Introduction
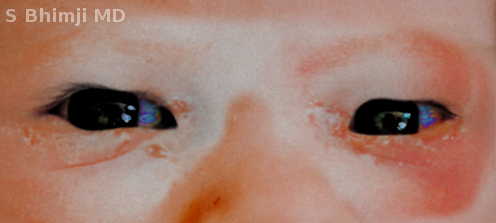
Ophthalmia neonatorum is a type of conjunctivitis that occurs in the neonatal period, affecting 1% to 12% of neonates (see Image. Ophthalmia Neonatorum). This condition commonly gets transmitted during vaginal delivery, and it correlates with severe complications (eg, corneal ulceration and perforation), which can potentially result in permanent blindness. Due to the significant morbidity associated with this disease, the United States Preventive Services Task Force (USPSTF) has issued new guidelines regarding antibiotic use in all newborns to prevent ophthalmia neonatorum. These guidelines were established to prevent the vertical transmission of gonococcal infection, which can occur in up to 50% of cases when prophylaxis is not administered.[1]
In 2010, The Centers for Disease Control and Prevention (CDC) developed the guidelines for the management of sexually transmitted infections (STIs), in which prophylaxis with erythromycin ointment (0.5%) or azithromycin solution 1% (if erythromycin not available) is recommended as a part of the routine newborn care for ophthalmia neonatorum prevention, considering that is effective and inexpensive. In these guidelines, routine screening and appropriate treatment, including for the partners of all pregnant women during the first trimester, are also recommended. During the third trimester, screening follow-up for those women considered high risk (eg, multiple sex partners and individuals aged 24 years or younger) is advisable. Silver nitrate effectively prevents gonococcal ophthalmia neonatorum; however, its use has been discontinued due to the high risk of developing chemical conjunctivitis in approximately 50% of the cases.[2][3]
Ophthalmia neonatorum, also known as neonatal conjunctivitis, is a severe form of conjunctival inflammation occurring within the first month of life. This condition represents a significant cause of neonatal ocular morbidity worldwide and, if not promptly recognized and treated, can lead to corneal ulceration, scarring, and permanent visual impairment. Historically, ophthalmia neonatorum was most commonly associated with Neisseria gonorrhoeae infection, but in modern practice, a broader spectrum of bacterial, viral, and chemical etiologies is recognized. Early identification and management are therefore critical to prevent complications that can have lifelong consequences.[4]
The incidence of ophthalmia neonatorum varies geographically, reflecting differences in maternal infection rates, availability of prenatal care, and adherence to prophylactic protocols. In high-resource settings, the incidence has declined markedly following the implementation of universal ocular prophylaxis—most commonly topical erythromycin ointment applied shortly after birth—and routine screening and treatment of maternal STIs during pregnancy. However, in low- and middle-income countries, limited access to prenatal screening and inconsistent application of prophylaxis contribute to higher rates of neonatal conjunctivitis. Recent epidemiological surveys estimate incidence rates ranging from <1% in well-resourced maternity services to >10% in underserved regions.[5]
Etiologically, ophthalmia neonatorum is classified according to the causative agent and the timing of onset. Early-onset cases (within 24–48 hours of birth) often result from chemical irritants, such as silver nitrate or povidone-iodine drops used for prophylaxis, or from viruses vertically transmitted in utero (notably herpes simplex virus). Bacterial causes, which may manifest between days 2 and 7 of life, include N. gonorrhoeae, Chlamydia trachomatis, Staphylococcus aureus, Streptococcus pneumoniae, and Haemophilus influenzae. Gonococcal conjunctivitis typically presents most aggressively, with copious purulent discharge, eyelid edema, and rapid progression to corneal involvement if untreated. Chlamydial conjunctivitis, in contrast, tends to have a more indolent course with watery or mucopurulent discharge emerging around day 5 to 14, often accompanied by nasopharyngeal colonization and potential otitis media. Viral etiologies, particularly herpes simplex virus type 2, may present later (days 7–14) with conjunctivitis often accompanied by systemic signs, eg, fever, irritability, and vesicular skin lesions.[6]
The pathophysiology of ophthalmia neonatorum centers on the immature anatomy and immunology of the newborn eye. The neonatal conjunctiva and cornea exhibit increased permeability, and tear film production is limited, thereby reducing the natural clearance of pathogens. In addition, maternal antibodies—while providing some passive immunity—may not fully protect against pathogens acquired during passage through the birth canal. For N. gonorrhoeae, bacterial adherence to conjunctival epithelial cells triggers a robust inflammatory response, characterized by the infiltration of polymorphonuclear leukocytes and the release of proteolytic enzymes that can damage the corneal stroma. Chlamydia induces a less fulminant but chronic inflammatory milieu that can lead to conjunctival scarring if untreated. Viral replication in epithelial cells contributes to cytopathic effects and secondary bacterial superinfection.[7]
Clinically, the diagnosis of ophthalmia neonatorum requires a high index of suspicion. Infants present with varying degrees of eyelid swelling, conjunctival redness, and ocular discharge. The nature of the discharge—watery, mucopurulent, or frankly purulent—guides the differential. Gonococcal infection is suspected when heavy purulence appears as early as 2 to 3 days of life; chlamydial infection should be considered with milder, delayed-onset discharge. Chemical conjunctivitis typically develops within hours of prophylactic instillation and resolves within 24 to 48 hours without the need for specific antimicrobial therapy. The presence of eyelid vesicles or systemic signs may distinguish viral conjunctivitis. A thorough history—particularly of maternal STI status, duration of membrane rupture, and prophylactic measures administered at birth—is essential. Laboratory confirmation involves gram staining and culture of conjunctival scrapings for gonorrhea, as well as nucleic acid amplification tests (NAATs) for chlamydia and gonococcus. Additionally, viral culture or polymerase chain reaction (PCR) is used for the herpes simplex virus.[8]
Management of ophthalmia neonatorum hinges on prompt, appropriate antimicrobial therapy tailored to the suspected or confirmed pathogen. For gonococcal conjunctivitis, systemic antibiotic therapy is mandatory—typically a single intramuscular dose of ceftriaxone, accompanied by saline eyelid cleansing and topical antibiotic drops to reduce surface bacterial load. Chlamydial conjunctivitis is treated with oral erythromycin or azithromycin, which reach therapeutic concentrations in tears and reduce the risk of nasopharyngeal and ear involvement. Chemical conjunctivitis typically requires only supportive care, including lubrication of the affected area. In suspected neonatal herpes infection, systemic acyclovir should be initiated urgently, given the risk of disseminated disease. Close ophthalmologic follow-up is necessary to monitor for corneal complications. Any sign of corneal ulceration or scarring requires intensive management, possibly including topical antibiotics, antiviral agents, or surgical intervention in severe cases.[9]
Prevention of ophthalmia neonatorum is equally paramount. Universal ocular prophylaxis remains a cornerstone of newborn care, with current guidelines favoring the application of erythromycin 0.5% ointment within 1 hour of birth. Some settings have adopted povidone-iodine as an alternative, given concerns about erythromycin resistance and availability. However, prophylaxis does not obviate the need for maternal STI screening and treatment: prenatal testing for N. gonorrhoeae and C. trachomatis, and treatment of positive cases, substantially reduces neonatal infection risk. Education of expectant mothers about safe sexual practices, diligent prenatal care, and early recognition of symptoms in neonates further enhances prevention efforts.[10]
Ophthalmia neonatorum poses a significant public health challenge, spanning the domains of obstetrics, neonatology, infectious diseases, and ophthalmology. Effective control requires an integrated approach, ensuring access to prenatal screening and treatment, guaranteeing the consistent application of ocular prophylaxis at birth, educating healthcare practitioners and parents about the early signs of infection, and establishing protocols for rapid diagnosis and treatment. In resource-limited settings, strengthening healthcare infrastructure, eg, supply chains for prophylactic agents, laboratory capacity for NAAT testing, and training of primary care clinicians, can markedly reduce the burden of neonatal conjunctivitis and its sequelae.[11][12]
Despite advances, challenges remain. Antimicrobial resistance among gonococcal strains poses a threat to undermine current treatment regimens, necessitating ongoing surveillance and potential adjustments to treatment regimens. The emergence of new enteric and respiratory pathogens capable of causing neonatal conjunctivitis underscores the need for vigilant epidemiological monitoring. Ultimately, disparities in healthcare access persist, driving uneven outcomes worldwide. Achieving universal coverage of prenatal STI screening and ocular prophylaxis, coupled with timely therapeutic interventions, is essential to safeguarding the vision and health of newborns worldwide.[12]
Ophthalmia neonatorum is a preventable and treatable condition whose successful management hinges on interprofessional collaboration, adherence to evidence-based guidelines, and equitable access to healthcare resources. By integrating robust preventive strategies with prompt, pathogen-specific treatments, clinicians can virtually eliminate the risk of vision-threatening complications in this vulnerable population, fulfilling the promise of modern neonatal care and preserving the gift of sight from the very first days of life.[13]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Research has identified various microorganisms as the causative agents of this eye disease, eg, Chlamydia trachomatis, Neisseria gonorrhoeae, viral infections, and bacteria from the gastrointestinal tract and the skin. The etiology of ophthalmia neonatorum is classified as either sexually transmitted bacteria, nonsexually transmitted bacteria, viral, or chemical (see Table 2. Ophthalmia Neonatorum Etiologies).
Neisseria gonorrhea accounts for <1% of ophthalmia cases worldwide; however, in babies born to mothers infected with N. gonorrhoeae, up to 48% develop ophthalmia neonatorum; in rare cases, if untreated or inadequately treated, meningitis and septicemia may develop. Nonsexually transmitted bacteria, eg, Staphylococcus aureus, Streptococcal species, and gram-negative bacteria, as well as Haemophilus, account for 30% to 50% of ophthalmia cases. Adenovirus and herpes simplex virus are the most common causes of viral conjunctivitis.[14][9][15]
Ophthalmia neonatorum—the onset of conjunctival inflammation within the first 28 days of life—has a well-characterized spectrum of causes. Etiologies are grouped into chemical, bacterial, viral, and other infectious agents. The timing of presentation, severity of inflammation, and risk of complications vary depending on the cause.[16]
Chemical Conjunctivitis
Chemical conjunctivitis is associated with the following characteristics:
- Agents: Silver nitrate (historical), povidone-iodine, preservatives in prophylactic drops
- Onset: Within 1 to 24 hours of application
- Pathogenesis: Direct epithelial irritation leading to transient conjunctival hyperemia and tearing
- Clinical course: Mild redness and tearing, resolves in 24 to 48 hours without antimicrobial therapy [17]
Bacterial Conjunctivitis
The clinical features associated with bacterial conjunctivitis vary depending on the etiologic organism (see Table 1. Bacterial Conjunctivitis Organism Features).
Table 1. Bacterial Conjunctivitis Organism Features
|
Organism |
Transmission |
Onset |
Key Features |
Complications |
|
Neisseria gonorrhoeae |
Vertical during passage through the birth canal |
2–5 days |
Profuse purulent discharge, eyelid edema, rapid corneal invasion |
Corneal ulceration, perforation |
|
Chlamydia trachomatis |
Vertical, often during birth or early contact |
5–14 days |
Mucopurulent discharge, mild eyelid edema, often bilateral, may accompany nasopharyngeal infection |
Conjunctival scarring, nasolacrimal obstruction |
|
Staphylococcus aureus |
Vertical or postnatal contact |
3–7 days |
Purulent or mucopurulent discharge, less aggressive than gonococcal |
Rare corneal involvement |
|
Streptococcus pneumoniae |
Vertical or postnatal contact |
3–7 days |
Purulent discharge, moderate inflammation |
Potential keratitis |
|
Haemophilus influenzae |
Vertical or postnatal contact |
3–7 days |
Mucopurulent discharge, conjunctival injection |
Rare invasive disease |
Viral Conjunctivitis
Conjunctivitis caused by viral infections is associated with the following characteristics:
- Herpes simplex virus (HSV) type 2
- Transmission: Vertical (intrapartum) or postnatal contact
- Onset: 5 to 14 days
- Features: Conjunctivitis often accompanied by eyelid vesicles, dendritic corneal ulcers, systemic signs (eg, fever and irritability)
- Complications: Corneal scarring, disseminated neonatal herpes [18]
- Enterovirus or adenovirus (rare)
- Onset: 1 to 2 weeks
- Features: Follicular conjunctivitis, watery discharge, adenopathy
- Complications: Typically self-limited; risk of keratitis [19]
Other Infectious Agents
Conjunctivitis caused by the following infectious agents is associated with these characteristics:
- Pseudomonas spp., gram-negative rods
- Context: Premature infants in neonatal intensive care, especially with indwelling devices
- Onset: Variable
- Features: Severe purulent conjunctivitis, high risk of systemic spread
- Complications: Conjunctival and corneal ulceration, sepsis [20]
- Fungal (Candida spp.)
- Context: Extremely premature or immunocompromised neonates
- Onset: Variable, often >7 days
- Features: White pseudomembranes on the conjunctiva, minimal discharge
- Complications: Membrane formation, risk of systemic candidiasis [21]
Table 2. Ophthalmia Neonatorum Etiologies
|
Category |
Agents |
Typical Onset |
Principal Features |
Management |
|
Chemical |
Silver nitrate, povidone-iodine |
< 24 hours |
Mild redness, tearing |
Supportive lubrication |
|
Bacterial |
N. gonorrhoeae, C. trachomatis, S. aureus, S. pneumoniae, H. influenzae |
2–14 days |
Purulent/mucopurulent discharge, edema |
Pathogen-specific antibiotics (eg, ceftriaxone, erythromycin) |
|
Viral |
HSV-2, adenovirus, enterovirus |
5–14 days |
Vesicles (HSV), watery/follicular discharge |
Systemic antivirals (acyclovir for HSV), supportive care |
|
Other Infectious |
Pseudomonas, Candida spp. |
Variable |
Severe purulence, pseudomembranes |
Broad-spectrum antibiotics/antifungals |
Epidemiology
Before 1880, ophthalmia neonatorum was the leading cause of permanent blindness in neonates, mainly caused by Neisseria gonorrhoeae. In 1881, Dr. Crede used 2% of silver nitrate for the first time at the time of birth for ophthalmia prophylaxis. After that time, the epidemiology of this eye disease changed, and the incidence of N. gonorrhoeae as the causal agent of ophthalmia decreased from 10% to 0.3%.[9] In the United States, ophthalmia neonatorum caused by N. gonorrhoeae has an incidence of 0.3 per 1000 live births, while Chlamydia trachomatis represents 8.2 per 1000 cases.
