Introduction
Despite its presence in clinical practice for the past 4 to 5 decades, neonatal hypertension has only recently gained recognition as a distinct neonatal morbidity. The absence of comprehensive normative data on neonatal blood pressure hindered earlier efforts to evaluate and manage this condition. Limitations in the availability of accurate invasive and noninvasive measurement techniques further complicated assessment. Additionally, significant variability in blood pressure based on gestational age, postnatal age, birth weight, and gender within the neonatal population created challenges in defining normal ranges and establishing a standardized definition of neonatal hypertension.
The clinical trajectory, long-term outcomes, and potential sequelae of neonatal hypertension remain incompletely understood. No clear consensus has emerged regarding the appropriate use or selection of antihypertensive medications in affected infants.[1] Current knowledge continues to evolve, with ongoing efforts to clarify the definition, risk factors, etiopathogenesis, and management strategies for hypertension in newborns.
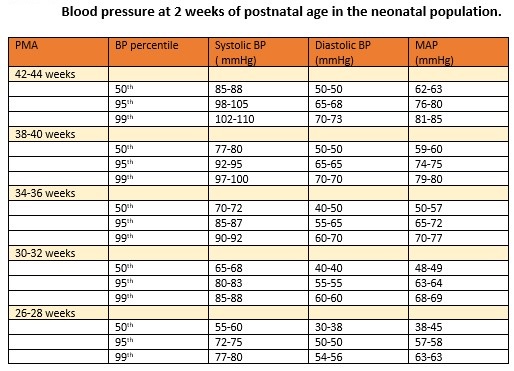
Diagnosis of neonatal hypertension requires systolic or diastolic blood pressure values at or above the 95th percentile for postconceptual age, recorded on three separate occasions.[2] Blood pressure values exceeding the 99th percentile suggest severe hypertension and warrant the initiation of antihypertensive treatment, along with further investigation to determine the underlying cause (see Image. Neonatal Normative Blood Pressure Data).[3]
Blood Pressure Measurements
Blood pressure can be measured via invasive or noninvasive methods.
Invasive methods
The invasive intra-arterial blood pressure measurement and continuous monitoring, regarded as the gold standard, are performed by utilizing an indwelling catheter in the umbilical, radial, or posterior tibial arteries, which is connected to a pressure transducer and, via that, to a multichannel display patient monitor. This method is generally reserved for sick, unstable, or extremely premature infants.[4]
The following steps and precautions should be taken while measuring the blood pressure intra-arterially via the transducer:
-
The transducer must remain positioned at the level of the heart to reflect actual pressure values.
-
Air bubbles in the tubing must be avoided, as their presence can elevate diastolic pressure and lower systolic pressure, distorting the readings.
-
A clear dicrotic notch should appear on the arterial waveform to confirm correct waveform representation.
-
Tubing should consist of low-compliance material and maintain the shortest acceptable length, since longer tubing can falsely reduce measured values.
-
The pressure transducer must be referenced to zero at atmospheric pressure to calibrate correctly.
-
A continuous heparin infusion should be used to irrigate the transducer and maintain line patency, thereby reducing the risk of clot formation.
-
The umbilical catheter must be appropriately sized, as narrow catheters may underestimate systolic pressure.
-
Removal of the umbilical artery catheter is recommended after 5 to 7 days of use. Prolonged catheterization increases the risk of thrombus formation, which may contribute to inaccurate readings and potential complications.
