Introduction
Papilledema, also known as choked disk, refers to swelling of the optic disc caused by elevated intracranial pressure (ICP). This condition must be distinguished from disc edema, which encompasses a broader category of optic disc swelling secondary to various local or systemic etiologies. Recent reviews have highlighted frequent misuse of the term “papilledema” when describing optic disc edema (ODE) unrelated to ICP elevation. Papilledema specifically denotes ODE secondary to raised ICP and represents a primary indication for referral to neuro-ophthalmologists. Accurate differentiation from other causes of ODE in any specialty setting is essential, as papilledema involves a distinct pathophysiology and management strategy, with inappropriate management potentially resulting in permanent vision loss.[1]
ICP elevation may occur without visible optic disc swelling. Papilledema typically presents bilaterally but may appear asymmetrically and, rarely, unilaterally. This sign may indicate serious conditions that increase ICP, including brain tumors, infectious or noninfectious cerebrospinal inflammation, and idiopathic intracranial hypertension (IIH).[2]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
The etiologies of papilledema are related to intracranial hypertension (see Image. Papilledema on Ultrasound). The Monro-Kellie hypothesis describes ICP as a function of the combined volumes of blood, cerebrospinal fluid (CSF), and brain parenchyma within the fixed cranial cavity. A compensatory reduction in volume of another intracranial component must occur to maintain equilibrium as the total intracranial volume rises. Failure to achieve this balance may result in clinical and radiologic manifestations, including diminished cerebral blood flow and headache.
Inadequate compensation for volume changes results in high ICP and may occur in the following scenarios:
- Space-occupying lesions, such as tumors or hemorrhage
- Accumulation of CSF, as in obstructive hydrocephalus
- Increased blood volume due to venous sinus thrombosis
- Cerebral edema
- Infectious or inflammatory processes
- Vascular optic neuropathies
- IIH [3][4][5]
In patients with intracranial tumors, tumor size is a primary predictor of papilledema development. However, a recent case report has described patients with large intracranial tumors without evidence of papilledema. The authors noted that tumors may alter surrounding tissue physiology, CSF dynamics, and cerebral venous outflow, while individual anatomic variations, particularly within the optic nerve sheath and optic canal, also influence papilledema development. These findings indicate that papilledema depends not only on the presence of ICP elevation but also on effective transmission of the heightened pressure to the subarachnoid space within the optic nerve sheath and to the optic nerve itself.[6]
A recent study has investigated the association between papilledema and venous stasis on susceptibility-weighted imaging (SWI) in cerebral venous sinus thrombosis. Higher venous stasis scores on SWI may serve as an imaging surrogate marker of raised ICP in patients with cerebral venous sinus thrombosis.[7]
Current literature indicates that IIH is the most common cause of papilledema in individuals younger than 50. Other etiologies include conditions that impede CSF outflow, either through CSF flow derangements or mechanical obstruction of CSF pathways, and, less frequently, disorders that increase CSF production.[8]
Epidemiology
The frequency of papilledema varies depending on the study and clinical setting. In the eyecare professional’s office, papilledema most often results from IIH, with an estimated annual incidence of 0.9 per 100,000 in the U.S. general population. IIH is most prevalent in women of childbearing age with obesity, with an incidence of 13 per 100,000 in women aged 20 to 44. The condition does not demonstrate a racial predilection but occurs much less frequently in men, children, and older persons.[9]
Pediatric papilledema is usually asymptomatic and typically detected during routine screening. Recent case reports indicate that IIH accounts for the majority of pediatric cases examined in these studies.[10]
Current evidence from France reports that the average age of patients with papilledema ranges from 7 to 79 years, with most cases presenting bilaterally rather than unilaterally. The majority of occurrences were attributable to inflammatory optic neuropathies, while other etiologies included arterial hypertension, central retinal vein occlusions, ocular trauma, hydrocephalus, and cerebral malaria.
