Introduction
Acute pancreatitis is a common and potentially serious condition marked by abrupt inflammation of the pancreas. This condition is the leading cause of hospitalization for gastrointestinal disease in the United States, accounting for more than 275,000 hospital admissions annually.[1] The clinical course varies from mild, self-limited inflammation to severe disease with necrosis, systemic inflammatory response syndrome, multiorgan dysfunction syndrome, and high mortality.[2]
Mortality depends on disease severity, ranging from approximately 3% in mild interstitial pancreatitis to 20% in necrotizing forms.[3] Although diagnosis is typically based on characteristic symptoms and elevated pancreatic enzymes, predicting clinical progression and long-term outcomes remains difficult. Early identification of high-risk patients is critical to determining the appropriate level of care, the need for intensive monitoring, and the timing of targeted interventions.[4]
The Revised Atlanta Classification provides standardized terminology for the morphological subtypes and severity of acute pancreatitis (see Table. Morphologic Subtypes of Acute Pancreatitis Based on the Revised Atlanta Classification). Interstitial edematous pancreatitis involves inflammation of the pancreatic parenchyma and peripancreatic tissue without necrosis. This form is more common and generally less severe. In contrast, necrotizing pancreatitis includes varying degrees of pancreatic parenchymal or peripancreatic necrosis and is associated with a higher risk of complications and poorer clinical outcomes (see Image. Necrotizing Pancreatitis with Extensive Peripancreatic Nodularity).
Table. Morphologic Subtypes of Acute Pancreatitis Based on the Revised Atlanta Classification
|
Subtype |
Definition |
Clinical Relevance |
|
Interstitial edematous pancreatitis |
Inflammation of the pancreatic parenchyma and peripancreatic tissues without necrosis |
More common; typically associated with a milder course |
|
Necrotizing pancreatitis |
Variable degrees of pancreatic and/or peripancreatic necrosis |
Higher risk of complications and worse clinical outcomes |
Severity is categorized into 3 clinical grades (see Table. Severity Staging of Acute Pancreatitis Based on the Revised Atlanta Classification). Mild acute pancreatitis is defined by the absence of organ failure and local or systemic complications. Moderately severe disease involves transient organ failure lasting less than 48 hours or local or systemic complications such as peripancreatic fluid collections. Severe acute pancreatitis is marked by persistent organ failure exceeding 48 hours, often involving multiple organ systems.[5]
Table. Severity Staging of Acute Pancreatitis Based on the Revised Atlanta Classification
|
Severity |
Definition |
Clinical Features |
|
Mild |
No organ failure and no local or systemic complications |
Generally self-limiting |
|
Moderately severe |
Transient organ failure (<48 hours) and/or local or systemic complications |
May present with peripancreatic fluid collections |
|
Severe |
Persistent organ failure (>48 hours), often involving multiple organ systems |
Associated with increased morbidity and mortality |
The pancreas is a retroperitoneal gland extending from the duodenum to the splenic hilum between the L1 and L2 vertebrae. This organ has 4 regions—the head, neck, body, and tail—and lies transversely along the posterior abdominal wall. The pancreas serves both exocrine and endocrine functions, with digestive enzymes delivered via the pancreatic ducts to the duodenum and hormonal products such as insulin, glucagon, and somatostatin secreted by the islets of Langerhans.[6] Thorough knowledge of acute pancreatitis's classification, anatomy, and clinical spectrum supports accurate diagnosis, enables early risk stratification, and guides appropriate interprofessional management. These core elements influence prognosis and inform inpatient and outpatient care decisions.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
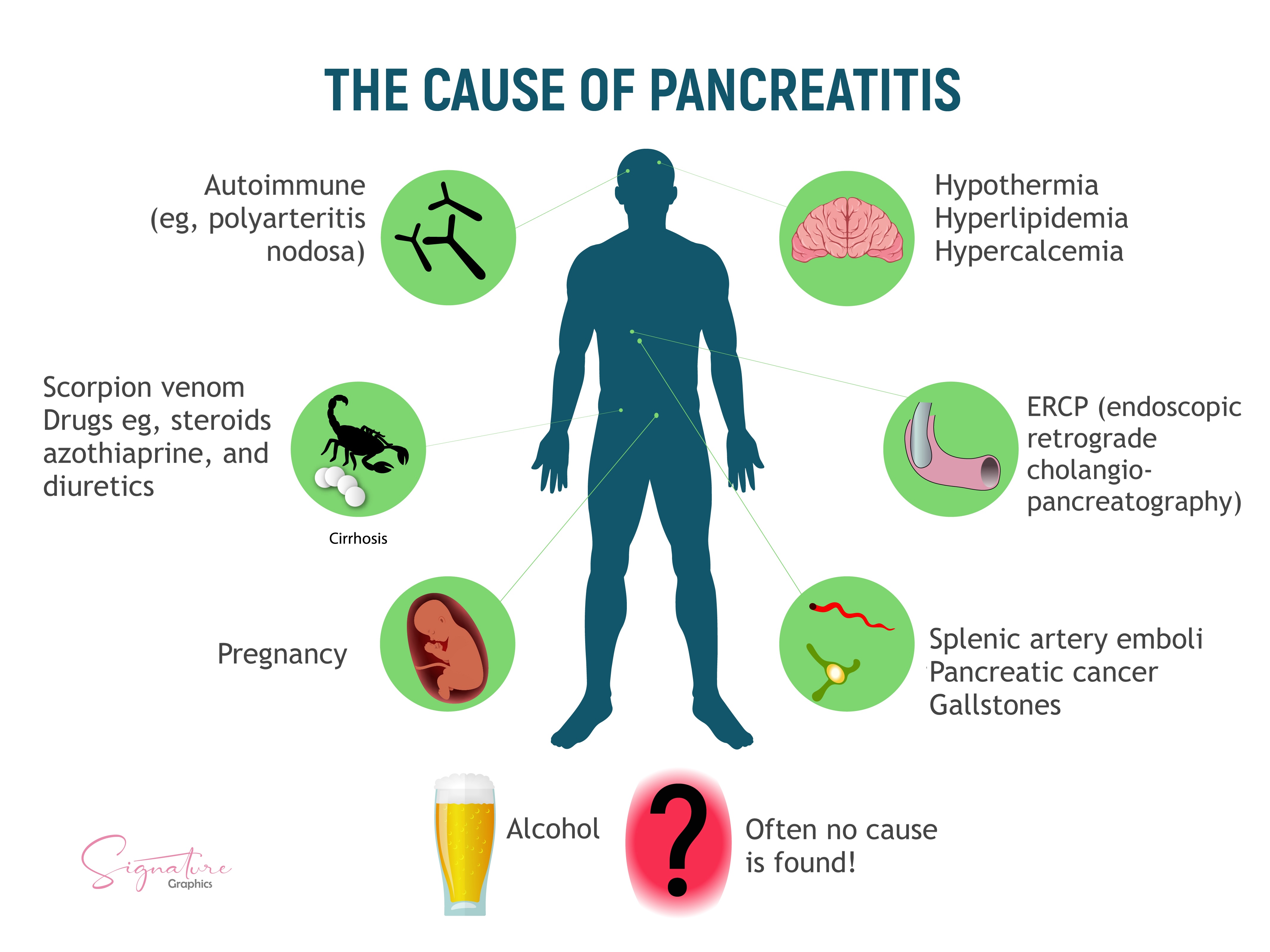
Gallstones and alcohol use account for approximately 35% to 40% and 17% to 25% of acute pancreatitis cases in the United States (US), respectively.[7][8] Less common etiologies span mechanical, metabolic, infectious, autoimmune, toxic, genetic, and idiopathic mechanisms (see Image. Causes of Pancreatitis). Determining the underlying cause is critical for immediate management and recurrence prevention.
Biliary Pancreatitis
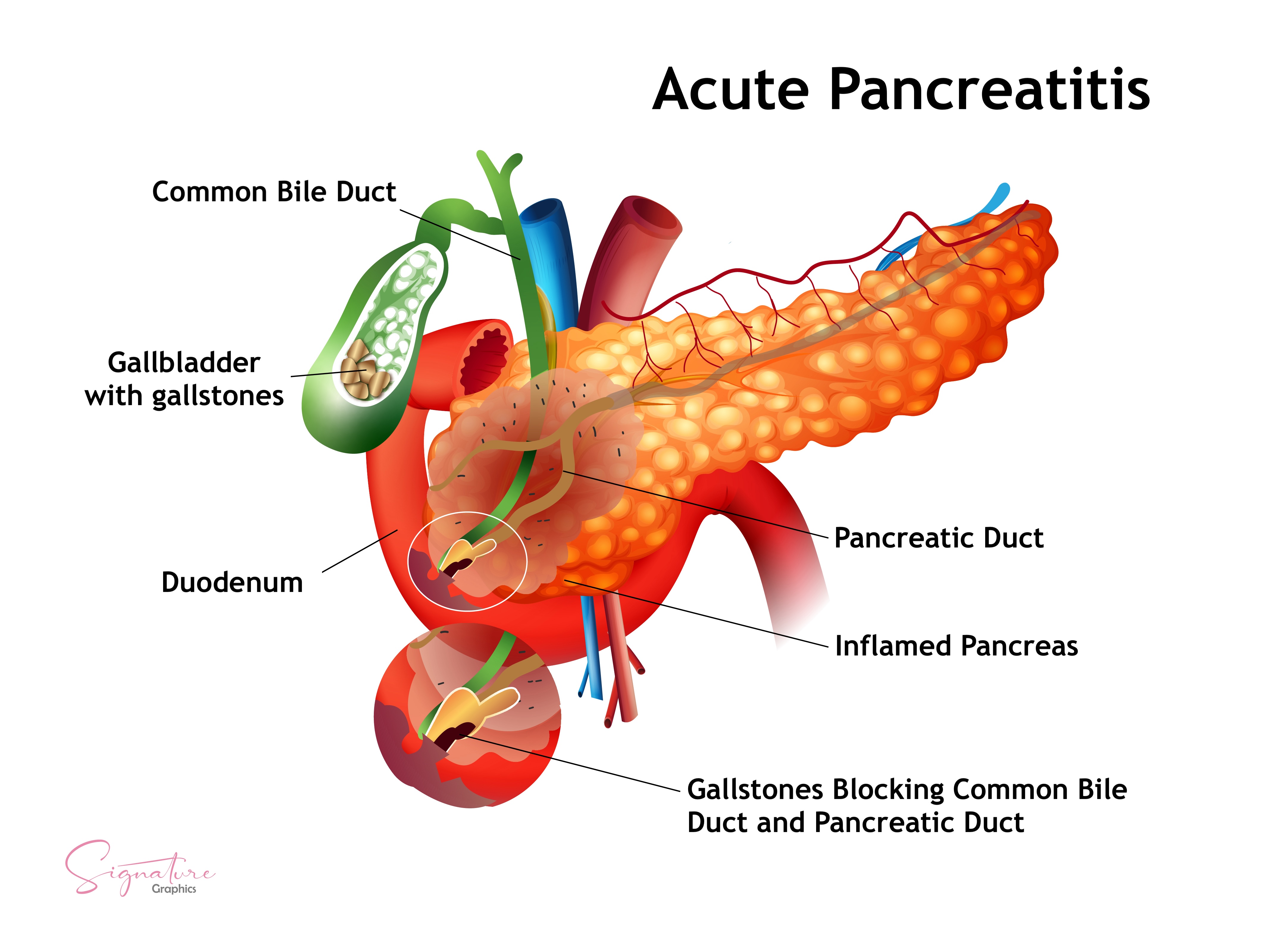
Gallstones and biliary sludge cause the largest proportion of acute pancreatitis cases in the United States (see Image. Gallstone-Induced Acute Pancreatitis). These entities trigger pancreatic inflammation when a stone transiently obstructs the ampulla of Vater, resulting in the reflux of bile alongside pancreatic secretions into the pancreatic duct. This process activates digestive enzymes prematurely, leading to local inflammation and pancreatic autodigestion. Smaller stones, typically less than 5 mm, and biliary sludge are more likely to migrate and cause obstruction.
