Papillomas: A Multisystem Overview of HPV-Associated and HPV-Independent Lesions
Papillomas: A Multisystem Overview of HPV-Associated and HPV-Independent Lesions
Introduction
A papilloma is a benign (noncancerous) tumor that arises from an epithelial surface and usually grows in an outward direction. The term "papilloma" most commonly refers to squamous cell papilloma, which appears as frond-like tumors that can develop on any area of the body lined by squamous epithelium, including:
- Skin, which presents as warts and cutaneous papillomas
- Lips
- Oral cavity
- Eyelid
- Tongue
- Pharynx
- Larynx
- Esophagus
- Cervix
- The genital tract, which is commonly known as genital warts
Most of these lesions are caused by human papillomavirus (HPV), and they are contagious through direct contact, except for cutaneous papilloma, also known as acrochordons or, more commonly, skin tags. Most of these lesions are usually self-limiting in immunocompetent individuals.[1]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Human Papillomavirus—Etiological Factors
The majority of papillomas are caused by HPV, which comprises over 170 identified subtypes. Among these, types 6, 7, and 11 are most commonly associated with papillomas and are considered low-risk, as they rarely cause precancerous lesions or progress to cancer. HPV can induce papilloma formation in various body parts lined with stratified squamous epithelium, including the skin, conjunctiva, oropharynx, larynx, upper trachea, and anogenital tract.[2]
Studies have detected HPV types 6 and 11 in 96% to 100% of all genital wart lesions.[3][4] The infection is highly contagious, primarily transmitted through direct and sexual contact. Genital HPV spreads mainly via sustained skin-to-skin contact, with vaginal, anal, and oral sex being the most common modes of transmission. Several studies have shown that most skin tags or cutaneous papillomas of the head and neck are noncontagious, unlike warts, despite occasionally containing low-risk HPV DNA, especially types 6 and 11.[5][6][7] However, a study by Pezeshkpoor et al. found no significant association between skin tags and HPV.[8] Therefore, nonviral growths are more accurately referred to as "acrochordons."
Evidence supports the vertical transmission of HPV from mothers to their infants.[9] This may lead to the development of several papillomas in the larynx and upper trachea, a condition known as "recurrent respiratory papillomatosis." This condition is particularly concerning, as the papillomas may progressively enlarge and obstruct the airway.
Non-Human Papillomavirus—Etiological Factors
Although HPV is a major cause of many papillomas, it is important to recognize that not all papillomas are virus-induced. Several important types develop through various mechanisms, highlighting the etiological heterogeneity of these lesions.[10]
- Intraductal papilloma of the breast: Current evidence does not support an association between intraductal breast papillomas and HPV infection. Their etiology remains largely unknown but is believed to result from an overgrowth of the epithelial cells lining the milk ducts. Hormonal factors, especially lifetime estrogen exposure, may contribute, as indicated by risk factors such as early menarche, late menopause, nulliparity, and the use of estrogen-containing contraceptives or hormone replacement therapy. A family history of breast cancer may also increase the risk.
- Urothelial papilloma: The involvement of HPV in the etiology of urothelial papillomas, particularly the inverted subtype, remains controversial and is generally considered unsubstantiated. Although some studies have reported p16 positivity—a surrogate marker often associated with high-risk HPV—specific detection of HPV DNA or RNA within these lesions has been largely unsuccessful or inconsistent. Alternative proposed etiological factors include chronic inflammation and irritation of the urothelium, potentially stemming from long-standing infections, smoking, urinary obstruction, or exposure to carcinogens. Some theories suggest that these lesions may originate from reactive hyperplasia of von Brunn nests, which are invaginations of normal urothelium. Molecular analyses have identified genetic alterations such as activating mutations in MAPK/ERK pathway genes (such as KRAS and HRAS) and occasional FGFR3 mutations. However, the overall genetic profile of urothelial papillomas appears distinct from that of HPV-related malignancies and conventional urothelial carcinoma.[11]
Sinonasal Papillomas
The etiology of sinonasal papillomas is complex and varies by histological subtype:
- Inverted papilloma: The role of HPV in inverted papillomas remains controversial. Both low-risk (6 and 11) and high-risk (16 and 18) HPV types have been detected, but the reported prevalence rates differ widely across studies, ranging from 0% to nearly 100%. The exact role of HPV in the initiation or progression of these lesions is unclear. A “hit and run” mechanism, where the virus triggers changes but is subsequently lost, has been proposed. Other strongly implicated factors include chronic inflammation, smoking, and occupational exposures such as welding fumes and organic solvents.
- Exophytic papilloma: This subtype demonstrates a clearer and more consistent association with low-risk HPV types 6 and 11.
Cutaneous Papillomas
These lesions are common and are also known as "acrochordons" (or skin tags). They often harbor detectable low-risk HPV DNA, particularly types 6 and 11. However, unlike HPV-induced warts, they are generally considered noncontagious. Their development is more closely associated with factors such as aging, obesity, insulin resistance, and mechanical friction in intertriginous areas. They frequently develop in areas where skin folds create repeated friction (see Image. Acrochordons or Skin Tags).
