Meningococcal Disease (Neisseria meningitidis Infection)
Meningococcal Disease (Neisseria meningitidis Infection)
Introduction
Neisseria meningitidis was first discovered by Anton Weichselbaum in 1887 during his analysis of the cerebrospinal fluid from a patient infected with meningitis.[1] N meningitidis is a human-specific bacterium that causes various illnesses, collectively termed meningococcal disease. Up to 10% of the general population carries the bacteria in their nose and throat without experiencing clinical symptoms. Risk factors for meningococcal carriage include adolescence and young adulthood, male sex, living in a communal setting, exposure to cigarette smoke, and frequent attendance at social events.[2][3] Transmission occurs through respiratory droplets and requires sustained close contact with the infected person or direct contact with the throat or nasal secretions.[4]
N meningitidis is associated with many infections. However, its most significant impact is associated with fulminant meningococcemia and meningococcal meningitis. More importantly, despite recent vaccine campaigns to help reduce complications of this disease, it continues to impact at-risk populations.[5] This activity offers a comprehensive clinical overview of meningococcal disease caused by N meningitidis. Please see StatPearls' companion resource, "Meningococcemia," for further information.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
N meningitidis is an aerobic or facultative anaerobic, gram-negative diplococcus that exclusively infects humans.[6] There are at least 12 serotypes based on unique capsular polysaccharides of N meningitidis, with serotypes A, B, C, W, X, and Y responsible for most meningococcal infections.[7][8] Serotypes A and C are Africa's main serotypes responsible for meningococcal disease. Serotypes B, C, and Y are the main serotypes causing disease in Europe, the United States (US), and Canada.[9] Serotype W is responsible for epidemic outbreaks worldwide and is associated with the Hajj pilgrimage to Saudi Arabia.[10]
N meningitidis is a common and significant cause of community-acquired bacterial meningitis in the US, affecting children and adults.[11] This infection can be devastating and has a high mortality rate; it is the second most common cause of community-acquired bacterial meningitis in adults after Streptococcus pneumoniae.[12] Other infections that N meningitidis can cause include meningococcemia in the bloodstream, pneumonia, septic arthritis, pericarditis, and urethritis. In addition, N meningitidis can also cause endemic and epidemic infections that affect young, healthy adults.[9]
Epidemiology
In the US, meningococcal disease is highest between November and March and has decreased with the routine administration of meningococcal vaccination.[13][14] The incidence has decreased from 1.2 cases per 100,000 individuals in 1996 to 0.1 cases per 100,000 individuals in 2018.[9] However, the US Centers for Disease Control and Prevention reported an increase in meningococcal disease in 2022 among individuals living with human immunodeficiency virus, which has historically had low meningococcal vaccine coverage rates.[15]
The highest incidence of meningococcal disease appears in infants younger than 1 year of age, with 2.45 cases reported per 100,000 individuals.[16] N meningitidis can cause endemic and epidemic outbreaks and is a significant cause of bacterial meningitis in sub-Saharan Africa, a region often referred to as the meningitis belt.[9] The mortality rate varies from approximately 10% to 14% among patients who receive treatment and can reach up to 50% among patients who do not receive treatment.[17]
Pathophysiology
Humans are the exclusive host of N meningitidis, carrying the pathogen in the nasopharynx before the onset of systemic infection, although colonized humans may remain asymptomatic carriers.[2][18] Nasopharyngeal colonization increases the risk of transmission to individuals in close contact with infected patients, such as family members, college roommates, and military recruits, placing them at higher risk for acquiring the infection.[19] Individuals involved in laboratory or clinical care are also at a heightened risk for infection, particularly when exposed to aerosolized particles during procedures such as intubation without appropriate protective equipment, including masks or face shields. The incubation period varies in length and ranges from 1 to 14 days.[20]
Following colonization, several virulence factors allow the bacterium to invade human cells and evade the immune system. The polysaccharide capsule enhances invasiveness by inhibiting phagocytosis and promoting the organism's survival during the invasion of the bloodstream and central nervous system. Pili mediate attachment to mucosal cells and colonization and invasion of the nasopharynx. The antigenic variation of pili by a cassette mechanism allows the bacterium to escape the host's immune system.[21] Lipooligosaccharide acts as an endotoxin and activates the proinflammatory cytokine pathway of the host to cause meningococcal sepsis.[22] Lipooligosaccharide interacts with host immune cells to initiate the release of inflammatory mediators such as tumor necrosis factor-alpha, interleukin-1 (IL-1), IL-6, and interferon-gamma to cause shock.[23] Once in the bloodstream, N meningitidis induces a robust immune response, leading to endothelial damage, capillary leakage, tissue necrosis, organ failure, and ultimately meningococcal sepsis. Bloodstream invasion is considered the primary route to the brain, but N meningitidis can also cross the cribriform plate of the ethmoid bone to invade the brain.[24]
