Introduction
Moyamoya disease was first described in Japanese literature in 1957; however, Suzuki and Takaku first coined the term “moyamoya disease” in 1969.[1] Moyamoya disease is an isolated chronic, usually bilateral, vasculopathy of undetermined etiology characterized by progressive narrowing of the terminal intracranial portion of the internal carotid artery (ICA) and circle of Willis. Moyamoya syndrome corresponds to the same moyamoya arteriopathy, but in the context of either neurological or extraneurological conditions, whether inherited or acquired. A fragile network of abundant collateral vessels develops in response to chronic brain ischemia, known as moyamoya vessels, which appear as a “puff of smoke” on angiography.[2] Patients most often present with ischemic strokes or transient ischemic attacks (TIAs); however, intracranial hemorrhages are also common due to the fragility of the abnormal moyamoya vessels. Surgical revascularization is the mainstay of treatment for both ischemic and hemorrhagic presentations of moyamoya disease.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Moyamoya disease is associated with several inherited and acquired conditions, including:
- Inherited conditions
- Acquired conditions
Epidemiology
Moyamoya disease occurs far more frequently in Asia, a trend reflected in the majority of available epidemiological data. The condition appears most commonly in East Asian countries, particularly Japan and Korea, though an increasing incidence has also been documented in Western nations. In Japan, incidence rates range from 0.35 to 0.94 per 100,000 individuals, with a reported prevalence of 3.16 per 100,000 population.[26][27][28] A positive family history has been identified in 11% to 12% of cases, according to prior studies.[29][27]
A population-based study conducted in California and Washington state involving 298 patients reported an incidence of 0.086 per 100,000 individuals.[30] Another study using data from the Nationwide Inpatient Sample Database found a higher incidence of 0.57 per 100,000 population.[31] This same study revealed a female predominance in the United States, with women accounting for 72% of cases. Moyamoya disease generally follows a bimodal age distribution, with incidence peaks between 5 and 9 years and 45 and 49 years of age.[27] Pediatric patients more frequently present with ischemic symptoms, while adults more commonly exhibit intracranial hemorrhage at onset.[31]
Pathophysiology
The pathophysiology of moyamoya disease remains unclear, though genetic predisposition is theorized in East Asian countries. The RNF213 gene (Ring Finger Protein) is a well-known susceptibility gene that is often found in patients of Japanese and Korean descent.[32] The homozygous variant has been shown to predict an earlier onset and a more severe presentation.[33]
In a study involving the Midwestern United States population, an unusually high prevalence of type 1 diabetes, autoimmune thyroid disorders, and other autoimmune disorders was found in the moyamoya cohort, which may suggest an autoimmune association.[34][35] Similar results were seen in the Western Chinese population, where 31% of patients with moyamoya disease also had an autoimmune disease, mostly diabetes mellitus type 1 and Graves’ disease.[36]
Chronic brain ischemia resulting from narrowing is believed to cause an overexpression of proangiogenic factors, including vascular endothelial growth factor, fibroblast growth factor, and hepatocyte growth factor, among others, which, in turn, promotes the development of a fragile network of collateral vessels.[37] Furthermore, the association between moyamoya disease and inflammatory disease has been studied, with inflammatory disease clusters seen in both pediatric and adult cohorts.[38]
The following types of moyamoya disease with the chromosome involved have been described in the literature:
- MYMY1 (chromosome 3p)
- MYMY2 (RNF213 gene on chromosome 17q25)
- MYMY3 (chromosome 8q23)
- MYMY4 (X-linked recessive condition characterized by moyamoya disease, short stature, hypergonadotropic hypogonadism, and facial dysmorphism)
- MYMY5 (ACTA2 gene on chromosome 10q23) [39]
- MYMY6 with achalasia (GUCY1A3 gene on chromosome 4q32)
Mutations in BRCC3/MTCP1 [40] and GUCY1A3 [41] genes have also been implicated in moyamoya syndrome.
Histopathology
Prior studies have shown that the arteries affected in moyamoya disease have fibrocellular thickening of the intimal layer, which contains smooth muscle cells, as well as duplicated internal elastic lamina.[42][43][44] The tunica media eventually becomes thinner, leading to a decrease in the outer vessel diameter, and is seen in conjunction with luminal stenosis.[45][46]
History and Physical
Cerebral ischemic events in the form of TIA or ischemic infarcts are the most common presentation of all moyamoya patients.[47] Intracerebral hemorrhage occurs more frequently among adult patients with moyamoya disease, while seizures can present in both adults and children. Symptoms can be categorized based on etiology: those due to cerebral ischemia (eg, stroke, TIA, and seizure) and those due to the growth of collateral vessels that compensate for ischemia (eg, hemorrhage and headache).
Cerebral Ischemia-Associated Presentations
In the pediatric population, moyamoya typically manifests with TIA or ischemic stroke. These events are often precipitated by hyperventilation (ie, crying). Hyperventilation decreases carbon dioxide, leading to cerebral vasoconstriction and worsening cerebral hypoperfusion. Children may also show intellectual disabilities of various degrees.[48][49][50] Deterioration of cognition is in a linear relationship with the number of strokes and chronic hypoxemia from a progressive narrowing of the cerebral vasculature.
Collateral Growth-Associated Presentations
In addition to TIA or ischemic strokes, adults also frequently present with hemorrhagic strokes. Hemorrhage primarily results from the rupture of fragile moyamoya collaterals and is typically observed in deep areas of the brain, eg, the basal ganglia and the periventricular deep white matter. Intraventricular hemorrhage is common due to the proximity of the primary site of intracerebral hemorrhage. Seizures are commonly seen after either ischemic or hemorrhagic events.[51]
Migraine-like headaches are common in both children and adults and are thought to occur from the stimulation of dural nociceptors by dilated transdural collaterals.[52][53] Tension-type headaches, hemiplegic migraines, and cluster headaches can also be seen.[19] Recent studies have also shown psychiatric symptoms, eg, depression, anxiety, and psychosis in adult patients with Moyamoya disease.[54][55][56]
Although rare, several reports have described choreoathetosis and other movement disorders as a manifestation of moyamoya disease.[57][58][59] A retrospective review of 316 consecutive patients found that only 3.2% of patients presented with chorea.[60]
Evaluation
Like all neurovascular conditions, the evaluation of moyamoya disease relies on different imaging modalities to help characterize the lesion, guide management strategies, and help predict postoperative recovery. A brief description of various imaging modalities is discussed here; however, a more thorough review was published by Du and colleagues.[61]
Magnetic Resonance Imaging
Magnetic resonance imaging (MRI) typically serves as an initial diagnostic tool due to its high sensitivity and noninvasive nature. MRI effectively detects hemorrhages and strokes, while older ischemic lesions frequently appear as white matter hyperintensities in distal vascular territories or border zone regions on FLAIR and T2-weighted sequences. A notable radiographic feature, the "ivy sign," reflects leptomeningeal enhancement or increased signal intensity on T2-FLAIR and appears in nearly half of pediatric patients with Suzuki stage III or IV disease.[62] This sign likely indicates slow cortical blood flow, and its resolution may serve as a prognostic marker following revascularization.[62] Incorporating vessel wall imaging into MRI protocols may also aid in differentiating Moyamoya disease from atherosclerotic lesions.[63]
Magnetic Resonance Angiography
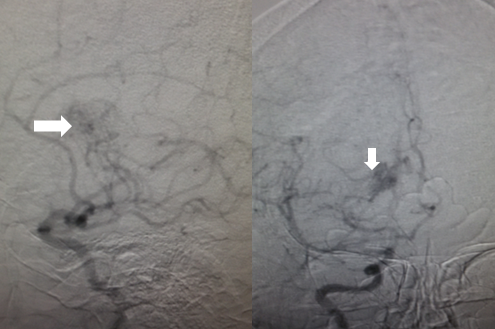
Magnetic resonance angiography (MRA) provides information on cerebral arteries and the degree of narrowing (see Image. Angiographic Findings in Moyamoya Disease). MRA also demonstrates the development of collaterals (ie, moyamoya vessels). In the 2021 Japanese guidelines, diagnosis can be made if the following are seen on MRI/MRA:
- Stenosis or occlusion of the terminal portion of the intracranial internal carotid artery.