Noninvasive methods
Automated oscillometry is the most common and widely used noninvasive method for measuring blood pressure in the neonatal intensive care unit. This device detects the maximum blood pressure oscillations from arterial blood flow as mean blood pressure, which is then converted into projected systolic and diastolic blood pressures using standard proprietary algorithms. Oscillometric blood pressure measurements generally correlate well with invasive intra-arterial readings. However, automated oscillometry may overestimate intra-arterial systolic blood pressure values by 3 to 8 mm Hg, thereby overdiagnosing hypertension, and becomes inaccurate when mean arterial pressure drops below 30 mm Hg, thus missing hypotension. The device may also underestimate systolic blood pressure in small-for-gestational-age infants. Variations in blood pressure accuracy across studies might stem from the fact that each manufacturer of oscillometric devices employs a unique algorithm to calculate blood pressure, and several studies have highlighted discrepancies among different devices.[5]
For accuracy, the optimum cuff width is suggested to be in a ratio of 0.44 to 0.55, with the arm circumference covering approximately 80% of the patient's arm length. The size should be standardized for uniformity in the results.[6] The blood pressure becomes erroneously high if the cuff size is too small. At the time of measurement, the infant should lie supine, quietly awake, calm, preferably sleeping, and about 1 to 1.5 hours postprandial. At least three readings, 2 minutes apart, should be taken in the right arm as the preferred site.[7] Using a sphygmomanometer is not recommended because the Korotkoff sounds are not loud enough to be reliably audible in this age group of infants.[8] Ultrasound Doppler is rarely used as a regular blood pressure monitoring device, as it can underestimate systolic blood pressure values.[9]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Prematurity and Genetic Etiologies
Prematurity plays a significant role in the development of neonatal hypertension, with approximately 75% of affected infants born preterm. Neonates diagnosed with hypertension tend to exhibit greater illness severity, higher neonatal illness scores, and prolonged neonatal intensive care unit (NICU) stays. The most frequent conditions associated with prematurity that can cause hypertension requiring pharmacologic intervention include respiratory illnesses, eg, bronchopulmonary dysplasia, medications (eg, caffeine and dexamethasone), extracorporeal membrane oxygenation (ECMO), and renal conditions, including parenchymal or renovascular disease, acute tubular necrosis, and renal failure. Additional etiologic factors include neurologic issues (eg, seizures); endocrine or cardiac disorders, particularly coarctation of the aorta or, less commonly, a patent ductus arteriosus; and thromboembolic complications associated with umbilical artery catheterization.[10]
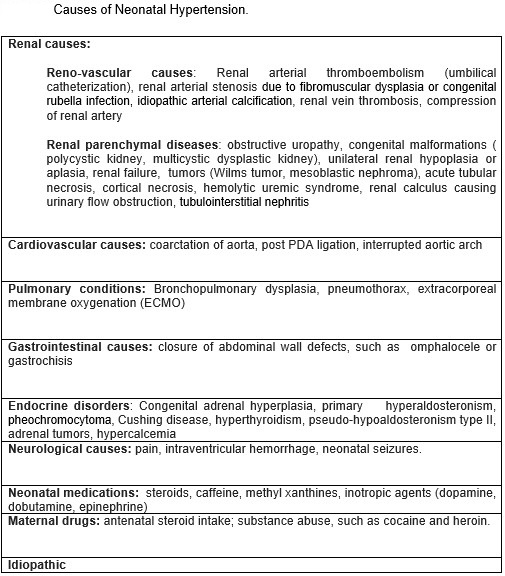
Among nonrenal contributors, bronchopulmonary dysplasia represents the most significant cause of neonatal hypertension in very low birth weight (VLBW) infants and functions as an independent risk factor. Premature infants with intraventricular hemorrhage may also demonstrate an elevated risk.[11][12] Isolated case reports have described hypertension in term infants with ductal aneurysms. Additional anecdotal causes include adrenal hemorrhage, vitamin D toxicity with renal calcinosis, and exposure to total parenteral nutrition. Rare genetic causes, eg, biallelic loss-of-function mutations in the NPR1 gene, have been linked to isolated neonatal-onset systemic hypertension. Approximately 57% of cases remain without a clearly identifiable cause and are classified as idiopathic (see Image. Neonatal Hypertension Etiologies).[13][14]
Phthalate-Associated Etiologies
Phthalates, particularly di-2-ethylhexyl phthalate (DEHP), are plasticizers used in medical equipment, eg, continuous positive airway pressure (CPAP) devices, endotracheal tubes, intravenous bags, and tubing. One study identified a possible association between phthalate exposure and idiopathic neonatal hypertension, typically emerging around 40 weeks postmenstrual age. This form presents with low plasma renin activity and responds favorably to spironolactone therapy.[15] The hypothesized mechanism involves the inhibition of 11β-hydroxysteroid dehydrogenase 2, resulting in the activation of the mineralocorticoid receptor. Researchers at the same center observed a decrease in neonatal hypertension rates, from 7.7% to 1.4%, following the discontinuation of DEHP-containing intravenous fluids, with a subsequent rise to 10.1% when those fluids were reintroduced. These findings, however, have yet to be independently confirmed.[16]
Epidemiology
The incidence of neonatal hypertension has been reported at 0.2% among term newborns and up to 3% in infants admitted to NICUs.[3][17] This wide variation likely reflects differences in study populations and the definitions of neonatal hypertension applied across studies. Among preterm infants, 1.4% require antihypertensive treatment during their NICU stay, compared to 1% of term infants who receive similar therapy.[11]
In infants diagnosed with bronchopulmonary dysplasia, the incidence of neonatal hypertension ranges from 13% to 43%, underscoring the increased risk in this subgroup. However, the precise prevalence of neonatal hypertension within the broader preterm population has not yet been established.
