Introduction
Gangrene is a condition characterized by tissue necrosis resulting from ischemia or infection. The condition is commonly classified into dry, wet, and gas gangrene. Dry gangrene typically results from progressive ischemia and is most pronounced in the digits, often causing circumferential necrosis. The affected tissue becomes dry and may undergo autoamputation. Wet gangrene refers to necrotic tissue complicated by secondary infection and is typically seen in the lower extremities. The condition presents with edema, erythema, and purulent drainage. Gas gangrene is a rapidly progressive, life-threatening necrotizing infection caused by gas-forming bacteria, most commonly Clostridium species.
Clinical features include edema, crepitance, and dishwater drainage involving the subcutaneous, fascial, and muscle compartments. Gas may be visible on radiographic imaging. Gas gangrene often arises in the setting of trauma, malignancy, intra-abdominal pathology, or in individuals with peripheral vascular disease, kidney disease, or diabetes.[1][2]
Gangrene can significantly impair the quality of life of patients due to pain, reduced mobility, and frequent hospitalizations. Amputations are common, with men affected more often than women. The resulting tissue loss can severely impact functional capacity. Morbidity and mortality are substantial, often involving multiple surgical interventions and an increased risk of death as the disease progresses.[3] Gas gangrene carries a high mortality rate, particularly in patients with leukocytosis, hyperglycemia, or sepsis.[4][5]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Dry gangrene is most commonly secondary to atherosclerosis and progressive occlusion of arterial perfusion to distal tissue, marking an end stage of peripheral vascular disease. The risk is higher in individuals with diabetes, hypertension, hyperlipidemia, and those who smoke. Arterial thrombosis may develop in situ in hypercoagulable states. Rupture of atherosclerotic plaques can lead to thrombosis and occlusion, while embolic events may result in distal occlusion. Conditions that increase local perfusion demands, such as focal infection, unsuccessful vascular surgery, intravenous drug use, and trauma, can exacerbate limb ischemia.[6] Additionally, vasculitis, adventitial cystic disease, popliteal artery entrapment, and Buerger disease can cause acute ischemia and contribute to the development of gangrene.[6][7][8]
Diabetes can lead to microvascular dysfunction, predisposing individuals to ischemia even when peripheral circulation appears grossly intact. Diabetic neuropathy may reduce awareness of developing gangrene.[9] Venous limb gangrene occurs when microthrombosis develops in the same limb as an acute large-vein thrombosis, often in the setting of a hypercoagulable state. Less common causes of dry gangrene include chronic myeloid leukemia (CML) and other hematological malignancies, sickle cell anemia, and autoimmune disorders. In addition, there is also a reported case of thrombocytosis leading to gangrene of a digit.[10][11]
Lymphoplasmacytic lymphoma, a type of B-cell lymphoma, can lead to vascular occlusion and dry gangrene when associated with type I cryoglobulinemia, which is caused by circulating immunoglobulin M (IgM).[12] Multiple limbs may also develop simultaneous gangrene despite adequate perfusion, as seen in purpura fulminans associated with septicemia secondary to Neisseria meningitidis.[8]
Dry gangrene is often aseptic, as bacteria do not thrive in mummified tissue.[13] Wet gangrene develops when tissue with impaired venous or arterial blood flow becomes infected. This condition most commonly affects areas prone to edema, such as the lower extremities, but can also occur in genitourinary and oral tissues. Patients with diabetes are more susceptible to wet gangrene due to hyperglycemia, which impairs wound healing and exacerbates inflammation.[13]
Gas gangrene is often caused by infection with Clostridium perfringens and other Clostridium species, resulting in myonecrosis. Clostridium is a genus of anaerobic, spore-forming bacterium that produces potent exotoxins. These toxins infect deeper muscle layers and spread rapidly, causing significant mortality. Exotoxins can cause rapid tissue necrosis and sepsis. This rapid progression contributes to the high mortality associated with gas gangrene.
Gas gangrene commonly occurs in the setting of trauma (historically associated with battlefield injuries), malignancy, retained placenta, intrauterine fetal death, intramuscular injections, and surgeries involving the bowel or biliary tract. C perfringens is often associated with trauma, whereas C septicum can be spontaneous or associated with gastrointestinal portals of entry. C novyi, C histolyticum, and C sordelli are associated with gynecological origins. Additionally, Vibrio vulnificus, commonly found in warm coastal waters, can cause infection through open wounds or ingestion of contaminated seafood.[14]