Ophthalmia neonatorum—neonatal conjunctivitis occurring within the first 28 days of life—remains an important cause of infant morbidity worldwide, despite advances in prenatal care and prophylactic interventions. Its overall incidence varies significantly by region, influenced by maternal STI prevalence, access to prenatal screening, and the routine implementation of topical ocular prophylaxis after birth. In high-income countries, where universal prenatal screening for chlamydia and gonorrhea is standard and where erythromycin ointment prophylaxis is mandated, the incidence of gonococcal ophthalmia neonatorum has fallen to less than 1 case per 100,000 live births. Chlamydial conjunctivitis, though more common than gonococcal disease in these settings, is still relatively rare, affecting approximately 2 to 5 per 10,000 live births.[22]
By contrast, in low- and middle-income countries (LMICs) where STI screening during pregnancy may be limited and topical prophylaxis inconsistently applied, rates are substantially higher. Surveys in sub-Saharan Africa report neonatal conjunctivitis rates ranging from 10% to 20% of all live births, with gonococcal cases comprising up to 5% of conjunctivitis presentations and chlamydial cases often falling within the 10% to 15% range. In South Asia, studies estimate a prevalence of neonatal conjunctivitis of 8% to 12%, with gonorrhea and chlamydia each contributing roughly one-third of cases. In Latin America, neonatal conjunctivitis affects about 5% to 10% of newborns, though comprehensive surveillance data are lacking in many rural areas.[23]
The age of onset helps distinguish etiologies: chemical irritation from prophylactic agents typically arises within the first 24 hours, gonococcal infections present between days 2 and 5, chlamydial infections between days 5 and 14, and herpes simplex virus (HSV) infections after day 7. HSV ophthalmia neonatorum is comparatively rare, occurring in 1 to 2 per 100,000 births in high-resource settings, but carries a high risk of corneal scarring and visual loss if left untreated. Globally, HSV eye involvement complicates approximately 10% of neonatal HSV infections, which occur in roughly 1 in 3,000 to 1 in 5,000 live births.[24]
Risk factors for ophthalmia neonatorum mirror those for maternal infection. Mothers with inadequate prenatal care, untreated STIs, younger maternal age (<25 years), multiple sexual partners, or a history of STIs are at increased risk of transmitting infection to the newborn (Williams and Clark, 2018). Vertical transmission rates for untreated maternal gonorrhea exceed 30%, and for chlamydia can reach 50%, underscoring the critical importance of screening and treatment during pregnancy.[25]
The burden of disease is not limited to acute conjunctivitis; up to 20% of affected infants in resource-limited settings develop corneal ulceration, and 5% to 10% may suffer permanent vision loss or globe perforation if not promptly diagnosed and treated. Even mild disease can lead to parental anxiety and increased healthcare utilization. Conversely, robust prophylaxis programs—whether penicillin eye drops, erythromycin ointment, or povidone-iodine in some low-resource contexts—can reduce overall conjunctivitis rates by 50% to 70%.[17]
Consequently, the epidemiology of ophthalmia neonatorum reveals stark disparities between high- and low-resource settings. While developed nations have nearly eliminated gonococcal neonatal eye infections and reduced chlamydial cases through routine prenatal STI screening and universal prophylaxis, LMICs continue to bear a heavy burden of disease. Strengthening prenatal care, expanding access to affordable STI diagnostics and treatment, and ensuring availability of effective ocular prophylaxis at birth are essential strategies for further reducing the global incidence of this preventable cause of neonatal ocular morbidity.[26]
Pathophysiology
Ophthalmia neonatorum occurs when pathogens or irritants come into contact with the newborn’s ocular surface, overcoming innate defenses and triggering a characteristic inflammatory cascade (see Table 4. Sequence of Conjunctival and Corneal Pathophysiologic Events). In the healthy eye, the tear film and blinking help clear debris and microbes, while conjunctival epithelial cells secrete antimicrobial peptides and immunoglobulins that neutralize invaders. In the neonate, however, tear production is immature, blink reflexes may be sluggish, and the conjunctival barrier is more permeable, creating an opportunity for organisms acquired during passage through the birth canal—or, less commonly, in utero—to colonize and infect the conjunctival epithelium.[1]
Within hours of birth, chemical agents used for prophylaxis (eg, silver nitrate or povidone-iodine) can irritate the delicate conjunctiva, causing transient hyperemia and tearing. This chemical conjunctivitis is usually self-limited, but it can lay the groundwork for secondary bacterial colonization. When Neisseria gonorrhoeae is present, its pili and outer membrane proteins mediate rapid adherence to and invasion of conjunctival epithelial cells. The organism’s potent endotoxin stimulates a brisk neutrophilic infiltrate, leading to the formation of thick, purulent exudate, epithelial necrosis, and potential corneal ulceration. Capillary permeability increases sharply under the influence of locally released vasoactive mediators, resulting in the eyelids becoming markedly swollen and red by the second or third day of life. Without prompt antibiotic therapy, gonococcal conjunctivitis can progress to frank corneal perforation within 24 to 48 hours of symptom onset.[4]
Chlamydia trachomatis gains entry to conjunctival cells via endocytosis, surviving within membrane-bound inclusion bodies that house the replicative reticulate form of the organism. After a latent interval of 5 to 14 days, infected cells release elementary bodies—highly infectious particles—that infect adjacent epithelial cells. The host immune response, dominated by lymphocytes and macrophages rather than neutrophils, produces a more mucopurulent but less immediately destructive discharge than that of the gonococcus. Over weeks, repeated infection and inflammation may lead to conjunctival scarring and symblepharon formation in severe cases, although most infants develop only mild to moderate hyperemia, eyelid swelling, and a watery to mucopurulent discharge.[27]
HSV type 2, acquired transplacentally or during passage through an infected birth canal, invades corneal epithelial cells and spreads via intraepithelial and stromal processes. Infected cells lyse, and the virus elicits both innate and adaptive responses, including natural-killer cell activity and cytotoxic T-cell–mediated killing of infected epithelial cells. This immune-mediated component contributes to the formation of dendritic ulcers, stromal inflammation, and, in some instances, endotheliitis. The result can be punctate keratitis, geographic epithelial defects, or deep stromal necrosis, with risk of scarring and vision loss.[28]
Throughout these infectious processes, the neonate’s immature immune system, characterized by lower complement levels, diminished neutrophil chemotaxis, and incomplete adaptive immunity, limits the rapid clearance of pathogens. At the same time, developing tissues are highly susceptible to damage from both microbial toxins and host inflammatory mediators. Tear flow, which is sluggish in newborns, fails to dilute and effectively wash away organisms. Conjunctival lymphatics and blood vessels can carry pathogens into the nasolacrimal system and, potentially, the bloodstream, contributing to systemic infection risk in particularly aggressive cases of gonococcal or herpetic ophthalmia.[29]
The pathophysiology of ophthalmia neonatorum is characterized by the interplay between pathogen virulence factors (eg, adhesins, endotoxins, inclusion body formation, and viral replication) and the neonate’s immature ocular defenses. Whether chemical, bacterial, or viral in origin, the resulting inflammatory cascade leads to hallmark signs—chemosis, conjunctival injection, eyelid edema, and discharge—and, if unchecked, may jeopardize corneal integrity and visual development (see Table 3. Pathogen-Specific Mechanisms of Conjunctival Injury). Prompt recognition and targeted therapy are therefore essential to interrupt these processes before permanent ocular damage ensues.[1]
Table 3. Pathogen-Specific Mechanisms of Conjunctival Injury
|
Pathogen |
Key Virulence Factors |
Primary Pathophysiologic Effects |
|
Neisseria gonorrhoeae |
|
|
|
Chlamydia trachomatis |
|
|
|
Staphylococcus aureus |
|
|
|
Pseudomonas aeruginosa |
|
|
|
Herpes simplex virus (HSV) |
|
|
Table 4. Sequence of Conjunctival and Corneal Pathophysiologic Events
|
Stage |
Microscopic/Cellular Changes |
Clinical Correlates |
|
Initial Colonization |
Pathogen adheres to conjunctival epithelium via pili/receptors or viral entry glycoproteins |
Redness, mild chemosis, initial discharge |
|
Epithelial Response |
Epithelial cells undergo necrosis or inclusion body formation, release of inflammatory mediators |
Tearing, photophobia, and the formation of pseudomembranes |
|
Inflammatory Infiltrate |
Neutrophils predominate (bacterial); lymphocytes and plasma cells in chronic (chlamydial) cases |
Purulent or mucopurulent discharge, follicles, papillae |
|
Stromal Involvement |
Protease-mediated collagen breakdown (Pseudomonas); stromal edema and early scar formation |
Corneal infiltrates, stromal haze, and thinning |
|
Healing/Repair |
Granulation tissue (fibroblasts, capillaries), re-epithelialization, goblet cell loss/regeneration |
Conjunctival scarring, potential symblepharon formation |
|
Sequelae |
Fibrosis, goblet cell depletion, and tear film instability |
Chronic dryness, recurrent inflammation, and vision impairment |
Histopathology
On microscopic examination, the conjunctival and corneal tissues of neonates with ophthalmia neonatorum reveal features that reflect both the causative agent and the intensity of the inflammatory response (see Table 5. Pathogen-Specific Histopathologic Findings in Ophthalmia Neonatorum). Overall, histopathology in ophthalmia neonatorum highlights the pathogen-specific cellular infiltrates—neutrophilic in gonococcal infection, mononuclear in chlamydial disease, and cytopathic in herpes, underscoring the importance of rapid etiologic diagnosis and therapy to prevent progressive tissue destruction and preserve ocular integrity (see Table 6. Common Tissue Reactions and Their Microscopic Correlates).[30]
Bacterial Histological Findings
In bacterial cases—particularly those caused by Neisseria gonorrhoeae—sections of conjunctival biopsy reveal a dense, often transmucosal neutrophilic infiltrate that fills the epithelium and subepithelial stroma. Large aggregates of polymorphonuclear leukocytes accumulate within dilated lymphatic-like channels in the substantia propria, and surface epithelial cells may exhibit foci of necrosis.[7]
Gram staining typically highlights numerous intracellular and extracellular gram-negative diplococci adherent to or invading the epithelial layer. Under higher magnification, one can appreciate epithelial microabscesses—small collections of neutrophils that blur the standard epithelial architecture—and extensive epithelial sloughing. The underlying lamina propria demonstrates vascular congestion, marked endothelial cell swelling, and perivascular cuffing by neutrophils. In severe, untreated cases, organisms and inflammatory cells may extend onto the corneal surface, producing a dense epithelial defect, underlying stromal infiltration by neutrophils, and early collagen breakdown that on histology appears as stromal edema and fragmentation of Bowman’s layer.[31]
In chlamydial ophthalmia, the histopathologic picture is more lymphoplasmacytic than neutrophilic. Conjunctival epithelium shows mild to moderate hyperplasia and spongiosis, with scattered intracytoplasmic inclusion bodies—round basophilic aggregates 0.3 to 1.0 µm in diameter—visible on hematoxylin and eosin stain or highlighted by Giemsa or iodine–periodic acid–Schiff (PAS) stains. These inclusions represent the intracellular elementary-body form of the organism. The subepithelial stroma is predominantly infiltrated by mononuclear cells, including lymphocytes, macrophages, and fewer plasma cells, often in a perivascular distribution. Follicle formation may be seen, especially along the tarsal conjunctiva, where aggregated lymphocytes form germinal-center-like structures. Stromal edema is typically mild, and vascular proliferation may be noted. Chronic cases can develop early fibrotic changes, with subepithelial collagen deposition and occasional symblepharon formation.[32]
Viral Histological Findings
When ophthalmia neonatorum is caused by HSV, corneal specimens show characteristic epithelial and stromal changes. Epithelial cells exhibit ballooning degeneration, characterized by enlarged nuclei with margination of chromatin and occasional multinucleation. Intranuclear Cowdry type A inclusion bodies—eosinophilic nuclear pads surrounded by clear halos—are diagnostic of this condition. The epithelial layer may be wholly or partially denuded in areas of ulceration. Beneath the ulcer bed, neutrophils accumulate along with fibrin, and in the deep stroma, patchy necrosis is present. Keratocytes adjacent to necrotic foci may show nuclear pyknosis. Viral antigens can be confirmed by immunofluorescence or immunohistochemistry, which label viral capsid proteins within epithelial and sometimes stromal cells.[33]
Chemical Conjunctivitis Histological Findings
In chemical conjunctivitis caused by prophylactic agents, histology reveals superficial epithelial toxicity rather than an actual infection. The epithelium shows vacuolar change and superficial sloughing, but without significant inflammatory cell infiltrates. Occasional subepithelial lymphocytes may be present, and mild vascular congestion is common. Neither inclusion bodies nor detectable organisms are noted. Chemical conjunctivitis (eg, silver nitrate) shows predominantly epithelial injury rather than heavy stromal inflammation.
Across all types, the neonatal conjunctival tissue exhibits more delicate and loosely organized collagen fibers in the substantia propria than in adult tissue, which predisposes it to rapid edema. Newborn vessels are also more permeable, which explains the pronounced chemosis and edema observed microscopically.
Table 5. Pathogen-Specific Histopathologic Findings in Ophthalmia Neonatorum
|
Pathogen |
Characteristic Microscopic Findings |
Key Stains/Markers |
|
Neisseria gonorrhoeae |
|
Gram stain: gram-negative diplococci |
|
Chlamydia trachomatis |
|
Giemsa or Giemsa-variant stain |
|
Staphylococcus aureus |
|
Gram stain: gram-positive cocci |
|
Pseudomonas aeruginosa |
|
Gram stain: gram-negative rods |
|
Herpes simplex virus |
|
|
|
Chemical conjunctivitis* |
|
— |
Table 6. Common Tissue Reactions and Their Microscopic Correlates
|
Tissue Reaction |
Microscopic Features |
Clinical Correlate |
|
Acute Suppurative Inflammation |
|
|
|
Follicular Hyperplasia |
|
|
|
Epithelial Necrosis/Ulceration |
|
|
|
Stromal Necrosis/Abscess |
|
|
|
Granulation Tissue |
|
|
|
Viral Cytopathic Change |
|
|
Toxicokinetics
In the context of ophthalmia neonatorum, toxicokinetics refers to the process by which prophylactic and therapeutic agents applied to the neonatal eye are absorbed, distributed, metabolized, and eliminated, as well as how these processes relate to their potential for local or systemic toxicity (see Table 7. Toxicokinetic Profile of Ophthalmia Neonatorum Therapeutic Agents). Because newborn skin and mucous membranes are uniquely permeable and their metabolic pathways immature, understanding the kinetic profile of ocular agents is critical to ensure both efficacy against pathogens and safety for the infant.[34]
Absorption
Topically applied antibacterial ointments or solutions, eg, erythromycin, tetracycline, or povidone–iodine, first encounter the tear film and conjunctival mucosa. The neonatal conjunctiva is thinner and more vascular than in older children or adults, and the corneal epithelium has a higher rate of cell turnover. As a result, some fraction of the drug penetrates through the epithelium into the stroma or is absorbed directly into the systemic circulation via conjunctival blood vessels. For water-soluble agents (eg, erythromycin base in a 0.5% ointment), the drug dissolves in the tears and enters epithelial cells through passive diffusion. Lipid-soluble drugs penetrate more readily but may also linger in the lipid layers of the tear film, thereby prolonging local exposure. In contrast, ionic compounds (eg, silver nitrate) dissociate in tears and deliver antimicrobial silver ions; however, these ions can bind to tissue proteins, limiting their deeper penetration.[35]
Distribution
Once absorbed across the conjunctiva, small amounts of the drug enter the periocular capillary network and can then be distributed systemically. Neonates’ body water content is high (approximately 75% to 80% of body weight), and their plasma protein binding capacity is significantly lower than in older children, meaning that free (unbound) drug concentrations in the bloodstream can be proportionately higher for a given absorbed dose. Nonetheless, the total absorbed mass is generally very low—often in the microgram range per eye drop or ointment application—so systemic concentrations remain minimal. In the eye itself, distribution to intraocular structures, including the anterior chamber, iris, or lens, is negligible at prophylactic concentrations; most of the drug remains confined to the conjunctival surface or superficial cornea.[36]
Metabolism
Neonates have immature hepatic enzyme systems, particularly the cytochrome P450 family, and reduced phase II conjugation capacity (eg, glucuronidation). However, because ocular antibiotic ointments are often composed of drugs delivered in their active form and because very little of the applied dose is absorbed systemically, hepatic metabolism generally plays a minor role in clearance. Erythromycin, for example, if absorbed, may undergo demethylation by CYP3A enzymes, but first-pass metabolism is limited since absorption occurs into conjunctival rather than portal circulation. Silver ions from silver nitrate combine with sulfhydryl groups in proteins to form insoluble silver–protein complexes. Although no enzymatic metabolism is noted, silver may be sequestered in tissues and slowly excreted.[37]
Elimination
Drugs absorbed into the circulation are eliminated primarily by renal excretion in neonates. Renal function in newborns is immature: glomerular filtration rate in the first week of life is approximately 30% to 40% of adult levels and improves over the first few months. Consequently, any systemically absorbed erythromycin or tetracycline may have a prolonged half-life compared to adults; however, the very low absorbed quantities mean that the overall systemic burden remains negligible. Locally, elimination from the ocular surface occurs via tear turnover (rate ~0.5–2 µL/min) and nasolacrimal drainage, whereby the drug enters the nasolacrimal duct and is swallowed, exposing the gastrointestinal tract to small residual doses.[38]
Toxicity Considerations
Because the neonatal conjunctiva and cornea are more permeable and tear turnover is slower than in adults, maintaining minimal effective concentrations on the ocular surface while avoiding local irritation is paramount. Erythromycin ointment is generally well tolerated, though occasional chemical conjunctivitis—characterized by transient redness and discharge—can occur if preservatives are present or if the ointment base is hyperosmolar. Silver nitrate has historically caused significant chemical conjunctivitis and corneal irritation due to its strong oxidative effects on surface proteins, leading to the replacement of 2% silver nitrate solution with more tolerable agents.[39]
Systemic toxicity from ocular prophylaxis is exceedingly rare. In theory, absorbed erythromycin could lead to gastrointestinal upset or contribute to hypertrophic pyloric stenosis, but epidemiologic data have not demonstrated a causal relationship at ophthalmic dosing. Tetracycline ointment theoretically raises concerns for tooth discoloration or bone deposition if systemically absorbed over prolonged periods; however, short-term use in neonates does not result in clinically significant exposure. In summary, the toxicokinetics of ophthalmia neonatorum prophylaxis are characterized by predominantly local drug action with minimal systemic absorption, limited metabolism due to the small amount of absorbed dose, and rapid local elimination via tear flow and nasolacrimal drainage. Understanding these processes ensures that agents chosen for neonatal eye care maximize antimicrobial efficacy while minimizing the risk of local irritation or systemic toxicity.[40]
Table 7. Toxicokinetic Profile of Ophthalmia Neonatorum Therapeutic Agents
|
Parameter |
Erythromycin Ophthalmic Ointment |
Tetracycline Ophthalmic Ointment |
2.5% Povidone–Iodine Solution |
2% Silver Nitrate Solution |
|
Formulation |
0.5% erythromycin base in petrolatum |
1% tetracycline hydrochloride in aqueous base |
2.5% w/v povidone–iodine in aqueous solution |
2% w/v silver nitrate in sterile water |
|
Absorption |
Partial conjunctival uptake; lipophilic diffusion |
Similar to erythromycin, moderate epithelial uptake |
Limited epithelial penetration; primarily surface action |
High local tissue binding; minimal systemic uptake |
|
Distribution |
Minimal intraocular; systemic free-drug fraction ↑ |
Minimal intraocular; systemic free-drug fraction ↑ |
Remains on the surface; negligible systemic levels |
Binds to proteins in the conjunctiva; negligible intraocular distribution |
|
Metabolism |
Limited hepatic demethylation if absorbed |
Limited hepatic metabolism if absorbed |
Non-enzymatic; iodine released reacts with microbes |
No enzymatic metabolism; silver–protein complex formation |
|
Elimination (Local) |
Tear turnover and nasolacrimal drainage |
Tear turnover and nasolacrimal drainage |
Tear turnover and nasolacrimal drainage |
Tear turnover; conjunctival sequestration |
|
Elimination (Systemic) |
Renal excretion; immature GFR prolongs half-life |
Renal excretion; immature GFR prolongs half-life |
Minimal systemic absorption; renal excretion is negligible |
Minimal systemic absorption; slow tissue release |
|
Local Toxicity |
Rare transient conjunctival irritation |
Rare, mild irritation in some infants |
Occasional mild stinging; transient redness |
Common chemical conjunctivitis, corneal irritation |
|
Systemic Toxicity |
Extremely rare; gastrointestinal upset is theoretical but unproven |
Extremely rare; tooth/bone deposition is theoretical but not seen |
None at ocular dosing |
None (systemic silver toxicity unlikely) |
|
Clinical Notes |
Well tolerated; preservative-free formulations preferred |
Alternative when erythromycin is unavailable |
Rapid broad-spectrum antisepsis; low irritation |
Largely abandoned in favor of gentler agents |
This toxicokinetic profile underscores why modern neonatal ocular prophylaxis emphasizes minimally absorbed, well-tolerated agents, maximizing local antimicrobial efficacy while virtually eliminating systemic risk.