Notably, some papillomas are nonviral in origin, such as inverted papillomas of the urinary tract, which have shown a strong association with smoking.[10] Nasal papillomas may result from local irritation or mucosal trauma, while certain cutaneous papillomas have been associated with skin irritation. For example, cutaneous papillomas have developed in rats, mice, and hamsters following local application of potent carcinogens, where they are believed to arise from the stratified squamous epithelium.[11] Additionally, certain types of papillomas, such as intraductal (breast duct) papillomas and choroid plexus papillomas, have unclear underlying mechanisms, and their exact causes remain poorly understood.
Table. HPV Types Associated With Various Papillomas and Their Malignant Potential
| HPV-Associated Lesion | HPV Genotype(s) | Risk Category (LR/HR) | Morphology/Common Names | Malignant Potential/Notes |
| Cutaneous warts (common, plantar, flat) | 1, 2, 3, 4, 7, 10, 27, 57 | LR | Verruca vulgaris, plantaris, plana | Very low; certain EV-HPV (5, 8) may pose a higher risk in EV or immunosuppressed patients. |
| Anogenital warts (condyloma acuminatum) | 6, 11 | LR | Papillary or cauliflower-like lesions, genital warts | LR; Buschke-Löwenstein tumors (giant condyloma) are locally destructive but rarely metastasize. Co-infection with HR-HPV is possible. |
| Recurrent respiratory papillomatosis | 6, 11 | LR | Laryngeal or tracheal papillomas, hoarseness, and airway obstruction | LR (~3%-6% risk of malignant transformation), risk increased with smoking, HR-HPV co-infection, and pulmonary spread. HPV-negative papillomas may have a higher risk. |
| Oral squamous papilloma | 6, 11 (~50% of cases) | LR | Small exophytic oral lesion | None or very low malignant potential. |
| Conjunctival papilloma (pedunculated or viral) | 6, 11 (common); 16, 18, 33 (also reported) | LR (6, 11) and HR (16, 18, 33) | Fleshy conjunctival growth | Low for LR types. HR types are associated with conjunctival dysplasia and squamous cell carcinoma. |
| Sinonasal exophytic papilloma | 6, 11 | LR | Papillary mass on the nasal septum | Rare risk of malignant transformation. |
| Sinonasal inverted papilloma | 6, 11, 16, 18 (variable or controversial) | LR/HR |
Endophytic mass involving the lateral nasal wall or sinuses |
Significant (~5%-15%) risk of synchronous or metachronous squamous cell carcinoma. The role of HPV in malignant transformation remains debated. |
| CIN or cancer | 16, 18 (major); 31, 33, 45, and others | HR | Precursor lesions (CIN 1-3), invasive cervical cancer | HR of progression from high-grade CIN (CIN 2/3) to invasive cancer if untreated. HPV causes >99% of cervical cancers. |
| PIN or cancer | 16 (major); 18, 6, 11 (also reported) | HR (especially, 16)/LR | Precursor lesions (PIN), invasive penile cancer | A significant proportion (~40%-50%) of penile cancers are HPV-related, primarily HPV 16. |
| Oropharyngeal cancer (especially, tonsil and base of tongue) | 16 (major) | HR | Invasive squamous cell carcinoma | Increasing incidence; ~70% of oropharyngeal cancers in the United States are HPV-positive (mostly HPV 16). HPV-positive tumors generally have a better prognosis than HPV-negative tumors. |
| Bladder papilloma or cancer | The role is controversial or unsubstantiated | N/A | Urothelial papilloma or carcinoma | The HPV link is not clearly established for most urothelial neoplasms. Some studies suggest a possible link in subsets (eg, squamous differentiation), but evidence remains weak. |
| Breast intraductal papilloma | No established association | N/A | Intraductal breast lesion | Not HPV-related. Malignant potential is related to the presence of atypia (eg, ADH/DCIS) or multiplicity, rather than HPV infection. |
Abbreviations: ADH, atypical ductal hyperplasia; CIN, Cervical intraepithelial neoplasia; DCIS, ductal carcinoma in situ; EV, Epidermodysplasia verruciformis; HPV, Human papillomavirus; HR, High risk; LR, Low risk; N/A, Not applicable; PIN, penile intraepithelial neoplasia.
Epidemiology
The global prevalence of HPV infection—the primary cause of papillomas—is estimated to be between 11% and 12%. However, obtaining reliable surveillance data on the prevalence of warty lesions remains challenging. For nongenital warts, 2 large population-based studies reported prevalence rates of 0.84% in the United States and 12.9% in Russia, with the highest rates observed among children and young adults.[12] Regarding genital warts, the annual incidence in developed countries ranges from 0.1% to 0.2% of the population, with peak prevalence occurring in teenagers and young adults.
Acrochordons, commonly known as skin tags, have a reported prevalence of 46% in the general population, with a higher prevalence observed in older age groups, unlike warty papillomas. These lesions are more common in individuals with obesity and typically develop in areas of skin-to-skin contact. They may be skin-colored or pigmented and can appear dome-shaped or papillomatous (elevated). Their occurrence is evenly distributed between males and females.
Pathophysiology
Papillomas develop from the skin or mucosal surfaces, depending on the specific HPV types involved and their affinity to different sites. For instance, conjunctival papillomas are associated with HPV types 6, 11, 16, 33, and 45. This differs somewhat from genital warts, which are typically linked to HPV types 2, 3, 6, 11, 16, 18, and 30 to 32, and from cutaneous papillomas, which are commonly associated with types 1 to 4 and 26 to 29.