- Decrease in the outer diameter of the terminal portion of the internal carotid artery and the horizontal portion of the middle cerebral artery bilaterally on heavy T2-weighted MRI.
- Abnormal vascular networks in the basal ganglia and periventricular white matter on MRA.[64]
"Quasi-moyamoya disease" (also known as moyamoya syndrome) is a unilateral or bilateral occlusive process associated with an underlying disease.[65]
Conventional cerebral angiography
Based on the 2021 Japanese guidelines, the diagnosis of moyamoya disease can be made with the following criteria:
- Stenosis or occlusion in the arteries centered on the terminal portion of the intracranial internal carotid artery.
- Moyamoya vessels (abnormal vascular networks) are present in the vicinity of the occlusive or stenotic lesions in the arterial phase.[64]
Both bilateral and unilateral cases can be diagnosed as moyamoya disease according to the following 2021 guidelines.[64] Cerebral angiography is considered the gold standard in the diagnosis of moyamoya disease. Suzuki and Kodoma classified the severity of moyamoya disease by progression of an occlusive process and the eventual appearance of collaterals based on serial cerebral angiographic evaluations and staged them, known as "Suzuki stages of moyamoya disease", which are mentioned under staging. Various types of collaterals have been described on angiography, each progressing from extracranial to the intracranial vasculature, including:
- Basal moyamoya: Abnormal dilation of the perforating arteries, such as the lenticulostriates and thalamo-perforating arteries
- Choroidal and pericallosal: Dilatation of the anterior choroidal and posterior pericallosal arteries
- Ethmoidal moyamoya: Collaterals are perfused from the ophthalmic artery and ethmoidal (anterior and posterior) arteries.
- Vault moyamoya: Last pathway in which transdural collaterals supply the pial arteries.[66]
Transcranial Doppler
Transcranial Doppler (TCD) is an adjunctive method for monitoring cerebral hemodynamics, and data on its ability to determine the stage and treatment method are scarce. Additionally, TCD is operator-dependent. Hence, this modality is not as useful as MRI, MRA, or conventional angiography. The primary parameters of monitoring using TCD are mean blood flow velocity and the pulsatility index.[67]
Electroencephalography
Electroencephalography (EEG) evaluations are necessary for patients presenting with seizures. Suzuki and Kodoma mentioned a distinctive EEG finding among ~50% of moyamoya patients, known as the "rebuild-up" phenomenon. The rebuild-up phenomenon is referred to as a reappearance of slow waves of higher amplitude (usually seen during hyperventilation), within 20 to 60 seconds following termination of hyperventilation, which is not seen in any other pathology. Rebuild-up is different than initial slowing due to hyperventilation and signifies the diminished blood flow. The slowdown due to rebuilding is expected to be resolved in approximately 10 minutes.[68]
Cerebral Perfusion Measurement
Important tools for measuring cerebral perfusion include single-photon emission computed tomography (SPECT), xenon-enhanced CT, and MRI-based methods. Xenon-enhanced CT and MRI-based methods include positron emission tomography (PET) scans and arterial spin labeling. Both typically show the following findings:
- Increase in oxygen fraction extraction
- Reduction of global cerebral blood flow with posterior cerebral flow distribution
- Impaired cerebrovascular reactivity to carbon dioxide and acetazolamide in ICA territory, suggesting low cerebrovascular reserve [69]
Treatment / Management
Management Approaches
Early diagnosis of moyamoya disease, coupled with timely surgical intervention, is of utmost importance as medical therapies act only as secondary prevention and do not halt disease progression. In asymptomatic patients, the risk of stroke has to be weighed against the risks of surgery. In a multicenter, nationwide survey in Japan, 40 patients were enrolled in a prospective cohort study, which demonstrated an annual risk of 3.2% of any stroke.[70] In another multicenter prospective cohort study, Kuroda et al found a yearly risk of stroke of 1.4% per person.[71] An interim analysis of the AMORE study revealed a 5.9% incidence of disease progression in asymptomatic patients, suggesting the need for careful decision-making when treating asymptomatic patients.[72] (B3)
In patients who present with ischemia, some studies suggest delaying revascularization, as patients who underwent surgery within 90 days after the last stroke had lower overall postoperative stroke incidence and lower mortality rates.[73] However, other authors suggest performing revascularization within 2 months of diagnosis, especially in patients younger than 4 years of age.[74] In patients presenting with intracranial hemorrhage, studies have demonstrated evidence that surgical revascularization may have a preventative effect against rebleeding, suggesting that surgical intervention is better than nonoperative management.[75] A thorough review of the recommendations from the Japanese guidelines, European Stroke Organization, and American Heart Association can be found in a review by Rifino and colleagues.[76](B3)
Medical Management
Acute therapy for strokes or intracranial bleeding is performed as per standard protocols.[77] Conservative management is directed towards maintaining cerebral blood flow and preventing further ischemic and hemorrhagic events. Aspirin has been conventionally used among patients with moyamoya disease. A meta-analysis evaluated the benefits of antiplatelet therapy and reported a reduced risk of hemorrhagic stroke, but did not decrease the risk of ischemic strokes.[78] The 2021 Japanese guidelines report that oral antiplatelet agents may be considered in patients with ischemic moyamoya disease, although they cite a low level of evidence.[77] Other studies have reported increased rates of bypass patency with perioperative aspirin use, without a significant increase in bleeding risk.[79][80][81](A1)
Headaches and seizures are usually managed by symptomatic treatments using analgesics and antiepileptic medications, respectively.[82]
Surgical Revascularization
Surgery is the mainstay of treatment for moyamoya disease to improve cerebral blood flow and prevent future strokes and hemorrhages. The main surgical approaches to revascularization include indirect, direct, or combined approaches.[83][84](A1)
Indirect revascularization
Indirect revascularization is the more common surgical approach in the pediatric population, as the donor and recipient vessels in children are much smaller than in adults, making a direct bypass technically more challenging. Indirect techniques include encephalomyosynangiosis (EMS), encephaloduroarteriosynangiosis (EDAS), encephalomyoarteriosynangiosis (EMAS), encephaloduroarteriomyosynangiosis (EDAMS), and encephalo-galeosynangiosis (EGS). Other methods include pial synangiosis, dural inversion, multiple burr holes, or omental transposition.
Direct revascularization
The superficial temporal artery is used as the donor vessel to the superficial branches of the MCA. The benefit of direct bypass is the immediate restoration of blood flow to the ischemic regions of the brain.
Perioperative Management
The precise management of patients with moyamoya disease both before and after surgical revascularization plays a vital role in optimizing outcomes for these patients. Because the affected cerebral hemispheres are at risk for ischemic events, special considerations must be made when undergoing anesthesia. The primary risk of undergoing anesthesia is the development of a mismatch between cerebral oxygen demand and supply. Consequently, the goals from an anesthesia standpoint include maintaining normocarbia, normotension, normovolemia, and normothermia.[85]
Expert consensus has recommended the following for pediatric patients undergoing indirect revascularization:
- Children with sickle cell disease should be preadmitted before surgical revascularization.
- Intravenous isotonic fluids should be given for at least 4 hours before and 24 hours after surgery.
- Antiplatelet therapy should not be stopped during the immediate preoperative and postoperative periods.
- Arterial lines should be continued for 24 hours after surgery for continuous blood pressure monitoring and until active interventions for blood pressure control are not needed.
- Hourly vital sign checks for 24 hours and hourly neurological assessments for at least 12 hours after surgery, with special attention to maintaining normoxia and normotherapy and avoiding hypotensive episodes.