History and Physical
A complete history and physical examination, including the chronological presentation of signs of conjunctivitis, is crucial but not specific for the diagnosis. The timing and characteristics of the clinical presentation of ophthalmia neonatorum may vary depending on the etiologic agent; however, specific clinical manifestations, eg, chemosis, erythema, and discharge, can be present regardless of the underlying etiology (see Table 8. Key Findings of Ophthalmia Neonatorum by Etiology).[41]
Chlamydia trachomatis and Neisseria gonorrhoeae are transmitted at the time of delivery. When these microorganisms are suspected of causing neonatal conjunctivitis, the clinician should obtain information about maternal infections during the prenatal period. Performing adequate screening for other STIs, including the human immunodeficiency virus, is also important.[42]
The timing of symptom onset can also serve as a guide in managing these patients. Chemical conjunctivitis should be suspected in patients who present with erythema, chemosis, eyelid edema, and discharge within the first 24 hours after birth. Gonococcal conjunctivitis typically presents between 2 and 5 days; the hallmark is a thick, purulent eye discharge accompanied by significant chemosis and eyelid edema. On the other hand, ophthalmia neonatorum due to Chlamydia trachomatis typically occurs 5 to 14 days after birth; both eyes may be affected, but unilateral involvement can also occur in some cases. The eye discharge is initially copious, then becomes purulent, and it is associated with eyelid swelling.[43]
Herpes simplex conjunctivitis is uncommon, accounting for <1% of ophthalmia cases; however, it warrants suspicion in patients presenting with unilateral chemosis, serosanguineous discharge that rarely becomes purulent, accompanied by vesicular lesions surrounding the eyelids or oral ulcers, and lymphadenopathy. Identification of this condition is crucial to prevent complications, including disseminated disease and meningoencephalitis, that may be lethal if left untreated.[18]
Ophthalmia neonatorum presents within the first 28 days of life as an acute conjunctival and often corneal infection. A systematic clinical evaluation is essential for distinguishing among causative pathogens, assessing disease severity, and guiding prompt management.
Clinical History
Perinatal and maternal factors associated with ophthalmia neonatorum include:
- Timing of onset
- Early (day 1–2): Suggests chemical conjunctivitis (irritant from prophylactic agents) or Neisseria gonorrhoeae infection.
- Intermediate (Day 3–7): Most consistent with N. gonorrhoeae
- Late (day 5–14): More typical of Chlamydia trachomatis
- Maternal infections and screening
- Known or suspected maternal STIs, eg, gonorrhea, chlamydia, HSV
- Prenatal screening results, adequacy of maternal antibiotic treatment if STI diagnosed
- Prophylactic agents: Type of ocular prophylaxis at birth (eg, silver nitrate, erythromycin ointment), chemical conjunctivitis risk on day 1
- Feeding and behavior: Tolerance of feeds, irritability, and sleep disturbance may indicate systemic involvement or discomfort.
- Systemic symptoms: Fever, lethargy, poor feeding, and respiratory distress—raising concern for systemic dissemination (particularly with N. gonorrhoeae or HSV).[44]
Physical Examination
A thorough physical examination for suspected ophthalmia neonatorum includes both ocular and general examinations.
General Examination
The general examination findings may include:
- Vital signs: Temperature (fever suggests systemic spread), heart rate, respiratory rate
- Skin and mucosa: Vesicular lesions on skin or mouth (eg, HSV), rash, hepatosplenomegaly (chlamydial or gonococcal sepsis) [45]
Ocular Examination
Findings consistent with ophthalmia neonatorum may include:
- Eyelids and periocular skin
- Swelling (chemosis), erythema, tenderness
- Presence of vesicles or ulcerations on eyelids (eg, HSV)
- Conjunctiva
- Degree and character of discharge
- Copious purulent discharge (N. gonorrhoeae)
- Mucoid or watery discharge (C. trachomatis or viral)
- Fine white, granular discharge (chemical)
- Conjunctival injection intensity
- Follicles versus papillae
- Follicles (small, translucent, central lymphoid aggregates) in chlamydia
- Papillae (raised, vascular) with bacterial infections [46]
- Cornea
- Presence of corneal clouding, epithelial defects, infiltrates, and ulceration
- Risk of perforation in gonococcal infection
- Pseudomembrane/membrane formation: Tenacious membranes that may bleed on removal (seen in C. trachomatis and severe bacterial infections)
- Lymphadenopathy: Preauricular lymph node enlargement (common in chlamydia and viral conjunctivitis) [47]
Table 8. Key Findings of Ophthalmia Neonatorum by Etiology
|
Feature |
Chemical |
Gonococcal |
Chlamydial |
Viral (HSV) |
|
Onset |
1st 24 hours |
Day 3–5 |
Day 5–14 |
Day 5–12 |
|
Discharge |
Mild, watery |
Profuse, purulent |
Mucoid, stringy |
Serous to purulent; may have pseudomembranes |
|
Conjunctival Injection |
Mild |
Severe |
Moderate |
Moderate |
|
Corneal Involvement |
Absent |
Common (ulcers, perforation) |
Rare |
Vesicular keratitis, dendrites |
|
Membrane/Pseudomembrane |
No |
Possible |
Common |
Possible |
|
Lymphadenopathy |
No |
Possible |
Preauricular lymph nodes |
Possible |
|
Systemic Signs |
No |
High fever, sepsis risk |
Low-grade fever |
Skin vesicles, systemic HSV |
Special Considerations
Other assessments that should be considered during clinical evaluation include:
- Severity assessment: Evaluate corneal integrity and risk of perforation—urgent ophthalmology referral if corneal ulceration or thinning is present.
- General well-being: Poor feeding or lethargy necessitates systemic evaluation and possible hospitalization.
- Differential diagnoses: Blepharitis, lacrimal duct obstruction (epiphora with minimal discharge), staphylococcal conjunctivitis.[48]
Clinical Pearls
Notable factors that clinicians should bear in mind when evaluating ophthalmia neonatorum include:
- Always correlate the timing of onset with likely pathogens.
- A “bright red eye with thick pus” in a day 3 to 5 neonate mandates immediate gram stain and culture for gonococcus.
- Persistent, follicular conjunctivitis beyond day 7 with minimal purulence suggests chlamydia—treat presumptively.
- HSV involvement is often accompanied by vesicular lesions elsewhere; treat with systemic antivirals.[49]
A structured history and meticulous ocular examination expedite pathogen-directed therapy, reduce the risk of complications (eg, corneal perforation and conjunctival scarring), and guide appropriate systemic workup in neonates with ophthalmia neonatorum.
Evaluation
When ophthalmia neonatorum is suspected, confirmation of the etiology is warranted. A sample from the eye discharge should be taken and sent for gram stain and culture in Thayer-Martin media and chocolate agar, especially if N gonorrhoeae is the possible causal agent; this guarantees that proper treatment is given and thus, ensures the prevention of potential complications which, is essential for a good prognosis and outcome. In Chlamydia trachomatis infection, polymerase chain reaction (PCR), direct fluorescent antibody staining, and Giemsa-stained epithelial cells from conjunctival scrapings can aid in making the diagnosis.[7]
Some authors have recommended nucleic acid amplification tests (NAATs) from conjunctival swabs when chlamydial ophthalmia is suspected; however, these tests do not have Food and Drug Administration approval for the detection of chlamydia in the conjunctiva. For herpetic conjunctivitis, the standard diagnostic tests for isolating the virus include viral culture and PCR-based detection of viral DNA. Patients with signs of systemic infection who appear unwell may have a disease that has spread, manifesting as meningitis, bacteremia, arthritis, or sepsis. In such cases, additional investigation, including blood culture and cerebrospinal fluid analysis for gram stain, is warranted.[50]
A structured, multistep evaluation is essential for accurately diagnosing and managing ophthalmia neonatorum, thereby minimizing the risk of vision-threatening complications and systemic spread. The evaluation encompasses a detailed clinical assessment, point-of-care diagnostics, laboratory confirmation, and targeted systemic work-up when indicated.
Detailed Clinical Assessment
Detailed clinical assessment and point-of-care diagnostic testing include the following evaluation steps (see Table 9. Bedside Point-of-Care Testing):
- Onset and chronology
- Chemical conjunctivitis: typically appears within the first 24 hours of life, often mild and transient, following prophylaxis with silver nitrate or erythromycin.
- Gonococcal infection: typically emerges between days 2 and 5, characterized by a rapid onset of marked purulent discharge.
- Chlamydial conjunctivitis: typically develops insidiously over days 5 to 14, presenting with mucoid or mucopurulent discharge and eyelid swelling.
- Viral (HSV): involvement arises days 5 to 12 and may manifest with vesicular eyelid lesions, serous to purulent discharge, and corneal dendrites.[51]
- Symptom and sign severity
- Discharge character: watery, mucoid, purulent
- Conjunctival appearance: chemosis, membrane/pseudomembrane formation
- Corneal status: inspect for clouding, epithelial defects, ulceration
- Eyelid lesions: vesicles suggest HSV
- Regional lymphadenopathy: preauricular nodes can enlarge in chlamydial or viral cases
- General health: fever, irritability, and feeding difficulty may indicate systemic involvement [47]
Table 9. Bedside Point-of-Care Testing
|
Test |
Specimen |
Technique |
Findings |
|
Gram stain |
Conjunctival swab |
Air-dried smear |
Gram-negative intracellular diplococci → N gonorrhoeae |
|
Giemsa stain |
Conjunctival scraping |
Fixed smear |
Intracellular basophilic inclusions → C trachomatis |
|
Tzanck smear |
Vesicle scrapings |
Wet mount |
Multinucleated giant epithelial cells → HSV |
|
Dry smear |
Conjunctival discharge |
Light microscopy |
Abundant neutrophils without organisms → consider chemical or viral etiology |
Clinical interpretation
A positive gram or Giemsa stain prompts immediate pathogen-specific treatment. Negative results with heavy inflammation suggest viral or chemical causes, guiding further testing.[52]
Laboratory Confirmation
Culture studies
Pathogen cultures are a diagnostic component utilized to identify potential underlying etiologies of ophthalmia neonatorum (see Table 10. Culture Methods).
Table 10. Culture Methods
|
Pathogen |
Culture Medium |
Incubation |
|
Neisseria gonorrhoeae |
Thayer-Martin agar |
24–48 hours |
|
Chlamydia trachomatis |
McCoy cell monolayers |
7–10 days |
|
Herpes simplex virus (HSV) |
Vero or HEp-2 cells |
3–7 days |
Molecular Diagnostics
PCR panels targeting Neisseria gonorrhoeae, Chlamydia trachomatis, and HSV allow for direct application to swabs, enabling rapid (within 48 hours) and highly sensitive detection. Direct fluorescent antibody testing or enzyme immunoassay also provides identification of chlamydial elementary bodies in epithelial cells, typically within 1 to 2 days.[53]
Ancillary and Systemic Evaluation
Further evaluation is indicated in cases involving severe purulent conjunctivitis, systemic signs, corneal ulceration, or suspected HSV encephalitis. Blood cultures and a complete blood count help assess for sepsis, especially in suspected gonococcal infections. Liver and renal function tests, along with cerebrospinal fluid analysis, assist in evaluating HSV cases presenting with neurologic symptoms. A chest x-ray may be warranted when systemic gonococcal dissemination is suspected. Furthermore, maternal medical records should be reviewed to confirm STI screening and treatment history, including testing for HSV.[54]
Imaging and Ophthalmic Examination
Slit-lamp biomicroscopy, when available and used with a neonatal speculum, can detect corneal epithelial defects, dendritic lesions, or deeper ocular involvement. Direct ophthalmoscopy, especially in severe or bilateral presentations, may uncover retinal hemorrhages or endophthalmitis.[55]
Diagnostic and Management Algorithm
The following diagnostic steps are recommended when managing a patient with suspected ophthalmia neonatorum:
- Begin with a thorough history and confirmation of perinatal prophylaxis and symptom onset.
- Perform bedside microscopy (gram, Giemsa, or Tzanck stain) to guide initial treatment rapidly.
- Initiate empiric therapy based on the suspected pathogen: IM or IV ceftriaxone for Neisseria gonorrhoeae, oral azithromycin for Chlamydia trachomatis, or IV acyclovir for HSV.
- Simultaneously obtain cultures and PCR to confirm the diagnosis and refine treatment.
- Conduct systemic evaluations—including CBC, blood cultures, CSF analysis, and organ function panels—when clinical findings warrant further investigation.
- Monitor clinical response daily, focusing on discharge quality, conjunctival inflammation, and corneal healing.[9]
Key Decision Points
Urgent referral to ophthalmology becomes necessary when corneal involvement, pseudomembranes, or any threat to vision is identified. Hospital admission ensures proper IV therapy for systemic gonococcal or HSV infections. Outpatient management remains appropriate for mild chlamydial conjunctivitis, provided close follow-up is arranged.
Through this layered approach—combining precise history, bedside cytology, culture, molecular testing, and systemic assessment—clinicians can accurately identify the causative pathogen, promptly initiate targeted therapy, and mitigate the ocular and systemic risks associated with ophthalmia neonatorum.
Treatment / Management
Patients with suspected neonatal conjunctivitis should be managed based on initial clinical assessment and evaluation of possible complications. Suppose the clinician has a high suspicion of neonatal conjunctivitis, but confirmatory tests for the infection are not available. In that case, treatment against both (Chlamydia trachomatis and Neisseria gonorrhoeae) should start to avoid complications.[9]
If the clinician has established the diagnosis of gonococcal ophthalmia neonatorum, immediate initiation of treatment, including hospitalization, is crucial. The first-line treatment of choice is a single dose of ceftriaxone 25 to 50 mg/kg/24 hr, with a maximum of 125 mg. Frequent eye irrigation with sterile isotonic saline is also recommended as an adjunct therapy. An alternative regimen is cefotaxime 100 mg/kg in a single dose. If chlamydial conjunctivitis is confirmed, oral erythromycin at 50 mg/kg every 24 hours for 2 weeks remains the regimen of choice; topical erythromycin can also be used as adjunctive therapy. Conjunctivitis secondary to Staphylococcal species and Pseudomonas requires treatment with systemic antibiotics. On the other hand, patients with herpes simplex conjunctivitis should receive treatment with systemic antiviral therapy, in addition to topical ophthalmic drugs, including 0.15% ganciclovir or 1% trifluridine, for 14 days. An ophthalmology consult is necessary in these cases.[56][57][58]
Recommendations about management in asymptomatic babies born to mothers infected with Chlamydia trachomatis infection exist; these babies require close monitoring for the appearance of clinical symptoms suggestive of chlamydia ocular or respiratory infections. Oral erythromycin is not recommended in asymptomatic babies due to an increased risk of developing pyloric stenosis.[59](B2)
Ophthalmia neonatorum is a potentially sight-threatening conjunctivitis that occurs in the first month of life. Immediate recognition and institution of appropriate therapy markedly reduce the risk of corneal damage, systemic complications, and long-term visual sequelae. Management must be tailored to the most likely etiological agent—chemical, bacterial (particularly Neisseria gonorrhoeae and Chlamydia trachomatis), or viral (herpes simplex virus)—and guided by local and international standards of care (see Table 10. First-Line Treatment for Common Causes of Ophthalmia Neonatorum). An interprofessional approach involving neonatologists, ophthalmologists, pediatric infectious disease specialists, and nursing staff is essential to ensure prompt diagnosis, adherence to treatment, and follow-up.[60]
Initial Assessment and Supportive Care
Upon presentation, the neonate’s general health status should be assessed, including vital signs, hydration, feeding tolerance, and signs of systemic infection. Before instituting pathogen-specific therapy, the following supportive measures should be performed to help maintain ocular comfort and hygiene:
- Eyewash and gentle cleansing: Using sterile saline or preservative-free artificial tears to remove purulent discharge and crusting from the eyelids and lashes 2 to 4 times daily aids in comfort and allows for more precise visualization of the conjunctiva and cornea.
- Lid hygiene: Soft cotton swabs dipped in sterile saline can gently cleanse the eyelid margins, decreasing bacterial load. Avoid povidone-iodine or harsh antiseptics on the newborn’s delicate skin.
- Lubrication: For cases with corneal epithelial compromise, frequent application of preservative-free ocular lubricants (drops or gels) helps protect the cornea and promotes healing.[61]
These measures do not substitute for antimicrobial therapy but create optimal conditions for drug penetration and reduce the risk of secondary skin irritation.
Etiology-Directed Antimicrobial Therapy
Gonococcal conjunctivitis (Neisseria gonorrhoeae)
Gonococcal ophthalmia neonatorum is a medical emergency due to rapid corneal infiltration and risk of perforation within 24 to 48 hours. Current international guidelines recommend the following:
- Systemic therapy: Administer a single intramuscular dose of ceftriaxone (25 to 50 mg/kg, maximum 125 mg), ensuring adequate serum and ocular tissue levels. If ceftriaxone is unavailable, cefotaxime 50 mg/kg can be administered intramuscularly (IM) or intravenously (IV).
- Topical therapy: High-concentration topical antibiotics, eg, 1% aqueous ceftriaxone or 0.5% aqueous penicillin drops, are instilled every 2 hours around the clock until clinical improvement is achieved. Then, they are instilled every 4 hours for an additional 2 to 3 days.
- Hospitalization: Due to the risk of systemic dissemination (eg, arthritis and meningitis), inpatient care with intravenous antibiotics (eg, ceftriaxone 50 mg/kg daily) may be warranted if a concern for sepsis or inadequate outpatient follow-up is present.