IIH typically presents with bilateral papilledema. However, a study reported 3 IIH cases meeting the modified Dandy criteria with unilateral papilledema. The authors proposed that variations in bony optic canal diameter, lamina cribrosa compliance, and optic nerve sheath anatomy may contribute to asymmetric presentation. Magnetic resonance imaging (MRI) findings demonstrated bilateral distention of the optic nerve sheaths, while computed tomography (CT) of the orbits revealed a smaller optic canal diameter on the side without papilledema.[11]
Pathophysiology
The optic nerve, composed of retinal ganglion cell axons and glial cells, is enveloped by all 3 meningeal layers and is continuous with the subarachnoid space of the brain. Therefore, CSF within the intracranial compartment is contiguous with the CSF surrounding the optic nerve, permitting transmission of raised ICP directly to the optic nerve. Transmission of heightened ICP disrupts the normal pressure gradient across the intraocular and orbital optic nerve, resulting in retrograde axoplasmic flow, ODE, and optic neuropathy. Competing theories exist regarding whether axoplasmic flow stasis arises primarily from mechanical compression of axons or microvascular ischemia.[12]
Recent studies support the concept that papilledema results from the transmission of increased ICP to the optic nerve’s subarachnoid space, which impedes axoplasmic transport within ganglion cell axons. Despite this mechanistic understanding, controversy persists as to whether flow stasis originates from direct axonal compression or ischemic insult.
History and Physical
A thorough history documenting visual complaints and potential underlying causes of raised ICP is essential. Patients may present with visual field loss or transient dim-outs. ICP elevation can produce horizontal binocular diplopia due to abducens nerve palsy and audible vascular bruits, known as pulsatile tinnitus. Headache is common and may worsen in positions that increase dependent intracranial volume. Nausea and vomiting may accompany acute rises in ICP. Medication history should include agents associated with ICP elevation, such as steroids, retinoids, tetracyclines, and oral contraceptives. Assessment of weight gain and obesity is important, as these factors increase the risk of IIH.
A standard ophthalmic and neurologic examination should be performed with careful evaluation of the optic disc. Absence of spontaneous venous pulsations at the disc indicates raised ICP when this parameter exceeds the intraocular pressure. Findings may include an elevated optic disc, dilated veins, hemorrhages overlying the disc, hyperemia, and peripapillary retinal folds, known as Paton lines. Blurring of the disc margins is a hallmark feature (see Image. Papilledema on Funduscopy).
A reproducible interobserver grading system developed by ophthalmologist Lars Friesen is widely used to assess papilledema severity. Grade 1 involves disruption of the normal radial nerve fiber arrangement with blurring of the nasal disc border, while the temporal margin remains normal. Grade 2 shows circumferential blurring of both nasal and temporal borders with more pronounced changes. Grade 3 is characterized by elevated, blurred disc margins that obscure 1 or more major retinal vessel segments. Grade 4 presents more severe changes with total obscuration of a segment of the central retinal artery or vein. Grade 5 is defined by complete obscuration of all disc vessels along with more pronounced disc elevation than in grade 4.[13][14]
Evaluation
Evaluation of papilledema aims to identify the underlying etiology. Initial workup should include contrast-enhanced MRI of the brain and orbits, as well as magnetic resonance venography (MRV), to detect masses or venous sinus thrombosis. If imaging confirms that lumbar puncture is safe and the risk of herniation is low, measurement of ICP via opening pressure in the left lateral decubitus position (normal < 25 cm H2O) is recommended. CSF analysis for protein, glucose, cell count with differential, and cultures provides additional diagnostic information.
Formal perimetry is indicated to document and monitor potential visual field loss. Fluorescein angiography and B-scan ultrasound may help identify other causes of disc edema or conditions that mimic papilledema. Imaging is critical in detecting pathologies responsible for papilledema, with contrast-enhanced MRI and MRV serving as key investigations for diagnosing IIH.
A recent review indicates that MRI of the brain and orbits with MRV sequences is the preferred neuroimaging modality to identify masses, venous sinus thrombosis, or indirect signs of raised ICP and exclude nonidiopathic causes. For patients outside the typical demographic for IIH, recent reviews suggest evaluating secondary causes of intracranial hypertension using MRI of the neck and spine, magnetic resonance angiography of the brain, CT of the chest, complete blood count, and creatinine testing.