Other gas-producing bacteria that can cause both local and systemic infections include Escherichia coli, Bacteroides, Staphylococcus epidermidis, and Streptococcus species.[15] Type I necrotizing fasciitis, characterized by friable superficial fascia, dishwater-gray exudate, and absence of pus, is a polymicrobial bacterial infection involving both aerobic and anaerobic organisms that can also produce gas within tissues.[16]
Epidemiology
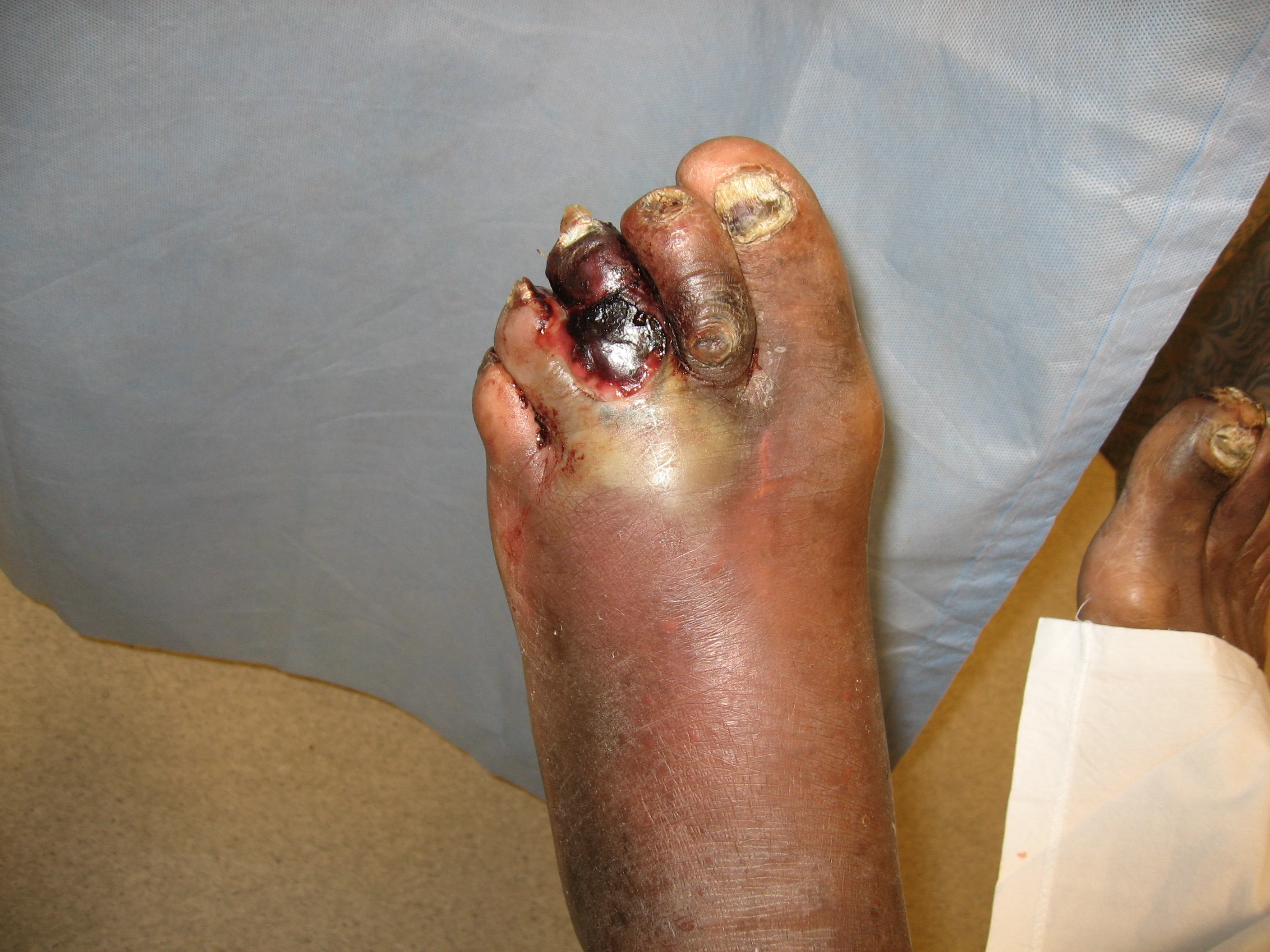
The annual incidence of gangrene due to peripheral vascular disease—most often from arterial insufficiency—is approximately 16.7%, and the risk increases with age and comorbidities such as diabetes, hypertension, and in individuals who smoke.[9] In the United States, the incidence of critical limb ischemia is 1% observed in individuals aged 50 or older, with a higher prevalence observed among those aged 70 or older.[6] Lower extremity peripheral artery disease affects more than 200 million people worldwide, and 10% of these patients develop critical limb ischemia. Over 5 years, 5% to 10% of individuals with asymptomatic peripheral arterial disease or intermittent claudication progress to critical ischemia (see Image. Peripheral and Arterial Disease With Gangrene of the Third Digit).[7]
In the United States, approximately 1000 cases of clostridial gas gangrene are reported every year, with half resulting from trauma, 30% following surgery, and 20% arising spontaneously.[14] The average age of affected individuals is around 70, often with comorbidities such as diabetes and peripheral vascular disease. Infections commonly arise in the context of trauma, contaminated surgery, intravenous drug use, animal or insect bites, enterocutaneous fistulae, abscesses, urinary tract infections, immunodeficiency, streptococcal infections of the throat, and anticoagulant use.
Nontraumatic gas gangrene has also been documented as a result of hematogenous spread, and multiple case studies have demonstrated an association with a metastatic gastrointestinal malignancy.[16][17] Infections caused by a single microbe, such as Staphylococcus or Streptococcus, are more common in younger patients, whereas polymicrobial infections typically affect individuals aged 60 or older.[18]
Pathophysiology
Gangrene can result from a range of mechanisms, including ischemia, tumor antigen–antibody complexes, hyperviscosity, immune dysregulation, circulating procoagulant factors, and damage to skin integrity. Increased blood viscosity may result from thromboxane–mediated platelet aggregation and platelet sludge.[10] In conditions such as lymphoplasmacytic lymphoma, intravascular deposits of IgM cryoglobulins contribute to the formation of gangrene. Additionally, the anaerobic environment created by penetrating injuries facilitates the proliferation of anaerobic bacteria.[12]
Atherosclerotic plaque, in combination with chronic hyperglycemia, leads to endothelial dysfunction and exacerbates atherosclerosis, thereby compromising the immune system and increasing susceptibility to infection. Reduced perfusion leads to compensatory arteriolar dilation, resulting in distal edema and endothelial damage. This cascade can initiate microthrombosis, further impairing tissue perfusion and exacerbating ischemia. Due to the ischemic environment, localized cellular dysregulation limits adequate wound healing, perpetuating tissue damage and increasing the risk of infection. The chemicals present in tobacco products directly harm blood vessels by causing vasoconstriction, plaque formation, and inflammation. Hypertension increases the risk of thromboembolism, and excess cholesterol contributes to plaque deposition, vessel narrowing, and blood hyperviscosity.[7][9][10]
Devitalized tissue is susceptible to microbial infiltration, including fungal pathogens, and is often accompanied by a weakened immune response, which increases the risk of abscess formation and sepsis. Defects in small bowel mucosa, caused by malignancy, radiation, chemotherapy, surgery (common pathway or entry points for C septicum), and deep wounds (typically associated with C perfringens), create portals for microbial invasion. Bacteria thrive in acidic, ischemic environments, releasing gases such as nitrogen, hydrogen, hydrogen sulfide, oxygen, and carbon dioxide, which can spread rapidly along muscle fiber planes. Bacteria such as C perfringens and group A Streptococcus can produce multiple toxins that lead to local tissue destruction and subsequent systemic infection, significantly increasing mortality. M proteins from group A beta-hemolytic Streptococcus promote bacterial adherence in tissue and evade immune mediators. They can also activate T cells, which can trigger a toxic inflammatory response.[14]