Serum alanine aminotransferase levels above 150 U/L and biliary dilation on imaging increase the likelihood of a biliary cause. This form of pancreatitis is more frequent in older adults, individuals with obesity, rapid weight loss, and the female sex. Microlithiasis may escape detection on standard ultrasonography but is more reliably identified via endoscopic ultrasound or magnetic resonance cholangiopancreatography.
The risk of recurrence is high without definitive management. In patients with mild disease, cholecystectomy is recommended during the same hospitalization. Surgery may be deferred in individuals with moderate-to-severe disease until the acute inflammatory phase resolves.
Alcohol-Induced Pancreatitis
Alcohol is the second most common cause of acute pancreatitis and typically occurs in individuals with chronic heavy alcohol use, often defined as 4 to 5 drinks daily for over 5 years. Binge drinking alone is rarely causative, although it can trigger acute episodes in those with preexisting pancreatic injury. Alcohol alters pancreatic physiology by increasing the viscosity of pancreatic secretions and promoting protein plug formation that obstructs small ducts.
The substance also enhances intrapancreatic enzyme activation through both oxidative (involving acetaldehyde accumulation and disruption in the nicotinamide adenine dinucleotide redox equilibrium) and nonoxidative (fatty acid ethyl ester formation) pathways, ultimately leading to acinar injury and inflammation. Fewer than 5% of chronic heavy drinkers develop pancreatitis. Still, those who do are at risk for recurrent episodes and progression to chronic pancreatitis with endocrine and exocrine insufficiency, diabetes mellitus, and increased pancreatic cancer risk. Alcohol is also the most common cause of chronic pancreatitis in the United States.
Metabolic and Nonalcohol-Related Toxic Causes
Hypertriglyceridemia, usually at levels exceeding 1000 mg/dL, is a well-recognized cause of acute pancreatitis. Current American College of Gastroenterology guidelines recommend measuring serum triglyceride levels in patients without gallstones or significant alcohol use. A level above 1000 mg/dL supports hypertriglyceridemia as the likely etiology.[9] Drug-induced pancreatitis is associated with agents such as azathioprine, 6-mercaptopurine, valproic acid, didanosine, mesalamine, and angiotensin-converting enzyme inhibitors. Additional metabolic triggers include hypercalcemia and toxins such as organophosphates or scorpion venom.
Postprocedural and Structural Etiologies
Acute pancreatitis may occur following procedures such as endoscopic retrograde cholangiopancreatography or abdominal surgery. Ampullary stenosis, previously termed "sphincter of Oddi dysfunction type I," can also obstruct outflow and lead to pancreatic injury. Congenital anomalies, including annular pancreas and choledochal cysts, may predispose individuals to pancreatic inflammation. Pancreas divisum, a common congenital variant with incomplete ductal fusion, may contribute to recurrent or idiopathic cases by impairing drainage through the minor papilla. Obstruction of the pancreatic duct by neoplasms, strictures, or postoperative scarring can also impair enzyme outflow, leading to ductal hypertension and intrapancreatic activation of digestive enzymes. A pancreatic neoplasm should be excluded as a possible obstructive etiology in adults older than 40 when common etiologies have been ruled out.
Infectious Causes
Viral infections associated with pancreatitis include Coxsackie virus, cytomegalovirus, Epstein-Barr virus, hepatitis A, B, or C, human immunodeficiency virus, mumps, rubella, and varicella. Bacterial pathogens such as Campylobacter jejuni, Legionella, Leptospira, Mycobacterium tuberculosis, and Mycoplasma pneumoniae have also been implicated. Parasitic infections, including Ascaris lumbricoides, Clonorchis sinensis, Cryptosporidium, and Microsporidia, are known causes in endemic regions.
Autoimmune and Genetic Etiologies
Autoimmune pancreatitis includes type I, an immunoglobulin G4-related disease, and type II, which involves granulocytic epithelial lesions. Genetic disorders that may predispose individuals to acute pancreatitis include mutations in PRSS1 (hereditary pancreatitis) and CFTR (cystic fibrosis), as well as α1-antitrypsin deficiency. These genetic variants are often associated with early-onset or recurrent disease. Genetic testing may be appropriate in patients younger than 30 with recurrent or idiopathic pancreatitis and a positive family history.
Idiopathic Cases
Despite appropriate diagnostic evaluation, no clear etiology is identified in 10% to 20% of patients. These cases are classified as idiopathic acute pancreatitis. Advanced imaging or molecular testing may later reveal occult microlithiasis, pancreas divisum, autoimmune disease, or hereditary pancreatitis. Referral to a gastroenterologist is often appropriate to facilitate further evaluation using endoscopic ultrasonography or magnetic resonance cholangiopancreatography.
Other Considerations
Pancreatitis has been reported in patients undergoing hemodialysis and in those with renal disease. Trauma, whether blunt abdominal, penetrating, or postoperative, can directly injure the pancreas and initiate inflammatory cascades. Ischemic injury due to vascular compromise or systemic hypotension can also lead to pancreatic inflammation. Vasculitides such as systemic lupus erythematosus and polyarteritis nodosa are additional rare causes.[10]
Cigarette smoking is recognized as an independent risk factor that may contribute to both acute and chronic pancreatitis. Tropical pancreatitis is a chronic idiopathic form observed in individuals from South and Southeast Asia and may occur in US immigrant populations. Environmental exposures such as malnutrition and dietary cyanogens, combined with genetic susceptibility, particularly SPINK1 mutations, contribute to the pathogenesis of this clinical entity.
Epidemiology
The incidence of acute pancreatitis is increasing in the US and globally. Whether this rise reflects a true increase in disease burden or improved diagnostic recognition remains uncertain, but the trend is likely multifactorial. Contributing factors include rising rates of obesity, metabolic syndrome, and associated hypertriglyceridemia, all of which are recognized risk factors for pancreatitis.[11]
In the US, acute pancreatitis accounts for an estimated 200,000 to 275,000 hospital admissions annually, making it the leading cause of hospitalization for gastrointestinal disease. The condition is common, but clinical severity varies. Approximately 80% of patients present with mild disease and are discharged within several days. The overall mortality rate ranges from 1% to 2%, but increases substantially in cases involving pancreatic necrosis or systemic organ failure.
The peak incidence of acute pancreatitis occurs during the fifth and sixth decades of life, with mortality increasing with age. Older adults have a higher risk of death due to reduced physiologic reserve, comorbid conditions, and delays in diagnosis.[12] Epidemiologic patterns vary by geographic region and socioeconomic status, reflecting differences in alcohol use, gallstone prevalence, and access to care. Alcohol-related pancreatitis is more common in lower-income populations, whereas gallstone-related cases are more frequent among older adults and populations with higher rates of obesity and metabolic syndrome.
The recurrence rate of acute pancreatitis depends on the underlying cause, ranging from 0.6% to 5.6%, with the highest rates observed in alcohol-related cases.[13] These figures underscore the importance of identifying and addressing the causative factor during the initial episode to prevent recurrence. Mortality in the US has declined despite rising hospitalization rates, likely due to earlier recognition, improved supportive care, and widespread use of standardized severity assessment tools such as the Revised Atlanta Classification and the Bedside Index for Severity in Acute Pancreatitis.
Pathophysiology
The pathophysiology of acute pancreatitis involves a complex cascade of local pancreatic injury and systemic inflammatory response. The central initiating event is the premature activation of digestive enzymes, particularly trypsin, within pancreatic acinar cells rather than in the intestinal lumen.[14] Under normal physiologic conditions, pancreatic enzymes are synthesized as inactive precursors (zymogens) and activated only upon entering the duodenum. In pancreatitis, however, trypsinogen is inappropriately converted to trypsin within the acinar cells, initiating a proteolytic cascade that activates enzymes such as elastase and phospholipase. These enzymes mediate pancreatic autodigestion and trigger the release of proinflammatory mediators.
Several intracellular disturbances contribute to the premature activation of digestive enzymes in acute pancreatitis. Elevated intraductal pressure, as seen in gallstone obstruction, disrupts acinar cell homeostasis. Other contributing factors include abnormalities in calcium signaling, intracellular acidosis, mitochondrial dysfunction, and adenosine triphosphate depletion. These insults impair the acinar cell’s enzyme trafficking system and compromise lysosomal membrane integrity, allowing colocalization of zymogens and lysosomal hydrolases, an essential step in pathologic enzyme activation.[15]
The resulting enzyme activation destroys pancreatic parenchyma and peripancreatic fat necrosis, releasing damage-associated molecular patterns (DAMPs). These DAMPs initiate a robust inflammatory response by stimulating cytokine release and recruiting neutrophils and monocytes. The ensuing inflammatory cascade causes endothelial injury, increased vascular permeability, and microvascular thrombosis, which drive systemic complications such as systemic inflammatory response syndrome and multiple organ dysfunction syndrome. The severity of inflammation ranges from mild, self-limited interstitial edema to extensive necrosis and hemorrhage. In necrotizing pancreatitis, these processes are prolonged and more severe, frequently resulting in superinfection, persistent organ failure, or death.