Pathophysiology
A renovascular mechanism is the most typical pathway and is operative in most conditions leading to neonatal hypertension. Systemic causes that might decrease renal perfusion vary. Catheterization of the umbilical artery causes endothelial cell injury and the formation of thrombi in the renal vessels. The resultant decrease in kidney perfusion activates the renin-angiotensin-aldosterone system (RAS), thus causing hypertension.[18]
An inability to excrete free water and an increase in serum aldosterone levels have been reported in bronchopulmonary dysplasia, which might contribute to systemic hypertension. An increase in peripheral arterial wall thickness and abnormal vasomotor functions have recently been demonstrated as the etiopathogenesis for neonatal hypertension in infants with bronchopulmonary dysplasia.[12][19]
Infants of diabetic mothers or those with Factor V Leiden mutation may present with renal vein thrombosis and decreased renal perfusion. Neonatal asphyxia may result in renal tubular necrosis, which may be secondary to neonatal hypertension. Neonatal tumors, eg, pheochromocytoma, neuroblastoma, Wilms tumor, and mesoblastic nephroma, can cause neonatal hypertension by compressing the renal vessels or ureters or by producing vasoactive substances, eg, catecholamines. Coarctation of the aorta and patent ductus arteriosus lead to decreased forward blood flow, diminished renal perfusion, and the activation of RAS. Closing abdominal wall defects increases intra-abdominal pressure, which may compress the renal vessels. Hypertension in ECMO is thought to be secondary to the increased stroke volume caused by the increased aortic return from the ECMO pump blood, as well as by abnormal sodium or water handling in response to the nonpulsatile arterial flow through the infants’ systems.
History and Physical
Clinical History
Neonates with hypertension typically remain asymptomatic, with diagnosis often occurring through routine continuous blood pressure monitoring in the NICU. When symptoms develop, they tend to be nonspecific and varied in nature. Affected infants may exhibit feeding intolerance, weak nipple efforts, irritability, hypotonia, hypertonia, vomiting, apnea, respiratory distress, and oxygen desaturation.
More severe presentations can include tachycardia, congestive heart failure, cardiogenic shock, and seizures. In term or older newborns, hypertension may also be identified during well-child visits or routine vital sign checks in the NICU. Newborns previously discharged from the nursery may return with signs (eg, irritability and failure to thrive).[20]
Physical Examination
A thorough, system-based physical examination plays a critical role in evaluation. A general inspection may reveal dysmorphic features suggestive of genetic syndromes associated with coarctation of the aorta, including Turner syndrome, Williams syndrome, or Noonan syndrome. Detection of a cardiac murmur, along with discrepancies in 4-limb blood pressures and diminished femoral pulses, raises suspicion for aortic coarctation. Signs of congestive heart failure, including tachycardia, murmur, mottling, and cyanosis, may also be present.
Abdominal palpation may uncover a mass associated with polycystic kidney disease, renal tumors, hydronephrosis, or renal vein thrombosis. Genitourinary examination could reveal congenital anomalies or ambiguous genitalia in cases of congenital adrenal hyperplasia. A prenatal history of oligohydramnios may suggest underlying congenital renal anomalies, often accompanied by characteristic physical features in severe cases.
