Introduction
The ovary has 2 primary functions. First, it produces hormones that drive the female reproductive system, principally estrogen and progesterone. Second, it controls the development, selection, and release of mature oocytes for fertilization. This process, known as ovarian folliculogenesis, begins while the female is in utero. After puberty, primordial follicles undergo a multi-step process to transform into mature, pre-ovulatory follicles.
Development
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Development
Ovarian follicular development begins while the female fetus is in utero. During the fifth week of pregnancy, a female fetus's ovary contains about 500 to 1300 primordial germ cells.[1] These cells undergo mitosis, and by the 20th week of pregnancy, the female fetus has approximately 6 to 7 million germ cells. Once mitosis is complete, the germ cells enter meiosis and arrest in meiotic prophase I, forming germ cell cysts. Peripartum, each germ cell cyst regresses to form a primordial follicle containing an oocyte and a single layer of nourishing granulosa cells. Many germ cells are lost during this process, and the female is born with 1 to 2 million primordial follicles. By the time she reaches puberty, approximately 400,000 to 500,000 primordial follicles remain. After menarche, approximately 1000 follicles are lost each month. After the age of 35, the rate of follicular loss increases.[2][3]
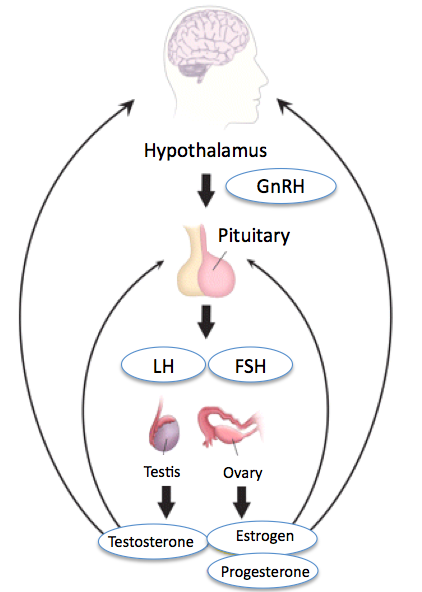
Ovarian follicle development occurs in 2 distinct phases—the gonadotropin-independent growth (pre-antral phase) and the gonadotropin-dependent growth (antral phase).[4] Gonadotropins, which include follicle-stimulating hormone (FSH) and luteinizing hormone (LH), are produced and released by the anterior pituitary gland in response to stimulation by gonadotropin-releasing hormone from the hypothalamus.[5] FSH promotes follicular growth by stimulating granulosa cell proliferation, whereas LH regulates ovulation.
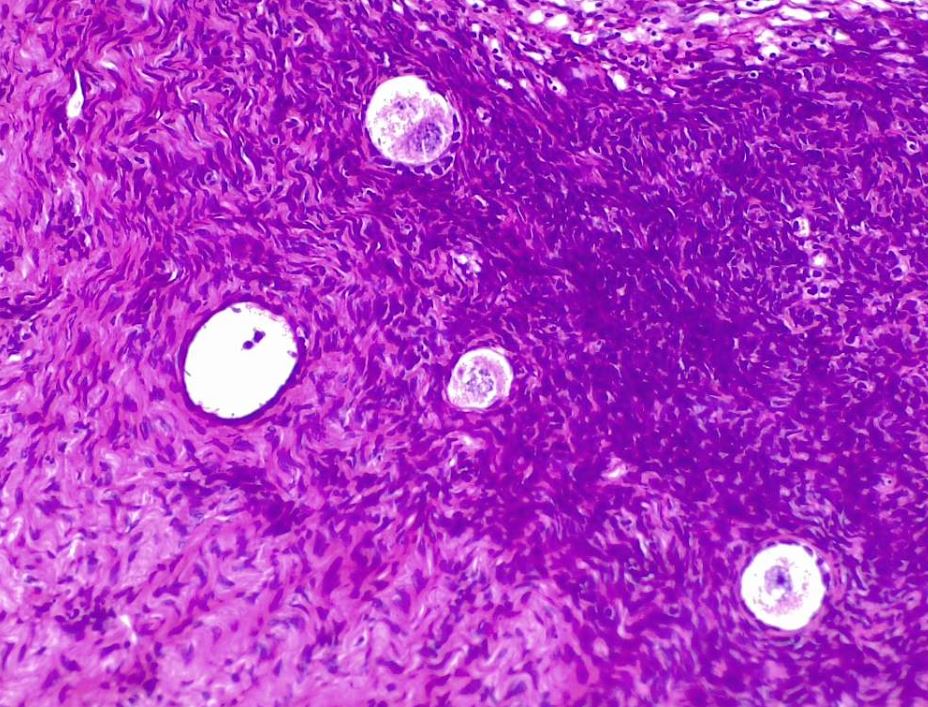
Ovarian follicular development occurs in the peripheral cortex of the ovary. Approximately 1 year is required for a primordial follicle to mature before ovulation (see Image. Ovarian Follicle Development: Oocytes and Primary Follicles in Early Stages). Most of this time is spent in the gonadotropin-independent (pre-antral) phase. Gonadotropin-independent growth relies on local growth factors, beginning with the maturation of the primordial follicle and ending before the follicle develops a fluid-filled space. Primordial follicles transition into primary follicles through oocyte enlargement and granulosa cell layer proliferation, which then start to express FSH receptors. As primary follicles become secondary follicles, the simple cuboidal epithelium of the primary follicle transforms into stratified columnar epithelium. The primary follicle stroma develops a blood supply and differentiates into the theca interna and theca externa. The theca interna develops LH receptors. The FSH and LH receptors enable the follicles to respond to their respective gonadotropins.[6]