The alpha toxin of Clostridium septicum, its main toxin, is secreted in an inactive form that binds to the host cell membrane, forms a pore, and causes cell lysis. The alpha toxin produced by C perfringens is a zinc-dependent metallophospholipase with phospholipase C and sphingomyelinase activity. This causes muscle necrosis and hemolysis, increases vascular permeability, and impairs myocardial function, leading to bradycardia and hypotension. Variants of alpha toxins, including beta-, gamma-, and delta toxins, cause extensive tissue necrosis and contribute to systemic hemolysis and gangrene.[17] In addition, endotoxins promote the aggregations of platelets, leukocytes, and endothelial cells, which leads to thrombosis. Endotoxins also prevent leukocyte migration into infected and hypoxic tissues, as well as impair the activity of neutrophils.[18]
History and Physical
Dry gangrene typically presents with significant pain, described as throbbing or burning, along with claudication, rest pain, pallor, cool skin temperature, paresthesias, neuropathy, and ulceration.[12] Patients often report progressive limb pain that worsens with elevation and improves when the limb is in a dependent position. In individuals with underlying neuropathy, tissue loss may be the first indication of ischemia.[6] Depending on the underlying syndrome, additional symptoms leading to dry gangrene include dizziness, altered mental status, headache, and priapism.[10]
On physical examination, findings may range from livedo reticularis to skin necrosis. Patients with ischemic gangrene may exhibit dependent rubor in the affected extremity when the limb is lowered and pallor upon elevation. Capillary refill is often delayed, overlying hair may be lost, and distal pulses are frequently non-palpable. Arterial ischemia often causes ulcerations on the toes and distal foot, while venous ulcers usually develop over the malleoli. Neuropathic ulcers are commonly found on pressure-bearing areas.[6][7] A comprehensive physical examination should assess sensory loss and evaluate the depth of any ulcerations.[19] Severe acute limb ischemia presents with intense lower extremity pain, sensory loss—ranging from mild at the toes to permanent, diffuse nerve damage—and paralysis of the affected limb. Arterial flow is initially inaudible, with venous flow also becoming undetectable as the condition progresses.[8]
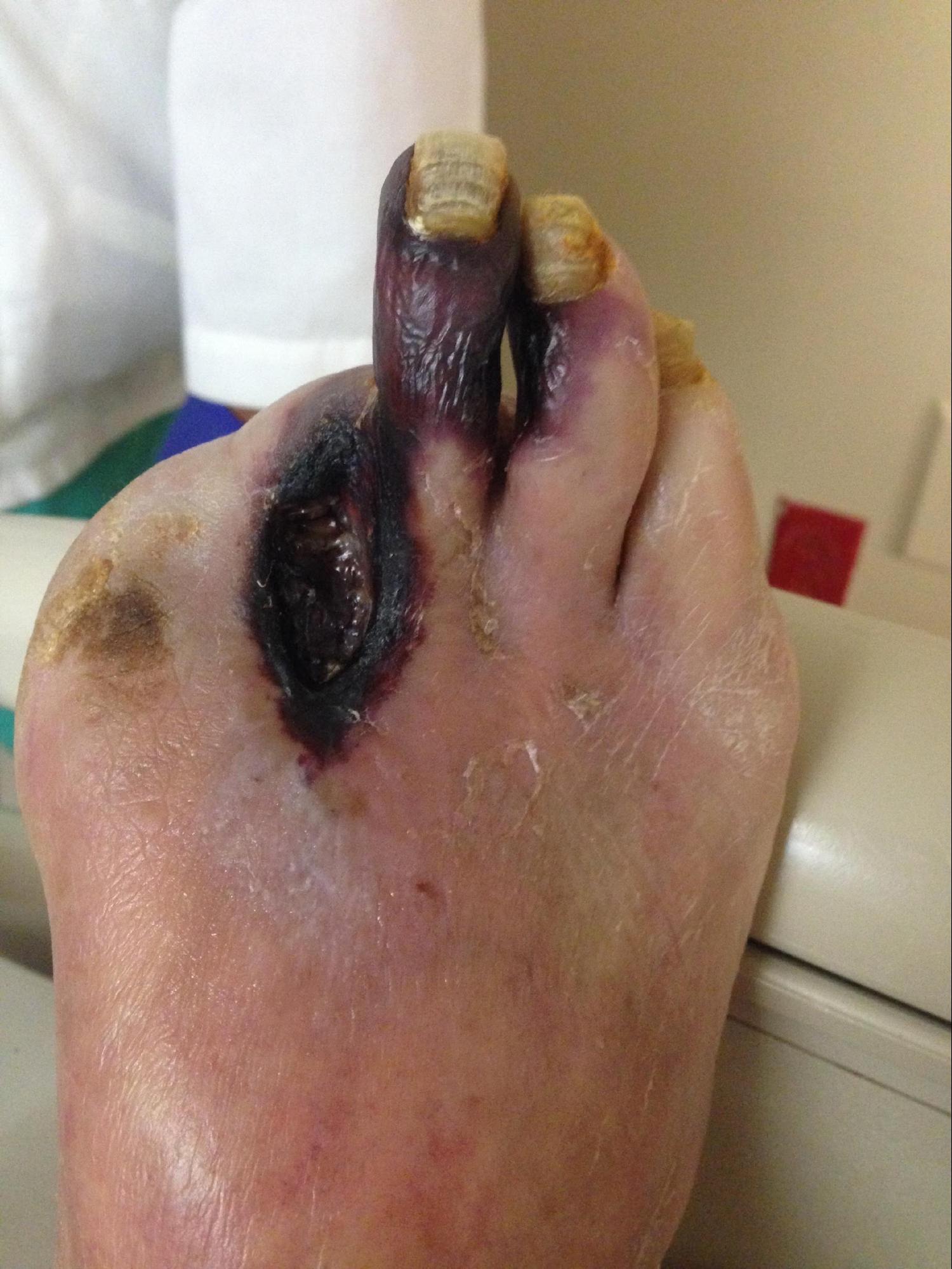
Wet gangrene presents with drainage and edema in the setting of a diabetic or ischemic ulcer or a comparable wound. The area may initially have been cellulitis or a contained purulent area that progressively becomes discolored, potentially accompanied by bullae, ecchymosis, crepitus, and paresthesia. Systemic signs of infection may also be present. The area is typically painful, and in some cases, focal tenderness over intact skin may indicate a deeper abscess. Any areas of concern should be thoroughly evaluated using appropriate laboratory testing and imaging (see Image. Gas Gangrene of the Right Leg and Pelvis).[3]
Gas gangrene has a variable presentation, ranging from marked skin changes and discomfort to nonspecific malaise or fulminant sepsis. Patients often have a recent history of trauma or surgery, though nonspecific early findings can delay diagnosis. A typical presentation may include severe pain disproportionate to physical findings, often extending beyond visible skin changes. Additional signs may include edema, erythema, hemorrhagic bullae, ecchymosis, necrosis, subcutaneous emphysema, and skin discoloration ranging from pale to purplish. As the condition progresses, findings may include crepitus, worsening erythema and edema, and paresthesias.[13] An erythematous lesion can progress to dusky skin with overlying hemorrhagic bullae within 24 to 72 hours of injury. Maintaining a high clinical suspicion is crucial to reduce mortality, which rises significantly once skin changes become more pronounced. Once soft tissue deterioration occurs, sepsis and substantial morbidity and mortality often follow.[16]
Evaluation
Multiple classification systems are available for staging critical or chronic limb ischemia. The Society for Vascular Surgery classification incorporates wound characteristics, the degree of ischemia, and the presence of foot infection to provide prognostic guidance and inform treatment decisions, including the anticipated success of revascularization.[6] Wounds are graded on a scale from 0 to 3.