- Intravenous fluid boluses should be considered first-line interventions for the treatment of new focal neurological deficits.[86]
A detailed care pathway has been described for pediatric patients undergoing revascularization.[87] Other protocols have been established specifically for perioperative nausea and vomiting in pediatric patients.[88] Another important consideration in the perioperative period is the management of relative anemia. One study analyzing 53 surgeries showed that larger decreases in hemoglobin, hematocrit, and blood urea nitrogen were associated with an increased risk of perioperative ischemic strokes.[89] Beyond assessing vitals and the neurological exam in the perioperative period, continuous EEG has been recommended by some authors to detect early clinical and subclinical ischemia, which may lead to the initiation of treatment before clinical symptoms manifest.[90](B3)
Differential Diagnosis
Differential diagnoses that should be considered when evaluating moyamoya disease include:
- Large vessel occlusion
- Cerebral vasculitis
- Intracranial atherosclerotic disease
- Basilar artery thrombosis
- Blood dyscrasia
- Cavernous sinus syndromes
- Cerebral aneurysms
- Dissection syndromes
- Fibromuscular dystrophies
- Spontaneous intracranial hemorrhage
Staging
The Suzuki staging system describes the progression from the initial stenosis in the terminal portion of the ICA to the formation of a deep but fragile network of collaterals (moyamoya) and the subsequent reduction of moyamoya vessels, accompanied by the development of supply from external carotid artery branches. This fragile network of collaterals mainly develops from thalamoperforating and lenticulostriate perforating arteries. Suzuki stages of moyamoya disease are the following:
- Stage 1
- Narrowing of carotid fork: On the angiographic exam, only the terminal portion of the internal carotid artery is stenosed.
- Stage 2
- Initiation and appearance of basal moyamoya: On the angiographic exam, stenosis of all the terminal branches of ICA (ACA and MCA) and deep moyamoya vessels are seen.
- Stage 3
- Intensification of basal moyamoya: On the angiographic exam, deep moyamoya vessels are intensified. MRA taken during this stage shows a "puff of smoke" appearance.
- The deflection of the anterior cerebral artery (ACA) and middle cerebral arteries (MCA) is noted.
- Stage 4
- Minimization of basal moyamoya: On the angiographic exam, deep moyamoya vessels begin to regress while transdural collaterals begin to appear.
- The deflection of the PCA is noted.
- Stage 5
- Reduction of moyamoya: On the angiographic exam, continued regression of deep moyamoya vessels and progression of transdural collateral vessels are noted.
- Stage 6
- Disappearance of moyamoya: On the angiographic exam, deep moyamoya vessels have vanished, and complete occlusion of the ICA is noted.
- Blood supply to the ACA and MCA areas is derived mainly from the external carotid artery.[2]
Prognosis
The prognosis of moyamoya disease depends on multiple factors. Patients initially presenting with ischemia, those diagnosed before the age of 3, individuals with diabetes mellitus, and those with PCA involvement face an increased risk of recurrent ischemic symptoms.[91] Identified risk factors for recurrent hemorrhages include an initial hemorrhagic presentation, smoking, choroidal collaterals, and decreased cerebrovascular reserve.[91] A large retrospective study from Korea demonstrated a reduced risk of death and recurrent hemorrhagic stroke in adult patients who underwent revascularization compared to those managed conservatively. However, an elevated risk of hemorrhagic stroke was observed in asymptomatic moyamoya disease patients who received revascularization.[92]
In a meta-analysis of pediatric moyamoya patients, revascularization correlated with lower odds of ischemic stroke and intracranial hemorrhage. Similar rates of ischemic stroke, TIAs, new or worsened seizures, and symptomatic improvement were observed when comparing direct or combined techniques to indirect methods. Another meta-analysis found superior long-term angiographic revascularization with direct or combined approaches, particularly when paired with indirect bypasses.[93] However, stroke recurrence, morbidity, and mortality did not significantly differ between techniques.[94] Postoperative monitoring remains essential, as contralateral progression frequently occurs and may require additional treatment.[95][96][97]
Complications
Complications that are associated with moyamoya disease include:
- Perioperative ischemic stroke
- Hemorrhagic stroke
- Hyperperfusion syndrome after direct vascularization
- New or worsening seizures
- Epidural hematoma
- Wound healing issues
Postoperative and Rehabilitation Care
Patients who experience perioperative ischemic or hemorrhagic strokes may require inpatient rehabilitation as they recover.
Consultations
Consultations that may be involved in the management of this condition:
- Neurosurgery
- Neurology
- Interventional radiology
- Genetics
- Rehab
- Physical, occupational, and speech therapies
Deterrence and Patient Education
Although no preventive treatments for moyamoya disease or moyamoya syndrome have been established, early recognition and treatment can improve long-term outcomes. Deterrence and patient education for moyamoya disease focus on early recognition, risk reduction, and proactive management to prevent neurological deterioration. Since no curative treatment exists, educating patients and caregivers about the importance of timely diagnosis and surgical intervention remains crucial.
Clinicians must emphasize adherence to follow-up appointments, imaging surveillance, and medication regimens, particularly antiplatelet therapy when indicated. Patients should receive counseling on modifiable risk factors, including blood pressure control, smoking cessation, weight management, and lipid regulation, to minimize the risk of ischemic and hemorrhagic events. For pediatric patients, families should understand the potential impact of hyperventilation-induced symptoms and the importance of monitoring for cognitive or behavioral changes. A clear explanation of the disease process, treatment options, and long-term expectations empowers patients and supports informed decision-making, fostering greater engagement and adherence to care plans.
Enhancing Healthcare Team Outcomes
Effective management of Moyamoya disease requires seamless collaboration across an interprofessional healthcare team, including physicians, advanced practitioners, nurses, pharmacists, radiologists, anesthesiologists, and surgical specialists. Neurologists and neurosurgeons play central roles in diagnosis, staging, and determining the appropriate surgical intervention, while advanced practitioners and nurses ensure timely follow-up, monitoring, and patient education. Accurate interpretation of advanced imaging modalities, such as MRI, MRA, and cerebral angiography, requires coordination between radiologists and treating clinicians to inform evidence-based decision-making. Pharmacists support optimal use of antiplatelet agents and seizure medications, carefully monitoring for drug interactions, adherence, and perioperative considerations. Effective communication during preoperative and postoperative phases—particularly around anesthetic risks and neurological status—enhances safety and minimizes complications.
Patient-centered care relies on aligning treatment strategies with the patient's age, presentation, and risk profile, while also integrating education on lifestyle modifications, such as smoking cessation, blood pressure control, and stroke prevention. Nurses and allied health professionals provide ongoing support and health coaching, especially for pediatric patients and families coping with neurocognitive or psychosocial challenges. Structured care coordination meetings and standardized care pathways—particularly for perioperative care—optimize team performance and reduce variability in outcomes. By fostering open dialogue, shared goals, and role clarity, interprofessional teams ensure safer interventions, more efficient transitions of care, and improved long-term outcomes for individuals affected by Moyamoya disease.