Further maturation of secondary follicles and selection of a dominant follicle for ovulation are gonadotropin-dependent.[7] FSH binds to FSH receptors in the granulosa cell layer of the secondary follicle. In response to FSH stimulation, follicles continue to grow and develop a fluid-filled space. This space is referred to as an antrum and contains secretory material from the growing oocyte and granulosa cells. LH binds to LH receptors of the theca interna, resulting in the production of androgens. These androgens facilitate not only follicular growth but also promote follicular loss through apoptosis. As ovarian follicles mature, the larger follicles can use the enzyme aromatase to convert these androgens into estrogen.
Increasing levels of estrogen send a negative feedback signal to the hypothalamic-pituitary-adrenal axis, decreasing the circulating levels of FSH (see Image. Hypothalamic-Pituitary-Gonadal Axis). Large follicles remain sensitive to the decreasing levels of FSH. A large follicle is selected as the dominant follicle and continues to mature through a rapid proliferation of granulosa and theca cells. The antrum also enlarges rapidly. Smaller developing follicles that are not selected as the dominant follicle during the cycle undergo degeneration due to reduced sensitivity to FSH associated with their smaller size. Granulosa cells of the dominant pre-ovulatory follicle develop high concentrations of LH receptors and become responsive to the LH surge. The LH surge is caused by further increases in circulating estrogen made by the pre-ovulatory follicle and results in ovulation. During ovulation, the follicle releases its mature oocyte, which is then available for fertilization (see Image. Mature Ovarian Follicle).[8]
After ovulation, the empty follicle develops into the corpus luteum, as granulosa and theca cells differentiate into granulosa luteal and theca luteal cells, respectively. The corpus luteum secretes progesterone and estrogen to support implantation and early pregnancy. If implantation does not occur, the corpus luteum degenerates into a connective tissue structure known as the corpus albicans.
Clinical Significance
Abnormal follicular development is a hallmark of polycystic ovarian syndrome (PCOS), a disorder characterized by enlarged polycystic ovaries, abnormal menstrual bleeding, and increased androgen production.[9] Compared to females without PCOS, the ovaries of patients with PCOS have at least twice the number of growing pre-antral and antral follicles. Additionally, these follicles do not develop properly. Many follicles do not progress through the antral stage; they accumulate excess fluid, lose their granulosa cell border, and degenerate into cystic structures.[10] Patients with PCOS also have increased levels of LH, androgens, and insulin and decreased levels of FSH.[11] The precise mechanism resulting in the cystic changes associated with PCOS remains unknown. The cystic changes can cause anovulatory infertility.[12]
Premature ovarian failure is rare and most commonly presents as a loss of ovarian reserve, defined as a decreased number of primordial follicles before the age of 40.[13] Patients present with absent menstruation and disrupted ovarian follicular development, leading to symptoms of menopause and infertility. Premature ovarian failure can also present in patients who never reach menarche because of ovarian dysgenesis.