Infection is established in the basal cell layers of the epithelium, but this involves the expression of a limited part of the viral genome. Viral replication begins as these infected basal cells differentiate and migrate outward through the stratum spinosum and stratum granulosum layers of the skin, at which point the lesion becomes infectious.
Papillomavirus typically infects the epidermis near the site of entry; however, self-inoculation can occur, allowing the virus to spread to more distant sites. This process is known as the Koebner phenomenon. Notably, the immune system has an important role in controlling the spread of the virus. Although papillomavirus infects intraepidermal cells that are relatively “hard-to-reach” by the immune system, papillomas often reactivate and become more extensive in immunocompromised individuals.
Histopathology
Microscopically, papillomas are typically characterized by papillary projections or fronds. Each projection contains a central fibrovascular core—composed of connective tissue and blood vessels—that provides structural support and nourishment to the overlying proliferating epithelium. The type of epithelium covering these cores reflects the tissue of origin, including:
- Stratified squamous epithelium in the skin or oral mucosa
- Urothelium in the bladder
- Ductal epithelium in the breast
Depending on the specific type of papilloma and contributing factors, such as viral infection or chronic irritation, the epithelium may exhibit varying degrees of hyperplasia, metaplasia, or cytological atypia. A critical histological feature of benign papillomas is the integrity of the basement membrane, indicating that the proliferating epithelial cells remain confined and do not invade the underlying stromal tissue. This