Media
(Click Image to Enlarge)
References
Suzuki J, Takaku A. Cerebrovascular "moyamoya" disease. Disease showing abnormal net-like vessels in base of brain. Archives of neurology. 1969 Mar:20(3):288-99 [PubMed PMID: 5775283]
Hertza J, Loughan A, Perna R, Davis AS, Segraves K, Tiberi NL. Moyamoya disease: a review of the literature. Applied neuropsychology. Adult. 2014:21(1):21-7. doi: 10.1080/09084282.2012.721147. Epub 2013 Jun 14 [PubMed PMID: 24826492]
Level 3 (low-level) evidenceRallo MS, Akel O, Kalakoti P, Sun H. Characteristics and outcomes of stroke hospitalizations in patients with sickle cell disease and moyamoya syndrome. Journal of stroke and cerebrovascular diseases : the official journal of National Stroke Association. 2022 Oct:31(10):106705. doi: 10.1016/j.jstrokecerebrovasdis.2022.106705. Epub 2022 Aug 11 [PubMed PMID: 35964532]
Newman S, Boulter JH, Malcolm JG, Pradilla I, Pradilla G. Outcomes in Patients with Moyamoya Syndrome and Sickle Cell Disease: A Systematic Review. World neurosurgery. 2020 Mar:135():165-170. doi: 10.1016/j.wneu.2019.11.137. Epub 2019 Nov 29 [PubMed PMID: 31790841]
Level 1 (high-level) evidenceKauv P, Gaudré N, Hodel J, Tuilier T, Habibi A, Oppenheim C, Edjlali M, Hervé D, Calvet D, Bartolucci P. Characteristics of Moyamoya Syndrome in Sickle-Cell Disease by Magnetic Resonance Angiography: An Adult-Cohort Study. Frontiers in neurology. 2019:10():15. doi: 10.3389/fneur.2019.00015. Epub 2019 Jan 22 [PubMed PMID: 30723452]
Hankinson TC, Bohman LE, Heyer G, Licursi M, Ghatan S, Feldstein NA, Anderson RC. Surgical treatment of moyamoya syndrome in patients with sickle cell anemia: outcome following encephaloduroarteriosynangiosis. Journal of neurosurgery. Pediatrics. 2008 Mar:1(3):211-6. doi: 10.3171/PED/2008/1/3/211. Epub [PubMed PMID: 18352765]
Dobson SR, Holden KR, Nietert PJ, Cure JK, Laver JH, Disco D, Abboud MR. Moyamoya syndrome in childhood sickle cell disease: a predictive factor for recurrent cerebrovascular events. Blood. 2002 May 1:99(9):3144-50 [PubMed PMID: 11964276]
See AP, Ropper AE, Underberg DL, Robertson RL, Scott RM, Smith ER. Down syndrome and moyamoya: clinical presentation and surgical management. Journal of neurosurgery. Pediatrics. 2015 Jul:16(1):58-63. doi: 10.3171/2014.12.PEDS14563. Epub 2015 Apr 3 [PubMed PMID: 25837890]
Cramer SC, Robertson RL, Dooling EC, Scott RM. Moyamoya and Down syndrome. Clinical and radiological features. Stroke. 1996 Nov:27(11):2131-5 [PubMed PMID: 8898827]
Brosius SN, Vossough A, Fisher MJ, Lang SS, Beslow LA, George BJ, Ichord R. Characteristics of Moyamoya Syndrome in Pediatric Patients With Neurofibromatosis Type 1. Pediatric neurology. 2022 Sep:134():85-92. doi: 10.1016/j.pediatrneurol.2022.05.013. Epub 2022 Jun 6 [PubMed PMID: 35849956]
Santoro C, Di Rocco F, Kossorotoff M, Zerah M, Boddaert N, Calmon R, Vidaud D, Cirillo M, Cinalli G, Mirone G, Giugliano T, Piluso G, D'Amico A, Capra V, Pavanello M, Cama A, Nobili B, Lyonnet S, Perrotta S. Moyamoya syndrome in children with neurofibromatosis type 1: Italian-French experience. American journal of medical genetics. Part A. 2017 Jun:173(6):1521-1530. doi: 10.1002/ajmg.a.38212. Epub 2017 Apr 19 [PubMed PMID: 28422438]
Rosser TL, Vezina G, Packer RJ. Cerebrovascular abnormalities in a population of children with neurofibromatosis type 1. Neurology. 2005 Feb 8:64(3):553-5 [PubMed PMID: 15699396]
Wang R, Xu Y, Lv R, Chen J. Systemic lupus erythematosus associated with Moyamoya syndrome: a case report and literature review. Lupus. 2013 May:22(6):629-33. doi: 10.1177/0961203313485828. Epub 2013 Apr 10 [PubMed PMID: 23574743]
Level 3 (low-level) evidenceJeong HC, Kim YJ, Yoon W, Joo SP, Lee SS, Park YW. Moyamoya syndrome associated with systemic lupus erythematosus. Lupus. 2008 Jul:17(7):679-82. doi: 10.1177/0961203307087375. Epub [PubMed PMID: 18625642]
El Ramahi KM, Al Rayes HM. Systemic lupus erythematosus associated with moyamoya syndrome. Lupus. 2000:9(8):632-6 [PubMed PMID: 11035439]
Nakamura H, Sato K, Yoshimura S, Hayashi Y, Izumo T, Tokunaga Y. Moyamoya Disease Associated with Graves' Disease and Down Syndrome: A Case Report and Literature Review. Journal of stroke and cerebrovascular diseases : the official journal of National Stroke Association. 2021 Jan:30(1):105414. doi: 10.1016/j.jstrokecerebrovasdis.2020.105414. Epub 2020 Oct 30 [PubMed PMID: 33130479]
Level 3 (low-level) evidenceGupta M, Choudhri OA, Feroze AH, Do HM, Grant GA, Steinberg GK. Management of moyamoya syndrome in patients with Noonan syndrome. Journal of clinical neuroscience : official journal of the Neurosurgical Society of Australasia. 2016 Jun:28():107-11. doi: 10.1016/j.jocn.2015.11.017. Epub 2016 Jan 6 [PubMed PMID: 26778511]
Hung PC, Wang HS, Wong AM. Moyamoya syndrome in a child with Noonan syndrome. Pediatric neurology. 2011 Aug:45(2):129-31. doi: 10.1016/j.pediatrneurol.2011.03.007. Epub [PubMed PMID: 21763956]
Gonzalez NR, Amin-Hanjani S, Bang OY, Coffey C, Du R, Fierstra J, Fraser JF, Kuroda S, Tietjen GE, Yaghi S, American Heart Association Stroke Council; Council on Cardiovascular and Stroke Nursing; and Council on Clinical Cardiology. Adult Moyamoya Disease and Syndrome: Current Perspectives and Future Directions: A Scientific Statement From the American Heart Association/American Stroke Association. Stroke. 2023 Oct:54(10):e465-e479. doi: 10.1161/STR.0000000000000443. Epub 2023 Aug 23 [PubMed PMID: 37609846]
Level 3 (low-level) evidenceScala M, Fiaschi P, Cama A, Consales A, Piatelli G, Giannelli F, Barra S, Satragno C, Pacetti M, Secci F, Tortora D, Garrè ML, Pavanello M. Radiation-Induced Moyamoya Syndrome in Children with Brain Tumors: Case Series and Literature Review. World neurosurgery. 2020 Mar:135():118-129. doi: 10.1016/j.wneu.2019.11.155. Epub 2019 Dec 2 [PubMed PMID: 31805403]
Level 2 (mid-level) evidenceWang C, Roberts KB, Bindra RS, Chiang VL, Yu JB. Delayed cerebral vasculopathy following cranial radiation therapy for pediatric tumors. Pediatric neurology. 2014 Jun:50(6):549-56. doi: 10.1016/j.pediatrneurol.2013.09.018. Epub 2014 Jan 25 [PubMed PMID: 24739378]