In a subset of patients, particularly those with genetic susceptibility, the inflammatory cycle of acute pancreatitis becomes recurrent. Mutations in several genes contribute to this predisposition. Gain-of-function mutations in PRSS1 lead to excessive trypsin activity, while SPINK1 mutations impair the function of pancreatic secretory trypsin inhibitor, a key regulator that prevents premature enzyme activation. Dysfunction of CTRC (chymotrypsin C) interferes with trypsin degradation, and mutations in CFTR disrupt bicarbonate secretion and impair ductal clearance.
These genetic alterations increase the risk of early-onset or recurrent acute pancreatitis and promote progression to chronic pancreatitis, a condition marked by irreversible fibrosis, acinar cell atrophy, and eventual endocrine and exocrine insufficiency. The pathogenesis of acute pancreatitis reflects a combination of premature enzyme activation, amplification of the inflammatory response, and systemic organ injury. Clinical outcomes depend on the intensity of the initial insult and the host’s genetic and physiologic capacity to contain inflammation and tissue damage.
Histopathology
The histologic appearance of acute pancreatitis reflects the severity of inflammation and the presence or absence of necrosis. The 2 principal forms, interstitial edematous pancreatitis and necrotizing pancreatitis, exhibit distinct microscopic and gross features.
Interstitial Edematous Pancreatitis
This form is more common and typically less severe. Histologic examination reveals edema of the pancreatic parenchyma and scattered inflammatory infiltrates composed primarily of neutrophils and mononuclear cells. Fat necrosis is minimal, and the overall acinar architecture remains intact. Grossly, the pancreas may appear swollen and pale in early or mild cases, without hemorrhage or necrosis. The inflammatory process in this form is generally self-limited and resolves without lasting structural damage.
Necrotizing Pancreatitis
Necrotizing pancreatitis represents a more severe form of disease, involving necrosis of the pancreatic parenchyma, peripancreatic fat, or both. Histologic features include extensive fat necrosis with saponification, focal or diffuse parenchymal destruction, areas of hemorrhage, and leukocytic infiltration with vascular thrombosis. In advanced cases, fat necrosis becomes confluent and parenchymal destruction widespread. Grossly, hemorrhagic regions appear dark red to black, and the affected tissue is friable. These findings often correlate with systemic complications and adverse clinical outcomes.
Comparison with Chronic Pancreatitis
Although not the focus of this discussion, chronic pancreatitis presents with histologic features distinct from acute inflammation. Typical findings include mononuclear cell infiltrates, progressive fibrosis, acinar atrophy, and islet cell loss. Ductal calcifications may also be observed. These changes are not seen in acute disease but may develop in patients with recurrent pancreatitis or those with predisposing genetic mutations, such as PRSS1 or SPINK1.
The Roles of Histopathology and Imaging in the Diagnosis of Pancreatitis
Histopathology can distinguish infected necrosis, which shows infiltration by neutrophils, liquefaction, and bacterial colonies, from sterile necrosis, which consists of inflammatory cells and cellular debris without microbial organisms. This distinction is clinically significant but rarely made histologically unless the tissue is obtained surgically or postmortem. However, histologic confirmation of pancreatitis is uncommon in clinical practice. Instead, contrast-enhanced computed tomography or magnetic resonance imaging typically provides a surrogate necrosis assessment, guiding clinical management and prognostication.
History and Physical
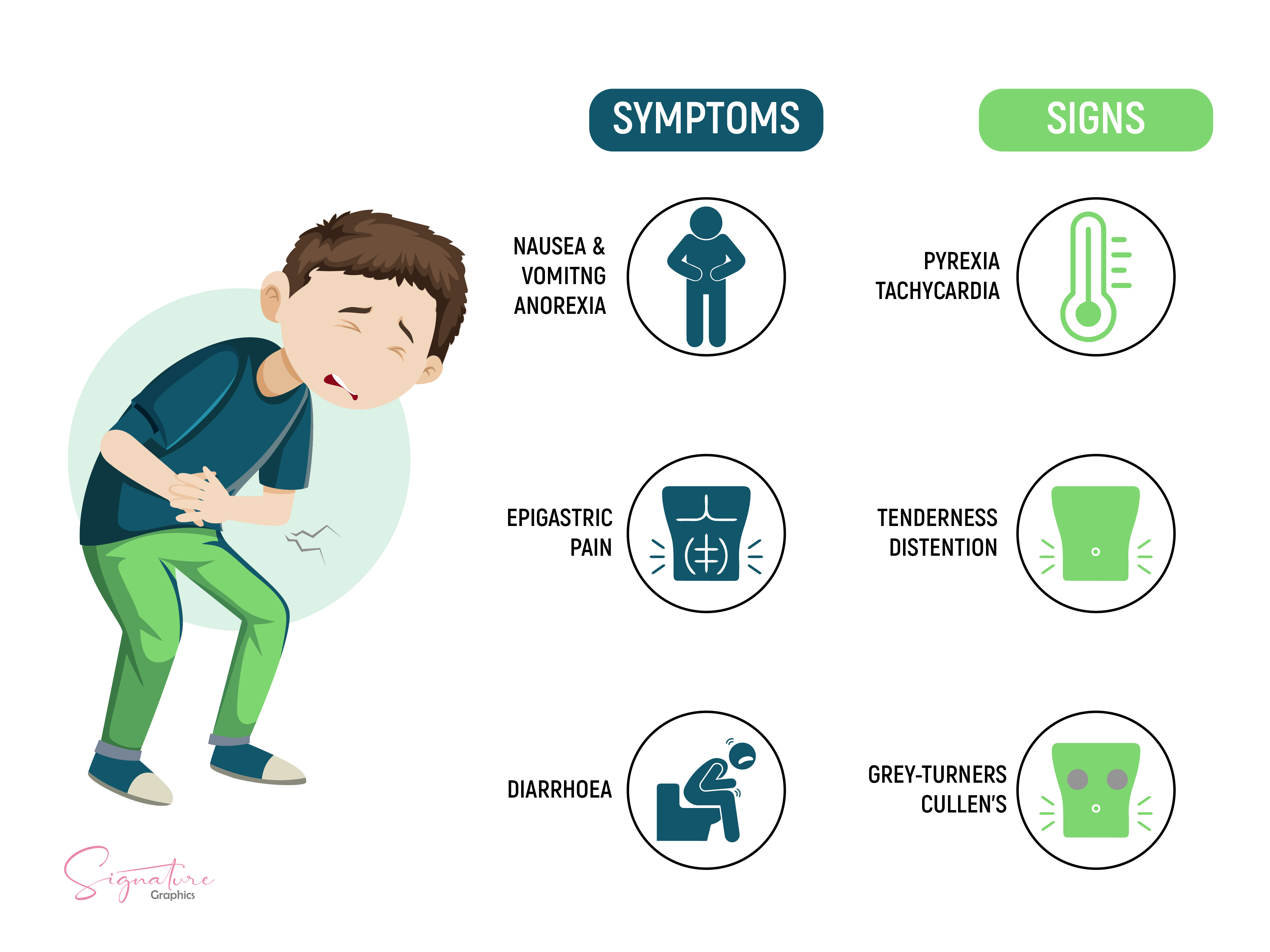
The classic clinical presentation of acute pancreatitis involves moderate-to-severe epigastric abdominal pain, often described as deep, burning, or stabbing, with radiation to the back. Pain onset is typically sudden, although it may develop more gradually and with less localization in alcohol-induced pancreatitis. Nausea, vomiting, and anorexia commonly accompany the pain, while fever and systemic signs, such as malaise or hemodynamic instability, may occur in more severe cases.
History
A detailed clinical history is critical for identifying potential etiologies and anticipating complications. Information about alcohol consumption, especially chronic heavy use spanning more than 5 years, is essential, as is a history of gallbladder disease, including known gallstones, prior episodes of biliary colic, or a history of cholecystectomy. Hyperlipidemia, particularly familial or untreated, should be elicited. Recent procedures, notably endoscopic retrograde cholangiopancreatography or abdominal surgeries, may suggest a postprocedural trigger. Medication review is also important to identify the use of drugs known to cause pancreatitis, such as azathioprine, valproic acid, didanosine, mesalamine, or angiotensin-converting enzyme inhibitors.
A history of cigarette smoking, an independent risk factor for both acute and chronic pancreatitis, should be obtained. Family history is relevant in patients younger than 30 who present without an identifiable cause, as genetic predispositions may underlie their disease. A history of autoimmune disease may point to autoimmune pancreatitis, while unexplained weight loss or newly diagnosed diabetes may suggest a pancreatic neoplasm. Any prior episodes of pancreatitis should be documented to help determine whether the current episode is isolated or part of a recurrent or chronic pattern. Finally, recent trauma, substance use, or changes in diet may reveal precipitating factors.
Physical Examination
The physical examination typically reveals signs of systemic inflammation and localized abdominal tenderness. Vital signs may show fever and tachycardia, with hypotension present in severe cases due to third-spacing or systemic inflammatory response syndrome (see Image. Signs and Symptoms of Acute Pancreatitis). Findings suggestive of hypovolemia, such as dry mucous membranes, prolonged capillary refill, and decreased skin turgor, are common.
Epigastric tenderness with guarding or mild rigidity is often present on abdominal examination. Decreased bowel sounds may indicate ileus, while abdominal distension is more frequently observed in severe or necrotizing disease. Cullen sign, characterized by periumbilical ecchymosis, and Grey-Turner sign, marked by flank ecchymosis, suggest hemorrhagic pancreatitis with retroperitoneal or peritoneal bleeding.[16] Jaundice may reflect biliary obstruction or concomitant cholangitis.
In some cases, a palpable epigastric mass may be appreciated, suggesting the presence of a pseudocyst or peripancreatic fluid collection. Altered mental status, particularly in older individuals, may indicate severe systemic involvement or underlying metabolic derangement. Historical and physical findings support early risk stratification. These clinical data also guide decisions regarding monitoring intensity, imaging studies, and potential intensive care unit admission.