lack of invasion is the primary feature distinguishing benign papillomas from invasive carcinomas. Although these general features characterize papillomas as a group, variations in epithelial type, growth pattern (exophytic vs. endophytic), and the presence or absence of cytological atypia are critical for accurate diagnosis and prognosis.[13] Please see StatPearls' companion resource, "Human Papillomavirus," for more information.
History and Physical
A detailed history and physical examination are essential for identifying papillomas and differentiating them based on location, symptoms, and potential risk factors.
History
Patients typically report a small, rough bump on the skin, which may be either painless or painful. They might also describe behaviors that increase the risk of transmission, such as using communal showers, handling meat products or farm animals without protection, or engaging in unprotected sex.
Additional symptoms often relate to the papilloma’s specific site of origin:
- Intraductal papillomas of the breast may present with spontaneous bloody nipple discharge.
- Recurrent respiratory papillomas involving the larynx and trachea may cause stridor and hoarseness of voice.
- Nasal papillomas: They can cause sinusitis and loss of smell.[14]
Physical Findings
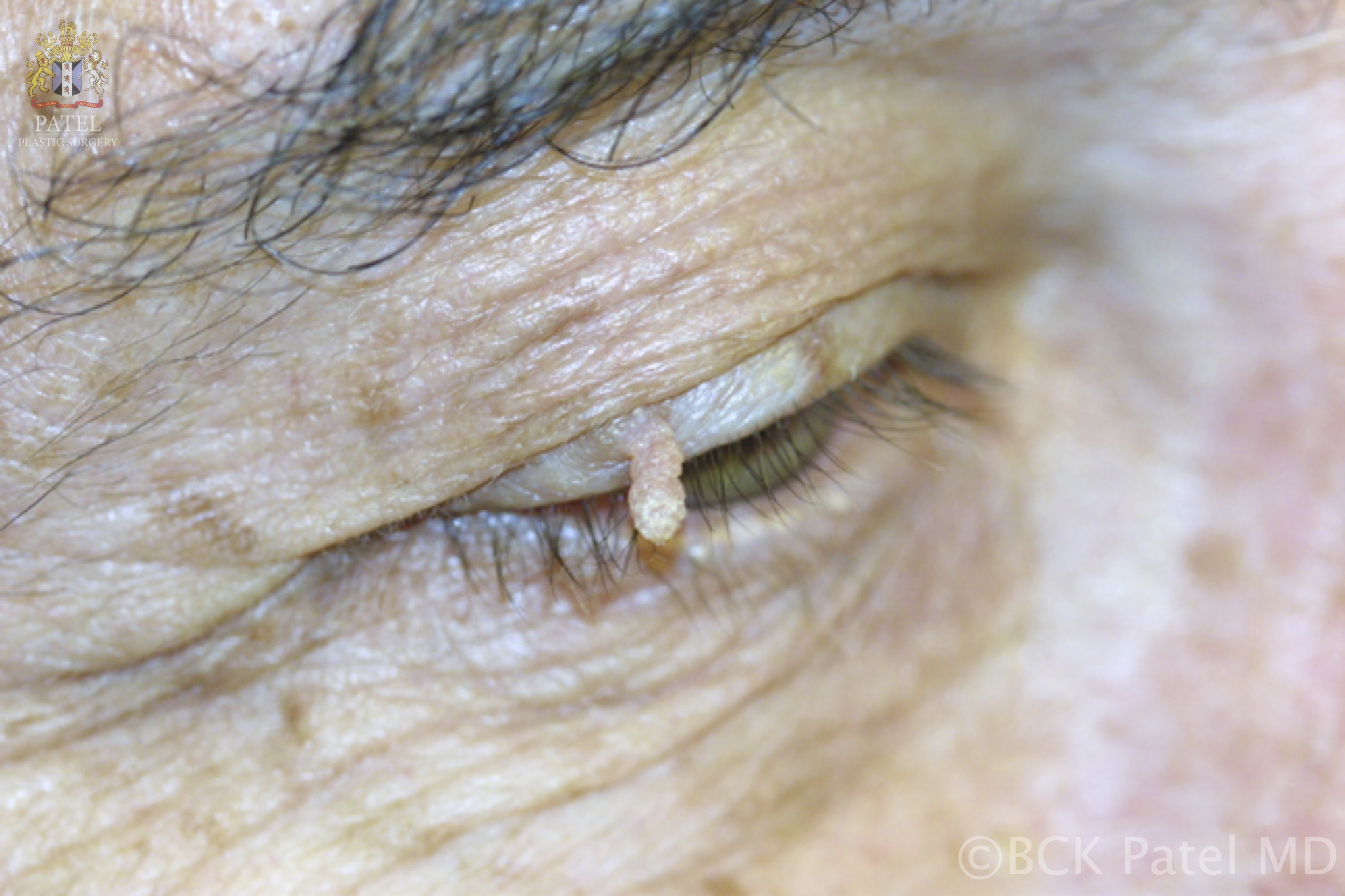
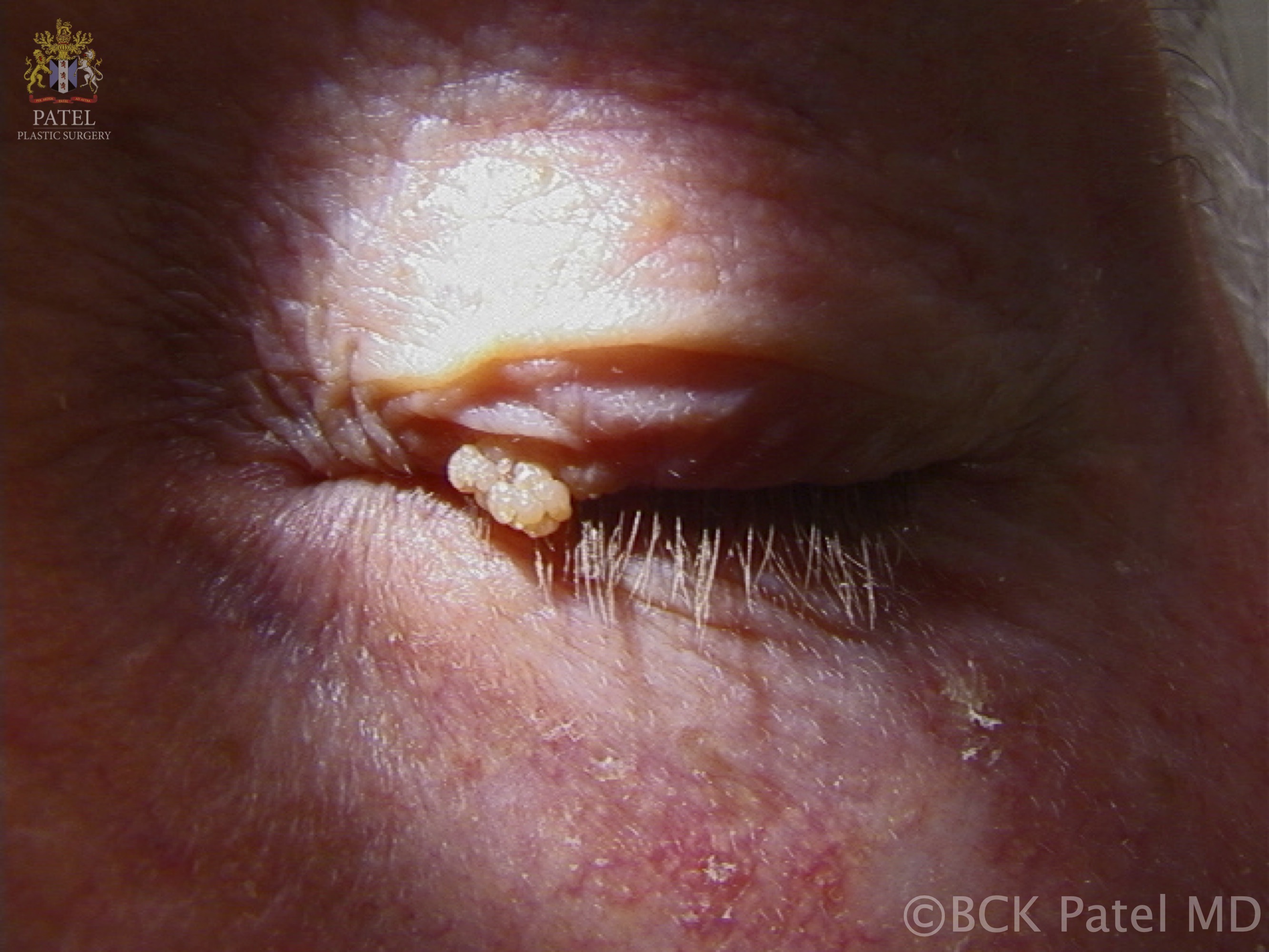
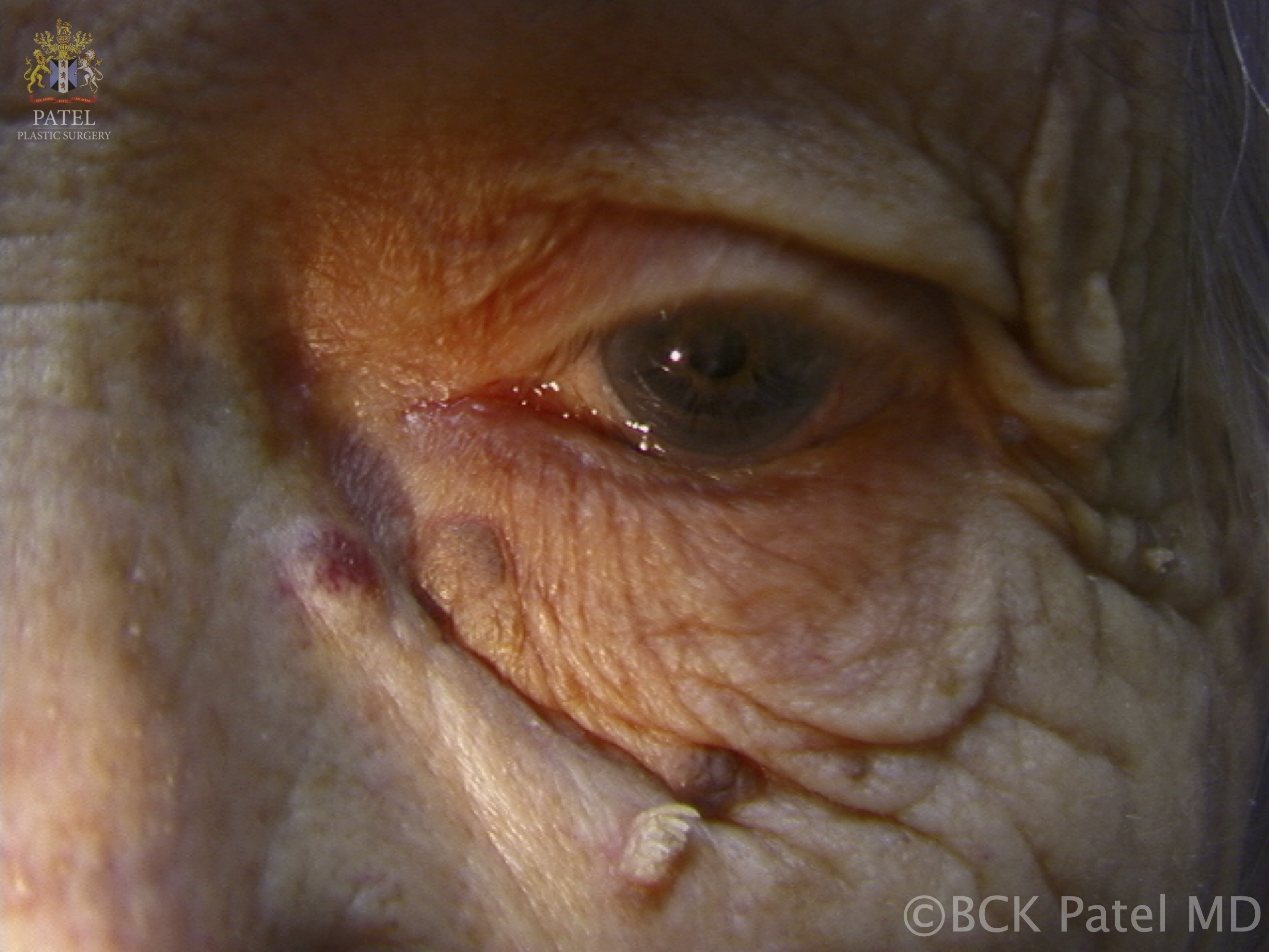
Papillomas may present as single or multiple solid papules. These lesions typically appear on the skin as rough and solid papules, which are often covered with hyperkeratinized skin. Lesions on mucosal surfaces are generally soft, pedunculated masses supported by a stalk, each bearing numerous finger-like projections (see Image. Cutaneous Horn or Cornu