Evaluation
Laboratory Studies
A thorough maternal and perinatal history and physical examination guide in identifying the potential etiology. As renovascular or renal parenchymal disorders account for most cases of neonatal hypertension, the initial investigations focus on this system and include urine analysis, blood urea nitrogen level, and serum values of creatinine, electrolytes, and calcium. The urine should be tested for vanillyl mandelic acid and homovanillic acid. The initial evaluation should include aortic and renal ultrasonography with a Doppler study.[10]
Further investigation depends on the clinical history and the level of clinical suspicion. Recommended laboratory evaluations include:
- Thyroid function tests
- Cortisol levels
- Aldosterone and plasma renin activity
- Plasma and urine catecholamines and metanephrines
- Serum 11-deoxycortisol and 11-deoxycorticosterone
- Urinary 17-hydroxysteroid and 17-ketosteroid
Imaging Studies
The following diagnostic imaging studies may also be utilized:
- Chest radiograph (eg, when assessing for bronchopulmonary dysplasia, congestive heart failure)
- Echocardiogram
- Voiding cystourethrogram
- Captopril renal scintigraphy
- Dimercaptosuccinic acid renal scan
- Computed tomographic angiography (to evaluate the renal artery and the aorta)
- Abdominal magnetic resonance imaging
- Head ultrasonography (to rule out intraventricular hemorrhage)
Treatment / Management
In most cases of neonatal hypertension, addressing the underlying correctable causes leads to resolution of the condition. Prompt removal of the umbilical catheter helps eliminate a common contributing factor to this condition. Correction of hypercalcemia or fluid overload can be achieved through fluid restriction or the use of diuretics. Adjusting doses or discontinuing medications, eg, inotropes, steroids, or caffeine, also contributes to blood pressure normalization.
Surgical conditions require timely intervention, and analgesia may be indicated to relieve pain that exacerbates hypertension. Endocrine disorders necessitate appropriate hormonal therapy. When systolic blood pressure remains above the 99th percentile despite these measures, initiation of antihypertensive treatment becomes necessary.
Mild Neonatal Hypertension Management
In cases of mild neonatal hypertension, defined as systolic blood pressure between the 95th and 99th percentile, careful monitoring often suffices for asymptomatic neonates. Antihypertensive therapy should be considered if blood pressure remains persistently elevated or if echocardiographic findings reveal end-organ damage, eg, left ventricular hypertrophy.
Moderate Neonatal Hypertension Management
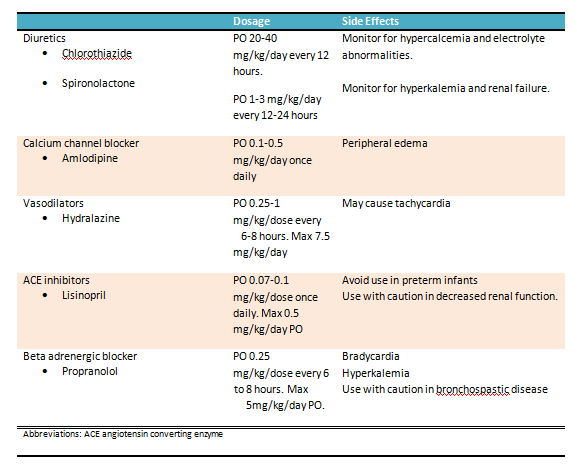
Moderate neonatal hypertension includes systolic pressure at or above the 99th percentile without evident end-organ damage. Although data remain limited, clinicians often prescribe calcium channel blockers, vasodilators, diuretics, or beta-blockers. Angiotensin-converting enzyme (ACE) inhibitors should be avoided in preterm infants younger than 40 to 42 weeks postmenstrual age due to their potential impact on nephron development (see Image. Oral Treatment for Neonatal Hypertension).[21]
Severe Neonatal Hypertension Management
Severe neonatal hypertension requires continuous intravenous infusion of antihypertensive agents. Rapid reductions in blood pressure must be avoided to prevent cerebral or renal complications. Intra-arterial monitoring provides the most accurate and continuous assessment of blood pressure in these critically ill patients. Specific intravenous agents, along with their recommended dosages, have been suggested (see Image. Intravenous Treatment for Neonatal Hypertension).
Surgical Management
Surgical management is indicated in cases involving structural or obstructive conditions, eg, coarctation of the aorta, renal artery or vein occlusion, ureteropelvic junction obstruction, polycystic kidney disease, neuroblastoma, or Wilms tumor.[22] Timely identification and correction of these abnormalities remain crucial for long-term blood pressure control and improved neonatal outcomes.