Like many other diseases, the immune system protects the host against meningococcal infection. Certain health conditions that compromise the human immune system increase the risk of contracting meningococcal diseases, such as meningococcemia and meningitis. These conditions include human immunodeficiency virus infection, acquired immune deficiency syndrome, asplenia, and complement deficiencies.[6]
History and Physical
The clinical presentation of N meningitidis can vary widely and may initially appear asymptomatic, making it difficult to diagnose, especially in areas with no reported epidemics. Typically, the initial presentation of meningococcal meningitis can include the sudden onset of fever, headache, nausea, vomiting, severe myalgias, nonspecific rash, sore throat, and other upper respiratory symptoms. These symptoms may be confused with a variety of other illnesses. The potential for rapid disease progression, particularly with the possibility of death within hours of symptom onset, makes the early identification of meningococcal disease challenging due to its low incidence and nonspecific early symptomatology. As the disease progresses, more specific signs may develop, including neck stiffness, photophobia, petechiae or hemorrhagic rash, altered mental status, shock, abnormal skin color, purpura fulminans, or even disseminated intravascular coagulation.[20]
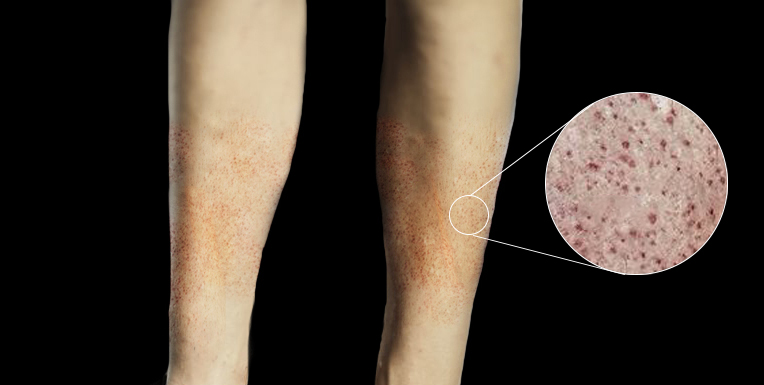
Vital sign abnormalities may include hypotension and tachycardia, which can indicate early signs of sepsis. A comprehensive physical examination should be performed, including a thorough skin inspection for any signs of a rash. The rash is a distinguishing feature and may initially appear as small lesions that are urticarial, macular, or papular in nature. The rash can develop into petechiae, purpura, or ecchymosis (see Image. Petechiae). These findings may be early signs of thrombocytopenia, purpura fulminans, and disseminated intravascular coagulation.[20]
Meningeal irritability can be confirmed by provocative tests such as the Kernig and Brudzinski signs. The Kernig sign is considered positive if the patient experiences muscular resistance or pain when the knee is extended beyond 135° while in the supine position with the hip flexed.[25] Similarly, the Brudzinski sign is positive when the supine individual shows reflex flexion of the hips and knees after passive neck flexion.[25] Although these signs are classic signs of meningitis, their respective sensitivities of 55.5% and 53.3% make them unreliable when excluding meningitis.[26] A thorough neurologic examination should be performed, focusing on alterations in mental status and focal neurological deficits.
When considering signs and symptoms of the disease, it is essential to note that the classic triad for meningitis of fever, altered mental status, and nuchal rigidity is rare, occurring in only about 44% of cases. However, when 2 of the 4 symptoms that include headache, altered mental status, neck stiffness, and fever were present, 95% of patients were diagnosed ultimately with meningitis.[27] Clinicians should consider N meningitidis infection in patients with a sudden onset of fever who display early signs of sepsis, a rapidly progressing disease, and rash.
Purpura fulminans, a meningococcal disease complication from vascular collapse, is characterized by cutaneous hemorrhage and skin necrosis due to vascular thrombosis and disseminated intravascular coagulation. Typically, petechiae and erythema are observed involving the skin but can progress to ecchymosis and eventually painful areas of necrosis with concurrent bullae and vesicles. Gangrenous necrosis can occur and be severe, potentially resulting in limb amputation. Atypical skin color, cold hands and feet, and painful legs may represent signs of vascular compromise.[20]
Meningococcal pneumonia occurs in 5% to 15% of individuals with meningococcal disease and typically presents with symptoms similar to a community-acquired pneumonia, such as fever, chills, and pleuritic chest pain. Less than 1 in 3 patients with meningococcal pneumonia present with a productive cough, and less than 1 in 4 will experience shortness of breath. Although some patients may have a rash, meningococcal pneumonia rarely coexists with meningococcemia.[28]
Since the mid-2010s, an increase in cases of meningococcal urethritis has been reported, predominantly in young heterosexual men with purulent urethral discharge and dysuria.[29][30][31] A sentinel surveillance site for gonococcal disease surveillance reported that 20% of men with urethritis and gram-negative intracellular diplococci were ultimately diagnosed with meningococcal urethritis.[31] Therefore, meningococcal urethritis should be considered in the differential diagnosis of urethritis.