Formal perimetry should be performed to document and monitor potential visual field loss. Fluorescein angiography and B-scan ultrasound may assist in identifying alternative causes of disc edema or conditions that mimic papilledema. However, a recent study has found that ocular ultrasonography alone is less sensitive in diagnosing papilledema and may not reliably distinguish papilledema from pseudopapilledema.[15]
Current literature suggests that artificial intelligence models demonstrate high diagnostic accuracy in detecting papilledema and differentiating it from other optic nerve head (ONH) conditions, often exceeding human experts in sensitivity, though not consistently in specificity. Despite limitations related to patient selection, image sourcing, and heterogeneity, artificial intelligence has the potential to enhance diagnostic accuracy and streamline clinical workflows in ophthalmology substantially.[16][17]
Treatment / Management
Treatment of papilledema is directed at addressing the underlying cause of raised ICP. The diagnosis of IIH is applied in patients with documented high ICP and no structural or other localizing causes. Recent reviews suggest that most patients with IIH respond to weight loss, and papilledema often resolves with acetazolamide therapy.
Surgical intervention may be required in cases of mass lesions. Venous sinus thrombosis warrants evaluation for precipitating factors and secondary prevention with anticoagulation.[18] Optic nerve sheath fenestration (ONSF) is considered when vision is severely threatened. Additional surgical options to reduce ICP include ventriculoperitoneal and lumboperitoneal shunts, which drain CSF. Venous sinus stenting may be indicated in patients with IIH exhibiting significant transverse venous sinus stenosis.
Recent reviews indicate that patients presenting with decreased central acuity and constricted visual fields, as well as those not responding to acetazolamide, should be considered for surgical treatment, with ventriculoperitoneal CSF shunting as the preferred procedure. A recent study has identified predictors of rapid papilledema improvement following venous sinus stenting in IIH, including lower preoperative pressure gradients, greater stenosis severity, and preoperative papilledema status.[19]
A retrospective review of adult patients with vision-threatening papilledema assessed outcomes under an interprofessional management protocol. Patients were admitted for lumbar drain placement and diuretics and monitored daily to determine whether medical management would suffice or surgical intervention (whether ONSF or CSF shunting) was required. Most patients demonstrated improvement in visual acuity, visual fields, and resolution of papilledema.[20]
Differential Diagnosis
Other causes of ODE, including intraocular inflammation, central retinal vein occlusion, compressive optic neuropathy, optic neuritis, diabetic papillopathy, and ischemic optic neuropathy, should be differentiated from papilledema. Blood pressure should be measured when papilledema is suspected clinically, and pseudopapilledema should be ruled out. Pseudopapilledema may result from congenital dysplasia, optic disc drusen, or a tilted optic disc, which can produce an elevated ONH appearance with blurred margins.
Recent studies have reported that papilledema can also occur in neurologic Lyme borreliosis. Underlying neurologic Lyme borreliosis should be considered in patients from endemic regions presenting with symptoms suggestive of intracranial hypertension, particularly cranial nerve deficits like facial nerve palsy.[21]
Another case report described bilateral papilledema due to neurobrucellosis, mimicking pseudotumor cerebri with isolated intracranial hypertension. The study highlighted that neurobrucellosis may be missed and diagnosis delayed when systemic symptoms are minimal and neurological signs like isolated papilledema are uncommon. Prompt recognition and treatment prevent complications.[22]
In most cases, papilledema is associated with vestibular schwannomas due to intracranial hypertension secondary to hydrocephalus (obstructive or communicating). An atypical case described bilateral papilledema revealing a vestibular schwannoma without hydrocephalus and with normal ICP. Ophthalmologic signs resolved completely after tumor removal, with tumor-induced hyperproteinorachia proposed as the underlying mechanism.[23]
Prognosis
The prognosis of papilledema depends on the chronicity of ICP elevation. Persistently high ICP can cause permanent dropout of the nerve fiber layer, leading to progressive visual field loss and impairment of central visual acuity.