Ullrich NJ, Robertson R, Kinnamon DD, Scott RM, Kieran MW, Turner CD, Chi SN, Goumnerova L, Proctor M, Tarbell NJ, Marcus KJ, Pomeroy SL. Moyamoya following cranial irradiation for primary brain tumors in children. Neurology. 2007 Mar 20:68(12):932-8 [PubMed PMID: 17372129]
Desai SS, Paulino AC, Mai WY, Teh BS. Radiation-induced moyamoya syndrome. International journal of radiation oncology, biology, physics. 2006 Jul 15:65(4):1222-7 [PubMed PMID: 16626890]
Pinardi F, Stracciari A, Spinardi L, Guarino M. Postpneumococcal Moyamoya syndrome case report and review of the postinfective cases. BMJ case reports. 2013 Feb 6:2013():. doi: 10.1136/bcr-2012-006726. Epub 2013 Feb 6 [PubMed PMID: 23391943]
Level 3 (low-level) evidenceCzartoski T, Hallam D, Lacy JM, Chun MR, Becker K. Postinfectious vasculopathy with evolution to moyamoya syndrome. Journal of neurology, neurosurgery, and psychiatry. 2005 Feb:76(2):256-9 [PubMed PMID: 15654044]
Baba T, Houkin K, Kuroda S. Novel epidemiological features of moyamoya disease. Journal of neurology, neurosurgery, and psychiatry. 2008 Aug:79(8):900-4 [PubMed PMID: 18077479]
Level 2 (mid-level) evidenceKuriyama S, Kusaka Y, Fujimura M, Wakai K, Tamakoshi A, Hashimoto S, Tsuji I, Inaba Y, Yoshimoto T. Prevalence and clinicoepidemiological features of moyamoya disease in Japan: findings from a nationwide epidemiological survey. Stroke. 2008 Jan:39(1):42-7 [PubMed PMID: 18048855]
Level 2 (mid-level) evidenceWakai K, Tamakoshi A, Ikezaki K, Fukui M, Kawamura T, Aoki R, Kojima M, Lin Y, Ohno Y. Epidemiological features of moyamoya disease in Japan: findings from a nationwide survey. Clinical neurology and neurosurgery. 1997 Oct:99 Suppl 2():S1-5 [PubMed PMID: 9409395]
Level 2 (mid-level) evidenceSato Y, Kazumata K, Nakatani E, Houkin K, Kanatani Y. Characteristics of Moyamoya Disease Based on National Registry Data in Japan. Stroke. 2019 Aug:50(8):1973-1980. doi: 10.1161/STROKEAHA.119.024689. Epub 2019 Jun 25 [PubMed PMID: 31234758]
Uchino K, Johnston SC, Becker KJ, Tirschwell DL. Moyamoya disease in Washington State and California. Neurology. 2005 Sep 27:65(6):956-8 [PubMed PMID: 16186547]
Starke RM, Crowley RW, Maltenfort M, Jabbour PM, Gonzalez LF, Tjoumakaris SI, Randazzo CG, Rosenwasser RH, Dumont AS. Moyamoya disorder in the United States. Neurosurgery. 2012 Jul:71(1):93-9. doi: 10.1227/NEU.0b013e318253ab8e. Epub [PubMed PMID: 22418580]
Duan L, Wei L, Tian Y, Zhang Z, Hu P, Wei Q, Liu S, Zhang J, Wang Y, Li D, Yang W, Zong R, Xian P, Han C, Bao X, Zhao F, Feng J, Liu W, Cao W, Zhou G, Zhu C, Yu F, Yang W, Meng Y, Wang J, Chen X, Wang Y, Shen B, Zhao B, Wan J, Zhang F, Zhao G, Xu A, Zhang X, Liu J, Zuo X, Wang K. Novel Susceptibility Loci for Moyamoya Disease Revealed by a Genome-Wide Association Study. Stroke. 2018 Jan:49(1):11-18. doi: 10.1161/STROKEAHA.117.017430. Epub [PubMed PMID: 29273593]
Miyatake S, Miyake N, Touho H, Nishimura-Tadaki A, Kondo Y, Okada I, Tsurusaki Y, Doi H, Sakai H, Saitsu H, Shimojima K, Yamamoto T, Higurashi M, Kawahara N, Kawauchi H, Nagasaka K, Okamoto N, Mori T, Koyano S, Kuroiwa Y, Taguri M, Morita S, Matsubara Y, Kure S, Matsumoto N. Homozygous c.14576G}A variant of RNF213 predicts early-onset and severe form of moyamoya disease. Neurology. 2012 Mar 13:78(11):803-10. doi: 10.1212/WNL.0b013e318249f71f. Epub 2012 Feb 29 [PubMed PMID: 22377813]
Asselman C, Hemelsoet D, Eggermont D, Dermaut B, Impens F. Moyamoya disease emerging as an immune-related angiopathy. Trends in molecular medicine. 2022 Nov:28(11):939-950. doi: 10.1016/j.molmed.2022.08.009. Epub 2022 Sep 14 [PubMed PMID: 36115805]
Bower RS, Mallory GW, Nwojo M, Kudva YC, Flemming KD, Meyer FB. Moyamoya disease in a primarily white, midwestern US population: increased prevalence of autoimmune disease. Stroke. 2013 Jul:44(7):1997-9. doi: 10.1161/STROKEAHA.111.000307. Epub 2013 May 7 [PubMed PMID: 23652271]
Chen JB, Liu Y, Zhou LX, Sun H, He M, You C. Prevalence of autoimmune disease in moyamoya disease patients in Western Chinese population. Journal of the neurological sciences. 2015 Apr 15:351(1-2):184-186. doi: 10.1016/j.jns.2015.02.037. Epub 2015 Feb 26 [PubMed PMID: 25743224]
He S, Zhou Z, Cheng MY, Hao X, Chiang T, Wang Y, Zhang J, Wang X, Ye X, Wang R, Steinberg GK, Zhao Y. Advances in moyamoya disease: pathogenesis, diagnosis, and therapeutic interventions. MedComm. 2025 Feb:6(2):e70054. doi: 10.1002/mco2.70054. Epub 2025 Jan 14 [PubMed PMID: 39822761]
Level 3 (low-level) evidenceMejia-Munne JC, Ellis JA, Feldstein NA, Meyers PM, Connolly ES. Moyamoya and Inflammation. World neurosurgery. 2017 Apr:100():575-578. doi: 10.1016/j.wneu.2017.01.012. Epub 2017 Jan 16 [PubMed PMID: 28093343]
Guo DC, Papke CL, Tran-Fadulu V, Regalado ES, Avidan N, Johnson RJ, Kim DH, Pannu H, Willing MC, Sparks E, Pyeritz RE, Singh MN, Dalman RL, Grotta JC, Marian AJ, Boerwinkle EA, Frazier LQ, LeMaire SA, Coselli JS, Estrera AL, Safi HJ, Veeraraghavan S, Muzny DM, Wheeler DA, Willerson JT, Yu RK, Shete SS, Scherer SE, Raman CS, Buja LM, Milewicz DM. Mutations in smooth muscle alpha-actin (ACTA2) cause coronary artery disease, stroke, and Moyamoya disease, along with thoracic aortic disease. American journal of human genetics. 2009 May:84(5):617-27. doi: 10.1016/j.ajhg.2009.04.007. Epub 2009 Apr 30 [PubMed PMID: 19409525]
Miskinyte S, Butler MG, Hervé D, Sarret C, Nicolino M, Petralia JD, Bergametti F, Arnould M, Pham VN, Gore AV, Spengos K, Gazal S, Woimant F, Steinberg GK, Weinstein BM, Tournier-Lasserve E. Loss of BRCC3 deubiquitinating enzyme leads to abnormal angiogenesis and is associated with syndromic moyamoya. American journal of human genetics. 2011 Jun 10:88(6):718-728. doi: 10.1016/j.ajhg.2011.04.017. Epub 2011 May 19 [PubMed PMID: 21596366]
Hervé D, Philippi A, Belbouab R, Zerah M, Chabrier S, Collardeau-Frachon S, Bergametti F, Essongue A, Berrou E, Krivosic V, Sainte-Rose C, Houdart E, Adam F, Billiemaz K, Lebret M, Roman S, Passemard S, Boulday G, Delaforge A, Guey S, Dray X, Chabriat H, Brouckaert P, Bryckaert M, Tournier-Lasserve E. Loss of α1β1 soluble guanylate cyclase, the major nitric oxide receptor, leads to moyamoya and achalasia. American journal of human genetics. 2014 Mar 6:94(3):385-94. doi: 10.1016/j.ajhg.2014.01.018. Epub 2014 Feb 27 [PubMed PMID: 24581742]