- Grade 1 indicates minor tissue loss that may be managed with local debridement or focal amputation.
- Grade 2 refers to gangrene limited to the digits, typically treated with multiple digital amputations or a transmetatarsal amputation.
- Grade 3 denotes extensive tissue loss requiring a more proximal amputation.
Patients are stratified based on their risk of amputation and the expected benefit of revascularization.[20]
Several additional staging systems are used to classify peripheral vascular disease. The Rutherford classification ranges from 0 to 6 and categorizes clinical presentations from intermittent claudication to ulceration and gangrene. The Fontaine classification uses a scale from 1 to 4 based on symptoms and functional impairment, where stage 1 represents early disease with minor symptoms and stage 4 indicates advanced disease, including gangrene. For diabetic ulcers, the Wagner ulcer classification is commonly used, ranging from 0 to 5, where 0 denotes no ulceration, and 5 represents extensive gangrene.[9]
The ankle-brachial index (ABI) is a key tool for the early identification of arterial insufficiency, and it is considered abnormal if it is less than 1.0. An ankle blood pressure reading below 40 to 60 mm Hg indicates ischemia, and a value below 70 mm Hg in ischemic tissue is also abnormal.[20] In cases where the ABI is inconclusive—particularly in individuals with diabetes or older adults due to vessel calcification and reduced compressibility—additional noninvasive assessments may include ankle pressures, toe pressures, and transcutaneous oxygen measurements.[7] Imaging modalities such as Doppler ultrasound, digital subtraction angiography, computed tomography (CT) angiography, and magnetic resonance angiography can help localize areas of ischemia.[6]
Laboratory tests, imaging, and clinical assessment are central to the evaluation of gangrene. When gas or wet gangrene is suspected, Gram stain and wound cultures can help identify the causative bacteria to guide antibiotic therapy, although diagnosis is primarily clinical. Cultures should be obtained through deep swabbing or aspiration of purulent discharge, as superficial swabs are often contaminated by skin flora.[21] Laboratory workup focuses on infection markers such as C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR), as well as blood and wound cultures. Additional clinical indicators of disease include renal failure, hyperlipidemia, and diabetes. Elevated CRP levels correlate with gas gangrene, while increased interleukin-6, procalcitonin, and low hematocrit are associated with higher mortality, especially in cases with truncal involvement (see Image. Gas Gangrene in a Diabetic Foot).[6][19]
Radiographic evaluation is crucial in diagnosing gangrene and associated complications. Plain x-rays may show subcutaneous gas, whereas CT can demonstrate fascial thickening or intramuscular fluid collections. Magnetic resonance imaging (MRI) offers high-resolution images of soft tissues.[13][16][21] CT with contrast may demonstrate a lack of fascial enhancement, and MRI may reveal abnormal signal intensity in the deep fascia. If the diagnosis remains unclear, patients can be evaluated through local exploration under bedside anesthesia. The presence of "dishwater" fluid and rapid auto-dissection of fascial planes is characteristic of necrotizing infections.[22]
Additional testing is warranted depending on the clinical scenario. An echocardiogram may be used to assess the right ventricular function and detect pulmonary hypertension or the presence of a thrombus. CT angiography helps evaluate tissue perfusion. A bone biopsy, when osteomyelitis is suspected, or a bone marrow biopsy, to evaluate for a suspected myeloproliferative disorder, aids in diagnosis. Hypercoagulability workup is indicated for thrombosis of unclear etiology. Further laboratory tests may include serum protein electrophoresis, beta-2 microglobulin, serum viscosity, HIV and hepatitis screening, as well as autoantibodies such as myelin-associated glycoprotein and ganglioside antibodies. Cytogenetic studies, including reverse transcriptase polymerase chain reaction (RT-PCR), can be used to detect genetic abnormalities, such as the BCR-ABL fusion gene, associated with CML.[10]
Treatment / Management
Treatment of gangrene ranges from debridement and advanced wound care to amputation and revascularization. Management of ischemic gangrene primarily aims to restore blood flow, relieve rest pain, and promote healing of ischemic wounds. In cases of acute ischemia, catheter-based intravascular thrombolysis may be used. Revascularization can be achieved through endovascular techniques such as balloon angioplasty (with or without stent placement) or surgical bypass, depending on the location of the lesion and the patient’s comorbidities.[6][7] Once ulcers progress to dry gangrene, full tissue recovery is unlikely, but medical and surgical interventions can help minimize tissue loss.[6][13] (A1)