- Follow-up: Daily ophthalmic examinations until resolution of purulence and corneal epithelial defects.[62]
Chlamydial conjunctivitis (Chlamydia trachomatis)
Chlamydia–associated ophthalmia develops more gradually and often presents after the first week of life. Treatment entails the following:
- Systemic macrolide: Oral azithromycin 20 mg/kg as a single dose, repeated once after 1 week, has become the preferred regimen due to its excellent tolerability and high compliance. Alternative regimens include erythromycin 50 mg/kg/day divided into 4 doses for 14 days, though this carries a risk of infantile hypertrophic pyloric stenosis.
- Topical silver nitrate or erythromycin ointment: Not effective as monotherapy for chlamydia, but may be continued for symptom relief.
- Maternal and partner therapy: Simultaneous treatment of the mother (azithromycin 1 g orally) and sexual partners is essential to prevent reinfection.
- Monitoring: Clinicians should assess the resolution of conjunctivitis within 7 to 14 days; if persistent, confirm compliance and consider a second course or an alternative macrolide.[63] (A1)
Herpes simplex virus (HSV) conjunctivitis
HSV–related disease can threaten both ocular and systemic health. Management includes:
- Systemic antiviral: Intravenous acyclovir (20 mg/kg every 8 hours) for 14 to 21 days if disseminated disease or corneal involvement is present; for isolated ocular disease, some experts recommend oral acyclovir (20 mg/kg 4 times daily for 14 days).
- Topical antiviral: Trifluridine 1% eye drops 5 times daily or vidarabine ointment 5 times daily for 10 to 14 days, in conjunction with systemic therapy, to inhibit viral replication at the ocular surface.
- Supportive measures: Frequent lubrication, antiviral mittens to prevent self-inoculation, and close monitoring for corneal dendritic lesions or stromal keratitis.
- Consultation and follow-up: Early involvement of a pediatric infectious disease specialist and serial ophthalmic exams to detect progression to keratitis or uveitis.[64]
Adjunctive and Preventive Strategies
Preventive measures are also a component of management for ophthalmia neonatorum (see Table 11. Adjunctive and Specialized Therapies). Universal prophylaxis using erythromycin 0.5% ointment, tetracycline 1% ointment, or povidone-iodine 2.5% solution, applied to both eyes within the first hour after birth, remains standard practice in many regions and effectively reduces gonococcal transmission.
Prenatal screening for gonorrhea, chlamydia, and genital herpes during the third trimester enables timely treatment of maternal infections before delivery, significantly lowering the risk of neonatal transmission. Parental counseling should include guidance on proper hand hygiene, avoiding contaminated bathing supplies, and seeking prompt medical care for any signs of recurrent ocular discharge.[35]
Management of Complications
Treatment of ophthalmia neonatorum can be associated with various complications (see Table 11. Adjunctive and Specialized Therapies). Management of these complications includes:
- Corneal ulceration and scarring
- Treatment involves intensive antimicrobial therapy based on the identified pathogen, combined with adjunctive cycloplegia and frequent ocular lubrication to enhance comfort and support corneal healing.
- Surgical intervention may be necessary in cases of impending corneal perforation, with options including conjunctival flap placement or therapeutic penetrating keratoplasty, performed in consultation with a pediatric corneal surgeon.[65][66]
(B3)
- Membrane or pseudomembrane formation: Mechanical removal under topical anesthesia in an operating theater helps prevent symblepharon formation. Follow-up with topical corticosteroids reduces inflammation and supports tissue recovery.[39][67] (A1)
Discharge Planning and Follow-Up
Before discharge, ensure caregivers understand the administration schedules, warning signs (eg, increased redness, discharge, or feeding difficulty), and the importance of follow-up visits. A typical follow-up schedule may include:
- Day 2 to 3 posttreatment initiation for clinical assessment.
- Weekly visits until complete resolution of conjunctivitis and corneal epithelial defects.
- Monthly vision and ocular health checks for the first 6 months if significant ocular involvement occurred.[70] (B2)
Quality Improvement and Guidelines
Adherence to national and international guidelines from reputable entities, including the World Health Organization, the American Academy of Pediatrics, and the Centers for Disease Control and Prevention, ensures adherence to best practices. Continuous quality improvement initiatives—tracking rates of ophthalmia neonatorum, timing of prophylaxis, and treatment outcomes—help institutions refine protocols and reduce incidence.
Effective management of ophthalmia neonatorum hinges on a rapid etiological diagnosis, the prompt institution of targeted systemic and topical therapy, and vigilant follow-up to prevent complications. A coordinated, evidence-based approach—integrating obstetric screening, neonatal prophylaxis, interprofessional collaboration, and caregiver education—safeguards newborn vision and lays the foundation for healthy ocular development.[71](B2)
Table 10. First-Line Treatment for Common Causes of Ophthalmia Neonatorum
|
Etiology |
Systemic Therapy |
Topical Therapy |
Follow-Up and Other Considerations |
|
Neisseria gonorrhoeae |
|
|
|
|
Chlamydia trachomatis |
|
|
|
|
Herpes simplex virus (HSV) |
|
|
|
|
Chemical conjunctivitis |
|
|
|
Table 11. Adjunctive and Specialized Therapies
|
Indication |
Intervention |
Frequency and Duration |
Special Considerations |
|
Persistent epithelial defects |
|
|
|
|
Membrane/pseudomembrane formation |
|
|
|
|
Severe scleral involvement |
|
|
|
|
Secondary uveitis or elevated intraocular pressure (IOP) |
|
|
|
Differential Diagnosis
Ophthalmia neonatorum—conjunctival inflammation occurring within the first 28 days of life—has a broad differential, encompassing both infectious and noninfectious etiologies (see. Table 12. Ophthalmia Neonatorum Differential Diagnoses) Early recognition of the specific cause is critical, as some agents (notably Neisseria gonorrhoeae and herpes simplex virus) can lead to rapid corneal damage and vision loss if not treated promptly.
Table 12. Ophthalmia Neonatorum Differential Diagnoses
| Condition | Age of Onset | Clinical Symptoms | Clinical and Diagnostic Assessment | Management |
| Chemical conjunctivitis | 1–2 days | Mild eyelid edema; scant serous discharge; conjunctival hyperemia resolves within 24–48 h | History of prophylactic agent (eg, silver nitrate) | Reassurance; topical lubricants |
| Neisseria gonorrhoeae | 2–5 days | Marked eyelid swelling; copious purulent discharge; pseudomembranes; rapid corneal ulceration | Gram-negative intracellular diplococci on smear/culture | Hospitalize; IV/IM ceftriaxone; saline lavage |
| Chlamydia trachomatis | 5–14 days | Gradual onset; moderate–copious mucopurulent discharge; follicles; eyelid edema | Giemsa stain or PCR of the conjunctival swab | Oral azithromycin (single dose) |
| Staphylococcus aureus / epidermidis | 3–7 days | Moderate purulent discharge; mild to moderate hyperemia; eyelid erythema | Gram-positive cocci in clusters on Gram stain | Topical broad-spectrum antibiotic drops/ointment |
| Streptococcus pneumoniae | 3–7 days | Purulent discharge; marked hyperemia; may form pseudomembranes | Gram-positive lancet-shaped cocci | Topical antibiotics; systemic therapy if severe |
| Gram-negative enteric bacteria | 3–7 days | Copious purulent discharge; risk of corneal involvement | Culture on MacConkey agar | Topical and/or systemic antibiotics |
| Herpes simplex virus (HSV-1/2) | 5–14 days | Conjunctivitis, eyelid vesicles, dendritic corneal ulcers; possible systemic signs | Tzanck smear, PCR, or viral culture | IV acyclovir; topical antivirals |
| Adenovirus | 7–14 days | Follicular conjunctivitis; watery to mucopurulent discharge; possible pseudomembranes | Rapid antigen test or PCR | Supportive; lubricants; cold compresses |
| Nasolacrimal duct obstruction | Variable (weeks–months) | Chronic tearing, mild mucous discharge, conjunctival hyperemia without actual infection | Dye-disappearance test; absence of purulence | Sac massage; antibiotic prophylaxis if needed |
| Dacryocystitis | 2–8 weeks | Tearing, mucopurulent discharge, redness/swelling over lacrimal sac; possible fever | Palpable, tender lacrimal sac; reflux on pressure | Systemic antibiotics, lacrimal sac massage, and surgical probing after the acute phase |
| Congenital glaucoma | Birth–2 months | Tearing, photophobia; blepharospasm, corneal haze/enlargement (“buphthalmos”) | Elevated IOP; corneal diameter increase; Haab’s striae | Urgent surgical intervention (goniotomy/trabeculotomy) |
| Keratitis | Any neonatal period | Redness, photophobia, discharge, corneal opacity, or ulceration | Slit-lamp exam showing epithelial defect; culture swab | Topical fortified antibiotics/antivirals; close monitoring |
| Cellulitis (pre- or postseptal) | 3–14 days | Eyelid erythema, warmth, tenderness; possible systemic signs | Clinical exam, ultrasound/CT if orbital involvement | Systemic broad-spectrum antibiotics; hospitalize if orbital |
Pertinent Studies and Ongoing Trials
The following summary highlights key studies and ongoing clinical trials that shape current recommendations for preventing and treating ophthalmia neonatorum. The focus centers on antibiotic prophylaxis and treatment strategies targeting the most common bacterial pathogens—Neisseria gonorrhoeae and Chlamydia trachomatis—alongside emerging therapeutic approaches.
Epidemiology of Ophthalmia Neonatorum: A Systematic Review and Meta-analysis
This global systematic review pooled data from 25 studies encompassing over 1.1 million births and found an overall incidence of ophthalmia neonatorum of 2.0% and a prevalence of 7.8%. Staphylococcus species accounted for 39% of positive cultures, while Serratia marcescens was also prominent. Alarmingly, more than 90% of S. aureus and 100% of E. coli isolates were penicillin-resistant, underscoring the need for effective universal prophylaxis to prevent vision-threatening sequelae.[8]
Ophthalmia Neonatorum in Central Ghana: Causative Agents and Antibiotic Susceptibility
In a prospective, multicenter study of 110 neonates over 17 months, bacterial pathogens were isolated in 52.4% of cases; Staphylococcus spp. Predominated (39.2%), and no Neisseria gonorrhoeae was found. Notably, 73% of isolates were tetracycline-resistant, which challenges the efficacy of standard prophylactic ointments and prompts calls for routine culture and sensitivity testing in similar settings.[72]
National Survey of Neonatal Prophylaxis Practices in Italy
Surveying 419 maternity centers covering over 1 million annual births, 82.3% of Italian newborns received ocular prophylaxis, but only 0.4% received WHO-recommended agents. Over the 3 years, 12 cases of chlamydial ophthalmia neonatorum and zero cases of gonococcal infection were reported. The findings, alongside low prenatal STI screening rates, led to the development of an intersociety guideline standardizing prophylaxis to optimize prevention and resource utilization.[73]
Chlamydial Ophthalmia Neonatorum in Botswana: Case Series and Review
Among infants born to untreated chlamydia-positive mothers, 12 cases of ON (8 PCR-confirmed) were identified despite routine tetracycline ointment prophylaxis. Four infants developed chlamydial pneumonia, and several cases persisted after maternal erythromycin therapy. The study highlights the inadequacy of current prophylaxis alone and recommends routine prenatal chlamydia screening and treatment to avert neonatal infection.[70]
Risk of Invasive Infection in Neonates With Ophthalmia Neonatorum
Retrospectively reviewing 52 term neonates presenting with ophthalmia neonatorum in an emergency setting, investigators found no instances of concurrent invasive bacterial infection (0%, 95% CI 0–6.9%). However, evaluation and treatment practices varied widely, with 87% receiving antibiotics. The authors suggest deescalating extensive sepsis workups in healthy neonates while still ensuring prompt ocular care.[17]
Neonatal Herpes Simplex Virus Infections in Germany: 2-Year Surveillance
A nationwide surveillance study conducted from 2017 to 2018 identified 37 cases of neonatal HSV (incidence is approximately 2.35/100,000 births). Most infants had mild or no symptoms and recovered fully, but 4 suffered severe neurological impairment and 2 died. Notably, most transmissions were postnatal from family members’ orolabial lesions rather than maternal genital disease, underscoring the need for family-member education alongside maternal screening.[74]
Treatment Planning
Management of ophthalmia neonatorum hinges on prompt recognition, appropriate empiric therapy, targeted treatment once causative organisms are identified, and careful follow-up to prevent complications. The following step-wise plan reflects current international guidelines and best practices approaches (see Table 13. Prophylaxis and Initial Empiric Therapy and Table 14. Targeted Therapy and Follow-Up).
Universal Prophylaxis at Birth
Apply a topical prophylactic agent within the first hour of life. Recommended options include:
-
Erythromycin ophthalmic ointment (0.5%–1%) applied once to each eye.
-
Povidone–iodine solution (1.25%–2.5%) as a single drop in each eye, especially when erythromycin is unavailable or resistance is a concern.
The rationale behind this recommendation is that prophylaxis significantly lowers the risk of gonococcal and chlamydial conjunctivitis without causing systemic adverse effects.[4]
Early Recognition and Diagnostic Evaluation
Clinicians should evaluate any neonate presenting within the first month of life with symptoms suspicious for ophthalmia neonatorum, including eyelid edema, purulent discharge, conjunctival injection, or signs of corneal involvement.
Before initiating topical therapy, the conjunctival discharge should be swabbed for Gram stain, culture, sensitivity testing (including testing for Neisseria gonorrhoeae and Chlamydia trachomatis), and PCR when available. Furthermore, clinicians should review maternal history for sexually transmitted infections, prolonged rupture of membranes, or intrapartum infections.[16]
Empiric (Initial) Therapy
Gonococcal coverage
For symptoms emerging 2 to 5 days after birth with highly purulent discharge, initiate immediate treatment for gonorrhea while awaiting culture results, consisting of ceftriaxone IV or IM at 25 to 50 mg/kg (maximum dose 125 mg), due to its rapid corneal penetration and favorable resistance profile.
Chlamydial coverage
For symptom onset between 5 and 14 days featuring mucopurulent discharge without marked conjunctival swelling, oral azithromycin at 20 mg/kg as a single dose, or erythromycin at 50 mg/kg/day divided into 4 daily doses for 14 days should be prescribed.
Combination cases or unclear etiology
In patients with unclear underlying etiologies or a combination of Neisseria gonorrhoeae and Chlamydia trachomatis, both ceftriaxone (single dose) and oral azithromycin (single dose) should be used to ensure coverage for both pathogens.[11]
Targeted (Organism-Specific) Therapy
For confirmed Neisseria gonorrhoeae infections, treatment requires a 7- to 10-day course of a systemic third-generation cephalosporin, eg, cefotaxime or ceftriaxone. Daily saline eye irrigation should continue until ocular discharge fully resolves. In cases involving Chlamydia trachomatis, oral erythromycin or azithromycin should be continued for the entire 14-day regimen.
To prevent recurrence, retesting and appropriate treatment of the mother and her sexual partner should be undertaken. When other organisms, eg, Staphylococcus or HSV, are identified, therapy must be guided by culture or PCR results, which may include topical fortified antibiotics or acyclovir, depending on the pathogen involved.[45]
Supportive Measures
Frequent and gentle saline irrigation of the eyes helps remove purulent discharge and maintain eyelid hygiene. Daily slit-lamp examinations are necessary to monitor for corneal involvement, eg, keratitis or ulceration. Upon detection of corneal changes, escalation of the antimicrobial regimen and possible hospital admission should be promptly considered to prevent permanent ocular damage.[17]
Follow-Up and Prevention of Sequelae
Ongoing inpatient care should include daily ophthalmology consultations until the patient demonstrates marked clinical improvement. Outpatient follow-up must occur at 1 week and again at the conclusion of therapy to confirm complete resolution and to identify any complications, eg, corneal scarring or vision impairment. Screening of the mother and her sexual contacts for N. gonorrhoeae and C. trachomatis remains essential, and any positive cases require full treatment to break the cycle of reinfection.[46]
Reporting and Public Health Measures
Confirmed diagnoses of N. gonorrhoeae or C. trachomatis must be reported to local public health authorities in compliance with regulatory requirements. Parental education plays a vital role in preventing recurrence; caregivers should receive clear instructions on maintaining hygiene, recognizing signs of relapse, and completing the full course of systemic treatment.
By systematically following this protocol—from universal prophylaxis through targeted therapy and vigilant follow-up—clinicians can effectively prevent, diagnose, and manage ophthalmia neonatorum, thereby safeguarding neonatal ocular health and preserving vision.[60]
Table 13. Prophylaxis and Initial Empiric Therapy
|
Step |
Timing |
Intervention |
Dose/Regimen |
Comments |
|
1. Prophylaxis |
Within 1 hr of birth |
Erythromycin ophthalmic ointment |
0.5%–1% ointment, single application to each eye |
CDC first-line; safe, effective against N. gonorrhoeae and C. trachomatis |
|
Povidone–iodine solution |
1.25%–2.5% drop, single application to each eye |
Alternative when erythromycin is unavailable; broad-spectrum |
||
|
2. Empiric therapy |
Onset ≤5 days (purulent, severe) |
IM/IV ceftriaxone |
25–50 mg/kg once (max 125 mg) |
Gonococcal coverage; rapid corneal penetration |
|
Onset 5–14 days (mucopurulent) |
Oral azithromycin |
20 mg/kg single dose |
Chlamydial coverage; easier dosing than erythromycin |
|
|
Oral erythromycin |
50 mg/kg/day divided 4 times a day for 14 days |
Alternative if macrolide resistance concerns |
||
|
3. Combined empiric* |
Variable |
Ceftriaxone and azithromycin |
Ceftriaxone 25–50 mg/kg once plus azithromycin 20 mg/kg once |
When etiology is unclear or dual coverage is indicated |
Use a combined regimen if awaiting diagnostics or are at high risk for mixed infection.