Differential Diagnosis
Neonatal hypertension is a manifestation of specific disorders of various organ systems. Appropriate investigations should differentiate the etiopathogenic disease entities leading to neonatal hypertension. As the clinical presentation of neonatal hypertension, with symptoms including respiratory distress, hypotonia, irritability, feeding intolerance, and tachycardia, among others, is nonspecific, all other neonatal causes for such symptoms should be ruled out.
Prognosis
The prognosis of neonatal hypertension is influenced by both the cause and the severity of the condition. Neonatal hypertension linked to renal venous thrombosis, umbilical catheterization, or acute renal tubular necrosis tends to be temporary and improves as the underlying issue is addressed. The presence of damage to end organs is linked to a poor prognosis. Typically, most newborns require medication for a brief period, with long-term treatment being rarely necessary. Xiao et al conducted a retrospective study on infants with idiopathic hypertension who were discharged from the NICU while receiving antihypertensive treatment. The study revealed that calcium channel blockers were the most commonly prescribed class of antihypertensives, accounting for 56% of prescriptions.
Following NICU discharge, 60% of the infants continued antihypertensive therapy, with 26% remaining on treatment after 1 year, and 7% after 2 years. This multicenter study indicates that the majority of infants diagnosed with idiopathic hypertension in the NICU will discontinue antihypertensive treatment within 2 years postdischarge.[23] Published data on the long-term outcomes of infants with hypertension as they progress into late childhood and adulthood are lacking. Consequently, monitoring patients with neonatal hypertension closely is crucial. Regular assessments of blood pressure and kidney function should be conducted, and an interprofessional team should oversee their management and treatment.
Complications
Infants with untreated severe, unrelenting hypertension may develop multiple end-organ damage and suffer from vascular injury, left ventricular hypertrophy, encephalopathy, and hypertensive retinopathy. Early, aggressive, and effective treatment should be provided in such conditions. Common complications of longstanding and severe hypertension are hypertensive nephropathy with variable renal dysfunction and hypertensive cardiomyopathy with left ventricular hypertrophy, dilation, or dysfunction. The long-term adverse effects and sequelae of untreated as compared to treated hypertension in infants are unknown. Recent epidemiological evidence suggests that childhood hypertension is associated with a higher risk for adulthood hypertension.
Deterrence and Patient Education
Neonatal hypertension frequently goes unrecognized in infants admitted to the newborn nursery and often surfaces incidentally during routine follow-up visits. Early identification and timely intervention remain critical in this population to reduce the risk of end-organ damage and minimize long-term complications. Pharmacologic treatment presents significant challenges for neonatologists, as limited data exist regarding the safety and efficacy of most antihypertensive agents in neonates. The absence of robust normative blood pressure data and a universally accepted definition of neonatal hypertension further complicates clinical decision-making during this developmental period.
No standardized guidelines currently outline the optimal timing for initiating antihypertensive therapy or managing mild cases in neonates. Since many affected infants require only short-term pharmacologic intervention and improve spontaneously over time, careful clinical judgment must guide treatment. Decisions should be individualized, based on the infant’s overall health status, the presence of end-organ involvement, and contributing risk factors. This cautious, case-by-case approach helps balance the benefits of treatment with the potential risks associated with unnecessary medication exposure in this vulnerable population.
Enhancing Healthcare Team Outcomes
Effective management of neonatal hypertension requires a coordinated, interprofessional approach in which physicians, advanced practitioners, nurses, pharmacists, and other health professionals share distinct yet interrelated responsibilities. Neonatologists and pediatric nephrologists play a crucial role in establishing accurate diagnoses by utilizing gestational and postnatal age-specific blood pressure percentiles and ensuring the proper interpretation of both invasive and non-invasive monitoring data. Advanced practitioners contribute by conducting thorough assessments, recognizing early signs of hypertension, and initiating appropriate evaluations, while ensuring consistent monitoring and documentation of trends. Nurses play a central role in the implementation of bedside care, including meticulous blood pressure measurement techniques, monitoring for clinical signs of hypertensive complications, and recognizing potential adverse effects of medications. Pharmacists ensure safe and effective medication management by adjusting dosages based on neonatal pharmacokinetics and guiding the selection of antihypertensive agents, particularly in complex cases involving renal or cardiovascular comorbidities.