Meningococcal arthritis may occur as septic arthritis caused by N meningitidis infection of the joint fluid or as a secondary reactive arthritis related to joint immune complexes.[32] The prevalence of arthritis among persons with meningococcal disease is not yet well understood but has been reported to range from 2% to 12.5%.[32] Meningococcal arthritis may occur with or without meningitis.[33] Joint symptoms include pain, redness, swelling, and warmth.[32] The knee is the most commonly affected joint, followed by the wrist and ankle.[34] Meningococcal pericarditis is a rare condition that may present either as primary pericarditis with meningococcal infection limited to the pericardium or as late-stage reactive pericarditis that resembles post-viral pericarditis. Accurate identification of meningococcal pericarditis is critical because treatment and prognosis depend on the ultimate cause.[35]
Evaluation
A lumbar puncture is the preferred diagnostic method for identifying meningitis and should be a priority to facilitate prompt evaluation and diagnosis. Contraindications to performing a lumbar puncture include evidence of elevated intracranial pressure, such as altered mental status, new-onset seizure within 1 week of symptom development, papilledema, an immunocompromised state, or focal neurologic deficit, coagulopathy, and cardiorespiratory insufficiency. Antibiotic therapy should be initiated promptly, even before the lumbar puncture is performed, for patients where meningitis is highly suspected.[17]
Although parenteral antibiotic therapy can eliminate meningococci from cerebral spinal fluid (CSF) in less than 6 hours, blood cultures and CSF studies can still be useful even after antibiotic initiation.[36] CSF analysis should include Gram staining, culture, glucose level, cell count, and protein concentration. CSF findings that suggest bacterial meningitis include high opening pressure, decreased glucose levels (less than 45 mg/dL or less than 2.5 mmol/L), a polymorphonuclear pleocytosis (white blood count more than 1000/µL), increased protein levels (greater than 500 mg/dL), and a CSF-to-serum glucose ratio of less than 0.4 (see Table. Expected CSF Findings in Bacterial versus Viral versus Fungal Meningitis).[20]
Table. Expected CSF Findings in Bacterial, Viral, and Fungal Meningitis
| Type of Meningitis | Appearance | Opening Pressure (mm Hg) | White Blood Cells (cells/µL) | Protein (mg/dL) | Glucose (mg/dL) |
| Normal | Clear | 90-180 | <8 | 15–45 | 50–80 |
| Bacterial | Turbid | Elevated | >1000–2000 | >200 | <40 |
| Viral | Clear | Normal | <300; lymphocytic predominance | <200 | Normal |
| Fungal | Clear | Normal-elevated | <500 | >200 | Normal-low |
CSF Gram staining, polymerase chain reaction (PCR), and latex agglutination may aid diagnostic confirmation, particularly in patients with negative CSF cultures after antibiotic pretreatment. Gram staining is diagnostic in 85% of patients with meningococcal meningitis. PCR detects strands of bacterial deoxyribonucleic acid, not requiring an intact bacterium for detection, whereas latex agglutination detects capsular antigens.[37] CSF culture is the gold standard for the diagnosis of bacterial meningitis.[37]
Computed tomography (CT) imaging may assist in the diagnosis of patients with altered mental status, mainly to rule out other diagnoses under consideration.[17] If an intracranial mass is a consideration, CT should be considered before performing a lumbar puncture to potentially avoid a brain herniation syndrome. If imaging is necessary, it is crucial to coordinate with the nursing team and imaging suite to avoid delaying antibiotic administration.
Treatment / Management
Early recognition and treatment of meningococcal infections are critical for improving clinical outcomes. The primary goals in treating meningococcal infection include administering antibiotics, implementing isolation and droplet precautions, consulting with infectious disease specialists, management in the intensive care unit, treatment of coagulopathies, and identifying individuals who are at risk who may have been exposed to N meningitidis.[17] Because meningococcal meningitis can present like other bacterial meningitides, empiric treatment should be initiated while awaiting culture results. This preemptive approach includes administering a third-generation cephalosporin such as ceftriaxone or cefotaxime and broad-spectrum antibiotics such as vancomycin while awaiting bacterial identification.[38]
If the culture confirms that the organism is penicillin-susceptible, treatment can be changed to penicillin G. However, continuing third-generation cephalosporin treatment is also an option. For patients who have significant allergies to penicillin and other beta-lactams, chloramphenicol may be used as an alternative. Chloramphenicol is rarely used in the US because of the potential for aplastic anemia and gray baby syndrome. The duration of antibiotic therapy is typically 5 to 6 days.[17][39][40] Droplet precautions should be maintained for at least 24 hours after initiating effective antibiotic therapy.(A1)
Dosages
- Ceftriaxone dosing is 2 g for adults and 50 mg/kg for pediatric patients older than 1 month, administered intravenously (IV) every 12 hours. In contrast, cefotaxime dosing is 2 g every 4 to 6 hours for adults and 50 mg/kg for pediatric patients older than 1 month, given every 6 hours.[17]
- Third-generation cephalosporins are generally preferable due to their high efficacy and simpler dosing regimens.