Evaluation
The diagnosis of acute pancreatitis is based on the Revised Atlanta Classification, which requires the presence of at least 2 of the following criteria:
- Characteristic abdominal pain, defined as epigastric discomfort that often radiates to the back
- Serum amylase or lipase level 3 or more times the upper limit of normal
- Imaging findings consistent with acute pancreatitis on ultrasound, computed tomography, or magnetic resonance imaging scans
Clinical presentation and elevated pancreatic enzymes are usually sufficient to establish the diagnosis. Imaging is typically reserved for patients with diagnostic uncertainty or a clinical course that is severe or worsening.[17]
Etiologic Differentiation
The etiology directly influences management and the risk of recurrence. Thus, early identification of the underlying cause is essential once acute pancreatitis is confirmed. A biliary etiology should be suspected in patients with a history of gallstones, recent biliary colic, or biochemical signs of liver involvement. An alanine aminotransferase (ALT) level greater than 150 U/L within the first 48 hours of symptom onset has a positive predictive value exceeding 85% for gallstone pancreatitis. All patients should undergo a right upper quadrant ultrasound to assess for gallstones, biliary dilation, or sludge. Endoscopic ultrasonography or magnetic resonance cholangiopancreatography may identify microlithiasis or choledocholithiasis if ultrasonography does not reveal a source despite a high clinical suspicion. Timely recognition of biliary pancreatitis allows for prompt therapeutic interventions, including endoscopic retrograde cholangiopancreatography and cholecystectomy.
An alcoholic cause should be considered in individuals with a history of chronic heavy alcohol use, typically defined as 4 to 5 drinks daily for at least 5 years. These patients often lack biliary pathology and may present with more insidious symptom onset. Although serum amylase and lipase are typically elevated, no specific laboratory marker confirms alcohol as the etiology, making history the primary diagnostic tool. A hepatic panel may reveal mild transaminitis, and serum triglycerides should be measured to evaluate for concurrent hypertriglyceridemia. Routine screening for alcohol use disorder is recommended, along with early referral for behavioral counseling when indicated.
Initial Evaluation
All patients admitted with acute pancreatitis should undergo early severity assessment, with specific attention to signs of organ failure involving the respiratory, cardiovascular, or renal systems. Clinical history and physical examination should determine the underlying etiology, evaluate disease severity, and identify potential complications.
Laboratory Evaluation
Initial laboratory testing should include serum lipase and amylase, which, when elevated, support the diagnosis of acute pancreatitis. Liver function tests, including ALT, aspartate aminotransferase, alkaline phosphatase, and bilirubin, are essential for identifying biliary involvement. Measurement of serum calcium and triglyceride levels aids in detecting metabolic causes. A complete blood count and basic metabolic panel, including blood urea nitrogen (BUN), should also be obtained. Serum lactate may help assess tissue perfusion and guide resuscitative efforts in patients with suspected systemic hypoperfusion.
Monitoring BUN and hematocrit levels provides important information about intravascular volume status. Rising values may reflect inadequate fluid resuscitation and correlate with poorer outcomes.[18] C-reactive protein levels can assist in estimating disease severity. Serum immunoglobulin G4 measurement is warranted when autoimmune pancreatitis is suspected. Genetic testing should be considered in patients with early-onset, recurrent, or idiopathic acute pancreatitis, especially if a family history of pancreatic disease has been elicited. Variants associated with increased susceptibility and the proteins they encode include the following:
- PRSS1: Cationic trypsinogen
- SPINK1: Serine protease inhibitor Kazal-type 1
- CFTR: Cystic fibrosis transmembrane conductance regulator
- CTRC: Chymotrypsin C
- CASR: Calcium-sensing receptor
- CLDN2: Claudin-2, a tight junction protein involved in paracellular ion transport within pancreatic ducts
These genes are implicated in dysregulated trypsin activation, impaired protective mechanisms, and abnormal ductal secretion, all contributing to pancreatic injury and chronic disease progression.
Routine Imaging
While acute pancreatitis is primarily diagnosed clinically, imaging studies help confirm etiology, assess disease severity, and guide intervention. The modality should be tailored to patient risk factors and the progression of the illness. Ultrasound is the preferred first-line modality and should be performed in all patients to evaluate for biliary causes. This imaging tool detects gallstones, biliary sludge, and common bile duct dilation. Abdominal radiographs have limited diagnostic utility but may reveal indirect signs of pancreatitis, such as a sentinel loop, colon cutoff sign, or extraluminal air in cases of bowel perforation. Chest radiographs may demonstrate pleural effusions or acute respiratory distress syndrome (ARDS), particularly in severe disease.
Cross-sectional imaging is not routinely required but becomes important when diagnostic uncertainty persists, or the patient fails to improve after 48 to 72 hours. Computed tomography (CT) with intravenous contrast is commonly used to evaluate for pancreatic necrosis, pseudocyst formation, or infection (see Image. Peripancreatic Necrotic Fluid on Computed Tomography). Magnetic resonance imaging and magnetic resonance cholangiopancreatography (MRCP) offer an alternative to CT and are particularly useful for characterizing pancreatic necrosis, fluid collections, and ductal anatomy. MRCP is noninvasive, does not require contrast administration, and carries no procedural risk, making it preferable in patients with renal insufficiency or contrast allergy.
Advanced Imaging
Advanced imaging modalities help clarify etiology and guide intervention in selected patients. MRCP provides noninvasive visualization of the pancreatic and biliary ducts but may miss stones smaller than 3 mm. Endoscopic ultrasonography (EUS) offers greater sensitivity for detecting microlithiasis, early neoplasms, and subtle ductal abnormalities. Endoscopic retrograde cholangiopancreatography (ERCP) is reserved for therapeutic purposes, such as stone removal or sphincterotomy, and should not be performed solely for diagnosis unless MRCP or EUS confirms biliary obstruction or cholangitis.[19][20][21]
Severity Assessment
Several clinical scoring systems and laboratory parameters assist in predicting the severity of acute pancreatitis. Common tools include the systemic inflammatory response syndrome criteria, the bedside index for severity in acute pancreatitis score, and the Ranson criteria. Serial BUN and hematocrit measurements can provide additional prognostic information. Rising values may indicate inadequate fluid resuscitation and are associated with worse outcomes. The Revised Atlanta Classification stratifies disease into mild, moderately severe, or severe categories based on clinical course and complications. Severe acute pancreatitis is defined by the presence of persistent organ failure lasting more than 48 hours, pancreatic or peripancreatic necrosis, or systemic complications. These cases typically require intensive care unit management.
Treatment / Management
Initial management of acute pancreatitis is supportive and focuses on preventing complications, identifying the underlying etiology, and addressing modifiable risk factors. Most cases are mild and resolve with conservative care, including fluid resuscitation, pain control, and nutritional support. However, moderate-to-severe cases require close monitoring, risk stratification, and interprofessional coordination to reduce morbidity and prevent deterioration.
Etiology-Specific Management Considerations
Urgent ERCP is indicated when clinical or diagnostic findings, including fever, jaundice, and elevated bilirubin or transaminases, suggest biliary obstruction or cholangitis. Laparoscopic cholecystectomy should be performed during the index hospitalization for most patients with gallstone-induced pancreatitis to reduce the risk of recurrence. Surgery may be deferred if the patient has ongoing organ failure or local complications, such as necrosis or fluid collections. Further evaluation with MRCP or EUS can confirm the diagnosis and guide management in suspected microlithiasis or biliary sludge, but no stones are visualized on ultrasound.
Supportive care is central to the management of alcohol-related pancreatitis, but structured counseling for alcohol use should also be provided. Inpatient brief interventions have been shown to reduce the risk of recurrent pancreatitis, hospital readmissions, and progression to chronic disease. Referral to addiction medicine or behavioral health services is recommended. Chronic alcohol use is often associated with nutritional deficiencies. Consultation with a dietitian can help address caloric and micronutrient deficits, particularly in patients with recurrent or chronic disease. Evaluation for chronic pancreatic injury, including signs of malabsorption (eg, steatorrhea) or new-onset diabetes mellitus, is appropriate. Pancreatic enzyme replacement therapy may be warranted in select cases.
Fluid Resuscitation
Early fluid resuscitation is a cornerstone of acute pancreatitis treatment. While aggressive hydration was previously recommended, the 2022 Early Weight-Based Aggressive versus Nonaggressive Goal-Directed Fluid Resuscitation in the Early Phase of Acute Pancreatitis (WATERFALL) trial demonstrated that early aggressive fluid administration, defined as a 20 mL/kg bolus followed by 3 mL/kg/hr, was associated with an increased risk of fluid overload without clinical benefit.[22][23] This finding prompted a paradigm shift toward moderate, individualized resuscitation guided by frequent reassessment.(A1)
Lactated Ringer solution is the preferred fluid. Resuscitation goals include maintaining urine output above 0.5 mL/kg per hour and normalizing heart rate and hematocrit. Key parameters such as blood urea nitrogen, hematocrit, lactate, and urine output should be regularly monitored to guide ongoing fluid therapy.
Pain Management
Pain management in acute pancreatitis should follow the World Health Organization's 4-step analgesic ladder, with most patients requiring step 3 agents such as intravenous opioids.[24] Hydromorphone and fentanyl are preferred. Meperidine is avoided due to neurotoxicity risk. Patient-controlled analgesia may improve pain control consistency and reduce total opioid use, particularly in moderate-to-severe cases.[23] Nonopioid adjuncts, including acetaminophen or regional nerve blocks, may provide additional benefit in select patients.(A1)
Nutritional Support
Early enteral nutrition should begin within 24 to 48 hours, even in moderate-to-severe cases, as it lowers the risk of infectious complications and organ failure.[25] Patients may safely resume a low-fat solid diet once symptoms improve, as this approach is preferable to initiating clear liquids. Enteral feeding through a nasogastric or nasojejunal tube should be started if oral intake is not tolerated. Both routes are equally effective and safe.[26] Parenteral nutrition is reserved for patients who cannot tolerate enteral feeding after 5 to 7 days.[27] Prolonged fasting or “gut rest” should be avoided, as it may lead to worse outcomes.(B2)
Antibiotics
Prophylactic antibiotics are not recommended in cases of sterile necrosis.[28] Antimicrobial therapy should be reserved for confirmed or strongly suspected infections, such as cholangitis, infected necrosis, pneumonia, or urinary tract infection.[29] When infected necrosis is suspected, CT-guided fine-needle aspiration can assist in confirming infection and determining the need for intervention or drainage.[30]
Etiology-Specific Therapy
For gallstone pancreatitis, urgent ERCP is indicated in patients with cholangitis or biliary obstruction. Elective cholecystectomy should be performed during the same hospitalization once the acute episode resolves. Alcohol-related pancreatitis requires counseling and treatment for alcohol use disorder as part of comprehensive care. Hypertriglyceridemia-induced pancreatitis should be managed with insulin infusion, heparin, or plasmapheresis if triglyceride levels exceed 1000 mg/dL.