Kraemer M, Keyvani K, Berlit P, Diesner F, Marquardt M. Histopathology of Moyamoya angiopathy in a European patient. Journal of neurology. 2019 Sep:266(9):2258-2262. doi: 10.1007/s00415-019-09406-w. Epub 2019 Jun 4 [PubMed PMID: 31165233]
Bersano A, Guey S, Bedini G, Nava S, Hervé D, Vajkoczy P, Tatlisumak T, Sareela M, van der Zwan A, Klijn CJ, Braun KP, Kronenburg A, Acerbi F, Brown MM, Calviere L, Cordonnier C, Henon H, Thines L, Khan N, Czabanka M, Kraemer M, Simister R, Prontera P, Tournier-Lasserve E, Parati E, European Moyamoya Disease Initiative. Research Progresses in Understanding the Pathophysiology of Moyamoya Disease. Cerebrovascular diseases (Basel, Switzerland). 2016:41(3-4):105-18. doi: 10.1159/000442298. Epub 2016 Jan 12 [PubMed PMID: 26756907]
Level 3 (low-level) evidenceFukui M, Kono S, Sueishi K, Ikezaki K. Moyamoya disease. Neuropathology : official journal of the Japanese Society of Neuropathology. 2000 Sep:20 Suppl():S61-4 [PubMed PMID: 11037190]
Chen T, Wei W, Yu J, Xu S, Zhang J, Li X, Chen J. The Progression of Pathophysiology of Moyamoya Disease. Neurosurgery. 2023 Sep 1:93(3):502-509. doi: 10.1227/neu.0000000000002455. Epub 2023 Mar 14 [PubMed PMID: 36912514]
Takagi Y, Kikuta K, Nozaki K, Hashimoto N. Histological features of middle cerebral arteries from patients treated for Moyamoya disease. Neurologia medico-chirurgica. 2007 Jan:47(1):1-4 [PubMed PMID: 17245006]
Research Committee on the Pathology and Treatment of Spontaneous Occlusion of the Circle of Willis, Health Labour Sciences Research Grant for Research on Measures for Infractable Diseases. Guidelines for diagnosis and treatment of moyamoya disease (spontaneous occlusion of the circle of Willis). Neurologia medico-chirurgica. 2012:52(5):245-66 [PubMed PMID: 22870528]
Kazumata K, Tokairin K, Sugiyama T, Ito M, Uchino H, Osanai T, Kawabori M, Nakayama N, Houkin K. Association of cognitive function with cerebral blood flow in children with moyamoya disease. Journal of neurosurgery. Pediatrics. 2020 Jan 1:25(1):62-68. doi: 10.3171/2019.7.PEDS19312. Epub 2019 Oct 11 [PubMed PMID: 31604320]
Kronenburg A, van den Berg E, van Schooneveld MM, Braun KPJ, Calviere L, van der Zwan A, Klijn CJM. Cognitive Functions in Children and Adults with Moyamoya Vasculopathy: A Systematic Review and Meta-Analysis. Journal of stroke. 2018 Sep:20(3):332-341. doi: 10.5853/jos.2018.01550. Epub 2018 Sep 30 [PubMed PMID: 30309228]
Level 1 (high-level) evidenceWilliams TS, Westmacott R, Dlamini N, Granite L, Dirks P, Askalan R, Macgregor D, Moharir M, Deveber G. Intellectual ability and executive function in pediatric moyamoya vasculopathy. Developmental medicine and child neurology. 2012 Jan:54(1):30-7. doi: 10.1111/j.1469-8749.2011.04144.x. Epub 2011 Nov 24 [PubMed PMID: 22117564]
Gatti JR, Penn R, Ahmad SA, Sun LR. Seizures in Pediatric Moyamoya: Risk Factors and Functional Outcomes. Pediatric neurology. 2023 Aug:145():36-40. doi: 10.1016/j.pediatrneurol.2023.04.025. Epub 2023 May 8 [PubMed PMID: 37271055]
Chiang CC, Shahid AH, Harriott AM, Tietjen GE, Savastano LE, Klaas JP, Lanzino G. Evaluation and treatment of headache associated with moyamoya disease - a narrative review. Cephalalgia : an international journal of headache. 2022 May:42(6):542-552. doi: 10.1177/03331024211056250. Epub 2021 Nov 17 [PubMed PMID: 34786968]
Level 3 (low-level) evidenceGao B, Kang K, Zhang J, Zhang D, Zhao X. Clinical Characteristics and Long-Term Outcome of Headaches Associated With Moyamoya Disease in the Chinese Population-A Cohort Study. Frontiers in neurology. 2020:11():605636. doi: 10.3389/fneur.2020.605636. Epub 2020 Nov 26 [PubMed PMID: 33324340]
Oakley CI, Lanzino G, Klaas JP. Neuropsychiatric Symptoms of Moyamoya Disease: Considerations for the Clinician. Neuropsychiatric disease and treatment. 2024:20():663-669. doi: 10.2147/NDT.S440975. Epub 2024 Mar 22 [PubMed PMID: 38532905]
Antonov A, Terraciano A, Essibayi MA, Javed K, Altschul DJ. Current Understanding of Moyamoya Disease (MMD) and Associated Neuropsychiatric Outcomes. Neuropsychiatric disease and treatment. 2023:19():2673-2680. doi: 10.2147/NDT.S402375. Epub 2023 Dec 5 [PubMed PMID: 38090021]
Level 3 (low-level) evidenceHaas P, Fudali M, Wang SS, Hurth H, Hauser TK, Ernemann U, Tatagiba M, Khan N, Roder C. Quality of life impairment in adult Moyamoya patients-preoperative neuropsychological assessment and correlation to MRI and H(2)(15)O PET findings. Neurosurgical review. 2022 Apr:45(2):1533-1541. doi: 10.1007/s10143-021-01660-9. Epub 2021 Oct 20 [PubMed PMID: 34671887]
Level 2 (mid-level) evidenceDemartini Z Jr, Teixeira BCA, Cardoso-Demartini AA. Choreoathetosis in Moyamoya Disease. World neurosurgery. 2021 Dec:156():103-104. doi: 10.1016/j.wneu.2021.09.100. Epub 2021 Oct 1 [PubMed PMID: 34601171]
Gonzalez-Alegre P, Ammache Z, Davis PH, Rodnitzky RL. Moyamoya-induced paroxysmal dyskinesia. Movement disorders : official journal of the Movement Disorder Society. 2003 Sep:18(9):1051-6 [PubMed PMID: 14502675]
Lyoo CH, Oh SH, Joo JY, Chung TS, Lee MS. Hemidystonia and hemichoreoathetosis as an initial manifestation of moyamoya disease. Archives of neurology. 2000 Oct:57(10):1510-2 [PubMed PMID: 11030805]
Ahn ES, Scott RM, Robertson RL Jr, Smith ER. Chorea in the clinical presentation of moyamoya disease: results of surgical revascularization and a proposed clinicopathological correlation. Journal of neurosurgery. Pediatrics. 2013 Mar:11(3):313-9. doi: 10.3171/2012.11.PEDS12199. Epub 2013 Jan 4 [PubMed PMID: 23289915]
Du L, Jiang H, Li J, Duan T, Zhou C, Yan F. Imaging methods for surgical revascularization in patients with moyamoya disease: an updated review. Neurosurgical review. 2022 Feb:45(1):343-356. doi: 10.1007/s10143-021-01596-0. Epub 2021 Aug 21 [PubMed PMID: 34417671]