Amputation may be required, which can range from a ray amputation of a digit or a guillotine amputation for demarcated necrosis to more extensive procedures, such as below-the-knee or above-the-knee amputation with flap closure or reconstruction, including muscle flap transfers when indicated.[6][13] Amputation before an attempt at revascularization is recommended when there is significant necrosis of the weight-bearing portion of the foot, refractory pain, uncontrolled infection or sepsis, paresis of the extremity, or limited life expectancy.[6][19] Often, above-ankle amputation is recommended if there is extensive foot necrosis.[7][3] Autoamputation—the spontaneous detachment of unviable tissue from viable tissue—may occur in approximately 1 of 12 cases.[2] If more than 2 digital ray amputations are recommended to treat the distribution of gangrenous tissue, performing a transmetatarsal amputation of the forefoot is generally more functionally advantageous, as multiple toe amputations can negatively alter pressure distribution and increase the risk of pressure injuries.[19](A1)
Gas gangrene can progress rapidly and carries a high mortality rate if treatment is delayed, largely due to the systemic effects of exotoxins. Management includes prompt surgical debridement or amputation, broad-spectrum antibiotics, and, in some cases, intravenous immunoglobulin to bind and neutralize toxins, as well as optimization of comorbid conditions. Patients are treated following sepsis protocols, including fluid resuscitation and support for organ dysfunction. Early surgical exploration and debridement are critical for source control and effective therapy, often requiring multiple re-evaluations and repeat debridements. Surgery within 24 hours of admission significantly improves survival.[16][22] Gangrene involving the trunk demands particularly aggressive surgical debridement. Intravenous immunoglobulin may also be beneficial in binding and neutralizing toxins produced by Staphylococcus and Streptococcus species.[18](A1)
Medical treatment of ischemic gangrene includes the use of antiplatelet therapy with aspirin or clopidogrel and management of hypertension with beta-blockers and angiotensin-converting enzyme inhibitors. Hyperlipidemia is treated with statins as appropriate, and patients with diabetes are treated to a target hemoglobin A1c (HbA1c) of less than 7%. Smoking cessation is vital for reducing the risk of disease progression.[6][7][13][19] Restoration of blood flow may involve angioplasty or surgical bypass, whereas amputation is performed for unsalvageable tissues.[6][13] (A1)
Dry gangrene resulting from myeloproliferative disorders is treated with hydroxyurea and subcutaneous heparin. Imatinib, a tyrosine kinase inhibitor, is used in cases of CML. Circulating immune complexes may be addressed with plasma exchange, bendamustine, rituximab, and prednisone. Leukapheresis is indicated for leukemia-induced thrombocytosis. Cytokine and adhesion molecule inhibitors may also offer benefit. Pressor support is used as needed in the context of sepsis.[10]
Antibiotic treatment for gas gangrene and necrotizing soft tissue infections should be tailored to the identified causative organism as soon as possible. Common regimens include ampicillin-sulbactam or imipenem, combined with metronidazole and clindamycin, or vancomycin with piperacillin-tazobactam, or a carbapenem, for clostridial infections. Initially, broad-spectrum coverage targeting both gram-positive and gram-negative bacteria, as well as anaerobic bacteria, is essential.[3][19] (A1)
If group A Streptococcus or Clostridium species are identified, the recommended treatment is penicillin combined with clindamycin for 10 to 14 days. Clindamycin helps inhibit the production of clostridial toxins and is especially important for reducing toxin-mediated damage. However, clindamycin monotherapy is not recommended due to the increased risk of inducible resistance. Empiric antibiotic selection should be based on patient risk factors and local susceptibility patterns, ensuring broadened coverage for gram-positive bacteria and expanded coverage for diabetic patients to include MRSA and other gram-negative pathogens.[16][21]
Treatment with hyperbaric oxygen therapy has been proposed to increase oxygen tension in ischemic tissue; however, no proven benefit has been demonstrated for critical limb ischemia. While it is postulated that hyperbaric oxygen may reduce mortality, definitive evidence is lacking. Other potential experimental therapies include growth factors and stem cell treatments aimed at promoting angiogenesis; however, clinical data are limited, and these treatments are currently restricted to clinical trials.[7] Intravenous immunoglobulin may help neutralize exotoxins, but its efficacy has not been established in randomized controlled trials.[14](A1)
Differential Diagnosis
Necrotizing fasciitis—often caused by group A Streptococcus—primarily affects the subcutaneous tissue and fascia, sparing the muscle. Other conditions that may present with similar features to gangrene include toxic shock syndrome, intra-abdominal abscesses, and infections caused by pathogens such as V vulnificus.[14]