- Penicillin G dosing is 4 million units every 4 hours IV for adults and pediatric patients older than 1 month.[17]
- Chloramphenicol dosing is 50 mg/kg IV 4 times daily.[17]
- Serum concentrations require monitoring because of the risk of chloramphenicol toxicity.[41]
- Persistently elevated serum concentrations of chloramphenicol above 50 mg/L have been associated with gray baby syndrome, consisting of ashen-gray skin discoloration, abdominal distention, respiratory distress, and hemodynamic collapse. Although neonates are at the highest risk, gray syndrome can occur in any age group if hepatic or renal function is impaired or excessive doses are administered.[42]
- In the US, chloramphenicol is classed as pregnancy category C, and its use should be avoided during breastfeeding.[43]
- High-dose dexamethasone should be administered at the initial suspicion of bacterial meningitis due to its protective benefits in pneumococcal meningitis.[17][44][45] Dexamethasone should be administered IV within 4 hours before but no more than 12 hours after the first dose of IV antibiotics. The dosage should be 0.15 mg/kg every 6 hours up to 10 mg every 6 hours.[17][39] However, dexamethasone has no therapeutic benefit in meningococcal meningitis and should be discontinued once this diagnosis is established.[39][44] (B3)
Patients with meningococcal infection require prompt and aggressive supportive care, particularly in cases of sepsis or septic shock. Management may include intravenous fluid resuscitation and the use of vasopressors such as norepinephrine.[39] Early intubation should be considered in patients with evidence of airway compromise, ongoing shock, intractable seizures, or elevated intracranial pressure.[46] Patients showing signs of disseminated intravascular coagulation may require aggressive hydration, blood transfusions, platelet replacement, and possibly coagulation factor replacement. Although researchers have proposed protein C as an adjuvant treatment, its use remains controversial and is not widely practiced.[17]
Differential Diagnosis
Streptococcus pneumoniae is the most common cause of bacterial meningitis in the US and should be included in the differential diagnosis for patients suspected of having meningococcal meningitis. Less common causes of bacterial meningitis include Haemophilus influenzae, group B Streptococcus, and Listeria monocytogenes.[37] Other potential causes of meningitis include viral, tuberculous, eosinophilic, fungal meningitis, and noninfectious causes such as malignancy.[47]
Clinicians examining adults with a maculopapular rash and fever should consider alternate etiologies, including infectious mononucleosis, West Nile virus, Zika virus, human immunodeficiency virus, Ebola virus, Rocky Mountain spotted fever, ehrlichiosis, and hypersensitivity drug reactions.[48] Children with a maculopapular rash and fever should be considered for a differential diagnosis that includes Kawasaki disease, measles, scarlet fever, rubella, parvovirus B19, roseola, Epstein-Barr virus, and hand, foot, and mouth disease.[49]
Prognosis
Although the mortality rate of meningococcal infection can be as high as 50% in untreated individuals, early and aggressive treatment can reduce the mortality rate to approximately 10% to 14%. Early administration of antibiotics is crucial for obtaining a good clinical outcome in meningococcal infection. Even with treatment, long-term complications can still occur in 11% to 19% of survivors.[17] Poor prognostic factors in patients with meningococcal meningitis include shock, presence of focal neurological deficits, mental obtundation or coma, purpuric or ecchymotic rash, absence of meningeal signs, low or normal blood leukocyte count, age older than 60 years, presence of anemia, thrombocytopenia, low erythrocyte sedimentation rate, low C-reactive protein level, low blood concentrations of antithrombin or proteins S and C, high blood levels of plasminogen activator inhibitor 1, malignancy, intracranial bleed, and cerebrovascular accident.[27]
Complications
Common complications of meningococcal disease include chronic pain, skin scarring, limb amputation, and neurological impairments, ranging from hearing loss and visual impairments to impaired motor function. Urgent complications of meningococcal disease include septic shock, purpura fulminans, seizures, hydrocephalus, cerebral venous sinus thrombosis, acute adrenal insufficiency due to adrenal hemorrhage (Waterhouse-Friderichsen syndrome), and subdural empyema.[39] Hearing impairment and extremity amputations occur in approximately 3% of cases, arthritis in 10% of cases, and post-infection inflammatory syndrome in 6% to 15% of cases.[17] Up to one-third of survivors of meningococcal disease experience psychological disorders, such as posttraumatic stress disorder, anxiety, and depression, that require follow-up care with psychologists and psychiatrists.[17] As with any critical illness, other complications may include prolonged ventilator support, tracheostomy care, feeding tube care, prolonged physical and occupational rehabilitation, critical illness polyneuropathy and myopathy, and secondary infections and skin ulceration.