Montaser AS, Lalgudi Srinivasan H, Staffa SJ, Zurakowski D, Slingerland AL, Orbach DB, Hausman-Kedem M, Roth J, Smith ER. Ivy sign: a diagnostic and prognostic biomarker for pediatric moyamoya. Journal of neurosurgery. Pediatrics. 2022 Apr 1:29(4):458-466. doi: 10.3171/2021.11.PEDS21384. Epub 2021 Dec 31 [PubMed PMID: 34972077]
Yang S, Wang X, Liao W, Li L, Tan Z, Zhu L, Hu P, Cui X, Xing W. High-resolution MRI of the vessel wall helps to distinguish moyamoya disease from atherosclerotic moyamoya syndrome. Clinical radiology. 2021 May:76(5):392.e11-392.e19. doi: 10.1016/j.crad.2020.12.023. Epub 2021 Feb 12 [PubMed PMID: 33583567]
Kuroda S, Fujimura M, Takahashi J, Kataoka H, Ogasawara K, Iwama T, Tominaga T, Miyamoto S, Research Committee on Moyamoya Disease (Spontaneous Occlusion of Circle of Willis) of the Ministry of Health, Labor, and Welfare, Japan. Diagnostic Criteria for Moyamoya Disease - 2021 Revised Version. Neurologia medico-chirurgica. 2022 Jul 15:62(7):307-312. doi: 10.2176/jns-nmc.2022-0072. Epub 2022 May 25 [PubMed PMID: 35613882]
Hishikawa T, Sugiu K, Date I. Moyamoya Disease: A Review of Clinical Research. Acta medica Okayama. 2016 Aug:70(4):229-36 [PubMed PMID: 27549666]
Pilgram-Pastor S, Chapot R, Kraemer M. The angiographic presentation of European Moyamoya angiopathy. Journal of neurology. 2022 Feb:269(2):997-1006. doi: 10.1007/s00415-021-10684-6. Epub 2021 Jul 8 [PubMed PMID: 34240321]
Yeh SJ, Tang SC, Tsai LK, Lee CW, Chen YF, Liu HM, Yang SH, Kuo MF, Jeng JS. Color Doppler ultrasonography as an alternative tool for postoperative evaluation of collaterals after indirect revascularization surgery in Moyamoya disease. PloS one. 2017:12(12):e0188948. doi: 10.1371/journal.pone.0188948. Epub 2017 Dec 8 [PubMed PMID: 29220356]
Frechette ES, Bell-Stephens TE, Steinberg GK, Fisher RS. Electroencephalographic features of moyamoya in adults. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2015 Mar:126(3):481-5. doi: 10.1016/j.clinph.2014.06.033. Epub 2014 Jul 5 [PubMed PMID: 25065300]
Level 2 (mid-level) evidenceHara S, Tanaka Y, Ueda Y, Hayashi S, Inaji M, Ishiwata K, Ishii K, Maehara T, Nariai T. Noninvasive Evaluation of CBF and Perfusion Delay of Moyamoya Disease Using Arterial Spin-Labeling MRI with Multiple Postlabeling Delays: Comparison with (15)O-Gas PET and DSC-MRI. AJNR. American journal of neuroradiology. 2017 Apr:38(4):696-702. doi: 10.3174/ajnr.A5068. Epub 2017 Feb 16 [PubMed PMID: 28209582]
Kuroda S, Hashimoto N, Yoshimoto T, Iwasaki Y, Research Committee on Moyamoya Disease in Japan. Radiological findings, clinical course, and outcome in asymptomatic moyamoya disease: results of multicenter survey in Japan. Stroke. 2007 May:38(5):1430-5 [PubMed PMID: 17395863]
Level 3 (low-level) evidenceKuroda S, Yamamoto S, Funaki T, Fujimura M, Kataoka H, Hishikawa T, Takahashi J, Endo H, Nariai T, Osato T, Saito N, Sato N, Hori E, Ito YM, Miyamoto S, AMORE Study Group. Five-Year Stroke Risk and Its Predictors in Asymptomatic Moyamoya Disease: Asymptomatic Moyamoya Registry (AMORE). Stroke. 2023 Jun:54(6):1494-1504. doi: 10.1161/STROKEAHA.122.041932. Epub 2023 May 22 [PubMed PMID: 37216455]
Kuroda S, Yamamoto S, Funaki T, Fujimura M, Kataoka H, Hishikawa T, Takahashi JC, Endo H, Nariai T, Osato T, Saito N, Sato N, Hori E, Kashiwazaki D, Ito YM, Miyamoto S. Disease progression, transient ischemic attack, and de novo parenchymal lesions in asymptomatic moyamoya disease: results of a 5-year interim analysis of the AMORE study. Journal of neurosurgery. 2025 Mar 1:142(3):658-666. doi: 10.3171/2024.6.JNS24736. Epub 2024 Oct 18 [PubMed PMID: 40367646]
Xu R, Xie ME, Khalifeh J, Feghali J, Yang W, Kim J, Liew J, Tamargo RJ, Huang J. Timing of Revascularization in Ischemic Moyamoya Disease: Association of Early Versus Delayed Surgery with Perioperative and Long-Term Outcomes. World neurosurgery. 2022 Oct:166():e721-e730. doi: 10.1016/j.wneu.2022.07.090. Epub 2022 Aug 2 [PubMed PMID: 35931338]
Hayashi T, Kimiwada T, Karibe H, Shirane R, Sasaki T, Metoki H, Tominaga T. Preoperative Risks of Cerebral Infarction in Pediatric Moyamoya Disease. Stroke. 2021 Jul:52(7):2302-2310. doi: 10.1161/STROKEAHA.120.032699. Epub 2021 May 11 [PubMed PMID: 33971740]
Miyamoto S, Yoshimoto T, Hashimoto N, Okada Y, Tsuji I, Tominaga T, Nakagawara J, Takahashi JC, JAM Trial Investigators. Effects of extracranial-intracranial bypass for patients with hemorrhagic moyamoya disease: results of the Japan Adult Moyamoya Trial. Stroke. 2014 May:45(5):1415-21. doi: 10.1161/STROKEAHA.113.004386. Epub 2014 Mar 25 [PubMed PMID: 24668203]
Rifino N, Hervè D, Acerbi F, Kuroda S, Lanzino G, Vajkoczy P, Bersano A. Diagnosis and management of adult Moyamoya angiopathy: An overview of guideline recommendations and identification of future research directions. International journal of stroke : official journal of the International Stroke Society. 2025 Jun:20(5):512-523. doi: 10.1177/17474930241297031. Epub 2024 Nov 4 [PubMed PMID: 39425621]
Level 3 (low-level) evidenceFujimura M, Tominaga T, Kuroda S, Takahashi JC, Endo H, Ogasawara K, Miyamoto S, Research Committee on Moyamoya Disease (Spontaneous Occlusion of Circle of Willis) of the Ministry of Health, Labor Welfare, Japan, Guideline Committee 2021 of the Japan Stroke Society. 2021 Japanese Guidelines for the Management of Moyamoya Disease: Guidelines from the Research Committee on Moyamoya Disease and Japan Stroke Society. Neurologia medico-chirurgica. 2022 Apr 15:62(4):165-170. doi: 10.2176/jns-nmc.2021-0382. Epub 2022 Feb 22 [PubMed PMID: 35197402]
Si J, Kang X, Li Z, Hao J, Zhang L. Benefits and risks of antiplatelet therapy after bypass surgery for moyamoya disease: A meta-analysis. Journal of clinical neuroscience : official journal of the Neurosurgical Society of Australasia. 2025 Feb:132():110956. doi: 10.1016/j.jocn.2024.110956. Epub 2024 Dec 6 [PubMed PMID: 39644520]
Level 1 (high-level) evidenceXiang Y, Zhang P, Zhao P, Sun T, Wang F, He Y, Wang D, Liu A. Effects of Aspirin Therapy on Bypass Efficacy and Survival of Patients Receiving Direct Cerebral Revascularization. Frontiers in pharmacology. 2022:13():841174. doi: 10.3389/fphar.2022.841174. Epub 2022 May 3 [PubMed PMID: 35592422]