Interprofessional communication and structured care coordination are essential for improving outcomes and patient safety. Regular interprofessional rounds facilitate collaborative decision-making, enable the timely identification of risk factors, and clearly define roles in both acute management and long-term planning. Clinical teams must coordinate diagnostic workups, integrate maternal and perinatal history, and ensure seamless transition of care from inpatient to outpatient follow-up. Education of families, supported by nurses and social workers, enhances understanding of the condition and reinforces adherence to monitoring and treatment plans. By promoting open communication, shared decision-making, and standardized care protocols, healthcare teams can reduce missed diagnoses, avoid inappropriate treatment delays, and improve both short- and long-term outcomes for neonates with hypertension.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Adelman RD. Neonatal hypertension. Pediatric clinics of North America. 1978 Feb:25(1):99-110 [PubMed PMID: 628572]
Seliem WA, Falk MC, Shadbolt B, Kent AL. Antenatal and postnatal risk factors for neonatal hypertension and infant follow-up. Pediatric nephrology (Berlin, Germany). 2007 Dec:22(12):2081-7 [PubMed PMID: 17874136]
Level 2 (mid-level) evidenceDionne JM, Abitbol CL, Flynn JT. Hypertension in infancy: diagnosis, management and outcome. Pediatric nephrology (Berlin, Germany). 2012 Jan:27(1):17-32. doi: 10.1007/s00467-010-1755-z. Epub 2011 Jan 22 [PubMed PMID: 21258818]
Flynn JT. Hypertension in the neonatal period. Current opinion in pediatrics. 2012 Apr:24(2):197-204. doi: 10.1097/MOP.0b013e32834f8329. Epub [PubMed PMID: 22426156]
Level 3 (low-level) evidenceO'Shea J, Dempsey EM. A comparison of blood pressure measurements in newborns. American journal of perinatology. 2009 Feb:26(2):113-6. doi: 10.1055/s-0028-1091391. Epub 2008 Nov 19 [PubMed PMID: 19021094]
Dionne JM, Bremner SA, Baygani SK, Batton B, Ergenekon E, Bhatt-Mehta V, Dempsey E, Kluckow M, Pesco Koplowitz L, Apele-Freimane D, Iwami H, Klein A, Turner M, Rabe H, International Neonatal Consortium. Method of Blood Pressure Measurement in Neonates and Infants: A Systematic Review and Analysis. The Journal of pediatrics. 2020 Jun:221():23-31.e5. doi: 10.1016/j.jpeds.2020.02.072. Epub [PubMed PMID: 32446487]
Level 1 (high-level) evidenceFlynn JT, Kaelber DC, Baker-Smith CM, Blowey D, Carroll AE, Daniels SR, de Ferranti SD, Dionne JM, Falkner B, Flinn SK, Gidding SS, Goodwin C, Leu MG, Powers ME, Rea C, Samuels J, Simasek M, Thaker VV, Urbina EM, SUBCOMMITTEE ON SCREENING AND MANAGEMENT OF HIGH BLOOD PRESSURE IN CHILDREN. Clinical Practice Guideline for Screening and Management of High Blood Pressure in Children and Adolescents. Pediatrics. 2017 Sep:140(3):. pii: e20171904. doi: 10.1542/peds.2017-1904. Epub 2017 Aug 21 [PubMed PMID: 28827377]
Level 1 (high-level) evidencede Swiet M, Dillon MJ, Littler W, O'Brien E, Padfield PL, Petrie JC. Measurement of blood pressure in children. Recommendations of a working party of the British Hypertension Society. BMJ (Clinical research ed.). 1989 Aug 19:299(6697):497 [PubMed PMID: 2507035]
Nascimento MC, Xavier CC, Goulart EM. Arterial blood pressure of term newborns during the first week of life. Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologica. 2002 Aug:35(8):905-11 [PubMed PMID: 12185382]