Cutaneum). These projections may be long and pointed or short and rounded, depending on keratin content. Less keratinized lesions tend to be pink or red, and they resemble a raspberry. In contrast, heavily keratinized lesions appear white and mimic the head of a cauliflower.
Evaluation
Most squamous cell papillomas are diagnosed clinically and typically do not require further investigation, especially in immunocompetent individuals. These lesions are usually self-limiting and rarely undergo malignant transformation. However, papillomas located in areas with a higher risk of malignant transformation, such as the anogenital and oropharyngeal tract, may be excised, and a biopsy sent for histopathological evaluation if there is diagnostic uncertainty or concern for dysplasia.[15][16]
Although genital warts are usually caused by low-risk HPV subtypes, 5% to 20% of affected individuals also carry other sexually transmitted infections. Notably, it is important to remain vigilant for high-risk HPV infections that can cause precancerous lesions in patients with genital warts, and early screening should be initiated. Screening for cervical dysplasia or malignancy is typically performed through speculum examination and Pap smear.
Treatment / Management
Management of papillomas varies widely, reflecting the heterogeneity of these lesions in terms of anatomical location, underlying etiology, symptom burden, risk of recurrence, and malignant potential. Treatment strategies range from simple observation to extensive surgical excision and long-term surveillance.[17] Please see StatPearls' companion resource, "Pulmonary Papilloma," for more information.
Observation Versus Excision
The decision to observe a papilloma or proceed with excision depends on several factors.