Lu J, Shi G, Zhao Y, Wang R, Zhang D, Chen X, Wang H, Zhao JZ. Effects and safety of aspirin use in patients after cerebrovascular bypass procedures. Stroke and vascular neurology. 2021 Dec:6(4):624-630. doi: 10.1136/svn-2020-000770. Epub 2021 May 26 [PubMed PMID: 34039715]
Zhao Y, Zhang Q, Zhang D, Zhao Y. Effect of Aspirin in Postoperative Management of Adult Ischemic Moyamoya Disease. World neurosurgery. 2017 Sep:105():728-731. doi: 10.1016/j.wneu.2017.06.057. Epub 2017 Jun 15 [PubMed PMID: 28625901]
Porras JL, Yang W, Xu R, Garzon-Muvdi T, Caplan JM, Colby GP, Coon AL, Ahn ES, Tamargo RJ, Huang J. Effectiveness of Ipsilateral Stroke Prevention Between Conservative Management and Indirect Revascularization for Moyamoya Disease in a North American Cohort. World neurosurgery. 2018 Feb:110():e928-e936. doi: 10.1016/j.wneu.2017.11.113. Epub 2017 Dec 2 [PubMed PMID: 29196251]
Nguyen VN, Motiwala M, Elarjani T, Moore KA, Miller LE, Barats M, Goyal N, Elijovich L, Klimo P, Hoit DA, Arthur AS, Morcos JJ, Khan NR. Direct, Indirect, and Combined Extracranial-to-Intracranial Bypass for Adult Moyamoya Disease: An Updated Systematic Review and Meta-Analysis. Stroke. 2022 Dec:53(12):3572-3582. doi: 10.1161/STROKEAHA.122.039584. Epub 2022 Sep 22 [PubMed PMID: 36134563]
Level 1 (high-level) evidenceNguyen VN, Parikh KA, Motiwala M, Erin Miller L, Barats M, Milton C, Khan NR. Surgical techniques and indications for treatment of adult moyamoya disease. Frontiers in surgery. 2022:9():966430. doi: 10.3389/fsurg.2022.966430. Epub 2022 Aug 19 [PubMed PMID: 36061058]
Scott RM, Smith ER. Moyamoya disease and moyamoya syndrome. The New England journal of medicine. 2009 Mar 19:360(12):1226-37. doi: 10.1056/NEJMra0804622. Epub [PubMed PMID: 19297575]
Sun LR, Jordan LC, Smith ER, Aldana PR, Kirschen MP, Guilliams K, Gupta N, Steinberg GK, Fox C, Harrar DB, Lee S, Chung MG, Dirks P, Dlamini N, Maher CO, Lehman LL, Hong SJ, Strahle JM, Pineda JA, Beslow LA, Rasmussen L, Mailo J, Piatt J, Lang SS, Adelson PD, Dewan MC, Mineyko A, McClugage S, Vadivelu S, Dowling MM, Hersh DS. Pediatric Moyamoya Revascularization Perioperative Care: A Modified Delphi Study. Neurocritical care. 2024 Apr:40(2):587-602. doi: 10.1007/s12028-023-01788-0. Epub 2023 Jul 20 [PubMed PMID: 37470933]
Gardner Yelton SE, Williams MA, Young M, Fields J, Pearl MS, Casella JF, Lawrence CE, Felling RJ, Jackson EM, Robertson C, Scafidi S, Lee JK, Cohen AR, Sun LR. Perioperative Management of Pediatric Patients with Moyamoya Arteriopathy. Journal of pediatric intensive care. 2023 Sep:12(3):159-166. doi: 10.1055/s-0041-1731667. Epub 2021 Jul 1 [PubMed PMID: 37565017]
Level 3 (low-level) evidenceJudge J, Kappel AD, Isibor C, O'Hara JE, Larson A, Kleinman M, See AP, Lehman LL, Smith ER. Prevention of postoperative stroke in pediatric moyamoya patients: a standardized perioperative care protocol. Journal of neurosurgery. Pediatrics. 2024 Feb 1:33(2):185-189. doi: 10.3171/2023.9.PEDS23313. Epub 2023 Nov 17 [PubMed PMID: 37976515]
Gatti JR, Ahmad SA, Gardner Yelton S, DiGiusto M, Leung D, Xu R, Cohen AR, Gottesman RF, Sun LR. Relative anemia and perioperative stroke in children with moyamoya. Journal of stroke and cerebrovascular diseases : the official journal of National Stroke Association. 2024 Jan:33(1):107476. doi: 10.1016/j.jstrokecerebrovasdis.2023.107476. Epub 2023 Nov 15 [PubMed PMID: 37976795]
Huguenard AL, Guerriero RM, Tomko SR, Limbrick DD, Zipfel GJ, Guilliams KP, Strahle JM. Immediate Postoperative Electroencephalography Monitoring in Pediatric Moyamoya Disease and Syndrome. Pediatric neurology. 2021 May:118():40-45. doi: 10.1016/j.pediatrneurol.2021.02.004. Epub 2021 Feb 15 [PubMed PMID: 33773289]
Hirano Y, Miyawaki S, Imai H, Hongo H, Teranishi Y, Dofuku S, Ishigami D, Ohara K, Koizumi S, Ono H, Nakatomi H, Saito N. Differences in Clinical Features among Different Onset Patterns in Moyamoya Disease. Journal of clinical medicine. 2021 Jun 25:10(13):. doi: 10.3390/jcm10132815. Epub 2021 Jun 25 [PubMed PMID: 34202349]
Lim YC, Lee E, Song J. Outcomes of Bypass Surgery in Adult Moyamoya Disease by Onset Type. JAMA network open. 2024 Jun 3:7(6):e2415102. doi: 10.1001/jamanetworkopen.2024.15102. Epub 2024 Jun 3 [PubMed PMID: 38842810]
Shahbandi A, Sattari SA, Azad TD, Xia Y, Lehner K, Yang W, Feghali J, Reynolds RA, Akbari SHA, Groves ML, Xu R, Caplan JM, Bettegowda C, Cohen AR, Huang J, Tamargo RJ, Gonzalez LF. The Management of Symptomatic Moyamoya Disease in Pediatric Patients: A Systematic Review and Meta-Analysis. Neurosurgery. 2024 Nov 14:97(1):65-81. doi: 10.1227/neu.0000000000003277. Epub 2024 Nov 14 [PubMed PMID: 39560368]
Level 1 (high-level) evidenceLee KS, Zhang JJY, Bhate S, Ganesan V, Thompson D, James G, Silva AHD. Surgical revascularizations for pediatric moyamoya: a systematic review, meta-analysis, and meta-regression analysis. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2023 May:39(5):1225-1243. doi: 10.1007/s00381-023-05868-6. Epub 2023 Feb 8 [PubMed PMID: 36752913]
Level 1 (high-level) evidenceWang XP, Zou ZX, Bao XY, Wang QN, Ren B, Yu D, Zhang Q, Liu JQ, Hao FB, Gao G, Guo QB, Fu HG, Li JJ, Wang MJ, Liu SM, Duan L. Clinical and genetic factors associated with contralateral progression in unilateral moyamoya disease: Longitudinal and Cross-Sectional Study. Heliyon. 2024 Feb 29:10(4):e26108. doi: 10.1016/j.heliyon.2024.e26108. Epub 2024 Feb 18 [PubMed PMID: 38404780]
Level 2 (mid-level) evidenceYeon JY, Shin HJ, Kong DS, Seol HJ, Kim JS, Hong SC, Park K. The prediction of contralateral progression in children and adolescents with unilateral moyamoya disease. Stroke. 2011 Oct:42(10):2973-6. doi: 10.1161/STROKEAHA.111.622522. Epub 2011 Aug 11 [PubMed PMID: 21836096]
Park EK, Lee YH, Shim KW, Choi JU, Kim DS. Natural history and progression factors of unilateral moyamoya disease in pediatric patients. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2011 Aug:27(8):1281-7. doi: 10.1007/s00381-011-1469-y. Epub 2011 May 7 [PubMed PMID: 21552998]