Complications
Complications may develop when ICP elevation is inadequately treated, either due to unrecognized increased pressure or unsuccessful surgery. ONSF may produce suboptimal outcomes due to scar tissue formation, while CSF shunts are vulnerable to malfunction, obstruction, or blockage. Any surgical procedure involving access to the cerebrospinal space carries a risk of severe infection.
Deterrence and Patient Education
Headache is the most common presenting symptom in patients with papilledema. Fundus assessment is essential for every patient with a headache, particularly when accompanied by visual changes. Headaches may improve with treatment. However, the optic disc should be monitored regularly to assess stability and regression of papilledema. Chronic papilledema may lead to persistent visual deficits and, if left untreated, result in blindness.
Pearls and Other Issues
Disc edema is frequently identified in the clinical setting and may indicate life- or vision-threatening conditions. Expedited workup, including hospital admission and aggressive management, is warranted in patients presenting with poor central visual acuity, high-grade disc edema, or frequent transient visual obscurations. Patients with a history suggestive of underlying malignancy or systemic symptoms should also be hospitalized for further evaluation.[24]
The ICP can increase even in the absence of disc edema. Direct measurement is the only reliable method to assess this parameter. Optic nerves that have undergone atrophy with axonal loss are less likely to develop edema during repeated episodes of ICP elevation.
Recent studies indicate that neurofilament light chain reflects axonal damage in neurological disorders. Neurofilament light chain levels in the CSF appear to correlate with papilledema severity in IIH, as well as CSF opening pressure, providing a potential predictor of optic nerve injury in these patients.[25]
Enhancing Healthcare Team Outcomes