Table 14. Targeted Therapy and Follow-Up
|
Pathogen |
Definitive Therapy |
Duration |
Supportive Care and Monitoring |
|
Neisseria gonorrhoeae |
Ceftriaxone IV/IM (25–50 mg/kg/day) or cefotaxime |
7–10 days |
|
|
Chlamydia trachomatis |
Oral azithromycin (20 mg/kg single dose) or erythromycin (50 mg/kg/day 4 times daily) |
14 days |
|
|
Other bacteria (eg, Staph spp.) |
Topical fortified antibiotics (eg, 5% cefazolin and 1% tobramycin every 2 hrs) |
7–14 days |
|
|
Herpes simplex virus |
Topical acyclovir ointment 3% 5 times daily plus oral acyclovir 20 mg/kg/dose 4 times daily |
10–14 days |
|
|
Fungal (rare) |
Topical natamycin 5% every 1–2 hrs plus systemic voriconazole (6 mg/kg BID load, then 4 mg/kg BID) |
14–21 days |
|
|
Follow-up |
Schedule |
Goals |
|
|
Day 2–3 inpatient review (if severe) |
|
||
|
Week 1 outpatient |
|
||
|
End of treatment |
|
||
|
1 month posttherapy (if complications) |
|
Expanded Treatment Planning
Universal prophylaxis
Prophylactic ocular instillation at birth is universally recommended to prevent gonococcal and chlamydial conjunctivitis. Erythromycin ophthalmic ointment remains the first choice due to its proven safety and efficacy. In resource-limited settings or during drug shortages, a single drop of dilute povidone–iodine provides broad-spectrum antimicrobial coverage without the risk of antibiotic resistance.
Early recognition and diagnostics
Any neonate presenting within the first month with eyelid swelling and conjunctival discharge should prompt immediate evaluation. Before topical therapy, swab the conjunctival sac for gram stain, culture, and PCR to guide therapy.
Empiric therapy
For suspected gonococcal infections (onset 2–5 days), a single dose of ceftriaxone is administered, given the risk of rapid corneal ulceration. For later-onset, more indolent chlamydial cases, oral macrolide antibiotics are started. If the causative organism is unclear or dual infection is likely, combined coverage with both agents is prudent.
Targeted therapy
Once culture or PCR identifies the pathogen, antimicrobials are tailored accordingly. Gonococcal cases require extended systemic treatment with third-generation cephalosporins; chlamydial infections require a full course of macrolides. For other bacterial, viral, or fungal pathogens, modify therapy based on susceptibility data and clinical response.
Supportive measures
Frequent saline irrigations and proper eyelid hygiene help prevent the accumulation of biofilm and facilitate the penetration of medications. Corneal integrity must be monitored daily; severe cases often warrant inpatient care until the risk of ulceration resolves.
Follow-up
Structured follow-up ensures infection clearance and early detection of sequelae, eg, corneal scarring, symblepharon, or vision impairment. Maternal and partner screening for STIs completes the cycle of care and prevents the risk of reinfection.
By combining universal prophylaxis, prompt empiric therapy, organism-specific treatment, and meticulous follow-up, this comprehensive treatment plan aims to eliminate ophthalmia neonatorum pathogens, prevent sight-threatening complications, and safeguard long-term visual outcomes in newborns.
Toxicity and Adverse Effect Management
While the cornerstone of therapy for ophthalmia neonatorum is prompt antimicrobial treatment, clinicians must remain vigilant for potential toxicities and adverse reactions associated with systemic and topical agents used in newborns (see Table 15. Common Neonatal Adverse Effects and Management of Systemic and Topical Therapeutic Agents). Common adverse effects and evidence-based strategies to prevent or manage them vary depending on the therapeutic agent used.
Table 15. Common Neonatal Adverse Effects and Management of Systemic and Topical Therapeutic Agents
|
Agent |
Adverse Effects |
Prevention and Management |
|
Topical Erythromycin Ointment |
Mild eye irritation, transient blurred vision |
|
|
Povidone–Iodine Drops |
Mild transient burning or conjunctival hyperemia |
|
|
Intramuscular/IV Ceftriaxone |
Injection-site pain, diarrhea, and biliary sludging in neonates |
|
|
Oral Azithromycin/Erythromycin |
Gastrointestinal (vomiting, diarrhea), hypertrophic pyloric stenosis (rare with erythromycin) |
|
|
Topical Fortified Antibiotics |
Corneal epithelial toxicity, drug-induced conjunctivitis |
|
|
Topical Acyclovir |
Minimal, possibly mild stinging |
|
|
Supportive Care (Saline Irrigations, Lubricants) |
Rare contamination leading to conjunctivitis |
|
Key Management Principles
Parental counseling should precede the start of treatment to address expected side effects, eg, mild ocular stickiness from topical ointments or gastrointestinal discomfort following the use of oral antibiotics. Setting these expectations helps build trust and promotes better adherence to the therapeutic regimen.
Weight-based dosing and strict adherence to recommended treatment durations remain critical to minimizing systemic toxicity. Prolonged erythromycin use, for example, may increase the risk of developing complications such as pyloric stenosis, particularly in neonates.
When significant adverse effects arise, switching to an alternative medication within the same drug class may be necessary. For instance, azithromycin may be used as an alternative to erythromycin for chlamydial infections, or cefotaxime may serve as an alternative to ceftriaxone for gonococcal coverage, with culture data guiding these substitutions.
Daily ocular examinations help detect early signs of epithelial toxicity in severe cases. In addition, clinicians should closely observe feeding behavior, track weight gain, and monitor stool patterns during systemic antibiotic use. For IM therapy, injection sites require assessment for erythema, tenderness, or induration to identify local complications promptly.
By anticipating these potential toxicities, educating caregivers, and promptly addressing adverse reactions, clinicians can optimize the safety profile of ophthalmia neonatorum treatments while preserving their efficacy in preventing sight-threatening sequelae.[80]
Staging
Ophthalmia neonatorum is typically staged by the time of onset after birth, which correlates closely with the most common etiologies. Early recognition of the timing of disease onset helps guide diagnostic evaluation and empirical therapy.
|
Stage |
Time of Onset |
Likely Etiology |
Key Clinical Features |
|
Stage I (Chemical) |
Within 0–24 hours |
Silver nitrate prophylaxis*; irritant drops |
|
|
Stage II (Gonococcal) |
2–5 days |
Neisseria gonorrhoeae |
|
|
Stage III (Chlamydial) |
5–14 days |
Chlamydia trachomatis |
|
|
Stage IV (Herpetic) |
5–14 days (variable) |
Herpes simplex virus |
|
|
Stage V (Other Bacterial) |
4–10 days |
Staphylococcus aureus, Streptococcus spp., Pseudomonas spp. |
|
*Many jurisdictions now use antibiotic eye ointment (eg, erythromycin) instead of silver nitrate; true “chemical” conjunctivitis has become uncommon, but staging retains historical importance.
Clinical Application of Ophthalmia Neonatorum Staging
Accurate identification of disease stage based on symptom timing and clinical presentation supports targeted empiric treatment while awaiting microbiologic confirmation. This approach minimizes the risk of vision-threatening complications and improves long-term visual outcomes in affected neonates.
Empiric therapy
Stage II onset requires immediate intervention, including gram staining and culture, as well as IV or IM administration of a third-generation cephalosporin, due to the potential for rapid corneal perforation. Clinical signs consistent with stage III should prompt oral macrolide therapy following appropriate swab collection. In cases of Stage IV presentation, severe disease warrants HSV PCR or Tzanck smear testing and intravenous acyclovir administration.[81]
Diagnostic evaluation
Conjunctival swabs should be obtained in stages II through V to perform gram staining, culture, and PCR testing based on clinical suspicion and availability. These diagnostic tools help identify the causative organism and guide targeted antimicrobial therapy.
Systemic involvement must be excluded when evaluating for gonococcal and herpetic infections. In such cases, a thorough clinical assessment and additional laboratory testing help determine the extent of the disease and ensure timely intervention to prevent serious complications.[82]
Prognosis
Stage I typically results in excellent outcomes without long-term sequelae. Prompt recognition and supportive care contribute to complete recovery in nearly all cases. Stage II presents the highest risk for corneal scarring and demands aggressive intervention to preserve ocular integrity. Early administration of appropriate systemic antibiotics and close ophthalmologic monitoring remain essential to prevent serious complications.
Stage III often responds favorably to macrolide therapy when initiated without delay. Timely treatment reduces the likelihood of associated nasopharyngeal colonization or secondary complications. Stage IV, if left unrecognized or untreated, may progress to chronic conjunctival scarring and permanent vision loss. Early detection and initiation of antiviral therapy are critical to mitigate these outcomes.[28]
Prognosis
Before 1880, ophthalmia neonatorum due to Neisseria gonorrhoeae was the most common cause of neonatal blindness. Early detection and timely treatment of infected mothers are crucial elements in preventing permanent ocular damage. If left untreated or partially treated, corneal ulceration, perforation, and blindness can occur. Approximately 10000 cases of blindness per year are secondary to ophthalmia neonatorum worldwide. Fortunately, in most cases, neonatal ophthalmia neonatorum caused by nongonococcal bacteria is a mild disease with a good prognosis. However, up to 50% of babies born to mothers with chlamydia infection may develop neonatal conjunctivitis, and among those, between 10% and 20% are at risk of developing pneumonia. Chemical conjunctivitis secondary to silver nitrate is self-limiting.[56][51][83]
The visual and systemic outlook for a neonate with ophthalmia neonatorum varies significantly depending on the etiology, timeliness of recognition, and appropriateness of therapy. Early and accurate diagnosis, along with the institution of targeted treatment, are critical for preventing long-term ocular morbidity. The following prognostic outcomes are associated with each etiology:
- Chemical conjunctivitis (stage I)
- Visual outcome: Excellent; virtually no risk of corneal involvement or scarring once irritant removed
- Course: Self-limited within 24 to 48 hours after cessation of the causative agent
- Sequelae: None [9]
- Gonococcal conjunctivitis (stage II)
- Visual outcome: Guarded without prompt treatment. Historical data report up to 20% risk of corneal ulceration, perforation, and potential blindness if therapy is delayed beyond 24 hours.
- Following aggressive therapy: Intravenous or intramuscular third-generation cephalosporins reduce corneal complications to under 5%. Timely intervention often preserves normal vision.
- Systemic risks: May develop concurrent septic arthritis or meningitis in up to 10% of untreated infants; early systemic antibiotic treatment minimizes these risks [5]
- Chlamydial conjunctivitis (stage III)
- Visual outcome: Generally favorable when treated with a full course of systemic macrolides (eg, oral erythromycin or azithromycin)
- Corneal involvement: Rare; mild punctate keratitis can occur but typically resolves without scarring
- Late sequelae: Without treatment, chronic follicular conjunctivitis may persist for weeks to months, but permanent damage is uncommon [84]
- Herpes simplex conjunctivitis (stage IV)
- Visual outcome: Variable. Corneal ulceration may lead to scarring and astigmatism; early administration of IV acyclovir and ophthalmic antivirals improves the prognosis.
- Systemic outcome: Neonatal HSV disseminated disease carries 30% to 50% mortality without treatment. With timely antiviral therapy, mortality falls below 10%, though neurological sequelae remain a concern.
- Long-term: Survivors require lifelong ophthalmic follow-up to monitor for potential recurrent keratitis and scarring.[18]
- Other bacterial pathogens (stage V)
- Visual outcome: Generally good when treated with culture-guided topical antibiotics; purulent infections caused by Staph or Streptococcus rarely result in corneal perforation.
- Complications: Pseudomonas species infections can progress rapidly; however, early antibiotic therapy typically prevents severe complications from developing.[85]
Factors Influencing Prognosis
Several key factors influence the prognosis of ophthalmia neonatorum, each carrying significant weight in determining clinical outcomes.
Time to treatment plays a critical role, particularly in cases involving suspected gonococcal or herpetic infections. Delays, even by a few hours, substantially elevate the risk of corneal damage, underscoring the need for immediate empiric intervention upon suspicion. Additionally, access to care also shapes prognosis. In low-resource settings, the absence of routine ocular prophylaxis and limited availability of effective antibiotics often leads to more severe disease progression and poorer visual outcomes. Strengthening healthcare infrastructure and ensuring consistent prophylactic practices can improve prognosis in these environments.
Furthermore, compliance with the full antibiotic course and adherence to scheduled ophthalmic follow-ups significantly reduce the likelihood of chronic ocular sequelae. Continued monitoring helps detect residual inflammation or complications such as corneal scarring early in the disease course. Systemic evaluation becomes essential in cases involving potential dissemination, especially with gonococcal or herpetic pathogens. Coordinated interprofessional care supports both survival and neurological function by addressing systemic involvement in conjunction with ocular management.[86]
With modern prophylaxis and prompt, pathogen-directed therapy, the vast majority of cases of ophthalmia neonatorum resolve without lasting visual impairment. Gonococcal and herpetic infections remain the most severe threats; aggressive early treatment and careful monitoring are essential to preserve sight and overall health.
Complications
Untreated ophthalmia neonatorum places infants at high risk for severe complications, including corneal ulceration, perforation of the ocular globe, and irreversible blindness. In rare instances, the infection may extend beyond the ocular surface, leading to meningitis, sepsis, or death.
Inadequate prevention or delayed treatment can result in a wide range of complications. Some infants may experience mild conjunctival scarring, while others may progress to significant vision loss due to corneal involvement. Certain pathogens, eg, Neisseria gonorrhoeae and herpes simplex virus, pose a heightened risk for systemic dissemination.
Prompt identification of clinical signs and initiation of targeted therapy remain essential to prevent irreversible damage. Aggressive management, especially during the early stages, reduces the likelihood of ocular morbidity and improves long-term visual and systemic outcomes (see Table 17. Long-Term Sequelae and Management Strategies). Complications of ophthalmia neonatorum include:
- Corneal involvement
- Punctate keratitis: Fine epithelial defects that cause irritation but rarely scar if treated promptly.[87]
- Corneal ulceration: Full-thickness epithelial and stromal loss; risk is highest with Neisseria gonorrhoeae and Pseudomonas (see Table 16. Ocular Complication Rates by Pathogen).[88]
- Corneal perforation: Extreme ulcerative progression may breach the corneal stroma, necessitating emergency surgical repair.[89]
- Conjunctival scarring and symblepharon: Chronic inflammation—especially from untreated chlamydial or herpetic infections—can lead to subconjunctival fibrosis, forniceal shortening, and adhesions between bulbar and palpebral conjunctiva.[47]
- Secondary glaucoma: Scarring around the ocular surface or angle structures may impede aqueous outflow, leading to elevated intraocular pressure and damage to the optic nerve.[90][91][92]
- Visual axis opacification: Persistent epithelial defects or stromal scarring centrally may decrease best-corrected acuity and induce irregular astigmatism.[93]
- Structural dyelid and lacrimal sequelae
- Cicatricial entropion or trichiasis from conjunctival fibrosis can abrade the cornea.
- Scarring of the puncta or canaliculi may cause chronic epiphora and predispose to dacryocystitis.[94]
- Systemic spread
- Neisseria gonorrhoeae: Risk of bacteremia, septic arthritis, meningitis.
- Herpes simplex virus: Risk of disseminated infection, neonatal encephalitis, and high mortality without antiviral therapy.
Table 16. Ocular Complication Rates by Pathogen
|
Pathogen |
Keratitis (%) |
Ulceration (%) |
Perforation (%) |
Conjunctival Scarring (%) |
Secondary Glaucoma (%) |
|
Neisseria gonorrhoeae |
35 |
20 |
5 |
3 |
2 |
|
Chlamydia trachomatis |
10 |
2 |
<1 |
15 |
1 |
|
Herpes simplex virus |
25 |
12 |
3 |
10 |
2 |
|
Other bacteria (Staph/Strep/Pseudo) |
15 |
5 |
1 |
2 |
<1 |
Table 17. Long-Term Sequelae and Management Strategies
|
Complication |
Clinical Finding |
Long-Term Effect |
Preventive/Management Approach |
|
Corneal scarring |
Central white stromal opacity |
Reduced visual acuity, astigmatism |
Early antibiotic/antiviral therapy; amniotic membrane graft for deep ulcers |
|
Symblepharon |
Forniceal adhesions |
Restricted motility; pain |
Surgical symblepharon lysis; mucous membrane grafting |
|
Secondary glaucoma |
Elevated IOP on tonometry |
Optic nerve damage |
Monitor IOP; topical/systemic IOP-lowering agents; surgery if needed |
|
Entropion/trichiasis |
Eyelid margin inversion, misdirected lashes |
Corneal abrasion, ulcer risk |
Lid surgery (entropion repair); lash epilation |
|
Lacrimal obstruction |
Epiphora; recurrent dacryocystitis |
Chronic tearing, infection risk |
Dacryocystorhinostomy or punctal reconstruction |
|
Encephalitis (HSV) |
Seizures, altered consciousness |
Neurodevelopmental impairment |
Prompt IV acyclovir; neurologic monitoring |
|
Systemic gonococcal spread |
Septic arthritis, meningitis |
Joint destruction, neurologic deficits |
Early systemic ceftriaxone; supportive care |
Key Points
Neisseria gonorrhoeae presents the most significant risk for severe corneal injury and systemic dissemination, often leading to complications such as ulceration, perforation, or sepsis. Chlamydia trachomatis and other bacterial pathogens tend to cause less aggressive disease but may still result in conjunctival scarring and chronic ocular issues when left untreated.
The timely administration of both topical and systemic antimicrobial therapy significantly reduces the likelihood of vision-threatening or life-threatening complications. Early therapeutic intervention remains the cornerstone of effective management.
Managing advanced or complicated cases often requires collaboration among multiple specialists. Ocular surgeons, pediatricians, infectious disease experts, and neurologists contribute essential perspectives to support recovery and mitigate systemic risks, particularly in cases involving gonococcal or herpetic infections.