Limb pain must be carefully distinguished from ischemia and other conditions that can lead to gangrene. Some causes of limb pain include diabetic neuropathy, complex regional pain syndrome, and nerve root compression. Underlying disorders that contribute to ischemia include Buerger disease, paraneoplastic syndrome, calciphylaxis, frostbite, and ergotism.[23][24]
Pertinent Studies and Ongoing Trials
A case of invasive group A streptococcal infection in an infant led to disseminated intravascular coagulation, myositis, and dry gangrene, ultimately requiring amputation.[25] In a study examining the relationship between physical findings of dry gangrene and Doppler ultrasound results, gangrene involving the proximal foot, leg, and toes was associated with ischemia in the femoral artery distribution. In contrast, gangrene limited to the toes and distal foot correlated with popliteal artery occlusion. Ischemic gangrene may also occur as a complication of prolonged vasopressor use and can necessitate amputation, followed by reconstructive efforts aimed at preserving maximum sensory and functional capacity.[26]
Diabetic wet gangrene of the foot frequently recurs, with ray amputations carrying a higher risk of subsequent amputations compared to isolated toe amputations. In cases of gas gangrene of the lower extremities, factors such as sepsis at presentation, leukocytosis, and hyperglycemia are significant predictors of mortality.[4]
Prognosis
Within 1 year of being diagnosed with critical limb ischemia or chronic limb-threatening ischemia, up to 50% of patients with diabetes will undergo amputation, and 20% to 25% will die.[6] Additional observational studies in nondiabetic patients with rest pain and ischemic ulcers or gangrene report amputation rates of 19% at 6 months and 23% at 12 months. The most common indication for amputation is an infection that cannot be treated.[20][27] Patients are typically monitored for at least 2 years following revascularization to assess for recurrence.[19]
Gas gangrene often manifests as early sepsis and has a high mortality rate. Up to 25% of trauma patients with gas gangrene may die, with mortality approaching 100% if diagnosis and treatment are delayed. Death can occur within 48 hours after admission if treatment is insufficient. Poor prognostic factors include age, comorbidities, and a truncal location.[18] Immunosuppression further increases the risk of morbidity and mortality.[14]
Complications
Sequelae of gangrene include prolonged hospitalization, amputations, and additional procedures that lead to tissue loss and functional impairment. Limb salvage is attempted in select cases; however, anatomically favorable amputations can facilitate the use of prostheses. Retrospective studies show that 65% of patients with below-the-knee amputations and 29% with above-the-knee amputations remain ambulatory at 1 year.[28] Observational studies following patients 2 years after below-the-knee amputation report that 15% underwent a contralateral amputation, 15% progressed to above-the-knee amputation, and 30% had died.[24]
Consultations
All patients with suspected ischemia should be referred to a vascular specialist for evaluation and possible revascularization. All newly diagnosed and chronic comorbidities require treatment by specialists in relevant fields, such as endocrinologists, cardiologists, and rheumatologists.[19]
For acute cases of gangrene requiring hospitalization, monitoring, and invasive intervention, care often involves a multidisciplinary team that includes vascular surgeons, general surgeons, plastic surgeons, wound care specialists, infectious disease experts, pain management specialists, physical therapists, occupational therapists, prosthetics specialists, and intensivists. Gangrenous soft tissue infections that require debridement or amputation benefit from collaborative surgical input, particularly from orthopedic and plastic surgeons. Urologists or colorectal surgeons may be required, depending on the location and extent of the infection, as well as the type of surgical intervention.[22]
Deterrence and Patient Education
Patients who have been treated for, or are at risk of, gangrene should receive education on proper foot and wound care to promote healing and prevent recurrence. Teaching early recognition of ischemic changes or signs of inflammation is essential to prevent unnoticed progression to gangrene.[19] Individuals experiencing tenderness, erythema, disproportionate pain, or fever should seek prompt medical evaluation and treatment. Patients with comorbidities or risk factors for gangrene require education about the signs, symptoms, and potential fatal complications associated with the condition.[29]
Pearls and Other Issues
The premature application of vacuum dressings to gangrenous tissue can exacerbate anaerobic infections. Achieving adequate antibiotic concentrations within infected tissue can be challenging in gangrenous infections. Strategies to enhance local antibiotic delivery include using vitamin D granules infused with antibiotics and applying fibrin spray to localize the antibiotics within the affected area.