Papilledema may influence aspects of a patient’s life other than their eyesight. Although intracranial hypertension is primarily a neurosurgical condition, ophthalmologists are often the first clinicians patients consult, frequently in a primary healthcare setting. Studies have noted that ophthalmologists may encounter challenges in differentiating papilledema from other ONH changes observed during ophthalmoscopic examination.[26]
Eyecare professionals should be familiar with optic disc appearance, evaluation techniques, and the proper collection of a relevant history and physical examination. Timely workup and appropriate referral to specialists are essential. Optimal patient care and safety depend on precise and efficient communication between referring and comanaging healthcare providers. For example, an ophthalmologist recognizing the need for surgical intervention in IIH should coordinate with neurosurgery regarding the appropriate procedure. Patient autonomy is maintained when risks, benefits, and alternative interventions, such as ONSF versus ventriculoperitoneal shunting, are clearly communicated across disciplines.
Media
(Click Image to Enlarge)
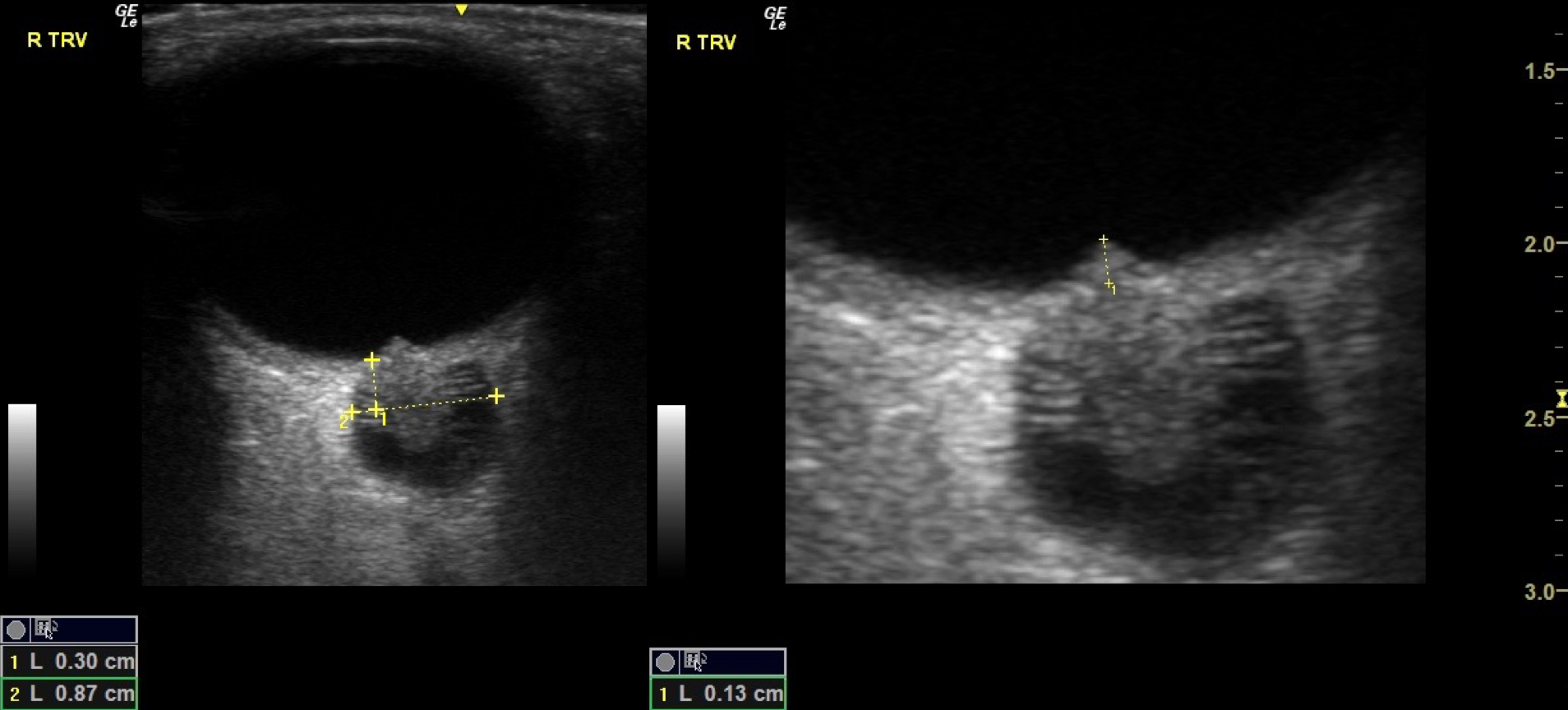
Papilledema on Ultrasound. The left image shows a transverse view of the optic nerve sheath with measurements indicating significant widening to 8.7 mm (normal usually <5 mm), a key sign of elevated intracranial pressure. The right image focuses on the optic nerve head, showing optic disc swelling with a measured elevation of 1.3 mm, consistent with papilledema. Together, these ultrasound images demonstrate characteristic findings of raised intracranial pressure affecting the optic nerve.
Contributed by Harry J. Goett, MD
(Click Image to Enlarge)
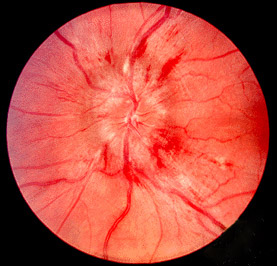
Papilledema on Funduscopy. This photograph shows optic disc swelling caused by increased intracranial pressure, characterized by blurred disc margins, elevation of the optic nerve head, and engorged retinal veins, which are hallmark signs of papilledema typically seen on fundoscopy.