Autoimmune pancreatitis may respond to corticosteroids, but treatment should only follow confirmation by imaging and serologic testing. For medication-induced pancreatitis, the suspected drug should be discontinued and reported if necessary. Common triggers include azathioprine, 6-mercaptopurine, valproic acid, and mesalamine. Pancreatic neoplasm should be considered in adults older than 40 when no clear etiology is identified. In patients younger than 30 years with a positive family history and no other cause, genetic testing for PRSS1, CFTR, or SPINK1 mutations is recommended.
Differential Diagnosis
Acute pancreatitis can mimic numerous intraabdominal and thoracic conditions. Early recognition is essential, but misdiagnosis may occur if the differential diagnosis is too narrow. A broad clinical perspective is necessary, particularly during the initial evaluation.
Peptic ulcer disease may present with epigastric pain similar to pancreatitis, and perforation can result in peritonitis. Acute cholecystitis shares overlapping features with biliary pancreatitis, including right upper quadrant pain and fever. Choledocholithiasis or cholangitis should be considered in patients with jaundice, fever, and systemic inflammatory response. Gastroenteritis presents with nausea, vomiting, and diarrhea, but typically lacks pancreatic enzyme elevation.
Mesenteric ischemia should be suspected when pain is severe and disproportionate to physical findings. CT angiography is required for diagnosis. Bowel obstruction can cause abdominal distension and vomiting, with air-fluid levels seen on imaging. Myocardial infarction, particularly inferior wall involvement, may present as epigastric pain and vomiting. Electrocardiography and troponin testing are critical for evaluation.
Diabetic ketoacidosis may resemble pancreatitis, as it can cause abdominal pain and elevated amylase levels. Aortic dissection must be suspected in patients with tearing chest or back pain, pulse deficits, or neurologic symptoms and is confirmed by CT angiography. Ruptured abdominal aortic aneurysm should be considered in hypotensive individuals with back or flank pain, given its high mortality if undiagnosed.
Perforated viscus presents with signs of peritonitis, and free intraperitoneal air on upright imaging is diagnostic. Pneumonia, particularly in the lower lobes, may refer to abdominal pain and cause systemic symptoms that mimic pancreatitis. Renal colic may present with flank or epigastric pain and hematuria, with noncontrast-enhanced CT confirming nephrolithiasis. Viral hepatitis should be considered in patients with recent travel, jaundice, or elevated transaminases. Confirming the diagnosis and ruling out critical mimics requires a thorough evaluation. Recommended studies include laboratory tests, electrocardiography, and imaging such as abdominal ultrasound, CT, and chest radiograph.
Pertinent Studies and Ongoing Trials
A recent multicenter randomized controlled trial, the WATERFALL trial, was conducted across 18 hospitals and published in the New England Journal of Medicine to evaluate optimal fluid resuscitation strategies in acute pancreatitis. Patients were randomized to receive either aggressive fluid resuscitation (20 mL/kg bolus followed by 3 mL/kg/hr of lactated Ringer solution) or a moderate regimen (10 mL/kg bolus only if hypovolemic, followed by 1.5 mL/kg/hr maintenance). Clinical assessments and fluid adjustments were performed at 12, 24, 48, and 72 hours based on patient status.
The trial’s primary outcome was the development of moderately severe or severe acute pancreatitis, while the primary safety endpoint was fluid overload. Interim analysis of 249 patients showed a significantly higher incidence of fluid overload in the aggressive group (20.5%) compared to the moderate group (3.3%), with no associated improvement in clinical outcomes (adjusted relative risk 2.85; 95% CI, 1.36 to 5.94; P = 0.004). The trial was terminated early due to safety concerns. These findings support a shift toward more cautious, individualized fluid resuscitation strategies, moving away from routine aggressive protocols. Ongoing trials are investigating emerging interventions such as probiotics, immunomodulation, endoscopic management of necrosis, and machine learning–based severity prediction models to improve early risk stratification and outcomes in acute pancreatitis.
Prognosis
The overall mortality of acute pancreatitis is approximately 1% to 2%, but this figure increases substantially in cases classified as severe, although reported rates vary across studies. Early assessment of disease severity is critical for determining the appropriate level of care, triage, intensive care unit admission, and timing of intervention. Numerous scoring systems have been developed to aid in risk stratification. Older tools, such as the Ranson criteria and the Acute Physiology and Chronic Health Evaluation (APACHE) II score, require data over the first 48 hours and may be impractical for routine use due to their complexity.
The International Association of Pancreatology and the American Pancreatic Association recommend using systemic inflammatory response syndrome (SIRS) as an early predictor of severity. The presence of SIRS on admission, especially if it persists beyond 48 hours, is associated with a significantly increased mortality risk.[31] Patients with persistent SIRS have an estimated mortality rate of 25%, compared to approximately 8% in those with transient SIRS.[32] SIRS at admission has 100% sensitivity but low specificity (31%) for predicting mortality, while persistent SIRS has 77% to 89% sensitivity and 79% to 86% specificity.[33][34]
A more recent and user-friendly scoring system is the bedside index for severity in acute pancreatitis, BISAP, which incorporates 5 simple clinical variables available at initial presentation:
- B: Blood urea nitrogen over 25 mg/dL (8.9 mmol/L)
- I: Impaired mental status (Glasgow Coma Scale <15)
- S: Presence of SIRS
- A: Age older than 60
- P: Pleural effusion on imaging
Each criterion scores 1 point. A BISAP score of 0 to 2 is associated with a mortality rate of less than 2%, whereas a score of 3 to 5 corresponds to a mortality rate exceeding 15%. While BISAP is convenient and specific, it has lower sensitivity than more complex models.[35]
Another validated prognostic tool is the Modified CT Severity Index (CTSI), which integrates the Balthazar grading system with measures of pancreatic necrosis and extrapancreatic complications.[36] This imaging-based score ranges from 0 to 10 and classifies disease severity as mild, moderate, or severe. Compared to clinical scoring systems, the Modified CTSI provides additional prognostic value by assessing anatomic and structural damage.
The Modified CTSI incorporates 3 components: pancreatic inflammation, pancreatic necrosis, and extrapancreatic complications. For pancreatic inflammation, a normal pancreas scores 0 points; intrinsic pancreatic abnormalities with or without peripancreatic fat inflammation score 2 points; and the presence of fluid collection or fat necrosis scores 4 points. For necrosis, absence of necrosis scores 0, necrosis involving 30% or less of the pancreas scores 2, and necrosis exceeding 30% scores 4. Extrapancreatic complications, such as ascites, pleural effusion, or involvement of vascular or gastrointestinal structures, contribute 2 additional points.
The total score ranges from 0 to 10. A score of 0 to 2 indicates mild disease, 4 to 6 indicates moderate disease, and 8 to 10 indicates severe disease. These scoring systems aid in prognostication and interprofessional management but should always be interpreted within the broader clinical context. Repeat imaging and reassessment may be necessary as the disease evolves.
Patients with alcohol-related pancreatitis have an elevated risk of recurrence and progression to chronic disease. Following a single episode, approximately 24% will experience recurrence, and 16% will develop chronic pancreatitis. Continued alcohol use strongly contributes to this progression, often resulting in permanent exocrine and endocrine dysfunction. The prognosis improves with early behavioral intervention and sustained abstinence. However, smoking, malnutrition, and genetic susceptibility may further worsen long-term outcomes.
Biliary pancreatitis generally carries a favorable prognosis when addressed promptly. Performing laparoscopic cholecystectomy during the index hospitalization significantly reduces the recurrence rate, which may otherwise approach 30% if surgery is delayed. ERCP reduces morbidity and mortality in cases involving choledocholithiasis, cholangitis, or ongoing biliary obstruction. With timely and definitive management, the risk of progression to chronic pancreatitis is low, particularly when compared to alcohol-induced disease.
Complications
Acute pancreatitis may lead to a broad range of local, systemic, and vascular complications. These sequelae are generally categorized as early, occurring within the first week, or late, developing thereafter. Prompt recognition of complications is essential, as many require intensive care or procedural intervention. Early complications often result from the systemic inflammatory response. SIRS can progress to multiple organ dysfunction syndrome and septic shock. Persistent SIRS beyond 48 hours correlates with increased mortality and typically warrants intensive care unit admission.
Cytokine-mediated pulmonary injury may precipitate ARDS, which presents with hypoxemia and radiographic changes. Mechanical ventilation is often necessary in patients who develop this complication. Acute kidney injury occurs frequently in severe cases and may be attributed to hypovolemia or widespread inflammation. Rising creatinine, persistent oliguria, or fluid overload may necessitate renal replacement therapy.
Inflammation, bowel dysmotility, or hypoperfusion may contribute to ileus and metabolic acidosis. Although generally managed supportively, these findings may indicate more extensive disease. Severe inflammation and the release of pancreatic enzymes may lead to disseminated intravascular coagulation, resulting in simultaneous bleeding and thrombosis. Clinically significant cases require close hemodynamic monitoring and supportive measures in the ICU. Increased intraabdominal pressure can progress to abdominal compartment syndrome, leading to hemodynamic instability and organ failure. Rapid surgical decompression is essential to prevent further deterioration.
Late complications of acute pancreatitis involve both structural and vascular consequences that typically develop after the first week. Pancreatic necrosis often emerges during this period and may be sterile or infected. Infected necrosis presents with systemic signs of sepsis and may necessitate intensive care, image-guided drainage, or surgical debridement, depending on clinical severity and response to antibiotics. Pancreatic abscesses and pseudocysts are localized fluid collections that can become secondarily infected or exert a mass effect. Although many pseudocysts resolve spontaneously, large or symptomatic ones often require drainage through endoscopic, percutaneous, or surgical approaches.