Sharma D, Farahbakhsh N, Shastri S, Sharma P. Neonatal hypertension. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstetricians. 2017 Mar:30(5):540-550. doi: 10.1080/14767058.2016.1177816. Epub 2016 May 5 [PubMed PMID: 27072362]
Sahu R, Pannu H, Yu R, Shete S, Bricker JT, Gupta-Malhotra M. Systemic hypertension requiring treatment in the neonatal intensive care unit. The Journal of pediatrics. 2013 Jul:163(1):84-8. doi: 10.1016/j.jpeds.2012.12.074. Epub 2013 Feb 7 [PubMed PMID: 23394775]
Level 2 (mid-level) evidenceAlagappan A, Malloy MH. Systemic hypertension in very low-birth weight infants with bronchopulmonary dysplasia: incidence and risk factors. American journal of perinatology. 1998 Jan:15(1):3-8 [PubMed PMID: 9475679]
Level 2 (mid-level) evidenceSharma D, Pandita A, Shastri S. Neonatal hypertension: an underdiagnosed condition, a review article. Current hypertension reviews. 2014:10(4):205-12 [PubMed PMID: 25694190]
Giri P, Roth P. Neonatal Hypertension. Pediatrics in review. 2020 Jun:41(6):307-311. doi: 10.1542/pir.2019-0159. Epub [PubMed PMID: 32482697]
Jenkins RD. Phthalates cause a low-renin phenotype commonly found in premature infants with idiopathic neonatal hypertension. Pediatric nephrology (Berlin, Germany). 2023 Jun:38(6):1717-1724. doi: 10.1007/s00467-022-05773-1. Epub 2022 Nov 2 [PubMed PMID: 36322257]
Jenkins R, Farnbach K, Iragorri S. Elimination of Intravenous Di-2-Ethylhexyl Phthalate Exposure Abrogates Most Neonatal Hypertension in Premature Infants with Bronchopulmonary Dysplasia. Toxics. 2021 Apr 2:9(4):. doi: 10.3390/toxics9040075. Epub 2021 Apr 2 [PubMed PMID: 33918157]
AlMaazmi A, Hagan J, Fernandes CJ, Gowda SH. Neonatal systemic hypertension across the PHIS database: An update. International journal of cardiology. 2023 Apr 1:376():49-53. doi: 10.1016/j.ijcard.2023.01.060. Epub 2023 Jan 20 [PubMed PMID: 36682689]
Kilian K. Hypertension in neonates causes and treatments. The Journal of perinatal & neonatal nursing. 2003 Jan-Mar:17(1):65-74; quiz 75-6 [PubMed PMID: 12661740]
Sehgal A, Malikiwi A, Paul E, Tan K, Menahem S. Systemic arterial stiffness in infants with bronchopulmonary dysplasia: potential cause of systemic hypertension. Journal of perinatology : official journal of the California Perinatal Association. 2016 Jul:36(7):564-9. doi: 10.1038/jp.2016.10. Epub 2016 Feb 25 [PubMed PMID: 26914016]
Skalina ME, Kliegman RM, Fanaroff AA. Epidemiology and management of severe symptomatic neonatal hypertension. American journal of perinatology. 1986 Jul:3(3):235-9 [PubMed PMID: 3718646]
Gantenbein MH, Bauersfeld U, Baenziger O, Frey B, Neuhaus T, Sennhauser F, Bernet V. Side effects of angiotensin converting enzyme inhibitor (captopril) in newborns and young infants. Journal of perinatal medicine. 2008:36(5):448-52. doi: 10.1515/JPM.2008.064. Epub [PubMed PMID: 18605972]
Stanley JC, Zelenock GB, Messina LM, Wakefield TW. Pediatric renovascular hypertension: a thirty-year experience of operative treatment. Journal of vascular surgery. 1995 Feb:21(2):212-26; discussion 226-7 [PubMed PMID: 7853595]
Xiao N, Starr M, Stolfi A, Hamdani G, Hashmat S, Kiessling SG, Sethna C, Kallash M, Matloff R, Woroniecki R, Sanderson K, Yamaguchi I, Cha SD, Semanik MG, Chanchlani R, Flynn JT, Mitsnefes M. Blood Pressure Outcomes in NICU-Admitted Infants with Neonatal Hypertension: A Pediatric Nephrology Research Consortium Study. The Journal of pediatrics. 2024 Jan:264():113765. doi: 10.1016/j.jpeds.2023.113765. Epub 2023 Sep 29 [PubMed PMID: 37778410]