Observation
A conservative approach may be appropriate for certain low-risk lesions when the diagnosis is well established—clinically or histologically—and the lesion is asymptomatic. Examples include common cutaneous warts expected to resolve spontaneously in immunocompetent individuals or small, asymptomatic intraductal papillomas of the breast identified on core biopsy without atypia. However, many clinicians still favor excision in the latter scenario due to concerns about sampling error. Observation requires confidence in the diagnosis and acceptance of the small risks related to lesion growth or potential misdiagnosis.
- Symptom relief: This is to alleviate symptoms caused by the papilloma, such as pain, irritation, bleeding (eg, nipple discharge, epistaxis, or hematuria), obstruction of the airway or urinary tract, or cosmetic concerns.
- Malignant potential: Lesions with known malignant potential or associations, such as sinonasal inverted or oncocytic papillomas, or papillomas exhibiting atypia, warrant removal.
- Diagnostic confirmation: Adequate tissue sampling is essential for a definitive histological diagnosis, especially when biopsy results are equivocal or malignancy cannot be excluded.
- Prevention of growth or complications: Removal of lesions may prevent further growth that could result in obstruction or other complications.
- Reduction of recurrence: Complete excision reduces the risk of local recurrence.
Excision
Surgical removal remains the primary treatment for most papillomas. The rationale for excision is multifaceted.
Excision is generally considered mandatory for papillomas exhibiting atypia or dysplasia on biopsy, those associated with suspected malignancy, multiple intraductal papillomas of the breast, symptomatic papillomas, and subtypes known for high recurrence rates or significant malignant potential (eg, sinonasal inverted and oncocytic papillomas). Decision-making involves weighing the generally benign nature of most papillomas against the potential risks associated with the specific lesion's type, location, and the inherent risks of surgery.[18][19][20][21]
Surgical Approach
The choice of surgical approach must be tailored to the anatomical location, size, histological subtype, and extent of the papilloma. The primary goals are achieving complete removal, preserving function, and minimizing morbidity.
- Cutaneous papillomas: Commonly used techniques include cryotherapy (liquid nitrogen), topical agents (eg, salicylic acid and imiquimod), electrosurgery, curettage, laser ablation (eg, CO2 laser), and simple surgical excision.[22]
- Recurrent respiratory papillomatosis: Management focuses on maintaining airway patency through repeated endoscopic surgical debulking. Microdebriders and lasers (eg, CO2 and KTP) are commonly used to remove papillomas from the larynx and trachea while preserving underlying structures as much as possible. Complete eradication is often challenging due to the diffuse nature of HPV infection. Adjuvant therapies, such as intralesional cidofovir, systemic bevacizumab, and interferon-alpha (IFN-α), have been used to reduce recurrence, although their success varies, and potential adverse effects must be considered.
- Sinonasal papillomas: Endoscopic sinus surgery is the preferred approach for most sinonasal papillomas, allowing for precise removal with minimal external incisions. Inverted and oncocytic papillomas, which require complete excision to reduce recurrence and address malignant potential, often necessitate procedures such as endoscopic medial maxillectomy (removal of the medial wall of the maxillary sinus) or more extensive resections based on tumor origin and extent. Traditional open approaches, including lateral rhinotomy or midfacial degloving, may still be required in complex or recurrent cases. Careful removal of the tumor base and involved mucosa is essential.[23]
- Breast intraductal papillomas: Treatment options range from minimally invasive vacuum-assisted excision for small, selected lesions to surgical excision (lumpectomy or microdochectomy or major duct excision) for solitary symptomatic papillomas or those exhibiting atypia. In rare cases of extensive papillomatosis with a high risk of cancer, mastectomy may be considered.
- Urothelial papillomas: Bladder papillomas are typically managed by transurethral resection of bladder tumor (TURBT), which facilitates both diagnosis and treatment. Papillomas located in the upper urinary tract (ureter or renal pelvis) are addressed through ureteroscopic visualization and resection or ablation, often utilizing laser energy.[24] (B3)
- Conjunctival papillomas: Treatment options include simple surgical excision, cryotherapy applied to the lesion base, or topical chemotherapeutic agents such as mitomycin C or IFN-α2b eye drops.[2]
- Oral/anogenital papillomas: Common treatments include simple surgical excision, laser ablation (eg, CO2 laser), cryotherapy, or electrosurgery.
Differential Diagnosis
A variety of conditions characterized by epithelial overgrowth and solid skin papules must be differentiated from papillomas. These include:
- Acrochordon
- Adenoma
- Basal cell carcinoma
- Chancroid
- Condyloma latum
- Corns and calluses
- Cysts
- Milia
- Herpes simplex virus
- Keratoacanthoma
- Molluscum contagiosum (caused by a poxvirus and presents as fleshy papules)
- Psoriasis
- Moles
- Neurofibromas
- Nevus lipomatosus
- Seborrheic keratoses
Prognosis
The prognosis for papillomas caused by HPV infection is generally favorable in immunocompetent individuals, although recurrences may occur. Certain high-risk HPV subtypes are associated with vulvar intraepithelial neoplasia, cervical dysplasia, and cervical cancer. Although these subtypes do not typically cause papillomas, they are often found alongside genital papillomas. In immunocompromised individuals, papillomas tend to increase in number and spread more rapidly, with a higher risk of malignant transformation.[25]
Complications
Papillomas rarely cause serious or life-threatening complications, and the severity largely depends on the anatomical location of the lesion.
- Recurrent respiratory papillomatosis can grow rapidly and extensively in the larynx and upper trachea, potentially leading to upper airway obstruction and stridor.