Hemorrhagic pancreatitis results from the enzymatic erosion of regional vasculature and carries a high mortality risk. Management typically involves emergent surgical intervention or angiographic embolization in cases of active bleeding. Pancreatic arterial pseudoaneurysms, commonly involving the splenic artery, may rupture and cause intraabdominal hemorrhage. These lesions are generally treated with embolization or surgery. Splenic or mesenteric venous thrombosis can arise from peripancreatic inflammation and endothelial injury. Although these thrombotic events may be asymptomatic, complications such as gastric varices or bowel ischemia may develop. Anticoagulation is appropriate in many cases, and surgical management is indicated when infarction or hemorrhage occurs.
Bowel infarction is an uncommon but life-threatening consequence, often resulting from prolonged hypotension or mesenteric ischemia, and requires immediate surgical intervention. Ascites may accumulate due to capillary leak or portal hypertension and are typically managed conservatively or with paracentesis when symptomatic. Chronic pancreatitis may evolve in patients who experience recurrent or severe acute episodes, ultimately leading to irreversible fibrosis, pain, and both exocrine and endocrine pancreatic insufficiency. Timely recognition of these complications, especially those requiring procedural or intensive care, is essential for appropriate monitoring and intervention.
Patients with recurrent alcohol-induced pancreatitis face a heightened risk of progressive pancreatic fibrosis, which can lead to chronic pancreatitis. This condition is defined by exocrine insufficiency, manifesting as malabsorption and steatorrhea, endocrine dysfunction such as diabetes, and persistent abdominal pain syndromes. Chronic alcohol use also increases the risk of pancreatic ductal adenocarcinoma, particularly in cases of long-standing disease. In addition, alcohol may impair nutritional status, hepatic function, and tissue healing, all of which can hinder recovery and contribute to higher hospital readmission rates.
Gallstone-induced pancreatitis may be complicated by ongoing biliary obstruction, ascending cholangitis, or recurrent episodes if cholecystectomy is not performed promptly. Patients with choledocholithiasis are at risk of developing postpancreatitis cholangitis or, in untreated cases, secondary biliary cirrhosis. Definitive removal of the biliary source significantly reduces the risk of recurrence and long-term complications, and outcomes are generally favorable when timely intervention is provided.
Consultations
Early, case-specific consultation with relevant specialists improves outcomes in patients with moderate-to-severe acute pancreatitis. The timing and type of involvement depend on disease severity, clinical progression, underlying etiology, and emerging complications. Gastroenterology input is recommended early in moderate-to-severe cases, particularly when biliary pancreatitis, autoimmune disease, or idiopathic causes are suspected. Gastroenterologists perform urgent ERCP in cases of cholangitis or biliary obstruction and manage pseudocysts or walled-off necrosis through endoscopic drainage when appropriate. Surgical consultation is indicated for cholecystectomy during the index admission in gallstone pancreatitis and becomes urgent in cases of suspected infected necrosis, bowel infarction, or abdominal compartment syndrome. Surgical expertise is also necessary when minimally invasive interventions are either ineffective or not feasible.
Critical care specialists should be involved when SIRS persists, organ dysfunction develops, or patients require vasopressors, mechanical ventilation, or renal replacement therapy. Early triage to an intensive care setting facilitates close monitoring and optimal resource utilization. Nutrition services play a key role in initiating and adjusting enteral feeding. Dietitians assist with transitioning to oral intake and tailoring long-term nutrition plans for patients at risk of malnutrition or pancreatic insufficiency.
In alcohol-induced pancreatitis, early involvement of behavioral health or addiction medicine enhances long-term sobriety, reduces recurrence risk, and supports recovery. Endocrinology consultation may be necessary for managing new-onset or worsening diabetes, severe hyperglycemia, or hypertriglyceridemia and can help guide long-term metabolic control. Interventional radiology provides image-guided drainage of infected collections, necrosis, or hemorrhage and offers minimally invasive alternatives that may delay or obviate the need for surgical intervention. Timely and coordinated interprofessional consultation promotes targeted therapy, reduces complications, and improves outcomes during care's acute and recovery phases. This collaborative approach facilitates prompt decision-making, minimizes delays in escalation of care, and supports comprehensive patient care.
Deterrence and Patient Education
Patient education is critical for preventing recurrence and identifying complications early. Counseling should be tailored to the specific etiology and individual risk factors. Patients with alcohol-related pancreatitis should abstain from alcohol, with referrals to addiction medicine or behavioral health services offered when appropriate. Smoking cessation should also be addressed, as tobacco worsens pancreatic injury. Nutrition counseling is recommended to support a low-fat diet, which may reduce symptom recurrence. Adherence to medications, particularly for hypertriglyceridemia, diabetes, or autoimmune disease, is essential.
All patients should be advised to seek prompt medical attention for concerning symptoms such as persistent abdominal pain, fever, jaundice, vomiting, dizziness, abdominal distension, tachypnea, and rapid heart rate. Individuals at higher risk may benefit from monitoring their pulse at home and keeping emergency instructions accessible. Driving during symptomatic episodes should be avoided. Genetic counseling may be appropriate in cases of hereditary or early-onset disease. Ongoing patient engagement helps reduce long-term complications and supports better outcomes.
Sustained abstinence offers the greatest benefit for patients with alcohol-induced pancreatitis. Even after a single episode, the risk of recurrence and progression to chronic pancreatitis rises markedly without intervention. Brief inpatient counseling, delivered by physicians, nurses, or behavioral health professionals, can reduce future episodes and alcohol use. Continued outpatient follow-up with addiction specialists or primary care providers reinforces long-term recovery. Smoking cessation should also be emphasized, as tobacco independently contributes to pancreatic injury. Patients must be educated on recognizing the early signs of recurrence, and selected individuals may benefit from structured recovery programs or peer support groups.
Patient education should emphasize the importance of definitive treatment, particularly laparoscopic cholecystectomy during the index admission, to prevent recurrence. Even small gallstones or biliary sludge can trigger recurrent or severe episodes, and patients should be made aware of this risk. For individuals awaiting elective surgery, clear instructions must be provided regarding symptoms such as biliary colic, jaundice, or signs of infection that warrant prompt medical evaluation. When endoscopic procedures such as ERCP are performed, patients should be informed of the indication, expected outcomes, and any required follow-up.
Pearls and Other Issues
Acute pancreatitis presents with variable severity and requires early recognition and targeted management. The following pearls highlight key diagnostic and therapeutic considerations:
- Acute pancreatitis is diagnosed when at least 2 of the following are present: lipase or amylase that is at least 3 times the upper limit of normal, characteristic clinical presentation, and imaging findings consistent with pancreatitis.
- The most common causes are gallstones and alcohol. Other etiologies include hypertriglyceridemia, medications, autoimmune pancreatitis, and post-ERCP injury.
- The WATERFALL trial (NEJM 2022) demonstrated that aggressive early fluid resuscitation increases the risk of fluid overload without improving outcomes, leading to a shift toward moderate, goal-directed hydration.
- Early enteral nutrition within 24 to 48 hours lowers the risk of infectious complications and is preferred over parenteral feeding.
- Persistent SIRS lasting beyond 48 hours correlates with significantly increased mortality and indicates the need for ICU-level monitoring.
- The BISAP score and the Modified CTSI are validated tools for early risk stratification and care planning.
- Complications such as pancreatic necrosis, ARDS, disseminated intravascular coagulation, abdominal compartment syndrome, and vascular events may require ICU care or surgical intervention.
- Prophylactic antibiotics should be avoided in sterile necrosis. Antibiotic therapy is reserved for confirmed or highly suspected infections.
Management strategies should be tailored to disease severity and etiology while anticipating complications. Judicious fluid resuscitation, early enteral feeding, and targeted intervention are key to optimizing recovery.
Enhancing Healthcare Team Outcomes
Acute pancreatitis is a complex condition requiring a coordinated, interprofessional approach for optimal management. The healthcare team may involve surgery, gastroenterology, radiology, endocrinology, critical care, pharmacy, nursing, nutrition, addiction medicine, and interventional radiology. Gallstones, alcohol use, and medications represent the 3 leading causes of acute pancreatitis. Prevention and early identification of at-risk individuals are central to effective care. Nurses and pharmacists contribute to patient education on modifiable risk factors such as alcohol cessation, low-fat dietary habits, weight management, and triglyceride control. Pharmacists also assist by identifying and discontinuing medications associated with pancreatitis risk.[37][38][39]
Unified, patient-centered care relies on timely documentation, effective communication, and shared decision-making across disciplines. Intensivists typically lead initial fluid resuscitation and monitoring, while surgical and interventional teams address complications requiring procedural intervention. Nutritionists provide tailored enteral support. Behavioral health professionals manage underlying substance use. Well-coordinated care transitions and adherence to evidence-based protocols reduce complications, promote recovery, and improve long-term outcomes.