Ongoing follow-up plays a critical role in the care of infants recovering from ophthalmia neonatorum. Periodic ophthalmologic evaluations help detect and address long-term complications, eg, glaucoma, corneal scarring, and refractive abnormalities, ensuring optimal visual development and minimizing permanent impairment. By understanding the full spectrum of potential complications and implementing preventive strategies, clinicians can preserve vision and overall health in neonates with ophthalmia neonatorum.
Postoperative and Rehabilitation Care
Even after the acute phase of ophthalmia neonatorum has resolved, meticulous postoperative and rehabilitative care are essential to ensure full recovery, prevent long-term sequelae, and optimize visual development. The key components include:
- Continued topical antimicrobial therapy
- Duration: Extend topical antibiotic (or antiviral) drops for at least 48 to 72 hours beyond clinical resolution of conjunctival and corneal inflammation. This “tail” reduces the risk of relapse and ensures the eradication of intracellular organisms (especially Chlamydia trachomatis).
- Dosing schedule: Maintain the same frequency used during acute therapy (eg, erythromycin ointment 4 times daily, or 1% azithromycin drops twice daily) but reassess daily to taper based on ocular surface findings.[95]
- Corneal healing and surface protection
- Frequent Lubrication: Use preservative-free artificial tears (0.1% sodium hyaluronate or carboxymethylcellulose) every 2 to 4 hours to foster epithelial regeneration and prevent punctate keratopathy.
- Patching/air exposure: If significant epithelial defects persist, a soft silicone hydrogel therapeutic contact lens or a bandage patch may be applied under close supervision to promote reepithelialization. Patching should be limited to 24 to 48 hours to minimize the risk of amblyopia.[1]
- Monitoring for secondary glaucoma
- IOP checks: In cases with deep anterior chamber inflammation or prolonged steroid use, measure intraocular pressure (with a handheld tonometer) at each postoperative visit. Early detection of steroid-induced ocular hypertension or synechial angle closure enables prompt medical or surgical intervention.
- Anterior chamber assessment: Slit-lamp (or portable handheld) exam to detect any new iris bombe, peripheral anterior synechiae, or hypopyon recurrence.[4]
- Management of lid and lacrimal sequelae
- Lid hygiene: Advise gentle eyelid cleansing with dilute baby shampoo twice daily to prevent blepharitis from chronic staphylococcal colonization.
- Punctal patency: Check the lower puncta for stenosis caused by conjunctival scarring. If necessary, perform punctal dilation in the clinic or refer to a specialist in oculoplastics.[5]
- Visual function assessment
- Refraction and amblyopia prevention: After age-appropriate milestones (around 6 to 8 weeks), assess fix-and-follow behavior. By 3 to 4 months, perform cycloplegic retinoscopy to detect anisometropia or astigmatism from corneal scarring. Prescribe corrective spectacles promptly to prevent amblyopia.
- Therapeutic occlusion: If a unilateral corneal scar significantly degrades vision, initiate part-time occlusion of the better eye under pediatric ophthalmology guidance.[45]
- Rehabilitation services
- Early intervention programs: For infants with bilateral or dense unilateral corneal scarring, enroll in early childhood visual rehabilitation. Services include home-based visual stimulation, orientation and mobility evaluation, and referral to low-vision specialists.
- Parent education: Instruct caregivers on recognizing strabismus or nystagmus, which may emerge if central corneal scars impair fixation, and on performing age-appropriate visual developmental exercises.[1]
- Interdisciplinary coordination
- Pediatrician and infectious-disease follow-up: Ensure that systemic antibiotic or antiviral courses—for example, oral erythromycin for Chlamydia or intravenous acyclovir for HSV—are completed and that liver function is monitored if required by regimen.
- Oculoplastic referral: For symblepharon or fornix foreshortening, arrange surgical lysis with amniotic membrane grafting once the eye is quiescent, ideally after 3 to 6 months.
- Long-term surveillance
- Schedule follow-up exams at 1 week, 1 month, 3 months, and then every 6 to 12 months until the child reaches age 2—monitoring for late-onset glaucoma, corneal haze progression, and refractive changes.
- Document corneal scar size and location serially with anterior-segment photography to guide future management decisions.[4]
In summary, postoperative care in ophthalmia neonatorum extends well beyond the control of acute infection, encompassing extended topical therapy, vigilant monitoring of corneal healing and intraocular pressure, proactive management of lid and lacrimal sequelae, early assessment of visual function, and rehabilitation services. Coordinated interprofessional follow-up maximizes the chances of preserving a clear visual axis, preventing amblyopia, and securing optimal long-term visual outcomes for the neonate
Consultations
Effective management of ophthalmia neonatorum often requires an interprofessional approach to address both ocular and systemic needs of the newborn and to prevent transmission to family members or healthcare workers (see Table 18. Consultations Involved with Ophthalmia Neonatorum Management). Therefore, several specialties may be involved in the evaluation and management of ophthalmia neonatorum. Through timely, coordinated consultations across these specialties, clinicians can address the full spectrum of ocular, systemic, and psychosocial needs associated with ophthalmia neonatorum, thereby optimizing outcomes for both the neonate and their family.
Neonatology and Pediatrics
Pediatricians and neonatologists oversee the newborn’s initial assessment, focusing on vital signs, feeding tolerance, and growth metrics. When systemic infection, eg, sepsis, is suspected, they coordinate blood tests and cultures to guide diagnosis. In cases requiring systemic antimicrobial therapy—such as oral erythromycin for chlamydial conjunctivitis or intravenous acyclovir for herpes simplex virus—neonatology specialists direct dosing protocols, monitor for adverse effects (eg, pyloric stenosis from erythromycin), and ensure full completion of the therapeutic course.[1]
Ophthalmology
Pediatric ophthalmologists conduct comprehensive eye evaluations using slit-lamp or handheld devices to assess the extent of conjunctival inflammation, identify corneal epithelial defects or ulcers, and measure intraocular pressure when necessary. They perform urgent ocular procedures, eg, corneal scrapings for microbiologic analysis, punctal dilation for stenosis, and apply therapeutic contact lenses or perform tarsorrhaphy in cases of severe exposure keratopathy or corneal ulceration.[4]
Microbiology and Infectious Diseases
Microbiology specialists handle the collection, transport, and culture of conjunctival swabs to identify bacterial, chlamydial, or gonococcal pathogens. They also conduct PCR testing for herpes simplex virus and interpret antimicrobial susceptibility results to support organism-specific treatment strategies. Infectious disease physicians oversee public health reporting of notifiable infections (eg, Neisseria gonorrhea), guide maternal and partner treatment, and establish screening protocols for neonates in shared hospital settings to control potential outbreaks.[16]
Obstetrics and Gynecology
Obstetricians assess maternal records for prenatal screening results related to sexually transmitted infections. When maternal infection with Chlamydia trachomatis or Neisseria gonorrhoeae has not been adequately addressed, they initiate maternal testing and treatment to lower the infant’s reinfection risk and reduce further transmission. In addition, they counsel mothers on breastfeeding safety when systemic medications are prescribed to the newborn and discuss strategies for preventing perinatal transmission in future pregnancies.[96]
Dermatology (HSV Cases)
In cases of neonatal HSV with ocular involvement, dermatologists perform thorough evaluations of the skin and mucous membranes to identify characteristic herpetic lesions in other body regions. They guide PCR testing of suspicious lesions and contribute to the management plan when signs of disseminated disease emerge.[97]
ENT and Pulmonology (Rarely)
When facial or mucosal HSV lesions raise concerns about airway compromise, ENT or pediatric pulmonology teams conduct airway assessments. These specialists provide respiratory support when disseminated HSV leads to severe systemic involvement, particularly in cases with facial swelling or mucosal obstruction affecting breathing.[98]
Social Work and Case Management
Social workers focus on educating families about treatment plans and coordinating outpatient follow-up appointments, eg, pediatric, ophthalmology, and infectious disease teams. They also help arrange practical support, eg, transportation and home health nursing when needed. Case managers oversee discharge readiness by confirming the availability of necessary prophylactic agents (eg, ointment) for other infants in the nursery and advising caregivers on hygiene practices to minimize the risk of cross-contamination in the home environment.[98]
Table 18. Consultations Involved with Ophthalmia Neonatorum Management
|
Consulting Service |
Primary Roles and Responsibilities |
|
Neonatology/Pediatrics |
|
|
Ophthalmology |
|
|
Microbiology/Infectious Diseases |
|
|
Obstetrics/Gynecology |
|
|
Dermatology |
|
|
ENT/Pulmonology |
|
|
Social Work/Case Management |
|
Deterrence and Patient Education
Mothers should be educated and counseled about the importance of regular prenatal visits. Screening for Neisseria gonorrhoeae and Chlamydia trachomatis infections is imperative, along with all other essential tests performed during these visits.
Effective prevention of ophthalmia neonatorum hinges on both deterring maternal transmission of pathogens and empowering caregivers through targeted education and training, including:
- Maternal screening and treatment
- Educate expectant mothers about the importance of prenatal STI screening (particularly for Neisseria gonorrhoeae, Chlamydia trachomatis, and HSV) and timely treatment to eradicate infection before delivery.
- Counsel on adherence to prescribed antibiotic or antiviral regimens, highlighting that incomplete treatment can lead to reinfection and neonatal risk.[1]
- Intrapartum prophylaxis
- Explain the rationale for universal ocular prophylaxis (eg, erythromycin ointment within 1 hour of birth) as a safety net against undiagnosed or occult maternal infections.
- Reassure parents that this prophylactic step is painless and has a very low risk of adverse effects.[4]
- Hygiene and contact precautions
- Instruct caregivers to practice strict hand hygiene—washing hands with soap and water or using alcohol-based hand rub—before and after any handling of the infant’s eyes or changing dressings.
- Advise against sharing towels, washcloths, or eye drops between infants or other family members to prevent cross-contamination.[5]
- Recognizing early signs
- Teach parents to watch for signs of redness, swelling of the eyelids, discharge, or crusting around an infant’s eyes, especially within the first 2 weeks of life.
- Emphasize that early presentation to a healthcare practitioner can prevent progression to corneal ulceration or vision loss.
- Safe breastfeeding practices
- Counsel mothers with active herpetic lesions on the breast or nipple to avoid direct contact with the infant’s mouth and eyes until lesions have fully crusted over and antiviral treatment is underway.
- Encourage expressing and discarding breast milk from the affected breast to maintain nutrition while preventing the transmission of HSV.[16]
- Follow-up and compliance
- Provide clear, written discharge instructions detailing medication schedules (frequency, dosage) and the necessity of completing the full course, even if symptoms improve.
- Arrange early outpatient follow-up (within 48 to 72 hours) to ensure therapeutic response and reinforce education.[10]
- Community and cultural considerations
- Tailor education to the family’s language and literacy level, using visual aids or interpreters as needed.
- Address cultural beliefs or practices that could delay care (eg, reliance on traditional eye remedies), respectfully explaining potential harms and offering culturally sensitive alternatives.[17]
By combining maternal prevention, prophylactic measures at birth, vigilant hygiene, caregiver recognition of early signs, and structured follow-up, patient education not only deters the occurrence of ophthalmia neonatorum but also fosters timely intervention, ultimately safeguarding neonatal ocular health and vision.
Pearls and Other Issues
The following factors should be kept in mind when managing ophthalmia neonatorum:
- Ophthalmia neonatorum is a type of conjunctivitis encountered in the neonatal period (the first 28 days after birth).
- In the United States, N. gonorrhoeae conjunctivitis has an incidence of 0.3 per 1000 live births, while Chlamydia trachomatis represents 8.2 per 1000 cases.
- Approximately 10000 cases of blindness per year are secondary to ophthalmia neonatorum worldwide.
- Prophylaxis with erythromycin ointment (0.5%) is part of routine newborn care and is currently the only medication approved for the prevention of ophthalmia neonatorum.
- Early detection and initiation of treatment are crucial elements for a good outcome.
- All pregnant women should have screening for sexually transmitted infections and be adequately treated.
- An interprofessional approach is critical for the successful management of patients with ophthalmia neonatorum.[5]
Timing of Onset Guides Etiology
Early-onset (<24 hours) typically implicates chemical conjunctivitis (prophylaxis-related) or Neisseria gonorrhoeae. Rapid, fulminant purulent discharge within 24 hours of birth should raise suspicion for gonococcal infection—a true ophthalmic emergency requiring immediate systemic antibiotics and ophthalmology consultation.
Intermediate-onset (2–7 days) is most often due to Chlamydia trachomatis. Erythromycin ointment prophylaxis does not prevent chlamydial infection, so persistent mucopurulent discharge in days 5 to 14 warrants chlamydia testing and oral macrolide therapy.
Late-onset (>7 days) suggests the presence of other bacterial pathogens (eg, Staphylococcus aureus, Streptococcus) or herpes simplex virus. HSV keratoconjunctivitis may present with vesicles, eyelid edema, or dendritic keratitis; maintain a high index of suspicion and obtain viral PCR when indicated.[4]
Chemical versus Infectious Conjunctivitis
Erythromycin or povidone–iodine prophylaxis can cause mild irritation, tearing, and transient conjunctival redness, which peaks at 24 to 48 hours. Unlike infectious etiologies, chemical conjunctivitis lacks significant purulence, lid swelling, or corneal involvement, and it resolves spontaneously within 3 to 5 days.[103]
Specimen Collection Best Practices
To optimize microbiologic yield, obtain conjunctival swabs before initiating topical antibiotics whenever feasible. Use separate swabs for gram stain, culture, and nucleic acid amplification tests to detect gonococcus, chlamydia, and HSV.[16]
Systemic Therapy Imperative for Gonococcal and Chlamydial Infections
Topical agents alone do not adequately penetrate ocular tissues for the treatment of gonorrhoeae or C. trachomatis. Systemic antibiotics (eg, IM ceftriaxone for gonorrhea; oral erythromycin for chlamydia) are mandatory. Concurrent evaluation and treatment of the mother and consideration of additional testing for other sexually transmitted infections are essential.
HSV Management Requires Prompt Antiviral Therapy
Neonatal HSV conjunctivitis can progress rapidly to keratitis or systemic disease. Empiric acyclovir should be started when HSV is suspected, even before confirmatory PCR results, especially if vesicular lesions or sepsis-like symptoms are present.[104]
Avoid Harmful Traditional Remedies
In some settings, folk remedies (eg, breast milk instillation, herbal eye washes) remain common but risk worsening infection or chemical injury. Gentle, nonjudgmental education on evidence-based care improves adherence.
Embrace Telemedicine for Early Triage
High-quality smartphone or video images of the neonate’s eyes can facilitate early decision-making in resource-limited or remote areas. Clear guidelines on when to escalate care (eg, referral to ophthalmology for suspected gonococcal cases) help avoid delays.
Breastfeeding and Maternal Lesions
Mothers with herpetic lesions on the breast or face should avoid direct contact until lesions have crusted; express and discard milk from affected breasts. This prevents neonatal HSV transmission while preserving breastfeeding benefits.
Long-term Surveillance
Even after apparent resolution, infants treated for gonococcal or chlamydial conjunctivitis benefit from follow-up to ensure no residual corneal scarring or conjunctival symblepharon development.
Interdisciplinary Collaboration
Optimal outcomes require coordination among neonatologists, pediatricians, obstetricians, infectious disease specialists, and ophthalmologists. Shared protocols for intrapartum prophylaxis, early recognition, and management streamline care and reduce complications.[105]
Enhancing Healthcare Team Outcomes
Effective management of ophthalmia neonatorum requires a highly coordinated, interprofessional team approach that integrates specialized skills, timely strategy, and seamless communication. Physicians—including pediatricians, neonatologists, ophthalmologists, and infectious disease specialists—play central roles in early recognition, diagnostic confirmation, and therapeutic decision-making. Team members require proficiency in neonatal eye examination techniques (eg, gentle eyelid eversion, safe specimen collection), awareness of current CDC and WHO–endorsed treatment algorithms, and competence in interpreting laboratory data. A standardized care pathway—beginning with maternal screening for sexually transmitted infections, through intrapartum reporting, to neonatal eye evaluation at birth and follow-up—streamlines decision-making and minimizes delays.[106]
Advanced practitioners assist in frontline assessments and reinforce treatment protocols. Prompt identification of the causative organism through culture or PCR testing remains a cornerstone of accurate diagnosis. Ophthalmologists provide guidance on ocular findings and the urgency of treatment, especially when signs such as corneal involvement suggest a high risk of complications.[107] Pharmacists contribute by verifying antibiotic dosages—especially for weight-based neonatal regimens—and by advising on drug interactions and safety. Nursing professionals administer medications, perform frequent ocular assessments, monitor for adverse reactions, and maintain detailed documentation to inform the clinical team’s decisions.