Different pathogens are associated with distinct clinical patterns—C septicum is associated with malignancy, whereas C perfringens usually requires a deep wound to cause infection. Immunosuppression generally leads to more devastating infections.[14] Digital ischemia may coincide with or precede a cancer diagnosis or indicate recurrence, seen most frequently with hematological malignancies and adenocarcinoma. Thrombocytosis is a notable risk factor for gangrene of the digits.[10]
Enhancing Healthcare Team Outcomes
Early identification of ischemic tissue, whether caused by infection or peripheral vascular disease, is essential for optimizing clinical outcomes. A detailed history and physical examination, supported by documented results of noninvasive testing and relevant imaging, can help identify individuals at risk for peripheral artery disease before tissue loss occurs.[24] Once ischemia is detected, validated classification systems facilitate standardized communication within interdisciplinary teams regarding disease staging and treatment planning.[20]
Caring for patients with gangrene requires coordinated resource management and a multidisciplinary approach involving skilled nursing, wound care specialists, and surgeons specializing in general, vascular, and plastic surgery.[9] Delays in diagnosis and treatment, especially those exceeding 24 hours after admission, significantly increase mortality. Timely collaboration and intervention are essential to improving patient outcomes.[12][18]
Media
(Click Image to Enlarge)
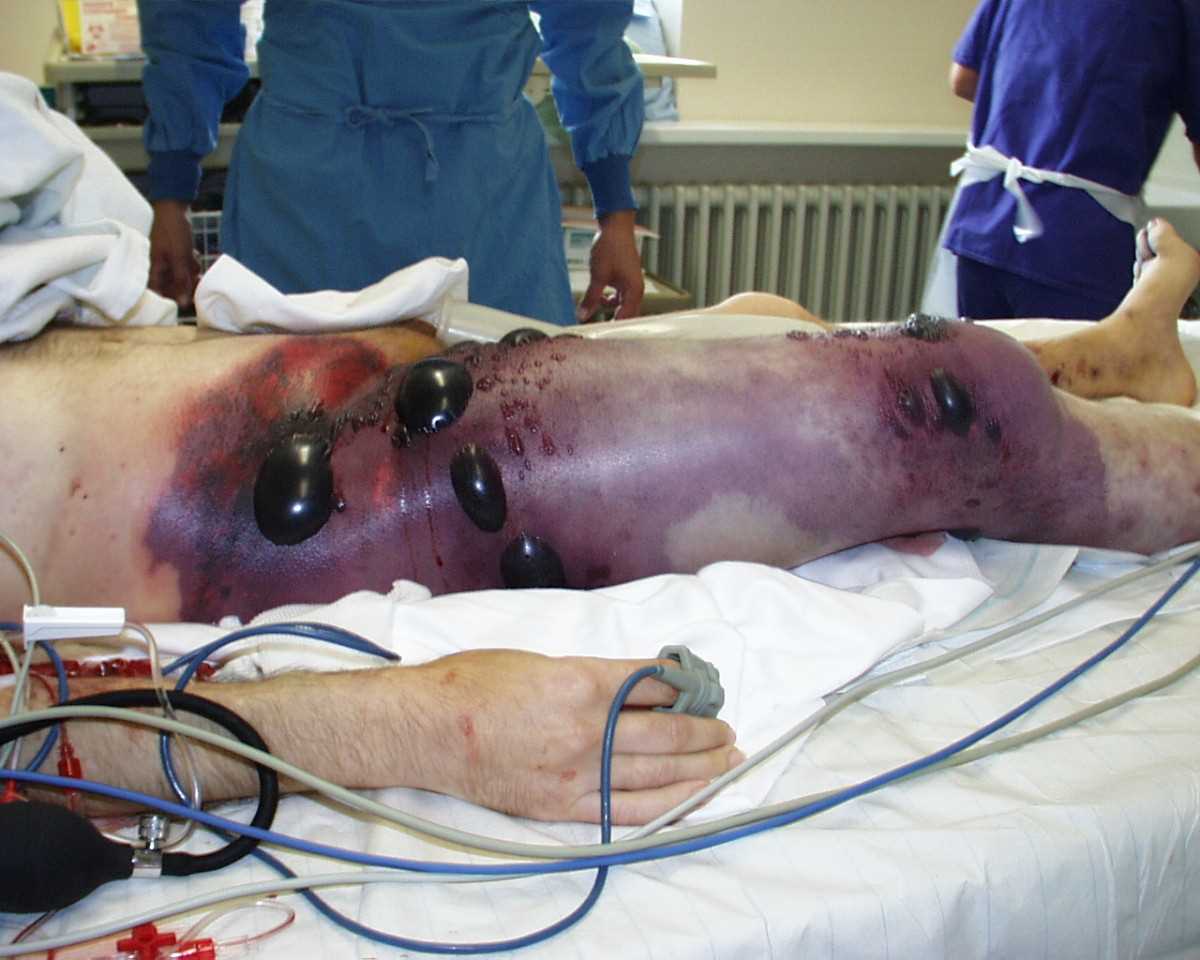
Gas Gangrene of the Right Leg and Pelvis. Gas gangrene involving the right thigh and pelvis, characterized by swelling, skin discoloration, bullae formation, and palpable crepitus. The patient was in shock at the time of the photograph and underwent hemipelvectomy but succumbed less than 8 hours later.
Engelbert Schröpfer, Stephan Rauthe, and Thomas Meyer, Public Domain, via Wikimedia Commons
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Bahebeck J, Sobgui E, Loic F, Nonga BN, Mbanya JC, Sosso M. Limb-threatening and life-threatening diabetic extremities: clinical patterns and outcomes in 56 patients. The Journal of foot and ankle surgery : official publication of the American College of Foot and Ankle Surgeons. 2010 Jan-Feb:49(1):43-6. doi: 10.1053/j.jfas.2009.08.011. Epub [PubMed PMID: 20123286]
Al Wahbi A. Operative versus non-operative treatment in diabetic dry toe gangrene. Diabetes & metabolic syndrome. 2019 Mar-Apr:13(2):959-963. doi: 10.1016/j.dsx.2018.12.021. Epub 2018 Dec 27 [PubMed PMID: 31336551]
Farber A. Chronic Limb-Threatening Ischemia. The New England journal of medicine. 2018 Jul 12:379(2):171-180. doi: 10.1056/NEJMcp1709326. Epub [PubMed PMID: 29996085]
Mahjoubi MF, Rezgui B, Maatouk M, Essid N, Karoui Y, Kandara H, Moussa MB. Tracking a Fatal Disease: Lower Limb Gas Gangrene's Mortality-Related Factors. The international journal of lower extremity wounds. 2025 Mar:24(1):192-197. doi: 10.1177/15347346231158858. Epub 2023 Feb 21 [PubMed PMID: 39996358]
Rusu E, Catrina EL, Brezean I, Georgescu AM, Vișinescu A, Georgescu DAV, Mioara CA, Dobra GM, Verde I, Stanciu S, Coșoreanu A, Rusu F, Nica A, Mihai DA, Radulian G. Lower Extremity Amputations Among Patients with Diabetes Mellitus: A Five-Year Analysis in a Clinical Hospital in Bucharest, Romania. Medicina (Kaunas, Lithuania). 2024 Dec 4:60(12):. doi: 10.3390/medicina60122001. Epub 2024 Dec 4 [PubMed PMID: 39768881]
Elsayed S, Clavijo LC. Critical limb ischemia. Cardiology clinics. 2015 Feb:33(1):37-47. doi: 10.1016/j.ccl.2014.09.008. Epub [PubMed PMID: 25439329]
Level 2 (mid-level) evidenceFarber A, Eberhardt RT. The Current State of Critical Limb Ischemia: A Systematic Review. JAMA surgery. 2016 Nov 1:151(11):1070-1077. doi: 10.1001/jamasurg.2016.2018. Epub [PubMed PMID: 27551978]
Level 1 (high-level) evidenceCreager MA, Kaufman JA, Conte MS. Clinical practice. Acute limb ischemia. The New England journal of medicine. 2012 Jun 7:366(23):2198-206. doi: 10.1056/NEJMcp1006054. Epub [PubMed PMID: 22670905]
Bhargava A, Mahakalkar C, Kshirsagar S. Understanding Gangrene in the Context of Peripheral Vascular Disease: Prevalence, Etiology, and Considerations for Amputation-Level Determination. Cureus. 2023 Nov:15(11):e49026. doi: 10.7759/cureus.49026. Epub 2023 Nov 18 [PubMed PMID: 38116352]
Level 3 (low-level) evidencePatil VL, Chavan DR, Thimmarayappa Y, Mathias LP. Digital Ischemia and Gangrene: An Unusual Presentation of Chronic Myeloid Leukemia. Cureus. 2024 Sep:16(9):e70426. doi: 10.7759/cureus.70426. Epub 2024 Sep 29 [PubMed PMID: 39473658]
Bhangu G, Uminski K, Roessner C, Goodyear D, Sale T, Rydz N. Dry gangrene in a patient with sickle cell disease on hydroxyurea: a case report. Annals of hematology. 2024 Aug:103(8):3277-3279. doi: 10.1007/s00277-024-05732-w. Epub 2024 Apr 17 [PubMed PMID: 38630130]