- Choroid plexus papillomas in the brain can increase cerebrospinal fluid production, resulting in elevated intracranial pressure and hydrocephalus, which may eventually lead to brain herniation and death.
- Papillomas are rarely premalignant in immunocompetent individuals; however, malignant transformation can still occur.
- A papilloma at the lacrimal punctum may extend into the punctum and canaliculus, potentially involving the lacrimal sac and nasolacrimal duct, resulting in an inverted papilloma. When located on the eyelids or eyelid margins, these usually skin-colored papillomas can shed, leading to the development of additional lesions (see Image. Viral Papilloma).
Deterrence and Patient Education
Prevention and Patient Education
Key preventive measures for papillomas include:
- Practicing safe sex
- Undergoing routine Pap smear screenings
- Wearing flip-flops or protective footwear in communal showers
- Using disposable gloves when handling meat in occupational settings
- Understanding that warts are contagious through direct contact, whereas skin tags are not
Vaccination
The HPV vaccine is recommended for individuals aged 9 to 12 to help prevent certain precancerous lesions in both males and females. The 9-valent vaccine protects against HPV subtypes 6, 11, 16, 18, 31, 33, 45, 52, and 58.
Enhancing Healthcare Team Outcomes
HPV is the most common cause of papillomas affecting the skin and mucous membranes. With over 175 identified subtypes of HPV, some of which are associated with an increased risk of malignancy, HPV is primarily transmitted through direct contact with infected individuals or animals. One of the most effective strategies for reducing the morbidity associated with HPV infection is comprehensive patient and public education.
Pharmacist-led patient counseling is essential to inform individuals about the available treatments for warts, including their benefits and potential adverse effects. School nurses or trained educators should provide guidance on safe sex practices to teens who are approaching sexual activity. Additionally, it is important to educate parents about the importance of vaccinating their children against HPV before they become sexually active.
Sexually active females with genital warts should be encouraged to undergo Pap smear screening to assess for cervical dysplasia and high-risk HPV infections. Individuals with genital warts are advised to abstain from sexual activity until the lesions are treated. Additionally, patients should avoid touching the warts to prevent self-inoculation and further spread of the virus.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
Acrochordons or Skin Tags. Acrochordons vary in appearance and may be skin-colored or pigmented, often presenting as dome-shaped or papillomatous elevations. Common warts, caused by human papillomavirus, are small skin growths that can be white, beige, or brown and appear on various body parts.
Contributed by BCK Patel, MD, FRCS
(Click Image to Enlarge)
References
Karani LW, Musyoki S, Orina R, Nyamache AK, Khayeka-Wandabwa C, Nyagaka B. Human papillomavirus genotype profiles and cytological grades interlinkages in coinfection with HIV. The Pan African medical journal. 2020:35():67. doi: 10.11604/pamj.2020.35.67.21539. Epub 2020 Mar 10 [PubMed PMID: 32537071]
Theotoka D, Morkin MI, Galor A, Karp CL. Update on Diagnosis and Management of Conjunctival Papilloma. Eye and vision (London, England). 2019:6():18. doi: 10.1186/s40662-019-0142-5. Epub 2019 Jun 18 [PubMed PMID: 31236424]
Ball SL, Winder DM, Vaughan K, Hanna N, Levy J, Sterling JC, Stanley MA, Goon PK. Analyses of human papillomavirus genotypes and viral loads in anogenital warts. Journal of medical virology. 2011 Aug:83(8):1345-50. doi: 10.1002/jmv.22111. Epub [PubMed PMID: 21678438]
Brown DR, Schroeder JM, Bryan JT, Stoler MH, Fife KH. Detection of multiple human papillomavirus types in Condylomata acuminata lesions from otherwise healthy and immunosuppressed patients. Journal of clinical microbiology. 1999 Oct:37(10):3316-22 [PubMed PMID: 10488198]
Dianzani C, Calvieri S, Pierangeli A, Imperi M, Bucci M, Degener AM. The detection of human papillomavirus DNA in skin tags. The British journal of dermatology. 1998 Apr:138(4):649-51 [PubMed PMID: 9640372]
Donà MG, Pichi B, Rollo F, Gheit T, Laquintana V, Covello R, Pescarmona E, Spriano G, Pellini R, Giuliani M, Tommasino M, Benevolo M. Mucosal and cutaneous human papillomaviruses in head and neck squamous cell papillomas. Head & neck. 2017 Feb:39(2):254-259. doi: 10.1002/hed.24575. Epub 2016 Sep 12 [PubMed PMID: 27618734]