Outcomes
Acute pancreatitis continues to carry a significant risk of morbidity and mortality, particularly when manifestations are severe or organ failure develops. While overall mortality ranges from 5% to 15%, this risk increases to more than 20% in patients with multiorgan dysfunction, infected necrosis, or persistent SIRS.[40] Prognosis is influenced by multiple factors, including patient age, underlying comorbidities, disease etiology, and initial severity. Outcomes in gallstone pancreatitis are generally worse than in alcohol-related cases. Type 2 diabetes is also linked to higher complication rates and increased mortality.[41]
Most deaths occur within the first 2 weeks and are frequently caused by progressive multiorgan failure or hypotensive shock. A smaller proportion of deaths result from late complications such as infected necrosis or hemorrhage. Several classification systems, including the Revised Atlanta Criteria and the BISAP score, have been developed to predict disease severity and inform triage decisions. However, complexity and predictive accuracy limitations may reduce their utility in routine practice.[42] Early identification of high-risk individuals and targeted supportive care during the first 48 to 72 hours remain essential to improving outcomes.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
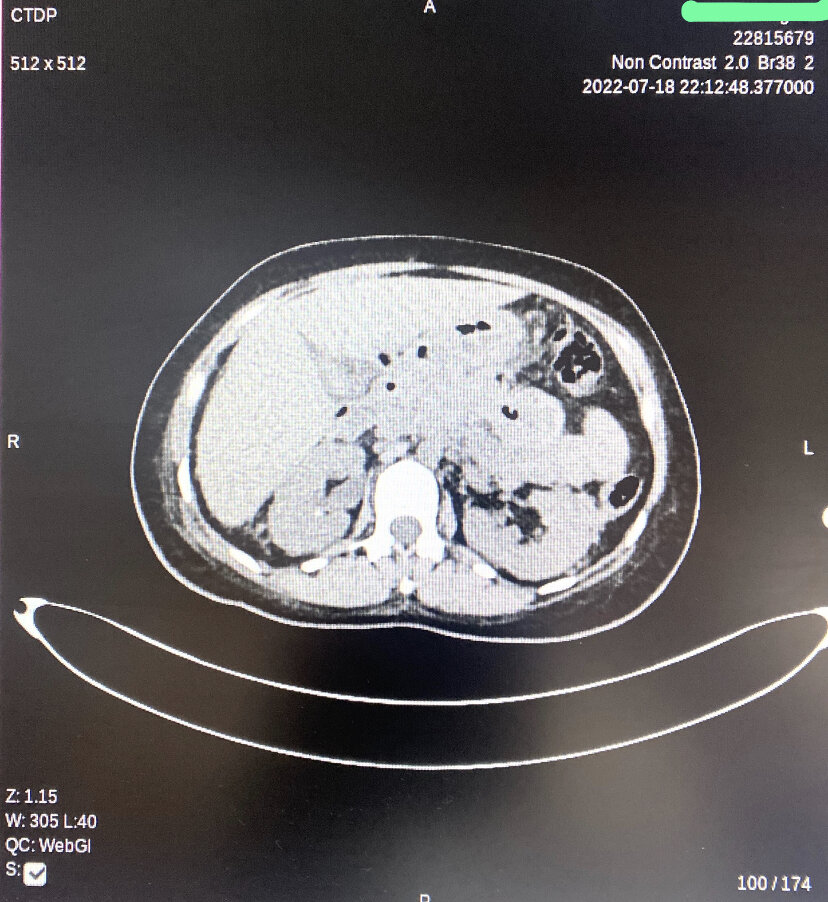
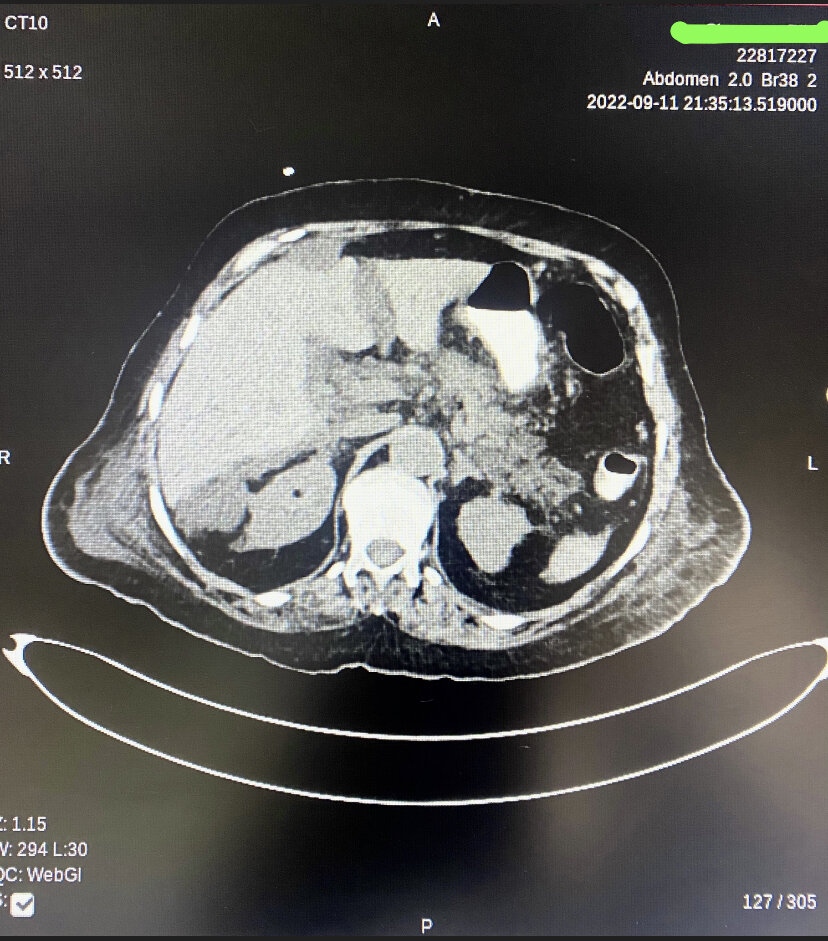
Peripancreatic Necrotic Fluid on Computed Tomography. This image demonstrates acute necrotizing pancreatitis with a diffusely hypoenhancing pancreas and patchy nonenhancing regions within the parenchyma. Multiple extensive peripancreatic necrotic fluid collections with internal air loculi are visible in the lesser sac, pancreatic bed, right hemiabdomen, and pelvis.
Contributed by A Tariq, MD
References
Werge M, Novovic S, Schmidt PN, Gluud LL. Infection increases mortality in necrotizing pancreatitis: A systematic review and meta-analysis. Pancreatology : official journal of the International Association of Pancreatology (IAP) ... [et al.]. 2016 Sep-Oct:16(5):698-707. doi: 10.1016/j.pan.2016.07.004. Epub 2016 Jul 9 [PubMed PMID: 27449605]
Level 1 (high-level) evidenceValverde-López F, Wilcox CM, Redondo-Cerezo E. Evaluation and management of acute pancreatitis in Spain. Gastroenterologia y hepatologia. 2018 Dec:41(10):618-628. doi: 10.1016/j.gastrohep.2018.06.012. Epub 2018 Aug 24 [PubMed PMID: 30149943]
Kahaleh M. Management of pancreatitis and pancreatic: fluid collections. Revista de gastroenterologia del Peru : organo oficial de la Sociedad de Gastroenterologia del Peru. 2018 Apr-Jun:38(2):169-182 [PubMed PMID: 30118464]
Bazerbachi F, Haffar S, Hussain MT, Vargas EJ, Watt KD, Murad MH, Chari S, Abu Dayyeh BK. Systematic review of acute pancreatitis associated with interferon-α or pegylated interferon-α: Possible or definitive causation? Pancreatology : official journal of the International Association of Pancreatology (IAP) ... [et al.]. 2018 Oct:18(7):691-699. doi: 10.1016/j.pan.2017.08.012. Epub 2017 Sep 21 [PubMed PMID: 30061072]
Level 1 (high-level) evidenceOrtiz Morales CM, Girela Baena EL, Olalla Muñoz JR, Parlorio de Andrés E, López Corbalán JA. Radiology of acute pancreatitis today: the Atlanta classification and the current role of imaging in its diagnosis and treatment. Radiologia. 2019 Nov-Dec:61(6):453-466. doi: 10.1016/j.rx.2019.04.001. Epub 2019 May 29 [PubMed PMID: 31153603]
Talathi SS, Zimmerman R, Young M. Anatomy, Abdomen and Pelvis, Pancreas. StatPearls. 2025 Jan:(): [PubMed PMID: 30422507]
Fonseca Sepúlveda EV, Guerrero-Lozano R. Acute pancreatitis and recurrent acute pancreatitis: an exploration of clinical and etiologic factors and outcomes. Jornal de pediatria. 2019 Nov-Dec:95(6):713-719. doi: 10.1016/j.jped.2018.06.011. Epub 2018 Aug 1 [PubMed PMID: 30075118]
Barbara M, Tsen A, Rosenkranz L. Acute Pancreatitis in Chronic Dialysis Patients. Pancreas. 2018 Sep:47(8):946-951. doi: 10.1097/MPA.0000000000001119. Epub [PubMed PMID: 30113429]
Tenner S, Baillie J, DeWitt J, Vege SS, American College of Gastroenterology. American College of Gastroenterology guideline: management of acute pancreatitis. The American journal of gastroenterology. 2013 Sep:108(9):1400-15; 1416. doi: 10.1038/ajg.2013.218. Epub 2013 Jul 30 [PubMed PMID: 23896955]
Forsmark CE, Vege SS, Wilcox CM. Acute Pancreatitis. The New England journal of medicine. 2016 Nov 17:375(20):1972-1981 [PubMed PMID: 27959604]
de Pretis N, Amodio A, Frulloni L. Hypertriglyceridemic pancreatitis: Epidemiology, pathophysiology and clinical management. United European gastroenterology journal. 2018 Jun:6(5):649-655. doi: 10.1177/2050640618755002. Epub 2018 Jan 22 [PubMed PMID: 30083325]
Kirkegård J, Cronin-Fenton D, Heide-Jørgensen U, Mortensen FV. Acute Pancreatitis and Pancreatic Cancer Risk: A Nationwide Matched-Cohort Study in Denmark. Gastroenterology. 2018 May:154(6):1729-1736. doi: 10.1053/j.gastro.2018.02.011. Epub 2018 Feb 9 [PubMed PMID: 29432727]
Lankisch PG, Breuer N, Bruns A, Weber-Dany B, Lowenfels AB, Maisonneuve P. Natural history of acute pancreatitis: a long-term population-based study. The American journal of gastroenterology. 2009 Nov:104(11):2797-805; quiz 2806. doi: 10.1038/ajg.2009.405. Epub 2009 Jul 14 [PubMed PMID: 19603011]
Level 2 (mid-level) evidenceJohnstone C. Pathophysiology and nursing management of acute pancreatitis. Nursing standard (Royal College of Nursing (Great Britain) : 1987). 2018 Jun 28:():. doi: 10.7748/ns.2018.e11179. Epub 2018 Jun 28 [PubMed PMID: 29952150]
Constantinoiu S, Cochior D. Severe Acute Pancreatitis - Determinant Factors and Current Therapeutic Conduct. Chirurgia (Bucharest, Romania : 1990). 2018 May-Jun:113(3):385-390. doi: 10.21614/chirurgia.113.3.385. Epub [PubMed PMID: 29981669]
Mookadam F, Cikes M. Images in clinical medicine. Cullen's and Turner's signs. The New England journal of medicine. 2005 Sep 29:353(13):1386 [PubMed PMID: 16192483]
Level 3 (low-level) evidenceBanks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, Tsiotos GG, Vege SS, Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013 Jan:62(1):102-11. doi: 10.1136/gutjnl-2012-302779. Epub 2012 Oct 25 [PubMed PMID: 23100216]
Level 3 (low-level) evidenceYang CJ, Chen J, Phillips AR, Windsor JA, Petrov MS. Predictors of severe and critical acute pancreatitis: a systematic review. Digestive and liver disease : official journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver. 2014 May:46(5):446-51. doi: 10.1016/j.dld.2014.01.158. Epub 2014 Mar 16 [PubMed PMID: 24646880]
Level 1 (high-level) evidenceChoi HW, Park HJ, Choi SY, Do JH, Yoon NY, Ko A, Lee ES. Early Prediction of the Severity of Acute Pancreatitis Using Radiologic and Clinical Scoring Systems With Classification Tree Analysis. AJR. American journal of roentgenology. 2018 Nov:211(5):1035-1043. doi: 10.2214/AJR.18.19545. Epub 2018 Aug 30 [PubMed PMID: 30160978]
Mandalia A, Wamsteker EJ, DiMagno MJ. Recent advances in understanding and managing acute pancreatitis. F1000Research. 2018:7():. pii: F1000 Faculty Rev-959. doi: 10.12688/f1000research.14244.2. Epub 2018 Jun 28 [PubMed PMID: 30026919]