Level 3 (low-level) evidenceRao AK, Syed F, Garrido D, Holladay CS, Saylors J. A Case of Type 1 Cryoglobulinemia With Lymphoplasmacytic Lymphoma and Dry Gangrene. Cureus. 2024 Jan:16(1):e52659. doi: 10.7759/cureus.52659. Epub 2024 Jan 21 [PubMed PMID: 38380210]
Level 3 (low-level) evidenceAl Wahbi A. Autoamputation of diabetic toe with dry gangrene: a myth or a fact? Diabetes, metabolic syndrome and obesity : targets and therapy. 2018:11():255-264. doi: 10.2147/DMSO.S164199. Epub 2018 Jun 1 [PubMed PMID: 29910628]
Leiblein M, Wagner N, Adam EH, Frank J, Marzi I, Nau C. Clostridial Gas Gangrene - A Rare but Deadly Infection: Case series and Comparison to Other Necrotizing Soft Tissue Infections. Orthopaedic surgery. 2020 Dec:12(6):1733-1747. doi: 10.1111/os.12804. Epub 2020 Oct 4 [PubMed PMID: 33015993]
Level 2 (mid-level) evidenceBrucato MP, Patel K, Mgbako O. Diagnosis of gas gangrene: does a discrepancy exist between the published data and practice. The Journal of foot and ankle surgery : official publication of the American College of Foot and Ankle Surgeons. 2014 Mar-Apr:53(2):137-40. doi: 10.1053/j.jfas.2013.10.009. Epub 2013 Dec 15 [PubMed PMID: 24345706]
Stevens DL, Bryant AE. Necrotizing Soft-Tissue Infections. The New England journal of medicine. 2017 Dec 7:377(23):2253-2265. doi: 10.1056/NEJMra1600673. Epub [PubMed PMID: 29211672]
Lehner PJ, Powell H. Gas gangrene. BMJ (Clinical research ed.). 1991 Jul 27:303(6796):240-2 [PubMed PMID: 1884064]
Level 3 (low-level) evidenceYang Z, Hu J, Qu Y, Sun F, Leng X, Li H, Zhan S. Interventions for treating gas gangrene. The Cochrane database of systematic reviews. 2015 Dec 3:2015(12):CD010577. doi: 10.1002/14651858.CD010577.pub2. Epub 2015 Dec 3 [PubMed PMID: 26631369]
Level 1 (high-level) evidenceConte MS, Bradbury AW, Kolh P, White JV, Dick F, Fitridge R, Mills JL, Ricco JB, Suresh KR, Murad MH, GVG Writing Group. Global vascular guidelines on the management of chronic limb-threatening ischemia. Journal of vascular surgery. 2019 Jun:69(6S):3S-125S.e40. doi: 10.1016/j.jvs.2019.02.016. Epub 2019 May 28 [PubMed PMID: 31159978]
Level 1 (high-level) evidenceMills JL Sr, Conte MS, Armstrong DG, Pomposelli FB, Schanzer A, Sidawy AN, Andros G, Society for Vascular Surgery Lower Extremity Guidelines Committee. The Society for Vascular Surgery Lower Extremity Threatened Limb Classification System: risk stratification based on wound, ischemia, and foot infection (WIfI). Journal of vascular surgery. 2014 Jan:59(1):220-34.e1-2. doi: 10.1016/j.jvs.2013.08.003. Epub 2013 Oct 12 [PubMed PMID: 24126108]
Singer AJ, Tassiopoulos A, Kirsner RS. Evaluation and Management of Lower-Extremity Ulcers. The New England journal of medicine. 2017 Oct 19:377(16):1559-1567. doi: 10.1056/NEJMra1615243. Epub [PubMed PMID: 29045216]
Bonne SL, Kadri SS. Evaluation and Management of Necrotizing Soft Tissue Infections. Infectious disease clinics of North America. 2017 Sep:31(3):497-511. doi: 10.1016/j.idc.2017.05.011. Epub [PubMed PMID: 28779832]
Warkentin TE. Ischemic Limb Gangrene with Pulses. The New England journal of medicine. 2015 Aug 13:373(7):642-55. doi: 10.1056/NEJMra1316259. Epub [PubMed PMID: 26267624]
Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG, TASC II Working Group, Bell K, Caporusso J, Durand-Zaleski I, Komori K, Lammer J, Liapis C, Novo S, Razavi M, Robbs J, Schaper N, Shigematsu H, Sapoval M, White C, White J, Clement D, Creager M, Jaff M, Mohler E 3rd, Rutherford RB, Sheehan P, Sillesen H, Rosenfield K. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). European journal of vascular and endovascular surgery : the official journal of the European Society for Vascular Surgery. 2007:33 Suppl 1():S1-75 [PubMed PMID: 17140820]
Level 3 (low-level) evidenceRoach AN, Kwong R, Sylvester S. Emergence of Invasive Group A Streptococcus Infection in an Infant: A Case Report. Clinical practice and cases in emergency medicine. 2025 Jan:9(1):45-48. doi: 10.5811/cpcem.21172. Epub [PubMed PMID: 39903622]
Level 3 (low-level) evidenceHenn MC, Hathaway BA, Lipira AB. Reconstructive Surgical Management of Vasopressor-Ischemia Related Distal Extremity Loss. Journal of orthopaedic case reports. 2025 Apr:15(4):45-51. doi: 10.13107/jocr.2025.v15.i04.5440. Epub [PubMed PMID: 40212481]
Level 3 (low-level) evidenceSchreuder SM, Hendrix YMGA, Reekers JA, Bipat S. Predictive Parameters for Clinical Outcome in Patients with Critical Limb Ischemia Who Underwent Percutaneous Transluminal Angioplasty (PTA): A Systematic Review. Cardiovascular and interventional radiology. 2018 Jan:41(1):1-20. doi: 10.1007/s00270-017-1796-9. Epub 2017 Sep 18 [PubMed PMID: 28924874]
Level 2 (mid-level) evidenceLandry GJ. Functional outcome of critical limb ischemia. Journal of vascular surgery. 2007 Jun:45 Suppl A():A141-8 [PubMed PMID: 17544035]
Level 2 (mid-level) evidenceDetermann C, Walker CA. Clostridium perfringens gas gangrene at a wrist intravenous line insertion. BMJ case reports. 2013 Oct 9:2013():. doi: 10.1136/bcr-2013-200242. Epub 2013 Oct 9 [PubMed PMID: 24108766]
Level 3 (low-level) evidence