Communication across this network of professionals must be structured, timely, and patient-centered. Handoff tools, such as SBAR (Situation, Background, Assessment, Recommendation), ensure the clear transmission of critical findings, including the onset of symptoms, character of discharge, and treatment response. Electronic health records with built-in alerts for positive PCR results expedite team response and support concurrent notification among clinicians, laboratory staff, and pharmacy. Nurses and midwives further support outcomes by educating caregivers about prophylaxis, signs of infection, and the importance of follow-up. Case managers and social workers assist with safe discharge planning and outpatient coordination. This collective, synchronized effort safeguards neonatal vision, promotes safety through standardized procedures and double-checks on antibiotics, and enhances both short-term and long-term health outcomes. Continuous quality improvement through interprofessional debriefs and protocol updates ensures the system evolves in step with clinical evidence, setting a high standard for neonatal care delivery.[108]
Media
References
Vielmetti L, Hibbs S, Yoon H. Neonatal Visual Impairment: Etiologies, Screening, and Management. NeoReviews. 2025 Jun 1:26(6):e391-e401. doi: 10.1542/neo.26-6-023. Epub [PubMed PMID: 40449916]
US Preventive Services Task Force, Curry SJ, Krist AH, Owens DK, Barry MJ, Caughey AB, Davidson KW, Doubeni CA, Epling JW Jr, Kemper AR, Kubik M, Landefeld CS, Mangione CM, Silverstein M, Simon MA, Tseng CW, Wong JB. Ocular Prophylaxis for Gonococcal Ophthalmia Neonatorum: US Preventive Services Task Force Reaffirmation Recommendation Statement. JAMA. 2019 Jan 29:321(4):394-398. doi: 10.1001/jama.2018.21367. Epub [PubMed PMID: 30694327]
Mallika P, Asok T, Faisal H, Aziz S, Tan A, Intan G. Neonatal conjunctivitis - a review. Malaysian family physician : the official journal of the Academy of Family Physicians of Malaysia. 2008:3(2):77-81 [PubMed PMID: 25606121]
Hong Z, Graham M, Daley AJ, Troutbeck R. Microbiology and Management of Neonatal Conjunctivitis Presenting to an Urban Australian Paediatric Hospital: An Eight-Year Retrospective Review. Clinical & experimental ophthalmology. 2025 May 19:():. doi: 10.1111/ceo.14557. Epub 2025 May 19 [PubMed PMID: 40390235]
Level 2 (mid-level) evidenceAzmi NH, Alias R, Vaiyavari V, Ch'ng H, Nasaruddin RA. Perforated Corneal Ulcer Arising From Gonococcal Keratoconjunctivitis: A Report of Three Cases. Cureus. 2025 Apr:17(4):e82150. doi: 10.7759/cureus.82150. Epub 2025 Apr 12 [PubMed PMID: 40370876]
Level 3 (low-level) evidenceHuber-Spitzy V, Arocker W, Schmidt C. [Ophthalmia neonatorum]. Klinische Monatsblatter fur Augenheilkunde. 1987 Nov:191(5):341-3 [PubMed PMID: 3430999]
Churchward CP, Alany RG, Kirk RS, Walker AJ, Snyder LAS. Prevention of Ophthalmia Neonatorum Caused by Neisseria gonorrhoeae Using a Fatty Acid-Based Formulation. mBio. 2017 Jul 25:8(4):. doi: 10.1128/mBio.00534-17. Epub 2017 Jul 25 [PubMed PMID: 28743809]
Asiamah R, Owusu G, Amoako PT, Amponsah R, Adator E, Kyei S. Epidemiology of ophthalmia neonatorum: a systematic review and meta-analysis. BMC pediatrics. 2025 Jan 14:25(1):31. doi: 10.1186/s12887-024-05382-x. Epub 2025 Jan 14 [PubMed PMID: 39810179]
Level 1 (high-level) evidenceMatejcek A, Goldman RD. Treatment and prevention of ophthalmia neonatorum. Canadian family physician Medecin de famille canadien. 2013 Nov:59(11):1187-90 [PubMed PMID: 24235191]
Nelson C, Deshler BJ, Bernstein B, Aronoff S. Variability in the Evaluation and Treatment of Healthy Neonates With Uncomplicated Conjunctivitis. Journal of pediatric ophthalmology and strabismus. 2025 Mar-Apr:62(2):143-149. doi: 10.3928/01913913-20241121-04. Epub 2024 Dec 30 [PubMed PMID: 39749988]
Mondì V, Caravetta J, Paolillo P, Salce N, Tzialla C, Vasapollo B, Valensise H, Bedetta M, Picone S. Are Chlamydia Trachomatis and Neisseria Gonorrhoeae Screenings in Pregnant Women Being Properly Performed? A Single-Center Retrospective Observational Study in Italy. Pathogens (Basel, Switzerland). 2024 Jul 8:13(7):. doi: 10.3390/pathogens13070570. Epub 2024 Jul 8 [PubMed PMID: 39057797]
Level 2 (mid-level) evidenceFranco S, Hammerschlag MR. Can we use azithromycin eye drops for gonococcal ophthalmia prophylaxis in the United States? Expert review of anti-infective therapy. 2024 Jun:22(6):373-377. doi: 10.1080/14787210.2024.2359725. Epub 2024 May 27 [PubMed PMID: 38781483]
Kapoor VS, Evans JR, Vedula SS. Interventions for preventing ophthalmia neonatorum. The Cochrane database of systematic reviews. 2020 Sep 21:9(9):CD001862. doi: 10.1002/14651858.CD001862.pub4. Epub 2020 Sep 21 [PubMed PMID: 32959365]
Level 1 (high-level) evidenceMoore DL, MacDonald NE, Canadian Paediatric Society, Infectious Diseases and Immunization Committee. Preventing ophthalmia neonatorum. Paediatrics & child health. 2015 Mar:20(2):93-6 [PubMed PMID: 25838784]
Thanathanee O, O'Brien TP. Conjunctivitis: systematic approach to diagnosis and therapy. Current infectious disease reports. 2011 Apr:13(2):141-8. doi: 10.1007/s11908-011-0167-y. Epub [PubMed PMID: 21365377]
Level 1 (high-level) evidenceZwicker P, Opitz N, Harris J, Stahl A, Kellner U, Koelb-Keerl R, Muether PS, Hunold A, Kramer A. In vitro efficacy of aqueous PVP-iodine solution below 5% as alternative to preoperative antisepsis in ophthalmology as the basis for an in vivo study. Journal of ophthalmic inflammation and infection. 2025 Apr 2:15(1):35. doi: 10.1186/s12348-025-00489-3. Epub 2025 Apr 2 [PubMed PMID: 40172756]
St-Onge-St-Hilaire A, Boutin A, Gravel J. Is Ophthalmia Neonatorum Associated With Invasive Bacterial Infection? A Single-Center Retrospective Study. Pediatric emergency care. 2023 Nov 1:39(11):858-862. doi: 10.1097/PEC.0000000000003066. Epub 2023 Oct 11 [PubMed PMID: 37820378]
Level 2 (mid-level) evidenceAhmad B, Gurnani B, Patel BC. Herpes Simplex Keratitis. StatPearls. 2025 Jan:(): [PubMed PMID: 31424862]
Länsivaara A, Palmroth M, Kaarela O, Hyöty H, Oikarinen S, Lehto KM. Virus detection in influent, activated sludge, and effluent from municipal wastewater treatment plants using composite and grab samples in Finland. Environmental research. 2025 Aug 15:279(Pt 2):121776. doi: 10.1016/j.envres.2025.121776. Epub 2025 May 3 [PubMed PMID: 40324624]
Jefferies JMC, Cooper T, Yam T, Clarke SC. Pseudomonas aeruginosa outbreaks in the neonatal intensive care unit--a systematic review of risk factors and environmental sources. Journal of medical microbiology. 2012 Aug:61(Pt 8):1052-1061. doi: 10.1099/jmm.0.044818-0. Epub 2012 Jun 8 [PubMed PMID: 22683659]
Level 1 (high-level) evidenceTinoco-Araujo JE, Araújo DF, Barbosa PG, Santos PS, Medeiros AM. Invasive candidiasis and oral manifestations in premature newborns. Einstein (Sao Paulo, Brazil). 2013 Jan-Mar:11(1):71-5 [PubMed PMID: 23579747]
Kaštelan S, Anić Jurica S, Orešković S, Župić T, Herman M, Gverović Antunica A, Marković I, Bakija I. A Survey of Current Prophylactic Treatment for Ophthalmia Neonatorum in Croatia and a Review of International Preventive Practices. Medical science monitor : international medical journal of experimental and clinical research. 2018 Nov 10:24():8042-8047. doi: 10.12659/MSM.910705. Epub 2018 Nov 10 [PubMed PMID: 30413681]
Level 3 (low-level) evidenceBabalola CM, Klausner JD. Antenatal Screening for Sexually Transmitted Infections to Improve Maternal and Newborn Outcomes: An Update From 11 Low- and Middle-Income Countries. Sexually transmitted diseases. 2025 Mar 1:52(3):141-145. doi: 10.1097/OLQ.0000000000002100. Epub 2024 Nov 25 [PubMed PMID: 39874241]
Rodrigues R, Vieira-Baptista P, Catalão C, Borrego MJ, Sousa C, Vale N. Chlamydial and Gonococcal Genital Infections: A Narrative Review. Journal of personalized medicine. 2023 Jul 21:13(7):. doi: 10.3390/jpm13071170. Epub 2023 Jul 21 [PubMed PMID: 37511783]
Level 3 (low-level) evidenceMsukwa G, Batumba N, Drucker M, Menezes L, Ranjit R. Maternal and neonatal risk factors associated with vertical transmission of ophthalmia neonatorum in neonates receiving health care in Blantyre, Malawi. Middle East African journal of ophthalmology. 2014 Jul-Sep:21(3):240-3. doi: 10.4103/0974-9233.134684. Epub [PubMed PMID: 25100909]
Fransen L, Klauss V. Neonatal ophthalmia in the developing world. Epidemiology, etiology, management and control. International ophthalmology. 1988 Jan:11(3):189-96 [PubMed PMID: 3047073]
Bibb LA, Htet KZ, Waldman CW, Sloan SB. Sexually Transmitted Infections and HIV in Ophthalmology. Clinics in dermatology. 2023 Oct 17:():. pii: S0738-081X(23)00178-5. doi: 10.1016/j.clindermatol.2023.10.013. Epub 2023 Oct 17 [PubMed PMID: 37858780]
Gurnani B, Kaur K. Pythium Keratitis. StatPearls. 2025 Jan:(): [PubMed PMID: 34424645]
Gurnani B, Christy J, Narayana S, Rajkumar P, Kaur K, Gubert J. Retrospective multifactorial analysis of Pythium keratitis and review of literature. Indian journal of ophthalmology. 2021 May:69(5):1095-1101. doi: 10.4103/ijo.IJO_1808_20. Epub [PubMed PMID: 33913840]
Level 2 (mid-level) evidenceMalik ANJ, Gilbert C. Cochrane corner: interventions for preventing ophthalmia neonatorum. Eye (London, England). 2022 Feb:36(2):356-357. doi: 10.1038/s41433-021-01803-8. Epub 2021 Oct 12 [PubMed PMID: 34642498]
Becerra SC, Roy DC, Sanchez CJ, Christy RJ, Burmeister DM. An optimized staining technique for the detection of Gram positive and Gram negative bacteria within tissue. BMC research notes. 2016 Apr 12:9():216. doi: 10.1186/s13104-016-1902-0. Epub 2016 Apr 12 [PubMed PMID: 27071769]
Church D, Melnyk E, Unger B. Quantitative gram stain interpretation criteria used by microbiology laboratories in Alberta, Canada. Journal of clinical microbiology. 2000 Nov:38(11):4266-8 [PubMed PMID: 11060107]
Maqsood N, Mahmood U. Herpes simplex ophthalmia neonatorum: a sight-threatening diagnosis. The British journal of general practice : the journal of the Royal College of General Practitioners. 2020 Oct:70(699):513-514. doi: 10.3399/bjgp20X712973. Epub 2020 Oct 1 [PubMed PMID: 33004377]
Ali Z, Khadije D, Elahe A, Mohammad M, Fateme Z, Narges Z. Prophylaxis of ophthalmia neonatorum comparison of betadine, erythromycin and no prophylaxis. Journal of tropical pediatrics. 2007 Dec:53(6):388-92 [PubMed PMID: 18055489]
David M, Rumelt S, Weintraub Z. Efficacy comparison between povidone iodine 2.5% and tetracycline 1% in prevention of ophthalmia neonatorum. Ophthalmology. 2011 Jul:118(7):1454-8. doi: 10.1016/j.ophtha.2010.12.003. Epub 2011 Mar 25 [PubMed PMID: 21439642]
Ruggiero A, Ariano A, Triarico S, Capozza MA, Ferrara P, Attinà G. Neonatal pharmacology and clinical implications. Drugs in context. 2019:8():212608. doi: 10.7573/dic.212608. Epub 2019 Oct 14 [PubMed PMID: 31692800]
Tayman C, Rayyan M, Allegaert K. Neonatal pharmacology: extensive interindividual variability despite limited size. The journal of pediatric pharmacology and therapeutics : JPPT : the official journal of PPAG. 2011 Jul:16(3):170-84. doi: 10.5863/1551-6776-16.3.170. Epub [PubMed PMID: 22479159]
Lu H, Rosenbaum S. Developmental pharmacokinetics in pediatric populations. The journal of pediatric pharmacology and therapeutics : JPPT : the official journal of PPAG. 2014 Oct-Dec:19(4):262-76. doi: 10.5863/1551-6776-19.4.262. Epub [PubMed PMID: 25762871]
Hashmi MF, Gurnani B, Benson S. Conjunctivitis. StatPearls. 2025 Jan:(): [PubMed PMID: 31082078]
Hauben M, Amsden GW. The association of erythromycin and infantile hypertrophic pyloric stenosis: causal or coincidental? Drug safety. 2002:25(13):929-42 [PubMed PMID: 12381214]
Solano D, Fu L, Czyz CN. Viral Conjunctivitis. StatPearls. 2025 Jan:(): [PubMed PMID: 29262100]
Pourabbas B, Rezaei Z, Mardaneh J, Shahian M, Alborzi A. Prevalence of Chlamydia trachomatis and Neisseria gonorrhoeae infections among pregnant women and eye colonization of their neonates at birth time, Shiraz, Southern Iran. BMC infectious diseases. 2018 Sep 24:18(1):477. doi: 10.1186/s12879-018-3382-4. Epub 2018 Sep 24 [PubMed PMID: 30249196]
Gurnani B, Kaur K, Chaudhary S, Kaur RP, Nayak S, Mishra D, Balakrishnan H, Parkash RO, Morya AK, Porwal A. Pediatric corneal transplantation: techniques, challenges, and outcomes. Therapeutic advances in ophthalmology. 2024 Jan-Dec:16():25158414241237906. doi: 10.1177/25158414241237906. Epub 2024 Mar 25 [PubMed PMID: 38533487]
Level 3 (low-level) evidenceMakker K, Nassar GN, Kaufman EJ. Neonatal Conjunctivitis. StatPearls. 2025 Jan:(): [PubMed PMID: 28722870]
Law NL, Tan VC, Lim TH, Nurul Rosli A. Ophthalmia neonatorum complicated with neonatal orbital cellulitis: A case series. Malaysian family physician : the official journal of the Academy of Family Physicians of Malaysia. 2024:19():5. doi: 10.51866/cr.438. Epub 2024 Jan 8 [PubMed PMID: 38371721]
Level 2 (mid-level) evidenceMondì V, Tzialla C, Aversa S, Merazzi D, Martinelli S, Araimo G, Massenzi L, Cavallaro G, Gagliardi L, Piersigilli F, Giuffrè M, Lozzi S, Manzoni P, Mosca F, Cetin I, Trojano V, Valensise H, Colacurci N, Orfeo L, Auriti C. Antibiotic prophylaxis for ophthalmia neonatorum in Italy: results from a national survey and the Italian intersociety new position statements. Italian journal of pediatrics. 2023 Sep 11:49(1):117. doi: 10.1186/s13052-023-01507-7. Epub 2023 Sep 11 [PubMed PMID: 37697419]
Level 3 (low-level) evidenceBurrow MK, Gurnani B, Patel BC. Keratoconjunctivitis. StatPearls. 2025 Jan:(): [PubMed PMID: 31194419]
Gurnani B, Kaur K. Bacterial Keratitis. StatPearls. 2025 Jan:(): [PubMed PMID: 34662023]
Wood M. Conjunctivitis: diagnosis and management. Community eye health. 1999:12(30):19-20 [PubMed PMID: 17491982]
Workowski KA, Berman S, Centers for Disease Control and Prevention (CDC). Sexually transmitted diseases treatment guidelines, 2010. MMWR. Recommendations and reports : Morbidity and mortality weekly report. Recommendations and reports. 2010 Dec 17:59(RR-12):1-110 [PubMed PMID: 21160459]
Moore DL, MacDonald NE, Canadian Paediatric Society, Infectious Diseases and Immunization Committee. Preventing ophthalmia neonatorum. The Canadian journal of infectious diseases & medical microbiology = Journal canadien des maladies infectieuses et de la microbiologie medicale. 2015 May-Jun:26(3):122-5 [PubMed PMID: 26236350]
Meyer T, Buder S. The Laboratory Diagnosis of Neisseria gonorrhoeae: Current Testing and Future Demands. Pathogens (Basel, Switzerland). 2020 Jan 31:9(2):. doi: 10.3390/pathogens9020091. Epub 2020 Jan 31 [PubMed PMID: 32024032]
Crotchfelt KA, Welsh LE, DeBonville D, Rosenstraus M, Quinn TC. Detection of Neisseria gonorrhoeae and Chlamydia trachomatis in genitourinary specimens from men and women by a coamplification PCR assay. Journal of clinical microbiology. 1997 Jun:35(6):1536-40 [PubMed PMID: 9163477]