https://en.wikipedia.org/wiki/Papilledema#/media/File:Papilledema.jpg
References
Tao B, Vosoughi A, Margolin E, Micieli JA. Inappropriate Use of the Term "Papilledema" in the Medical Literature: A Systematic Review of Case Reports across Specialties. Ophthalmology. 2023 Feb:130(2):129-136. doi: 10.1016/j.ophtha.2022.09.017. Epub 2022 Oct 3 [PubMed PMID: 36195254]
Level 1 (high-level) evidenceRigi M, Almarzouqi SJ, Morgan ML, Lee AG. Papilledema: epidemiology, etiology, and clinical management. Eye and brain. 2015:7():47-57. doi: 10.2147/EB.S69174. Epub 2015 Aug 17 [PubMed PMID: 28539794]
Level 2 (mid-level) evidenceMokri B. The Monro-Kellie hypothesis: applications in CSF volume depletion. Neurology. 2001 Jun 26:56(12):1746-8 [PubMed PMID: 11425944]
Guarnizo A, Albreiki D, Cruz JP, Létourneau-Guillon L, Iancu D, Torres C. Papilledema: A Review of the Pathophysiology, Imaging Findings, and Mimics. Canadian Association of Radiologists journal = Journal l'Association canadienne des radiologistes. 2022 Aug:73(3):557-567. doi: 10.1177/08465371211061660. Epub 2022 Jan 19 [PubMed PMID: 35044276]
Koki G, Aboubakar H, Mukam WN, Biangoup PN, Tépéresna P, Epée E, Bella AL. [Etiologies of papilledema in Cameroonian hospitals]. The Pan African medical journal. 2023:45():66. doi: 10.11604/pamj.2023.45.66.36676. Epub 2023 May 29 [PubMed PMID: 37637400]
Donaldson L, Margolin E. Absence of papilledema in large intracranial tumours. Journal of the neurological sciences. 2021 Sep 15:428():117604. doi: 10.1016/j.jns.2021.117604. Epub 2021 Aug 5 [PubMed PMID: 34384969]
Park MG, Roh J, Ahn SH, Park KP, Baik SK. Papilledema and venous stasis in patients with cerebral venous and sinus thrombosis. BMC neurology. 2023 Apr 28:23(1):175. doi: 10.1186/s12883-023-03228-0. Epub 2023 Apr 28 [PubMed PMID: 37118674]
Xie JS, Donaldson L, Margolin E. Papilledema: A review of etiology, pathophysiology, diagnosis, and management. Survey of ophthalmology. 2022 Jul-Aug:67(4):1135-1159. doi: 10.1016/j.survophthal.2021.11.007. Epub 2021 Nov 20 [PubMed PMID: 34813854]
Level 3 (low-level) evidenceChen J, Wall M. Epidemiology and risk factors for idiopathic intracranial hypertension. International ophthalmology clinics. 2014 Winter:54(1):1-11. doi: 10.1097/IIO.0b013e3182aabf11. Epub [PubMed PMID: 24296367]
Maheswaran M, Dheera MS, Kumar M, Kowsalya A. Pediatric Papilledema at a Tertiary Care Ophthalmological Center. Indian pediatrics. 2020 Oct 15:57(10):966-967 [PubMed PMID: 33089814]
Swinkin E, Jabehdar Maralani P, Sundaram AN. Unilateral Papilledema in Idiopathic Intracranial Hypertension: A Case Series. The Canadian journal of neurological sciences. Le journal canadien des sciences neurologiques. 2022 Mar:49(2):278-281. doi: 10.1017/cjn.2021.79. Epub 2021 Apr 23 [PubMed PMID: 33888167]
Level 2 (mid-level) evidenceTrobe JD. Papilledema: the vexing issues. Journal of neuro-ophthalmology : the official journal of the North American Neuro-Ophthalmology Society. 2011 Jun:31(2):175-86. doi: 10.1097/WNO.0b013e31821a8b0b. Epub [PubMed PMID: 21593630]
Jacks AS, Miller NR. Spontaneous retinal venous pulsation: aetiology and significance. Journal of neurology, neurosurgery, and psychiatry. 2003 Jan:74(1):7-9 [PubMed PMID: 12486256]
Frisén L. Swelling of the optic nerve head: a staging scheme. Journal of neurology, neurosurgery, and psychiatry. 1982 Jan:45(1):13-8 [PubMed PMID: 7062066]
Kohli AA, Pistilli M, Alfaro C, Ross AG, Jivraj I, Bagchi S, Chan J, May D, Liu GT, Shindler KS, Tamhankar MA. Role of Ocular Ultrasonography to Distinguish Papilledema From Pseudopapilledema. Journal of neuro-ophthalmology : the official journal of the North American Neuro-Ophthalmology Society. 2021 Jun 1:41(2):206-211. doi: 10.1097/WNO.0000000000000984. Epub [PubMed PMID: 33296160]