Gupta S, Aggarwal R, Gupta S, Arora SK. Human papillomavirus and skin tags: is there any association? Indian journal of dermatology, venereology and leprology. 2008 May-Jun:74(3):222-5 [PubMed PMID: 18583787]
Pezeshkpoor F, Jafarian AH, Ghazvini K, Yazdanpanah MJ, Sadeghian A, Esmaili H, Karrabi M, Rohani F, Joushan B. An association of human papillomaviruses low risk and high risk subtypes with skin tag. Iranian journal of basic medical sciences. 2012 May:15(3):840-4 [PubMed PMID: 23493098]
Smith EM, Parker MA, Rubenstein LM, Haugen TH, Hamsikova E, Turek LP. Evidence for vertical transmission of HPV from mothers to infants. Infectious diseases in obstetrics and gynecology. 2010:2010():326369. doi: 10.1155/2010/326369. Epub 2010 Mar 14 [PubMed PMID: 20300545]
Brown AL, Cohen RJ. Inverted papilloma of the urinary tract. BJU international. 2011 Apr:107 Suppl 3():24-6. doi: 10.1111/j.1464-410X.2011.10046.x. Epub [PubMed PMID: 21492372]
Sanders JM, Bucher JR, Peckham JC, Kissling GE, Hejtmancik MR, Chhabra RS. Carcinogenesis studies of cresols in rats and mice. Toxicology. 2009 Mar 4:257(1-2):33-9. doi: 10.1016/j.tox.2008.12.005. Epub 2008 Dec 9 [PubMed PMID: 19114085]
Level 3 (low-level) evidenceLoo SK, Tang WY. Warts (non-genital). BMJ clinical evidence. 2009 Sep 24:2009():. pii: 1710. Epub 2009 Sep 24 [PubMed PMID: 21726478]
Level 1 (high-level) evidenceMichaels L, Young M. Histogenesis of papillomas of the nose and paranasal sinuses. Archives of pathology & laboratory medicine. 1995 Sep:119(9):821-6 [PubMed PMID: 7668940]
De Luca LM, Sly L, Jones CS, Chen LC. Effects of dietary retinoic acid on skin papilloma and carcinoma formation in female SENCAR mice. Carcinogenesis. 1993 Mar:14(3):539-42 [PubMed PMID: 8453733]
Level 3 (low-level) evidenceNunes EM, Talpe-Nunes V, Sichero L. Epidemiology and biology of cutaneous human papillomavirus. Clinics (Sao Paulo, Brazil). 2018 Aug 20:73(suppl 1):e489s. doi: 10.6061/clinics/2018/e489s. Epub 2018 Aug 20 [PubMed PMID: 30133564]
Heo I, Kwak HJ, Nah EH, Cho S, Kim S, Cho HI. Evaluation of the LC-1000 Flow Cytometry Screening System for Cervical Cancer Screening in Routine Health Checkups. Acta cytologica. 2018:62(4):279-287. doi: 10.1159/000489079. Epub 2018 May 29 [PubMed PMID: 29843120]
Tu S, Yin Y, Yuan C, Chen H. Management of Intraductal Papilloma of the Breast Diagnosed on Core Needle Biopsy: Latest Controversies. Phenomics (Cham, Switzerland). 2023 Apr:3(2):190-203. doi: 10.1007/s43657-022-00085-8. Epub 2023 Feb 14 [PubMed PMID: 37197642]
Moynihan A, Quinn EM, Smith CS, Stokes M, Kell M, Barry JM, Walsh SM. Benign breast papilloma: Is surgical excision necessary? The breast journal. 2020 Apr:26(4):705-710. doi: 10.1111/tbj.13642. Epub 2019 Oct 14 [PubMed PMID: 31612568]
Moseley T, Desai B, Whitman GJ, Robinson EK, Saunders T, Gonzalez A, He H. Benign Breast Intraductal Papillomas Without Atypia at Core Needle Biopsies: Is Surgical Excision Necessary? Annals of surgical oncology. 2021 Mar:28(3):1347-1355. doi: 10.1245/s10434-020-09061-w. Epub 2020 Aug 28 [PubMed PMID: 32860176]
Rozentsvayg E, Carver K, Borkar S, Mathew M, Enis S, Friedman P. Surgical excision of benign papillomas diagnosed with core biopsy: a community hospital approach. Radiology research and practice. 2011:2011():679864. doi: 10.1155/2011/679864. Epub 2011 Nov 30 [PubMed PMID: 22191029]
Uusküla A, Tisler A, DeHovitz J, Murenzi G, Castle PE, Clifford G. Prevention and control of HPV-related cancers in people living with HIV. The lancet. HIV. 2025 Apr:12(4):e293-e302. doi: 10.1016/S2352-3018(25)00011-6. Epub 2025 Mar 11 [PubMed PMID: 40086453]
Dall'oglio F, D'Amico V, Nasca MR, Micali G. Treatment of cutaneous warts: an evidence-based review. American journal of clinical dermatology. 2012 Apr 1:13(2):73-96. doi: 10.2165/11594610-000000000-00000. Epub [PubMed PMID: 22292461]
Ivancic R, Iqbal H, deSilva B, Pan Q, Matrka L. Current and future management of recurrent respiratory papillomatosis. Laryngoscope investigative otolaryngology. 2018 Feb:3(1):22-34. doi: 10.1002/lio2.132. Epub 2018 Jan 14 [PubMed PMID: 29492465]
Abdel Gawad AM, Rabie A, Abdelwahed MS, Hasan A. Urothelial Papilloma of the Urinary Bladder: A Case Report and Literature Review of a Rare Entity. Cureus. 2022 Feb:14(2):e22046. doi: 10.7759/cureus.22046. Epub 2022 Feb 9 [PubMed PMID: 35340489]
Level 3 (low-level) evidenceJung K, Narwal M, Min SY, Keam B, Kang H. Squamous cell carcinoma of head and neck: what internists should know. The Korean journal of internal medicine. 2020 Sep:35(5):1031-1044. doi: 10.3904/kjim.2020.078. Epub 2020 Jul 14 [PubMed PMID: 32663913]