Li S, Jiang W, Peng JM, Du B, Weng L. Herpes simplex virus associated sepsis in an immunocompetent adult: the value of next-generation sequencing. Chinese medical journal. 2020 Jul 20:133(14):1727-1728. doi: 10.1097/CM9.0000000000000893. Epub [PubMed PMID: 32568872]
Martin R. Cornea and anterior eye assessment with slit lamp biomicroscopy, specular microscopy, confocal microscopy, and ultrasound biomicroscopy. Indian journal of ophthalmology. 2018 Feb:66(2):195-201. doi: 10.4103/ijo.IJO_649_17. Epub [PubMed PMID: 29380757]
Hammerschlag MR. Chlamydial and gonococcal infections in infants and children. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2011 Dec:53 Suppl 3():S99-102. doi: 10.1093/cid/cir699. Epub [PubMed PMID: 22080275]
Zloto O, Gharaibeh A, Mezer E, Stankovic B, Isenberg S, Wygnanski-Jaffe T. Ophthalmia neonatorum treatment and prophylaxis: IPOSC global study. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2016 Mar:254(3):577-82. doi: 10.1007/s00417-016-3274-5. Epub 2016 Jan 26 [PubMed PMID: 26810921]
Zuppa AA, D'Andrea V, Catenazzi P, Scorrano A, Romagnoli C. Ophthalmia neonatorum: what kind of prophylaxis? The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstetricians. 2011 Jun:24(6):769-73. doi: 10.3109/14767058.2010.531326. Epub [PubMed PMID: 21534852]
Eberly MD, Eide MB, Thompson JL, Nylund CM. Azithromycin in early infancy and pyloric stenosis. Pediatrics. 2015 Mar:135(3):483-8. doi: 10.1542/peds.2014-2026. Epub [PubMed PMID: 25687145]
Level 2 (mid-level) evidenceInaba S, Aizawa Y, Kataoka S, Saitoh A. Purulent nasal discharge due to gonococcal nasopharyngitis in a neonate. Journal of infection and chemotherapy : official journal of the Japan Society of Chemotherapy. 2023 Dec:29(12):1164-1166. doi: 10.1016/j.jiac.2023.08.005. Epub 2023 Aug 7 [PubMed PMID: 37558089]
Singh M, Alsaleem M, Gray CP. Neonatal Sepsis. StatPearls. 2025 Jan:(): [PubMed PMID: 30285373]
Costumbrado J, Ng DK, Ghassemzadeh S. Gonococcal Conjunctivitis. StatPearls. 2025 Jan:(): [PubMed PMID: 29083770]
Zikic A, Schünemann H, Wi T, Lincetto O, Broutet N, Santesso N. Treatment of Neonatal Chlamydial Conjunctivitis: A Systematic Review and Meta-analysis. Journal of the Pediatric Infectious Diseases Society. 2018 Aug 17:7(3):e107-e115. doi: 10.1093/jpids/piy060. Epub [PubMed PMID: 30007329]
Level 1 (high-level) evidenceJames SH, Whitley RJ. Treatment of herpes simplex virus infections in pediatric patients: current status and future needs. Clinical pharmacology and therapeutics. 2010 Nov:88(5):720-4. doi: 10.1038/clpt.2010.192. Epub 2010 Sep 29 [PubMed PMID: 20881952]
Byrd LB, Gurnani B, Martin N. Corneal Ulcer. StatPearls. 2025 Jan:(): [PubMed PMID: 30969511]
Gurnani B, Narayana S, Christy J, Rajkumar P, Kaur K, Gubert J. Successful management of pediatric pythium insidiosum keratitis with cyanoacrylate glue, linezolid, and azithromycin: Rare case report. European journal of ophthalmology. 2022 Sep:32(5):NP87-NP91. doi: 10.1177/11206721211006564. Epub 2021 Mar 28 [PubMed PMID: 33779337]
Level 3 (low-level) evidenceMeena A, Agrawal A, Parmar G, Gurnani B. Subconjunctival dexamethasone-assisted conjunctival autograft harvesting versus normal saline during pterygium surgery - A randomized clinical trial. Indian journal of ophthalmology. 2024 Feb 1:72(2):217-222. doi: 10.4103/IJO.IJO_969_23. Epub 2023 Dec 15 [PubMed PMID: 38099381]
Level 1 (high-level) evidenceGurnani B, Kaur K. Bilateral Morgagnian Cataract Post Neoadjuvant Chemotherapy for Oral Carcinoma- A Potentially Blinding Sequelae. Indian journal of surgical oncology. 2022 Dec:13(4):824-825. doi: 10.1007/s13193-022-01570-2. Epub 2022 Jul 8 [PubMed PMID: 36687240]
Murugan SRB, Sanjay S, Somanath A, Mahendradas P, Patil A, Kaur K, Gurnani B. Artificial Intelligence in Uveitis: Innovations in Diagnosis and Therapeutic Strategies. Clinical ophthalmology (Auckland, N.Z.). 2024:18():3753-3766. doi: 10.2147/OPTH.S495307. Epub 2024 Dec 14 [PubMed PMID: 39703602]
Yang K, Babalola CM, Mussa A, Ryan R, Wynn A, Simon S, Bame B, Morroni C, Klausner JD. Case series and literature review of chlamydial ophthalmia neonatorum in Botswana. International journal of STD & AIDS. 2023 Oct:34(12):860-868. doi: 10.1177/09564624231173028. Epub 2023 Jun 20 [PubMed PMID: 37338101]
Level 2 (mid-level) evidenceMcCalman J, Bailie R, Bainbridge R, McPhail-Bell K, Percival N, Askew D, Fagan R, Tsey K. Continuous Quality Improvement and Comprehensive Primary Health Care: A Systems Framework to Improve Service Quality and Health Outcomes. Frontiers in public health. 2018:6():76. doi: 10.3389/fpubh.2018.00076. Epub 2018 Mar 22 [PubMed PMID: 29623271]
Level 2 (mid-level) evidenceBoadi-Kusi SB, Kyei S, Holdbrook S, Abu EK, Ntow J, Ateko AM. A study of Ophthalmia Neonatorum in the Central Reion of Ghana: Causative Agents and Antibiotic Susceptibility Patterns. Global pediatric health... 2021:8():2333794X211019700. doi: 10.1177/2333794X211019700. Epub 2021 May 28 [PubMed PMID: 34104699]
Tang H, Sun Q, Huang J, Wen G, Han L, Wang L, Zhang Y, Dong M, Wang W. Residue behaviors, degradation, processing factors, and risk assessment of pesticides in citrus from field to product processing. The Science of the total environment. 2023 Nov 1:897():165321. doi: 10.1016/j.scitotenv.2023.165321. Epub 2023 Jul 6 [PubMed PMID: 37419352]
Kidszun A, Bruns A, Schreiner D, Tippmann S, Winter J, Pokora RM, Urschitz MS, Mildenberger E. Characteristics of neonatal herpes simplex virus infections in Germany: results of a 2-year prospective nationwide surveillance study. Archives of disease in childhood. Fetal and neonatal edition. 2022 Mar:107(2):188-192. doi: 10.1136/archdischild-2021-321940. Epub 2021 Jul 13 [PubMed PMID: 34257101]
Bremond-Gignac D, Chiambaretta F, Milazzo S. A European perspective on topical ophthalmic antibiotics: current and evolving options. Ophthalmology and eye diseases. 2011:3():29-43. doi: 10.4137/OED.S4866. Epub 2011 Oct 24 [PubMed PMID: 23861622]
Level 3 (low-level) evidenceCasemiro JH, Oguido APMT, Casella AMB. Using 2% PVPI topical solution for serial intravitreous injections and ocular surface findings: a case control study. International journal of retina and vitreous. 2024 May 29:10(1):41. doi: 10.1186/s40942-024-00557-1. Epub 2024 May 29 [PubMed PMID: 38812063]
Level 2 (mid-level) evidenceDonnelly PC, Sutich RM, Easton R, Adejumo OA, Lee TA, Logan LK. Ceftriaxone-Associated Biliary and Cardiopulmonary Adverse Events in Neonates: A Systematic Review of the Literature. Paediatric drugs. 2017 Feb:19(1):21-34. doi: 10.1007/s40272-016-0197-x. Epub [PubMed PMID: 27718120]
Level 1 (high-level) evidenceKhan H, Chebolu A, Richards AB, Barry GP. Risk of corneal epithelial defects with and without postoperative erythromycin ointment after laser photocoagulation for retinopathy of prematurity. Indian journal of ophthalmology. 2021 Aug:69(8):2178-2181. doi: 10.4103/ijo.IJO_216_21. Epub [PubMed PMID: 34304205]
Rahman MQ, Tejwani D, Wilson JA, Butcher I, Ramaesh K. Microbial contamination of preservative free eye drops in multiple application containers. The British journal of ophthalmology. 2006 Feb:90(2):139-41 [PubMed PMID: 16424520]
El Hachem M, Diociaiuti A, Bonamonte D, Brena M, Lospalluti L, Magnoni C, Neri I, Peris K, Tadini G, Zambruno G, Delphi Study Group. Taking care of patients with recessive dystrophic epidermolysis bullosa from birth to adulthood: a multidisciplinary Italian Delphi consensus. Orphanet journal of rare diseases. 2025 Mar 16:20(1):128. doi: 10.1186/s13023-025-03635-1. Epub 2025 Mar 16 [PubMed PMID: 40091088]
Level 3 (low-level) evidenceHarrison RF, Ouyang H. Fever and the rational use of antimicrobials in the emergency department. Emergency medicine clinics of North America. 2013 Nov:31(4):945-68. doi: 10.1016/j.emc.2013.07.007. Epub 2013 Sep 12 [PubMed PMID: 24176473]
Albuquerque C, Dias ME, Pelicano M, Banganho D, Morais RB. Neisseria meningitidis: The Unforeseen Agent of Acute Neonatal Conjunctivitis. Cureus. 2024 Jul:16(7):e65681. doi: 10.7759/cureus.65681. Epub 2024 Jul 29 [PubMed PMID: 39205736]
Darville T. Chlamydia trachomatis infections in neonates and young children. Seminars in pediatric infectious diseases. 2005 Oct:16(4):235-44 [PubMed PMID: 16210104]
Balamurugan S, Das D, Hasanreisoglu M, Toy BC, Akhter M, Anuradha VK, Anthony E, Gurnani B, Kaur K. Interleukins and cytokine biomarkers in uveitis. Indian journal of ophthalmology. 2020 Sep:68(9):1750-1763. doi: 10.4103/ijo.IJO_564_20. Epub [PubMed PMID: 32823391]
Badger-Emeka L, Emeka P, Thirugnanasambantham K, Alatawi AS. The Role of Pseudomonas aeruginosa in the Pathogenesis of Corneal Ulcer, Its Associated Virulence Factors, and Suggested Novel Treatment Approaches. Pharmaceutics. 2024 Aug 16:16(8):. doi: 10.3390/pharmaceutics16081074. Epub 2024 Aug 16 [PubMed PMID: 39204419]
Gurnani B, Kaur K, Venugopal A, Srinivasan B, Bagga B, Iyer G, Christy J, Prajna L, Vanathi M, Garg P, Narayana S, Agarwal S, Sahu S. Pythium insidiosum keratitis - A review. Indian journal of ophthalmology. 2022 Apr:70(4):1107-1120. doi: 10.4103/ijo.IJO_1534_21. Epub [PubMed PMID: 35325996]
Gurnani B, Feroze KB, Patel BC. Neurotrophic Keratitis. StatPearls. 2025 Jan:(): [PubMed PMID: 28613758]
Gurnani B, Kaur K, Gireesh P, Balakrishnan L, Mishra C. Evaluating the novel role of ChatGPT-4 in addressing corneal ulcer queries: An AI-powered insight. European journal of ophthalmology. 2025 Apr 28:():11206721251337290. doi: 10.1177/11206721251337290. Epub 2025 Apr 28 [PubMed PMID: 40295112]
Gurnani B, Christy J, Narayana S, Kaur K, Moutappa F. Corneal Perforation Secondary to Rosacea Keratitis Managed with Excellent Visual Outcome. Nepalese journal of ophthalmology : a biannual peer-reviewed academic journal of the Nepal Ophthalmic Society : NEPJOPH. 2022 Jan:14(27):162-167. doi: 10.3126/nepjoph.v14i1.36454. Epub [PubMed PMID: 35996914]
Gurnani B, Kaur K, Chaudhary S, Gandhi AS, Balakrishnan H, Mishra C, Gosalia H, Dhiman S, Joshi S, Nagtode AH, Jain S, Aguiar M, Rustagi IM. Nystagmus in Clinical Practice: From Diagnosis to Treatment-A Comprehensive Review. Clinical ophthalmology (Auckland, N.Z.). 2025:19():1617-1657. doi: 10.2147/OPTH.S523224. Epub 2025 May 17 [PubMed PMID: 40401036]
Gurnani B, Kaur K. Re: Dot et al.: Incidence of retinal detachment, macular edema, and ocular hypertension after neodymium:yttrium-aluminum-garnet capsulotomy: a population-based nationwide study-The French YAG 2 Study (Ophthalmology. 2022;130:478-487). Ophthalmology. 2023 Dec:130(12):e43. doi: 10.1016/j.ophtha.2023.08.012. Epub 2023 Sep 20 [PubMed PMID: 37737811]
Gurnani B, Srinivasan K, Venkatesh R, Kaur K. Do motivational cards really benefit sibling screening of primary open-angle glaucoma probands? Indian journal of ophthalmology. 2022 Dec:70(12):4158-4163. doi: 10.4103/ijo.IJO_1346_22. Epub [PubMed PMID: 36453305]
Ray A, Gurnani B. Pediatric Cataract. StatPearls. 2025 Jan:(): [PubMed PMID: 34283446]
Gurnani B, Kaur K. Inflammatory cytokines in tears of patients with lacrimal duct obstruction. The Indian journal of medical research. 2023 Sep:158(3):317. doi: 10.4103/ijmr.ijmr_1487_22. Epub [PubMed PMID: 37815066]
Gurnani B, Kaur K. Anti-infective therapies for Pythium insidiosum keratitis. Expert review of anti-infective therapy. 2024 Oct:22(10):805-817. doi: 10.1080/14787210.2024.2403146. Epub 2024 Sep 13 [PubMed PMID: 39268901]
Niles JK, Kaufman HW, Peterman TA, Tao G, Gift TL, Alagia DP. Chlamydia trachomatis and Neisseria gonorrhoeae in Pregnancy: Trends in United States, 2010 to 2018. Sexually transmitted diseases. 2021 Dec 1:48(12):932-938. doi: 10.1097/OLQ.0000000000001504. Epub [PubMed PMID: 34192725]
Muller WJ, Zheng X. Laboratory Diagnosis of Neonatal Herpes Simplex Virus Infections. Journal of clinical microbiology. 2019 May:57(5):. doi: 10.1128/JCM.01460-18. Epub 2019 Apr 26 [PubMed PMID: 30602444]
Pata R, Datar P. The Diagnosis and Management of Herpes Simplex Pneumonia in the Critical Care Setting: A Comprehensive Review. Cureus. 2023 Aug:15(8):e43224. doi: 10.7759/cureus.43224. Epub 2023 Aug 9 [PubMed PMID: 37692679]
De Rose DU, Ronchetti MP, Martini L, Rechichi J, Iannetta M, Dotta A, Auriti C. Diagnosis and Management of Neonatal Bacterial Sepsis: Current Challenges and Future Perspectives. Tropical medicine and infectious disease. 2024 Aug 28:9(9):. doi: 10.3390/tropicalmed9090199. Epub 2024 Aug 28 [PubMed PMID: 39330888]
Level 3 (low-level) evidenceMiller JM, Binnicker MJ, Campbell S, Carroll KC, Chapin KC, Gilligan PH, Gonzalez MD, Jerris RC, Kehl SC, Patel R, Pritt BS, Richter SS, Robinson-Dunn B, Schwartzman JD, Snyder JW, Telford S 3rd, Theel ES, Thomson RB Jr, Weinstein MP, Yao JD. A Guide to Utilization of the Microbiology Laboratory for Diagnosis of Infectious Diseases: 2018 Update by the Infectious Diseases Society of America and the American Society for Microbiology. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2018 Aug 31:67(6):e1-e94. doi: 10.1093/cid/ciy381. Epub [PubMed PMID: 29955859]
Fowler JR, Jenkins SM, Jack BW. Preconception Counseling. StatPearls. 2025 Jan:(): [PubMed PMID: 28722910]
Giardino AP, De Jesus O. Case Management. StatPearls. 2025 Jan:(): [PubMed PMID: 32965885]
Level 3 (low-level) evidenceTzialla C, Auriti C, Aversa S, Merazzi D, Martinelli S, Araimo G, Massenzi L, Cavallaro G, Gagliardi L, Giuffrè M, Mosca F, Cetin I, Trojano V, Valensise H, Colacurci N, Orfeo L, Mondì V, On Behalf Of Their Respective Scientific Societies. Intersociety Position Statement on the Prevention of Ophthalmia Neonatorum in Italy. Microorganisms. 2023 Dec 21:12(1):. doi: 10.3390/microorganisms12010015. Epub 2023 Dec 21 [PubMed PMID: 38276184]
Bibb LA, Htet KZ, Waldman CW, Sloan SB. Sexually transmitted infections and HIV in ophthalmology. Clinics in dermatology. 2024 Jan-Feb:42(1):25-37. doi: 10.1016/j.clindermatol.2023.08.011. Epub 2023 Aug 13 [PubMed PMID: 37582453]
Sim TF, Sherriff J, Hattingh HL, Parsons R, Tee LB. The use of herbal medicines during breastfeeding: a population-based survey in Western Australia. BMC complementary and alternative medicine. 2013 Nov 13:13():317. doi: 10.1186/1472-6882-13-317. Epub 2013 Nov 13 [PubMed PMID: 24219150]
Level 3 (low-level) evidenceKaur K, Zeppieri M, Gurnani B. Primary Congenital Glaucoma. StatPearls. 2025 Jan:(): [PubMed PMID: 34662067]
Gurnani B, Kaur K. Recent Advances in Refractive Surgery: An Overview. Clinical ophthalmology (Auckland, N.Z.). 2024:18():2467-2472. doi: 10.2147/OPTH.S481421. Epub 2024 Sep 2 [PubMed PMID: 39246558]
Level 3 (low-level) evidenceGurnani B, Kaur K. Comment on "Impact of the COVID-19 pandemic on research publications in emergency medicine". World journal of emergency medicine. 2025:16(2):172-173. doi: 10.5847/wjem.j.1920-8642.2025.026. Epub [PubMed PMID: 40135213]
Level 3 (low-level) evidence