Łajczak PM, Sirek S, Wyględowska-Promieńska D. Unveiling AI's role in papilledema diagnosis from fundus images: A systematic review with diagnostic test accuracy meta-analysis and comparison of human expert performance. Computers in biology and medicine. 2025 Jan:184():109350. doi: 10.1016/j.compbiomed.2024.109350. Epub 2024 Nov 7 [PubMed PMID: 39515271]
Level 1 (high-level) evidenceGirard MJA, Panda S, Tun TA, Wibroe EA, Najjar RP, Aung T, Thiéry AH, Hamann S, Fraser C, Milea D. Discriminating Between Papilledema and Optic Disc Drusen Using 3D Structural Analysis of the Optic Nerve Head. Neurology. 2023 Jan 10:100(2):e192-e202. doi: 10.1212/WNL.0000000000201350. Epub 2022 Sep 29 [PubMed PMID: 36175153]
Sader N, de Lotbinière-Bassett M, Tso MK, Hamilton M. Management of Venous Sinus Thrombosis. Neurosurgery clinics of North America. 2018 Oct:29(4):585-594. doi: 10.1016/j.nec.2018.06.011. Epub [PubMed PMID: 30223971]
Yang H, Raynald, Tong X, Huo X, Wang Z, Li X, Liu L, Wang S, Miao Z, Mo D. Predicting the Rapid Improvement of Papilledema After Stenting in Idiopathic Intracranial Hypertension. Clinical neuroradiology. 2023 Jun:33(2):537-544. doi: 10.1007/s00062-022-01243-1. Epub 2022 Dec 19 [PubMed PMID: 36536160]
Brady T, Vegunta S, Crum AV, Marx D, Patel BCK, Seay MD, Schmidt RH, Warner JEA, Digre KB, Katz BJ. Interdisciplinary Protocol for the Management of Vision-Threatening Papilledema. Journal of neuro-ophthalmology : the official journal of the North American Neuro-Ophthalmology Society. 2022 Dec 1:42(4):495-501. doi: 10.1097/WNO.0000000000001594. Epub 2022 Apr 19 [PubMed PMID: 35439211]
Vaysbrot EE, Bannuru RR, Christopher MC, Osani MC, Halperin JJ. Papilledema Secondary to Neurologic Lyme Borreliosis: A Meta-Case Series. Journal of neuro-ophthalmology : the official journal of the North American Neuro-Ophthalmology Society. 2021 Dec 1:41(4):e498-e508. doi: 10.1097/WNO.0000000000000983. Epub [PubMed PMID: 34788244]
Level 2 (mid-level) evidenceMengi T, Çelebisoy M. Bilateral papilledema caused by brucellosis mimicking pseudotumor cerebri. Agri : Agri (Algoloji) Dernegi'nin Yayin organidir = The journal of the Turkish Society of Algology. 2022 Jul:34(3):213-216. doi: 10.14744/agri.2020.20053. Epub [PubMed PMID: 35792692]
Gavotto A, Feuillade V, Bresch S, Guevara N, Mondot L, Almairac F. Papilledema secondary to vestibular schwannoma: An atypical case without intracranial hypertension. Neuro-Chirurgie. 2022 Apr:68(3):327-330. doi: 10.1016/j.neuchi.2021.04.017. Epub 2021 May 11 [PubMed PMID: 33989639]
Level 3 (low-level) evidenceWall M, Falardeau J, Fletcher WA, Granadier RJ, Lam BL, Longmuir RA, Patel AD, Bruce BB, He H, McDermott MP, NORDIC Idiopathic Intracranial Hypertension Study Group. Risk factors for poor visual outcome in patients with idiopathic intracranial hypertension. Neurology. 2015 Sep 1:85(9):799-805. doi: 10.1212/WNL.0000000000001896. Epub 2015 Aug 5 [PubMed PMID: 26245929]
Knoche T, Gaus V, Haffner P, Kowski A. Neurofilament light chain marks severity of papilledema in idiopathic intracranial hypertension. Neurological sciences : official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology. 2023 Jun:44(6):2131-2135. doi: 10.1007/s10072-023-06616-z. Epub 2023 Jan 23 [PubMed PMID: 36689008]
Serova NK, Eliseeva NM. [Papilledema as a sign of intracranial hypertension]. Vestnik oftalmologii. 2022:138(4):87-93. doi: 10.17116/oftalma202213804187. Epub [PubMed PMID: 36004596]