A proper understanding of ovarian follicular development is essential when counseling patients about fertility. Healthcare providers must understand and explain an assessment of a patient's ovarian follicular reserve and the potential benefit of assisted reproductive technology, including in vitro fertilization and controlled ovarian hyperstimulation.[14] As previously discussed, women younger than 35 lose approximately 1000 primordial follicles per month, and this rate increases in females older than 35. If a patient needs or desires assisted reproductive technology, it is essential to assess their ovarian reserve to maintain patient safety and define expectations. Ovarian reserve status is related to the production of antral follicles, which can be measured through transvaginal ultrasound. A decreased antral follicle count correlates with a lower ovarian reserve and decreased fertility. Patients with a smaller ovarian reserve are less likely to respond to ovarian stimulation, and women with a large ovarian reserve are at an increased risk of severe adverse effects. Understanding a patient's ovarian reserve enables healthcare providers to determine reproductive capability before and during assisted reproductive technology and establish an appropriate treatment plan.[15]
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
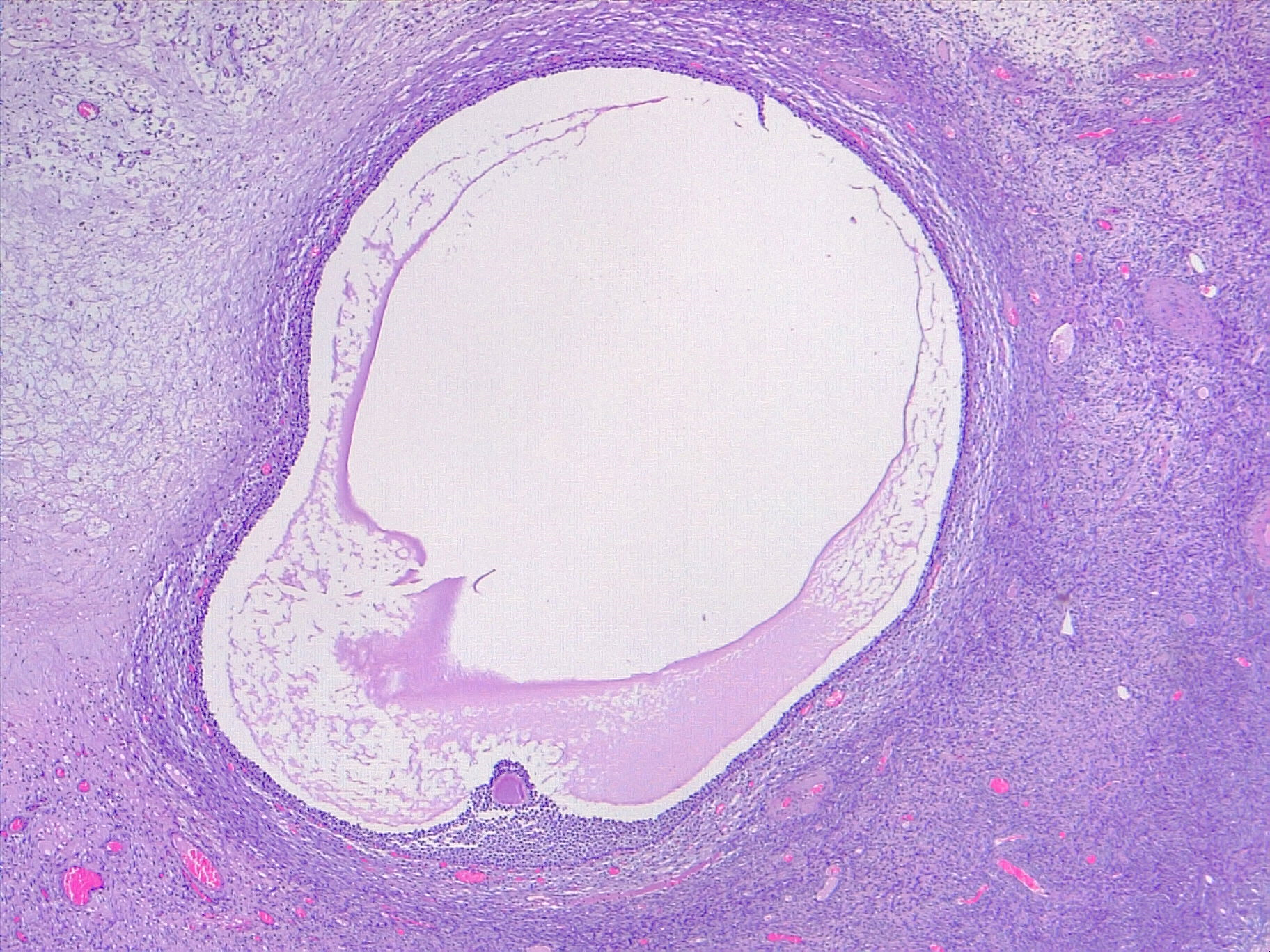
Mature Ovarian Follicle. The image depicts a mature ovarian follicle, also known as a Graafian follicle. In this stage, the oocyte has completed the first meiotic division (2N haploid) and is surrounded by granulosa cells. The cavity formed in the middle is known as the follicular antrum. The antrum contains hyaluronic acid and proteoglycans. The theca interna, a layer of cells surrounding the follicle, produces estrogen. Upon ovulation, the oocyte is released into the oviduct (hematoxylin-eosin, original magnification ×4).