Level 3 (low-level) evidenceSmeets XJNM, Litjens G, Gijsbers K, Prokop M, Drenth JPH, Hermans J, van Geenen EJM. The Accuracy of Pancreatic Perfusion Computed Tomography and Angiography in Predicting Necrotizing Pancreatitis: A Systematic Review. Pancreas. 2018 Jul:47(6):667-674. doi: 10.1097/MPA.0000000000001067. Epub [PubMed PMID: 29894416]
Level 1 (high-level) evidenceBarrie J, Jamdar S, Smith N, McPherson SJ, Siriwardena AK, O'Reilly DA. Mis-use of antibiotics in acute pancreatitis: Insights from the United Kingdom's National Confidential Enquiry into patient outcome and death (NCEPOD) survey of acute pancreatitis. Pancreatology : official journal of the International Association of Pancreatology (IAP) ... [et al.]. 2018 Oct:18(7):721-726. doi: 10.1016/j.pan.2018.05.485. Epub 2018 May 24 [PubMed PMID: 30075909]
Level 3 (low-level) evidencede-Madaria E, Buxbaum JL, Maisonneuve P, García García de Paredes A, Zapater P, Guilabert L, Vaillo-Rocamora A, Rodríguez-Gandía MÁ, Donate-Ortega J, Lozada-Hernández EE, Collazo Moreno AJR, Lira-Aguilar A, Llovet LP, Mehta R, Tandel R, Navarro P, Sánchez-Pardo AM, Sánchez-Marin C, Cobreros M, Fernández-Cabrera I, Casals-Seoane F, Casas Deza D, Lauret-Braña E, Martí-Marqués E, Camacho-Montaño LM, Ubieto V, Ganuza M, Bolado F, ERICA Consortium. Aggressive or Moderate Fluid Resuscitation in Acute Pancreatitis. The New England journal of medicine. 2022 Sep 15:387(11):989-1000. doi: 10.1056/NEJMoa2202884. Epub [PubMed PMID: 36103415]
Level 1 (high-level) evidenceSellers ZM, Abu-El-Haija M, Husain SZ, Morinville V. New Management Guidelines for Both Children and Adults With Acute Pancreatitis. Gastroenterology. 2018 Jul:155(1):234-235. doi: 10.1053/j.gastro.2018.03.068. Epub 2018 Jun 8 [PubMed PMID: 29890113]
Reynolds PT, Brady EK, Chawla S. The utility of early cross-sectional imaging to evaluate suspected acute mild pancreatitis. Annals of gastroenterology. 2018 Sep-Oct:31(5):628-632. doi: 10.20524/aog.2018.0291. Epub 2018 Jul 13 [PubMed PMID: 30174401]
Level 2 (mid-level) evidenceGardner TB. Fluid Resuscitation in Acute Pancreatitis - Going over the WATERFALL. The New England journal of medicine. 2022 Sep 15:387(11):1038-1039. doi: 10.1056/NEJMe2209132. Epub [PubMed PMID: 36103418]
Petrov MS, Yadav D. Global epidemiology and holistic prevention of pancreatitis. Nature reviews. Gastroenterology & hepatology. 2019 Mar:16(3):175-184. doi: 10.1038/s41575-018-0087-5. Epub [PubMed PMID: 30482911]
Samarasekera E, Mahammed S, Carlisle S, Charnley R, Guideline Committee. Pancreatitis: summary of NICE guidance. BMJ (Clinical research ed.). 2018 Sep 5:362():k3443. doi: 10.1136/bmj.k3443. Epub 2018 Sep 5 [PubMed PMID: 30185473]
Crockett SD, Wani S, Gardner TB, Falck-Ytter Y, Barkun AN, American Gastroenterological Association Institute Clinical Guidelines Committee. American Gastroenterological Association Institute Guideline on Initial Management of Acute Pancreatitis. Gastroenterology. 2018 Mar:154(4):1096-1101. doi: 10.1053/j.gastro.2018.01.032. Epub 2018 Feb 3 [PubMed PMID: 29409760]
Leppäniemi A, Tolonen M, Tarasconi A, Segovia-Lohse H, Gamberini E, Kirkpatrick AW, Ball CG, Parry N, Sartelli M, Wolbrink D, van Goor H, Baiocchi G, Ansaloni L, Biffl W, Coccolini F, Di Saverio S, Kluger Y, Moore E, Catena F. 2019 WSES guidelines for the management of severe acute pancreatitis. World journal of emergency surgery : WJES. 2019:14():27. doi: 10.1186/s13017-019-0247-0. Epub 2019 Jun 13 [PubMed PMID: 31210778]
Working Group IAP/APA Acute Pancreatitis Guidelines. IAP/APA evidence-based guidelines for the management of acute pancreatitis. Pancreatology : official journal of the International Association of Pancreatology (IAP) ... [et al.]. 2013 Jul-Aug:13(4 Suppl 2):e1-15. doi: 10.1016/j.pan.2013.07.063. Epub [PubMed PMID: 24054878]
Level 1 (high-level) evidenceMofidi R, Duff MD, Wigmore SJ, Madhavan KK, Garden OJ, Parks RW. Association between early systemic inflammatory response, severity of multiorgan dysfunction and death in acute pancreatitis. The British journal of surgery. 2006 Jun:93(6):738-44 [PubMed PMID: 16671062]
Level 2 (mid-level) evidenceButer A, Imrie CW, Carter CR, Evans S, McKay CJ. Dynamic nature of early organ dysfunction determines outcome in acute pancreatitis. The British journal of surgery. 2002 Mar:89(3):298-302 [PubMed PMID: 11872053]
Singh VK, Wu BU, Bollen TL, Repas K, Maurer R, Mortele KJ, Banks PA. Early systemic inflammatory response syndrome is associated with severe acute pancreatitis. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2009 Nov:7(11):1247-51. doi: 10.1016/j.cgh.2009.08.012. Epub 2009 Aug 15 [PubMed PMID: 19686869]
Gao W, Yang HX, Ma CE. The Value of BISAP Score for Predicting Mortality and Severity in Acute Pancreatitis: A Systematic Review and Meta-Analysis. PloS one. 2015:10(6):e0130412. doi: 10.1371/journal.pone.0130412. Epub 2015 Jun 19 [PubMed PMID: 26091293]
Level 1 (high-level) evidenceMortele KJ, Wiesner W, Intriere L, Shankar S, Zou KH, Kalantari BN, Perez A, vanSonnenberg E, Ros PR, Banks PA, Silverman SG. A modified CT severity index for evaluating acute pancreatitis: improved correlation with patient outcome. AJR. American journal of roentgenology. 2004 Nov:183(5):1261-5 [PubMed PMID: 15505289]
Gódi S, Erőss B, Gyömbér Z, Szentesi A, Farkas N, Párniczky A, Sarlós P, Bajor J, Czimmer J, Mikó A, Márta K, Hágendorn R, Márton Z, Verzár Z, Czakó L, Szepes Z, Vincze Á, Hegyi P. Centralized care for acute pancreatitis significantly improves outcomes. Journal of gastrointestinal and liver diseases : JGLD. 2018 Jun:27(2):151-157. doi: 10.15403/jgld.2014.1121.272.pan. Epub [PubMed PMID: 29922760]
Branquinho D, Ramos-Andrade D, Elvas L, Amaro P, Ferreira M, Sofia C. Drug-Induced Acute Pancreatitis and Pseudoaneurysms: An Ominous Combination. GE Portuguese journal of gastroenterology. 2016 Nov-Dec:23(6):309-313. doi: 10.1016/j.jpge.2016.06.002. Epub 2016 Aug 12 [PubMed PMID: 28868485]
Srinivasan G, Venkatakrishnan L, Sambandam S, Singh G, Kaur M, Janarthan K, John BJ. Current concepts in the management of acute pancreatitis. Journal of family medicine and primary care. 2016 Oct-Dec:5(4):752-758. doi: 10.4103/2249-4863.201144. Epub [PubMed PMID: 28348985]
Jin T, Jiang K, Deng L, Guo J, Wu Y, Wang Z, Shi N, Zhang X, Lin Z, Asrani V, Jones P, Mittal A, Phillips A, Sutton R, Huang W, Yang X, Xia Q, Windsor JA. Response and outcome from fluid resuscitation in acute pancreatitis: a prospective cohort study. HPB : the official journal of the International Hepato Pancreato Biliary Association. 2018 Nov:20(11):1082-1091. doi: 10.1016/j.hpb.2018.05.018. Epub 2018 Aug 29 [PubMed PMID: 30170979]
Amas Gómez L, Zubia Olaskoaga F. Results of the modification of an acute pancreatitis management protocol in Intensive Care medicine. Medicina intensiva. 2019 Dec:43(9):546-555. doi: 10.1016/j.medin.2018.05.004. Epub 2018 Jul 30 [PubMed PMID: 30072142]
Garret C, Péron M, Reignier J, Le Thuaut A, Lascarrou JB, Douane F, Lerhun M, Archambeaud I, Brulé N, Bretonnière C, Zambon O, Nicolet L, Regenet N, Guitton C, Coron E. Risk factors and outcomes of infected pancreatic necrosis: Retrospective cohort of 148 patients admitted to the ICU for acute pancreatitis. United European gastroenterology journal. 2018 Jul:6(6):910-918. doi: 10.1177/2050640618764049. Epub 2018 Mar 1 [PubMed PMID: 30023069]
Level 2 (mid-level) evidence