Consultations
The interprofessional team managing meningococcal infection includes internists, pediatricians, infectious disease specialists, hematologists, neurologists, and nursing staff. Hearing tests are recommended within 4 weeks of hospital discharge. Orthopedic follow-up and prosthetic fitting are necessary for patients who have undergone limb amputations.[39]
Deterrence and Patient Education
Timely recognition and treatment are essential because meningococcal infection is a severe disease that has caused epidemics and has the potential for recurrence. All cases of meningococcal infection are reportable, and local health departments must be notified.[9] Vaccination is the most effective strategy for preventing meningococcal disease, particularly among high-risk populations. See Table. Routine Meningococcal Vaccine Recommendations for Healthy Adolescents and Young Adults Aged 11 through 23 in the US. The infectious disease nurse should educate patients regarding vaccination. Vaccines against N meningitidis serogroups A, C, W, Y, and B are routinely recommended in the US for adolescents, young adults, and individuals at increased risk for meningococcal disease. The overall burden of meningococcal infection worldwide has diminished due to these vaccinations.[9] Individuals at risk, including college students, military recruits, immunocompromised patients such as those with HIV/AIDS, complement deficiency, and those with asplenia, should be vaccinated. Individuals who work with N meningitidis in laboratories, travelers to endemic areas such as the meningitis belt, and those exposed to meningococcal disease outbreaks should also be vaccinated based on local epidemiology.[9]
Table. Routine Meningococcal Vaccine Recommendations for Healthy Adolescents and Young Adults Aged 11 through 23 in the US
| Meningococcal Vaccine | Primary Dosage(s) | Booster Dosage(s) |
| MenACWY (for ages 11–21 years)* |
|
|
| MenB (for ages 16–23 years) |
Preferred age: 16–18 years
|
|
*Although the Advisory Committee on Immunization Practices recommends routine MenACWY vaccination only for individuals aged 11 through 18, it may be administered to individuals aged 19 through 21 if they have not been vaccinated.
In addition, the MenACWY vaccine is mandatory for all individuals visiting Mecca for the Hajj or Umrah.[9] Please see StatPearls' companion resource, "Meningococcal Vaccine," for further information.
Pearls and Other Issues
Individuals who have close contact with patients infected with meningococcal disease may become infected themselves and develop symptoms within 14 days of exposure. Close contact refers to the proximity to the patient for more than 4 hours within the past 7 days. Examples of close contacts include family members, roommates, military recruits, and individuals in daycare centers.[20] Individuals exposed to infected patients' oral secretions (through kissing), respiratory secretions (during intubation or endotracheal suctioning), or who had prolonged proximity to an infected person on a long flight are also at risk. Antimicrobial chemoprophylaxis of exposed contacts should be initiated as soon as possible. Due to its high efficacy, chemoprophylaxis should be strongly considered for patients who may have had questionable close contact with an infected patient.
Several antimicrobial chemoprophylaxis options are listed below.
- Ceftriaxone is administered at 250 mg intramuscularly as a one-time dose for adults and 125 mg for pediatric patients younger than 15.
- For adults, ifampin is prescribed at a dosage of 600 mg orally or intravenously twice daily for 2 days. The recommended dose for pediatric patients younger than 1 month is 5 mg/kg, whereas for those older than 1 month, it is 10 mg/kg. Rifampin is not recommended for pregnant patients.
- Ciprofloxacin is given orally at 500 mg as a one-time dose for adults, and for pediatric patients older than 1 month, the dose is 20 mg/kg (maximum 500 mg).
- Azithromycin, typically reserved as a last choice, is given orally at 500 mg as a one-time dose in adults and 10 mg/kg (maximum 500 mg) in pediatric patients older than 1 month.[17]
Enhancing Healthcare Team Outcomes
Meningococcal infection is a highly contagious and fatal disease that can progress rapidly. Early recognition is one of the most challenging aspects of diagnosis and management, requiring a coordinated interprofessional approach. The interprofessional team should include clinicians, infectious disease specialists, intensive care unit nurses, and pharmacists, all adhering to proper isolation protocols and collaborating with the local health department. Meningococcal disease is reportable, and local health departments can assist in identifying exposed close contacts and other individuals at risk and manage outbreaks.[9]
Clinicians initiating treatment benefit from the expertise of an infectious disease board-certified pharmacist, who can verify antimicrobial selection based on the latest antibiogram data, confirm appropriate dosing, and identify potential drug interactions that may affect therapy. Nurses typically administer these agents and should comprehensively understand appropriate isolation protocols. They are also responsible for monitoring therapeutic effectiveness, identifying adverse reactions, and promptly notifying the healthcare team of any issues. The interprofessional team approach is the best way to optimize therapeutic results while minimizing the risk of infection transmission and adverse reactions.
