait characteristics and falls in Parkinson's disease: A systematic review and meta-analysis. Parkinsonism & related disorders. 2018 Dec:57():1-8. doi: 10.1016/j.parkreldis.2018.07.008. Epub 2018 Jul 17 [PubMed PMID: 30041848]
Level 1 (high-level) evidenceOdin P, Chaudhuri KR, Volkmann J, Antonini A, Storch A, Dietrichs E, Pirtošek Z, Henriksen T, Horne M, Devos D, Bergquist F. Viewpoint and practical recommendations from a movement disorder specialist panel on objective measurement in the clinical management of Parkinson's disease. NPJ Parkinson's disease. 2018:4():14. doi: 10.1038/s41531-018-0051-7. Epub 2018 May 10 [PubMed PMID: 29761156]
Shen Y, Huang JY, Li J, Liu CF. Excessive Daytime Sleepiness in Parkinson's Disease: Clinical Implications and Management. Chinese medical journal. 2018 Apr 20:131(8):974-981. doi: 10.4103/0366-6999.229889. Epub [PubMed PMID: 29664059]
Goodarzi Z, Hanson HM, Jette N, Patten S, Pringsheim T, Holroyd-Leduc J. Barriers and Facilitators for Guidelines with Depression and Anxiety in Parkinson's Disease or Dementia. Canadian journal on aging = La revue canadienne du vieillissement. 2018 Jun:37(2):185-199. doi: 10.1017/S0714980818000053. Epub 2018 Apr 5 [PubMed PMID: 29618389]
Zhang X, Molsberry SA, Schwarzschild MA, Ascherio A, Gao X. Association of Diet and Physical Activity With All-Cause Mortality Among Adults With Parkinson Disease. JAMA network open. 2022 Aug 1:5(8):e2227738. doi: 10.1001/jamanetworkopen.2022.27738. Epub 2022 Aug 1 [PubMed PMID: 35984656]
Rogers G, Davies D, Pink J, Cooper P. Parkinson's disease: summary of updated NICE guidance. BMJ (Clinical research ed.). 2017 Jul 27:358():j1951. doi: 10.1136/bmj.j1951. Epub 2017 Jul 27 [PubMed PMID: 28751362]
Biundo R, Weis L, Fiorenzato E, Antonini A. Cognitive Rehabilitation in Parkinson's Disease: Is it Feasible? Archives of clinical neuropsychology : the official journal of the National Academy of Neuropsychologists. 2017 Nov 1:32(7):840-860. doi: 10.1093/arclin/acx092. Epub [PubMed PMID: 28961738]
Rosenquist PB, Youssef NA, Surya S, McCall WV. When All Else Fails: The Use of Electroconvulsive Therapy for Conditions Other than Major Depressive Episode. The Psychiatric clinics of North America. 2018 Sep:41(3):355-371. doi: 10.1016/j.psc.2018.04.002. Epub 2018 Jun 15 [PubMed PMID: 30098650]
Aquino CH, Moscovich M, Marinho MM, Barcelos LB, Felício AC, Halverson M, Hamani C, Ferraz HB, Munhoz RP. Fundamentals of deep brain stimulation for Parkinson's disease in clinical practice: part 1. Arquivos de neuro-psiquiatria. 2024 Apr:82(4):1-9. doi: 10.1055/s-0044-1786026. Epub 2024 Apr 23 [PubMed PMID: 38653485]
Hariz M, Blomstedt P. Deep brain stimulation for Parkinson's disease. Journal of internal medicine. 2022 Nov:292(5):764-778. doi: 10.1111/joim.13541. Epub 2022 Jul 13 [PubMed PMID: 35798568]
Wanneveich M, Moisan F, Jacqmin-Gadda H, Elbaz A, Joly P. Projections of prevalence, lifetime risk, and life expectancy of Parkinson's disease (2010-2030) in France. Movement disorders : official journal of the Movement Disorder Society. 2018 Sep:33(9):1449-1455. doi: 10.1002/mds.27447. Epub 2018 Aug 25 [PubMed PMID: 30145805]
Ophey A, Eggers C, Dano R, Timmermann L, Kalbe E. Health-Related Quality of Life Subdomains in Patients with Parkinson's Disease: The Role of Gender. Parkinson's disease. 2018:2018():6532320. doi: 10.1155/2018/6532320. Epub 2018 Aug 1 [PubMed PMID: 30155238]
Level 2 (mid-level) evidenceEggers C, Dano R, Schill J, Fink GR, Timmermann L, Voltz R, Golla H, Lorenzl S. Access to End-of Life Parkinson's Disease Patients Through Patient-Centered Integrated Healthcare. Frontiers in neurology. 2018:9():627. doi: 10.3389/fneur.2018.00627. Epub 2018 Jul 30 [PubMed PMID: 30105000]
Espay AJ, Aybek S, Carson A, Edwards MJ, Goldstein LH, Hallett M, LaFaver K, LaFrance WC Jr, Lang AE, Nicholson T, Nielsen G, Reuber M, Voon V, Stone J, Morgante F. Current Concepts in Diagnosis and Treatment of Functional Neurological Disorders. JAMA neurology. 2018 Sep 1:75(9):1132-1141. doi: 10.1001/jamaneurol.2018.1264. Epub [PubMed PMID: 29868890]