Contributed by F Farci, MD
References
Overland MR, Li Y, Derpinghaus A, Aksel S, Cao M, Ladwig N, Cunha GR, Himelreich-Perić M, Baskin LS. Development of the human ovary: Fetal through pubertal ovarian morphology, folliculogenesis and expression of cellular differentiation markers. Differentiation; research in biological diversity. 2023 Jan-Feb:129():37-59. doi: 10.1016/j.diff.2022.10.005. Epub 2022 Oct 19 [PubMed PMID: 36347737]
Sun YC, Sun XF, Dyce PW, Shen W, Chen H. The role of germ cell loss during primordial follicle assembly: a review of current advances. International journal of biological sciences. 2017:13(4):449-457. doi: 10.7150/ijbs.18836. Epub 2017 Mar 11 [PubMed PMID: 28529453]
Level 3 (low-level) evidenceVollenhoven B, Hunt S. Ovarian ageing and the impact on female fertility. F1000Research. 2018:7():. pii: F1000 Faculty Rev-1835. doi: 10.12688/f1000research.16509.1. Epub 2018 Nov 22 [PubMed PMID: 30542611]
Orisaka M, Miyazaki Y, Shirafuji A, Tamamura C, Tsuyoshi H, Tsang BK, Yoshida Y. The role of pituitary gonadotropins and intraovarian regulators in follicle development: A mini-review. Reproductive medicine and biology. 2021 Apr:20(2):169-175. doi: 10.1002/rmb2.12371. Epub 2021 Feb 13 [PubMed PMID: 33850449]
Casati L, Ciceri S, Maggi R, Bottai D. Physiological and pharmacological overview of the gonadotropin releasing hormone. Biochemical pharmacology. 2023 Jun:212():115553. doi: 10.1016/j.bcp.2023.115553. Epub 2023 Apr 17 [PubMed PMID: 37075816]
Level 3 (low-level) evidenceJo M, Brännström M, Akins JW, Curry TE Jr. New insights into the ovulatory process in the human ovary. Human reproduction update. 2025 Jan 1:31(1):21-47. doi: 10.1093/humupd/dmae027. Epub [PubMed PMID: 39331957]
Gershon E, Dekel N. Newly Identified Regulators of Ovarian Folliculogenesis and Ovulation. International journal of molecular sciences. 2020 Jun 26:21(12):. doi: 10.3390/ijms21124565. Epub 2020 Jun 26 [PubMed PMID: 32604954]
Soygur B, Laird DJ. Ovary Development: Insights From a Three-Dimensional Imaging Revolution. Frontiers in cell and developmental biology. 2021:9():698315. doi: 10.3389/fcell.2021.698315. Epub 2021 Jul 26 [PubMed PMID: 34381780]
Stener-Victorin E, Teede H, Norman RJ, Legro R, Goodarzi MO, Dokras A, Laven J, Hoeger K, Piltonen TT. Polycystic ovary syndrome. Nature reviews. Disease primers. 2024 Apr 18:10(1):27. doi: 10.1038/s41572-024-00511-3. Epub 2024 Apr 18 [PubMed PMID: 38637590]
Su P, Chen C, Sun Y. Physiopathology of polycystic ovary syndrome in endocrinology, metabolism and inflammation. Journal of ovarian research. 2025 Feb 20:18(1):34. doi: 10.1186/s13048-025-01621-6. Epub 2025 Feb 20 [PubMed PMID: 39980043]
Chauvin S, Cohen-Tannoudji J, Guigon CJ. Estradiol Signaling at the Heart of Folliculogenesis: Its Potential Deregulation in Human Ovarian Pathologies. International journal of molecular sciences. 2022 Jan 3:23(1):. doi: 10.3390/ijms23010512. Epub 2022 Jan 3 [PubMed PMID: 35008938]
Liu X, Zhang J, Wang S. Global, regional, and national burden of infertility attributable to PCOS, 1990-2019. Human reproduction (Oxford, England). 2024 Jan 5:39(1):108-118. doi: 10.1093/humrep/dead241. Epub [PubMed PMID: 38011904]
França MM, Mendonca BB. Genetics of ovarian insufficiency and defects of folliculogenesis. Best practice & research. Clinical endocrinology & metabolism. 2022 Jan:36(1):101594. doi: 10.1016/j.beem.2021.101594. Epub 2021 Oct 14 [PubMed PMID: 34794894]
Graham ME, Jelin A, Hoon AH Jr, Wilms Floet AM, Levey E, Graham EM. Assisted reproductive technology: Short- and long-term outcomes. Developmental medicine and child neurology. 2023 Jan:65(1):38-49. doi: 10.1111/dmcn.15332. Epub 2022 Jul 18 [PubMed PMID: 35851656]
Fleming R, Seifer DB, Frattarelli JL, Ruman J. Assessing ovarian response: antral follicle count versus anti-Müllerian hormone. Reproductive biomedicine online. 2015 Oct:31(4):486-96. doi: 10.1016/j.rbmo.2015.06.015. Epub 2015 Jul 3 [PubMed PMID: 26283017]