Media
(Click Image to Enlarge)
References
Rouphael NG, Stephens DS. Neisseria meningitidis: biology, microbiology, and epidemiology. Methods in molecular biology (Clifton, N.J.). 2012:799():1-20. doi: 10.1007/978-1-61779-346-2_1. Epub [PubMed PMID: 21993636]
Level 3 (low-level) evidenceSantos-Neto JF, Ferreira VM, Feitosa CA, Martinez-Silveira MS, Campos LC. Carriage prevalence of Neisseria meningitidis in the Americas in the 21st century: a systematic review. The Brazilian journal of infectious diseases : an official publication of the Brazilian Society of Infectious Diseases. 2019 Jul-Aug:23(4):254-267. doi: 10.1016/j.bjid.2019.06.006. Epub 2019 Jul 22 [PubMed PMID: 31344352]
Level 1 (high-level) evidenceSpyromitrou-Xioufi P, Tsirigotaki M, Ladomenou F. Risk factors for meningococcal disease in children and adolescents: a systematic review and META-analysis. European journal of pediatrics. 2020 Jul:179(7):1017-1027. doi: 10.1007/s00431-020-03658-9. Epub 2020 May 13 [PubMed PMID: 32405695]
Level 1 (high-level) evidenceMorello BR, Milazzo A, Marshall HS, Giles LC. Public health management of invasive meningococcal disease outbreaks: worldwide 1973-2018, a systematic review. BMC public health. 2024 Aug 20:24(1):2254. doi: 10.1186/s12889-024-19740-y. Epub 2024 Aug 20 [PubMed PMID: 39164680]
Level 1 (high-level) evidenceRead RC. Neisseria meningitidis and meningococcal disease: recent discoveries and innovations. Current opinion in infectious diseases. 2019 Dec:32(6):601-608. doi: 10.1097/QCO.0000000000000606. Epub [PubMed PMID: 31567569]
Level 3 (low-level) evidenceTakada S, Fujiwara S, Inoue T, Kataoka Y, Hadano Y, Matsumoto K, Morino K, Shimizu T. Meningococcemia in Adults: A Review of the Literature. Internal medicine (Tokyo, Japan). 2016:55(6):567-72. doi: 10.2169/internalmedicine.55.3272. Epub 2016 Mar 15 [PubMed PMID: 26984070]
Pardo de Santayana C, Tin Tin Htar M, Findlow J, Balmer P. Epidemiology of invasive meningococcal disease worldwide from 2010-2019: a literature review. Epidemiology and infection. 2023 Mar 6:151():e57. doi: 10.1017/S0950268823000328. Epub 2023 Mar 6 [PubMed PMID: 37052295]
Chhabria D, Anjankar A. An Overview of Meningococcal Disease's Recent Diagnostic and Treatment Model. Cureus. 2023 Nov:15(11):e48509. doi: 10.7759/cureus.48509. Epub 2023 Nov 8 [PubMed PMID: 38073961]
Level 3 (low-level) evidenceMbaeyi SA, Bozio CH, Duffy J, Rubin LG, Hariri S, Stephens DS, MacNeil JR. Meningococcal Vaccination: Recommendations of the Advisory Committee on Immunization Practices, United States, 2020. MMWR. Recommendations and reports : Morbidity and mortality weekly report. Recommendations and reports. 2020 Sep 25:69(9):1-41. doi: 10.15585/mmwr.rr6909a1. Epub 2020 Sep 25 [PubMed PMID: 33417592]
Badur S, Khalaf M, Öztürk S, Al-Raddadi R, Amir A, Farahat F, Shibl A. Meningococcal Disease and Immunization Activities in Hajj and Umrah Pilgrimage: a review. Infectious diseases and therapy. 2022 Aug:11(4):1343-1369. doi: 10.1007/s40121-022-00620-0. Epub 2022 May 19 [PubMed PMID: 35585384]
. Meningitis (bacterial) and meningococcal disease: recognition, diagnosis and management. 2024 Mar 19:(): [PubMed PMID: 38843370]
Hasbun R. Progress and Challenges in Bacterial Meningitis: A Review. JAMA. 2022 Dec 6:328(21):2147-2154. doi: 10.1001/jama.2022.20521. Epub [PubMed PMID: 36472590]
Sharip A, Sorvillo F, Redelings MD, Mascola L, Wise M, Nguyen DM. Population-based analysis of meningococcal disease mortality in the United States: 1990-2002. The Pediatric infectious disease journal. 2006 Mar:25(3):191-4 [PubMed PMID: 16511378]
Parikh SR, Campbell H, Bettinger JA, Harrison LH, Marshall HS, Martinon-Torres F, Safadi MA, Shao Z, Zhu B, von Gottberg A, Borrow R, Ramsay ME, Ladhani SN. The everchanging epidemiology of meningococcal disease worldwide and the potential for prevention through vaccination. The Journal of infection. 2020 Oct:81(4):483-498. doi: 10.1016/j.jinf.2020.05.079. Epub 2020 Jun 3 [PubMed PMID: 32504737]
Rubis AB, Howie RL, Marasini D, Sharma S, Marjuki H, McNamara LA. Notes from the Field: Increase in Meningococcal Disease Among Persons with HIV - United States, 2022. MMWR. Morbidity and mortality weekly report. 2023 Jun 16:72(24):663-664. doi: 10.15585/mmwr.mm7224a4. Epub 2023 Jun 16 [PubMed PMID: 37319021]
MacNeil JR, Blain AE, Wang X, Cohn AC. Current Epidemiology and Trends in Meningococcal Disease-United States, 1996-2015. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2018 Apr 3:66(8):1276-1281. doi: 10.1093/cid/cix993. Epub [PubMed PMID: 29126310]
Nadel S. Treatment of Meningococcal Disease. The Journal of adolescent health : official publication of the Society for Adolescent Medicine. 2016 Aug:59(2 Suppl):S21-8. doi: 10.1016/j.jadohealth.2016.04.013. Epub [PubMed PMID: 27449146]
McMillan M, Chandrakumar A, Wang HLR, Clarke M, Sullivan TR, Andrews RM, Ramsay M, Marshall HS. Effectiveness of Meningococcal Vaccines at Reducing Invasive Meningococcal Disease and Pharyngeal Neisseria meningitidis Carriage: A Systematic Review and Meta-analysis. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2021 Aug 2:73(3):e609-e619. doi: 10.1093/cid/ciaa1733. Epub [PubMed PMID: 33212510]
Level 1 (high-level) evidenceSchaffer DeRoo S, Torres RG, Fu LY. Meningococcal disease and vaccination in college students. Human vaccines & immunotherapeutics. 2021 Nov 2:17(11):4675-4688. doi: 10.1080/21645515.2021.1973881. Epub 2021 Oct 6 [PubMed PMID: 34613863]
Bosis S, Mayer A, Esposito S. Meningococcal disease in childhood: epidemiology, clinical features and prevention. Journal of preventive medicine and hygiene. 2015 Aug 31:56(3):E121-4 [PubMed PMID: 26788732]
Coureuil M, Jamet A, Bille E, Lécuyer H, Bourdoulous S, Nassif X. Molecular interactions between Neisseria meningitidis and its human host. Cellular microbiology. 2019 Nov:21(11):e13063. doi: 10.1111/cmi.13063. Epub 2019 Jun 13 [PubMed PMID: 31167044]
Lin LY, Rakic B, Chiu CP, Lameignere E, Wakarchuk WW, Withers SG, Strynadka NC. Structure and mechanism of the lipooligosaccharide sialyltransferase from Neisseria meningitidis. The Journal of biological chemistry. 2011 Oct 28:286(43):37237-48. doi: 10.1074/jbc.M111.249920. Epub 2011 Aug 31 [PubMed PMID: 21880735]
Johswich K. Innate immune recognition and inflammation in Neisseria meningitidis infection. Pathogens and disease. 2017 Mar 1:75(2):. doi: 10.1093/femspd/ftx022. Epub [PubMed PMID: 28334203]
Rausch-Phung EA, Siddiqui JA, Gulick PG. Meningococcemia. StatPearls. 2025 Jan:(): [PubMed PMID: 30521270]
Verghese A, Gallemore G. Kernig's and Brudzinski's signs revisited. Reviews of infectious diseases. 1987 Nov-Dec:9(6):1187-92 [PubMed PMID: 3321367]
Ala A, Rahmani F, Abdollahi S, Parsian Z. Accuracy of Neck stiffness, Kernig, Brudzinski, and Jolt Accentuation of Headache Signs in Early Detection of Meningitis. Emergency (Tehran, Iran). 2018:6(1):e8 [PubMed PMID: 29503833]
van de Beek D, de Gans J, Spanjaard L, Weisfelt M, Reitsma JB, Vermeulen M. Clinical features and prognostic factors in adults with bacterial meningitis. The New England journal of medicine. 2004 Oct 28:351(18):1849-59 [PubMed PMID: 15509818]
Vossen M, Mitteregger D, Steininger C. Meningococcal pneumonia. Vaccine. 2016 Aug 17:34(37):4364-70. doi: 10.1016/j.vaccine.2016.07.013. Epub 2016 Jul 18 [PubMed PMID: 27443594]
Toh E, Gangaiah D, Batteiger BE, Williams JA, Arno JN, Tai A, Batteiger TA, Nelson DE. Neisseria meningitidis ST11 Complex Isolates Associated with Nongonococcal Urethritis, Indiana, USA, 2015-2016. Emerging infectious diseases. 2017 Feb:23(2):336-339. doi: 10.3201/eid2302.161434. Epub [PubMed PMID: 28098538]
Bazan JA, Peterson AS, Kirkcaldy RD, Briere EC, Maierhofer C, Turner AN, Licon DB, Parker N, Dennison A, Ervin M, Johnson L, Weberman B, Hackert P, Wang X, Kretz CB, Abrams AJ, Trees DL, Del Rio C, Stephens DS, Tzeng YL, DiOrio M, Roberts MW. Notes from the Field: Increase in Neisseria meningitidis-Associated Urethritis Among Men at Two Sentinel Clinics - Columbus, Ohio, and Oakland County, Michigan, 2015. MMWR. Morbidity and mortality weekly report. 2016 Jun 3:65(21):550-2. doi: 10.15585/mmwr.mm6521a5. Epub 2016 Jun 3 [PubMed PMID: 27254649]
Bazan JA, Turner AN, Kirkcaldy RD, Retchless AC, Kretz CB, Briere E, Tzeng YL, Stephens DS, Maierhofer C, Del Rio C, Abrams AJ, Trees DL, Ervin M, Licon DB, Fields KS, Roberts MW, Dennison A, Wang X. Large Cluster of Neisseria meningitidis Urethritis in Columbus, Ohio, 2015. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2017 Jul 1:65(1):92-99. doi: 10.1093/cid/cix215. Epub [PubMed PMID: 28481980]
Masson-Behar V, Jacquier H, Richette P, Ziza JM, Zeller V, Rioux C, Coustet B, Dieudé P, Ottaviani S. Arthritis secondary to meningococcal disease: A case series of 7 patients. Medicine. 2017 Jul:96(29):e7573. doi: 10.1097/MD.0000000000007573. Epub [PubMed PMID: 28723791]
Level 2 (mid-level) evidenceCabellos C, Nolla JM, Verdaguer R, Pelegrin I, Ribera A, Ariza J, Viladrich PF. Arthritis related to systemic meningococcal disease: 34 years' experience. European journal of clinical microbiology & infectious diseases : official publication of the European Society of Clinical Microbiology. 2012 Oct:31(10):2661-6 [PubMed PMID: 22476361]
Weisfelt M, van de Beek D, Spanjaard L, de Gans J. Arthritis in adults with community-acquired bacterial meningitis: a prospective cohort study. BMC infectious diseases. 2006 Mar 29:6():64 [PubMed PMID: 16571115]
Finkelstein Y, Adler Y, Nussinovitch M, Varsano I, Amir J. A new classification for pericarditis associated with meningococcal infection. European journal of pediatrics. 1997 Aug:156(8):585-8 [PubMed PMID: 9266185]
Crosswell JM, Nicholson WR, Lennon DR. Rapid sterilisation of cerebrospinal fluid in meningococcal meningitis: Implications for treatment duration. Journal of paediatrics and child health. 2006 Apr:42(4):170-3 [PubMed PMID: 16630316]
Brouwer MC, Tunkel AR, van de Beek D. Epidemiology, diagnosis, and antimicrobial treatment of acute bacterial meningitis. Clinical microbiology reviews. 2010 Jul:23(3):467-92. doi: 10.1128/CMR.00070-09. Epub [PubMed PMID: 20610819]
. Evidence review for antibiotics for meningococcal disease: Meningitis (bacterial) and meningococcal disease: recognition, diagnosis and management: Evidence review F. 2024 Mar:(): [PubMed PMID: 38829973]
McGill F, Heyderman RS, Michael BD, Defres S, Beeching NJ, Borrow R, Glennie L, Gaillemin O, Wyncoll D, Kaczmarski E, Nadel S, Thwaites G, Cohen J, Davies NW, Miller A, Rhodes A, Read RC, Solomon T. The UK joint specialist societies guideline on the diagnosis and management of acute meningitis and meningococcal sepsis in immunocompetent adults. The Journal of infection. 2016 Apr:72(4):405-38. doi: 10.1016/j.jinf.2016.01.007. Epub 2016 Feb 2 [PubMed PMID: 26845731]
Tunkel AR, Hartman BJ, Kaplan SL, Kaufman BA, Roos KL, Scheld WM, Whitley RJ. Practice guidelines for the management of bacterial meningitis. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2004 Nov 1:39(9):1267-84 [PubMed PMID: 15494903]
Level 1 (high-level) evidenceOong GC, Tadi P. Chloramphenicol. StatPearls. 2025 Jan:(): [PubMed PMID: 32310426]
Wiest DB, Cochran JB, Tecklenburg FW. Chloramphenicol toxicity revisited: a 12-year-old patient with a brain abscess. The journal of pediatric pharmacology and therapeutics : JPPT : the official journal of PPAG. 2012 Apr:17(2):182-8. doi: 10.5863/1551-6776-17.2.182. Epub [PubMed PMID: 23118672]
Level 3 (low-level) evidenceNahum GG, Uhl K, Kennedy DL. Antibiotic use in pregnancy and lactation: what is and is not known about teratogenic and toxic risks. Obstetrics and gynecology. 2006 May:107(5):1120-38 [PubMed PMID: 16648419]
Runde TJ, Anjum F, Hafner JW. Bacterial Meningitis. StatPearls. 2025 Jan:(): [PubMed PMID: 29261975]
. Evidence review for corticosteroids in meningococcal disease: Meningitis (bacterial) and meningococcal disease: recognition, diagnosis and management: Evidence review H. 2024 Mar:(): [PubMed PMID: 38838175]
Tacon CL, Flower O. Diagnosis and management of bacterial meningitis in the paediatric population: a review. Emergency medicine international. 2012:2012():320309. doi: 10.1155/2012/320309. Epub 2012 Sep 20 [PubMed PMID: 23050153]
Young N, Thomas M. Meningitis in adults: diagnosis and management. Internal medicine journal. 2018 Nov:48(11):1294-1307. doi: 10.1111/imj.14102. Epub [PubMed PMID: 30387309]
Muzumdar S, Rothe MJ, Grant-Kels JM. The rash with maculopapules and fever in adults. Clinics in dermatology. 2019 Mar-Apr:37(2):109-118. doi: 10.1016/j.clindermatol.2018.12.004. Epub 2018 Dec 5 [PubMed PMID: 30981291]
Muzumdar S, Rothe MJ, Grant-Kels JM. The rash with maculopapules and fever in children. Clinics in dermatology. 2019 Mar-Apr:37(2):119-128. doi: 10.1016/j.clindermatol.2018.12.005. Epub 2018 Dec 5 [PubMed PMID: 30981292]